
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 08 January 2018
Sec. Microbiotechnology
Volume 8 - 2017 | https://doi.org/10.3389/fmicb.2017.02528
 Rok Tkavc1,2
Rok Tkavc1,2 Vera Y. Matrosova1,2
Vera Y. Matrosova1,2 Olga E. Grichenko1,2
Olga E. Grichenko1,2 Cene Gostinčar3
Cene Gostinčar3 Robert P. Volpe1,2
Robert P. Volpe1,2 Polina Klimenkova1,2
Polina Klimenkova1,2 Elena K. Gaidamakova1,2
Elena K. Gaidamakova1,2 Carol E. Zhou4
Carol E. Zhou4 Benjamin J. Stewart5
Benjamin J. Stewart5 Mathew G. Lyman5
Mathew G. Lyman5 Stephanie A. Malfatti5
Stephanie A. Malfatti5 Bonnee Rubinfeld5
Bonnee Rubinfeld5 Melanie Courtot6
Melanie Courtot6 Jatinder Singh7
Jatinder Singh7 Clifton L. Dalgard8,9
Clifton L. Dalgard8,9 Theron Hamilton10
Theron Hamilton10 Kenneth G. Frey10
Kenneth G. Frey10 Nina Gunde-Cimerman3
Nina Gunde-Cimerman3 Lawrence Dugan5
Lawrence Dugan5 Michael J. Daly1*
Michael J. Daly1*Highly concentrated radionuclide waste produced during the Cold War era is stored at US Department of Energy (DOE) production sites. This radioactive waste was often highly acidic and mixed with heavy metals, and has been leaking into the environment since the 1950s. Because of the danger and expense of cleanup of such radioactive sites by physicochemical processes, in situ bioremediation methods are being developed for cleanup of contaminated ground and groundwater. To date, the most developed microbial treatment proposed for high-level radioactive sites employs the radiation-resistant bacterium Deinococcus radiodurans. However, the use of Deinococcus spp. and other bacteria is limited by their sensitivity to low pH. We report the characterization of 27 diverse environmental yeasts for their resistance to ionizing radiation (chronic and acute), heavy metals, pH minima, temperature maxima and optima, and their ability to form biofilms. Remarkably, many yeasts are extremely resistant to ionizing radiation and heavy metals. They also excrete carboxylic acids and are exceptionally tolerant to low pH. A special focus is placed on Rhodotorula taiwanensis MD1149, which was the most resistant to acid and gamma radiation. MD1149 is capable of growing under 66 Gy/h at pH 2.3 and in the presence of high concentrations of mercury and chromium compounds, and forming biofilms under high-level chronic radiation and low pH. We present the whole genome sequence and annotation of R. taiwanensis strain MD1149, with a comparison to other Rhodotorula species. This survey elevates yeasts to the frontier of biology's most radiation-resistant representatives, presenting a strong rationale for a role of fungi in bioremediation of acidic radioactive waste sites.
Between 1945 and 1986, immense volumes of radioactive waste were generated from the production of 46,000 nuclear weapons in the United States. This was a period of history when national security priorities often surmounted concerns over the environment. Many Cold War wastes contained mixtures of inorganic contaminants including radionuclides (e.g., U and Tc), heavy metals (e.g., Cr and Hg), and nitrate, which were disposed directly to the ground at 120 sites across the United States (Daly, 2000). As the processing of uranium ores involved dissolution and extraction with nitric acid, this led to large volumes of highly acidic radioactive waste, which were stored in subterranean holding tanks or ponds. Over the past six decades, low levels of widespread contamination originating from such waste sites have contaminated over 7.0 × 107 m3 of surface and subsurface soils, and over 3.0 × 1012 L of groundwater (McCullough et al., 1999; Daly, 2000). As a result of the chemical reprocessing of 1.1 × 108 kg of nuclear fuel at the Hanford Site (WA, USA) alone, 2.1 × 105 m3 of radioactive waste were produced at nine reactors and stored in 177 underground tanks. These storage tanks with a lifespan of 10–20 years have been used since 1943, and the first leaks were confirmed in 1959. The amount of waste leakage from the Hanford tanks continues to grow, with estimates in 2004 ranging from 2.3 to 3.7 × 106 L (Fredrickson et al., 2004). The scale of these waste environments leaves few options for cleanup other than bioremediation (Brim et al., 2000).
In 2000, more than 110 distinct aerobic heterotrophic bacteria were isolated from below Hanford tank SX-108, which has been leaking extremely radioactive waste since the 1960s (Fredrickson et al., 2004). Among the numerous bacteria identified, Arthrobacter spp. were the most prevalent and Deinococcus spp. the most radiation-resistant. Both bacterial genera are known for their ability to survive harsh environmental conditions and reduce a variety of metals, and for their dependence on Mn for growth and resistance (Daly et al., 2004; Fredrickson et al., 2004; Ehrlich and Newman, 2008). The isolation of Deinococcus radiodurans from sediments under tank SX-108 focused research on this extremophile: first, to engineer metal-reducing and organic toxin-degrading capabilities into this bacterium; and second, to test the ability of engineered D. radiodurans to reduce/immobilize different metals, and to couple those reactions to solvent degradation while growing under high-level chronic ionizing radiation (CIR). Metal reduction coupled to toluene degradation as a bioremediation strategy for radioactive sites was successfully demonstrated in D. radiodurans at near-neutral pH under CIR (60 Gy/h) (Brim et al., 2006). However, D. radiodurans strain R1 and its engineered counterparts cannot grow at pH values below 4.8 (unpublished results).
To determine whether or not radiation-resistant acidophilic microorganisms exist, we first screened approximately 60 different environmental samples (desert sands, acid mine drainages, soils) for microorganisms that are able to grow under 36 Gy/h at pH 2.3. This yielded the basidiomycetous yeast Rhodotorula taiwanensis MD1149, which can grow under 66 Gy/h at pH 2.3. Fungi play an important role in the biogeochemical cycling of manganese and other redox-active metals (Ehrlich and Newman, 2008; Culotta and Daly, 2013), which is related to their ability to survive radiation and other oxidative challenges (Gadd, 2007; Daly, 2009; Sharma et al., 2017). Nevertheless, any prospect of yeasts in bioremediation of radioactive waste sites has been neglected, mainly due to the lack of research in this nascent field of radiomycology; preliminary fungal isolates from beneath tank SX-108 were dismissed as contaminants (Fredrickson et al., 2004). We therefore screened 26 additional yeasts of the Microbial Culture Collection EX1. These EX yeast strains (EXF) and MD1149 were tested for their resistance to ionizing radiation (chronic and acute), heavy metal resistance, their pH minima and temperature maxima, and for their ability to form biofilms. From among the numerous CIR- and heavy metal-resistant yeasts identified, we judged MD1149 as the most suitable for bioremediation of acidic radioactive sites, therefore justifying its whole genome sequencing. We present a comparative analysis of MD1149 with three other Rhodotorula spp. Our analysis of the core metabolic and stress-resistance characteristics of MD1149, together with the identification of several yeasts capable of growth at low pH under high-level chronic γ-irradiation, strengthens the rationale for the important role of fungi in bioremediation of radioactive Cold War environmental waste sites.
All experimental work was performed under standard laboratory safety conditions, and all radiological, chemical, and biological safety precautions were observed following rules and regulations established for respective research institutions.
The ascomycetous and basidiomycetous yeasts used in this study and their isolation sites are presented in Table 1.
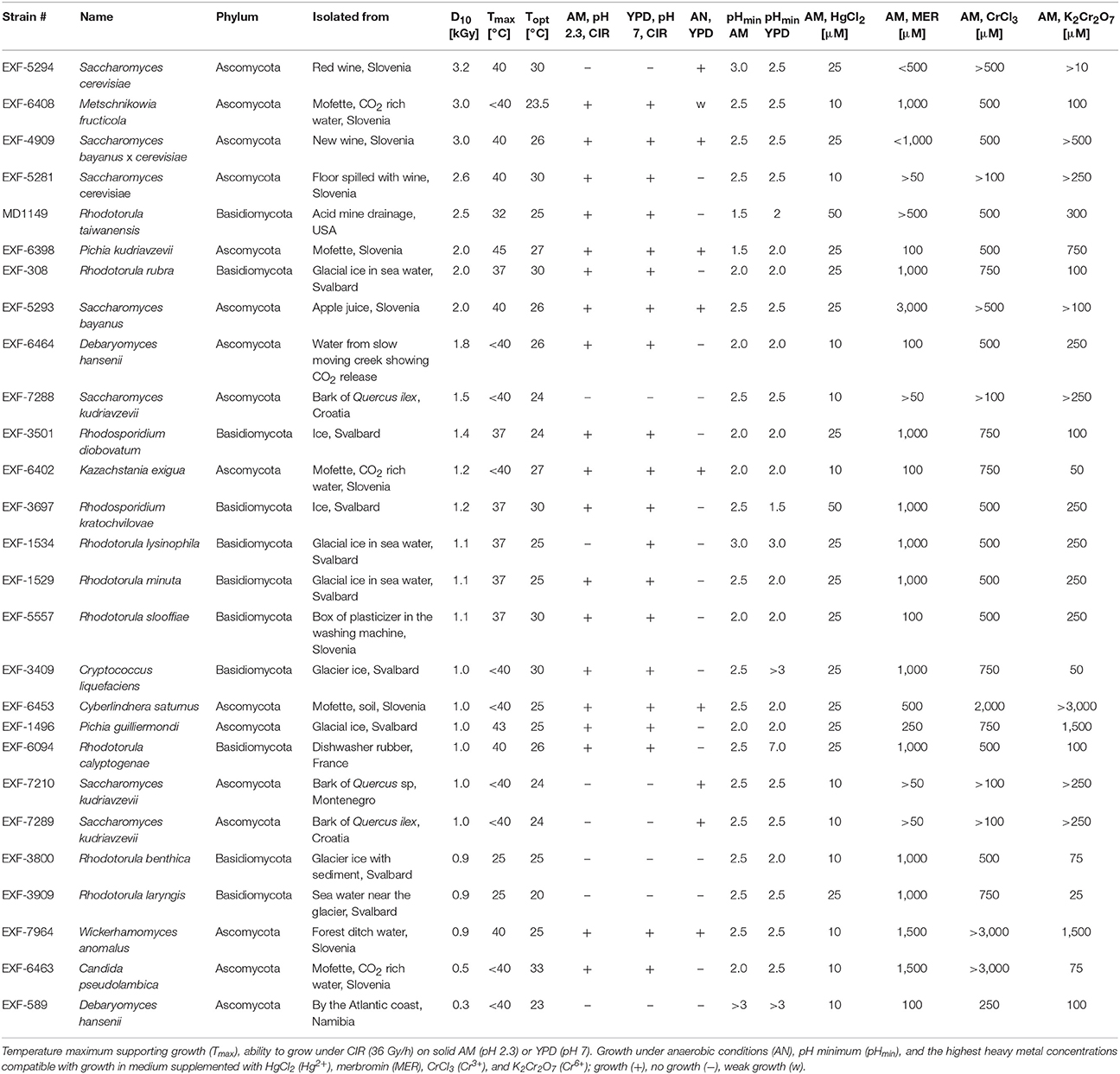
Table 1. Ranking of representative fungi by the survival index D10 together with other characteristics.
