- 1State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops, Fujian University Key Laboratory for Plant-Microbe Interaction, Fujian Agriculture and Forestry University, Fuzhou, China
- 2School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China
Xanthomonas citri subsp. citri (Xcc) is the major causal agent of citrus canker disease. The XAC1347 gene, which encodes a conserved membrane protein in Xcc, is required for virulence during infection. However, the molecular events mediated by XAC1347 remain unclear. In this study, we reported that XAC1347 gene is positively regulated by two component regulatory system ColRS and required for type III secretion system function. A non-polar deletion mutant of the XAC1347 gene resulted in a Hrp minus phenotype in plants and reduced copper homeostasis. Real-time PCR experiments indicated that XAC1347 gene is induced by copper ions. The expression levels of representative genes from four hrp operons, including hrpB1, hrcV, hrpF, and hrpD6, were reduced in XAC1347 mutant, indicating that XAC1347 is involved hrp gene expression.
Introduction
Xanthomonas citri subsp. citri (syn. Xanthomonas axonopodis pv. citri; Xcc) is a causal agent of canker disease in citrus plants (Vauterin et al., 1995). Citrus canker is a serious disease which causes great concern to citrus producers worldwide. Xcc is naturally spread by rain splash, invades host plants through natural openings, and multiplies in the intercellular spaces (Graham et al., 1992). The typical symptoms of citrus canker include necrotic lesions surrounded by oily, water-soaked margins, and yellow chlorotic rings (Brunings and Gabriel, 2003).
The two-component system colRS was originally identified from Pseudomonas fluorescens strain WCS365, and was shown to play a critical role in root-colonization (Dekkers et al., 1998). Subsequently, it was found to be involved in the regulation of membrane function (Hõrak et al., 2004; de Weert et al., 2006; Kivistik et al., 2006; Yan and Wang, 2011; Mumm et al., 2016), transposition of a transposon (Kivistik et al., 2006), and phenol tolerance (Kivistik et al., 2006). Its role in tolerance to excess iron stress has been extensively studied by addition of zinc, manganese, or cadmium into culturing medium (Hu and Zhao, 2007; Ainsaar et al., 2014). In X. oryzae pv. oryzae, colRS mutants exhibit a decrease in virulence, a inability to grow on low-iron media and inability to induced HR on non-host tomato (Subramoni et al., 2012). The colRS system of Xcc exerts multiple regulatory roles in biological processes, including in planta growth, biofilm formation, catalase activity, lipopolysaccharide production, and resistance to environmental stress (Yan and Wang, 2011). Mutation in this system results in reduced tolerance to copper iron stress.
The type III secretion system (T3SS) is essential for pathogenic bacteria to deliver type III effectors into plant cells, and in determining effector-triggered susceptibility (ETS) or effector-triggered immunity (ETI) (Alfano and Collmer, 1997). In Xcc, T3SS is encoded by a cluster of HR and pathogenicity (hrp) genes organized into six hrp operons (Dunger et al., 2005). The hrp genes located in the hrpB, hrpD, and hrpF operons are essential for pathogenicity in citrus and the HR in non-host plants (Dunger et al., 2005). In addition to the key transcriptional activator hrpX (Guo et al., 2011), the ColRS system is also required for the optimal expression of hrpD6 and hpaF genes encoding components of T3SS (Yan and Wang, 2011). The hrpX is located outside of the hrp gene cluster, while hrpD6 in the hrpD operon has been reported to have a regulatory role on hpa2, hpa1, hpaB, hrcC, and hrcT in X. oryzae pv. oryzicola, which causes a bacterial stripe disease in rice plants (Li et al., 2011b).
Membrane bound proteins are the fundamental structure of bacterial cell membranes involved in cell signaling, adhesion and transportation (Vinothkumar and Henderson, 2010). To build a functional T3SS, HrcQ, HrcR, HrcS, HrcT, HrcU, and HrcV are localized to integral inner membrane proteins to form the inner core of the T3SS, while HrcJ and HrcC are localized to membrane periplasm (Deng and Huang, 1999; Tampakaki et al., 2004; Berger et al., 2010). An Xcc periplasmic protein VrpA physically interacts with T3SS components HrcJ and HrcC to mediate efficient effector protein secretion (Zhou et al., 2015). The outer membrane protein OprB is more abundant in Xcc biofilm. An oprB mutant is impaired in biofilm formation, adherence to the host, and virulence (Ficarra et al., 2017). Through proteomics and transposon-based analysis, a large number of outer membrane proteins, transporters and membrane-bound receptors have been identified to be associated with Xcc infection (Yan and Wang, 2012; Facincani et al., 2014; Song et al., 2015; Ferreira et al., 2016; Carnielli et al., 2017).
The contribution of the outer membrane protein XAC1347 to canker development was previously discovered by random Tn5 mutagenesis of Xcc (Yan and Wang, 2012; Song et al., 2015). Tn5 insertion in XAC1347 gene did not affect Xcc growth on minimal medium but led to a remarkable reduction in canker symptom (Yan and Wang, 2012), and two XAC1347 insertion mutants of strain Xcc 29-1 were impaired in pathogenicity (Song et al., 2015). In this study, a non-polar deletion mutant of XAC1347 was constructed to determine how XAC1347 affected bacterial virulence and whether it was involved in environmental adaptation.
Materials and Methods
Bacterial Strains, Plasmids, and Culture Conditions
Bacterial strains and plasmids used in this study are listed in Supplementary Table S1. The Xcc strains were cultivated in nutrient broth (NB) medium or NB added with 1.5 % agar (NA) at 28°C (Song et al., 2015). To study protein secretion, western blotting was performed by culturing Xcc in XVM2, which mimics a plant-like environment (Guo et al., 2011). The minimal medium M9 was used to test whether the mutants were autotrophic. Escherichia coli strains were routinely cultured in Luria–Bertani medium at 37°C. Agrobacterium tumefaciens GV3101 was cultured in yeast extract and beef extract medium at 28°C. Antibiotics were applied at the following concentrations: kanamycin (Km) at 50 μg/ml, rifampin (Rif) at 50 μg/ml, spectinomycin (Sp) at 50 μg/ml, and gentamycin (Gm) at 10 μg/ml.
Mutant Generation
The non-polar mutant of ΔXAC1347 was constructed using homologous recombination in the Xcc 29-1 genetic background (Gicharu et al., 2016). The flanking DNA fragments were PCR amplified from Xcc 29-1 genomic DNA using the primer pairs 1347.1.F/1347.1.R and 1347.2.F/1347.2.R (Supplementary Table S2). These two flanking fragments were ligated into the vector pKMS1 at the XbaI and PstI sites, resulting in pKMS-1347 (Supplementary Table S1). The recombinant plasmid was introduced into wild-type Xcc 29-1 for deletion mutant isolation (Gicharu et al., 2016). The primer sets 1347.1.F/1347.2.R was used to identify the desired deletion mutants ΔXAC1347. The deletion of targeted fragments was confirmed by sequencing the PCR products obtained from each mutant.
A site-directed mutagenesis was employed to construct the response regulator ColR insertion mutant. The 399-bp DNA fragment of partial ColR gene was PCR amplified and cloned into suicide vector pK18mob at EcoRI and HindIII, generating pK18-ColR. The resulted construct was introduced into wild type Xcc 29-1 to generate the insertion mutant ΔColR (Schäfer et al., 1994).
