
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol., 09 May 2018
Sec. Virology
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.00925
This article is part of the Research TopicMolecular Pathology of HTLV-1View all 22 articles
 Francesca Marino-Merlo1
Francesca Marino-Merlo1 Antonio Mastino2,3*
Antonio Mastino2,3* Sandro Grelli4
Sandro Grelli4 Olivier Hermine5
Olivier Hermine5 Ali Bazarbachi6,7†
Ali Bazarbachi6,7† Beatrice Macchi1†
Beatrice Macchi1†Human T cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T cell leukemia/lymphoma (ATL), HTLV-1 associated myelopathy (HAM/TSP), and of a number of inflammatory diseases with an estimated 10–20 million infected individuals worldwide. Despite a number of therapeutic approaches, a cure for ATL is still in its infancy. Conventional chemotherapy has short-term efficacy, particularly in the acute subtype. Allogeneic stem cell transplantation offers long-term disease control to around one third of transplanted patients, but few can reach to transplant. This prompted, over the past recent years, the conduction of a number of clinical trials using novel treatments. Meanwhile, new data have been accumulated on biological and molecular bases of HTLV-1 transforming and infecting activity. These data offer new rational for targeted therapies of ATL. Taking into account the double-face of ATL as an hematologic malignancy as well as a viral infectious disease, this Mini-Review seeks to provide an up-to-date overview of recent efforts in the understanding of the mechanisms involved in already used therapeutic regimens showing promising results, and in selecting novel drug targets for ATL.
Human T cell leukemia virus type 1 (HTLV-1) is the first identified human retrovirus endemic in southwestern Japan, the Caribbean islands, inter-tropical Africa, South America, Romania and the Middle East, with an estimated 10–20 million infected individuals worldwide. HTLV-1 is known to cause adult T cell leukemia/lymphoma (ATL), HTLV-1 associated myelopathy (HAM/TSP), and a number of inflammatory diseases. Firstly detected and isolated from a cutaneous T cell lymphoma almost 40 years ago, HTLV-1 still represents a significant challenge for the scientific community engaged to disclose its oncogenic potential and to identify a focused therapy (Gallo, 2005; Tagaya and Gallo, 2017). HTLV-1 transforms CD4+ lymphocytes in vitro and in vivo, and complex mechanisms control virus spreading, expression of viral proteins and host immune response in