
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 18 October 2017
Sec. Aquatic Microbiology
Volume 8 - 2017 | https://doi.org/10.3389/fmicb.2017.02032
This article is part of the Research Topic The Responses of Marine Microorganisms, Communities and Ecofunctions to Environmental Gradients View all 22 articles
 Yanying Zhang1,2,3
Yanying Zhang1,2,3 Qingsong Yang1,4
Qingsong Yang1,4 Juan Ling1
Juan Ling1 Joy D. Van Nostrand3
Joy D. Van Nostrand3 Zhou Shi3
Zhou Shi3 Jizhong Zhou3
Jizhong Zhou3 Junde Dong1,2*
Junde Dong1,2*Diazotrophic communities make an essential contribution to the productivity through providing new nitrogen. However, knowledge of the roles that both mangrove tree species and geochemical parameters play in shaping mangove rhizosphere diazotrophic communities is still elusive. Here, a comprehensive examination of the diversity and structure of microbial communities in the rhizospheres of three mangrove species, Rhizophora apiculata, Avicennia marina, and Ceriops tagal, was undertaken using high-throughput sequencing of the 16S rRNA and nifH genes. Our results revealed a great diversity of both the total microbial composition and the diazotrophic composition specifically in the mangrove rhizosphere. Deltaproteobacteria and Gammaproteobacteria were both ubiquitous and dominant, comprising an average of 45.87 and 86.66% of total microbial and diazotrophic communities, respectively. Sulfate-reducing bacteria belonging to the Desulfobacteraceae and Desulfovibrionaceae were the dominant diazotrophs. Community statistical analyses suggested that both mangrove tree species and additional environmental variables played important roles in shaping total microbial and potential diazotroph communities in mangrove rhizospheres. In contrast to the total microbial community investigated by analysis of 16S rRNA gene sequences, most of the dominant diazotrophic groups identified by nifH gene sequences were significantly different among mangrove species. The dominant diazotrophs of the family Desulfobacteraceae were positively correlated with total phosphorus, but negatively correlated with the nitrogen to phosphorus ratio. The Pseudomonadaceae were positively correlated with the concentration of available potassium, suggesting that diazotrophs potentially play an important role in biogeochemical cycles, such as those of nitrogen, phosphorus, sulfur, and potassium, in the mangrove ecosystem.
Mangroves are unique intertidal ecosystems along tropical and subtropical coastlines and play an essential role in maintaining sea levels and protecting coasts in tropical and subtropical regions (Duke et al., 2007). In tropical marine environments, mangroves are thought to be important as primary producers of organic matter, providing the basis of a large and complex food web (Holguin et al., 2001). Although mangrove ecosystems are rich in organic matter, in general they are nutrient-deficient (Sengupta and Chaudhuri, 1991; Holguin et al., 1992; Alongi et al., 1993; Vazquez et al., 2000). Microorganisms are an important component of the mangrove ecosystem, and there is increasing evidence that microbes are crucial to the biogeochemical productivity of the mangrove ecosystem (Holguin et al., 2001; Thatoi et al., 2013).
In tropical mangroves, bacteria and fungi constitute 91% of the total microbial biomass, whereas algae and protozoa represent only 7 and 2%, respectively (Alongi, 1988). Bacterial communities play an important role in nutrient transformation in mangrove ecosystems (Holguin et al., 2001). Nitrogen fixation [converting gaseous nitrogen (N2) to biologically available forms such as ammonia (NH3)] by diazotrophs is considered to be the major source of combined nitrogen input in mangrove forest habitats (Kyaruzi et al., 2003). Free-living diazotrophs are widely distributed within the mangrove ecosystem, with high rates of nitrogen fixation detected in association with dead and decomposing leaves, pneumatophores, rhizosphere soil, tree bark, cyanobacterial mats covering the surface of the sediment, and the sediments themselves (Zuberer and Silver, 1978, 1979; Hicks and Silvester, 1985; Holguin et al., 1992, 2001; Lugomela and Bergman, 2002). The high productivity of mangrove ecosystems might be partially attributable to the high rate of biological nitrogen-fixing activity of diazotrophs in sediments and in the rhizosphere of mangrove trees (Holguin et al., 2001).
In recent years, high-throughput sequencing has offered a more comprehensive perspective on microbial communities and has been employed to study the bacterial community associated with mangroves (Dos Santos et al., 2011; Andreote et al., 2012; Gomes et al., 2014; Loganathachetti et al., 2015; Alzubaidy et al., 2016; Wu et al., 2016). The functional diversity and structure of the microbial communities in mangrove wetlands is largely shaped by environmental variables, and each habitat harbors unique microbial functional communities (Bai et al., 2013). Mangrove trees can influence the growth and distribution of microbial communities by enriching the organic carbon pool and changing the redox conditions of the sediments (Holguin et al., 2001). Previous results obtained using acetylene reduction and molecular methods [denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism (T-RFLP)] showed that the composition and activity of diazotrophs in mangrove ecosystems are strongly influenced by both root–bacterial interactions (Zuberer and Silver, 1978, 1979; Ravikumar et al., 2004; Flores-Mireles et al., 2007) and geochemical parameters (Zhang et al., 2008; Romero et al., 2012). However, the functional nifH gene, a marker for diazotrophs, has seldom been examined using high-throughput sequencing in the mangrove rhizosphere (Jing et al., 2014), and little is known comprehensively about the roles that mangrove tree species and environmental variables play in shaping mangove rhizosphere diazotrophic communities. Therefore, it is necessary to examine the diversity, composition, and structure of sediment communities based on both 16S rRNA and nifH genes and their links with environmental factors in order to improve our understanding of mangrove ecosystem functioning.
In this study, high-throughput Illumina sequencing was used to investigate microbial communities in the rhizosphere sediments of three species of urban mangrove trees located in Sanya River Mangrove Nature Reserve: Rhizophora apiculata, Avicennia marina, and Ceriops tagal. The aims were to: (i) investigate the diversity and abundance of microbial taxa associated with these three mangrove habitats based on identification of the 16S rRNA and nifH genes; (ii) determine the differences in the total microbial composition and the diazotrophic composition among different mangrove species; and (iii) explore the possible relationships between the 16S rRNA and nifH gene communities and environmental variables, given the important roles of these microbes in driving biogeochemical cycles in mangrove ecosystems.
Three species of mangrove trees were investigated, R. apiculata, A. marina, and C. tagal, which are the dominant species in Sanya River Mangrove Nature Reserve, a typical tropical urban mangrove ecosystem located in the southernmost part of Hainan Island in China. In June 2013, six different sediment cores were collected randomly at each sampling point during low tide. Sediments adjacent to the rhizosphere were collected down to approximately 10 cm. After roots were removed, the sediment was packed on-site into sealed polythene bags. Samples were maintained on ice until transfer to the laboratory. The wet sediments of each core were thoroughly mixed, and subsamples used for nucleic acid extraction were stored at -20°C prior to DNA extraction. Subsamples for environmental parameter analysis were stored at 4°C prior to analysis. Environmental variables including total carbon (TC), total nitrogen (TN), total phosphorus (TP), and available potassium (AK) were measured as described by Bao (1999).
Total community DNA was extracted from 1.0 g of wet sediment using an E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, United States). The DNA was then purified with a Promega Wizard DNA Clean-Up System (Madison, WI, United States). DNA concentration was measured by Pico Green using a FLUOstar OPTIMA fluorescence plate reader (BMG Labtech, Jena, Germany).
