- 1Department of Molecular Cell Biology, VU University Amsterdam, Amsterdam, Netherlands
- 2Yoba for Life Foundation, Amsterdam, Netherlands
- 3Canadian R&D Centre for Human Microbiome and Probiotics, Lawson Health Research Institute, London, ON, Canada
- 4Department of Microbiology and Immunology, and Surgery, Western University, London, ON, Canada
- 5Department of Microbiology and Systems Biology, Netherlands Organization for Applied Scientific Research, Zeist, Netherlands
- 6ARTIS-Micropia, Amsterdam, Netherlands
The probiotic Lactobacillus rhamnosus GG (LGG) can play a role in establishing a harmless relationship with Helicobacter pylori and reduce gastric pathology in East African populations. H. pylori has the ability to inhabit the surface of the mucous layer of the human stomach and duodenum. In the developing world, an estimated 51% of the population is carrier of H. pylori, while in some Western countries these numbers dropped below 20%, which is probably associated with improved sanitation and smaller family sizes. Colonization by H. pylori can be followed by inflammation of the gastric mucus layer, and is a risk factor in the development of atrophic gastritis, peptic ulcers and gastric cancer. Notwithstanding the higher prevalence of H. pylori carriers in developing countries, no equal overall increase in gastric pathology is found. This has been attributed to a less pro-inflammatory immune response to H. pylori in African compared to Caucasian populations. In addition, a relatively low exposure to other risk factors in certain African populations may play a role, including the use of non-steroidal anti-inflammatory drugs, smoking, and diets without certain protective factors. A novel approach to the reduction of H. pylori associated gastric pathology is found in the administration of the probiotic bacterium Lactobacillus rhamnosus yoba 2012 (LRY), the generic variant of LGG. This gastro-intestinal isolate inhibits H. pylori by competition for substrate and binding sites as well as production of antimicrobial compounds such as lactic acid. In addition, it attenuates the host’s H. pylori-induced apoptosis and inflammation responses and stimulates angiogenesis in the gastric and duodenal epithelium. The probiotic LRY is not able to eradicate H. pylori completely, but its co-supplementation in antibiotic eradication therapy has been shown to relieve side effects of this therapy. In Uganda, unlike other African countries, gastric pathology is relatively common, presumably resulting from the lack of dietary protective factors in the traditional diet. Supplementation with LRY through local production of probiotic yogurt, could be a solution to establish a harmless relationship with H. pylori and reduce gastric pathology and subsequent eradication therapy treatment.
Introduction
During the last decade of our probiotic yogurt programs in the East-African countries Uganda, Tanzania, and Kenya, we took note of the high incidence of self-reported ulcers and accompanying stomach pains, as reported decades ago for in particular Northern Tanzania and South-Western Uganda and some regions in Kenya (Tovey and Tunstall, 1975; Balint, 1998). Interestingly, during our fieldwork aimed at the stimulation of local production and consumption of probiotic yogurt to improve health and wealth in local communities, we observed a decrease of complaints among consumers and realized that there might be an effect from the intake of probiotic yogurt containing Lactobacillus rhamnosus yoba 2012 (LRY), the generic variant of Lactobacillus rhamnosus GG (LGG) (Kort and Sybesma, 2012; Sybesma et al., 2013), on the reduction of symptoms associated with ulcers.
Over the last years a number of reviews have been published on H. pylori ulcers (Blaser and Atherton, 2004; Cover and Blaser, 2009), the prevalence of H. pylori and ulcers in different populations (Kidd et al., 1999b; Roberts et al., 2016), the interaction between H. pylori and LGG (Hamilton-Miller, 2003; Gotteland et al., 2006), and effects of LGG administration on ulcers (Lam et al., 2007a,b). The present paper is, however, the first review that summarizes the incidence of H. pylori in East-Africa and its pathology affected by immune responses, genetic factors and environmental factors, including specific regional diets, emphasizing the importance of this topic for East-Africa. In addition, this article reviews effects of the probiotic bacterium LGG on H. pylori colonization as well as on inflammation and associated injury of gastric mucosa, in either presence or absence of other treatments. Finally, this review provides substantiation for our on-going probiotic yogurt programs in East-Africa, as reported in this Research Topic in Frontiers in Microbiology in another contribution by Westerik et al. (2018), to likely reduce H. pylori-associated pathology.
Previous studies also suggested that LGG prevented H. pylori colonization, which often occurs in early childhood (Misiewicz et al., 1995; Peña and Versalovic, 2003; Myllyluoma et al., 2008). It is therefore expected that administration of probiotic yogurt containing LGG to children in resource-poor countries from early childhood, can reduce the incidence of H. pylori colonization in the general population. Besides prevention of H. pylori, LGG may present an approach to establish and manage a harmless relationship between the host and H. pylori when the latter one is already present, counteracting the need for H. pylori eradication therapy. Kort et al. (2015) and Sybesma et al. (2015) have shown that yogurt containing LRY could be made accessible for people in resource-poor countries in a sustainable way. This is done through providing freeze-dried yogurt starter cultures containing LRY at cost price, in combination with a training program for the local population on standardized and safe yogurt production with locally available equipment.
Prevalence of H. pylori in East Africa
Helicobacter pylori is a Gram-negative spiral bacterium that inhabits the surface of the mucous layer of the human stomach and duodenum (upper intestine), usually in a chronic manner, though H. pylori colonization can also be temporarily (Hestvik et al., 2010). The majority of carriers have acquired this bacterium through contact with other human beings before the age of 10 years old, more so in cramped housing areas with poor sanitation practices. When present, H. pylori is the dominant microorganism, as very few other bacteria can survive in the stomach.
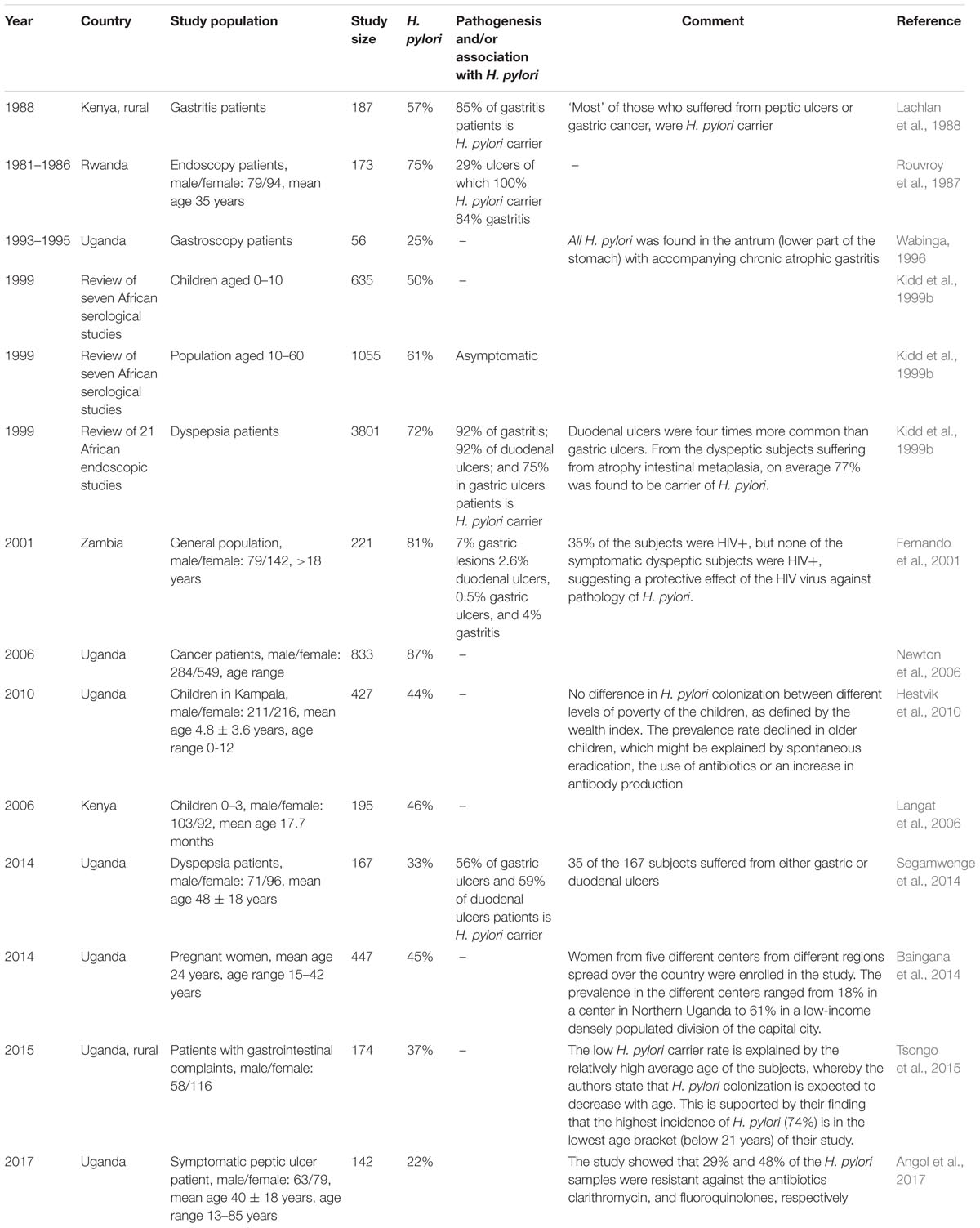
Helicobacter pylori is found in more than 50% of world’s population, spread all around the globe (Kodaman et al., 2014). In 10–20% of the carriers, H. pylori is believed to be a factor in the development of gastric pathology (Fernando et al., 2001; Gotteland et al., 2006; Kate et al., 2013). Gastric pathology often starts with gastritis (inflammation of the stomach lining), which can become atrophic (degenerating cells) and via gastric metaplasia (abnormal change in tissue) can lead to peptic ulcers and in rare cases to gastric cancer. Duodenal ulcers are much more common than gastric (stomach) ulcers. Over the course of the 20th century, H. pylori colonization has become less common in Westernized populations, and in some countries the incidence has dropped below 20% (Go, 2002; Roberts et al., 2016). This is probably associated with improved sanitation, smaller family sizes, and frequent use of antibiotics from childhood (Cover and Blaser, 2009). In contrast, general estimates on the prevalence of H. pylori still indicate that over 51% of people living in developing countries, and even 57% of people living on the African continent, are H. pylori positive. When taking into account the age of subjects, 52% of African children less than 10 years of age, and over 64% of the African adults are H. pylori positive (Zamani et al., 2018). Hence, the high incidence of H. pylori in East-Africa might be a risk factor in the development of gastric pathology among the local population. A summary of studies on the incidence of H. pylori and gastric pathology in East-Africa can be found in Table 1. Table 1 shows great variety in the incidence rate between 25 and 87%. This may be due to community-specific differences, but also different diagnostic methods used to determine the presence of H. pylori (Ricci et al., 2007; Zamani et al., 2018). Notwithstanding, we can conclude that H. pylori is a significant risk factor in the development of gastric pathology in East-Africa.
