- 1Department of Agrotechnology, Faculty of Agriculture, Universitas Sumatera Utara, Medan, Indonesia
- 2Department of Entomology and Plant Pathology, Faculty of Agriculture, Gadjah Mada University, Yogyakarta, Indonesia
- 3Research Center for Biotechnology, Gadjah Mada University, Yogyakarta, Indonesia
- 4Agriculture Victoria, Department of Economic Development, Jobs, Transport and Resources, Bundoora, VIC, Australia
Ralstonia solanacearum species complex phylotype IV strains, which have been primarily isolated from Indonesia, Australia, Japan, Korea, and Malaysia, have undergone recent taxonomic and nomenclatural changes to be placed in the species Ralstonia syzygii. This species contains three subspecies; Ralstonia syzygii subsp. syzygii, a pathogen causing Sumatra disease of clove trees in Indonesia, Ralstonia syzygii subsp. indonesiensis, the causal pathogen of bacterial wilt disease on a wide range of host plants, and Ralstonia syzygii subsp. celebesensis, the causal pathogen of blood disease on Musa spp. In Indonesia, these three subspecies have devastated the cultivation of susceptible host plants which have high economic value. Limited knowledge on the ecology and epidemiology of the diseases has hindered the development of effective control strategies. In this review, we provide insights into the ecology, epidemiology and disease control of these three subspecies of Ralstonia syzygii.
Introduction
Indonesian agriculture is dedicated to the production of food crops for local consumption by an ever expanding population (Maulana and Sayaka, 2007), agriculture also plays a significant role in the Indonesian economy (Cervantes-Godoy and Dewbre, 2010). From 2001 to 2008, national spending on agriculture increased from 11 billion rupiah to 53 billion rupiah, which is an average increase of 11% annually (Armas et al., 2012). Plant and animal diseases are primary constraints affecting agricultural production, especially in tropical countries such as Indonesia (Magarey et al., 2010; Prabaningrum and Moekasan, 2014; Drenth and Guest, 2016). Bacterial wilt disease, caused by members of the Ralstonia solanacearum species complex, is a serious disease of crop plants in Indonesia. Geddes (1992) ranked bacterial wilt as the 6th most detrimental plant pest and disease in Indonesia after the damage caused by rats (Ratus spp.), stem borers (Scirpophaga innotata, S. incertula, and Chilo suppressalis), bacterial rice blight (Xanthomonas oryzae pv. oryzae), the brown planthopper (Nilaparvata lugens) and the oriental leafworm moth (Spodoptera litura and S. exigua).
Bacterial wilt disease on Musa spp., called blood disease, was first reported on Selayar Island, South Sulawesi (formerly Celebes) in 1906 by Gäumann (1921). The local people named the disease “blood disease” to reflect the reddish brown bacterial exudate secreted from internal vascular tissue of pseudostems and fruits of infected bananas (Gäumann, 1924). Bacterial wilt affecting Syzygium aromaticum trees, Sumatra disease or wooden vessel bacteria on S. aromaticum, was first observed in Sumatra, Indonesia in 1975 (Waller and Sitepu, 1975).
In Indonesia, bacterial wilt disease occurs on a wide varieties of crops and both blood disease of banana and Sumatra disease of clove have significantly impacted the banana and clove industries, respectively. The impact on banana production due to blood disease was estimated to be approximately 36% in 1991 (Muharam and Subijanto, 1991). In Lampung, Southern Sumatra, losses due to blood disease have been estimated to reach 64% (Cahyaniati et al., 1997). Production of S. aromaticum has decreased rapidly since 1996, mainly due to Sumatra disease (Suryana et al., 2004).
Phylogeny, Classification and Geographic Distribution of R. syzygii Subspecies
Members of the R. solanacearum species complex have the most diverse host range and widest geographic distribution of any plant pathogenic bacterium (Elphinstone, 2005). The term “species complex” was introduced by Gillings and Fahy (1994) to indicate the high degree of phenotypic and genotypic diversity within the species R. solanacearum. Within the R. solanacearum species complex, four genetic groups, termed phylotypes, have been defined (Fegan and Prior, 2005; Prior and Fegan, 2005). Phylotypes I, II, and III are composed of strains mainly from Asia, America, and Africa, respectively, while Phylotype IV is primarily composed of strains from Indonesia but also occurs in a number of other Asian countries (Fegan and Prior, 2005).
Prior to the phylotyping classification system, R. solanacearum species complex strains were grouped into five races on the basis of host range (Buddenhagen, 1962; He et al., 1983) and five biovars based on the metabolism of three disaccharides (maltose, lactose, and cellobiose) and three hexose alcohols (sorbitol, mannitol, and dulcitol), the production of nitrite from nitrate and the production of gas from nitrate (Hayward, 1964, 1991, 1994a,b). While the biovar concept is applicable to R. solanacearum strains the biovar typing system has not been applied to R. syzygii or banana blood disease strains. The race and biovar classification system do not relate to each other with the exception that race 3 strains causing brown rot of Solanum tuberosum L. are generally considered to be equivalent to biovar 2 (Li et al., 2014).
