
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 15 May 2018
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.00993
 Ye Jin1†
Ye Jin1† Meilan Li2†
Meilan Li2† Yongpeng Shang3
Yongpeng Shang3 Li Liu1
Li Liu1 Xiaofei Shen1
Xiaofei Shen1 Zhihui Lv3
Zhihui Lv3 Zhihao Hao1
Zhihao Hao1 Jingjing Duan1
Jingjing Duan1 Yang Wu3
Yang Wu3 Chun Chen4*
Chun Chen4* Jingye Pan1*
Jingye Pan1* Fangyou Yu5*
Fangyou Yu5*Mupirocin, a topical antibiotic, has been utilized for decades to treat Staphylococcus aureus skin infections, as well as to decolonize patients at risk of methicillin-resistant S. aureus (MRSA) infection. The aims of this study were to investigate the expression of α-toxin (encoded by the hla gene) in ten clinical MRSA strains (MIC = 1024 μg/ml) in response to a sub-inhibitory concentration of mupirocin (1/32 minimum inhibitory concentration [MIC]) (32 μg/ml) by using α-toxin activity determination and enzyme-linked immune sorbent assay (ELISA). Subsequently, real-time polymerase chain reaction (RT-PCR) was used to examine the expression of saeR, agrA, RNAIII, and sarA genes under sub-inhibitory concentration of mupirocin in order to investigate the mechanism of action of this treatment regarding its strong inhibition of α-toxin expression. For all the strains tested, mupirocin dramatically reduced mRNA levels of α-toxin. The results indicated that α-toxin activity in mupirocin-treated groups was significantly lower than that in untreated groups. The results show that the levels of agrA, RNAIII, saeR, and sarA expression significantly decrease by 11.82- to 2.23-fold (P < 0.01). Moreover, we speculate that mupirocin-induced inhibition of α-toxin expression may be related to the inhibition of regulatory loci, such as agr, sarA and saeRS. More specifically, we found that the mechanism involves inhibiting the expression of agrA and RNAIII. In conclusion, sub-inhibitory concentrations of mupirocin strongly inhibit alpha-toxin production in high-level mupirocin-resistant MRSA by down-regulating agr, saeRS and sarA, which could potentially be developed as a supplemental treatment to control high-level mupirocin-resistant MRSA infection and reduce the risk of infection and colonization.
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the major causes of hospital- and community-acquired infections, including those associated with burn injury, sepsis, pneumonia, keratitis, and nasosinusitis (David and Daum, 2010). Moreover, colonization with MRSA greatly increases the risk of MRSA infection (Coello et al., 1997; Davis et al., 2004).
In the aforementioned infections, alpha-toxin (α-toxin) plays an indispensable role in the pathogenicity of S. aureus by causing tissue damage. α-toxin is a 33.2-kDa extracellularly secreted protein encoded by the hla gene (Bhakdi and Tranum-Jensen, 1991). It is a pore-forming toxin that causes damage and death of cells (Bhakdi and Tranum-Jensen, 1991). This protein has been documented as a virulence factor in many severe infections, including keratitis, mastitis, nasosinusitis, peritonitis, and skin and soft tissue infections (SSITs) (Bayer et al., 1997). As shown in Figure 1, three regulatory systems, comprising the accessory gene regulator (agr), staphylococcal accessory protein effector (saeRS) and staphylococcal accessory gene regulator (sarA) systems, appear to regulate hla expression in a coordinated manner in vitro (Xiong et al., 2006). The agr locus activates hla expression directly and positively, while sarA exerts a positive impact on hla expression by both agr-dependent and agr-independent pathways. In addition, the sae locus includes a two-component signal-transduction system encoded by saeS and saeR that positively regulates the expression of hla at the transcriptional level. Moreover, sae activation is also affected by agr, as well as by some stress environment.
