- 1Univ. Manouba, ISBST, BVBGR-LR11ES31, Biotechpole of Sidi Thabet, Ariana, Tunisia
- 2Laboratory of Microorganisms and Active Biomolecules, MBA-LR03ES03, Faculty of Sciences of Tunis, University of Tunis El Manar, Tunis, Tunisia
- 3Department of Civil, Chemical, Environmental and Materials Engineering (DICAM), University of Bologna, Bologna, Italy
A number of Pseudomonas strains function as inoculants for biocontrol, biofertilization, and phytostimulation, avoiding the use of pesticides and chemical fertilizers. Here, we present a new metabolically versatile plant growth-promoting rhizobacterium, Pseudomonas rhizophila S211, isolated from a pesticide contaminated artichoke field that shows biofertilization, biocontrol and bioremediation potentialities. The S211 genome was sequenced, annotated and key genomic elements related to plant growth promotion and biosurfactant (BS) synthesis were elucidated. S211 genome comprises 5,948,515 bp with 60.4% G+C content, 5306 coding genes and 215 RNA genes. The genome sequence analysis confirmed the presence of genes involved in plant-growth promoting and remediation activities such as the synthesis of ACC deaminase, putative dioxygenases, auxin, pyroverdin, exopolysaccharide levan and rhamnolipid BS. BS production by P. rhizophila S211 grown on olive mill wastewater based media was effectively optimized using a central-composite experimental design and response surface methodology (RSM). The optimum conditions for maximum BS production yield (720.80 ± 55.90 mg/L) were: 0.5% (v/v) inoculum size, 15% (v/v) olive oil mill wastewater (OMWW) and 40°C incubation temperature at pH 6.0 for 8 days incubation period. Biochemical and structural characterization of S211 BS by chromatography and spectroscopy studies suggested the glycolipid nature of the BS. P. rhizophila rhamnolipid was stable over a wide range of temperature (40–90°C), pH (6–10), and salt concentration (up to 300 mM NaCl). Due to its low-cost production, emulsification activities and high performance in solubilization enhancement of chemical pesticides, the indigenous BS-producing PGPR S211 could be used as a promising agent for environmental bioremediation of pesticide-contaminated agricultural soils.
Introduction
Overuse of chemical fertilizers and pesticides to meet the growing demand of food supply has undoubtedly cause pollution and severe damage to soil organisms and insect pollinators (Savci, 2012; Mahanty et al., 2017). Nonetheless, the outcome of using excess chemical inputs has made the crops more susceptible to diseases and decreased soil fertility (Tilman et al., 2002; Aktar et al., 2009). Considering the hazardous effects of chemical fertilizers, biofertilizers are supposed to be a safe alternative to chemical inputs and minimizes ecological disturbance to a great extent. Biofertilizers are ecofriendly agro-input, more cost-effective than chemical fertilizers, and their prolonged use enhances soil fertility substantially (Mahdi et al., 2010; Singh et al., 2011). It was mentioned that the use of biofertilizers enhances crop yield by 10–40% (Bhardwaj et al., 2014). The use of biofertilizers has many benefits, including cheap source of nutrients, excellent suppliers of micronutrients and organic matter, secretion of growth hormones, no adverse effects to ecosystem and longer shelf life (Gaur, 2010; Mahanty et al., 2017).
Plant growth promoting rhizobacteria (PGPR) are the soil bacteria that colonize the root surface and promote plant growth via secretion of regulatory chemicals in the vicinity of the rhizosphere (Cherif et al., 2017). PGPR enhance the plant growth directly by facilitating water and nutrient uptake and modulating phytohormone levels, or indirectly by inhibiting pathogens in the forms of biocontrol agents (Ahemad and Kibret, 2014). Besides offering economically and ecologically attractive means for increasing the nutrient supply and protecting against soil borne pathogens, PGPR are the key players in efforts to alleviate plan stress and to enhance bioremediation of polluted soils (Zhuang et al., 2007; El-Daim and Moustafa, 2015). In fact, BS-producing PGPR capable of solubilizing and degrading certain kind of pollutants (i.e., explosives, herbicides or hydrocarbons) have been isolated from different contaminated sites and the potential degradation pathways, and enzyme-encoding genes have been studied (Burd et al., 2000; Germaine et al., 2006; Sheng et al., 2008; Kruijt et al., 2009; Singh and Cameotra, 2013).
Microbial BS include a large number of chemical structures, such as lipopeptides, phospholipids, glycolipids, and polymeric macromolecules like exopolysaccharides (Mukherjee et al., 2006; Jadhav et al., 2013). Recently, there has been considerable interest in the properties of microbial BS such as biodegradability, low toxicity, biocompatibility, digestibility, diversity for chemical structure and activities, and effectiveness even at extreme conditions including heat, alkalinity and salinity (Fakruddin, 2012; Sarubbo et al., 2015a,b). BS are expected to reach more than 2 billion USD by 2020, with different bioremediation applications such as enhanced oil recovery, heavy metals removal, and pesticides detoxification in contaminated sites (Maier, 2003; Ying, 2006; Franzetti et al., 2009; Pacwa-Płociniczak et al., 2011; Bustamante et al., 2012; Sarubbo et al., 2015b).
Currently, one of the major problems in the production of microbial BS at a large-scale is the high production costs (Geys et al., 2014). In order to make it economically competitive, it is necessary to reduce substrate cost, optimize culture conditions using experimental designs, improve recovery process, and use overproducing strains for high yields. As fermentation medium can represent about 30% of the production cost, BS can be produced from inexpensive waste substrates, thereby dramatically reducing the microbial culture cost (Sarubbo et al., 2015a). Olive oil mill wastewater (OMWW) is a phenolic-rich industrial effluent that can be considered as a serious environmental problem, particularly in olive oil producing Mediterranean countries. The high content of polyphenols in OMWW is problematic for biological processing. However, the presence of polysaccharides, free sugars and residual oil, suggest that OMWW could be advantageously valorized and used as a carbon source for microbial biosurfactant (BS) production (Colak and Kahraman, 2013; Ramírez et al., 2015; Neifar et al., 2017).
The present study investigates the ability of a novel species of Pseudomonas, isolated from pesticide-contaminated artichoke farm soil, to promote plant growth and to produce BS. The in vitro tests and the genome sequencing, assembly and annotation revealed that the S211 strain has a wide spectrum of PGP traits including biocontrol, biofertilization and rhizoremediation activities. This study also describes the statistical optimization of BS production, the biochemical characterization and structural identification of BS from S211, as well as the evaluation of its ability to enhance pesticide solubilization. Further characterization of multi-trait PGPR such as P. rhizophila S211 will be promising to design an effective strategy for a sustainable agriculture development.
Materials and Methods
Reagents and Chemicals
All chemicals and reagents used in this study were of pure analytical-grade and available commercially.
Bacterial Isolation and PGP Activities
Rhizospheric soil sample was collected from pesticides-contaminated field in Sidi Thabet, an agricultural region of northern Tunisia “36°54′31.1″N; 10°2′32.89″E.” Bacterium isolation was carried out by an enrichment culture technique in mineral salt medium (MSM) supplemented with 100 mg/L of dimethoate as a sole carbon source. MSM medium contained the following components at the specified concentrations (in g/L): (NH4)2SO4, 2; MgSO4 7H2O, 0.2; CaCl2 2H2O, 0.01; FeSO4 7H2O, 0.001; Na2HPO4 12H2O, 1.5; KH2PO4, 1.5; pH8 (Cycon et al., 2009). Growth of S211 strain was performed in Nutrient Agar (NA) at different pH values of 7, 9, and 11. The same culture medium supplemented with 0, 5, 10, and 20% of NaCl (w/v) was used to test the growth behavior of the strain after 3 days of incubation at 30°C. S211 isolate was screened in vitro for various plant growth promoting properties. Screening for N2-fixing activity of the pure bacterial culture was determined on Jensen's N-free medium as reported by Jensen (1942). The ability of the isolate to solubilize the inorganic tricalcium phosphate [Ca3 (PO4)2] was checked on to the National Botanical Research Institute's medium Phosphate (NBRIP) according to the method of Nautiyal (1999). Siderophore production ability of S211 was determined in CAS agar medium (Chrome Azurol S medium) as described by Alexander and Zuberer (1991). Indole Acetic Acid (IAA) production was carried out using Salkowaski's method (Gordon and Weber, 1951). Production of ammonia (NH3) by S211 was qualitatively tested as reported by Cappuccino and Sherman (1992). The method described by Alström and Burns (1989) was adopted to evaluate the ability of S211 strain to produce hydrogen cyanide (HCN). Siderophores-pyoverdine was detected in King's B (KB), succinate medium (SM), and Casamino Acid media (CAA) according to the method of Page and Tingerstrom (1988). The inhibitory power between the bacterial strain (P. aeruginosa ATCC27853) and the pyoverdine extract was checked in TSA. S211 strain was also screened for their EPS-producing potential as described by Vijayabaskar et al. (2011). The assay plate used for the detection of anionic-BS was performed in solid MSM medium supplemented with OMWW as a sole carbon source, cetyltrimethyl ammonium bromide (CTAB) and methylene blue (MB) (Satpute et al., 2008). The oil displacement activity was determined according to Techaoei et al. (2011).
S211 Genome Sequencing, Assembly, and Annotation
Genomic DNA extraction was performed using the MagNA Pure LC DNA isolation KitIII (Roche) and was sent to Inqaba Biotechnical Industries, a commercial NGS service provider, for whole genome sequencing. Briefly, genomic DNA sample were fragmented using an ultrasonication approach (Covaris), size-selected and end repaired. Each generated fragment was ligated to illumina specific adapter sequence, quantified, indexed, size selected (AMPure XP Bead-based) and then sequenced on illumina's MiSeq platform, using a MiSeq v3 (600 cycle) kit. 250 Mb of data (2 × 300 bp long paired end reads) were produced for each sample. Quality of sequence reads was first analyzed using the FastQC tool (Andrews, 2011). Then, adaptor sequence removal, trimming, error correction, and assembly were performed using the A5-miseq pipeline, an integrated pipeline for de novo assembly of microbial genomes (Tritt et al., 2012), and finally analyzed with QUAST (Gurevich et al., 2013). These contigs were finally ordered using CONTIGuator v2.3 (Galardini et al., 2011) with its closely related genomes, P. lini (NZ_LT629746.1), P. frederiksbergensis (NZ_CP017886.1) P. mandelii JR-1 (NZ_CP005960.1), and P. brassicacearum (CP012680.1) using CONTIGuator (Galardini et al., 2011). The assembled genome of P. rhizophila S211 consists of 5.98 Mbp distributed over 26 contigs and organized in one scaffold with fold coverage of 80X. Gene predictions and annotations were performed with Rapid Annotations using Subsystems Technology (RAST) database (Aziz et al., 2008), Integrated Microbial Genomes/Expert Review (IMG/ER) (Markowitz et al., 2009) and Prokka version 1.1 (Seemann, 2014), which predicts coding DNA sequence (CDS) using Prodigal (Hyatt et al., 2010). tRNA genes and rRNA genes were predicted by tRNAScan-SE software (Lowe and Eddy, 1997) and RNAmmer, respectively (Lagesen et al., 2007). The scaffolds were searched against the KEGG database to analyze metabolic pathways and gene functions (Kanehisa and Goto, 2000). Glimmer3 was used for the prediction of structural genes (Delcher et al., 2007). The predicted ORFs were annotated by searching against the COG and SEED databases. Functional annotation was performed by searching the National Center for Biotechnology Information (NCBI) protein database and the Kyoto Encyclopedia of Genes and Genomes (KEGG) protein database, the cluster of orthologous groups (COG) (Tatusov et al., 2003) database, and the TIGRfam database (Haft et al., 2003).