Two genes were amplified for each sample. To characterize the general 16S rRNA gene community (total microbial composition), the V4 region of the 16S rRNA gene was amplified with the primer pair 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The diazotrophic microbial community was characterized using the nifH gene, which was amplified with primers PolF and PolR (Poly et al., 2001). Both sets of amplicons were modified with Illumina adapter and barcode sequences (Caporaso et al., 2012). Sample libraries were generated from purified PCR products. The Miseq 300 Cycle Kit was used for paired-end sequencing on a Miseq benchtop sequencer (Illumina, San Diego, CA, United States).
The 16S rRNA and nifH gene sequences were separated by sample based on their barcodes, permitting up to one mismatch. Quality trimming was done using Btrim (Kong, 2011). Forward and reverse reads were merged into full-length sequences using FLASH (Magoč and Salzberg, 2011). Sequences were removed if they were too short or contained ambiguous bases. For the 16S rRNA gene, operational taxonomic units (OTUs) were classified using UCLUST at the 97% similarity level. Samples were rarefied to 20,000 sequences per sample. OTUs that were only present in a single sample were removed. Taxonomic assignment was conducted using the RDP classifier (release 5.0) (Wang et al., 2013).
For the nifH gene, the sequences were analyzed using the FRAMEBOT program (Wang et al., 2013). Sequences having frameshift errors were removed. Error-free sequences were then translated into conceptual protein sequences. The NifH protein sequences were grouped into OTUs using the DOTUR program with a 0.05 sequence distance cutoff (Schloss and Handelsman, 2005; Dang et al., 2009, 2013; Zhou et al., 2016). Samples were rarefied to 12,000 sequences per sample, and singletons were removed. Taxonomic assignment for nifH OTUs was carried out by searching representative sequences against reference nifH sequences with known taxonomic information. A neighbor-joining (NJ) phylogenetic tree was built by the molecular evolutionary genetics analysis (MEGA6) software (Tamura et al., 2013) for all NifH protein sequences together with selected reference sequences from different diazotrophic groups.
All analyses were performed using the package vegan in R (R Foundation for Statistical Computing, Vienna, Austria) or our R-based pipeline1. Total microbial and diazotrophic species richness and diversity were calculated using the Chao1, Shannon–Wiener (H′), and Simpson evenness (E) indices. Principal coordinates analysis (PCoA) was used to visualize changes in overall microbial and diazotrophic community structure. Three non-parametric tests [multiple-response permutation procedure (MRPP), permutational multivariate analysis of variance (Adonis), and analysis of similarity (ANOSIM)] were performed based on Bray–Curtis distances to test dissimilarity of 16S rRNA and nifH gene communities among mangrove species. Analysis of variance (ANOVA) was performed to identify significant variation in 16S rRNA and nifH gene groups among mangrove species. Redundancy analysis (RDA) and the Mantel test were performed to determine the relationships between 16S rRNA and nifH gene communities and environmental parameters. All sequences obtained from this study were deposited in the NCBI sequence read archive (SRA) under accession number SRP103888.
The environmental characteristics of the rhizosphere sediment samples from three mangrove species are shown in Table 1. All of the measured environmental characteristics including TC, TN, TP, and AK were higher in R. apiculata rhizosphere samples than in those from the other two mangrove species. The sediment samples from the A. marina rhizosphere had the lowest concentrations of TC, TN, and TP, and had the highest nitrogen to phosphorus ratio (N/P). The sediment samples from the C. tagal rhizosphere had the lowest N/P (Table 1). The concentrations of TN and AK were higher than observed in our previous investigation (Zhang et al., 2008). The concentration of TP was almost the same as that reported in the Sungei Mandai mangrove ecosystem of Singapore (Jing et al., 2014).
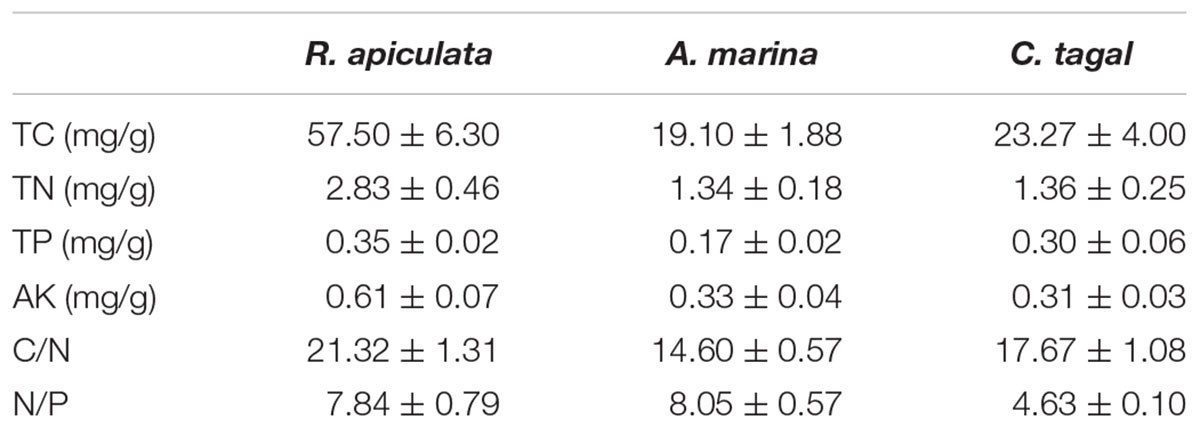
TABLE 1. Environmental characteristics of rhizosphere sediment samples (n = 6) from the three mangrove species (expressed as mean value and standard error, SE).
A total of 406,272 high-quality 16S rRNA gene sequences with lengths of 245–260 bp were obtained. Samples were rarefied to 20,000 sequences per sample. All sequences obtained could be assigned to 24,015 OTUs using UClust (grouped based on 97% similarity). These sequences were classified into 27 bacterial and 2 archaeal phyla (Figure 1A). Sequences related to bacteria within the phylum Proteobacteria were the most abundant. Within the Proteobacteria, Gammaproteobacteria, and Deltaproteobacteria sequences made up an average of 25 and 21% of the samples, respectively. Bacteroidetes was the second most abundant phylum observed in this study, followed by Chloroflexi, Acidobacteria, and Firmicutes (Figure 1A).
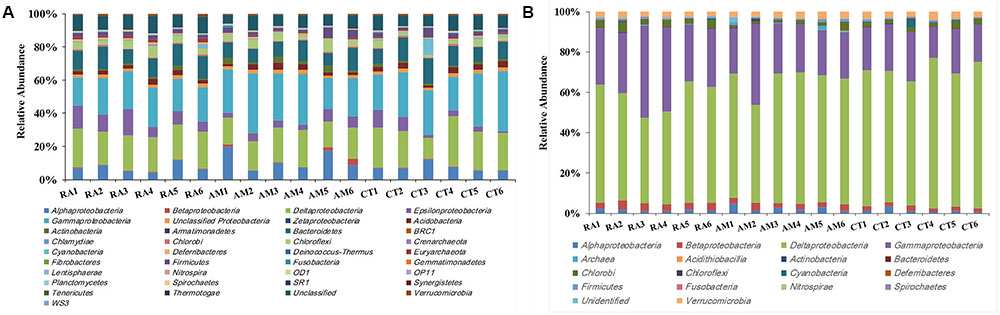
FIGURE 1. Total 16S rRNA (A) and nifH (B) gene community composition profiles. General 16S rRNA and nifH gene taxa were categorized at the phylum level except for the Proteobacteria, which were categorized by class. RA, R. apiculata. AM, A. marina. CT, C. tagal.
