
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 17 August 2018
Sec. Virology
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.01783
More and more dogs have been used as a disease model for medical research and drug safety evaluation. Therefore, it is important to make sure that the dogs and their living houses are special pathogen free. In this study, the development and evaluation of a Luminex xTAG assay for simultaneous detection of five canine viruses was carried out, including canine distemper virus, canine parvovirus, canine parainfluenza virus, canine adenovirus, and rabies virus. Assay specificity was accomplished by targeting conserved genomic regions for each virus. Hybridization between multiplexed PCR products and the labeled fluorescence microspheres was detected in a high throughput format using a Luminex fluorescence reader. The Luminex xTAG assay showed high sensitivity with limits of detection for the five viruses was 100 copies/μL. Specificity of the xTAG assay showed no amplification of canine coronavirus, pseudorabies virus and canine influenza virus indicating that the xTAG assay was specific. Seventy-five clinical samples were tested to evaluate the xTAG assay. The results showed 100% coincidence with the conventional PCR method. This is the first report of a specific and sensitive multiplex Luminex xTAG assay for simultaneous detection of five major canine viral pathogens. This assay will be a useful tool for quality control and environmental monitoring for dogs used as laboratory animals, may even be applied in laboratory epidemiological investigations.
Dogs has been extensively used as animal models for medical research and drug evaluation, such as studying human breast cancer carcinogenesis (Abdelmegeed and Mohammed, 2018), Duchenne muscular dystrophy (DMD) (Wells, 2018), management of furcation defect (Afifi et al., 2018), chronic myocardial infarction (MI) (Xiong et al., 2018), comparative pathogenesis study of H3N2 canine influenza virus (CIV) challenged (Luo et al., 2018), long term safety and efficacy of AAV gene therapy (Lee et al., 2018). Therefore, it is critical to make sure that the dogs used as laboratory animals are free of special pathogens. In China, according to the national standard of laboratory dogs, the rabies virus (RV), canine parvovirus (CPV), canine adenovirus (CAV), and canine distemper virus (CDV) are the items that must be tested. All the laboratory dogs and their living houses should be checked up with these viruses regularly for quality monitoring. However, the currently existing detection method recommended by the industry standard is conventional PCR. To complete the whole check routine, four individual PCR should be carried out, which is time-consuming, especially when dealing with a large scale of samples.
A rapid and sensitive multiplex pathogens detection technology is needed to meet the detection requirement of monitoring the animal health status and the sanitary of their living environment. Unlike the existing multiplex PCR methods that only focus on two or three targets and cannot be adapted to high-throughput testing. The luminescent beads are a novel platform for multiplex high-throughput detection and can simultaneously detect up to 100 targets in a single reaction (Dunbar, 2006; Liu et al., 2012). This tool had been adapted for use in genetic analysis, gene expression analysis, SNP analysis, and pathogen identification (Chen et al., 2000; Ocheretina et al., 2013; Zhang et al., 2016).
The Luminex xTAG assay was designed for multiple nucleic acid detection in a single reaction through the hybridization of magnetic microspheres and PCR products (Chen et al., 2015; Reslova et al., 2017). The magnetic microspheres pre-coupled with anti-MagPlex-TAG oligo-nucleotides capture the matching oligo-nucleotide presented on the 5′ end of PCR amplicons. A Luminex fluorescence reader is then used for analytical measurements of bead types. This technology is commercially available for respiratory viral pathogens and gastrointestinal pathogens for human clinical diagnoses (Pabbaraju et al., 2011; Duong et al., 2016), and barely reports on applications in veterinary research.
To fulfill the testing needs of the laboratory dog suppliers for these four viruses and canine parainfluenza virus (CPIV), a multiplex assay for rapid detection of CDV, CPV, RV, CAV, and CPIV in a single tube based on the Luminex xTAG technology was developed and validated.
Canine distemper virus and CPV were obtained from the Institute of Animal Health, Guangdong Academy of Agricultural Sciences (Guangzhou, China). Nucleic acid genomes of canine coronavirus (CCV), pseudorabies virus (PRV), CIV subtype H3N8 and the Nobivac DHPPi vaccine against CDV, CPV, CPIV, and CAV-1 (Intervet, Boxmeer, Netherlands) were preserved by our laboratory. The rabies vaccine and the polyvalent vaccine against CDV, CPV, RV, and CPIV (WuXin Pharmaceutical, Changchun, China) were obtained from the Veterinary Epidemic Prevention Station (Dongguan, China). All viruses, nucleic acids and vaccines were stored at -80°C until use.