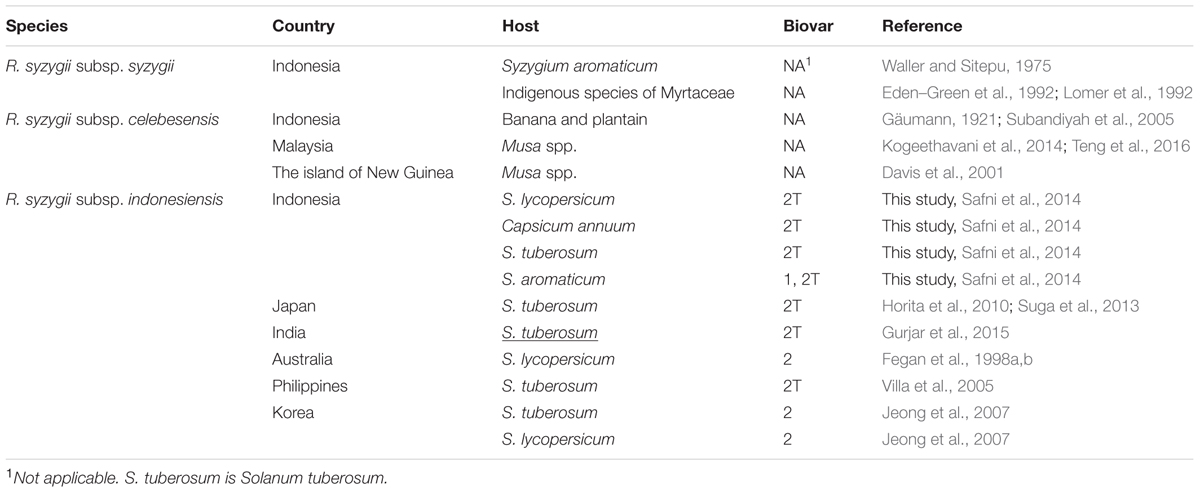
Recently, members of the R. solanacearum species complex have undergone a taxonomic revision (Safni et al., 2014). The reclassification of members of the R. solanacearum species complex based on a polyphasic study of phenotypic and genotypic characteristics led to the description of three species, R. solanacearum, R. pseudosolanacearum, and R. syzygii which are comprised of phylotype II, Phylotypes I and III, and phylotype IV strains, respectively (Safni et al., 2014). This classification has been confirmed by further proteomic and genomic characterisation of R. solanacearum species complex strains (Prior et al., 2016). Of the three species R. syzygii, as defined by Safni et al. (2014), is the most diverse group and contains three subspecies R. syzygii subsp. syzygii, R. syzygii subsp. indonesiensis and R. syzygii subsp. celebesensis. DNA-DNA Hybridization (DDH) is a molecular approach used to compare the overall similarity of whole genomes among different organisms (Rossello-Mora, 2006). The DDH value is expressed as a percentage homology, which a value of greater than 70% relatedness has been proposed as a recommended standard for species delineation (Wayne et al., 1987). However, a more relaxed boundary value The DDH values among the three subspecies of R. syzygii ranges from 67 to 100%, each subspecies can also be differentiated using phenotypic and genotypic characteristics in combination with pathogenicity (Safni et al., 2014). R. syzygii subsp. syzygii is the pathogen which causes Sumatra disease of clove trees and has only been described to occur in Indonesia. This subspecies, which contains the type strain of the species, was originally described as Pseudomonas syzygii by Roberts et al. (1990). This subspecies contains group of strains which are able to utilize only a small number of carbon sources (Roberts et al., 1990; Safni et al., 2014). R. syzygii subsp. celebesensis, the causal agent of blood disease on banana and plantain, occurs in Indonesia but has also been observed on the island of New Guinea (Davis et al., 2001) and has recently been identified in Malaysia (Kogeethavani et al., 2014; Teng et al., 2016). R. syzygii subsp. celebesensis strains are more metabolically active than R. syzygii subsp. syzygii strains but are less metabolically active than R. syzygii subsp. indonesiensis strains. Strains of R. syzygii subsp. indonesiensis cause bacterial wilt of a range of solanaceous host plants and have been recorded as being present in Indonesia, Australia, and Japan (Horita et al., 2010; Suga et al., 2013), Korea (Jeong et al., 2007), India (Gurjar et al., 2015) and the Philippines (Villa et al., 2005) (Figure 1 and Table 1).
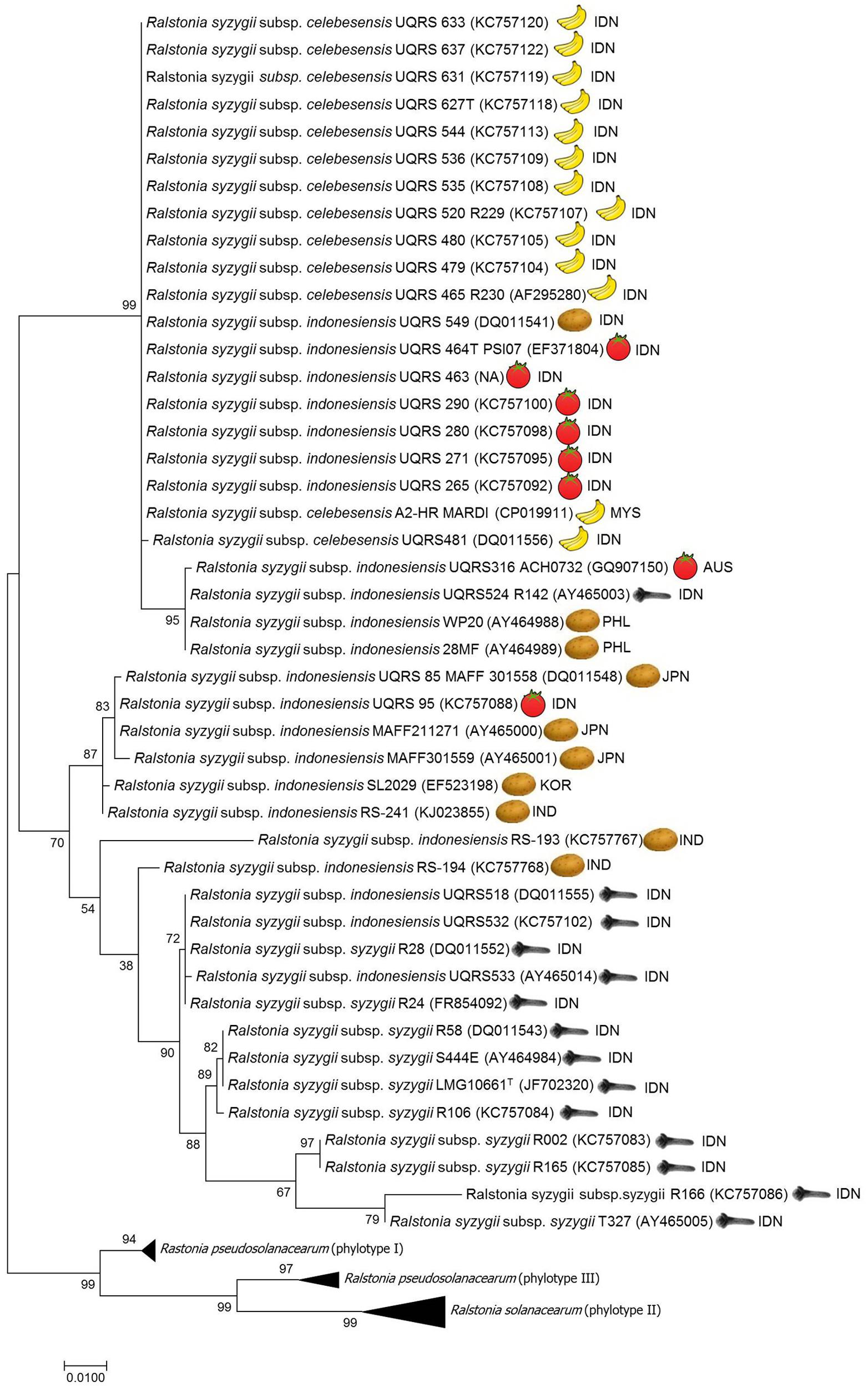
FIGURE 1. Molecular Phylogenetic analysis of egl gene sequences data using the Maximum Likelihood method. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. There were a total of 794 positions in the final dataset. Evolutionary analyses were conducted in MEGA7.0 (Kumar et al., 2016). ISO geographical codes: AUS, Australia; IDN, Indonesia; IND, India; JPN, Japan; KOR, Korea; MYS, Malaysia; PHL, Philippines.