Pathogenesis and Other Consequences of H. pylori Colonization
The high incidence of H. pylori among East African populations per se does not necessarily cause increased levels of gastric pathology, as H. pylori induced gastric pathology is influenced by genetic factors of the H. pylori strain as well as by specific immune responses of the host. At the same time, it has been reported that H. pylori might exert beneficial functions in African populations (Blaser and Atherton, 2004; Cover and Blaser, 2009).
In the context of pathology, Blaser and Atherton (2004) distinguish two topographic distributions of H. pylori induced gastric inflammation, both having different outcomes. Firstly, inflammation could be in the lower part of the stomach only (antral-gastritis), which via a cascade of cell signaling leads to increased gastric acid output and subsequently increases the risk for duodenal ulcers. Secondly, inflammation of the whole stomach (pan-gastritis) could induce cascades of signaling that lead to reduced acid output and associated increased risk for gastric ulcers. This topographic distribution might be determined by genetic factors of H. pylori strains as well as environmental factors.
Helicobacter pylori strains exhibit a high level of genetic diversity. Certain genetic characteristics of this bacterium have been associated with increased interactions with the host cells. One of the best documented characteristics is the presence of the pathogenicity island cagA, which expresses the effector protein CagA that can be injected into epithelial cells (Crabtree et al., 1991; Bravo et al., 2002). Connected to the presence of cagA is the presence of an active VacA protein (Kidd et al., 1999a). All H. pylori strains contain the vacA gene but with a great variance in nucleotide sequence, and not all expressed VacA proteins have the same functional activities. Active VacA causes alterations in epithelial cells and immune cells due to massive vacuolation (Cover and Blanke, 2005). Strains that both express CagA and active VacA are more likely to cause gastric inflammations and subsequent pathology including peptic ulcers and gastric cancer (Cover and Blaser, 2009). Other authors identified the dupA gene as a virulence factor in H. pylori strains that is associated with duodenal ulcers (Kate et al., 2013). Immune responses against these virulent factors involve Th1 cells, which paradoxically play a major role in H. pylori associated pathogenesis by enhancing inflammation (Blaser and Atherton, 2004).
With regards to the virulence factors, people in developed countries are found to be colonized by almost equal proportions of cagA+ and cagA- strains (50% cagA+), whereas people in developing countries are predominantly colonized by cagA+ strains (79% cagA+) (Parsonnet et al., 1997). However, it has been suggested that people in developing countries respond to H. pylori in a way that is associated with a relatively low risk for pathogenesis (Holcombe, 1992; Mitchell et al., 2009). For example, Segal et al. (2001) found that among people in several African countries, gastric cancer accounts only for 2–3% of all cancers, whereas the international average is 9% (Parkin, 2004). This might be due to modulated immune responses to H. pylori as a result of infections with a myriad of gastrointestinal pathogens from early life, which primarily occur in developing countries. Mitchell et al. (2009) quantified the immune response by measuring the IgG1/IgG2 ratio (a marker of the T helper cell response) in Sowetan (South-African), German and Australian symptomatic H. pylori-positive subjects. A less pro-inflammatory, IgGI predominant response (IgG1/IgG2 ratio > 1) was observed in 81% of Sowetans, but only in 4.7% of Australians and 4.4% of Germans.
The realization that H. pylori has colonized in the gut of mankind for thousands of years (Ghose et al., 2002), creating long-standing dynamic equilibriums, led to the assumption that there must be a mutual beneficial relationship (Blaser and Atherton, 2004; Cover and Blaser, 2009). Kodaman et al. (2014) postulate that H. pylori co-evolves with host populations and as a consequence, H. pylori is less virulent upon colonization in its ‘natural host’ compared to its colonization in populations from other ethnic origins. The authors reported a strong correlation between H. pylori virulence in people of Amerindian origin and the presence of H. pylori of African origin and concluded that members of multicultural societies were at higher risk of H. pylori associated pathology (Kodaman et al., 2014).
In addition to being harmless in many cases, it has even been proposed that cagA+ H. pylori could exert beneficial functions in the body. Over the past decennia, gastroesophageal reflux disease (Vicari et al., 1998), Barrett’s esophagus (Vaezi et al., 2000), esophageal adenocarcinoma (Ye et al., 2004) and dysfunctional responses to common allergens leading to subsequent childhood asthma and allergic disorders (Cover and Blaser, 2009) have become more common in the developed world. Recent research has associated the increase of those diseases with the decrease of cagA+ H. pylori colonized persons. Mechanisms have been proposed on how the absence of cagA+ H. pylori induced pan-gastritis could lead to an increase in acid production (Blaser and Atherton, 2004), which in turn could lead to an increased risk of the above mentioned diseases. Indeed, Segal et al. (2001) found that incidence of gastro-oesophageal reflux and its complications were low in black populations where the incidence of cagA+ H. pylori is high. However, there was no uniform agreement on the topic, as for example Moon et al. (2009) found that there is an increase in reflux disease among H. pylori positive children in America. However, considering the differences in topographic distribution of gastritis and subsequent different pathologies (Blaser and Atherton, 2004), the study by Moon et al. (2009) did not necessarily contradict those of others.
Another possible beneficial effect of H. pylori is protection against tuberculosis, as a study in West Africa indicated that persons with latent tubercular infections have lower chances of re-activating their infections when they are H. pylori positive. This is attributed to the fact that H. pylori induces the release of IFN-y, an interferon that activates defenses against different types of infections including tuberculosis (Perry et al., 2010). Finally, H. pylori is proposed to play a positive role in body weight regulation: H. pylori positive persons produce reduced levels of ghrelin, a weight-regulating hormone that is produced for 60–80% in the stomach. H. pylori eradication leads to an increased production of ghrelin and a subsequent increase in body weight (Nwokolo et al., 2003).
Correlation Between Diet and Lifestyle, and H. pylori Associated Pathology
There is evidence for a connection between H. pylori and dyspeptic symptoms (suffering from indigestion), as it has been shown that the odds ratio of a person with dyspeptic symptoms being a carrier of H. pylori is higher than the H. pylori incidence in the general population (Kidd et al., 1999b). Furthermore, H. pylori eradication leads to long-term cure in the majority of peptic ulcer patients, whereas the natural relapse rate is 70% (Kate et al., 2013).
However, this argument is not a definite proof for a causal relationship between H. pylori and dyspepsia. It has been suggested that genetic predisposition and environmental factors such as diet, smoking, age and the use of non-steroidal anti-inflammatory drugs (NSAIDs) can lead to high gastric acid production, which in turn can lead to gastric metaplasia and subsequent development of duodenal ulcers (Tovey, 2009; Kate et al., 2013). Similarly, the intake of iron supplements has been associated with gastric pathology through the induction of oxidative stress (Naito et al., 1995; Fisher and Naughton, 2004). The positive correlation between H. pylori and dyspeptic symptoms is attributed to the fact that treatment of duodenal ulcer with acid-reducing medicine facilitates H. pylori colonization in the stomach. Hence, the self-reported high incidence of ulcers in Uganda might be partly a result of lifestyle and dietary habits of the local population.
The hypothesis that H. pylori colonization is a result rather than a cause of ulcers, is supported by a retrospective case review of 208 persons, of which 37 were diagnosed with duodenal ulcers. A total number of 32 of the ulcer patients had been suffering from the ulcer for more than 6 months, and all 32 were found to be H. pylori positive. Only five patients suffered from ulcers less than 6 months, but all five were found to be H. pylori negative (Boulos et al., 2002). Upon colonization, H. pylori might produce toxic substances that inhibit the natural healing of the ulcers, which explains why H. pylori eradication leads to highly increased chances of long-term cure of ulcers (Tovey, 2009; Kate et al., 2013).
Tovey (2009) emphasizes the role of dietary protective factors. Dietary fiber intake was one of the first factors that was suspected to be negatively associated with the incidence of ulcers. In an intervention study among 42 culturally rice-eating subjects with a history of duodenal ulcers, 21 were changed to a predominantly unrefined wheat diet, whereas the remaining 21 continued the rice diet. Over a period of 5 years, 81% of the subjects in the rice-eating group reported ulcers, versus only 14% in the wheat-eating group (Malhotra, 1978). In a Norwegian study, 73 subjects who recently healed from an ulcer (but by whom H. pylori was not necessarily eradicated) were assigned to either a high-fiber diet or a low-fiber diet. After 6 months, ulcers had reoccurred in 80% of the subjects on the low-fiber diet, versus only in 45% of the subjects on high-fiber diet (Rydning et al., 1982).
