- 1Institute of Microbiology and Epizootics, Centre for Infection Medicine, Department of Veterinary Medicine, Freie Universität Berlin, Berlin, Germany
- 2Institute of Farm Animal Genetics, Friedrich-Loeffler-Institut, Neustadt, Germany
- 3Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 4State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin, China
Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) isolates of the clonal complex 398 are often resistant to a number of antimicrobial agents. Studies on the genetic basis of antimicrobial resistance in these bacteria identified SCCmec cassettes, various transposons and plasmids of different sizes that harbor antimicrobial resistance genes. While large plasmids that carry multiple antimicrobial resistance genes – occasionally together with heavy metal resistance genes and/or virulence genes – are frequently seen in LA-MRSA ST398, certain resistance genes are also associated with small plasmids of up to 15 kb in size. These small resistance plasmids usually carry only one, but in rare cases also two or three antimicrobial resistance genes. In the current review, we focus on small plasmids that carry the macrolide-lincosamide-streptogramin B resistance genes erm(C) or erm(T), the lincosamide resistance gene lnu(A), the pleuromutilin-lincosamide-streptogramin A resistance genes vga(A) or vga(C), the spectinomycin resistance gene spd, the apramycin resistance gene apmA, or the trimethoprim resistance gene dfrK. The detailed analysis of the structure of these plasmids allows comparisons with similar plasmids found in other staphylococci and underlines in many cases an exchange of such plasmids between LA-MRSA ST398 and other staphylococci including also coagulase-negative staphylococci.
Introduction
Isolates of the clonal complex 398 (CC398) are the most frequently encountered livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) in Europe as well as Northern America (Cuny et al., 2013). It is assumed to have developed from methicillin-susceptible S. aureus (MSSA), which has gained methicillin and tetracycline resistance after its introduction into animal hosts (Price et al., 2012). In contrast, LA-MRSA ST9 is the most widespread and most important LA-MRSA type in China (Li et al., 2017).
Many antimicrobial resistance genes in staphylococci of human and animal origin are located on plasmids (Wendlandt et al., 2013a; Schwarz et al., 2014). These include original plasmid-borne resistance genes, but also transposon-borne resistance genes in cases when the corresponding transposon has integrated into a plasmid or recombination between a resistance gene-carrying transposon and a plasmid has occurred. Plasmids play an important role in the dissemination of antimicrobial resistance genes among staphylococci (Schwarz et al., 2014). In this regard, LA-MRSA isolates do not differ from other staphylococci. Previous studies showed that LA-MRSA of CC398 can act as a donor and as a recipient in the dissemination of antimicrobial resistance plasmids, and thereby plays an important role in the mobilome of firmicutes (Schwarz et al., 2014). Over the years, several novel or unusual resistance genes have been found in LA-MRSA CC398 (Kadlec et al., 2012b).
In this review, we describe selected small antimicrobial resistance plasmids (<15 kb in size) that have been identified in LA-MRSA of CC398 and their relationships to similar plasmids of other staphylococcal species (Table 1).
Small Plasmids Carrying erm(C) Genes
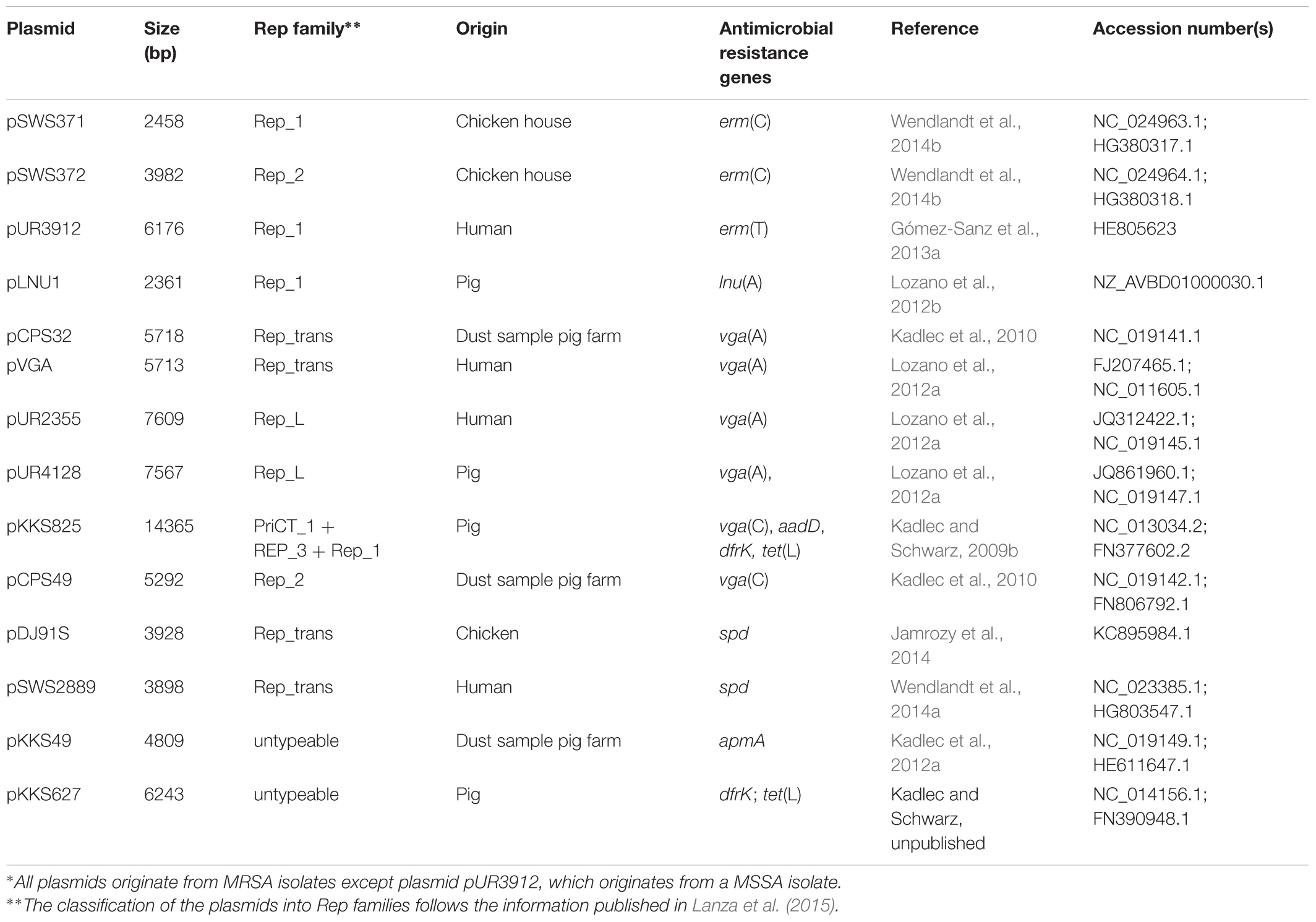
The gene erm(C) is the most widespread erm gene among staphylococci (Schwarz et al., 2014; Feßler et al., 2018). It is mainly located on plasmids. The erm(C) gene codes for a rRNA methyltransferase that targets the adenine residue at position 2048 in 23S rRNA and confers resistance to macrolides, lincosamides, and streptogramin B (MLSB) antibiotics. Its expression can be inducible or constitutive, based on the completeness of the translational attenuator that is located upstream of the erm(C) gene. So far, three types of small plasmids that carry solely the erm(C) gene have been identified in staphylococci (Schwarz et al., 2014; Feßler et al., 2018) – two of them also among LA-MRSA from a chicken house environment (Wendlandt et al., 2014b; Figure 1A and Table 1).