Clinical samples were obtained from the local veterinary clinic in Guangzhou, China. DNA and RNA were extracted using a commercial automatic nucleic acid extraction instrument (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions.
Viral genomes from the NCBI database were aligned to determine the most conserved genomic regions for each virus. Primers were designed using Primer Premier 5.0 software and inspected with the vector NTI software (Table 1). All the forward primers were modified with specific oligo-nucleotide tags that enabled magnetic microsphere conjugation. The 5′ ends of reverse primers were biotinylated to enable detection using streptavidin-R-phycoerythrin (SAPE). All primers and oligo-nucleotides were analyzed with Tag-It Oligo Design Software v.3.00 and purified by high-performance liquid chromatography (Invitrogen, Guangzhou, China).
The multiplex amplification was performed using a one-step RT-PCR assay kit (QIAGEN, Hilden, Germany) in a total volume of 50 μL as suggested by the manufacturer. The amplification steps were: 50°C for 30 min, 95°C for 15 min and 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s and a final step of 10 min at 72°C. All samples were tested in triplicate and all assays were run with positive and negative controls.
MagPlex-TAG microspheres were purchased from Luminex (Austin, TX, United States) and suspended in 1.1× tetramethylammonium chloride as outlined in the manufacturer’s protocol. Nucleic acid hybridization was carried out in a 100 μL volume that included 5 μL of amplified product, 20 μL of the working microsphere mixture that contained 2500 beads of each target-specific microsphere and 75 μL SAPE. Hybridization was carried out at 45°C for 30 min. The products were analyzed using the Luminex 200 reader (Luminex) immediately after hybridization and the results were expressed as mean fluorescence intensity (MFI).
Pseudorabies virus, CIV, and CCV samples were used as templates in amplification reactions to determine assay specificity. All amplified products were sequenced for verification (Sangon Biotech, Guangzhou, China). Sensitivity testing was carried out using PCR products from CDV, CPV, CAV, RV, and CPIV amplification that were cloned into the pGEM T easy vector (Promega, Madison, WI, United States). CDV, RV, and CPIV were in vitro transcribed using T7 RNA polymerase with the RiboMAX Large Scale RNA production kit (Promega) according to the manufacturer’s instructions. RNase-free DNase (Promega) was used to remove plasmid DNA after transcription. TRIzol LS reagent (Invitrogen, Carlsbad, CA, United States) was used for RNA isolation according to the manufacturer’s instructions. Genome copy numbers were calculated based on the nucleic acid concentration and molecular weight. Tenfold serial dilutions of DNA or RNA standards were used as assay templates. All the samples were tested in triplicate.
Considering that most of the samples from SPF dogs or their living houses were tested negative, clinical samples from the animal hospital were needed to evaluate the xTAG assay. Clinical samples that included feces, saliva, throat swab, and urine from dogs were tested using conventional PCR (Table 2) and the Luminex xTAG assay. Besides, artificial mixed infection samples (by mixing the positive nucleic acid together, or mixing the positive samples together before nucleic acid extraction) were tested for further validation of the xTAG assay. All samples were tested in duplicate. In addition, a comparative test was carried out using the CDV/CPV test card (Quicking Biotech, Shanghai, China) followed by the manufacturer’s instructions.
Mean fluorescence intensity values were calculated using software supplied with the Luminex reader after counting at least 100 beads for each bead set in a sample (Ponzoni et al., 2013). Background calculations used blank controls containing all the hybridization components except target DNA or RNA. The cutoff value of each bead set was defined as the net MFI value of negative controls +3 SD as previously defined (Dangoudoubiyam et al., 2011).
Nucleic acid of PRV, CIV, and CCV were used as templates in this study to evaluate the specificity of the multiplex-PCR primers. The results showed that, all primers pairs were found to detect their specific viral target with no amplification of non-target samples. The fluorescence signals were observed only in the positive controls (Figure 1), revealing a good specificity of the assay.