The 16S rRNA gene communities were highly diverse. The number of OTUs ranged from 8,329 to 14,359 per sample, with A. marina harboring the fewest OTUs among the three mangrove species. For individual samples the Shannon–Wiener (H′) index ranged from 6.99 to 8.04, and Simpson evenness (E) ranged from 0.02 to 0.14. The average Shannon–Wiener (H′) of 16S rRNA gene sequence diversity from R. apiculata was higher than those from A. marina and C. tagal (Table 2). Three non-parametric tests (MRPP, Adonis, and ANOSIM) were performed using the Bray–Curtis dissimilarity index and consistently showed that microbial communities were significantly different among mangrove species (P < 0.02) (Table 3). PCoA was also used to compare microbial communities among mangrove species, and the results confirmed that the microbial communities could be divided into three groups corresponding to the mangrove species (Figure 2A).
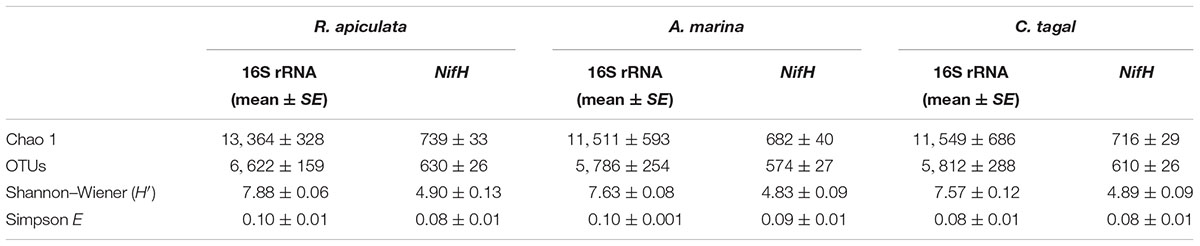
TABLE 2. Diversity indices of 16S rRNA gene and nifH sequences from rhizosphere sediments (n = 6) of three mangrove species (expressed as mean value and standard error, SE).
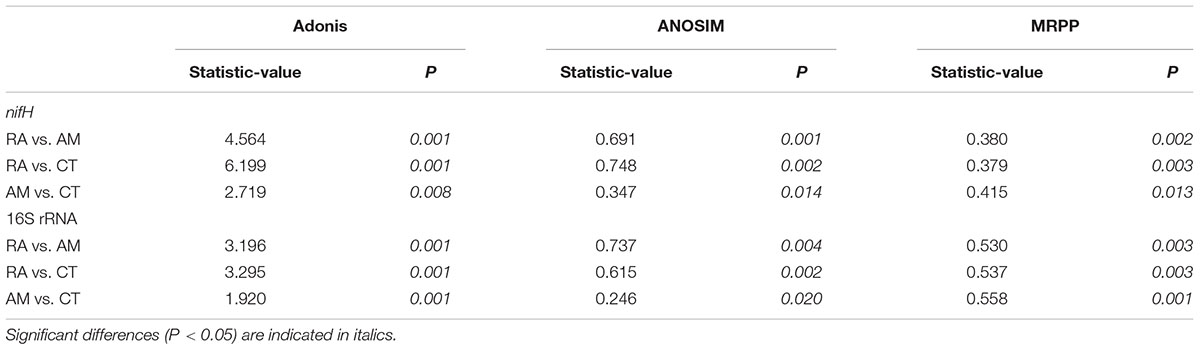
TABLE 3. Non-parametric analyses to test dissimilarity of 16S rRNA and nifH gene communities between any two mangrove rhizosphere sediments (RA, R. apiculata; AM, A. marina; CT, C. tagal).
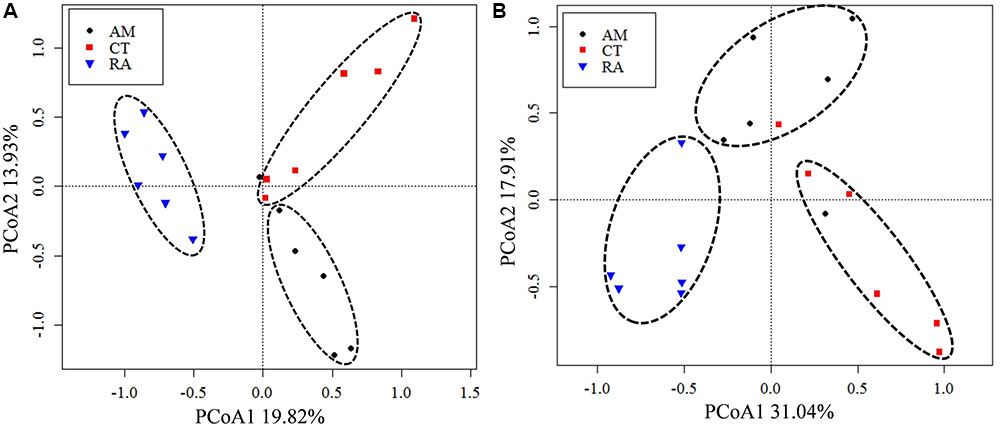
FIGURE 2. Principal coordinates analysis (PCoA) of total 16S rRNA (A) and nifH (B) gene communities based on high-throughput sequencing data. The percentage of variation explained by each axis is shown. RA, R. apiculata. AM, A. marina. CT, C. tagal.
After processing, 216,000 high-quality nifH sequences (283–323 bp) were retrieved from the mangrove rhizosphere sediments of R. apiculata, A. marina, and C. tagal. Samples were rarefied to 12,000 sequences per sample. All sequences obtained could be assigned to 1,334 OTUs at the 95% protein sequence similarity level (Dang et al., 2009, 2013; Zhou et al., 2016). The 1,334 unique nifH protein sequences shared 51–100% sequence identity with the top-match sequences obtained from GenBank. Among these, 591 unique protein sequences (comprising up to 82.82% of total sequences) shared quite high sequence identity (>90%) with nifH sequences of known bacteria or archaea, such as Deltaproteobacteria, Acidithiobacillia, Alphaproteobacteria, Gammaproteobacteria, Bacteroidetes, Cyanobacteria, Chlorobi, Deferribacteres, Epsilonproteobacteria, Firmicutes, Spirochaetes, Verrucomicrobia, and Euryarchaeota. Phylogenetic types of nifH genes were defined according to Zehr et al. (2003). The deduced nifH sequences were affiliated with four major groups in the reconstructed nifH phylogenetic tree (Figure 3 and Supplementary Figure S1). The nifH community was dominated by sequences belonging to Cluster I and Cluster III nifH clades, which accounted for 35.33 and 64.61% of the total captured sequences and 16.56 and 82.23% of the total OTUs, respectively. Only seven OTUs accounting for 0.52% of total sequences were found to belong to Cluster II, and nine OTUs accounting for 0.67% of total sequences were found in Cluster IV (Figure 3 and Supplementary Figure S1). R. apiculata harbored significantly more nifH sequences belonging to Cluster I and fewer nifH sequences belonging to Cluster III than did A. marina and C. tagal (Supplementary Tables S1, S2).