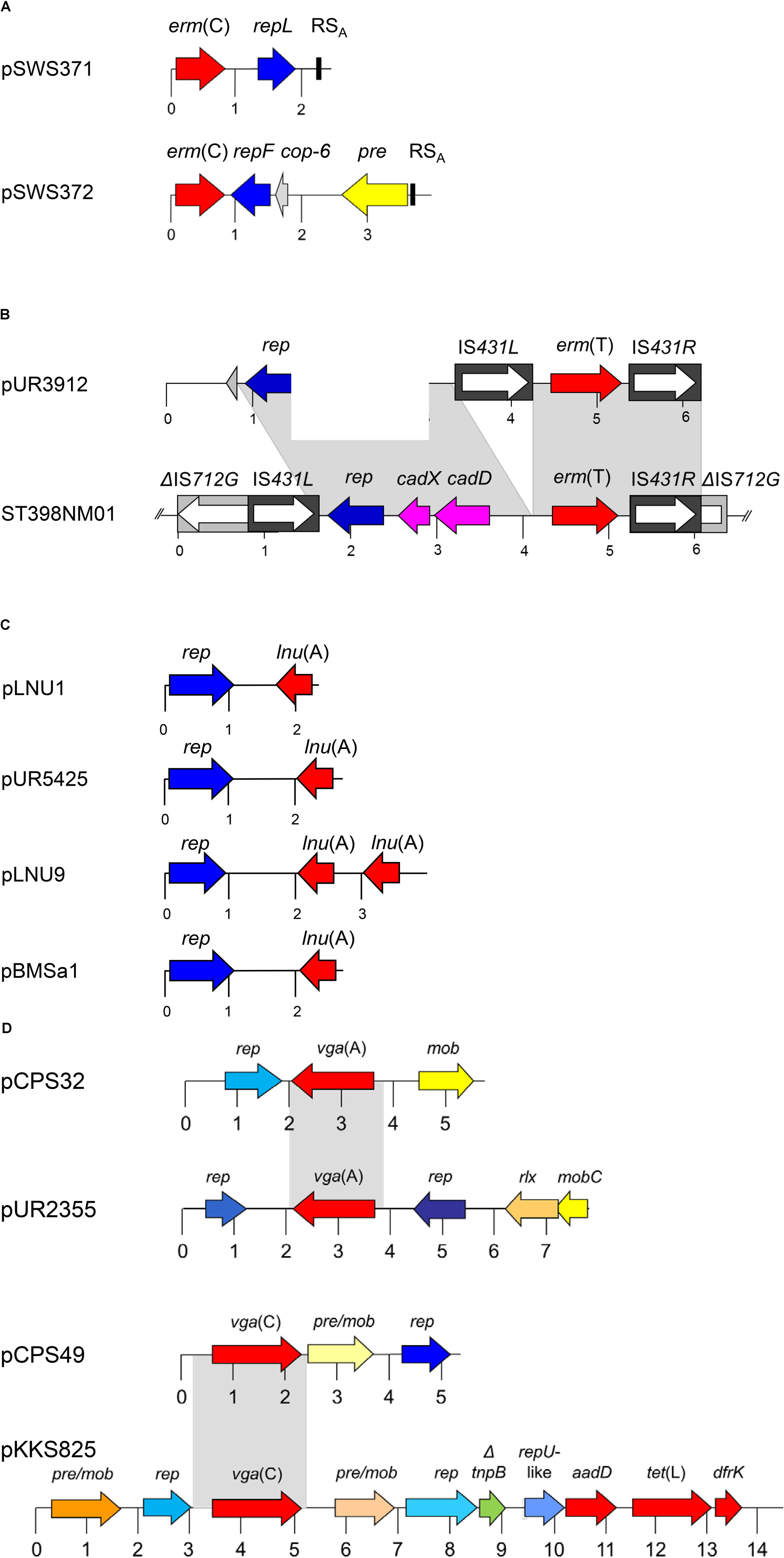
FIGURE 1. Schematic presentation of the organization of (A) the two erm (C)-carrying plasmids pSWS371 and pSWS372, (B) the erm(T)-carrying plasmid pUR3912 and the chromosomal erm(T) region of MRSA ST398 isolate ST398NM01, (C) the lnu(A)-carrying plasmids pLNU1, pLNU9, pUR5425, and pBMSa1, and (D) the vga(A)- and the vga(C)-carrying plasmids pCPS32, pUR2355, pCPS49 and pKKS825 from MRSA ST398. The reading frames are presented as arrows with the arrowhead indicating the direction of transcription. A distance scale in kb is given below the maps. The resistance genes are indicated in red, plasmid replication genes rep in different shades of blue, and genes involved in plasmid recombination, mobilization and relaxation pre, mob, pre/mob, and rlx in different shades of yellow/-orange. The different blue and yellow-orange shadings indicate differences in the respective genes. In panel (A), the plasmid copy control gene cop-6 is displayed in gray and the staphylococcal recombination site A (RSA) is indicated by a black box. In panel (B), the cadmium resistance operon cadDX is shown in pink and the IS431 elements and the IS712G element are displayed as black or gray boxes, respectively, with the white arrow inside representing the transposase gene tnp. The gray-shaded areas represent areas of at least > 95% sequence identity. This figure is modified from Lüthje et al. (2007) and Kadlec et al. (2010), Gómez-Sanz et al. (2013a), and Wendlandt et al. (2014b).