Sixty environmental samples were collected between 2001 and 2015 as a part of a larger study. These samples represent desert sands (Arizona, Nevada, New Mexico); dried plant debris from deserts (Arizona, Nevada, New Mexico); water, sediments, and soil from abandoned mines and mine drainages (coal mine in Maryland, silver and gold mines in Colorado, mercury mine in Idrija, Slovenia); hot springs (Colorado; Radenci, Slovenia); water and sediments from acidic river (Rio Tinto, Spain); and radioactive waste storage tanks (Uniformed Services University of the Health Sciences, Maryland). One gram of each environmental sample was resuspended in 10 ml of MQ purified water and allowed to settle for 30 min. One milliliter of the supernatant was added to 10 ml of the oligotrophic medium AM (complex Acidiphilium Medium) (San Martin-Uriz et al., 2014) adjusted to pH 2.3 with HNO3, and incubated in a shaker incubator (200 rpm) at 25°C for 4 days. One hundred microliters were then spread on AM plates (pH 2.3) and incubated at 25°C under 36 Gy/h. After 3 days of continuous CIR, the plates were inspected for growth. Single colonies were re-inoculated on fresh AM solid medium.
Throughout this work, CIR exposures specified under 36 Gy/h (~22°C) were performed in a 137Cs irradiator (GammaCell 40, J. L. Shepard and Associates). For all other CIR exposures, we used a second adjustable dose rate 137Cs irradiator (Mark 1 Model 68 A, J. L. Shepard and Associates), also at ~22°C. Acute exposures were performed in a 60Co irradiator (10 kGy/h) (J. L. Shepard and Associates) at 0°C.
The minimum pH and the highest Hg2+, merbromin, Cr6+, and Cr3+ concentrations supporting growth were determined in liquid AM and Yeast Extract-Peptone-Dextrose (YPD)2 medium. The overnight (O/N) cultures pre-grown at optimal temperatures were washed twice in sterile MQ and used to inoculate fresh liquid media adjusted for pH with HNO3, and/or supplemented with different concentrations of heavy metals to a final OD600 ~0.1. The strains were incubated in a shaker incubator, 200 rpm, at optimal temperatures. After inoculation, the OD600 was measured every 24 h for 1 week.
Maximum growth temperature and anaerobic growth were determined by observing colony formation on solid YPD medium incubated at various temperatures (25–65°C; temperature maxima); and for anaerobic growth, at a given strain's optimal temperature, in the presence or absence of atmospheric oxygen for 1 week.
Survival following acute forms of γ-radiation was determined on solid YPD medium by colony forming unit (CFU) assay as described previously (Daly et al., 2004). The ability of cells to grow under CIR on YPD pH 7.0 and AM pH 2.3 was monitored visually. The ability of a strain to form biofilms was tested in 96-well microtiter plates, as described by others (O'Toole, 2011), with eight replicate wells for every strain and each condition, and eight wells for blank controls. Pulsed-field gel electrophoresis (PFGE) with MD1149 genomic DNA was performed as described previously (Saracli et al., 2003).
The OD600 of an O/N culture of MD1149 in the Yeast Mold Broth (YM, Difco) was adjusted to 0.05 in modified Hommel's Minimal Salts (HMS; 0.3% (NH4)2SO4, 0.05% NaCl, 0.07% MgSO4, 0.04% Ca(NO3)2, 0.04% K2HPO4, 0.25% KH2PO4, 0.06% yeast extract, 5% glucose). At the indicated time (2, 4, 6, and 8 days), OD600 was assessed, and 10 ml of culture were centrifuged twice at 5,000 × g to obtain spent liquid medium (SLM).
Organic acids in SLM were identified and measured using a Waters Xevo G2-XS QTOF mass spectrometer (Waters Corporation, Milford, MA USA) coupled with a Waters Acquity H Class chromatography system. Organic acids were separated on a Waters Acquity UPLC HSS C18 1.8 μm 2.1 × 100 mm column using a modification of a previously published method (Fernández-Fernández et al., 2010). Mobile phases were methanol (solvent A) and water with 0.5% formic acid (solvent B). The separation method was as follows: initial, 90% B; 0.1 min, 90% B; 6 min, 70% B; 6.1 min, 90% B; 12 min, 90% B. The flow rate was 125 μl/min. The column compartment thermostat was set at 35°C, and the autosampler tray temperature was maintained at 4°C. Detection was accomplished by mass spectrometry with the electrospray ion source operating in negative ion, resolution mode. Data acquisition was performed using MassLynx Version 4.1 data acquisition software (Waters Corp.), with MSe data-independent centroid acquisition and leucine enkephalin lockmass correction.
Liquid chromatography mass spectrometry (LC-MS) and liquid chromatography tandem-mass spectrometry (LC-MS/MS) settings were as follows: Low Energy, 50–1000 Da, 6 V collision energy; High Energy, 50–1000 Da, collision energy ramp from 10–40 V; Scan time: 0.5 s; Source: 120°C; Desolvation, 450°C; Desolvation gas flow: 800 l/h; capillary voltage: 1.90 kV; Sampling cone voltage: 40 V. Experimental samples were compared with authentic standards (Sigma-Aldrich, St. Louis, MO) to identify organic acids present in cell culture media. Quantitation was performed with Waters TargetLynx software with quadratic calibration curve fitting.
MD1149 was first identified at the genus level based on micro- and macro morphology and assimilation test (YT MicroPlate™, BIOLOG Inc.), and then to the species level using genetic molecular identification (Mohamed et al., 2014).
Total DNA was isolated from MD1149 using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) and quantified by NanoDrop 2000 (Thermo Fisher Scientific).
The ITS1-5.8S rDNA-ITS2 and 18S rDNA sequences were matched to the GenBank non-redundant nucleotide database with the BLASTN algorithm (Altschul et al., 1990). MD1149 and related sequences were analyzed for similarity within the Geneious software package (Kearse et al., 2012) by using MUSCLE alignment (Edgar, 2004). The aligned sequences of representative strains were used to construct a phylogenetic tree with the PhyML 3.0 software (Guindon et al., 2010) with approximate likelihood-ratio test for branch supports, and with six substitution rate categories. The substitution model, alpha parameter of the gamma distribution and the proportion of invariable sites, was estimated by jModelTest 2.0 (Darriba et al., 2012).
The draft genome was generated using a combination of Illumina and 454 technologies. Two short-insert paired-end libraries, a fragment, 625-bp insert size (2 × 300 bp reads) and an overlapping fragment, 405-bp insert size (2 × 300 bp reads) were sequenced using version 3 chemistry on the MiSeq (Illumina, Inc., San Diego, CA, USA) (Bennett, 2004). Two large-insert paired-end libraries (8-kbp and 20-kbp insert size) were constructed and sequenced on the 454 GS FLX (Roche/454 Life Sciences, Branford, CT, USA) (Margulies et al., 2005). The draft data was assembled de novo with CLC Genomics Workbench v9.0 (QIAGEN Aarhus, Denmark). Repetitive sequences were identified using RepeatMasker (Smit et al., 2013–2015) and RepBase library (Jurka et al., 2005). The genome assembly completeness was evaluated with the Benchmarking Universal Single-Copy Orthologs (BUSCO 1.22) (Simão et al., 2015) software using the dataset for fungi.
For pairwise genome alignments, the following genomes were used: Rhodotorula sp. (Goordial et al., 2016), R. mucilaginosa (Deligios et al., 2015), R. glutinis (Paul et al., 2014), R. toruloides (Zhang et al., 2016), R. graminis (Firrincieli et al., 2015), Puccinia graminis (Duplessis et al., 2011). The genome alignments of contigs longer than 100 kbp were calculated with the PROmer algorithm, as implemented in MUMmer 3.23, and plotted with the MUMmerplot utility (Kurtz et al., 2004) as described by Hane et al. (2011).
MD1149 total RNA was isolated, then pooled and sequenced from cells grown under the following conditions: YPD (25°C, O/N and 3 days), YNB + 2% glucose (25°C, O/N and 3 days), YNB + 2% glucose (6°C and 37°C, O/N), YNB + 2% glycerol (25°C, O/N), AM pH 2.3 (25°C, O/N, 0 and 36 Gy/h), and AM pH 7 (25°C, O/N, 0 and 36 Gy/h). The RNA was isolated from MD1149 using RiboPure RNA Purification Kit for Yeasts (Thermo Fisher Scientific). RNA integrity was assessed by Fragment Analyzer (Advanced Analytical Technologies Inc., Ankeny, IA, USA). Sequencing libraries were prepared from 500 ng of total RNA input using the TruSeq Stranded mRNA Library Preparation Kit (Illumina) with barcoded adapters. Sequencing libraries yield and concentration were determined using the Illumina/Universal Library Quantification Kit (KAPA Biosystems, Wilmington, MA, USA) on the Light Cycler 480 (Roche Diagnostics Co., Indianapolis, IN, USA). Library size distribution was determined using the Fragment Analyzer™ (Advanced Analytical Technologies Inc.). Clustering and sequencing was performed on the NextSeq 500 (Illumina) with paired-end reads of 75 bp length.
RNAseq reads were quality trimmed with Sickle (Joshi and Fass, 2011) and aligned to the assembled genome with TopHat 2.1.1 (Kim et al., 2013). The alignment was then used for the transcriptome assembly with Trinity 2.2.0 (Haas et al., 2013) in Genome Guided mode with jaccard clipping and a maximum intron length of 1,500 bp. Protein-coding and tRNA genes were annotated using MAKER 2.31.8 (Campbell et al., 2014). The complete Swissprot database was used as evidence, along with the database of BUSCO, a set of Basidiomycete fungal proteomes and the sequenced transcriptome of MD1149. Three gene predictors were used in the MAKER pipeline: SNAP (Korf, 2004; Campbell et al., 2014), GeneMark-ET (Lomsadze et al., 2014), and Augustus (Stanke and Waack, 2003). This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession PJQD00000000. The version described in this paper is version PJQD01000000.
