- 1UCIBIO, Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, Caparica, Portugal
- 2School of Food Science and Environmental Health, College of Sciences and Health, Dublin Institute of Technology, Dublin, Ireland
- 3Department of Microbiology, Moyne Institute of Preventive Medicine, Schools of Genetics and Microbiology, Trinity College Dublin, University of Dublin, Dublin, Ireland
- 4Nuritas Limited, Dublin, Ireland
Infectious diseases remain one of the leading causes of morbidity and mortality worldwide. The WHO and CDC have expressed serious concern regarding the continued increase in the development of multidrug resistance among bacteria. Therefore, the antibiotic resistance crisis is one of the most pressing issues in global public health. Associated with the rise in antibiotic resistance is the lack of new antimicrobials. This has triggered initiatives worldwide to develop novel and more effective antimicrobial compounds as well as to develop novel delivery and targeting strategies. Bacteria have developed many ways by which they become resistant to antimicrobials. Among those are enzyme inactivation, decreased cell permeability, target protection, target overproduction, altered target site/enzyme, increased efflux due to over-expression of efflux pumps, among others. Other more complex phenotypes, such as biofilm formation and quorum sensing do not appear as a result of the exposure of bacteria to antibiotics although, it is known that biofilm formation can be induced by antibiotics. These phenotypes are related to tolerance to antibiotics in bacteria. Different strategies, such as the use of nanostructured materials, are being developed to overcome these and other types of resistance. Nanostructured materials can be used to convey antimicrobials, to assist in the delivery of novel drugs or ultimately, possess antimicrobial activity by themselves. Additionally, nanoparticles (e.g., metallic, organic, carbon nanotubes, etc.) may circumvent drug resistance mechanisms in bacteria and, associated with their antimicrobial potential, inhibit biofilm formation or other important processes. Other strategies, including the combined use of plant-based antimicrobials and nanoparticles to overcome toxicity issues, are also being investigated. Coupling nanoparticles and natural-based antimicrobials (or other repurposed compounds) to inhibit the activity of bacterial efflux pumps; formation of biofilms; interference of quorum sensing; and possibly plasmid curing, are just some of the strategies to combat multidrug resistant bacteria. However, the use of nanoparticles still presents a challenge to therapy and much more research is needed in order to overcome this. In this review, we will summarize the current research on nanoparticles and other nanomaterials and how these are or can be applied in the future to fight multidrug resistant bacteria.
Introduction
Multidrug resistant (MDR) bacteria remain the greatest challenge in public health care. The numbers of infections produced by such resistant strains are increasing globally. This acquired resistance of pathogens presents a key challenge for many antimicrobial drugs. Recent advances in nanotechnology offer new prospects to develop novel formulations based on distinct types of nanoparticles (NPs) with different sizes and shapes and flexible antimicrobial properties.
NPs may offer a promising solution as they can not only combat bacteria themselves but can also act as carriers for antibiotics and natural antimicrobial compounds (Wang et al., 2017a). While various materials have been explored from liposomal to polymer based nano-drug carriers, metallic vectors, such as gold NPs, are attractive as core materials due to their essentially inert and nontoxic nature (Burygin et al., 2009). Arguably the most attractive aspect of NPs drug delivery systems is their ability to introduce a wide range of therapeutics, either bound to their large surface area or contained within the structure, to the site of infection effectively and safely by having a controlled rate of targeted delivery (Pissuwan et al., 2011; Gholipourmalekabadi et al., 2017). By improving the pharmacokinetic profile and therapeutic index of encapsulated drugs compared to free drug equivalents, the dose required to achieve clinical effects can be dramatically decreased (Gao et al., 2018). This in turn, can reduce the toxicity and the adverse side effects associated with high systemic drug concentrations and frequent dosing (Liu et al., 2009).
This review covers the latest approaches in the development of new nanobiotechnology approaches that may challenge the medical practice to fight bacteria and particularly MDR bacteria.
Nanomaterials Against Bacteria
Nanomaterials have at least one dimension in the nanometer scale range (1–100 nm) that convey particular physical and chemical properties considerably different from those of bulk materials (Wang et al., 2017a). Among the wide range of nanomaterials, particular interest has been directed toward NPs. NPs have a number of features, which make them favorable as vectors for drugs to combat disease-causing pathogens. These include their enhancement of drug solubility and stability (Huh and Kwon, 2011); their ease of synthesis (Gholipourmalekabadi et al., 2017); their biocompatibility with target agents; and their modulated release, which can be controlled by stimuli, such as light, pH and heat (Wang Z. et al., 2017). Their distinctive functionality in drug delivery is achieved by their ultra-small size and vast surface to volume ratios. This is a key competitive advantage over conventional therapies in the treatment of infections caused by intracellular pathogens and MDR strains. Their functionalization with different (bio)molecules is another important feature. These comprise NPs containing Ag, Au, Al, Cu, Ce, Cd, Mg, Ni, Se, Pd, Ti, Zn, and super-paramagnetic Fe (Hemeg, 2017; Slavin et al., 2017). AgNPs are considered the most effective nanomaterial against bacteria but other metallic NPs, such as CuONPs, TiONPs, AuNPs, and Fe3O2NPs, have also demonstrated bactericidal effects (Dakal et al., 2016; Hemeg, 2017; Slavin et al., 2017).
While poor membrane transport limits the potency of many antibiotics (Andrade et al., 2013), drug loaded NPs vehicles can enter host cells via endocytosis, facilitating their intracellular entry (Wang Z. et al., 2017). Membrane penetration can also be achieved through interactions with surface lipids, for example, using gold NPs in the co-administration of protein-based drugs (Huang et al., 2010). The therapeutic appeal of NPs is enhanced by their ability to confer physical protection against bacterial resistance mechanisms (Huh and Kwon, 2011). Furthermore, the potential to load multiple drug combinations into NPs presents a highly complex antimicrobial mechanism of action, to which, bacteria are unlikely to develop resistance (Huh and Kwon, 2011). Although, this is usually believed to be the case, there are some studies reporting development of bacterial resistance against silver NPs (Panáček et al., 2018). There is also evidence that exposure of bacteria to this type of NPs may increase its antibiotic tolerance (Kaweeteerawat et al., 2017).
NPs can exert their antibacterial activity via a multitude of mechanisms, such as: (1) direct interaction with the bacterial cell wall; (2) inhibition of biofilm formation; (3) triggering of innate as well as adaptive host immune responses; (4) generation of reactive oxygen species (ROS); and (5) induction of intracellular effects (e.g., interactions with DNA and/or proteins). Because they do not present the same mechanisms of action of standard antibiotics (Figure 1), they can be of extreme use against MDR bacteria (Singh K. et al., 2014; Aderibigbe, 2017; AlMatar et al., 2017; Hemeg, 2017; Natan and Banin, 2017; Rai et al., 2017; Slavin et al., 2017; Zaidi et al., 2017; Bassegoda et al., 2018; Katva et al., 2018; Siddiqi et al., 2018).
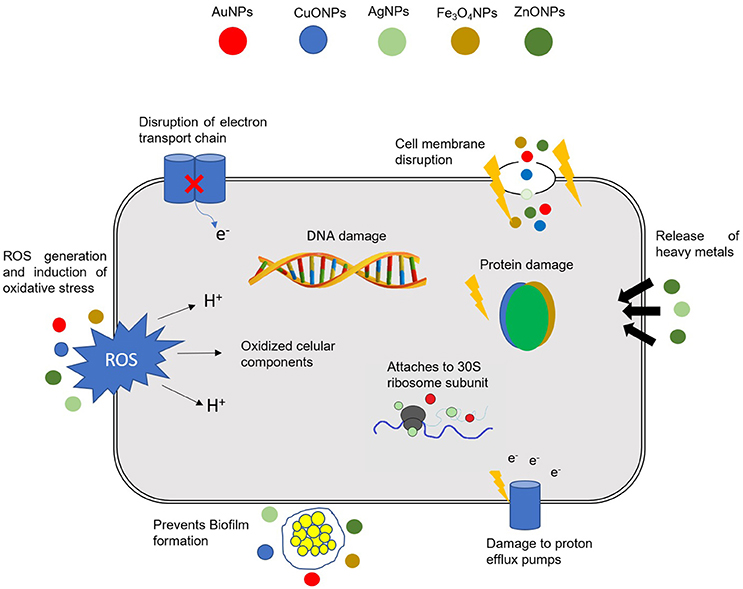
Figure 1. Different mechanisms of action of NPs in bacterial cells. The combination in a single nanomaterial of a multitude of cellular effects may have a tremendous impact in fighting MDR bacteria. DNA, deoxyribonucleic acid; ROS, Reactive oxygen species; AuNPs, gold NPs; CuONPs, Copper oxide NPs; AgNPs, silver NPs; Fe3O4NPs, iron oxide NPs; ZnONPs, zinc oxide NPs.
Besides the broad-spectrum antibacterial properties that NPs have against Gram-positive and -negative bacteria, NPs have been used as vectors for the delivery of antimicrobial moieties that greatly improve their biocidal properties (Beyth et al., 2015; Rai A. et al., 2016; Singh J. et al., 2016; Esmaeillou et al., 2017; Wang et al., 2017a; Zaidi et al., 2017; Hadiya et al., 2018). Some of the advantages of using NPs as vectors are due to their small and controllable size; their protective action against enzymes that would otherwise destroy antimicrobial compounds; their ability to actively deliver antibiotics; and their capability to combine several therapeutic modalities onto a single nanomaterial (e.g., several antibiotics/compounds onto the same NPs for combined action; combining silencing agents and drugs, etc.) (Turos et al., 2007; Huh and Kwon, 2011; Mohammed Fayaz et al., 2011; Liu et al., 2013; Qi et al., 2013; Li et al., 2014; Ranghar et al., 2014; Thomas et al., 2014; Wang et al., 2014; Payne et al., 2016; Rai A. et al., 2016; Singh J. et al., 2016; Yeom et al., 2016; Esmaeillou et al., 2017; Zaidi et al., 2017; Zong et al., 2017; Hadiya et al., 2018).
NPs carriers can tackle bacterial threats “passively,” through prolonged drug retention at the specific infection site, or “actively,” through surface conjugation with active molecules that bind a certain target (Wang Z. et al., 2017). The balance between the surface modification interaction strength, the compound release rate and the stability of the conjugate should be carefully considered for the design of an effective “active” delivery strategy (Burygin et al., 2009; Pissuwan et al., 2011). In an attempt to overcome their therapeutic limitations, various research groups have investigated the conjugation of antibiotics to NPs (Tiwari et al., 2011). For example, Saha et al. describe the direct conjugation of ampicillin, streptomycin and kanamycin to gold NPs (Saha et al., 2007). The resulting complexes were shown to have lower minimum inhibitory concentration (MIC) than the free drug counterparts against both Gram -negative and -positive bacteria. While the detailed mechanism of these effects are not explained by the authors in the above case, Fayaz et al. has attempted to uncover how their vancomycin functionalized gold NPs demonstrated activity against strains which are usually vancomycin resistant based either on mutations (vancomycin resistant Staphylococcus aureus), or membrane structure (Escherichia coli) (Mohammed Fayaz et al., 2011). They propose that only when the antibiotic was complexed with the NPs could this result in nonspecific, multivalent interactions and anchoring of the carrier to the cell wall synthesis proteins. Based on the presence of pits in the cells, which was observed using transmission electron microscopy, the authors concluded that the consequence of the non-specific binding was compromised membrane integrity, and subsequent cell death (Mohammed Fayaz et al., 2011; Gao et al., 2018).
Antibacterial Mechanism of NPs
The antibacterial activity of NPs against MDR bacteria and biofilms depends on a number of factors, namely, their large surface area in contact with bacteria through electrostatic attraction, van der walls forces or hydrophobic interactions; on the nanoparticle size and stability; together with the drug concentration (Chen et al., 2014; Gao et al., 2014; Li et al., 2015). The interaction of NPs with bacteria generally triggers oxidative stress mechanisms, enzymatic inhibition, protein deactivation and changes in gene expression. Still, the most common antibacterial mechanisms are related to oxidative stress, metal ion release, and non-oxidative mechanisms (Wang et al., 2017a; Zaidi et al., 2017 see Figure 1).
Oxidative stress induced by ROS is one of the most important mechanisms assisting the antibacterial activity of NPs (Dwivedi et al., 2014; Rudramurthy et al., 2016). ROS are natural byproducts of cellular oxidative metabolism and have significant important roles in the modulation of cell survival and death, differentiation and cell signaling. In bacteria, ROS are formed from aerobic respiration, and their production is balanced by the cell antioxidant machinery but upon an additional ROS insult, oxidation of biomolecules, and cell components result in severe cellular damage (Li et al., 2012b). The excessive production of ROS leads to a disturbed redox homeostasis resulting in oxidative stress, affecting membrane lipids and altering the structure of DNA and proteins (Dwivedi et al., 2014). It has been shown that while and H2O2 can be neutralized by endogenous antioxidants, ·OH and singlet oxygen (1[O2]) lead to acute microbial death (Zaidi et al., 2017). Different NPs may generate distinctive ROS, such as superoxide () or hydroxyl radical (·OH), hydrogen peroxide (H2O2), and 1[O2]) (Wang et al., 2017a). In this manner, the level of ROS generated by NPs is dependent on the chemical nature of the NPs themselves. Application of metallic NPs is currently being considered to overcome bacterial infections since they have shown antimicrobial efficacy due to their high surface-to-volume ratio. An increase ratio is usually accompanied by increased production of ROS, including free radicals. Zhang et al. (2013) demonstrated that ROS generation and metal ion release significantly enhanced the antibacterial activity through uncoated AuNPs in aqueous suspension under UV irradiation (365 nm). Umamaheswari (Umamaheswari et al., 2014) demonstrated that the antibacterial activity of AuNPs against E. coli, Salmonella Typhi, Pseudomonas aeruginosa and Klebsiella pneumoniae were due to oxidative stress caused by increased intracellular ROS. A recent study (Zhang et al., 2013) evaluated AuNPs and AuNPs -laser combined therapy against C. pseudotuberculosis and suggested that the mechanism of action is related with ROS production, that causes an increase of oxidative stress of microbial cells in the form of vacuole formation as an indication of potent activity. This effect was higher with AuNPs-laser, causing a rapid loss of bacterial cell membrane integrity due to the fact that laser light enhances at least one fold antimicrobial activity of AuNPs. Several other studies have addressed the role of metal NPs to induce MDR bacteria death via oxidative stress (Table 1) (Foster et al., 2011; Li et al., 2012b; Rai et al., 2012; Zhang et al., 2013; Reddy L. S. et al., 2014; Singh R. et al., 2014; Pan et al., 2016; Courtney et al., 2017; Ulloa-Ogaz et al., 2017; Zaidi et al., 2017). Indeed, titanium dioxide NPs were shown to adhere to the surface of the bacterial cell and trigger the production of ROS, which in turns lead to damage of the structure of cellular components and consequent cell death (Foster et al., 2011). In another important study using different metal NPs, AgNPs were shown to generate superoxide radicals and hydroxyl radicals, whereas Au, Ni, and Si NPs generated only singlet oxygen, which upon entering the cell produced an antibacterial effect (Zhang et al., 2013). More recently, Reddy and co-workers demonstrated that ZnONPs alone can also act as an effective antibacterial agent via the generation of ROS (Reddy L. S. et al., 2014). Exposure to UV irradiation may also potentiate the action of NPs. Li et al. (2012b) reported the augmented antibacterial effects of zinc oxide (ZnO) and titanium oxide (TiO) NPs triggered by UV irradiation as the results of the increased production of superoxide, hydroxyl and singlet oxygen radicals that potentiated bacteria mortality by severe oxidative stress. Graphene oxide–iron oxide NPs have also demonstrated maximum antibacterial activity due to the generation of hydroxyl radicals and diffusion into bacterial cells (Pan et al., 2016). More recently, Ulloa-Ogaz and collaborators demonstrated that copper oxide NPs interact with bacteria, generating an intracellular signaling cascade that trigger oxidative stress and, thus, an antibacterial effect (Ulloa-Ogaz et al., 2017).
Metal oxides slowly release metal ions that are up taken by the cell, reaching the intracellular compartment where they can interact with functional groups of proteins and nucleic acids, such as amino (–NH), mercapto (–SH), and carboxyl (–COOH) groups (Wang et al., 2017a). This interaction alters the cell structure, hampers enzymatic activity and interferes with the normal physiological processes in the bacterial cell. It has been shown that copper oxide (CuO) NPs cause a significant alteration of the expression of key proteins and may inhibit denitrification. Proteomic analysis showed that CuONPs cause an alteration of proteins involved in nitrogen metabolism, electron transfer and transport (Su et al., 2015). Also, the interaction of gold–superparamagnetic iron oxide NPs with bacterial proteins via disulfide bonds affects the metabolism and redox system of bacterial cells (Niemirowicz et al., 2014). NPs may also enter bacteria through absorption, releasing metal ions to the surrounding medium and/or binding to the negatively charged functional groups of the bacterial cell membrane. For example, silver ions (from silver NPs) are adsorbed on the cell membrane, leading to protein coagulation (Jung et al., 2008). Jankauskaitl and collaborators described the bactericidal effect of graphene oxide/Cu/Ag NPs against E. coli, P. aeruginosa, K. pneumoniae, S. aureus, and Methicillin-resistant S. aureus (MRSA) through a possible synergy between multiple toxic mechanisms (Jankauskaite et al., 2016).