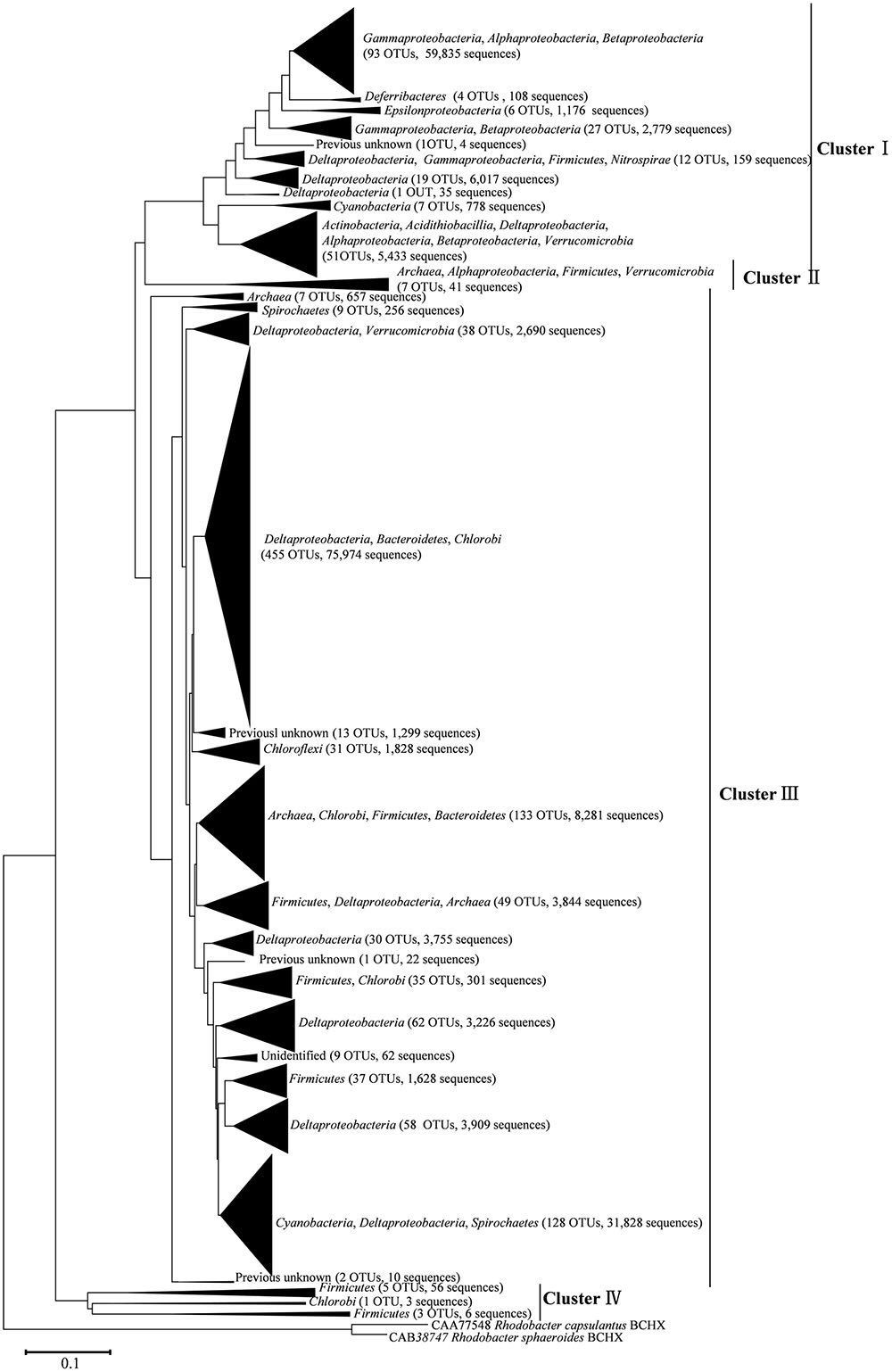
FIGURE 3. Skeleton phylogenetic tree of the nifH sequences reconstructed with the neighbor-joining method from aligned nifH sequences as shown in Supplementary Figure S1 in the Supplementary Material. Bootstrap values higher than 50% of 100 resamplings are shown near the corresponding nodes. The chlorophyllide reductase iron protein subunit BchX sequences from Rhodobacter capsulatus and Rhodobacter sphaeroides were used as an outgroup.
Family or higher taxonomic information was then assigned to the 1,334 nifH OTUs, according to their nearest taxonomic matches, for further analyses. All nifH sequences were classified into 1 archaeal and 11 bacterial phyla (Figure 1B). Sequences related to diazotrophs within the phylum Proteobacteria were the most abundant. Within the Proteobacteria, Deltaproteobacteria-related sequences were the most abundant group, making up an average of 60.59% of diazotrophic sequences. Gammaproteobacteria were the second major diazotrophic group, making up an average of 26.07% diazotrophic sequences (Figure 1B). The number of diazotrophic OTUs ranged from 490 to 715 across all the samples, with C. tagal harboring the highest number of OTUs among the three mangrove species. The Shannon–Wiener (H′) index ranged from 4.51 to 5.21, and Simpson evenness (E) ranged from 0.05 to 0.12 for individual samples. The average Shannon–Wiener (H′) index of diazotrophic diversity from A. marina was relatively lower than those of A. marina and C. tagal (Table 2). Dissimilarity tests showed that the diazotrophic communities were significantly different among mangrove species (P < 0.01) (Table 3). PCoA also showed significant variations in the diazotrophic communities from different mangrove species (Figure 2B).
The effect of mangrove tree species on 16S rRNA and nifH gene distribution was further investigated using the most dominant 16S rRNA (n = 17) and nifH (n = 15) gene groups (Figure 4). The results showed that most of the dominant microbial groups had no significant differences in relative abundance in the communities from the three mangrove species. Of these dominant 16S rRNA gene groups, Epsilonproteobacteria, Deferribacteres, and Euryarchaeota were highly abundant in rhizosphere sediment from R. apiculata, while Actinobacteria dominated in sediment from A. marina. However, Gammaproteobacteria and Deltaproteobacteria, the two most dominant bacterial groups across all of the mangrove species, were almost invariable (Figure 4A).
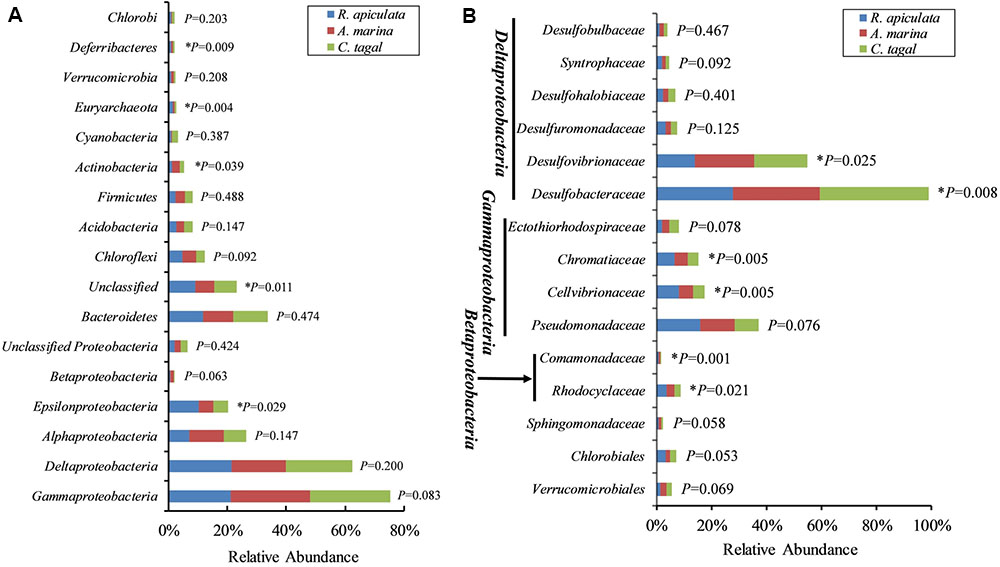
FIGURE 4. Relative abundance of the most abundant 16S rRNA and nifH gene groups. (A) The dominant 16S rRNA gene groups were categorized at the phylum level, except for the Proteobacteria, which were categorized by class. (B) The dominant nifH gene groups were classified to the family level. Asterisks (∗) represent significant differences among sediments of the three mangrove species (P < 0.05).