Bacterial Identification and Phylogenetic Analysis
Assessment of phylogenetic affiliation of bacterial isolate was first based on 16S rRNA gene sequence analysis according to the procedure described previously (Guesmi et al., 2013) and then confirmed by whole genome sequence analyses. Identification of closely related strains to S211 was performed based on Basic Local Alignment Search Tool (BLAST) searches. 16S rRNA gene sequences were aligned with T-Coffee (v11.00.8cbe486) using Lalign_pair and slow_pair alignments (Weng et al., 2013). The phylogenetic tree was reconstructed using the bayesian inference method implemented in the MrBayes program (v3.2.3). The number of substitution types was fixed to 6. The standard (4 by 4) model of nucleotide substitution was used, while rates variation across sites was fixed to “invgamma.” Four Markov Chain Monte Carlo (MCMC) chains were run for 10,000 generations, sampling every 10 generations, with the first 250 sampled trees discarded as “burn-in.” Finally, a 50% majority rule consensus tree was constructed. Graphical representation and edition of the phylogenetic tree were performed using iTOL v3 (Letunic and Bork, 2016).
In Silico DNA-DNA Hybridization and Average Nucleotide Identity
The draft genome of S211 strain was used to evaluate its similarity to closely related species based on (i) in silico DNA-DNA hybridization (isDDH) determination, using the Genome-to-Genome Distance Calculator (GGDC) version 2.0 (http://ggdc.dsmz.de/distcalc2.php; Auch et al., 2010; Meier-Kolthoff et al., 2013b). isDDH values were calculated using the recommended formula 2 for draft genome assemblies. The isDDH values between genomes of the same species are above 70% (Auch et al., 2010; Meier-Kolthoff et al., 2013b), and (ii) the estimation of the Average Nucleotide Identity (ANI) using best hits and reciprocal best hits between two genomic datasets as described by Goris et al. (2007). Bacterial strains that exhibited more than 95% ANI should belong to the same species (Rodriguez and Konstantinidis, 2014).
Statistical Optimization of BS Production by S211 Using OMWW as Low-Cost Substrate
Response surface methodology (RSM) and central composite design (CCD) were applied to study the BS production variables by P. rhizophila S211 (Myers et al., 2009). This methodology is suitable for fitting a quadratic surface and optimizing the effective variables with a minimum number of experiments, as well as to analyse the interaction effects between factors (Goupy, 1999; Myers et al., 2009). A CCD with 31 experiments was applied for the optimization of BS production. S211 inoculum was prepared in 50 ml tryptic soy broth (TSB) with overnight incubation at 30°C at 150 rpm. BS production was conducted in 250 ml conical flasks containing 100 ml MSM supplemented with OMWW. The effect of five independent variables; pH (X1), temperature (X2), OMWW (X3), inoculum size (X4), and incubation time (X5) on the rhamnolipid production yield (Response Y), were evaluated at three levels (Table 1). After each incubation time, an aliquot of 10 ml was taken from each flask; 2 ml was used to estimate the bacterial growth by measuring the OD600. Eight milliliter was centrifuged at 10,000 rpm for 15 min to remove bacterial cells and the supernatant was used for BS production yield determination according to the Orcinol assay method (Tuleva et al., 2002). This last was used for the direct assessment of the amount of rhamnolipids in the sample as rhamnose (mg/L). To 100 μl of each sample, 900 μl of a solution containing 0.2% orcinol in concentrated sulfuric acid was added. Samples were heated for 30 min at 80°C, cooled at room temperature then OD421 was measured. Control was prepared with distilled water. The BS yields were calculated from a standard curve prepared with L-rhamnose.
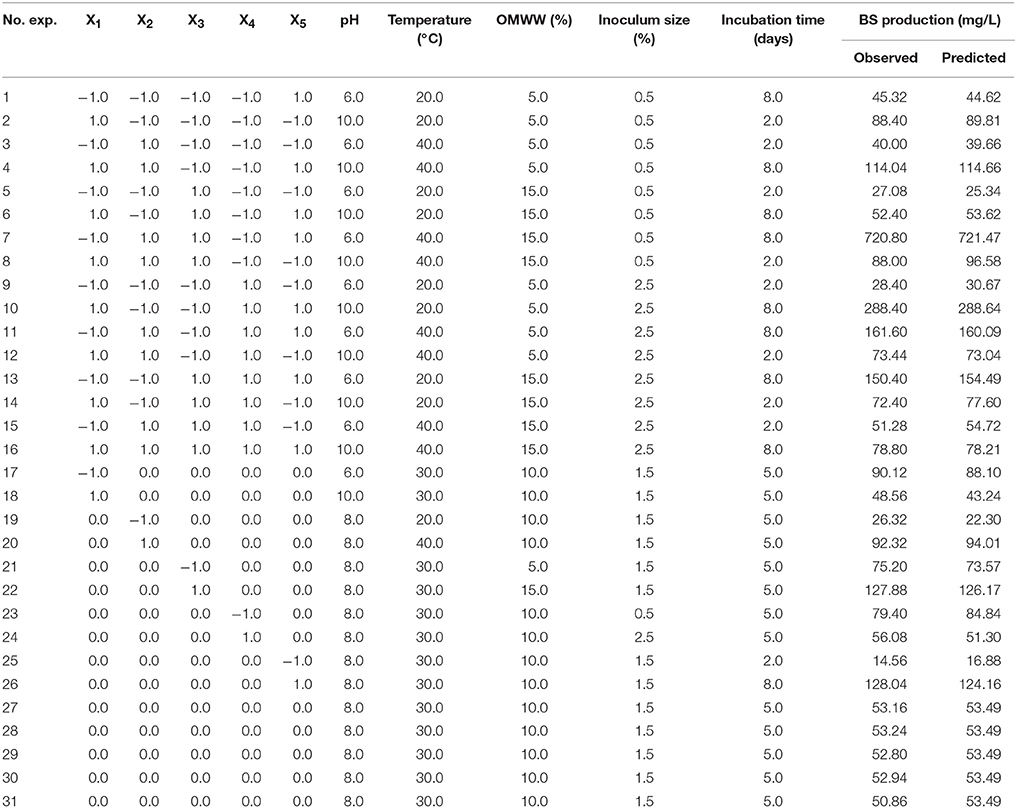
Table 1. Experimental conditions of the CCD design in coded and natural variables and the corresponding observed and predicted responses.
The five-significant variables can be approximated by the quadratic model equation as follows:
Where Y are the response (BS production and oil displacement activity (ODA), respectively); Xj: system variables (correspond to the different factors influencing the production of BS) and b0, bj, bjk, and bjj: model coefficients.
Validation of the optimum BS production predicted by the CCD model was conducted in triplicate. The generation and the data treatment of the five factors CCD were performed using NemrodW software (Mathieu et al., 2000).
BS Purification, Properties, and Structural Characterization
The supernatant of optimized fermentation culture was collected after centrifugation (12,000 rpm/20 min at 4°C), and was adjusted to pH 2.0 with 6N HCl. Then, the acidified supernatant was left overnight at 4°C in order to precipitate the BS. The precipitate was collected by centrifugation at 12,000 rpm for 30 min at 4°C to obtain the crude BS. For additional purification, the crude BS was extracted at three successive washes with a mixture of the chloroform-methanol solvent (2:1, v/v). Finally, the combined extracts were then dried with anhydrous sodium sulfate and were concentrated using a rotary evaporation at 40°C. The resulting product was obtained as a viscous brown matter. A portion of the viscous brown extract was then purified by silica gel column chromatography using sequential washes of chloroform and methanol (Smyth et al., 2010). The active fraction (5 ml each) was confirmed through two different tests [emulsification activity (E24) and ODA].
For emulsifying activity essays, a volume of 2 ml of vegetable oil was vigorously votexed with 2 ml of BS fraction in a screw-top glass tube for 2 min and allowed to stand for 24 h, and then the emulsification index (E24%) was calculated (Cooper and Goldenberg, 1987). A BS-producing strain having high emulsion stability (E24 ≥ 50%) was considered as an efficient emulsifying strain. The oil displacement activity was determined according to Techaoei et al. (2011), a volume of 20 μl of crude oil was placed on the surface of 20 ml of distilled water into the culture dish of diameter (9 cm) and a 10 μl of each fraction was gently placed on the surface of the oil film. Diameter of the clear halo viewed on oil surface under visible light was measured.
Rhamnolipid BS detection was performed by thin-layer chromatography TLC on pre-coated silica gel of standard 20 × 20 Kiesel-gel 60 F254 Merck plates using chloroform: methanol: acetic acid (65:15:2, v/v/v) as a solvent system and antrone as a visualization agent (Antoniou et al., 2015).
To confirm the glycolipid nature of the BS produced, Fourier Transform Infra-Red spectroscopy FTIR (Perkin Elmer FTIR model 2000) was applied as described by Rahman et al. (2010). This technique makes it possible to explore the functional groups and the chemical bonds present in the purified extract of BS. Infrared absorption spectra were obtained over the range of 400–4,000 cm−1 with a resolution of 4 cm1.
