- 1Department of Biological Sciences, University of Calgary, Calgary, AB, Canada
- 2Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad, Novi Sad, Serbia
- 3Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
- 4Department of Biochemistry and Molecular Biology in the Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 5Department of Geoscience, University of Calgary, Calgary, AB, Canada
- 6Institute of Computational Biotechnology, Graz University of Technology, Graz, Austria
Oil sands process-affected water (OSPW), produced by surface-mining of oil sands in Canada, is alkaline and contains high concentrations of salts, metals, naphthenic acids, and polycyclic aromatic compounds (PAHs). Residual hydrocarbon biodegradation occurs naturally, but little is known about the hydrocarbon-degrading microbial communities present in OSPW. In this study, aerobic oxidation of benzene and naphthalene in the surface layer of an oil sands tailings pond were measured. The potential oxidation rates were 4.3 μmol L−1 OSPW d−1 for benzene and 21.4 μmol L−1 OSPW d−1 for naphthalene. To identify benzene and naphthalene-degrading microbial communities, metagenomics was combined with stable isotope probing (SIP), high-throughput sequencing of 16S rRNA gene amplicons, and isolation of microbial strains. SIP using 13C-benzene and 13C-naphthalene detected strains of the genera Methyloversatilis and Zavarzinia as the main benzene degraders, while strains belonging to the family Chromatiaceae and the genus Thauera were the main naphthalene degraders. Metagenomic analysis revealed a diversity of genes encoding oxygenases active against aromatic compounds. Although these genes apparently belonged to many phylogenetically diverse taxa, only a few of these taxa were predominant in the SIP experiments. This suggested that many members of the community are adapted to consuming other aromatic compounds, or are active only under specific conditions. 16S rRNA gene sequence datasets have been submitted to the Sequence Read Archive (SRA) under accession number SRP109130. The Gold Study and Project submission ID number in Joint Genome Institute IMG/M for the metagenome is Gs0047444 and Gp0055765.
Introduction
The Athabasca oil sands reserves, located in northern Alberta, Canada, contribute more than 50% of the total crude oil production in Canada (National Energy Board, 2015). It is estimated that bitumen production could reach 5–6 million barrels per day by 2050 if demand persists (Rahnama et al., 2013), although the availability of shale oil and alternative fuels may depress this demand. For every cubic meter of bitumen extracted, 4 m3 of fluid tailings are produced. This consists of oil sands process-affected water (OSPW), sand, clays, residual bitumen and dissolved inorganic and organic compounds (Holowenko et al., 2002; Quagraine et al., 2005). Fluid tailings are deposited into open ponds to allow settling of particles, reuse of surface water for extraction, and long-term pollutant containment for on-site reclamation (Government of Alberta, 2009).
In general, OSPW is alkaline (pH 7.8–8) with high contents of salts (2.2 g L−1), metals, sulfides, naphthenic acids (NAs), and polycyclic aromatic compounds (PAHs) (Quagraine et al., 2005; Kelly et al., 2009, 2010; Saidi-Mehrabad et al., 2013). Some of the organic contaminants are derived from the naphtha (usually a mixture of C3–C14 alkanes, BTEX, and iso-paraffins) used as a diluent in processing of bitumen, and others are derived from bitumen (Siddique et al., 2007). The NAs and PAHs are particular compounds of concern (Rogers et al., 2002; Quagraine et al., 2005; Allen, 2008; Wayland et al., 2008; Grewer et al., 2010), and questions have been raised about the potential movement of these compounds from tailings ponds into surrounding environments (Wayland et al., 2008). PAHs and heterocyclic aromatic compounds account for 0.01–10 mg kg−1 of fine tails in Alberta tailings ponds (Fine Tailings Fundamentals Consortium (FTFC), 1995; Wayland et al., 2008). Tailings ponds are predominantly anoxic, except for a (roughly 1-m-deep) surface water cap that is partially oxygenated and supports some aerobic microbial activities such as methane oxidation (Saidi-Mehrabad et al., 2013). Previous studies on biodegradation of compounds of concern in tailings ponds have therefore concentrated on anoxic conditions. Relatively little is known about activities in the more oxic surface water (Foght et al., 2017).
The major aerobic degradation pathways for both saturated and aromatic hydrocarbons involve the addition of oxygen atoms in reactions carried out by oxygenase enzymes (Atlas, 1981). Genes encoding dioxygenases, monooxygenases, and extradiol ring-cleavage enzymes were abundant in metagenomes constructed from surface water samples of oil sands tailings ponds (An et al., 2013; Saidi-Mehrabad et al., 2013), showing a diversity of putative hydrocarbon degradation mechanisms. Many of the bacterial genera detected in the surface water are known to have potential for aerobic hydrocarbon degradation, including Brevundimonas (Caulobacterales), Methylocaldum (Methylococcales); Xanthobacter (Rhizobiales), Flavobacterium (Flaviobacteriales), and diverse members of the order Burkholderiales (An et al., 2013; Saidi-Mehrabad et al., 2013). Aerobic microbial communities in oil sands tailings ponds therefore may have potential in bioremediation (Golby et al., 2012; Saidi-Mehrabad et al., 2013).