A case–control study in the United Kingdom compared 78 subjects with duodenal ulcer with 156 matching control subjects (two matches for every ulcer subject). After controlling for total calorie intake, it was found that sugar intake correlated positively and high vegetable fiber intake negatively with ulcers, whereas cereal fibers did not show a specific correlation. After further controlling for smoking, social class and body weight, only the intake of refined sugar remained a significant factor that correlated with ulcer incidence (Katschinski et al., 1990).
From an anecdotal study on the incidence of peptic ulcers in sub-Saharan Africa, a correlation between areas with a high incidence of ulcers and high intake of starchy foods, such as bananas, cassava, sweet potato, white wheat flour, white maize flour, and white rice has been suggested. This was opposed to regions with high intake of millet and home-pounded (unrefined) maize, where the incidence of ulcers was low (Tovey, 2009). However, Tovey (2009) suggests that it is not the fiber from the unrefined cereal in itself, but rather the phospholipids, sterol esters, and sterol fractions of the lipid components from the cereal fiber fraction that exercises the protective action.
Other studies identified the plant-derived polyunsaturated fatty acids lipid fraction, such as linoleic and linolenic acid, to have inhibitory effects on H. pylori. In vitro studies with these components showed to cause cell death to H. pylori by damaging the bacterial outer lipid membrane (Thompson et al., 1994). Besides, certain fatty acids are precursors for prostaglandins which protect the gastric mucosa against injury through increased mucus secretion. Furthermore, arachidonic acid has been shown to improve gastric blood vessel synthesis, thereby speeding up gastric healing processes (Hollander and Tarnawski, 1990).
Also in more dated review studies on the relationship between diet and duodenal ulcers Misciagna et al. (2000) concluded that there were strong indications for a negative correlation between duodenal ulcers and the intake of fiber, mainly soluble fiber from fruit and vegetables, and perhaps polyunsaturated fatty-acids, vitamin A, and vitamin C. However, the study made note of the overall poor quality from the studies that led to this conclusion.
Other studies suggest a correlation between intake of salt and gastric pathology, partly modulated through H. pylori, most commonly in the form of gastric cancer (Wang et al., 2009). Ulceration has also been mentioned as an outcome of high salt intake (Kato et al., 1992), though another study found no effect of salt intake on gastric inflammation (Lee et al., 2014). An in vitro study suggested that salt induces expression of virulence factors in H. pylori (Xu et al., 2011), which provides a possible explanation for increased gastric pathology upon high salt intake.
The Probiotic LGG in H. pylori Eradication Therapy
One of the few other bacteria that can abide under the same gastric circumstances as H. pylori (Bezkorovainy, 2001), being resistant to acid and bile (Fernandez et al., 2003), is LGG and its generic variant LRY. The probiotic bacterium LGG is world’s best documented probiotic bacterium, with many unique characteristics and reported health benefits (Segers and Lebeer, 2014). Especially to provide access to this bacterium for people in developing countries, the LRY has been incorporated in an affordable probiotic starter culture that enables local communities to make their own probiotic fermented food (Kort et al., 2015; Westerik et al., 2016). H. pylori can be inhibited in a dose dependent manner by LRY through several pathways, as discussed below, and hence may play a role in the reduction of H. pylori related gastric pathology in East Africa (Angol et al., 2017).
Currently, the standard method to eradicate H. pylori is a three-component therapy treatment that combines acid suppression with two antibiotics for 1 week. This method is also used in East Africa for those who can afford it (Angol et al., 2017). However, H. pylori becomes increasingly resistant against this therapy, and the success rates are dropping below 70% (Malfertheiner et al., 2012). Furthermore, the therapy typically has low patient compliance due to many side effects such as diarrhea, nausea, vomiting, bloating, and abdominal pain (Armuzzi et al., 2001a). The European Helicobacter Study Group recently acknowledged that certain probiotics show promising results as an adjuvant treatment in reducing side effects of the antibiotic therapy (Malfertheiner et al., 2012).
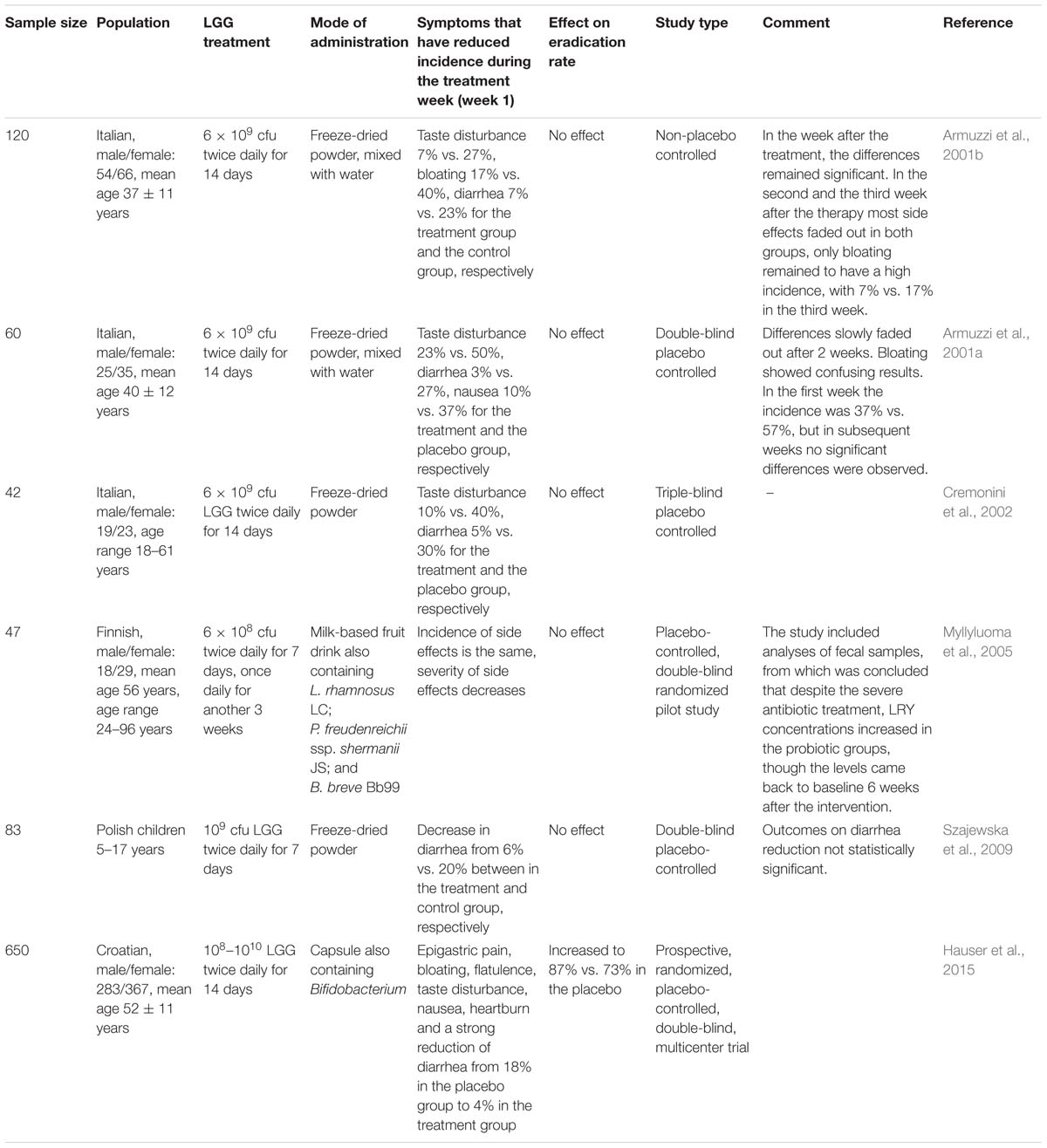
A summary of studies on the role of LGG in H. pylori eradication therapy can be found in Table 2. All studies confirmed a beneficial effect of LGG in reducing side effects of eradication therapy. Five studies with an average sample size of 70, showed no significant effect on the H. pylori eradication rate. However, the sixth study with a sample size of 650 showed H. pylori eradication rates of 87% and 73% in the LGG-supplemented group and the control group, respectively.
Ulcer Prevention and Suppression by LGG
Apart from its role as co-supplement during H. pylori eradication therapy, LGG has a direct inhibitory effect on H. pylori and has the potential to directly prevent and reverse gastric pathology in East African populations. Accordingly, in a review of 13 clinical trials reporting on the activity of probiotics on H. pylori, Hamilton-Miller (2003) concludes that probiotics can reduce the severity of H. pylori induced pathology, but are not able to eradicate H. pylori completely. Three distinct pathways in which LGG counteracts H. pylori-induced gastric pathology in the stomach and duodenum can be discriminated: (i) competition for binding sites between LGG and H. pylori, (ii) attenuation of the host’s H. pylori-induced apoptosis, inflammation responses and stimulation of angiogenesis, and (iii) production of anti-microbial substances such as lactic acid.
Competition for Binding Sites
In vitro pre-treatment of epithelial glandular cells (coca-2 cell culture) with 107 cfu/ml LGG was found to inhibit subsequent adhesion by H. pylori with 53%, and at 109 cfu/ml LGG inhibited H. pylori with 66%. Competition for binding sites on the epithelial cells was cited as a probable cause (Myllyluoma et al., 2008) (Figure 1), which appeared to be unrelated to adhesion capacity or organic acid production. Note that binding alone is not a marker for anti-H. pylori activity (Peña and Versalovic, 2003; Myllyluoma et al., 2008).
