- 1Jiangsu Key Laboratory for Microbes and Functional Genomics, Jiangsu Engineering and Technology Research Center for Microbiology, College of Life Sciences, Nanjing Normal University, Nanjing, China
- 2National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, China
The purpose of this study was to elucidate the characteristics and mechanisms of adsorption and desorption for heavy metals by micro and nano-sized biogenic CaCO3 induced by Bacillus subtilis, and the pH effect on adsorption was investigated. The results showed that the adsorption characteristics of Cd2+ and Pb2+ are well described by the Langmuir adsorption isothermal equation, and the maximum adsorption amounts for Cd2+ and Pb2+ were 94.340 and 416.667 mg/g, respectively. The maximum removal efficiencies were 97% for Cd2+, 100% for Pb2+, and the desorption rate was smaller than 3%. Further experiments revealed that the biogenic CaCO3 could maintain its high adsorption capability for heavy metals within wide pH ranges (3–8). The FTIR and XRD results showed that, after the biogenic CaCO3 adsorbed Cd2+ or Pb2+, it did not produce a new phase, which indicated that biogenic CaCO3 and heavy metal ions were governed by a physical adsorption process, and the high adsorptive capacity of biogenic CaCO3 for Cd2+ and Pb2+ were mainly attributed to its large total specific surface area. The findings could improve the state of knowledge about biogenic CaCO3 formation in the environment and its potential roles in the biogeochemical cycles of heavy metals.
Introduction
The main current environmental problems are the increasing atmospheric greenhouse effect and environmental pollution of large areas. Moreover, the increase of population and industrial or agricultural production makes such environmental issues more prominent. Heavy metal pollution in water and soil is already a global problem, and it is especially serious in soils (Zhao et al., 2014a). For example, the proportion of heavy metal pollution has exceeded 2.5% by land area, covering 2.32 million hectares in China, and the exceedances of permissible threshold values for Cd, Hg, As, Cu, Pb, Cr, Zn, and Ni are 7, 1.6, 2.7, 2.1, 1.5, 1.1, 0.9, and 4.8%, respectively (The Ministry of Environmental Protection, 2014; Zhao et al., 2014a; The Ministry of Land and Resources, 2015).
Microbial methods are become favored due to their low cost and environmentally—friendly nature (Lian et al., 2008; Kavamura and Esposito, 2010; Moreau et al., 2013; Santos et al., 2017), but the adsorbed heavy metals may re-enter the environment and cause re-pollution with the change in environmental conditions (Pavasant et al., 2006; Pan et al., 2007; Apiratikul and Pavasant, 2008; Tsekova et al., 2010). A sequestration method seems to be the most convenient and most commonly chosen method (Lagadic et al., 2001; Babel and Kurniawan, 2003; Kobya et al., 2005; Ren et al., 2013); however, currently available heavy metals adsorbents remain limited, and most traditional adsorbents come with high utilization costs. Therefore, it is necessary to develop a new high-efficiency, low-cost, environmentally-friendly heavy metal adsorbent.
CaCO3 is one of the most abundant bio-minerals found in nature, and it has aroused great interest in many branches of science. The biosynthesis of CaCO3 is of great significance. It can promote carbon deposition, thus contributing to mitigate global warming (Dupraz et al., 2009; Mitchell et al., 2010; Phillips et al., 2013). CaCO3 is reported to adsorb heavy metal ions in water or soil with good effect, and increasing the amount of CaCO3 in the soil or water can significantly reduce the migration of heavy metals (Al-Degs et al., 2006; Yavuz et al., 2007; Aziz et al., 2008; Cai et al., 2010; Zhao et al., 2014a). However, the use of biogenic CaCO3 combined with microbial technology to remediate heavy metal pollution, including the related process and the microscopic mechanism, has not yet been reported.
Natural CaCO3 from limestone has limited further development prospects as a result of its low purity and efficiency. The traditional CO2-bubble method for synthesizing CaCO3 cannot sufficiently regulate the product size, morphology, or crystal type, and the cost is higher. But it is feasible to produce biogenic CaCO3 particles with morphological diversity (such as: spherical, rhabditiform, flower, dumbbell-shape, reticular structure aggregates, etc.) and low cost by microbial mineralisation technology (Lian et al., 2006; Han et al., 2013; Cao et al., 2016). On this basis, developing the application of biogenic CaCO3 in the treatment of heavy metals can not only deepen our understanding of the environmental significance of bio-mineralisation, but also develop a potential means with which to control heavy metal pollution.
The adsorption of heavy metal ions is subject to many factors, such as contact time, temperature, pH, and so on. Since the surface charge of an adsorbent in a solution could be altered by changing its pH value, the pH is one of the most important factors affecting the adsorption process of metal ions (Farrah and Pickering, 1979; Chen et al., 1997; Abollino et al., 2003; Üçer et al., 2006; Wolthers et al., 2008; Meng et al., 2009; Ma et al., 2012). Here, the adsorption-desorption properties of Cd2+ and Pb2+ by CaCO3 induced by Bacillus subtilis and the pH effect on adsorption were investigated. This study will improve our knowledge of biogenic CaCO3 formation in the environment and its potential role in the remediation of heavy metals.