Construction of Complementary Plasmids
The primer pairs C1347.F/C1347.R and CColR.F/CColR.R were used to PCR amplify XAC1347 and ColR with their promoter region, respectively (Supplementary Table S2). The PCR products were separately inserted into pBBR1MCS-5, generating pBB-1347 and pBB-ColR. Each complementary plasmid was transformed into corresponding mutant for phenotype restoration analysis. The pBB-1347 was additionally transformed into a Tn5 insertion mutant Mxac111-54 to make comparison with XAC1347 deletion mutant. The mutant Mxac111-54 carried a Tn5 insertion site at position 40 from translation start codon in XAC1347, and showed a loss of pathogenicity on citrus plants (Song et al., 2015).
Analysis of Bacterial Homeostasis to Copper
Xcc 29-1, ΔXAC1347, Tn5 insertion mutant Mxac111-54 and the corresponding complementation strains were cultured in NB broth and grown at 28°C for 24–36 h until the OD600 = 0.8. The cells were sub-cultured (1:100) in 4 ml fresh NB and incubated for another 16 h until the OD600 reached 0.6. After centrifugation at 6,000 rpm for 10 min at 4°C, the cell pellets were washed twice with sterilized water, and then re-suspended with sterilized water to an OD600 = 1.0. Cell suspensions were sub-cultured (1:100) in NB supplemented with gradient concentrations of copper ion (0.1, 0.2, 0.3, 0.4, and 0.5 mM) generated by adding copper sulfate to media. Growth rates were assessed by measuring OD600 values every 4 h over 2 days after sub-culturing. All of the experiments were repeated at least three times.
Pathogenicity and HR Assays
The cultured Xcc strains were suspended in sterile distilled water to a final concentration of 108 colony forming units (CFU)/ml (OD600 = 0.3). For the pathogenicity assay, bacterial suspensions were injected into fully expanded grapefruit (Citrus paradise Macf. cv Duncan) leaves with a needleless syringe. Disease symptoms were scored and photographed 5 days post inoculation. For the HR assay, bacteria were infiltrated into Lycopersicon esculentum leaves. Plant reactions were viewed 2 days post inoculation. Each test was repeated at least three times.
For the growth assay in planta, 0.8-cm-diameter leaf disks were cut with a cork borer. After surface sterilization twice with 75% ethanol, the disks were ground completely in 1 ml of sterile double distilled water. Serial dilutions of the suspension were plated on NA plates supplemented with appropriate antibiotics. Plates were incubated at 28°C for 3–4 days and the individual colonies were counted to determine the approximate CFU/cm2 of leaf area. The standard deviation was calculated based on the colony counts for three triplicate disks taken from each of the three samples per time point per inoculum. Experiments were repeated three times.
Western Blot
To determine whether XAC1347 protein was secreted by Xcc, c-Myc-tagged XAC1347 was expressed in the broad host vector pBBR1MCS-5. A fused c-Myc-tag was introduced at the 3′-terminal in the reverse primer 1347.S.R (Supplementary Table S2). The 670-bp fused DNA fragment containing the full-length XAC1347 gene was cloned into pBBR1MCS-5 at the XbaI and SacI sites. The resulting recombinant plasmid pBB-1347S was introduced into wild-type Xcc 29-1. The Xcc 29-1(1347S) was cultured in NB medium to the logarithmic phase. Bacterial cells were harvested and re-suspended in fresh NB (OD600 = 0.2) or XVM2 (OD600 = 1.0) medium. After suspension, cells were cultured at 28°C for 16 h, bacterial cells and the corresponding supernatant fractions were separated by centrifugation, and the protein in the supernatant fraction was precipitated with 12.5% trichloroacetic acid (Li et al., 2011a). Proteins were separated on 10% SDS-PAGE gels and were transferred to membranes for immunoblotting using anti-c-Myc primary antibodies (HuaAn Biotechnology, Hangzhou, China). Primary antibodies were recognized by anti-rabbit secondary antibodies (HuaAn Biotechnology) and were visualized on autoradiographs using the Western-Light chemiluminescence system (Transgene, Beijing, China).
GUS Activity Assay
To construct XAC1347 promoter–GUS fusion, the promoter region upstream of the XAC1347 open reading frame was PCR amplified from genomic DNA using primers 1347.P.F and 1347.P.R (Supplementary Table S2). The 542-bp PCR product was fused with the gusA gene in pRG960 vector at PstI-BamHI sites (Supplementary Table S2). The resulting construct was introduced into wild-type Xcc 29-1, ColR mutant ΔColR, and complementary strain CΔColR, and the transformed strains were cultured in NB until the OD600 reached 0.8. After centrifugation at 6,000 rpm for 10 min at 4°C, the cell pellets were re-suspended in NB to OD600 = 1.0. Copper sulfate was added to cell suspension to produce a final copper concentration of 0.3 mM. After the cultures were cultivated for 4 h, cells were collected for GUS activity analysis (Li et al., 2011b). The GUS activities were measured using the GloMax Multi Detection System (Promega, Madison, WI, United States) with p-nitrophenyl-D-glucuronide as the substrate in a 1-h reaction. The GUS activity for each sample was read, and empty NB media were used as blank. The relative GUS activity was calculated as the GUS activities from ΔColR and CΔColR divided by that from wild type 29-1. Assays were independently repeated three times.
RNA Isolation and qRT-PCR
To evaluate gene expression levels in the bacteria cultured in liquid media, RNA was extracted from Xcc cells using the RNAprep pure Kit for Cell/Bacteria (Tiangen Biotech, Beijing, China). To study the expression of XAC1347 under copper stress condition, the Xcc cells were cultured in NB medium till OD600 = 1.5, and then copper sulfate was supplemented and co-cultured for 4 h. To obtain RNA from cells growing in citrus, Xcc strains were infiltrated into fully expanded citrus leaves. At 24 h post inoculation, leaves were collected for RNA extraction using the Plant RNA Kit (Omega, Norcross, GA, United States) to isolate RNAs from grapefruit leaves. The total RNAs were quantified by measuring the OD260/OD280 ratio and RNA quality was analyzed using gel electrophoresis. Genomic DNA contamination was removed using the PrimeScript RT reagent Kit with gDNA Eraser (perfect Real Time) (Takara, Dalian, China) before reverse transcription. All of the primers used for qRT-PCR are listed in Supplementary Table S3. Assays were performed using the Applied Biosystems 7500 real-time PCR system with SYBR Premix Ex Taq (Takara). The PCR thermal cycle conditions were as follows: denaturation at 95°C for 30 s and 40 cycles of 95°C for 5 s and 60°C for 30 s. The expression of gyrA was used as the internal control.
Transient Expression in Tobacco Leaves
To investigate the membrane bound trait of XAC1347, the XAC1347 open reading frame was inserted into the pGDG vector (Goodin et al., 2002), generating an N-terminal fusion with the GFP gene under the control of the double cauliflower mosaic virus 35S promoter (Supplementary Table S2). The resulting pGDG-1347 and the pGDG empty vector control were transformed into A. tumefaciens strain GV3101. The infiltration manipulation was performed as described previously (Li et al., 2011b). Two days after inoculation, samples were imaged under a confocal laser scanning microscope (CLSM, Leica TCS SP5 II, Germany). Experiments were independently repeated three times.
Results
XAC1347 Is Alanine-Rich and Conserved in Plant Pathogenic Xanthomonas
XAC1347 is a 339-bp-long gene coding for a 113-amino acid small protein, which does not contain phenylalanine, histidine, valine, tyrosine, or tryptophan, but contains 49 alanines, accounting for approximately 43.3% of the total amino acids. Particularly, there are six –AAA- and nine –AA- repeats in this small protein (Figure 1). The first 20 amino acids at the N-terminus comprises a putative a putative signal peptide, possibly required for translocation to cell membrane. The XAC1347 protein shows 100% identity among sequenced Xcc strains and one X. axonopodis pv. citrumelo F1 strain. Using multiple sequence alignment, nine amino acids are found different among homologs in X. campestris, X. oryzae, X. vesicatoria, X. arboricola, X. fragariae, and X. gardneri. The XAC1347 protein shows over 97% identity between those homologs (Figure 1).