Two small erm(C)-carrying plasmids, pSWS371 and pSWS372, were identified in the same LA-MRSA CC398 isolate of dru type dt11 and spa type t3015. Plasmid pSWS371 has a size of 2458 bp and harbored – besides the erm(C) gene – only the plasmid replication gene repL. Plasmid pSWS372 has a size of 3982 bp and carried the plasmid replication gene repF, a new type of plasmid recombination and mobilization gene pre/mob, and the cop-6 gene possibly involved in the control of the copy number of this plasmid. The expression of both erm(C) genes was constitutive as explained by 16-bp (pSWS371) and 74-bp (pSWS372) deletions in the respective erm(C)-associated translational attenuators. After separate transformation of each plasmid into S. aureus RN4220, both plasmids were functionally active and conferred the expected MLSB resistance phenotype. The observation that both erm(C)-carrying plasmids stably coexist in the same bacterium may be explained by the fact that they belong to different incompatibility groups because they belong to different plasmid replication families (Wendlandt et al., 2014b).
Plasmid pSWS371 resembles in its structure and nucleotide sequence a number of small erm(C)-carrying plasmids which have been identified not only in S. aureus, but also in various coagulase-negative staphylococci from humans and animals (Schwarz et al., 2014; Feßler et al., 2018), including the prototype plasmid pNE131 from human Staphylococcus epidermidis (Lampson and Parisi, 1986). In contrast, plasmid pSWS372 is closely related to the prototype plasmid pE194 from human S. aureus (Horinouchi and Weisblum, 1982). Plasmids related to pE194 have so far only rarely been detected among staphylococci from humans and animals (Lodder et al., 1996; Schwarz et al., 2014).
It should be noted that small erm(C)-encoding plasmids can be integrated in part or completely into larger plasmids. In the case of the approximately 25-kb erm(T)-carrying plasmid pUR2940 of human LA-MRSA CC398, a complete 2363-bp erm(C)-carrying plasmid was integrated via insertion sequences of the type ISSau10 (Gómez-Sanz et al., 2013b). Moreover, the 7057-bp plasmid pSS-03 identified in various CoNS species of pigs in China carried an erm(C) gene together with the multidrug resistance gene cfr (Wang et al., 2011). The closely related 7054-bp plasmid pMSA16, in which, the erm(C) gene was replaced by a Tn554-analogous erm(A) gene, was identified in a LA-MRSA ST9 of bovine origin in China (Wang et al., 2012). Most recently, another related plasmid, the 8558-bp plasmid pSEM13-0451, which carried the genes erm(T) and cfr was detected in methicillin-resistant S. epidermidis of human origin (Lazaris et al., 2017).
Small Plasmids Carrying erm(T) Genes
Like erm(C), the gene erm(T) also confers inducible or constitutive MLSB resistance and is also preceded by a translational attenuator. The erm(T) gene has been found in LA-MRSA and MSSA of ST398 and can be located either in the chromosomal DNA or on plasmids of different sizes (Kadlec and Schwarz, 2010a; Vandendriessche et al., 2011; Gómez-Sanz et al., 2013a,b).
The erm(T) gene was first described in staphylococci on the approximately 40-kb plasmid pKKS25 from a porcine LA-MRSA ST398 in Germany. In this plasmid, the erm(T) gene was found together with the trimethoprim resistance gene dfrK and the tetracycline resistance gene tet(L) on an approximately 4.6 kb segment that was flanked by ISSau10 elements in the same orientation (Kadlec and Schwarz, 2010a). A smaller plasmid, the 6176-bp plasmid pUR3912, was isolated from a human MSSA ST398-t571 isolate in 2011 in Spain (Table 1). This plasmid harbors the erm(T) gene flanked by two IS elements in the same orientation, a plasmid replication gene rep and a functionally cadDX operon for cadmium resistance (Gómez-Sanz et al., 2013a). This plasmid showed striking homology to a chromosomal segment found in the MSSA strain ST398NM01 (Uhlemann et al., 2012) (Figure 1B). In a different study, the original strain carrying plasmid pUR3912 was analyzed in more detail and a chromosomal copy of plasmid pUR3912 was found in addition to the extrachromosomal location (Gómez-Sanz et al., 2013c). Plasmid pUR3912 was able to integrate into and excise from the chromosome of the corresponding MSSA isolate via the IS elements (Gómez-Sanz et al., 2013c). The closely related insertion sequences IS257, IS431, and ISSau10, play an important role in the integration of small resistance plasmids into the chromosomal DNA or into other plasmids (Schwarz et al., 2014).
Small Plasmids Carrying lnu(A) Genes
The gene lnu(A) codes for a lincosamide nucleotidyltransferase that confers solely low-level lincosamide resistance. The first complete sequence of an lnu(A)-carrying plasmid was from a bovine S. aureus isolate from Mexico. The corresponding plasmid pBMSa1 had a size of 2750 bp and carried only the lnu(A) gene and a plasmid replication gene rep (Loeza-Lara et al., 2004). In later studies on CoNS from bovine mastitis cases in Germany, nine novel types of lnu(A)-carrying small plasmids – pLNU1 to pLNU9 – have been identified (Lüthje et al., 2007). Plasmids pLNU1 to pLNU9 were similar to each other and to pBMSa1 in their structures and in their organization. They varied in size between 2278 bp and 3783 bp (Lüthje et al., 2007). In a study on MRSA and other staphylococci of human and animal origin conducted in Spain, Lozano and co-workers found a plasmid identical to pLNU1 (Figure 1C) in a porcine LA-MRSA ST398-t108 isolate (Table 1) and in a porcine methicillin-resistant Staphylococcus sciuri isolate. Moreover, they identified a novel type of lnu(A)-carrying plasmid, the 2690-bp plasmid pUR5425, which was next related to plasmid pLNU4, in a human MRSA ST125-t067 isolate (Lozano et al., 2012b).