infected individuals (Figure 1). As a consequence, ATL patients are often refractory to intensive, conventional chemotherapy regimens. Classically used regimen are CHOP, CHOEP dose-adjusted EPOCH, and hyper-CVAD, alternating with high-dose methotrexate and cytarabine (Dittus and Sloan, 2017). An intensive treatment consisting of VCAP-AMP-VECP with the prophylactic use of G-CSF, has been introduced in Japan (Watanabe et al., 2011). In addition, results of preclinical studies, such as the high expression of CCR4 in ATL cells (Ishida et al., 2003), led to hypothesize new targets for biological therapy in ATL. Indeed, clinical trials using humanized monoclonal antibodies such as mogamulizumab (anti-CCR4) (Ishida et al., 2004; Yamamoto et al., 2010), alemtuzumab (anti-CD52) (Sharma et al., 2017), or daclizumab (anti-CD25) (Berkowitz et al., 2014) have been conducted on ATL patients (Figure 2). However, an important hurdle emerged in practically all the completed studies, is the limited duration of the response. Allogeneic stem transplantation could represent an alternative, potentially curative approach (Zell et al., 2016). Unfortunately, its use is limited to a small percentage of ATL patients. Recently published review articles provide detailed information on the state-of-the-art of treatment strategies until today adopted in clinical trials for ATL patients and on related results (Kato and Akashi, 2015; Hermine et al., 2018), and this mini-review will not address these aspects.
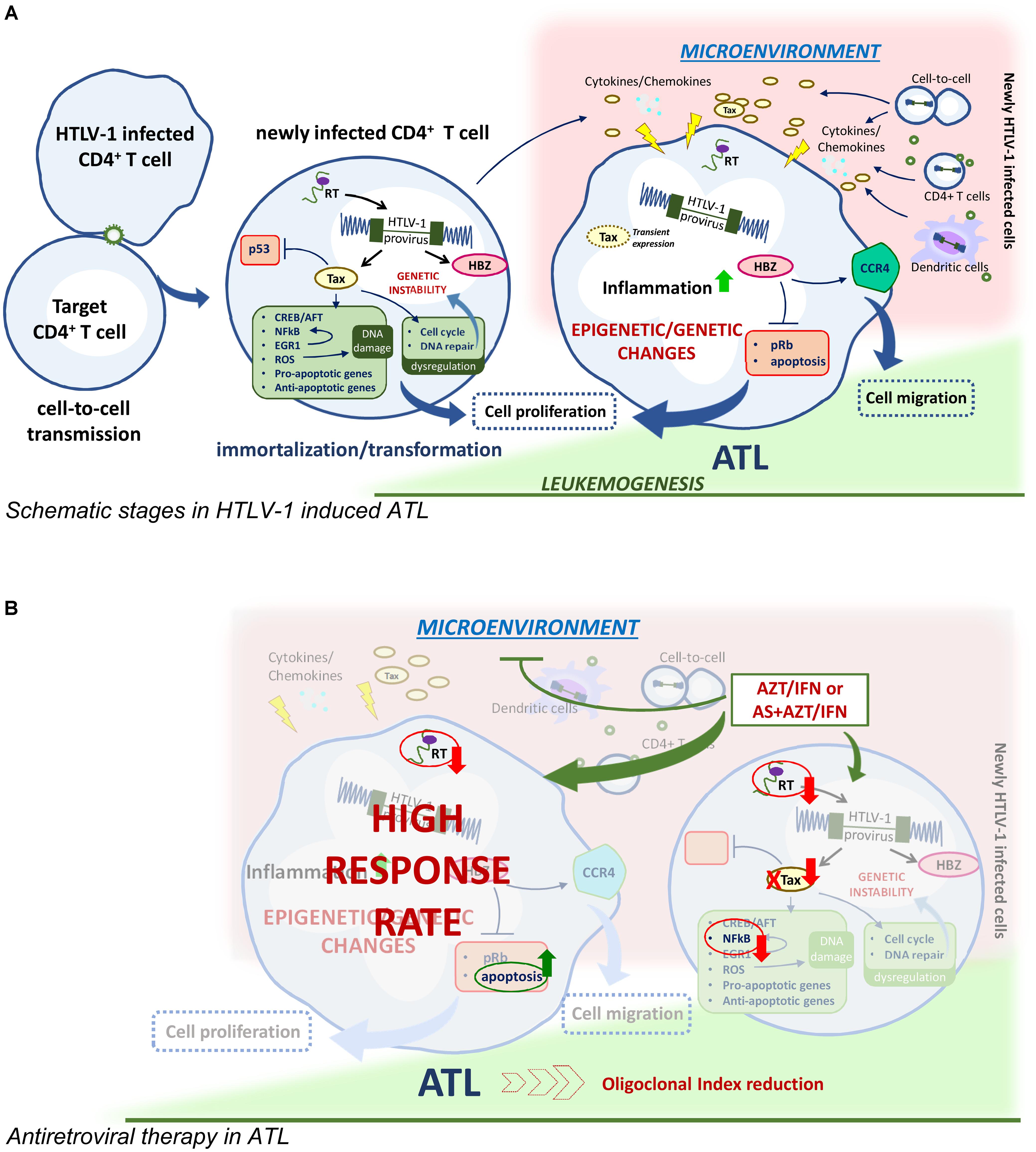
FIGURE 1. HTLV-1 driven leukomogenesis and possible mechanism of antiviral therapy. (A) Main immortalization/transformation process activated by HTLV-1 regulatory protein Tax and HBZ in newly infected CD4+ cells transmit virus to uninfected CD4+ cells and leading to epigenetic and genetic changes in ATL transformed cells. Cell migration and cell-to-cell virus transmission, presumably involving also dendritic cells, favor the release of inflammatory cytokines and chemokines milieu in the microenvironment and contribute to the maintaining of the infected clones. (B) AZT/IFN with or without arsenic trioxide (AS) could interrupt the maintaining route of ATL.
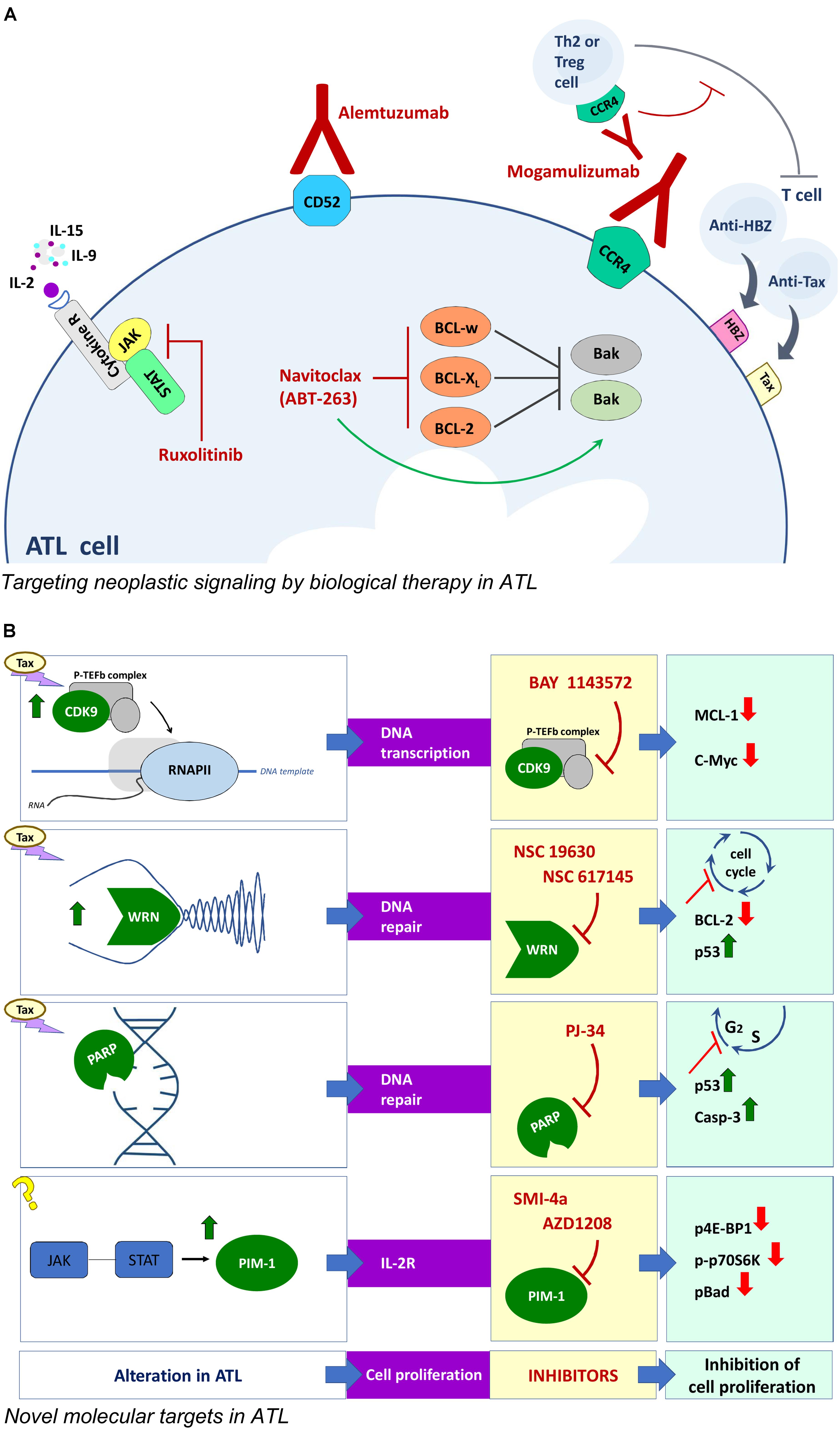
FIGURE 2. Novel approaches for ATL therapy. (A) Biological therapy with monoclonal antibodies and corresponding targets involved in neoplastic signaling in ATL cells. (B) Compounds and corresponding targets potentially useful for innovative targeted therapies in ATL.