FIGURE 1. Sequence analysis of XAC1347 protein. Amino acid sequence alignment of XAC1347 homologs from eight Xanthomonas species. The 20 amino acids of signal peptide are underlined at N-terminus. Proteins involved are listed as follows: Xcc 29-1 (X. citri subsp. citri 29-1, AGH76857.1), Xcc 306 (X. citri subsp. citri 306, AAM36218.1), Xcc Aw (X. citri subsp. citri Aw, AGI08775.1), Xa citumelo (X. axonopodis pv. citrumelo F1, AEO41615.1), X. oryzae (WP_019302275.1), X. vesicatoria (WP_005992008.1), X. campestris (NP_636670.1), X. gardneri (WP_006449860.1), X. arboricola (WP_016904096.1), and X. fragariae (WP_002810337.1).
The Non-polar Deletion Mutant ΔXAC1347 Was Impaired in Pathogenicity in Citrus and HR Induction in Tomato
A non-polar deletion mutant ΔXAC1347 was produced by two steps of homologous recombination (Supplementary Figure S1). A 631-bp DNA fragment comprised of the XAC1347 gene and its promoter was cloned into pBBR1MCS-5 to generate a recombinant pBB-1347 for complementation analysis. The wild-type strain 29-1, the deletion mutant ΔXAC1347 and the complemented strain CΔXAC1347 were individually infiltrated into the leaves of grapefruit and tomato. Similar to the phenotypes of Tn5 insertion mutants (Song et al., 2015), the deletion mutant ΔXAC1347 produced no canker symptoms in citrus leaves (Figure 2A). The complemented CΔXAC1347 strain restored the virulence in citrus to the level of that in the wild-type strain, producing typical canker symptoms (Figure 2A). At 48 h after inoculation into L. esculentum leaves, ΔXAC1347 did not induce the HR, and the HR defect was restored by the XAC1347 gene under the control of its own promoter (Figure 2B).
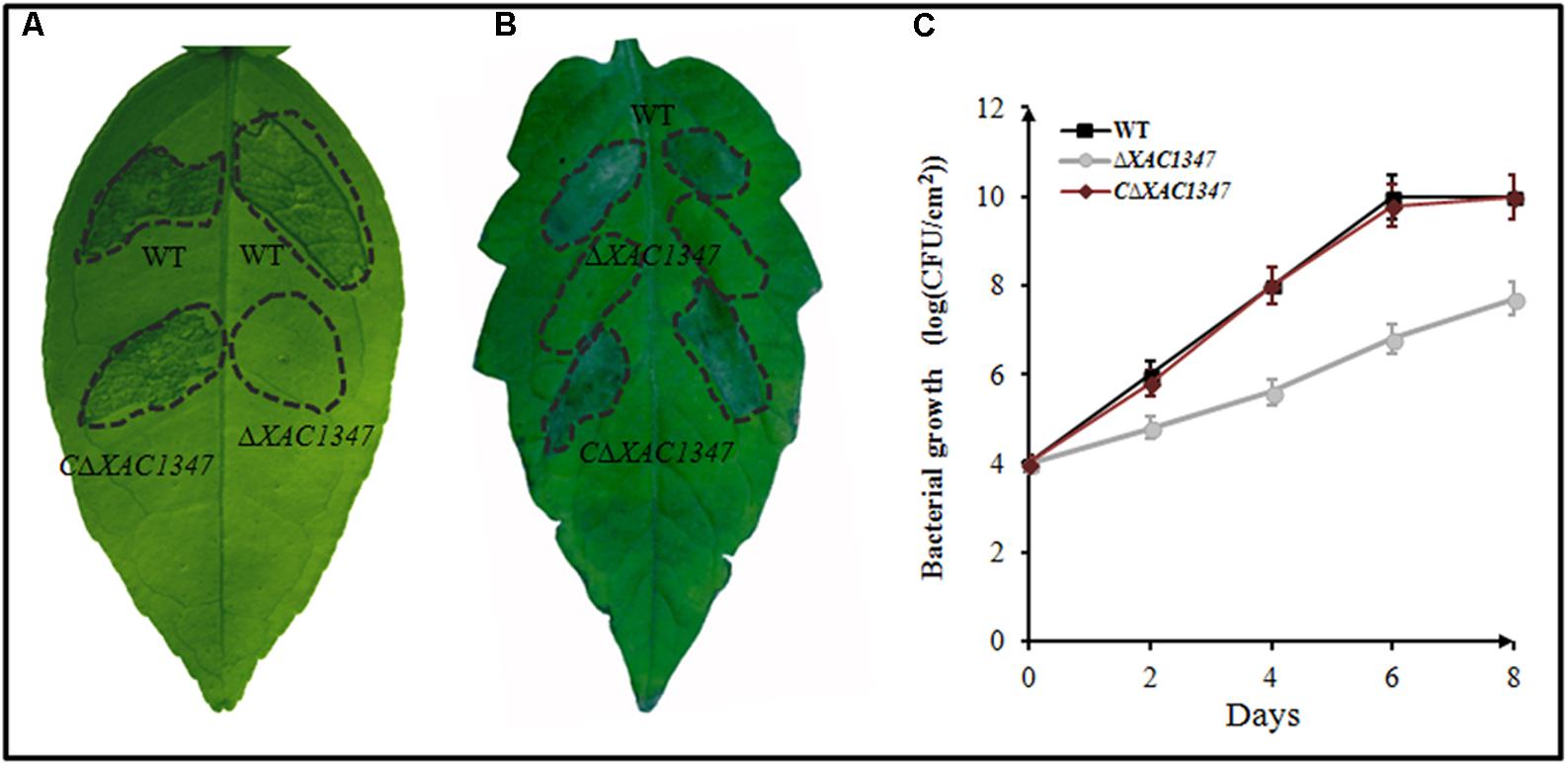
FIGURE 2. The phenotypes of non-polar deletion mutant ΔXAC1347 in Citrus paradise and Lycopersicon esculentum. (A) Citrus canker on C. paradise Macf. cv Duncan. (B) The hypersensitive response on L. esculentum. (C) Bacterial growth in citrus plants. The bacterial cells were resuspended in sterile water at a concentration of 108 CFU/ml and used to infiltrate cv. Duncan grapefruit leaves with a needleless syringe. The values shown are the means of three technical repeats with standard deviations.
To evaluate the bacterial growth in host plants, the cell numbers were measured every 2 days after inoculation into citrus leaves. At 2 days after inoculation, the cell density of wild-type Xcc 29-1 reached 106 CFU/cm2, exhibiting a high proliferation rate in the host plants. It reached 108 CFU/cm2 at 4 days after inoculation, and reached 1010 CFU/cm2 at 6 days after inoculation. By contrast, the cell number of the deletion mutant ΔXAC1347 was ∼105 CFU/cm2 at 2 days after inoculation, nearly 10-fold lower than that of the wild type. At 4, 6, and 8 days after inoculation, the mutant’s growth rate remained slow, and the cell number was less than 108 CFU/cm2 at 8 days after inoculation. The complementary strain CΔXAC1347 restored the bacterial growth capability in citrus plants (Figure 2C).
