- 1College of Animal Science and Technology, Shandong Agricultural University, Tai’an, China
- 2Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Shandong Agricultural University, Tai’an, China
- 3Shandong Provincial Engineering Technology Research Center of Animal Disease Control and Prevention, Shandong Agricultural University, Tai’an, China
Duck Tembusu virus (DTMUV) is an emerging pathogenic flavivirus that has resulted in large economic losses to the duck-rearing industry in China since 2010. Therefore, an effective diagnostic approach to monitor the spread of DTMUV is necessary. Here, a novel diagnostic immunochromatographic strip (ICS) assay was developed to detect DTMUV. The assay was carried out using colloidal gold coated with purified monoclonal antibody A12D3 against envelope E protein. Purified polyclonal C12D1 antibodies from BALB/c mice against the envelope E protein were used as the capture antibody. Goat anti-mouse IgG was used to detect DTMUV, which was also assembled on the ICS. Results showed that the ICS could specifically detect DTMUV within 10 min. It also could be stored 25 and 4°C for 4 and 6 months, respectively. The sensitivity of the ICS indicated that the dilution multiples of positive allantoic fluid of DTMUV (LD50: 104.33/0.2 ml) was up to 200. Its specificity and sensibility showed no significant change under the above storage situations. Fifty clinical samples were simultaneously detected by ICS and reverse-transcription polymerase chain reaction with a 93.9% coincidence rate between them. It proved that the ICS in the present study was highly specific, sensitive, repeatable, and more convenient to rapidly detect DTMUV in clinical samples.
Introduction
Like other flaviviruses, Duck Tembusu virus (DTMUV) from the Flaviviridae family has a 10,990-nt positive-sense single-stranded RNA genome and belongs to the Ntaya antigenic group. The virus was initially isolated from Culex tritaeniorhynchus mosquitoes in Malaysia in 1957 and subsequently detected in Thailand and numerous Southeastern Asian areas (Homonnay et al., 2014; Chakritbudsabong et al., 2015; Thontiravong et al., 2015). In China, it was originally detected in Shanghai in April 2010 and then spread rapidly throughout most duck-producing provinces such as Guangdong, Fujian, Jiangsu, Anhui, Henan, and Shandong (Su et al., 2011; Tang et al., 2013). This virus can cause retarded growth, high fever, loss of appetite, severe duck-drop syndrome, and death of the ducks (Yan et al., 2011). Over 10 million ducks have been infected, and more than 1 million ducks died in 2010, which caused massive economic losses in duck-producing industries (Li et al., 2012; He et al., 2017). Moreover, DTMUV poses a potential threat to public health, and it is no longer detectable after the infected fowls recover (Li et al., 2012; Liu et al., 2013; Tang et al., 2013). Therefore, a rapid detection method is necessary to monitor viral occurrence and prevalence.
The colloidal-gold immunochromatography strip (ICS) assay has been widely used for detection of as bacteria (Kong et al., 2017), viruses (Kim et al., 2015; Deng et al., 2017), parasites (Wang et al., 2014), hormones (Nara et al., 2010), and antibodies (Li et al., 2017). ICS uses percolation of the microporous membrane and capillarity, which provides a quick antigen–antibody reaction on the immobilon-P, and then colors for colloidal gold-labeled antibody with a positive reaction appear red. The ICS is suitable for a basic clinical application, which takes a short time and does not require special equipment (Kusano et al., 2014).
Immunochromatographic strip also has been generally applied to detect pathogens in fowls, including the Newcastle disease virus (NDV) (Gao et al., 2004), goose plague virus (Li et al., 2007), avian influenza virus (AIV) (Peng et al., 2008), goose parvovirus (Shaozhou et al., 2015), duck plague virus (DPV) (Zhao et al., 2016), but less applied to DTMUV. Therefore, to satisfy above requirements, an ICS based for E protein was prepared to detect DTMUV from clinical specimens. This study laid the foundation for the further development of a commercial kit.
Materials and Methods
Reference Virus Strains and Antibodies
Avian reovirus virus (ARV), H9N2 AIV, DTMUV, DPV, Duck hepatitis A virus type-1 (DHAV-1), egg drop syndrome virus (EDSV), Muscovy duck parovirus (MPV), and NDV reference strains were obtained from our laboratory, the Poultry Disease Lab of Shandong Agricultural University, and used to test the specificity of ICS. The monoclonal antibody, A12D3, against the envelope E protein was produced in our previous study (Ou, 2013), while the polyclonal antibody C12D1 against the envelope E protein was prepared according to the following procedures (Table 1). The specificity and sensitivity of above two antibodies were also detected using an indirect ELISA (Supplementary Figures 1, 2; Bai et al., 2015; Lin, 2015). The goat anti-mouse IgG antibody (1 mg/ml) was purchased from Beijing Dingguo Changsheng Biotechnology Co. Ltd., China.
Preparation of C12D1
In this study, the polyclonal antibody for C12D1 was prepared by injecting purified DTMUV-E protein into 6 week-BALB/c female mice (purchased from the New Drug Evaluation Center of Shandong University, China) subcutaneously or intraperitoneally following the immunization procedures in Table 1. After 1 week, 100 μg of the antigen, E protein, with Freund’s incomplete or complete adjuvant (Becton Dickinson, Franklin Lakes, NJ, United States) was injected intraperitoneally.
After three immunizations per week, the tails were cut on the mice to collect blood samples. E protein (200 ng/well) was used to coat enzyme-labeled plates, and the serum titer was measured as an indirect ELISA (Bai et al., 2015). Mice with a serum titer up to 1:5000 were chosen for the fourth immunization. Blood samples from these mice were collected retro-orbitally in sterilized tubes the third day after the fourth immunization. After leaving the samples undisturbed for 30 min at 37°C, the supernatant was collected and centrifuged at 3,000 rpm for 10 min. The resultant supernatants were used as the crude extracts of C12D1. C12D1 was purified using saturated ammonium sulfate [(NH4)2SO4], and the concentration of C12D1 was determined using an ultraviolet spectrophotometer (Eppendorf BioSpectrometer®, Germany) based on the following formula: concentration of protein (mg/ml) = 1.45 × OD280 - 0.74 × OD260.
Preparation of the Colloidal Gold Solution
In this study, we prepared colloidal gold solution with an average particle diameter of 20 nm using a tri-sodium citrate reduction method (Liu et al., 2012; Zhao et al., 2016). Before the preparation of the colloidal gold, all glassware used were siliconized with sigmacote, cleaned with aqua regia [HNO3/HCL (v/3v)], washed with ultrapure water, and oven-dried (Meng et al., 2014). Subsequently, 1.0 ml of 1% HAuCl4 (Aladdin, Shanghai, China) solution was added to 99-ml ultrapure water and boiled thoroughly for 3 min. Then, 2.0 ml of 1% trisodium citrate (Aladdin, Shanghai, China) solution was dropped into the boiled solution, stirred, and heated continuously. When the color of the solution changed from black–blue into red–purple, it was heated for an additional 15 min. Then supplemented ultrapure water was added to the solution until it reached the original volume. Finally, the OD520 value of the colloidal gold solution at 500–600 nm was detected by an ultraviolet spectrophotometer (Eppendorf BioSpectrometer®, Germany), and the prepared colloidal gold solution was available only when the OD520 value was between 0.8 and 1.2 (Wang et al., 2004).
Optimum pH of the Colloidal Gold Solution
One milliliter of colloidal gold solution was added to eight EP tubes (1.5 ml) and tested with special pH indicator paper, respectively. The pH values were debugged for 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5, and 10.0 with 0.2 mol/l K2CO3. After that, 10 μl of A12D3 (1.6 mg/ml) was added into each of the tubes with gentle stirring for 10 min, and then left static for another 10 min at room temperature. 100 μl of NaCl (10%) was added to the above tubes with gentle stirring and left static for 2 h at room temperature. The OD520 values were measured using an ultraviolet spectrophotometer at 500–600 nm (Liu et al., 2012; Guo et al., 2017). Finally, the optimum pH of the colloidal gold was analyzed by diagraph.
