
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 19 April 2018
Sec. Virology
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.00751
This article is part of the Research Topic New and Innovative Strategies in The Development of Antivirals View all 13 articles
 Chenggang Liu1,2
Chenggang Liu1,2 Lei Jiang1,2
Lei Jiang1,2 Liangliang Liu1,2
Liangliang Liu1,2 Li Sun1
Li Sun1 Wenjun Zhao1,2
Wenjun Zhao1,2 Yuqiu Chen1,2
Yuqiu Chen1,2 Tianming Qi1,2
Tianming Qi1,2 Zongxi Han2
Zongxi Han2 Yuhao Shao2
Yuhao Shao2 Shengwang Liu2*
Shengwang Liu2* Deying Ma1*
Deying Ma1*The study was conducted to evaluate whether avian β-defensins (AvBDs) could be induced by Newcastle disease virus (NDV) infection, and to investigate the potential signaling pathway of AvBD2 induction in response to NDV infection as well. First, mRNA expression of AvBDs (1–14) was evaluated in the chicken embryo fibroblasts (CEFs) infected with NDV strain F48E9 at 6, 12, 24, 36, and 48 h post-inoculation (hpi), respectively. The results demonstrated a significant induction of AvBD2 in CEFs elicited by the NDV strain. Then, we expressed and purified the AvBD2 proteins in both eukaryotic cells and prokaryotic cells. Of the two recombinant AvBD2 proteins, only the protein expressed in eukaryotic cells showed directly antiviral activity against NDV strain F48E9 in vitro. Ligands of toll-like receptors (TLRs) were chosen as alternatives to NDV to further study signaling pathway of AvBD2 induction here, due to insufficient upregulation of AvBD2 expression elicited by NDV. We found that the mRNA expression of AvBD2 was highly upregulated by Pam3CSK4, FLA-ST, and ODN-M362. Then, four inhibitors of signaling pathway, including inhibitors of JNK, ERK1/2, p38 MAPK, and NF-κB, were used in this study. Of the four inhibitors, only inhibition of the p38 MAPK signaling pathway significantly reduced AvBD2 expression after stimulation with Pam3CSK4, FLA-ST and ODN-M362, respectively. Taken together, these results revealed that AvBD2 play a pivotal role in host innate immunity response to NDV infection. The mRNA expression of AvBD2 might be regulated in a p38 MAPK-dependent manner.
Newcastle disease virus (NDV), also known as avian paramyxovirus type 1, belongs to the genus Avulavirus in the family paramyxoviridae (Mayo, 2002) and is the etiological agent of Newcastle disease (ND), which is an important disease hazardous to the poultry industry. As a negative-sense, single-stranded, non-segmented, enveloped RNA virus, the NDV genome consists of 15,586 nucleotides (Liu et al., 2012). ND has a global distribution with a wide host range that not only results in high morbidity and mortality in poultry, but also has a great negative effect on the productivity of surviving birds (Wang et al., 2012). Numerous studies have focused on characterizing the pathogenesis of different NDV isolates in past years, while few studies have been done to evaluate host response to NDV infection. Our recent study demonstrated that NDV infection induces strong innate immune responses and intense inflammatory responses at early stage in goose (Xu et al., 2016). Similarly, it has been also reported that pigeon paramyxovirus type 1, a variant of NDV, induces immune responses characterized by activation of TLRs (TLR3 and TLR7), iNOS, and avian β-defensin (AvBD) 2 and 10 of pigeons post infection (Li et al., 2015).
The innate immune system is the first line of host defense against invading pathogens. Upon viral infection, the host cell detects the presence of viral components by means of germline-encoded pattern-recognition receptors (PRRs). In vertebrate species, three types of PRRs engage to recognize viruses: nucleotide oligomerization domain (NOD)-like receptors, Toll-like receptors (TLRs), and retinoic acid-inducible gene-1-like receptors (Yoneyama et al., 2004; Le Goffic et al., 2006; Allen et al., 2009; Barjesteh et al., 2016). TLRs are important PRRs that recognize essential components of microorganisms, such as membrane proteins, lipids, and nucleic acids (Rock et al., 1998). To date, at least 13 TLRs have been identified in mammals. Although TLR9 is absent and TLR8 is disrupted by a retroviral-like insertion element in avian species, other TLR orthologs have been described in avian species (Cheng et al., 2015). Additionally, two uncommon avian TLRs, TLR15 and TLR21, have been also identified (Keestra et al., 2010; Ramasamy et al., 2012). Interactions of TLRs with their specific ligands lead to an innate immune response through activation of MyD88-dependent or -independent intracellular pathways to activate the transcriptional factors mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB), resulting in the expression of host defense peptides (HDPs, also known as antimicrobial peptides) and cytokines (Sato et al., 2005; Yamamoto and Takeda, 2010; Abdel-Mageed et al., 2014).
The MAPK signal transduction pathways are evolutionarily conserved in eukaryotes and involved in many cellular processes, including immune response, apoptosis, and proliferation (Chang and Karin, 2001; Whitmarsh, 2007; Kyriakis and Avruch, 2012). The MAPK family of serine/threonine kinases consists of at least three subfamilies: c-Jun N-terminal kinase (JNK), p38 MAPK, and extracellular signal-regulated kinase 1/2 (ERK1/2) (Kogut et al., 2012). As a regulator of the transcription factor c-Jun and a mediator of intra- or extra-cellular stress, the JNK cascade is the stress-activated protein kinase cascade (Davis, 1994; Plotnikov et al., 2011). The p38 cascade is another MAPK pathway that demonstrates considerable cross-talk and shares components with the other stress-induced cascade of JNKs (Plotnikov et al., 2011). The ERK1/2 cascade was the first MAPK pathway elucidated (Seger and Krebs, 1995). In mammalian cells, HDPs could be induced by activation of MAPKs during stimulation by extracellular stress signals (Krisanaprakornkit et al., 2002; Lee et al., 2008; Lewis et al., 2016).
The HDPs are important components of the innate immune response. Being the most studied family of cysteine-rich HDPs, defensins are critical to innate immunity and subsequent protection against infection (Yang et al., 1999). They can be divided into three subfamilies, named α, β, and θ-defensins according to their structural properties (Ganz, 2003). α-Defensins are only present in mammalian species and form disulfide bridges between Cys1–Cys6, Cys2–Cys4, and Cys3–Cys5. β-Defensins can be found in all vertebrate species and form disulfide bridges between Cys1–Cys5, Cys2–Cys4, and Cys3–Cys6. θ-Defensins are cyclic defensins, with cystine bridges between Cys1-Cys6, Cys2-Cys5, and Cys3-Cys4 and are found in rhesus monkeys and baboons (Lehrer and Ganz, 2002; Yang et al., 2004; Klotman and Chang, 2006; Lehrer et al., 2012; Cuperus et al., 2013). Of the three defensin subfamilies, only β-defensins have been found in birds (Cuperus et al., 2013). In addition to antibacterial activities, defensins from various species have been demonstrated previously to display antiviral activity. It has been reported that human β-defensins show direct inhibitory activities against various viruses, including human immunodeficiency virus (HIV) (Quinones-Mateu et al., 2003), adeno-associated virus (Virella-Lowell et al., 2000), adenovirus (Bastian and Schafer, 2001), influenza virus (Leikina et al., 2005), and respiratory viruses (Zhao et al., 2016). It is also reported that rainbow trout β-defensin exhibit antiviral activity against the enveloped viral hemorrhagic septicemia virus (Falco et al., 2008). In addition to their direct antimicrobial activity, some β-defensins are capable of promoting local innate and systemic adaptive immune responses (Yang et al., 1999; Barabas et al., 2013; Cuperus et al., 2013). It has been shown that β-defensins from various species can be induced by various viruses following infection both in vivo and in vitro (Grubor et al., 2004; Chong et al., 2008; Ma et al., 2011). Sheep β-defensin-1 was increased following parainfluenza virus type 3 infection (Grubor et al., 2004). In mice, influenza virus could induce β-defensin-1, -2, and -3 upregulation in the lungs (Chong et al., 2006). Human epithelial cells highly expressed β-defensins when infected virus (Proud et al., 2004). To date, 14 AvBDs have been identified in chicken (Lynn et al., 2007). In addition to their antibacterial potential, AvBDs have also been demonstrated direct antiviral activity against various viruses, including pigeon Paramyxovirus type 1 (Li et al., 2015), infectious bronchitis virus (IBV) (Xu et al., 2015), and duck hepatitis virus (DHV) (Ma et al., 2012).
Based on the antiviral activity of AvBDs and its importance in the response to viral infections, as well as the need for new antiviral drugs, the purpose of this study was to assess induction of AvBD2 by NDV infection, and then speculate the signaling pathway of AvBD production.