XAC1347 Is Involved in the Expression of hrp Genes
Since the XAC1347 mutant showed a typical T3SS defection phenotype, we therefore evaluate its effect on hrp gene expression. Because the hrp genes located in the hrpB, hrpC, hrpE, and hrpF operons are essential for pathogenicity in citrus (Dunger et al., 2005), four representative genes hrpB1, hrcV, hrpF, and hrpD6 are chosen for further analysis. The representative hrp genes were all down regulated in XAC1347 deletion mutant ΔXAC1347 and Tn5 insertion mutant Mxac111-54. Specifically, the expression levels of hrpB1 and hrpF were reduced over 90%, and the expression levels of hrcV and hrpD6 were reduced over 85% (Figure 3). Our results indicate that XAC1347 is required for the full expression of hrp genes.
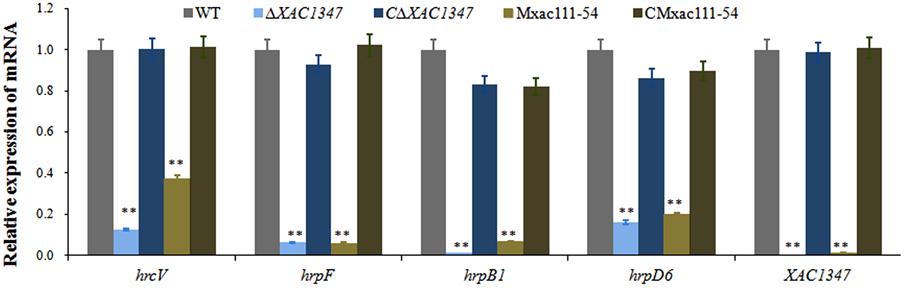
FIGURE 3. qRT-PCR analysis of hrp gene expression in XAC1347 mutant. Total RNAs were extracted from Xcc cells growing in citrus at 24 h post inoculation, The gyrA was used as the internal control. Statistical analysis was conducted using Student’s t-test. Asterisks denote statistical significance as compared to wild type. ∗∗P < 0.01; n = 3.
XAC1347 Is Involved in Copper Homeostasis
XAC1347 is annotated as a membrane protein in the genome database (da Silva et al., 2002). We therefore tested whether it is involved in maintaining cell osmotic balance. Bacterial growth was assessed in nutrient NB liquid media by adding a gradation of copper sulfate concentrations, ranging from 0.1 to 0.5 mM. Wild-type Xcc 29-1 could grow in all five concentrations of copper ions, but with the increasing copper concentration, the growth rate declined (Figure 4). In contrast, the deletion mutant ΔXAC1347 had reduced growth ability in 0.1 and 0.2 mM copper sulfate, and could not grow in media containing 0.3, 0.4, or 0.5 mM copper (Figure 4). The introduction of pBB-1347 into the mutant ΔXAC1347 partially restored its homeostasis to copper. Similarly, the growth rate of Tn5 insertion mutant Mxac111-54 also declined as the copper concentration increased (Figure 4). Even though the copper homeostasis of the complementation strains was not restored to that of the wild type, the experiment indicated that XAC1347 plays a role in copper homeostasis.
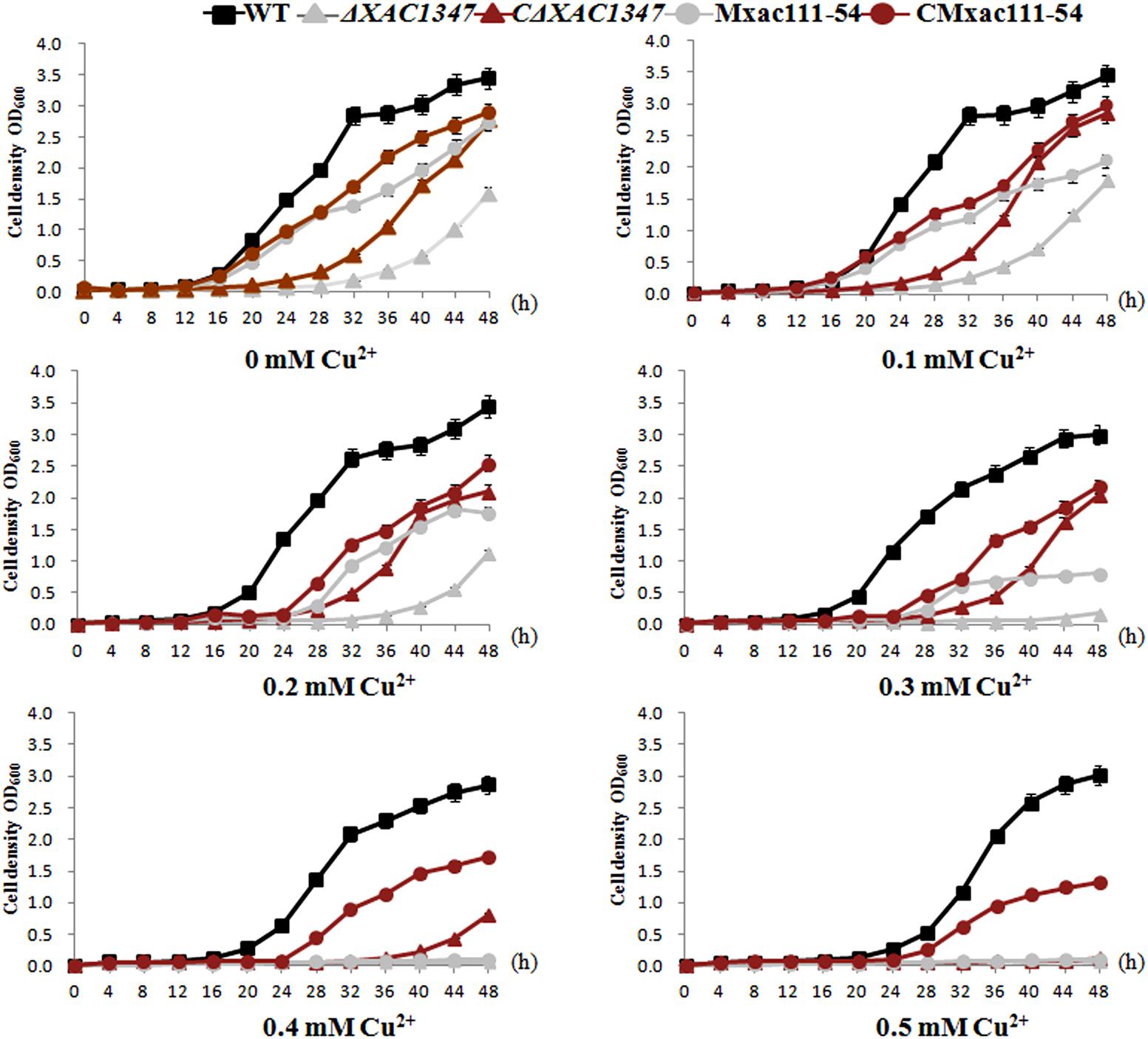
FIGURE 4. Growth curves of XAC1347 mutants in response to copper stress. The cultured Xcc cells were adjusted to OD60 = 1.0 and sub-cultured (1:100) in NB medium supplemented with gradient concentrations of copper ions. Growth rates were assessed by measuring OD600 values every 4 h. All of the experiments were performed in triplicate and repeated three times with similar results. Error bars denote standard deviation of three experimental replicates.
The Transcription of XAC1347 Gene Is Induced by Copper Ion
The promoter region of XAC1347 was predicted using the Berkeley Drosophila Genome Project1. A putative promoter with a 90% possibility score was predicted from 37 bp upstream of the transcriptional ATG start codon (Figure 5A). The promoter region also showed a high level of conservation in plant pathogenic Xanthomonas species, implying that its homologs may have similar expression patterns in other Xanthomonas strains.