Materials and Methods
Preparation of Micro and Nano-sized Biogenic CaCO3 and Its Morphology and Chemical Composition Analysis
Experimental Strain
B. subtilis (GenBank accession number KT343639), derived from the National Research and Extension Centre of Microbial Fertilizer Technology of China, is the legal functional microbial fertilizer strain in China.
We inoculated two or three rings with B. subtilis in 200 mL LB liquid culture medium [tryptone 0.1% (W/V), yeast extract 0.5% (W/V), NaCl 1% (W/V), 6.5 ≤ pH ≤ 7.5], shaking-cultured for 10 h at 30°C and 180 rpm, to prepare the bacterial liquid [(7.75 ± 1.19) × 107 cfu/mL]. We added 100 mL LB liquid medium (containing CaCl2 0.2 g) to a clean 250 mL conical flask. Afterwards, we inoculated 2 mL strain from the aforementioned bacterial liquid to form the experimental group, and set up 20 parallel, shaking-cultured samples (30°C, 180 rpm, for 7 days) to induce CaCO3 synthesis. The culture solution was centrifuged at 8000 rpm for 15 min at 4°C, and then the centrifuged sediments were collected and dried at 55°C, then milled to 200 mesh size or finer by agate mortar in readiness for testing. To verify whether the acquisition of micro- and nano-sized biogenic CaCO3 was successful, or not, we smeared the precipitate evenly on clean cover-glasses, drying naturally, then, subjected them to field emission scanning electron microscopy and energy dispersive spectrometry (FESEM-EDS) analysis. In addition, the XRD and TEM-SAED (selected area electron diffraction) methods were used to analyse the crystal structure of the precipitate.
The Adsorption and Desorption of Cd2+ and Pb2+ by Micro and Nano-sized Biogenic CaCO3
To investigate the environmental remediation benefits of biogenic CaCO3, the adsorption and desorption characteristics of two common heavy metal ions (Cd2+ and Pb2+) under the action of biogenic CaCO3 were investigated. The Langmuir and Freundlich equations were used to fit an adsorption model, and this was then employed to obtain the maximum adsorption capacity of such heavy metals (Wang et al., 2007b; Mikutta et al., 2012; Musso et al., 2014).
Adsorption Experiments
Some 0.10 g biogenic CaCO3 was added to a 50 mL polyethylene centrifuge tube containing 20 mL solution with different Cd2+ (CdCl2), and Pb2+ (Pb(NO3)2) concentrations (0, 5, 10, 30, 60, 100, 150, 180, 220, and 260 mg/L: concentration based on actual measurements). The mixture was shaken at 25°C, and 100 rpm in a shaker for 24 h, and each group was tested as a set of three replicates. After shaking, the supernatant was separated by centrifuging at 8000 rpm for 15 min. The concentration of metal ions was determined by atomic absorption spectrometer (AAS, AA-6300C, Shimadzu). The adsorption amount of Cd2+ and Pb2+ by biogenic CaCO3 (Qe) was calculated based on Equation (1), the adsorption isotherms were obtained by use of Ce with Qe, and the heavy metal adsorption rates were calculated by using of Equation (2), the formulae are as follows (Argun et al., 2007; Wang et al., 2007b; Lian et al., 2008; Ma et al., 2012; Yao et al., 2013; Zhao et al., 2014b; Liu et al., 2016):
Where C0 and Ce are the initial, and equilibrium concentrations of the metal ions (mg/L), respectively; V represents the volume of equilibrium liquid in the centrifuge tube (L), and W1 is the mass of biogenic CaCO3 (g).
Experimental results were analyzed with reference to the Langmuir and Freundlich isotherms (Equations 3, 4), respectively (Grimm et al., 2008; Ma et al., 2012; Mikutta et al., 2012; Wang et al., 2015):
Where Ce denotes the equilibrium concentration of metal ions in the supernatant (mg/L), Qe is the adsorption amount of metal ions by biogenic CaCO3 (mg/g), Qm denotes the maximum adsorption amount of metal ions (mg/g), KL is the adsorption coefficient of the Langmuir model (L/mg), Kf is the Freundlich constant, and nf is the adsorption intensity constant of the Freundlich equation.
Desorption Experiments
We added 20 mL desorption liquid (Dong-Mei et al., 2003; Arias et al., 2006; Gherasim and Bourceanu, 2013; 1 mol/L NaNO3, pH = 7.0) to the centrifugal tube with the precipitates therein after adsorbing any Cd2+ or Pb2+, then the samples were shocked at 25°C, and 100 rpm for 12 h. Afterwards, the samples were centrifuged at 8000 rpm for 15 min, and AAS was used to determine the Cd2+ or Pb2+ concentrations in supernatant (C1). Each desorption experiment was conducted in triplicate.
The desorption amount of heavy metals (Qde) (Equation 5) and the rate of desorption (Equation 6) were calculated as follows (Gao et al., 2003; Wang et al., 2007a; Zhao et al., 2014b):
Where Qde is the desorption amount of heavy metals (mg/g), V is the volume of desorption solution (L), C1 represents the metal ion concentration of desorption supernatant (mg/L), and W1 is the mass of biogenic CaCO3 (g).
