- College of Forestry, Beijing Forestry University, Beijing, China
The fungus Colletotrichum gloeosporiodes infects plant hosts with a specialized cell called an appressorium, which is melanized and required for plant cell wall penetration. Here, we show that the mitogen-activated protein kinase CgMK1 governs appressorium formation and virulence in the poplar anthracnose fungus C. gloeosporioides. Deletion of CgMK1 impairs aerial hyphal growth and biomass accumulation, and CgMK1 is responsible for the expression of melanin biosynthesis-associated genes. CgMK1 deletion mutants are unable to form appressorium and lose the capacity to colonize either wounded or unwounded poplar leaves, leading to loss of virulence. We demonstrate that the exogenous application of cAMP fails to restore defective appressorium formation in the CgMK1 deletion mutants, suggesting that CgMK1 may function downstream or independent of a cAMP-dependent signal for appressorium formation. Moreover, CgMK1 mutants were sensitive to high osmosis, indicating that CgMK1 plays an important role in stress response. We conclude that CgMK1 plays a vital role in regulating appressorium formation, melanin biosynthesis, and virulence in C. gloeosporiodes.
Introduction
Various genes in phytopathogenic fungi are involved in certain signaling pathways associated with the recognition of chemical substances from the host and in response to other extracellular stimuli. The mitogen-activated protein kinase (MAPK) signaling pathway is a downstream cellular signaling event that is remarkably conserved and mainly consists of MAPK cascades, including three-tiered protein kinase modules by which phosphorylation sites of tyrosine and threonine deliver signals, ultimately activating the downstream components of the pathway (Marshall, 1994; Seger and Krebs, 1995; Hamel et al., 2012). MAPK signaling pathways are divided into five categories that are classified according to the different functions of Saccharomyces cerevisiae, including conditioning the response of mating pheromones, controlling homeostasis under high osmolarity stress, and maintaining cell wall integrity (Herskowitz, 1995). The interplay of these signaling pathways in certain external stress conditions has previously been illustrated (Baltanás et al., 2013). Among the five types of MAPK signaling pathways, the functions of a Fus3/Kss1-related gene in phytopathogenic fungi include, in addition to filamentous growth, a vital role in virulence and in host surface colonization and the formation of infection structures, as well as penetration and development into the cell tissues of the host.
Fus3/Kss1 orthologs are denominated as “pathogenic MAPKs,” which are vital genes that are significant for infection structure differentiation and various processes in pathogenic fungi. Increasing evidence has indicated that the sensor genes orchestrate virulence via Fus3/Kss1 orthologs, such as SHO1 and MSB2 (Leroch et al., 2015). Various components that converge downstream of fus3/Kss1 have also been identified, such as STE12 (Park et al., 2002) and SLF1 (Li et al., 2011). Some genes associated with other signaling pathways are also implicated in fus3/kss1-dependent patterns such as the inactivation of the TOR pathway (Marroquin-Guzman and Wilson, 2015), as well as the thioredoxin-associated gene, TRX2 (Wang et al., 2016). Previous studies have shown that the activation of fus3/kss1-associated genes is necessary for the development of a unique infection structure for endangering hosts in phytopathogenic fungi. For instance, in Magnaporthe oryzae, deletion of PMK1 (Fus3/Kss1 ortholog) disrupts the capacity of appressoria differentiation, thereby resulting in non-pathogenicity (Xu and Hamer, 1996). Additionally, strains lacking PMK1 are also prevented from attacking the host via wounded tissues, suggesting that PMK1 is vital for various invasive stages of fungal attack toward the host. Subsequently, ortholog analysis has demonstrated that Fus3/Kss1 is essential for pathogenicity in phytopathogens, not only in appressorium-forming fungi, such as CMK1 in Colletotrichum orbiculare (Takano et al., 2000) and ChMK1 in C. higginsianum (Wei et al., 2016), but also in non-classical appressorium-forming fungi such as VMK1 in Verticillium dahliae (Rauyaree et al., 2005) and CsFUS3 in Bipolaris sorokiniana (Leng and Zhong, 2015). Indeed, these MAPKs have been verified to be closely associated with the infection morphology and pathogenicity of fungi.
Anthracnose, a typical poplar foliage disease generated by the hemibiotrophic ascomycete fungus Colletotrichum gloeosporioides, is highly prevalent in northeast China and results in substantial economic losses. During pathogenesis process, an infectious structure characterized by the appearance of dome-like structures called forming at the hyphal apex (Kleemann et al., 2008). Previous studies have explored the significance of MAPK components and the cAMP/PKA pathway in appressorium formation in C. gloeosporioides. The functions of certain MAPK modules have been reported, such as the deletion of Cgl-SLT2 results in deficient sporulation, disruption of appressorium formation, and a reduction in pathogenicity in mango (Yong et al., 2013). CgMEK1 deletion mutants exhibit defective in appressoria development and the loss of virulence (Kim et al., 2000). Moreover, CgPKAC, a cAMP pathway-related protein kinase in C. gloeosporioides, is an imperative gene in virulence and appressorium formation (Priyatno et al., 2012). Together, these findings could facilitate the elucidation of the molecular mechanism underlying appressorium formation. However, details investigations on the signaling cascades in C. gloeosporioides are not well-studied to date.
We therefore aimed to explore the multiple functions of CgMK1. Here, we demonstrated that CgMK1 (an ortholog of Fus3/Kss1) in C. gloeosporioides plays an important role in appressorium formation and pathogenesis. The results also indicated that CgMK1 affects aerial hyphal growth and governs the expression of melanin production-related genes. Furthermore, based on the exogenous application of cAMP, we reveal that CgMK1 may function downstream or independent of a cAMP-dependent signal in appressorium formation.
Materials and Methods
Strains and Growth Tests
The wild-type of C. gloeosporioides is CFCC80308, isolated from Populus × beijingensis. The wild-type and its derivatives were cultured in complete medium (CM) or on potato dextrose agar (PDA) plates at 25°C with day/night cycles as previously described (Xu et al., 2016). To assay for defects in stress responses, the size and morphology of the colonies were measured with cultures grown on CM with 1.2 M NaCl, 1 M sorbitol, Congo Red (100 μg/ml), or Calcofluor (60 μg/ml). Statistical analyses of all data were performed by using the Duncan’s range test of SPSS20.0. The P-value < 0.05 was considered as statistically significant. All assays were performed in triplicate, in three independent experiments.
Bioinformatics Analysis of CgMK1
The sequence of CgMK1 was retrieved from the C. gloeosporioides genome database1. A BLASTp search on the National Center for Biotechnology Information (NCBI) website was used to find all annotated Fus3 genes from other fungal genomes. The InterPro2 was used to predict domain of CgMK1. Amino-acid sequences of CgMK1 and Fus3/Kss1 homologs from other fungi were aligned by the ClustalX 2.1. For homology analysis, we constructed a phylogenetic tree in MEGA 7.0 with 1000 bootstrap replicates and the neighbor-joining method as previously described (Sun et al., 2016).
Targeted Gene Knockout and Complementation
The CgMK1 gene replacement constructs were established using the split-marker gene replacement strategy. To construct the upstream (∼1.2 kb) and downstream (∼1.4 kb) flanking sequences of CgMK1, the primers CgMK1-5Ffor, CgMK1-5Frev, CgMK1-3Ffor, and CgMK1-3Frev were used for the 5′-flanking sequence and the 3′-flanking sequence, respectively. The next step required fusing the fragments and two-thirds of the hygromycin cassette by overlapping PCR using the primers CgMK1-5Ffor/HY-R and YG-F/CgMK1-3Frev, respectively. The CgMK1 gene replacement and transformation methods were performed as described in a previous study (Xu et al., 2016). The PCR assays were used to confirm whether the CgMK1 gene had been replaced using the primers External-CgMK1for/External-CgMK1rev and RT-CgMK1for/RT-CgMK1rev. To analyze homologous recombination events in the transformants, southern blotting was performed to confirm the deletion of CgMK1 with the DIG High PrimeDNALabeling and Detection Starter Kit I in conformity to the manufacturers’ protocol (Roche, Germany). ScaI was used to digest the genomic DNA of wild-type and transformants. The probe amplified with primers ProbeHPHfor, ProbeHPHrev from HPH and the primers ProbeCgMK1for, ProbeCgMK1rev from the C. gloeosporioides CFCC80308 and labeled with DIG primer (Table 1).