Optimum A12D3-Labeled Dose of the Colloidal Gold
One milliliter of optimum pH of colloidal gold solution was added into nine EP tubes, respectively. Then 0–8 μl of A12D3 was added (1.6 mg/ml) into each of the above tubes. After remaining static for 15 min, 100 μl of NaCl (10%) was added to the tubes. Next, after gentle stirring, and then being static for 2 h, the OD520 value was measured using an ultraviolet spectrophotometer at 500–600 nm. Finally, diagraph analysis was carried out on the optimum A12D3-labeled dose of the colloidal gold. The stable dose of A12D3 was 1.1–1.2 times the optimum-labeled dose (Hampl et al., 2001).
Preparation of the Colloidal Gold–A12D3 Conjugate
One milliliter of purified A12D3 (1.6 mg/ml) was added into 50 ml of colloidal gold solution (pH 8.5, slightly more by 0.5 than the optimum pH of colloidal gold solution) with gentle stirring for 1 h. Then, 2.0 ml of 10% of bovine serum albumin (BSA; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was added to the solution with gentle stirring for 5 min. Next, 1.0 ml of 10% of PEG2000 (Sigma, United States) was added with gentle stirring for another 5 min. It was centrifuged at 12,000 rpm for 30 min. After centrifugation, the sediment was suspended in 5.0 ml dilution buffer (0.002 mol/l sodium carbonate solution containing 1% BSA and 0.2% sodium azide) and stored at 4°C.
Preparation of Conjugate Pad
In this study, the colloidal gold–A12D3 conjugate was diluted with 0.02 M PBS containing 1.0% (w/v) BSA and 5.0% (w/v) sucrose at a pH of 8.5. The glass fiber (GL0194; Shanghai JieYi Biotechnology Co. Ltd., China) was incubated with 0.1% Tween-20 for 10 h and dried at 37°C for 2 h. Then, the above colloidal gold–A12D3 conjugate (7.5 μl/cm) was added to the conjugate pad and dried at 37°C for 1 h. It was stored at 4°C for future use.
Preparation of the Nitrocellulose Membrane
The diluted C12D1 and goat anti-mouse IgG were transferred onto the NC membrane (Millipore, United States) at 1 μl/cm to form the test and the control lines, respectively. The test line was 6 mm apart from the control line. Subsequently, the NC membrane was incubated with blocking buffer containing 1.0% BSA at 37°C for 1 h and then washed two times with wash buffer. The NC membrane was then dried for 2 h at 37°C and stored at 4°C for future use.
Assembly of the ICS
The structure component of ICS is shown in Figure 1A. A12D3, C12D1, and goat anti-mouse IgG were coated on the conjugated pad, test line, and control line, respectively. The sample pad, conjugate pad, NC membrane, and absorbent pad were then assembled on the PVC pad (Jiang et al., 2011). Five millimeters of wide strips were cut and then installed in the test card (Liu et al., 2012). Finally, the ICS was stored with desiccant in a sealed bag at 4°C for future use.
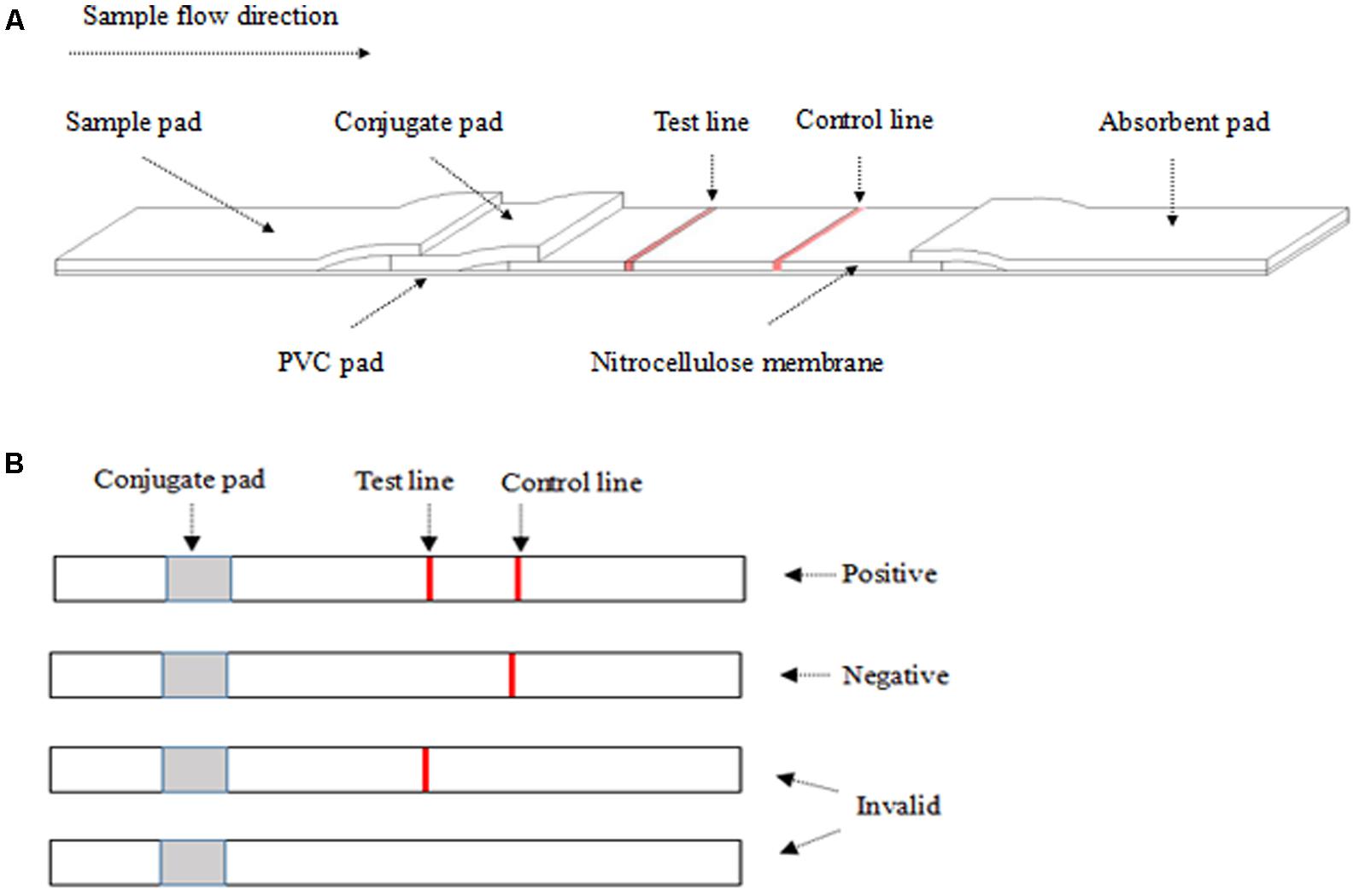
FIGURE 1. The structure of the colloidal gold test strip (A) and the result from ICS (B). (A) The liquid sample was added to the sample pad. The test line (polyclonal antibody C12D1) and control line (goat anti-mouse IgG antibody) were examined visually. (B) The appearance of two lines indicates a positive result, whereas a valid negative test produces only the control line.
ICS Result
After dropping 100–200 μl of the sample on the sample pad for 10 min at room temperature, a positive result was determined by the appearance of two red lines in the test and the control regions. A negative result was only one red line in the control region. An invalid result was one red line in the test region or no red line on NC membrane (Figure 1B).