In contrast to the total microbial pattern at the phylum level, most of the dominant diazotrophic groups were significantly different among the three mangrove species (P < 0.05). The abundance of the two most dominant diazotrophic groups, Desulfobacteraceae and Desulfovibrionaceae, belonging to the Deltaproteobacteria, was significantly different among the mangrove species (P < 0.05), and these groups accounted for an average of 33.02 and 18.29% of total diazotrophic sequences, respectively. Desulfobacteraceae were highly abundant in rhizosphere sediment from C. tagal, while Desulfovibrionaceae dominated in A. marina. The Pseudomonadaceae of the Gammaproteobacteria, the third most dominant diazotrophic group, were almost invariable, accounting for an average of 12.38% of total diazotrophic sequences. Two dominant Betaproteobacteria diazotrophic groups, Rhodocyclaceae and Comamonadaceae, were relatively highly abundant in rhizosphere sediment from R. apiculata. The diazotrophic groups belonging to the Verrucomicrobia and Firmicutes were almost invariable (Figure 4B).
Redundancy analysis was performed to explore the relationship between 16S rRNA and nifH gene community structures with different environmental characteristics. The results showed that the composition of diazotrophic communities was significantly correlated with all investigated environmental factors (Monte Carlo test, P < 0.05; Table 4) except the concentration of TN. The total 16S rRNA gene community did not significantly correlate with TN or N/P ratios of mangrove sediments (Table 4). However, the diversity of both 16S rRNA and nifH genes did not significantly correlate with investigated environmental factors (Figure 5).
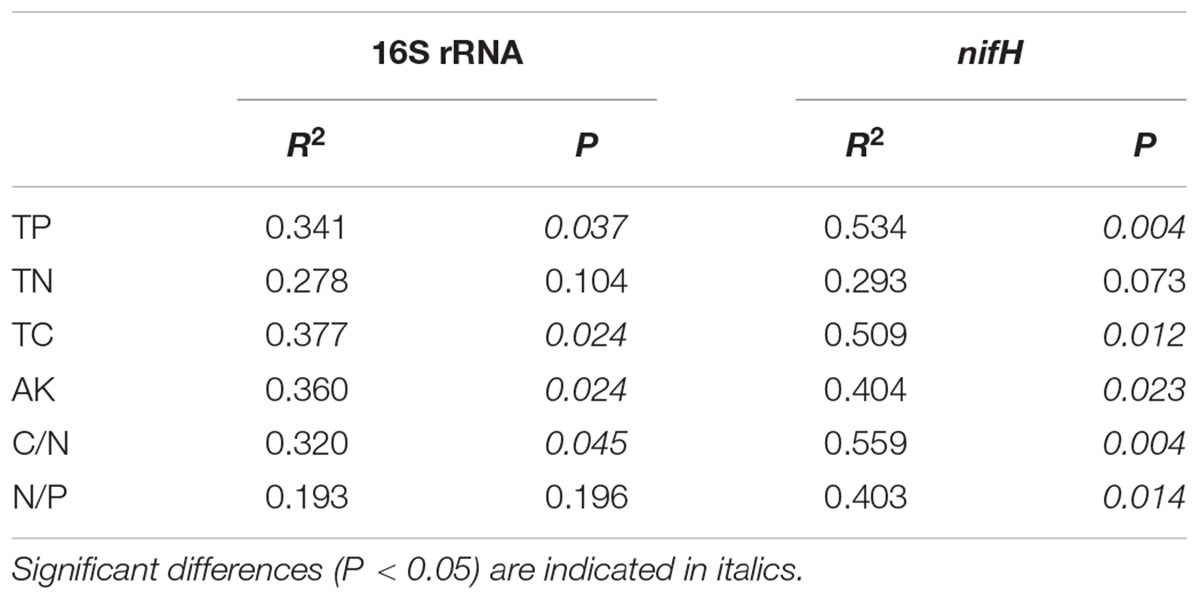
TABLE 4. Monte Carlo permutation test of relationship between environmental attributes and 16S rRNA and nifH gene high-throughput sequencing data.
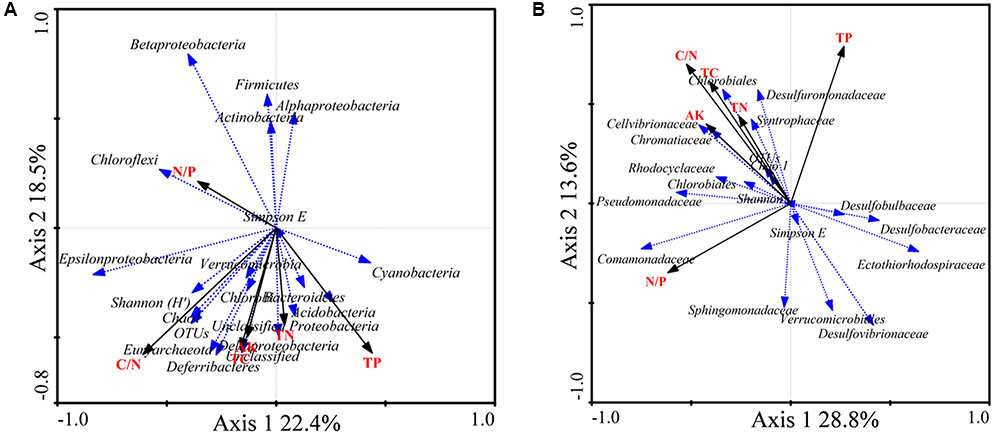
FIGURE 5. Redundancy analysis (RDA) ordination biplot showing the relationships between environmental variables and total 16S rRNA (A) and nifH (B) gene groups. Only the most abundant groups are shown in the biplot.
The RDA biplot showed that the abundance of sequences from the 16S rRNA groups Chloroflexi, Betaproteobacteria, Alphaproteobacteria, Firmicutes, and Actinobacteria was positively correlated with the N/P ratio. Gammaproteobacteria, Cyanobacteria, Acidobacteria, and Deltaproteobacteria were positively correlated with TP. In addition, Epsilonproteobacteria, Deferribacteres, and Euryarchaeota were positively correlated with the C/N ratio, TC, and AK (Figure 5A).
The diazotrophic groups Deltaproteobacteria, Desulfobulbaceae, and Desulfuromonadaceae were positively correlated with TP. The diazotrophic gammaproteobacterial families Pseudomonadaceae, Cellvibrionaceae, and Chromatiaceae were positively correlated with the C/N ratio, TC, TN, and AK. The diazotrophic groups Deltaproteobacteria, Desulfovibrio, and Desulfobacca were negatively correlated with the C/N ratio, TC, TN, and AK. Diazotrophic Deltaproteobacteria and Desulfobacter were positively correlated with the N/P ratio (Figure 5B).
Although high-throughput sequencing approaches are now commonly applied to investigate bacterial community structures in mangroves (Dos Santos et al., 2011; Andreote et al., 2012; Gomes et al., 2014; Loganathachetti et al., 2015; Alzubaidy et al., 2016; Wu et al., 2016), they have not been widely used to target nitrogen-fixing functional genes (nifH) to explore diazotrophic communities in urban mangrove rhizospheres (Jing et al., 2014). Compared with previous high-throughput sequencing studies on diazotrophic communities from the mangroves along the coastline of Singapore (Jing et al., 2014), we found a much greater diversity of bacteria and archaea having the potential to fix nitrogen from the three rhizosphere sediments from R. apiculata, A. marina, and C. tagal, which might be attributable to differences in mangrove species, environmental conditions, and primers used for analysis. The primers we used have a very broad coverage for both bacterial and archaeal nifH genes, and have been widely used in various environmental studies (Bernard et al., 2014; Hoppe et al., 2014; Keshri et al., 2015; Zhang et al., 2015; Newell et al., 2016; Penton et al., 2016; Tu et al., 2016; Rodrigues et al., 2017).