The fragment for complementing the CgMK1 deletion strains, which contains the entire CgMK1 coding region, its native promoter, and terminator regions, was PCR-amplified using primers CgMK1-Compfor/CgMK1-Comprev, and inserted into a modified pBC-phleo vector conferring phleomycin resistance (provided by FGSC). After harvesting the transformants, we selected the strain from the TB3 medium (with 100 μg/mL phleomycin) and confirmed the results using RT-PCR.
Generation of the CgMK1/CgMK1::eGFP Fusion Construct
To explore the subcellular location of CgMK1, the sequence was amplified from the wild-type strain using the primers CgMK1-5Ffor/promoter + CgMK1rev, CgMK1-5Ffor/promoter + CgMK1 + eGFPrev and a pKD6-GFP plasmid. The resulting DNA product was then transferred into the protoplasts of the ΔCgMK1-13 mutant. Upon harvesting the transformants, we selected the strain from TB3 medium (with 100 μg/mL phleomycin), examined the eGFP signals, and confirmed using qPCR. These tests were conducted as previously described (Xu et al., 2016).
Quantitative RT-PCR Assays
To obtain whole RNA of the vegetative mycelia, TRIzol reagent (Invitrogen) was used to isolate RNA from the conidia of the wild-type and transformants. The RNA was purified with the PureLink RNA Mini Kit (Ambion). The RNA products were incubated with DNase I (TaKaRa) to reduce DNA contamination. For the quantitative real-time PCR (qRT-PCR) assays, Oligo-DT and SuperScript III reverse transcriptase (Invitrogen) were used to transcribe the mRNA into cDNA. The qRT-PCR reactions were conducted to analyze the transcript levels. The Cg18S gene served as an internal reference in this study. The 2-ΔΔCT method was used to analyze gene expression levels of the genes (Livak and Schmittgen, 2001). These tests were carried out as previously described (Xu et al., 2016). Quantitative real-time PCR was performed in triplicate with three independent biological experiments. All primers used in this study are listed in Table 1.
Conidiation and Appressorium Formation Tests
For the conidiation assays, we selected 5 mm diameter mycelial plugs from the edge of a 3-days-old colony that had been placed onto PDA medium and grown at 25°C for 7 days. Conidial suspensions from each strain were selected from the PDA plates after applying 5 mL of sterile water. Conidial suspensions were placed at 25°C for 12 h on a GelBond membrane to allow germination and growth. The suspensions were monitored, and the colony-forming efficiency was assessed under the microscope.
Equal numbers of spores were placed on the hydrophobic surface of the GelBond film (Lonza) at 28°C for 12 h to examine appressoria formation. The appressoria from germinated spores were counted. At least 200 conidia were surveyed in experiments. The rates of conidiation and appressorium formation assays were performed in triplicate, in three independent experiments.
Pathogenicity Assays
To test the virulence, approximately 3-week-old detached leaves of Populus × beijingensis were incubated with 20 μL conidium suspension (105 conidia/mL) at 25°C. Sufficient moisture was maintained, and the morphology and size of the lesions were observed and measured at 4–5 dpi. For the wound-inoculation assays, the conidia of the strain were placed on the wounded sites of the poplar leaves, and the morphology and size of the lesions were observed and measured at 2–3 dpi. At least three virulence experiments were performed, with seven replicates each.
Results
Deletion of CgMK1 Impairs Aerial Hyphae Growth
We explored a gene in the C. gloeosporioides genome database (JGI) by BLASTp search that displayed high identity with PMK1 of M. oryzae, thus designated as CgMK1. CgMK1 contained the protein kinase domain with a threonine and tyrosine phosphorylation site, and displayed high identity with Fus3/Kss1 from C. orbiculare (99.4%), V. dahliae (98.0%), Botrytis cinerea (94.6%), and Fusarium graminearum (98.6%) (Supplementary Figure S1A). Furthermore, phylogenetic analysis demonstrated that CgMK1 is homologous to Fus3/Kss1 MAPKs of other fungi and the Fus3/Kss1 is highly conserved among fungi (Supplementary Figure S1B).
To investigate the role of CgMK1 in C. gloeosporioides, CgMK1 deletion mutants (ΔCgMK1-8, ΔCgMK1-13) were obtained by replacing the wild-type CgMK1 gene with a hygromycin cassette (Supplementary Figure S2). For mutant complementation, we reintroduced the wild-type CgMK1 into the ΔCgMK1-13 mutant to produce the complementation strain (ΔCgMK1/CgMK1; Supplementary Figure S2E).
While the ΔCgMK1 mutants showed no obvious defects of growth rate on potato dextrose agar (PDA), aerial hyphal growth was remarkably reduced (Figures 1A,B). Likewise, radial growth of the ΔCgMK1 mutants on solid media showed no difference to the wild-type, but biomass production in the liquid was apparently reduced (Figures 1C,D). These results imply that CgMK1 is essential to aerial hyphal growth.
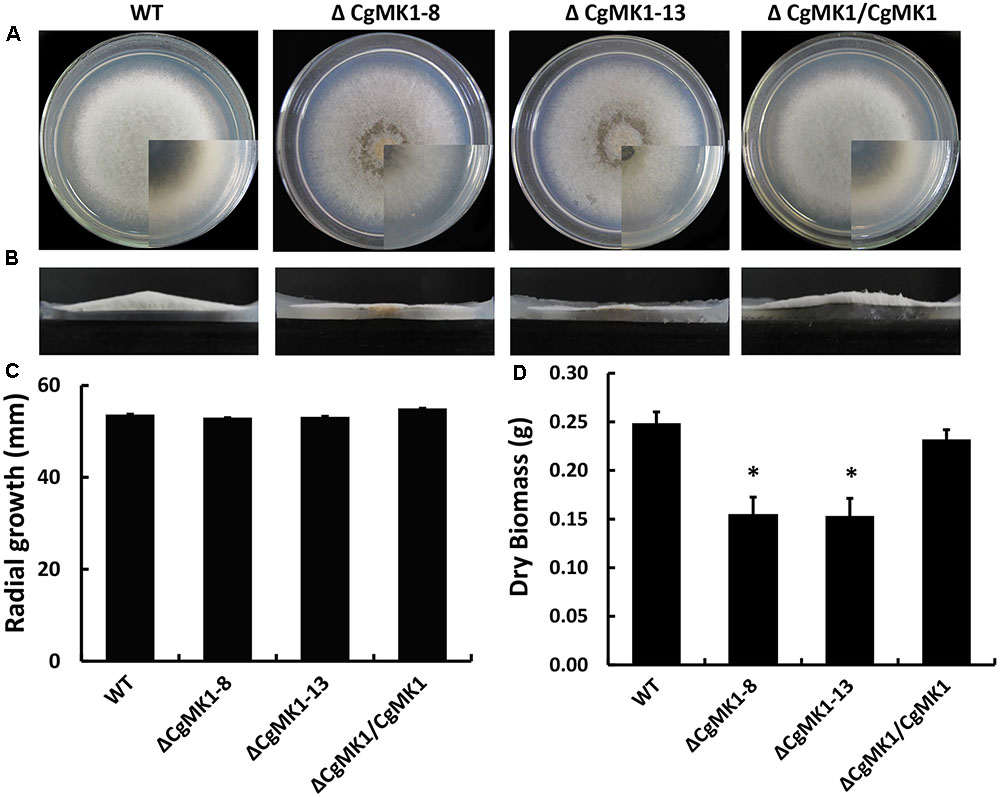
FIGURE 1. Loss of CgMK1 impairs aerial hyphae growth. (A) The CgMK1 mutants (ΔCgMK1-8 and ΔCgMK1-13), the wild-type (WT), and complement (ΔCgMK1/CgMK1) were inoculated on PDA plates at 25°C for 5 days. (B) Aerial hyphae growth is reduced in the CgMK1 deletion mutants. Colony side elevations of the indicated strains placed on PDA solid culture medium. (C) Relative mycelial growth rate of the strains placed on PDA plates after 4 days. (D) Relative dry biomass of the strains in liquid CM after 5 days. Error bars represent the standard deviations based on three independent replicates with three technical replicates each. The data were performed by using the Duncan’s range test. The asterisk indicates significant difference at P = 0.05.