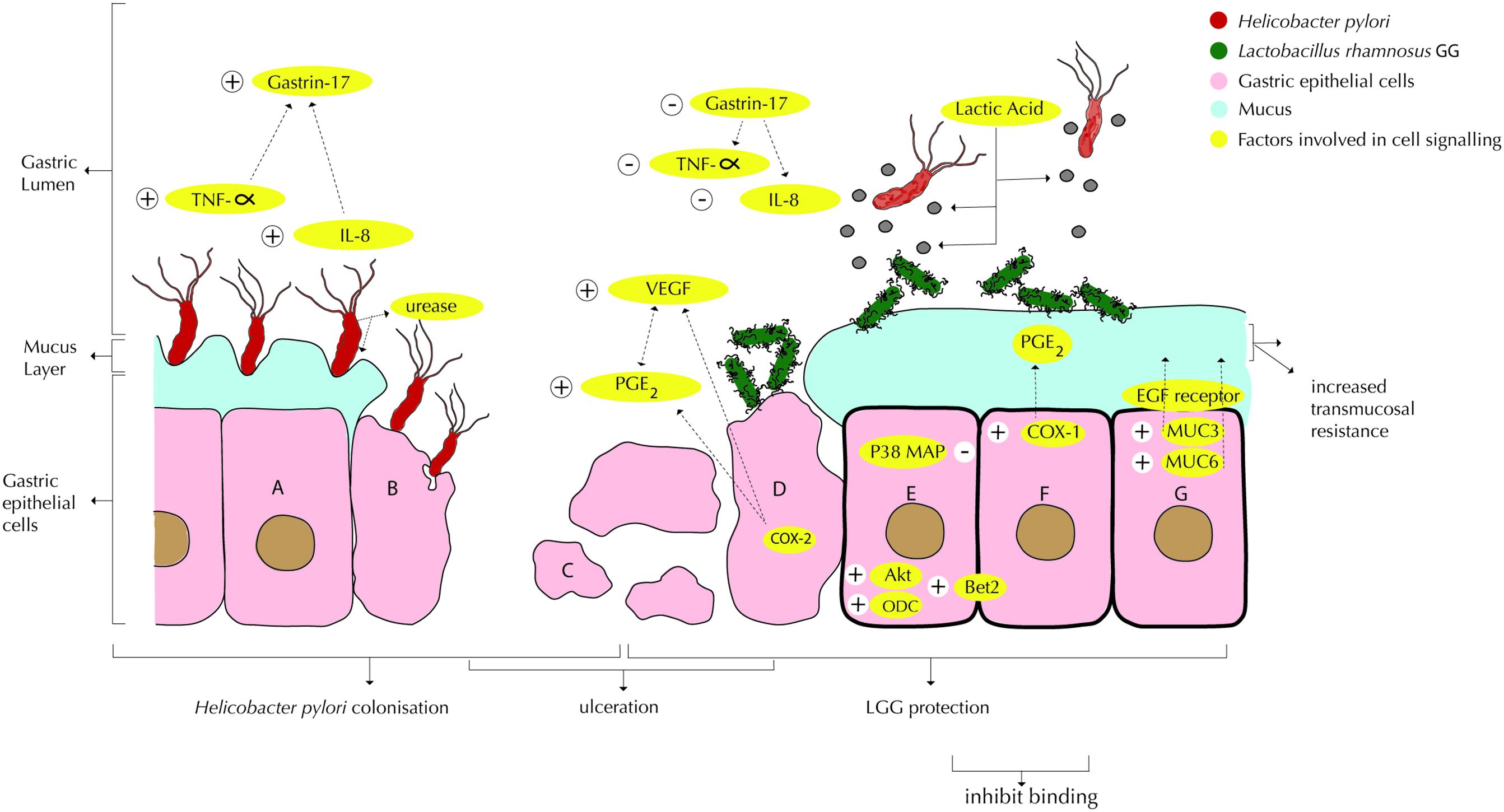
FIGURE 1. Interactions of Lactobacillus rhamnosus GG or LRY (green) with Helicobacter pylori (red) and damaged epithelial cells at the gastric mucosal surface. (A) H. pylori upregulates TNF-α (Peña and Versalovic, 2003) and IL-8 (Myllyluoma et al., 2008; Rokka et al., 2008), which in turn upregulate gastrin-17 (Myllyluoma et al., 2007). H. pylori produces urease to reduce the pH in its immediate environment as means of survival (Chen et al., 2010). (B) Interactions under A are causing inflammation and apoptosis. (C) Gastric inflammation and apoptosis lead to ulceration. (D) LRY binds specifically at affected (mucosal) tissue due to change in microbiota and inflammatory markers (immunoglobulins and cytokines) and qualitative and quantitative changes in the mucus (Lam et al., 2007b). LRY upregulates COX-2 protein expression in damaged (ulcerated) stomachs (Jackson et al., 2000), which induces PGE2 modulated vascular endothelial growth factor (VEGF) expression, causing angiogenesis (Korhonen et al., 2004; Tamura et al., 2006; Lam et al., 2007b). LRY inhibits H pylori induced IL-8 (interleukin-8) release (Myllyluoma et al., 2008; Rokka et al., 2008) and TNF-α release (Peña and Versalovic, 2003; Kim et al., 2006). Through suppression of TNF-α and IL-8, H pylori induced elevated gastrin-17 levels are attenuated (Myllyluoma et al., 2007). (E) LRY inhibits H pylori adhesion (Myllyluoma et al., 2008), which appears to be related to competition for binding sites (Peña and Versalovic, 2003; Myllyluoma et al., 2008). LRY activates Akt/protein kinase B, which is an anti-apoptotic signaling pathway (Yan and Polk, 2002). LRY blocks p38 MAP, which is a pro-apoptosis (cell survival) signaling pathway (Yan and Polk, 2002). LRY induces upregulation of ornithine decarboxylase (ODC) (enzyme essential in DNA stabilization and thus cell growth) and B-cell lymphoma 2 (Bcl-2) causing attenuation of apoptosis (Lam et al., 2007b). LRY shows antagonistic activity against H. pylori in vitro, possibly associated with lactic acid production (De Keersmaecker et al., 2006; Hütt et al., 2006). Lactic acid increases the cell wand permeability of H. pylori. Besides, production of exopolysaccharide (Allonsius et al., 2017), lectin (Petrova et al., 2016), and/or peptides with antimicrobial activity (Lu et al., 2009) by LRY has been shown to inhibit other pathogenic bacteria, however, this effect has not been shown for H. pylori specifically. LRY inhibits urease activity of H. pylori (Chen et al., 2010). (F) LRY upregulates COX-1 (cyclooxygenase-1) protein expression (Jackson et al., 2000; Lam et al., 2007a) in normal stomachs (ulcer prevention), which increases PGE2, which in turn protects mucus cells and increases trans mucosal resistance, thereby protecting mucosal cells from apoptosis (Jackson et al., 2000; Lam et al., 2007a). LRY upregulates the expression of MUC3 and MUC6 mucin gene, causing significant increase in thickness of the basal mucus layer (Mack et al., 2003; Lam et al., 2007a). (G) LRY causes upregulation of phosphorylation level of epidermal growth factor receptor (EGF receptor), leading to cell proliferation and angiogenesis and suppresses cell apoptosis (Lam et al., 2007b). LRY initially improves epithelial (mucosal) barrier function (Gotteland et al., 2001; Myllyluoma et al., 2008) but increases H. pylori induced barrier deterioration after incubation for 24–42 h, hence causes delayed cell membrane damage and leakage (Myllyluoma et al., 2008).
Secondly, a study on mice found that during a pre-treatment with a combination of the probiotic bacteria L. acidophilus R0052 and L. rhamnosus R0011 and subsequent administration of H. pylori, colonization was reduced from 100% in the control group to 50% in the probiotic pre-treated group (Johnson-Henry et al., 2004). Note that this effect has not been proven for LGG specifically.
In a third study, a decrease in H. pylori of 27% was measured in a probiotic intervention among 13 Finnish H. pylori positive subjects, based on the well accepted 13C-Urea Breath Test, which values were an indirect indicator of H. pylori density in gastric mucosa. The subjects daily consumed a drink with four probiotic strains, including LGG, with each of the strains in a concentration of 2.5 × 109 cfu, for the duration of 56 days, for the duration of 56 days (Myllyluoma et al., 2007).
Upon the formation of an ulcer, probably regardless of the causative factor of the ulceration, a spectrum of bacteria other than H. pylori has been found to colonize the ulcer surface and to subsequently impair ulcer healing (Elliott et al., 1998). This has been attributed to a local increase of the pH around the ulcer surface, allowing for strongly increased bacterial growth as compared to normal stomach tissue. The same study found that antibiotic treatment, preferably with a mixture of different antibiotics, increased ulcer healing. Secondly, promotion of lactic acid bacteria (non-specified) in the stomach competed with outgrowth of other possibly harmful bacteria, and increased the rate of ulcer healing. Other studies have indicated specifically the suppressing effect of LGG on several pathogens (Rinkinen et al., 2003; Hütt et al., 2006) and its site-specific binding to damaged tissue (Ouwehand et al., 2003).
Modulation of Immune Responses
LGG has shown to modulate local immune responses upon colonization with H. pylori or ulceration through the pathways as summarized in Figure 1.
In vitro pre-treatment of epithelial glandular cells (coca-2 cell culture) with 107 cfu/ml LGG was shown to counteract acute H. pylori cell membrane leakage by initially tightening the barrier function (Gotteland et al., 2001; Myllyluoma et al., 2008). However, over time H. pylori induced barrier deterioration after incubation for 24–42 h, causing delayed cell membrane damage and leakage (Myllyluoma et al., 2008).