The Mechanism of Adsorption
To elucidate the adsorption mechanism of biogenic CaCO3, we collected the biogenic CaCO3 before and after adsorbing Cd2+ or Pb2+, and dried it at 55°C, Afterwards, using FTIR (NEXUS670, Thermo Nicolet), XRD (Ultima IV Multipurpose, Rigaku), FESEM-EDS, and soft X-ray microscopy techniques were used to analyse the changes in structures, morphologies and elemental compositions. Meanwhile, the adsorption and desorption of Cd2+ (74 mg/L) and Pb2+ (94 mg/L) by vaterite biogenic CaCO3 (prepared in LB liquid medium containing 0.8 g CaCl2, referenced in Section Preparation of Micro and Nano-sized
Biogenic CaCO3 and Its Morphology and Chemical Composition Analysis) were also studied, and the structural changes of vaterite before, and after, adsorbing Cd2+ or Pb2+ were analyzed by XRD.
The Comparison of Adsorption and Desorption for Heavy Metals by Biogenic CaCO3 and Bacterial Cells
We added 100 mL LB liquid medium to a clean 250 mL conical flask, sterilized it at 115°C for 20 min, then inoculated 2 mL strain from the bacterial liquid mentioned above in the experimental group, set up 10 parallel trials, and subjected them to shaking-culturation at 30°C and 180 rpm for 7 days. Then, the culture solution was centrifuged at 8000 rpm for 15 min at 4°C, whereafter, the bacterial cells were collected and dried at 55°C, and then milled to 200 mesh or finer, by agate mortar in readiness for testing.
The biogenic CaCO3 including CaCO3 and bacterial cells was used to clarify the advantages of biogenic CaCO3 for heavy metals the adsorption. The adsorption and desorption experiments of Cd2+ (74 mg/L), and Pb2+ (94 mg/L) by biogenic CaCO3 and bacterial cells were carried out using the method described in section The Adsorption and Desorption of Cd2+ and Pb2+ by Micro and Nano-sized Biogenic CaCO3.
The Effect of pH on Adsorption of Biogenic CaCO3 for Heavy Metals
To study the influence of pH on the removal efficiency, 0.05 g biogenic CaCO3 was placed into a 50 mL polyethylene centrifuge tube containing 20 mL solution with different pH values (1, 2, 3, 4, 5, 6, 7, and 8) of 83.13 mg/g Cd2+ (CdCl2), or 99.30 mg/g Pb2+ (Pb(NO3)2), respectively. The mixture was shaken at 25°C and 100 rpm for 24 h, and each group was replicated three times. The supernatant was obtained by centrifuging at 8000 rpm for 15 min. The concentrations of Cd2+ or Pb2+ in the supernatant were determined by AAS, and the heavy metal adsorption rates were calculated by use of Equation (2).
Results and Discussion
The Morphological and Elemental Composition Analysis of Biogenic CaCO3 Sediments
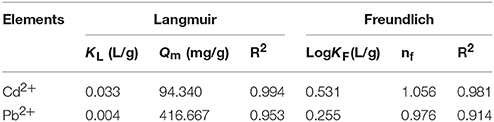
Different morphologies of crystals in the sediments were observed by using FESEM. These morphologies included cauliflower-like forms, scaly aggregates, and various irregular aggregates of sediment (part of them shown in Figures 1A,B). EDS was used to determine the main component as being CaCO3 (Figure 1C). The biogenic CaCO3 exhibited its porous surface, corner-incomplete form, and visible irregular fine lines on its surface, thus it had both a larger internal and external specific surface area and pore volume.
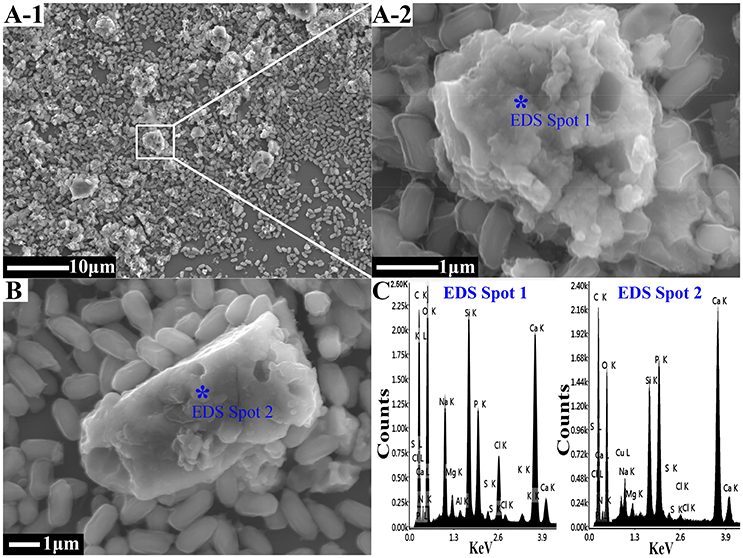
Figure 1. The FESEM-EDS analysis of biogenic CaCO3. (A,B) The FESEM images of the biogenic CaCO3; among them, A–2 is the magnification for the marked area in A–1. (C) The EDS results for the asterisk site. The “*” showed the site of the EDS analysis.