Small Plasmids Carrying vga(A) Genes
The gene vga(A) codes for an ABC-F protein that mediates resistance by protecting the ribosome against lincosamides, pleuromutilins, and streptogramin A antibiotics (Sharkey et al., 2016). Among the various vga genes so far identified in staphylococci, the vga(A) genes are most widespread. They may be located either on plasmids of variable sizes or on transposon Tn5406 (Haroche et al., 2002). The vga(A) genes have also been found on small plasmids, including the 5713-bp plasmid pVGA from a human S. aureus of unknown MLST type in Portugal (Gentry et al., 2008) and the closely related 5718-bp plasmid pCPS32 from an LA-MRSA ST398 which originated from a dust sample taken at a swine farm in Portugal (Kadlec et al., 2010; Figure 1D and Table 1). These plasmids harbor three genes: the vga(A) gene, a plasmid mobilization gene mob, and a plasmid replication gene rep. In a study from Spain, Lozano et al. (2012a) detected plasmid pVGA in two LA-MRSA ST398 from humans. In addition, they identified four novel vga(A)-carrying plasmids among LA-MRSA ST398 from humans and pigs, but also in a human methicillin-resistant S. epidermidis ST83, a feline methicillin-resistant S. epidermidis ST60, and human methicillin-susceptible S. epidermidis ST100 (Lozano et al., 2012a). These four novel plasmids ranged in size between 7209 and 7609 bp. The two larger plasmids pUR4128 and pUR2355 had a similar structure which comprised – in addition to the vga(A) gene – a mobilization gene mobC, a relaxase gene rlx, two plasmid replication genes rep, and a small ORF of unknown function. The two smaller plasmids pUR3937 and pUR3036 carried – besides the vga(A) gene – a rlx gene, three mob genes, a single rep gene, and three small ORFs of unknown function (Lozano et al., 2012a). In a study from China on dogs and cats and their owners, Deng et al. (2017) found only 13 staphylococcal isolates with elevated pleuromutilin MICs. One of them, a human S. epidermidis isolate, harbored the vga(A) gene on plasmid p132R (7209 bp) which shared 99% nucleotide sequence identity with the same-sized plasmid pUR3036 of feline origin (Lozano et al., 2012a). Another small vga(A)-carrying plasmid, pSWS581 (6311 bp) was identified in a bovine S. epidermidis isolate from Germany (Wendlandt et al., 2015a).
A variant of the gene vga(A), designated vga(A)LC, was found on plasmids p131R and p131A, both 6056 bp in size and originating from S. haemolyticus isolates of human and feline origin in China (Deng et al., 2017). Both plasmids differed only by 9 bp from each other and shared 99% nucleotide sequence identity with plasmid pUR2355 from a human S. aureus ST398-t011 isolate in Spain (Deng et al., 2017).
Small Plasmids Carrying vga(C) Genes
The vga(C) gene was described in a LA-MRSA isolate from pig origin in Germany. In this isolate, the vga(C) gene was located on the plasmid pKKS825 (Kadlec and Schwarz, 2009b). This plasmid has a size of 14365 bp. Besides the vga(C) gene that confers resistance to pleuromutilins, lincosamides, and streptogramin A antibiotics, plasmid pKKS825 harbored the gene aadD for kanamycin/neomycin resistance, the gene dfrK for trimethoprim resistance, and the gene tet(L) for tetracycline resistance. In addition, plasmid pKKS825 carried two pre/mob genes and three rep genes (Kadlec and Schwarz, 2009b). The vga(C) gene was also found on smaller plasmids, such as the 5292-bp plasmid pCPS49 which originated from a dust sample taken in a breeding pig farm in Portugal (Kadlec et al., 2010; Figure 1D and Table 1). This plasmid harbored a rep gene and a pre/mob gene which were unrelated to the corresponding genes usually present on staphylococcal plasmids. Based on the analysis of the rep and pre/mob genes, it is assumed that plasmid pCPS49 may have developed in bacteria other than staphylococci. Sequence homology between plasmids pCPS49 and pKKS825 was limited to the vga(C) gene and 404 bp in the upstream and 249 bp in the downstream region (Kadlec et al., 2010).
Small Plasmids Carrying spd Genes
The gene spd codes for a spectinomycin adenyltransferase and is one of the three so far known spectinomycin resistance genes in staphylococci (Murphy, 1985; Wendlandt et al., 2013b, 2014c; Jamrozy et al., 2014). The gene spd is usually located on small plasmids of <5 kb in size (Jamrozy et al., 2014; Wendlandt et al., 2014a, 2015b).
The gene spd was initially identified in 2014 on the 3928-bp plasmid pDJ91S from a LA-MRSA ST398 of chicken origin. This plasmid was also detected in several other LA-MRSA ST398 isolates of chicken, pig, cattle, rat, and horse origins (Jamrozy et al., 2014). Plasmid pDJ91S consisted of a rep gene related to repN, a plasmid recombination gene rec (pre/mob) and the spd gene. Soon after its first description, the spd gene was also identified in porcine LA-MRSA ST398 and in porcine MSSA ST433 (Wendlandt et al., 2014a). In this latter strain collection, plasmid pDJ91S, but but also a slightly smaller plasmid of 3898 bp, designated pSWS2889, were present. Plasmid pSWS2889 showed the same overall structure as pDJ91S (Table 1). While the spd genes and the rec (pre/mob) genes of both plasmids were identical, the rep genes differed (Figure 2A). Moreover, the isolates in this latter study dated back to the year 2005, which suggested that the spd gene was present among LA-MRSA/MSSA isolates from animals for longer than initially thought (Wendlandt et al., 2014a).