Non-oxidative mechanisms involve interaction of the NPs with the cell wall. In bacteria, the cell wall and membrane behave as defensive barriers that protect against environmental insults. Cell membrane components provide different adsorption pathways for the NPs (Lesniak et al., 2013). The cell wall of Gram-negative bacteria is composed of lipoproteins, phospholipids and lipid polysaccharides (LPS), which form a barrier only allowing the entry of certain macromolecules (Zaidi et al., 2017). In Gram-positive bacteria, the cell wall is composed of a thin layer of peptidoglycans and abundant pores that allow the penetration of foreign molecules, leading to covalent binding with proteins and cellular components, interrupting the proper functioning of the bacterial cell (Sarwar et al., 2015). In addition, Gram-positive bacteria have a highly negative charge on the surface of the cell wall. For example, LPS provides negatively charged regions on the cell wall of Gram-negative bacteria that attracts NPs; and, since teichoic acid is only expressed in Gram-positive bacteria, the NPs are distributed along the phosphate chain. As such, the antimicrobial effect is more foreshadowed in Gram-positive than -negative bacteria (Wang et al., 2017a). Indeed, Yu and colleagues synthesized a novel hydroxyapatite whisker (HAPw)/zinc oxide (ZnO) NPs and evaluated the antimicrobial effect against S. aureus, E. coli, and Streptococcus mutans. The authors demonstrate that the antibacterial effect depends on the components and structure of the bacterial cell wall. The antibacterial action of these NPs could be improved for Gram-positive bacteria and certain components could prevent the adhesion of ZnO NPs to the bacterial cell barrier (Yu et al., 2014). Ansari et al. reported that the accumulation on NPs in the bacterial cell wall causes irregularly shaped pit, perforation and disturbs metabolic processes (Ansari et al., 2014). In a study carried out by Joost and co-workers, it was demonstrated that a treatment with TiO2 NPs increased the bacterial cell volume, resulting in membrane leakage (Joost et al., 2015).
Biofilm Formation and Quorum-Sensing
Biofilm formation plays an important role in bacterial resistance protecting bacteria and allowing then to evade the action of antibiotics (Lebeaux et al., 2014; Khameneh et al., 2016). The most active fractions of bacteria are now recognized to occur as biofilms, where cells are adhered to each other on surfaces within a self-produced matrix of extracellular polymeric substance (EPS). EPS provide a barrier allowing to inhibit the penetration of antibiotics and further promote antibiotic resistance leading to a serious health threat worldwide since biofilms are resistant to antibiotics penetration and escape innate immune system by phagocytes (Hall-Stoodley et al., 2004; Bjarnsholt, 2013). Numerous experimental evidence show that NPs are capable of disrupting the bacterial membranes and can hinder biofilm formation thus reducing the survival of the microorganism (Peulen and Wilkinson, 2011; Leuba et al., 2013; Pelgrift and Friedman, 2013; Slomberg et al., 2013; Chen et al., 2014; Miao et al., 2016; Yu et al., 2016; Kulshrestha et al., 2017). This way, NPs provide an alternative strategy to target bacterial biofilms with potential to use both antibiotic-free and antibiotic-coated approaches (Gu et al., 2003; Li et al., 2012a; Sathyanarayanan et al., 2013). Earlier reports demonstrated that NPs are able to interfere with biofilm integrity by interacting with EPS and with the bacterial communication - quorum sensing (QS). The properties of NPs must be designed to be able to inhibit biofilm formation namely through size and surface chemistry. The size of NPs is important to it since they must be able to penetrate the EPS matrix and surface chemistry will command the amount of interactions with the EPS (Lundqvist et al., 2008). The majority of the strategies to achieve inhibition of biofilm formation are to target and interfere with QS molecules (Singh et al., 2017).
QS systems in bacterial populations act as major regulatory mechanisms of pathogenesis, namely in the formation of biofilm structures. These systems help bacteria to “communicate” with each other, through the production and detection of signal molecules (Rutherford and Bassler, 2012; Papenfort and Bassler, 2016). Using this cell-to-cell communication, bacterial populations are able to synchronize the expression of their genes, acquiring competitive advantage to respond to changes in the environment (Rutherford and Bassler, 2012). Therefore, QS systems are known to promote the formation of antibiotic tolerant biofilm communities. It is known that biofilm structures are a recalcitrant mode of bacterial growth that increases bacterial resistance to conventional antibiotics (Reen et al., 2018). This way, bacterial biofilms pose a significant challenge to the efficacy of conventional antibiotics being considered an essential platform for antibiotic resistance (Høiby et al., 2011). Taking this into account, it isn't surprising that the targeting and disruption of QS signaling systems and consequently, of the biofilm production, set the pillar for future next-generation anti-virulence therapies to be developed (LaSarre and Federle, 2013; Venkatesan et al., 2015; Jakobsen et al., 2017).
Surface-functionalized NPs with β-cyclodextrin (β-CD) or N-acylated homoserine lactonase proteins (AiiA) are able to interfere with signaling molecules preventing these molecules from reaching its cognate receptor, therefore inhibiting the signal/receptor interaction. This process will “turn off” QS and obstructing the bacterial communication (Kato et al., 2006; Ortíz-Castro et al., 2008). Several papers reported inhibition of biofilm formation namely by gold NPs (AuNPs). Acyl homoserine lactones (AHL) are signaling molecules with a role in bacterial QS and bind directly to transcription factors to regulate gene expression Recently, Gopalakrishnan and colleges synthesized (Vinoj et al., 2015) AuNPs coated AiiA purified from Bacillus licheniformis. These AiiA AuNPs inhibited EPs production and demonstrated potent antibiofilm activity against Proteus species at 2–8 μM concentrations without being harmful for the host cells at the 2μM concentration. Sathyanarayanan et al. (2013) demonstrated that using AuNPs there is a significant reduction of S. aureus and P. aeruginosa biofilms applied in high concentration (exceeding 50 mg/L). A recent study by Yu et al. (2016) demonstrated that AuNPs were able to strongly attenuate biofilm formation of P. aeruginosa. The inhibition observed in this study was related with interruption of adhesin- mediated interaction between the bacteria and the substrate surface due to electrostatic attractions between the AuNPs and cell wall surface of P. aeruginosa, instead of QS-related molecules. Positive charge AuNPs inhibited significantly S. aureus and P. aeruginosa biofilm formation (while minimizing mammalian cytotoxicity) (Ramasamy et al., 2016). The use of NPs demonstrates an exclusive approach to penetrate infectious biofilms and target bacterial communication, overcoming this major health issue related with biofilm infections.
Because most of these NPs-based platforms exert their action via distinct mechanisms/structures/pathways of those used by traditional antibiotics, combined therapeutic regimens are promising strategies to tackle the surge of multidrug resistant (MDR) bacteria bypassing their defense mechanisms (Pelgrift and Friedman, 2013; Singh K. et al., 2014; Hemeg, 2017; Zaidi et al., 2017). Additionally, NPs have been shown to activate macrophages in a dose dependent manner (Patel and Janjic, 2015) which promotes the host defenses (Hemeg, 2017; Jagtap et al., 2017).
This multi-target action of NPs may overcome multidrug resistance by circumventing several obstacles encountered by traditional antibiotics (Pelgrift and Friedman, 2013; Chen et al., 2014; Singh K. et al., 2014; Hemeg, 2017; Jagtap et al., 2017; Rai et al., 2017; Zaidi et al., 2017). Table 1 highlights several types of NPs that have shown effective bactericidal activity when administered isolated; combined with standard antibiotics; and/or radiation or as vectors for biocidal delivery allowing killing of MDR bacteria, and in some cases also inhibiting biofilm production.
We will now focus on the different types of metal NPs highlighting their most relevant mechanism/effects against MDR bacteria and/or biofilms structures.
Silver Nanoparticles (AgNPs)
Since the ancient times, silver has been recognized as having antimicrobial effects (Rai et al., 2009; Reidy et al., 2013). Based on all the evidence to date, AgNPs are probably one of the most promising inorganic NPs that can be used for the treatment of bacterial infections (Natan and Banin, 2017). These NPs may be synthesized by traditional chemical reduction or via “green” chemistry approaches using plant and/or microbial extracts (Iravani et al., 2014; Ribeiro et al., 2018).
Several mechanisms have been proposed to understand how AgNPs mediate cell death, including cell wall disruption (Lok et al., 2007; Bondarenko et al., 2013), oxidation of cellular components, inactivation of the respiratory chain enzymes, production of ROS, and decomposition of the cellular components (Chen et al., 2014; Rizzello and Pompa, 2014; Dakal et al., 2016). The permeability of the membrane increases after incorporation of AgNPs into the cell membrane. The adsorption of the NPs leads to the depolarization of the cell wall, altering the negative charge of the cell wall to become more permeable. It was demonstrated disruption of the cell wall with subsequent penetration of the NPs. The entry of AgNPs induces ROS that will inhibit ATP production and DNA replication (Zhang et al., 2013; Dakal et al., 2016; Durán et al., 2016; Ramalingam et al., 2016). However, there is evidence that AgNPs can release Ag+, known to exhibit antimicrobial activity, when interacting with thiol-containing proteins, which weaken their functions (Durán et al., 2010). The precise method of the antibacterial mechanism of AgNPs is still not completely understood (Franci et al., 2015; Durán et al., 2016). All the existing data indicates that AgNPs exert several bactericidal mechanisms in parallel, which may explain why bacterial resistance to silver is rare (Karimi et al., 2016). Concerns regarding the cytotoxicity and genotoxicity of AgNPs have been raised (Chopra, 2007) but various authors have conducted clinical trials based on AgNPs and no important clinical alterations have been detected (Munger et al., 2014a,b; Smock et al., 2014). Interestingly, AgNPs have been found to exhibit higher antimicrobial activity than antibiotics like gentamicin or vancomycin against P. aeruginosa and MRSA (Saeb et al., 2014). Lara et al. showed the potential bactericidal effect of AgNPs against MDR P. aeruginosa, ampicillin-resistant E. coli O157:H7 and erythromycin-resistant Streptococcus pyogenes (Lara et al., 2010). Nagy et al., reported that AgNPs were capable of inhibiting the growth of S. aureus and E. coli via the up-regulation of the expression of several antioxidant genes and ATPase pumps (Nagy et al., 2011). Dolman et al. also showed that the Ag-containing Hydrofiber® dressing and nanocrystalline Ag-containing dressing are effective agents against antibiotic sensitive Gram-negative and -positive bacteria as well as antibiotic resistant bacteria, such as MRSA, Vancomycin-resistant Enterococci (VRE) and Serratia marcescens, avoiding the formation of biofilms on biomaterials (Percival et al., 2007). Su and collaborators showed that AgNPs immobilized on the surface of nanoscale silicate platelets (AgNP/NSPs) have strong antibacterial activity against MRSA and silver-resistant E. coli via generation of ROS (Su et al., 2011). Singh and collaborators showed that AgNPs from P. amarus extract exhibited excellent antibacterial potential against MDR strains of P. aeruginosa (Singh K. et al., 2014). Recently, two different shaped AgNPs (spheres and rods) were used against Gram-positive and -negative bacteria, both showing promising antibacterial activity against different strains (Acharya et al., 2018).
An emerging practice is to combine AgNPs with antibiotics to enhance antimicrobial potency. Recently, Katya and collaborators showed that the combination of gentamicin and chloramphenicol with AgNPs has a better antibacterial effect in MDR E. faecalis than both antibiotics alone (Katva et al., 2018). McShan et al. described that AgNPs combined with either one of two-different class of antibiotics (tetracycline and neomycin) can exhibit a synergistic effect, showing an enhanced antibacterial activity at concentrations below the MIC of either the NPs or the antibiotic (McShan et al., 2015). Other authors also reported similar results (Thomas et al., 2014; Panáček et al., 2016a,b; Salomoni et al., 2017). Djafari and collaborators described the synthesis of water-soluble AgNPs using the antibiotic tetracycline as co-reducing and stabilizing agent (AgNPs@TC) and demonstrated their effectiveness against tetracycline-resistant bacteria (Djafari et al., 2016).
Antimicrobial peptides (AMPs) represent one of the forms of defense strategy against infections in living organisms and are emerging as essential tools to kill pathogenic bacteria, since they exhibit broad-spectrum activity and low resistance development (Yeaman, 2003). Lytic peptides are AMPs produced by all organisms. In mammals, they are an innate host defense mechanism against pathogens (Bahar and Ren, 2013). The mechanism of action of AMPs relies on the ability to interact with bacterial membranes or the cell wall, thus inhibiting cellular biochemical pathways and ultimately killing the bacteria (Zhang and Gallo, 2016). Defensins and cathelicidin are two of the larger families of lytic peptides that kill bacteria by disrupting the membrane. Unfortunately, AMPs have poor enzymatic stability, low permeability across biological barriers and may be rapidly degraded in the human body by proteases, which greatly limits their application (Wang, 2014). Immobilization of the peptides onto NPs can increase their stability, enhancing the antimicrobial properties of the NPs and therefore, has the potential to be used as a new tool to tackle antibiotic resistant bacteria (Brandelli, 2012; Rai A. et al., 2016). Indeed, the first author to demonstrate that functionalized AgNPs with peptides increased their antibacterial activity was Ruden and co-workers (Ruden et al., 2009). Based on this strategy several researchers functionalized AgNPs with AMPs (AgNP@AMP) with increases in the antimicrobial activity compared with free AMPs (Ruden et al., 2009; Liu et al., 2013; Mohanty et al., 2013). Polymyxin B is the most used AMP and exhibits antibacterial activity via interaction with the endotoxin LPS in the outer membrane of Gram-negative bacteria (Morrison and Jacobs, 1976; Lambadi et al., 2015). It was proved that AgNPs functionalized with polymyxin-B removed almost completely endotoxins from solutions and hindered the formation of biofilm onto surgical blades (Jaiswal et al., 2015; Lambadi et al., 2015). Liu et al., demonstrated that the immobilization of peptides with AgNPs enhanced their antimicrobial activity compared to an unbound peptide and also minimized toxicity of AgNPs compared to using the AgNPs alone (Liu et al., 2013). A recent study by Pal et al. describes a system consisting of a cysteine containing AMP conjugated with AgNPs, which demonstrated that the Ag-S bonds increased stability and enhanced antimicrobial activity than conjugation using electrostatic interactions (Pal et al., 2016).
Other methods have been used to improve the antibacterial activity of AgNPs. One of these methods relies on the use of visible blue light, which was previously shown to exhibit strong antibacterial activity (Dai T. et al., 2013; Maclean et al., 2014). El Din and collaborators demonstrated that blue light combined with AgNPs exhibits therapeutic potential to treat MDR infections and can represent an alternative to conventional antibiotic therapy, since the antimicrobial activity of the combination was greater than the components alone. Moreover, this approach proved to be synergistic in the treatment of an unresponsive antibiotic-resistant bacteria responsible for a wound in a horse (El Din et al., 2016). Spherical shaped thioglycolic acid-stabilized AgNPs (TGA-AgNPs) conjugated with vancomycin were used as drug delivery systems and demonstrated to possess increased antimicrobial activity against MDR bacteria such as MRSA and VRE (Esmaeillou et al., 2017).
Gold Nanoparticles (AuNPs)
Metallic gold is considered inert and non-toxic, which may vary when it shifts form metallic bulk to oxidation states (I and II) (Merchant, 1998). Gold NPs (AuNPs) may be synthesized by traditional chemical reduction of a gold salt or via “green” chemistry approaches using plant and/or microbial extracts (Shah et al., 2014). The most used and described method is the chemical synthesis based on the reduction of chloroauric acid by citrate (Lee and Meisel, 1982; Fernandes and Baptista, 2017). Some studies have addressed the potential of using AuNPs as antibacterial agents, but some controversy still exists (Cui et al., 2012; Bresee et al., 2014; Shah et al., 2014; Shareena Dasari et al., 2015; Zhang et al., 2015a; Shamaila et al., 2016).
According to Yu H and collaborators, AuNPs are usually not bactericidal at low concentrations and weakly bactericidal at high concentrations (Shareena Dasari et al., 2015; Zhang et al., 2015a). This is possibly due to the effect of co-existing chemicals, such as gold ions, surface coating agents, and chemicals involved in the synthesis that were not completely removed (Shareena Dasari et al., 2015; Zhang et al., 2015a). However, other authors describe that the antibacterial mechanism of AuNPs is associated to (i) the collapse in the membrane potential, hindering ATPase activity causing a deterioration of the cell metabolism; (ii) hindering of the binding subunit of the ribosome to tRNA (Cui et al., 2012); and (iii) Shamaila and co-workers showed that AuNPs may affect the bacterial respiratory chain by attacking nicotinamide (Shamaila et al., 2016). Since AuNPs are non-toxic to the host (Conde et al., 2014; Li et al., 2014; Rajchakit and Sarojini, 2017), the possibility of fine tuning their conjugation chemistry to act as carriers or delivery vehicles of antibiotics or other antibacterial moieties may enhance their bactericidal effect and potentiate the effect of antibiotics (Zhao and Jiang, 2013; Conde et al., 2014; Li et al., 2014; Uma Suganya et al., 2015; Zhang et al., 2015a; Fernandes et al., 2017).