FIGURE 1. Specificity analysis of the 5-plex Luminex xTAG assay. Each bar represents the average MFI of duplicate samples. CDV, canine distemper virus; CPV, canine parvovirus; CAV, canine adenovirus; CPIV, canine parainfluenza virus; RV, rabies virus; PRV, pseudorabies virus; CCV, canine coronavirus; CIV, canine influenza virus; NC, negative control (water). The result of the 5-plex Luminex xTAG assay showed high specificity.
Sensitivity was tested using 10-fold serial dilutions of standards and the limit of detection was defined as the highest dilution that resulted in an MFI value above the cutoff value. In this study, these five viruses shared different cutoff values, which were based on the MFI values of their own negative controls. Data analysis showed that the limit of detection for each of the five viruses was about 100 copies/μL (Figure 2 and Table 3).
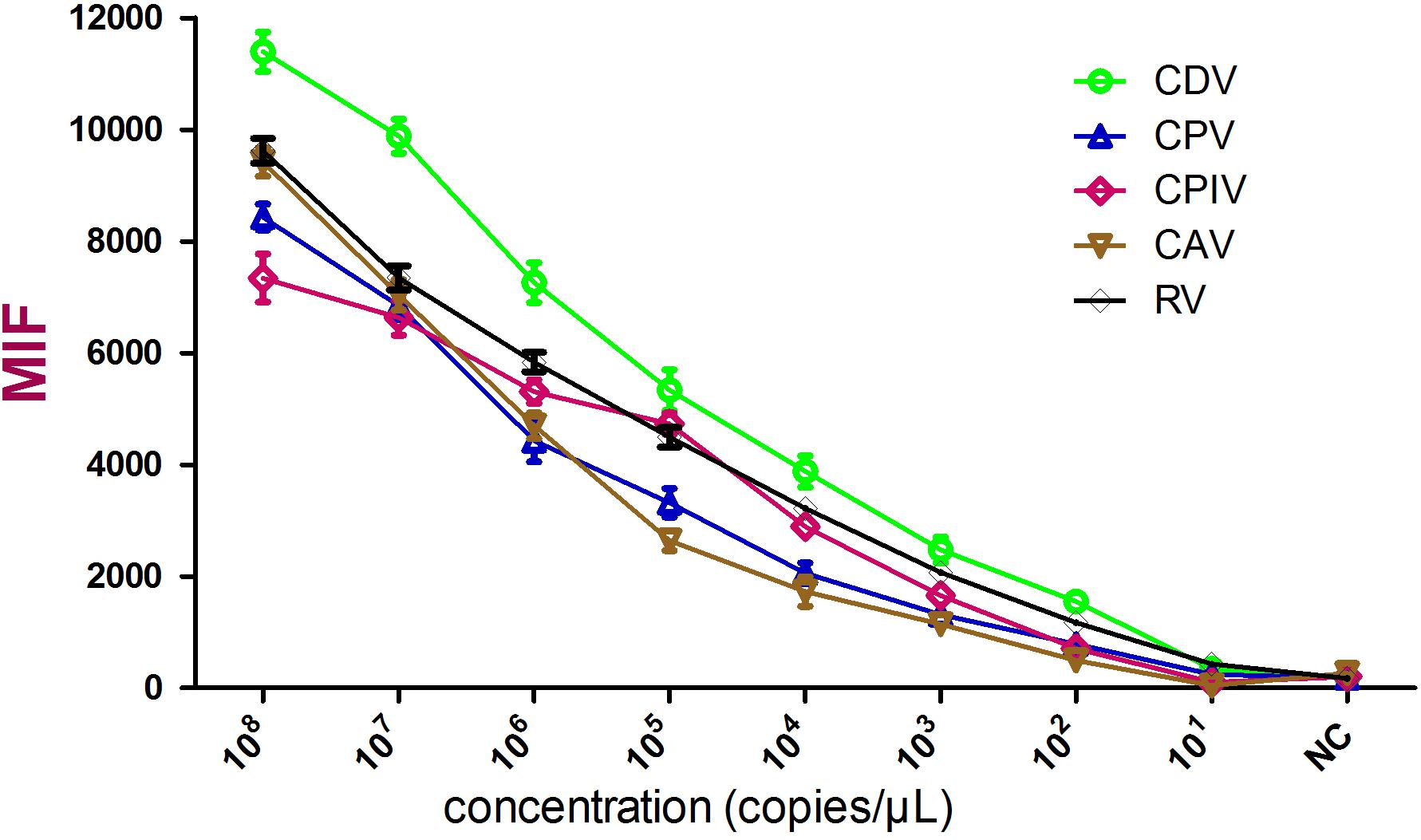
FIGURE 2. Sensitivity analysis of the xTAG assay. Cloned gene copies from the indicated viruses were transcribed in vitro and used in triplicate. The detection limit was 100 copies/μL for these five viruses.