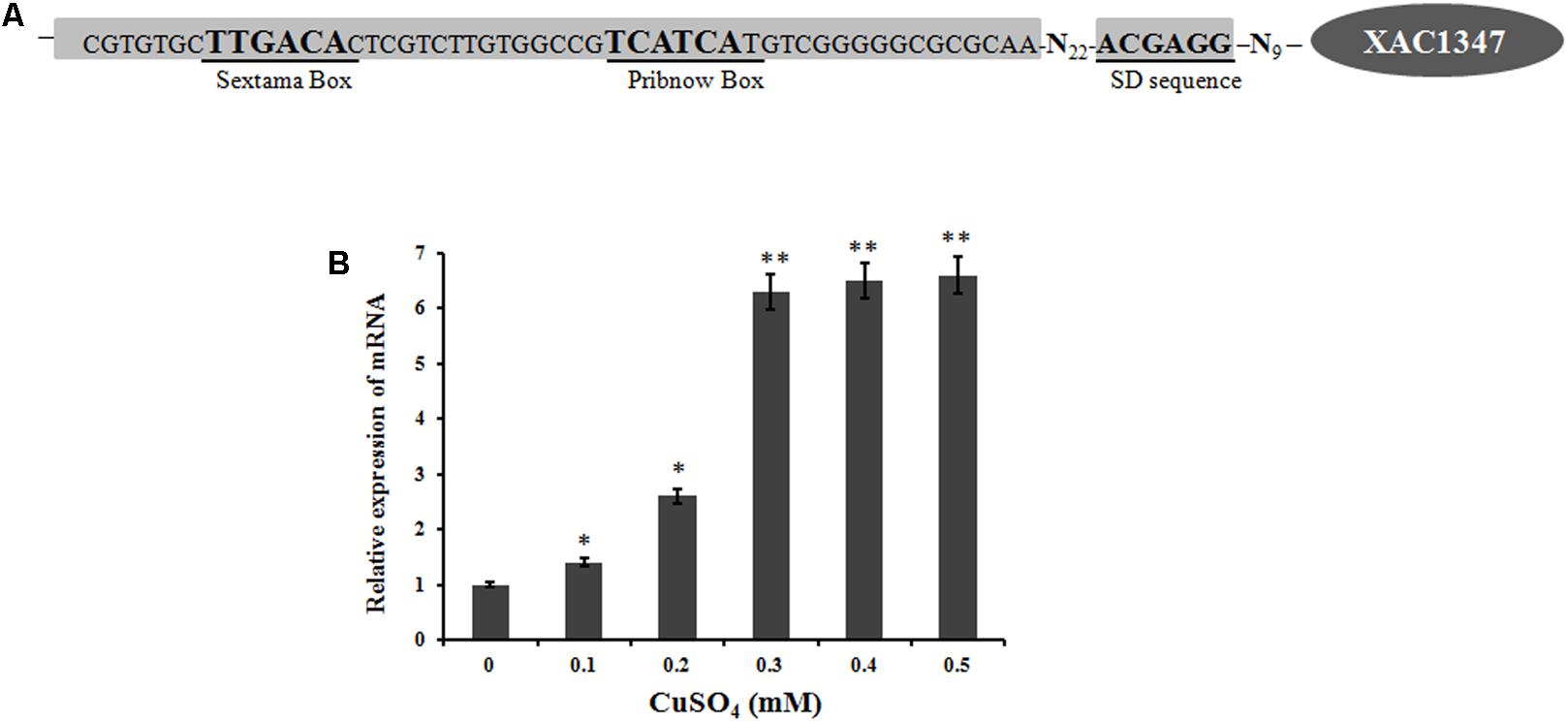
FIGURE 5. The transcription of XAC1347 gene induced by copper. (A) Nucleotide sequence of XAC1347 promoter region. Sextama box, Pribnow box, and SD sequence were underlined. (B) qRT-PCR analysis of XAC1347 mRNA levels. The gyrA was used as the internal control. Statistical analysis was conducted using Student’s t-test. Asterisks denote statistical significance as compared to the control culturing in copper-free NB. ∗P < 0.05; ∗∗P < 0.01, n = 3.
A real-time qRT-PCR analysis was carried out to determine the mRNA level of XAC1347 under copper-stress conditions. Based on a comparison with the expression level in copper-free NB medium, the XAC1347 gene’s mRNA level was increased when copper sulfate was added. In NB media supplemented with 0.1 mM copper sulfate, the mRNA level increased 40%. The mRNA level was 2.6-fold in media supplemented with 0.2 mM copper sulfate relative to that in copper-free medium. At the 0.3, 0.4, and 0.5 mM concentrations of copper sulfate, the mRNA levels were increased by 6.3, 6.5, and 6.6-fold, respectively (Figure 5B). These data indicate that expression of XAC1347 is induced by copper.
XAC1347 Gene Is Under Control of ColRS System
The two-component system ColRS plays important roles in several biological processes, including the pathogenicity and adaptation to copper stress (Yan and Wang, 2011). To determine whether XAC1347 gene is regulated by ColRS system, a null mutant of response regulator ColR was constructed to mutagenize the ColRS system. The ΔColR did not produce canker symptom in citrus plants (Figure 6A). Next, XAC1347 promoter-GUS fusion construct was introduced into ΔColR and its complementary strain CΔColR. Compared with wild type, GUS activities were reduced in ColR mutant culturing either in NB medium or in media containing 0.3 mM copper sulfate (Figure 6B). Then, transcription level of XAC1347 gene was assessed by qRT-PCR analysis. In Figure 6C transcription of XAC1347 was reduced in ΔColR compared with that in wild type cultured in NB medium with or without copper. In citrus plants, the transcription of XAC1347 gene showed similar pattern of reduced expression in ColR mutant (Figure 6C). Furthermore, the expression levels of the representative hrp genes were all reduced in the ColR mutant (Figure 6D). This indicated that XAC1347 gene was positively regulated by ColRS in Xcc. We additionally assessed the ColR and ColS expression in XAC1347 mutant in planta. Both genes did not show distinct expression level changes compared to expression in the wild type (Supplementary Figure S2).