The Adsorption and Desorption of Biogenic CaCO3 for Cd2+ and Pb2+
Although both Freundlich and Langmuir equations could be used to fit the isothermal adsorption process of Cd2+ and Pb2+ by biogenic CaCO3, the fitting effect of Langmuir adsorption isotherm equation was more favorable, which suggested that the adsorption process was a single-molecule adsorption process (Mikutta et al., 2012; Wang et al., 2015). The maximum adsorption amounts of Cd2+ and Pb2+ by biogenic CaCO3 were 94.340 and 416.667 mg/g, respectively (Table 1). CaCO3 is an important mineral that is ubiquitous in soils, shallow grand water aquifers and marine sediments which has good adsorption properties for heavy metals (Davis et al., 1987; Garcia-Sánchez and Alvarez-Ayuso, 2002; Al-Degs et al., 2006; Lee et al., 2007; Yavuz et al., 2007). Yavuz et al. (2007) found that the maximum adsorption capacities of Cd2+ and Pb2+ by natural CaCO3 were determined as 18.52 and 19.92 mg/g, respectively. This research on the adsorption of heavy metals with biogenic CaCO3 induced by the strain (as a legal strain used in microbial fertilizer) is the first report, and the maximum adsorption capacities of biogenic CaCO3 for heavy metals are apparently higher than that of natural calcite (p < 0.01), which suggests a considerable potential to immobilize or passivate heavy metals in contaminated soil.
Figure 2A showed that the adsorption amount (Qe) of Cd2+ or Pb2+ on biogenic CaCO3 increased with increasing Cd2+ or Pb2+ concentration in the equilibrium solution (Ce). When the concentration of Cd2+ or Pb2+ was between 5 and 260 mg/L, the rate of adsorption of heavy metals on biogenic CaCO3 was as high as 87–100%, while the rate of desorption remained steady at 0.1–3% (Figure 2B), which suggest that biogenic CaCO3 has a high adsorption capacity for heavy metals and carries little environmental risk. The results provide evidence that bacterial fertilizer and biogenic CaCO3 may play important roles in various environments, and indeed more than previously acknowledged.
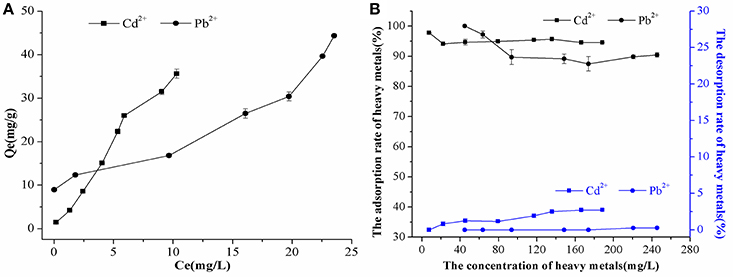
Figure 2. The adsorption and desorption characteristics of Cd2+ and Pb2+ by biogenic CaCO3. (A) The adsorption isotherm curves. (B) The adsorption and desorption rates. The black line represents the adsorption rate data, and the blue line represents the desorption rate data. Data represent the mean ± standard deviation (SD) of three independent experiments.
The Adsorption Mechanism
The FTIR results showed that it did not undergo a chemical precipitation reaction to produce new substances after the CaCO3 had adsorbed Cd2+ (74 mg/L) or Pb2+ (94 mg/L) (Figure 3A); which indicated that the reaction between the biogenic CaCO3 and Cd2+ or Pb2+ was mainly based on physical adsorption. The FESEM-EDS analysis showed that the adsorbed Cd2+ and Pb2+ were visible on the surface of biogenic CaCO3 (Figures 3C,E1,D,E2). Chemical CaCO3 morphology is essentially a diamond-shaped cubic structure with smooth surfaces (Lian et al., 2006; Xiao et al., 2015; Cao et al., 2016), but the biogenic CaCO3 surface is porous, micro and nano-sized, the edge is incomplete, and it can stack to form a fragmented structure or form a reticular aggregate with other different forms of CaCO3 according to FESEM and soft X-ray microscopy analysis (Figures 1A,B,3B, Supplementary Video 1); thus it has a larger internal and external specific surface area and pore volume, which can provide more adsorption sites and accommodation spaces for heavy metals. The XRD and TEM-SAED results indicated that the biogenic CaCO3 used in this test was mainly amorphous CaCO3 (Figure 4), and according to the reports that the amorphous CaCO3 surface area is 20 times that of other crystalline forms of CaCO3 (Yan and Lu, 2012), therefore, it exhibits strong adsorption properties for Cd2+ and Pb2+.
Figure 3. The adsorptive mechanism analysis of Cd2+ and Pb2+ by biogenic CaCO3. (A) The results of FTIR spectra of biogenic CaCO3 before, and after, adsorbing Cd2+ or Pb2+ (CK: before adsorbing Cd2+ or Pb2+ by biogenic CaCO3). (B) The reticular structure of biogenic CaCO3 by FESEM. (C,E1) The result of biogenic CaCO3 after adsorbing Cd2+ (74 mg/L) by FESEM-EDS: Cd2+ is visible on the surface of the biogenic CaCO3. (D,E2) The result of biogenic CaCO3 after adsorbing Pb2+ (94 mg/L) by FESEM-EDS: Pb2+ is visible on the surface of the biogenic CaCO3. (F) The XRD results of biogenic vaterite before, and after, adsorbing Cd2+ and Pb2+ (CK: before adsorbing Cd2+ and Pb2+ by biogenic CaCO3). The “*” inside the figure is the site of the EDS spot.
