- 1College of Food and Biological Engineering, Henan University of Science and Technology, Luoyang, China
- 2Changbai Mountains Food and Drug Inspection Testing Center, Baishan, China
- 3Department of Market Supervision and Management, MuLing Food Inspection Testing Center, Mudanjiang, China
- 4Anda Department of Animal Husbandry and Veterinary, Anda, China
- 5Foodmicrobe.com, Nottingham, United Kingdom
Cronobacter sakazakii is an opportunistic pathogen that causes severe infections in neonates and infants through contaminated powdered infant formula (PIF). Therefore, the aim of this study was a large-scale study on determine the prevalence, molecular characterization and antibiotic susceptibility of C. sakazakii isolates from PIF purchased from Chinese retail markets. Two thousand and twenty PIF samples were collected from different institutions. Fifty-six C. sakazakii strains were isolated, and identified using fusA sequencing analysis, giving a contamination rate of 2.8%. Multilocus sequence typing (MLST) was more discriminatory than other genotyping methods. The C. sakazakii isolates were divided into 14 sequence types (STs) by MLST, compared with only seven clusters by ompA and rpoB sequence analysis, and four C. sakazakii serotypes by PCR-based O-antigen serotyping. C. sakazakii ST4 (19/56, 33.9%), ST1 (12/56, 21.4%), and ST64 (11/56, 16.1%) were the dominant sequence types isolated. C. sakazakii serotype O2 (34/56, 60.7%) was the primary serotype, along with ompA6 and rpoB1 as the main allele profiles, respectively. Antibiotic susceptibility testing indicated that all C. sakazakii isolates were susceptible to ampicillin-sulbactam, cefotaxime, ciprofloxacin, meropenem, tetracycline, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole. The majority of C. sakazakii strains were susceptible to chloramphenicol and gentamicin (87.5 and 92.9%, respectively). In contrast, 55.4% C. sakazakii strains were resistant to cephalothin. In conclusion, this large-scale study revealed the prevalence and characteristics of C. sakazakii from PIF in Chinese retail markets, demonstrating a potential risk for neonates and infants, and provide a guided to effective control the contamination of C. sakazakii in production process.
Introduction
Cronobacter spp. are emerging foodborne opportunistic pathogens that can infect neonates and infants resulting in necrotizing enterocolitis, bacteremia, and meningitis, with a 40–80% mortality rate (Holy and Forsythe, 2014; Li et al., 2016). These organisms have be isolated from various food sources, including spiced meat, ready-to-eat foods, dehydrated rice powder, retail foods, and powdered infant formula (PIF) (Iversen and Forsythe, 2004; Hochel et al., 2012; Joseph et al., 2012a; Huang et al., 2015; Xu et al., 2015; Zhang et al., 2016; Brandão et al., 2017).
The genus Cronobacter has been divided into seven species: Cronobacter sakazakii, Cronobacter malonaticus, Cronobacter turicensis, Cronobacter muytjensii, Cronobacter dublinensis, Cronobacter universalis, and Cronobacter condimenti (Joseph et al., 2012a,b; Yan et al., 2012). Among them, C. sakazakii is considered as the predominant species associated with neonatal infections (Forsythe et al., 2014). The consumption of contaminated PIF is the main reason for the occurrence of neonatal infections (Drudy et al., 2006). In production process of PIF, the addition of heat sensitive material, spray drying, fluidized-bed-drying, filling, and packing are the possible links with C. sakazakii contamination (Nazarowec-White and Farber, 1997; Pan et al., 2014; Fei et al., 2015). Because of the strong ability to resist desiccation environment, C. sakazakii strains can persist in PIF for more than 1 year (Osaili and Forsythe, 2009). Therefore, the presence of C. sakazakii in commercial PIF needs to be monitored.
Multilocus sequence typing (MLST), O-antigen serotyping, ompA analysis, and rpoB analysis can be used to reveal the molecular characterization of Cronobacter spp. (Joseph et al., 2012c; Cui et al., 2014; Forsythe et al., 2014; Fei et al., 2015). More than 2,000 Cronobacter isolates have been divided into >600 sequence types (STs) using MLST, details of which are recorded in the open access MLST database (http://pubmlst.org/cronobacter/; Forsythe et al., 2014; Ogrodzki and Forsythe, 2017). O-antigen serotyping associated with lipopolysaccharide (LPS) structure is used to type Cronobacter strains for epidemiological purposes (Jarvis et al., 2013; Blažková et al., 2015). The O-antigen serotyping scheme based on multiplex polymerase chain reaction (PCR) has been designed, but this method appears to be less discriminatory than MLST which has >600 defined STs (Sun et al., 2012; Mueller et al., 2013; Ogrodzki and Forsythe, 2015). The outer membrane protein A (ompA) of C. sakazakii plays an important role in invading human intestinal epithelial cells and brain microvascular endothelial cells (Mohan Nair and Venkitanarayanan, 2007; Singamsetty et al., 2008). The sequence analysis of ompA gene has been applied to identify and type this pathogen for purposes of pathogenicity (Mohan Nair and Venkitanarayanan, 2006; Fei et al., 2015). Furthermore, rpoB allele sequence is also included in the international PubMLST database (Fei et al., 2015). Therefore, a comprehensive comparative analysis of C. sakazakii strains isolated from PIF using MLST, O-antigen serotyping, ompA scheme, and rpoB scheme is warranted.