Predicted protein sequences from the MD1149 genome were processed through automated functional annotation using the PSAT metaserver (Leung et al., 2016), which ran EFICAz 2.5 (Kumar and Skolnick, 2012), blastp against the KEGG, MetaCyc, BRENDA, and STRING databases (Caspi et al., 2014; Chang et al., 2015; Szklarczyk et al., 2015; Kanehisa et al., 2016), and Interproscan (Jones et al., 2014). Additionally, protein sequences were processed through online servers running SignalP 4.0 (Petersen et al., 2011) and TMHMM 2.0 (Krogh et al., 2001). The pepstats utility (EMBOSS suite) was used to calculate protein properties (Rice et al., 2000).
GO annotations were also quantified and then projected into the full GO hierarchy using slim-o-matic (Courtot et al., 2016) and reviewed using Protege (Munsen, 2015). High-level categories were selected, into which the MD1149 GO annotations were mapped (slimmed). Categories corresponding to GO molecular function and biological process were quantified and expressed as percent of annotations across the MD1149 genome.
Predicted proteins from MD1149, R. graminis, R. sp. JG-1b, and R. toruloides were compared by all-against-all blastp at identity cutoff 95% and query coverage ≥95% using CGP3, and post-processed to identify fasta sequences unique or in common among the species and putatively duplicated within each species. Sequence logos of conserved nucleotide positions in all introns of median length were drawn using WebLogo 3 (Crooks et al., 2004).
In order to find a suitable candidate for bioremediation of acidic radioactive environmental waste sites, we first screened a variety of aquatic and terrestrial environments (desert sands, acid mine drainages, soils, and water samples) for strains that are both acid- and CIR-resistant. This strategy yielded only one strain, named MD1149, isolated from a sediment sample from an abandoned acid mine drainage facility in Maryland, USA (39°31′34.22″N, 79°1′12.16″W). MD1149 is a red-pigmented, unicellular, non-sporulating, ovoidal, obligately aerobic, budding yeast (Figure 1A, Table 1), which became pleomorphic under 36 Gy/h (Figure 1B). Phylogenetic analysis based on the internal transcribed spacer (ITS) and small subunit rRNA (SSU) sequences identified MD1149 as the basidiomycetous yeast R. taiwanensis [closely related to the type strain BCRC 23118(T) = CBS 11729(T)] (Figure 2), and confirmed by its micromorphological, macromorphological, and physiological characteristics (data not shown). Based on PFGE, the genome size of MD1149 was estimated to be >13 Mbp (Figure 1F). MD1149 was deposited with the Microbial Culture Collection EX as EXF-12971. Since other environmental samples screened did not yield additional acid- and CIR-resistant strains, we extended our study by including 26 distinct yeasts from the Microbial Culture Collection EX (Table 1).
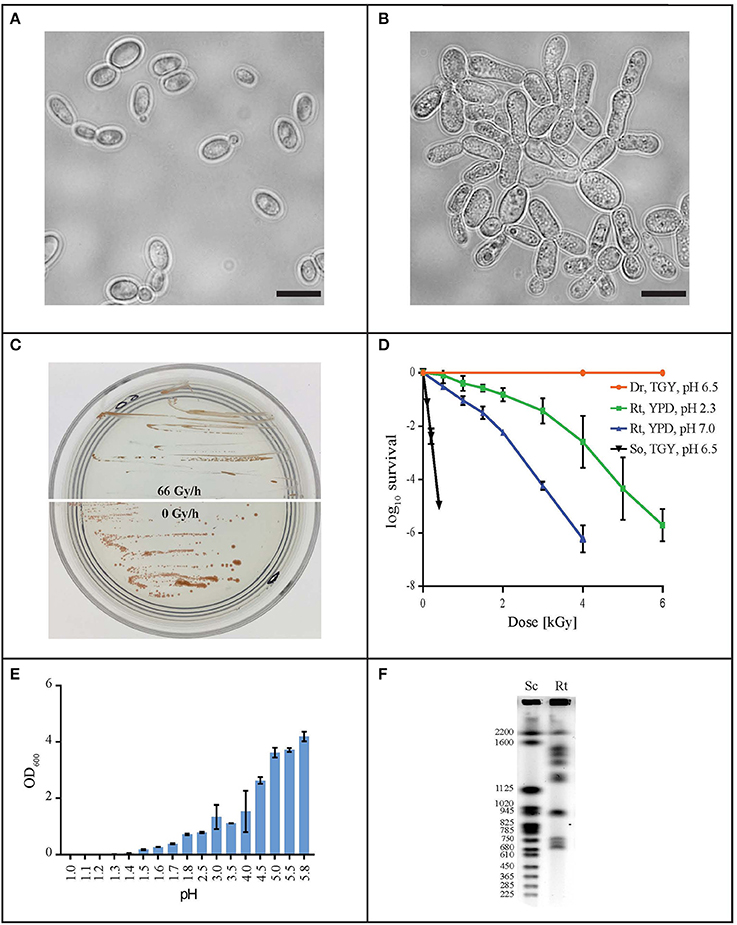
Figure 1. Characterization of R. taiwanensis MD1149. Light microscopy of liquid O/N culture of MD1149 grown (A) without CIR and (B) under 36 Gy/h (137Cs). Scale bars: 5 μm. (C) Growth of MD1149 on AM plates at pH 2.3 under 66 Gy/h, and without CIR. (D) Survival after acute gamma irradiation (60Co) of MD1149 (Rt) pre-grown in and recovered on YPD at pH 2.3 and 7.0. Model bacteria D. radiodurans (Dr) and Shewanella oneidensis (So) were pre-grown in and recovered on TGY, pH 6.5. (E) pH-dependent growth of MD1149 in YPD (pH adjusted with HNO3) after 48 h. (F) Chromosomal partitioning in MD1149 by PFGE (Rt) and in S. cerevisiae size ladder (Sc).
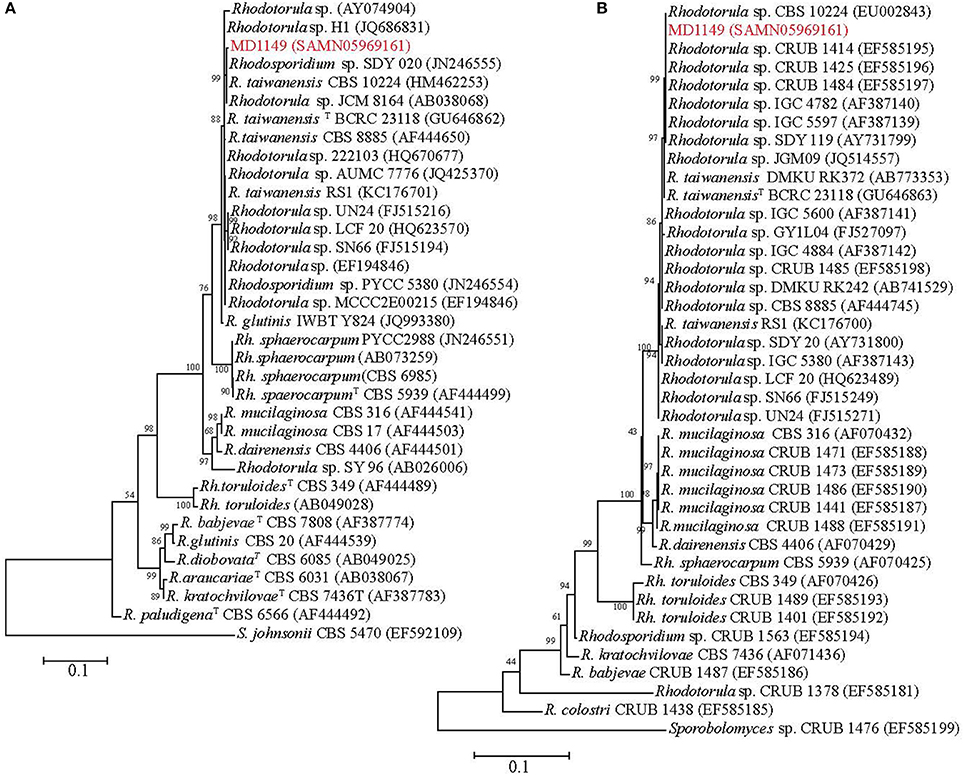
Figure 2. Phylogenetic analysis of R. taiwanensis MD1149 and related strains, and Sporobolomyces spp. as the root. The phylogenetic tree was constructed based on (A) ITS1-5.8S rDNA-ITS2 and (B) 18S rDNA sequences. GenBank accession number of each strain's sequence is in parentheses. TType strain.
Like most of the tested yeasts in this study, MD1149 was capable of growing luxuriantly at pH 2.3 and 7.0 under 36 Gy/h (Table 1). However, it was the only strain capable of growth under 66 Gy/h (Figure 1C).
Survival assays yield a radiation resistance metric named D10, which represents the acute radiation dose (Gy) giving 10% CFU survival (Daly et al., 2007, 2010; Sharma et al., 2017). Among tested strains, the most resistant was Saccharomyces cerevisiae EXF-5294 (D10, 3.2 kGy), and the most sensitive was Debaryomyces hansenii (D10, 0.3 kGy). The D10 of MD1149 is 2.5 kGy and ranks among the most radiation-resistant yeasts identified both for acute and chronic exposures (Table 1). Importantly, the radiation resistance of MD1149 increased with decreasing pH, from D10 0.8 kGy at pH 7.0 to D10 2.5 kGy at pH 2.3 (Figure 1D).
Optimal and maximum growth temperatures for the strains are reported in Table 1. Pichia kudriavzevii was the most thermotolerant and could grow at 45°C. The temperature maximum of MD1149, which optimally grows at 20–25°C, was 32°C. The most temperature-sensitive strains were R. benthica and R. larynges, which could grow at 25°C or below.
Over the course of 7 days, growth of strains in YPD and AM media adjusted to different pH values was montiored spectrophotometrically. Growth was considered as increasing when the OD600 rose above 0.1. The pH minima for growth of the yeasts are presented in Table 1. A full pH-dependent growth response curve for MD1149 is presented (Figure 1E). As shown in Table 1, the pH minima supporting growth of the yeast in rich (YPD) or oligotrophic (AM) media were very similar. Rhodotorula calyptogenae was the only strain that could not grow in YPD at low pH, whereas it grew well in AM at pH 2.5. Remarkably, growth responses of MD1149 and Pichia kudriavzevii in AM and Rhodosporidium kratochvilovae in YPD showed that their pH-minima approximate 1.5 (Figure 1E, Table 1).