The stability studies of BS produced by the selected strain were carried out with respect to temperature, pH and salinity (Sharma et al., 2015). To determine the thermal stability of BS, the produced glycolipid BS was maintained at different temperature range of 20–90°C at neutral pH for 24 h; then the activity was measured. To evaluate the effect of pH on BS activity, BS solutions with different pH values (ranging from 2 to 10) were prepared using 100 mM buffers (tris-HCl pH 2–3, sodium acetate pH 4–5, phosphate pH 6–8 and tris-NaOH pH 9–10). Subsequently, the mixtures were vortexed and the ODA was measured after 24 h at room temperature. The effect of addition of different concentrations of salt (ranging from 0 to 600 mM NaCl) on the activity of produced BS was investigated at pH 7 and at room temperature.
Enhancement of Pesticide Solubilization Using S211 BS
The enhanced solubilization of Pentachlorophenol (PCP) into aqueous phase was evaluated using different BS concentrations (between 5 and 20 g/L) in comparison with three control preparations: a sample without BS and a sample with various concentrations of the synthetic surfactants, sodium dodecyl sulfate (SDS) and Tween 80 (between 5 and 20 g/L). For each experiment, in a 20 ml screw-capped glass vial, 0.5 g/L of PCP was suspended in deionized water (to a final volume of 10 ml). The vials were covered with aluminum foil to protect the surfactant samples from photolysis and were vigorously vortexed at room temperature (30°C). After 24 h, the residual pesticide remaining in the bottom of the tube was separated by centrifugation (15 min/12,000 rpm) and the aqueous phase was then transferred to clean vials until analysis. The PCP concentration was carried out using a reverse phase Ultra-High Performance Liquid Chromatography (UHPLC) equipped with a UV detector (at 265 nm). The separation was performed at 27°C on C18 column (250 × 4.6 mm; Inertsil ODS-4, GL Sciences, Japan) using an isocratic method with acetonitrile-water mixture of 50: 50 (v/v) as the mobile phase at a flow rate of 1 ml/min. The volume of the sample injected was 10 μl. The retention time was about 3.1 min. The enhanced apparent solubility of PCP by surfactant addition is expressed by an enhanced solubility ratio (ESR) (Wattanaphon et al., 2008), calculated according to the following formula:
Where C: the pesticide concentration in surfactant based solution and C0: the pesticide concentration in surfactant free solution.
Statistical Analysis
The statistical software package, NemrodW was performed to conduct a regression analysis of the obtained experimental data and to plot the response surface graphs (Mathieu et al., 2000). Student's t-test permitted to evaluate the statistical significance of the CCD model coefficients. Analysis of variance (ANOVA) using Fisher's F-test was performed on experimental data to check the statistical significance of the model.
Nucleotide Sequence Accession Number
This Whole Genome Shotgun project of P. rhizophila S211 has been deposited at DDBJ/ENA/GenBank under the accession CP024081.
Results
Isolation, Phylogenetic Assignment, General Feature, and Genomic Insights Inferred from S211 Genome Sequence
S211 is a free-living rhizobacterium isolated from a pesticide-contaminated soil through consecutive exposure to elevated concentrations of dimethoate and PCP in minimal salt medium. In vitro PGP experiments showed that S211 strain was able to grow under high salinity (10% NaCl) and alkalinity (pH 9), fix nitrogen, solubilize inorganic phosphate, synthetize auxine, HCN, ammonia, pyoverdine-siderophores and produce exopolysaccharides and anionic BS (Supplementary Figure 1).
The phylogenetic relatedness to the closest relative species, based on almost-complete 16S rDNA gene sequences, showed that S211 is most closely to P. frederiksbergensis (NR_117177.1) and P. brassicacearum (NR_024950.1) with 99% sequence similarities for both (Figure 1). This value is higher than the 98.7% identity threshold to propose a new species. However, such comparison has the disadvantage that 16S rRNA sequence similarities do not always accurately reflect similarities at the whole-genome level and cannot distinguish between recently diverged species (Richter and Rosselló-Móra, 2009; Zhang and Qiu, 2016). Two methods, isDDH and ANI values, known for species delineation were used to confirm the phylogentic assignement. S211 strain genome is close to those of P. brassicacearum (CP012680.1) and P. frederiksbergensis (NZ_CP017886.1), with isDDH values of 35.50 and 26.20, and ANI values of 87.96 and 81.14%, respectively. Digital DDH values, lower than the threshold of 70% for species delineation, together with the ANI values, lower than the cut-off value of 95% distinguishing different species (Meier-Kolthoff et al., 2013a,b), indicate that P. rhizophila S211 forms a separate novel species within the genus Pseudomonas.
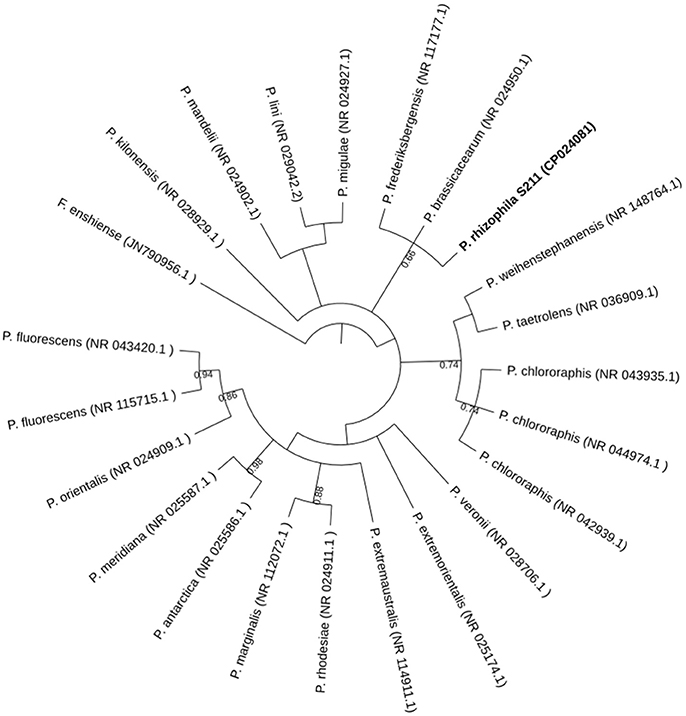
Figure 1. Phylogenetic tree generated with Mr. Bayes based on 16S rRNA gene sequences of P. rhizophila S211 isolate and related species. The tree was rooted with Flavobacterium enshiense (N790956.1) as an out-group. Bootstrap values are expressed as randomization of 1,000. GenBank accession numbers of the reference strains are indicated.
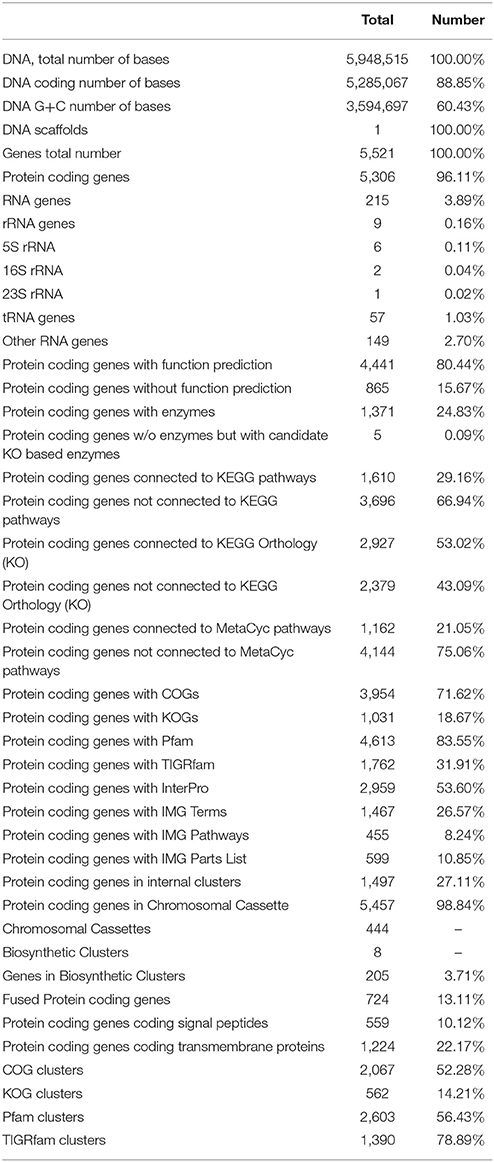
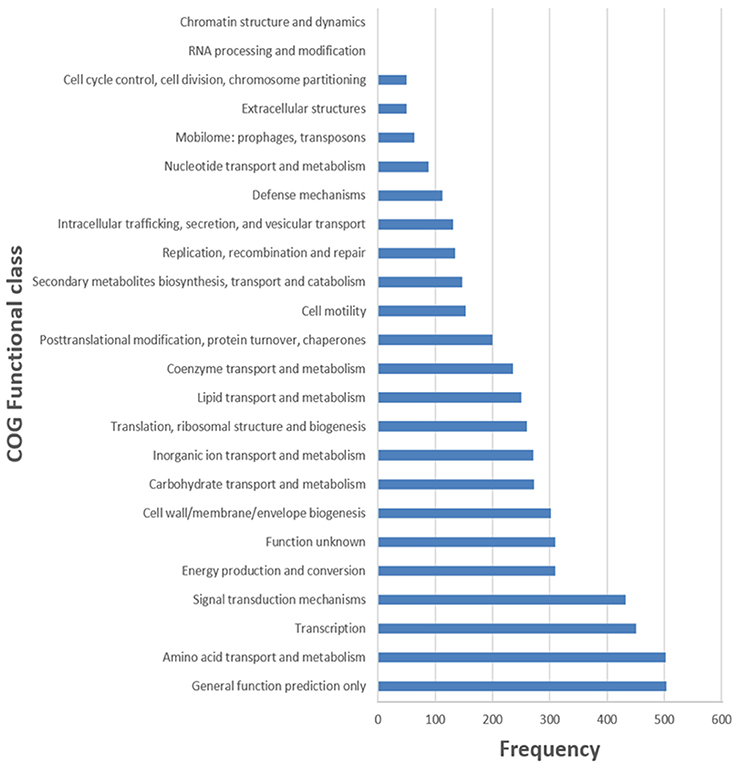
The general features of S211 genome sequence are shown in Table 2 and Supplementary Table 1. The genome consists of one scaffold with 5,948,515 bp (Figure 2) with an average GC content of 60.4%. Among the 5306 predicted genes, 4441 were identified as protein coding genes where 865 (15.67%) without function prediction. The CDSs were classified into 24 functional categories according to the COG database (Figure 3). Beside the predicted genes, a total of 57 tRNA, 9 rRNA loci (5S, 16S, 23S), and 547 SEED subsystem features were predicted in S211 genome sequence.