All animal experimental procedures were approved by the Ethical and Animal Welfare Committee of Heilongjiang Province, China (License No. SQ20160408).
Specific-pathogen-free (SPF) embryonated chicken eggs were supplied by the Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Harbin, China). Chicken embryo fibroblasts (CEFs) were prepared with 9-day-old SPF embryonated chicken eggs as previously described (Shahsavandi et al., 2013). The CEFs were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine, at 37°C in a humidified atmosphere of 95% air/5% CO2. Expi293 FreeStyle cells were incubated at 37°C in a humidified atmosphere of 90% air/10% CO2 on an orbital shaker platform rotating at 125 rpm and then maintained in Expi293 Expression medium (Gibco, Carlsbad, CA, United States). The virulent NDV strain F48E9 was used in this study in the biosafety level-3 laboratory (Guo et al., 2014). The intracerebral pathogenicity index of the NDV strain is 2.0 (Guo et al., 2014). The median cell culture infective dose (TCID50) of the virus in CEFs was 10-6.75/100 μl (tested in this study).
The CEFs were cultured at an initial density of 1 × 104 cells per well in a 96-well cell culture plate and kept overnight. Firstly, survival rate of CEFs infected with NDV was measured by the Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s protocol. In brief, CEFs in different multiplicity of infection (MOI) (0.01, 0.1, 1, 10, and 100) at different time points (24, 36, and 48 h post-infection, hpi) was added to the CCK-8 solution (5 mg/ml, 10 μl/well) before incubation at 37°C for 1 h. Absorbance was then measured at a wavelength of 450 nm by using a microplate reader (model 680; Bio-Rad Laboratories, Hercules, CA, United States). Then, CEFs were cultured in 12-well cell culture plates at a density of 1 × 106 cells per well, and were used at 90% confluence. The cells were infected with NDV strain F48E9 at MOI of 1 at 37°C for 1.5 h. After an absorption period of 1.5 h, the wells were supplemented with DMEM containing 2% FBS after washing off the unattached virus. The cytopathic effect (CPE) was observed for 48 h in succession (Li et al., 2014). At 6, 12, 24, 36, and 48 hpi, the supernatants of the cell cultures were harvested and assayed for virus titer using the hemagglutination (HA) and TCID50, as previously described. Both the infected and uninfected cells were harvested for RNA isolation. Uninfected cells were used as negative controls in these experiments. The experiment was performed in triplicate. The challenge test was conducted in isolators in biosafety level-3 facilities under negative pressure.
The HA assay was performed according to standard method as described by reference (Sano and Ogawa, 2014). The end-point dilution is known as 1 HA unit (HAU) and the number of HAUs in each 50 μl is the reciprocal of the highest dilution (Sano and Ogawa, 2014). The TCID50 of NDV F48E9 strain in CEFs was calculated by using the Reed and Muench method (Reed and Muench, 1938). The experiment was performed in triplicate.
The RNA extraction and One-step Real-Time (RT)-PCR were conducted as described in previous studies (Xu et al., 2015, 2016). Primers of 18S rRNA and AvBDs (1–14) used in this study were described by reference (Xu et al., 2015).
The pCAG (+)-AvBD2-His plasmid, in which AvBD2 cDNA was in frame fused to the C-terminal His tag in the pCAG (+) vector plasmid (stored in our laboratory), was constructed by standard molecular biology techniques. Briefly, cDNA sequences that encoded AvBD2 bases (GenBank Accession No. NM_204992.2) (amino acid sequence: RDMLFCKGGSCHFGGCPSHLIKVGSCFGFRSCCKWPWNA) were amplified by PCR from the plasmid of pProEX-HTa-AvBD2 (Xu et al., 2015) using the following primers: forward, 5′–CCGCT CGAGGCCACCATGAGGATTCTTTACCTGCTTT–3′; reverse, 5′–CGGGATCCTTAATGGTGATGGTGATGATGTGCATTCCAAGGCCATTTG–3′. The PCR products flanked by the Xho I and Bam HI restriction sites were inserted into the same sites of the pCAG (+) vector. The resulting constructs were confirmed to contain AvBD2 by sequencing and were then transformed into Expi293 cells using the ExpiFectamine 293 Transfection system. A total of 5 days after transfection, the cells were harvested by centrifugation (750 × g, 25°C, 5 min) and then lysed by RIPA Lysis Buffer (Beyotime, Shanghai, China). After centrifugation (13,000 × g, 4°C, 30 min), the supernatant, including the soluble AvBD2 protein, was purified using the Ni-NTA Purification System (Invitrogen), and the purified AvBD2 protein was desalted using the Amicon® Ultra-15 3K Centrifugal Filter Device (EMD Millipore, Temecula, CA, United States). The released mature AvBD2 was examined by 12% SDS–PAGE (Li et al., 2015). The concentration of the protein was measured using the NanoVue Plus spectrophotometer (GE Healthcare, Cleveland, OH, United States). Then, AvBD2 protein band was cut out and analyzed by mass spectrometry (Beijing Protein Innovation Co., Ltd., Beijing, China).
The construct of pProEX-HTa-AvBD2 plasmid (Xu et al., 2015) was transformed into Escherichia coli BL21 (DE3) competent cells and induced as described previously (Xu et al., 2015). The protein was expressed, purified and refolded as described previously (Xu et al., 2015). Purified AvBD2 protein was desalted using the 3K centrifugal filter device. The protein was examined by 12% SDS-PAGE (Li et al., 2015). The concentration of the protein was measured using the NanoVue Plus spectrophotometer (GE Healthcare, Cleveland, OH, USA).
Three experiments were conducted to evaluate antiviral activities of both recombinant AvBD2 proteins against NDV by using CCK-8 assay (Gong et al., 2010). (1) The NDV strain (1 MOI) was pre-incubated with various concentrations of AvBD2 (3.75-120 ng/μl) freshly diluted in PB for1.5 h at 37°C. To begin infection, the cells were washed twice with PBS in order to remove FBS, followed by adding 100 μl of the AvBD2 protein-virus mixture and further incubated for1.5 h at 37°C. (2) CEFs were infected with NDV at MOI of 1 and incubated for 1.5 h at 37°C, followed by addition of various concentrations of AvBD2 (3.75-120 ng/μl) further incubated for 1.5 h at 37°C. (3) AvBD2 protein (3.75-120 ng/μl) was incubated with CEFs for 1.5 h at 37°C, followed by addition of NDV (1 MOI) and incubation for another 1.5 h at 37°C. Then, the cells were washed two times with PBS and 100 μl of fresh DMEM was added to each well and plates were incubated at 37°C for 48 h. Ten μl of CCK-8 solution (5 mg/ml) was added into each well of the cell culture plate and incubated further for 40 min at 37 °C. The absorbance at 450 nm was measured as described above. Cells without virus and AvBD2 mixture were used as a negative control. Cells infected with the virus only served as a positive control. The antiviral activity of AvBD2 was calculated as follows: protective rate = [(mean optical density of test – mean optical density of positive controls) / (optical density of negative controls – mean optical density of positive controls)] × 100% (Pauwels et al., 1988; Gong et al., 2010). The experiments were performed in triplicate. Furthermore, the IC50 value was calculated based on the log values by using GraphPad Prism 5 software in the present study (Motulsky, 2007).
The CEFs were cultured in 12-well cell culture plates and were used at 90% confluence. All TLR ligands were purchased from InvivoGen (San Diego, CA, USA) and were dissolved in double distilled H2O. The cells were stimulated with Pam3CSK4 (synthetic triacylated lipoprotein) (0.4 μg/ml), LPS (standard preparation of lipopolysaccharide from E. coli 055: B5) (2 μg/ml), poly I:C (polyinosinic-polycytidylic acid) (50 μg/ml), FLA-ST (flagellin from Salmonella typhimurium) (2 μg/ml), R848 (imidazoquinoline compound) (5 μg/ml), and ODN-M362 (class C CpG oligonucleotide) (2.5 μM), respectively, and then harvested at 6, 24, and 48 h post-stimulation (hpt) for RNA isolation and AvBD2 mRNA evaluation as described above. The negative controls were treated with the same volume of endotoxin-free H2O. All experiments were performed in triplicate.