The importance of aerobic hydrocarbon degradation has been demonstrated in many environments (DeLaune et al., 1980; Atlas, 1991; Das and Chandran, 2011). Aerobic microbial populations are known to be pivotal in the reclamation of industrially affected waters (Oller et al., 2011; Tocchi et al., 2012). Although oil sands tailings ponds are largely anoxic at present, understanding aerobic degradation processes is valuable because some of the reclamation strategies proposed for them involve increasing the depth of the oxic water cap. The “wet-landscape” approach proposes the conversion of tailings ponds to End-Pit Lakes or other wetlands (Allen, 2008; Wayland et al., 2008). In an End-Pit Lake strategy, freshwater (and OSPW) is used to form a deeper water cap on a tailings pond, in order to increase O2 supply (thereby accelerating biodegradation), provide seed microbes and algae, and dilute the contaminants present (Allen, 2008; Wayland et al., 2008). It is expected that tailings will settle, density, release pore water into the water column, and detoxify naturally over time.
In this study, we combined metagenomics, Stable Isotope Probing (SIP), high-throughput sequencing of 16S rRNA gene amplicons, and cultivation to examine bacterial communities responsible for the degradation of two model aromatic hydrocarbons, benzene and naphthalene, in the oxic surface OSPW layer of an oil sands tailings pond.
Materials and Methods
Sampling and Water Chemistry
The West-In-Pit (WIP) of Syncrude Canada, Ltd, established in 1997, was primarily used as a storage facility for fluid fine tailings and recycle water supply before its closure in December 2012 and repurposing as the first model End-Pit Lake for the oil sands industry in Canada (Syncrude, 2012). Before closure, the pond stored approximately 200 Mm3 of fluid fine tailings, capped with a minimum 5-m-deep layer (30 Mm3) of OSPW. Tailings porewater from this operation has previously been noted to contain 2,600 ng L−1 of total PAH, including 101 ng L−1 (780 nM) naphthalene (Madill et al., 1999). The OSPW used in this study was surface water (0–10 cm) sampled in August 2011. The chemical composition of the OSPW and the water sampling methods were described previously (Saidi-Mehrabad et al., 2013). Throughout this article, OSPW sampled from the WIP tailings pond is termed WIP-OSPW.
Potential Hydrocarbon Oxidation Rates
Potential rates of benzene and naphthalene oxidation (i.e., rates with excess substrate added) were measured in 20 mL (benzene) or 40 mL (naphthalene) amounts of OSPW in 120-mL serum vials, which were sealed with butyl rubber stoppers (20 mm). Vials containing MilliQ water were used as abiotic controls, and vials containing unamended OSPW were used as controls with no substrate addition. There is a small amount of hydrocarbon and other substrates in OSPW, therefore a slow CO2 increase is expected. Hydrocarbon-amended samples, unfiltered OSPW controls, and MilliQ water controls were run in triplicates.
For benzene oxidation, 1 μl (= 11 μmol) of benzene (Sigma-Aldrich, St Louis, MO, USA) was added to 20 mL OSPW. Using a Henry's law constant of 0.18 M atm−1, the initial benzene concentration was 0.30 mM in the liquid phase and 0.075 mmol L−1 in the gas phase. For naphthalene degradation, a 4.0 mM naphthalene (Sigma-Aldrich) stock was prepared in a 20-mL inert non-degradable carrier 2, 2, 4, 4, 6, 8, 8-heptamethylnonane (HMN; Sigma-Aldrich) and 10-mL aliquots were added to 40-mL OSPW sample volumes. The concentration of naphthalene in HMN is well above the saturation point of naphthalene in water (about 0.24 mM), and therefore it is assumed that naphthalene is saturating, and that calculations of naphthalene degradation in the system need only account for the HMN phase.
Bottles were incubated at 20°C on a rotary shaker at 180 rpm to ensure aeration, for 23 days (benzene) or 14 days (naphthalene). At regular intervals, benzene and naphthalene were measured by gas chromatography as previously described (Berdugo-Clavijo et al., 2012; Fowler et al., 2012). Oxidation rates were calculated by linear regression of benzene and naphthalene depletion over time. Headspace CO2 and O2 were monitored by injection of gas samples into a Varian 450-GC gas chromatograph equipped with a thermal conductivity detector (150°C), after separation in a 2 mm × 0.5 m Hayesep N and a 2 mm × 1.2 m Molecular Sieve 16X column in series (70°C). CO2 production rates were calculated by linear regression of CO2 production vs. time.