Phylogenetic analysis of endoglucanase (egl) gene sequence data has been used to establish the evolutionary history of the R. solanacearum species complex and to determine the phylotype to which a strain belongs (Villa et al., 2005; Fegan and Prior, 2006). Using phylogenetic analysis of egl gene sequence data on R. syzygii strains reveals that the genetic diversity of these three subspecies varies (see Figure 1). All strains of R. syzygii subsp. celebesensis cluster together into a monophyletic group with certain R. syzygii subsp. indonesiensis strains from Indonesia. In contrast, strains of R. syzygii subsp. syzygii exhibit a greater degree of diversity in egl sequences but the largest level of genetic diversity in the species is present in strains belonging to R. syzygii subsp. indonesiensis. Analysis of egl gene sequence data using different phylogenetic methods, including Maximum likelihood, Neighbor joining, unweighted pair group method (UPGMA), and minimum evolution, produce congruent phylogenies (results not shown) but are not able to resolve the three subspecies with certain strains of R. syzygii subsp. indonesiensis being closely related to R. syzygii subsp. celebesensis and others more closely related to R. syzygii subsp. syzygii (Safni, 2014) (Figure 1). By employing multilocus sequence analysis (MLSA) Wicker et al. (2012) were able to identify eight groups, or clades, among the four phylotypes. Although only a few strains of R. syzygii were examined by MLSA results similar to the analysis of egl sequence data were found. MLSA was not able to clearly delineate the three subspecies of R. syzygii and certain R. syzygii subsp. indonesiensis strains from Indonesia clustered with R. syzygii subsp. celebesensis or R. syzygii subsp. syzygii strains (Wicker et al., 2012). It is interesting to note that the R. syzygii subsp. indonesiensis strains clustering closely with R. syzygii subsp. syzygii are also isolated from clove trees.
While a low level of genetic diversity has been described between R. syzygii subsp. celebesensis strains by sequence analysis of egl genes (Fegan and Prior, 2006; Safni et al., 2014) (Figure 1) and the 16S–23S intergenic spacer (ITS) region (Safni et al., 2014) and also genomic DNA fingerprinting patterns by rep-PCR and random amplified polymorphic DNA analysis (Thwaites et al., 1999), pulsed-field gel electrophoresis has revealed a degree of diversity within strains of the pathogen (Hadiwiyono et al., 2011).
Ecology and Epidemiology
Sumatra Disease of Clove Trees: R. syzygii subsp. syzygii
Sumatra disease of clove usually affects productive trees over 10 years of age (Hadiwijaya, 1983). Externally the initial symptom of Sumatra disease of cloves is unseasonal yellowing of leaves followed by leaf-drop from the tips of branches high in the crown (Figure 2). However, the leaves may also wilt suddenly and turn brown, but stay attached to the branch. Affected twigs turn reddish brown and progressively die back (Figures 2a,b). Symptoms typically progress to lower branches until the whole crown is affected, and the tree dies within 6–18 months (Bennett et al., 1985). Artificial inoculation of R. syzygii subsp. syzygii on 3 months old S. aromaticum seedling leads to symptom appearance beginning with leaf yellowing and drying at 28 days after inoculation and the death of the seedling 56 days after inoculation (Danaatmadja et al., 2009).

FIGURE 2. (a) Field infection of Sumatra Disease of Clove caused by Ralstonia syzygii subsp. syzygii in Magelang, Central Java, Indonesia. (b) Infected twig. (c) Horizontal section of infected twig. (d) Bacterial ooze oozing from the infected twig section. Reprinted with permission from Bambang Trianom.
Internally, the newly formed wood adjacent to the cambium becomes discolored a pale grayish-brown. When cut infected branches often produce a milky white to pale brown bacterial ooze from the cut surface (Figure 2c). The discolouration of the xylem can be traced down the trunk into one or more major roots (Bennett et al., 1985).
Sumatra disease of clove was initially observed in 1975 (Waller and Sitepu, 1975) and further reported in 1985 (Bennett et al., 1985). The disease affects S. aromaticum and some species of Myrtaceae including some indigenous species in native forests in Indonesia (Lomer et al., 1992), such as Syzygium aqueum (Eden–Green et al., 1992). The disease, which was initially confined to the Indonesian provinces of Sumatra and West Java, has now spread to Central Java and East Java and causes economic losses of up to 5–10% per year (Tjahyono, 2013).
Initially Sumatra disease of clove was assumed to be caused by nutritional disorders (Hadiwidjaja, 1956), mineral toxicities (Finck, 1972) as well as disease causing agents such as Ralstonia solanacearum [Xanthomonas solanacearum, Pseudomonas solanacearum (Hidir, 1973)], Phytophthora spp. (Djafaruddin et al., 1979), leaf spot fungi Phyllostictina sp. (Kranz, 1976), and the fastidious-xylem limited bacteria (XLB) (Bennett et al., 1985; Hunt et al., 1985) prior to the causative agent being taxonomically described as Pseudomonas syzygii (Roberts et al., 1990). Although R. syzygii subsp. indonesiensis strains have been isolated from the roots and lower trunk of trees only R. syzygii subsp. syzygii can systemically colonize and kill S. aromaticum trees.
Ralstonia syzygii subsp. syzygii is included as one of the xylem-restricted or xylem-limited bacteria, which live in xylem cells or tracheary elements of plants (Purcell and Hopkins, 1996). Similar to other diseases caused by xylem-restricted bacteria, R. syzygii subsp. syzygii is transmitted by insect vectors that feed on xylem sap (Bové and Garnier, 2002). The tube-building cercopoid (Hemiptera), Hindola fulva was found to be the natural insect vector in Sumatra whereas H. striata (Hemiptera: Machaerotidae) has been described as the primarily vector in Java (Eden–Green et al., 1992). Vector transmission of R. syzygii subsp. syzygii is persistent with a short latent period between acquisition and transmission of the pathogen (Eden–Green et al., 1992). Lomer et al. (1992) suggested that Sumatra disease of cloves may have been transferred to clove trees from an unknown forest hosts because of the localized initial distribution of the disease and the corresponding localized distribution of the vector species. If this hypothesis is correct then the pathogen may have a wider host range than has been previously identified (Purcell and Hopkins, 1996).
Sumatra disease of clove has a distinct pattern of disease expression and distribution in the field. Seedlings less than 2 years old are rarely affected, with trees over 10 years of age being the first to show symptoms and the first to die. The disease advances on a broad front, at an estimated rate of 1–2 km per year and then disappears for years until young trees mature and the cycle repeats (Bennett et al., 1985). As would be expected of an insect transmitted disease the disease spreads in a jump-spread pattern and spreads rapidly in all directions uphill, downhill, and across rivers (Bennett et al., 1985). Bennett et al. (1985) also reported that the rate of spread and symptom expression was partially affected by altitude possibly due to the lower temperature at higher altitudes where rapid decline symptoms are most commonly observed.
Blood Disease of Banana: R. syzygii subsp. celebesensis
Symptoms of Blood Disease are quite similar to Moko disease caused by insect-transmitted strains, the male flower bud and peduncle discolor and shrivel, the fruit pulp shows a reddish dry rot (Figure 3), and the vascular tissue throughout the plant exhibits a reddish discoloration, which emits reddish-brown bacterial ooze when cut (Sequeira and Averre, 1961; Buddenhagen, 1962). Moko disease of Musa spp. is caused by R. solanacearum strains which belong to phylotype II of the R. solanacearum species complex (Fegan and Prior, 2006). The older leaves of blood disease-infected Musa spp. become yellow, followed by wilting, necrosis and collapse; younger leaves turn bright yellow before becoming necrotic and dry. The pathogen rapidly colonizes the entire plant, and suckers will also wilt and die (Eden-Green, 1994b).