What makes the design of therapeutic approaches for ATL problematic is the double routes of HTLV-1 transmission in vivo, i.e., the mitotic and the RT-dependent ones, that might require a combined approach targeting both chronically infected host cells and direct viral replication. Actually, in the past recent years a lot of data have been accumulated on biological and molecular bases of HTLV-1 transforming as well as infecting activity. These data offer new rational for targeted therapies of ATL. Thus, taking into account the double-face of ATL as an hematologic malignancy as well as a viral infectious disease, this mini-review seeks to specifically focus on providing an up-to-date overview of recent efforts in: (i) understanding mechanisms involved in already used therapeutic regimens showing promising results, (ii) identifying and selecting novel drug targets for ATL.
Adult T cell leukemia/lymphoma is a severe, aggressive leukemia, unequivocally associated to HTLV-1 infection, which develop in 2–4% of the infected individuals after a long time of latency. Four clinical forms/subtypes of ATL have been recognized: acute, lymphomatous, chronic, and smoldering. (Shimoyama, 1991). The smoldering is the mildest form while the acute type represents the most aggressive form, but the life expectancy for each subtype is very poor reaching a maximum of 24 months. Although the molecular mechanism of virus transformation is highly complex and not entirely clarified, several studies highlighted that the viral regulatory proteins Tax and HTLV-1 bZIP factor (HBZ) play key roles in driving oncogenesis in HTLV-1 infected cells (Figure 1). The Tax protein has been demonstrated to trigger a number of immortalization/transformation related events in the early phase after infection, such as activation of the interaction with cAMP-responsive element-binding protein/activating transcription factor (CREB/ATF), the activation of NF-κB transcription factor, inhibition of p53, dysregulation of the cell cycle by interfering with the cellular checkpoint, impairment of cellular DNA repair mechanisms resulting in genetic instability, induction of DNA damage through production of reactive oxygen species, induction of both pro-apoptotic and anti-apoptotic activities (Chlichlia and Khazaie, 2010). In particular, Tax has been recently shown to intervene at an early phase of cell transformation by upregulating a family of early growth factor1 (EGR1), which upregulate the NF-κB system, establishing a positive feedback loop (Huang et al., 2017). Thus, Tax is likely required to initiate leukemogenesis while its expression is not routinely detected later in fresh cells from at least 50% of patients with established ATL. Recent data, however, demonstrated that survival of ATL cells depend on transient tax expression (Dassouki et al., 2015; Mahgoub et al., 2018) Conversely, HBZ is persistently expressed, even if at low level, in vivo in ATL cells, and interacts with elongation factors, Rb/E2F-1 complex, for cell cycle progression (Kawatsuki et al., 2016), inhibits apoptosis and upregulates expression of CCR4, thus promoting proliferation and migration of T cells (Sugata et al., 2016) and finally inducing global epigenetic changes in infected cells. In addition epigenetic dysregulation plays a role in ATL transformation consisting in GpC methylation of cell cycle, p53, apoptotic genes and histone modification of epigenetic reprogramming genes (Watanabe, 2017).
Similarly to leukemic cells of different types, ATL cells exhibit high expression of genes associated to cell proliferation/death, cytokines, chemokines and/or markers of cell transformation. Therefore, shared potential pharmacological targets can justify in ATL the use of biological therapy set up for other malignancies. As observed in other neoplasia, the balance between pro and anti apoptotic response is subverted in ATL cells. Preclinical studies have shown that HTLV-1 infection in vitro gives rise, in a first phase, to high proliferation and a concomitant high apoptosis rate in infected cells until, in a successive phase, the selection of immortalized clones lead to outgrowth of cells preferentially exhibiting anti-apoptotic gene expression (Matteucci et al., 2004). Coherently, transformed clones from ATL patients over-express in culture the anti-apoptotic Bcl-2, Bcl-xL, and Bcl-w proteins and exhibit ex vivo a 10- to 20-fold higher sensitivity to navitoclax (ABT-263), an orally bio-available mimetic of the Bcl-2 homology domain 3 small molecule, as compared to non-HTLV-1-associated leukemic cells (Tse et al., 2008). Interestingly, molecular studies showed that the efficacy of navitoclax in ATL cells in vitro was increased by Tax induced upregulation of the pro-apoptotic Bax gene. However, the side effects of navitoclax limit its therapeutic use in vivo. Another crucial target in ATL can be detected in the complex network of autocrine (IL-2-IL-2Rα/IL-15-IL-15Rα) and paracrine (IL-9) loops, able to drive ex vivo spontaneous proliferation of ATL cells at an early stage (Chen et al., 2010). The three involved cytokines share in common a γc receptor whose expression is regulated by a family of kinase (JAK/STAT). Interestingly, JAK/STAT selective inhibitors suppressed the proliferation of smoldering/chronic ATL cells ex vivo (Ju et al., 2011). Given that combining inhibitors of the same signaling pathway can increase the chance to block cancer cell growth, a combination of navitoclax and of the JAK/STAT inhibitor ruxolitinib, was tested on ATL cells ex vivo and in animals. The combination provided additive/synergistic activity in inhibiting proliferation of ATL cells, delayed tumor growth and prolonged survival in tumor –bearing mice. This was associated to increasing inhibition of Bcl-xL which favored the upregulation of the pro apoptotic gene expression (Zhang et al., 2015). In vivo, the immune response has a remarkable impact on the turnover of HTLV-1 infected clones. Actually, cytotoxic T lymphocytes recognizing the immunodominant viral protein Tax and HBZ are both critical to determine the proviral load (Bangham and Matsuoka, 2017). Ruxolitinib is currently under evaluation in phase 2 clinical trials in ATL patients.