H. pylori upregulates TNF-α (Peña and Versalovic, 2003) and IL-8 (Myllyluoma et al., 2008; Rokka et al., 2008), which in turn upregulate gastrin-17. IL-8 is a chemokine which induces inflammation (Rokka et al., 2008) and TNF-α is a cytokine which induces apoptosis (Peña and Versalovic, 2003). Gastric inflammation and apoptosis lead to ulceration. Gastrin-17 has been associated with gastric cancer (Myllyluoma et al., 2007). LGG inhibits H pylori induced IL-8 (interleukin-8) release (Myllyluoma et al., 2008; Rokka et al., 2008) and TNF-α release (Peña and Versalovic, 2003; Kim et al., 2006), although it must be noted that another in vitro study (Zhang et al., 2005) showed that high doses of LGG (1010 cfu) can increase IL-8 production in the absence of H. pylori. Through suppression of TNF-α and IL-8, H pylori induced elevated gastrin-17 levels were attenuated (Myllyluoma et al., 2007). A decrease in the hormone gastrin-17 levels was measured in subjects who daily consumed a drink with four probiotic strains including LGG. This study concluded that gastrin-17 could be seen as a maker for non-atrophic gastritis, that probiotics can have a moderate positive influence on non-atrophic gastritis (Myllyluoma et al., 2007).
Apart from H. pylori, alcohol has been associated with gastric mucosal damage, and alcoholism can be another cause of peptic ulcers. Rats pre-treated with LGG for 3 days responded to the administration of ethanol in a dose-dependent manner: 1 h after administering 10 ml/kg bodyweight of 60% v/v ethanol, the 2 × 108 cfu/day pre-treated group did not show any difference, while in the 2 × 109 cfu/day group showed 45% smaller gastric lesions as compared to the control group (Lam et al., 2007a). The study concluded that LGG significantly increases the mucosal layer and mucosal integrity (trans mucosal resistance) through upregulated expression of MUC3 (Mack et al., 2003) and MUC6 mucin genes, thereby counteracting the effects that ethanol normally has on the mucus layer (Lam et al., 2007a). Nevertheless, LGG regulates COX-1 (cyclooxygenase-1) protein expression (Jackson et al., 2000; Lam et al., 2007a) in normal stomachs, which increases PGE2, which protects mucus cells and increases trans mucosal resistance, thereby protecting mucosal cells from apoptosis (Jackson et al., 2000; Lam et al., 2007a) and reducing chances of ulceration.
In a subsequent study of Lam et al. (2007b), ulcers were induced by luminal application of acetic acid solution, and LGG was administered in the same manner as described for the previous study, but this time after the ulcer-induction. LGG supplementation had no obvious effects in the control group, but for the ulcer-induced group the larger dose at 109 cfu/day induced enhanced cell proliferation of 54%, increased blood vessels generation (angiogenesis) by 41% at the ulcer margins and reduced cell death (apoptosis) by 33%, thereby obtaining significantly reduction of gastric ulcer area by 32% after 3 days of LGG administration. These effects were found to be modulated firstly by the upregulation of phosphorylation level of epidermal growth factor receptor (EGF receptor) causing angiogenesis, cell proliferation and attenuation of apoptosis. Secondly, COX-2 protein expression was upregulated, which induces PGE2 modulated vascular endothelial growth factor (VEGF) expression, causing angiogenesis (Korhonen et al., 2004; Tamura et al., 2006; Lam et al., 2007b). Thirdly, LGG upregulates ornithine decarboxylase (ODC) (enzyme essential in DNA stabilization and thus cell growth) and B-cell lymphoma 2 (Bcl-2), thereby causing attenuation of apoptosis (Lam et al., 2007b). The healing continued upon administration of LRY for more days. The study concludes that LGG does not affect the normal gastric mucosa, but normalizes gastric mucosa that is altered by events such as ulcers. LGG has shown to bind specifically at affected (mucosal) tissue due to a change in microbiota and inflammatory markers (immunoglobulins and cytokines) and qualitative and quantitative changes in the mucus. The study has not shown whether it is the live LGG, its metabolites, its cell wall components or other results of gene expression that exert the healing properties of the organism (Lam et al., 2007b).
Similarly, in an intervention among 16 human subjects, a 5-day pre-treatment with a probiotic dairy product containing among others 2.4 × 109 cfu LGG per day, has been proven to stabilize the intestinal barrier function against increased permeability normally induced by NSAIDs by 77%, thereby preventing the alterations from future pathology such as ulcers (Gotteland et al., 2001). Heat-killed LGG did not show protective effects. Interestingly, a study by Kamil et al. (2007) on ulceration in the small intestine of rats as induced by NSAIDs, found that although LGG increased cell proliferation and reduced cell apoptosis, it still aggravated the NSAID induced ulcer size, possibly through increased inflammation.
Other pathways include the activation of Akt/protein kinase B by LGG, which is an anti-apoptotic signaling pathway (Yan and Polk, 2002). Secondly, LGG blocks p38 MAP, which is a pro-apoptosis (cell survival) signaling pathway (Yan and Polk, 2002). Lastly, H. pylori produces urease to reduce the pH in its immediate environment as means of survival, which is inhibited by LGG (Chen et al., 2010), thereby reducing its chances for survival.
The Probiotic LGG Produces Antimicrobial Substances Against H. pylori
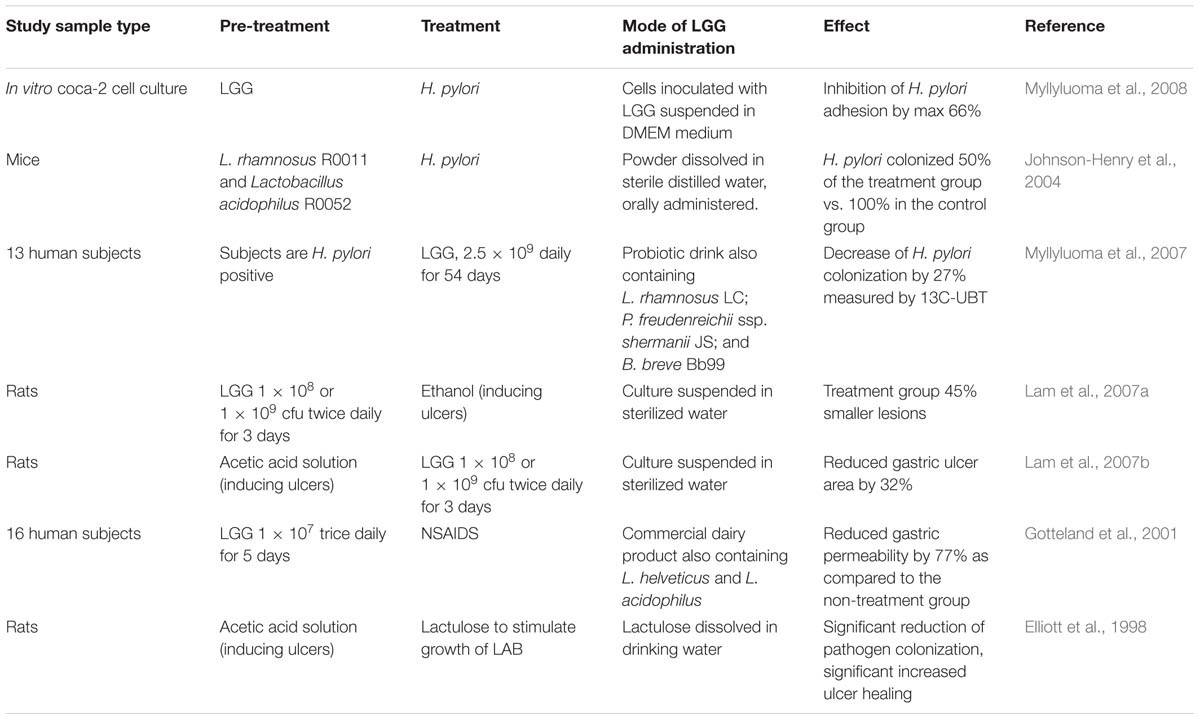
Antagonistic activity against H. pylori in vitro has been associated with lactic acid production (De Keersmaecker et al., 2006; Hütt et al., 2006; Segers and Lebeer, 2014) and to a lesser extent with the production of other short-chain fatty acids (SCFAs) (Gotteland et al., 2006). Lactic acid increases the cell wall permeability of H. pylori (Figure 1). Besides, production of exopolysaccharide (Allonsius et al., 2017), lectin (Petrova et al., 2016), and/or peptides with antimicrobial activity (Lu et al., 2009) by LGG has been shown to inhibit other pathogenic bacteria, however, this effect has not been shown for H. pylori specifically. A summary of studies on the effect of LGG on H. pylori and gastric pathology can be found in Table 3.
A Sustainable Nutritional Intervention in East Africa – the Case Study of Uganda
The absence of dietary protective factors in the traditional starch-based diet in Uganda which mainly consists of bananas, roots and refined cereals, might be a major factor causing the high incidence of ulcers in this country. In rural Uganda, when one experiences dull, sharp or burning pain in the upper abdomen that might indicates dyspepsia, subsequent self-medication with simple and cheap acid suppressors (magnesium trisilicate) is the common practice. Many clinics lack any form of diagnostic equipment and will diagnose ulcers based on description of symptoms only, and subsequently mainly prescribe acid suppressors, though other medicine like histamine antagonists (H2 blockers) and proton-pump inhibitors that suppress acid secretion are also common. The frequent advice is to not use NSAIDs, spices, cigarettes, alcohol or carbonated drinks. Some clinics can perform serum-blood test to detect the presence of H. pylori antibodies. Only the minority of the population that enjoys a better economic status might visit private clinics that can perform endoscopy, and might opt for the more expensive eradication therapy. In its clinical guidelines, the Uganda ministry of health advises to primarily treat ulcers with acid suppressors and encourages regular, small and frequent meals, as well as the consumption of milk (Ministry of Health, 2010).