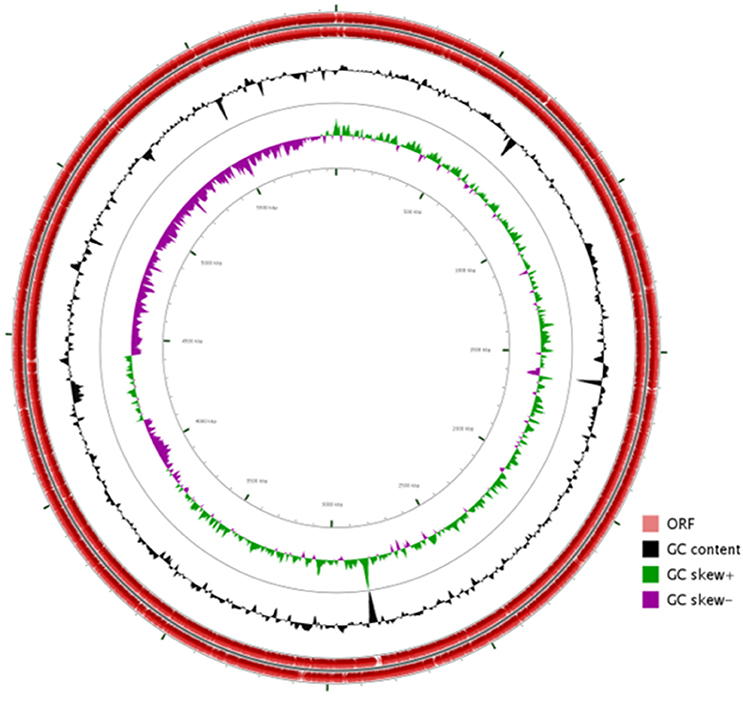
Figure 2. Circular representation of P. rhizophila S211 genome generated by CG viewer. Circles from outside to inside: first, scale bar in kilobases; second and third, predicted coding sequences of chromosome on leading and lagging strands; fourth, GC content; fifth, GC skew.
Annotation of the draft genome sequence confirmed the presence of genes involved in plant-growth promoting, biological control and bioremediation activities (Supplementary Table 2). S211 genome contained several genes specific to nitrogen fixation and others encoding alkaline phosphatases, which convert insoluble phosphorus into a bioavailable form for plant growth. The genome of S211 also contained putative 1-aminocyclopropane-1-carboxylate (ACC) deaminase. Different kinds of defenses are revealed in the genome of the PGPR strain S211, including genes involved in resistance/tolerance to antibiotics, heavy metals and toxic compounds (90), oxidative stress (99), osmotic stress (34), temperature stress (23), and multiple drugs (28). P. rhizophila S211 can produce bacteriocins, ribosomally synthesized antibacterial peptides as well as several antagonistic molecules such as phenazines. S211 can also synthesize pyroverdine, a fluorescent siderophore, and chelate the ferric iron Fe(III) under low-iron conditions and thereby make it available for microbial and plant cells. The genome of S211 harbors 18 genes encoding dioxygenases (DOs) related to degradation of aromatic compounds. P. rhizophila is able also to produce and excrete the exopolysaccharide levan mediated by extracellular levan sucrase. The rhamnolipid synthesis proceeds by sequential glycosyl transfer reactions, catalyzed by glycosyl transferases with TDP-rhamnose acting as a rhamnosyl donor and 3-hydroxydecanoyl-3-hydroxydecanoate acting as the acceptor. All enzymatic steps required for the synthesis of rhamnolipids by P. rhizophila S211 are available in Figure 4.
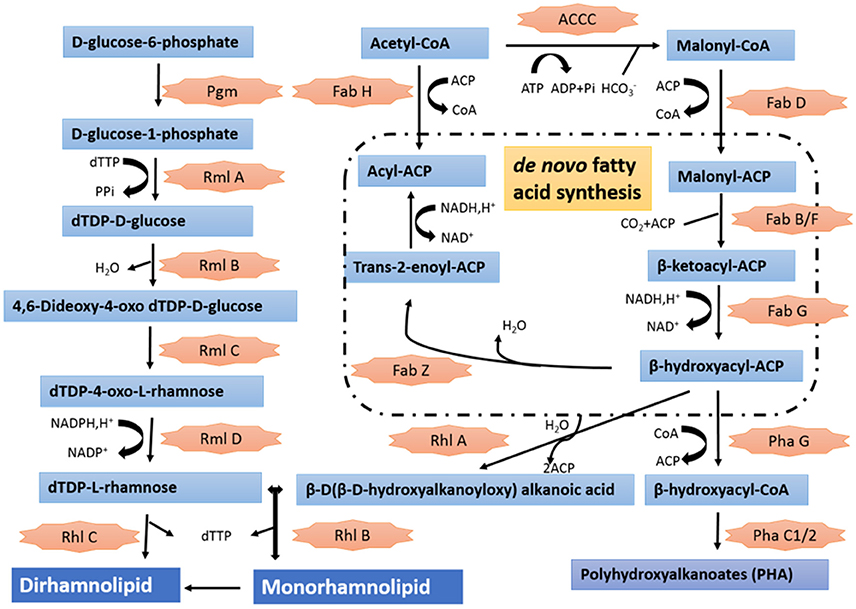
Figure 4. Putative metabolic pathway involved in the synthesis of rhamnolipid in genome of P. rhisophila S211. Pgm, Phosphoglucomutase, EC 5.4.2.2; RmlA, Glucose-1-phosphate thymidylyltransferase, EC 2.7.7.24; RmlB, dTDP-glucose 4,6-dehydratase, EC 4.2.1.46; RmlC, dTDP-4-dehydrorhamnose 3,5-epimerase, EC 5.1.3.13; RmlD, dTDP-4-dehydrorhamnose reductase, EC 1.1.1.133; RhlB and RhlC, Glycosyltransferase, EC 2.4.1.; PhaG, (R)-3-hydroxydecanoyl-ACP:CoA transacylase PhaG (3-hydroxyacyl-CoA-acyl carrier protein transferase), EC 2.4.1.-; PhaC1/2: Polyhydroxyalkanoic acid synthases, EC 2.3.1-; ACCC, Acetyl-coenzyme A carboxyl transferase alpha chain, EC 6.4.1.2; FabD, Malonyl CoA-acyl carrier protein transacylase, EC 2.3.1.39; Fab H, 3-oxoacyl-[acyl-carrier-protein] synthase, KASIII, EC 2.3.1.180; FabB/F, 3-oxoacyl-[ACP] synthase, EC 2.3.1.41; FabG, 3-oxoacyl-[acyl-carrier protein] reductase, EC 1.1.1.100.
Optimization of BS Production by P. rhizophila S211 on OMWW Based Medium using Response Surface Methodology
In this investigation, the power of response surface method to optimize BS production by P. rhizophila S211 was explored using a five variables CCD (Table 1). The experimental results were modeled with a second-order polynomial equation to predict the response for given experimental conditions within the design space:
where Y was the estimated BS production and X1, X2, X3, X4, and X5 were the coded values for pH, temperature, OMWW concentration, inoculum size, and incubation time, respectively.
Statistical analysis of experimental results was performed with NomrodW statistical software to determine the significant differences between the independent variables. The significance of CCD model coefficients was determined by t-values and p-values which indicates the pattern of interactions between the five variables. The Student t distribution and the corresponding p-values, along with the variable estimate, were illustrated in Table 3. In this case, the linear effect of the parameters X2 and X5 and the interactions X1X2, X1X3, X1X4, X1X5, X2X3, X2X4, and X3X4 were statistically significant. By considering only these significant factors, BS production by P. rhizophila S211 can be predicted by the following equation:
The significance of the CCD model was checked by F-test and the corresponding statistical results were presented in Supplementary Table 3. ANOVA analysis for BS production showed that the regression model was significant and the lack of fit was insignificant (Supplementary Table 3). The fit of the models was evaluated by the determination of coefficient R2. The regression equations obtained indicated the R2 values of 0.938 suggesting an adequate adjustment of the quadratic model to the experimental data and indicating that the model could explain 93.80% of the variability in the response.
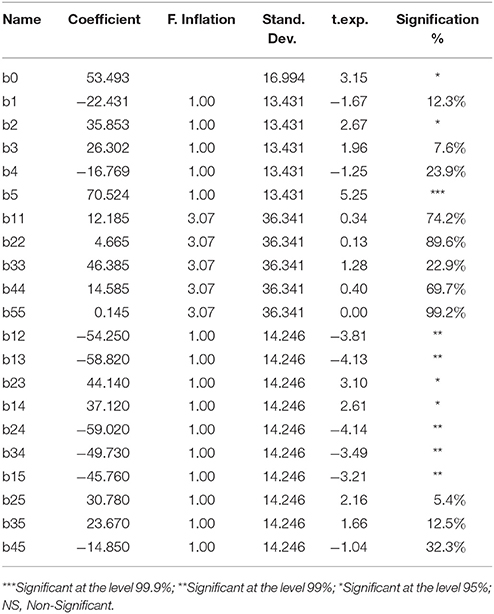
Table 3. Estimated effect, regression coefficient, and corresponding t- and P-values for BS production in central composite design experiments.
The interaction effects and optimal levels of the selected culture variables on BS production by P. rhizophila S211 were determined by plotting the response surface curves against the two significant variables X2 and X5, while fixing the other variables at constant levels (pH 6, OMWW 15% and inoculum size 0.5%). The contour plots and response surface curves for the predicted response Y (BS production yield), based on the second-order model were shown in Figure 5. They provided useful information about interactions between variables and allowed an easy interpretation of the CCD results and prediction of the optimal levels of each variable for maximum BS production (the highlighted zone in yellow). Indeed, S211 BS yield increased with the increase in temperature and incubation time from their low levels to their high levels (Figure 5). As a result, based on the response surface and contour plots, the optimum operating culture conditions, carried out numerically by using NemrodW software were: OMWW concentration 15%, inoculum size 0.5%, temperature 40°C, pH 6.0 and incubation time 8 days. The expected value of the BS production yield was Y1 = 721.47 ± 43 mg/L. A supplementary experiment was performed under the selected optimal production conditions. It led to BS production yield equal to 720.80 ± 55.90 mg/L, which was well in close agreement with predicted value (Figure 6A).