Deletion of CgMK1 Causes the Repression of Melanin Biosynthesis Genes
Because the deletion of CgMK1 clearly affected melanin deposition in the vegetative hyphae, we then investigated whether the melanin defect of the ΔCgMK1 mutants could result from the downregulation of melanin biosynthesis genes. To verify the hypothesis, we identified key genes participated in melanin biosynthesis in C. gloeosporioides using comparative genomics. Here, three genes associated with melanin production via the dihydroxy naphthalene (DHN) pathway were identified as follows, β-ketoacyl synthase (PKS1), scytalone dehydratase (SCD1), and trihydroxy naphthalene reductase (THR1). The transcript levels of these three genes were measured through quantitative real-time PCR method to compare their expression between wild-type and ΔCgMK1 mutant. The 2-ΔΔCT method of data statistics showed that the transcription levels of the melanin biosynthesis genes were strongly repressed in the ΔCgMK1 mutants. The relative expression levels of these genes in the ΔCgMK1 mutants were at least 50 fold lower gene transcript abundance compared to that in the wild-type strain (Figure 2). These results thus indicate that CgMK1 plays a key role in controlling melanin production.
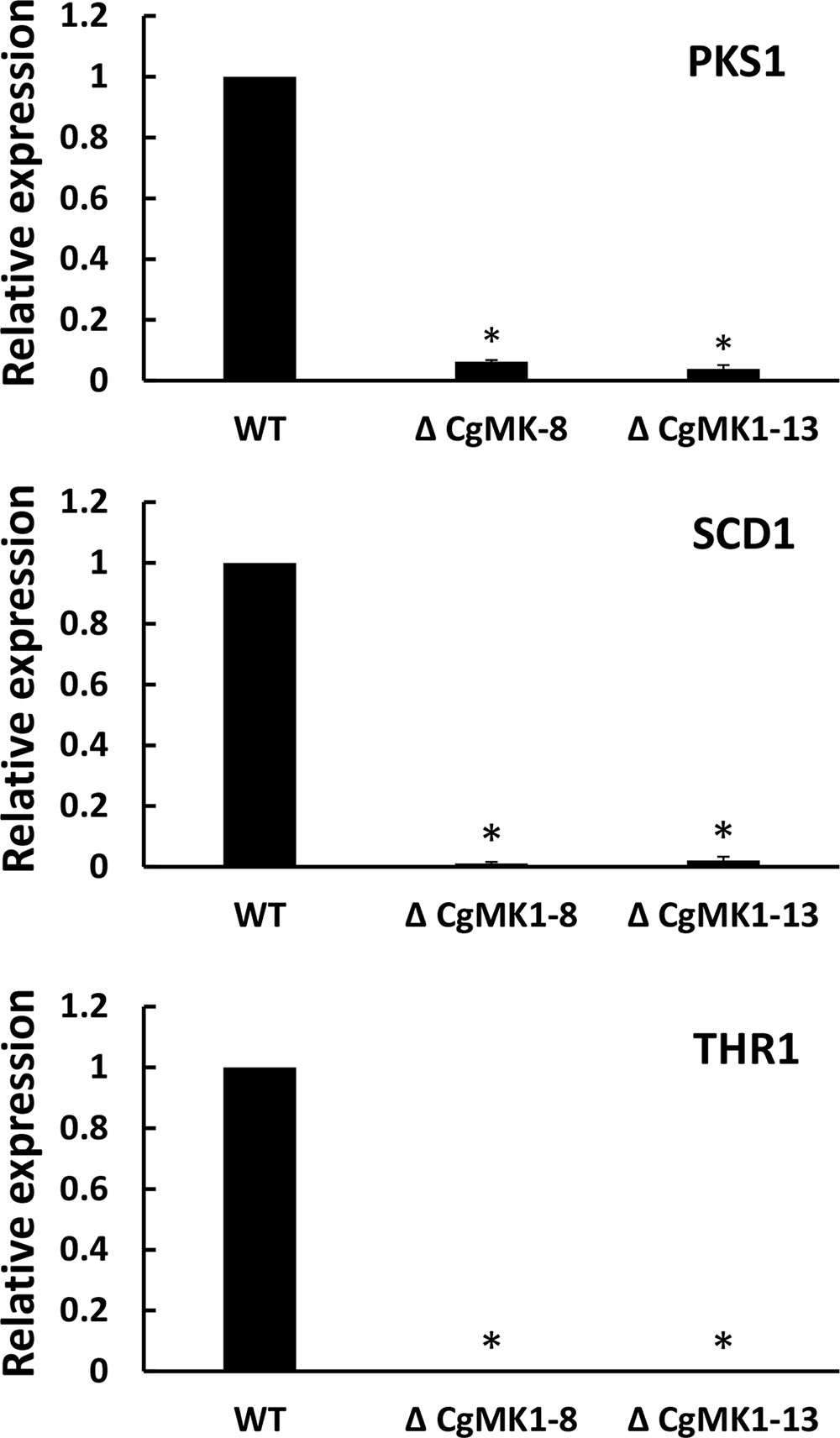
FIGURE 2. Knockout of CgMK1 affects the expression of three melanin biosynthesis-related genes. RT-PCR was used for analyzing the transcript levels of the ΔCgMK1 mutants and wild-type cultured on PDA for 4 days. Transcript levels of PKS1, SCD1, and THR1 are expressed in ΔCgMK1 mutants relative to those of the wild-type. The Cg18S gene served as an internal reference for the analysis. Error bars represent the standard deviations based on three independent replicates with four technical replicates each. The data were performed by using the Duncan’s range test. Asterisks mean statistically significant differences at P = 0.05.
CgMK1 Is Necessary for Appressorium Formation
We also examined the role of CgMK1 in appressorium formation and conidia germination using a GelBond membrane. Firstly, we determined that the ΔCgMK1 mutant and the wild-type exhibit a similar conidia germination phenotypes (data not shown), though the germ tube of the ΔCgMK1 mutants continued to lengthen instead of forming appressoria at 12 hpi (Figure 3A). Based on the data statistics, about 70% of the appressoria were produced in the wild-type strain and ΔCgMK1/CgMK1; however, none were produced by the ΔCgMK1 mutants (Figure 3B). These findings demonstrate that CgMK1 is required for appressoria development.
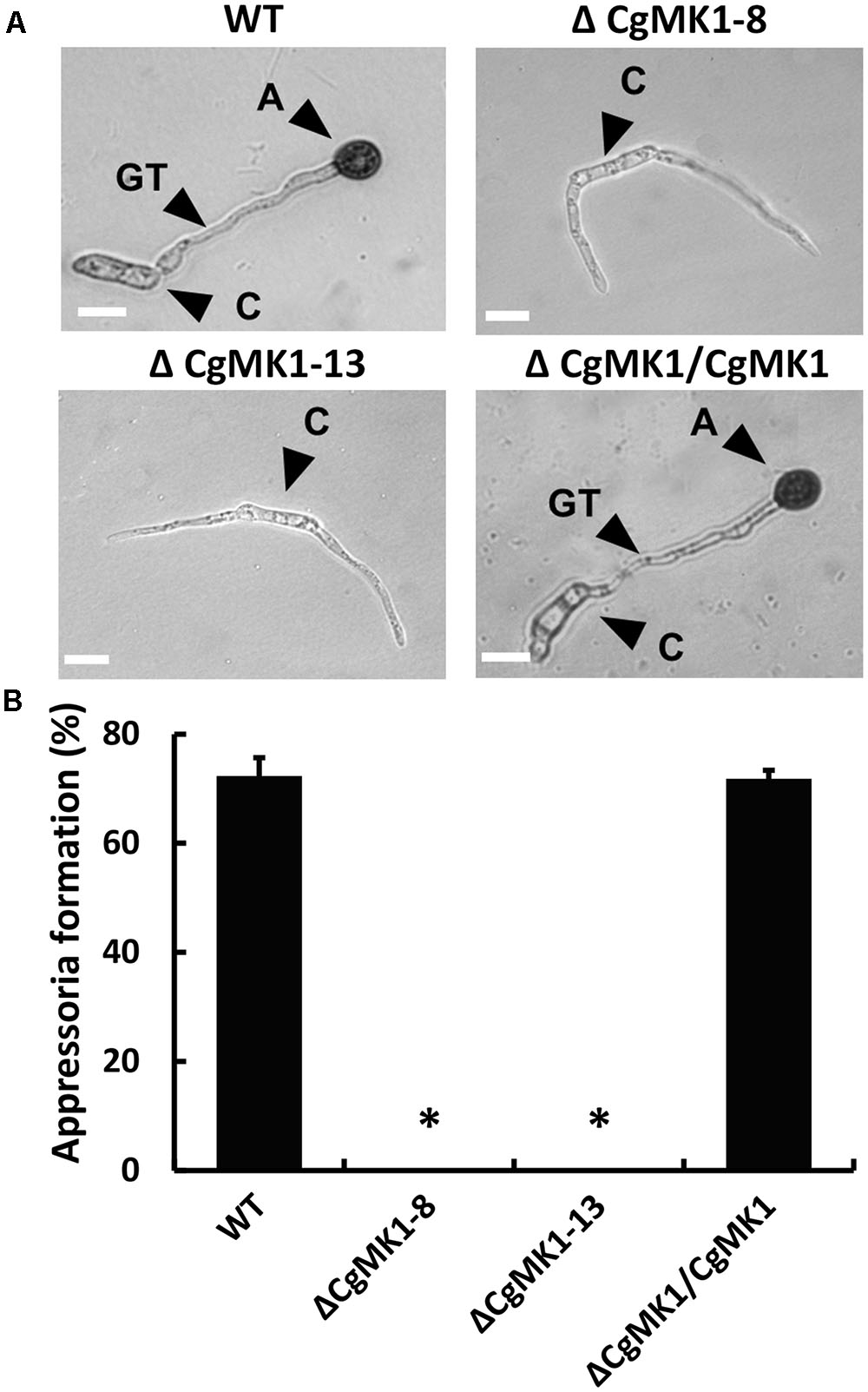
FIGURE 3. CgMK1 is essential for appressorium formation. (A) The conidial suspension from WT, ΔCgMK1 mutants, and the ΔCgMK1/CgMK1 strains were cultured on the hydrophobic surface of the GelBond membrane at 25°C and were observed at 12 h. A: appressorium; C: conidium; GT: germ tube. Scale bar: 10 μm. (B) Bar chart showing the appressoria formation rate percentage on the GelBond membrane. The value represents the mean of the three tests. Error bars represent the standard deviations based on three independent replicates with three technical replicates each. The data were performed by using the Duncan’s range test. Asterisks mean statistically significant differences at P = 0.05.