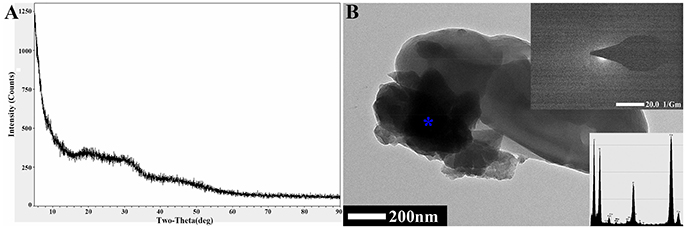
Figure 4. The detection and analysis of biogenic CaCO3 by XRD and TEM-SAED. (A) XRD detection. (B) TEM-SAED analysis. The “*” in the figure is the site of the EDS analysis.
Since there were no diffraction peaks observed from the amorphous biogenic CaCO3 in the XRD result (Figure 4A), to clarify the adsorptive mechanism of biogenic CaCO3 for heavy metals, vaterite-type biogenic CaCO3 was used to adsorb Cd2+ (74 mg/L) or Pb2+ (94 mg/L), and the adsorption rates were 98.42 and 100%, respectively, moreover, the desorption rates were all zero. The XRD results revealed that, there was no new phase diffraction peak after the biogenic vaterite had adsorbed the Cd2+ and Pb2+ (Figure 3F), which also showed that the reaction between the biogenic CaCO3 and Cd2+ or Pb2+ was mainly based on physical adsorption. It was also indicated that the Cd2+ or Pb2+ was stable in the mineral as a result of binding to the CaCO3 surface adsorption sites, or entry to the CaCO3 crystal pores. Consequently, biogenic CaCO3 offered better adsorption properties for heavy metals. Our findings suggested that the biogenic CaCO3 could be expected to be developed into a new type of heavy metals adsorbent, and might achieve the dual environmental benefits of carbon sequestration and heavy metal immobilization.
The Comparison of Adsorption and Desorption of Cd2+ and Pb2+ by Biogenic CaCO3 and Bacterial Cells
Figure 5 illustrates that the adsorption rate of Cd2+ (74 mg/L) and Pb2+ (94 mg/L) by biogenic CaCO3, was significantly higher than that of the bacterial cells, and the desorption rate was significantly smaller than that of the desorption rate of bacterial cells (p < 0.01), it suggested that the adsorption of CaCO3 crystal for heavy metals was dominant and its environmental risk was very low, but the adsorption rate of bacterial cells for heavy metals was not only low, but also the adsorbed heavy metals would be released to the environment easily, therefore, it posed a higher environmental risk. This also suggested that the biogenic CaCO3 had a larger specific surface area and rich reticular structures which contributed to its high adsorption and low desorption performance. This significant retaining ability of heavy metal ions indicates the remarkable efficiency of biogenic CaCO3 as an adsorbent.
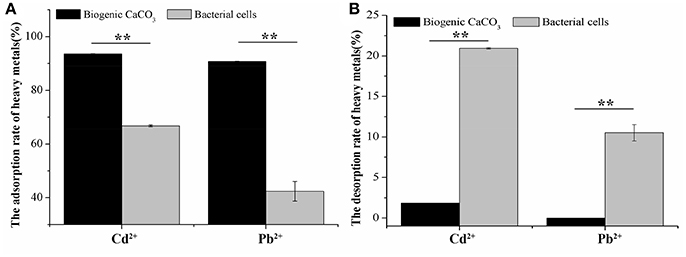
Figure 5. The adsorption and desorption of Cd2+ or Pb2+ by biogenic CaCO3 and bacterial cells. (A) The rate of adsorption (%). (B) The rate of desorption (%). The “*” indicates statistically significant differences between the two treatments (t-test, *p < 0.05, **p < 0.01). Data represent the mean ± SD of three independent experiments.
The Effect of pH on Adsorption of Biogenic CaCO3 for Heavy Metals
The results in Figure 6A demonstrate the effects of pH on Cd2+ and Pb2+ adsorption by biogenic CaCO3. The adsorption rate of these heavy metals was quite low at pH ≤ 1, as biogenic CaCO3 could not exist at such a low pH value. At pH values from 1.0 to 4.0, the adsorption percentage increased rapidly with increasing pH; thereafter (pH >4) it did not change to any significant extent with further increases in pH and the adsorption percentage was stable at around 95% (Figure 6A). Similar experimental results, such as those from Ma et al. (2012) who used use chemogenic CaCO3 for the adsorption of Cd2+ and Pb2+ and Merrikhpour and Jalali (2012) who used natural CaCO3 for Cd2+, Pb2+, Cu2+, Zn2+ adsorption, etc., can also obtain good adsorption effect when starting from an acidic pH value. Furthermore, we found that the pH value of the adsorption system was increased after adding biogenic CaCO3, and the final pH value after adsorption is around 8.61 (Figure 6B), which should be attributable by the biogenic CaCO3 and alkaline metabolites produced by B. subtilis. In addition, the adsorption percentages of Cd2+ and Pb2+ at pH 8 were 16.22 and 41.23% when we did not add biogenic CaCO3, which were significantly lower than biogenic CaCO3 adsorption percentages (p < 0.01). This indicated that the adsorption rate of heavy metals was mainly influenced by the biogenic CaCO3 rather than the formation of heavy metal hydroxides in alkaline conditions. In summary, the high adsorption capability of the biogenic CaCO3 within a wide pH range (3–8) indicated its potential application in the control of the fate of heavy metals in the natural environment.