The CEFs were cultured in 12-well cell culture plates and were used at 90% confluence. CEFs were pre-treated for 1 h with various inhibitors (Beyotime, Shanghai, China) of the signaling pathway dissolved in dimethyl sulfoxide (DMSO). The following concentration were used: JNK inhibitor (SP600125), 50 μM; ERK1/2 inhibitor (PD98059), 50 μM; p38 MAPK inhibitor (SB203580), 50 μM; NF-κB inhibitor (PDTC), 50 μM; the negative control (DMSO, volume < 0.1%), and an untreated control, respectively. Two experiments were conducted to evaluate inhibition of the signaling pathway on AvBD2 expression elicited by either of NDV, or ligands of TLRs. After pre-treatment with inhibitors for 1 h, (1) The cells were infected with NDV (1 MOI) at 37°C for 1.5 h. The wells were supplemented with DMEM containing 2% FBS after washing off the unattached virus and the CEFs were cultured for another 36 or 48 h. (2) Either of ODN-M362 (2.5 μM), FLA-ST (2 μg/ml), or Pam3CSK4 (0.4 μg/ml) were added to the plates and the CEFs were cultured for another 24 h. Then, the cells were harvested for RNA isolation and AvBD2 mRNA evaluation as described above. All experiments were performed in triplicate.
Statistical analysis was used GraphPad Prism software v5.01 (GraphPad Software Inc., San Diego, CA, United States). One-way analysis of variance followed by the Tukey–Kramer post-test was used. A p < 0.05 was considered statistically significant.
The CEFs were infected with NDV strain F48E9 at different MOI (0.01, 0.1, 1, 10, and 100). Cell survival rate was measured using the CCK-8 assay at different hours post-infection (24, 36, and 48 hpi). The results showed that the survival rate of cells was significantly increased in treatments of 1–100 MOI of NDV infection at 24 hpi, compared to the other treatments (p < 0.05) (Figure 1A). Then, the CPE (1 MOI) was monitored for 48 h. The results showed no obvious CPE in NDV-infected CEFs until 24 hpi. The CPE was characterized by syncytia owing to coalescence with adjacent infected cells (Figure 1B). Similarly, HA titers of cell supernatants were undetectable at 6 and 12 hpi, but it was detectable at 24 hpi and peaked at 48 hpi (Figure 1C). To confirm the presence of NDV in the infected cells, viral loads of NDV in CEFs were examined by RT-PCR (Figure 1D). Viral replication was time-dependent, occurring as early as 6 hpi, and peaked at 36 hpi. In contrast, no viral RNA was detected in the uninfected cells (data not shown). Collectively, these results confirmed successful NDV infection of cells. Furthermore, the supernatant was tested for TCID50 in CEF and the result showed that the titers were detectable at 12 hpi and reached the highest at 48 hpi (Figure 1E).
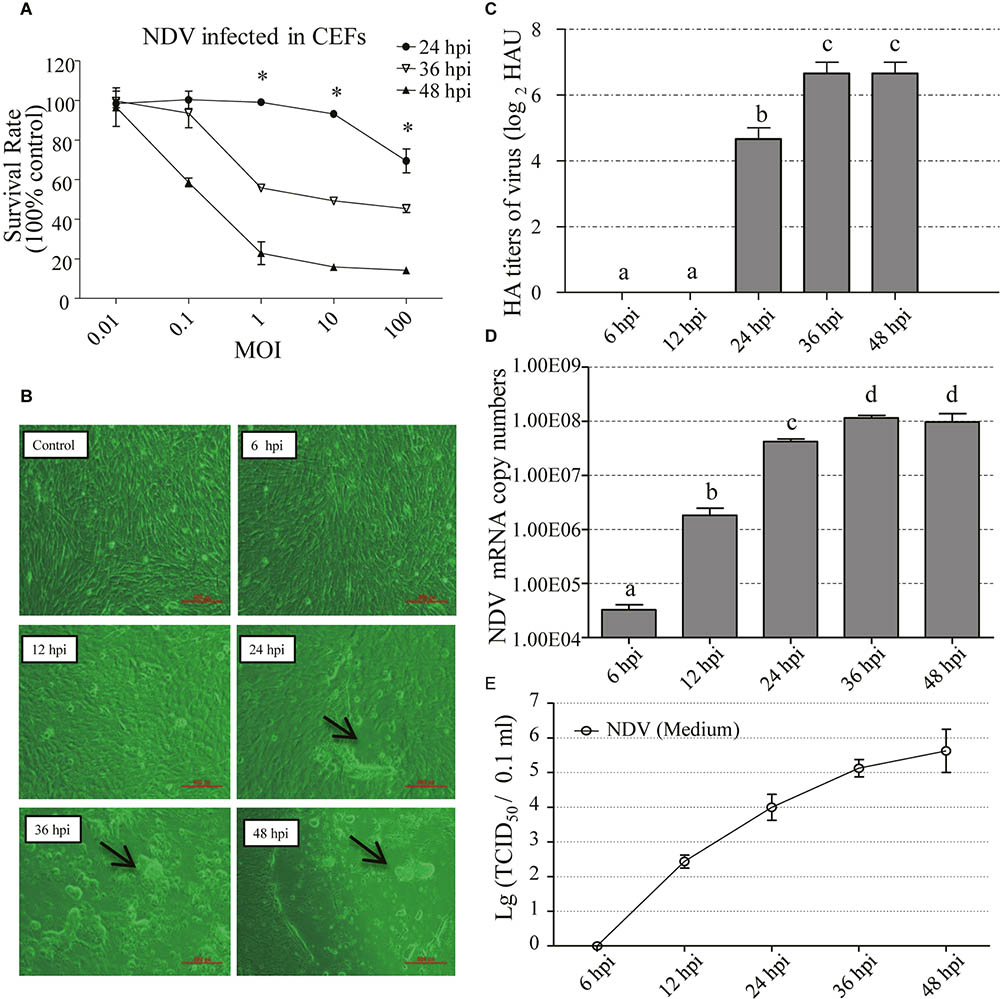
FIGURE 1. Biological characterization of NDV-F48E9 infection in CEFs. (A) Survival rate of NDV infected CEFs in different MOI at different time points. CCK-8 was used to detect cell viability. Absorbance was measured at a wavelength of 450 nm by using a microplate reader (model 680; Bio-Rad Laboratories, Hercules, CA, United States). Protective rate was described as previous reports (Pauwels et al., 1988; Gong et al., 2010). Each group has three replicates. The difference between the same MOI at three different infection time points is represented by ∗p < 0.05. (B) Cytopathic effect of NDV at 6, 12, 24, 36, and 48 hpi. Black arrow indicated the syncytia induced by NDV. (C) HA titer of the supernatants of NDV-infected cells. (D) Viral RNA copy numbers measured by RT-PCR in CEFs infected with NDV. (E) TCID50 of the supernatants of NDV-infected cells. All assays were performed in triplicates. Each bar is the mean ± SEM. a,b,c,dThe values with different letters are significantly different (p < 0.05)
In order to determine whether mRNA expression of AvBDs in CEFs was induced in response to NDV infection, expression of AvBDs 1–14 in the CEFs was evaluated at 6, 12, 24, 36, and 48 hpi, respectively, using RT-PCR. In general, most of the AvBDs measured were detectable in both the control and the infected CEFs at each time point. In contrast, little expression of AvBD1, 7, 8, 13, and 14 was detected in CEFs during the experimental period (Figure 2). Interestingly, of the 14 AvBDs, only AvBD2 expression was significantly upregulated in CEFs at 36 hpi, and peaked at 48 hpi, as compared with the control (p < 0.05). However, upregulation of the other AvBDs expression was less significant in NDV infected CEFs at each time point, as compared to the control (p > 0.05).
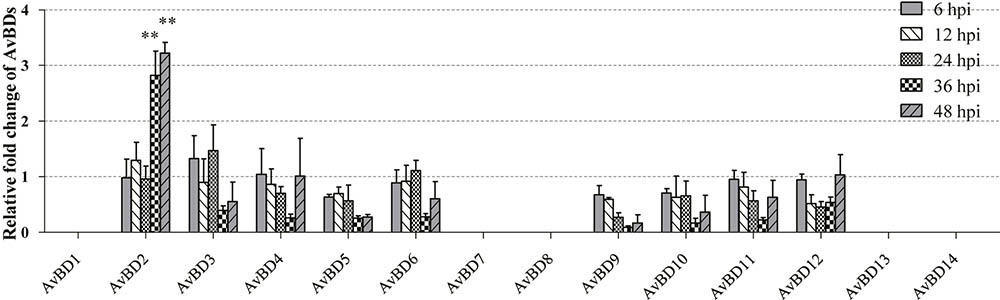
FIGURE 2. Relative gene expression of AvBDs in response to NDV infection in CEFs. Fold change of mRNA expression in CEFs of each group were measured by RT-PCR at 6, 12, 24, 36, and 48 hpi, respectively. Transcript levels of AvBDs were normalized to 18S rRNA and mRNA expression is shown as the fold change relative to the control. All assays were performed in triplicate, and each bar is the mean ± SEM. Statistically significant differences between groups are indicated by ∗p < 0.05 or ∗∗p < 0.01.