Resistance to conventional chemotherapy prompted a reconsideration of therapy in ATL. Taking into account the retroviral etiology of the disease and that antiretroviral drugs proved their effectiveness in counteracting HIV infection, the use of AZT was tested in ATL. The rationale for this therapeutic intervention was to keep a low level of virus spreading and, possibly, also to limit the onset of inflammatory processes. In fact, successful results were initially reported in two preliminary phase II studies, using the combination of AZT and alpha interferon (IFN) showing an unexpected high response rate, particularly in previously untreated acute ATL patients (Gill et al., 1995; Hermine et al., 1995, 1998). A prospective phase II study on 13 patients who received AZT/IFN treatment as initial therapy, showed nine complete response (CR) and four partial remission with mild toxicity. The CR patients survived more than three years, after which most of the patients relapse underlying the need for additional therapy with AZT/IFN (Hermine et al., 2002). The impact of first-line AZT/IFN therapy on long-term survival was reported in a worldwide meta-analysis showing a significant improvement of survival of the leukemic subtypes with an unprecedented 100% 5-year overall-survival in chronic and smoldering ATL, at a median follow-up time of 5 years (Bazarbachi et al., 2010). Although AZT/IFN therapy provided reasonable management of ATL, most patients relapse. To counteract this aspect, based on previous experience in acute promyelocytic leukemia, arsenic trioxide (AS) was tested in ATL. The arsenic/IFN combination induced proteasomal degradation of Tax through stepwise poly-sumoylation and SUMO-dependent ubiquitination (El-Sabban et al., 2000; Dassouki et al., 2015). This combination cured murine ATL derived from tax-transgenic through selective targeting of ATL leukemia initiating cells (El Hajj et al., 2010). The triple combination of arsenic, IFN and AZT resulted in a high rate of response in chronic ATL patients (Kchour et al., 2009). However, the mechanisms involved in the therapeutic effectiveness of AZT/IFN are not clear, although in vitro studies demonstrated that this combination differently affects HTLV-1 mRNA and viral protein expression and activates the p53 pathway and apoptosis in HTLV-1 infected cells (Kinpara et al., 2013). Nevertheless, no clear evidence was provided concerning the inhibition of viremia by AZT/IFN in ATL patients. However, viremia in ATL patients is low and viral load, reverse transcriptase (RT) activity and/or other virus related assays, carried out in lymphocytes from patients, could be more reliable parameters for assessing HTLV-1 replicative potential in ATL patients. Interestingly, we have recently reported that long-term in vivo therapy with AZT and IFN actually caused complete inhibition of RT activity, reduction of p19 release and viral mRNA, and a dramatic decrease of the oligoclonal index, in short-term cultures of PBMCs from ATL patients who responded to therapy, but not in those who did not respond (Macchi et al., 2017). Thus, the above reported data sustain that the therapeutic efficacy of AZT/IFN combination in ATL is actually mediated, at least in part, by the inhibition of RT-dependent viral replication. Consequently, we can hypothesize that the AZT/IFN combination in ATL patients targets viral replication presumably outside leukemic cells. This could occur in cells such as dendritic cells or in newly infected T lymphocytes, immediately after their first contact with the virus, or other cell types in which a dynamic, continuous viral replication occurs. In this case, AZT/IFN treatment can impede HTLV-1 viral replication that could provide a microenvironment that is mandatory for survival and/or renewal of ATL cells (Figure 1). This could occur through direct cell-to-cell communication or paracrine stimulation through secreted Tax or various cytokines/chemokines as reported for chronic lymphocytic leukemia (Bazarbachi et al., 2011). Moreover, these findings could explain the impossibility to set-up long-term culture of ATL cells in vitro and why the AZT/IFN combination exerts a beneficial effect in vivo but not ex vivo on ATL cells. A recent trial using EPOCH chemotherapy in combination with bortezomib, to block NF-κB activation, and raltegravir, as antiviral drug, in acute ATL and in ATL lymphoma showed that this regiment was well tolerated, leading to 67% response rate. Changes in RNA viral load and HBZ viral expression ex vivo were found as reliable parameters of response as well as inhibition of viral replication and repression of NF-κB activation through proteasome inhibition (Ratner et al., 2016). Thus, accumulating evidence sustains that controlling virus spread is a crucial aspect in ATL therapy.