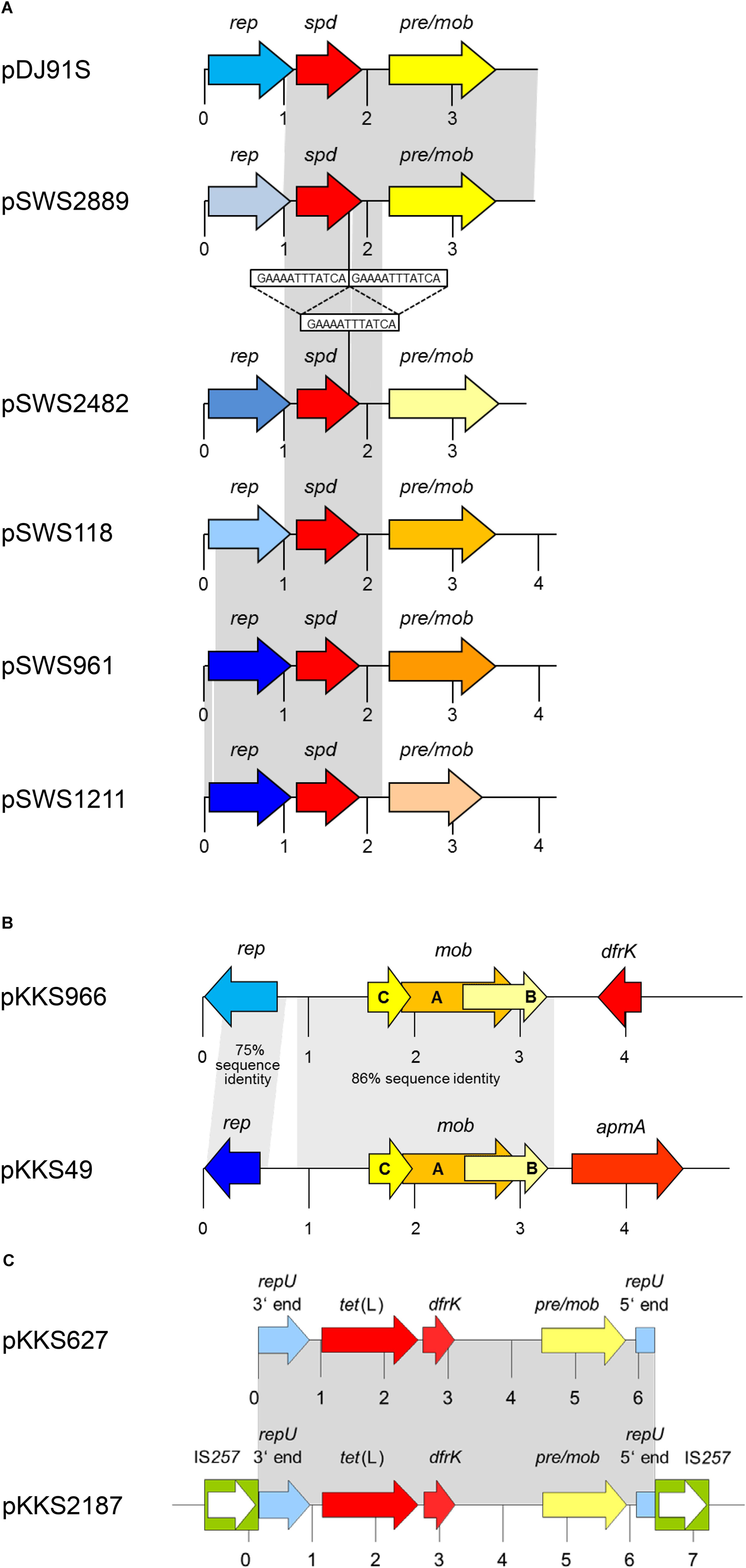
FIGURE 2. Schematic presentation of (A) the spd-carrying plasmids pDJ91S and pSWS2889 from MRSA ST398 in comparison to closely related plasmids pSWS2482 and pSWS1211 from S. hyicus, pSWS118 from S. chromogenes, and pSWS961 from S. equorum, (B) the apmA-carrying plasmid pKKS49 from MRSA ST398 and the dfrK-carrying plasmid pKKS966 from S. hyicus, and (C) the dfrK-carrying plasmid pKKS627 and the dfrK region of plasmid pKKS2187, both from MRSA ST398. The reading frames are presented as arrows with the arrowhead indicating the direction of transcription. A distance scale in kb is given below the maps. The resistance genes are indicated in red, plasmid replication genes rep in different shades of blue, and genes involved in plasmid recombination and mobilization, pre/mob and mob, in different shades of yellow/-orange. The different blue and yellow-orange shadings indicate differences in the respective genes. The IS257 elements in panel (C) are displayed as green boxes with the white arrow inside indicating the transposase gene tnp. The gray-shaded areas represent areas of at least > 99% sequence identity unless otherwise indicated. This figure is modified from Kadlec et al. (2012a) and Wendlandt et al. (2015b).
A variant of the gene spd was identified on four small plasmids from porcine S. hyicus, S. chromogenes, and S. equorum (Wendlandt et al., 2015b). They ranged in size between 3780 and 4229 bp and had the same overall structure as pDJ91S and pSWS2889 (Figure 2A). However, the rep and pre/mob genes of all four plasmids differed from each other and from those of the two spd-carrying plasmids of LA-MRSA ST398. Moreover, all four novel plasmids carried a variant of the spd gene, which had a 12 bp deletion in the terminal part of the gene. This deletion, however, had no impact on the high spectinomycin MIC conferred by the corresponding Spd variant (Wendlandt et al., 2015b).
Small Plasmids Carrying apmA Genes
The gene apmA is the first and so far only apramycin resistance gene described in staphylococci. This gene codes for an acetyltransferase, which is only distantly related to other acetyltransferases. It confers resistance to the aminocyclitol apramycin and also elevates the MIC values for gentamicin. The apmA gene was initially found on plasmid pAFS11 from a bovine LA-MRSA ST398-t2576-dt11a isolate (Feßler et al., 2011). In that study, the gene was also detected in one bovine and four porcine MRSA ST398-t011-dt11a isolates. Plasmid pAFS11 has recently been completely sequenced (Feßler et al., 2017). Its size is 49189 bp and it has a small plasmid, that harbors the genes aadD for kanamycin/neomycin resistance, tet(L) for tetracycline resistance, and dfrK for trimethoprim resistance, but also a repU gene and a pre/mob gene, integrated via IS431 into the pAFS11 backbone (Feßler et al., 2017).
The results of another study revealed that the gene apmA can also reside on small plasmids. One such plasmid, the 4809-bp plasmid pKKS49, was identified in a LA-MRSA ST398 isolate that originated from a dust sample taken in a pig farm in Portugal (Kadlec et al., 2012a; Table 1). This plasmid had a simple composition consisting of a plasmid replication gene rep, three in part overlapping mobilization genes mobA, mobB, and mobC, and the apmA gene (Figure 2B). The corresponding Rep and Mob proteins were only distantly related to Rep and Mob proteins of staphylococci. The pKKS49-associated ApmA protein differed in 12/274 amino acids from the original ApmA protein. Based on the high apramycin MIC of 64 mg/L seen in S. aureus RN4220 carrying pKKS49, these amino acid substitutions seem to have no impact on the activity of the ApmA protein. Homology between both plasmids, pKKS49 and pAFS11, included only the apmA gene and 64 bp in the apmA downstream region as well as 72 bp upstream of apmA (Kadlec et al., 2012a).