Currently, antibiotic therapy is the most common and effective method to treat Cronobacter infections (Depardieu et al., 2007). A majority of Cronobacter spp. stains are reported to be susceptible to frequently-used antibiotics, however, long-term use or abuse of antibiotics is likely to lead to the development of Cronobacter antibiotic resistance (Yoneyama and Katsumata, 2006; McMahon et al., 2007). Cronobacter strains resistant to amoxicillin-clavulanate, ampicillin, cefazolin, cephalothin, cefotaxime, and streptomycin have been isolated from food samples (Molloy et al., 2009; Ye et al., 2010; Chon et al., 2012; Lee et al., 2012; Pan et al., 2014; Fei et al., 2017). Therefore, it is necessary to evaluate the antibiotic resistance of Cronobacter spp. isolated from PIF. PIF is a major food product in China, and the safety of PIF is of particular concern. Our previous study isolated and typed C. sakazakii and C. malonaticus strains from PIF and production environment of PIF from 2009 to 2012 (Fei et al., 2015). As a continuing research project, the aim of this large-scale study was to determine the prevalence and molecular characterization and of C. sakazakii isolates from PIF purchased from Chinese retail markets from January 2015 to March 2017. In addition, the antibiotic susceptibility of these strains was determined to assess any changes in C. sakazakii antibiotic resistance compared with earlier studies.
Materials and Methods
Sample Collection
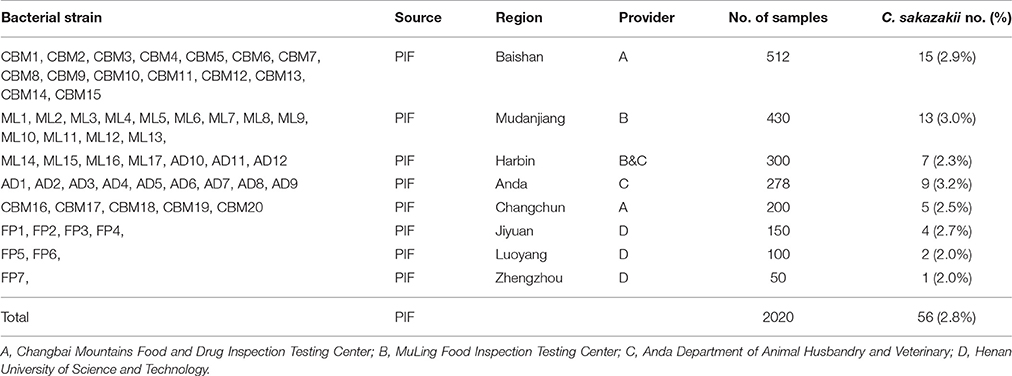
A total of 2,020 PIF samples were collected from Chinese retail markets for the isolation and identification of C. sakazakii strains from January 2015 to March 2017. These PIF samples were from eight cities in three provinces (512 PIF samples from Baishan, 430 PIF samples from Mudanjiang, 300 PIF samples from Harbin, 278 samples from Anda, 200 PIF samples from Changchun, 150 samples from Jiyuan, 100 samples from Luoyang, 50 samples from Zhengzhou; Figure 1, Table S1). Samples were transported to laboratories, and stored cool until further analysis.
Isolation and Identification of Bacterial Strains
C. sakazakii strains were isolated and identified as according to the national food safety standard method for food microbiological examination as used in China GB4789.40-2010 (Ministry of Health of the People's Republic of China, 2010). One hundred gram portions of PIF samples were dissolved in 900 mL of buffered peptone water (BPW, Beijing Obostar Biotechnology Co. Ltd., China), and incubated at 37 ± 1°C for 18 ± 2 h. One milliliter overnight culture was inoculated into 10 mL modified lauryl sulfate tryptose broth-vancomycin medium (mLST-Vm, Beijing Obostar Biotechnology Co. Ltd., China), followed by further selective cultivation at 44 ± 0.5°C or 24 ± 2 h. The cultures were streaked onto Druggan-Forsythe-Iversen (DFI, Beijing Obostar Biotechnology Co. Ltd., China) and incubated at 36 ± 1°C for 24 ± 2 h. Typical Cronobacter colonies (blue-green colored colonies) were selected and presumptively identified using the API 20E system. Finally, the identity of the strains was confirmed as C. sakazakii using fusA sequencing (Joseph et al., 2012c; Forsythe et al., 2014).
DNA Extraction
All isolates were incubated in brain–heart infusion (BHI) broth at 37°C for 18 h, and streaked on Tryptic Soy Agar (TSA) plates, followed by incubation at 37°C for 24 h to obtain isolated colonies. A single colony of each strain was inoculated into the BHI and cultivated at 37°C for 18 h. Approximately 2 mL above-mentioned culture was used to extract genomic DNA of isolates by TIANamp Bacterial DNA Kit (TIANGEN BIOTECH (BEIJING) Co., Ltd., Beijing, China).
MLST Analysis
The MLST scheme was carried out according to Baldwin et al. (2009). Seven housekeeping genes (atpD, fusA, glnS, gltB, gyrB, infB, and ppsA) was amplified and sequenced in Beijing Genomics Institute (BGI, Beijing China). The sequences were aligned in the Cronobacter PubMLST database (http://www.pubmlst.org/cronobacter) to determine type sequence (ST) of C. sakazakii isolates. The phylogenetic relationship based on the concatenated sequences composed of seven loci (3,036 bp length) was analyzed using Neighbor-joining algorithm in MEGA6, with 1,000 bootstrap replicates. The equivalent concatenated sequences from C. sakazakii ATCC29544T, C. sakazakii ATCC BAA-894, C. sakazakii ATCC29004, C. sakazakii ATCC12868, C. malonaticus CDC 105877T, C. dublinensis LMG 23823T, C. turicensis LMG 23827T, C. universalis NCTC 9529T, C. condimenti LMG 26250T, and C. muytjensii ATCC 51329T were used as species specific reference strains.