Regarding in vitro studies, AZT and tenofovir inhibit virus transmission to PBMC in short-term cultures and interfere with immortalization in the long run at a drug concentration which was poorly toxic toward uninfected cells (Macchi et al., 1997; Balestrieri et al., 2005). Conversely, lamivudine was not effective in inhibiting HTLV-1 infection in vitro, presumably owed to the presence of an isoleucine at position 118 in HTLV-1 RT, conferring natural resistance to 3TC (Balestrieri et al., 2002; Toro et al., 2003). Different not licensed compounds, such as the PCOAN phosphonates, were able to inhibit cell-to-cell HTLV-1 transmission directly inhibiting the HTLV-1 RT activity, as demonstrated by a cell-free assay (Balestrieri et al., 2008b). Inhibition of HTLV-1 infection could also rely on other still unclear mechanisms, as shown in case of compounds of natural origin such as carbohydrate-binding agents (Balestrieri et al., 2008a) and an extract from the seeds of bergamot, which remarkably blocked virus horizontal transmission in vitro (Balestrieri et al., 2011). In addition, raltegravir and diketo acid, MK-2048, were active inhibitors of viral transmission as well as viral immortalization of HTLV-1 in lymphoid and non lymphoid cells, in vitro (Seegulam and Ratner, 2011).
Further targets are being investigated to find new therapeutic approaches in ATL (Figure 2). Tax is known to remarkably affect the host cell proliferation by directly intervening in the processes regulating DNA transcription, replication and repair. On the basis of this regulatory role of Tax in HTLV-1 transformation, a few druggable targets have been demonstrated in preclinical studies. CDK9 is a component of a transcription factor, P-TEFb, essential for transcription of most MHC class II genes and for the transcriptional elongation by phosphorylating the C-terminal domain of RNA polymerase II. The importance of CDK9 for a targeted therapy was demonstrated in advanced stage of chronic lymphocytic leukemia and multiple myeloma (Tong et al., 2010). An additional reason to investigate CDK9 as a possible molecular target in ATL is that P-TEFb is present within HTLV-1 transformed cells in Tax-regulated complexes (Cho et al., 2010). In fact, the P-TEFb/CDK-9 inhibitor, BAY 1143572, was able to block the growth of ATL cells ex vivo, and to decrease MCL-1 and c-Myc expression levels. In addition, BAY 1143572 decreased ATL cell migration in the liver and bone marrow in a model of ATL in vivo xenograft, in immunocompromised NOG mice (Narita et al., 2017). Tax was reported to affect the replicative fork during DNA replication by blocking the progression of the process (Chaib-Mezrag et al., 2014). Helicases are deeply involved in DNA double-strand break repair through the homologous repair as well as the non-homologous end-joining pathway. In particular, the WRN helicases are mutated in cancer and are generally highly expressed in human leukemia (Sallmyr et al., 2008). This finding prompted to assay WRN helicases inhibitors in ATL cells. The results showed that the WRN inhibitors NSC 19630 and NSC 617145 efficiently killed HTLV-1-transformed and patient-derived cells, by inducing cell cycle arrest, downregulation of BCl-2, caspase 3 activation, and apoptosis in a dose-dependent manner, without affecting HTLV-1 expression (Moles et al., 2016). Tax was also recognized to induce genomic double strand breaks during DNA replication and alteration in the subsequent use of non-homologous end joining pathway for repair during the S phase (Baydoun et al., 2012). Thus, the genomic instability afforded by Tax represents a possible target within the repair enzymes family. PJ-34, a small molecule inhibitor of poly (ADP-ribose) polymerase (PARP), arrested cell cycle at S/G2M phase, inducing reactivation of p53 and caspase 3 activation in HTLV-1 infected cells. However, MT-2 chronically infected cells were resistant to PJ-34, showing reduced caspase 3 cleavage and increased RelA/p65 expression. These results suggest that the NF-κB system might be involved in resistance to PJ-34 (Bai et al., 2015). The inhibitors of JAK/STAT pathway regulating expression of IL-2R common γ chain reported as therapeutic option in ATL, were found to be highly immunosuppressive. Thus more recent data proposed a different JAK/STAT-pathway associated target, relying on the Pim 1 downstream target of JAK, whose expression is regulated by miRNA124a. The Pim 1 gene was found to be constitutively expressed in 71% of freshly isolated ATL cells and in chronically HTLV-1 infected cell lines, while PBMC from healthy donors were negative. Treatment with the Pim 1 inhibitors, SMI-4a or AZD1208, decreased ATL cells proliferation and decreased Pim 1 activity as demonstrated by downregulation of p4E-BP1, p-p70S6K, and p-Bad. The AZD 1208 was found more efficacious than SMI-4a. Moreover, AZD 1208 significantly inhibited ATL tumors in the pre-clinical NOG mice model (Bellon et al., 2016), showing that the JAK/STAT-Pim1 pathway could be a novel therapeutic target for the treatment of ATL. In addition the ATL cells exhibited a downregulation of miRNA and Dicer expression. The suitability of these target was demonstrated by the fact that the in vitro effect of deacetylase inhibitor, valproate, on ATL cells was owed to the rescue of the pre-miRNA maturation pathway (Gazon et al., 2016).
Theoretically, ATL cells exhibit a number of potential, different viral and cellular pharmacological targets. A number of studies suggests to take under consideration the suitability of numerous, known drugs to counteract ATL. However, it is hard to explain why, despite broad chances of potentially druggable targets, success is still limited in ATL clinical studies. Possible reasons could reside in the long latency of HTLV-1 infection, allowing the virus to escape host response, as well as the lack of suitable markers of disease progression. Hopefully, more deep knowledge of how the virus affects the regulation of host immune response and the metabolic requirements of transformed cells could represent new issues for future challenges in ATL therapy.