Cationic and hydrophobic functionalized AuNPs were shown to be effective against both Gram-negative and -positive uropathogens, including MRSA. These AuNPs exhibited low toxicity to mammalian cells (biocompatibility) and the development of resistance to these NPs was very low (Li et al., 2014). Vinoj et al. demonstrated that coating AuNPs with N-acylated homoserine lactonase proteins (AiiA AuNPs) resulted in a nanocomposite with activity against MDR species compared with AiiA proteins alone (Vinoj et al., 2015). Other approaches were also studied, as adsorbing AuNPs to PVA-lysozyme micro bubbles potentiate the antibacterial activity due to the interaction of AuNPs with cells membranes causing bacterial lysis (Mahalingam et al., 2015). Galic acid capped AuNPs have also been found to be active against Gram-negative and -positive bacteria (Kim D. et al., 2017). Recently, Ramasamy and collaborators described the direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles (CGNPs) with effective biofilm inhibition of more than 80% against Gram-positive bacteria (methicillin-sensitive and -resistant strains of S. aureus, MSSA and MRSA, respectively) and Gram-negative (E. coli and P. aeruginosa) in vitro and in vivo (Ramasamy et al., 2017a,b). Also, the integration of AuNPs with ultrathin graphitic carbon nitride was described as having high bactericidal performance against both MDR Gram-negative and -positive bacteria, and a high effectiveness in eliminating existing MDR-biofilms and preventing the formation of new biofilms in vitro (Wang Z. et al., 2017). Also, conjugation of antibiotics to AuNPs, such as vancomycin, methicillin, etc., increases their intrinsic activity against MDR strains (Mohammed Fayaz et al., 2011; Lai et al., 2015; Roshmi et al., 2015; Payne et al., 2016). Recently Payne and collaborators develop a single-step synthesis of kanamycin-capped AuNPs (Kan-AuNPs) with high antibacterial activity against both Gram-positive and -negative bacteria, including kanamycin resistant bacteria. The authors observed a significant reduction in the MIC against all the bacterial strains tested for Kan-AuNPs when compared to the free drug. This higher efficacy was due to the disruption of the bacterial envelope, resulting in leakage of the cytoplasmic content and consequent cell death (Payne et al., 2016). Pradeepa and collaborators synthesized AuNPs with bacterial exopolysaccharide (EPS) and functionalized them with antibiotics (levofloxacin, cefotaxime, ceftriaxone and ciprofloxacin). They observed that these AuNPs exhibited excellent bactericidal activity against MDR Gram-positive and -negative bacteria compared to free drugs. E. coli was the most susceptible MDR bacteria followed by K. pneumoniae and S. aureus (Pradeepa et al., 2016). Recently, Yang and collaborators described the effect of small molecule (6-aminopenicillanic acid, APA)-coated AuNPs to inhibit MDR bacteria (Yang et al., 2017). They doped AuNPs into electrospun fibers of poly(ε-caprolactone) (PCL)/gelatin to produce materials that avoid wound infection by MDR bacteria and demonstrated in vitro and in vivo that APA-AuNPs reduced MDR bacterial infections (Yang et al., 2017). Shaker and Shaaban evaluated the surface functionalization of AuNPs with carbapenems [imipenem (Ipm) and meropenem (Mem)] as a delivering strategy against carbapenem resistant Gram-negative bacteria isolated from an infected human. Both Ipm-AuNPs and Mem-AuNPs, with 35 nm diameter showed a significant increase in antibacterial activity against all the tested isolates (Shaker and Shaaban, 2017). Also, Shaikh and collaborators described recently the synthesis and characterization of cefotaxime conjugated AuNPs to target drug-resistant CTX-M-producing bacteria. The authors could invert resistance in cefotaxime resistant bacterial strains (i.e., E. coli and K. pneumoniae) by using cefotaxime-AuNPs. Their results reinforce the efficacy of conjugating an unresponsive antibiotic with AuNPs to restore its efficacy against otherwise resistant bacterial pathogens (Shaikh et al., 2017).
Combination of AuNPs with other approaches has also been demonstrated. Indeed, one of the most extraordinary properties of AuNPs is the capability to transform light into heat under laser irradiation (Mendes et al., 2017; Mocan et al., 2017). This property is extremely important because it can be exploited to develop photothermal nanovectors to destroy MDR bacteria at a molecular level (for a complete review see Mocan et al., 2017). For example, Khan and collaborators showed that the combination of Concanavalin-A (ConA) directed dextran capped AuNPs (GNPDEX-ConA) conjugated with methylene blue (MB) (MB@GNPDEX-ConA) and photodynamic therapy (PDT) enhanced the efficacy and selectivity of MB induced killing of MDR clinical isolates, including E. coli, K. pneumoniae, and E. cloacae (Khan et al., 2017). Gil-Tomas and collaborators described that the functionalization of AuNPs covalently with toluidine blue O–tiopronin forms an enhanced, exceptionally potent antimicrobial agent when activated by white light or 632 nm laser light (Gil-Tomás et al., 2007). Hu and collaborators prepared a mixed charged zwitterion-modified AuNPs consisting of a weak electrolytic 11-mercaptoundecanoic acid (HS-C10-COOH) and a strong electrolytic (10-mercaptodecyl)trimethylammonium bromide (HS-C10-N4) that exhibited in vivo and under near-infrared (NIR) light irradiation an enhanced photothermal ablation of MRSA biofilm with no damage to the healthy tissues around the biofilm (Hu et al., 2017). Also, the antibacterial activity of glucosamine-gold nanoparticle-graphene oxide (GlcN-AuNP-GO) and UV-irradiated GlcN-AuNP-GO was evaluated against E. coli and E. faecalis. Results show that UV irradiation of GlcN-AuNP-GO results in higher antibacterial activity than the standard drug kanamycin (Govindaraju et al., 2016). Ocsoy et al. reported the development of DNA aptamer-functionalized AuNPs (Apt@AuNPs) and gold nanorods (Apt@AuNRs) for inactivation of MRSA with targeted PTT (Ocsoy et al., 2017). The authors showed that although both NPs could specifically bind to MRSA cells, Apt@AuNPs and Apt@AuNRs increased resistant cell death for 5% and for more than 95%, respectively through PTT. This difference in induction of cell death was based on the relatively high longitudinal absorption of NIR radiation and strong photothermal conversion capability for the Apt@AuNRs compared to the Apt@AuNPs. However, with the new developments of using AuNPs for hyperthermia in the visible light (Mendes et al., 2017) might additionally potentiate the Apt@AuNPs results observed for these authors (Ocsoy et al., 2017). Recently, Mocan et al. also described the synthesis of AuNPs by wet chemistry, their functionalization with IgG molecules following laser irradiation. Their results indicate that administration of IgG-AuNPs following laser irradiation provided an extended and selective bacterial death in a dose dependent manner (Mocan et al., 2016).
In recent years, a new approach relying on the conjugation of AuNPs with AMPs has shown interesting results (Rajchakit and Sarojini, 2017). Indeed, Kuo and collaborators mixed synthetic-peptides containing arginine, tryptophan and cysteine termini [(DVFLG)2REEW4C and (DVFLG)2REEW2C], with aqueous tetrachloroauric acid to generate peptide-immobilized AuNPs [i.e., (DVFLG)2REEW4C-AuNPs and (DVFLG)2REEW2C-AuNPs] that were effective antibacterial agents against Staphylococci, Enterococci, and antibiotic-resistant bacterial strains (Kuo et al., 2016). Rai and co-workers demonstrated that the use of cecropin-melittin (CM-SH) a known peptide with antibacterial properties (Boman et al., 1989), functionalized in the surface of AuNPs through Au-S bond, showed higher antimicrobial activity and higher stability in media compared with an in vitro and in vivo infection animal model with the MIC of CM-SH AuNPs four times lower than free CM-SH (Rai A. et al., 2016). Conjugation of AMP with AuNPs usually involves the formation of the Au-S coordinate covalent bond, relying on the amine or thiol groups in peptides or conjugating specific linkers to AMPs with a terminal (N- or C-terminal) cysteine which helps conjugation with gold (Tielens and Santos, 2010; Xue et al., 2014). However, there is one example where covalent conjugation of an AMP to AuNPs has been achieved via Au-O bond (Lai et al., 2015). Other approaches use a linker for the conjugation to AuNPs, Poly(ethylene glycol) carboxylic acid (PEGCOOH) covalently bound to AMP showed a significantly increase of the antibacterial and anti-biofilm activity for resistant Gram-negative bacteria (Casciaro et al., 2017). Yeom and co-workers demonstrated the most advanced in vivo clinical application for AuNPs@AMP using infected mice and resulting in the inhibition of Salmonella Typhimurium colonization in the organs of the animals (Yeom et al., 2016). The reason behind the increased antimicrobial activity of AuNPs@AMP over the free components is that AuNPs can get a higher concentration of the antibiotic at the site of action. These NPs can interact with LPS, proteins in the membrane of the bacteria and in some cases, penetrate the bacterial membrane through the porin channel. This way they can interact with the inner membrane making the AuNPs@AMP conjugate more efficient than the non-conjugated form (Katz and Willner, 2004; Wangoo et al., 2008; Chen J. et al., 2009).
Bimetallic NPs
Ag and Au may be used in a single NP to enhance the effects of a drug and reduce the required dose. Alternatively, they can be used alone since they possess antimicrobial properties that are enhanced when combined in the form of bimetallic NPs (Arvizo et al., 2010; Singh R. et al., 2016). The role of Ag against MDR pathogens has been previously described. However, AgNPs are difficult to functionalize with biomolecules and drugs. Such limitation may be circumvented by means of alloy/bimetallic NPs that excel their monometallic counterparts providing improved electronic, optical and catalytic properties (Cho et al., 2005; Shah et al., 2012). As reported above, AuNPs constitute good vectors to the delivery of pharmacologic compounds. Gold(Au)-silver(Ag) alloys are an optimal solution since they combine the antimicrobial effect of silver with the ease of functionalization and improved stability in complex biological media provided by gold (Doria et al., 2010; dos Santos et al., 2012). Fakhri and co-workers synthetized and functionalized AgAuNPs with a tetracycline and concluded that there exists a synergetic effect of the antibiotic with the bimetallic nanoparticle, with greater bactericidal activity of this form in detriment of its free forms. The mechanism of action was established as being the generation of ROS (Fakhri et al., 2017). Also recently, Baker and collaborators described the synthesis and antimicrobial activity of bimetallic AgAuNPs from the cell free supernatant of Pseudomonas veronii strain AS41G inhabiting Annona squamosa L. The authors showed their synergistic effect with standard antibiotics with 87.5, 18.5, 11.15, 10, 9.7, and 9.4% fold increased activity with bacitracin, kanamycin, gentamicin, streptomycin, erythromycin and chloramphenicol, respectively, against bacitracin resistant strains of Bacillus subtilis, E. coli, and K. pneumoniae (Baker et al., 2017). Zhao and collaborators have demonstrated the antibacterial activity of AuPtNPs bimetallic NPs against sensitive and drug-resistant bacteria via the dissipation of the bacterial membrane potential and the elevation of adenosine triphosphate (ATP) levels (Zhao et al., 2014).
Other types of bimetallic NPs have been studied and their antibacterial activity explored, but in most cases as coating agents and not as a delivery approach and antibacterial activity (Argueta-Figueroa et al., 2014).
Metal Oxides
Metal oxides NPs are among one of the most explored and studied family of NPs and are known to effectively inhibit the growth of a wide range of sensitive and resistant Gram-positive and -negative bacteria, emerging as hopeful candidates to challenge antimicrobial resistance (Raghunath and Perumal, 2017; Reshma et al., 2017; Kadiyala et al., 2018). Iron oxide (Fe3O4), Zinc oxide (ZnO), and Copper oxide (CuO) possess antimicrobial properties and can be applied in clinical care (Sinha et al., 2011). Due to the intrinsic photocatalytic activity of the metal oxides they generate ROS and become powerful agents against bacteria (Tong et al., 2013; Singh R. et al., 2014). These will be described in more detail on the following sections.
Iron Oxide (Fe3O4)
The synthesis of iron oxide NPs may be achieved via different routes (Babes et al., 1999; Berry and Curtis, 2003). The antibacterial mechanism of these NPs is mainly attributed to dissolved metal ions and the generation of ROS (Wang et al., 2017a). It was shown that superparamagnetic iron oxide NPs interact with microbial cells by penetrating the membrane and interfering with the electron transfer (Behera et al., 2012; El-Zowalaty et al., 2015). Additionally, it has been described that iron oxide NPs can damage macromolecules, including DNA and proteins, through the formation of ROS (Leuba et al., 2013). Pan et al. developed a system of reduced graphene oxide (rGO)-iron oxide nanoparticles (rGO-IONP) by the chemical deposition of Fe2+/Fe3+ ions on nanosheets of rGO in aqueous ammonia. The in vivo results showed maximum antibacterial activity due to the generation of hydroxyl radicals that can cause physical and chemical damage, which inactivated MRSA (Pan et al., 2016).
Zinc Oxide (ZnO)
ZnO NPs are often used to restrict microorganism growth, being effective against planktonic bacteria, and also inhibiting the formation of biofilms (Hsueh et al., 2015; Sarwar et al., 2016) (Espitia et al., 2012). These NPs can be synthesized by various methods, from green chemistry to sonochemistry (Salem et al., 2015; Ali et al., 2016; Nagvenkar et al., 2016). The antibacterial mechanism of the NPs is partially attributed to two principal factors, the dissolution of metal ion and the generation of ROS (Gelabert et al., 2016; Nagvenkar et al., 2016; Sarwar et al., 2016). ZnO releases Zn2+ in liquid medium and is adsorbed on the surface of bacteria or may entry the cell, where it interacts with functional groups in proteins and nucleic acids, hindering enzyme activity and the normal physiological processes (Yu et al., 2014). However, some authors demonstrated that Zn ions have little antimicrobial activity, implying that dissolution of Zn2+ might not be the main mechanism of action (Aydin Sevinç and Hanley, 2010). Sarwar and co-workers demonstrated that nanosized ZnO caused significant oxidative stress to Vibrio cholera, the damage inflicted was DNA degradation, protein leakage, membrane depolarization and fluidity (Sarwar et al., 2016). Ehsan and Sajjad, described that ZnO NPs impregnated with antibiotics showed good antibacterial activities against S. aureus, Proteus, Acinetobacter, P. aeruginosa, and E. coli, being that these were resistant to antibiotics but became sensitive in the presence of these NPs with antibiotics (Ehsan and Sajjad, 2017). It was also discovered that these NPs induce the production of ROS even in the dark, and this happens due to the surface defects on the NPs. The different shapes function as enzyme inhibitors, where nanopyramids are the most effective (Cha et al., 2015; Lakshmi Prasanna and Vijayaraghavan, 2015). Recently, Aswathanarayan and Vittal described the antimicrobial effect of ZnO NPs against MDR Gram-positive and -negative pathogens in comparison to gold and iron NPs and these could be used at concentrations less toxic to mammalian cells (Aswathanarayan and Vittal, 2017). ZnO NPs are also known for inhibiting biofilm formation and production of quorum-sensing-dependent virulence factors in P. aeruginosa (Lee et al., 2014; García-Lara et al., 2015).
Copper Oxide (CuO)
Copper containing NPs have been shown effective against animal and plant pathogens (LewisOscar et al., 2015), impeding formation of MDR biofilms, and showing the potential to serve as antimicrobial coating agents (LewisOscar et al., 2015). Kruk et al. and Zhang et al. showed that copper NPs are capable of inhibiting the growth of MDR bacteria, namely, P. aeruginosa and MRSA (Zhang et al., 2014, 2015b; Kruk et al., 2015). The antimicrobial activity of these NPs is comparable to that of AgNPs but at a lower cost (Kruk et al., 2015). Copper oxide NPs generate ROS that often leads to chromosomal DNA degradation, which seems to highlight a “particle-specific” action rather than resulting from the release of metallic ions (Chakraborty et al., 2015). Su and collaborators investigated the effects of CuONPs on bacterial denitrification and explored the effect on the expression of intracellular proteins. When CuONPs entry into bacteria metabolic functions are affected, such as active transport, electron transfer, and nitrogen metabolism (Su et al., 2015).
NPs can also be complexed with other metals, like gallium. Gallium NPs have been described to facilitate phagosome maturation of macrophages infected with virulent M. tuberculosis and therefore being able to inhibit growth of this pathogen (Choi et al., 2017).
The Potential for Nanotheranostics
NPs applications in biodetection is huge and more insights on pathogen detection using NPs platforms can be seen in Veigas et al. (2013, 2014, 2015); Costa et al. (2014); Weng et al. (2015); Kim J. et al. (2017); Wang et al. (2017b); Galvan and Yu (2018), and Yang et al. (2018).
Theranostics is a combination of diagnosis and therapy onto a single platform, which allow for timely biodetection and/or real-time monitoring of therapy. By using NPs, this can be translated to the nanoscale—Nanotheranostics. NPs have been applied for multiplex high-throughput diagnostics to assist precision therapy. For example, Verigene® is an AuNPs test commercialized for diagnosis. It is an automated microarray-based system that identifies Gram-negative pathogens from positive blood cultures. Verigene® BC-GN also detects key resistance mechanisms (Walker et al., 2016; Claeys et al., 2018). Others have used, magnetic and functionalized magnetic iron oxide NPs as affinity probes to capture Gram-positive and -negative bacteria. The analyses of captured bacteria using matrix-assisted laser desorption/ionization mass spectrometry was <1 h (Reddy P. M. et al., 2014). One pioneer work on nanotheranostics against bacterial infection was the development of a method for in vivo photoacoustic detection and photothermal eradication of S. aureus. Two-color gold and multilayer magnetic nanoparticles were functionalized with an antibody cocktail for the targeting of S. aureus. These platform demonstrated ultrasensitive detections for circulating bacterial cells (CBCs), in vivo magnetic enrichment and PT eradication of CBCs (Galanzha et al., 2012). Recently, Zhou and collaborators developed a silicon 2,3-naphthalocyanine dihydroxide (Nc) and Vancomycin functionalized silica-encapsulated, silver-coated gold NPs (Au@AgNP@SiO2@Nc-Van) as a novel theranostic system for surface-enhanced Raman scattering (SERS) detection and antimicrobial photodynamic therapy (aPDT) of vancomycin (Van)-resistant enterococci (VRE) strains (Zhou et al., 2018). These authors observed a 4–5 logs reduction of bacteria upon in vitro aPDT of VRE treated with a nanomolar concentration of the Au@AgNP@SiO2@Nc-Van and an infection regression and even complete eradication of VRE in vivo using infected mice (Zhou et al., 2018).