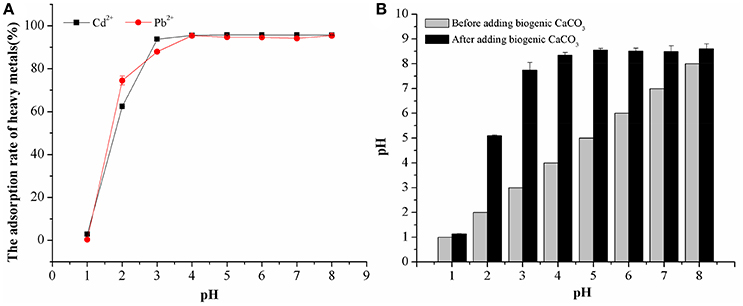
Figure 6. The effect of pH on the adsorption of heavy metals by biogenetic CaCO3. (A) The adsorption rate of biogenetic CaCO3 for heavy metals under different pH conditions (%). (B) The variation of pH before, and after, adding biogenetic CaCO3. Data represent the mean ±SD of three independent experiments.
Conclusions
The Langmuir isotherm was preferred to describe the adsorption characteristics of Cd2+ and Pb2+ by biogenic CaCO3 which suggested that the adsorption process was a single molecule layer adsorption process, and the maximum adsorption amounts (Qm) of Cd2+ and Pb2+ were 94.340 and 416.667 mg/g, respectively. Moreover, biogenic CaCO3 could maintain a high adsorption capability for heavy metals within a wide pH range. The biogenic CaCO3 and heavy metal ions formed a physical adsorption process, and the high efficiency and stability of the adsorption of biogenic CaCO3 for Cd2+ and Pb2+ were mainly attributed to its large total specific surface area and their porous structure. These findings revealed a new perspective on the remediation of heavy metal pollution by using biogenic CaCO3.
Author Contributions
BL: designed this study; RL, YG, and LC: performed the laboratory work; RL, YG, LC, and BL: analyzed the data; RL and BL: wrote this manuscript; All authors have read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China (Grant No. 41373078) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00041/full#supplementary-material
Supplementary Video 1. The video of biogenic CaCO3 by soft x-ray microscopy.
References
Abollino, O., Aceto, M., Malandrino, M., Sarzanini, C., and Mentasti, E. (2003). Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res. 37, 1619–1627. doi: 10.1016/S0043-1354(02)00524-9
Al-Degs, Y. S., El-Barghouthi, M. I., Issa, A. A., Khraisheh, M. A., and Walker, G. M. (2006). Sorption of Zn (II), Pb (II), and Co (II) using natural sorbents: equilibrium and kinetic studies. Water Res. 40, 2645–2658. doi: 10.1016/j.watres.2006.05.018
Apiratikul, R., and Pavasant, P. (2008). Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour. Technol. 99, 2766–2777. doi: 10.1016/j.biortech.2007.06.036
Argun, M. E., Dursun, S., Ozdemir, C., and Karatas, M. (2007). Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics. J. Hazard. Mater. 141, 77–85. doi: 10.1016/j.jhazmat.2006.06.095
Arias, M., Pérez-Novo, C., López, E., and Soto, B. (2006). Competitive adsorption and desorption of copper and zinc in acid soils. Geoderma 133, 151–159. doi: 10.1016/j.geoderma.2005.07.002
Aziz, H. A., Adlan, M. N., and Ariffin, K. S. (2008). Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour. Technol. 99, 1578–1583. doi: 10.1016/j.biortech.2007.04.007
Babel, S., and Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J. Hazard. Mater. 97, 219–243. doi: 10.1016/S0304-3894(02)00263-7
Cai, G. B., Zhao, G. X., Wang, X. K., and Yu, S. H. (2010). Synthesis of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water. J. Phys. Chem C 114, 12948–12954. doi: 10.1021/jp103464p
Cao, C., Jiang, J., Sun, H., Huang, Y., Tao, F., and Lian, B. (2016). Carbonate mineral formation under the influence of limestone-colonizing actinobacteria: morphology and polymorphism. Front. Microbiol. 7:366. doi: 10.3389/fmicb.2016.00366
Chen, X. B., Judith, V. W., Conca, J. L., and Peurrung, L. M. (1997). Effects of pH on heavy metal sorption on mineral apatite. Environ. Sci. Technol. 31, 624–631. doi: 10.1021/es950882f
Davis, J. A., Fuller, C. C., and Cook, A. D. (1987). A model for trace metal sorption processes at the calcite surface: adsorption of Cd2+ and subsequent solid solution formation. Geochim. Cosmochim. Acta 51, 1477–1490. doi: 10.1016/0016-7037(87)90330-9
Dong-Mei, Z., Huai-Man, C., Shen-Qiang, W., and Chun-Rong, Z. (2003). Effects of organic acids, o-phenylenediamine and pyrocatechol on cadmium adsorption and desorption in soil. Water Air Soil Pollut. 145, 109–121. doi: 10.1023/A:1023636330221
Dupraz, C., Reid, R. P., Braissant, O., Decho, A. W., Norman, R. S., and Visscher, P. T. (2009). Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 96, 141–162. doi: 10.1016/j.earscirev.2008.10.005
Farrah, H., and Pickering, W. F. (1979). pH effects in the adsorption of heavy metal ions by clays. Chem. Geol. 25, 317–326. doi: 10.1016/0009-2541(79)90063-9
Gao, Y., Kan, A. T., and Tomson, M. B. (2003). Critical evaluation of desorption phenomena of heavy metals from natural sediments. Environ. Sci. Technol. 37, 5566–5573. doi: 10.1021/es034392w
Garcia-Sánchez, A., and Alvarez-Ayuso, E. (2002). Sorption of Zn, Cd and Cr on calcite. Application to purification of industrial wastewaters. Miner. Eng. 15, 539–547. doi: 10.1016/S0892-6875(02)00072-9