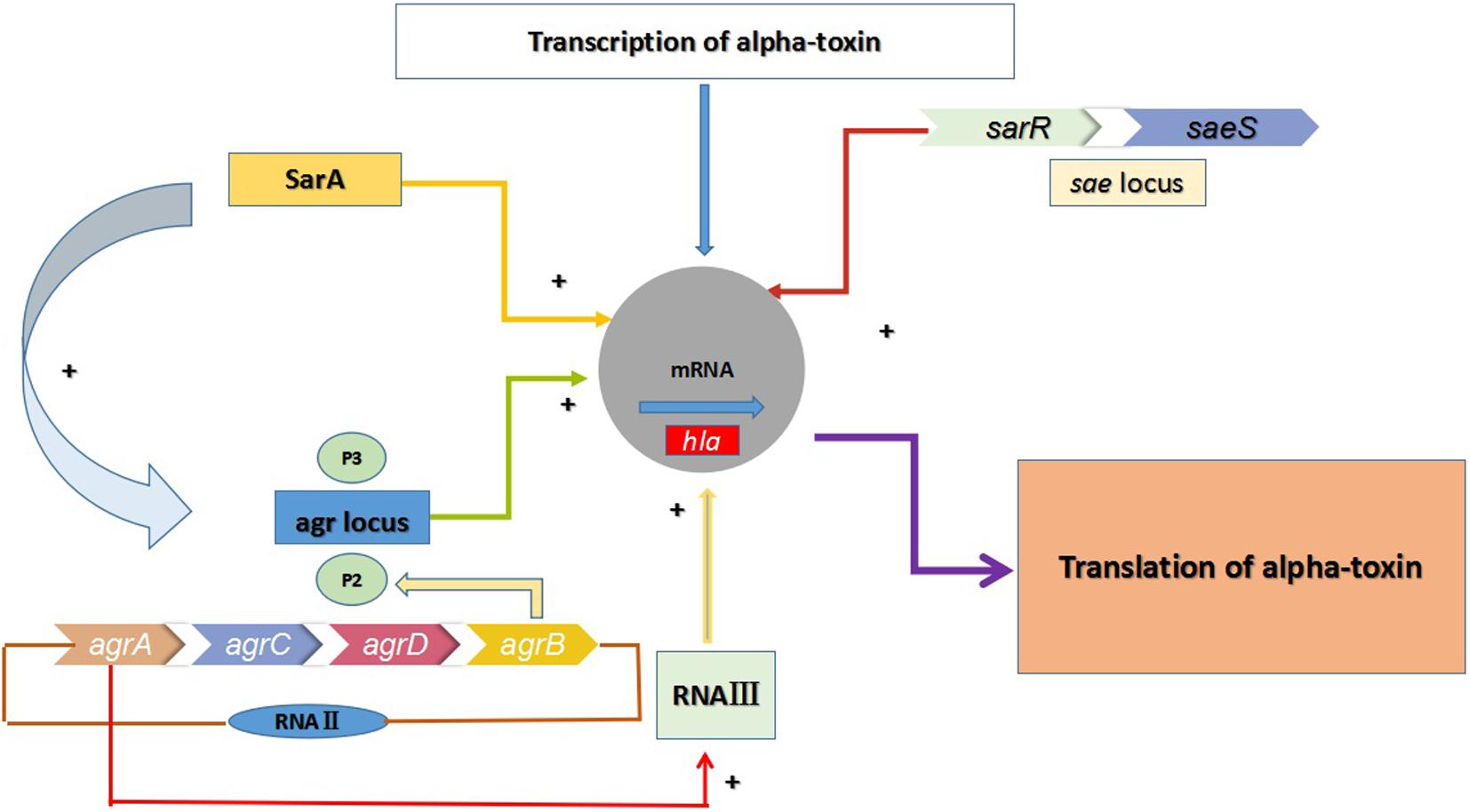
FIGURE 1. Multiple regulatory factors regulate the expression of hla. The agr locus encodes two transcripts known as RNAII and RNAIII, which are transcribed from the P2 and P3 promoters. The regulatory RNA molecule RNAIII can up-regulate the expression of hla, and agrA is an essential transcription factor for RNAIII. RNAII encodes AgrB, AgrD, AgrC, and AgrA. Meanwhile, the expression of hla in MRSA is also regulated by the two-component regulatory system SaeRS (saeR and saeS)and the SarA protein family.
Mupirocin (pseudomonic acid A), a competitive inhibitor of isoleucyl-tRNA synthetase (IRS), is an active antibiotic against most of gram-positive bacteria (Fuller et al., 1971; Thomas et al., 2010). It mediates the inhibition of ribosomal IRS binding, thereby impeding protein and RNA synthesis and leading to cell death (Hughes and Mellows, 1978). Mupirocin first became available in 1985, and it has been widely used for the management of topical MRSA infection and colonization in both patients and healthcare personnel (Boelaert, 1994).
Two categories of mupirocin resistance are recognized: high-level, plasmid-mediated resistance (minimum inhibitory concentration [MIC] ≥512 μg/ml) and low-level, chromosomally encoded resistance (MIC ≤256 μg/ml) (Eltringham, 1997). In recent years, various studies have shown that high-level mupirocin resistance is associated with MRSA colonization and treatment failure (Simor, 2011; Poovelikunnel et al., 2015), partially due to the inability to attain mupirocin concentrations ≥512 μg/ml in clinical settings. However, as previous reports have shown, sub-inhibitory concentrations of antibacterial agents can modulate the expression of virulence factors in S. aureus (Ohlsen et al., 1998; Herbert et al., 2001; Deneve et al., 2009; Otto et al., 2013; Li et al., 2014), and thereby impact the outcome of severe infections. Despite this, limited work has been undertaken to investigate the effect of sub-inhibitory concentrations of mupirocin on virulence factors produced by S. aureus.
In this study, therefore, we investigated the hypothesis that sub-inhibitory concentrations of mupirocin can prevent the release of the virulence factors, such as α-toxin.
All ten clinical mupirocin-resistant S. aureus strains (SA001–SA010) in this study were isolated from the First Affiliated Hospital of Wenzhou Medical University located in Wenzhou, east China, from 2013 to 2016 (Table 1). Identification and antimicrobial susceptibility testing of the isolates were carried out using a Vitek-2 Microbiology Analyzer (bioMerieux, Marcy l’Etoile, France) in accordance with the manufacturer’s instructions. The S. aureus ATCC25923 was used as the control strain for the identification of bacterial clinical isolates.
Polymerase chain reaction was used to detect whether the strains tested harbored mecA and mecC, with MRSA N315 as the positive control strain. The strains carrying mecA or mecC were defined as MRSA. Moreover, all the clinical strains with high-level mupirocin resistance were targeted mupA by PCR assays.
Multilocus sequence typing of the S. aureus strains was determined by amplifying seven housekeeping genes (arc, aroE, glpF, gmk, pta, tpi, and yqiL) as described previously (Saunders and Holmes, 2007). The sequences were compared with the existing sequences available on the online database1, and sequence types (STs) were assigned according to the allelic profiles.
Staphylococcal cassette chromosome mec typing of all the high-level mupirocin-resistant strains was determined by multiplex PCR with eight unique and specific primer pairs for SCCmec I, II, III, IVa, IVb, IVc, IVd, and V, as described previously (Zhang et al., 2005).