A selenium nanoplatform (Se@PEP-Ru) was designed with excellent fluorescent properties for imaging bacteria and with high antimicrobial properties (Huang et al., 2017). Zhao and co-workers developed an activated theranostics nanoprobe for near-infrared fluorescence imaging and photothermal therapy of MRSA infections, based on SiO2/PAH-cypate nanosystems modified with PEG and Vancomycin-conjugated poly(acrylic acid) molecules (PAAPEG-Van). This probe is activated by bacteria-responsive polyelectrolyte dissociation from silica NPs. The authors believe that this concept can be used as an approach to design and for production of bacteria responsive multifunctional nanomaterials and constitute their ultimate functions in the treatment of drug-resistant bacterial infections (Zhao et al., 2017). Kuo and collaborators developed a nanotheranostics system using Au nanorods conjugated with a hydrophilic photosensitizer, toluidine blue O, that acted as dual-function agents in photodynamic inactivation and hyperthermia against MRSA (Kuo et al., 2009).
Clinical Translation
At present, there are a few metal NPs-based strategies against bacterial infections undergoing clinical trials. The costs associated to the use of nanotechnology platforms are very high, and therefore conventional treatments are preferred. However, these platforms might be preferable in specific situations, with direct impact on the quality of patients life (Caster et al., 2017).
Bio-kil® [3-(Trimethoxysilyl) propyloctadecyldimethyl ammonium chloride] (Cargico Group, Taiwan) is a patented technology that is based on affixing nano-sized antimicrobials onto a large surface area through covalent chemical bonding to form a durable polymer. Bio-kil® eliminates microorganism through a physical biocide process. This type of nanomaterial consists in inorganic metal components and organic quaternary ammonium components. Recently, Bio-Kil® has been shown to reduce the environmental bacterial burden and MDR organisms (Lee et al., 2017).
AgTive (NCT00337714) is a silver-impregnated central venous catheter and has been marketed with the claim to improved bactericidal activity. AgTive catheters are made of polyurethanes impregnated with silver NPs, and their interaction with body fluids and intravenous solutions results in the release of significantly larger amounts of silver ions from the catheter reducing bloodstream infection (Antonelli et al., 2012).
Acticoat is a nanocrystalline silver dressing that acts as an antimicrobial topical, releasing silver into the wound. This nanoformulation has been shown to inhibit in vitro biofilms formation in P. aeruginosa and Acinetobacter baumannii by more than 90% (Potgieter and Meidany, 2017). Madigan Army Medical Center is studying the efficacy of a silver NPs gel SilvaSorb (NCT00659204) and currently is in phase III of the clinical trials. The aim of this study is to compare the antimicrobial efficacy of a one-time application of SilverSorb (AcryMed, Inc., Portland) against the standard antibacterial hand gel Purell (GoJo Industries, Akron), in reducing transient bacterial counts isolated from the hands of 40 patients seeded with S. marcescens.
Nano Silver Fluoride is a new formulation that combines silver NPs, chitosan and fluoride and was developed with antimicrobial properties. This nanoformulation has excellent results as antibacterial agent against S. mutans and Lactobacilli. Currently, is used to prevent dental caries in children (Dos Santos et al., 2014).
Despite this review do not concern liposomal formulations since it refers to clinical translation other formulations involving NPs, such as liposomal formulations, have been also identified as antimicrobial agents. Most of these formulations rely on the incorporation of traditional antibiotics into nanoliposomes to improve distribution and circulation times (Caster et al., 2017). Table 2 summarizes antimicrobial liposomes, which are undergoing clinical trials. For example, Amikacin (NCT01315691) is a potent aminoglycoside antibiotic that is useful for the treatment of MDR Gram-negative bacteria. Arikace is an inhaled liposomal formulation that encapsulates amikacin composed of dipalmitoyl-phosphatidylcholine (DPPC) and cholesterol (Meers et al., 2008). These formulation have high drug loading and stability when administrated and in phase II trial, there was no notable difference in toxicity between liposomal drug treatment and placebo (Clancy et al., 2013). Another two-inhaled liposomal formulation are currently in clinical trials. Linhaliq (NCT02104245) is a combination of liposomal and aqueous phase ciprofloxacin, whereas Lipoquin (NCT00889967) is a liposomal ciprofloxacin that allows prolonged drug release. Both of these nanoformulation were developed for the treatment of non-cystic fibrosis bronchiectasis (NCFBE) patients with chronic lung infections with P. aeruginosa. Phase II in patients with both CF and non-CF bronchiectasis have been completed. After analysis of clinical data from the two different formulations, Linhaliq showed better performance. The Food and Drug Administration (FDA) has designated Linhaliq as a qualified infection disease product and made it eligible for Fast track designation. In 2016, Pulmanic completed two phases III clinical trials, but has not yet been approved by the FDA. The Hadassah Medical Organization (Jerusalem, Israel) has incorporated quaternary ammonium polyethyleneimine (QA-PEI) based polymers into dental composites. The bacterial membrane may be disturbed by the charged quaternary moiety, it also has potent activity against a series of Gram-positive and -negative pathogens (Ortega et al., 2015). In 2013, these nanoformulation completed phase II trials but no data on outcome have been released to date.
MAT2501 is designed to targeted delivery of the antibiotic amikacin while providing an improved safety and tolerability profile. Currently, Matinas Biopharma has reported positive data from the Phase I study in healthy volunteers for the treatment of MDR Gram-negative bacterial infections and is in preparation for a phase II in patients.
Other Potential Applications of NPs
In the case of non-antibiotic therapy, combinations of NPs with essential oils, peptides and other natural compounds have featured as promising antimicrobial strategies. The therapeutic applications of these substances are often limited by their toxicity and volatility (Chen F. et al., 2009; Allahverdiyev et al., 2011). A recent study has shown that chitosan NPs vectors, modified with eugenol and carvacrol essential oils on their surface, were active against E. coli and S. aureus at concentrations better or equal to unmodified NPs versions (Chen F. et al., 2009). Furthermore, the toxicity of the conjugates toward mouse fibroblasts was significantly less than the pure oils alone. With regards to peptides, the active sequences can be vulnerable to denaturation, aggregation or hydrolysis within end products or in the human body. Colloidal systems containing NPs are at the forefront of peptide research, as they can be designed to encapsulate and protect peptides during biological transit. Water in oil micelles have been successfully used to increase the potency of antimicrobial peptides against E. coli (Gontsarik et al., 2016). In another example, liposomes have been used to improve the stability of encapsulated nisin against pH and temperature extremes thereby increasing its potential in food processing (Taylor et al., 2007). Popular NPs vehicle materials for peptides include phytoglycogen NPs (Bi et al., 2011), chitosan (Wu et al., 2017), pectin (Krivorotova et al., 2017), and alginate (Khaksar et al., 2014).
NPs have also been applied with tremendous success in biodetection systems, namely as sensors and diagnostics platforms with increased sensitivity and selectivity. Due to the decrease in size of the transduction mechanisms provided by NPs, most of these platforms have found applications at point-of-need and/or point-of-care (Costa et al., 2014; Veigas et al., 2014; Weng et al., 2015; Kim J. et al., 2017; Wang et al., 2017b; Galvan and Yu, 2018; Yang et al., 2018). In some cases, diagnostics/sensing and therapeutic properties have been combined onto single NPs, providing for innovative tools – Nanotheranostics. Recently, several nanotheranostics strategies against bacteria have been described (Kuo et al., 2009; DeGrasse, 2012; Dai X. et al., 2013; Khlebtsov et al., 2013; Kim et al., 2013; Gamella et al., 2014; Pei et al., 2014; Setyawati et al., 2014; Patel and Janjic, 2015; Thompson et al., 2015; Jagtap et al., 2017; Mocan et al., 2017; Zhao et al., 2017).
Bottlenecks and Future Challenges of NPs
Despite the foreseen potential of NPs for medical applications, there are still several bottlenecks related with their acute and long-term exposure in humans. Several routes of exposure must be considered when evaluating NPs exposure, such as oral and gastrointestinal tract, dermal, respiratory system, and endovenous administration directly to the bloodstream (De Matteis, 2017). It is well known also that the physicochemical properties of NPs (e.g., size, shape and surface chemistry) affect their interaction with biological systems, influencing cellular uptake, pharmacokinetics, biodistribution, all of them with direct impact on final biological effects (for recent reviews see Bakand and Hayes, 2016; Xia et al., 2016; De Matteis, 2017; Warheit, 2018). These aspects have been addressed over the past years via the evaluation of the in vitro and in vivo toxicity of metal and metal oxide NPs (Dobrovolskaia et al., 2007; Asharani et al., 2010; Li et al., 2010; Baek and An, 2011; Hackenberg et al., 2011; Conde et al., 2012, 2014; Bondarenko et al., 2013; Ivask et al., 2014; Larsen et al., 2016; Sukwong et al., 2016; Rai et al., 2017), whose conclusions concerning their nanosafety differ depending on the type of assessment. This poses a major concern to effectively draw critical conclusions on NPs safety due to the vast number of different types/shapes/surface modified nanoparticles, the different methods used to evaluate their safety and environmental effects, and also by the fact most of these in vitro/in vivo studies present acute studies rather than long-term exposure (Bakand and Hayes, 2016; Xia et al., 2016; De Matteis, 2017; Warheit, 2018). Nevertheless, these in vivo and in vitro studies have been providing clues to the specific mechanisms by which NPs trigger an adverse effect enabling future surface modification of NPs to make them safer and less toxic (De Matteis, 2017). These concerns relating to nanosafety have been addressed and implemented via European Commission FP7 and H2020-sponsored programs followed by some relevant conclusions issued by the US National Academy of Science Committee on Research Progress of Environmental Health and Safety Aspects of Engineered Nanomaterials (Warheit, 2018).
Due to the 3Rs (Replacement, Reduction and Refinement) policies of in vivo studies, the future challenge of Regulatory Agencies is the standardization of the in vitro methodologies to establish the toxicology profile of NPs based on good laboratory practice (GLP) and the construction of flexible and reliable databases in which NPs are classified according to the data derived from these toxicological investigations. Together, these efforts might provide information on the dosage at which a particular NP can be considered safe and thus appropriate for medical use.
Challenges of Current Research
As mentioned above, nanomaterials have great potential to prevent and treat bacterial infection, but several challenges remain for the translation to the clinics. Some of these include assessing the interactions of nanoantibiotics with cells, tissues and organs, for dose recalibration and identification of appropriate routes of administration (Sandhiya et al., 2009). The biocompatibility of NPs is generally evaluated through in vitro assays, using cell culture. Because NPs, used as antimicrobial agents can enter through skin contact, ingestion, inhalation, oral and intravenous injection, in vivo models must also be applied to better understand their effects, including potential toxicity, clearance and metabolism (Beyth et al., 2015). Several studies have shown that intravenously injected NPs accumulate in the colon, lung, bone marrow, liver, spleen and lymphatics (Hagens et al., 2007). Inhalation has also been shown to cause cytotoxicity at the lung, and in the liver, heart and spleen through systemic circulation (Poma and Di Giorgio, 2008; Leucuta, 2013). This is of particular relevance for small NPs because of efficient cellular uptake and transcytosis across epithelial and endothelial cells into the blood and lymphatic circulation. Several NPs systems have demonstrated toxicity in multiple organs, such as free radical-mediated oxidative stress generated by the interaction of antimicrobial NPs with cell components that can result in hepatotoxicity and nephrotoxicity (De Jong and Borm, 2008; Lei et al., 2008).
The effective translation to the clinics will require appropriate guidelines for production and scale-up of manufacturing these nanomaterials, for characterization of the physico-chemical properties and their impact on biocompatibility, for standardization of nanotoxicology assays and protocols to assist easy comparison of data originating from in vitro and in vivo studies, for the evaluation of their metabolism and mode of action (Duncan and Gaspar, 2011; Bertrand and Leroux, 2012; Beyth et al., 2015; Cordeiro et al., 2016; Rai M. et al., 2016; Zazo et al., 2016). Finally, the community still needs to address the economic impact of translation of these nanomaterials to the clinics.
Conclusions
Given their vast therapeutic potential, it is becoming increasingly important to understand the mechanisms by which NPs complexes can impact bacterial viability. While one of the beneficial aspects of NPs drug carriers involves “macro-targeting,” i.e., specific delivery to the site of infection, understanding the “micro-targeting” of bacterial mechanisms is imperative for the widespread future use of these vectors. Their impact of cell functions such as cell wall permeability, efflux activity, formation of reactive species, and inhibition of essential cellular metabolism and reproduction is of utmost importance.
Author Contributions
PB supervision and correction of Nanoparticles part of the manuscript; MPM supervision and correction of MDR bacteria part of the manuscript; AC draft Nanoparticles part of the manuscript, figure draw, tables design; DF draft MDR bacteria part of the manuscript; NM draft MDR bacteria part of the manuscript; MM coordination of MDR bacteria part of the manuscript and final correction and integration; AF coordination of Nanoparticles part of the manuscript and final correction and integration.
Conflict of Interest Statement
NM was employed by the company Nuritas limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Unidade de Ciências Biomoleculares Aplicadas-UCIBIO, which is financed by National Funds from FCT/MEC (PTDC_CVT-EPI_6685_2014; UID/Multi/04378/2013) and co-financed by the ERDF under the PT2020 Partnership Agreement (POCI-01-0145-FEDER-007728). DF is funded by the Trinity College Dublin Postgraduate Research (1252) Studentship. NM is funded by the Irish Research Council under the employment-based programme EBPPG/2015/233 in conjunction with Nuritas limited. We would like to acknowledge Ana Sofia Santos for preliminary revision of the manuscript.