Stable Isotope Probing (SIP)
To identify microbial communities actively assimilating the model aromatic compounds, isotopically fully-labeled benzene (13C6H6 99 atom%, Sigma-Aldrich) and naphthalene (13C10H8 99 atom%) were used in DNA-SIP. To concentrate bacteria, 150 mL of OSPW were filtered through a 0.2-μm polysulfone filter (Pall Life Sciences, East Hills, NY, USA). The filter and 20 mL of OSPW were added to 100-mL serum vials and supplemented with 5 μM of labeled benzene or naphthalene. Vials containing 20 mL of filtered OSPW without added substrate were used as controls. Headspace CO2 was monitored for all SIP-enrichments and incubations were terminated when CO2 levels in the microcosms increased by around 1.0 μmol of CO2. After experimenting with different incubation times (3, 7, 9, and 14 days, data not shown), we chose the earliest times at which a shift toward heavy density fraction was visible in CsCl gradients, indicating 13C incorporation into microbial genomes. Shifts were visible after 7 days (naphthalene) and 9 days (benzene), of incubation. Cells were collected by centrifugation for 10 min at 10,000 × g. DNA was extracted, purified, separated by isopycnic centrifugation in CsCl, and fractionated as described previously (Sharp et al., 2012). DNA extracted from OSPW incubated for the same time without addition of substrates was used as a control to determine the expected position of unlabeled DNA in CsCl gradients. Heavy 13C-labeled DNA fractions of labeled samples were selected as shown in Supplementary Figure S1. The corresponding highest-density fractions of OSPW receiving the same treatments (OSPW-heavy), and unfractionated DNA from fresh OSPW (OSPW-control) were used as controls.
Amplification of 16S rRNA genes, sequencing on a Roche 454 GS FLX, and analysis using QIIME were all carried out as described previously (Sharp et al., 2014). Briefly, 16S rRNA genes were amplified from the gradient fractions using FLX Titanium amplicon primers 454T_RA_X and 454T_F containing the 16S rRNA gene targeted primers 926f and 1392r at their 3′- ends, along with adaptors necessary for the Roche Titanium chemistry (Sharp et al., 2014). The QIIME software platform was used to analyze the sequences (Caporaso et al., 2010). Low-quality sequences were removed based on a minimum quality score of 25, Operational Taxonomic Units (OTUs) clustered based on 97% sequence identity, chimeras removed via ChimeraSlayer, and sequences classified via BLAST against the Greengenes database. Taxonomic identifications were verified by manual BLAST of representative OTU sequences against the NCBI database.
Metagenomics
Metagenomic DNA from OSPW was prepared and extracted as described previously (Saidi-Mehrabad et al., 2013). DNA concentration was determined with a Qubit Fluorometer using a Quant-iT dsDNA HS Assay Kit (Invitrogen, Carlsbad, CA, USA). The DNA (12 μg) was sequenced using a combination of Roche 454 GS FLX and paired-end Illumina HiSeq2000 platforms at the Genome Quebec and McGill University Innovation Centre, Montreal, Quebec. Quality control and assembly of metagenomic data was performed at the University of Calgary Visual Genomics Center, Calgary, Canada according to previous reports (Saidi-Mehrabad et al., 2013; Tan et al., 2013; Aguilar et al., 2016). Annotation was conducted by submission to the Joint Genome Institute IMG platform (Markowitz et al., 2012).
Unassembled read based analysis was performed on the Illumina HiSeq2000 output due to the higher sequencing depth, although similar results were obtained when 454 reads were used. Clipping of the Illumina adapters, quality-trimming and size-filtering for the raw reads was performed using a BBDuk tool (trimk = 27, trimq = 20; minlen = 80, http://jgi.doe.gov/data-and-tools/bbtools/). Curated databases for key genes encoding enzymes for aerobic benzene and naphthalene degradation were constructed in a two-step process. First, core datasets for each gene were created by retrieving DNA sequences from NCBI Gene database using Enzyme Commission numbers and gene names. Next, the core datasets were extended by querying their entries using BLASTN against the NCBI non-redundant and environmental samples databases (Coordinators, 2013). Sequences with nucleotide identities of 70% and above were added to the corresponding datasets. Processed metagenomic sequences were then queried against the reference datasets using BLASTN. Reads with minimum nucleotide sequence identities of 50% and alignment lengths of 75 bp were recruited. Custom python and bash scripts were created to run BLASTN, process the output, and quantify and recruit reads matching the specified criteria. Scripts are available at the GitHub repository (release v1.14.3, https://github.com/dunfieldlab/mg_wrapser). Recruited reads, corresponding to each dataset, were then queried by BLASTN against the NCBI non-redundant database to validate functional identity and processed with MEtaGenome ANalyzer (MEGAN v.5.11.3) for taxonomic assignment (Huson et al., 2007).