Mupirocin was obtained from Sigma-Aldrich (St. Louis, MO, United States). MICs were determined with the standard broth microdilution method recommended by the Clinical and Laboratory Standards Institute [CLSI] (2017). The S. aureus ATCC 29213 was used as the control strain in accordance with CLSI breakpoints (Clinical and Laboratory Standards Institute [CLSI], 2017). Thereafter, tryptic soy broth (TSB; Sigma-Aldrich) culture medium containing either 1/8 MIC (128 μg/ml), 1/16 MIC (64 μg/ml), 1/32 MIC (32 μg/ml), or 1/64 MIC (16 μg/ml) of mupirocin, or no mupirocin, was prepared for each strain to measure virulence factor expression.
The strains tested were grown in TSB (Sigma-Aldrich) at 37°C overnight. After 24 h of incubation, the bacteria were diluted to a concentration 5 × 105 CFU/ml. Different concentrations of mupirocin were then each added to the TSB. Overnight cultures containing TSB with 1/8, 1/16, 1/32, and 1/64 MIC of mupirocin or without mupirocin were normalized to the same optical density values at 600 nm (OD600) and then centrifuged at 4000 × g to harvest the bacterial cells. Aliquots of the supernatants were added to a 1% suspension of washed rabbit red blood cells (RRBCs) in 0.01 M phosphate-buffered saline (PBS; pH 7.2) containing 0.1% bovine serum albumin (Sigma-Aldrich), and they were then incubated for 1 h at 37°C. We used Triton X-100 as a positive control and RRBCs with 0.9% NaCl solution as a negative control. The absorbance at 600 nm (A600) of the complete hemolysis group (positive control) is set to 100. The A600 percentage experimental group is the ratio of A600 to complete hemolysis groups in each group and multiplied by 100. All data have been calibrated with negative controls. Each test was performed independently in triplicate.
The strains tested were cultured at 37°C in 96-well microtiter plates containing TSB with 1/32 MIC of mupirocin or without mupirocin overnight. Aliquots of each culture were then centrifuged at 4,000 × g for 10 min. The α-toxin level in the supernatant was then assessed. The concentration of α-toxin in the supernatant was quantified using a specific sandwich-type ELISA (Sigma-Aldrich) targeting hla with an anti-hla monoclonal antibody as primary antibodies. We then detected the antibody–antigen complex by using an anti-hla polyclonal rabbit antibody and a peroxidase-conjugated goat anti-rabbit antibody as secondary antibodies. The concentration of α-toxin in each sample was calculated using the linear regression method with a standard curve, y = ax+b. The absorbance readings were obtained by subtracting the absorbance of the blank from the absorbance of the samples subjected to ELISA. Untreated supernatant was used as the negative control. The cut-off value was defined as less than twice the value of the negative control absorbance.
A total of 4 ml of bacterial culture (grown with 1/32 MIC of mupirocin, as well as without mupirocin) was incubated at 37°C with shaking at 220 rpm for 16 h. Each solution was centrifuged at 12000 × g for 2 min. After centrifugation, the supernatant was discarded. We then added each pellet plus 1 ml 0.1-mm zirconia-silica beads to a vial tube and filled it with normal saline without bubbles. The tube was then shaken at 4000 rpm for 1 min using a Mini-Bead Beater (BioSpec Products, Bartlesville, OK, United States), followed by 1 min of cooling on ice. This procedure was repeated for five times.
The total RNA was then purified using an RNeasy Plus Mini Kit (QIAGEN, Berlin, Germany) following the manufacturer’s instructions. The purified RNA (1 μg) was used to generate cDNA using a PrimeScript RT Reagent Kit (TaKaRa, Tokyo, Japan), and the resulting cDNA was amplified using a SYBR Green Premix Kit (TaKaRa, Tokyo, Japan) with a Mastercycler ep realplex instrument (Eppendorf, Hamburger, Germany). The hla gene and regulatory genes (agr, sarA, saeR, and RNAIII) were determined by RT-PCR. Table 2 shows the oligonucleotide primers. Cultures of the S. aureus strains grown without mupirocin were used as positive controls (relative expression = 1), and gyrB was used as an endogenous control to investigate target genes. RNA transcript levels were calculated using the expression 2-ΔΔCT. Each reaction was performed in triplicate.
Chi-square or Fisher’s exact tests were used to compare the differences in α-toxin activity and hla expression among the groups treated with 1/8 to 1/32 MIC of mupirocin or no mupirocin. SPSS statistical software (version 19, IBM Corp, Armonk, NY, United States) was used, and a 2-sided p-value <0.05 was considered statistically significant. The ELISA and RT-PCR data were analyzed using GraphPad Prism software (version 7.00, La Jolla, CA, United States), and a p-value <0.05 was considered statistically significant.
The details of the strains used in this study are described in Table 1. All clinical isolates were isolated from different wards, comprising the intensive care unit (ICU), nephrology ward, temporary turnover ward, brain intensive care ward, urinary surgery ward, and operation room. The sources of the isolates comprised sputum, blood, pus, and skin. All clinical isolates possessed high-level resistance to mupirocin (MIC = 1024 μg/ml) and were positive for mupA. Regarding the molecular characteristics, the SA001–SA004 were identified as ST5-MRSA-II, ST239-MRSA-III, ST7-MRSA-un, and ST5-MRSA-II, respectively. The information of strains SA005–SA010 was shown in Table 1.
As it is difficult to achieve the concentrations of mupirocin >256 μg/ml (1/4MIC7) in serum, we explored the effects of mupirocin on α-toxin expression using concentrations of 16–128 μg/ml (1/64 to 1/8 MIC).