References
Acharya, D., Singha, K. M., Pandey, P., Mohanta, B., Rajkumari, J., and Singha, L. P. (2018). Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 8:201. doi: 10.1038/s41598-017-18590-6
Aderibigbe, B. A. (2017). Metal-based nanoparticles for the treatment of infectious diseases. Molecules 22:E1370. doi: 10.3390/molecules22081370
Ali, K., Dwivedi, S., Azam, A., Saquib, Q., Al-Said, M. S., Alkhedhairy, A. A., et al. (2016). Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 472, 145–156. doi: 10.1016/j.jcis.2016.03.021
Allahverdiyev, A. M., Kon, K. V., Abamor, E. S., Bagirova, M., and Rafailovich, M. (2011). Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev. AntiInfect. Ther. 9, 1035–1052. doi: 10.1586/eri.11.121
AlMatar, M., Makky, E. A., Var, I., and Koksal, F. (2017). The role of nanoparticles in the inhibition of multidrug-resistant bacteria and biofilms. Curr. Drug Deliv. 15, 470–484. doi: 10.2174/1567201815666171207163504
Andrade, F., Rafael, D., Videira, M., Ferreira, D., Sosnik, A., and Sarmento, B. (2013). Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 65, 1816–1827. doi: 10.1016/j.addr.2013.07.020
Ansari, M. A., Khan, H. M., Khan, A. A., Cameotra, S. S., Saquib, Q., and Musarrat, J. (2014). Interaction of Al(2)O(3) nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Appl. Microbiol. 116, 772–783. doi: 10.1111/jam.12423
Ansari, M. A., Khan, H. M., Khan, A. A., Pal, R., and Cameotra, S. S. (2013). Antibacterial potential of Al2O3 nanoparticles against multidrug resistance strains of Staphylococcus aureus isolated from skin exudates. J. Nanoparticle Res. 15:1970. doi: 10.1007/s11051-013-1970-1
Antonelli, M., De Pascale, G., Ranieri, V. M., Pelaia, P., Tufano, R., Piazza, O., et al. (2012). Comparison of triple-lumen central venous catheters impregnated with silver nanoparticles (AgTive®) vs conventional catheters in intensive care unit patients. J. Hosp. Infect. 82, 101–107. doi: 10.1016/j.jhin.2012.07.010
Argueta-Figueroa, L., Morales-Luckie, R. A., Scougall-Vilchis, R. J., and Olea-Mejía, O. F. (2014). Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu-Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 24, 321–328. doi: 10.1016/j.pnsc.2014.07.002
Arvizo, R., Bhattacharya, R., and Mukherjee, P. (2010). Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 7, 753–763. doi: 10.1517/17425241003777010
Asharani, P. V., Xinyi, N., Hande, M. P., and Valiyaveettil, S. (2010). DNA damage and p53-mediated growth arrest in human cells treated with platinum nanoparticles. Nanomedicine 5, 51–64. doi: 10.2217/nnm.09.85
Ashfaq, M., Verma, N., and Khan, S. (2016). Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: a novel potential antibiotic material. Mater. Sci. Eng. C 59, 938–947. doi: 10.1016/j.msec.2015.10.079
Aswathanarayan, J. B., and Vittal, R. R. (2017). Antimicrobial, biofilm inhibitory and anti-infective activity of metallic nanoparticles against pathogens MRSA and Pseudomonas aeruginosa PA01. Pharm. Nanotechnol. 5, 148–153. doi: 10.2174/2211738505666170424121944
Aydin Sevinç, B., and Hanley, L. (2010). Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 94, 22–31. doi: 10.1002/jbm.b.31620
Babes, L., Denizot, B., Tanguy, G., Le Jeune, J. J., and Jallet, P. (1999). Synthesis of iron oxide nanoparticles used as MRI contrast agents: a parametric study. J. Colloid Interface Sci. 212, 474–482. doi: 10.1006/jcis.1998.6053
Baek, Y.-W., and An, Y.-J. (2011). Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 409, 1603–1608. doi: 10.1016/j.scitotenv.2011.01.014
Bahar, A., and Ren, D. (2013). Antimicrobial peptides. Pharmaceuticals 6, 1543–1575. doi: 10.3390/ph6121543
Bakand, S., and Hayes, A. (2016). Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 17:E929. doi: 10.3390/ijms17060929
Baker, S., Pasha, A., and Satish, S. (2017). Biogenic nanoparticles bearing antibacterial activity and their synergistic effect with broad spectrum antibiotics:emerging strategy to combat drug resistant pathogens. Saudi Pharm. J. 25, 44–51. doi: 10.1016/j.jsps.2015.06.011
Bassegoda, A., Ivanova, K., Ramon, E., and Tzanov, T. (2018). Strategies to prevent the occurrence of resistance against antibiotics by using advanced materials. Appl. Microbiol. Biotechnol. 102, 2075–2089. doi: 10.1007/s00253-018-8776-0
Behera, S. S., Patra, J. K., Pramanik, K., Panda, N., and Thatoi, H. (2012). Characterization and evaluation of antibacterial activities of chemically synthesized iron oxide nanoparticles. World J. Nano Sci. Eng. 2, 196–200. doi: 10.4236/wjnse.2012.24026
Berry, C. C., and Curtis, A. S. G. (2003). Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D. Appl. Phys. 36, R198–R206. doi: 10.1088/0022-3727/36/13/203
Bertrand, N., and Leroux, J. C. (2012). The journey of a drug-carrier in the body: an anatomo-physiological perspective. J. Control. Release 161, 152–163. doi: 10.1016/j.jconrel.2011.09.098
Beyth, N., Houri-Haddad, Y., Domb, A., Khan, W., and Hazan, R. (2015). Alternative antimicrobial approach: nano-antimicrobial materials. Evid. Based Complement. Altern. Med. 2015, 1–16. doi: 10.1155/2015/246012
Bi, L., Yang, L., Narsimhan, G., Bhunia, A., and Yao, Y. (2011). Designing carbohydrate nanoparticles for prolonged efficacy of antimicrobial peptide. J. Control Release 150, 150–156. doi: 10.1016/j.jconrel.2010.11.024
Bjarnsholt, T. (2013). The role of bacterial biofilms in chronic infections. APMIS 121, 1–58. doi: 10.1111/apm.12099
Boman, H. G., Wade, D., Boman, I. A., Wåhlin, B., and Merrifield, R. B. (1989). Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 259, 103–106. doi: 10.1016/0014-5793(89)81505-4
Bondarenko, O., Ivask, A., Käkinen, A., Kurvet, I., and Kahru, A. (2013). Particle-cell contact enhances antibacterial activity of silver nanoparticles. PLoS ONE 8:e64060. doi: 10.1371/journal.pone.0064060
Brandelli, A. (2012). Nanostructures as promising tools for delivery of antimicrobial peptides. Mini Rev. Med. Chem. 12, 731–741. doi: 10.2174/138955712801264774
Bresee, J., Bond, C. M., Worthington, R. J., Smith, C. A., Gifford, J. C., Simpson, C. A., et al. (2014). Nanoscale structure–activity relationships, mode of action, and biocompatibility of gold nanoparticle antibiotics. J. Am. Chem. Soc. 136, 5295–5300. doi: 10.1021/ja408505n
Brown, A. N., Smith, K., Samuels, T. A., Lu, J., Obare, S. O., and Scott, M. E. (2012). Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 78, 2768–2774. doi: 10.1128/AEM.06513-11
Burygin, G., Khlebtsov, B., Shantrokha, A., Dykman, L., Bogatyrev, V., Khlebtsov, N., et al. (2009). On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res. Lett. 4, 794–801. doi: 10.1007/s11671-009-9316-8
Casciaro, B., Moros, M., Rivera-Fernández, S., Bellelli, A., de la Fuente, J. M., and Mangoni, M. L. (2017). Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a(1-21)NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 47, 170–181. doi: 10.1016/j.actbio.2016.09.041
Caster, J. M., Patel, A. N., Zhang, T., and Wang, A. (2017). Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9:e1416. doi: 10.1002/wnan.1416
Cavassin, E. D., de Figueiredo, L. F. P., Otoch, J. P., Seckler, M. M., de Oliveira, R. A., Franco, F. F., et al. (2015). Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J. Nanobiotechnol. 13, 1–16. doi: 10.1186/s12951-015-0120-6
Cha, S. H., Hong, J., McGuffie, M., Yeom, B., Vanepps, J. S., and Kotov, N. A. (2015). Shape-dependent biomimetic inhibition of enzyme by nanoparticles and their antibacterial activity. ACS Nano 9, 9097–9105. doi: 10.1021/acsnano.5b03247
Chakraborti, S., Mandal, A. K., Sarwar, S., Singh, P., Chakraborty, R., and Chakrabarti, P. (2014). Bactericidal effect of polyethyleneimine capped ZnO nanoparticles on multiple antibiotic resistant bacteria harboring genes of high-pathogenicity island. Colloids Surf. B. Biointerfaces 121, 44–53. doi: 10.1016/j.colsurfb.2014.03.044
Chakraborty, R., Sarkar, R. K., Chatterjee, A. K., Manju, U., Chattopadhyay, A. P., and Basu, T. (2015). A simple, fast and cost-effective method of synthesis of cupric oxide nanoparticle with promising antibacterial potency: unraveling the biological and chemical modes of action. Biochim. Biophys. Acta Gen. Subj. 1850, 845–856. doi: 10.1016/j.bbagen.2015.01.015
Chang, T. Y., Chen, C. C., Cheng, K. M., Chin, C. Y., Chen, Y. H., Chen, X. A., et al. (2017). Trimethyl chitosan-capped silver nanoparticles with positive surface charge: their catalytic activity and antibacterial spectrum including multidrug-resistant strains of Acinetobacter baumannii. Colloids Surf. B. Biointerfaces 155, 61–70. doi: 10.1016/j.colsurfb.2017.03.054
Chaurasia, A. K., Thorat, N. D., Tandon, A., Kim, J. H., Park, S. H., and Kim, K. K. (2016). Coupling of radiofrequency with magnetic nanoparticles treatment as an alternative physical antibacterial strategy against multiple drug resistant bacteria. Sci. Rep. 6, 1–13. doi: 10.1038/srep33662
Chen, C. W., Hsu, C. Y., Lai, S. M., Syu, W. J., Wang, T. Y., and Lai, P. S. (2014). Metal nanobullets for multidrug resistant bacteria and biofilms. Adv. Drug Deliv. Rev. 78, 88–104. doi: 10.1016/j.addr.2014.08.004
Chen, F., Shi, Z., Neoh, K., and Kang, E. (2009). Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol Bioeng. 104, 30–39. doi: 10.1002/bit.22363
Chen, J., Hessler, J. A., Putchakayala, K., Panama, B. K., Khan, D. P., Hong, S., et al. (2009). Cationic nanoparticles induce nanoscale disruption in living cell plasma membranes. J. Phys. Chem. B 113, 11179–11185. doi: 10.1021/jp9033936
Cho, K. H., Park, J. E., Osaka, T., and Park, S. G. (2005). The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 51, 956–960. doi: 10.1016/j.electacta.2005.04.071
Choi, S. R., Britigan, B. E., Moran, D. M., and Narayanasamy, P. (2017). Gallium nanoparticles facilitate phagosome maturation and inhibit growth of virulent Mycobacterium tuberculosis in macrophages. PLoS ONE 12:e0177987. doi: 10.1371/journal.pone.0177987
Chopra, I. (2007). The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J. Antimicrob. Chemother. 59, 587–590. doi: 10.1093/jac/dkm006
Claeys, K. C., Heil, E. L., Pogue, J. M., Lephart, P. R., and Johnson, J. K. (2018). The Verigene dilemma: gram-negative polymicrobial bloodstream infections and clinical decision making. Diagn. Microbiol. Infect. Dis. 91, 144–146. doi: 10.1016/j.diagmicrobio.2018.01.012
Clancy, J. P., Dupont, L., Konstan, M. W., Billings, J., Fustik, S., Goss, C. H., et al. (2013). Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 68, 818–825. doi: 10.1136/thoraxjnl-2012-202230
Conde, J., Doria, G., and Baptista, P. V. (2012). Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012:751075. doi: 10.1155/2012/751075
Conde, J., Larguinho, M., Cordeiro, A., Raposo, L. R., Costa, M., Santos, S., et al. (2014). Gold-Nanobeacons for gene therapy : evaluation of genotoxicity, cell toxicity and proteome profiling analysis. Nanotocicology 8, 521–532. doi: 10.3109/17435390.2013.802821
Cordeiro, M., Ferreira Carlos, F., Pedrosa, P., Lopez, A., and Baptista, P. V. (2016). Gold nanoparticles for diagnostics: advances towards points of care. Diagnostics 6:43. doi: 10.3390/diagnostics6040043
Costa, M. N., Veigas, B., Jacob, J. M., Santos, D. S., Gomes, J., Baptista, P. V., et al. (2014). A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: lab-on-paper. Nanotechnology 25:94006. doi: 10.1088/0957-4484/25/9/094006
Courtney, C. M., Goodman, S. M., Nagy, T. A., Levy, M., Bhusal, P., Madinger, N. E., et al. (2017). Potentiating antibiotics in drug-resistant clinical isolates via stimuli-activated superoxide generation. Sci. Adv. 3:e1701776. doi: 10.1126/sciadv.1701776
Cui, Y., Zhao, Y., Tian, Y., Zhang, W., Lu, X., and Jiang, X. (2012). The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33, 2327–2333. doi: 10.1016/j.biomaterials.2011.11.057
Dai, T., Gupta, A., Murray, C. K., Vrahas, M. S., Tegos, G. P., and Hamblin, M. R. (2013). Blue Light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. 15, 223–236. doi: 10.1016/j.drup.2012.07.001.Blue
Dai, X., Fan, Z., Lu, Y., and Ray, P. C. (2013). Multifunctional nanoplatforms for targeted multidrug-resistant-bacteria theranostic applications. ACS Appl. Mater. Interfaces 5, 11348–11354. doi: 10.1021/am403567k
Dakal, T. C., Kumar, A., Majumdar, R. S., and Yadav, V. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 7:1831. doi: 10.3389/fmicb.2016.01831
De Jong, W. H., and Borm, P. J. a (2008). Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomedicine 3, 133–149. doi: 10.2147/IJN.S596
De Matteis, V. (2017). Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics 5:E29. doi: 10.3390/toxics5040029
DeGrasse, J. A. (2012). A single-stranded DNA aptamer that selectively binds to Staphylococcus aureus enterotoxin B. PLoS ONE 7:e33410. doi: 10.1371/journal.pone.0033410
Ding, F., Songkiatisak, P., Cherukuri, P. K., Huang, T., and Xu, X.-H. N. (2018). Size-dependent inhibitory effects of antibiotic drug nanocarriers against Pseudomonas aeruginosa. ACS Omega 3, 1231–1243. doi: 10.1021/acsomega.7b01956
Djafari, J., Marinho, C., Santos, T., Igrejas, G., Torres, C., Capelo, J. L., et al. (2016). New synthesis of gold- and silver-based nano-tetracycline composites. ChemistryOpen 5, 206–212. doi: 10.1002/open.201600016
Dobrovolskaia, M. A., McNeil, S. E., and Neil, S. E. M. (2007). Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2, 469–478. doi: 10.1038/nnano.2007.223
Doria, G., Larguinho, M., Dias, J. T., Pereira, E., Franco, R., and Baptista, P. (2010). Gold–silver-alloy nanoprobes for one-pot multiplex DNA detection. Nanotechnology 21:255101. doi: 10.1088/0957-4484/21/25/255101
dos Santos, M. M., Queiroz, M. J., and Baptista, P. V. (2012). Enhancement of antibiotic effect via gold:silver-alloy nanoparticles. J. Nanoparticle Res. 14:859. doi: 10.1007/s11051-012-0859-8
Dos Santos, V. E., Filho, A. V., Ribeiro Targino, A. G., Pelagio Flores, M. A., Galembeck, A., Caldas, A. F., et al. (2014). A new “silver-Bullet” to treat caries in children - Nano Silver Fluoride: a randomised clinical trial. J. Dent. 42, 945–951. doi: 10.1016/j.jdent.2014.05.017
Duncan, R., and Gaspar, R. (2011). Nanomedicine(s) under the microscope. Mol. Pharm. 8, 2101–2141. doi: 10.1021/mp200394t
Durán, N., Durán, M., de Jesus, M. B., Seabra, A. B., Fávaro, W. J., and Nakazato, G. (2016). Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 12, 789–799. doi: 10.1016/j.nano.2015.11.016
Durán, N., Marcato, P. D., De Conti, R., Alves, O. L., Costa, F. T. M., and Brocchi, M. (2010). Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 21, 949–959. doi: 10.1590/s0103-50532010000600002
Dwivedi, S., Wahab, R., Khan, F., Mishra, Y. K., Musarrat, J., and Al-Khedhairy, A. A. (2014). Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE 9:e111289. doi: 10.1371/journal.pone.0111289
Ehsan, S., and Sajjad, M. (2017). Bioinspired synthesis of zinc oxide nanoparticle and its combined efficacy with different antibiotics against multidrug resistant bacteria. J. Biomater. Nanobiotechnol. 8, 159–175. doi: 10.4236/jbnb.2017.82011
El Din, S. N., El-Tayeb, T. A., Abou-Aisha, K., and El-Azizi, M. (2016). In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int. J. Nanomedicine 11, 1749–1758. doi: 10.2147/IJN.S102398
El-Zowalaty, M. E., Al-Ali, S. H. H., Husseiny, M. I., Geilich, B. M., Webster, T. J., and Hussein, M. Z. (2015). The ability of streptomycin-loaded chitosan-coated magnetic nanocomposites to possess antimicrobial and antituberculosis activities. Int. J. Nanomed. 10, 3269–3274. doi: 10.2147/IJN.S74469
Esmaeillou, M., Zarrini, G., Rezaee, M. A., Mojarrad, J. S., and Bahadori, A. (2017). Vancomycin capped with silver nanoparticles as an antibacterial agent against multi-drug resistance bacteria. Adv. Pharm. Bull. 7, 479–483. doi: 10.15171/apb.2017.058
Espitia, P. J. P., Soares, N., de, F. F., Coimbra, J. S., dos, R., de Andrade, N. J., Cruz, R. S., and Medeiros, E. A. A. (2012). Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 5, 1447–1464. doi: 10.1007/s11947-012-0797-6
Fakhri, A., Tahami, S., and Naji, M. (2017). Synthesis and characterization of core-shell bimetallic nanoparticles for synergistic antimicrobial effect studies in combination with doxycycline on burn specific pathogens. J. Photochem. Photobiol. B Biol. 169, 21–26. doi: 10.1016/j.jphotobiol.2017.02.014
Fernandes, A. R., and Baptista, P. V. (2017). Gene silencing using multifunctionalized gold nanoparticles for cancer therapy. Methods Mol. Biol. 1530, 319–336. doi: 10.1007/978-1-4939-6646-2_19
Fernandes, A. R., Jesus, J., Martins, P., Figueiredo, S., Rosa, D., Martins, L. M., et al. (2017). Multifunctional gold-nanoparticles: a nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J. Control. Release 245, 52–61. doi: 10.1016/j.jconrel.2016.11.021
Foster, H. A., Ditta, I. B., Varghese, S., and Steele, A. (2011). Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 90, 1847–1868. doi: 10.1007/s00253-011-3213-7
Franci, G., Falanga, A., Galdiero, S., Palomba, L., Rai, M., Morelli, G., et al. (2015). Silver nanoparticles as potential antibacterial agents. Molecules 20, 8856–8874. doi: 10.3390/molecules20058856
Galanzha, E. I., Shashkov, E., Sarimollaoglu, M., Beenken, K. E., Basnakian, A. G., Shirtliff, M. E., et al. (2012). In vivo magnetic enrichment, photoacoustic diagnosis, and photothermal purging of infected blood using multifunctional gold and magnetic nanoparticles. PLoS ONE 7:e45557. doi: 10.1371/journal.pone.0045557
Galvan, D. D., and Yu, Q. (2018). Surface-enhanced raman scattering for rapid detection and characterization of antibiotic-resistant bacteria. Adv. Healthc. Mater. doi: 10.1002/adhm.201701335. [Epub ahead of print].