In order to evaluate the antiviral activity of AvBD2 against NDV in vitro, recombinant AvBD2 proteins were expressed and purified in both prokaryotic cells (Figure 3A) and eukaryotic cells (Figure 3B). The two kinds of recombinant AvBD2 protein sample were separated on 12% SDS–PAGE. The gel band was then confirmed by mass spectrometry. The result showed that the molecular weight of the recombinant protein was approximately 7.5 kDa, and pI value was 9.38. The Mascot Score of the gel band confirmed by mass spectrometry was 342. Proteins with significant score of > 21 (p < 0.05) were considered successfully identified. This result confirmed that AvBD2 protein was successfully expressed in eukaryotic cells. Detailed information about the result of mass spectrometry is shown in Table 1.
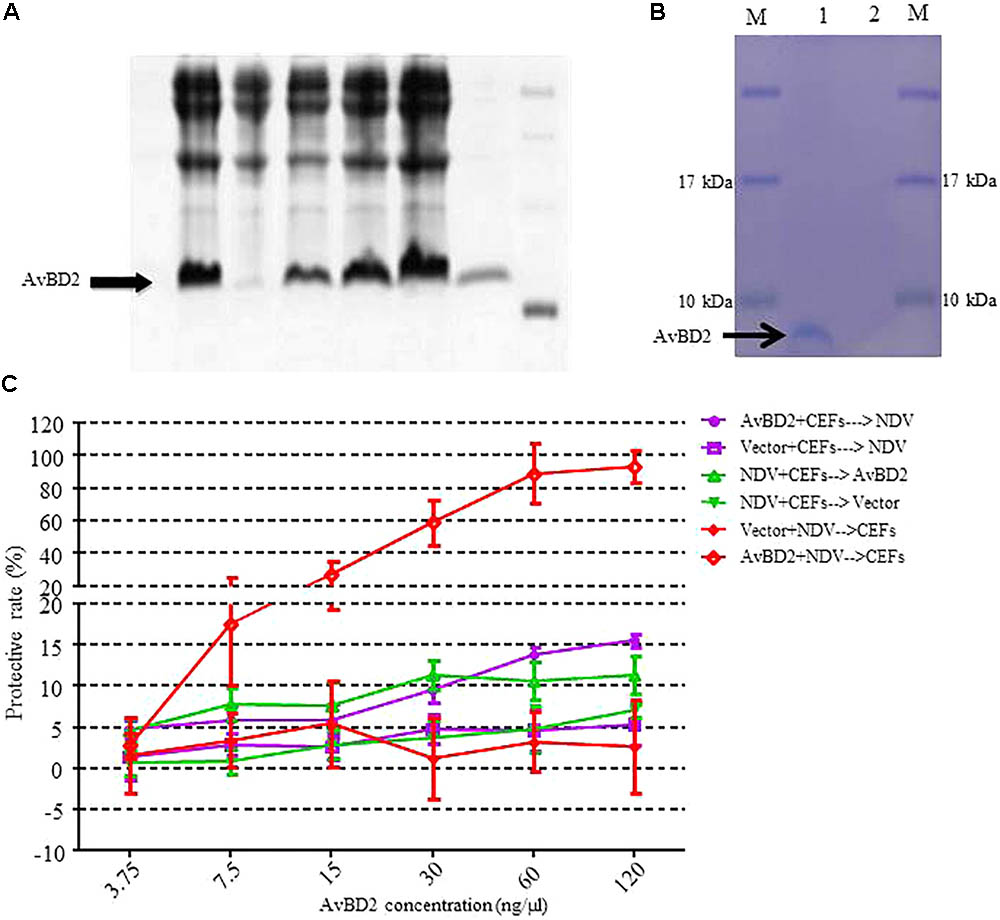
FIGURE 3. Recombinant expression of AvBD2 protein and antiviral activity of AvBD2 against NDV. (A) 12% SDS–PAGE analysis of His-tagged recombinant AvBD2 protein expressed in E. coli BL21 (DE3) cells. Lane 1: Supernatant; lane 2: Inclusion body; lane 3: Total protein of Rosetta containing AvBD2 without IPTG induction; lanes 4–6: Total protein of Rosetta containing AvBD2 at 2, 4, 6 h after induction with IPTG, respectively; lane 7: Purified recombinant AvBD2 protein; lane M: Protein marker. (B) 12% SDS–PAGE analysis of His-tagged AvBD2 protein expressed in Expi293 cells. Lane 1: purified AvBD2 with a His-tag on the C-terminal; lane 2: purified vector with a His-tag on the C-terminal; lane M: protein molecular weight marker. (C) Protection of CEFs from NDV infection by using the CCK-8 assay. Red curve, NDV (1 MOI) and AvBD2 protein (3.75–120 ng/μl) were mixed for 1.5 h at 37°C. Cells were washed and then incubated with the NDV-AvBD2 protein mixture for another 1.5 h at 37°C. Green curve, NDV (1 MOI) and equal volume of PB were mixed and then incubated with CEFs for 1.5 h at 37°C. Cells were washed and incubated with AvBD2 protein and equal volume of DMEM without serum for another 1.5 h. Purple curve, AvBD2 protein (3.75–120 ng/μl) and equal volume of DMEM without serum were mixed to incubate with CEFs for 1.5 h at 37°C. Cells were washed and then the mixtures of NDV (1 MOI) and equal volume of PB were added and incubated with CEFs for another 1.5 h at 37°C. Then, the cells were washed, and 2% DMEM were added and incubated for 48 h at 37°C. Then CCK-8 solution (5 mg/ml, 10 μl/well) was added to the plate. The absorbance at 450 nm was measured using a microplate reader after the incubation at 37°C for 1.5 h. The vector was used as control for each group. Cells without virus-protein mixture were also used as a negative control. Cells only infected with the virus served as a positive control. The experiment was performed in triplicate.
To evaluate the antiviral activity of the two kinds of recombinant AvBD2 proteins against NDV, three experiments were conducted by measuring cell viability with the CCK-8 assay. The protective rate of cells is significantly increased in the treatment in which NDV was pre-incubated with AvBD2 expressed in eukaryotic cells, compared to that of the other two treatments. The result demonstrated that the recombinant AvBD2 showed directly antiviral activity against the NDV strain. The protective rate reached 93.35% at the concentration of 120 ng/μl (Figure 3C). The IC50 of the recombinant AvBD2 was 19.61 ± 4.76. In addition, the results of the cytotoxicity by CCK-8 assay indicated that the recombinant AvBD2 showed little cytotoxicity in CEFs, even at concentration of 120 ng/μl (data not shown). In contrast, little significant protection against NDV was found by recombinant AvBD2 expressed in prokaryotic cells (even at concentration of 300 ng/μl), compared with the control group (data not shown).
In order to elucidate the regulatory mechanism of AvBD2 expression in response to NDV infection, CEFs were infected with NDV for 36 and 48 h after pre-treatment with MAPK and NF-κB pathway inhibitors, respectively. However, AvBD2 mRNA expression was not detectable at both time points (Figure 4), which may be due to insufficient upregulation of AvBD2 mRNA expression induced by NDV (the fold change relative to the uninfected group is less to 3). Alternatives to NDV, such as ligands of TLRs, needed to be identified for further study.