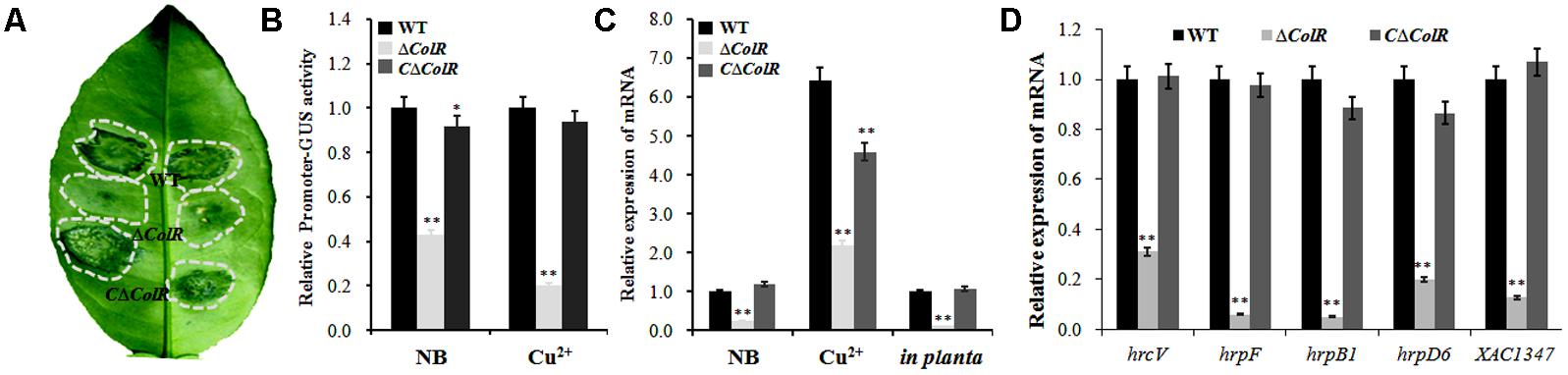
FIGURE 6. The transcriptions of XAC1347 and hrp genes in ColR mutant. (A) Pathogenicity of ColR mutant in citrus plants. (B) XAC1347 promoter activity monitored by β-glucuronidase (GUS). The relative GUS activities were calculated from the GUS activity of mutant divided by the GUS activity in wild type. (C) qRT-PCR analysis of XAC1347 mRNA levels. (D) qRT-PCR analysis of hrp gene expressions. The gyrA was used as the internal control. ( ), Wild type Xcc 291-1; (
), Wild type Xcc 291-1; ( ) Mutant ΔColR; (
) Mutant ΔColR; ( ) Complementary strain CΔColR. Statistical analysis was conducted using Student’s t-test. Asterisks denote statistical significance as compared to the control culturing in copper-free NB. ∗∗P < 0.01, n = 3.
) Complementary strain CΔColR. Statistical analysis was conducted using Student’s t-test. Asterisks denote statistical significance as compared to the control culturing in copper-free NB. ∗∗P < 0.01, n = 3.
Discussion
The contribution of XAC1347 to bacterial virulence has been reported in several previous publications (Yan and Wang, 2012; Song et al., 2015), but the molecular mechanisms have not been fully understood. In our previous work, two Tn5 insertion mutants of XAC1347 were identified to have abolished virulence in citrus plants (Song et al., 2015). To gain a better understanding of this alanine-rich protein, a non-polar deletion mutant of XAC1347 was constructed in this study. In addition to the loss of pathogenicity in citrus host and HR reaction in tomato, the mutant ΔXAC1347 had reduced homeostasis to copper iron. The expression of XAC1347 was copper-induced and controlled by the two-component system ColRS.
A previous study reported that an Tn5 insertion mutant of XAC1347 gene derived from strain Xcc 306 did not show distinct growth alteration in minimal medium M9 (Yan and Wang, 2011). Our deletion mutant ΔXAC1347 showed reduced growth rate in nutrient rich medium. To confirm phenotypic alteration in bacterial growth, the Tn5 insertion mutant Mxac111-54 was additionally studied. Like the deletion mutant ΔXAC1347, Mxac111-54 also reduced the growth in nutrient medium. In minimal medium M9, the colonies of ΔXAC1347 and Mxac111-54 were smaller than those of wild type (Supplementary Figure S3). This demonstrated that mutation in XAC1347 did lead to a reduction in bacterial growth in our research. The inconsistent growth phenotype may have been due to that the mutants were constructed from different wild type Xcc strains.
The ΔXAC1347 mutant did not induce canker symptom in citrus or HR reaction in tomato plant. This phenotype is resulted from the fact that the hrp genes were down regulated in XAC1347 mutants. It has to be mentioned that the Hrp phenotype alteration is not caused by the slow growth, because 102–103 CFU/ml Xcc cells are sufficient to induce canker lesions through wounds (Goto, 1962; Zubrzycki and Diamante, 1987). In our study, the cell number of ΔXAC1347 reached 105 CFU/ml at 2 days post inoculation and 107 CFU/ml at 6 days post inoculation in citrus tissue. Through promoter-GUS fusion and Real-time PCR experiments, XAC1347 was found to be down regulated in ColR mutant cells cultured in NB medium, copper stress condition, or in planta. The hrpC and hrpE operons were positively regulated by the ColRS system in X. campestris pv. campestris and X. oryzae pv. oryzicola (Zhang et al., 2008; Li et al., 2011b). In Xcc, ColRS system controls multiple bacterial traits, like hrp gene expression, LPS production, catalase activity, and copper resistance (Yan and Wang, 2011). Four representative hrp genes, hrcV, hrpD6, hrpF, and hrpB1, which were all down-regulated in XAC1347 mutant, were also regulated by ColRS. The collected data suggest that XAC1347 is positively regulated by the ColRS system.
Proteins delivered by secretion systems in Xcc play important roles in canker development. The T3SS is responsible for the secretion of a repertoire of effector proteins, some of which are translocated directly into plant cells (Alfano and Collmer, 1997). There are at least 24 effector protein coding genes in the sequenced strain Xcc 29-1, including four avr/pthA genes, pthA1, pthA2, pthA3, and pthA4 (Song et al., 2015). The pthA4 homolog that carries a 17.5-tandem-repeat domain has been proposed as the main virulence determinant during infection (Song et al., 2015). Although the XAC1347 mutant showed a Hrp deficiency similar phenotype, it remains unclear whether XAC1347 is required for effector efficient secretion, functioning like the periplasmic protein VrpA (Zhou et al., 2015).
XAC1347 shows certain traits of a typical integral protein. Although it only contains 113-amino acids, the N-terminus 20 amino acids were predicted to form a signal peptide, which makes it possible that XAC1347 may function as an integral membrane protein (Wallin and von Heijne, 1998). By fusion with GFP, we did found that most of XAC1347 protein is bonded to cell membrane (Supplementary Figure S4A). As a biotrophic pathogen, Xcc penetrates the apoplast and releases a large number of molecules into the apoplastic space, some of which are pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharides, flagellin or elongation factor Tu (Facincani et al., 2014; Ferreira et al., 2016; Carnielli et al., 2017). The western blot results indicated that the XAC1347-C-Myc protein was detected from the total cell extract, but not present in the culture supernatant (Supplementary Figure S4B). Thus, XAC1347 may not be secreted outside to plant apoplastic space during infection.
Because membrane integral proteins usually act as pathways for ions and molecules, we suspected that XAC1347 might be involved in maintaining cell integrity and osmotic balance (Cybulski and de Mendoza, 2011; Kar et al., 2017). We therefore studied the role of XAC1347 in copper homeostasis. Copper is an important transition metal for most organisms, but can be toxic at high levels. The mechanism for copper homeostasis has been studied in many prokaryotes. In Pseudomonas syringae, the plasmid-borne copper resistance operon copABCD is regulated by a copper-inducible promoter that is recognized by the regulatory genes copR and copS located downstream of copD (Cha and Cooksey, 1991; Mills et al., 1993). This suggests that bacteria employ a two-component system to mediate gene expression in response to copper stimuli and to regulate copper-resistance gene expression. Seven copper-resistance genes have been molecularly identified from Xcc A44; however, only three genes (copL, copA, and copB) were found in the sequenced strains Xcc 306 and Xcc 29-1 (Teixeira et al., 2008; Behlau et al., 2011). Additionally, copR/copS were not found in all Xcc strains (da Silva et al., 2002; Behlau et al., 2011). It is still unclear which member of the two-component system is responsible for regulating copper homeostasis in Xcc. Even though mutations in ColRS reduced bacterial tolerance to copper, no evidence has been presented if copLAB was directly regulated by ColRS (Yan and Wang, 2011). In this study we found that the transcription of XAC1347 was increased under copper stress and XAC1347 was essential for copper homeostasis in Xcc. Further studies are needed to elucidate the exact mechanism employed by XAC1347 to mediate bacterial copper homeostasis.