With respect to observed differences in α-toxin activity between untreated strains and strains exposed to 1/64 MIC (16 μg/ml), 1/32 MIC (32 μg/ml), 1/16 MIC (64 μg/ml), and 1/8 MIC (128 μg/ml) mupirocin, we found that α-toxin activity among the latter groups was significantly reduced (P < 0.05) except for the 1/64 MIC group. When cultured without mupirocin, 74.26 – 88.6% (SA001–SA004) of the RRBCs were lysed by α-toxin (Figure 2). When cultured in the presence of 1/8 MIC mupirocin, these percentages significantly decreased (P < 0.05) to 7.20 – 10.74%, respectively. When cultured with 1/16 and 1/32 MIC mupirocin, they also decreased significantly compared with the untreated strains (P < 0.05) (data not shown). However, 1/64 MIC group did not produce a significant decrease in α-toxin activity (P > 0.05). In summary, compared with culture without mupirocin, mupirocin with 1/8, 1/16 and 1/32 MIC induced significant reductions in α-toxin activity, resulting in 13.03- to 6.44-fold in strains SA001–SA004. The results show that mupirocin treatment is concentration-independent. As a result, regarding strains SA005–SA010, we investigated the effects of mupirocin on α-toxin expression using concentration of 32 μg/ml (1/32 MIC). As shown in Supplementary Figure S1, when cultured with 1/32 MIC, the treatment group shows significant decrease (P < 0.05) compared with the untreated group.
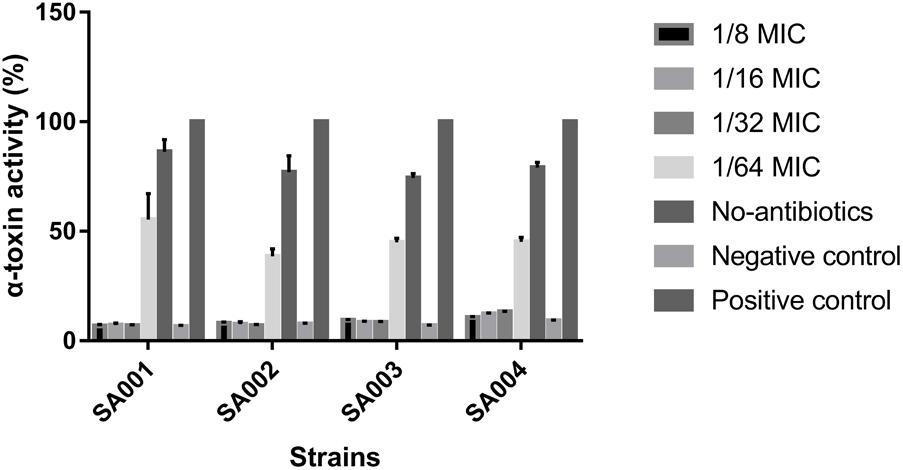
FIGURE 2. Effects of sub-inhibitory concentrations of mupirocin (1/64–1/8 MIC) on α-toxin activity in high-level mupirocin-resistant MRSA strains. We used Triton X-100 (which causes complete hemolysis) as a positive control and RRBCs with 0.9% NaCl solution as a negative control. The absorbance at 600 nm (A600 nm) of the positive control was set to 100. The α-toxin activity percentage for each experimental group is the ratio of the A600 nm for that group to the A600 nm of the positive control multiplied by 100. All data were calibrated with negative controls. Each test was performed independently in triplicate. Values are means ± SD (three repeated experiments).
As described above, we conclude that mupirocin can reduce α-toxin production in the clinical strains SA001–SA010. To investigate this further, we used ELISA to investigate the nature of α-toxin production in S. aureus when cultured with or without sub-inhibitory concentrations of mupirocin (1/32 MIC). As shown in Figure 3, exposure to sub-inhibitory concentrations of mupirocin resulted in large decreases in α-toxin production, with antibody titers 7.74–2.20-fold lower than those of cultures not exposed to mupirocin. However, the 1/64 MIC of mupirocin is not as strongly not responding to α-toxin secretion (P > 0.05). These findings demonstrate that 1/32 MIC of mupirocin can greatly reduce external α-toxin secretion. As a result, to analyze the degree of production further, we finally selected a certain concentration of mupirocin (1/32 MIC) to explore the effects of this sub-inhibitory concentration of mupirocin on α-toxin.
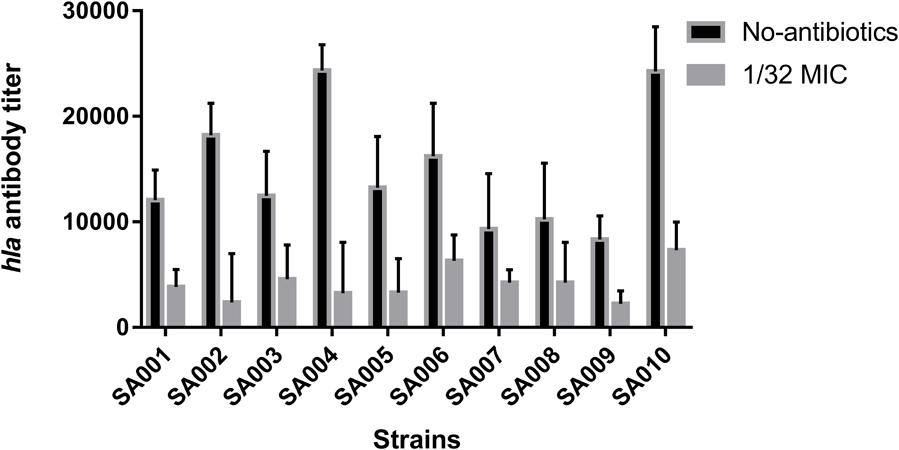
FIGURE 3. Effects of a sub-inhibitory concentration of mupirocin on α-toxin release. All the high-level mupirocin-resistant clinical isolates (SA001–SA010) were cultured with mupirocin at 1/32 MIC or without mupirocin. The α-toxin concentration in the supernatant was quantified by ELISA. The α-toxin concentration in each sample was calculated using the linear regression method with a standard curve, y = ax+b. The absorbance readings were obtained by subtracting the absorbance of the blank from the absorbance of the samples subjected to ELISA. Untreated supernatant was used as the negative control. The cut-off value was defined as less than twice the value of the negative control absorbance.