Small Plasmids Carrying dfrK Genes
The trimethoprim resistance gene dfrK was first described on the ca. 40-kb plasmid pKKS2187 from a porcine LA-MRSA ST398 isolate (Kadlec and Schwarz, 2009a). A closer look at the genetic environment of the dfrK gene revealed the apparent presence of a small plasmid consisting of a repU gene, a pre/mob gene, the tetracycline resistance gene tet(L) and the dfrK gene. This small plasmid was integrated via IS257 elements into the pKKS2187 backbone. Further screening of porcine LA-MRSA ST398 isolates from Germany identified the 6243-bp plasmid pKKS627 (Kadlec and Schwarz, unpublished), which is likely to represent the progenitor plasmid of the one integrated in plasmid pKKS2187 (Figure 2C and Table 1). The dfrK gene has been detected not only as part of diverse larger multiresistance plasmids among MRSA ST398 isolates (Kadlec et al., 2009; Kadlec and Schwarz, 2010a; Feßler et al., 2017), but also as part of the 4289-bp non-conjugative transposon Tn559 (Kadlec and Schwarz, 2010b; López et al., 2012).
In addition to plasmid pKKS627, the dfrK gene was also detected on a structurally different small staphylococcal plasmid. This plasmid, pKKS966, had a size of 4957 bp and was found in a S. hyicus isolate from a sow (Kadlec et al., 2012a) (Figure 2B). It was the first description of the dfrK gene in a staphylococcal species other than S. aureus. The plasmid, however, carried – besides dfrK – three mob genes mobA, mobB, and mobC as well as a rep gene and thus, resembled the unusual apmA-carrying plasmid pKKS49 (Kadlec et al., 2012a) (Figure 2B).
Concluding Remarks
The data presented in this review showed that small antimicrobial resistance plasmids play a role in the dissemination of certain antimicrobial resistance genes. The observation that small plasmids similar or even identical to the ones found in LA-MRSA ST398 are present in other staphylococci, including CoNS, underlines the role of LA-MRSA ST398 as donor and/or recipient of such plasmids. Moreover, the finding that the same small plasmids occur in isolates of different geographic regions, e.g., plasmid pLNU1 in bovine CoNS from Germany and in porcine LA-MRSA ST398 and porcine S. sciuri from Spain, confirms that these plasmids are disseminated across animal species, bacterial species, and geographic boundaries. The observation that small staphylococcal resistance plasmids can integrate or be integrated via insertion sequences into larger plasmids or the chromosomal DNA renders them highly versatile mobile genetic elements and underlines their important role in the dissemination of antimicrobial resistance.
Author Contributions
AF and SS wrote the first draft. All other authors provided additions and corrections. All authors listed have approved the manuscript for publication.
Funding
The work of AF and SS on staphylococci was funded by the Federal Ministry of Education and Research (BMBF) under project number 01KI1727D as part of the Research Network Zoonotic Infectious Diseases. In addition, research on LA-MRSA by AF, YW, CW, and SS was supported by the German Research Foundation (DFG) and the National Natural Science Foundation of China (NSFC) under grant numbers SCHW382/11-1 and 31761133022, respectively.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Cuny, C., Köck, R., and Witte, W. (2013). Livestock associated MRSA (LA-MRSA) and its relevance for humans in Germany. Int. J. Med. Microbiol. 303, 331–337. doi: 10.1016/j.ijmm.2013.02.010
Deng, F., Wang, H., Liao, Y., Li, J., Feßler, A. T., Michael, G. B., et al. (2017). Detection and genetic evironment of pleuromutilin-lincosamide-streptogramin a resistance genes in staphylococci isolated from pets. Front. Microbiol. 8:234. doi: 10.3389/fmicb.2017.00234
Feßler, A. T., Kadlec, K., and Schwarz, S. (2011). Novel apramycin resistance gene apmA in bovine and porcine methicillin-resistant Staphylococcus aureus ST398 isolates. Antimicrob. Agents Chemother. 55, 373–375. doi: 10.1128/AAC.01124-10
Feßler, A. T., Wang, Y., Wu, C., and Schwarz, S. (2018). Mobile macrolide resistance genes in staphylococci. Plasmid. pii:S0147-619X(18)30025-8. doi: 10.1016/j.plasmid.2018.05.001
Feßler, A. T., Zhao, Q., Schoenfelder, S., Kadlec, K., Brenner Michael, G., Wang, Y., et al. (2017). Complete sequence of a plasmid from a bovine methicillin-resistant Staphylococcus aureus harbouring a novel ica-like gene cluster in addition to antimicrobial and heavy metal resistance genes. Vet. Microbiol. 200, 95–100. doi: 10.1016/j.vetmic.2016.07.010
Gentry, D. R., McCloskey, L., Gwynn, M. N., Rittenhouse, S. F., Scangarella, N., Shawar, R., et al. (2008). Genetic characterization of Vga ABC proteins conferring reduced susceptibility to pleuromutilins in Staphylococcus aureus. Antimicrob. Agents Chemother. 52, 4507–4509. doi: 10.1128/AAC.00915-08
Gómez-Sanz, E., Kadlec, K., Feßler, A. T., Billerbeck, C., Zarazaga, M., Schwarz, S., et al. (2013a). Analysis of a novel erm(T)- and cadDX-carrying plasmid from methicillin-susceptible Staphylococcus aureus ST398-t571 of human origin. J. Antimicrob. Chemother. 68, 471–473. doi: 10.1093/jac/dks411
Gómez-Sanz, E., Kadlec, K., Feßler, A. T., Zarazaga, M., Torres, C., and Schwarz, S. (2013b). Novel erm(T)-carrying multiresistance plasmids from porcine and human isolates of methicillin-resistant Staphylococcus aureus ST398 that also harbor cadmium and copper resistance determinants. Antimicrob. Agents Chemother. 57, 3275–3282. doi: 10.1128/AAC.00171-13
Gómez-Sanz, E., Zarazaga, M., Kadlec, K., Schwarz, S., and Torres, C. (2013c). Chromosomal integration of the novel plasmid pUR3912 from methicillin-susceptible Staphylococcus aureus ST398 of human origin. Clin. Microbiol. Infect. 19, E519–E522. doi: 10.1111/1469-0691.12279
Haroche, J., Allignet, J., and El Solh, N. (2002). Tn5406, a new staphylococcal transposon conferring resistance to streptogramin A and related compounds including dalfopristin. Antimicrob. Agents Chemother. 46, 2337–2343. doi: 10.1128/AAC.46.8.2337-2343.2002
Horinouchi, S., and Weisblum, B. (1982). Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J. Bacteriol. 150, 804–814.