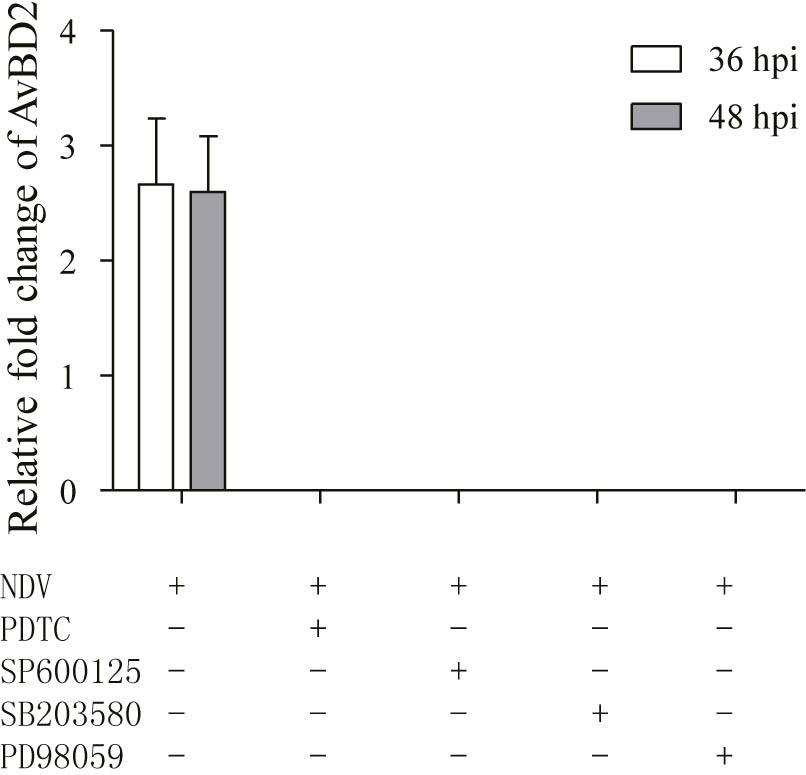
FIGURE 4. Inhibitors of MAPK/NF-κB inhibit AvBD2 expression mediated by NDV. CEFs were pre-treated with 50 μM PDTC (NF-κB inhibitor), 50 μM SP600125 (JNK inhibitor), 50 μM SB203580 (p38 MAPK inhibitor), or 50 μM PD98059 (ERK1/2 inhibitor) for 1 h, then infected with or without NDV (1 MOI) for another 36 or 48 h. Fold change of mRNA expression in CEFs of each group were measured at 36 or 48 hpi using RT-PCR. Transcript level of AvBD2 was normalized to the levels of 18S rRNA in the same samples and gene expression is presented as the fold change relative to the uninfected group. All assays were performed in triplicate, and each bar is the mean ± SEM.
In order to further understand the molecular basis of the regulation of AvBD2 secretion in response to NDV infection, ligands of TLRs were chosen as alternatives to NDV in the study. Different TLR ligands, namely, Pam3CSK4 (TLR1/2), polyI:C (TLR3), LPS (TLR4), FLA-ST (TLR5), R848 (TLR7), and ODN-M362 (TLR21) were employed in this study. The mRNA expression of AvBD2 was evaluated in CEFs stimulated with each TLR ligands at 6, 24, and 48 hpt, respectively. The results demonstrated that mRNA expression of AvBD2 in CEFs was significantly up-regulated by Pam3CSK4, FLA-ST, and ODN-M362 stimulation at both 24 and 48 hpi, compared with respective control (Figure 5). In contrast, little significant regulation on AvBD2 expression was observed by polyI:C, LPS, and R848 in this study.
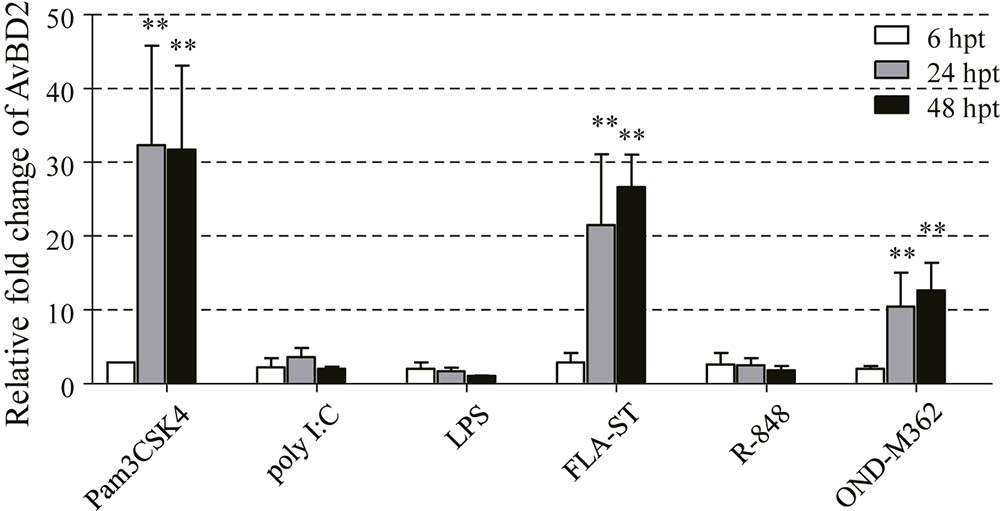
FIGURE 5. Induction of AvBD2 mRNA expression following TLR ligands stimulation in CEFs. CEFs were treated with Pam3CSK4 (0.4 μg/ml), polyI:C (50 μg/ml), LPS (2 μg/ml), FLA-ST (2 μg/ml), R848 (5 μg/ml), and ODN-M362 (2.5 μM), respectively. CEFs in the control groups received medium. Fold change of mRNA expression in CEFs of each group were measured at 6, 24, and 48 h post-treatment using RT-PCR. Transcript levels of AvBD2 were normalized to 18s rRNA and mRNA expression is presented as the fold change relative to the control group. All assays were performed in triplicate, and each bar is the mean ± SEM. Statistical significant difference between cells without and with TLR ligands are indicated by ∗ p < 0.05 or ∗∗p < 0.01.
In order to elucidate which signaling pathway regulates secretion of AvBD2, we have succeeded in detecting ERK, p38, JNK kinases, and NF-κB 1 in CEFs in this study. Then, inhibition of transcriptional factor in CEFs was performed. As shown in Figure 6, expression of AvBD2 was significantly inhibited by SB203580 in TLR ligands-induced cells (p < 0.05) (including Pam3CSK4, FLA-ST, and ODN-M362, respectively) (Figures 6A–C). Both SP600125 and PD98059 had only a partial effect on AvBD2 expression (Figures 6A,C). However, the mRNA expression of AvBD2 was undetectable in which only be pre-treated with various inhibitors (no TLR ligands added) (Figures 6A–C). These results suggested that the p38 MAPK signaling pathway exhibited a more important role on AvBD2 expression than both the ERK1/2 and JNK signaling pathways (p < 0.05). In addition, PDTC treatment had little effect on AvBD2 expression (Figures 6A–C). Together, these data revealed that transcription factor p38 MAPK may play an important role in the regulation of AvBD2 production in response to stimulation with the TLR ligands (Pam3CSK4, FLA-ST, and ODN-M362).
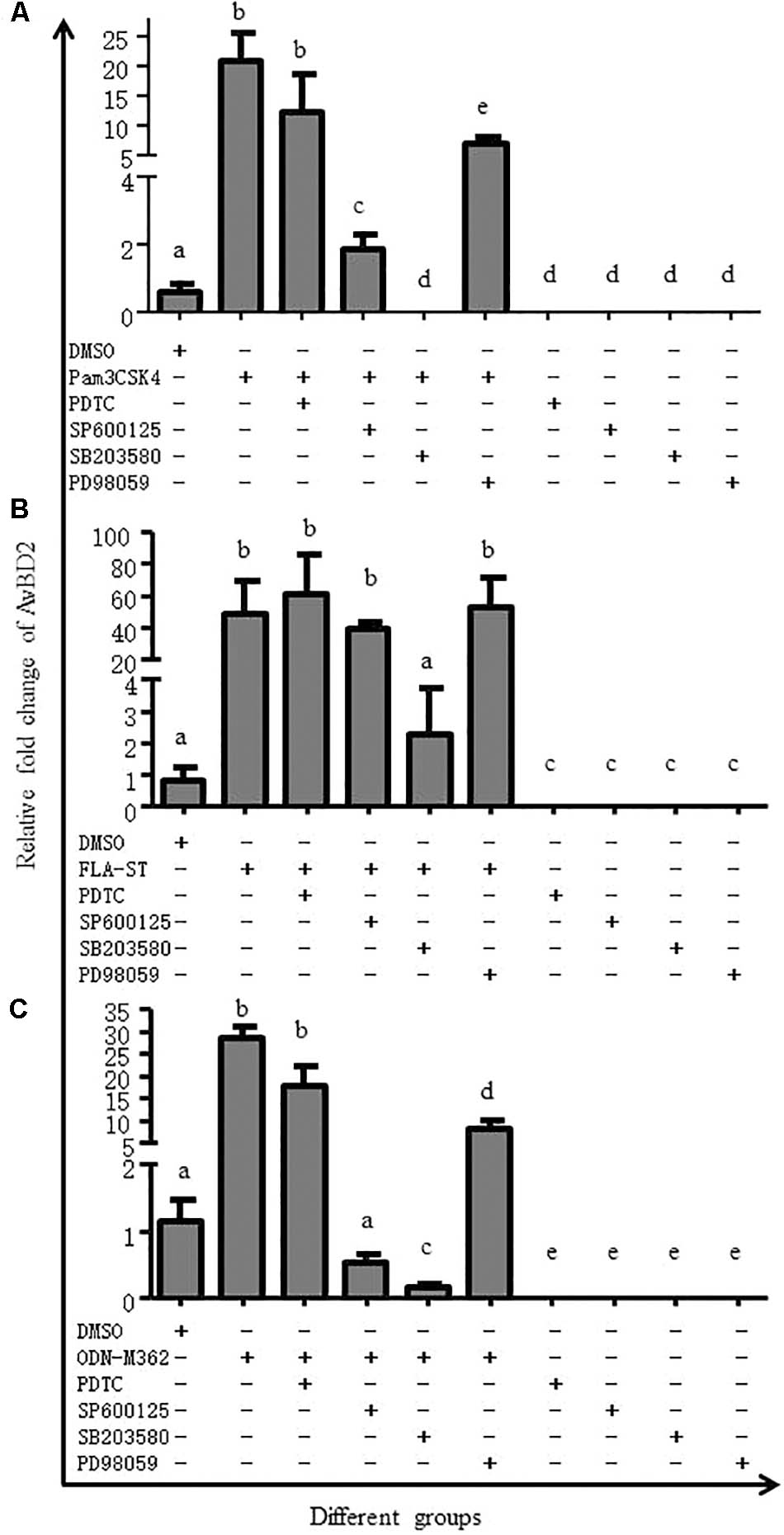
FIGURE 6. MAPK signaling pathway is involved in AvBD2 induction mediated by TLR ligands. CEFs were pre-treated with 50 μM PDTC (NF-κB inhibitor), 50 μM SP600125 (JNK inhibitor), 50 μM SB203580 (p38 MAPK inhibitor), or 50 μM PD98059 (ERK1/2 inhibitor) for 1 h, followed by stimulation with or without (A) Pam3CSK4 (0.4 μg/ml), (B) FLA-ST (2 μg/ml), or (C) ODN-M362 (2.5 μM) for another 24 h. The DMSO group was used as a negative control. Fold change of mRNA expression in CEFs of each group were measured by RT-PCR at 24 h post-treatment. Transcript level of AvBD2 was normalized to the levels of 18S rRNA in the same samples and gene expression is presented as the fold change relative to the control group. All assays were performed in triplicate, and each bar is the mean ± SEM. a,b,c,d,eThe values with different letters are significantly different (p < 0.05).