Previous studies have reported Deltaproteobacteria and Gammaproteobacteria as the dominant bacterial groups in rhizosphere sediments from many mangrove species (Gomes et al., 2014; Loganathachetti et al., 2015; Alzubaidy et al., 2016; Wu et al., 2016). In this present study, Deltaproteobacteria and Gammaproteobacteria were the dominant groups of both total bacterial and diazotrophic communities from all three mangrove species studied. These two groups constituted an average of 45.87 and 86.66% of total 16S rRNA and nifH gene communities, respectively (Figure 1), suggesting that these groups play an important functional role in the anaerobic conditions of the mangrove rhizosphere. Furthermore, there was a high relative abundance of diazotrophic groups from the Deltaproteobacteria in this study, such as Desulfobacteraceae, Desulfovibrionaceae, and Desulfuromonadaceae, belonging to the sulfate-reducing bacteria, which are known to play key roles in sedimentary cycling of N, C, and S (Lyimo et al., 2002; Varon-Lopez et al., 2014; Romero et al., 2015). Sulfate-reducing bacteria were also reported to be prevalent in pristine, anthropogenic, and oil-contaminated mangrove sediments (Santos et al., 2011; Jing et al., 2014; Wu et al., 2016), which suggests that sulfate-reducing bacteria may contribute substantially to both nitrogen fixation and sulfate reduction in the mangrove rhizosphere. Furthermore, our findings reinforce the prominence of sulfate-reducing bacteria as the main diazotrophic group in mangrove samples. In addition, diazotrophs from the Gammaproteobacteria were reported to be widespread in tropical and subtropical oceans (Bird et al., 2005; Jing et al., 2014). The family Pseudomonadaceae is a common constituent of mangrove rhizosphere diazotrophs (Zhang et al., 2008; Liu et al., 2012; Jing et al., 2014), and it was detected as the dominant group of the class Gammaproteobacteria in this study.
In addition, Bacteroidetes and Firmicutes, including a high abundance of sulfate reducers and methanogens, were dominant in sediments of A. marina (Alzubaidy et al., 2016). Bacteroidetes are very frequent in tidal mudflats or near-shore sediments, and an increased abundance of Bacteroidetes in the rhizospheres of mangroves has been noted for Rhizophora mangle, Avicennia schaueriana, and Laguncularia racemosa located in Guanabara Bay (Rio de Janeiro, Brazil) (Gomes et al., 2010). Actinobacteria, including mostly soil-borne microbes, were reported to be enriched in mangrove sediments in the Red Sea (Alzubaidy et al., 2016). Chloroflexi was the second most dominant phylum in three mangrove species in Beilun Estuary (Wu et al., 2016). Consistent with previous reports, bacteria from the phyla Bacteroidetes, Chloroflexi, Acidobacteria, and Firmicutes were also widespread in the rhizosphere sediments of R. apiculata, A. marina, and C. tagal.
Several factors shaping mangrove rhizosphere microbial communities have been proposed (Alzubaidy et al., 2016). Mangrove tree species and geochemical parameters were widely reported to be influential (Flores-Mireles et al., 2007; Zhang et al., 2008; Gomes et al., 2010, 2014; Romero et al., 2012; Jing et al., 2014; Wu et al., 2016). Mangrove roots have been suggested to be able to impose a selective force on the mangrove rhizosphere microbial communities. This phenomenon appeared to be plant species-specific (Gomes et al., 2014), resulting in the mangrove tree species playing an important role in shaping the rhizosphere microbial communities (Gomes et al., 2010, 2014; Pires et al., 2012; Wu et al., 2016). Mangrove root exudates may not only stimulate microbial respiration and create suboxic and anoxic microenvironments to facilitate nitrogen fixation, but also directly stimulate nitrogen fixation by providing metabolizable organic matter as carbon and energy sources to the diazotrophs (Dang and Lovell, 2016). The significant influence of the mangrove tree species on the rhizosphere microbial community was further confirmed in this study. Three non-parametric tests based on the Bray–Curtis distance matrix showed that both the total 16S rRNA and the nifH gene communities were significantly different among mangrove species (P < 0.02) (Table 2). The PCoA results also confirmed that the 16S rRNA and nifH gene communities could be divided into three groups corresponding to the respective mangrove species (Figure 2). The differences in 16S rRNA gene community composition from the three investigated mangrove species showed that the dominant 16S rRNA gene groups Epsilonproteobacteria, Actinobacteria, Deferribacteres, and Euryarchaeota were significantly different in abundance in association with different mangrove species (Figure 4A). Our results are consistent with the idea that root exudates select for specific groups at both the taxonomic level and the functional level (Alzubaidy et al., 2016). Most of the dominant diazotrophic groups were significantly different among mangrove species (P < 0.05). The possible reason for plant species-specific diazotrophic communities is that mangroves of different species and under different physiological conditions may secrete different types of organic matter, which selects different diazotrophic species to be functional (Dang and Lovell, 2016).
The importance of geochemical parameters in structuring 16S rRNA and nifH gene communities has been previously shown in mangrove ecosystems (Flores-Mireles et al., 2007; Zhang et al., 2008; Romero et al., 2012; Jing et al., 2014). Our results showed that both total 16S rRNA and nifH gene communities significantly correlated with most of the investigated environmental factors (Table 4). Mangrove root exudates provide a valuable source of carbon, while microorganisms that colonize the rhizosphere help plants acquire phosphorus and potassium; enhance nitrogen uptake; or even help the plants to cope with infection, toxic compounds, and other sources of stress (Singh et al., 2004; Kristensen et al., 2008). In this study, the fact that the highest 16S rRNA and nifH gene diversity was found in samples from the R. apiculata rhizosphere is not surprising, as these samples have a relatively high TC concentration. It has been reported that long-term fertilization with nitrogen and phosphorus not only affects the community structure and activity of diazotrophs, but also potentially plant–microbe interactions (Romero et al., 2012, 2015). Diazotroph diversity is reduced or completely eliminated by high levels of ammonia caused by anthropogenic activities, while relatively high phosphorus concentrations provide favorable conditions for nitrogen fixation (Jing et al., 2014). The abundance of the dominant diazotroph Desulfatibacillum in this study was positively correlated with TP, but negatively correlated with the N/P ratio. Since plants require potassium for numerous physiological processes such as growth and development, and protein synthesis, there is interest in bacteria such as Pseudomonas, which are capable of solubilizing potassium to an accessible form in the soil (Alzubaidy et al., 2016). In the present study, Pseudomonadaceae abundance was positively correlated with the concentration of AK. Among the family Pseudomonadaceae, most protein sequences shared quite high sequence identity (>90%) with those of the known bacteria Pseudomonas stutzeri, which further hints at the important role that diazotrophic Pseudomonas play in potassium cycling. It is notable that more than half of the variability (>50%) was unexplained by the host species or environmental variables investigated in this study, for both the 16S rRNA and nifH gene communities. Other unknown factors such as redox, pH, oxygen content, organic matter content, and salinity may be important in influencing the diversity, abundance, structure, and spatial distribution of the sediment nifH gene communities (Dang et al., 2009, 2013; Zhou et al., 2016).
Our study comprehensively characterized the diversity and structure of 16S rRNA and nifH gene communities in the rhizosphere of three mangrove species. Both the mangrove species and various environmental variables played important roles in shaping these communities. Most of the dominant diazotrophs were significantly different among mangrove species. However, our study was based on DNA abundance, so activity of nitrogenase cannot be confirmed. Therefore, expression-based studies such as mRNA-based microarray hybridization and metagenomic studies, together with in situ nitrogen-fixing measurements, are required to elucidate the role that diazotrophs play in the mangrove rhizosphere.
YZ, JL, and JD conceived the research. YZ, QY, and JL performed the experiments. YZ wrote the manuscript. JVN and JZ edited the manuscript. QY, JL, and ZS contributed sampling or data analysis pipelines. All authors reviewed and approved the manuscript.