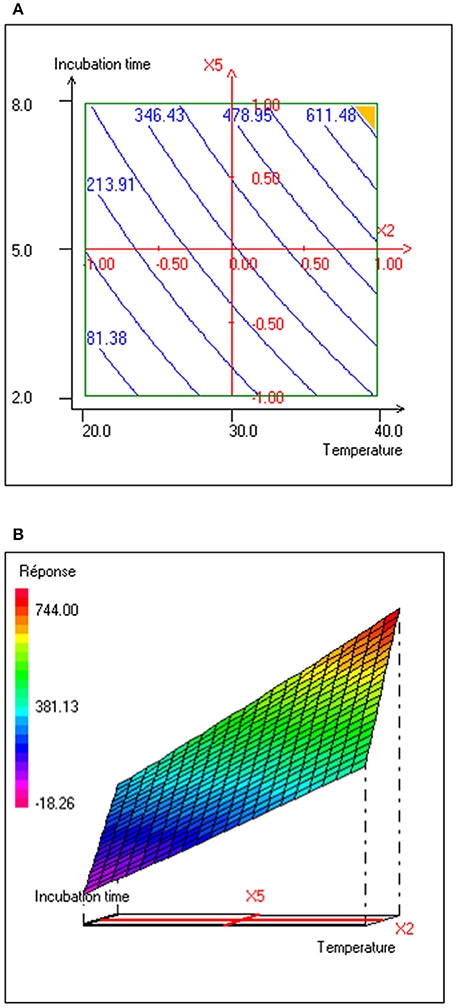
Figure 5. (A) Contour plot and (B) response surface plot of interaction effect between temperature (°C) and incubation time (days) on BS production yield (mg/L) with pH, OMWW concentration and inoculum size kept at 6.0, 15% and 0.5%, respectively.
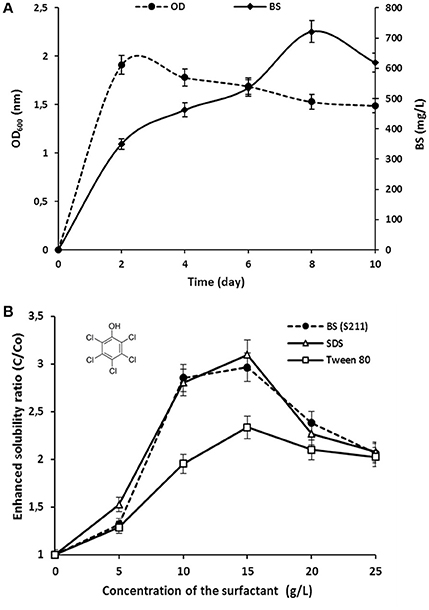
Figure 6. (A) Growth (OD600nm) kinetics and BS production by P. rhizophila S211 under optimized conditions; (B) Effect of chemical surfactants and P. rhizophila rhamnolipid BS concentrations on the solubility of PCP.
Biochemical Characterization of Purified BS from P. rhizophila S211 and Its Application in Enhancement of Pesticide Solubilization
The purified BS of P. rhizophila S211 showed important emulsification activity (E24 = 90%) and oil displacement area (ODA = 63.58 cm2). The separation of purified BS products on TLC plate indicate the presence of two characteristic spots, when anthrone reagent was sprayed. According to the commercially available purified rhamnolipid, the lower spot consisted of di-rhamnolipids with a retention factor value equal to 0.43, while the higher spot consisted of mono-rhamnolipids with a retention factor value equal to 0.67.
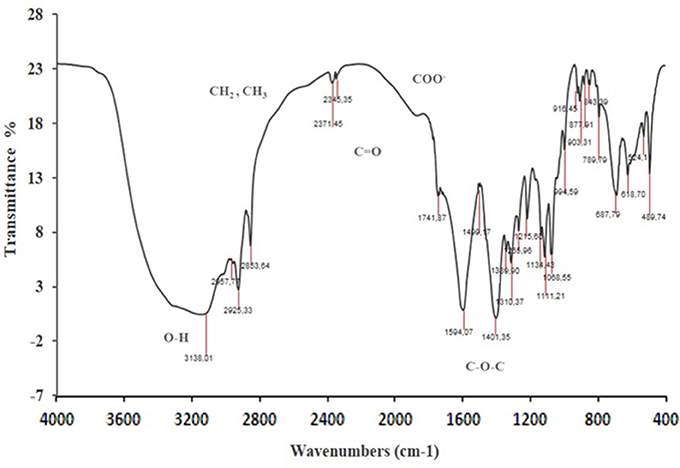
FTIR characteristic peaks of P. rhizophila BS observed at 3138 denoted the presence of –OH stretching (free hydroxyl groups of rhamnose rings) of hydroxyl group. The strong adsorption peaks at 2345, 2925, and 2853 showed the presence of methylene and the terminal methyl group of aliphatic (-CH, -CH2, -CH3) stretching bands confirmed the glycolipid type of produced BS (Figure 7). The carbonyl functional group (C = O) had a peak in the region of 1,741 cm−1. Peaks recorded in the range of 1,200–1,000 cm−1 indicate the presence of bonds between carbon atoms and the hydroxyl groups found in the rhamnose units.
The stability of P. rhizophila BS at different salinity, pH and temperature values was measured and the results are illustrated in Figure 8. The ODA of purified BS showed a remarkable increase at high temperatures and remained without any significant decrease on the oil displacement capacity in a range of 60–80°C, therefore it was found that the P. rhizophila BS is thermally stable. In addition, the salinity and pH stability analysis were carried out, revealing that P. rhizophila BS conserves its surfactant activity up to 300 mM NaCl. On the other hand, the purified BS produced by P. rhizophila S211 showed higher stability at alkaline conditions than acidic conditions.
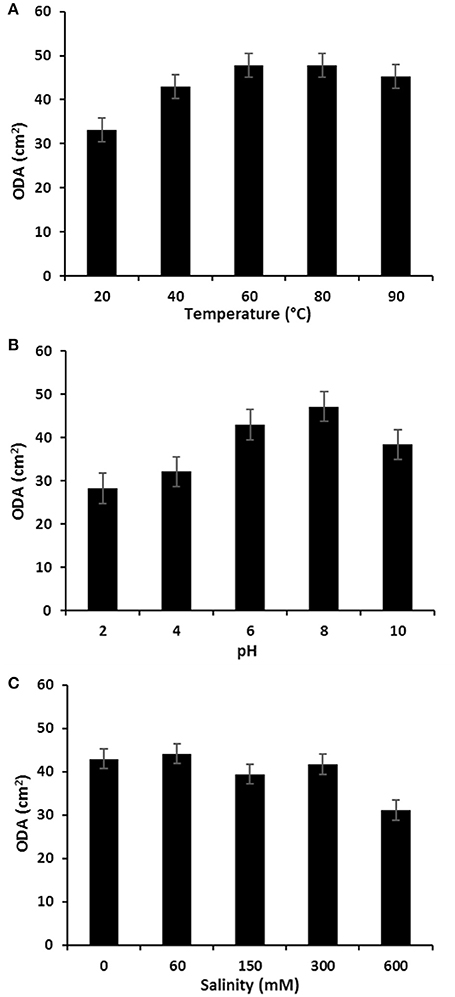
Figure 8. (A) Temperature; (B) pH, and (C) salinity effects on the stability of glycolipid BS produced by P. rhizophila S211.
The ability of purified BS from P. rhizophila S211 to enhance PCP solubility was performed in comparison with those of synthetic surfactants, i.e., a nonionic surfactant (Tween 80) and an anionic surfactant (SDS) (Figure 6B). The aqueous solubility of PCP was evaluated by test tube solubilization assays in the presence of different concentrations of surfactants ranging from 5 to 25 g/L. For all surfactants tested, the apparent PCP solubility increased with an increase in BS concentration up to 15 g/L. With an initial PCP concentration of 500 mg/L, enhanced PCP solubility ratios were about 2.32 ± 0.01, 3.15 ± 0.03, and 2.96 ± 0.02 folds higher than the pure water solubility for Tween 80, SDS and S211 BS, respectively. SDS was more effective than Tween 80 in enhancing PCP solubilization. Under these conditions, BS from P. rhizophila S211 showed better solubilization efficiency than Tween 80, while exhibited fairly similar behavior to an anionic surfactant, SDS, which confirm the anionic nature of BS from S211 strain.
Discussion
Although PGPR are mainly considered for promoting the plant growth and disease control, much attention has recently been focused on xenobiotic bioremediation using PGPR (Bishnoi, 2015). In an effort to develop innovative technological and management strategies for wastewater treatment and efficient reuse in agriculture, the MADFORWATER project (http://www.madforwater.eu/fr/) has been established. In the framework of this project, an extensive collection of PGPR isolates with bioremediation potential has been conducted. Among the group of PGPR, the genus Pseudomonas is strongly represented in literature. For example, Berendsen et al. (2015) reported that over 300 publications in the past 30 years described biological mechanisms involved in the ability of three PGPR strains P. putida WCS358, P. fluorescens WCS374, and P. fluorescens WCS417 to enhance plant growth and protect plants against diseases. In the present work, a novel species of Pseudomonas, namely P. rhizophila S211 has been isolated from a pesticide-contaminated artichoke farm soil in Tunisia using enrichment culture technique. P. rhizophila S211 appeared to be taxonomically very closely related to other plant growth-promoting Pseudomonas strains such as P. putida (Berendsen et al., 2015), P. fluorescens (Alsohim et al., 2014), and P. aeruginosa (Bhakthavatchalu et al., 2013; Singh and Cameotra, 2013).
In vitro biochemical experiments and genomic analysis showed that the free-living rhizobacterium, P. rhizophila S211 has a strong capacity to enhance plant growth mainly by increasing nitrogen fixation, phosphate solubilization, ACC deaminase and phytohormones production. Such plant growth-promoting activities of native Pseudomonas strains isolated from rhizospheric soils have been previously reported by many researchers (Beneduzi et al., 2012; Santoro et al., 2015; Kaundal et al., 2017). The biological control activity of this strain was mainly linked to siderophore-mediated competition for iron (Berendsen et al., 2015; Santoro et al., 2015). Among most of siderophore-producing bacteria studied, pyoverdine-synthesizing pseudomonads are known for their high affinity to the ferric ion. Several studies reported that fluorescent pseudomonads can efficiently colonize roots and suppress soil-borne fungal pathogens through the release of iron-chelating pyoverdines (Beneduzi et al., 2012).