The reproducibility and stability of the assay was determined using parallel reactions at concentrations of 108, 105, and 103 copies/μL of standard DNA/RNA. The inter-assay variance was less than 3%, while the intra-assay variance was less than 4%. The low inter-assay and intra-assay coefficient of variations demonstrated a high repeatability of the xTAG assay (Table 4).
A total number of 75 clinical samples were evaluated by both the Luminex xTAG assay and conventional PCR. The positive rates for CDV, CPV, CAV, and CPIV were 8% (6/75), 50.7% (38/75), 2.7% (2/75), and 4% (3/75), respectively. No sample tested positive for RV and no mixed infections were identified. The detection results of these two methods were in 100% agreement. The positive detection rate of CDV test card was 8% (6/75), which was fully consistent with the xTAG assay. However, only 35 samples were tested positive with the CPV test card, three positive samples (confirmed by sequencing) were falsely detected as negative. To a certain extent, the xTAG assay was more sensitive than the colloidal gold test strip, as revealed by the comparison results (Supplementary Table S1).
To determine the utility of the multiplex xTAG assay in multiplex infection, artificial mixed infection samples were tested. Three samples were found triple infection and four samples were found double infection (Figure 3). Indicating that, the new developed multiplex xTAG assay had the ability to identify different targets in co-infection samples.
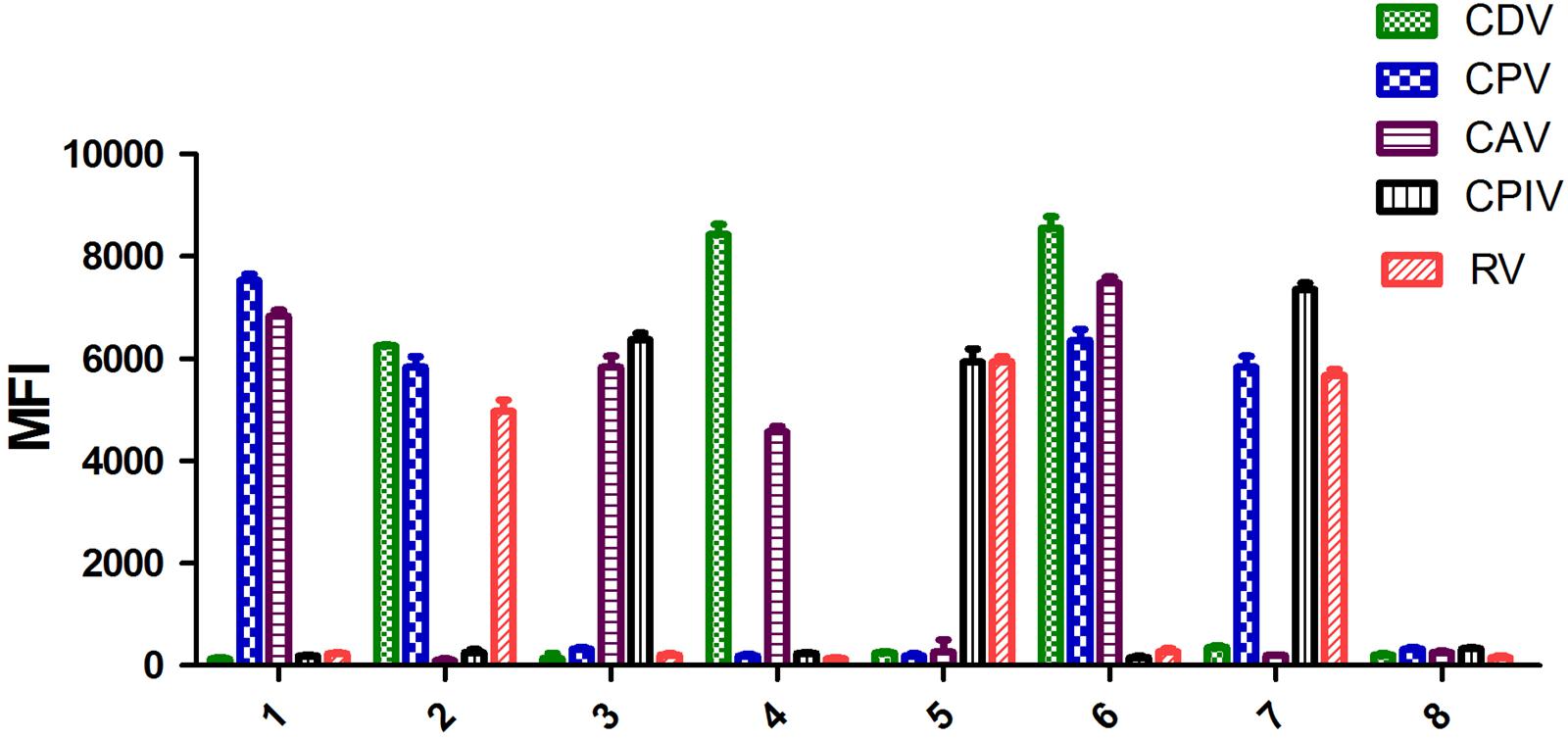
FIGURE 3. Validation of the artificial mixed infection samples. 1, CPV and CAV positive sample; 2, CDV, CPV, and RV positive sample; 3, CAV and CPIV positive samples; 4, CDV and CAV positive sample; 5, CPIV and RV positive sample; 6, CDV, CPV, and CAV positive sample; 7, CPV, CPIV, and RV positive sample; 8, negative control.
The multiplex PCR format allows for the simultaneous detection of different nucleic acid sequences in a single reaction. This is time- and labor-saving, but the interpretation of conventional multiplex PCR relies on amplicon size, restricting its use in a high-throughput format (Piewbang et al., 2017). The Luminex xTAG platform was used in this study to develop specific and sensitive high-throughput assays that can be expanded to include more pathogens as needed (Jiang et al., 2017). This format has been widely used in human disease diagnosis but not available in dogs (Wessels et al., 2014; Dunbar et al., 2015; Laamiri et al., 2016).