Kort and Sybesma (2012) and Kort et al. (2015) described an intervention with the generic variant of LGG, in form of an LRY containing yogurt drink, which is locally produced (Westerik et al., 2016) and subsequently consumed by resource-poor communities in rural Uganda. We propose such an intervention as a preferred option to alleviate the burden of H. pylori induced pathology in resource poor communities. Blaser and Atherton (2004) mentioned that a beneficial function of H. pylori colonization is the reduction of childhood diarrhea. However, when H. pylori is in part replaced by LRY, this beneficial function of H. pylori might not be lost: LRY has the proven ability to reduce certain types diarrhea (De Roos and Katan, 2000; Szajewska et al., 2007; Allen et al., 2010; Guandalini, 2011; Guarino et al., 2015), but without the adverse risks that are associated with H. pylori colonization. The local production of probiotic fermented foods can be extended to cereal fermentations, most importantly as a variation on the already popular fermented millet drink (obushera) for the case of Uganda (Westerik et al., 2016). As it has been suggested that cereal fibers provide additional protection against gastric pathology (Malhotra, 1978; Tovey, 2009), this high-fiber drink may exhibit dual protective action against ulcer formation.
Conclusion
Studies on the incidence of H pylori in East Africa showed widely varying outcomes, ranging between 25 and 87% in various population groups, possibly due to different detection methods used, or differences in study population. Pathology upon H. pylori colonization is modulated by several factors including the presence of virulence factors in the H. pylori strain, the ‘ethnic’ origin of the strain, and the specific immune responses of the host. Apart from H. pylori colonization, gastric pathology including ulceration is also affected by lifestyle factors, including diet. The right dietary factors have been shown to directly inhibit H. pylori as well as reduce H. pylori induced pathology.
It is expected that administering probiotic yogurt to children in developing countries from early childhood can reduce the incidence of H. pylori colonization in the general population. Besides prevention of H. pylori, LGG or its generic variant LRY may present an approach to establish and manage a harmless relationship between the host and H. pylori when the latter one is already present, counteracting the need for H. pylori eradication therapy. This alternative approach is cheaper and does not carry the risk of extensive antibiotic resistance (Michetti, 2001), and is feasible to be implemented sustainably through locally produced yogurt containing LRY.
It should be noted that major changes in diet might play an equally important role in the prevention and relieve of gastric pathology. In Uganda, locally available foods with protective factors include unrefined wheat, unrefined maize, unrefined rice, millet, soy beans, full-cream milk, spinach, and cabbage (Tovey, 2009). However, consumption of these products would require education of the population and a change in attitude, since even the rural population as a rule brings produced cereals to an electrical mill, in which the bran is separated from the cereal and subsequently the bran fraction is being used solely for animal feeds. An intervention with the mentioned locally produced yogurt could capture the dietary protective benefits of milk as well as the those of LRY.
Author Contributions
NW reviewed the cited literature and drafted the manuscript under guidance of RK. WS, GR, and RK critically read and corrected the draft versions of the article.
Funding
Funding for this study was provided by the Yoba for Life Foundation, Amsterdam, Netherlands.
Conflict of Interest Statement
RK and WS are co-founders of the Yoba for Life Foundation (2009), a non-profit organization, accredited by the Dutch Tax Authorities as a Public Benevolent Institution (PBI), which aims to promote local production and consumption of fermented products in Africa. NW is the Country Coordinator of the Yoba for Life Foundation in Uganda. African fermented products made with the Yoba starter culture, are not marketed by the foundation as such, but the Yoba for Life Foundation stimulates local production and ownership, allowing income-generating activities for African small-scale entrepreneurs in the food sector. The Yoba for Life Foundation distributes and sells ready-to-use sachets with dried bacterial starter cultures at cost price, through a network of partners and volunteers to facilitate the local production of dairy and cereal-based products by controlled bacterial fermentation. The Yoba starter culture contains Lactobacillus rhamnosus yoba 2012, which is a generic variant of Lactobacillus rhamnosus GG.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge all the small-scale probiotic yogurt producers who may contribute to the alleviation of Helicobacter pylori-associated gastric pathology in East Africa.
References
Allen, S. J., Martinez, E. G., Gregorio, G. V., Dans, L. F. (2010). Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst. Rev. 11:11. doi: 10.1002/14651858.CD003048.pub3
Allonsius, C. N., van den Broek, M. F. L., De Boeck, I., Kiekens, S., Oerlemans, E. F. M., Kiekens, F., et al. (2017). Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 1, 1753–1763. doi: 10.1111/1751-7915.12799
Angol, D. C., Ocama, P., Ayazika Kirabo, T., Okeng, A., Najjingo, I., Bwanga, F. (2017). Helicobacter pylori from Peptic Ulcer Patients in Uganda Is Highly Resistant to Clarithromycin and Fluoroquinolones: Results of the GenoType HelicoDR Test Directly Applied on Stool. BioMed. Res. Int. 2017:9. doi: 10.1155/2017/5430723
Armuzzi, A., Cremonini, F., Bartolozzi, F., Canducci, F., Candelli, M., Ojetti, V., et al. (2001a). The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 5, 163–169. doi: 10.1046/j.1365-2036.2001.00923.x
Armuzzi, A., Cremonini, F., Ojetti, V., Bartolozzi, F., Canducci, F., Candelli, M., et al. (2001b). Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion 63, 1–7. doi: 10.1159/000051865
Baingana, R. K., Kiboko Enyaru, J., and Davidsson, L. (2014). Helicobacter pylori infection in pregnant women in four districts of Uganda: role of geographic location, education and water sources. BMC Public Health. 4:915. doi: 10.1186/1471-2458-14-915
Balint, G. A. (1998). Selected gastrointestinal pathologies in tropical sub-Saharan Africa. Bull. World Health Organ. 76, 207–212.
Bezkorovainy, A. (2001). Probiotics: determinants of survival and growth in the gut. Am. J. Clin. Nutr. 73, 399s–405s. doi: 10.1093/ajcn/73.2.399s
Blaser, M. J., and Atherton, J. C. (2004). Helicobacter pylori persistence: biology and disease. J. Clin. Invest. 1, 321–333. doi: 10.1172/JCI20925
Boulos, P. B., Botha, A., Hobsley, M., Holton, J., Oshowo, A. O., and Tovey, F. I. (2002). Possible absence of Helicobacter pylori in the early stages of duodenal ulceration. QJM 1, 749–752. doi: 10.1093/qjmed/95.11.749
Bravo, L. E., van Doorn, L.-J., Realpe, J. L., and Correa, P. (2002). Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma? Am. J. Gastroenterol. 97, 2839–2842. doi: 10.1111/j.1572-0241.2002.07031.x
Chen, X., Tian, F., Liu, X., Zhao, J., Zhang, H.-P., Zhang, H., et al. (2010). In vitro screening of Lactobacilli with antagonistic activity against Helicobacter pylori from traditionally fermented foods. J. Dairy Sci. 93, 5627–5634. doi: 10.3168/jds.2010-3449
Cover, T. L., and Blanke, S. R. (2005). Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3, 320–332. doi: 10.1038/nrmicro1095
Cover, T. L., and Blaser, M. J. (2009). Helicobacter pylori in health and disease. Gastroenterology 136, 1863–1873. doi: 10.1053/j.gastro.2009.01.073
Crabtree, J. E., Taylor, J. D., Heatley, R. V., Shallcross, T. M., Rathbone, B. J., Wyatt, J. I., et al. (1991). Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet 10, 332–335. doi: 10.1016/0140-6736(91)90477-7
Cremonini, F., Di Caro, S., Covino, M., Armuzzi, A., Gabrielli, M., Santarelli, L., et al. (2002). Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am. J. Gastroenterol. 97, 2744–2749. doi: 10.1111/j.1572-0241.2002.07063.x
De Keersmaecker, S. C., Verhoeven, T. L. A., Desair, J., Marchal, K., Vanderleyden, J., and Nagy, I. (2006). Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett. 1, 89–96. doi: 10.1111/j.1574-6968.2006.00250.x
De Roos, N. M., and Katan, M. B. (2000). Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am. J. Clin. Nutr. 71, 405–411. doi: 10.1093/ajcn/71.2.405
Elliott, S. N., Buret, A., McKnight, W., Miller, M. J., and Wallace, J. L. (1998). Bacteria rapidly colonize and modulate healing of gastric ulcers in rats. Am. J. Physiol. Gastrointest Liver Physiol. 275, G425–G432. doi: 10.1152/ajpgi.1998.275.3.G425
Fernandez, M. F., Boris, S., and Barbes, C. (2003). Probiotic properties of human Lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94, 449–455. doi: 10.1046/j.1365-2672.2003.01850.x
Fernando, N., Holton, J., Zulu, I., Vaira, D., Mwaba, P., and Kelly, P. (2001). Helicobacter pylori Infection in an Urban African population. J. Clin. Microbiol. 1, 1323–1327. doi: 10.1128/JCM.39.4.1323-1327.2001
Fisher, A. E., and Naughton, D. P. (2004). Iron supplements: the quick fix with long-term consequences. Nutr. J. 16:2. doi: 10.1186/1475-2891-3-2
Ghose, C., Perez-Perez, G. I., Dominguez-Bello, M.-G., Pride, D. T., Bravi, C. M., and Blaser, M. J. (2002). East Asian genotypes of Helicobacter pylori strains in amerindians provide evidence for its ancient human carriage. Proc. Natl. Acad. Sci. U.S.A. 99, 15107–15111. doi: 10.1073/pnas.242574599
Go, M. F. (2002). Natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol. Ther. 1, 3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x
Gotteland, M., Brunser, O., and Cruchet, S. (2006). Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol. Ther. 1, 1077–1086. doi: 10.1111/j.1365-2036.2006.02868.x
Gotteland, M., Cruchet, S., and Verbeke, S. (2001). Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment Pharmacol. Ther. 17, 11–17. doi: 10.1046/j.1365-2036.2001.00898.x
Guandalini, S. (2011). Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 45, S149–S153. doi: 10.1097/MCG.0b013e3182257e98
Guarino, A., Guandalini, S., and Vecchio, A. L. (2015). Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 49, S37–S45. doi: 10.1097/MCG.0000000000000349
Hamilton-Miller, J. M. T. (2003). The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int. J. Antimicrob. Agents. 22, 360–366. doi: 10.1016/S0924-8579(03)00153-5
Hauser, G., Salkic, N., Vukelic, K., JajacKnez, A., and Stimac, D. (2015). Probiotics for standard triple Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial. Medicine (Baltimore) 94:17. doi: 10.1097/MD.0000000000000685
Hestvik, E., Tylleskar, T., Kaddu-Mulindwa, D. H., Ndeezi, G., Grahnquist, L., Olafsdottir, E., et al. (2010). Helicobacter pylori in apparently healthy children aged 0-12 years in urban Kampala. Uganda: a community-based cross sectional survey. BMC Gastroenterol. 10:62. doi: 10.1186/1471-230X-10-62
Holcombe, C. (1992). Helicobacter pylori: the African enigma. Gut 33, 429–431. doi: 10.1136/gut.33.4.429
Hollander, D., and Tarnawski, A. (1990). Is there a role for dietary essential fatty acids in gastroduodenal mucosal protection? J. Clin. Gastroenterol. 13(Suppl. 1), S72–S74.