Gamella, M., Guz, N., Mailloux, S., Pingarrón, J. M., and Katz, E. (2014). Antibacterial drug release electrochemically stimulated by the presence of bacterial cells - theranostic approach. Electroanalysis 26, 2552–2557. doi: 10.1002/elan.201400473
Gao, W., Chen, Y., Chang, Y., Zhang, Y., Chang, Q., and Zhang, L. (2018). Nanoparticle-based local antimicrobial drug delivery. Adv Drug Deliv Rev. 127, 46–57. doi: 10.1016/j.addr.2017.09.015
Gao, W., Thamphiwatana, S., Angsantikul, P., and Zhang, L. (2014). Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 6, 532–547. doi: 10.1002/wnan.1282
García-Lara, B., Saucedo-Mora, M. Á., Roldán-Sánchez, J. A., Pérez-Eretza, B., Ramasamy, M., Lee, J., et al. (2015). Inhibition of quorum-sensing-dependent virulence factors and biofilm formation of clinical and environmental Pseudomonas aeruginosa strains by ZnO nanoparticles. Lett Appl Microbiol. 61, 299–305. doi: 10.1111/lam.12456
Gelabert, A., Sivry, Y., Gobbi, P., Mansouri-Guilani, N., Menguy, N., Brayner, R., et al. (2016). Testing nanoeffect onto model bacteria: impact of speciation and genotypes. Nanotoxicology 10, 216–225. doi: 10.3109/17435390.2015.1048323
Gholipourmalekabadi, M., Mobaraki, M., Ghaffari, M., Zarebkohan, A., Omrani, V., Urbanska, A., et al. (2017). Targeted drug delivery based on gold nanoparticle derivatives. Curr. Pharm. Des. 23, 2918–2929. doi: 10.2174/1381612823666170419105413
Gil-Tomás, J., Tubby, S., Parkin, I. P., Narband, N., Dekker, L., Nair, S. P., et al. (2007). Lethal photosensitisation of Staphylococcus aureus using a toluidine blue O–tiopronin–gold nanoparticle conjugate. J. Mater. Chem. 17:3739. doi: 10.1039/b706615e
Gontsarik, M., Buhmann, M., Yaghmur, A., Ren, Q., Maniura-Weber, K., and Salentinig, S. (2016). Antimicrobial peptide - driven colloidal transformations in liquid crystalline nanocarriers. J. Phys. Chem. Lett. 7, 3482–3486. doi: 10.1021/acs.jpclett.6b01622
Govindaraju, S., Samal, M., and Yun, K. (2016). Superior antibacterial activity of GlcN-AuNP-GO by ultraviolet irradiation. Mater. Sci. Eng. C 69, 366–372. doi: 10.1016/j.msec.2016.06.052
Gu, H., Ho, P. L., Tong, E., Wang, L., and Xu, B. (2003). Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 3, 1261–1263. doi: 10.1021/nl034396z
Hackenberg, S., Scherzed, A., Kessler, M., Hummel, S., Technau, A., Froelich, K., et al. (2011). Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 201, 27–33. doi: 10.1016/j.toxlet.2010.12.001
Hadiya, S., Liu, X., Abd El-Hammed, W., Elsabahy, M., and Aly, S. A. (2018). Levofloxacin-loaded nanoparticles decrease emergence of fluoroquinolone resistance in Escherichia coli. Microb. Drug Resist. doi: 10.1089/mdr.2017.0304. [Epub ahead of print].
Hagens, W. I., Oomen, A. G., de Jong, W. H., Cassee, F. R., and Sips, A. J. A. M. (2007). What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul. Toxicol. Pharmacol. 49, 217–229. doi: 10.1016/j.yrtph.2007.07.006
Hall-Stoodley, L., Costerton, J. W., and Stoodley, P. (2004). Bacterial biofilms: from the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108. doi: 10.1038/nrmicro821
Hemeg, H. A. (2017). Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 12, 8211–8225. doi: 10.2147/IJN.S132163
Høiby, N., Ciofu, O., Johansen, H. K., Song, Z.-J., Moser, C., Jensen, P. Ø., et al. (2011). The clinical impact of bacterial biofilms. Int J Oral Sci. 3, 55–65. doi: 10.4248/IJOS11026
Hsueh, Y. H., Ke, W. J., Hsieh, C., Te, L.in, K. S., Tzou, D. Y., and Chiang, C. L. (2015). ZnO nanoparticles affect bacillus subtilis cell growth and biofilm formation. PLoS ONE 10:e0128457. doi: 10.1371/journal.pone.0128457
Hu, D., Li, H., Wang, B., Ye, Z., Lei, W., Jia, F., et al. (2017). Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant Staphylococcus aureus biofilm. ACS Nano 11, 9330–9339. doi: 10.1021/acsnano.7b04731
Huang, N., Chen, X., Zhu, X., Xu, M., and Liu, J. (2017). Ruthenium complexes/polypeptide self-assembled nanoparticles for identification of bacterial infection and targeted antibacterial research. Biomaterials 141, 296–313. doi: 10.1016/j.biomaterials.2017.07.005
Huang, Y., Yu, F., Park, Y., Wang, J., Shin, M., Chung, H., et al. (2010). Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery. Biomaterials 31, 9086–9091. doi: 10.1016/j.biomaterials.2010.08.046
Huh, A. J., and Kwon, Y. J. (2011). “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 156, 128–145. doi: 10.1016/j.jconrel.2011.07.002
Iravani, S., Korbekandi, H., Mirmohammadi, S. V., and Zolfaghari, B. (2014). Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci. 9, 385–406.
Ivask, A., Juganson, K., Bondarenko, O., Mortimer, M., Aruoja, V., Kasemets, K., et al. (2014). Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology 8, 57–71. doi: 10.3109/17435390.2013.855831
Jagtap, P., Sritharan, V., and Gupta, S. (2017). Nanotheranostic approaches for management of bloodstream bacterial infections. Nanomedicine 13, 329–341. doi: 10.1016/j.nano.2016.09.005
Jaiswal, S., Bhattacharya, K., McHale, P., and Duffy, B. (2015). Dual effects of β-cyclodextrin-stabilised silver nanoparticles: enhanced biofilm inhibition and reduced cytotoxicity. J. Mater. Sci. Mater. Med. 26, 1–10. doi: 10.1007/s10856-014-5367-1
Jakobsen, T. H., Tolker-Nielsen, T., and Givskov, M. (2017). Bacterial biofilm control by perturbation of bacterial signaling processes. Int. J. Mol. Sci. 18:E1970. doi: 10.3390/ijms18091970
Jankauskaite, V., Vitkauskiene, A., Lazauskas, A., Baltrusaitis, J., Prosyčevas, I., and Andrulevičius, M. (2016). Bactericidal effect of graphene oxide/Cu/Ag nanoderivatives against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus. Int. J. Pharm. 511, 90–97. doi: 10.1016/j.ijpharm.2016.06.121
Joost, U., Juganson, K., Visnapuu, M., Mortimer, M., Kahru, A., Nommiste, E., et al. (2015). Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B 142, 178–185. doi: 10.1016/j.jphotobiol.2014.12.010
Jung, W. K., Koo, H. C., Kim, K. W., Kim, S. H., Park, Y. H., Jung, W. K., et al. (2008). Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 74, 2171–2178. doi: 10.1128/AEM.02001-07
Kadiyala, U., Kotov, N. A., and VanEpps, J. S. (2018). Antibacterial metal oxide nanoparticles: challenges in interpreting the literature. Curr. Pharm. Des. 24, 896–903. doi: 10.2174/1381612824666180219130659
Karimi, F., Dabbagh, S., Alizadeh, S., and Rostamnia, S. (2016). Evaluation of AgClNPs@SBA-15/IL nanoparticle-induced oxidative stress and DNA mutation in Escherichia coli. Appl. Microbiol. Biotechnol. 100, 7161–7170. doi: 10.1007/s00253-016-7593-6
Kato, N., Morohoshi, T., Nozawa, T., Matsumoto, H., and Ikeda, T. (2006). Control of gram- negative bacterial quorum sensing with cyclodextrin immobilized cellulose ether gel. J. Incl. Phenom. Macrocycl. Chem. 56, 55–59. doi: 10.1007/s10847-006-9060-y
Katva, S., Das, S., Moti, H. S., Jyoti, A., and Kaushik, S. (2018). Antibacterial synergy of silver nanoparticles with gentamicin and chloramphenicol against Enterococcus faecalis. Pharmacogn. Mag. 13, S828–S833. doi: 10.4103/pm.pm_120_17
Katz, E., and Willner, I. (2004). Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew. Chem. Int. Ed. 43, 6042–6108. doi: 10.1002/anie.200400651
Kaweeteerawat, C., Na Ubol, P., Sangmuang, S., Aueviriyavit, S., and Maniratanachote, R. (2017). Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J. Toxicol. Environ. Health A. 80, 1276–1289. doi: 10.1080/15287394.2017.1376727
Khaksar, R., Hosseini, S., Hosseini, H., Shijaee-Aliabadi, S., Mohammadifa, M., Mortazavian, A. M., et al. (2014). Nisin-loaded alginate-high methoxy pectin microparticles: preparation and physicochemical characterisation. Int. J. Food Sci. Technol. 49, 2076–2082. doi: 10.1111/ijfs.12516
Khameneh, B., Diab, R., Ghazvini, K., and Fazly Bazzaz, B. S. (2016). Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 95, 32–42. doi: 10.1016/j.micpath.2016.02.009
Khan, S., Khan, S. N., Meena, R., Dar, A. M., Pal, R., and Khan, A. U. (2017). Photoinactivation of multidrug resistant bacteria by monomeric methylene blue conjugated gold nanoparticles. J. Photochem. Photobiol. B 174, 150–161. doi: 10.1016/j.jphotobiol.2017.07.011
Khlebtsov, B. N., Tuchina, E. S., Khanadeev, V. A., Panfilova, E. V., Petrov, P. O., Tuchin, V. V., et al. (2013). Enhanced photoinactivation of Staphylococcus aureus with nanocomposites containing plasmonic particles and hematoporphyrin. J. Biophotonics 6, 338–351. doi: 10.1002/jbio.201200079
Kim, D., Kim, M., Shinde, S., Sung, J., and Ghodake, G. (2017). Cytotoxicity and antibacterial assessment of gallic acid capped gold nanoparticles synthesized at ambient temperature. Coll. Surfaces B Biointerfaces 149, 162–167. doi: 10.1016/j.colsurfb.2016.10.017
Kim, J., Kim, H., Park, J. H., and Jon, S. (2017). Gold nanorod-based photo-PCR system for one-step, rapid detection of bacteria. Nanotheranostics 1, 178–185. doi: 10.7150/ntno.18720
Kim, J.-W., Galanzha, E. I., Zaharoff, D. A., Griffin, R. J., and Zharov, V. P. (2013). Nanotheranostics of circulating tumor cells, infections and other pathological features in vivo. Mol. Pharm. 10, 813–830. doi: 10.1021/mp300577s
Krivorotova, T., Staneviciene, R., Luksa, J., Serviene, E., and Sereikaite, J. (2017). Impact of pectin esterification on the antimicrobial activity of nisin-loaded pectin particles. Biotechnol. Prog. 33, 245–251. doi: 10.1002/btpr.2391
Kruk, T., Szczepanowicz, K., Stefanska, J., Socha, R. P., and Warszynski, P. (2015). Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Coll. Surfaces B Biointerfaces 128, 17–22. doi: 10.1016/j.colsurfb.2015.02.009
Kulshrestha, S., Qayyum, S., and Khan, A. U. (2017). Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: a comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 103, 167–177. doi: 10.1016/j.micpath.2016.12.022
Kuo, W.-S., Chang, C.-N., Chang, Y.-T., and Yeh, C.-S. (2009). Antimicrobial gold nanorods with dual-modality photodynamic inactivation and hyperthermia. Chem. Commun. 28, 4853–4855. doi: 10.1039/b907274h
Kuo, Y.-L., Wang, S.-G., Wu, C.-Y., Lee, K.-C., Jao, C.-J., Chou, S.-H., et al. (2016). Functional gold nanoparticle-based antibacterial agents for nosocomial and antibiotic-resistant bacteria. Nanomedicine 11, 2497–2510. doi: 10.2217/nnm-2016-0232
Lai, H. Z., Chen, W. Y., Wu, C. Y., and Chen, Y. C. (2015). Potent antibacterial nanoparticles for pathogenic bacteria. ACS Appl. Mater. Interfaces 7, 2046–2054. doi: 10.1021/am507919m
Lakshmi Prasanna, V., and Vijayaraghavan, R. (2015). Insight into the mechanism of antibacterial activity of ZnO: surface defects mediated reactive oxygen species even in the dark. Langmuir 31, 9155–9162. doi: 10.1021/acs.langmuir.5b02266
Lambadi, P. R., Sharma, T. K., Kumar, P., Vasnani, P., Thalluri, S. M., Bisht, N., et al. (2015). Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int. J. Nanomed. 10, 2155–2171. doi: 10.2147/IJN.S72923
Lara, H. H., Ayala-Núñez, N. V., Ixtepan Turrent, L., del, C., and Rodríguez Padilla, C. (2010). Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 26, 615–621. doi: 10.1007/s11274-009-0211-3
Larsen, S. T., Jackson, P., Poulsen, S. S., Levin, M., Jensen, K. A., Wallin, H., et al. (2016). Airway irritation, inflammation, and toxicity in mice following inhalation of metal oxide nanoparticles. Nanotoxicology 10, 1254–1262. doi: 10.1080/17435390.2016.1202350
LaSarre, B., and Federle, M. J. (2013). Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 77, 73–111. doi: 10.1128/MMBR.00046-12
Lebeaux, D., Ghigo, J.-M., and Beloin, C. (2014). Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 78, 510–543. doi: 10.1128/MMBR.00013-14
Lee, J. H., Kim, Y. G., Cho, M. H., and Lee, J. (2014). ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol Res. 169, 888–896. doi: 10.1016/j.micres.2014.05.005
Lee, P. C., and Meisel, D. (1982). Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 86, 3391–3395. doi: 10.1021/j100214a025
Lee, W. S., Hsieh, T. C., Shiau, J. C., Ou, T. Y., Chen, F. L., Liu, Y. H., et al. (2017). Bio-Kil, a nano-based disinfectant, reduces environmental bacterial burden and multidrug-resistant organisms in intensive care units. J. Microbiol. Immunol. Infect. 50, 737–746. doi: 10.1016/j.jmii.2016.04.008
Lei, R., Wu, C., Yang, B., Ma, H., Shi, C., Wang, Q., et al. (2008). Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity. Toxicol. Appl. Pharmacol. 232, 292–301. doi: 10.1016/j.taap.2008.06.026
Lellouche, J., Kahana, E., Elias, S., Gedanken, A., and Banin, E. (2009). Antibiofilm activity of nanosized magnesium fluoride. Biomaterials 30, 5969–5978. doi: 10.1016/j.biomaterials.2009.07.037
Lesniak, A., Salvati, A., Santos-Martinez, M. J., Radomski, M. W., Dawson, K. A., and Åberg, C. (2013). Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 135, 1438–1444. doi: 10.1021/ja309812z
Leuba, K. D., Durmus, N. G., Taylor, E. N., and Webster, T. J. (2013). Short communication: carboxylate functionalized superparamagnetic iron oxide nanoparticles (SPION) for the reduction of S. aureus growth post biofilm formation. Int. J. Nanomedicine 8, 731–736. doi: 10.2147/IJN.S38256
Leucuta, S. E. (2013). Systemic and biophase bioavailability and pharmacokinetics of nanoparticulate drug delivery systems. Curr. Drug Deliv. 10, 208–240. doi: 10.2174/1567201811310020007
LewisOscar, F., MubarakAli, D., Nithya, C., Priyanka, R., Gopinath, V., Alharbi, N. S., et al. (2015). One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling 31, 379–391. doi: 10.1080/08927014.2015.1048686
Li, H., Chen, Q., Zhao, J., and Urmila, K. (2015). Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci. Rep. 5:11033. doi: 10.1038/srep11033
Li, J. J., Hartono, D., Ong, C. N., Bay, B. H., and Yung, L. Y. L. (2010). Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 31, 5996–6003. doi: 10.1016/j.biomaterials.2010.04.014
Li, X., Robinson, S. M., Gupta, A., Saha, K., Jiang, Z., Moyano, D. F., et al. (2014). Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8, 10682–10686. doi: 10.1021/nn5042625
Li, Y., Yu, S., Wu, Q., Tang, M., Pu, Y., and Wang, D. (2012a). Chronic Al2O3-nanoparticle exposure causes neurotoxic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode Caenorhabditis elegans. J. Hazard Mater. 219–220, 221–230. doi: 10.1016/j.jhazmat.2012.03.083
Li, Y., Zhang, W., Niu, J., and Chen, Y. (2012b). Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal oxide nanoparticles. ACS Nano 6, 1–22. doi: 10.1021/nn300934k
Lin, Y., and Hamme, I. I. A. T (2015). Gold nanoparticle labeling based ICP-MS detection/measurement of bacteria, and their quantitative photothermal destruction. J. Mater. Chem. B 3, 3573–3582. doi: 10.1039/C5TB00223K
Liu, L., Yang, J., Xie, J., Luo, Z., Jiang, J., Yang, Y. Y., et al. (2013). The potent antimicrobial properties of cell penetrating peptide-conjugated silver nanoparticles with excellent selectivity for Gram-positive bacteria over erythrocytes. Nanoscale 5:3834. doi: 10.1039/c3nr34254a
Liu, P. F., Lo, C. W., Chen, C. H., Hsieh, M. F., and Huang, C. M. (2009). Use of nanoparticles as therapy for methicillin-resistant Staphylococcus aureus infections. Curr. Drug Metab. 10, 875–884. doi: 10.2174/138920009790274522
Lok, C.-N., Ho, C.-M., Chen, R., He, Q.-Y., Yu, W.-Y., Sun, H., et al. (2007). Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 12, 527–534. doi: 10.1007/s00775-007-0208-z
Lundqvist, M., Stigler, J., Elia, G., Lynch, I., Cedervall, T., and Dawson, K. A. (2008). Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. U.S.A. 105, 14265–14270. doi: 10.1073/pnas.0805135105