The banana blood disease pathogen is disseminated in soil and water and on farm tools, and has been hypothesized to enter host roots through natural openings or wounds (Gäumann, 1921, 1924). Gäumann (1921) reported that the pathogen survived in soil for at least a year in infested plant residues and infected fruits after entering the plant through its roots. Infested soil, tools, and vehicles move the pathogen within plantations, and movement of infected fruit and planting material enable long-distance spread. Insects that visit Musa spp. inflorescences, particularly those of cultivars with dehiscent bracts and an ABB genome (a plantain with one set of chromosomes donated by Musa acuminata and two by Musa balbisiana), can spread the pathogen rapidly over great distances (Mairawita et al., 2012). R. syzygii subsp. celebesensis has been isolated from the insect species Trigona minangkabau (Mairawita et al., 2012) and Erionota thrax (Suharjo et al., 2008). Erionota thrax has been observed to visit banana flowers 2–3 times a day (Suharjo et al., 2008). The bracts of ABB/BBB genotype Musa spp. are non-persistent and as they fall off they leave abscission scars which provide sites for pathogen entry into the vascular tissue of the plant. Also the male buds of the highly susceptible cultivar “Pisang Kepok” appear to be particularly attractive to insects such as wasps, bees and flies possibly because the male flower nectar has a high sugar content (Setyobudi and Hermanto, 1999).
The transmigration of people from Java to less populated islands in the country appears to be associated with the spread of the disease. R. syzygii subsp. celebesensis is thought to have originated on Selayar Island near Sulawesi, as the disease was first reported in the early 1900’s after the introduction of dessert bananas (Eden–Green, 1994a). The disease spread to Java in the late 1980’s and has become common on local M. paradisiaca cultivars in Sulawesi (Stover and Espinoza, 1992). Unfortunately, the pathogen has spread to most of the larger Indonesian islands, with average yield losses exceeding 35% (Supriadi, 2005), and has also been reported on the island of New Guinea (Davis et al., 2001).
The host range of R. syzygii subsp. celebesensis is not as wide as the R. solanacearum strains causing Moko and Bugtok diseases on Musa spp. Baharuddin (1994) showed that Heliconia sp. and Strelitzia reginae, both relatives of the Musaceae, are susceptible to R. syzygii subsp. celebesensis as are Canna indica, Datura stramonium, Asclepias currassiva, and Solanum nigrum. Unlike R. solanacearum strains causing Moko disease, R. syzygii subsp. celebesensis is not pathogenic on S. lycopersicum and Solanum melongena seedlings (Cellier and Prior, 2010; Eden–Green, 1994a; Supriadi, 2005). Strains of R. syzygii subsp. celebesensis are also not able to infect Arachis hypogaea, Capsicum sp., Nicotiana tabacum, S. tuberosum, and Zingiber officinale (Baharuddin, 1994).
Bacterial Wilt: R. syzygii subsp. indonesiensis
Ralstonia syzygii subsp. indonesiensis causes disease in a number of solanaceous plants in Indonesia and other countries in Asia but has also has been isolated from clove plants in Indonesia (Table 1). The disease symptoms caused by R. syzygii subsp. indonesiensis strains on solanaceous crops are no different from those described in the past for R. solanacearum (Kelman, 1953). The external symptoms of the infected plants are wilting, stunting and yellowing of the foliage with the disease progressing until the plant completely collapses from wilt (Figure 4). Internally the vascular tissue becomes progressively discolored in the early stages of infection with portions of the pith and cortex becoming involved as disease develops until complete necrosis occurs.

FIGURE 4. Potato infected by Ralstonia syzygii subsp. indonesiensis in Magelang, Central Java, Indonesia.
In Japan R. syzygii subsp. indonesiensis strains have only been isolated from S. tuberosum (Horita et al., 2014). The R. syzygii subsp. indonesiensis strains isolated from S. tuberosum in Japan have been reported to show varying levels of pathogenicity for S. lycopersicum, A. hypogaea and N. tabacum (Suga et al., 2013). However, none of the strains tested by Suga et al. (2013) were pathogenic for S. melongena. A R. syzygii subsp. indonesiensis strain isolated from S. lycopersicum in Indonesia (PSI07) was found to not to cause wilting of Cucumis melo, Anthurium andraeanum, Musa spp. and S. tuberosum but retained pathogenicity for S. lycopersicum (Ailloud et al., 2015).
There is little literature describing the epidemiology and ecology of R. syzygii subsp. indonesiensis strains. However, as is commonly described for other R. solanacearum species complex strains, the bacterium is reported to be able to survive in the field for long periods of time (Suga et al., 2013). Unlike R. solanacearum strains which cause brown rot of S. tuberosum R. syzygii subsp. indonesiensis strains have been shown to only cause disease in S. tuberosum in tropical but not temperate conditions (Cellier and Prior, 2010). In comparison to R. pseudosolanacearum strains Habe (2016) showed that R. syzygii subsp. indonesiensis exhibited high pathogenicity for S. tuberosum at a lower temperature (26°C) than R. pseudosolanacearum strains (28°C).
Disease Management
Sumatra Disease of Clove Trees: R. syzygii subsp. syzygii
All known clove varieties appear equally susceptible (Bennett et al., 1985). As indicated above the spread of the disease in the field is via an insect vector, Hindola spp., and most probably contaminated agricultural tools. Therefore local agricultural departments in Indonesia recommend that agricultural tools used for field work should be disinfected between uses, infected plants should be eradicated and insecticide should be applied to minimize the spread of the disease by insect vectors. Although several insecticides have been tested without effective control, aldicarb and carbofuran granules have provided effective control from 7 to 35 and 28 to 217 days after treatment, respectively (Stride and Nurmansyah, 1991). It has further been suggested that if resistant rootstocks could be identified for grafting the degeneration of the roots may be controlled (Bennett et al., 1985). Antibiotics have been shown to be useful in controlling the disease in mature trees for short periods but their use as an effective disease management tool is not recommended (Hunt et al., 1985). Biological control of the pathogen using antagonistic bacterial endophytes and rhizobacteria has also been suggested. Dwimartina et al. (2017) found that endophytic strains of Bacillus subtilis subsp. subtilis and rhizobacteria which produce indole acetic acid and dissolved phosphate could inhibit the growth of R. syzygii subsp. syzygii. However, the agropolitical challenges of S. aromaticum cultivation in Indonesia has made the application of any disease management strategies difficult (Baharuddin, 1994).
Some natural enemies of Hindola spp that may be potential as biological control of the insect vector were identified. Nuhardiyati et al. (1990) reported that H. fulva was found in the population with the unknown species of Hindola, Hindola sp. in Bengkulu, Sumatra, and Indonesia. Stylops sp. was found to parasitize the nymphs and adults of Hindola spp. The nymph of family Tettigoniidae was also found and assumed as the predator of Hindola sp. nymphs (Nuhardiyati et al., 1990). On the other hand, Hemipterian parasitoid paratized the nymph and eggs of Hindola spp. (Balfas et al., 1990). A member of the genus Acmopolynema was the parasite of Hindola spp. in Java, Indonesia (Balfas et al., 1990). This parasitoid of the insect vector of R. syzygii subsp. syzygii is a potential natural biological control agent of the insect vector. Balfas et al. (1990) reported that 30% of Hindola spp. eggs collected from clove and 60–80% of eggs collected from the clove related tree, Xanthostemon chrysanthus, were parasitized by Acmopolynema.