To explore the influence of a sub-inhibitory concentration (1/32 MIC) of mupirocin on hla expression, we performed relative quantitative PCR to assess the hla/gyrB ratios in cultures grown with and without mupirocin for 16 h. As expected, mupirocin induced a strong decrease of hla mRNA levels in strains SA001–SA010 after 16 h incubation. As shown in Figure 4, when cultured with 1/32 MIC of mupirocin compared with culture without mupirocin, the level of hla expression significantly (P < 0.05) decreased by 73.52–4.12-fold depending on the strain (see the concrete data in Supplementary Table S1). Therefore, when considering these concentrations together (1/8, 1/16 and 1/32 MIC), we can conclude that mupirocin significantly decrease the α-toxin level in the bacterial external secretion by affecting the expression of the hla gene.
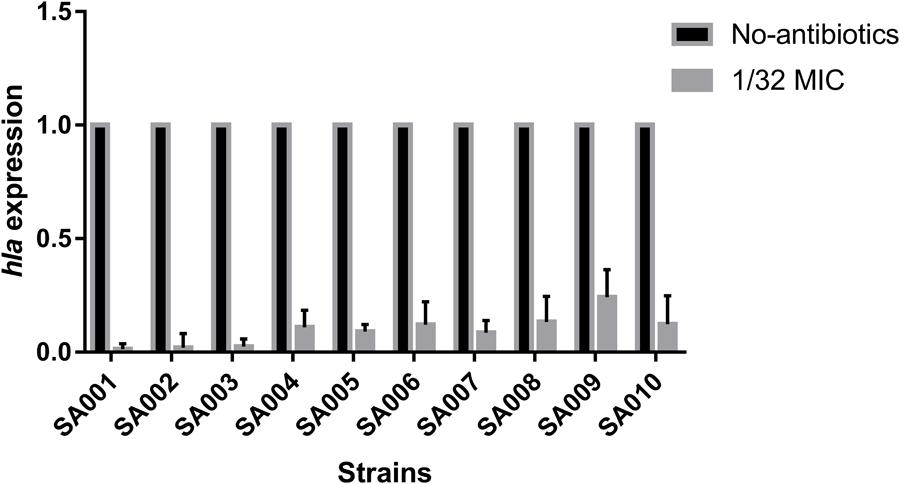
FIGURE 4. Real-time polymerase chain reaction (RT-PCR) analysis of hla expression after exposure to a sub-inhibitory concentration of mupirocin (1/32 MIC). All the high-level mupirocin-resistant clinical isolates (SA001–SA010) were cultured with mupirocin at 1/32 MIC or without mupirocin. The results are expressed as n-fold differences in the hla/gyrB ratio in the presence of mupirocin relative to the hla/gyrB ratio without mupirocin. Values are means+SDs (based on three repeated assays). There were significant differences with the control group (grown without mupirocin) for each strain (P < 0.05).
We have shown that sub-inhibitory concentrations of mupirocin reduce the level of α-toxin in S. aureus. To elucidate the inhibition mechanism of mupirocin on hla mRNA expression, we investigated the expression of the major virulence regulatory genes in S. aureus (agr, saeRS, and sarA) under the same conditions. As shown in Figure 5, when the strains were clutured with 1/32 MIC of mupirocin for 16 h, the levels of agrA expression significantly decrease (P < 0.01) by 11.82- to 3.46-fold (P < 0.01). The levels of RNAIII expression significantly decrease (P < 0.01) by 7.80- to 3.46-fold (P < 0.01). About the levels of saeR and sarA, the expressions significantly decrease by 2.58- to 5.32-fold (P < 0.01) and 3.38- to 2.23-fold (P < 0.01) respectively (see the concrete data in Supplementary Table S1). These results demonstrate that sub-inhibitory concentrations of mupirocin most likely act by repressing the expression of these regulatory genes, especially at the agr regulatory locus.