Maclean, M., McKenzie, K., Anderson, J. G., Gettinby, G., and MacGregor, S. J. (2014). 405 Nm light technology for the inactivation of pathogens and its potential role for environmental disinfection and infection control. J. Hosp. Infect. 88, 1–11. doi: 10.1016/j.jhin.2014.06.004
Mahalingam, S., Xu, Z., and Edirisinghe, M. (2015). Antibacterial activity and biosensing of PVA-lysozyme microbubbles formed by pressurized gyration. Langmuir 31, 9771–9780. doi: 10.1021/acs.langmuir.5b02005
Mahmoudi, M., and Serpooshan, V. (2012). Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano 6, 2656–2664. doi: 10.1021/nn300042m
McShan, D., Zhang, Y., Deng, H., Ray, P. C., and Yu, H. (2015). Synergistic antibacterial effect of silver nanoparticles combined with ineffective antibiotics on drug resistant Salmonella typhimurium DT104. J. Environ. Sci. Heal. Part C 33, 369–384. doi: 10.1080/10590501.2015.1055165
Meers, P., Neville, M., Malinin, V., Scotto, A. W., Sardaryan, G., Kurumunda, R., et al. (2008). Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 61, 859–868. doi: 10.1093/jac/dkn059
Mendes, R., Pedrosa, P., Lima, J. C., Fernandes, A. R., and Baptista, P. V. (2017). Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of gold nanoparticles. Sci. Rep. 7, 1–9. doi: 10.1038/s41598-017-11491-8
Merchant, B. (1998). Gold, the noble metal and the paradoxes of its toxicology. Biologicals 26, 49–59. doi: 10.1006/biol.1997.0123
Miao, L., Wang, C., Hou, J., Wang, P., Ao, Y., Li, Y., et al. (2016). Aggregation and removal of copper oxide (CuO) nanoparticles in wastewater environment and their effects on the microbial activities of wastewater biofilms. Bioresour. Technol. 216, 537–544. doi: 10.1016/j.biortech.2016.05.082
Millenbaugh, N. J., Baskin, J. B., DeSilva, M. N., Elliott, W. R., and Glickman, R. D. (2015). Photothermal killing of Staphylococcus aureus using antibody-targeted gold nanoparticles. Int. J. Nanomed. 10, 1953–1960. doi: 10.2147/IJN.S76150
Mocan, L., Matea, C., Tabaran, F. A., Mosteanu, O., Pop, T., Puia, C., et al. (2016). Selective in vitro photothermal nano-therapy of MRSA infections mediated by IgG conjugated gold nanoparticles. Sci. Rep. 6:39466. doi: 10.1038/srep39466
Mocan, L., Tabaran, F. A., Mocan, T., Pop, T., Mosteanu, O., Agoston-Coldea, L., et al. (2017). Laser thermal ablation of multidrug-resistant bacteria using functionalized gold nanoparticles. Int. J. Nanomed. 12, 2255–2263. doi: 10.2147/IJN.S124778
Mohammed Fayaz, A., Girilal, M., Mahdy, S. A., Somsundar, S. S., Venkatesan, R., and Kalaichelvan, P. T. (2011). Vancomycin bound biogenic gold nanoparticles: a different perspective for development of anti VRSA agents. Process Biochem. 46, 636–641. doi: 10.1016/j.procbio.2010.11.001
Mohanty, S., Jena, P., Mehta, R., Pati, R., Banerjee, B., Patil, S., et al. (2013). Cationic antimicrobial peptides and biogenic silver nanoparticles kill mycobacteria without eliciting dna damage and cytotoxicity in mouse macrophages. Antimicrob. Agents Chemother. 57, 3688–3698. doi: 10.1128/AAC.02475-12
Morrison, D. C., and Jacobs, D. M. (1976). Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry 13, 813–818. doi: 10.1016/0019-2791(76)90181-6
Munger, M. A., Hadlock, G., Stoddard, G., Slawson, M. H., Wilkins, D. G., Cox, N., et al. (2014a). Assessing orally bioavailable commercial silver nanoparticle product on human cytochrome P450 enzyme activity. Nanotoxicology 5390, 1–8. doi: 10.3109/17435390.2014.948092
Munger, M. A., Radwanski, P., Hadlock, G. C., Stoddard, G., Shaaban, A., Falconer, J., et al. (2014b). In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomedicine Nanotechnology, Biol. Med. 10, 1–9. doi: 10.1016/j.nano.2013.06.010
Muniyan, A., Ravi, K., Mohan, U., and Panchamoorthy, R. (2017). Characterization and in vitro antibacterial activity of saponin-conjugated silver nanoparticles against bacteria that cause burn wound infection. World J. Microbiol. Biotechnol. 33:147. doi: 10.1007/s11274-017-2309-3
Nagvenkar, A. P., Deokar, A., Perelshtein, I., and Gedanken, A. (2016). A one-step sonochemical synthesis of stable ZnO–PVA nanocolloid as a potential biocidal agent. J. Mater. Chem. B 4, 2124–2132. doi: 10.1039/C6TB00033A
Nagy, A., Harrison, A., and Dutta, P. K. (2011). Silver nanoparticles embedded in zeolite membranes : release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 6, 1833–1852. doi: 10.2147/IJN.S24019
Natan, M., and Banin, E. (2017). From nano to micro: using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 41, 302–322. doi: 10.1093/femsre/fux003
Niemirowicz, K., Swiecicka, I., Wilczewska, A. Z., Misztalewska, I., Kalska-Szostko, B., Bienias, K., et al. (2014). Gold-functionalized magnetic nanoparticles restrict growth of Pseudomonas aeruginosa. Int. J. Nanomed. 9, 2217–2224. doi: 10.2147/IJN.S56588
Ocsoy, I., Yusufbeyoglu, S., Yilmaz, V., McLamore, E. S., Ildiz, N., and Ulgen, A. (2017). DNA aptamer functionalized gold nanostructures for molecular recognition and photothermal inactivation of methicillin-Resistant Staphylococcus aureus. Coll. Surf. B. Biointerfaces 159, 16–22. doi: 10.1016/j.colsurfb.2017.07.056
Ortega, A., Farah, S., Tranque, P., Ocana, A. V., Nam-Cha, S. H., Beyth, N., et al. (2015). Antimicrobial evaluation of quaternary ammonium polyethyleneimine nanoparticles against clinical isolates of pathogenic bacteria. IET Nanobiotechnol. 9, 342–348. doi: 10.1049/iet-nbt.2014.0078
Ortíz-Castro, R., Martínez-Trujillo, M., and López-Bucio, J. (2008). N -acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ. 31, 1497–1509. doi: 10.1111/j.1365-3040.2008.01863.x
Otari, S. V., Patil, R. M., Waghmare, S. R., Ghosh, S. J., and Pawar, S. H. (2013). A novel microbial synthesis of catalytically active Ag–alginate biohydrogel and its antimicrobial activity. Dalt. Trans. 42, 9966–9975. doi: 10.1039/c3dt51093j
Pal, I., Brahmkhatri, V. P., Bera, S., Bhattacharyya, D., Quirishi, Y., Bhunia, A., et al. (2016). Enhanced stability and activity of an antimicrobial peptide in conjugation with silver nanoparticle. J. Colloid Interface Sci. 483, 385–393. doi: 10.1016/j.jcis.2016.08.043
Pallavicini, P., Donà, A., Taglietti, A., Minzioni, P., Patrini, M., Dacarro, G., et al. (2014). Self-assembled monolayers of gold nanostars: a convenient tool for near-IR photothermal biofilm eradication. Chem. Commun. 50, 1969–1971. doi: 10.1039/C3CC48667B
Pan, W. Y., Huang, C. C., Lin, T. T., Hu, H. Y., Lin, W. C., Li, M. J., et al. (2016). Synergistic antibacterial effects of localized heat and oxidative stress caused by hydroxyl radicals mediated by graphene/iron oxide-based nanocomposites. Nanomed. Nanotechnol. Biol. Med. 12, 431–438. doi: 10.1016/j.nano.2015.11.014
Panáček, A., Kvítek, L., Smékalová, M., Večerová, R., Kolár, M., Röderová, M., et al. (2018). Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 13, 65–71. doi: 10.1038/s41565-017-0013-y
Panáček, A., Smékalová, M., Kilianová, M., Prucek, R., Bogdanová, K., Věcěrová, R., et al. (2016a). Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 21, 1–17. doi: 10.3390/molecules21010026
Panáček, A., Smékalová, M., Večerová, R., Bogdanová, K., Röderová, M., Kolár, M., et al. (2016b). Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids Surfaces B Biointerfaces 142, 392–399. doi: 10.1016/j.colsurfb.2016.03.007
Papenfort, K., and Bassler, B. (2016). Quorum-sensing signal-response systems in gram-negative bacteria. Nat Rev Microbiol. 14, 576–588. doi: 10.1038/nrmicro.2016.89
Patel, S. K., and Janjic, J. M. (2015). Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases. Theranostics 5, 150–172. doi: 10.7150/thno.9476
Payne, J. N., Waghwani, H. K., Connor, M. G., Hamilton, W., Tockstein, S., Moolani, H., et al. (2016). Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front. Microbiol. 7:607. doi: 10.3389/fmicb.2016.00607
Pei, H., Zuo, X., Zhu, D., Huang, Q., and Fan, C. (2014). Functional DNA nanostructures for theranostic applications. Acc. Chem. Res. 47, 550–559. doi: 10.1021/ar400195t
Pelgrift, R. Y., and Friedman, A. J. (2013). Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 65, 1803–1815. doi: 10.1016/j.addr.2013.07.011
Percival, S. L., Bowler, P. G., and Dolman, J. (2007). Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int. Wound J. 4, 186–191. doi: 10.1111/j.1742-481X.2007.00296.x
Peulen, T.-O., and Wilkinson, K. J. (2011). Diffusion of nanoparticles in a biofilm. Environ. Sci. Technol. 45, 3367–3373. doi: 10.1021/es103450g
Pissuwan, D., Niidome, T., and Cortie, M. (2011). The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Control Release 149, 65–71. doi: 10.1016/j.jconrel.2009.12.006
Poma, A., and Di Giorgio, M. (2008). Toxicogenomics to improve comprehension of the mechanisms underlying responses of in vitro and in vivo systems to nanomaterials: a review. Curr. Genomics 9, 571–585. doi: 10.2174/138920208786847962
Potgieter, M. D., and Meidany, P. (2017). Evaluation of the penetration of nanocrystalline silver through various wound dressing mediums: an in vitro study. Burns 44, 596–602. doi: 10.1016/j.burns.2017.10.011
Pradeepa, Vidya S. M., Mutalik, S., Udaya Bhat, K., Huilgol, P., and Avadhani, K. (2016). Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 153, 171–179. doi: 10.1016/j.lfs.2016.04.022
Qi, G., Li, L., Yu, F., and Wang, H. (2013). Vancomycin-modified mesoporous silica nanoparticles for selective recognition and killing of pathogenic gram-positive bacteria over macrophage-like cells. ACS Appl. Mater. Interfaces 5, 10874–10881. doi: 10.1021/am403940d
Raghunath, A., and Perumal, E. (2017). Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int. J. Antimicrob. Agents 49, 137–152. doi: 10.1016/j.ijantimicag.2016.11.011
Rai, A., Pinto, S., Velho, T. R., Ferreira, A. F., Moita, C., Trivedi, U., et al. (2016). One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 85, 99–110. doi: 10.1016/j.biomaterials.2016.01.051
Rai, M. K., Deshmukh, S. D., Ingle, A. P., and Gade, A. K. (2012). Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 112, 841–852. doi: 10.1111/j.1365-2672.2012.05253.x
Rai, M., Ingle, A. P., Gaikwad, S., Gupta, I., Gade, A., and Silvério da Silva, S. (2016). Nanotechnology based anti-infectives to fight microbial intrusions. J. Appl. Microbiol. 120, 527–542. doi: 10.1111/jam.13010
Rai, M., Ingle, A. P., Pandit, R., Paralikar, P., Gupta, I., Chaud, M. V., et al. (2017). Broadening the spectrum of small-molecule antibacterials by metallic nanoparticles to overcome microbial resistance. Int. J. Pharm. 532, 139–148. doi: 10.1016/j.ijpharm.2017.08.127
Rai, M., Yadav, A., and Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27, 76–83. doi: 10.1016/j.biotechadv.2008.09.002
Rajchakit, U., and Sarojini, V. (2017). Recent developments in antimicrobial-peptide-conjugated gold nanoparticles. Bioconjug. Chem. 28, 2673–2686. doi: 10.1021/acs.bioconjchem.7b00368
Ramalingam, B., Parandhaman, T., and Das, S. K. (2016). Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 8, 4963–4976. doi: 10.1021/acsami.6b00161
Ramasamy, M., Lee, J. H., and Lee, J. (2017a). Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Coll. Surfaces B Biointerfaces 160, 639–648. doi: 10.1016/j.colsurfb.2017.10.018
Ramasamy, M., Lee, J.-H., and Lee, J. (2016). Potent antimicrobial and antibiofilm activities of bacteriogenically synthesized gold-silver nanoparticles against pathogenic bacteria and their physiochemical characterizations. J. Biomater. Appl. 31, 366–378. doi: 10.1177/0885328216646910
Ramasamy, M., Lee, J.-H., and Lee, J. (2017b). Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomed. 12, 2813–2828. doi: 10.2147/IJN.S132784
Ranghar, S., Sirohi, P., Verma, P., and Agarwal, V. (2014). Nanoparticle-based drug delivery systems : promising approaches against infections. Braz. Arch. Biol. Technol. 57, 209–222. doi: 10.1590/S1516-89132013005000011
Reddy, L. S., Nisha, M. M., Joice, M., and Shilpa, P. N. (2014). Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 52, 1388–1397. doi: 10.3109/13880209.2014.893001
Reddy, P. M., Chang, K.-C., Liu, Z.-J., Chen, C.-T., and Ho, Y.-P. (2014). Functionalized magnetic iron oxide (Fe3O4) nanoparticles for capturing gram-positive and gram-negative bacteria. J. Biomed. Nanotechnol. 10, 1429–1439. doi: 10.1166/jbn.2014.1848
Reen, F. J., Gutiérrez-Barranquero, J. A., Parages, M. L., and O'Gara, F. (2018). Coumarin: a novel player in microbial quorum sensing and biofilm formation inhibition. Appl Microbiol Biotechnol. 102, 2063–2073. doi: 10.1007/s00253-018-8787-x
Reidy, B., Haase, A., Luch, A., Dawson, K. A., and Lynch, I. (2013). Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials 6, 2295–2350. doi: 10.3390/ma6062295
Reshma, V. G., Syama, S., Sruthi, S., Reshma, S. C., Remya, N. S., and Mohanan, P. V. (2017). Engineered nanoparticles with antimicrobial property. Curr. Drug Metab. 18, 1040–1054. doi: 10.2174/1389200218666170925122201
Ribeiro, A. P. C., Anbu, S., Alegria, E. C. B. A., Fernandes, A. R., Baptista, P. V., Mendes, R., et al. (2018). Evaluation of cell toxicity and DNA and protein binding of green synthesized silver nanoparticles. Biomed. Pharmacother. 101, 137–144. doi: 10.1016/j.biopha.2018.02.069
Rizzello, L., and Pompa, P. P. (2014). Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 43, 1501–1518. doi: 10.1039/c3cs60218d
Roshmi, T., Soumya, K. R., Jyothis, M., and Radhakrishnan, E. K. (2015). Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull. 48, 63–71. doi: 10.1007/s13404-015-0162-4
Ruden, S., Hilpert, K., Berditsch, M., Wadhwani, P., and Ulrich, A. S. (2009). Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob. Agents Chemother. 53, 3538–3540. doi: 10.1128/AAC.01106-08
Rudramurthy, G. R., Swamy, M. K., Sinniah, U. R., and Ghasemzadeh, A. (2016). Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules 21:E836. doi: 10.3390/molecules21070836
Rutherford, S. T., and Bassler, B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2:a012427. doi: 10.1101/cshperspect.a012427
Roy, A., Parveen, A. R., Koppalkar, A., and Prasad, M. V. N. A. (2010). Effect of nano - titanium dioxide with different antibiotics against methicillin-resistant Staphylococcus Aureus. J. Biomater. Nanobiotechnol. 1, 37–41. doi: 10.4236/jbnb.2010.11005
Saeb, A. T. M., Alshammari, A. S., Al-brahim, H., and Al-rubeaan, K. A. (2014). Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2:704708. doi: 10.1155/2014/704708
Saha, B., Bhattacharya, J., Mukherjee, A., Ghosh, A., Santra, C., Anjan, K., et al. (2007). In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2, 614–622. doi: 10.1007/s11671-007-9104-2
Salem, W., Leitner, D. R., Zingl, F. G., Schratter, G., Prassl, R., Goessler, W., et al. (2015). Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 305, 85–95. doi: 10.1016/j.ijmm.2014.11.005
Salomoni, R., Léo, P., Montemor, A. F., Rinaldi, B. G., and Rodrigues, M. (2017). Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol. Sci. Appl. 10, 115–121. doi: 10.2147/NSA.S133415
Sandhiya, S., Dkhar, S. A., and Surendiran, A. (2009). Emerging trends of nanomedicine - an overview. Fundam. Clin. Pharmacol. 23, 263–269. doi: 10.1111/j.1472-8206.2009.00692.x
Sarwar, A., Katas, H., Samsudin, S. N., and Zin, N. M. (2015). Regioselective sequential modification of chitosan via azide-alkyne click reaction: synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS ONE 10:e0123084. doi: 10.1371/journal.pone.0123084
Sarwar, S., Chakraborti, S., Bera, S., Sheikh, I. A., Hoque, K. M., and Chakrabarti, P. (2016). The antimicrobial activity of ZnO nanoparticles against Vibrio cholerae: variation in response depends on biotype. Nanomedicine Nanotechnology, Biol. Med. 12, 1499–1509. doi: 10.1016/j.nano.2016.02.006