P. rhizophila S211 exhibited bioremediation potentialities by synthesizing dioxygenases (DOs) and producing rhamnolipid BSs. The DOs including extradiol dioxygenase, homogentisate 1,2-dioxygenase, protocatechuate 3,4-dioxygenase, and aromatic ring-cleaving dioxygenases. The DOs play key roles in modifying diverse recalcitrant aromatic compounds to common intermediates, that can feed into central pathways. The ability to catabolize various recalcitrant aromatic compounds such as chemical pesticides in exudates represents one possible strategy that could confer a selective advantage in the plant rhizosphere (Shen et al., 2013). Rhamnolipids may also enhance bioremediation of pesticides and other pollutants in the rhizosphere by increasing the substrate availability for microorganisms, or by enhancing the hydrophobicity of the cell surface, allowing hydrophobic substrates to associate more easily with bacterial cells (Pacwa-Płociniczak et al., 2014). Rhamnolipid biosynthesis pathway is divided into biosynthesis of the fatty acid; sugar moieties and link the sugar and lipid (Wittgens et al., 2011; Irorere et al., 2017). In genome of strain S2111, the lipid moiety of the BS was generated through the classical pathway of fatty acid synthesis. All the genes potentially involved in the biosynthesis of dTDP-L-rhamnose were found based on S211 genome analysis including pgm, rmlA, rmlB, rmlC, and rmlD. The genes encoding rhamnosyltransferases that participate in the final steps of rhamnolipid biosynthesis were also identified in S211 genome. Rhamnolipid BS producers belonging to Pseudomonas species have been previously reported for P. putida (Kaskatepe et al., 2017), P. aeruginosa (Pansiripat et al., 2010), P. stutzeri (Joshi and Shekhawat, 2014), P. luteala (Onbasli and Aslim, 2009), P. fluorescens (Peter and Singh, 2014), P. chlorophis (Lan et al., 2015), P. alcaligenes (Oliveira et al., 2009). However, this is the first study to describe PGP potential and BS synthesis by P. rhizophila.
Pseudomonas strains are able to use different substrates, such as glucose, fructose, glycerol, mannitol, n-paraffins and vegetable oils, cheaper agro-industrial wastes and by-products to produce rhamnolipid-type BS (Ben Belgacem et al., 2015; Gudiña et al., 2015a,b). In this study, the BS production by S211 was performed in low-cost liquid medium formulated with OMWW. The effects of pH, temperature, OMWW concentration, inoculum size, and incubation time and their interactions on maximization of P. rhizophila BS production were evaluated and validated, experimentally using a CCD and RSM. Based on this experimental design, the BS yields under each set of conditions were determined and compared with the corresponding predicted levels suggested by NemrodW software. The obtained results showed that the model can be used for the navigation of BS model space. The optimum conditions for maximum BS yield by P. rhizophila S211 (720 mg/L) were predicted from the produced model as follows: 15% for OMWW concentration, 0.5% for inoculum size, 40°C for temperature, 6.0 for pH and8 days for incubation time. The maximum BS production by P. rhizophila S211 cultivated on OMWW as an alternative low-cost substrate was relatively higher than those of P. fluorescens strains cultivated on high-cost hydrophobic substrates such as soybean oil (437 mg/L), coconut oil (299 mg/L), palm oil (289 mg/L), mustard oil (233 mg/L), sunflower oil (187 mg/L), and olive oil (108 mg/L) (Peter and Singh, 2014). Other researchers reported BS production by different P. aeruginosa strains using culture media containing agro-industrial by-products and vegetable oils, such as olive oil, sunflower oil, Babassu oil, palm oil and soybean oil, leading to BS yields between 200 and 800 mg/L (Kaskatepe and Yildiz, 2016).
Purified BS of S211 was initially characterized by TLC revealing double spots when being visualized under UV light, which confirmed the presence of glycolipids. The replica plate when sprayed with anthrone, produced dark and light brown spots indicating the presence of mono- and di-rhamnolipids. The molecular composition of the purified S211 BS was evaluated by FTIR, which revealed the presence of saccharides and lipid in combination. Similar results have been reported for other glycolipid BS produced by Pseudomonas strains (Banat et al., 2000; Rahman et al., 2010; Wittgens et al., 2011; Ibrahim et al., 2013; Nalini and Parthasarathi, 2014; Elazzazy et al., 2015).
Applicability of glycolipid BS in several fields depends on their activity and stability at harsh conditions (Banat et al., 2000; Mata-Sandoval et al., 2002; Manivasagan et al., 2014). The BS produced by P. rhizophila S211 exhibited good stability over higher temperatures, a large range of pHs and salt concentrations, making it a potential candidate for bioremediation of soils contaminated by xenobiotics. It was shown to be highly thermostable. In fact, heating of the BS sample to 100°C (or its autoclaving at 120°C) caused little significant decrease on the BS performance (Rahman et al., 2010; Techaoei et al., 2011). Little changes were also observed with addition of up to 300 mM sodium chloride. pH increase has a positive effect on S211 BS performance. This is due to higher stability of fatty acids-surfactant micells in the presence of sodium hydroxide and the precipitation of secondary metabolites at high pH values. The effect of pH on BS activities of different Pseudomonas strains has been well reported (Manivasagan et al., 2014; Elazzazy et al., 2015).
This study demonstrated that BS produced by plant-growth promoting P. rhizophila could enhance the solubility of PCP under different contamination conditions indicating the feasibility of BS application as an efficient biological tool for remediation of pesticide-contaminated site. Similar results were reported by Wattanaphon et al. (2008) who evaluated the solubilization potential of three pesticides, i.e., methyl parathion, trifluralin and ethyl parathion, in the presence of three surfactants namely Tween 80, SDS and a glycolipid produced by Burkholderia cenocepacia BSP3.
Conclusion
Given their ability to enhance soil nutrient availability, produce plant growth–stimulating compounds, and protect against pathogens, PGPR are widely used as bioinoculants to support survival and development of plants even under various stressing conditions, such as pesticide contamination of soil. In this work, the new bacterium P. rhizophila S211, isolated from an agricultural contaminated soil, both displayed pesticide solubilizing and plant-growth-promoting activities. By analyzing the complete S211 genome, we identified the key genes that potentially promote plant growth as well as genes involved in xenobiotic biodegradation and BS synthesis. BS production by the new producer P. rhizophila S211 was performed in low-cost liquid medium supplemented with OMWW and optimized by RSM. P. rhizophila BS not only exhibited excellent yield (720 mg/L) but also showed high emulsification index (90%) and oil displacement area (63.58 cm2). S211 BS characterization using TLC and FT-IR confirmed the presence of the rhamnolipid type BS. It exhibited also good stability over higher temperatures, different pHs and sodium chloride. This glucolipid BS from P. rhizophila S211 noticeably enhanced PCP solubilization suggesting its role in environmental pesticide bioremediation. Further comparative genomic analysis with phylogenetically closely related strains together with structural and functional studies will allow for a more comprehensive understanding of the mechanisms used by this bacterium to remediate rhizospheric pollutants and to promote plant growth especially for the crops grown in contaminated soils. A future research is also needed to elucidate glycolipid BS-pesticide interactions in soil to find out predictive and mechanistic models and thus better real-scale bioremediation design.
Author Contributions
Conceived and designed the experiments: MN, HbC, HnC, YS, NR, FF, and AC. Analyzed the data: WH, MN, AN, RC, AS, FN, AM, and NR. Contributed reagents, materials, analysis: WH, HnC, AS, YS, and NR. Manuscript preparation and revision: WH, MN, NR, HO, and AC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge financial support from the European Union in the ambit of the project MADFORWATER (H2020, GA 688320) and the Tunisian Ministry of Higher Education and Scientific Research in the ambit of the laboratory project LR11ES31.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00034/full#supplementary-material
References
Ahemad, M., and Kibret, M. (2014). Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J. King Saud. Univ. Sci. 26, 1–20. doi: 10.1016/j.jksus.2013.05.001
Aktar, W., Sengupta, D., and Chowdhury, A. (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2, 1–12. doi: 10.2478/v10102-009-0001-7
Alexander, D. B., and Zuberer, D. A. (1991). Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertility Soils 12, 39–45. doi: 10.1007/BF00369386
Alsohim, A. S., Taylor, T. B., Barrett, G. A., Gallie, J., Zhang, X. X., Altamirano-Junqueira, A. E., et al. (2014). The biosurfactant viscosin produced by Pseudomonas fluorescens SBW25 aids spreading motility and plant growth promotion. Environ. Microbiol. 16, 2267–2281. doi: 10.1111/1462-2920.12469
Alström, S., and Burns, R. G. (1989). Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biol. Fertility Soils. 7, 232–238. doi: 10.1007/BF00709654
Andrews, S. (2011). FastQ Screen. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastq_screen/
Antoniou, E., Fodelianakis, S., Korkakaki, E., and Kalogerakis, N. (2015). Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source. Front. Microbiol. 6:274. doi: 10.3389/fmicb.2015.00274
Auch, A. F., Jan, M., Klenk, H. P., and Göker, M. (2010). Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genomic Sci. 2, 117–134. doi: 10.4056/sigs.531120
Aziz, R. K., Bartels, D., Best, A. A., DeJongh, M., Disz, T., Edwards, R. A., et al. (2008). The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75
Banat, I. M., Makkar, R. S., and Cameotra, S. S. (2000). Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 53, 495–508. doi: 10.1007/s002530051648
Ben Belgacem, Z., Bijttebier, S., Verreth, C., Voorspoels, S., Van de Voorde, I., Aerts, G., et al. (2015). Biosurfactant production by Pseudomonas strains isolated from floral nectar. J. Appl. Microbiol. 118, 1370–1384. doi: 10.1111/jam.12799
Beneduzi, A., Ambrosini, A., and Passaglia, L. M. (2012). Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 35, 1044–1051. doi: 10.1590/S1415-47572012000600020
Berendsen, R. L., Verk, M. C., Stringlis, I. A., Zamioudis, C., Tommassen, J., Pieterse, C. M., et al. (2015). Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genomics 16:539. doi: 10.1186/s12864-015-1632-z
Bhakthavatchalu, S., Shivakumar, S., and Sullia, S. B. (2013). Characterization of multiple plant growth promotion traits of Pseudomonas aeruginosa FP6, a potential stress tolerant biocontrol agent. Ann. Biol. Res. 4, 214–223.
Bhardwaj, D., Ansari, M. W., Sahoo, R. K., and Tuteja, N. (2014). Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 13:1. doi: 10.1186/1475-2859-13-66
Bishnoi, U. (2015). Chapter four-PGPR interaction: an ecofriendly approach promoting the sustainable agriculture system. Adv. Bot. Res. 75, 81–113. doi: 10.1016/bs.abr.2015.09.006
Burd, G. I., Dixon, D. G., and Glick, B. R. (2000). Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 46, 237–245. doi: 10.1139/w99-143
Bustamante, M., Duran, N., and Diez, M. (2012). Biosurfactants are useful tools for the bioremediation of contaminated soil: a review. J. Soil. Sci. Plant. Nut. 12, 667–687. doi: 10.4067/S0718-95162012005000024
Cappuccino, J. C., and Sherman, N. (1992). Microbiology: A Laboratory Manual, 3rd Edn. New York, NY: Benjamin/Cummings Pub. Co.