OmpA and rpoB Sequence Analysis
The ompA and rpoB of C. sakazakii were amplified as described by previous studies (Mohan Nair and Venkitanarayanan, 2007; Stoop et al., 2009). The PCR products of ompA and rpoB were sequenced (BGI, Beijing China), and the sequencing results were aligned in Cronobacter PubMLST database to determine the allele of ompA and rpoB.
O-antigen Serotype Analysis
C. sakazakii isolates were serotyped using multiplex serotyping PCR, mainly according to the previous reports (Jarvis et al., 2011; Sun et al., 2012). Five pairs of primers representing C. sakazakii serotypes O1, O2, O3, O4, and O7 were mixed to perform the multiplex serotyping PCR (Sun et al., 2012; Blažková et al., 2015). The sizes of the PCR products were used to determine the serotype of C. sakazakii isolates.
Antibiotic Susceptibility Testing
The Kirby-Bauer disc diffusion method on the basis of the guidelines of the Clinical Laboratory Standards Institute (CLSI, 2015) was used to evaluate the antibiotic susceptibility of 56 C. sakazakii isolates. Ampicillin-sulbactam (10:10 μ g), cephalothin (30 μg), cefotaxime (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), meropenem (10 μg), piperacillin-tazobactam (100:10 μg), tetracycline (30 μg), and trimethoprim-sulfamethoxazole (1.25:23.75 μg) were selected for the susceptibility test. The results were expressed as sensitive (S), intermediate (I), and resistant (R) according to the CLSI guidelines. Escherichia coli ATCC 25922 was used as the quality control organism.
Results
Prevalence of C. sakazakii in PIF from Chinese Retail Markets
C. sakazakii strains were isolated from 56 out of 2,020 (2.8%) PIF samples in Chinese retail markets, and were provisionally identified using API 20E system and confirmed using fusA sequencing analysis. As shown in Table 1, the highest percentage of C. sakazakii isolates was detected in PIF from Anda (3.2%, 9/78), followed by Mudanjiang (3.0%, 13/430), Baishan (2.9%, 15/512), Jiyuan (2.7%, 4/150), Changchun (2.5%, 7/200), Harbin (2.3%, 7/300), Luoyang (2.0%, 2/100), and Zhengzhou (2.0%, 1/50).
MLST Analysis
Fifty-six C. sakazakii strains were divided into 14 sequence types, including ST4 (19/56, 33.93%), ST1 (12/56, 21.43%), ST64 (9/56, 16.07%), ST8 (3/56, 5.36%), ST12 (2/56, 3.57%), ST17 (2/56, 3.57%), ST83 (2/56, 3.57%), ST21 (1/56, 1.79%), ST22 (1/56, 1.79%), ST31 (1/56, 1.79%), ST40 (1/56 1.79%), ST50 (1/56, 1.79%), ST259 (1/56, 1.79%), ST261 (1/56, 1.79%), respectively, shown in Table 2. Therefore, ST4, ST1, and ST 64 were considered to be the dominant type sequences of C. sakazakii in PIF from Chinese retail markets. The information of all 56 C. sakazakii strains were submitted to the Cronobacter PubMLST database (http://www.pubmlst.org/cronobacter) with PubMLST IDs 2005 to 2060. A Neighbor-Joining tree based on the concatenated sequences of the seven loci (3,036 bp) for the 56 C. sakazakii isolates and 10 reference strains was constructed (Figure 2). The phylogenetic tree showed a clear relatedness between 14 sequence types; ST4, ST1, ST64, ST8, ST12, ST17, ST83, ST21, ST22, ST31, ST40, ST50, ST259, and ST261.
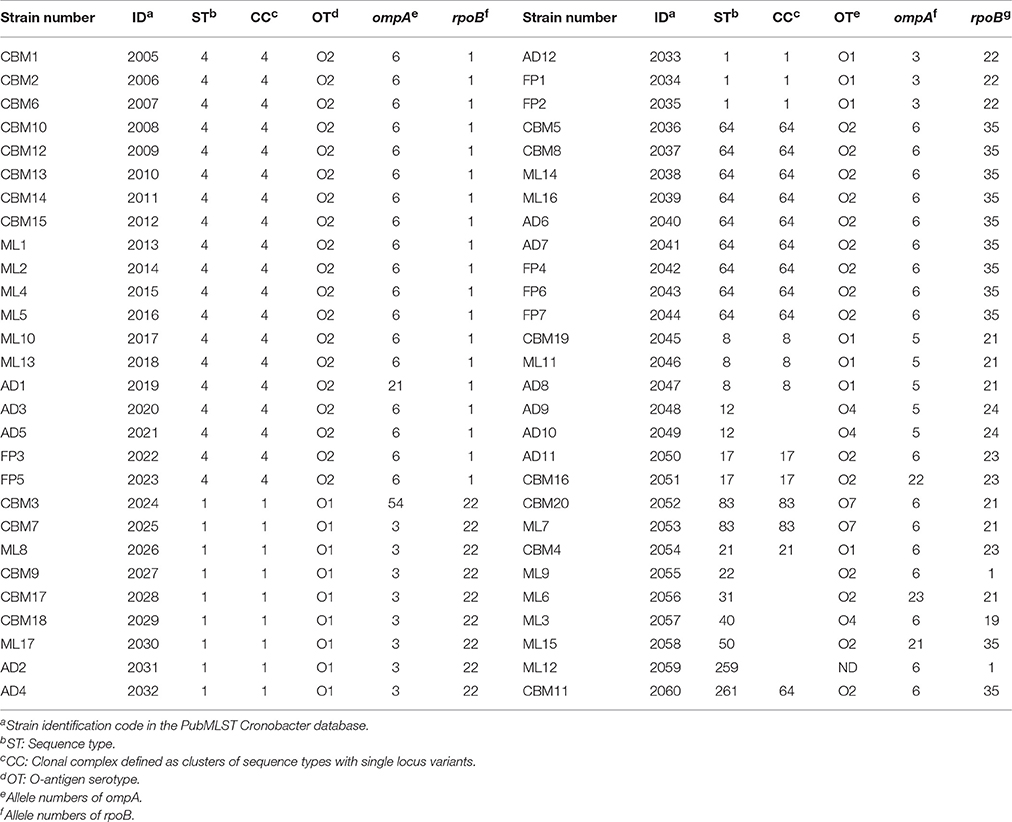
Table 2. Molecular characterization of C. sakazakii strains isolated from PIF in Chinese retail markets.