Conclusion
XAC1347 in Xanthomonas citri subsp. citri is alanine-rich membrane protein involved in copper homeostasis. The transcription of XAC1347 gene is induced by copper iron and under the control of two component regulatory system ColRS. Mutation in XAC1347 resulted in significantly reduced expression of hrp genes, which may be the reason why the ΔXAC1347 mutant fails to cause canker symptom in citrus.
Author Contributions
XF and JG performed the research. YZ, TZ, and XH analyzed the data. HZ designed the research and wrote the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31671988 and 31701752) and the Guiding Project from Fujian Province (2016N0006).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01171/full#supplementary-material
FIGURE S1 | Molecular analysis of the ΔXAC1347 mutant of Xanthomonas citri subsp. citri. (A) PCR analysis of the ΔXAC1347 mutant. The size difference of PCR products from wild-type Xcc 29-1 and ΔXAC1347 was revealed using primers 1347.1.F and 1347.2.R. (B) Southern blot of the ΔXAC1347 mutant. The Southern blot was carried out using the 342-bp XAC1347 gene as the probe against genomic DNA digested with NotI.
FIGURE S2 | qRT-PCR analysis of the transcription of ColR and ColS in XAC1347 mutant. The gyrA was used as the internal control. Statistical analysis was conducted using Student’s t-test. Asterisks denote statistical significance as compared to wild type. ∗P < 0.05; ∗∗P < 0.01, n = 3.
FIGURE S3 | XAC1347 mutants grown on solid minimal medium M9. The cultured Xcc cells were adjusted to OD60 = 1.0 in liquid M9 medium, and then serially diluted by 10-fold to make further concentrations of OD600 = 0.1, 0.01, 0.001. For each series, 2 μl cell suspension was dropped on M9 plates. Cell colonies were viewed at 6 days post inoculation
FIGURE S4 | Membrane bound trait and secretion analysis of XAC1347 protein. (A) Subcellular localization of XAC1347 in Nicotiana benthamiana. Bars, 15 μm; LM, light microscopy; FM, fluorescence microscopy, Mer, Merge. (B) The secretion of XAC1347 in Xanthomonas citri spp. citri. A c-Myc-tagged XAC1347 was expressed under the control of its own promoter. The cell pellets were ultrasonicated for total extraction (TE) and the protein in the supernatant fraction (SN) was precipitated with 12.5% trichloroacetic acid.
TABLE S1 | Bacterial strains and plasmids used in this study.
TABLE S2 | Primers for molecular cloning in this study.
TABLE S3 | Primers for real time PCR analysis in this study.
Footnotes
References
Ainsaar, K., Mumm, K., Ilves, H., and Hõrak, R. (2014). The ColRS signal transduction system responds to the excess of external zinc, iron, manganese, and cadmium. BMC Microbiol. 14:162. doi: 10.1186/1471-2180-14-162
Alfano, J. R., and Collmer, A. (1997). The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J. Bacteriol. 179, 5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997
Behlau, F., Canteros, B., Minsavage, G. V., Jones, J. B., and Graham, J. H. (2011). Molecular characterization of copper resistance genes from Xanthomonas citri subsp. citri and Xanthomonas alfalfa subsp. citrumelonis. Appl. Environ. Microbiol. 77, 4089–4096. doi: 10.1128/AEM.03043-10
Berger, C., Robin, G. P., Bonas, U., and Koebnik, R. (2010). Membrane topology of conserved components of the type III secretion system from the plant pathogen Xanthomonas campestris pv. vesicatoria. Microbiology 156, 1963–1974. doi: 10.1099/mic.0.039248-0
Brunings, A. M., and Gabriel, D. W. (2003). Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. doi: 10.1046/j.1364-3703.2003.00163.x
Carnielli, C. M., Artier, J., de Oliveira, J. C., and Novo-Mansur, M. T. (2017). Xanthomonas citri subsp. citri surface proteome by 2D-DIGE: ferric enterobactin receptor and other outer membrane proteins potentially involved in citric host interaction. J. Proteomics 151, 251–263. doi: 10.1016/j.jprot.2016.05.007
Cha, J. S., and Cooksey, D. A. (1991). Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 88, 8915–8919. doi: 10.1073/pnas.88.20.8915
Cybulski, L. E., and de Mendoza, D. (2011). Bilayer hydrophobic thickness and integral membrane protein function. Curr. Protein Pept. Sci. 12, 760–766. doi: 10.2174/138920311798841681
da Silva, A. C., Ferro, J. A., Reinach, F. C., Farah, C. S., Furlan, L. R., Quaggio, R. B., et al. (2002). Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417, 459–463. doi: 10.1038/417459a
de Weert, S., Dekkers, L. C., Bitter, W., Tuinman, S., Wijfjes, A. H., van Boxtel, R., et al. (2006). The two-component colR/S system of Pseudomonas fluorescens WCS365 plays a role in rhizosphere competence through maintaining the structure and function of the outer membrane. FEMS Microbiol. Ecol. 58, 205–213. doi: 10.1111/j.1574-6941.2006.00158.x
Dekkers, L. C., Bloemendaal, C. J., de Weger, L. A., Wijffelman, C. A., Spaink, H. P., and Lugtenberg, B. J. (1998). A two-component system plays an important role in the root-colonizing ability of Pseudomonas fluorescens strain WCS365. Mol. Plant Microbe Interact. 11, 45–56. doi: 10.1094/MPMI.1998.11.1.45
Deng, W. L., and Huang, H. C. (1999). Cellular locations of Pseudomonas syringae pv. syringae HrcC and HrcJ proteins, required for harpin secretion via the type III pathway. J. Bacteriol. 181, 2298–2301.