Isolation of Hydrocarbon Degrading Bacteria
OSPW samples were initially enriched for 14 days with either 5 mM of benzene or naphthalene added (99.9%, Sigma-Aldrich). To cultivate bacterial strains from these enrichments, both complex media targeting versatile chemotrophs and basal mineral salts media containing the model hydrocarbons as sole carbon and energy sources were used. Enriched OSPW was diluted 1 : 10 with MilliQ water and 100-μl aliquots spread on 20% strength R2A (20R2A) plates adjusted to pH 8 with NaOH, as well as on a modification of the basal mineral salts medium M10 (Reasoner and Geldreich, 1985). M10 was prepared as described previously (Heyer et al., 2002), except that agar was substituted with 1.5% phytagel (Sigma-Aldrich), and MilliQ water was substituted with 0.2-μm-filtered OSPW to reproduce the natural conditions of tailings water. We named these modified media containing filtered tailings water 20R2A-T and M10-T. To specifically grow naphthalene-degrading microorganisms, a 10-fold serial dilution of the initial enrichment was applied (1 mL enrichment sample and 9 mL MilliQ water). Dilutions up to (10−10) were plated onto solid medium M10-T prepared in 125-mL wide-mouth jars (I-Chem, VWR, Radnor, PA, USA). These growth vessels were inverted and 3 g of naphthalene placed on the lids to diffuse naphthalene vapor as previously described (Jeon et al., 2004). For benzene-degrading microorganisms, OSPW samples enriched with 5 mM benzene were serially diluted as above, spread onto 20R2A-T in wide-mouth jars, and incubated with the addition of 0.1 mL of benzene per jar at 20°C for 60–70 days.
Colonies were picked and streaked onto new plates of the same medium until pure colonies were obtained. Colony PCR was performed to screen the isolates. Single colonies were picked with autoclaved toothpicks and frozen at –80°C overnight in 0.2-mL tubes to lyse cells. 16S rRNA genes were amplified using the universal primer set 9f and 1492b (Weisburg et al., 1991). PCR reaction conditions were: initial denaturation at 94°C for 10 min, followed by 35 cycles of 1 min at 94°C, 1 min at 56°C and 2 min at 72°C, and a 10-min final elongation at 72°C. PCR products were visualized on a 1% agarose gel and purified with an EZ-10 Spin Column PCR Purification Kit (BioBasic Inc., Markham, ON, Canada). DNA concentration was determined as described above. Sanger sequencing was performed at the University of Calgary Core DNA Services using Applied Biosystems 3730xl (96 capillary) genetic analyzer (Applied Biosystems, Foster City, CA, USA). Forward and reverse sequences were merged via EMBOSS 6.3.1 merger (Néron et al., 2009) and >1,300 bp sequences were identified via BLASTN (Altschul et al., 1997) against the EzTaxon server (Kim et al., 2012).
A number of isolates were tested for their benzene degradation. Exponentially growing bacteria in 20R2A-T medium (pH 8.5) were transferred to 20R2A liquid medium (20 mL in a 100-mL serum vial), and sealed with butyl rubber stoppers. Benzene (6 μM) was added to the cultures. Each sample was run in triplicate. Loss of benzene was measured in duplicate pure cultures as described above. Growth was monitored by measuring OD600 on an Ultrospec 10 Cell Density Meter (Amersham Biosciences, Piscataway, NJ).
Results
Potential Hydrocarbon Oxidation Rates
The benzene and naphthalene oxidation rates measured were roughly linear over time (Supplementary Figures S2, S3). Therefore we assume 0-order kinetics (i.e., the enzymes are saturated and the rates reported are Vmax values not dependent on substrate concentration). The potential benzene oxidation rate was 4.3 μmol L−1 OSPW d−1 in the first 23 d, and the naphthalene oxidation rate was 21.4 μmol L−1 OSPW d−1 over 14 d (Supplementary Figures S2, S3). Conditions remained oxic throughout the incubations (the maximum O2 decline was from 21% to 17% v/v). In the benzene enrichment 5.6 μmol of CO2 L−1 OSPW d−1 was produced, 1.3 times of the expected CO2 production based on the reaction: C6H6 + 2.5O2 + NH3 → C5H7O2N + CO2 + H2O (Shuler and Kargi, 2002). In the naphthalene enriched samples, 144.1 μmol of CO2 L−1 OSPW d−1 was produced, or 1.3 times of the expected CO2 production based on the reaction: C10H8 + 7O2 + NH3 → C5H7O2N + 5CO2 + 2H2O (Shuler and Kargi, 2002). The slightly higher than expected CO2 production indicates that less C is assimilated from these compounds than predicted by simple models, or that added benzene and naphthalene enhance the degradation of other hydrocarbon substrates available in OSPW (Suthersan and McDonough, 1996).