The most common metal contaminants at DOE sites are U, Sr, Cs, Tc, Cr, Pb, and Hg. Among these, U, Tc, Hg, and Cr are significantly less mobile when reduced, and are capable of being immobilized by microorganisms (Daly, 2000). We tested yeasts for their resistance to Hg and Cr: mercury in the form of HgCl2 (Hg2+) and merbromin (organo-Hg), and chromium in the form of CrCl3 (Cr3+) and K2Cr2O7 (Cr6+). Table 1 summarizes heavy metal tolerances for strains grown in oligotrophic medium (AM) instead of YPD; YPD contains phosphates and myriad small organic molecules (e.g., peptides) that can mask metal toxicity (e.g., Mergeay, 1995). As expected, Hg2+ in HgCl2 is considerably more toxic than Cr3+ and Cr6+. The two strains most resistant to Hg2+, Cr3+, and Cr6+, were MD1149 and R. kratochvilovae (Figure 3 and Table 1), which could grow in AM supplemented with 50 μM HgCl2, and at significantly higher concentrations of Cr3+ and Cr6+. In contrast, most strains were resistant to millimolar concentrations of Hg when added as merbromin. Wickerhamomyces anomalus and Candida pseudolambica could grow in liquid medium supplemented with 3 mM Cr3+, whereas MD 1149 was resistant only to 0.5 mM Cr3+ (Table 1). The growth responses of MD1149 in AM supplemented with increasing concentrations of HgCl2 or K2Cr2O7 were distinct. Whereas increasing the concentration of Hg2+ increased the length of the lag-phase before the onset of exponential growth, increasing the concentration of Cr6+ slowed the growth of MD1149 (Figures 3A,B). Furthermore, in contrast to Cr6+/Cr3+, we showed that Hg2+ had a significant detrimental effect on MD1149 growth under CIR or not (Figures 3C,D).
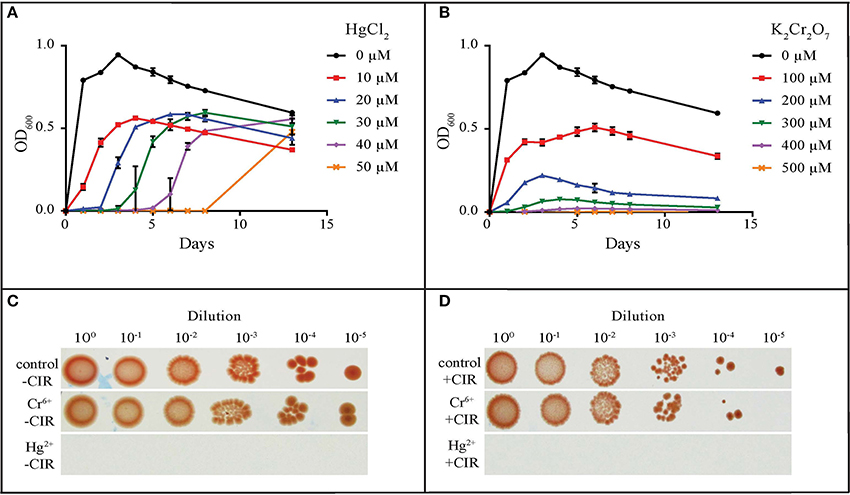
Figure 3. Resistance of R. taiwanensis MD1149 to HgCl2 and K2Cr2O7. Growth in liquid AM supplemented with (A) HgCl2 and (B) K2Cr2O7. (C) Growth of diluted cell suspension (OD600 ~0.9) on solid AM with no metals added (control), with 100 μM K2Cr2O7 (Cr6+), and with 30 μM HgCl2 (Hg2+), no CIR. (D) As for Panel (C), under 36 Gy/h (+CIR). For corresponding CrCl3 (Cr3+) results, see Table 1.
Biofilms are very important in bioremediation, since they offer sorption sites for many divalent cations that are toxic, and thus prevent their migration in the environment. The biofilm-forming capacity in yeasts was estimated with crystal violet assay (O'Toole, 2011) after 24 h incubation (in case of MD1149 it was additionally monitored over 5 days) at pH values 2–6, in the presence and absence of chronic gamma-radiation (36 Gy/h), and in oligotrophic (AM) and rich medium (YPD). This assay was performed on 8 parallels for each strain. The results are summarized in Figures 4, 5. The average absorbance of negative control (no inoculum) was subtracted from each measurement. Difference in A570 between the negative control and the sample above 0.2 was interpreted as an indication of biofilm formation.
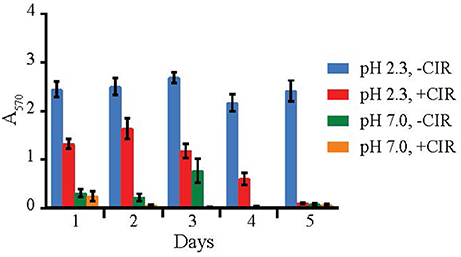
Figure 4. Biofilm formation by R. taiwanensis MD1149. Biofilm formation in YPD at pH 2.3 and 7.0 without CIR (-CIR) or under 36 Gy/h (+CIR) was quantified by crystal violet assay.
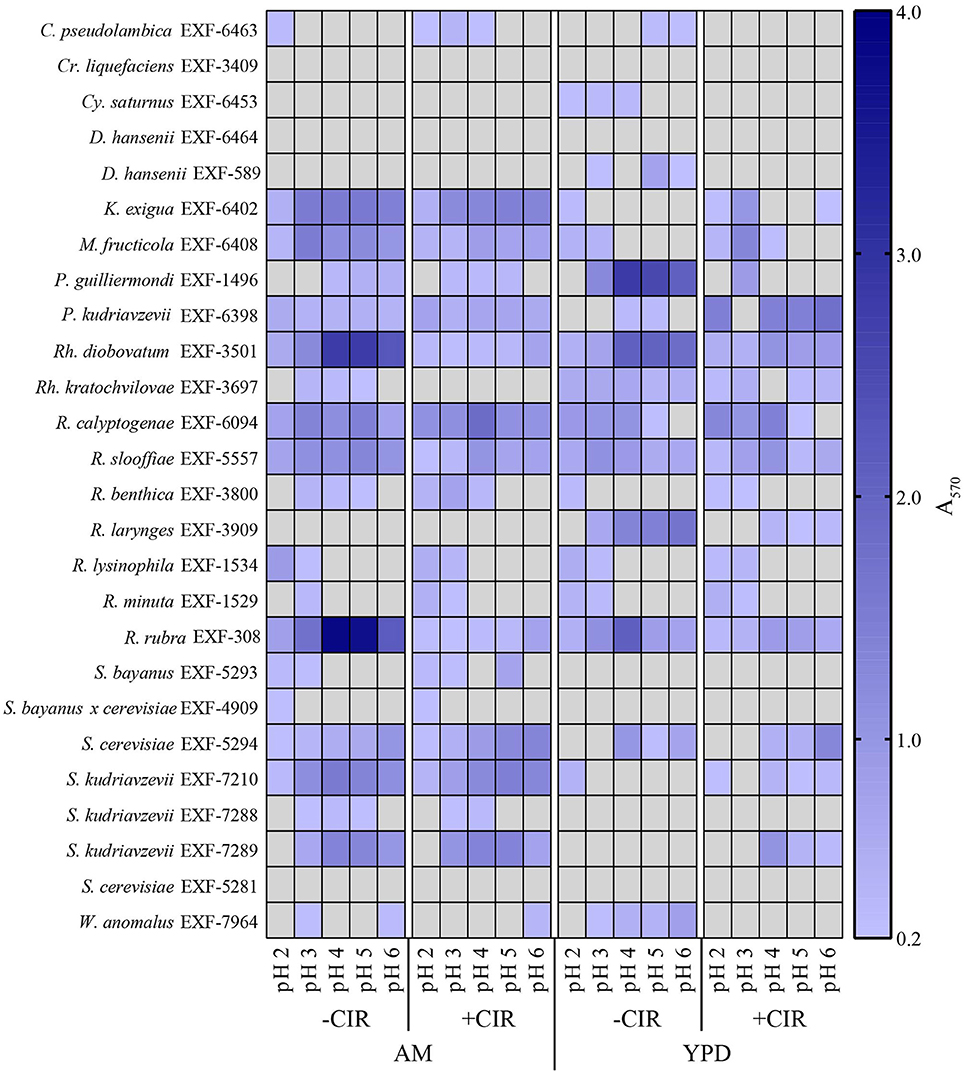
Figure 5. Heat map showing biofilm formation in yeasts. Growth in liquid AM and YPD at pH 2.0, 3.0, 4.0, 5.0, and 6.0. Without CIR and under 36 Gy/h. Biofilms were stained with crystal violet and quantified. Gray area indicates the absence of detectable biofilm, determined by threshold-spectrophotometry at A570 < 0.2.
Out of 27 strains, 3 strains were unable to form biofilms: Cryptococcus liquefaciens, Debariomyces hansenii, and S. cerevisiae EXF-5281. For the remaining strains, biofilm forming capacity was strongly dependent on the species and physical parameters. For most strains, biofilm formation was inhibited by CIR. However, under specified conditions, biofilm formation in 7 species (C. pseudolambica, M. fruticola, P. kudriavzevii, R. benthica, S. bayanus, S. cerevisiae, and S. kudriavzevii) was moderately enhanced under CIR, based on A570 values (Figure 5). In the absence of CIR, pH values below 4 stimulated biofilm formation in 4 yeasts, but inhibited biofilms in the remainder. As pH values decreased to 2.3, in the presence or absence of CIR, MD1149 increasingly formed dense biofilms (Figure 4).
While monitoring the growth of MD1149 in YM medium (Figure 6A), we noted unusually high OD600 values in stationary phase cultures compared to growth in YPD or AM. This was not due to high cell concentrations in YM, but instead was caused by secretion of metabolites that absorb at 600 nm, which accompanied the drop in pH from 6 to 2.0–2.5 (Figure 6A). These metabolites are now being further investigated.
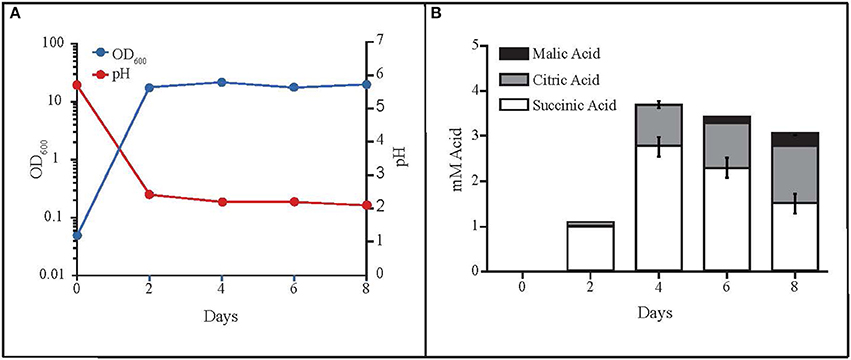
Figure 6. Production of organic acids by R. taiwanensis MD1149. (A) Growth curve of MD1149 and the pH of the medium over an 8-day time period. (B) Quantitation of three organic acids in the SLM (spent liquid medium) for which authentic standards were available (citric, malic, and succinic acids).