Jamrozy, D. M., Coldham, N. G., Butaye, P., and Fielder, M. D. (2014). Identification of a novel plasmid-associated spectinomycin adenyltransferase gene spd in methicillin-resistant Staphylococcus aureus ST398 isolated from animal and human sources. J. Antimicrob. Chemother. 69, 1193–1196. doi: 10.1093/jac/dkt510
Kadlec, K., Ehricht, R., Monecke, S., Steinacker, U., Kaspar, H., Mankertz, J., et al. (2009). Diversity of antimicrobial resistance pheno- and genotypes of methicillin-resistant Staphylococcus aureus ST398 from diseased swine. J. Antimicrob. Chemother. 64, 1156–1164. doi: 10.1093/jac/dkp350
Kadlec, K., Feßler, A. T., Couto, N., Pomba, C. F., and Schwarz, S. (2012a). Unusual small plasmids carrying the novel resistance genes dfrK or apmA isolated from methicillin-resistant or -susceptible staphylococci. J. Antimicrob. Chemother. 67, 2342–2345. doi: 10.1093/jac/dks235
Kadlec, K., Feßler, A. T., Hauschild, T., and Schwarz, S. (2012b). Novel and uncommon antimicrobial resistance genes in livestock-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 18, 745–755. doi: 10.1111/j.1469-0691.2012.03842.x
Kadlec, K., Pomba, C. F., Couto, N., and Schwarz, S. (2010). Small plasmids carrying vga(A) or vga(C) genes mediate resistance to lincosamides, pleuromutilins and streptogramin A antibiotics in methicillin-resistant Staphylococcus aureus ST398 from swine. J. Antimicrob. Chemother. 65, 2692–2693. doi: 10.1093/jac/dkq365
Kadlec, K., and Schwarz, S. (2009a). Identification of a novel trimethoprim resistance gene, dfrK, in a methicillin-resistant Staphylococcus aureus ST398 strain and its physical linkage to the tetracycline resistance gene tet(L). Antimicrob. Agents Chemother. 53, 776–778. doi: 10.1128/AAC.01128-08
Kadlec, K., and Schwarz, S. (2009b). Novel ABC transporter gene, vga(C), located on a multiresistance plasmid from a porcine methicillin-resistant Staphylococcus aureus ST398 strain. Antimicrob. Agents Chemother. 53, 3589–3591. doi: 10.1128/AAC.00570-09
Kadlec, K., and Schwarz, S. (2010a). Identification of a plasmid-borne resistance gene cluster comprising the resistance genes erm(T), dfrK, and tet(L) in a porcine methicillin-resistant Staphylococcus aureus ST398 strain. Antimicrob. Agents Chemother. 54, 915–918. doi: 10.1128/AAC.01091-09
Kadlec, K., and Schwarz, S. (2010b). Identification of the novel dfrK-carrying transposon Tn559 in a porcine methicillin-susceptible Staphylococcus aureus ST398 strain. Antimicrob. Agents Chemother. 54, 3475–3477. doi: 10.1128/AAC.00464-10
Lampson, B. C., and Parisi, J. T. (1986). Nucleotide sequence of the constitutive macrolide-lincosamide-streptogramin B resistance plasmid pNE131 from Staphylococcus epidermidis and homologies with Staphylococcus aureus plasmids pE194 and pSN2. J. Bacteriol. 167, 888–892. doi: 10.1128/jb.167.3.888-892.1986
Lanza, V. F., Tedim, A. P., Martínez, J. L., Baquero, F., and Coque, T. M. (2015). The plasmidome of firmicutes: impact on the emergence and the spread of resistance to antimicrobials. Microbiol Spectr. 3:PLAS-0039-2014.
Lazaris, A., Coleman, D. C., Kearns, A. M., Pichon, B., Kinnevey, P. M., Earls, M. R., et al. (2017). Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J. Antimicrob. Chemother. 72, 3252–3257. doi: 10.1093/jac/dkx292
Li, J., Jiang, N., Ke, Y., Feßler, A. T., Wang, Y., Schwarz, S., et al. (2017). Characterization of pig-associated methicillin-resistant Staphylococcus aureus. Vet. Microbiol. 201, 183–187. doi: 10.1016/j.vetmic.2017.01.017
Lodder, G., Schwarz, S., Gregory, P., and Dyke, K. (1996). Tandem duplication in ermC translational attenuator of the macrolide-lincosamide-streptogramin B resistance plasmid pSES6 from Staphylococcus equorum. Antimicrob. Agents Chemother. 40, 215–217.