Dogs are important experimental animals with strict quality control. CDV, CPV, CAV, and RV are the pathogens that must be excluded according to the national standard of laboratory animal in China. The xTAG technology was used to develop high throughput assays for the rapid and sensitive simultaneous detection of these viruses to help with laboratory dog’s daily monitoring.
In this study, a 5-plex Luminex xTAG assay for the simultaneous detection of CDV, CPV, CPIV, CAV, and RV in a single reaction was developed and validated. This is the first report of a Luminex xTAG assay for use in canine pathogen identification, and it’s expected to replace the currently existing single PCR method for canine daily monitoring. However, this panel may not be a good tool for clinical diagnosis, compared with the fast and easy point-of-care testing (POCT).
Primer design is the key for the establishment of Luminex xTAG assay. Unwanted hairpin structures would increase the MFI background. The bio-informatics analysis was applied to evaluate specificity and avoid primer dimers. Specific primers targeted conserved genomic regions for each virus were selected. This approach yielded an assay without cross-reactions between target and non-target viruses.
The limit of detection for the multiplex Luminex xTAG assay was estimated using viral DNA/RNA generated from cloned viral fragments. The assay sensitivity was 100 copies/μL, which might be slightly lower than real-time PCR (Schlottau et al., 2017). Validation of 75 clinical samples revealed a 100% coincidence rate with conventional PCR detection, indicating that the assay was sufficient for the detection of these five pathogens in clinical samples. Artificial mixed infection samples were verified, revealing that the assay was capable of identifying specific targets in co-infection samples.
In this study, a novel multiplex platform for the simultaneous detection of five important canine viral pathogens was established. This assay will be useful for animal health status and their living house monitoring. Additional targets can be integrated into the existing multiplex platform as needed and allows increased flexibility in disease investigation and environmental monitoring.