Gherasim, C., and Bourceanu, G. (2013). Removal of chromium (VI) from aqueous solutions using a polyvinyl-chloride inclusion membrane: experimental study and modelling. Chem. Eng. J. 220, 24–34. doi: 10.1016/j.cej.2013.01.058
Grimm, A., Zanzi, R., Björnbom, E., and Cukierman, A. L. (2008). Comparison of different types of biomasses for copper biosorption. Bioresour. Technol. 99, 2559–2565. doi: 10.1016/j.biortech.2007.04.036
Han, J., Lian, B., and Ling, H. (2013). Induction of calcium carbonate by Bacillus cereus. Geomicrobiol. J. 30, 682–689. doi: 10.1080/01490451.2012.758194
Kavamura, V. N., and Esposito, E. (2010). Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol. Adv. 28, 61–69. doi: 10.1016/j.biotechadv.2009.09.002
Kobya, M., Demirbas, E., Senturk, E., and Ince, M. (2005). Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 96, 1518–1521. doi: 10.1016/j.biortech.2004.12.005
Lagadic, I. L., Mitchell, M. K., and Payne, B. D. (2001). Highly effective adsorption of heavy metal ions by a thiol-functionalized magnesium phyllosilicate clay. Environ. Sci. Technol. 35, 984–990. doi: 10.1021/es001526m
Lee, M., Paik, I. S., Kim, I., Kang, H., and Lee, S. (2007). Remediation of heavy metal contaminated groundwater originated from abandoned mine using lime and calcium carbonate. J. Hazard. Mater. 144, 208–214. doi: 10.1016/j.jhazmat.2006.10.007
Lian, B., Chen, Y., Zhao, J., Teng, H. H., Zhu, L., and Yuan, S. (2008). Microbial flocculation by Bacillus mucilaginosus: applications and mechanisms. Bioresour. Technol. 99, 4825–4831. doi: 10.1016/j.biortech.2007.09.045
Lian, B., Hu, Q., Chen, J., Ji, J., and Teng, H. H. (2006). Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim. Cosmochim. Acta 70, 5522–5535. doi: 10.1016/j.gca.2006.08.044
Liu, J., Ge, X., Ye, X., Wang, G., Zhang, H., Zhou, H., et al. (2016). 3D graphene/δ-MnO 2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 4, 1970–1979. doi: 10.1039/C5TA08106H
Ma, X., Li, L., Yang, L., Su, C., Wang, K., Yuan, S., et al. (2012). Adsorption of heavy metal ions using hierarchical CaCO3-maltose meso/macroporous hybrid materials: adsorption isotherms and kinetic studies. J. Hazard. Mater. 209–210, 467. doi: 10.1016/j.jhazmat.2012.01.054
Meng, Y. T., Zheng, Y. M., Zhang, L. M., and He, J. Z. (2009). Biogenic Mn oxides for effective adsorption of Cd from aquatic environment. Environ. Pollut. 157, 2577–2583. doi: 10.1016/j.envpol.2009.02.035
Merrikhpour, H., and Jalali, M. (2012). Waste calcite sludge as an adsorbent for the removal of cadmium, copper, lead, and zinc from aqueous solutions. Clean Technol. Environ. 14, 845–855. doi: 10.1007/s10098-012-0450-0
Mikutta, R., Baumgärtner, A., Schippers, A., Haumaier, L., and Guggenberger, G. (2012). Extracellular polymeric substances from Bacillus subtilis associated with minerals modify the extent and rate of heavy metal sorption. Environ. Sci. Technol. 46, 3866–3873. doi: 10.1021/es204471x
Mitchell, A. C., Dideriksen, K., Spangler, L. H., Cunningham, A. B., and Gerlach, R. (2010). Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci. Technol. 44, 5270–5276. doi: 10.1021/es903270w
Moreau, J. W., Fournelle, J. H., and Banfield, J. F. (2013). Quantifying heavy metals sequestration by sulfate-reducing bacteria in an Acid mine drainage-contaminated natural wetland. Front. Microbiol. 4:43. doi: 10.3389/fmicb.2013.00043
Musso, T. B., Parolo, M. E., Pettinari, G., and Francisca, F. M. (2014). Cu (II) and Zn (II) adsorption capacity of three different clay liner materials. J. Environ. Manage. 146, 50–58. doi: 10.1016/j.jenvman.2014.07.026
Pan, J., Liu, R., and Tang, H. (2007). Surface reaction of Bacillus cereus biomass and its biosorption for lead and copper ions. J. Environ. Sci. China 19, 403–408. doi: 10.1016/S1001-0742(07)60067-9
Pavasant, P., Apiratikul, R., Sungkhum, V., Suthiparinyanont, P., Wattanachira, S., and Marhaba, T. F. (2006). Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 97, 2321–2329. doi: 10.1016/j.biortech.2005.10.032
Phillips, A. J., Lauchnor, E., Eldring, J., Esposito, R., Mitchell, A. C., Gerlach, R., et al. (2013). Potential CO2 leakage reduction through biofilm-induced calcium carbonate precipitation. Environ. Sci. Technol. 47, 142–149. doi: 10.1021/es301294q
Ren, Y., Abbood, H. A., He, F., Peng, H., and Huang, K. (2013). Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 226, 300–311. doi: 10.1016/j.cej.2013.04.059
Santos, D. K., Resende, A. H., and de Almeida, D. G. (2017). Candida lipolytica UCP0988 biosurfactant: potential as a bioremediation agent and in formulating a commercial related product. Front. Microbiol. 8:767. doi: 10.3389/fmicb.2017.00767
The Ministry of Environmental Protection (2014). The Ministry of Land and Resources Report on the National Soil Contamination Survey. Available online at: http://www.mep.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm (Accessed April 17, 2014).