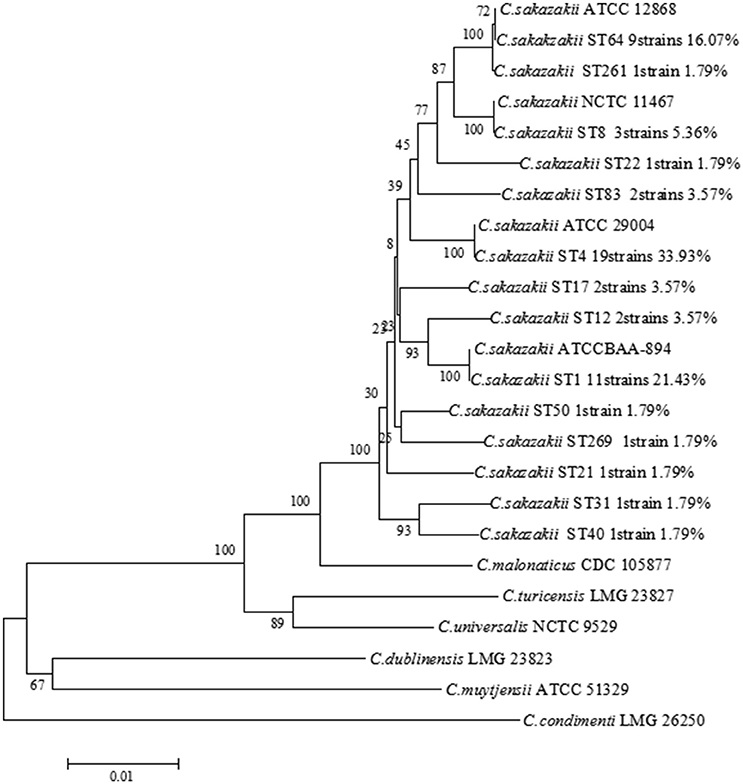
Figure 2. Neighbor-joining tree of MLST 7 loci (3,036 bp) of C. sakazakii strains isolated from PIF in Chinese retail markets. C. sakazakii ATCC29544T, C. sakazakii ATCC BAA-894, C. sakazakii ATCC29004, C. sakazakii ATCC12868, C. malonaticus CDC 105877T, C. dublinensis LMG 23823T, C. turicensis LMG 23827T, C. universalis NCTC 9529T, C. condimenti LMG 26250T, and C. muytjensii ATCC 51329T were used as the reference strains. The tree was generated using MEGA 6.0 with 1,000 bootstrap replicates.
OmpA and rpoB Analysis
The nucleotide sequences of ompA and rpoB were compared with the Cronobacter PubMLST database to obtain their allele numbers (Table 2). The 56 C. sakazakii strains contained 7 ompA allele numbers (ompA6, ompA3, ompA5, ompA21, ompA22, ompA23, and ompA54) and 7 rpoB allele numbers (rpoB1, rpoB19, rpoB21, rpoB22, rpoB23, rpoB24, and rpoB35), respectively. OmpA allele 6 (35/56, 62.5%) was dominant, and included nine sequence types; ST4, ST64, ST17, ST21, ST22, ST40, ST83, ST259, and ST261. Meanwhile, rpoB allele 1 (21/56, 37.5%) included three sequence types; ST4, ST22, and ST259 was the main allele number.
O-antigen Serotype Analysis
According to the size of the target gene, 56 C. sakazakii isolates were divided into several C. sakazakii serotypes, including C. sakazakii serotype O2 (34/56, 60.71%), C. sakazakii serotype O1 (16/56, 28.57%), C. sakazakii serotype O4 (3/56, 5.36%), and C. sakazakii serotype O7 (2/56, 3.57%; Table 2). The C. sakazakii serotype O2 was the dominant serotype for PIF from Chinese retail markets, and was composed of C. sakazakii ST4, ST64, ST17, ST22, ST31, ST50, and ST261. In addition, C. sakazakii serotype O1 included C. sakazakii ST1, ST8, ST21, C. sakazakii serotype O4 was composed of C. sakazakii ST12 and ST40, C. sakazakii serotype O7 contained two strains which belonged to ST83. The serotype of C. sakazakii ML12 (ST259) could not be determined using the standard multiplex serotyping PCR method.
Antibiotic Resistance Profiles
The antibiotic susceptibility of the 56 C. sakazakii strains isolated from PIF is shown in Table 3. All C. sakazakii isolates were susceptible to ampicillin-sulbactam, cefotaxime, ciprofloxacin, meropenem, tetracycline, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole. The majority of C. sakazakii strains were susceptible to chloramphenicol and gentamicin, with sensitive rates of 87.5 and 92.9%, respectively. In contrast, most C. sakazakii strains were resistant to cephalothin, with resistance and intermediate rates of 55.4 and 41.0%, respectively.