Hütt, P., Shchepetova, J., Lõivukene, K., Kullisaar, T., and Mikelsaar, M. (2006). Antagonistic activity of probiotic Lactobacilli and bifidobacteria against entero-and uropathogens. J. Appl. Microbiol. 1, 1324–1332. doi: 10.1111/j.1365-2672.2006.02857.x
Jackson, L. M., Wu, K. C., Mahida, Y. R., Jenkins, D., and Hawkey, C. J. (2000). Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut 47, 762–770. doi: 10.1136/gut.47.6.762
Johnson-Henry, K. C., Mitchell, D. J., Avitzur, Y., Galindo-Mata, E., Jones, N. L., and Sherman, P. M. (2004). Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-Infected mice. Dig. Dis. Sci. 49, 1095–1102. doi: 10.1023/B:DDAS.0000037794.02040.c2
Kamil, R., Geier, M. S., Butler, R. N., and Howarth, G. S. (2007). Lactobacillus rhamnosus GG exacerbates intestinal ulceration in a model of indomethacin-induced enteropathy. Dig. Dis. Sci. 1, 1247–1252. doi: 10.1007/s10620-006-9443-3
Kate, V., Ananthakrishnan, N., and Tovey, F. I. (2013). Is Helicobacter pylori infection the primary cause of duodenal ulceration or a secondary factor? A review of the evidence. Gastroenterol. Res. Pract. 27:e425840.
Kato, I., Nomura, A. M. Y., Stemmermann, G. N., and Chyou, P.-H. (1992). A prospective study of gastric and duodenal ulcer and its relation to smoking, alcohol, and diet. Am. J. Epidemiol. 1, 521–530. doi: 10.1093/oxfordjournals.aje.a116319
Katschinski, B. D., Logan, R. F., Edmond, M., and Langman, M. J. (1990). Duodenal ulcer and refined carbohydrate intake: a case-control study assessing dietary fibre and refined sugar intake. Gut 1, 993–996. doi: 10.1136/gut.31.9.993
Kidd, M., Lastovica, A. J., Atherton, J. C., and Louw, J. A. (1999a). Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut 45, 499–502.
Kidd, M., Louw, J. A., and Marks, I. N. (1999b). Helicobacter pylori in Africa: observations on an ‘enigma within an enigma. J. Gastroenterol. Hepatol. 1, 851–858. doi: 10.1046/j.1440-1746.1999.01975.x
Kim, S. O., Sheikh, H. I., Ha, S.-D., Martins, A., and Reid, G. (2006). G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 8, 1958–1971. doi: 10.1111/j.1462-5822.2006.00763.x
Kodaman, N., Pazos, A., Schneider, B. G., Piazuelo, M. B., Mera, R., Sobota, R. S., et al. (2014). Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc. Natl. Acad. Sci. U.S.A. 111, 1455–1460. doi: 10.1073/pnas.1318093111
Korhonen, R., Kosonen, O., Korpela, R., and Moilanen, E. (2004). The expression of COX2 protein induced by Lactobacillus rhamnosus GG, endotoxin and lipoteichoic acid in T84 epithelial cells. Lett. Appl. Microbiol. 1, 19–24. doi: 10.1111/j.1472-765X.2004.01531.x
Kort, R., and Sybesma, W. (2012). Probiotics for every body. Trends Biotechnol. 30, 613–615. doi: 10.1016/j.tibtech.2012.09.002
Kort, R., Westerik, N., Serrano, L. M., Douillard, F., Gottstein, W., Ananta, E., et al. (2015). A novel consortium of Lactobacillus rhamnosus and Streptococcus thermophilus for increased access to functional fermented foods. Microb. Cell Fact. 14:195. doi: 10.1186/s12934-015-0370-x
Lachlan, G. W., Gilmour, H. M., and Jass, J. J. (1988). Campylobacter pylori in central Africa. Br. Med. J. Clin. Res. Ed. 296:66. doi: 10.1136/bmj.296.6614.66-b
Lam, E. K. Y., Tai, E. K. K., Koo, M. W. L., Wong, H. P. S., Wu, W. K. K., Yu, L., et al. (2007a). Enhancement of gastric mucosal integrity by Lactobacillus rhamnosus GG. Life Sci. 16, 2128–2136. doi: 10.1016/j.lfs.2007.03.018
Lam, E. K. Y., Yu, L., Wong, H. P. S., Wu, W. K. K., Shin, V. Y., Tai, E. K. K., et al. (2007b). Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur. J. Pharmacol. 22 565, 171–179. doi: 10.1016/j.ejphar.2007.02.050
Langat, A. C., Ogutu, E., Kamenwa, R., and Simiyu, D. E. (2006). Prevalence of Helicobacter pylori in children less than three years of age in health facilities in Nairobi Province. East Afr. Med. J. 83, 471–477.
Lee, J. Y., Kim, N., Nam, R. H., Choi, Y. J., Seo, J. H., Lee, H. S., et al. (2014). No correlation of inflammation with colonization of Helicobacter pylori in the stomach of mice fed high-salt diet. J. Cancer Prev. 19, 144–151. doi: 10.15430/JCP.2014.19.2.144
Lu, R., Fasano, S., Madayiputhiya, N., Morin, N. P., Nataro, J., and Fasano, A. (2009). Isolation, identification, and characterization of small bioactive peptides from Lactobacillus GG conditional media that exert both anti-gram-negative and gram-positive bactericidal activity. J. Pediatr. Gastroenterol. Nutr. 49:23. doi: 10.1097/MPG.0b013e3181924d1e
Mack, D. R., Ahrné, S., Hyde, L., Wei, S., and Hollingsworth, M. A. (2003). Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52, 827–833. doi: 10.1136/gut.52.6.827
Malfertheiner, P., Megraud, F., O’Morain, C. A., Atherton, J., Axon, A. T. R., Bazzoli, F., et al. (2012). Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut 1, 646–664. doi: 10.1136/gutjnl-2012-302084
Malhotra, S. L. (1978). A comparison of unrefined wheat and rice diets in the management of duodenal ulcer. Postgrad. Med. J. 1, 6–9. doi: 10.1136/pgmj.54.627.6
Michetti, P. (2001). Lactobacilli for the management of Helicobacter pylori. Nutrition. 17, 268–269. doi: 10.1016/S0899-9007(00)00475-5
Misciagna, G., Cisternino, A. M., and Freudenheim, J. (2000). Diet and duodenal ulcer. Dig Liver Dis. 32, 468–472. doi: 10.1016/S1590-8658(00)80002-X
Misiewicz, G., Misiewicz, J. J., and Harris, A. (1995). Clinician’s Manual on Helicobacter pylori. ıWuhan: Science Press.
Mitchell, H. M., Ally, R., Wadee, A., Wiseman, M., and Segal, I. (2009). Major differences in the IgG subclass response to Helicobacter pylori in the first and third worlds. Scand. J. Gastroenterol. 37, 517–522. doi: 10.1080/00365520252903044
Moon, A., Solomon, A., Beneck, D., and Cunningham-Rundles, S. (2009). Positive association between Helicobacter pylori and gastroesophageal reflux disease in children. J. Pediatr. Gastroenterol. Nutr. 49:283. doi: 10.1097/MPG.0b013e31818eb8de
Myllyluoma, E., Ahonen, A.-M., Vapaatalo, H., and Kankuri, E. (2008). Effects of multispecies probiotic combination on Helicobacter pylori infection in vitro. Clin. Vaccine Immunol. 15, 1472–1482. doi: 10.1128/CVI.00080-08
Myllyluoma, E., Kajander, K., Mikkola, H., Kyrönpalo, S., Rasmussen, M., Kankuri, E., et al. (2007). Probiotic intervention decreases serum gastrin-17 in Helicobacter pylori infection. Dig. Liver Dis. 39, 516–523. doi: 10.1016/j.dld.2007.02.015
Myllyluoma, E., Veijola, L., Ahlroos, T., Tynkkynen, S., Kankuri, E., Vapaatalo, H., et al. (2005). Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy – a placebo-controlled, double-blind randomized pilot study. Aliment Pharmacol. Ther. 1, 1263–1272. doi: 10.1111/j.1365-2036.2005.02448.x
Naito, Y., Yoshikawa, T., Yoneta, T., Yagi, N., Matsuyama, K., Arai, M., et al. (1995). A new gastric ulcer model in rats produced by ferrous iron and ascorbic acid injection. Digestion 56, 472–478. doi: 10.1159/000201278
Newton, R., Ziegler, J. L., Casabonne, D., Carpenter, L., Gold, B. D., Owens, M., et al. (2006). Helicobacter pylori and cancer among adults in Uganda. Infect Agent Cancer 1:5. doi: 10.1186/1750-9378-1-5
Nwokolo, C. U., Freshwater, D. A., O’hare, P., and Randeva, H. S. (2003). Plasma ghrelin following cure of Helicobacter pylori. Gut. 52, 637–640. doi: 10.1136/gut.52.5.637
Ouwehand, A. C., Salminen, S., Roberts, P. J., Ovaska, J., and Salminen, E. (2003). Disease-dependent adhesion of lactic acid bacteria to the human intestinal mucosa. Clin. Diagn. Lab. Immunol. 1, 643–646. doi: 10.1128/CDLI.10.4.643-646.2003
Parsonnet, J., Replogle, M., Yang, S., and Hiatt, R. (1997). Seroprevalence of CagA-Positive Strains among Helicobacter pylori—Infected, healthy young adults. J. Infect Dis. 1, 1240–1242. doi: 10.1086/593680
Peña J. A., and Versalovic, J. (2003). Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol. 1, 277–285.