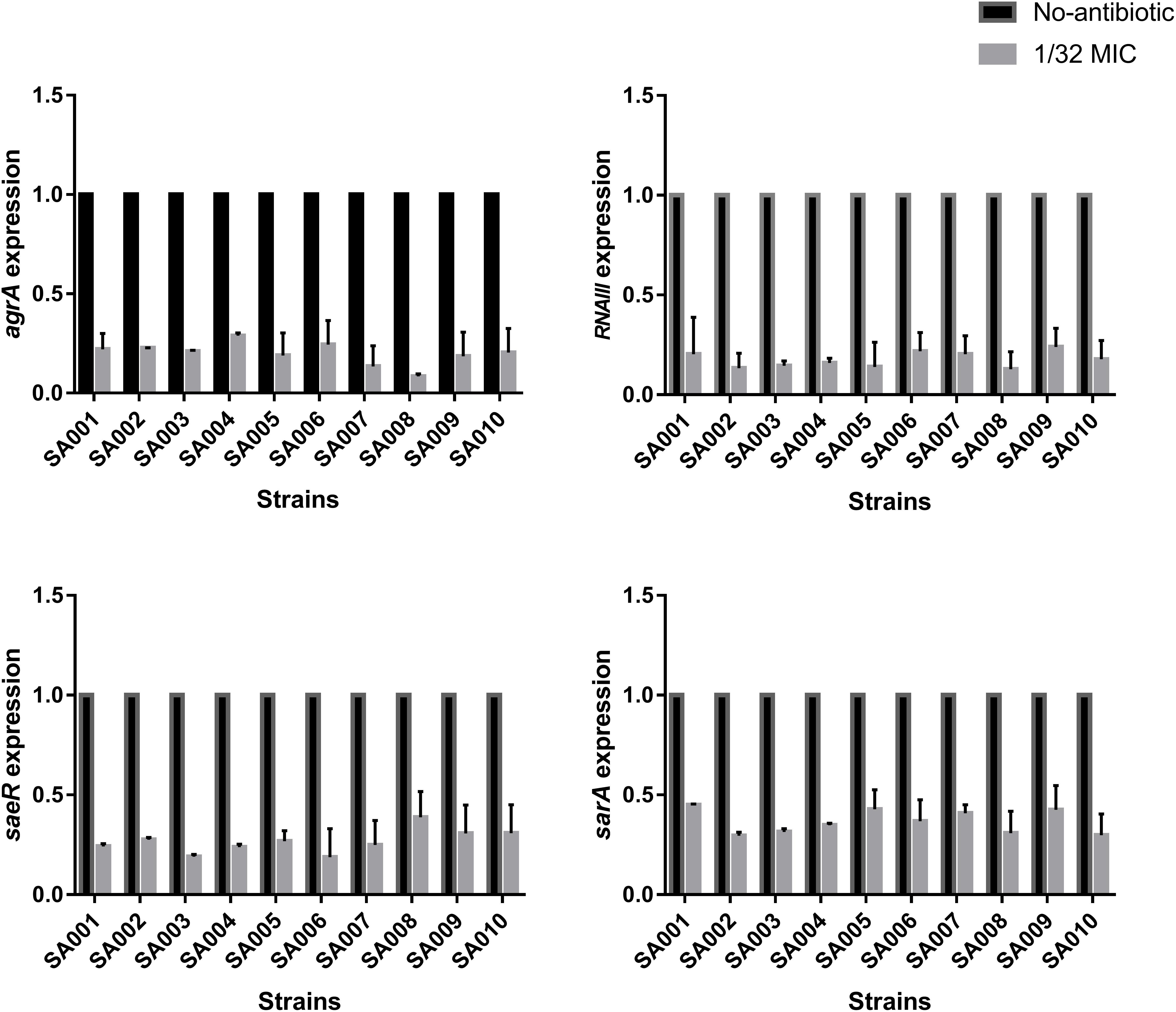
FIGURE 5. Effects of sub-inhibitory a concentration of mupirocin (1/32 MIC) on the expression of regulatory genes. All the high-level mupirocin-resistant clinical isolates (SA001–SA010) were cultured with mupirocin at 1/32 MIC or without mupirocin. The results are expressed as n-fold differences in the RNAIII/gyrB, sarA/gyrB, agrA/gyrB, and saeR/gyrB ratios in the presence of mupirocin relative to the same ratios of the strain grown without mupirocin. Values are means+SDs (based on three repeated assays). There were significant differences with the control group (grown without mupirocin) for each strain (P < 0.05).
The rapid global dissemination of antibiotic-resistant S. aureus strains has rendered treatment of serious infections increasingly difficult (Tong et al., 2015). As a result, a number of studies have focused on the effects of sub-inhibitory concentrations of antibiotics on bacterial cell functions, including virulence factor expression (Otto et al., 2013). In this study, we first investigated the effect of sub-inhibitory concentrations of mupirocin on hla expression, utilizing clinical MRSA isolates that exhibit high-level mupirocin resistance.
The clinical performance of antibiotics is usually evaluated based firstly on their bactericidal or bacteriostatic effect and secondly on their impact on the release of virulence factors. Moreover, sub-inhibitory concentrations of antibiotics against S. aureus have been shown to interfere with the expression of virulence factors such as regulatory gene products that in turn affect the transcription of exoprotein-encoding genes (Otto et al., 2013). Recently, the effects of sub-inhibitory concentrations of β-lactams and fluoroquinolones on the expression of virulence factors in pathogenic Gram-positive bacteria have been investigated by several studies (Otto et al., 2013). These studies have demonstrated that the changes of virulence factor expression induced by different sub-inhibitory concentrations of antibiotics are diverse, and can be detected by different methods (Davies et al., 2006). In S. aureus, exposure to different sub-inhibitory concentrations of antibiotics (such as β-lactams, macrolides, aminoglycosides, glycopeptides, and fluoroquinolones) leads to varying degrees of hla expression (Ohlsen et al., 1998). More precisely, sub-inhibitory concentrations of β-lactams result in almost complete inhibition of α-toxin production (Otto et al., 2013), while macrolides and aminoglycosides result in partial inhibition (Ohlsen et al., 1998), glycopeptides have no effect, and fluoroquinolones lead to slightly increased expression (Ohlsen et al., 1998; Otto et al., 2013).