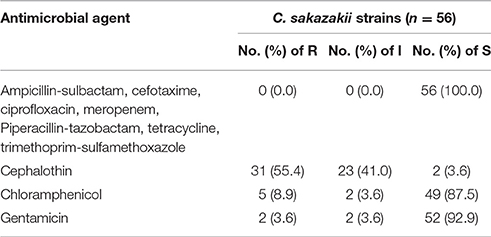
Table 3. Antibiotic susceptibility of 56 C. sakazakii strains isolated from PIF in Chinese retail markets.
Discussion
C. sakazakii is the dominant species in Cronobacter spp. associated with the infection of newborns through contaminated PIF, therefore, the issue of PIF contamination by C. sakazakii is a matter of continuing concern. Many studies have focused on the isolation and identification of Cronobacter spp. in PIF for evaluating the contamination of PIF by C. sakazakii and related species (FAO/WHO, 2004, 2008; Hoque et al., 2010; Pan et al., 2014; Xu et al., 2014). In our previous study, 66 C. sakazakii strains and 4 C. malonaticus strains were isolated from 1,228 PIF samples and a wet processing factory of PIF between 2009 to 2012 (Fei et al., 2015). As a continuing study, 56 C. sakazakii strains were isolated and identified from 2,020 PIF samples from Chinese retail markets sampled between July 2015 and March 2017. Giving a contamination rate of 2.8%. The contamination rate in this study is lower than the previous data provided by Pan et al. (2014) (12.3%, 49 out of 399) and Xu et al. (2014) (4.3%, 23 out of 530). Our results can contribute toward to an improved understanding and improvement in the surveillance of C. sakazakii in commercial PIF available in China.
The samples used for this test were collected from eight cities in three provinces. In the three provinces, the main sequence types of isolates from PIF in retail markets were ST4, ST1, and ST64, which agrees with previous studies and two of which (ST1 and ST4) are major Cronobacter pathovars (Sonbol et al., 2013; Fei et al., 2015; Ogrodzki and Forsythe, 2017). However, there were some difference in the composition of STs between three provinces. A total of 12 C. sakazakii STs were found in PIF from Heilongjiang province, among them, ST12, ST22, ST31, ST40, ST50, and ST259 were not detected in both Jilin province and Henan province. Eight C. sakazakii STs were isolated from PIF collected from Jinlin province, ST21 and ST261 were unique in this region. In Henan province, only three C. sakazakii STs (ST4, ST1, and ST64) were found. These finding revealed the relationship between C. sakazakii STs and regions, which contribute to make better targeted prevention and control measures in the different regions.
A total of 56 C. sakazakii isolates were genotyped into 14 STs by MLST, among them, C. sakazakii ST4 was the main sequence type of Cronobacter spp., and was associated with neonatal meningitis (Joseph and Forsythe, 2011; Joseph et al., 2012c; Forsythe et al., 2014). Meanwhile, C. sakazakii isolates belonging to ST4 had a stronger ability to resistance to desiccation than ST1, ST8, ST12, ST21, ST64, ST201, and ST258, which may be one of reasons that ST4 was the main sequence type recovered from PIF (Fei et al., 2017). C. sakazakii ST83 is another major sequence type with a strong capacity to resistance to desiccation in PIF factories (Chase et al., 2017). C. sakazakii ST1 is reported to be a major sequence type of strains from PIF, while C. sakazakii ST8 strains are primarily isolated from clinical sources (Sonbol et al., 2013). In addition, C. sakazakii ST12 can infect neonates and infants to suffer from necrotizing enterocolitis (Forsythe et al., 2014). The C. sakazakii strains with these STs have been isolated from commercial PIF, which suggests that ST4, ST1, ST8, ST12, and ST83 should be more risk for neonates and infants.
OmpA and rpoB analysis can be used to identify and genotype the Cronobacter spp. OmpA6 was the main cluster of C. sakazakii isolated from PIF in Chinese retail markets, and corresponded with C. sakazakii ST4 associated with neonatal meningitis, besides, ompA21 also been found in C. sakazakii ST4 strains. Meanwhile, rpoB1 containing ST4, ST22, and ST259 was the predominant, and overlapped with those in ompA6. In addition, compared with MLST, the ompA and rpoB analysis were less discriminatory.
O-antigen serotype analysis can improve the understanding of C. sakazakii on pathogenicity. Previously, C. sakazakii species had been classified into seven O-antigen serotypes (Sun et al., 2012). However, a new report indicated C. sakazakii serotype O5 and O6 should be classified as C. malonaticus serotype O2 and O3, respectively (Blažková et al., 2015). Therefore, in this study, five pairs of primers representing C. sakazakii serotypes O1, O2, O3, O4, and O7 were mixed to perform the multiplex serotyping PCR. Meanwhile, C. sakazakii serotype O2 and O1 were the main O-antigen serotypes, which had been confirmed to be particularly predominant in clinical cases by Blažková et al. (2015). C. sakazakii ST83 and C. sakazakii O7 strains can survive in PIF and PIF processing environment for several years, and infect neonates with a high risk (Chase et al., 2017). Our result indicated there was a correlation between O-antigen serotype O7 and ST 83, which was consistent with the finding of Mueller et al. (2013).