Blood Disease of Banana: R. syzygii subsp. celebesensis
It is thought that all edible Musa spp. may be susceptible to blood disease as no Indonesian cultivars of Musa spp. have been found to be resistant (Gäumann, 1921; Supriadi, 2005). However, some tolerance to blood disease has been reported to occur that may be a source of genetic material for resistance breeding (Supriadi, 2005).
Restricting the movement of planting material from infected areas has been successful in limiting the spread of the disease. A quarantine imposed by the Dutch to limit the spread the disease from Sulawesi was effective for over 60 years until the disease eventually spread to Java around 1987. From this point onward the pathogen has spread rapidly over the Indonesian archipelago and more recently Malaysia.
Removal of the male flower has been found to be effective in controlling the spread of disease as has the use of cultivars that abort the male bud (Hermanto et al., 2013). The cultivar “Pisang Puju,” an acceptable resistant Musa paradisiaca variety from Sulawesi, and “Pisang Sepatu Amora” may be suitable because these cultivars abort the male bud, blocking insect transmission (Hermanto et al., 2013).
A combination of basic quarantine and sanitation practices has been suggested to reduce the spread of blood disease (Davis et al., 2001). These measures include prohibiting movement of Musa spp. plants or plant parts out of infected areas, using disease free-planting materials, removing male buds immediately after the emergence of the last fruit, pesticide application as soon as the symptoms appear to reduce vector related spread and sterilizing the knives for harvesting.
Biological control of blood disease was suppressed by the application of endophytic bacteria including Bacillus sp and Bacillus subtilis isolated from Musa troglodytarum (Hadiba et al., 2010; Laturapeissa et al., 2014). Since the disease is insect-spread through the bacterial contaminated body of insects visiting Musa spp., therefore the biological control of insects associated with Musa spp. is potential for limiting the rate of blood disease spread. Cosmopolites sordidus, the Musa spp. and plantain root and corn borer insect, is not only potential in damaging banana plantation, but also increases the spread rate of blood disease (Subandiyah et al., 2005). This insect pest was reported to be effectively controlled by the application of Steinernematid nematodes (Figueroa, 1988) and Beauveria bassiana (Fancelli et al., 2013).
Bacterial Wilt: R. syzygii subsp. indonesiensis
Habe (2016) identified that there is a degree of resistance in S. tuberosum cultivars to R. syzygii subsp. indonesiensis. Suga et al. (2013) assessed pathogenic differences between R. pseudosolanacearum and R. syzygii subsp. indonesiensis strains against several Japanese varieties of S. tuberosum and breeding lines and indicated that R. syzygii subsp. indonesiensis strains show high virulence to the breeding lines carrying bacterial wilt resistance conferred from the wild Solanum sp., Solanum phureja, which is resistant to R. pseudosolanacearum strains. Several wild species of S. tuberosum such as S. phureja, Solanum stenostomum, and Solanum commersonii have been used as genetic resources to breed for resistance to bacterial wilt worldwide, and certain new S. tuberosum varieties with a high level of resistance have been identified. However, the high levels of resistance of these new S. tuberosum varieties have only been confirmed against R. pseudosolanacearum or R. solanacearum strains. R. syzygii subsp. indonesiensis strains have not been commonly used as targets in breeding for bacterial wilt resistance, most probably because this organism has only recently been identified as a taxonomic group within the R. solanacearum species complex and the restricted distribution [Japan, Korea, the Philippines, India, Indonesia, and Australia (Arwiyanto et al., 2015)] of this organism. Investigations to determine the pathogenicity of R. syzygii subsp. indonesiensis strains toward for new S. tuberosum varieties are needed to address the issue of variability in pathogenicity among different strains. Furthermore, the identification of new genetic resources for breeding need to consider resistance to R. syzygii subsp. indonesiensis strains in the future.
Conclusion
Ralstonia syzygii with its three subspecies, R. syzygii subsp. syzygii, R. syzygii subsp. celebesensis, and R. syzygii subsp. indonesiensis, is a phenotypically, genotypically and pathogenically diverse member of the R. solanacearum species complex. The members of this species have devastated agricultural commodities including S. aromaticum, Musa spp., and solanaceous vegetables in Indonesia. As the diseases caused by these pathogens continue to constrain agricultural production, effective disease management strategies are required.
While R. syzygii subsp. syzygii, which causes Sumatra disease of cloves, and R. syzygii subsp. celebesensis, the causal pathogen of banana blood disease, have restricted host ranges and geographic distribution, R. syzygii subsp. indonesiensis affects many solanaceous crops in several countries in Asia.
Information related to the epidemiology and ecology of R. syzygii subsp. indonesiensis is limited although it is assumed to behave similarly to R. solanacearum and R. pseudosolanacearum. Several disease management strategies have been developed and deployed to exclude, prevent and eliminate the pathogen. However, further work is required to confirm the efficacy of current control strategies and to improve implementation to achieve sustainable disease management solutions.
Both R. syzygii subsp. syzygii and R. syzygii subsp. celebesensis are insect transmitted pathogens. The dissemination of R. syzygii subsp. syzygii depends primarily on transmission via the insect vectors H. fulva and H. striata that feed on xylem sap while R. syzygii subsp. celebesensis can be disseminated non-specifically by insects visiting Musa spp. male buds of infected plants. While control of the diseases caused by R. syzygii subsp. syzygii and R. syzygii subsp. celebesensis is possible by the use of insecticides this approach is not widely used in Indonesia. Both R. syzygii subsp. celebesensis and R. syzygii subsp. syzygii can also spread through contaminated farm tools, plant material, and other human activities.
As bacterial wilt pathogens are also soil-borne they are difficult to control and successful management usually depends on the eradication and sanitation practices. The application of biosafety practices on infected farms is highly recommended for the management of bacterial wilt diseases. In the face of the high demand of clove for national cigarette production the Indonesian government has preferred to import clove from other countries. This policy has adversely affected the industry and the management of Sumatra disease of clove tree has been hindered because the Indonesian policy does not prioritize the expansion of domestic clove plantations. For blood disease of banana, preventing the spread of the disease by prohibiting the movement of Musa spp. plants or plant parts out of infected areas has not been applied effectively in Indonesia due to the difficulties in enforcing quarantine restrictions. The lack of success in the management of these bacterial diseases should become a lesson that promotes the improvement of future control strategies for these important plant diseases.
Author Contributions
IS and MF: study conception and design of the work. IS, SS, and MF: analysis and interpretation of data. IS and MF: drafting of manuscript and revising it critically for important intellectual content. IS, SS, and MF: final approval of the version to be published. IS, SS, and MF: agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research was supported by the Ministry of Research, Technology and Higher Education (KEMENRISTEKDIKTI) of the Republic of Indonesia through the World Class Professor Program (No. 168.A10/D2/KP/2017) organized at Research Center for Biotechnology Gadjah Mada University, Yogyakarta, Indonesia.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We also thank Prof. André Drenth (The University of Queensland) for helpful discussions.