SIP and 16S rRNA Gene Sequencing
Density gradient separation of extracted DNA after 9 days (benzene) or 7 days (naphthalene) of incubation indicated that 13C-substrate incubations led to a small shift in the DNA distribution toward heavier densities (Supplementary Figure S1). The positions of the “heavy” DNA fractions representing the active 13C-assimilating communities were determined by comparing density gradients of the DNA extracted from the SIP incubations to that of the control samples, as indicated in Supplementary Figure S1.
Community analysis of 16S rRNA genes amplified from OSPW DNA prior to the SIP incubations showed both methanogenic archaea and diverse bacteria. The combination of anaerobes, aerobes, and facultative anaerobes has been noted before (An et al., 2013; Saidi-Mehrabad et al., 2013) and is probably the result of active water circulation between anoxic and oxic water zones. The dominant classes/phyla of bacteria were Betaproteobacteria, Alphaproteobacteria, and Flavobacteria (Supplementary Figure S4). The predominant genera were Hydrogenophaga, Luteibacter, and Enhydrobacter (Figure 1). After fractionation of this DNA extract in a CsCl gradient, generally the highest density from which a PCR product could be obtained was 1.74–1.75 g/mL, which corresponded to the expected peak of the 13C-labeled DNA in the SIP incubations. The community detected in this heaviest fraction of unincubated OSPW was much simpler, and dominated by the genera Devosia, Methylomonas, Thauera, and Brevundimonas (Figure 1). This OSPW-heavy community is a simplified subset of the OSPW-control community (Figure 1, Supplementary Table S1), suggesting that a few species have high G+C contents and therefore high DNA densities. This is supported by reported G+C contents for isolates of these genera: which range from 47 to 58% for Methylomomas (Hoefman et al., 2014), 64–6% for Devosia (Nakagawa et al., 1996), and 66–69% for Thauera (Mechichi et al., 2002).
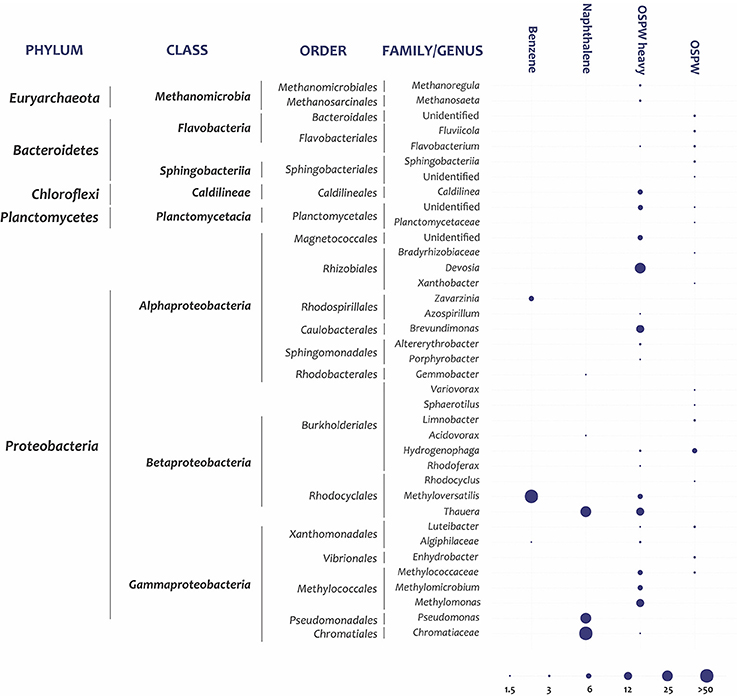
Figure 1. Predominant taxa detected in DNA of control OSPW samples, and in heavy DNA fractions extracted after incubation with 13C benzene or 13C naphthalene. Data are relative abundances of taxa within sequenced 16S rRNA gene amplicons (only OTUs >1% of the total reads are shown). The lowest taxonomic level confidently assigned is based on 16S rRNA identity thresholds defined by Yarza et al. (2014). Phylum, class, order, and either family or genus is indicated. Benzene: heavy-DNA fraction of benzene-amended OSPW incubated for 9 days; Naphthalene: heavy-DNA fraction of naphthalene- amended OSPW incubated for 7 days; OSPW-heavy: heavy fraction of OSPW incubated for the same amount of time as the amended samples; and OSPW-control: complete, unfractionated DNA from OSPW. The bubbles show 6 abundance classes (1–1.75%; 1.76–4.5%; 4.6–9%; 9.1–18.5%; 18.6–37.5%; >37.6%).