Antibiotic susceptibility tests showed that all 56 C. sakazakii strains were susceptible to ampicillin-sulbactam, cefotaxime, ciprofloxacin, meropenem, piperacillin-tazobactam, tetracycline, and trimethoprim-sulfamethoxazole. Similarly, the resistance of these antibiotics in Cronobacter spp. isolates from PIF, ready-to-eat foods, Brazilian retail foods, and desiccated foods in Korea is common (Chon et al., 2012; Hochel et al., 2012; Xu et al., 2015; Fei et al., 2017). In addition, 8.9 and 3.6% isolates were resistant to chloramphenicol and gentamicin, respectively. This ratio was greater than previous reports (Al-Nabulsi et al., 2011; Lee et al., 2012; Zhang et al., 2016), which may be due to the continued use of antibiotics in clinical practice (Yoneyama and Katsumata, 2006).
In conclusion, the contamination of C. sakazakii strains in PIF was still evident in products from Chinese retail markets. The finding of our study detected the prevalence and levels of C. sakazakii strains in PIF from Chinese retail markets, and revealed the molecular characterization and antibiotic resistance of these isolates. These results contributes to monitoring the contamination of commercial PIF for C. sakazakii, and provide a basis for improved control and reduce neonatal exposure to the organism.
Author Contributions
Conceived and designed the experiments: PF, HK, and SF. Performed the experiments: PF, YiJ, YaJ, XY, and ZW. Generated and analyzed the data: TY and JC. Wrote the paper: PF, HK, and SF.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the Doctor Scientific Research Start-up Fund of Henan University of Science and Technology (13480066), also thanks to the supports of Changbai mountains food and drug inspection testing center, MuLing food inspection testing center, and Anda department of animal husbandry and veterinary.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02026/full#supplementary-material
References
Al-Nabulsi, A. A., Osaili, T. M., Elabedeen, N. A., Jaradat, Z. W., Shaker, R. R., Kheirallah, K. A., et al. (2011). Impact of environmental stress desiccation, acidity, alkalinity, heat or cold on antibiotic susceptibility of Cronobacter sakazakii. Int. J. Food Microbiol. 146, 137–143. doi: 10.1016/j.ijfoodmicro.2011.02.013
Baldwin, A., Loughlin, M., Caubilla-Barron, J., Kucerova, E., Manning, G., Dowson, C., et al. (2009). Multilocus sequence typing of Cronobacter sakazakii and Cronobacter malonaticus reveals stable clonal structures with clinical significance which do not correlate with biotypes. BMC Microbiol. 9:223. doi: 10.1186/1471-2180-9-223
Blažková, M., Javurková, B., Vlach, J., Göselová, S., Karamonová, L., Ogrodzki, P., et al. (2015). Diversity of O antigens within the genus Cronobacter: from disorder to order. Appl. Environ. Microbiol. 81, 5574. doi: 10.1128/AEM.00277-15
Brandão, M. L., Umeda, N. S., Jackson, E., Forsythe, S. J., and De, F. I. (2017). Isolation, molecular and phenotypic characterization, and antibiotic susceptibility of Cronobacter spp. from Brazilian retail foods. Food Microbiol. 63, 129–138. doi: 10.1016/j.fm.2016.11.011
Chase, H. R., Gopinath, G. R., Eshwar, A. K., Stoller, A., Fricker-Feer, C., Gangiredla, J., et al. (2017). Comparative genomic characterization of the highly persistent and potentially virulent Cronobacter sakazakii ST83, CC65 strain H322 and other ST83 strains. Front. Microbiol. 8:1136. doi: 10.3389/fmicb.2017.01136
Chon, J.-W., Song, K.-Y., Kim, S.-Y., Hyeon, J.-Y., and Seo, K.-H. (2012). Isolation and characterization of Cronobacter from desiccated foods in Korea. J. Food Sci. 77, 354–358. doi: 10.1111/j.1750-3841.2012.02750.x
CLSI (2015). “Disk diffusion,” in Performance Standards for Antimicrobial Susceptibility Testing; 15th Informational Supplement, Vol. 35 (Wayne, PA).
Cui, J., Du, X., Liu, H., Hu, G., Lv, G., Xu, B., et al. (2014). The Genotypic Characterization of Cronobacter spp. isolated in China. PLoS ONE 9:e102179. doi: 10.1371/journal.pone.0102179
Depardieu, F., Podglajen, I., Leclercq, R., Collatz, E., and Courvalin, P. (2007). Modes and modulations of antibiotic resistance gene expression. Clin. Microbiol. Rev. 20, 79–114. doi: 10.1128/CMR.00015-06
Drudy, D., Mullane, N. R., Quinn, T., Wall, P. G., and Fanning, S. (2006). Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin. Infect. Dis. 42, 996–1002. doi: 10.1086/501019