Exogenous cAMP Does Not Restore the Appressoria Defective of ΔCgMK1 Mutants
Previous studies have shown that the extracellular application of cAMP induces appressorium formation on hydrophilic surfaces, indicating that the cAMP-dependent signal is important for appressorium development. Here, the application of extracellular cAMP to the ΔCgMK1 mutants induced conidia germination in a similar manner as in the wild-type, but exogenous cAMP was unable to recover the appressorium formation defect in the ΔCgMK1 mutants (Figure 4). This suggests that CgMK1 controls the formation of appressoria in a cAMP-independent manner or functions downstream of cAMP signaling.
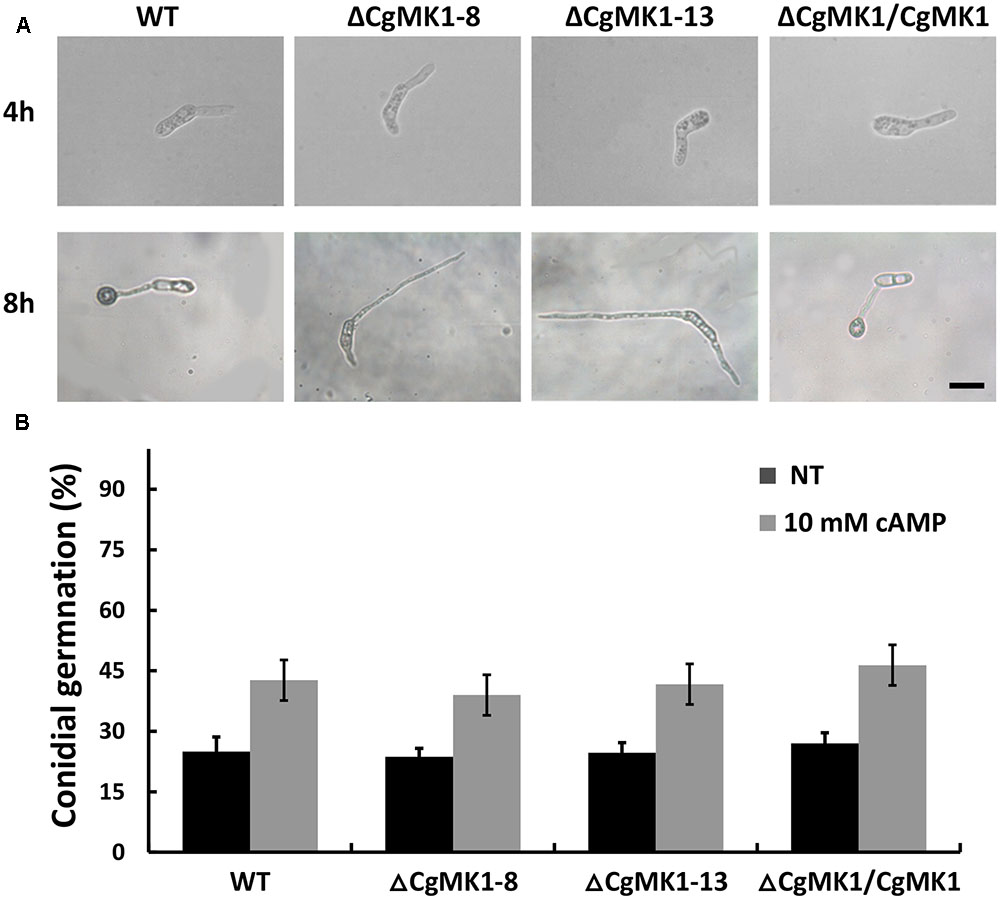
FIGURE 4. Exogenous application of cAMP fails to restore appressoria formation in the CgMK1 mutants. (A) Conidial germination and appressoria phenotype induced by addition of 10 mM cAMP on the hydrophobic surface of GelBond membrane. Scale bar: 15 μm. (B) The germination rate of the strains on the hydrophilic surface of the GelBond membrane in the presence or absence of 10 mM cAMP at 25°C for 4 h. Error bars represent the standard deviations based on three independent replicates with three technical replicates each.
Disruption of CgMK1 Results in a Loss of Virulence
The ΔCgMK1 mutants lost the capability to form appressoria on artificial hydrophobic surfaces. To determine whether this results in defective pathogenicity, the strains were inoculated on detached but intact poplar (Populus × beijingensis) leaves. At 7 dpi, the ΔCgMK1 mutants appeared to completely lose virulence compared to the distinct lesion symptoms of the leaves placed with the ΔCgMK1/CgMK1 and wild-type (Figures 5A,C). Since the ΔCgMK1 mutants could not form appressoria, the wounded poplar leaves were inoculated with the above strain as well. Similar to the symptoms of the intact leaves, the wild-type strain and ΔCgMK1/CgMK1 caused typical disease symptoms; in contrast, the ΔCgMK1 mutants failed to infect the wounded leaves (Figures 5B,C). Although the wild-type and ΔCgMK1/CgMK1 inoculated on the leaf surfaces produced masses of invasive hyphae by 2 dpi, the ΔCgMK1 mutants did not (Figure 5D). Microscopic observations showed that the ΔCgMK1 mutants extended the germ tube but hardly penetrated into the plant cell. Conversely, in the wild-type, the majority of the conidia developed melanized appressoria, which invaded the epidermal cells and then formed swollen invading hyphae (Figure 5D).