The rapid pH drop suggested that MD1149 produced significant quantities of organic acids, theoretically in excess of 10 mM depending on pKa values of the acids present. Therefore, we analyzed the SLM by LC-MS to identify any excreted organic acids. We detected the presence of at least six organic acids by LC-MS and LC-MS/MS as well as elemental composition prediction using Waters MassLynx 4.1 software. These acids included citric, homoaconitic, homocitric/homoisocitric (constitutional isomers), malic, rhodotorulic, and succinic. It is noteworthy that organic acids with available reference spectra (citric, malic, and succinic) matched the precursor and product ions from LC-ESI-QTOF (liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry) spectra published in MassBank (Horai et al., 2010). Furthermore, we procured standards for three of the six organic acids detected (citric, malic, and succinic) and quantitated their abundance (Figure 6B). The combined total concentration of these three acids was ~4 mM, consistent with the idea that these three acids contributed to the decrease in media pH, and that the other identified organic acids (which we were unable to characterize) contribute to the full pH change.
The estimated size of the genome assembly (without mitochondrial DNA) was 19.58 Mbp, and the final assembly of 181 scaffolds is based on 19.935 Gbp of draft sequence data, which provides 230× coverage of the genome. We identified a total of 26 scaffolds containing either 5′ or 3′ tandem DNA repeats with a sequence TTAGGG, which correspond to the most prevalent telomeric repeats (Teixeira and Gilson, 2005). Based on this result, we can conclude that the genome of MD1149 is organized in at least 13 chromosomes.
The size of the assembled mitochondrial genome was 38.20 kbp, slightly less than 40.39 kb reported by Zhao et al. (2013a). An alignment of both mitochondrial genomes showed that the sequences were largely syntenic (Figure 7D).
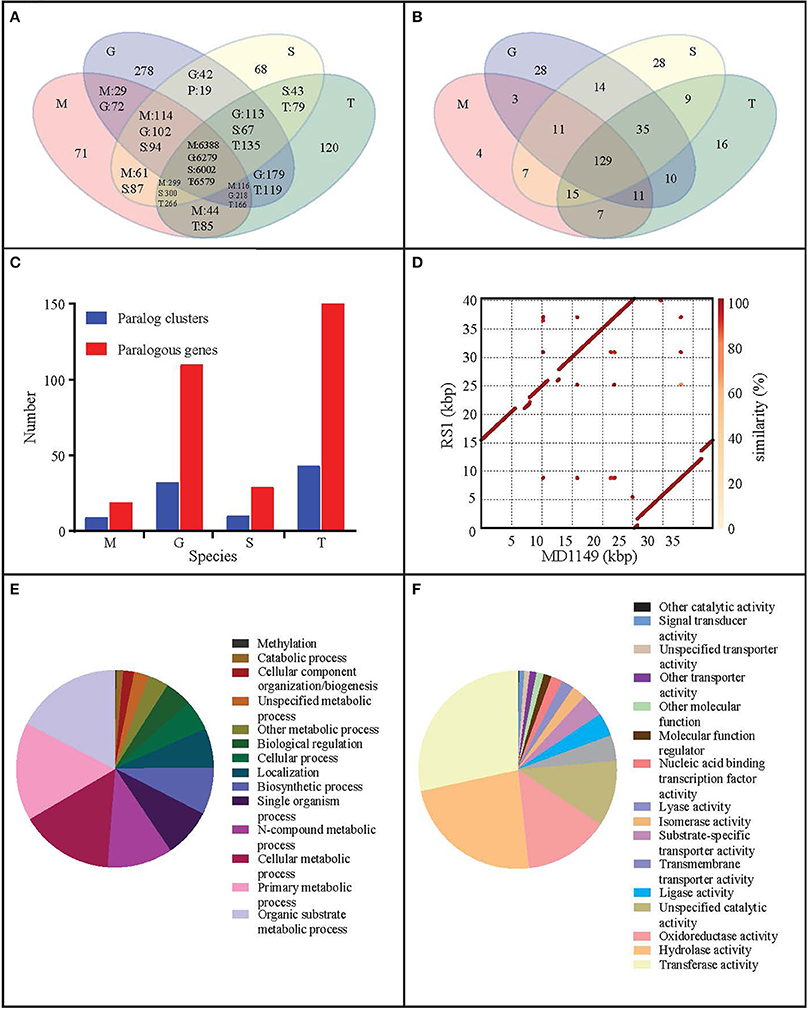
Figure 7. Genome analysis of R. taiwanensis MD1149. Venn diagram representation of (A) shared/unique genes and (B) OrthoMLC groups in R. taiwanensis MD1149 (M), R. graminis (G), R. sp. JG-1b (S), and R. toruloides (T). (C) Numbers of genes/clusters determined to occur in at least two copies. (D) Alignment of mitochondrial DNA of MD1149 and R. taiwanensis RS1 (GeneBank: HF558455.1). Percentage of mapped GO annotation translated proteins of MD1149 belonging to two yeast GO-slim functional categories: (E) biological process and (F) molecular function.
The content of GC pairs was 40.85% in the mitochondrial genome and 61.69% in the nuclear DNA. This finding is comparable to R. glutinis (61.87%) and R. mucilaginosa (60.54%), but lower than in R. graminis (67.76%), which has one of the most GC-rich genomes among available fungal genomes. The number of repetitive sequences was relatively low at 1.49%.
The number of genes annotated in the genome was 7,122. The genome completeness was estimated by searching the predicted proteome for 1,438 groups of BUSCO. We found 91% complete matches, 7% were fragmented and 2% were missing. More than 97% of MD1149 genes contained introns (Table 2), with an average of 6.2 exons and 5.2 introns per gene (Figure 8C, Table 2) (R. graminis: 6.2). The median length of introns was 69 bp (R. graminis: 101 bp), and they contained the typical 5′ and 3′ consensus sequences (Figures 8A,B). The median length of the exons was 151 bp (Figure 8D). The average length of the predicted proteins was 531, and their amino acid composition and isoelectric points were comparable to those of other Rhodotorula spp. (Figure 9).

Figure 8. Intron and exon statistics of R. taiwanensis MD1149. (A) The size distribution of introns. (B) The consensus sequence of all median-length introns. (C) The distribution of the number of exons per gene. (D) The size distribution of exons. The arrow indicates the median.
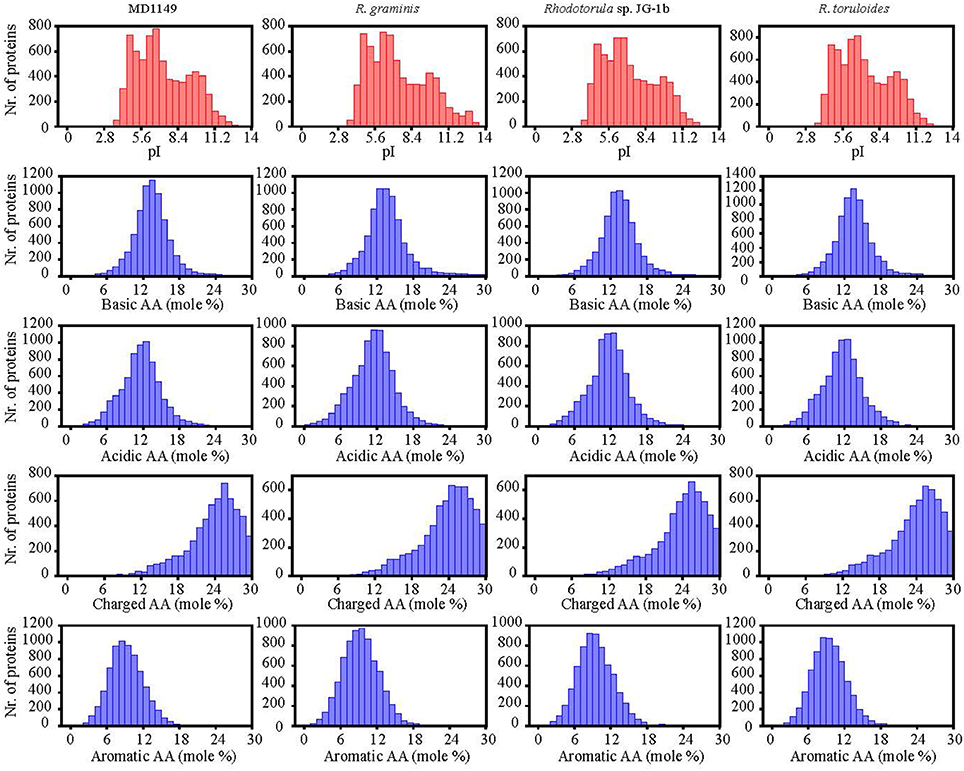
Figure 9. Isoelectric points and amino acid composition of predicted proteins of R. taiwanensis MD1149 and related species with sequenced genome.
When compared to related species, the distributions of gene families were similar. Only 71 predicted MD1149 proteins and 4 OrthoMLC groups were unique (Figures 7A,B). Although the number of duplicated genes in MD1149 was similar to that in Rhodotorula sp. JG-1b, it was much lower than in R. graminis and R. toruloides (Figure 7C). GO-slim analysis revealed the expected distribution of functional categories (Figures 7E,F) for MD1149 genes. To better understand the remarkable radiation resistance of MD1149, we further analyzed the genome for the presence of genes involved in homologous DNA recombination, non-homologous end joining, oxidative stress response, Mn homeostasis, heavy metal resistance, and hydrolases; results are presented (Table 3). The set of genes and their copy number are comparable to other fungi.