FAO/WHO (2004). Enterobacter sakazakii and Other Organisms in Powdered Infant Formula. Microbiological Risk Assessment Series 6. Geneva. Available online at: http://www.who.int/foodsafety/publications/mra6-enterobacter-sakazakii/en/
Fei, P., Jiang, Y., Feng, J., Forsythe, S. J., Li, R., Zhou, Y., et al. (2017). Antibiotic and desiccation resistance of Cronobacter sakazakii and C. malonaticus isolates from powdered infant formula and processing environments. Front. Microbiol. 8:316. doi: 10.3389/fmicb.2017.00316
Fei, P., Man, C., Lou, B., Forsythe, S. J., Chai, Y., Li, R., et al. (2015). Genotyping and source tracking of Cronobacter sakazakii and C. malonaticus isolates from powdered infant formula and an infant formula production factory in China. Appl. Environ. Microbiol. 81, 5430–5439. doi: 10.1128/AEM.01390-15
Forsythe, S. J., Dickins, B., and Jolley, K. A. (2014). Cronobacter, the emergent bacterial pathogen Enterobacter sakazakii comes of age; MLST and whole genome sequence analysis. BMC Genomics 15:1121. doi: 10.1186/1471-2164-15-1121
Hochel, I., Ružičkova, H., Krásný, L., and Demnerová, K. (2012). Occurrence of Cronobacter spp. in retail foods. J. Appl. Microbiol. 112:1257. doi: 10.1111/j.1365-2672.2012.05292.x
Holy, O., and Forsythe, S. (2014). Cronobacter spp. as emerging causes of healthcare-associated infection. J. Hosp. Infect. 86, 169–177. doi: 10.1016/j.jhin.2013.09.011
Hoque, A., Ahmed, T., Shahidullah, M., Hossain, A., Mannan, A., Noor, K., et al. (2010). Isolation and molecular identification of Cronobacter spp. from powdered infant formula (PIF) in Bangladesh. Int. J. Food Microbiol. 142:375. doi: 10.1016/j.ijfoodmicro.2010.07.019
Huang, Y., Pang, Y., Wang, H., Tang, Z., Zhou, Y., Zhang, W., et al. (2015). Occurrence and characterization of Cronobacter spp. in dehydrated rice powder from Chinese supermarket. PLoS ONE 10:e0131053. doi: 10.1371/journal.pone.0131053
Iversen, C., and Forsythe, S. (2004). Isolation of Enterobacter sakazakii and other Enterobacteriaceae from powdered infant formula milk and related products. Food Microbiol. 21, 771–777. doi: 10.1016/j.fm.2004.01.009
Jarvis, K. G., Grim, C. J., Franco, A. A., Gopinath, G., Sathyamoorthy, V., Hu, L., et al. (2011). Molecular characterization of Cronobacter lipopolysaccharide O-Antigen gene clusters and development of serotype-specific PCR assays. Appl. Environ. Microbiol. 77, 4017–4026. doi: 10.1128/AEM.00162-11
Jarvis, K. G., Yan, Q. Q., Grim, C. J., Power, K. A., Franco, A. A., Hu, L., et al. (2013). Identification and characterization of five new molecular serogroups of Cronobacter spp. Foodborne Pathog. Dis. 10, 343–352. doi: 10.1089/fpd.2012.1344
Joseph, S., and Forsythe, S. J. (2011). Predominance of Cronobacter sakazakii sequence type 4 in neonatal infections. Emerg. Infect. Dis. 17, 1713–1715. doi: 10.3201/eid1709.110260
Joseph, S., Cetinkaya, E., Drahovska, H., Levican, A., Figueras, M. J., and Forsythe, S. J. (2012a). Cronobacter condimenti sp nov., isolated from spiced meat, and Cronobacter universalis sp nov., a species designation for Cronobacter sp genomospecies, recovered from a leg infection, water and food ingredients. Int. J. Syst. Evol. Microbiol. 62, 1277–1283. doi: 10.1099/ijs.0.032292-0
Joseph, S., Desai, P., Ji, Y., Cummings, C. A., Shih, R., Degoricija, L., et al. (2012b). Comparative analysis of genome sequences covering the seven Cronobacter species. PLoS ONE 7:e49455. doi: 10.1371/journal.pone.0049455
Joseph, S., Sonbol, H., Hariri, S., Desai, P., McClelland, M., and Forsythe, S. J. (2012c). Diversity of the Cronobacter genus as revealed by multilocus sequence typing. J. Clin. Microbiol. 50, 3031–3039. doi: 10.1128/JCM.00905-12
Lee, Y. D., Park, J. H., and Chang, H. I. (2012). Detection, antibiotic susceptibility and biofilm formation of Cronobacter spp. from various foods in Korea. Food Control 24, 225–230. doi: 10.1016/j.foodcont.2011.09.023
Li, R., Fei, P., Man, C. X., Lou, B. B., Niu, J. T., Feng, J., et al. (2016). Tea polyphenols inactivate Cronobacter sakazakii isolated from powdered infant formula. J. Dairy Sci. 99, 1019–1028. doi: 10.3168/jds.2015-10039
McMahon, M. A. S., Xu, J., Moore, J. E., Blair, I. S., and McDowell, D. A. (2007). Environmental stress and antibiotic resistance in food-related pathogens. Appl. Environ. Microbiol. 73, 211–217. doi: 10.1128/AEM.00578-06
Ministry of Health of the People's Republic of China (2010). GB4789.40-2010. National Food Safety Standard: Food Microbiological Examnation: Enterobacter sakazakii[S]. Beijing: China Standard Press.