References
Ailloud, F., Lowe, T., Cellier, G., Roche, D., Allen, C., and Prior, P. (2015). Comparative genomic analysis of Ralstonia solanacearum reveals candidate genes for host specificity. BMC Genomics 16:270. doi: 10.1186/s12864-015-1474-8
Armas, E., Osorio, C. G., Moreno-Dodson, B., and Abriningrum, D. (2012). Agriculture Public Spending and Growth in Indonesia. Policy Research Working Paper No. 5977. Washington, DC: World Bank. doi: 10.1596/1813-9450-5977
Arwiyanto, T., Nurcahyanti, S. D., Indradewa, D., and Widada, J. (2015). “Grafting local commercial tomato cultivars with H-7996 and Eg-203 to suppress bacterial wilt,” in Proceedings of the 4th International Conference on Potato Diseases (Orlando, FL: International Society for Horticultural Science (ISHS)).
Baharuddin, B. (1994). Pathological, Biochemical and Serological Characterization of the Blood Disease Bacterium Affecting Banana and Plantain (Musa spp.) in Indonesia. Göttingen: Cuvillier Verlag.
Balfas, R., Nuhardiyati, M., and Lomer, C. J. (1990). Distribution and parasitism of Acmopolynema (Hymenoptera: Machaerotidae) on clove and Xanthostemon chrysanthus. Ind. Crops Res. J. 3, 6–10.
Bennett, C. P. A., Hunt, P., and Asman, A. (1985). Association of a xylem-limited bacterium with Sumatra disease of cloves in Indonesia. Plant Pathol. 34, 487–494. doi: 10.1111/j.1365-3059.1985.tb01398.x
Bové, J. M., and Garnier, M. (2002). Phloem-and xylem-restricted plant pathogenic bacteria. Plant Sci. 163, 1083–1098. doi: 10.1016/S0168-9452(02)00276-5
Buddenhagen, I. (1962). Strains of Pseudomonas solanacearum in indigenous hosts in banana plantations of Costa Rica, and their relationship to bacterial wilt of bananas. Phytopathology 50, 660–664.
Cahyaniati, C., Mortensen, N., and Mathur, S. B. (1997). Bacterial Wilt of Banana in Indonesia. Technical Bulletin. Jakarta: Directorate Plant Protection of Indonesia.
Cellier, G., and Prior, P. (2010). Deciphering phenotypic diversity of Ralstonia solanacearum strains pathogenic to potato. Phytopathology 100, 1250–1261. doi: 10.1094/PHYTO-02-10-0059
Cervantes-Godoy, D., and Dewbre, J. (2010). Economic importance of agriculture for sustainable development and poverty reduction: findings from a case study of Indonesia. Paper Presented to the OEDC Global Forum on Agriculture, Paris.
Danaatmadja, Y., Subandiyah, S., Joko, T., and Sari, C. U. (2009). Isolasi dan karakterisasi Ralstonia syzygii (Isolation and characterization of Ralstonia syzygii). J. Perlindungan Tanaman Indonesia 15, 7–12.
Davis, R., Moore, N., and Fegan, M. (2001). “Blood disease and Panama disease: two newly introduced and grave threats to banana production on the island of New Guinea,” in Food Security for Papua New Guinea. Proceedings of the Papua New Guinea Food and Nutrition 2000 Conference PNG University of Technology, Lae, 26–30 June 2000, eds R. M. Bourke, M. G. Allen, and J. G. Salisbury (Lae: ACIAR), 816–821.
Djafaruddin, A., Hanafiah, D., Su’ud, M. M., Syafruddin, and Mardinus. (1979). Penelitian Penyebab Utama Mass Decline (Mati Massal) pada Tanaman Cengkeh di Sumatera Barat. II. Padang: Universitas Andalas.
Drenth, A., and Guest, D. I. (2016). Fungal and Oomycete diseases of tropical tree fruit crops. Annu. Rev. Phytopathol. 543, 373–395. doi: 10.1146/annurev-phyto-080615-095944
Dwimartina, F., Arwiyanto, T., and Joko, T. (2017). Potential of endophytic and rhizobacteria as an effective biocontrol for Ralstonia syzygii subsp. syzygii. Asian J. Plant Pathol. 11, 191–198. doi: 10.3923/ajppaj.2017.191.198
Eden–Green, S. J. (1994a). Diversity of Pseudomonas solanacearum and Related Bacteria in South East Asia: New Directions for Moko Disease. Wallingford: CAB International, 25–34.
Eden–Green, S. J., Balfas, R., Sutarjo, T., and Jamalius. (1992). Characteristics of the transmission of Sumatra disease of cloves by tube-building cercopoids, Hindola spp. Plant Pathol. 41, 702–712. doi: 10.1111/j.1365-3059.1992.tb02553.x
Elphinstone, J. G. (2005). “The current bacterial wilt situation: a global perspective,” in Bacterial Wilt: The Disease and the Ralstonia solanacearum Species Complex, eds C. Allen, P. Prior, and A. C. Hayward (St. Paul, MN: American Phytopathological Society Press), 9–28.
Fancelli, M., Dias, A. B., Delalibera Júnior, I., de Jesus, S. C., do Nascimento, A. S., de Oliveira e Silva, S., et al. (2013). Beauveria bassiana strains for biological control of Cosmopolites sordidus (Germ.) (Coleoptera: Curculionidae) in plantain. BioMed Res. Int. 2013:184756. doi: 10.1155/2013/184756
Fegan, M., Holoway, G., Hayward, A. C., and Timmis, J. (1998a). “Development of a diagnostic test based on the polymerase chain reaction (PCR) to identify strains of R. solanacearum exhibiting the biovar 2 genotype,” in Bacterial Wilt Disease: Molecular and Ecological Aspects, eds P. Prior, C. Allen, and J. Elphinstone (Berlin: Springer-Verlag), 34–43. doi: 10.1007/978-3-662-03592-4_5
Fegan, M., and Prior, P. (2005). “How complex is the Ralstonia solanacearum species complex?,” in Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex, eds C. Allen, P. Prior, and A. C. Hayward (St. Paul, MN: APS Press), 449–461.
Fegan, M., and Prior, P. (2006). Diverse members of the Ralstonia solanacearum species complex cause bacterial wilts of banana. Australas. Plant Pathol. 35, 93–101. doi: 10.1071/AP05105
Fegan, M., Taghavi, M., Sly, L. I., and Hayward, A. C. (1998b). “Phylogeny, diversity and molecular diagnostics of Ralstonia solanacearum,” in Bacterial Wilt Disease: Molecular and Ecological Aspects, eds P. Prior, C. Allen, and J. Elphinstone (Paris: INRA Editions), 19–23.
Figueroa, W. (1988). Biocontrol of the banana root borer weevil, Cosmopolites sordidus (Gerinar), with Steinernematid nematodes. J. Agric. Univ. Puerto Rico 74, 15–19.
Finck, A. (1972). Nutrient disorder as a possible cause of a clove disease in West Sumatra. Potash Rev. 23/40, 1–2.
Gäumann, E. (1921). Onderzoekingen over de bloedziekte der bananen op Celebes I. (Investigations into the blood disease of bananas on Celebes Island) Mededeelingen van het Instituut voor Plantenziekten No. 50, 47p. Rev. Appl. Mycol. 1, 225–227.