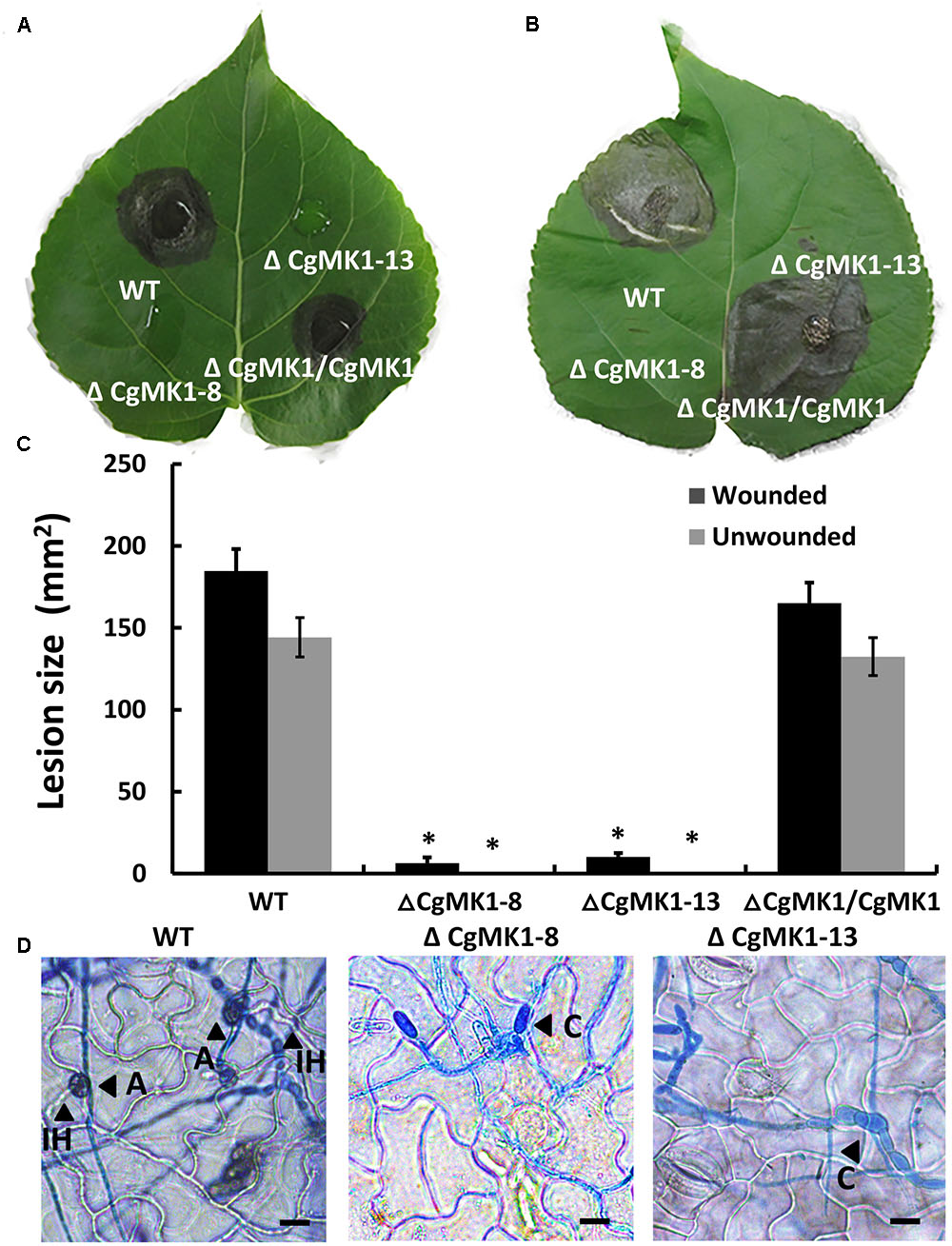
FIGURE 5. The CgMK1 deletion mutants lose pathogenicity on poplar leaves, indicating that CgMK1 governs virulence. (A) Spores (105/mL) from WT, ΔCgMK1 mutants and the ΔCgMK1/CgMK1 were inoculated on the detached leaves of 1-year-old poplar seedlings. Ten healthy intact poplar leaves were used in each independent experiment with three replicates. The images were captured at 7 dpi. (B) Spores (105/mL) of WT, ΔCgMK1 mutants and the ΔCgMK1/CgMK1 were inoculated on detached leaves of 1-year-old poplar seedlings. Ten poplar leaves were wounded and were used in each independent experiment with three replicates. The images were captured at 7 dpi. (C) Quantification of lesion sizes caused by the strains in each independent experiment. Values represent the mean of three repetitions. The data were performed by using the Duncan’s range test. Asterisks mean statistically significant differences at P = 0.05. (D) Infection hyphae development of the strains on the detached poplar leaves at 48 hpi. Invasive hyphae were treated with lactophenol aniline blue. A: appressorium; C: conidium; IH: invasive hyphae. Scale bar: 10 μm.
We also performed a penetration assay on onion epidermal cells and cellophane membranes. The results showed that the ΔCgMK1 mutants produced massive amounts of hyphae on the onion epidermis, but very few hyphae could penetrate into the onion epidermal cells. Nevertheless, the wild-type and ΔCgMK1/CgMK1 strains infected the epidermal cells and expanded into the epidermal tissues at 24 hpi (Figure 6A). We also found that, in contrast to the ΔCgMK1/CgMK1 and wild-type, the ΔCgMK1 mutants failed to penetrate the cellophane membranes and were then unable to grow (Figure 6B). Collectively, the ΔCgMK1 mutants lost virulence due to their failure to penetrate into the plant tissues.
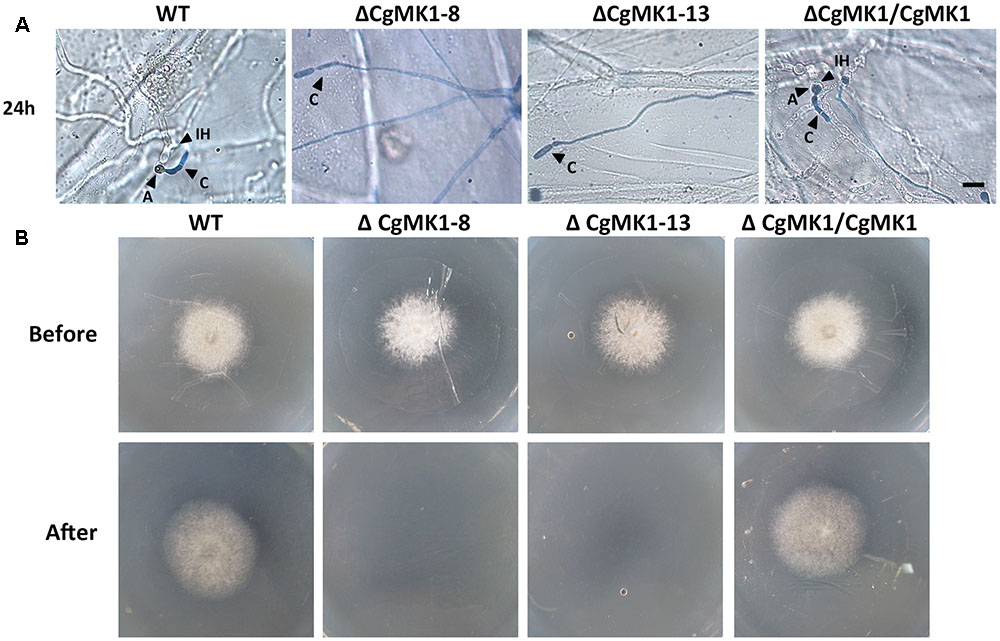
FIGURE 6. Assays for penetration and colonization defects in the CgMK1 mutants. (A) Onion epidermal cell penetration assay of the ΔCgMK1 mutants. The assay was performed by inoculating conidial suspensions from the indicated strains. Examination under a light microscope was performed after aniline-blue staining. A: appressorium; C: conidium; IH: invasive hyphae. Scale bar: 10 μm. (B) Cellophane membranes penetration assay was performed by the conidial suspensions from strains. Colonies of the indicated strains grown on PDA plates covered with a cellophane membrane (before). Then the medium was removed the cellophane membrane and placed for additional days (after). Photos in the first row were taken at 3 dpi, the second row were taken at 5 dpi that showed growth of strains after penetration from cellophane membrane.
Subcellular Localization of the CgMK1
To investigate the cellular localization of CgMK1 in C. gloeosporioides, we generated a fragment containing the CgMK1 and enhanced green fluorescent protein (eGFP), hereby designated as CgMK1_C::eGFP. The CgMK1_C::eGFP and phleomycin resistance cassette were introduced into ΔCgMK1-13 protoplasts by co-transformation. GFP signals were detected under the active expression of the native promoter and whole CgMK1 sequence. In our study, the fluorescence was detected at different stages of conidium, germinating conidia, germ tube, and appressorium formation (Figure 7) Moreover, the expression was relatively higher in the mature appressoria than the other stages. These results indicate that CgMK1 is induced during appressorium formation.