Cherif, H., Neifar, M., Chouchane, H., Soussi, A., Hamdi, C., Guesmi, A., et al. (2017). “Extremophile diversity and biotechnological potential from desert environments and saline systems of southern Tunisia,” in Extremophiles: From Biology to Biotechnology, eds V. Ravi, D. Durvasula, and V. Subba Rao (Boca Raton, FL: CRC Publishers), 33–64.
Colak, A. K., and Kahraman, H. (2013). The use of raw cheese whey and olive oil mill wastewater for rhamnolipid production by recombinant Pseudomonas aeruginosa. Environ. Experi. Biol. 11, 125–130.
Cooper, D. G., and Goldenberg, B. G. (1987). Surface-active agents from two Bacillus species. Appl. Environ. Microbial. 53, 224–229.
Cycon, M., Wójcik, M., and Piotrowska-Seget, Z. (2009). Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere 76, 494–501. doi: 10.1016/j.chemosphere.2009.03.023
Delcher, A. L., Bratke, K. A., Powers, E. C., and Salzberg, S. L. (2007). Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23, 673–679. doi: 10.1093/bioinformatics/btm009
Elazzazy, A. M., Abdelmoneim, T. S., and Almaghrabi, O. A. (2015). Isolation and characterization of biosurfactant production under extreme environmental conditions by alkali-halo-thermophilic bacteria from Saudi Arabia. Saudi. J. Biological. Sci. 22, 466–475. doi: 10.1016/j.sjbs.2014.11.018
El-Daim, A., and Moustafa, I. A. (2015). Use of rhizobacteria for the alleviation of plant stress. ACTA Swedish Univ. Agr. Sci. 2015, 48–58. doi: 10.1371/journal.pone.0096086
Fakruddin, M. D. (2012). Biosurfactant: production and application. J. Pet. Environ. Biotechnol. 3:124. doi: 10.4172/2157-7463.1000124
Franzetti, A., Caredda, P., Ruggeri, C., La Colla, P., Tamburini, E., Papacchini, M., et al. (2009). Potential applications of surface active compounds by Gordonia sp. strain BS29 in soil remediation technologies. Chemosphere 75, 801–807. doi: 10.1016/j.chemosphere.2008.12.052
Galardini, M., Biondi, E. G., Bazzicalupo, M., and Mengoni, A. (2011). CONTIGuator: a bacterial genomes finishing tool for structural insights on draft genomes. Source Code Biol. Med. 6:11. doi: 10.1186/1751-0473-6-11
Germaine, K. J., Liu, X., Cabellos, G. G., Hogan, J. P., and Ryan, D. (2006). Bacterial endophyte- enhanced phytoremediation of the organochlorine herbicide 2,4-dichlorophenoxyacetic acid. FEMS Microb. Ecol. 57, 302–310. doi: 10.1111/j.1574-6941.2006.00121.x
Geys, R., Soetaert, W., and Van Bogaert, I. (2014). Biotechnological opportunities in biosurfactant production. Curr. Opin. Biotechnol. 30, 66–72. doi: 10.1016/j.copbio.2014.06.002
Gordon, S. A., and Weber, R. P. (1951). Colorimetric estimation of indoleacetic acid. Plant Physiol. 26, 192–195. doi: 10.1104/pp.26.1.192
Goris, J., Konstantinidis, K. T., Klappenbach, J. A., Coenye, T., Vandamme, P., and Tiedje, J. M. (2007). DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbial. 57, 81–91. doi: 10.1099/ijs.0.64483-0
Goupy, J. (1999). Plans d'experiences pour surfaces de reponse. [Experimental Plans for Response Surfaces]. Dunod, Paris.
Gudiña, E. J., Fernandes, E. C., Rodrigues, A. I., Teixeira, J. A., and Rodrigues, L. R. (2015a). Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 6:59. doi: 10.3389/fmicb.2015.00059
Gudiña, E. J., Rodrigues, A. I., Alves, E., Domingues, M. R., Teixeira, J. A., and Rodrigues, L. R. (2015b). Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresour. Technol. 177, 87–93. doi: 10.1016/j.biortech.2014.11.069
Guesmi, A., Ettoumi, B., El Hidri, D., Essanaa, J., Cherif, H., Mapelli, F., et al. (2013). Uneven distribution of Halobacillus trueperi species in arid natural saline systems of southern Tunisian Sahara. Microb. Ecol. 66, 831–839. doi: 10.1007/s00248-013-0274-4
Gurevich, A., Saveliev, V., Vyahhi, N., and Tesler, G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075. doi: 10.1093/bioinformatics/btt086
Haft, D. H., Selengut, J. D., and White, O. (2003). The TIGRFAMs database of protein families. Nucleic Acid Res. 31, 371–373. doi: 10.1093/nar/gkg128
Hyatt, D., Chen, G. L., Locascio, P. F., Land, M. L., Larimer, F. W., and Hauser, L. J. (2010). Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 11:119. doi: 10.1186/1471-2105-11-119
Ibrahim, M. L., Ijah, U. J. J., Manga, S. B., Bilbis, L. S., and Umar, S. (2013). Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Inter. Biodeterior. Biodegrad. 81, 28–34. doi: 10.1016/j.ibiod.2012.11.012
Irorere, V. U., Tripathi, L., Marchant, R., McClean, S., and Banat, I. M. (2017). Microbial rhamnolipid production: a critical re-evaluation of published data and suggested future publication criteria. Appl. Microbiol. Biotechnol. 101, 3941–3951. doi: 10.1007/s00253-017-8262-0
Jadhav, V., Yadav, A., Shouche, Y., Aphale, S., Moghe, A., Pillai, S., et al. (2013). Studies on biosurfactant from Oceanobacillus sp. BRI 10 isolated from Antarctic sea water. Desalination 318, 64–71. doi: 10.1016/j.desal.2013.03.017
Jensen, H. L. (1942). Nitrogen fixation in leguminous plants. l. General characters of root-nodule bacteria isolated from species of Medicago and Trifoliwn in Australia. Proc. Linnean. Soc. NSW. 67, 98–108.
Joshi, P. A., and Shekhawat, D. B. (2014). Screening and isolation of biosurfactant producing bacteria from petroleum contaminated soil. Eur. J. Exp. Biol. 4, 164–169.
Kanehisa, M., and Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Kaskatepe, B., and Yildiz, S. (2016). Rhamnolipid biosurfactants produced by pseudomonas species. Braz. arch. biol. technol. 59:e16160786. doi: 10.1590/1678-4324-2016160786
Kaskatepe, B., Yildiz, S., Gumustas, M., and Ozkan, S. (2017). Rhamnolipid production by Pseudomonas putida IBS036 and Pseudomonas pachastrellae LOS20 with using pulps. Current. Pharmaceutical. Anal. 13, 138–144. doi: 10.2174/1573412912666161018144635
Kaundal, K., Sharma, S., Manorma, K., and Kaur, M. (2017). Evaluation of plant growth promoting (PGP) efficacy of rhizospheric Pseudomonas strains. Int. J. Farm Sci. 7, 165–169.
Kruijt, M., Tran, H., and Raaijmakers, J. M. (2009). Functional, genetic and chemical characterization of biosurfactants produced by plant growth-promoting Pseudomonas putida 267. J. Appl. Microbiol. 107, 546–556. doi: 10.1111/j.1365-2672.2009.04244.x
Lagesen, K., Hallin, P., Rødland, E. A., Stærfeldt, H. H., Rognes, T., and Ussery, D. W. (2007). RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic acids Res. 35, 3100–3108. doi: 10.1093/nar/gkm160
Lan, G., Fan, Q., Liu, Y., Chen, C., Li, G. X., Liu, Y., et al. (2015). Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochemical. Eng. J. 101, 44–54. doi: 10.1016/j.bej.2015.05.001
Letunic, I., and Bork, P. (2016). Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, 242–245. doi: 10.1093/nar/gkw290
Lowe, T. M., and Eddy, S. R. (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964. doi: 10.1093/nar/25.5.0955
Mahanty, T., Bhattacharjee, T. M. S., Goswami, M., Bhattacharyya, P., Das, B., Ghosh, A., et al. (2017). Biofertilizers: a potential approach for sustainable agriculture development, Env. Sci. Pollut. Res. Int. 24, 3315–3335. doi: 10.1007/s11356-016-8104-0
Mahdi, S. S., Hassan, G. I., Samoon, S. A., Rather, H. A., Dar, S. A., and Zehra, B. (2010). Bio-fertilizers in organic agriculture. J. Phytol. 2, 42–54.
Maier, R. M. (2003). Biosurfactants: evolution and diversity in bacteria. Adv. Appl. Microbiol. 52, 101–121. doi: 10.1016/S0065-2164(03)01004-9
Manivasagan, P., Sivasankar, P., Venkatesan, J., Sivakumar, K., and Kim, S. K. (2014). Optimization, production and characterization of glycolipid biosurfactant from the marine actinobacterium, Streptomyces sp. MAB36. Bioprocess. Biosystems. Eng. 37, 783–797. doi: 10.1007/s00449-013-1048-6
Markowitz, V. M., Mavromatis, K., Ivanova, N. N., Chen, I. M. A., Chu, K., and Kyrpides, N. C. (2009). IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 25, 2271–2278. doi: 10.1093/bioinformatics/btp393
Mata-Sandoval, J., Karns, J., and Torrents, A. (2002). Influence of rhamnolipids and Triton X-100 on the desorption of pesticides from soils. Environ. Sci. Technol. 36, 4669–4675. doi: 10.1021/es011260z
Meier-Kolthoff, J. P., Auch, A. F., Klenk, H. P., and Göker, M. (2013a). Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60
Meier-Kolthoff, J. P., Göker, M., Spröer, C., and Klenk, H. P. (2013b). When should a DDH experiment be mandatory in microbial taxonomy? Arch. Microbiol. 195, 413–418. doi: 10.1007/s00203-013-0888-4
Mukherjee, S., Das, P., and Sen, S. (2006). Towards commercial production of microbial surfactants. Trends. Biotechnol. 24, 509–515. doi: 10.1016/j.tibtech.2006.09.005
Myers, R., Montgomery, D. C., and Anderson-Cook, C. M. (2009). Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 3rd Edn. New York, NY: Wiley.