Evaluation of the ICS Performance
In this study, to evaluate the performance of ICS objectively, we verified the specificity, sensibility, repeatability, stability, and practicability of prepared ICS, and the details were as follows.
To confirm the specificity of ICS, the positive allantoic fluid samples of DTMUV, NDV, DPV, H9N2 AIV, and ARV were detected at the same time, which were obtained from above virally infected eggs.
For its sensibility, we first diluted the positive allantoic fluid of DTMUV (median lethal dose, LD50 = 10-4.33/0.2 ml) in multiples dilution of 10, 50, 100, 200, and 500, and then added the same dose dilution on five ICSs repeated five times.
For the repeatability, we detected the same DTMUV positive allantoic fluid (median tissue culture infective dose, TCID50 = 101.69/0.2 ml) with five batches of ICSs (i.e., the ICSs were assembled from the same components at five different stages).
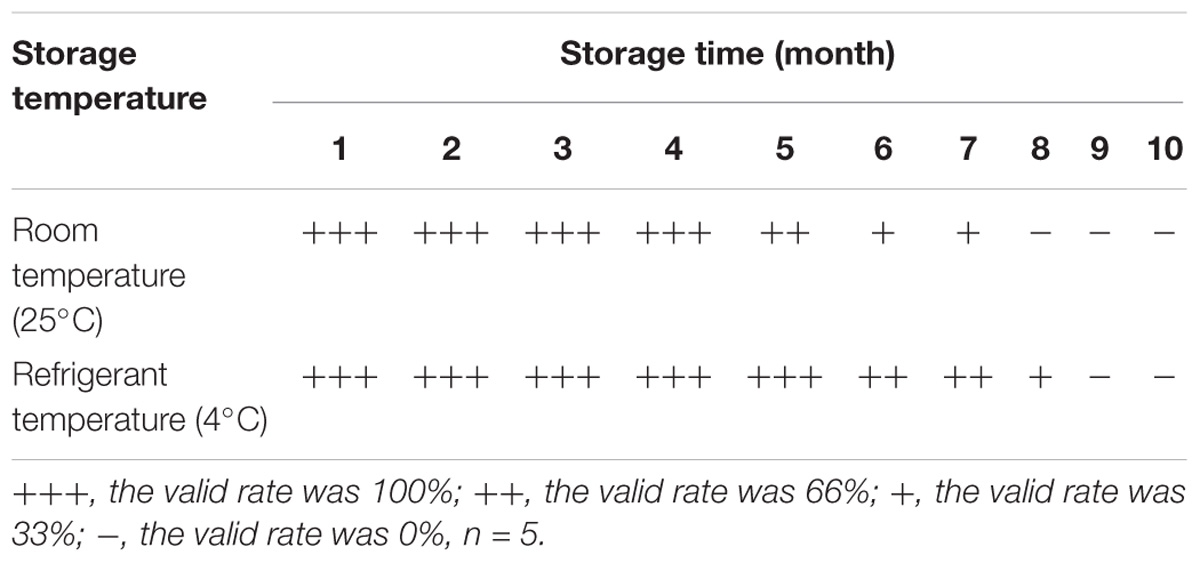
For the stability, we stored the ICS for 10 months at room temperature (25°C) and at 4°C, respectively. During the storage period, we detected the same DTMUV positive allantoic fluid (TCID50 = 101.69/0.2 ml) by ICS monthly.
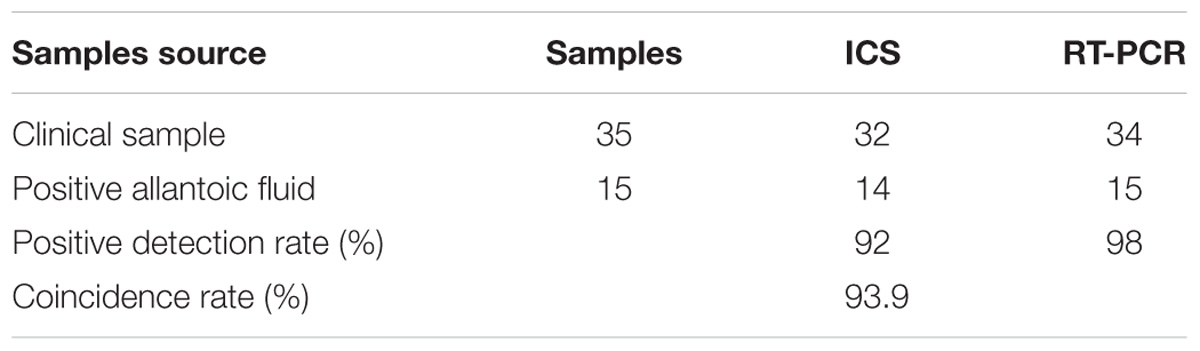
For the coincidence rate of ICS and reverse-transcription polymerase chain reaction (RT-PCR) (Sun et al., 2014; Zhang et al., 2015a; Siu et al., 2016; Zeng et al., 2016), a total of 50 clinical samples (i.e., 35 clinical samples and 15 positive allantoic fluid samples) collected in the field from different duck farms were tested by the ICS. Before detection, the samples were homogenized with an electrical tissue homogenizer (ARTMICCRA D-9, Germany) in PBS containing antibiotics (20% w/v). The sample RNA was extracted with Trizol reagent (TransGen Biotech, Beijing, China) according to the manufacturer’s instruction. The extracted RNA was then used for RT-PCR to evaluate the coincidence rate of the ICS. After the experiment, all of the clinical or viral samples were incinerated.
Results
Preparation of C12D1
In this study, the polyclonal antibody for C12D1 was prepared using the immune DTMUV-E protein method. After purification by saturated ammonium sulfate [(NH4)2SO4], the concentration of C12D1 was 1.2 mg/ml.
Optimization of ICS
From Figure 2, the maximum absorption wavelength of the colloidal gold solution was 520 nm. According to the following regression equation, Y = 0.786X + 505.53 (Y, maximum absorption wavelength; X, particle diameter) (He et al., 2005), the particle diameter of the prepared colloidal gold solution was 18.4 nm. Both parameters satisfied the requirements for colloidal gold preparation (Safenkova et al., 2010; Liu et al., 2012).
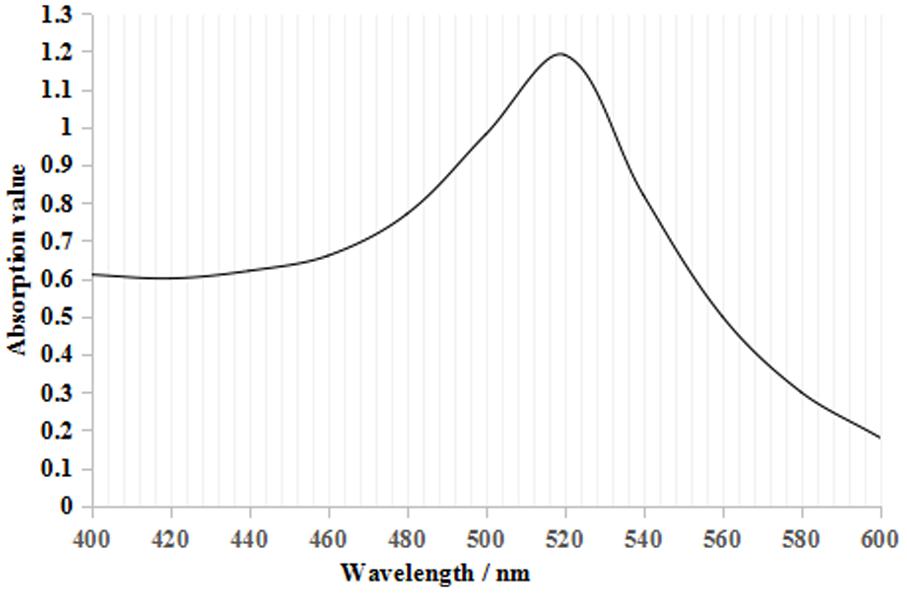
FIGURE 2. Colloidal gold UV–visible scanning curve. There was only one maximum absorption value at 520 nm, which is the characteristic absorbance peak for colloidal gold.