Sathyanarayanan, M. B., Balachandranath, R., Genji Srinivasulu, Y., Kannaiyan, S. K., and Subbiahdoss, G. (2013). The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens. ISRN Microbiol. 2013:272086. doi: 10.1155/2013/272086
Setyawati, M. I., Kutty, R. V., Tay, C. Y., Yuan, X., Xie, J., and Leong, D. T. (2014). Novel theranostic DNA nanoscaffolds for the simultaneous detection and killing of Escherichia coli and Staphylococcus aureus. ACS Appl. Mater. Interfaces 6, 21822–21831. doi: 10.1021/am502591c
Shah, A., Latif-Ur-Rahman, Qureshi R., and Zia-Ur-Rehman (2012). Synthesis, characterization, and application of Au-Ag alloy nanoparticles for the sensing of an environmental toxin, pyrene. Rev. Adv. Mater. Sci. 30, 133–149. doi: 10.1007/s10800-015-0807-2
Shah, M., Badwaik, V., Kherde, Y., Waghwani, H. K., Modi, T., Aguilar, Z. P., et al. (2014). Gold nanoparticles: various methods of synthesis and antibacterial applications. Front. Biosci. 19, 1320–1344. doi: 10.2741/4284
Shaikh, S., Rizvi, S. M. D., Shakil, S., Hussain, T., Alshammari, T. M., Ahmad, W., et al. (2017). Synthesis and characterization of cefotaxime conjugated gold nanoparticles and their use to target drug-resistant CTX-M-producing bacterial pathogens. J. Cell. Biochem. 118, 2802–2808. doi: 10.1002/jcb.25929
Shaker, M. A., and Shaaban, M. I. (2017). Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: in vitro antibacterial study. Int. J. Pharm. 525, 71–84. doi: 10.1016/j.ijpharm.2017.04.019
Shamaila, S., Zafar, N., Riaz, S., Sharif, R., Nazir, J., and Naseem, S. (2016). Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 6:71. doi: 10.3390/nano6040071
Shareena Dasari, T. P., Zhang, Y., and Yu, H. (2015). Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem. Pharmacol. 4:199. doi: 10.4172/2167-0501.1000199
Siddiqi, K. S., Husen, A., and Rao, R. A. K. (2018). A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnology 16:14. doi: 10.1186/s12951-018-0334-5
Singh, B. N., Prateeksha Upreti, D. K., Singh, B. R., Defoirdt, T., Gupta, V. K., De Souza, A. O., et al. (2017). Bactericidal, quorum quenching and anti-biofilm nanofactories: a new niche for nanotechnologists. Crit. Rev. Biotechnol. 37, 525–540. doi: 10.1080/07388551.2016.1199010
Singh, J., Garg, T., Rath, G., and Goyal, A. K. (2016). Advances in nanotechnology-based carrier systems for targeted delivery of bioactive drug molecules with special emphasis on immunotherapy in drug resistant tuberculosis - a critical review. Drug Deliv. 23, 1676–1698. doi: 10.3109/10717544.2015.1074765
Singh, K., Panghal, M., Kadyan, S., Chaudhary, U., and Yadav, J. P. arkas (2014). Green silver nanoparticles of Phyllanthus amarus: as an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnology 12:40. doi: 10.1186/s12951-014-0040-x
Singh, R., Nawale, L., Arkile, M., Wadhwani, S., Shedbalkar, U., Chopade, S., et al. (2016). Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int. J. Nanomed. 11, 1889–1897. doi: 10.2147/IJN.S102488
Singh, R., Smitha, M. S., and Singh, S. P. (2014). The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 14, 4745–4756. doi: 10.1166/jnn.2014.9527
Sinha, R., Karan, R., Sinha, A., and Khare, S. K. (2011). Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 102, 1516–1520. doi: 10.1016/j.biortech.2010.07.117
Slavin, Y. N., Asnis, J., Hafeli, U. O., and Bach, H. (2017). Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 15:65. doi: 10.1186/s12951-017-0308-z
Slomberg, D. L., Lu, Y., Broadnax, A. D., Hunter, R. A., Carpenter, A. W., and Schoenfisch, M. H. (2013). Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 5, 9322–9329. doi: 10.1021/am402618w
Smock, K. J., Schmidt, R. L., Hadlock, G., Stoddard, G., Grainger, D. W., and Munger, M. A. (2014). Assessment of orally dosed commercial silver nanoparticles on human ex vivo platelet aggregation. Nanotoxicology 8, 328–333. doi: 10.3109/17435390.2013.788749
Su, H.-L., Lin, S.-H., Wei, J.-C., Pao, I.-C., Chiao, S.-H., Huang, C.-C., et al. (2011). Novel nanohybrids of silver particles on clay platelets for inhibiting silver-resistant bacteria. PLoS ONE 6:e21125. doi: 10.1371/journal.pone.0021125
Su, Y., Zheng, X., Chen, Y., Li, M., and Liu, K. (2015). Alteration of intracellular protein expressions as a key mechanism of the deterioration of bacterial denitrification caused by copper oxide nanoparticles. Sci. Rep. 5:15824. doi: 10.1038/srep15824
Sukwong, P., Pissuwan, D., Somkid, K., Kongseng, S., and Yoovathaworn, K. (2016). Respiratory tract toxicity of titanium dioxide nanoparticles and multi-walled carbon nanotubes on mice after intranasal exposure. Micro Nano Lett. 11, 183–187. doi: 10.1049/mnl.2015.0523
Taylor, T., Gaysinsky, S., Davidson, P., Bruce, B., and Weiss, J. (2007). Characterization of antimicrobial bearing liposomes by zeta potential, vesicle size, and encapsulation efficiency. Food Biophys. 2, 1–9. doi: 10.1007/s11483-007-9023-x
Thapa, R., Bhagat, C., Shrestha, P., Awal, S., and Dudhagara, P. (2017). Enzyme-mediated formulation of stable elliptical silver nanoparticles tested against clinical pathogens and MDR bacteria and development of antimicrobial surgical thread. Ann. Clin. Microbiol. Antimicrob. 16, 1–10. doi: 10.1186/s12941-017-0216-y
Thomas, R., Nair, A. P., Kr, S., Mathew, J., and Ek, R. (2014). Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl. Biochem. Biotechnol. 173, 449–460. doi: 10.1007/s12010-014-0852-z
Thompson, M., Blaszykowski, C., Sheikh, S., and Romaschin, A. (2015). A true theranostic approach to medicine: towards tandem sensor detection and removal of endotoxin in blood. Biosens. Bioelectron. 67, 3–10. doi: 10.1016/j.bios.2014.07.008
Tielens, F., and Santos, E. (2010). AuS and SH bond formation/breaking during the formation of alkanethiol SAMs on Au(111): a theoretical study. J. Phys. Chem. C 114, 9444–9452. doi: 10.1021/jp102036r
Tiwari, P., Vig, K., Dennis, V., and Singh, S. (2011). Functionalised gold nanoparticles and their biomedical applications. Nanomaterials 1, 31–63. doi: 10.3390/nano1010031
Tong, G., Du, F., Wu, W., Wu, R., Liu, F., and Liang, Y. (2013). Enhanced reactive oxygen species (ROS) yields and antibacterial activity of spongy ZnO/ZnFe2O4 hybrid micro-hexahedra selectively synthesized through a versatile glucose-engineered co-precipitation/annealing process. J. Mater. Chem. B 1, 2647–2657. doi: 10.1039/c3tb20229a
Turos, E., Reddy, G. S. K., Greenhalgh, K., Ramaraju, P., Abeylath, S. C., Jang, S., et al. (2007). Penicillin-bound polyacrylate nanoparticles: restoring the activity of beta-lactam antibiotics against MRSA. Bioorg. Med. Chem. Lett. 17, 3468–3472. doi: 10.1016/j.bmcl.2007.03.077
Ulloa-Ogaz, A. L., Piñón-Castillo, H. A., Muñoz-Castellanos, L. N., Athie-García, M. S., Ballinas-Casarrubias, M. D. L., Murillo-Ramirez, J. G., et al. (2017). Oxidative damage to Pseudomonas aeruginosa ATCC 27833 and Staphylococcus aureus ATCC 24213 induced by CuO-NPs. Environ. Sci. Pollut. Res. 24, 22048–22060. doi: 10.1007/s11356-017-9718-6
Uma Suganya, K. S., Govindaraju, K., Ganesh Kumar, V., Stalin Dhas, T., Karthick, V., Singaravelu, G., et al. (2015). Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater. Sci. Eng. C 47, 351–356. doi: 10.1016/j.msec.2014.11.043
Umamaheswari, K., Baskar, R., Chandru, K., Rajendiran, N., and Chandirasekar, S. (2014). Antibacterial activity of gold nanoparticles and their toxicity assessment. BMC Infect. Dis. 14:P64. doi: 10.1186/1471-2334-14-S3-P64
Veigas, B., Fernandes, A. R., and Baptista, P. V. (2014). AuNPs for identification of molecular signatures of resistance. Front. Microbiol. 5:455. doi: 10.3389/fmicb.2014.00455
Veigas, B., Pedrosa, P., Carlos, F. F., Mancio-Silva, L., Grosso, A. R., Fortunato, E., et al. (2015). One nanoprobe, two pathogens: gold nanoprobes multiplexing for point-of-care. J. Nanobiotechnology 13:48. doi: 10.1186/s12951-015-0109-1
Veigas, B., Pedrosa, P., Couto, I., Viveiros, M., and Baptista, P. V (2013). Isothermal DNA amplification coupled to Au-nanoprobes for detection of mutations associated to Rifampicin resistance in Mycobacterium tuberculosis. J. Nanobiotechnology 11:38. doi: 10.1186/1477-3155-11-38
Venkatesan, N., Perumal, G., and Doble, M. (2015). Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 10, 1743–1750. doi: 10.2217/fmb.15.69
Vinoj, G., Pati, R., Sonawane, A., and Vaseeharan, B. (2015). In vitro cytotoxic effects of gold nanoparticles coated with functional acyl homoserine lactone lactonase protein from Bacillus licheniformis and their antibiofilm activity against proteus species. Antimicrob. Agents Chemother. 59, 763–771. doi: 10.1128/AAC.03047-14
Walker, T., Dumadag, S., Lee, C. J., Lee, S. H., Bender, J. M., Abbott, J. C., et al. (2016). Clinical impact of laboratory implementation of verigene BC-GN microarray-based assay for detection of gram-negative bacteria in positive blood cultures. J. Clin. Microbiol. 54, 1789–1796. doi: 10.1128/JCM.00376-16
Wang, G. (2014). Human antimicrobial peptides and proteins. Pharmaceuticals 7, 545–594. doi: 10.3390/ph7050545
Wang, L., Chen, Y. P., Miller, K. P., Cash, B. M., Jones, S., Glenn, S., et al. (2014). Functionalized nanoparticles complexed with antibiotic efficiently kill MRSA and other bacteria. Chem. Commun. 50, 12030–12033. doi: 10.1039/c4cc04936e
Wang, L., Hu, C., and Shao, L. (2017a). The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 12, 1227–1249. doi: 10.2147/IJN.S121956
Wang, L., Huang, F., Cai, G., Yao, L., Zhang, H., and Lin, J. (2017b). An electrochemical aptasensor using coaxial capillary with magnetic nanoparticle, urease catalysis and PCB electrode for rapid and sensitive detection of Escherichia coli O157:H7. Nanotheranostics 1, 403–414. doi: 10.7150/ntno.22079
Wang, Z., Dong, K., Liu, Z., Zhang, Y., Chen, Z., Sun, H., et al. (2017). Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 113, 145–157. doi: 10.1016/j.biomaterials.2016.10.041
Wangoo, N., Suri, C. R., and Shekhawat, G. (2008). Interaction of gold nanoparticles with protein: a spectroscopic study to monitor protein conformational changes. Appl. Phys. Lett. 92, 1–4. doi: 10.1063/1.2902302
Warheit, D. B. (2018). Hazard and risk assessment strategies for nanoparticle exposures: how far have we come in the past 10 years? F1000Res. 7:376. doi: 10.12688/f1000research.12691.1
Weng, C.-I., Chang, H.-T., Lin, C.-H., Shen, Y.-W., Unnikrishnan, B., Li, Y.-J., et al. (2015). One-step synthesis of biofunctional carbon quantum dots for bacterial labeling. Biosens. Bioelectron. 68, 1–6. doi: 10.1016/J.BIOS.2014.12.028
Wu, T., Wu, C., Fang, Z., Ma, X., Chen, S., and Hu, Y. (2017). Effect of chitosan microcapsules loaded with nisin on the preservation of small yellow croaker. Food Control 72, 43–52. doi: 10.1016/j.foodcont.2017.04.016
Xia, Q., Li, H., and Xiao, K. (2016). Factors affecting the pharmacokinetics, biodistribution and toxicity of gold nanoparticles in drug delivery. Curr. Drug Metab. 17, 849–861. doi: 10.2174/1389200217666160629114941
Xue, Y., Li, X., Li, H., and Zhang, W. (2014). Quantifying thiol-gold interactions towards the efficient strength control. Nat. Commun. 5:4348. doi: 10.1038/ncomms5348
Yang, X., Dang, Y., Lou, J., Shao, H., and Jiang, X. (2018). D-alanyl-D-alanine-modified gold nanoparticles form a broad-spectrum sensor for bacteria. Theranostics 8, 1449–1457. doi: 10.7150/thno.22540
Yang, X., Yang, J., Wang, L., Ran, B., Jia, Y., Zhang, L., et al. (2017). Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 11, 5737–5745. doi: 10.1021/acsnano.7b01240
Yeaman, M. R. (2003). Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55, 27–55. doi: 10.1124/pr.55.1.2
Yeom, J. H., Lee, B., Kim, D., Lee, J., kook Kim, S., Bae, J., et al. (2016). Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar Typhimurium. Biomaterials 104, 43–51. doi: 10.1016/j.biomaterials.2016.07.009
Yu, J., Zhang, W., Li, Y., Wang, G., Yang, L., Jin, J., et al. (2014). Synthesis, characterization, antimicrobial activity and mechanism of a novel hydroxyapatite whisker/nano zinc oxide biomaterial. Biomed. Mater. 10:015001. doi: 10.1088/1748-6041/10/1/015001
Yu, Q., Li, J., Zhang, Y., Wang, Y., Liu, L., and Li, M. (2016). Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 6:26667. doi: 10.1038/srep26667
Zaidi, S., Misba, L., and Khan, A. U. (2017). Nano-therapeutics : a revolution in infection control in post antibiotic era. Nanomed. Nanotechnol. Biol. Med. 13, 2281–2301. doi: 10.1016/j.nano.2017.06.015
Zazo, H., Colino, C. I., and Lanao, J. M. (2016). Current applications of nanoparticles in infectious diseases. J. Control. Release 224, 86–102. doi: 10.1016/j.jconrel.2016.01.008
Zhang, L., and Gallo, R. L. (2016). Antimicrobial peptides. Curr. Biol. 26, R14–R19. doi: 10.1016/j.cub.2015.11.017
Zhang, W., Li, Y., Niu, J., and Chen, Y. (2013). Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir 29, 4647–4651. doi: 10.1021/la400500t
Zhang, Y., Shareena Dasari, T. P., Deng, H., and Yu, H. (2015a). Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev. 33, 286–327. doi: 10.1080/10590501.2015.1055161
Zhang, Y., Zhu, P., Li, G., Wang, W., Chen, L., Lu, D. D., et al. (2015b). Highly stable and re-dispersible nano Cu hydrosols with sensitively size-dependent catalytic and antibacterial activities. Nanoscale 7, 13775–13783. doi: 10.1039/C5NR03414K
Zhang, Y., Zhu, P., Li, G., Zhao, T., Fu, X., Sun, R., et al. (2014). Facile preparation of monodisperse, impurity-free, and antioxidation copper nanoparticles on a large scale for application in conductive ink. ACS Appl. Mater. Interfaces 6, 560–567. doi: 10.1021/am404620y
Zhao, Y., and Jiang, X. (2013). Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale 5:8340. doi: 10.1039/c3nr01990j
Zhao, Y., Ye, C., Liu, W., Chen, R., and Jiang, X. (2014). Tuning the composition of AuPt bimetallic nanoparticles for antibacterial application. Angew Chem Int 53, 8127–8131. doi: 10.1002/anie.201401035
Zhao, Z., Yan, R., Yi, X., Li, J., Rao, J., Guo, Z., et al. (2017). Bacteria-activated theranostic nanoprobes against methicillin-resistant Staphylococcus aureus infection. ACS Nano 11, 4428–4438. doi: 10.1021/acsnano.7b00041
Zhou, Z., Peng, S., Sui, M., Chen, S., Huang, L., Xu, H., et al. (2018). Multifunctional nanocomplex for surface-enhanced Raman scattering imaging and near-infrared photodynamic antimicrobial therapy of vancomycin-resistant bacteria. Coll. Surfaces B Biointerfaces 161, 394–402. doi: 10.1016/j.colsurfb.2017.11.005
Keywords: antimicrobial resistance, multidrug resistance, nanomaterials, nanoparticles, plant-based compounds, novel antimicrobial agents, nanotheranostics
Citation: Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M and Fernandes AR (2018) Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 9:1441. doi: 10.3389/fmicb.2018.01441
Received: 31 March 2018; Accepted: 11 June 2018;
Published: 02 July 2018.
Edited by:
Rebecca Thombre, Pune University, IndiaReviewed by:
Shahper Nazeer Khan, Aligarh Muslim University, IndiaRodolfo García-Contreras, Universidad Nacional Autónoma de México, Mexico
Copyright © 2018 Baptista, McCusker, Carvalho, Ferreira, Mohan, Martins and Fernandes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro V. Baptista, cG12YkBmY3QudW5sLnB0
Marta Martins, bW1hcnRpbnNAdGNkLmll
Alexandra R. Fernandes, bWEuZmVybmFuZGVzQGZjdC51bmwucHQ=
†Present Address: Matthew P. McCusker, Kerry Europe, Global Technology & Innovation Centre, Naas, Ireland
 Pedro V. Baptista
Pedro V. Baptista Matthew P. McCusker
Matthew P. McCusker Andreia Carvalho
Andreia Carvalho Daniela A. Ferreira
Daniela A. Ferreira Niamh M. Mohan
Niamh M. Mohan Marta Martins
Marta Martins