Mohan Nair, M. K., and Venkitanarayanan, K. (2007). Role of bacterial OmpA and host cytoskeleton in the invasion of human intestinal epithelial cells by Enterobacter sakazakii. Pediatr. Res. 62, 664–669. doi: 10.1203/PDR.0b013e3181587864
Mohan Nair, M. K., and Venkitanarayanan, K. S. (2006). Cloning and sequencing of the ompA gene of Enterobacter sakazakii and development of an ompA-targeted PCR for rapid detection of Enterobacter sakazakii in infant formula. Appl. Environ. Microbiol. 72:2539. doi: 10.1128/AEM.72.4.2539-2546.2006
Molloy, C., Cagney, C., O'Brien, S., Iversen, C., Fanning, S., and Duffy, G. (2009). Surveillance and characterisation by pulsed-field gel electrophoresis of Cronobacter spp. in farming and domestic environments, food production animals and retail foods. Int. J. Food Microbiol. 136, 198–203. doi: 10.1016/j.ijfoodmicro.2009.07.007
Mueller, A., Stephan, R., Fricker-Feer, C., and Lehner, A. (2013). Genetic diversity of Cronobacter sakazakii isolates collected from a Swiss infant formula production facility. J. Food Prot. 76, 883–887. doi: 10.4315/0362-028X.JFP-12-521
Nazarowec-White, M., and Farber, J. M. (1997). Thermal resistance of Enterobacter sakazakii in reconstituted dried-infant formula. Lett. Appl. Microbiol. 24, 9–13. doi: 10.1046/j.1472-765X.1997.00328.x
Ogrodzki, P., and Forsythe, S. (2015). Capsular profiling of the Cronobacter genus and the association of specific Cronobacter sakazakii and C. malonaticus capsule types with neonatal meningitis and necrotizing enterocolitis. BMC Genomics 16:758. doi: 10.1186/s12864-015-1960-z
Ogrodzki, P., and Forsythe, S. J. (2017). DNA-sequence based typing of the Cronobacter genus using MLST, CRISPR-cas array and capsular profiling. Front. Microbiol. 8:1875. doi: 10.3389/fmicb.2017.01875
Osaili, T., and Forsythe, S. (2009). Desiccation resistance and persistence of Cronobacter species in infant formula. Int. J. Food Microbiol. 136, 214–220. doi: 10.1016/j.ijfoodmicro.2009.08.006
Pan, Z., Cui, J., Lyu, G., Du, X., Qin, L., Guo, Y., et al. (2014). Isolation and molecular typing of Cronobacter spp. in commercial powdered infant formula and follow-up formula. Foodborne Pathog. Dis. 11, 456–461. doi: 10.1089/fpd.2013.1691
Singamsetty, V. K., Wang, Y., Shimada, H., and Prasadarao, N. V. (2008). Outer membrane protein a expression in Enterobacter sakazakii is required to induce microtubule condensation in human brain microvascular endothelial cells for invasion. Microb. Pathog. 45, 181–191. doi: 10.1016/j.micpath.2008.05.006
Sonbol, H., Joseph, S., McAuley, C. M., Craven, H. M., and Forsythe, S. J. (2013). Multilocus sequence typing of Cronobacter spp. from powdered infant formula and milk powder production factories. Int. Dairy J. 30, 1–7. doi: 10.1016/j.idairyj.2012.11.004
Stoop, B., Lehner, A., Iversen, C., Fanning, S., and Stephan, R. (2009). Development and evaluation of rpoB based PCR systems to differentiate the six proposed species within the genus Cronobacter. Int. J. Food Microbiol. 136, 165–168. doi: 10.1016/j.ijfoodmicro.2009.04.023
Sun, Y., Wang, M., Wang, Q., Cao, B., He, X., Li, K., et al. (2012). Genetic analysis of the Cronobacter sakazakii O4 to O7 O-antigen gene clusters and development of a PCR Assay for identification of all C. sakazakii O-serotypes. Appl. Environ. Microbiol. 78, 3966–3974. doi: 10.1128/AEM.07825-11
Xu, X., Li, C., Wu, Q., Zhang, J., Huang, J., and Yang, G. (2015). Prevalence, molecular characterization, and antibiotic susceptibility of Cronobacter spp. in Chinese ready-to-eat foods. Int. J. Food Microbiol. 204, 17–23. doi: 10.1016/j.ijfoodmicro.2015.03.003
Xu, X., Wu, Q., Zhang, J., Ye, Y., Yang, X., and Dong, X. (2014). Occurrence and characterization of Cronobacter spp. in powdered formula from Chinese retail markets. Foodborne Pathog. Dis. 11, 307–312. doi: 10.1089/fpd.2013.1657
Yan, Q. Q., Condell, O., Power, K., Butler, F., Tall, B. D., and Fanning, S. (2012). Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: a review of our current understanding of the biology of this bacterium. J. Appl. Microbiol. 113, 1–15. doi: 10.1111/j.1365-2672.2012.05281.x
Ye, Y., Wu, Q., Xu, X., Yang, X., Dong, X., and Zhang, J. (2010). The phenotypic and genotypic characterization of Enterobacter sakazakii strains from infant formula milk. J. Dairy Sci. 93, 2315–2320. doi: 10.3168/jds.2009-2662
Yoneyama, H., and Katsumata, R. (2006). Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci. Biotechnol. Biochem. 70, 1060–1075. doi: 10.1271/bbb.70.1060
Keywords: C. sakazakii, prevalence, genotyping, antibiotic susceptibility, powdered infant formula (PIF)
Citation: Fei P, Jiang Y, Jiang Y, Yuan X, Yang T, Chen J, Wang Z, Kang H and Forsythe SJ (2017) Prevalence, Molecular Characterization, and Antibiotic Susceptibility of Cronobacter sakazakii Isolates from Powdered Infant Formula Collected from Chinese Retail Markets. Front. Microbiol. 8:2026. doi: 10.3389/fmicb.2017.02026
Received: 23 July 2017; Accepted: 04 October 2017;
Published: 17 October 2017.
Edited by:
Javier Carballo, University of Vigo, SpainReviewed by:
Ondřej Holý, Palacký University, CzechiaGonçalo Nieto Almeida, Instituto Nacional de Investigação Agrária e Veterinária, Portugal
Copyright © 2017 Fei, Jiang, Jiang, Yuan, Yang, Chen, Wang, Kang and Forsythe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaibin Kang, a2hiaW4wMDFAMTYzLmNvbQ==
Stephen J. Forsythe, c2ZvcnN5dGhlNGpAZ21haWwuY29t
 Peng Fei
Peng Fei Yichao Jiang2
Yichao Jiang2 Stephen J. Forsythe
Stephen J. Forsythe