MW carried out data analysis and manuscript writing. FC, YZ, and YL participated in manuscript preparation. MC, RH, and PG designed the experiments. All authors read and approved the final manuscript.
This research was supported by the Science and Technology Program of Guangzhou, China (No. 201504291050534) and the Science and Technology Planning Project of Guangdong Province, China (No. 2017B030314171 and 2017A070702001). This study was also supported by the National Key Technology R&D Program (2015BAI07B01). The external funding body had no role in study design, data collection and analysis, and decision to publish or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01783/full#supplementary-material
Abdelmegeed, S. M., and Mohammed, S. (2018). Canine mammary tumors as a model for human disease. Oncol. Lett. 15, 8195–8205. doi: 10.3892/ol.2018.8411
Afifi, M. M., Kotry, G. S., El-Kimary, G. I., and Youssef, H. A. (2018). Immuno-histopathologic evaluation of Drynaria fortunei rhizome extract in the management of grade II furcation defects in a canine model. J. Periodontol. doi: 10.1002/JPER.17-0655 [Epub ahead of print].
Chen, J., Iannone, M. A., Li, M. S., Taylor, J. D., Rivers, P., Nelsen, A. J., et al. (2000). A microsphere-based assay for multiplexed single nucleotide polymorphism analysis using single base chain extension. Genome Res. 10, 549–557. doi: 10.1101/gr.10.4.549
Chen, R., Yu, X. L., Gao, X. B., Xue, C. Y., Song, C. X., Li, Y., et al. (2015). Bead-based suspension array for simultaneous differential detection of five major swine viruses. Appl. Microbiol. Biotechnol. 99, 919–928. doi: 10.1007/s00253-014-6337-8
Dangoudoubiyam, S., Vemulapalli, R., Ndao, M., and Kazacos, K. R. (2011). Recombinant antigen-based enzyme-linked immunosorbent assay for diagnosis of Baylisascaris procyonis larva migrans. Clin. Vaccine Immunol. 18, 1650–1655. doi: 10.1128/CVI.00083-11
Dunbar, S. A. (2006). Applications of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta 363, 71–82. doi: 10.1016/j.cccn.2005.06.023
Dunbar, S. A., Ritchie, V. B., Hoffmeyer, M. R., Rana, G. S., and Zhang, H. (2015). Luminex((R)) multiplex bead suspension arrays for the detection and serotyping of Salmonella spp. Methods Mol. Biol. 1225, 1–27. doi: 10.1007/978-1-4939-1625-2_1
Duong, V. T., Phat, V. V., Tuyen, H. T., Dung, T. T., Trung, P. D., Minh, P. V., et al. (2016). Evaluation of Luminex xTAG gastrointestinal pathogen panel assay for detection of multiple diarrheal pathogens in fecal samples in Vietnam. J. Clin. Microbiol. 54, 1094–1100. doi: 10.1128/JCM.03321-15
Jiang, L., Ren, H., Zhou, H., Qin, T., and Chen, Y. (2017). Simultaneous detection of nine key bacterial respiratory pathogens using Luminex xTAG(R) technology. Int. J. Environ. Res. Public Health 14:223. doi: 10.3390/ijerph14030223
Laamiri, N., Fallgren, P., Zohari, S., Ben Ali, J., Ghram, A., Leijon, M., et al. (2016). Accurate detection of avian respiratory viruses by use of multiplex PCR-based Luminex suspension microarray assay. J. Clin. Microbiol. 54, 2716–2725. doi: 10.1128/JCM.00610-16
Lee, Y. M., Conlon, T. J., Specht, A., Coleman, K. E., Brown, L. M., Estrella, A. M., et al. (2018). Long-term safety and efficacy of AAV gene therapy in the canine model of glycogen storage disease type Ia. J. Inherit. Metab. Dis. doi: 10.1007/s10545-018-0199-7 [Epub ahead of print].
Liu, Y., Xu, Z. Q., Zhang, Q., Jin, M., Yu, J. M., Li, J. S., et al. (2012). Simultaneous detection of seven enteric viruses associated with acute gastroenteritis by a multiplexed Luminex-based assay. J. Clin. Microbiol. 50, 2384–2389. doi: 10.1128/JCM.06790-11
Luo, J., Lu, G., Ye, S., Ou, J., Fu, C., Zhang, X., et al. (2018). Comparative pathogenesis of H3N2 canine influenza virus in beagle dogs challenged by intranasal and intratracheal inoculation. Virus Res. doi: 10.1016/j.virusres.2018.05.023 [Epub ahead of print].