Gäumann, E. (1924). Onderzoekingen over de bloedziekte der bananen op Celebes II. (Investigations on the blood disease of banana in Celebes II). Mededeelingen van het Instituut voor Plantenziekten No. 59, 45p. Rev. Appl. Mycol. 1, 344–346.
Geddes, A. M. W. (1992). The relative importance of preharvest crop pests in Indonesia. Bulletin No. 47. Chatham: Natural Resources Institute, 70.
Gillings, M. R., and Fahy, P. (1994). “Genomic fingerprinting: towards a unified view of the Pseudomonas solanacearum species complex,” in Bacterial Wilt the Disease and Its Causative Agent, Pseudomonas solanacearum, eds A. C. Hayward and G. L. Hartman (Wallington: CAB International).
Gurjar, M. S., Sagar, V., Bag, T. K., Singh, B. P., Sharma, S., Jeevalatha, A., et al. (2015). Genetic diversity of Ralstonia solanacearum strains causing bacterial wilt of potato in the Meghalaya state of India. J. Plant Pathol. 97, 135–142.
Habe, I. (2016). Pathogenic characteristics by temperature of Ralstonia solanacearum phylotypes I and IV using in vitro screening assay for resistance to bacterial wilt in potato. Kyushu Plant Prot. Res. 62, 20–26. doi: 10.4241/kyubyochu.62.20
Hadiba, N., Wibowo, A., Widada, J., and Subandiyah, S. (2010). “Endophytic bacteria for suppressing Banana Blood Disease bacterium,” in Proceedings of the 12th ICPPB 7-11 June 2010, St. Denis.
Hadiwidjaja, T. (1956). Mati Budjang Disease of the Clove Trees. Bogor No. 143. Bogor: Contributions of the General Agricultural Research Station, 74.
Hadiwijaya, T. (1983). Cengkih, Data dan Petunjuk ke Arah Swasembada, 6th Edn. Jakarta: PT. Gunung Agung.
Hadiwiyono, Widada, J., Subandiyah, S., and Fegan, M. (2011). Pulsed Field Gel Electrophoresis (PFGE): a DNA finger printing technique to study the genetic diversity of blood disease bacterium of banana. Biodiversitas 12, 12–16. doi: 10.13057/biodiv/d120103
Hayward, A. C. (1964). Characteristics of Pseudomonas solanacearum. J. Appl. Bacteriol. 27, 265–277. doi: 10.1111/j.1365-2672.1964.tb04912.x
Hayward, A. C. (1991). Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 29, 65–87. doi: 10.1146/annurev.py.29.090191.000433
Hayward, A. C. (1994a). “Systematics and phylogeny of Pseudomonas solanacearum and related bacteria,” in Bacterial Wilt: The Disease and Its Causative Agent, Pseudomonas solanacearum, eds A. C. Hayward and G. L. Hartman (Wallingford: CAB International), 123–135.
Hayward, A. C. (1994b). “The hosts of Pseudomonas solanacearum,” in Bacterial Wilt: The Disease and Its Causative Agent, Pseudomonas solanacearum, eds A. C. Hayward and G. L. Hartman (Wallington: CAB International).
He, L. Y., Sequeira, L., and Kelman, A. (1983). Characteristics of strains of Pseudomonas solanacearum from China. Plant Dis. 67, 1357–1361. doi: 10.1094/PD-67-1357
Hermanto, C., Eliza, E., and Emilda, D. (2013). Bunch management of banana to control blood disease. Australas. Plant Pathol. 42, 653–658. doi: 10.1007/s13313-013-0248-5
Hidir, S. (1973). “Penyakit-penyakit cengkeh di Indonesia dan usaha penanggulangannya di lapangan (Diseases on clove and the disease management in the field),” in Proceedings of the Workshop of Directorate General of Plantation Ciawi, Bogor.
Horita, M., Suga, Y., Ooshiro, A., and Tsuchiya, K. (2010). Analysis of genetic and biological characters of Japanese potato strains of Ralstonia solanacearum. J. Gen. Plant Pathol. 76, 196–207. doi: 10.1007/s10327-010-0229-2
Horita, M., Tsuchiya, K., Suga, Y., Yano, K., Waki, T., Kurose, D., et al. (2014). Current classification of Ralstonia solanacearum and genetic diversity of the strains in Japan. J. Gen. Plant Pathol. 80, 455–465. doi: 10.1007/s10327-014-0537-z
Hunt, P., Bennett, C. P. A., and Asman, A. (1985). Suppression of Sumatra disease symptoms in cloves treated with antibiotics. Plant Pathol. 34, 495–501. doi: 10.1111/j.1365-3059.1985.tb01399.x
Jeong, Y., Kim, J., Kang, Y., Lee, S., and Hwang, I. (2007). Genetic diversity and distribution of Korean isolates of Ralstonia solanacearum. Plant Dis. 91, 1277–1287. doi: 10.1094/PDIS-91-10-1277
Kelman, A. (1953). The Bacterial Wilt Caused by Pseudomonas solanacearum. A Literature Review and Bibliography. Technical Bulletin No. 99. Davis, CA: North Carolina Agricultural Experiment Station.
Kogeethavani, R., Sulastri, N. J., Mazanah, M., Rozeita, L., and Roff, M. N. M. (2014). First Report of Blood Disease Bacterium on Banana in Malaysia. Selangor: Malaysian Agricultural Research and Development Institute.
Kranz, J. (1976). Phyllostictina sp. and Pycnothyrium sp. on elove trees (Eugenia aromatica) in Sumatra (Indonesia). Z. Pflanzenkrankheiten Pflarizenschutz 81, 138–140.
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Laturapeissa, Y., Nawangsih, A. A., and Mutaqin, K. H. (2014). Biocontrol of the banana root borer weevil, Cosmopolites sordidus (Gerinar), with Steinernemotid nematodes. J. ISSAAS 20, 110–120.
Li, X., Nie, J., Hammill, D. L., Smith, D., Xu, H., and De Boer, S. H. (2014). A comprehensive comparison of assays for detection and identification of Ralstonia solanacearum race 3 biovar 2. J. Appl. Microbiol. 117, 1132–1143. doi: 10.1111/jam.12585
Lomer, C. J., Eden-Green, S. J., Boa, E. R., and Supriadi (1992). Evidence for a forest origin of Sumatra disease of cloves. Trop. Sci. 32, 95–98.
Magarey, R. C., Kristini, A., Sallam, N., Samson, P. R., Achadian, E., McGuire, P. J., et al. (2010). IPM Strategies for pest and disease control in Indonesia: project overview and outcomes from recent ACIA-funded research. Proc. Aust. Soc. Sugar Cane Technol. 32, 169–180.
Mairawita, Suswati, Habazar, T., Hasyim, A., and Nasir, N. (2012). “Trigona minangkabau potential as bacterial spreader agent of Ralstonia solanacearum phylotype IV cause blood disease on banana plants,” in Proceedings of the 2012 International Conference on Biological and Life Sciences (Singapore: IACSIT Press), 109–116.
Maulana, M., and Sayaka, B. (2007). The features of vegetables in Indonesia and the current policy in the framework of agricultural development. Anal. Kebijakan Pertanian 5, 267–284.