The research was supported by the National Natural Science Foundation of China (41676107, 41276114, 41676163, and 41406191), National Key Research and Development Program of China (2017YFC0506301), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA13020300), Guangdong Province Public Welfare Research and Capacity Building Project (2015A020216016), and Science and Technology Planning Project of Guangdong Province, China (2014B030301064).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all of the members of the Tropical Marine Biological Research Station in Hainan for their assistance in field sample collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02032/full#supplementary-material
Alongi, D. M. (1988). Bacterial productivity and microbial biomass in tropical mangrove sediments. Microb. Ecol. 15, 59–79. doi: 10.1007/BF02012952
Alongi, D. M., Christoffersen, P., and Tirendi, F. (1993). The influence of forest type on microbial-nutrient relationships in tropical mangrove sediments. J. Exp. Mar. Bio. Ecol. 171, 201–223. doi: 10.1016/0022-0981(93)90004-8
Alzubaidy, H., Essack, M., Malas, T. B., Bokhari, A., Motwalli, O., Kamanu, F. K., et al. (2016). Rhizosphere microbiome metagenomics of gray mangroves (Avicennia marina) in the Red Sea. Gene 576, 626–636. doi: 10.1016/j.gene.2015.10.032
Andreote, F. D., Jimenez, D. J., Chaves, D., Dias, A. C. F., Luvizotto, D. M., Diniandreote, F., et al. (2012). The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLOS ONE 7:e38600. doi: 10.1371/journal.pone.0038600
Bai, S., Li, J., He, Z., Van Nostrand, J. D., Tian, Y., Lin, G., et al. (2013). GeoChip-based analysis of the functional gene diversity and metabolic potential of soil microbial communities of mangroves. Appl. Microbiol. Biotechnol. 97, 7035–7048. doi: 10.1007/s00253-012-4496-z
Bernard, R. J., Mortazavi, B., Wang, L., Ortmann, A. C., Macintyre, H., and Burnett, W. C. (2014). Benthic nutrient fluxes and limited denitrification in a sub-tropical groundwater-influenced coastal lagoon. Mar. Ecol. Prog. Ser. 504, 13–26. doi: 10.3354/meps10783
Bird, C., Martinez, M. J., O’Donnell, A. G., and Wyman, M. (2005). Spatial distribution and transcriptional activity of an uncultured clade of planktonic diazotrophic gamma-proteobacteria in the Arabian sea. Appl. Environ. Microbiol. 71, 2079–2085. doi: 10.1128/AEM.71.4.2079-2085.2005
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624. doi: 10.1038/ismej.2012.8
Dang, H., and Lovell, C. R. (2016). Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 80, 91–138. doi: 10.1128/MMBR.00037-15
Dang, H. Y., Luan, X. W., Zhao, J. Y., and Li, J. (2009). Diverse and novel nifH and nifH-like gene sequences in the deep-sea methane seep sediments of the Okhotsk Sea. Appl. Environ. Microbiol. 75, 2238–2245. doi: 10.1128/AEM.02556-08
Dang, H. Y., Yang, J. Y., Li, J., Luan, X. W., Zhang, Y. B., Gu, G. Z., et al. (2013). Environment-dependent distribution of the sediment nifH-harboring microbiota in the Northern South China Sea. Appl. Environ. Microbiol. 79, 121–132. doi: 10.1128/AEM.01889-12
Dos Santos, H. F., Cury, J. C., do Carmo, F. L., dos Santos, A. L., Tiedje, J., van Elsas, J. D., et al. (2011). Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: bacterial proxies for oil pollution. PLOS ONE 6:e16943. doi: 10.1371/journal.pone.0016943
Duke, N. C., Meynecke, J.-O., Dittmann, S., Ellison, A. M., Anger, K., Berger, U., et al. (2007). A world without mangroves? Science 317, 41–42. doi: 10.1126/science.317.5834.41b
Flores-Mireles, A. L., Winans, S. C., and Holguin, G. (2007). Molecular characterization of diazotrophic and denitrifying bacteria associated with mangrove roots. Appl. Environ. Microbiol. 73, 7308–7321. doi: 10.1128/AEM.01892-06
Gomes, N. C. M., Cleary, D. F. R., Pires, A. C., Almeida, A., Cunha, A., Mendoncahagler, L. C., et al. (2014). Assessing variation in bacterial composition between the rhizospheres of two mangrove tree species. Estuar. Coast. Shelf Sci. 139, 40–45. doi: 10.1016/j.ecss.2013.12.022
Gomes, N. C. M., Flocco, C. G., Costa, R., Junca, H., Vilchez, R., Pieper, D. H., et al. (2010). Mangrove microniches determine the structural and functional diversity of enriched petroleum hydrocarbon-degrading consortia. FEMS Microbiol. Ecol. 74, 276–290. doi: 10.1111/j.1574-6941.2010.00962.x
Hicks, B. J., and Silvester, W. B. (1985). Nitrogen fixation associated with the New Zealand mangrove (Avicennia marina (Forsk.) Vierh. var. resinifera (Forst. f.) Bakh.). Appl. Environ. Microbiol. 49, 955–959.
Holguin, G., Guzman, M. A., and Bashan, Y. (1992). Two new nitrogen-fixing bacteria from the rhizosphere of mangrove trees: their isolation, identification and in vitro interaction with rhizosphere Staphylococcus sp. FEMS Microbiol. Lett. 101, 207–216. doi: 10.1111/j.1574-6968.1992.tb05777.x
Holguin, G., Vazquez, P., and Bashan, Y. (2001). The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol. Fertil. Soils 33, 265–278. doi: 10.1007/s003740000319
Hoppe, B., Kahl, T., Karasch, P., Wubet, T., Bauhus, J., Buscot, F., et al. (2014). Network analysis reveals ecological links between N-fixing bacteria and wood-decaying fungi. PLOS ONE 9:e88141. doi: 10.1371/journal.pone.0088141
Jing, H., Xia, X., Liu, H., Zhou, Z., Wu, C., and Nagarajan, S. (2014). Anthropogenic impact on diazotrophic diversity in the mangrove rhizosphere revealed by nifH pyrosequencing. Front. Microbiol. 6:1172. doi: 10.3389/fmicb.2015.01172
Keshri, J., Yousuf, B., Mishra, A., and Jha, B. (2015). The abundance of functional genes, cbbl, nifH, amoA and apsA, and bacterial community structure of intertidal soil from arabian sea. Microbiol. Res. 175, 57–66. doi: 10.1016/j.micres.2015.02.007
Kong, Y. (2011). Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98, 152–153. doi: 10.1016/j.ygeno.2011.05.009
Kristensen, E., Bouillon, S., Dittmar, T., and Marchand, C. (2008). Organic carbon dynamics in mangrove ecosystems: a review. Aquat. Bot. 89, 201–219. doi: 10.1016/j.aquabot.2007.12.005
Kyaruzi, J., Kyewalyanga, M., and Muruke, M. (2003). Cyanobacteria composition and impact of seasonality on their in situ nitrogen fixation rate in a mangrove ecosystem adjacent to Zanzibar town. West. Indian Ocean J. Mar. Sci. 2, 35–44.