In our study, to detect α-toxin expression, we first calculated mupirocin MIC values using broth microdilution according to CLSI breakpoints. After confirming the mupirocin MICs, we chose 1/32 MIC (32 μg/ml) as a suitable concentration of mupirocin for culturing S. aureus strains for the remaining experiments. Based on the results, we conclude that sub-inhibitory concentrations of mupirocin lead to reductions of α-toxin production by suppressing the expression of hla. To confirm the phenotypic effect, we first investigated the α-toxin activity for each group, demonstrating that hla activity of the mupirocin-untreated groups was significantly lower than that of the untreated group. ELISA was then used to confirm the degree of α-toxin production. To explore what caused the decrease of hla expression, we determined the effects of a sub-inhibitory concentration of mupirocin on hla mRNA levels.
As a topical antimicrobial agent, mupirocin mediates the inhibition of isoleucyl-tRNA synthetase (IRS), thereby impeding protein and RNA synthesis (Fuller et al., 1971). The expression of virulence factors is controlled in a coordinated fashion by a network of regulatory systems, such as agr, sarA, and saeRS. The most likely mechanism for decreased production of exocrine proteins (such as α-toxin) involves blocking mRNA translation at the ribosomal level. Moreover, it is tempting to speculate that mupirocin-induced inhibition of hla may result from the suppression of regulatory systems such as agr, saeRS, and sarA. The agr locus encodes two transcripts known as RNAII and RNAIII, which are transcribed from the P2 and P3 promoters (Morfeldt et al., 1996). The regulatory RNA molecule RNAIII can up-regulate the expression of virulence genes such as hla, and agrA is an essential transcription factor for RNAIII. Meanwhile, the expression of hla in MRSA is also regulated by the two-component regulatory system SaeRS and the SarA protein family. Therefore, we speculate that sub-inhibitory concentrations of mupirocin strongly inhibit hla expression in high-level mupirocin-resistant MRSA by down-regulating a network of regulatory systems, namely, agr, saeRS, and sarA.
As a result, we demonstrate that sub-inhibitory concentrations of mupirocin can reduce the expression of the virulence gene hla in high-level mupirocin-resistant clinical strains. We find that sub-inhibitory concentrations of mupirocin reduce hla expression by interfering with at least three key regulatory loci: agr, saeRS, and sarA. We successfully show that reduction in RNAIII and agrA expression, and RNAIII-controlled virulence factors, is a result of a direct or indirect interaction between mupirocin and hla. Thus, mupirocin may modulate hla expression by blocking Agr’s ability to bind to the P2-P3 promoter region of the agr locus. Furthermore, we find that mupirocin plays a role in the inhibition of saeRS and sarA, which is essential to the pathogenicity of S. aureus. Although the exact mechanisms involved with the inhibition of these key virulence traits by mupirocin remain to be unascertained, we demonstrate that a sub-inhibitory concentration of mupirocin can be an efficient repressor of virulence gene expression.
This conjecture was supported by the findings that, when treated with a sub-inhibitory concentration of mupirocin, the expression levels of RNAIII, agrA, saeR, and sarA were all significantly reduced. Several investigators have reported that sub-inhibitory concentrations of clindamycin result in almost complete inhibition of α-toxin (Ohlsen et al., 1998; Herbert et al., 2001; Otto et al., 2013), while others have reported that agr can not be responsible for the clindamycin effect, although saeRS could conceivably have a role with respect to certain exoproteins (Herbert et al., 2001). In contrast, in our study, agrA, RNAIII, saeR, and sarA expression levels were all significantly reduced following exposure to a sub-inhibitory concentration of mupirocin. However, regarding the inhibition mechanism of action of sub-inhibitory concentrations of mupirocin, there are a lot work to be done.
In conclusion, in this study, we demonstrate mupirocin can suppress alpha-toxin in a dose-independent manner by phenotypic and transcriptional expression analysis. In addition, the findings in our study indicate that sub-inhibitory concentrations of mupirocin may be of great importance as supplemental treatment for controlling high-level mupirocin-resistant MRSA infection and preventing infection and colonization, as this treatment could be used as a targeted-therapy against α-toxin.
YJ, ML, YS, ZH, LL, JD, and XS designed of the work and analyzed and interpreted of data for the work. FY and CC drafted the work and revised it critically for important intellectual content. JP provided approval for publication of the content. YS, ZL, and YW participated in the experimental design and data analysis. FY agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
This study was supported by a grant from the National Natural Science Foundation of China (81672078).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00993/full#supplementary-material
FIGURE S1 | Effects of a sub-inhibitory concentration of mupirocin (1/32 MIC) on α-toxin activity in high-level mupirocin-resistant MRSA strains. We used Triton X-100 (which causes complete hemolysis) as a positive control and RRBCs with 0.9% NaCl solution as a negative control. The absorbance at 600 nm (A600nm) of the positive control was set to 100. The α-toxin activity percentage for each experimental group is the ratio of the A600nm for that group to the A600nm of the positive control multiplied by 100. All data were calibrated with negative controls. Each test was performed independently in triplicate. Values are means ± ±SD (three repeated experiments). ∗Significantly different from the control (strain grown without mupirocin).
TABLE S1 | The reduction coefficient (1/32 MIC) of α-toxin activity and the transcriptional levels of hla, RNAIII, agrA, saeR, and sarA (compared to the group without mupirocin).
Bayer, A. S., Ramos, M. D., Menzies, B. E., Yeaman, M. R., Shen, A. J., and Cheung, A. L. (1997). Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect. Immun. 65, 4652–4660.
Bhakdi, S., and Tranum-Jensen, J. (1991). Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 55, 733–751.