Perry, S., de Jong, B. C., Solnick, J. V., de la Luz Sanchez, M., Yang, S., Lin, P. L., et al. (2010). Infection with Helicobacter pylori Is associated with protection against tuberculosis. PLoS One 5:e8804. doi: 10.1371/journal.pone.0008804
Petrova, M. I., Imholz, N. C. E., Verhoeven, T. L. A., Balzarini, J., Van Damme, E. J. M., Schols, D., et al. (2016). Lectin-Like molecules of Lactobacillus rhamnosus GG inhibit pathogenic Escherichia coli and Salmonella biofilm formation. PLoS One 11:e0161337. doi: 10.1371/journal.pone.0161337
Ricci, C., Holton, J., and Vaira, D. (2007). Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract. Res. Clin. Gastroenterol. 21, 299–313.
Rinkinen, M., Jalava, K., Westermarck, E., Salminen, S., and Ouwehand, A. C. (2003). Interaction between probiotic lactic acid bacteria and canine enteric pathogens: a risk factor for intestinal Enterococcus faecium colonization? Vet. Microbiol. 20 92, 111–119. doi: 10.1016/S0378-1135(02)00356-5
Roberts, S. E., Morrison-Rees, S., Samuel, D. G., Thorne, K., Akbari, A., and Williams, J. G. (2016). Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol. Ther. 1, 334–345. doi: 10.1111/apt.13474
Rokka, S., Myllykangas, S., and Joutsjoki, V. (2008). Effect of specific colostral antibodies and selected Lactobacilli on the adhesion of Helicobacter pylori on AGS cells and the Helicobacter-induced IL-8 production. Scand. J. Immunol. 1, 280–286. doi: 10.1111/j.1365-3083.2008.02138.x
Rouvroy, D., Bogaerts, J., Nsengiumwa, O., Omar, M., Versailles, L., and Haot, J. (1987). gastritis, and peptic ulcer disease in central Africa. BMJ 295, 1174–1174. doi: 10.1136/bmj.295.6607.1174
Rydning, A., Aadland, E., Berstad, A., and Odegaard, B. (1982). Prophylactic effect of dietary fibre in duodenal ulcer disease. Lancet 320, 736–739. doi: 10.1016/S0140-6736(82)90922-9
Segal, I., Ally, R., and Mitchell, H. (2001). Helicobacter pylori—an African perspective. QJM 1, 561–565. doi: 10.1093/qjmed/94.10.561
Segamwenge, I. L., Kagimu, M., Ocama, P., and Opio, K. (2014). The Utility of the Helicobacter pylori stool antigen test in managing dyspepsia: an experience from a low resource setting. Afr. Health Sci. 1, 829–834. doi: 10.4314/ahs.v14i4.9
Segers, M. E., and Lebeer, S. (2014). Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microb. Cell Fact. 29 13:S7. doi: 10.1186/1475-2859-13-S1-S7
Sybesma, W., Kort, R., and Lee, Y.-K. (2015). Locally sourced probiotics, the next opportunity for developing countries? Trends Biotechnol. 33, 197–200. doi: 10.1016/j.tibtech.2015.01.002
Sybesma, W., Molenaar, D., IJcken, W., van Venema, K., and Kort, R. (2013). Genome instability in Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 1, 2233–2239. doi: 10.1128/AEM.03566-12
Szajewska, H., Albrecht, P., and Topczewska-Cabanek, A. (2009). Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J. Pediatr. Gastroenterol. Nutr. 48, 431–436. doi: 10.1097/MPG.0b013e318182e716
Szajewska, H., Skórka, A., Ruszczyński, M., and Gieruszczak-Białek, D. (2007). Meta-analysis: Lactobacillus GG for treating acute diarrhoea in children. Aliment Pharmacol. Ther. 1, 871–881. doi: 10.1111/j.1365-2036.2007.03282.x
Tamura, K., Sakurai, T., and Kogo, H. (2006). Relationship between prostaglandin E 2 and vascular endothelial growth factor (VEGF) in angiogenesis in human vascular endothelial cells. Vascul. Pharmacol. 44, 411–416. doi: 10.1016/j.vph.2006.02.009
Thompson, L., Cockayne, A., and Spiller, R. C. (1994). Inhibitory effect of polyunsaturated fatty acids on the growth of Helicobacter pylori: a possible explanation of the effect of diet on peptic ulceration. Gut 1, 1557–1561. doi: 10.1136/gut.35.11.1557
Tovey, F. I. (2009). Staple diets and duodenal ulcer prevalence. Int. Health 1, 124–132. doi: 10.1016/j.inhe.2009.09.004
Tovey, F. I., and Tunstall, M. (1975). Duodenal ulcer in black populations in Africa south of the Sahara. Gut 1, 564–576. doi: 10.1136/gut.16.7.564
Tsongo, L., Nakavuma, J., Mugasa, C., and Kamalha, E. (2015). Helicobacter pylori among patients with symptoms of gastroduodenal ulcer disease in rural Uganda. Infect Ecol. Epidemiol. 5:26785. doi: 10.3402/iee.v5.26785
Vaezi, M. F., Falk, G. W., Peek, R. M., Vicari, J. J., Goldblum, J. R., Perez-Perez, G. I., et al. (2000). CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am. J. Gastroenterol. 95, 2206–2211. doi: 10.1111/j.1572-0241.2000.02305.x
Vicari, J. J., Peek, R. M., Falk, G. W., Goldblum, J. R., Easley, K. A., Schnell, J., et al. (1998). The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology 115, 50–57. doi: 10.1016/S0016-5085(98)70364-6
Wabinga, H. (1996). Frequency of Helicobacter pylori in gastroscopic biopsy of Ugandan Africans. East Afr. Med J. 73, 691–693.
Wang, X.-Q., Terry, P. D., and Yan, H. (2009). Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J. Gastroenterol. 14, 2204–2213. doi: 10.3748/wjg.15.2204
Westerik, N., Kort, R., Sybesma, W., Reid, G. (2018). Lactobacillus rhamnosus probiotic food as a tool for empowerment across the value Chain in Africa. Front Microbiol. 9:1501. doi: 10.3389/fmicb.2018.01501
Westerik, N., Wacoo, A. P., Sybesma, W., and Kort, R. (2016). Novel production protocol for small-scale manufacture of probiotic fermented foods. J. Vis. Exp. 115:e54365. doi: 10.3791/54365
Xu, Y., Jing, J.-J., Gong, Y.-H., Xu, Q., Zhang, W.-L., Piao, Y., et al. (2011). Changes in biological and virulent characteristics of Helicobacter pylori exposed to high salt. Asian Pac. J. Cancer Prev. 12, 2637–2641.
Yan, F., and Polk, D. B. (2002). Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 27, 50959–50965. doi: 10.1074/jbc.M207050200
Ye, W., Held, M., Lagergren, J., Engstrand, L., Blot, W. J., McLaughlin, J. K., et al. (2004). Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J. Natl. Cancer Inst. 96, 388–396. doi: 10.1093/jnci/djh057
Zamani, M., Ebrahimtabar, F., Zamani, V., Miller, W. H., Alizadeh-Navaei, R., Shokri-Shirvani, J., et al. (2018). Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol. Ther. 47, 868–876. doi: 10.1111/apt.14561
Keywords: Lactobacillus rhamnosus yoba 2012, Lactobacillus rhamnosus GG, Helicobacter pylori, gastric pathology, ulcer, eradication therapy, Uganda, East Africa
Citation: Westerik N, Reid G, Sybesma W and Kort R (2018) The Probiotic Lactobacillus rhamnosus for Alleviation of Helicobacter pylori-Associated Gastric Pathology in East Africa. Front. Microbiol. 9:1873. doi: 10.3389/fmicb.2018.01873
Received: 30 May 2018; Accepted: 25 July 2018;
Published: 14 August 2018.
Edited by:
Teresa Zotta, Consiglio Nazionale delle Ricerche (CNR), ItalyReviewed by:
Yanmei Zhang, Fudan University, ChinaJennifer McMillan Noto, Vanderbilt University Medical Center, United States
Copyright © 2018 Westerik, Reid, Sybesma and Kort. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Remco Kort, ci5rb3J0QHZ1Lm5s
 Nieke Westerik
Nieke Westerik Gregor Reid
Gregor Reid Wilbert Sybesma
Wilbert Sybesma Remco Kort
Remco Kort