Liu, J., Peng, M., and Li, Y. (2012). Phylogenetic diversity of nitrogen-fixing bacteria and the nifH gene from mangrove rhizosphere soil. Can. J. Microbiol. 58, 531–539. doi: 10.1139/w2012-016
Loganathachetti, D. S., Sadaiappan, B., Poosakkannu, A., and Muthuraman, S. (2015). Pyrosequencing-based seasonal observation of prokaryotic diversity in pneumatophore-associated soil of Avicennia marina. Curr. Microbiol. 72, 68–74. doi: 10.1007/s00284-015-0920-9
Lugomela, C., and Bergman, B. (2002). Biological N2-fixation on mangrove pneumatophores: preliminary observations and perspectives. AMBIO 31, 612–613. doi: 10.1579/0044-7447-31.7.612
Lyimo, T. J., Pol, A., Op, and den Camp, H. J. M. (2002). Sulfate reduction and methanogenesis in sediments of Mtoni mangrove forest, Tanzania. AMBIO 31, 614–616. doi: 10.1579/0044-7447-31.7.614
Magoč, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Newell, S. E., Mccarthy, M. J., Gardner, W. S., and Fulweiler, R. W. (2016). Sediment nitrogen fixation: a call for re-evaluating coastal N budgets. Estuar. Coast. 39, 1–13. doi: 10.1007/s12237-016-0116-y
Penton, C. R., Yang, C., Wu, L., Wang, Q., Zhang, J., Liu, F., et al. (2016). NifH-harboring bacterial community composition across an alaskan permafrost thaw gradient. Front. Microbiol. 7:e42149. doi: 10.3389/fmicb.2016.01894
Pires, A. C. C., Cleary, D. F. R., Almeida, A., Cunha,Â, Dealtry, S., Mendonça-Hagler, L. C. S., et al. (2012). Denaturing gradient gel electrophoresis and barcoded pyrosequencing reveal unprecedented archaeal diversity in mangrove sediment and rhizosphere samples. Appl. Environ. Microbiol. 78, 5520–5528. doi: 10.1128/AEM.00386-12
Poly, F., Monrozier, L. J., and Bally, R. (2001). Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 152, 95–103. doi: 10.1016/S0923-2508(00)01172-4
Ravikumar, S., Kathiresan, K., Ignatiammal, S. T. M., Babu Selvam, M., and Shanthy, S. (2004). Nitrogen-fixing azotobacters from mangrove habitat and their utility as marine biofertilizers. J. Exp. Mar. Bio. Ecol. 312, 5–17. doi: 10.1016/j.jembe.2004.05.020
Rodrigues, R. R., Moon, J., Zhao, B., and Williams, M. A. (2017). Microbial communities and diazotrophic activity differ in the root-zone of alamo and dacotah switchgrass feedstocks. GCB Bioenergy 9, 1057–1070. doi: 10.1111/gcbb.12396
Romero, I. C., Jacobson, M., Fuhrman, J. A., Fogel, M., and Capone, D. G. (2012). Long-term nitrogen and phosphorus fertilization effects on N2 fixation rates and nifH gene community patterns in mangrove sediments. Mar. Ecol. 33, 117–127. doi: 10.1111/j.1439-0485.2011.00465.x
Romero, I. C., Jacobson-Meyers, M. E., Fuhrman, J. A., and Capone, D. G. (2015). Phylogenetic diversity of diazotrophs along an experimental nutrient gradient in mangrove sediments. J. Mar. Sci. Eng. 3, 699–719. doi: 10.3390/jmse3030699
Santos, H. F. D., Cury, J. C., Carmo, F. L. D., Santos, A. L. D., Tiedje, J., Elsas, J. D. V., et al. (2011). Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: bacterial proxies for oil pollution. PLOS ONE 6:e16943. doi: 10.1371/journal.pone.0016943
Schloss, P. D., and Handelsman, J. (2005). Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71, 1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005
Sengupta, A., and Chaudhuri, S. (1991). Ecology of heterotrophic dinitrogen fixation in the rhizosphere of mangrove plant community at the Ganges river estuary in India. Oecologia 87, 560–564. doi: 10.1007/BF00320420
Singh, B. K., Millard, P., Whiteley, A. S., and Murrell, J. C. (2004). Unravelling rhizosphere-microbial interactions: opportunities and limitations. Trends Microbiol. 12, 386–393. doi: 10.1016/j.tim.2004.06.008
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Thatoi, H., Behera, B. C., Mishra, R. R., and Dutta, S. K. (2013). Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Ann. Microbiol. 63, 1–19. doi: 10.1007/s13213-012-0442-7
Tu, Q., Zhou, X., He, Z., Xue, K., Wu, L., Reich, P., et al. (2016). The diversity and co-occurrence patterns of N2-fixing communities in a CO2-enriched grassland ecosystem. Microb. Ecol. 71, 604–615. doi: 10.1007/s00248-015-0659-7
Varon-Lopez, M., Dias, A. C. F., Fasanella, C. C., Durrer, A., Melo, I. S., Kuramae, E. E., et al. (2014). Sulphur-oxidizing and sulphate-reducing communities in Brazilian mangrove sediments. Environ. Microbiol. 16, 845–855. doi: 10.1111/1462-2920.12237
Vazquez, P., Holguin, G., Puente, M., Lopez-Cortes, A., and Bashan, Y. (2000). Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils 30, 460–468. doi: 10.1007/s003740050024
Wang, Q., Iii, J. F. Q., Fish, J. A., Lee, T. K., Sun, Y., Tiedje, J. M., et al. (2013). Ecological patterns of nifH genes in four terrestrial climatic zones explored with targeted metagenomics using FrameBot, a new informatics tool. mbio 4:e00592. doi: 10.1128/mBio.00592-13
Wu, P., Xiong, X., Xu, Z., Lu, C., Cheng, H., Lyu, X., et al. (2016). Bacterial communities in the rhizospheres of three mangrove tree species from Beilun Estuary, China. PLOS ONE 11:e0164082. doi: 10.1371/journal.pone.0164082
Zehr, J. P., Jenkins, B. D., Short, S. M., and Steward, G. F. (2003). Nitrogenase gene diversity and microbial community structure: a cross system comparison. Environ. Microbiol. 5, 539–554. doi: 10.1046/j.1462-2920.2003.00451.x
Zhang, X., Song, Y., Liu, D., Keesing, J. K., and Gong, J. (2015). Macroalgal blooms favor heterotrophic diazotrophic bacteria in nitrogen-rich and phosphorus-limited coastal surface waters in the Yellow Sea. Estuar. Coast. Shelf Sci. 163, 75–81. doi: 10.1016/j.ecss.2014.12.015
Zhang, Y., Dong, J., Yang, Z., Zhang, S., and Wang, Y. (2008). Phylogenetic diversity of nitrogen-fixing bacteria in mangrove sediments assessed by PCR–denaturing gradient gel electrophoresis. Arch. Microbiol. 190, 19–28. doi: 10.1007/s00203-008-0359-5
Zhou, H., Dang, H. Y., and Klotz, M. G. (2016). Environmental conditions outweigh geographical contiguity in determining the similarity of nifH-harboring microbial communities in sediments of two disconnected marginal seas. Front. Microbiol. 7:1111. doi: 10.3389/fmicb.2016.01111
Zuberer, D. A., and Silver, W. (1978). Biological dinitrogen fixation (acetylene reduction) associated with Florida mangroves. Appl. Environ. Microbiol. 35, 567–575.
Keywords: mangroves, microbial community, diazotrophs, nifH, high-throughput sequencing, sulfate-reducing bacteria
Citation: Zhang Y, Yang Q, Ling J, Van Nostrand JD, Shi Z, Zhou J and Dong J (2017) Diversity and Structure of Diazotrophic Communities in Mangrove Rhizosphere, Revealed by High-Throughput Sequencing. Front. Microbiol. 8:2032. doi: 10.3389/fmicb.2017.02032
Received: 31 March 2017; Accepted: 04 October 2017;
Published: 18 October 2017.
Edited by:
Hongyue Dang, Xiamen University, ChinaReviewed by:
Wei Xie, Tongji University, ChinaCopyright © 2017 Zhang, Yang, Ling, Van Nostrand, Shi, Zhou and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junde Dong, ZG9uZ2pkQHNjc2lvLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.