The ND is an infectious, highly contagious and pathogenic avian viral disease caused by virulent NDV strains. Despite considerable understanding of the pathogenesis of the virus, a little is known about the host-virus interaction. Recent studies have shown upregulation of AvBDs, cytokines and iNOS in goose and chickens infected with virulent NDV, respectively (Ecco et al., 2011; Rasoli et al., 2014; Li et al., 2015; Xu et al., 2016). However, limited researches have been reported on mechanism of AvBD induction in chickens infected with NDV. It is well documented that HDPs, including AvBDs, are key components of the host innate immune response against pathogens and potentially provide a link between innate and adaptive immunity (Cuperus et al., 2013; Garcia-Valtanen et al., 2014). The present study showed that only AvBD2 expression was significantly upregulated in CEFs in response to NDV infection in vitro, comparing to those of other 13 AvBDs. Consistent with this observation, our previous reports also showed that AvBDs, including AvBD2, from various avian species can be induced by several viruses, including DHV (Ma et al., 2011, 2012), IBV (Xu et al., 2015), PPMV-1 (Li et al., 2015), and a genotype VIId NDV (Xu et al., 2016) strain of goose origin (go/CH/LHLJ/1/06) in vivo. Overall, these studies suggested that AvBDs, especially AvBD2, are key components of the innate response and may play pivotal role in the host defense against viral infection.
Furthermore, the current results also demonstrated that the recombinant AvBD2 expressed in eukaryotic cells had directly antiviral activity against the NDV strain in vitro. Consistent with the present results, the antiviral activity of other HDPs against different viruses, including human α-defensins (HNP1–4, HD5, and HD6) against Herpes Simplex Virus (HSV), Rabbit α-defensins against HSV-1 and -2 (Lehrer et al., 1985), and Rhesus θ-defensin 2 against HSV-2 (Yasin et al., 2004), has been well documented in past years. The antiviral activity of β-defensins from different species against viruses, including HSV (Hazrati et al., 2006), spring viremia of carp virus (Garcia-Valtanen et al., 2014), HIV-1 (Quinones-Mateu et al., 2003), parainfluenza virus 3 (Grubor et al., 2004), and influenza virus (Gong et al., 2010) have also been described previously. Similarly, several AvBDs, including AvBD2, exhibited directly antiviral activity against DHV (Ma et al., 2011, 2012), IBV (Xu et al., 2015), and PPMV-1 (Li et al., 2015) in our recent studies. These findings further confirmed the importance of HDPs in the host defense response against viral infection and open the possibility of developing novel agents based on HDPs to treat viral infections.
Notably, in contrast to the recombinant AvBD2 protein expressed in eukaryotic cells, the recombinant AvBD2 protein expressed in prokaryotic cells exhibited little directly antiviral activity against NDV in this study. The results were partly different from previous study on the other virus (Xu et al., 2015). The possible reason needed to be further study. It is considered that structural organization of most defensins is essential for their antimicrobial activity (Hoover et al., 2003). Previous study showed that antimicrobial activity of linear AvBD2-Acm protein is lower than the fully oxidized protein, indicating the critical role of the tertiary structure on antimicrobial activity (Derache et al., 2012). Eukaryotic cell expression system could provide post-translational modifications, such as glycosylation and correct protein folding, which is critical to biological activity of protein (de la Salle et al., 1985). In this study, Expi293 FreeStyle cells were used to transfect pCAG (+)-AvBD2-His plasmid in the eukaryotic expression system. The antiviral activity of the recombinant AvBD2 against NDV might be related to its fully modified and folded tertiary structure.
The MAPK family is responsible for important signaling pathways which regulate immune responses (Xing et al., 2010). It was reported that the responses of HDPs to TLR ligands rely on the NF-κB or/and MAPK signaling pathways in mammals in vitro (Frigo et al., 2004; Roux and Blenis, 2004, Lee et al., 2008; Han et al., 2009, Lewis et al., 2016). Previous study also reported that avian influenza virus activates ERK, p38 and JNK in avian species (Xing et al., 2010). In this study, we found that AvBD2 expression was significantly suppressed by inhibitor of p38 MAPK. The results revealed that AvBD2 expression might mainly responds to TLR ligands through the p38 MAPK signaling pathway. Consistent with the current results, it was also reported that, in most cases, regulation of TLRs was independent of NF-κB but dependent on one or more of the MAPK pathway components (Krisanaprakornkit et al., 2002; Peroval et al., 2013). Similarly, inhibition of p38 MAPK by SB203580 could abolish HBD2 up-regulation by NTHi in human middle ear epithelial cells (Lee et al., 2008). These results showed that β-defensin 2 has a similar regulatory mechanism in different species.
This study reports that the mRNA expression of AvBD2 was upregulated in CEFs by NDV strain F48E9 infection in vitro and the recombinant AvBD2 protein expressed by eukaryotic cells demonstrated direct antiviral activity against the NDV strain. The AvBD2 expression is also induced by ligands of TLRs (Pam3CSK4, FLA-ST, and ODN-M362), and suppressed by inhibitor of p38 MAPK. Our results revealed that AvBD2 play a pivotal role in host innate immunity response to NDV infection. The AvBD2 expression might be regulated in a p38 MAPK-dependent manner.
CL performed the experiments and wrote the manuscript. LJ, LL, WZ, YC, and TQ performed the experiments and the calculation. LS, ZH, and YS collected the samples. DM and SL designed and conducted the study, and wrote the manuscript.