Muharam, A., and Subijanto (1991). “Status of banana diseases in Indonesia,” in Proceedings of the Technical Meeting in Diseases Affecting Banana and Plantain in Asia and Pacific, eds R. V. Valmayar, B. E. Umali, and C. P. Bejosano (Brisbane, QLD: INIBAP), 44–49.
Nuhardiyati, M., Lomer, C. J., Wikardi, J., and Wikardi, E. A. (1990). Preliminary observations on species of Hindola and their natural enemy complex in Bengkulu. Ind. Crops Res. J. 2, 37–42.
Prabaningrum, L., and Moekasan, T. K. (2014). Pengelolaan organisme pengganggu tumbuhan utama pada budidaya cabai merah di dataran tinggi (Pest and disease management on hot pepper cultivation in high land). J. Hortik. 24, 179–188. doi: 10.21082/jhort.v24n2.2014.p179-188
Prior, P., Ailloud, F., Dalsing, B. L., Remenant, B., Sanchez, B., and Allen, C. (2016). Genomic and proteomic evidence supporting the division of the plant pathogen Ralstonia solanacearum into three species. BMC Genomics 17:90. doi: 10.1186/s12864-016-2413-z
Prior, P., and Fegan, M. (2005). “Recent developments in the phylogeny and classification of Ralstonia solanacearum,” in Proceedings of the 1st International Symposium on Tomato Diseases (Acta Horticulturae), eds M. T. Momol, P. Ji, and J. B. Jones (Orlando, FL: International Society for Horticultural Science), 127–136. doi: 10.17660/ActaHortic.2005.695.14
Purcell, A. H., and Hopkins, D. L. (1996). Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34, 131–151. doi: 10.1146/annurev.phyto.34.1.131
Roberts, S. J., Eden-Green, S. J., Jones, P., and Ambler, D. J. (1990). Pseudomonas syzygii, sp. nov., the cause of Sumatra disease of cloves. Syst. Appl. Microbiol. 13, 34–43. doi: 10.1016/S0723-2020(11)80178-5
Rossello-Mora, R. (2006). “DNA-DNA reassociation methods applied to microbial taxonomy and their critical evaluation,” in Molecular Identification, Systematics, and Population Structure of Prokaryotes, ed. E. Stackebrandt (Berlin: Springer), 23–50.
Safni, I. (2014). Studies of the Taxonomy of Banana Blood Disease Bacterium and Related Bacteria. Doctoral dissertation, The University of Queensland, Brisbane, QLD.
Safni, I., Cleenwerck, I., De Vos, P., Fegan, M., Sly, L., and Kappler, U. (2014). Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int. J. Syst. Evol. Microbiol. 64, 3087–3103. doi: 10.1099/ijs.0.066712-0
Sequeira, L., and Averre, C. W. (1961). Distribution and pathogenicity of strains of Pseudomonas solanacearum from virgin soils in Costa Rica. Plant Dis. Report. 45, 435–440.
Setyobudi, L., and Hermanto, C. (1999). Rehabilitation of cooking bananas from blood disease: baseline status of distribution and infestation in Sumatera. Paper Presented on the RISBAP Meeting on September 13-17, 1999, Phitsanulok, 6.
Stride, A. B., and Nurmansyah (1991). Evaluation of insecticides against Hindola fulva, a vector of Sumatra disease of cloves in Indonesia. Ann. Appl. Biol. 118, 10–11.
Subandiyah, S., Indarti, S., Harjaka, T., Utami, S. N. H., Sumardiyono, C., and Mulyadi (2005). “Bacterial wilt disease complex of banana in Indonesia,” in Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex, eds C. Allen, P. Prior, and A. C. Hayward (St. Paul, MN: APS Press), 415–422.
Suga, Y., Horita, M., Umekita, M., Furuya, N., and Tsuchiya, K. (2013). Pathogenic characters of Japanese potato strains of Ralstonia solanacearum. J. Gen. Plant Pathol. 79, 110–114. doi: 10.1007/s10327-013-0429-7
Suharjo, R., Subandiyah, S., and Martono, E. (2008). Hubungan antara frekuensi kedatangan imago Erionota thrax pada bunga pisang dan keterjadian penyakit layu bakteri pisang pada lahan sawah, tegalan dan pekarangan. J. Hama Penyakit Tumbuhan Trop. 8, 47–54.
Supriadi (2005). Present Status of Blood Disease in Indonesia. St. Paul, MN: American Phytopathological Society (APS Press), 395–404.
Suryana, A., Allorerung, D., Wahid, P., Manohara, D., Pribadi, R., Indrawanto, C., et al. (2004). Prospek dan Pengembangan Agibisnis Cengkeh. Jakarta: Research and Development Institute.
Teng, S. K., Aziz, N. A. A., Mustafa, M., Laboh, R., Ismail, I. S., Sulaiman, S. R., et al. (2016). The occurrence of blood disease of banana in Selangor, Malaysia. Int. J. Agric. Biol. 18, 92–97. doi: 10.17957/IJAB/15.0067
Thwaites, R., Mansfield, J., Eden-Green, S., and Seal, S. (1999). RAPD and rep PCR-based fingerprinting of vascular bacterial pathogens of Musa spp. Plant Pathol. 48, 121–128. doi: 10.1046/j.1365-3059.1999.00321.x
Tjahyono, E. (2013). Penanganan serangan penyakit bakteri Pseudomonas syzygii, pembuluh kayu cengkeh Di Jawa Timur. Dinamika Perkebunan 24–25.
Villa, J. E., Tsuchiya, K., Horita, M., Natural, M., Opina, N., and Hyakumachi, M. (2005). Phylogenetic relationships of Ralstonia solanacearum species complex strains from Asia and other continents based on 16S rDNA, endoglucanase, and hrpB gene sequences. J. Gen. Plant Pathol. 71, 39–46. doi: 10.1007/s10327-004-0156-1
Waller, J. M., and Sitepu, D. (1975). Sumatra disease of cloves in Indonesia. Pest Articles News Summ. 21, 141–147. doi: 10.1080/09670877509411385
Wayne, L. G., Brenner, D. J., Colwell, R. R., Grimont, P. A. D., Kandler, O., Krichevsky, M. I., et al. (1987). Report of the ad-hoc-committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37, 463–464. doi: 10.1099/00207713-37-4-463
Keywords: Ralstonia syzygii, bacterial wilt, Indonesia, ecology, epidemiology, disease management
Citation: Safni I, Subandiyah S and Fegan M (2018) Ecology, Epidemiology and Disease Management of Ralstonia syzygii in Indonesia. Front. Microbiol. 9:419. doi: 10.3389/fmicb.2018.00419
Received: 06 July 2017; Accepted: 21 February 2018;
Published: 13 March 2018.
Edited by:
Alice Guidot, Institut National de la Recherche Agronomique (INRA), FranceReviewed by:
Massimiliano Morelli, Istituto per la Protezione Sostenibile delle Piante (CNR), ItalyYucheng Zhang, University of Florida, United States
Copyright © 2018 Safni, Subandiyah and Fegan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siti Subandiyah, c2l0aXN1YmFuZGl5YWhAdWdtLmFjLmlk
 Irda Safni
Irda Safni Siti Subandiyah
Siti Subandiyah Mark Fegan
Mark Fegan