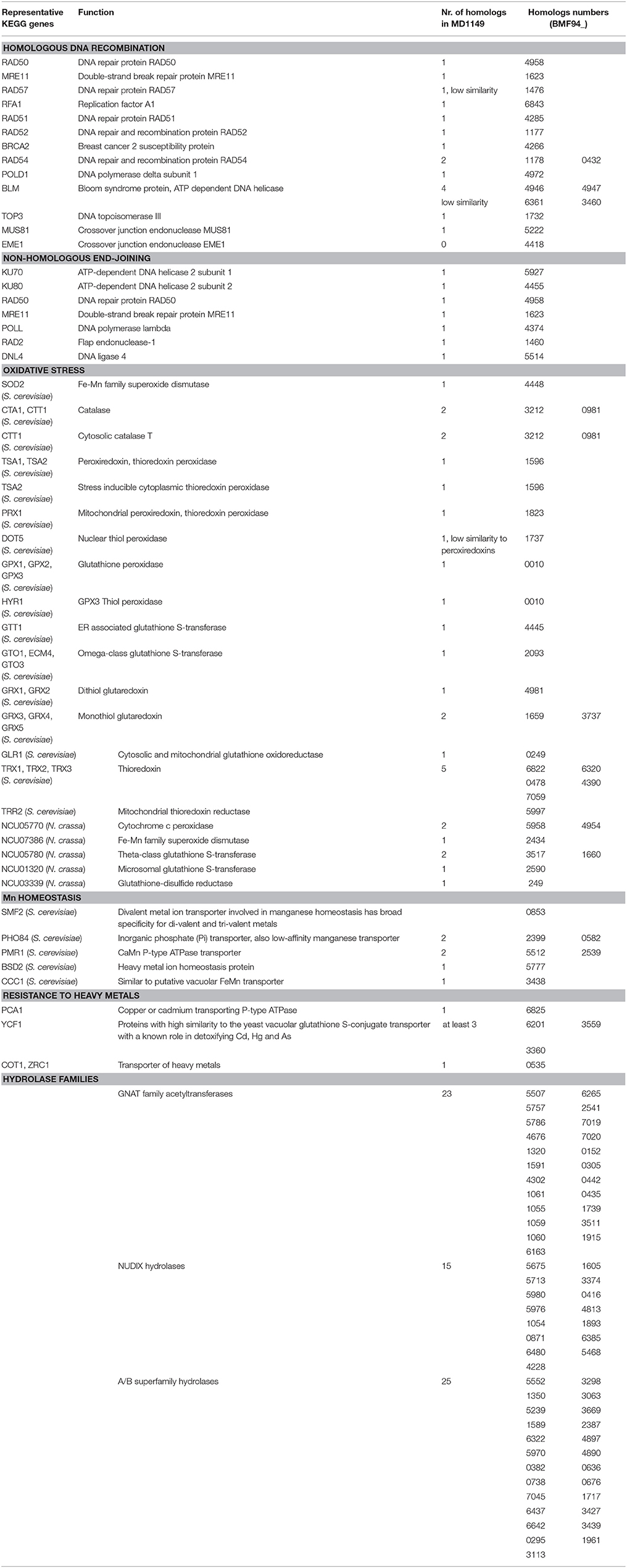
Table 3. R. taiwanensis MD1149 homologs of genes that are known from other fungi to be involved in DNA repair, oxidative stress, Mn homeostasis, resistance to heavy metals, and selected hydrolase genes.
The pairwise genome alignments showed a high level of macrosynteny between MD1149, R. mucilaginosa and Rhodotorula sp. JG-1b (Figure 10). With R. toruloides, R. glutinis, and R. graminis the order of the alignable regions was mixed, but the genomic rearrangements appear to have occurred within the same DNA molecules and not between them (Figure 10). This form of evolution is known as mesosynteny, and it was previously thought to be restricted only to filamentous ascomycetes (Hane et al., 2011).
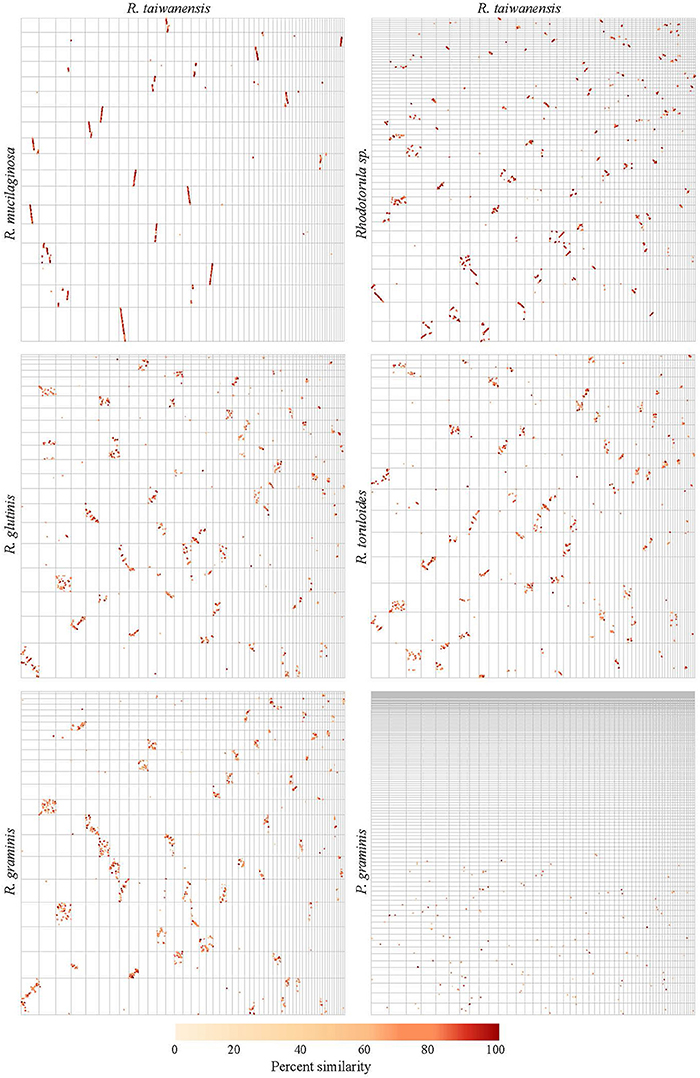
Figure 10. Pairwise genome alignments of R. taiwanensis MD1149 and related species. Contigs longer than 100 kbp from the genomes of MD1149 (x-axes) and related species (y-axes) were ordered by length and aligned with Mummer software.
The US Department of Energy (DOE) is the steward of the United States' nuclear waste legacy, comprised of immense volumes of long-lived radioactive environmental waste produced during the Cold War and stored at DOE sites. Over the last six decades, these radioactive wastes have been leaking into the environment, including mixtures of radionuclides, heavy metals and strong acids (e.g., HNO3) at levels (e.g., pH < 2.5) that exceed those tolerated by most microorganisms (Brim et al., 2000; Daly, 2000). Despite attempts to neutralize these acidic sites, low pH contamination zones persist, greatly diminishing the prospects for bioremediation at locations close to the originating leaks where the potential benefits are greatest, and where radiation levels are highest (Daly, 2000; Shelobolina et al., 2003).
We studied 16 ascomycetous and 11 basidiomycetous yeasts isolated from diverse environments including arctic ice, acid mine drainage, red wine, and apple juice, as well as dry environments with elevated temperatures (Table 1). Whereas many yeasts and filamentous fungi are reported to be resistant to various extreme environments, there are no reports of yeasts being resistant to high-level CIR. All 27 yeasts were able to survive an acute exposure to gamma-rays over the range 0.3–3.2 kGy: 8 extremely resistant yeasts displayed D10 values between 2.0 and 3.2 kGy; 14 yeasts were moderately resistant with D10 values between 1–2 kGy; and 5 yeasts were relatively sensitive, with D10 values as low as 300 Gy, but still more resistant than many bacteria (Daly, 2012). For comparison, the D10 of the soil bacterium Shewanella oneidensis is 70 Gy (Daly, 2012). Thus, this survey elevates yeast to the frontier of biology's most radiation-resistant representatives (Daly, 2012). In the context of bioremediation of DOE sites, CIR resistance is most relevant: 18/27 strains were able to grow under 36 Gy/h at pH 2.3, comparable to dose rates and pH values reported for sediments beneath Hanford tank SX-108 (Fredrickson et al., 2004). Surprisingly, among the surveyed yeasts, we show that chronic and acute radiation responses are not always aligned: S. cerevisiae strain EXF-5294 (D10, 3.2 kGy) did not grow under 36 Gy/h, and similarly for S. kudriavzevii EXF-7288 (D10, 1.5 kGy). A special focus is placed on R. taiwanensis MD1149, isolated from an acid mine drainage facility. MD1149 is capable of growth under 66 Gy/h at pH 2.3 (Figure 1C).
The concentration of contaminant heavy metals at DOE sediments can reach 10–30 μM (Fredrickson et al., 2004). Many microorganisms are reported to resist the toxic effects of metals by immobilizing and/or transforming those metals to less toxic chemical states (Brim et al., 2000; Fredrickson et al., 2000). Far fewer microorganisms are known to be able to transform metals at low pH, and there have been no published reports on any organism capable of transforming metals at low pH under high-level CIR. We ranked the 27 yeasts for their resistance to two heavy metals that predominate at DOE waste sites: 1. ionic Hg2+ in the form of HgCl2, and Hg as an organo-Hg compound merbromin; and 2. chromium in the form of CrCl3 (Cr3+) and K2Cr2O7 (Cr6+), as presented in Table 1. Redox-active heavy metals propagate ROS in cells and typically are more toxic than their covalently-bound counterparts. Consistently, we show Hg2+ and Cr6+ were the most toxic, followed by Cr3+, then merbromin. The ability of many of the tested yeasts to grow in the presence of 50–100 μM concentrations of Hg or Cr thus elevates these radiation-resistant simple eukaryotes to the forefront of metal resistances encountered in the natural world: 14 of the strains were able to grow in the presence of 25 μM HgCl2; 2 strains, MD1149 and R. kratochvilovae, grew in 50 μM HgCl2; and 14 strains grew in 1 mM merbromin (Table 1). Unlike Hg0 and Hg2+, redox-active Cr can cycle between several oxidation states between +2 to +6, with the most stable forms in the environment being hexavalent Cr6+ and trivalent Cr3+. These oxidation states have different chemical properties. For example, Cr3+ is relatively insoluble in the environment and is far less toxic than Cr6+, which is highly soluble and generates ROS in cells (Viti et al., 2014). Indeed, most yeasts were able to grow at concentrations of 500 μM merbromin or CrCl3; and three species, W. anomalus, Cyberlindnera saturnus and C. pseudolambica grew at even higher concentrations of these heavy metals (Table 1).
In bioremediation, biofilm formation is a highly desirable characteristic because the polysaccharide/protein extracellular matrix can bind/adsorb cations and reduce their migration in the environment. In the past few decades, most research on biofilms has focused on medically important bacteria and a few yeasts (Niemira and Solomon, 2005). Importantly, we report that biofilm formation in some yeasts is facilitated by chronic gamma radiation (Figure 5). In particular, MD1149 is capable of forming biofilms and growing in the presence of heavy metals under 36 Gy/h (Figures 3, 4). We also show that MD1149 produces abundant carboxylic acids (e.g., succinic acid) (Figure 6), similarly to Rhodotorula glutinis (Glass and Bhattacharjee, 1971), which is expected to facilitate metal transformation and metal accumulation in biofilms formed at low pH under CIR, but more evidence is needed.