The study was partly supported by the Specialized Research Fund for the Science and Technological Innovation Talent of Harbin (2013RFXXJ019), the Research Program for Applied Technology of Heilongjiang Province (PC13S02), and grants from the China Agriculture Research System (No. CARS-41-K12).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdel-Mageed, A. M., Isobe, N., and Yoshimura, Y. (2014). Effects of different TLR ligands on the expression of proinflammatory cytokines and avian beta-defensins in the uterine and vaginal tissues of laying hens. Vet. Immunol. Immunopathol. 162, 132–141. doi: 10.1016/j.vetimm.2014.10.013
Allen, I. C., Scull, M. A., Moore, C. B., Holl, E. K., McElvania-TeKippe, E., Taxman, D. J., et al. (2009). The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565. doi: 10.1016/j.immuni.2009.02.005
Barabas, N., Röhrl, J., Holler, E., and Hehlgans, T. (2013). Beta-defensins activate macrophages and synergize in pro-inflammatory cytokine expression induced by TLR ligands. Immunobiology 218, 1005–1011. doi: 10.1016/j.imbio.2012.11.007
Barjesteh, N., Alkie, T. N., Hodgins, D. C., Nagy, E., and Sharif, S. (2016). Local innate responses to TLR ligands in the chicken trachea. Viruses 8:207. doi: 10.3390/v8070207
Bastian, A., and Schafer, H. (2001). Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 101, 157–161. doi: 10.1016/S0167-0115(01)00282-8
Chang, L., and Karin, M. (2001). Mammalian MAP kinase signalling cascades. Nature 410, 37–40. doi: 10.1038/35065000
Cheng, Y., Wang, H., Yan, Y., Ding, C., and Sun, J. (2015). Two myeloid differentiation factor 88 (MyD88) isoforms identified in ducks. Dev. Comp. Immunol. 52, 144–154. doi: 10.1016/j.dci.2015.03.015
Chong, K. T., Thangavel, R. R., and Tang, X. H. (2008). Enhanced expression of murine beta-defensins (MBD-1,-2,-3, and -4) in upper and lower airway mucosa of influenza virus infected mice. Virology 380, 136–143. doi: 10.1016/j.virol.2008.07.024
Chong, K. T., Xiang, L., Wang, X., Jun, E. L., Xi, L. F., and Schweinfurth, J. M. (2006). High level expression of human epithelial beta-defensins (hBD-1, 2 and 3) in papillomavirus induced lesions. Virol. J. 3:75. doi: 10.1186/1743-422x-3-75
Cuperus, T., Coorens, M., van Dijk, A., and Haagsman, H. P. (2013). Avian host defense peptides. Dev. Comp. Immunol. 41, 352–369. doi: 10.1016/j.dci.2013.04.019
Davis, R. J. (1994). MAPKs: new JNK expands the group. Trends Biochem. Sci. 19, 470–473. doi: 10.1016/0968-0004(94)90132-5
de la Salle, H., Altenburger, W., Elkaim, R., Dott, K., Dieterle, A., Drillien, R., et al. (1985). Active gamma-carboxylated human factor IX expressed using recombinant DNA techniques. Nature 316, 268–270. doi: 10.1038/316268a0
Derache, C., Meudal, H., Aucagne, V., Mark, K. J., Cadene, M., Delmas, A. F., et al. (2012). Initial insights into structure-activity relationships of avian defensins. J. Biol. Chem. 287, 7746–7755. doi: 10.1074/jbc.M111.312108
Ecco, R., Brown, C., Susta, L., Cagle, C., Cornax, I., Pantin-Jackwood, M., et al. (2011). In vivo transcriptional cytokine responses and association with clinical and pathological outcomes in chickens infected with different Newcastle disease virus isolates using formalin-fixed paraffin-embedded samples. Vet. Immunol. Immunopathol. 141, 221–229. doi: 10.1016/j.vetimm.2011.03.002
Falco, A., Chico, V., Marroqui, L., Perez, L., Coll, J. M., and Estepa, A. (2008). Expression and antiviral activity of a beta-defensin-like peptide identified in the rainbow trout (Oncorhynchus mykiss) EST sequences. Mol. Immunol. 45, 757–765. doi: 10.1016/j.molimm.2007.06.358
Frigo, D. E., Tang, Y., Beckman, B. S., Scandurro, A. B., Alam, J., Burow, M. E., et al. (2004). Mechanism of AP-1-mediated gene expression by select organochlorines through the p38 MAPK pathway. Carcinogenesis 25, 249–261. doi: 10.1093/carcin/bgh009
Ganz, T. (2003). Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720. doi: 10.1038/nri1180
Garcia-Valtanen, P., Martinez-Lopez, A., Ortega-Villaizan, M., Perez, L., Coll, J. M., and Estepa, A. (2014). In addition to its antiviral and immunomodulatory properties, the zebrafish beta-defensin 2 (zfBD2) is a potent viral DNA vaccine molecular adjuvant. Antiviral Res. 101, 136–147. doi: 10.1016/j.antiviral.2013.11.009
Gong, T., Jiang, Y., Wang, Y., Yang, D., Li, W., Zhang, Q., et al. (2010). Recombinant mouse beta-defensin 2 inhibits infection by influenza A virus by blocking its entry. Arch. Virol. 155, 491–498. doi: 10.1007/s00705-010-0608-1
Grubor, B., Gallup, J. M., Meyerholz, D. K., Crouch, E. C., Evans, R. B., Brogden, K. A., et al. (2004). Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin. Diagn. Lab. Immunol. 11, 599–607. doi: 10.1128/cdli.11.3.599-607.2004
Guo, H., Liu, X., Xu, Y., Han, Z., Shao, Y., Kong, X., et al. (2014). A comparative study of pigeons and chickens experimentally infected with PPMV-1 to determine antigenic relationships between PPMV-1 and NDV strains. Vet. Microbiol. 168, 88–97. doi: 10.1016/j.vetmic.2013.11.002
Han, S. H., Kim, Y.-E., Park, J.-A., Park, J.-B., Kim, Y.-S., Lee, Y., et al. (2009). Expression of human β-defensin-2 gene induced by CpG-DNA in human B cells. Biochem. Biophys. Res. Commun. 389, 443–448. doi: 10.1016/j.bbrc.2009.08.162
Hazrati, E., Galen, B., Lu, W., Wang, W., Ouyang, Y., Keller, M. J., et al. (2006). Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 177, 8658–8666. doi: 10.4049/jimmunol.177.12.8658
Hoover, D. M., Wu, Z., Tucker, K., Lu, W., and Lubkowski, J. (2003). Antimicrobial characterization of human β-defensin 3 derivatives. Antimicrob. Agents Chemother. 47, 2804–2809. doi: 10.1128/AAC.47.9.2804-2809.2003
Keestra, A. M., de Zoete, M. R., Bouwman, L. I., and van Putten, J. P. (2010). Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. J. Immunol. 185, 460–467. doi: 10.4049/jimmunol.0901921
Klotman, M. E., and Chang, T. L. (2006). Defensins in innate antiviral immunity. Nat. Rev. Immunol. 6, 447–456. doi: 10.1038/nri1860
Kogut, M. H., Chiang, H. I., Swaggerty, C. L., Pevzner, I. Y., and Zhou, H. (2012). Gene expression analysis of toll-like receptor pathways in heterophils from genetic chicken lines that differ in their susceptibility to Salmonella enteritidis. Front. Genet. 3:121. doi: 10.3389/fgene.2012.00121
Krisanaprakornkit, S., Kimball, J. R., and Dale, B. A. (2002). Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J. Immunol. 168, 316–324. doi: 10.4049/jimmunol.168.1.316
Kyriakis, J. M., and Avruch, J. (2012). Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 92, 689–737. doi: 10.1152/physrev.00028.2011
Le Goffic, R., Balloy, V., Lagranderie, M., Alexopoulou, L., Escriou, N., Flavell, R., et al. (2006). Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2:e53. doi: 10.1371/journal.ppat.0020053
Lee, H. Y., Takeshita, T., Shimada, J., Akopyan, A., Woo, J. I., Pan, H., et al. (2008). Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC Infect. Dis. 8:87. doi: 10.1186/1471-2334-8-87
Lehrer, R. I., Cole, A. M., and Selsted, M. E. (2012). θ-Defensins: cyclic peptides with endless potential. J. Biol. Chem. 287, 27014–27019. doi: 10.1074/jbc.R112.346098
Lehrer, R. I., Daher, K., Ganz, T., and Selsted, M. E. (1985). Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J. Virol. 54, 467–472.