The Ministry of Land Resources (2015). Geological Survey Bureau of China, The Report on the Geochemical Survey of Cultivated Land in China. Available online at: http://www.cgs.gov.cn/xwl/ddyw/201603/t20160309_302254.html (Accessed June 25, 2015).
Tsekova, K., Todorova, D., Dencheva, V., and Ganeva, S. (2010). Biosorption of copper(II) and cadmium(II) from aqueous solutions by free and immobilized biomass of Aspergillus niger. Bioresour. Technol. 101, 1727–1731. doi: 10.1016/j.biortech.2009.10.012
Üçer, A., Uyanik, A., and Aygün, S. F. (2006). Adsorption of Cu (II), Cd (II), Zn (II), Mn (II) and Fe (III) ions by tannic acid immobilised activated carbon. Sep. Purif. Technol. 47, 113–118. doi: 10.1016/j.seppur.2005.06.012
Wang, B., Lehmann, J., Hanley, K., Hestrin, R., and Enders, A. (2015). Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 138, 120–126. doi: 10.1016/j.chemosphere.2015.05.062
Wang, L. K., Hung, Y.-T., and Shammas, N. K. (2007a). Advanced Physicochemical Treatment Technologies. Totowa, NJ: Humana Press.
Wang, S., Nan, Z., Zeng, J., and Hu, T. (2007b). Desorption of zinc by the kaolin from Suzhou, China. Appl. Clay Sci. 37, 221–225. doi: 10.1016/j.clay.2006.12.003
Wolthers, M., Charlet, L., and Van Cappellen, P. (2008). The surface chemistry of divalent metal carbonate minerals; a critical assessment of surface charge and potential data using the charge distribution multi-site ion complexation model. Am. J. Sci. 308, 905–941. doi: 10.2475/08.2008.02
Xiao, L., Lian, B., Hao, J., Liu, C., and Wang, S. (2015). Effect of carbonic anhydrase on silicate weathering and carbonate formation at present day CO2 concentrations compared to primordial values. Sci. Rep. 5:7733. doi: 10.1038/srep07733
Yan, X., and Lu, Y. (2012). The Key Technologies of Light and Nano Calcium Carbonate. Beijing: Chemical Industry Press.
Yao, M., Lian, B., Dong, H., Hao, J., and Liu, C. (2013). Iron and lead ion adsorption by microbial flocculants in synthetic wastewater and their related carbonate formation. J. Environ. Sci. China 25, 2422–2428. doi: 10.1016/S1001-0742(12)60151-X
Yavuz, Ö., Guzel, R., Aydin, F., Tegin, I., and Ziyadanogullari, R. (2007). Removal of cadmium and lead from aqueous solution by calcite. Pol. J. Environ. Stud. 16, 467–471. Available online at: https://www.researchgate.net/publication/279574155
Zhao, F., Ma, Y., Zhu, Y., Tang, Z., and McGrath, S. P. (2014a). Soil contamination in China: current status and mitigation strategies. Environ. Sci. Technol. 49, 750–759. doi: 10.1021/es5047099
Keywords: biogenic CaCO3, heavy metals, adsorption, desorption, mechanism
Citation: Liu R, Guan Y, Chen L and Lian B (2018) Adsorption and Desorption Characteristics of Cd2+ and Pb2+ by Micro and Nano-sized Biogenic CaCO3. Front. Microbiol. 9:41. doi: 10.3389/fmicb.2018.00041
Received: 10 July 2017; Accepted: 09 January 2018;
Published: 26 January 2018.
Edited by:
Alain F. Plante, University of Pennsylvania, United StatesReviewed by:
Mustafa Yucel, Middle East Technical University, TurkeyRachel Narehood Austin, Columbia University, United States
Copyright © 2018 Liu, Guan, Chen and Lian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Lian, YmluMjM2OEB2aXAuMTYzLmNvbQ==
 Renlu Liu
Renlu Liu Yong Guan
Yong Guan Liang Chen2
Liang Chen2 Bin Lian
Bin Lian