The yeast we judged most suitable for bioremediation of acidic radioactive DOE waste sites was MD1149. To further develop this basidiomycete as a bioremediating platform, we subjected MD1149 to whole genome sequencing, then compared the genome to three other Rhodotorula species (R. graminis, Rhodotorula sp. JG-1b, R. toruloides). The complete sequence of the MD1149 genome is organized into at least 13 chromosomes (Figure 1F). The sequence-based features are summarized (Table 2), and when compared to the other Rhodotorula spp., the genome is unremarkable with respect to its size and GC content. Moreover, compared to other basidiomycetes the genome and the predicted proteome are relatively small (Mohanta and Bae, 2015). Viewed from the perspective of radiation resistance, the MD1149 genome and the predicted proteome exemplify characteristics found in many other sequenced species across the tree of life (Paul et al., 2014; Deligios et al., 2015; Goordial et al., 2016; Zhang et al., 2016; Matrosova et al., 2017), viz. The predicted DSB homologous recombination and non-homologous end-joining repair functions of MD1149, as well as its enzymatic antioxidant enzymes, are unremarkable (Table 3). Further, MD1149 encodes numerous genes commonly implicated in generating low molecular weight (LMW) metabolites (e.g., orthophosphate). They include acetyltransferases of the GNAT family, Nudix hydrolases, a/b superfamily hydrolases and calcineurin family phosphoesterases, which are present in many fungi (Zhao et al., 2013b). For most of these predicted hydrolases, and phosphatases in particular, their substrate specificities are either unknown or the affinity of known substrates is extremely low. It is likely that these predicted MD1149 enzymes, similar to D. radiodurans, participate in the degradation of nucleic acids, proteins and lipids (Makarova et al., 2001). The prediction of so many hydrolase functions in MD1149, which also encodes systems for Mn accumulation (Table 3), is expected to give rise to high intracellular concentrations of low molecular weight Mn2+ antioxidants. The hydrolase genes may therefore play a role in MD1149's extreme radiation resistance, yielding high intracellular concentrations of the organic and inorganic ligand-precursors of Mn2+ antioxidants that maintain proteome functionality under oxidative stress (Daly et al., 2007, 2010; Sharma et al., 2017).
Physicochemical cleanup technologies that could be used to decontaminate the immense volumes of soils, sediments and groundwaters at DOE facilities are prohibitively expensive and dangerous. Thus, the use of microorganisms to stabilize and/or detoxify such waste environments may be a viable alternative (Prakash et al., 2013). A bioremediation strategy based on the basidiomycete MD1149 and other yeasts (this study; Chandran and Das, 2012) now offers a more promising path to stabilization of DOE sites than Deinococcus spp., which are intolerant of low pH and heavy metals. Remarkably, MD1149 is highly resistant to Hg, Cr and CIR, capable of forming biofilms under 36 Gy/h at pH 2.3, and surviving acute doses of 2.5 kGy at pH 2.3. Importantly, it is reported that Rhodotorula spp. are genetically tractable (Takahashi et al., 2014), and we anticipate that MD1149 could be a good candidate for fungal-based CRISPR/Cas9 technologies (DiCarlo et al., 2013). Thus, the proposed use of MD1149 and other fungi for treatment of environments where radiation, low pH, and heavy metals are the principle factors limiting microbial survival and function appears to be a realistic approach given these early data.
RT, MD, and LD: designed research; RT, VM, OG, RV, PK, EG, CZ, BS, ML, SM, BR, JS, CD, TH, KF, and NG-C: performed research; RT, CG, SM, MC, and CZ: analyzed data; and RT, LD, and MD: wrote the paper.
The opinions expressed herein are those of the authors, and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD); or, the United States Army, Navy, or Air Force. This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by the U.S. Department of Energy, Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 and at the Uniformed Services University of the Health Sciences under Contract DE-NA0002322/0006. The authors would also like to acknowledge Ms. Constance L. Loucks and Mr. Jaron Hawkins (Maryland Department of the Environment) for collecting samples, Mr. Michael E. Woolbert (USUHS) for technical support and maintenance of irradiation facilities, Dr. Tine Grebenc (Slovenian Forestry Institute, Slovenia) and Mr. John W. Hobson for proofreading, The American Genome Center (Bethesda, MD, USA) for services in library preparation and sequencing, and the Defense Threat Reduction Agency (HDTRA-18774-M), the Slovenian Research Agency (BI-US/12-13-003, BI-US/14-15-009, Infrastructural Centre Mycosmo, MRIC UL), the National Human Genome Research Institute (Gene Ontology Consortium P41, grant 5U41HG002273-14), and EMBL-EBI (core funds) for financial support.
1. ^http://www.ex-genebank.com
2. ^http://www.bd.com/europe/regulatory/Assets/IFU/Difco_BBL/242820.pdf
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Brim, H., McFarlan, S. C., Fredrickson, J. K., Minton, K. W., Zhai, M., Wackett, L. P., et al. (2000). Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat. Biotechnol. 18, 85–90. doi: 10.1038/71986
Brim, H., Osborne, J. P., Kostandarithes, H. M., Fredrickson, J. K., Wackett, L. P., and Daly, M. J. (2006). Deinococcus radiodurans engineered for complete toluene degradation facilitates Cr(VI) reduction. Microbiology 152, 2469–2477. doi: 10.1099/mic.0.29009-0
Campbell, M. S., Holt, C., Moore, B., and Yandell, M. (2014). Genome annotation and curation using MAKER and MAKER-P. Curr. Protoc. Bioinformatics 48, 4.11.1–39. doi: 10.1002/0471250953.bi0411s48
Caspi, R., Altman, T., Billington, R., Dreher, K., Foerster, H., Fulcher, C. A., et al. (2014). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 42, D459–D471. doi: 10.1093/nar/gkt1103
Chandran, P., and Das, N. (2012). Role of plasmid in diesel oil degradation by yeast species isolated from petroleum hydrocarbon-contaminated soil. Environ. Technol. 33, 645–652. doi: 10.1080/09593330.2011.587024
Chang, A., Schomburg, I., Placzek, S., Jeske, L., Ulbrich, M., Xiao, M., et al. (2015). BRENDA in 2015: exciting developments in its 25th year of existence. Nucleic Acids Res. 43, D439–D446. doi: 10.1093/nar/gku1068
Courtot, M., Mitchell, A., Scheremetjew, M., Pi-ero, J., Furlong, L. I., Finn, R., et al. (2016). “Slim-o-matic: a semi-automated way to generate Gene Ontology slims,” in Proceedings of SWAT4LS 2016 (Amsterdam).
Crooks, G. E., Hon, G., Chandonia, J. M., and Brenner, S. E. (2004). WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190. doi: 10.1101/gr.849004
Culotta, V. C., and Daly, M. J. (2013). Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid. Redox Signal. 19, 933–944. doi: 10.1089/ars.2012.5093
Daly, M. J. (2000). Engineering radiation-resistant bacteria for environmental biotechnology. Curr. Opin. Biotechnol. 11, 280–285. doi: 10.1016/S0958-1669(00)00096-3
Daly, M. J. (2009). A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 7, 237–245. doi: 10.1038/nrmicro2073
Daly, M. J. (2012). Death by protein damage in irradiated cells. DNA Repair 11, 12–21. doi: 10.1016/j.dnarep.2011.10.024
Daly, M. J., Gaidamakova, E. K., Matrosova, V. Y., Kiang, J. G., Fukumoto, R., Lee, D. Y., et al. (2010). Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS ONE 5:e12570. doi: 10.1371/journal.pone.0012570
Daly, M. J., Gaidamakova, E. K., Matrosova, V. Y., Vasilenko, A., Zhai, M., Leapman, R. D., et al. (2007). Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 5:e92. doi: 10.1371/journal.pbio.0050092
Daly, M. J., Gaidamakova, E. K., Matrosova, V. Y., Vasilenko, A., Zhai, M., Venkateswaran, A., et al. (2004). Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306, 1025–1028. doi: 10.1126/science.1103185
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. doi: 10.1038/nmeth.2109
Deligios, M., Fraumene, C., Abbondio, M., Mannazzu, I., Tanca, A., Addis, M. F., et al. (2015). Draft genome sequence of rhodotorula mucilaginosa, an emergent opportunistic pathogen. Genome Announc. 3:e00201-15. doi: 10.1128/genomeA.00201-15
DiCarlo, J. E., Norville, J. E., Mali, P., Rios, X., Aach, J., and Church, G. M. (2013). Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41, 4336–4343. doi: 10.1093/nar/gkt135
Duplessis, S., Cuomo, C. A., Lin, Y. C., Aerts, A., Tisserant, E., Veneault-Fourrey, C., et al. (2011). Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc. Natl. Acad. Sci. U.S.A. 108, 9166–9171. doi: 10.1073/pnas.1019315108
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Ehrlich, H. L., and Newman, D. K. (2008). “Geomicrobiology of manganese,” in Geomicrobiology, 5th Edn., eds H. L. Ehrlich and D. K. Newman (Boca Raton, FL: CRC Press), 347–420.
Fernández-Fernández, R., López-Martínez, J. C., Romero-González, R., Martínez-Vidal, J. L., Alarcón Flores, M. I., and Garrido Frenich, A. (2010). Simple LC–MS Determination of Citric and Malic Acids in Fruits and Vegetables. Chromatographia. 72, 55–62. doi: 10.1365/s10337-010-1611-0
Firrincieli, A., Otillar, R., Salamov, A., Schmutz, J., Khan, Z., Redman, R. S., et al. (2015). Genome sequence of the plant growth promoting endophytic yeast Rhodotorula graminis WP1. Front. Microbiol. 6:978. doi: 10.3389/fmicb.2015.00978
Fredrickson, J. K., Kostandarithes, H. M., Li, S. W., Plymale, A. E., and Daly, M. J. (2000). Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl. Environ. Microbiol. 66, 2006–2011. doi: 10.1128/AEM.66.5.2006-2011.2000
Fredrickson, J. K., Zachara, J. M., Balkwill, D. L., Kennedy, D., Li, S. M., Kostandarithes, H. M., et al. (2004). Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the hanford site, Washington state. Appl. Environ. Microbiol. 70, 4230–4241. doi: 10.1128/AEM.70.7.4230-4241.2004
Gadd, G. M. (2007). Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 111, 3–49. doi: 10.1016/j.mycres.2006.12.001
Glass, J., and Bhattacharjee, J. K. (1971). Biosynthesis of lysine in Rhodotorula: accumulation of homocitric, homoaconitic, and homoisocitric acids in a leaky mutant. Genetics 67, 365–376.