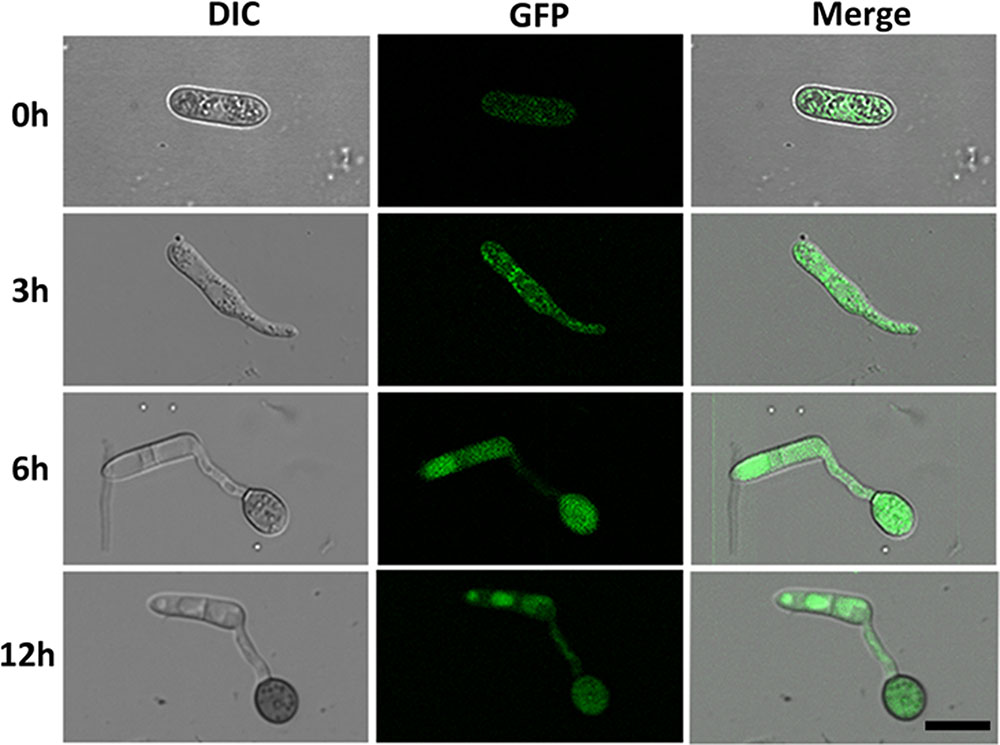
FIGURE 7. Subcellular localization of CgMK1 in appressorium formation. Fluorescence of CgMK1 was detected by C-terminal eGFP fusion under its promoter. Green fluorescence was detected during the stages of germination and appressorium formation on the hydrophobic surface of the GelBond membrane. Scale bar: 10 μm.
CgMK1 Is Involved in the Response to High Osmotic Stress
To determine the function of CgMK1 in stress responses, we observed the wild-type, ΔCgMK1 and ΔCgMK1/CgMK1 strains on complete medium (CM) with NaCl, sorbital, calcofluor white (CFW) and Congo red (CR). The ΔCgMK1 mutants showed sensitivity to 1.2 M NaCl, 1 M Sorbitol (Figure 8A). We also tested the growth rate of the strains on plates containing NaCl and sorbitol. The rate of growth inhibition of ΔCgMK1 mutants when grown on CM containing NaCl and sorbitol was higher than that of wild-type and ΔCgMK1/CgMK1 strain, respectively (Figure 8B). On the other hand, the ΔCgMK1 mutants showed no obvious difference from wild-type and ΔCgMK1/CgMK1 strain in growth in response to CFW and CR (Supplementary Figure S3). These results suggest that CgMK1 is involved in the response to high osmotic stress, but not important in the maintenance of cell wall integrity.
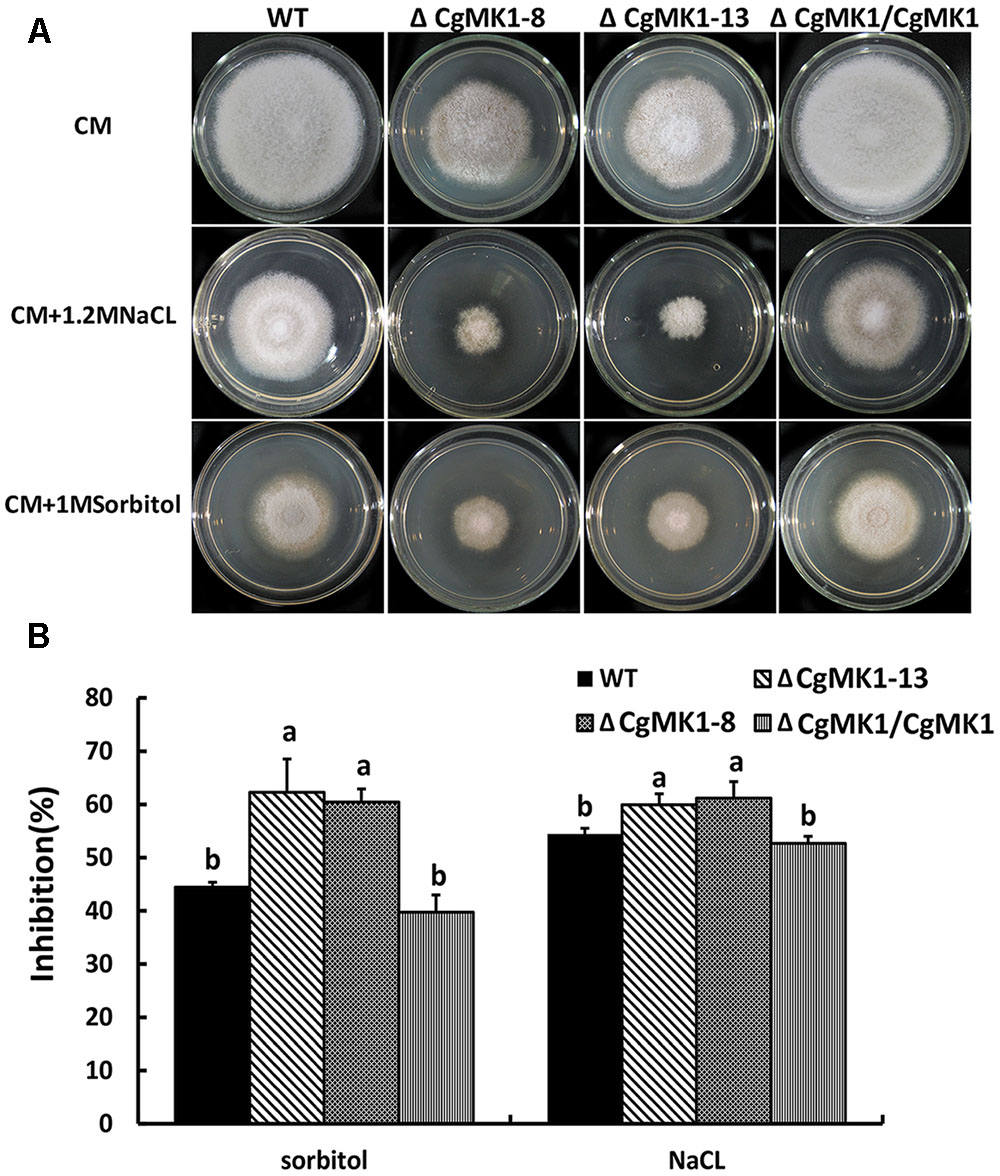
FIGURE 8. CgMK1 in response to osmotic stresses. (A) Colony morphology of the wild-type strain, ΔCgMK1 mutants (ΔCgMK1-8, ΔCgMK1-13) and complemented strain after 3 days of growth on CM or CM containing 1 M Sorbitol, 1.2 M NaCl. (B) The bar chart showed the colony diameter of wild-type, mutants and complementation strain under different chemical stresses. Data sets were calculated from the picture (A). “a” and “b” indicate a significant difference between wild-type, mutant and complementation strain at P = 0.05 according to Duncan’s range test.
Discussion
Here, we examined the functions of CgMK1 in appressorium formation and virulence of poplar anthracnose fungus, C. gloeosporioides. The results demonstrated that CgMK1 plays key roles in appressorium formation, melanin production, and plant infection.