For the optimum pH and labeled dose of A12D3, we measured the peak values of the solution at the OD520 under different pH conditions and labeled doses of A12D3 using an ultraviolet spectrophotometer (Paek et al., 2000; Nara et al., 2010). The lower the OD520, the more dispersed the colloidal gold particle was and the more stable the colloidal gold solution was. The optimum pH was 8 (Figure 3A), and the optimum of labeled dose of A12D3 was 48 μg (Figure 3B).
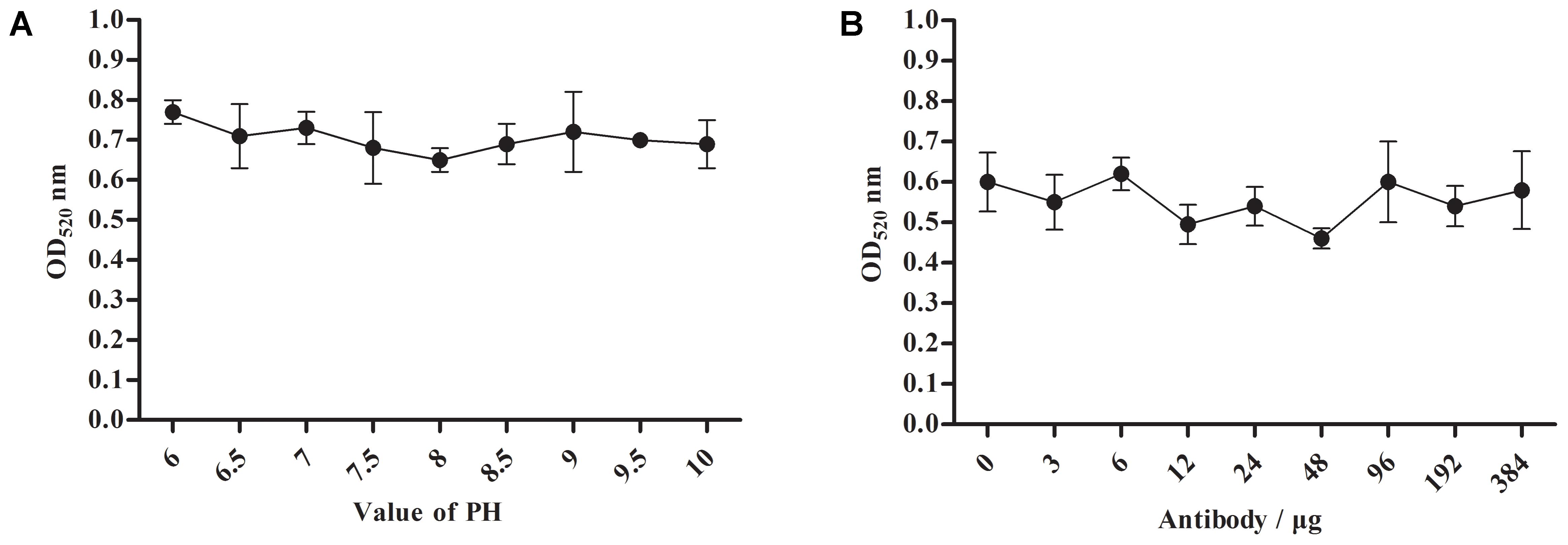
FIGURE 3. Optimum pH selection for colloidal gold solution (A) and a labeled dose selection for A12D3 (B). The optimal pH was 8 and the optimal labeled dose was 48 μg. The error bars correspond to the standard deviations form the data points (n = 5).
Specificity
To determine the specificity of ICS, we tested four usual poultry virus allantoic fluids (i.e., NDV, DPV, H9N2-AIV, and ARV). The results showed that the ICS only reacted to the positive allantoic fluid samples of DTMUV, and had no cross reaction with NDV, DPV, H9N2-AIV, or ARV (Figure 4).
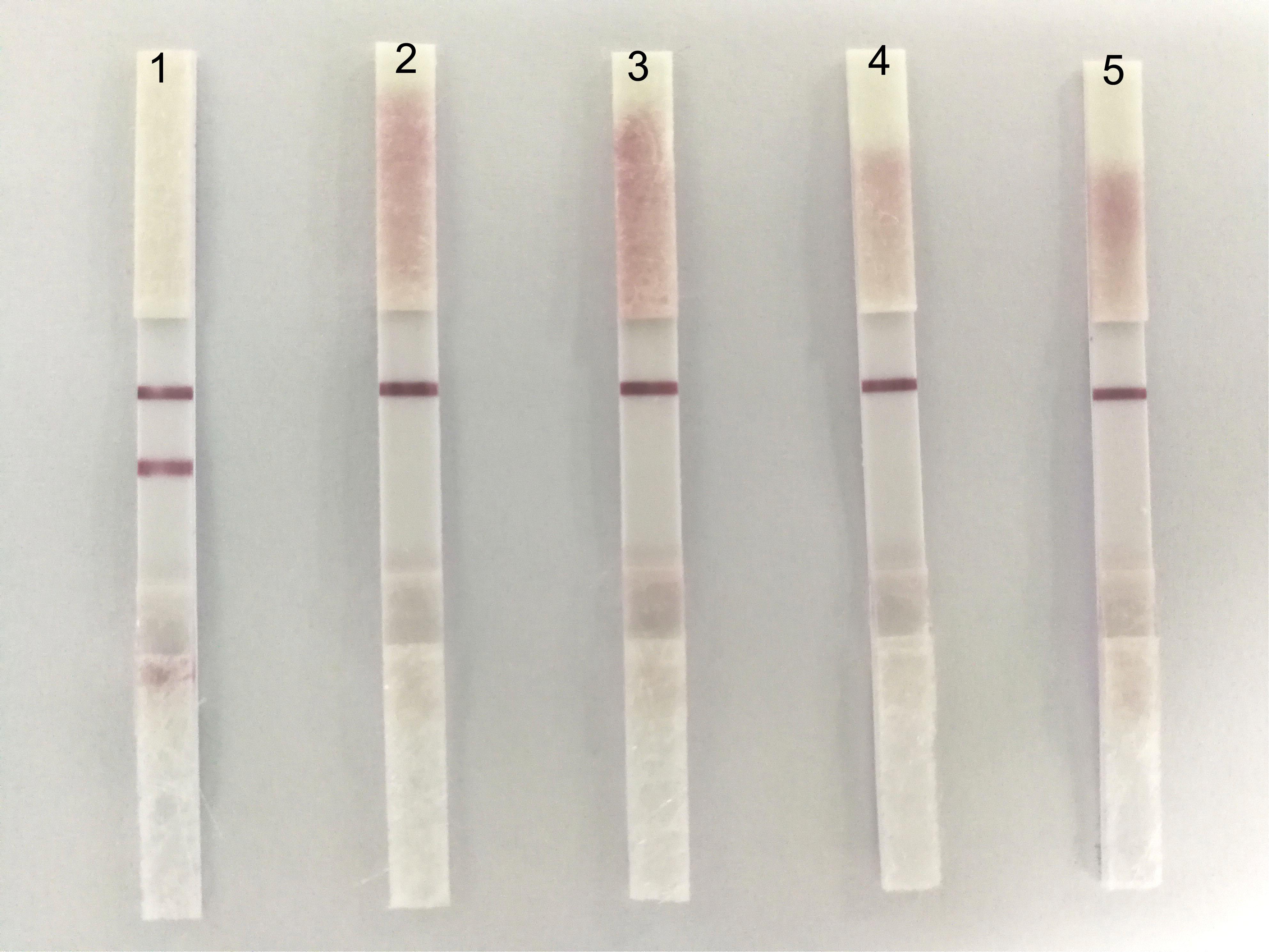
FIGURE 4. The specificity of the ICS. 1: DTMUV, 2: NDV, 3: DPV, 4: H9N2-AIV, and 5: ARV. ICS only reacted with DTMUV, and there was no cross reaction with NDV, DPV, H9N2-AIV, or ARV.