Lehrer, R. I., and Ganz, T. (2002). Defensins of vertebrate animals. Curr. Opin. Immunol. 14, 96–102. doi: 10.1016/S0952-7915(01)00303-X
Leikina, E., Delanoe-Ayari, H., Melikov, K., Cho, M. S., Chen, A., Waring, A. J., et al. (2005). Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 6, 995–1001. doi: 10.1038/ni1248
Lewis, S. B., Prior, A., Ellis, S. J., Cook, V., Chan, S. S., Gelson, W., et al. (2016). Flagellin Induces beta-Defensin 2 in Human Colonic Ex vivo Infection with Enterohemorrhagic Escherichia coli. Front. Cell Infect. Microbiol. 6:68. doi: 10.3389/fcimb.2016.00068
Li, B., Ye, J., Lin, Y., Wang, M., and Zhu, J. (2014). Preparation and identification of a single-chain variable fragment antibody against Newcastle diseases virus F48E9. Vet. Immunol. Immunopathol. 161, 258–264. doi: 10.1016/j.vetimm.2014.08.009
Li, Y., Xu, Q., Zhang, T., Gao, M., Wang, Q., Han, Z., et al. (2015). Host avian beta-defensin and toll-like receptor responses of pigeons following infection with pigeon paramyxovirus type 1. Appl. Environ. Microbiol. 81, 6415–6424. doi: 10.1128/aem.01413-15
Liu, W. Q., Tian, M. X., Wang, Y. P., Zhao, Y., Zou, N. L., Zhao, F. F., et al. (2012). The different expression of immune-related cytokine genes in response to velogenic and lentogenic Newcastle disease viruses infection in chicken peripheral blood. Mol. Biol. Rep. 39, 3611–3618. doi: 10.1007/s11033-011-1135-1
Lynn, D. J., Higgs, R., Lloyd, A. T., O’Farrelly, C., Herve-Grepinet, V., Nys, Y., et al. (2007). Avian beta-defensin nomenclature: a community proposed update. Immunol. Lett. 110, 86–89. doi: 10.1016/j.imlet.2007.03.007
Ma, D., Lin, L., Zhang, K., Han, Z., Shao, Y., Liu, X., et al. (2011). Three novel Anas platyrhynchos avian beta-defensins, upregulated by duck hepatitis virus, with antibacterial and antiviral activities. Mol. Immunol. 49, 84–96. doi: 10.1016/j.molimm.2011.07.019
Ma, D., Zhang, K., Zhang, M., Xin, S., Liu, X., Han, Z., et al. (2012). Identification, expression and activity analyses of five novel duck beta-defensins. PLoS One 7:e47743. doi: 10.1371/journal.pone.0047743
Mayo, M. A. (2002). A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 147, 1655–1663. doi: 10.1007/s007050200039
Motulsky. (2007). GraphPad Prism(R) Version 5.0 Statistics Guide [M]. San Diego, CA: GraphPad Software, Inc.
Pauwels, R., Balzarini, J., Baba, M., Snoeck, R., Schols, D., Herdewijn, P., et al. (1988). Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20, 309–321. doi: 10.1016/0166-0934(88)90134-6
Peroval, M. Y., Boyd, A. C., Young, J. R., and Smith, A. L. (2013). A critical role for MAPK signalling pathways in the transcriptional regulation of toll like receptors. PLoS One 8:e51243. doi: 10.1371/journal.pone.0051243
Plotnikov, A., Zehorai, E., Procaccia, S., and Seger, R. (2011). The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 1813, 1619–1633. doi: 10.1016/j.bbamcr.2010.12.012
Proud, D., Sanders, S. P., and Wiehler, S. (2004). Human rhinovirus infection induces airway epithelial cell production of human beta-defensin 2 both in vitro and in vivo. J. Immunol. 172, 4637–4645. doi: 10.4049/jimmunol.172.7.4637
Quinones-Mateu, M. E., Lederman, M. M., Feng, Z. M., Chakraborty, B., Weber, J., Rangel, H. R., et al. (2003). Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 17, F39–F48. doi: 10.1097/01.aids.0000096878.73209.4f
Ramasamy, K. T., Reddy, M. R., Verma, P. C., and Murugesan, S. (2012). Expression analysis of turkey (Meleagris gallopavo) toll-like receptors and molecular characterization of avian specific TLR15. Mol. Biol. Rep. 39, 8539–8549. doi: 10.1007/s11033-012-1709-6
Rasoli, M., Yeap, S. K., Tan, S. W., Moeini, H., Ideris, A., Bejo, M. H., et al. (2014). Alteration in lymphocyte responses, cytokine and chemokine profiles in chickens infected with genotype VII and VIII velogenic Newcastle disease virus. Comp. Immunol. Microbiol. Infect. Dis. 37, 11–21. doi: 10.1016/j.cimid.2013.10.003
Reed, L. J., and Muench, H. (1938). A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27, 493–497. doi: 10.1093/oxfordjournals.aje.a118408
Rock, F. L., Hardiman, G., Timans, J. C., Kastelein, R. A., and Bazan, J. F. (1998). A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. U.S.A. 95, 588–593. doi: 10.1073/pnas.95.2.588
Roux, P. P., and Blenis, J. (2004). ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344. doi: 10.1128/mmbr.68.2.320-344.2004
Sano, K., and Ogawa, H. (2014). Hemagglutination (inhibition) assay. Methods Mol. Biol. 1200, 47–52. doi: 10.1007/978-1-4939-1292-6_4
Sato, S., Sanjo, H., Takeda, K., Ninomiya-Tsuji, J., Yamamoto, M., Kawai, T., et al. (2005). Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095. doi: 10.1038/ni1255
Seger, R., and Krebs, E. G. (1995). The MAPK signaling cascade. FASEB J. 9, 726–735. doi: 10.1096/fasebj.9.9.7601337
Shahsavandi, S., Ebrahimi, M. M., Mohammadi, A., and Zarrin Lebas, N. (2013). Impact of chicken-origin cells on adaptation of a low pathogenic influenza virus. Cytotechnology 65, 419–424. doi: 10.1007/s10616-012-9495-5
Virella-Lowell, I., Poirier, A., Chesnut, K. A., Brantly, M., and Flotte, T. R. (2000). Inhibition of recombinant adeno-associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients. Gene Ther. 7, 1783–1789. doi: 10.1038/sj.gt.3301268
Wang, Y., Liu, W., Tian, M., Lin, Y., Zhao, F., Shi, M., et al. (2012). Downregulation of IL-15 and IL-18 in chicken embryo fibroblasts in response to virulent or lentogenic newcastle disease virus infection. Inform. Technol. Agric. Eng. 789–800. doi: 10.1007/978-3-642-27537-1_93
Whitmarsh, A. J. (2007). Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta 1773, 1285–1298. doi: 10.1016/j.bbamcr.2006.11.011
Xing, Z., Cardona, C. J., Anunciacion, J., Adams, S., and Dao, N. (2010). Roles of the ERK MAPK in the regulation of proinflammatory and apoptotic responses in chicken macrophages infected with H9N2 avian influenza virus. J. Gen. Virol. 91(Pt 2), 343–351. doi: 10.1099/vir.0.015578-0
Xu, Q., Chen, Y., Zhao, W., Zhang, T., Liu, C., Qi, T., et al. (2016). Infection of goose with genotype VIId Newcastle disease virus of goose origin elicits strong immune responses at early stage. Front. Microbiol. 7:1587. doi: 10.3389/fmicb.2016.01587
Xu, Y., Zhang, T., Xu, Q., Han, Z., Liang, S., Shao, Y., et al. (2015). Differential modulation of avian beta-defensin and Toll-like receptor expression in chickens infected with infectious bronchitis virus. Appl. Microbiol. Biotechnol. 99, 9011–9024. doi: 10.1007/s00253-015-6786-8
Yamamoto, M., and Takeda, K. (2010). Current views of toll-like receptor signaling pathways. Gastroenterol. Res. Pract. 2010:240365. doi: 10.1155/2010/240365
Yang, D., Biragyn, A., Hoover, D. M., Lubkowski, J., and Oppenheim, J. J. (2004). Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 22, 181–215. doi: 10.1146/annurev.immunol.22.012703.104603
Yang, D., Chertov, O., Bykovskaia, S. N., Chen, Q., Buffo, M. J., Shogan, J., et al. (1999). β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528. doi: 10.1126/science.286.5439.525
Yasin, B., Wang, W., Pang, M., Cheshenko, N., Hong, T., Waring, A. J., et al. (2004). Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78, 5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004
Yoneyama, M., Kikuchi, M., Natsukawa, T., Shinobu, N., Imaizumi, T., Miyagishi, M., et al. (2004). The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737. doi: 10.1038/ni1087
Keywords: avian β-defensin 2, Newcastle disease virus, antiviral activity, ligands of toll-like receptors, p38 MAPK signaling pathway
Citation: Liu C, Jiang L, Liu L, Sun L, Zhao W, Chen Y, Qi T, Han Z, Shao Y, Liu S and Ma D (2018) Induction of Avian β-Defensin 2 Is Possibly Mediated by the p38 MAPK Signal Pathway in Chicken Embryo Fibroblasts After Newcastle Disease Virus Infection. Front. Microbiol. 9:751. doi: 10.3389/fmicb.2018.00751
Received: 23 January 2018; Accepted: 03 April 2018;
Published: 19 April 2018.
Edited by:
Oliver Planz, Universität Tübingen, GermanyReviewed by:
Yan Li, Experimental Therapeutics Centre (A∗STAR), SingaporeCopyright © 2018 Liu, Jiang, Liu, Sun, Zhao, Chen, Qi, Han, Shao, Liu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengwang Liu, c3dsaXVAaHZyaS5hYy5jbg== Deying Ma, bWFkZXlpbmdAbmVhdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.