Fungal MAPK cascades play key roles in governing fungal development and signaling fungal responses to a variety of stress stimuli. In search of the component of MAPK, Fus3/Kss1-type MAPK was found to be required for fungi to attack plant tissues. Studies on the Fus3/Kss1-type MAPK in pathogenic fungi has revealed the importance in the production of infection structures (Zhao and Xu, 2007; Zhao et al., 2007). In pathogenic fungi, appressoria are special infection structures that impart high turgor pressure and excrete plant cell degradation enzymes that aid the penetration of the infection peg into plant cells (Mendgen et al., 1996; van Kan, 2006). Previous studies have demonstrated that many conserved signal modules are involved in appressorium formation and pathogenicity, at least including small GTPases (Fukada and Kubo, 2015; Xu et al., 2016) and the MAPK pathway (Hamel et al., 2012). In C. gloeosporioides, loss of CgRhoB resulted in shorter germ tubes and enhanced appressoria formation after germination on the hydrophobic surface. However, the present study demonstrated that CgMK1 is crucial for appressorium formation rather than conidia germination in C. gloeosporioides. The ΔCgMK1 mutants exhibited no obviously defective in conidia germination, indicating that CgMK1 does not contribute to conidia germination, which differs from C. orbiculare (Takano et al., 2000; Xiong et al., 2015). Subcellular localization of the CgMK1 showed that CgMK1 is induced during appressorium formation.
The C. gloeosporioides strain lacking the CgPKAC gene displayed delayed appressorium formation (Priyatno et al., 2012). The CMK1 cooperated with cAMP to control appressorium formation and germination in C. orbiculare (Takano et al., 2000). The PMK1 deletion mutants failed to develop appressoria on Teflon membranes in M. oryzae, and formed swollen germ tubes when exogenous cAMP was applied to the hydrophilic surfaces (Xu and Hamer, 1996). The results indicated that cAMP is necessary for appressorium formation and functions upstream of MAPK in appressoria-forming fungi. To explore the reason why the ΔCgMK1 mutants failed to form the appressorium, we also revealed the function of exogenous cAMP in ΔCgMK1 mutants. The results showed that exogenous cAMP application does not restore the defect of appressorium formation in the ΔCgMK1 mutants. This suggested that CgMK1 controls the formation of appressoria in a cAMP-independent manner or functions downstream of cAMP signaling.
Appressorium melanization is vital for the high turgor pressure necessary for the invasion and growth of appressoria-forming pathogens (Howard and Ferrari, 1989). In C. orbiculare (Takano et al., 2000), several genes involved in melanin biosynthesis have been isolated, such as PKS1, THR1, and SCD1. CgPKS1, a melanin biosynthesis gene in C. graminicola, is not required for appressoria turgor generation, but is important for penetration and melanin synthesis (Ludwig et al., 2014). In this study, the ΔCgMK1 mutants exhibited slightly darkened colonies. The expression of three melanin biosynthesis genes including PKS1, THR1, and SCD1 were significantly down-regulated in the ΔCgMK1 mutants.
Previous studies have demonstrated that deletion of the Fus3/Kss1-related pathway results in a loss of pathogenesis owing to an inability of several pathogenic fungi to colonize host tissues or form infection structures, for example VMK1 in V. dahliae, and PMK1 in M. oryzae (Xu and Hamer, 1996). Knockout of CgMK1 resulted in mutants that were non-pathogenic and unable to form lesions on poplar leaves. On the poplar epidermis, ΔCgMK1 mutants formed elongated germ tubes, but were unable to infect the epidermal cells. In addition, penetration assays with cellophane membranes and onion epidermal cells suggested that the ΔCgMK1 mutants were unable to penetrate the cellophane membranes. Furthermore, we discovered that the hyphae of the ΔCgMK1 mutants could only marginally infect onion epidermal cells. This result differs from that displayed in other fungi such as C. orbiculare (Takano et al., 2000), where the Fus3/Kss1 ortholog mutant completely lost the ability to attack the host via the wounded sites. The results indicated that CgMK1 is important for penetration and infectious growth once inside the plant. The relationship of Fus3/Kss1 MAPK cascade and plant immunization pathways has also been examined. A thioredoxin gene, TRX2, which is essential for intracellular ROS signaling, affects invasive growth via the Mst11-Mst7-PMK1 pathway (Wang et al., 2016). Recently, some genes from other families that are related to ROS and pathogenicity in C. gloeosporioides, such as CgAP1 and CgRhoB have been identified. Deletion of CgAP1 results enhances a strain sensitivity to oxidative stress, and a reduction in virulence (Sun et al., 2016). The ΔCgRhoB mutants affect cAMP levels and stress pathways, resulting in a significant reduction in poplar leaf virulence (Xu et al., 2016). Further investigations should therefore focus on elucidating the relationship between the CgMK1 gene of C. gloeosporioides and host defense. Previously, upstream components of the Fus3/Kss1 MAPK cascade have been identified and proved to be critical to appressorium formation and pathogenicity of Colletotrichum, such as CoMEKK1 of C. orbiculare (Sakaguchi et al., 2010) and ChSte7 in C. higginsianum (Yuan et al., 2016). Systematic studies of the Fus3/Kss1 signaling cascades in C. gloeosporioides are not well-characterized.
In summary, CgMK1 acts as a key regulator of appressorium formation and plant infection in C. gloeosporiodes. Furthermore, ΔCgMK1 mutants exhibit deficiency in invasive growth on media or onion epidermal cells. These findings improve our understanding toward the functions of the MAPK signaling pathway in C. gloeosporioides.
Author Contributions
YW, CT, and PH designed the experiments. PH, XW, and XZ performed the experiments and the data analyses. YW and PH prepared the figures and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PP-N and handling Editor declared their shared affiliation.
Acknowledgments
The research was supported by the National Natural Science Foundation of China (No. 31470647), Fundamental Research Funds for the Central Universities (No. 2017ZY17), and Project of Universities in Beijing supported by Beijing Government (No. 2050205).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02216/full#supplementary-material
FIGURE S1 | Phylogenetic analysis of CgMK1 and other homologs from other fungi. (A) Prediction of domains in CgMK1 used the InterPro website (http://www.ebi.ac.uk/interpro/). Amino-acid sequences of CgMK1 in Colletotrichum gloeosporioides and Fus3/Kss1 homologs in other fungi were aligned by the ClustalX 2.1. The conserved TEY sequence needed for kinase activation is marked by asterisks. aa, amino acid. (B) Phylogenetic tree of CgMK1 and its homologs in Magnaporthe oryzae (PMK1, AAC49521), Gaeumannomyces graminis (GMK1, AAG44657), Fusarium graminearum (GPMK1, AAL73403), Nectria haematococca (FsMAPK, Q00859), Colletotrichum orbiculare (CMK1, AAD50496), Mycosphaerella graminicola (MgFus3, XP_003851863), Aspergillus nidulans (AnFUS3, AN3719), Fusarium oxysporum (FMK1, AAG01162), Botrytis cinerea (BMP1, AAG23132). The phylogenetic tree was constructed by MEGA 7.0 with full-length protein sequences and neighbor-joining with 1000 bootstrap replicates.
FIGURE S2 | Disruption and complementation of CgMK1 in C. gloeosporioides. (A,B) Replacement method for the deletion of CgMK1. The 1.0 kb sequence of CgMK1 was substituted for a sequence including the hygromycin cassette. Filtration of the ΔCgMK1 mutants with primers External-CgMK1for and External-CgMK1rev (A) and primers RT-CgMK1for and RT-CgMK1rev (B). (C) Confirmation of the ΔCgMK1 mutants and complementation of CgMK1 by semiquantitative reverse transcription PCR. (D,E) Southern blot of wild-type strain and two independent ΔCgMK1 mutants (ΔCgMK1-8, ΔCgMK1-13). DNA specimens of transformants were digested by ScaI. The enzyme-digested product was probed with a DNA sequence from a hph gene (Probe 3′ of the HPH) (D) and CgMK1 (A 493 bp fragment of the 3′flanking sequence of CgMK1) (E).
FIGURE S3 | Sensitivity of the CgMK1 mutants to cell wall integrity stresses. (A) Colony morphology of the wild-type strain, ΔCgMK1 mutants (ΔCgMK1-8, ΔCgMK1-13) and complemented strain after 3 days of growth on CM or CM containing congo red, CFW. (B) The bar chart showed the colony diameter of wild-type, mutants and complementation strain under different chemical stresses. Data sets were calculated from the picture (A). “a” and “b” indicate a significant difference between wild-type, mutant and complementation strain at P = 0.05 according to Duncan’s range test.