Ocheretina, O., Merveille, Y. M., Mabou, M. M., Escuyer, V. E., Dunbar, S. A., Johnson, W. D., et al. (2013). Use of Luminex MagPlex magnetic microspheres for high-throughput spoligotyping of Mycobacterium tuberculosis isolates in Port-au-Prince, Haiti. J. Clin. Microbiol. 51, 2232–2237. doi: 10.1128/JCM.00268-13
Pabbaraju, K., Wong, S., Tokaryk, K. L., Fonseca, K., and Drews, S. J. (2011). Comparison of the Luminex xTAG respiratory viral panel with xTAG respiratory viral panel fast for diagnosis of respiratory virus infections. J. Clin. Microbiol. 49, 1738–1744. doi: 10.1128/JCM.02090-10
Piewbang, C., Rungsipipat, A., Poovorawan, Y., and Techangamsuwan, S. (2017). Development and application of multiplex PCR assays for detection of virus-induced respiratory disease complex in dogs. J. Vet. Med. Sci. 78, 1847–1854. doi: 10.1292/jvms.16-0342
Ponzoni, E., Breviario, D., Mautino, A., Giani, S., and Morello, L. (2013). A multiplex, bead-based array for profiling plant-derived components in complex food matrixes. Anal. Bioanal. Chem. 405, 9849–9858. doi: 10.1007/s00216-013-7434-8
Reslova, N., Michna, V., Kasny, M., Mikel, P., and Kralik, P. (2017). xMAP technology: applications in detection of pathogens. Front. Microbiol. 8:55. doi: 10.3389/fmicb.2017.00055
Schlottau, K., Freuling, C. M., Muller, T., Beer, M., and Hoffmann, B. (2017). Development of molecular confirmation tools for swift and easy rabies diagnostics. Virol. J. 14:184. doi: 10.1186/s12985-017-0853-y
Wells, D. J. (2018). Tracking progress: an update on animal models for Duchenne muscular dystrophy. Dis. Model Mech 11:dmm035774. doi: 10.1242/dmm.035774
Wessels, E., Rusman, L. G., van Bussel, M. J., and Claas, E. C. (2014). Added value of multiplex Luminex Gastrointestinal Pathogen Panel (xTAG(R) GPP) testing in the diagnosis of infectious gastroenteritis. Clin. Microbiol. Infect. 20, O182–O187. doi: 10.1111/1469-0691.12364
Xiong, L., Liu, Y., Zhou, M., Wang, G., Quan, D., Shen, C., et al. (2018). Targeted ablation of cardiac sympathetic neurons improves ventricular electrical remodelling in a canine model of chronic myocardial infarction. Europace doi: 10.1093/europace/euy090 [Epub ahead of print].
Zhang, H., Brankovics, B., van der Lee, T. A., Waalwijk, C., van Diepeningen, A. A., Xu, J., et al. (2016). A single-nucleotide-polymorphism-based genotyping assay for simultaneous detection of different carbendazim-resistant genotypes in the Fusarium graminearum species complex. PeerJ 4:e2609. doi: 10.7717/peerj.2609
Keywords: canine distemper virus, canine parvovirus, canine parainfluenza virus, canine adenovirus, rabies virus, multiplex PCR, xTAG
Citation: Wu M, Cong F, Zhu Y, Lian Y, Chen M, Huang R and Guo P (2018) Multiplex Detection of Five Canine Viral Pathogens for Dogs as Laboratory Animals by the Luminex xTAG Assay. Front. Microbiol. 9:1783. doi: 10.3389/fmicb.2018.01783
Received: 09 May 2018; Accepted: 16 July 2018;
Published: 17 August 2018.
Edited by:
Akio Adachi, Kansai Medical University, JapanReviewed by:
Xing-Quan Zhu, Lanzhou Veterinary Research Institute (CAAS), ChinaCopyright © 2018 Wu, Cong, Zhu, Lian, Chen, Huang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengju Guo, dmV0YmlvMjAxNkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.