Communities detected in the heavy DNA fractions of the SIP incubations were compared to two controls: the complete native OSPW community and the native community only in the 1.74–1.75 g/mL fraction, to verify that the species detected by SIP were truly enriched with 13C. Only OTUs that were enriched at least 10-fold in the SIP heavy fractions compared to both controls, or OTUs that were enriched at least 2-fold and were predominant at >10% of the total DNA in the heavy fraction, were considered to be enriched in the added 13C-substrate.
The 13C-labeled, heavy DNA fraction from the benzene-SIP experiment was dominated by a close relative of Methyloversatilis universalis (Betaproteobacteria) that accounted for 86.9% of the community and was enriched 20-fold compared to the OSPW-heavy control (Figure 1, Supplementary Table S1). Zavarzinia (Alphaproteobacteria) was the second most predominant genus, enriched 26-fold compared to OSPW-heavy controls. This result was reproducible: in a second SIP experiment of 13C-benzene incubated for 14 d Methyloversatilis was also predominant in the heavy DNA fraction (Supplementary Table S2). A SIP with 13C-methanol also verified the methylotrophic phenotype of this OTU (data not shown).
The three most dominant taxa in the 13C-naphthalene SIP heavy fraction were identified as Chromatiaceae, Thauera, and Pseudomonas. Chromatiaceae and Pseudomonas OTUs were enriched >25-fold compared to the OSPW-heavy control (Supplementary Table S1). The predominant Thauera OTU was enriched only 2-fold, but was the second most abundant OTU in this fraction. Although this Thauera OTU probably has a high G+C content and is therefore also abundant in the OSPW-heavy control, we conclude that its 2x enrichment in the benzene-heavy fraction may indicate a labeling of this organism by 13C for 2 reasons: (i) the absolute amount of DNA in the 13C-naphthalene SIP heavy fraction is much greater than the amount of DNA in the control OSPW-heavy fraction (31.6 vs. 0.17 ng/μl), indicating that this fraction is mostly newly synthesized DNA; and (ii) considering only OTUs that are many times enriched is a good approach for less common OTUs but may overlook common species, since abundances are relative and therefore the higher the initial abundance, the less it can increase (i.e., a greater than 10-fold increase is impossible for an OTU already present at 10% of the control, as seen for Thauera). Therefore the 2x enrichment of this OTU in the heavy fraction, combined with its predominance and the obvious increase in total DNA in the 1.74–1.75 fraction of the naphthalene-SIP, likely indicate that Thauera also metabolized the naphthalene.
WIP-OSPW Metagenome
An overview of the OSPW metagenome is given in the Supplementary Table S3. A predicted 7.5% of the annotated genes belonged to the KEGG category “xenobiotics biodegradation and metabolism.” The metagenome shows the genetic machinery for aerobic degradation of diverse petroleum hydrocarbons. Aromatic and polyaromatic compounds are predicted to be broken down via three different aerobic pathways: dioxygenase and dehydrogenase reactions, ring removal from polycyclic aromatic ring structures, and two-monooxygenase reactions (Figure 2). Each process forms catechol as an intermediate, which is broken down through the catechol meta-cleavage pathway into Acetyl-CoA. Benzene is attacked in this scenario by either benzene/toluene dioxygenase, or by a monooxygenase then a phenol hydroxylase, while naphthalene is attacked by dihydroxynaphthalene dioxygenases (Figure 2).