Nalini, S., and Parthasarathi, R. (2014). Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour. Technol. 173, 231–238. doi: 10.1016/j.biortech.2014.09.051
Nautiyal, C. S. (1999). An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbial. Lett. 170, 265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x
Neifar, M., Arous, F., Hassen, W., Chouchane, H., Cherif, A., and Jaouani, A. (2017). “An overview on olive oil mill wastes management and bio-valorization,” in Handbook of Olive Oil: Phenolic Compounds, Production and Health Benefits, ed J. Miloš (New York, NY: Nova Science Publishers), 395–422.
Oliveira, F., Vasquez, L., De Campos, N. P., and De Franca, F. P. (2009). Production of rhamnolipids by a Pseudomonas alcaligenes strain. Process. Biochem. 44, 383–389. doi: 10.1016/j.procbio.2008.11.014
Onbasli, D., and Aslim, B. (2009). Biosurfactant production in sugar beet molasses by some Pseudomonas spp. J. Environ. Biol. 30, 161–163.
Pacwa-Płociniczak, M., Płaza, G. A., Piotrowska-Seget, Z., and Cameotra, S. S. (2011). Environmental applications of biosurfactants: recent advances. Int. J. Mol. Sci. 12, 633–654. doi: 10.3390/ijms12010633
Pacwa-Płociniczak, M., Płaza, G. A., Poliwoda, A., and Piotrowska-Seget, Z. (2014). Characterization of hydrocarbon-degrading and biosurfactant producing Pseudomonas sp. P-1 strain as a potential tool for bioremediation of petroleum-contaminated soil. Environ. Sci. Pollut. Res. Int. 21, 9385–9395. doi: 10.1007/s11356-014-2872-1
Page, W. J., and Tingerstrom, V. M. (1988). Dual regulation of catecholate siderophore biosynthesis in Azotobacter vinelandii by Fe and Oxidative stress. J. Gen. Microbiol. 134, 453–460.
Pansiripat, S., Pornsunthorntawee, O., Rujiravanit, R., Kitiyanan, B., Somboonthanate, P., and Chavadej, S. (2010). Biosurfactant production by Pseudomonas aeruginosa SP4 using sequencing batch reactors: effect of oil-to-glucose ratio. Biochem. Eng. J. 49, 185–191. doi: 10.1016/j.bej.2009.12.011
Peter, J., and Singh, D. (2014). Characterization of emulsification activity of partially purified Rhamnolipids from Pseudomonas fluorescens. Int. J. Innovation. Sci. Res. 3, 88–100.
Rahman, P., Pasirayi, G., Auger, V., and Ali, Z. (2010). Production of rhamnolipid biosurfactants by Pseudomonas aeruginosa DS10-129 in a microfluidic bioreactor. Biotechnol. Appl. Biochem. 55, 45–52. doi: 10.1042/BA20090277
Ramírez, I. M., Tsaousi, K., Rudden, M., Marchant, R., Alameda, E. J., Román, M. G., et al. (2015). Rhamnolipid and surfactin production from olive oil mill waste as sole carbon source. Bioresour. Technol. 198, 231–236. doi: 10.1016/j.biortech.2015.09.012
Richter, M., and Rosselló-Móra, R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U.S.A. 106, 19126–19131. doi: 10.1073/pnas.0906412106
Rodriguez, -R. L. M., and Konstantinidis, K. T. (2014). Bypassing cultivation to identify bacterial species. Microbe 9, 111–118. doi: 10.1128/microbe.9.111.1
Santoro, M. V., Cappellari, L. R., Giordano, W., and Banchio, E. (2015). Plant growth-promoting effects of native Pseudomonas strains on Mentha piperita (peppermint): an in vitro study. Plant Biol. 17, 1218–1226. doi: 10.1111/plb.12351
Sarubbo, L., Luna, J. M., and Rufino, R. D. (2015a). Application of a biosurfactant produced in low-cost substrates in the removal of hydrophobic contaminants. Chem. Eng. Transactions. 43, 295–300. doi: 10.3303/CET1543050
Sarubbo, L., Rocha, R. B. Jr., Luna, J. M., Rufino, R. D., Santos, V. A., and Banat, I. M. (2015b). Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 31, 707–723. doi: 10.1080/02757540.2015.1095293
Satpute, S. K., Bhawsar, B. D., Dhakephalkar, P. K., and Chopade, B. A. (2008). Assessment of different screening methods for selecting biosurfactant producing marine bacteria. J. Marine. Sci. 37, 243–250.
Savci, S. (2012). An agricultural pollutant: chemical fertilizer. Int. J. Environ. Sci. Development. 3, 73–80. doi: 10.7763/IJESD.2012.V3.191
Seemann, T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. doi: 10.1093/bioinformatics/btu153
Sharma, D., Ansari, M. J., Gupta, S., Al Ghamdi, A., Pruthi, P., and Pruthi, V. (2015). Structural characterization and antimicrobial activity of a biosurfactant obtained from Bacillus pumilus DSVP18 grown on potato peels. Jundishapur J. Microbiol. 8:e21257. doi: 10.5812/jjm.21257
Shen, X., Hu, H., Peng, H., Wang, W., and Zhang, X. (2013). Comparative genomic analysis of four representative plant growth-promoting rhizobacteria in Pseudomonas. BMC Genomics 14:271. doi: 10.1186/1471-2164-14-271
Sheng, X., He, L., Wang, Q., Ye, H., and Jiang, C. (2008). Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J. Hazard. Mater. 155, 17–22. doi: 10.1016/j.jhazmat.2007.10.107
Singh, A. K., and Cameotra, S. S. (2013). Rhamnolipids production by multi-metal-resistant and plant-growth-promoting rhizobacteria. Appl. Biochem Biotechnol. 170, 1038–1056. doi: 10.1007/s12010-013-0244-9
Singh, J. S., Pandey, V. C., and Singh, D. P. (2011). Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 140, 339–353. doi: 10.1016/j.agee.2011.01.017
Smyth, T. J. P., Perfumo, A., Marchant, R., and Banat, I. M. (2010). “Isolation and analysis of low molecular weight microbial glycolipids,” in Handbook of Hydrocarbon and Lipid Microbiology, ed K. N. Timmis (Berlin: Springer-Verlag), 3705–3723.
Tatusov, R., Fedorova, N., Jackson, J., Jacobs, A., Kiryutin, B., Koonin, E., et al. (2003). The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4:41. doi: 10.1186/1471-2105-4-41
Techaoei, S., Lumyong, S., Prathumpai, W., Santiarwarn, D., and Leelapornpisid, P. (2011). Screening characterization and stability of biosurfactant produced by Pseudomonas aeruginosa SCMU106 isolated from soil in northern Thialand. Asian J. Biol. Sci. 4, 340–351. doi: 10.3923/ajbs.2011.340.351
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R., and Polasky, S. (2002). Agricultural sustainability and intensive production practices. Nature 418, 671–677. doi: 10.1038/nature01014
Tritt, A., Eisen, J. A., Facciotti, M. T., and Darling, A. E. (2012). An integrated pipeline for de novo assembly of microbial genomes. PLoS ONE 7:e42304. doi: 10.1371/journal.pone.0042304
Tuleva, B. K., Ivanov, G. R., and Christova, N. E. (2002). Biosurfactant production by a new Pseudomonas putida strain. Z. Naturforsch. C. 57, 356–360. doi: 10.1515/znc-2002-3-426
Vijayabaskar, P., Babinastarlin, S., Shankar, T., Sivakumar, T., and Anandapandian, K. T. K. (2011). Quantification and characterization of exopolysaccharides from Bacillus subtilis (MTCC 121). Adv. Biol. Res. 5, 71–76.
Wattanaphon, H. T., Kerdsin, A., Thammacharoen, C., Sangvanich, P., and Vangnai, A. S. (2008). A biosurfactant from Burkholderia cenocepacia BSP3 and its enhancement of pesticide solubilization. J. Appl. Microbiol. 105, 416–423. doi: 10.1111/j.1365-2672.2008.03755.x
Weng, H., Shen, W., Liu, Y., He, L., Niu, B., Meng, Z., et al. (2013). Cloning and characterization of two EcR isoforms from Japanese pine sawyer, Monochamus alternates. Arch. Insect Biochem. Physiol. 84, 27–42. doi: 10.1002/arch.21111
Wittgens, A., Tiso, T., Arndt, T. T., Wenk, P., Hemmerich, J., Müller, C., et al. (2011). Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb. Cell Fact. 10:80. doi: 10.1186/1475-2859-10-80
Ying, G. (2006). Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 32, 417–431. doi: 10.1016/j.envint.2005.07.004
Zhang, Y., and Qiu, S. (2016). Phylogenomic analysis of the genus Ralstonia based on 686 single-copy genes. Antonie Leeuwenhoek 109, 71–82. doi: 10.1007/s10482-015-0610-4
Keywords: Pseudomonas rhizophila, plant-growth promotion rhizobacterium, biofertilization, phytostimulator, pesticide bioremediation, biosurfactant production
Citation: Hassen W, Neifar M, Cherif H, Najjari A, Chouchane H, Driouich RC, Salah A, Naili F, Mosbah A, Souissi Y, Raddadi N, Ouzari HI, Fava F and Cherif A (2018) Pseudomonas rhizophila S211, a New Plant Growth-Promoting Rhizobacterium with Potential in Pesticide-Bioremediation. Front. Microbiol. 9:34. doi: 10.3389/fmicb.2018.00034
Received: 03 November 2017; Accepted: 09 January 2018;
Published: 23 February 2018.
Edited by:
George Tsiamis, University of Patras, GreeceReviewed by:
Spyridon Ntougias, Democritus University of Thrace, GreeceDimitris Tsaltas, Cyprus University of Technology, Cyprus
Copyright © 2018 Hassen, Neifar, Cherif, Najjari, Chouchane, Driouich, Salah, Naili, Mosbah, Souissi, Raddadi, Ouzari, Fava and Cherif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ameur Cherif, Y2hlcmlmLmFtZXVyQGdtYWlsLmNvbQ==
 Wafa Hassen
Wafa Hassen Mohamed Neifar
Mohamed Neifar Hanene Cherif
Hanene Cherif Afef Najjari
Afef Najjari Habib Chouchane
Habib Chouchane Rim C. Driouich1
Rim C. Driouich1 Fatma Naili
Fatma Naili Amor Mosbah
Amor Mosbah Yasmine Souissi
Yasmine Souissi Noura Raddadi
Noura Raddadi Ameur Cherif
Ameur Cherif