Boelaert, J. R. (1994). Staphylococcus aureus infection in haemodialysis patients. Mupirocin as a topical strategy against nasal carriage: a review. J. Chemother. 6(Suppl 2), 19–24.
Clinical and Laboratory Standards Institute [CLSI] (2017). Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute.
Coello, R., Glynn, J. R., Gaspar, C., Picazo, J. J., and Fereres, J. (1997). Risk factors for developing clinical infection with methicillin-resistant Staphylococcus aureus (MRSA) amongst hospital patients initially only colonized with MRSA. J. Hosp. Infect. 37, 39–46. doi: 10.1016/S0195-6701(97)90071-2
David, M. Z., and Daum, R. S. (2010). Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23, 616–687. doi: 10.1128/CMR.00081-09
Davies, J., Spiegelman, G. B., and Yim, G. (2006). The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 9, 445–453. doi: 10.1016/j.mib.2006.08.006
Davis, K. A., Stewart, J. J., Crouch, H. K., Florez, C. E., and Hospenthal, D. R. (2004). Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 39, 776–782. doi: 10.1086/422997
Deneve, C., Bouttier, S., Dupuy, B., Barbut, F., Collignon, A., and Janoir, C. (2009). Effects of subinhibitory concentrations of antibiotics on colonization factor expression by moxifloxacin-susceptible and moxifloxacin-resistant Clostridium difficile strains. Antimicrob. Agents Chemother. 53, 5155–5162. doi: 10.1128/AAC.00532-09
Eltringham, I. (1997). Mupirocin resistance and methicillin-resistant Staphylococcus aureus (MRSA). J. Hosp. Infect. 35, 1–8. doi: 10.1016/S0195-6701(97)90162-6
Fuller, A. T., Mellows, G., Woolford, M., Banks, G. T., Barrow, K. D., and Chain, E. B. (1971). Pseudomonic acid: an antibiotic produced by Pseudomonas fluorescens. Nature 234, 416–417. doi: 10.1038/234416a0
Herbert, S., Barry, P., and Novick, R. P. (2001). Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect. Immun. 69, 2996–3003. doi: 10.1128/IAI.69.5.2996-3003.2001
Hughes, J., and Mellows, G. (1978). Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem. J. 176, 305–318. doi: 10.1042/bj1760305
Li, G., Qiao, M., Guo, Y., Wang, X., Xu, Y., and Xia, X. (2014). Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog. Dis. 11, 677–683. doi: 10.1089/fpd.2013.1731
Morfeldt, E., Tegmark, K., and Arvidson, S. (1996). Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol. Microbiol. 21, 1227–1237. doi: 10.1046/j.1365-2958.1996.751447.x
Ohlsen, K., Ziebuhr, W., Koller, K. P., Hell, W., Wichelhaus, T. A., and Hacker, J. (1998). Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 42, 2817–2823.
Otto, M. P., Martin, E., Badiou, C., Lebrun, S., Bes, M., Vandenesch, F., et al. (2013). Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 68, 1524–1532. doi: 10.1093/jac/dkt073
Poovelikunnel, T., Gethin, G., and Humphreys, H. (2015). Mupirocin resistance: clinical implications and potential alternatives for the eradication of MRSA. J. Antimicrob. Chemother. 70, 2681–2692. doi: 10.1093/jac/dkv169
Saunders, N. A., and Holmes, A. (2007). Multilocus sequence typing (MLST) of Staphylococcus aureus. Methods Mol. Biol. 391, 71–85. doi: 10.1007/978-1-59745-468-1_6
Simor, A. E. (2011). Staphylococcal decolonisation: an effective strategy for prevention of infection? Lancet Infect. Dis. 11, 952–962. doi: 10.1016/S1473-3099(11)70281-X
Thomas, C. M., Hothersall, J., Willis, C. L., and Simpson, T. J. (2010). Resistance to and synthesis of the antibiotic mupirocin. Nat. Rev. Microbiol. 8, 281–289. doi: 10.1038/nrmicro2278
Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L., et al. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi: 10.1128/CMR.00134-14
Xiong, Y. Q., Willard, J., Yeaman, M. R., Cheung, A. L., and Bayer, A. S. (2006). Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J. Infect. Dis. 194, 1267–1275. doi: 10.1086/508210
Zhang, K., Mcclure, J. A., Elsayed, S., Louie, T., and Conly, J. M. (2005). Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43, 5026–5033. doi: 10.1128/JCM.43.10.5026-5033.2005
Keywords: MRSA, sub-inhibitory concentrations, mupirocin, alpha-toxin, agr, saeRS, sarA
Citation: Jin Y, Li M, Shang Y, Liu L, Shen X, Lv Z, Hao Z, Duan J, Wu Y, Chen C, Pan J and Yu F (2018) Sub-Inhibitory Concentrations of Mupirocin Strongly Inhibit Alpha-Toxin Production in High-Level Mupirocin-Resistant MRSA by Down-Regulating agr, saeRS, and sarA. Front. Microbiol. 9:993. doi: 10.3389/fmicb.2018.00993
Received: 24 February 2018; Accepted: 27 April 2018;
Published: 15 May 2018.
Edited by:
Dongsheng Zhou, Beijing Institute of Microbiology and Epidemiology, ChinaReviewed by:
Xu Jia, Chengde Medical College, ChinaCopyright © 2018 Jin, Li, Shang, Liu, Shen, Lv, Hao, Duan, Wu, Chen, Pan and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Chen, Q2FuZHkuMzU1QDE2My5jb20= Jingye Pan, cGFuamluZ3llQGhvc3AxLmFjLmNu Fangyou Yu, d3pqeHlmeUAxNjMuY29t
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.