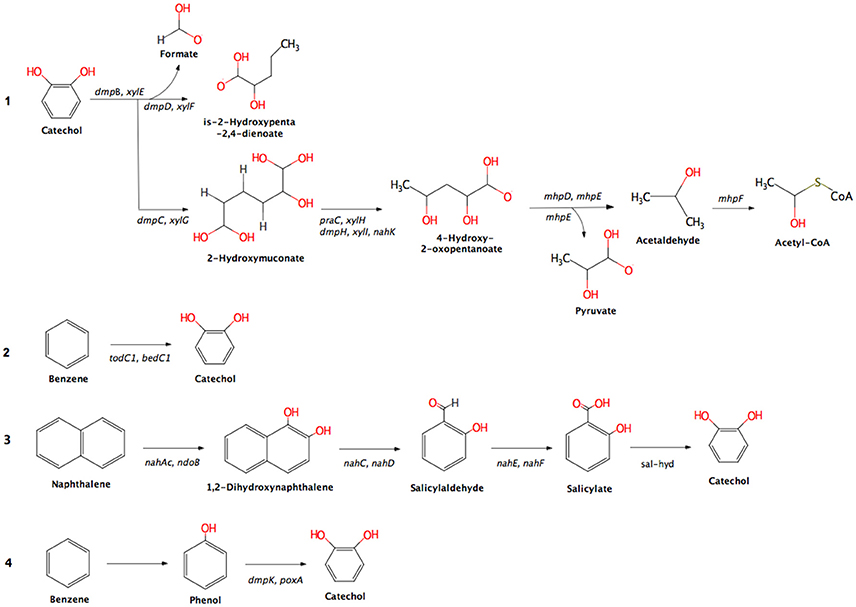
Figure 2. Detected pathways (substrates) for aerobic hydrocarbon metabolism in the OSPW metagenome and their associated genes. Pathway descriptions: 1, meta-cleavage of catechol; 2, dioxygenase and dehydrogenase reactions; 3, ring removal from polycyclic aromatic ring; and 4, two monooxygenase reactions. Enzyme names abbreviations and their synonyms: dmpB, xylE, catechol 2,3-dioxygenase; dmpD, xylF, hydroxymuconate-semialdehyde hydrolase; dmpC, xylG, aminomuconate-semialdehyde/2-hydroxymuconate-6-semialdehyde dehydrogenase; praC, xylH, oxalocrotonate tautomerase; dmpH, xylI, nahK, 2-oxo-3-hexenedioate decarboxylase; mhpD, 2-keto-4-pentenoate hydratase; mhpE, 4-hydroxy 2-oxovalerate aldolase; mhpF, acetaldehyde dehydrogenase; todC1, bedC1, benzene/toluene dioxygenase; nahAc, ndoB, naphthalene 1,2-dioxygenase; nahC, dihydroxynaphthalene dioxygenase; nahD, hydroxychromene-2-carboxylate isomerase; nahE, trans-o-hydroxybenzylidenepyruvate hydratase-aldolase; nahF, salicylaldehyde dehydrogenase; sal-hyd, salicylate hydroxylase; dmpK, poxA, phenol hydroxylase.
A quantitative analysis of unassembled Illumina metagenome reads for the key genes shown in Figure 2 suggested that these were predominantly found in Betaproteobacteria (particularly Burkholderiales and Rhodocyclales) and Gammaproteobacteria (particularly Pseudomonadales and unidentified orders) and a lesser extent in Alphaproteobacteria and Actinobacteria (Figure 3; finer taxonomic resolution of these results is shown for each gene in the Supplementary Figures S5A–P). The most unusual patterns were obtained for dmpB encoding for catechol 2,3-dioxygenase as well as todC1 encoding benzene/toluene dioxygenase. Both showed a large number of hits to bacteria that could not be identified reliably, suggesting that at the enzyme level and possibly also the taxonomic level uncharacterized species are involved in the biodegradation of aromatics The unassembled Illumina read analysis was also used to calculate the most prevalent taxa within the set of key genes encoding aromatic-compound degradation (Figure 4). Prevalence of a taxon was determined as the proportion of the 14 raw read datasets (i.e., the 14 genes in Figure 2) a taxon was detected in. The taxa identified in Figure 4 are those with >60% of the key genes. They are shown to a higher taxonomic resolution than in Figure 3 because of the relative simplicity of the dataset. The most prevalent taxa were similar to the most abundant taxa, dominated by Burkholderiales, Rhodocyclales, Pseudomonadales.
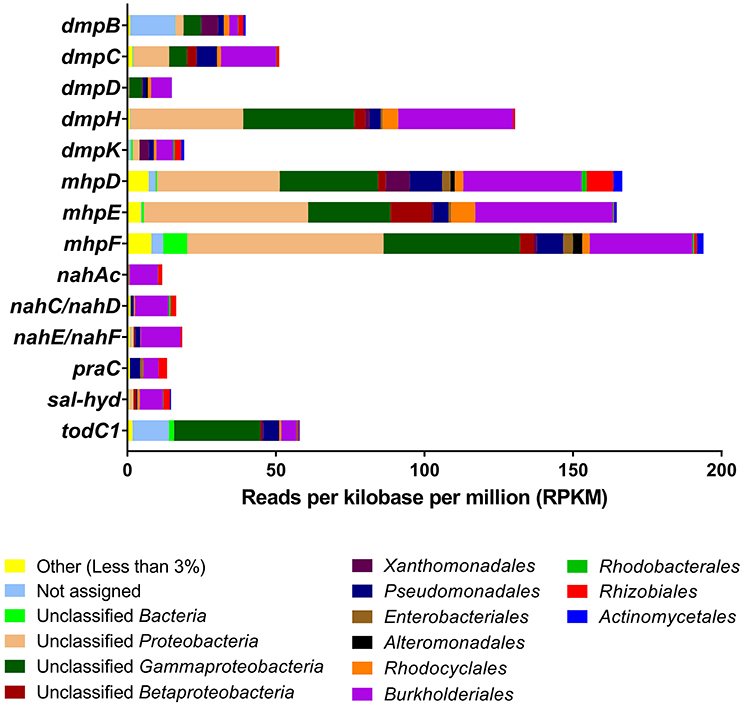
Figure 3. Unassembled metagenomic read distributions of the key genes from Figure 2. Bars represent reads per kilobase per million (RPKM) mapped to the corresponding gene using BLASTN. Taxonomic assignment on recruited reads that exceeded the 1% abundance cut-off was performed with MEGAN to the level of order. “Unclassified” groups include all members of a higher-level taxon that cannot be assigned at a more refined level. “Unassigned” represents reads MEGAN could not assign unambiguously to any taxon. RPKM values of genes nahC and nahD, and nahE and nahF are combined due to their identical functions and low RPKM levels.