FM-M searched for literature, wrote the paper, and performed the elaboration and graphical representation of the figures. AM, AB, and BM searched for literature and wrote the paper. SG searched for literature. OH revised the paper.
This work was supported by the Italian Ministry of University and Research, Projects of National Interest (PRIN; AM and BM); Istituto Superiore di Sanità, AIDS Project; the University of Rome “Tor Vergata” (BM); and the University of Messina and the Institute of Translational Pharmacology (AM). FM-M is a recipient of a fellowship from Centro Neurolesi Bonino Pulejo (IRCCS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bai, X. T., Moles, R., Chaib-Mezrag, H., and Nicot, C. (2015). Small PARP inhibitor PJ-34 induces cell cycle arrest and apoptosis of adult T-cell leukemia cells. J. Hematol. Oncol. 8:117. doi: 10.1186/s13045-015-0217-2
Balestrieri, E., Ascolani, A., Igarashi, Y., Oki, T., Mastino, A., Balzarini, J., et al. (2008a). Inhibition of cell-to-cell transmission of human T-cell lymphotropic virus type 1 in vitro by carbohydrate-binding agents. Antimicrob. Agents Chemother. 52, 2771–2779. doi: 10.1128/AAC.01671-07
Balestrieri, E., Forte, G., Matteucci, C., Mastino, A., and Macchi, B. (2002). Effect of lamivudine on transmission of human T-cell lymphotropic virus type 1 to adult peripheral blood mononuclear cells in vitro. Antimicrob. Agents Chemother. 46, 3080–3083. doi: 10.1128/AAC.46.9.3080-3083.2002
Balestrieri, E., Matteucci, C., Ascolani, A., Piperno, A., Romeo, R., Romeo, G., et al. (2008b). Effect of phosphonated carbocyclic 2′-oxa-3′-aza-nucleoside on human T-cell leukemia virus type 1 infection in vitro. Antimicrob. Agents Chemother. 52, 54–64.
Balestrieri, E., Pizzimenti, F., Ferlazzo, A., Giofre, S. V., Iannazzo, D., Piperno, A., et al. (2011). Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg. Med. Chem. 19, 2084–2089. doi: 10.1016/j.bmc.2011.01.024
Balestrieri, E., Sciortino, M. T., Mastino, A., and Macchi, B. (2005). Protective effect of the acyclic nucleoside phosphonate tenofovir toward human T-cell leukemia/lymphotropic virus type 1 infection of human peripheral blood mononuclear cells in vitro. Antiviral Res. 68, 154–162. doi: 10.1016/j.antiviral.2005.09.001
Bangham, C. R. M., and Matsuoka, M. (2017). Human T-cell leukaemia virus type 1: parasitism and pathogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160272. doi: 10.1098/rstb.2016.0272
Baydoun, H. H., Bai, X. T., Shelton, S., and Nicot, C. (2012). HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS One 7:e42226. doi: 10.1371/journal.pone.0042226
Bazarbachi, A., Plumelle, Y., Carlos Ramos, J., Tortevoye, P., Otrock, Z., Taylor, G., et al. (2010). Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 28, 4177–4183. doi: 10.1200/JCO.2010.28.0669
Bazarbachi, A., Suarez, F., Fields, P., and Hermine, O. (2011). How I treat adult T-cell leukemia/lymphoma. Blood 118, 1736–1745. doi: 10.1182/blood-2011-03-345702
Bellon, M., Lu, L., and Nicot, C. (2016). Constitutive activation of Pim1 kinase is a therapeutic target for adult T-cell leukemia. Blood 127, 2439–2450. doi: 10.1182/blood-2015-11-685032
Berkowitz, J. L., Janik, J. E., Stewart, D. M., Jaffe, E. S., Stetler-Stevenson, M., Shih, J. H., et al. (2014). Safety, efficacy, and pharmacokinetics/pharmacodynamics of daclizumab (anti-CD25) in patients with adult T-cell leukemia/lymphoma. Clin. Immunol. 155, 176–187. doi: 10.1016/j.clim.2014.09.012
Chaib-Mezrag, H., Lemacon, D., Fontaine, H., Bellon, M., Bai, X. T., Drac, M., et al. (2014). Tax impairs DNA replication forks and increases DNA breaks in specific oncogenic genome regions. Mol. Cancer 13:205. doi: 10.1186/1476-4598-13-205
Chen, J., Petrus, M., Bryant, B. R., Nguyen, V. P., Goldman, C. K., Bamford, R., et al. (2010). Autocrine/paracrine cytokine stimulation of leukemic cell proliferation in smoldering and chronic adult T-cell leukemia. Blood 116, 5948–5956. doi: 10.1182/blood-2010-04-277418
Chlichlia, K., and Khazaie, K. (2010). HTLV-1 Tax: linking transformation, DNA damage and apoptotic T-cell death. Chem. Biol. Interact. 188, 359–365. doi: 10.1016/j.cbi.2010.06.005
Cho, W. K., Jang, M. K., Huang, K., Pise-Masison, C. A., and Brady, J. N. (2010). Human T-lymphotropic virus type 1 Tax protein complexes with P-TEFb and competes for Brd4 and 7SK snRNP/HEXIM1 binding. J. Virol. 84, 12801–12809. doi: 10.1128/JVI.00943-10
Dassouki, Z., Sahin, U., El Hajj, H., Jollivet, F., Kfoury, Y., Lallemand-Breitenbach, V., et al. (2015). ATL response to arsenic/interferon therapy is triggered by SUMO/PML/RNF4-dependent Tax degradation. Blood 125, 474–482. doi: 10.1182/blood-2014-04-572750
Dittus, C., and Sloan, J. M. (2017). Adult T-cell leukemia/lymphoma: a problem abroad and at home. Hematol. Oncol. Clin. North Am. 31, 255–272. doi: 10.1016/j.hoc.2016.11.005
El Hajj, H., El-Sabban, M., Hasegawa, H., Zaatari, G., Ablain, J., Saab, S. T., et al. (2010). Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 207, 2785–2792. doi: 10.1084/jem.20101095
El-Sabban, M. E., Nasr, R., Dbaibo, G., Hermine, O., Abboushi, N., Quignon, F., et al. (2000). Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood 96, 2849–2855.