Goordial, J., Raymond-Bouchard, I., Riley, R., Ronholm, J., Shapiro, N., Woyke, T., et al. (2016). Improved high-quality draft genome sequence of the Eurypsychrophile Rhodotorula sp. JG1b, isolated from permafrost in the hyperarid upper-elevation McMurdo dry Valleys, Antarctica. Genome Announc. 4:e00069-16. doi: 10.1128/genomeA.00069-16
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. doi: 10.1093/sysbio/syq010
Haas, B. J., Papanicolaou, A., Yassour, M., Grabherr, M., Blood, P. D., Bowden, J., et al. (2013). De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512. doi: 10.1038/nprot.2013.084
Hane, J. K., Rouxel, T., Howlett, B. J., Kema, G. H., Goodwin, S. B., and Oliver, R. P. (2011). A novel mode of chromosomal evolution peculiar to filamentous Ascomycete fungi. Genome Biol. 12:R45. doi: 10.1186/gb-2011-12-5-r45
Horai, H., Arita, M., Kanaya, S., Nihei, Y., Ikeda, T., Suwa, K., et al. (2010). MassBank: a public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 45, 703–714. doi: 10.1002/jms.1777
Jones, P., Binns, D., Chang, H. Y., Fraser, M., Li, W., McAnulla, C., et al. (2014). InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240. doi: 10.1093/bioinformatics/btu031
Joshi, N. A., and Fass, J. N. (2011). Sickle: A Sliding-Window, Adaptive, Quality-Based Trimming tool for FastQ files. Available online at: https://github.com/najoshi/sickle
Jurka, J., Kapitonov, V. V., Pavlicek, A., Klonowski, P., Kohany, O., and Walichiewicz, J. (2005). Repbase update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 110, 462–467. doi: 10.1159/000084979
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M., and Tanabe, M. (2016). KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462. doi: 10.1093/nar/gkv1070
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kim, D., Pertea, G., Trapnell, C., Pimentel, H., Kelley, R., and Salzberg, S. L. (2013). TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14:R36. doi: 10.1186/gb-2013-14-4-r36
Korf, I. (2004). Gene finding in novel genomes. BMC Bioinformatics 5:59. doi: 10.1186/1471-2105-5-59
Krogh, A., Larsson, B., von Heijne, G., and Sonnhammer, E. L. (2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580. doi: 10.1006/jmbi.2000.4315
Kumar, N., and Skolnick, J. (2012). EFICAz2.5: application of a high-precision enzyme function predictor to 396 proteomes. Bioinformatics 28, 2687–2688. doi: 10.1093/bioinformatics/bts510
Kurtz, S., Phillippy, A., Delcher, A. L., Smoot, M., Shumway, M., Antonescu, C., et al. (2004). Versatile and open software for comparing large genomes. Genome Biol. 5:R12. doi: 10.1186/gb-2004-5-2-r12
Leung, E., Huang, A., Cadag, E., Montana, A., Soliman, J. L., and Zhou, C. L. (2016). Protein Sequence Annotation Tool (PSAT): a centralized web-based meta-server for high-throughput sequence annotations. BMC Bioinformatics 17:43. doi: 10.1186/s12859-016-0887-y
Lomsadze, A., Burns, P. D., and Borodovsky, M. (2014). Integration of mapped RNA-Seq reads into automatic training of eukaryotic gene finding algorithm. Nucleic Acids Res. 42, e119. doi: 10.1093/nar/gku557
Makarova, K. S., Aravind, L., Wolf, Y. I., Tatusov, R. L., Minton, K. W., Koonin, E. V., et al. (2001). Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol. Mol. Biol. Rev. 65, 44–79. doi: 10.1128/MMBR.65.1.44-79.2001
Margulies, M., Egholm, M., Altman, W. E., Attiya, S., Bader, J. S., Bemben, L. A., et al. (2005). Genome sequencing in microfabricated high-density picolitre reactors. Nature 437, 376–380. doi: 10.1038/nature03959
McCullough, J., Hazen, T., and Benson, S. (1999). Bioremediation of Metals and Radionuclides: What It Is And How it Works. UC-Berkley, CA: Lawrence Berkeley National Laboratory.
Matrosova, V. Y., Gaidamakova, E. K., Makarova, K. S., Grichenko, O., Klimenkova, P., Volpe, R. P., et al. (2017). High-quality genome sequence of the radioresistant bacterium Deinococcus ficus KS 0460. Stand Genomic. Sci. 12:46. doi: 10.1186/s40793-017-0258-y
Mergeay, M. (1995). “Heavy metal resistances in microbial ecosystems,” in Molecular Microbial Ecology Manual, eds A. D. L. Akkermans, J. D. Van Elsas, and F. J. De Bruijn (Dordrecht: Springer), 439–455.
Mohamed, M. A., Abdelrazik, A. B., and Ibrahim, S. A.-A. (2014). Identification of different species of Rhodotorula using Internal Transcribed Spacers. Open Sci. Reposit. Agric. e45011822. doi: 10.7392/openaccess.45011822
Mohanta, T. K., and Bae, H. (2015). The diversity of fungal genome. Biol. Proced. Online 17:8. doi: 10.1186/s12575-015-0020-z
Munsen, M. A. (2015). The Protégé project: A look back and a look forward. AI Matters 1, 4–12. doi: 10.1145/2757001.2757003
Niemira, B. A., and Solomon, E. B. (2005). Sensitivity of planktonic and biofilm-associated Salmonella spp. to ionizing radiation. Appl. Environ. Microbiol. 71, 2732–2736. doi: 10.1128/AEM.71.5.2732-2736.2005
O'Toole, G. A. (2011). Microtiter dish biofilm formation assay. J. Vis. Exp. e2437. doi: 10.3791/2437
Paul, D., Magbanua, Z., Arick, M. II., French, T., Bridges, S. M., Burgess, S. C., et al. (2014). Genome sequence of the oleaginous yeast Rhodotorula glutinis ATCC 204091. Genome Announc. 2:e00046-14. doi: 10.1128/genomeA.00046-14
Petersen, T. N., Brunak, S., von Heijne, G., and Nielsen, H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 8, 785–786. doi: 10.1038/nmeth.1701
Prakash, D., Gabani, P., Chandel, A. K., Ronen, Z., and Singh, O. V. (2013). Bioremediation: a genuine technology to remediate radionuclides from the environment. Microb. Biotechnol. 6, 349–360. doi: 10.1111/1751-7915.12059
Rice, P., Longden, I., and Bleasby, A. (2000). EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16, 276–277. doi: 10.1016/S0168-9525(00)02024-2
San Martin-Uriz, P., Mirete, S., Alcolea, P. J., Gomez, M. J., Amils, R., and Gonzalez-Pastor, J. E. (2014). Nickel-resistance determinants in Acidiphilium sp. PM identified by genome-wide functional screening. PLoS ONE 9:e95041. doi: 10.1371/journal.pone.0095041
Saracli, M. A., Sener, K., Gonlum, A., Yildiran, S. T., and Wickes, B. L. (2003). Genotyping of clinical Rhodotorula mucilaginosa isolates by pulsed field gel electrophoresis. Mycoses 46, 487–491. doi: 10.1046/j.0933-7407.2003.00925.x
Sharma, A., Gaidamakova, E. K., Grichenko, O., Matrosova, V. Y., Hoeke, V., Klimenkova, P., et al. (2017). Across the tree of life, radiation resistance is governed by antioxidant Mn2+, gauged by paramagnetic resonance. Proc. Natl. Acad. Sci. U.S.A. 114, E9253–E9260. doi: 10.1073/pnas.1713608114
Shelobolina, E. S., O'Neill, K., Finneran, K. T., Hayes, L. A., and Lovley, D. R. (2003). Potential for in situ bioremediation of a Low-pH, High-Nitrate Uranium-contaminated groundwater. Soil Sediment Contaminat. Int. J. 12, 865–884. doi: 10.1080/10588330390254928
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V., and Zdobnov, E. M. (2015). BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212. doi: 10.1093/bioinformatics/btv351
Smit, A. F. A., Hubley, R., and Green, P. (2013–2015). RepeatMasker Open-4.0. Available online at: http://www.repeatmasker.org/
Stanke, M., and Waack, S. (2003). Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 19(Suppl. 2), ii215–ii225. doi: 10.1093/bioinformatics/btg1080
Szklarczyk, D., Franceschini, A., Wyder, S., Forslund, K., Heller, D., Huerta-Cepas, J., et al. (2015). STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452. doi: 10.1093/nar/gku1003
Takahashi, S., Okada, H., Abe, K., and Kera, Y. (2014). Genetic transformation of the yeast Rhodotorula gracilis ATCC 26217 by electroporation. Appl. Biochem. Microbiol. 50, 624–628. doi: 10.1134/S0003683814110040
Teixeira, M. T., and Gilson, E. (2005). Telomere maintenance, function and evolution: the yeast paradigm. Chromosome Res. 13, 535–548. doi: 10.1007/s10577-005-0999-0
Viti, C., Marchi, E., Decorosi, F., and Giovannetti, L. (2014). Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiol. Rev. 38, 633–659. doi: 10.1111/1574-6976.12051
Zhang, S., Skerker, J. M., Rutter, C. D., Maurer, M. J., Arkin, A. P., and Rao, C. V. (2016). Engineering Rhodosporidium toruloides for increased lipid production. Biotechnol. Bioeng. 113, 1056–1066. doi: 10.1002/bit.25864
Zhao, X. Q., Aizawa, T., Schneider, J., Wang, C., Shen, R. F., and Sunairi, M. (2013a). Complete mitochondrial genome of the aluminum-tolerant fungus Rhodotorula taiwanensis RS1 and comparative analysis of Basidiomycota mitochondrial genomes. Microbiologyopen 2, 308–317. doi: 10.1002/mbo3.74
Keywords: bioremediation, yeasts, radiation resistance, heavy metal resistance, pH minimum, temperature maximum, Rhodotorula taiwanensis, genome
Citation: Tkavc R, Matrosova VY, Grichenko OE, Gostinčar C, Volpe RP, Klimenkova P, Gaidamakova EK, Zhou CE, Stewart BJ, Lyman MG, Malfatti SA, Rubinfeld B, Courtot M, Singh J, Dalgard CL, Hamilton T, Frey KG, Gunde-Cimerman N, Dugan L and Daly MJ (2018) Prospects for Fungal Bioremediation of Acidic Radioactive Waste Sites: Characterization and Genome Sequence of Rhodotorula taiwanensis MD1149. Front. Microbiol. 8:2528. doi: 10.3389/fmicb.2017.02528
Received: 23 August 2017; Accepted: 05 December 2017;
Published: 08 January 2018.
Edited by:
Haluk Beyenal, Washington State University, United StatesReviewed by:
Santosh Kr Karn, Sardar Bhagwan Singh Post Graduate Institute of Biomedical Science & Research, Dehradun, IndiaCopyright © 2018 Tkavc, Matrosova, Grichenko, Gostinčar, Volpe, Klimenkova, Gaidamakova, Zhou, Stewart, Lyman, Malfatti, Rubinfeld, Courtot, Singh, Dalgard, Hamilton, Frey, Gunde-Cimerman, Dugan and Daly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. Daly, bWljaGFlbC5kYWx5QHVzdWhzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.