Loeza-Lara, P. D., Soto-Huipe, M., Baizabal-Aguirre, V. M., Ochoa-Zarzosa, A., Valdez-Alarcón, J. J., Cano-Camacho, H., et al. (2004). pBMSa1, a plasmid from a dairy cow isolate of Staphylococcus aureus, encodes a lincomycin resistance determinant and replicates by the rolling-circle mechanism. Plasmid 52, 48–56. doi: 10.1016/j.plasmid.2004.03.001
López, M., Kadlec, K., Schwarz, S., and Torres, C. (2012). First detection of the staphylococcal trimethoprim resistance gene dfrK and the dfrK-carrying transposon Tn559 in enterococci. Microb. Drug Resist. 18, 13–18. doi: 10.1089/mdr.2011.0073
Lozano, C., Aspiroz, C., Rezusta, A., Gómez-Sanz, E., Simon, C., Gómez, P., et al. (2012a). Identification of novel vga(A)-carrying plasmids and a Tn5406-like transposon in methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis of human and animal origin. Int. J. Antimicrob. Agents 40, 306–312. doi: 10.1016/j.ijantimicag.2012.06.009
Lozano, C., Aspiroz, C., Sáenz, Y., Ruiz-García, M., Royo-García, G., Gómez-Sanz, E., et al. (2012b). Genetic environment and location of the lnu(A) and lnu(B) genes in methicillin-resistant Staphylococcus aureus and other staphylococci of animal and human origin. J. Antimicrob. Chemother. 67, 2804–2808. doi: 10.1093/jac/dks320
Lüthje, P., von Köckritz-Blickwede, M., and Schwarz, S. (2007). Identification and characterization of nine novel types of small staphylococcal plasmids carrying the lincosamide nucleotidyltransferase gene lnu(A). J. Antimicrob. Chemother. 59, 600–606. doi: 10.1093/jac/dkm008
Murphy, E. (1985). Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3”) (9). Mol. Gen. Genet. 200, 33–39. doi: 10.1007/BF00383309
Price, L. B., Stegger, M., Hasman, H., Aziz, M., Larsen, J., Andersen, P. S., et al. (2012). Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 3, e305–e311. doi: 10.1128/mBio.00305-11
Schwarz, S., Shen, J., Wendlandt, S., Feßler, A. T., Wang, Y., Kadlec, K., et al. (2014). Plasmid-mediated antimicrobial resistance in staphylococci and other firmicutes. Microbiol. Spectr. 2:PLAS-0020-2014. doi: 10.1128/microbiolspec.PLAS-0020-2014.
Sharkey, L. K., Edwards, T. A., and O’Neill, A. J. (2016). ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio 7:e01975. doi: 10.1128/mBio.01975-15
Uhlemann, A. C., Porcella, S. F., Trivedi, S., Sullivan, S. B., Hafer, C., Kennedy, A. D., et al. (2012). Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. mBio 3:e27-12. doi: 10.1128/mBio.00027-12
Vandendriessche, S., Kadlec, K., Schwarz, S., and Denis, O. (2011). Methicillin-susceptible Staphylococcus aureus ST398-t571 harbouring the macrolide-lincosamide-streptogramin B resistance gene erm(T) in Belgian hospitals. J. Antimicrob. Chemother. 66, 2455–2459. doi: 10.1093/jac/dkr348
Wang, Y., Zhang, W., Wang, J., Wu, C., Shen, Z., Fu, X., et al. (2011). Distribution of the multidrug resistance gene cfr in Staphylococcus species isolates from swine farms in China. Antimicrob. Agents Chemother. 56, 1485–1490. doi: 10.1128/AAC.05827-11
Wang, X. M., Zhang, W. J., Schwarz, S., Yu, S. Y., Liu, H., Si, W., et al. (2012). Methicillin-resistant Staphylococcus aureus ST9 from a case of bovine mastitis carries the genes cfr and erm(A) on a small plasmid. J. Antimicrob. Chemother. 67, 1287–1289. doi: 10.1093/jac/dks028
Wendlandt, S., Feßler, A. T., Kadlec, K., van Duijkeren, E., and Schwarz, S. (2014a). Identification of the novel spectinomycin resistance gene spd in a different plasmid background among methicillin-resistant Staphylococcus aureus CC398 and methicillin-susceptible S. aureus ST433. J. Antimicrob. Chemother. 69, 2000–2003. doi: 10.1093/jac/dku067
Wendlandt, S., Feßler, A. T., Monecke, S., Ehricht, R., Schwarz, S., and Kadlec, K. (2013a). The diversity of antimicrobial resistance genes among staphylococci of animal origin. Int. J. Med. Microbiol. 303, 338–349. doi: 10.1016/j.ijmm.2013.02.006
Wendlandt, S., Kadlec, K., Feßler, A. T., and Schwarz, S. (2015a). Identification of ABC transporter genes conferring combined pleuromutilin-lincosamide-streptogramin A resistance in bovine methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococci. Vet. Microbiol. 177, 353–358. doi: 10.1016/j.vetmic.2015.03.027
Wendlandt, S., Kadlec, K., Feßler, A. T., van Duijkeren, E., and Schwarz, S. (2014b). Two different erm(C)-carrying plasmids in the same methicillin-resistant Staphylococcus aureus CC398 isolate from a broiler farm. Vet. Microbiol. 171, 382–387. doi: 10.1016/j.vetmic.2014.01.009
Wendlandt, S., Kadlec, K., and Schwarz, S. (2015b). Four novel plasmids from Staphylococcus hyicus and CoNS that carry a variant of the spectinomycin resistance gene spd. J. Antimicrob. Chemother. 70, 948–949. doi: 10.1093/jac/dku461
Wendlandt, S., Li, J., Ho, J., Porta, M. A., Feßler, A. T., Wang, Y., et al. (2014c). Enterococcal multiresistance gene cluster in methicillin-resistant Staphylococcus aureus from various origins and geographical locations. J. Antimicrob. Chemother. 69, 2573–2575. doi: 10.1093/jac/dku137
Wendlandt, S., Li, B., Lozano, C., Ma, Z., Torres, C., and Schwarz, S. (2013b). Identification of the novel spectinomycin resistance gene spw in methicillin-resistant and methicillin-susceptible Staphylococcus aureus of human and animal origin. J. Antimicrob. Chemother. 68, 1679–1680. doi: 10.1093/jac/dkt081
Keywords: LA-MRSA, erm, lnu(A), cfr, vga, spd, apmA, dfrK
Citation: Feßler AT, Kadlec K, Wang Y, Zhang W-J, Wu C, Shen J and Schwarz S (2018) Small Antimicrobial Resistance Plasmids in Livestock-Associated Methicillin-Resistant Staphylococcus aureus CC398. Front. Microbiol. 9:2063. doi: 10.3389/fmicb.2018.02063
Received: 02 June 2018; Accepted: 13 August 2018;
Published: 19 September 2018.
Edited by:
Patrick Rik Butaye, Ross University School of Veterinary Medicine, Saint Kitts and NevisCopyright © 2018 Feßler, Kadlec, Wang, Zhang, Wu, Shen and Schwarz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Schwarz, c3RlZmFuLnNjaHdhcnpAZnUtYmVybGluLmRl
 Andrea Feßler1
Andrea Feßler1 Kristina Kadlec
Kristina Kadlec Yang Wang
Yang Wang Jianzhong Shen
Jianzhong Shen Stefan Schwarz
Stefan Schwarz