Footnotes
References
Baltanás, R., Bush, A., Couto, A., Durrieu, L., Hohmann, S., and Colman-Lerner, A. (2013). Pheromone-induced morphogenesis improves osmoadaptation capacity by activating the HOG MAPK pathway. Sci. Signal. 6, ra26. doi: 10.1126/scisignal.2003312
Fukada, F., and Kubo, Y. (2015). Colletotrichum orbiculare regulates cell cycle G1/S progression via a two-component GAP and a GTPase to establish plant infection. Plant Cell 27, 2530–2544. doi: 10.1105/tpc.15.00179
Hamel, L. P., Nicole, M. C., Duplessis, S., and Ellis, B. E. (2012). Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell 24, 1327–1351. doi: 10.1105/tpc.112.096156
Herskowitz, I. (1995). MAP kinase pathways in yeast: for mating and more. Cell 80, 187–197. doi: 10.1016/0092-8674(95)90402-6
Howard, R. J., and Ferrari, M. A. (1989). Role of melanin in appressorium function. Exp. Mycol. 13, 403–418. doi: 10.1016/0147-5975(89)90036-4
Kim, Y. K., Kawano, T., Li, D., and Kolattukudy, P. E. (2000). A mitogen-activated protein kinase kinase required for induction of cytokinesis and appressorium formation by host signals in the conidia of Colletotrichum gloeosporioides. Plant Cell 12, 1331–1343. doi: 10.1105/tpc.12.8.1331
Kleemann, J., Takahara, H., Stüber, K., and O’Connell, R. (2008). Identification of soluble secreted proteins from appressoria of Colletotrichum higginsianum by analysis of expressed sequence tags. Microbiology 154, 1204–1217. doi: 10.1099/mic.0.2007/014944-0
Leng, Y., and Zhong, S. (2015). The role of mitogen-activated protein (MAP) kinase signaling components in the fungal development, stress response and virulence of the fungal cereal pathogen Bipolaris sorokiniana. PLOS ONE 10:e0128291. doi: 10.1371/journal.pone.0128291
Leroch, M., Mueller, N., Hinsenkamp, I., and Hahn, M. (2015). The signalling mucin Msb2 regulates surface sensing and host penetration via BMP1 MAP kinase signalling in Botrytis cinerea. Mol. Plant Pathol. 16, 787–798. doi: 10.1111/mpp.12234
Li, G. T., Zhou, X. Y., Kong, L. G., Wang, Y. L., Zhang, H., Zhu, H., et al. (2011). MoSfl1 is important for virulence and heat tolerance in Magnaporthe oryzae. PLOS ONE 6:e19951. doi: 10.1371/journal.pone.0019951
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ludwig, N., Löhrer, M., Hempel, M., Mathea, S., Schliebner, I., Menzel, M., et al. (2014). Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola. Mol. Plant Microbe Interact. 27, 315–327. doi: 10.1094/MPMI-09-13-0267-R
Marroquin-Guzman, M., and Wilson, R. A. (2015). GATA-dependent glutaminolysis drives appressorium formation in Magnaporthe oryzae by suppressing TOR inhibition of cAMP/PKA signaling. PLOS Pathog. 11:e1004851. doi: 10.1371/journal.ppat.1004851
Marshall, C. J. (1994). MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4, 82–89. doi: 10.1016/0959-437X(94)90095-7
Mendgen, K., Hahn, M., and Deising, H. (1996). Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. phytopathol. 34, 367–386. doi: 10.1146/annurev.phyto.34.1.367
Park, G., Xue, C., Zheng, L., Lam, S., and Xu, J. R. (2002). MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol. Plant Microbe Interact. 15, 183–192. doi: 10.1094/MPMI.2002.15.3.183
Priyatno, T. P., Abu Bakar, F. D., Kamaruddin, N., Mahadi, N. M., and Abdul Murad, A. M. (2012). Inactivation of the catalytic subunit of cAMP-dependent protein kinase A causes delayed appressorium formation and reduced pathogenicity of Colletotrichum gloeosporioides. Sci. World J. 2012, 545784. doi: 10.1100/2012/545784
Rauyaree, P., Ospina-Giraldo, M. D., Kang, S., Bhat, R. G., Subbarao, K. V., Grant, S. J., et al. (2005). Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr. Genet. 48, 109–116. doi: 10.1007/s00294-005-0586-0
Sakaguchi, A., Tsuji, G., and Kubo, Y. (2010). A yeast STE11 homologue CoMEKK1 is essential for pathogenesis-related morphogenesis in Colletotrichum orbiculare. Mol. Plant Microbe Interact. 23, 1563–1572. doi: 10.1094/Mpmi-03-10-0051
Sun, Y., Wang, Y., and Tian, C. (2016). bZIP transcription factor CgAP1 is essential for oxidative stress tolerance and full virulence of the poplar anthracnose fungus Colletotrichum gloeosporioides. Fungal Genet. Biol. 95, 58–66. doi: 10.1016/j.fgb.2016.08.006
Takano, Y., Kikuchi, T., Kubo, Y., Hamer, J. E., Mise, K., and Furusawa, I. (2000). The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant Microbe Interact. 13, 374–383. doi: 10.1094/MPMI.2000.13.4.374
van Kan, J. A. L. (2006). Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11, 247–253. doi: 10.1016/j.tplants.2006.03.005
Wang, J., Yin, Z., Tang, W., Cai, X., Gao, C., Zhang, H., et al. (2016). The thioredoxin MoTrx2 protein mediates reactive oxygen species (ROS) balance and controls pathogenicity as a target of the transcription factor MoAP1 in Magnaporthe oryzae. Mol. Plant Pathol. 95, 58–66. doi: 10.1111/mpp.12484
Wei, W., Xiong, Y., Zhu, W., Wang, N., Yang, G., and Peng, F. (2016). Colletotrichum higginsianum mitogen-activated protein kinase ChMK1: role in growth, cell wall integrity, colony melanization, and pathogenicity. Front. Microbiol. 7:1212. doi: 10.3389/fmicb.2016.01212
Xiong, Q., Xu, J., Zhao, Y., and Wang, K. (2015). CtPMK1, a mitogen-activated-protein kinase gene, is required for conidiation, appressorium formation, and pathogenicity of Colletotrichum truncatum on soybean. Ann. Appl. Biol. 167, 63–74. doi: 10.1111/aab.12209
Xu, J. R., and Hamer, J. E. (1996). MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10, 2696–2706. doi: 10.1101/gad.10.21.2696
Xu, X., Wang, Y., Tian, C., and Liang, Y. (2016). The Colletotrichum gloeosporioides RhoB regulates cAMP and stress response pathways and is required for pathogenesis. Fungal Genet. Biol. 96, 12–24. doi: 10.3389/fmicb.2017.01416
Yong, H. Y., Bakar, F. D. A., Illias, R. M., Mahadi, N. M., and Murad, A. M. A. (2013). Cgl-SLT2 is required for appressorium formation, sporulation and pathogenicity in Colletotrichum gloeosporioides. Braz. J. Microbiol. 44, 1241–1250. doi: 10.1590/S1517-83822013000400031
Yuan, Q. F., Chen, M. J., Yan, Y. Q., Gu, Q. N., Huang, J. B., and Zheng, L. (2016). ChSte7 is required for vegetative growth and various plant infection processes in Colletotrichum higginsianum. Biomed Res. Int. 2016:7496569. doi: 10.1155/2016/7496569
Zhao, X., Mehrabi, R., and Xu, J. R. (2007). Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 6, 1701–1714. doi: 10.1128/EC.00216-07
Keywords: Colletotrichum gloeosporiodes, MAPK, appressorium formation, virulence, penetration
Citation: He P, Wang Y, Wang X, Zhang X and Tian C (2017) The Mitogen-Activated Protein Kinase CgMK1 Governs Appressorium Formation, Melanin Synthesis, and Plant Infection of Colletotrichum gloeosporioides. Front. Microbiol. 8:2216. doi: 10.3389/fmicb.2017.02216
Received: 11 August 2017; Accepted: 27 October 2017;
Published: 10 November 2017.
Edited by:
Hector Mora Montes, Universidad de Guanajuato, MexicoReviewed by:
Patricia Ponce-Noyola, Universidad de Guanajuato, MexicoMaría Guadalupe Zavala Páramo, Universidad Michoacana de San Nicolás de Hidalgo, Mexico
Copyright © 2017 He, Wang, Wang, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengming Tian, Y2hlbmdtdEBiamZ1LmVkdS5jbg==
†These authors have contributed equally to this work.
 Puhuizhong He
Puhuizhong He Yonglin Wang
Yonglin Wang Xiaolian Wang
Xiaolian Wang Xiaolin Zhang
Xiaolin Zhang Chengming Tian
Chengming Tian