Gallo, R. C. (2005). The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology 2:17. doi: 10.1186/1742-4690-2-17
Gazon, H., Belrose, G., Terol, M., Meniane, J. C., Mesnard, J. M., Cesaire, R., et al. (2016). Impaired expression of DICER and some microRNAs in HBZ expressing cells from acute adult T-cell leukemia patients. Oncotarget 7, 30258–30275. doi: 10.18632/oncotarget.7162
Gill, P. S., Harrington, W. Jr., Kaplan, M. H., Ribeiro, R. C., Bennett, J. M., Liebman, H. A., et al. (1995). Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N. Engl. J. Med. 332, 1744–1748. doi: 10.1056/NEJM199506293322603
Hermine, O., Allard, I., Levy, V., Arnulf, B., Gessain, A., Bazarbachi, A., et al. (2002). A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol. J. 3, 276–282. doi: 10.1038/sj.thj.6200195
Hermine, O., Bouscary, D., Gessain, A., Turlure, P., Leblond, V., Franck, N., et al. (1995). Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N. Engl. J. Med. 332, 1749–1751. doi: 10.1056/NEJM199506293322604
Hermine, O., Ramos, J. C., and Tobinai, K. (2018). A review of new findings in adult t-cell leukemia-lymphoma: a focus on current and emerging treatment strategies. Adv. Ther. 35, 135–152. doi: 10.1007/s12325-018-0658-4
Hermine, O., Wattel, E., Gessain, A., and Bazarbachi, A. (1998). Adult T cell leukaemia: a review of established and new treatments. BioDrugs 10, 447–462. doi: 10.2165/00063030-199810060-00003
Huang, Q., Niu, Z., Han, J., Liu, X., Lv, Z., Li, H., et al. (2017). HTLV-1 Tax upregulates early growth response protein 1 through nuclear factor-kappaB signaling. Oncotarget 8, 51123–51133. doi: 10.18632/oncotarget.17699
Ishida, T., Iida, S., Akatsuka, Y., Ishii, T., Miyazaki, M., Komatsu, H., et al. (2004). The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-Cell leukemia/lymphoma. Clin. Cancer Res. 10, 7529–7539. doi: 10.1158/1078-0432.CCR-04-0983
Ishida, T., Utsunomiya, A., Iida, S., Inagaki, H., Takatsuka, Y., Kusumoto, S., et al. (2003). Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 9, 3625–3634.
Ju, W., Zhang, M., Jiang, J. K., Thomas, C. J., Oh, U., Bryant, B. R., et al. (2011). CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood 117, 1938–1946. doi: 10.1182/blood-2010-09-305425
Kato, K., and Akashi, K. (2015). Recent advances in therapeutic approaches for adult t-cell leukemia/lymphoma. Viruses 7, 6604–6612. doi: 10.3390/v7122960
Kawatsuki, A., Yasunaga, J. I., Mitobe, Y., Green, P. L., and Matsuoka, M. (2016). HTLV-1 bZIP factor protein targets the Rb/E2F-1 pathway to promote proliferation and apoptosis of primary CD4(+) T cells. Oncogene 35, 4509–4517. doi: 10.1038/onc.2015.510
Kchour, G., Tarhini, M., Kooshyar, M. M., El Hajj, H., Wattel, E., Mahmoudi, M., et al. (2009). Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood 113, 6528–6532. doi: 10.1182/blood-2009-03-211821
Kinpara, S., Kijiyama, M., Takamori, A., Hasegawa, A., Sasada, A., Masuda, T., et al. (2013). Interferon-alpha (IFN-alpha) suppresses HTLV-1 gene expression and cell cycling, while IFN-alpha combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology 10:52. doi: 10.1186/1742-4690-10-52
Macchi, B., Balestrieri, E., Frezza, C., Grelli, S., Valletta, E., Marcais, A., et al. (2017). Quantification of HTLV-1 reverse transcriptase activity in ATL patients treated with zidovudine and interferon-alpha. Blood Adv. 1, 748–752. doi: 10.1182/bloodadvances.2016001370
Macchi, B., Faraoni, I., Zhang, J., Grelli, S., Favalli, C., Mastino, A., et al. (1997). AZT inhibits the transmission of human T cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J. Gen. Virol. 78, 1007–1016. doi: 10.1099/0022-1317-78-5-1007
Mahgoub, M., Yasunaga, J. I., Iwami, S., Nakaoka, S., Koizumi, Y., Shimura, K., et al. (2018). Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. U.S.A. 115, E1269–E1278. doi: 10.1073/pnas.1715724115
Matteucci, C., Balestrieri, E., Macchi, B., and Mastino, A. (2004). Modulation of apoptosis during HTLV-1-mediated immortalization process in vitro. J. Med. Virol. 74, 473–483. doi: 10.1002/jmv.20201
Moles, R., Bai, X. T., Chaib-Mezrag, H., and Nicot, C. (2016). WRN-targeted therapy using inhibitors NSC 19630 and NSC 617145 induce apoptosis in HTLV-1-transformed adult T-cell leukemia cells. J. Hematol. Oncol. 9:121. doi: 10.1186/s13045-016-0352-4