Sensibility
With regard to the sensibility of ICS, the positive allantoic fluid of DTMUV (LD50:10-4.33/0.2 ml) was diluted in 10, 50, 100, 200, and 500 dilution multiples, respectively. The results showed that the ICS could detect highly diluted allantoic fluid from DTMUV (LD50: 104.33/0.2 ml) at 1:200 (Figure 5).
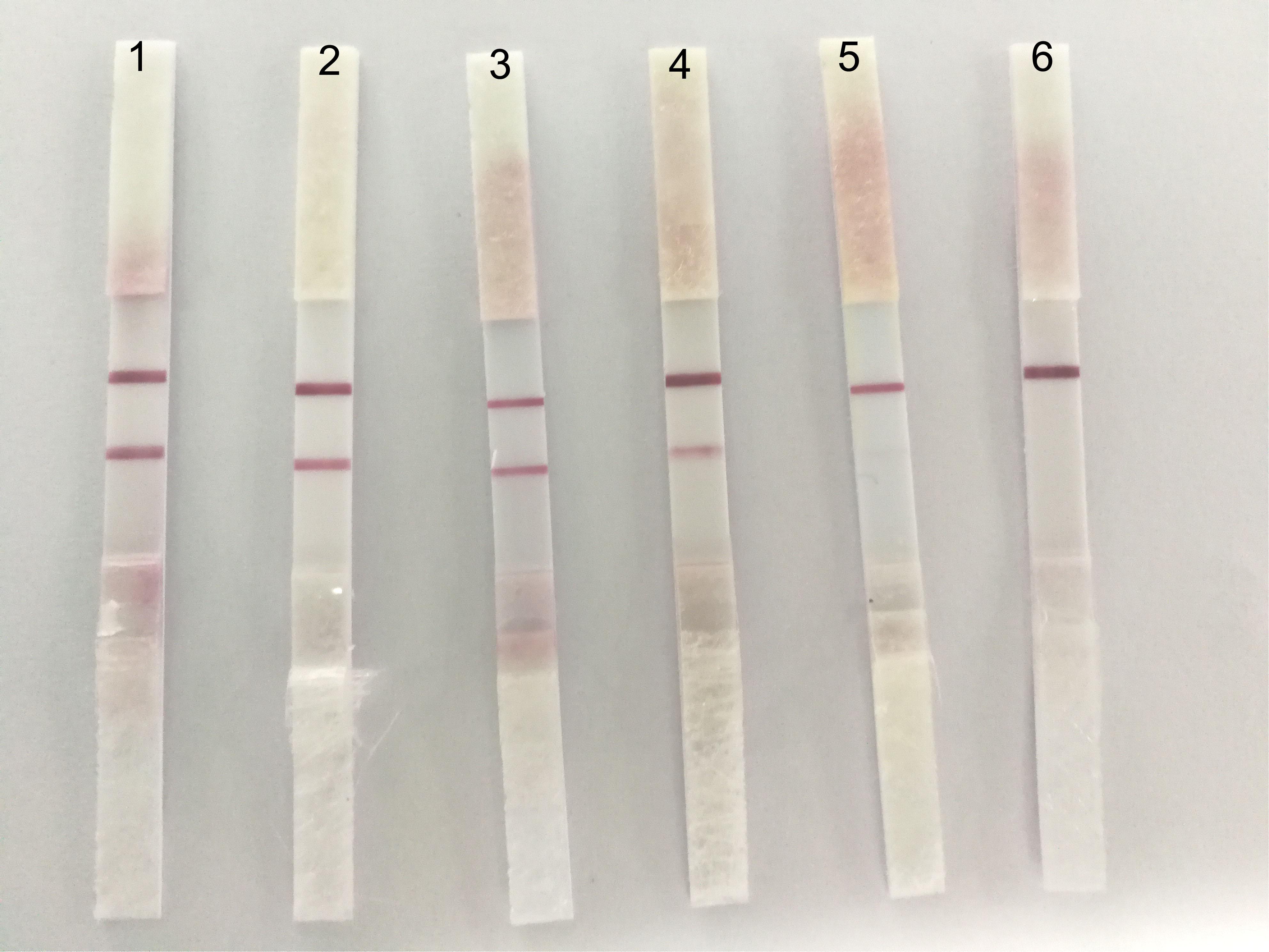
FIGURE 5. The sensibility of the ICS. The dilution multiples were prepared as follows; 1: 1/10, 2: 1/50, 3: 1/100, 4: 1/200, 5: 1/500, and 6: PBS. A fainter test line appeared at 1:200.
Repeatability
To evaluate the repeatability of ICS, we detected the same strain positive allantoic fluid of DTMUV with different batches of ICS, and the results showed that there were no obvious differences among the five batches of ICS (Figure 6).
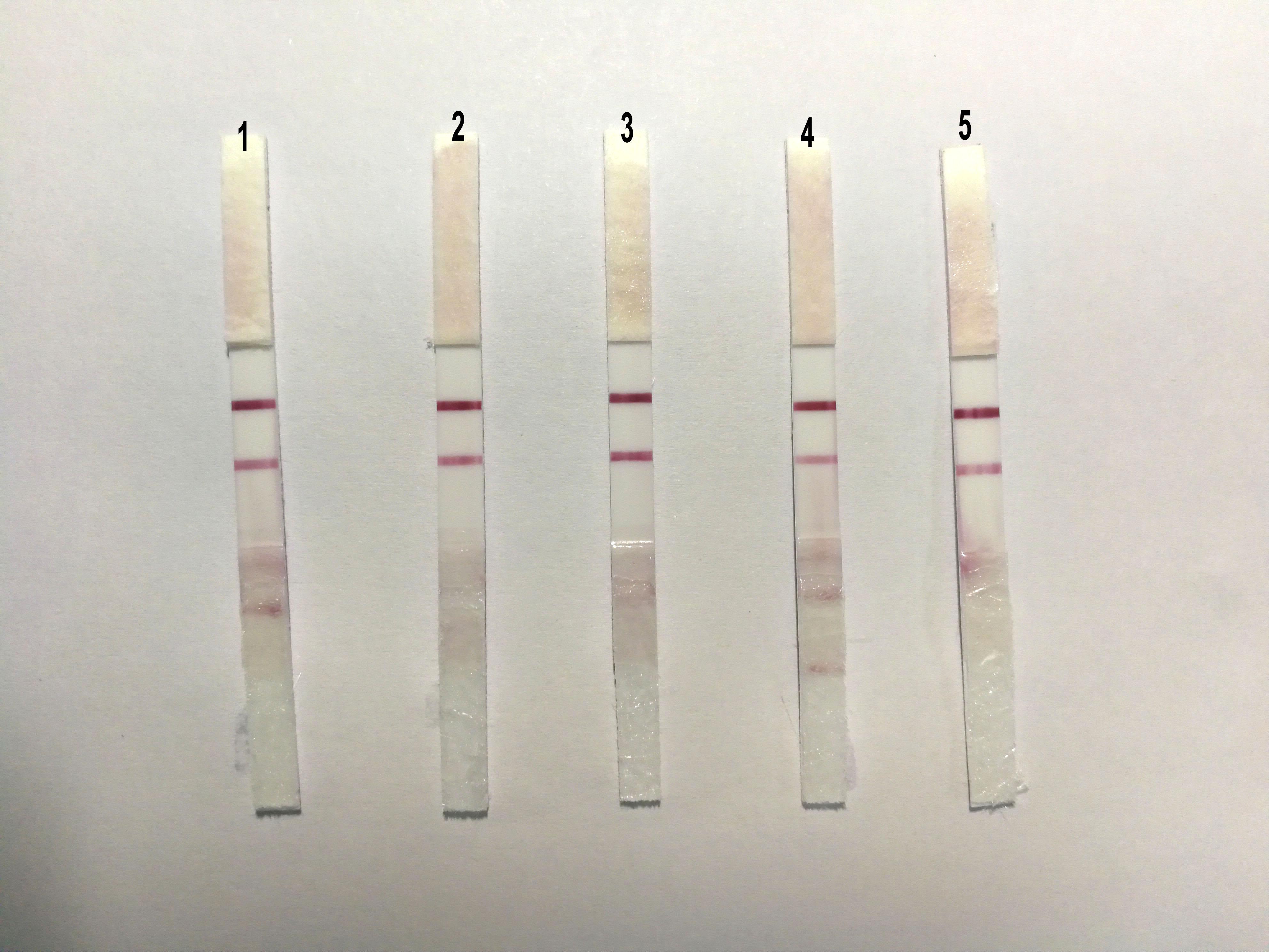
FIGURE 6. The sensibility of the ICS. The same strain positive allantoic fluid of DTMUV was detected by five different batches of ICS, and there were no obvious differences among the five batches of ICS.