Dunger, G., Arabolaza, A. L., Gottig, N., Orellano, E. G., and Ottado, J. (2005). Participation of Xanthomonas axonopodis pv. citri hrp cluster in citrus canker and nonhost plant responses. Plant Pathol. 54, 781–788. doi: 10.1111/j.1365-3059.2005.01284.x
Facincani, A. P., Moreira, L. M., Soares, M. R., Ferreira, C. B., Ferreira, R. M., Ferro, M. I., et al. (2014). Comparative proteomic analysis reveals that T3SS, Tfp, and xanthan gum are key factors in initial stages of Citrus sinensis infection by Xanthomonas citri subsp. citri. Funct. Integr. Genomics 14, 205–217. doi: 10.1007/s10142-013-0340-5
Ferreira, R. M., Moreira, L. M., Ferro, J. A., Soares, M. R. R., Laia, M. L., Varani, A. M., et al. (2016). Unravelling potential virulence factor candidates in Xanthomonas citri subsp. citri by secretome analysis. PeerJ 4:e1734. doi: 10.7717/peerj.1734
Ficarra, F. A., Grandellis, C., Galván, E. M., Ielpi, L., Feil, R., Lunn, J. E., et al. (2017). Xanthomonas citri ssp. citri requires the outer membrane porin OprB for maximal virulence and biofilm formation. Mol. Plant Pathol. 18, 720–733. doi: 10.1111/mpp.12433
Gicharu, G. K., Sun, D. L., Hu, X., Fan, X. J., Zhuo, T., Wu, C. W., et al. (2016). The sigma 54 genes rpoN1 and rpoN2 of Xanthomonas citri subsp. citri play different roles in virulence, nutrient utilization and cell motility. J. Integr. Agric. 15, 2032–2039. doi: 10.1016/S2095-3119(15)61317-X
Goodin, M. M., Dietzgen, R. G., Schichnes, D., Ruzin, S., and Jackson, A. O. (2002). pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31, 375–383. doi: 10.1046/j.1365-313X.2002.01360.x
Graham, J. H., Gottwald, T. R., Riley, T. D., and Achor, D. (1992). Penetration through leaf stomata and strains of Xanthomonas campestris in citrus cultivars varying in susceptibility to bacterial diseases. Phytopathology 82, 1319–1325. doi: 10.1094/Phyto-82-1319
Guo, Y. P., Figueiredo, F., Jones, J., and Wang, N. (2011). HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol. Plant Microbe Interact. 24, 649–661. doi: 10.1094/MPMI-09-10-0209
Hõrak, R., Ilves, H., Pruunsild, P., Kuljus, M., and Kivisaar, M. (2004). The colR colS two-component signal transduction system is involved in regulation of Tn4652 transposition in Pseudomonas putida under starvation conditions. Mol. Microbiol. 54, 795–807. doi: 10.1111/j.1365-2958.2004.04311.x
Hu, N., and Zhao, B. (2007). Key genes involved in heavy-metal resistance in Pseudomonas putida CD2. FEMS Microbiol. Lett. 267, 17–22. doi: 10.1111/j.1574-6968.2006.00505.x
Kar, U. K., Simonian, M., and Whitelegge, J. P. (2017). Integral membrane proteins: bottom-up, top-down and structural proteomics. Expert Rev. Proteomics 14, 715–723. doi: 10.1080/14789450.2017.1359545
Kivistik, P. A., Putrins, M., Püvi, K., Ilves, H., Kivisaar, M., and Hõrak, R. (2006). The ColRS two-component system regulates membrane functions and protects Pseudomonas putida against phenol. J. Bacteriol. 188, 8109–8117. doi: 10.1128/JB.01262-06
Li, Y. R., Che, Y. Z., Zou, H. S., Cui, Y. P., Guo, W., Zou, L. F., et al. (2011a). Hpa2 required by HrpF to translocate Xanthomonas oryzae transcriptional activator-like effectors into rice for pathogenicity. Appl. Environ. Microbiol. 77, 3809–3818. doi: 10.1128/AEM.02849-10
Li, Y. R., Zou, H. S., Che, Y. Z., Cui, Y. P., Guo, W., Zou, L. F., et al. (2011b). A novel regulatory role of HrpD6 in regulating hrp-hrc-hpa genes in Xanthomonas oryzae pv. oryzicola. Mol. Plant Microbe Interact. 24, 1086–1101. doi: 10.1094/MPMI-09-10-0205
Mills, S. D., Jasalavich, C. A., and Cooksey, D. A. (1993). A two-component regulatory system required for copper-inducible expression of the copper resistance operon of Pseudomonas syringae. J. Bacteriol. 175, 1656–1664. doi: 10.1128/jb.175.6.1656-1664.1993
Mumm, K., Ainsaar, K., Kasvandik, S., Tenson, T., and Hõrak, R. (2016). Responses of Pseudomonas putida to zinc excess determined at the proteome level: pathways dependent and independent of ColRS. J. Proteome Res. 15, 4349–4368. doi: 10.1021/acs.jproteome.6b00420
Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G., and Pühler, A. (1994). Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145, 69–73. doi: 10.1016/0378-1119(94)90324-7
Song, X., Guo, J., Ma, W. X., Ji, Z. Y., Zou, L. F., Chen, G. Y., et al. (2015). Identification of seven novel virulence genes from Xanthomonas citri subsp. citri by Tn5-based random mutagenesis. J. Microbiol. 53, 330–336. doi: 10.1007/s12275-015-4589-3
Subramoni, S., Pandey, A., Vishnu Priya, M. R., Patel, H. K., and Sonti, R. V. (2012). The ColRS system of Xanthomonas oryzae pv. oryzae is required for virulence and growth in iron-limiting conditions. Mol. Plant Pathol. 13, 690–703. doi: 10.1111/j.1364-3703.2011.00777.x
Tampakaki, A. P., Fadouloglou, V. E., Gazi, A. D., Panopoulos, N. J., and Kokkinidis, M. (2004). Conserved features of type III secretion. Cell. Microbiol. 6, 805–816. doi: 10.1111/j.1462-5822.2004.00432.x
Teixeira, E. C., Franco de Oliveira, J. C., Marques Novo, M. T., and Bertolini, M. C. (2008). The copper resistance operon copAB from Xanthomonas axonopodis pathovar citri: gene inactivation results in copper sensitivity. Microbiology 154, 402–412. doi: 10.1099/mic.0.2007/013821-0
Vauterin, L., Hoste, B., Kersters, K., and Swings, J. (1995). Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 45, 472–489. doi: 10.1099/00207713-45-3-472
Vinothkumar, K. R., and Henderson, R. (2010). Structures of membrane proteins. Q. Rev. Biophys. 43, 65–158. doi: 10.1017/S0033583510000041
Wallin, E., and von Heijne, G. (1998). Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 7, 1029–1038. doi: 10.1002/pro.5560070420
Yan, Q., and Wang, N. (2011). The ColR/ColS two-component system plays multiple roles in the pathogenicity of the citrus canker pathogen Xanthomonas citri subsp. citri. J. Bacteriol. 193, 1590–1599. doi: 10.1128/JB.01415-10
Yan, Q., and Wang, N. (2012). High-throughput screening and analysis of genes of Xanthomonas citri subsp. citri involved in citrus canker symptom development. Mol. Plant Microbe Interact. 25, 69–84. doi: 10.1094/MPMI-05-11-0121
Zhang, S. S., He, Y. Q., Xu, L. M., Chen, B. W., Jiang, B. L., Liao, J., et al. (2008). A putative colR(XC1049)-colS(XC1050) two-component signal transduction system in Xanthomonas campestris positively regulates hrpC and hrpE operons and is involved in virulence, the hypersensitive response and tolerance to various stresses. Res. Microbiol. 159, 569–578. doi: 10.1016/j.resmic.2008.06.010
Zhou, X. F., Hu, X. F., Li, J. Y., and Wang, N. (2015). A novel periplasmic protein, VrpA, contributes to efficient protein secretion by the type III secretion system in Xanthomonas spp. Mol. Plant Microbe Interact. 28, 143–153. doi: 10.1094/MPMI-10-14-0309-R
Zubrzycki, H. M., and Diamante, D. Z. A. (1987). “Relationship between the amount of the inoculum and the infection caused by Xanthomonas campestris pv. citri on citrus seedlings through natural infections in the field,” in Proceedings of the International Society of Citriculture, São Paulo, 379–382.
Keywords: Xanthomonas citri subsp. citri, XAC1347, membrane protein, hrp gene, regulation
Citation: Fan X, Guo J, Zhou Y, Zhuo T, Hu X and Zou H (2018) The ColRS-Regulated Membrane Protein Gene XAC1347 Is Involved in Copper Homeostasis and hrp Gene Expression in Xanthomonas citri subsp. citri. Front. Microbiol. 9:1171. doi: 10.3389/fmicb.2018.01171
Received: 06 November 2017; Accepted: 15 May 2018;
Published: 11 June 2018.
Edited by:
Brigitte Mauch-Mani, University of Neuchâtel, SwitzerlandReviewed by:
Chenglong Liu, Texas A&M University, United StatesJeffrey Jones, University of Florida, United States
Copyright © 2018 Fan, Guo, Zhou, Zhuo, Hu and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huasong Zou, aHN6b3VAZmFmdS5lZHUuY24=
†These authors have contributed equally to this work.
 Xiaojing Fan
Xiaojing Fan Jing Guo2†
Jing Guo2† Yinghui Zhou
Yinghui Zhou Huasong Zou
Huasong Zou