Narita, T., Ishida, T., Ito, A., Masaki, A., Kinoshita, S., Suzuki, S., et al. (2017). Cyclin-dependent kinase 9 is a novel specific molecular target in adult T-cell leukemia/lymphoma. Blood 130, 1114–1124. doi: 10.1182/blood-2016-09-741983
Ratner, L., Rauch, D., Abel, H., Caruso, B., Noy, A., Barta, S. K., et al. (2016). Dose-adjusted EPOCH chemotherapy with bortezomib and raltegravir for human T-cell leukemia virus-associated adult T-cell leukemia lymphoma. Blood Cancer J. 6:e408. doi: 10.1038/bcj.2016.21
Sallmyr, A., Tomkinson, A. E., and Rassool, F. V. (2008). Up-regulation of WRN and DNA ligase IIIalpha in chronic myeloid leukemia: consequences for the repair of DNA double-strand breaks. Blood 112, 1413–1423. doi: 10.1182/blood-2007-07-104257
Seegulam, M. E., and Ratner, L. (2011). Integrase inhibitors effective against human T-cell leukemia virus type 1. Antimicrob. Agents Chemother. 55, 2011–2017. doi: 10.1128/AAC.01413-10
Sharma, K., Janik, J. E., O’Mahony, D., Stewart, D., Pittaluga, S., Stetler-Stevenson, M., et al. (2017). Phase II study of alemtuzumab (CAMPATH-1) in patients with HTLV-1-associated Adult T-cell leukemia/ lymphoma. Clin. Cancer Res. 23, 35–42. doi: 10.1158/1078-0432.CCR-16-1022
Shimoyama, M. (1991). Diagnostic-criteria and classification of clinical subtypes of adult t-cell leukemia-lymphoma - a report from the lymphoma-study- group (1984-87). Br. J. Haematol. 79, 428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x
Sugata, K., Yasunaga, J., Kinosada, H., Mitobe, Y., Furuta, R., Mahgoub, M., et al. (2016). HTLV-1 viral factor HBZ induces CCR4 to promote T-cell migration and proliferation. Cancer Res. 76, 5068–5079. doi: 10.1158/0008-5472.CAN-16-0361
Tagaya, Y., and Gallo, R. C. (2017). The exceptional oncogenicity of HTLV-1. Front. Microbiol. 8:1425. doi: 10.3389/fmicb.2017.01425
Tong, W. G., Chen, R., Plunkett, W., Siegel, D., Sinha, R., Harvey, R. D., et al. (2010). Phase I and pharmacologic study of SNS-032, a potent and selective Cdk2, 7, and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia and multiple myeloma. J. Clin. Oncol. 28, 3015–3022. doi: 10.1200/JCO.2009.26.1347
Toro, C., Rodes, B., Mendoza, C., and Soriano, V. (2003). Lamivudine resistance in human T-cell leukemia virus type 1 may be due to a polymorphism at codon 118 (V–>I) of the reverse transcriptase. Antimicrob. Agents Chemother. 47, 1774;author rely 1774–1775. doi: 10.1128/AAC.47.5.1774-1775.2003
Tse, C., Shoemaker, A. R., Adickes, J., Anderson, M. G., Chen, J., Jin, S., et al. (2008). ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428. doi: 10.1158/0008-5472.CAN-07-5836
Watanabe, T. (2017). Adult T-cell leukemia: molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood 129, 1071–1081. doi: 10.1182/blood-2016-09-692574
Watanabe, T., Tobinai, K., Shibata, T., Tsukasaki, K., Morishima, Y., Maseki, N., et al. (2011). Phase II/III study of R-CHOP-21 versus R-CHOP-14 for untreated indolent B-cell non-Hodgkin’s lymphoma: JCOG 0203 trial. J. Clin. Oncol. 29, 3990–3998. doi: 10.1200/JCO.2011.34.8508
Yamamoto, K., Utsunomiya, A., Tobinai, K., Tsukasaki, K., Uike, N., Uozumi, K., et al. (2010). Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J. Clin. Oncol. 28, 1591–1598. doi: 10.1200/JCO.2009.25.3575
Zell, M., Assal, A., Derman, O., Kornblum, N., Battini, R., Wang, Y., et al. (2016). Adult T-cell leukemia/lymphoma in the Caribbean cohort is a distinct clinical entity with dismal response to conventional chemotherapy. Oncotarget 7, 51981–51990. doi: 10.18632/oncotarget.10223
Keywords: HTLV-1, ATL, antiviral agents, biological therapy, targeted therapy
Citation: Marino-Merlo F, Mastino A, Grelli S, Hermine O, Bazarbachi A and Macchi B (2018) Future Perspectives on Drug Targeting in Adult T Cell Leukemia-Lymphoma. Front. Microbiol. 9:925. doi: 10.3389/fmicb.2018.00925
Received: 29 January 2018; Accepted: 20 April 2018;
Published: 09 May 2018.
Edited by:
Maria Grazia Romanelli, University of Verona, ItalyReviewed by:
Mathias Lichterfeld, Massachusetts General Hospital and Harvard Medical School, United StatesCopyright © 2018 Marino-Merlo, Mastino, Grelli, Hermine, Bazarbachi and Macchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Mastino, YW50b25pby5tYXN0aW5vQHVuaW1lLml0
†These authors have contributed equally to this work as co-last authors.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.