Stability
The persistent stability of the ICS is an important consideration for field application. The stability of the antibodies (i.e., A12D3 and C12D1) immobilized on the ICS was tested by storing the assay at 4 and 25°C for 1–10 months. From Table 2, the ICS could be stored for 4 months at 25°C and for 6 months at 4°C.
Practicability
Fifty clinical samples (i.e., 35 clinical samples and 15 positive allantoic fluid samples) were tested in parallel using the ICS and RT-PCR. The results showed that the positive detection rate of both detection methods was 92 [(32+14)/50] and 98% [(34+15)/50], respectively. The coincidence rate of these two methods was 93.9% [(32+14)/(34+15)] (Table 3).
Discussion
Duck Tembusu virus is a contagious pathogen from fowl that includes ducks and geese with symptoms of high fever, loss of appetite, retarded growth, neurological signs, and severe duck-drop syndrome (Cao et al., 2011). To date, DTMUV has caused an enormous loss to many egg-laying and breeder duck farms in China (Yan et al., 2011). Thus, an efficient method to detect DTMUV is one of the important measures to monitor the spread of this viral contagious disease.
Current approaches for clinical diagnosis of DTMUV infection are mainly classified into three categories: virus isolation, a PCR-based assay, and an immunological method. Examples include virus isolation (Yan et al., 2011), RT-PCR (Cao et al., 2011), real-time PCR (Yun et al., 2012), semi-nested PCR (Tang et al., 2015), loop-mediated isothermal amplification (Tang et al., 2012), blocked ELISA (Li et al., 2012), competition ELISA (Fu et al., 2015), and indirect immunofluorescent assay (IFA) (Wu et al., 2016). The above diagnostic methods have their advantages and disadvantages, regarding sensitivity, accuracy, operability, and cost, and it takes to do them. For example, ELISAs are commonly used for filed detection due to its high sensitivity and specificity (Chen et al., 2014; Wang et al., 2014; Lin, 2015), but it usually requires expensive reagents, special equipment, well-trained staff, several hours, and only be carried out in laboratories. All of these detection methods are inconvenient and not easy enough for field diagnoses or small hospitals. Therefore, to solve these issues, we prepared a rapid and simple diagnostic technique for DTMUV the Colloid Gold Immunochromatographic Assay, which is cheap, easy to prepare, and does not skilled staff or specialized equipment.
In this study, the developed ICS was based on antibodies of E protein of this virus. E protein, as a major envelop protein of flavivirus, has multi-antigenic determinants and plays an important role in mediating the host-related immune response and producing neutralizing antibodies (Allison et al., 2001; Chen et al., 2005, 2014; Mukhopadhyay et al., 2005; Pierson et al., 2008; Chavez et al., 2010; Han et al., 2016). Therefore, we utilized the purified monoclonal antibody A12D3 against the envelop E protein as a gold conjugate antibody, while the preparation of purified polyclonal antibody C12D1 from BALB/c mice against the envelope E protein served as the capture antibody. The specificity and sensibility results showed that the ICS only reacted to DTMUV within 10 min and the diluted test samples were higher as well. Thus, it can be seen that the ICS showed high specificity and sensibility.
The low number of test steps in assay minimizes the risk of contamination, thereby allowing better understanding of outbreaks of the infectious disease (Kim et al., 2007; Nakayama et al., 2014). The rapid and accurate detection is crucial to help determine whether to initiate treatment, and it will also reduce economic losses in the animal industry (Kim et al., 2015). In our study, in order to evaluate how practical for detecting DTMUV, we detected 50 samples with ICS and RT-PCR (Zhang et al., 2015b; Siu et al., 2016; Zeng et al., 2016; Zhao et al., 2016). As expected, the results detected by ICS were generated within 10 min, which was significantly shorter than the time taken to do RT-PCR (about 5 h). Importantly, the results generated by ICS can be read directly with the naked eye. The shorter detection time is essential for monitoring the spread of DTMUV. Moreover, the coincidence rate of these two detection methods reached 93.9%, which implies ICS has better accuracy and reliability for the rapid detection of DTMUV. The long-term stability of ICS is also a critical factor for clinical use. As expected, it could be stored for up to 6 months at 4°C. Hence, ICS has an optimal clinical practicability for its convenience and reliability.
Conclusion
The present study provided an ICS for the rapid detection of DTMUV. The ICS demonstrated the advantages of high specificity and sensibility, good repeatability, convenience, and clinical application. Above all, it can be easily performed by unskilled staff, and the result is visualized with the naked eye within 10 min. In the future development of viral detection by ICS, quantitative, or semi-quantitative assay, and a multifunctional strip to detect two or more agents will be the next step.
Ethics Statement
In this study, all experiments were performed according to the guidelines of the Committee on the Ethics of Animals of Shandong and the appropriate biosecurity guidelines. The protocol was approved by Shandong Agricultural University Animal Care and Use Committee (No. SDAUA-2016-099).
Author Contributions
GYu and GYa completed most of the works. XY designed the tables and figures. YT and YD gave experimental instruction. Thank all the authors’ contribution to the study.
Funding
This research was funded by the National Natural Science Foundation of China (31472199); the National Key Research and Development Program of China (2016YFD0500107); the China Agriculture Research System (CARS-42-19); Science and Technology Development Plan of Shandong Province (2014GNC111023); and Funds of Shandong “Double Tops” Program.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01001/full#supplementary-material
References
Allison, S. L., Schalich, J., Stiasny, K., Mandl, C. W., and Heinz, F. X. (2001). Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. J. Virol. 75, 4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001
Bai, X. F., Shaozhou, W. L., Zhang, Q. S., Li, C. X., Qiu, N., Meng, R. Z., et al. (2015). Characterization of monoclonal antibodies against duck Tembusu virus E protein: an antigen-capture ELISA for the detection of Tembusu virus infection. Arch. Virol. 160, 757–764. doi: 10.1007/s00705-014-2312-z
Cao, Z., Zhang, C., Liu, Y., Liu, Y., Ye, W., Han, J., et al. (2011). Tembusu virus in ducks, China. Emerg. Infect. Dis. 17, 1873–1875. doi: 10.3201/eid1710.101890
Chakritbudsabong, W., Taowan, J., Lertwatcharasarakul, P., Phattanakunanan, S., Munkhong, A., Songserm, T., et al. (2015). Genomic characterization of a new Tembusu flavivirus from domestic ducks in Thailand. Thai J. Vet. Med. 45, 419–425.
Chavez, J. H., Silva, J. R., Amarilla, A. A., and Moraes Figueiredo, L. T. (2010). Domain III peptides from flavivirus envelope protein are useful antigens for serologic diagnosis and targets for immunization. Biologicals. 38, 613–618. doi: 10.1016/j.biologicals.2010.07.004
Chen, C. T., Yi, Y. C., Chiang, S. C., Liu, K. C., Lin, Y. L., and Lee, S. T. (2005). Selection of immunodominant fragments from envelope gene for vaccine against Japanese encephalitis virus in DNA priming-protein boosting protocols. Microb. Pathog. 38, 53–62. doi: 10.1016/j.micpath.2004.12.001
Chen, H., Ou, Q. B., Tang, Y., Gao, X. H., Wu, L. L., Xue, C., et al. (2014). Development and evaluation of a DAS-ELISA for rapid detection of tembusu virus using monoclonal antibodies against the envelope protein. PLoS One 9:e96366. doi: 10.1371/journal.pone.0096366
Deng, J., Liu, Y. H., Jia, R. Y., Wang, M. S., Chen, S., Zhu, D. K., et al. (2017). Development of an immunochromatographic strip for detection of antibodies against duck Tembusu virus. J. Virol. Methods 249, 137–142. doi: 10.1016/j.jviromet.2017.08.022
Fu, Y., Ji, Y., Liu, B., Dafallah, R. M., and Zhu, Q. (2015). Development of a solid-phase competition ELISA to detect antibodies against newly emerged duck Tembusu virus. J. Virol. Methods 224, 73–76. doi: 10.1016/j.jviromet.2015.08.017
Gao, L. M., Tian, F. L., Niu, Z. X., Wang, G. S., and Ding, S. Y. (2004). Development and preliminary application of rapid test strip for newcastle disease. Chin. J. Anim. Quarantine 21, 22–24.
Guo, J. B., Liu, W. P., Chen, H. L., Zhang, M. Y., and Lan, X. Q. (2017). Development and evaluation of a broad-specific immunochromatographic assay for screening of both tadalafil and its analogues in functional foods. Food Agric. Immunol. 28, 652–667. doi: 10.1080/09540105.2017.1309360
Hampl, J., Hall, M., Mufti, N. A., Yao, Y. M., Wright, W. H., and Cooper, D. E. (2001). Upconverting phosphor reporters in immunochromatographic assays. Anal. Biochem. 288, 176–187. doi: 10.1006/abio.2000.4902
Han, K., Zhao, D., Kiu, Y., Liu, Q., Huang, X., Yang, J., et al. (2016). Generation and characterization of a monoclonal antibody against duck Tembusu virus envelope protein. Pol. J. Vet. Sci. 19, 877–883. doi: 10.1515/pjvs-2016-0109
He, X., Xiong, X. D., Liang, J. B., Li, Z. Y., and Zhang, X. (2005). Preparation of colloidal gold used in immunoassay and control of particle size. Chin. J. Rare Met. 29, 471–474. doi: 10.13373/j.cnki.cjrm.2005.04.021
He, Y., Wang, A. Q., Chen, S., Wu, Z., Zhang, J. Y., Wang, M. S., et al. (2017). Differential immune-related gene expression in the spleens of duck Tembusu virus-infected goslings. Vet. Microbiol. 212, 39–47. doi: 10.1016/j.vetmic.2017.08.002
Homonnay, Z. G., Kovacs, E. W., Banyai, K., Albert, M., Feher, E., Mato, T., et al. (2014). Tembusu-like flavivirus (Perak virus) as the cause of neurological disease outbreaks in young Pekin ducks. Avian Pathol. 43, 552–560. doi: 10.1080/03079457.2014.973832
Jiang, T., Liang, Z., Ren, W. W., Chen, J., Zhi, X. Y., Qi, G. Y., et al. (2011). A sample and rapid colloidal gold-based immunochromatographic strip test for detection of FMDV serotype A. Virol. Sin. 26, 30–39. doi: 10.1007/s12250-011-3166-5
Kim, S. H., Kim, I. J., Pyo, H. M., Tark, D. S., Song, J. Y., and Hyun, B. H. (2007). Multiplexreal-time RT-PCR for the simultaneous detection and quantification oftransmissible gastroenteritis virus and porcine epidemic diarrhea virus. J. Virol. Methods 146, 172–177. doi: 10.1016/j.jviromet.2007.06.021
Kim, Y. K., Lim, S. I., Cho, I. S., Cheong, K. M., Lee, E. J., Lee, S. O., et al. (2015). A novel diagnostic approach to detecting porcine epidemic diarrhea virus: the lateral immunochromatography assay. J. Virol. Methods 225, 4–8. doi: 10.1016/j.jviromet.2015.08.024
Kong, M. M., Yang, B., Gong, C. J., Wang, H., Zhao, K. S., Li, J. J., et al. (2017). Development of immunochromatographic colloidal gold test strip for rapid detection of Haemophilus influenzae in clinical specimens. J. Appl. Microbiol. 123, 289–294. doi: 10.1111/jam.13489
Kusano, N., Iwanami, T., Narahara, K., and Tanaka, M. (2014). Production of monoclonal antibodies specific for the recombinantviral coat protein of Apple stem grooving virus-citrus isolate and theirapplication for a simple, rapid diagnosis by an immunochromatographic assay. J. Virol. Methods 195, 86–91. doi: 10.1016/j.jviromet.2013.09.015
Li, C. Y., Zhou, T. Z., Zhang, X. Y., Wang, Y., and Hu, G. X. (2007). Development of gold immunochromatography calorie for detection of goose plague virus. Chin. J. Prev. Vet. Med. 29, 625–628.
Li, H. W., Yang, J. F., Bao, D. K., Hou, J., Zhi, Y. B., Yang, Y. Y., et al. (2017). Development of an immunochromatographic strip for detection of antibodies against porcine reproductive and respiratory syndrome virus. J. Vet. Sci. 18, 307–316. doi: 10.4142/jvs.2017.18.3.307
Li, X., Li, G., and Teng, Q. (2012). Development of a blocking ELISA for detection of serum neutralizing antibodies against newly emerged duck Tembusu virus. PLoS One 7:e53026. doi: 10.1371/journal.pone.0053026
Liu, X., Xiang, J. J., Tang, Y., Zhang, X. L., Fu, Q. Q., Zou, J. H., et al. (2012). Colloidal gold nanoparticle probe-based immunochromatographic assay for the rapid detection of chromium ions in water and serum samples. Anal. Chim. Acta 745, 99–105. doi: 10.1016/j.aca.2012.06.029
Liu, Z., Ji, Y., Huang, X., Fu, Y., Wei, J., Cai, X., et al. (2013). An adapted duck Tembusu virus induces systemic infection and mediates antibody-dependent disease severity in mice. Virus Res. 176, 216–222. doi: 10.1016/j.virusres.2013.06.010
Meng, K., Sun, W. J., Zhao, P., Zhang, L. M., Cai, D. J., Cheng, Z. Q., et al. (2014). Development of colloidal gold-based immunochromatographic assay for rapid detection of Mycoplasma suis in porcine plasma. Biosens. Bioelectron. 55, 396–399. doi: 10.1016/j.bios.2013.12.048
Mukhopadhyay, S., Kuhn, R. J., and Rossmann, M. G. (2005). A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3, 13–22. doi: 10.1038/nrmicro1067
Nakayama, T., Zhao, J. Z., Takeuchi, D., Kerdsin, A., Chiranairadul, P., Areeratana, P., et al. (2014). Colloidal gold-based immunochromatographic strip test compromising optimised combinations of anti-S. sui capsular polysaccharide polyclonal antibodies for detection of Streptococcus suis. Biosens. Bioelectron. 60, 175–179. doi: 10.1016/j.bios.2014.03.074
Nara, S., Tripathi, V., Singh, H., and Shrivastav, T. G. (2010). Colloidal gold probe based rapid immunochromatographic strip assay for cortisol. Anal. Chim. Acta 682, 66–71. doi: 10.1016/j.aca.2010.09.041
Ou, Q. B. (2013). Preparation of Monoclonal Antibodies Against TMUV Envelope Protein and Establishment of the Rapid Detection Methods. Master thesis, Shandong Agricultural University, Tai’an.
Paek, S. H., Lee, S. H., Cho, J. H., and Kim, Y. S. (2000). Development of rapid one-step immunochromatographic assay. Methods 22, 53–60. doi: 10.1006/meth.2000.1036
Peng, F., Wang, Z., Zhang, S., Wu, R., Hu, S., Li, Z., et al. (2008). Development of an immunochromatographic strip for rapid detection of H9 subtype avian influenza viruses. Clin. Vaccine Immunol. 15, 569–574. doi: 10.1128/CVI.00273-07
Pierson, T. C., Fremont, D. H., Kuhn, R. J., and Diamond, M. S. (2008). Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4, 229–238. doi: 10.1016/j.chom.2008.08.004
Safenkova, I. V., Zherdev, A. V., and Dzantiev, B. B. (2010). Correlation between the composition of multivalent antibody conjugates with colloidal gold nanoparticles and their affinity. J. Immunol. Methods 357, 17–25. doi: 10.1016/j.jim.2010.03.010
Shaozhou, W. L., Bai, X. F., Liu, M., and Zhang, Y. (2015). Establishment of colloidal gold immunochromatographic assay for detection of goose parvovirus. Chin. Vet. Sci. 45, 486–489. doi: 10.16656/j.issn.1673-4696.2015.05.009
Siu, L. K., Tsai, Y. K., Lin, J. C., Chen, T. L., Fung, C. P., and Chang, F. Y. (2016). Development of a colloidal gold-based immunochromatographic strip for rapid detection of Klebsiella pneumoniae serotypes K1 and K2. J. Clin. Microbiol. 54, 3018–3021. doi: 10.1128/JCM.01608-16
Su, J., Li, S., Hu, X., Yu, X., Wang, Y., Liu, P., et al. (2011). Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106. doi: 10.1371/journal.pone.0018106
Sun, L., Li, Y. X., Zhang, Y., Han, Z. X., Xu, Y., Kong, X. G., et al. (2014). Adaptation and attenuation of duck tembusu virus strain du/ch/lsd/110128 following serial passage in chicken embryos. Clin. Vaccine Immunol. 21, 1046–1053. doi: 10.1128/CVI.00154-14
Tang, Y., Diao, Y., Chen, H., Ou, Q., Liu, X., Gao, X., et al. (2015). Isolation and genetic characterization of a Tembusu virus strain isolated from mosquitoes in Shandong, China. Transbound. Emerg. Dis. 62, 209–216. doi: 10.1111/tbed.12111
Tang, Y., Diao, Y., Yu, C., Gao, X., Chen, L., and Zhang, D. (2012). Rapid detection of Tembusu virus by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP). Transbound. Emerg. Dis. 59, 208–213. doi: 10.1111/j.1865-1682.2011.01257.x
Tang, Y., Gao, X., Diao, Y., Feng, Q., Chen, H., Liu, X., et al. (2013). Tembusu virus in human, China. Transbound. Emerg. Dis. 60, 193–196. doi: 10.1111/tbed.12085
Thontiravong, A., Ninvilai, P., Tunterak, W., Nonthabenjawan, N., Chaiyavong, S., Angkabkingkaew, K., et al. (2015). Tembusu-Related Flavivirus in Ducks, Thailand. Emerg. Infect. Dis. 21, 2164–2167. doi: 10.3201/eid2112.150600
Wang, Y., Wang, L., Zhang, J., Wang, G., Chen, W., Chen, L., et al. (2014). Preparation of colloidal gold immunochromatographic strip for detection of Paragonimiasis skrjabini. PLoS One 9:e92034. doi: 10.1371/journal.pone.0092034
Wang, Z. L., Song, X. H., Chen, X. Z., and Chen, G. S. (2004). The research of gold-immunochromatography for CPV diagnostic. Chin. J. Prev. Vet. Med. 26, 62–66.
Wu, X., Shi, Y., Yan, D., Li, X., Yan, P., Gao, X., et al. (2016). Development of a PCR-Based reverse genetics system for an attenuated duck Tembusu virus strain. PLoS One 11:e0156579. doi: 10.1371/journal.pone.0156579
Yan, P., Zhao, Y., Zhang, X., Xu, D., Dai, X., and Teng, Q. (2011). An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417, 1–8. doi: 10.1016/j.virol.2011.06.003
Yun, T., Ni, Z., Hua, J., Ye, W., Chen, L., Zhang, S., et al. (2012). Development of a one-step real-time RT-PCR assay using a minor-groove-binding probe for the detection of duck Tembusu virus. J. Virol. Methods 181, 148–154. doi: 10.1016/j.jviromet.2012.01.019
Zeng, H. J., Guo, W. B., Liang, B. B., Li, J. W., Zhai, X. Z., Song, C. M., et al. (2016). Self-paired monoclonal antibody lateral flow immunoassay strip for rapid detection of Acidovorax avenae subsp. citrulli. Anal. Bioanal. Chem. 408, 6071–6078. doi: 10.1007/s00216-016-9715-5
Zhang, Y., Li, X. L., Chen, H., Ti, J. F., Yang, G. P., Zhang, L., et al. (2015a). Evidence of possible vertical transmission of Tembusu virus in ducks. Vet. Microbiol. 179, 149–154. doi: 10.1016/j.vetmic.2015.06.004
Zhang, Y., Wang, Y. J., Yang, W. R., Xie, Z. K., Wang, R. Y., and Guo, Z. H. (2015b). A rapid immunochromatographic test to detect the lily mottle virus. J. Virol. Methods 220, 43–48. doi: 10.1016/j.jviromet.2015.04.010
Keywords: DTMUV, monoclonal antibody, virus detection, colloid gold, ICS
Citation: Yu G, Yu X, Yang G, Tang Y and Diao Y (2018) A Novel Diagnostic Method to Detect Duck Tembusu Virus: A Colloidal Gold-Based Immunochromatographic Assay. Front. Microbiol. 9:1001. doi: 10.3389/fmicb.2018.01001
Received: 28 October 2017; Accepted: 27 April 2018;
Published: 15 May 2018.
Edited by:
John P. Carr, University of Cambridge, United KingdomReviewed by:
Venkataramana M., DRDO – Bharathiar University (BU) – Center for Life Science (CLS), IndiaDabing Zhang, China Agricultural University, China
Copyright © 2018 Yu, Yu, Yang, Tang and Diao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Tang, dHljazI4OEAxNjMuY29t Youxiang Diao, eXhkaWFvQDEyNi5jb20=
 Guanliu Yu
Guanliu Yu Xianglong Yu1,2,3
Xianglong Yu1,2,3 Yi Tang
Yi Tang Youxiang Diao
Youxiang Diao