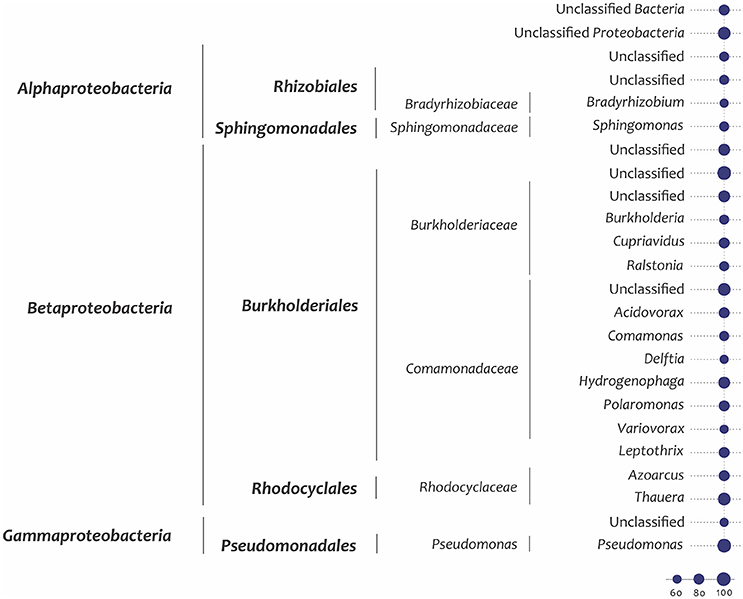
Figure 4. Prevalence of the 14 selected key marker genes encoding aromatic compound degradation within taxa. The prevalence parameter is calculated as the percentage of the gene sets in which a taxon is identified, and only taxa showing >60% prevalence (i.e.,>8 genes) are shown. “Unclassified” groups include all members of a higher-level taxon that cannot be assigned at a more refined level. The percent prevalence corresponds to the size of the node.
Two of the bacterial taxa identified as important in the SIP studies, Pseudomonas (Pseudomonadales) and Thauera (Rhodocyclales), show high abundance of some genes (Figure 3 and Supplementary Figure S5) and high prevalence of the overall gene set (Figure 4). The three other taxa identified as important in the SIP studies, Methyloversatilis (Rhodocyclales), Chromatiaceae (Chromatiales), and Zavarzinia (Rhodospirilalles), were neither abundant nor prevalent (Figures 3,4). However, in the case of Methyloversatilis, a large number of genes could be mapped to closely related sister genera within the Rhodocyclales/Rhodocyclaceae (Azoarcus and Thauera) (Figure 4; Supplementary Figures S5). MEGAN is limited by the NCBI database and is unable to resolve taxonomy unambiguously (Huson et al., 2007), so some of these sequences may in fact represent gene content of the important Methyloversatilis OTU. One member of the Chromatiaceae (Chromatiales) was detected as the closest BLAST hit to a catechol 2,3-dioxygenase and a benzene/toluene dioxygenase (data not shown).
Isolated Bacterial Strains and Their Degradation Potentials
Pure cultures of Xanthobacter (strain OSPW1) and Zavarzinia (strain OSPW2) were isolated using 20R2A-T medium (Supplementary Table S4). These were able to perform complete degradation of 6 μM benzene in 14 and 8 days, respectively, when growing on complex 20R2A medium (Supplementary Figure S6). Thauera sp. strain OSPW4 was isolated on complex media incubated under naphthalene vapor, which shows that it tolerates naphthalene, although it may not grow on naphthalene as a sole substrate. Pseudomonas sp. strain OSPW3 was isolated on mineral salts medium M10-T with naphthalene vapor as a sole substrate, and therefore was capable of growth on naphthalene as the sole substrate. No relatives of the two other predominant bacteria detected in 13C-benzene and 13C-naphthalene SIPs were isolated (i.e., Methyloversatilis and Chromatiaceae).
Discussion
One plan for reclaiming oil sands tailings ponds in Canada is to convert them into End-Pit Lakes or other wetlands (Allen, 2008; Wayland et al., 2008; COSIA, 2016). This method uses a layer of freshwater in order to increase O2 supply (Allen, 2008; Wayland et al., 2008). It is expected that increased aeration will stimulate indigenous microbial decontamination rates, and that these systems will undergo natural biological remediation (Allen, 2008; COSIA, 2016). The capability of an OSPW community to aerobically degrade the model aromatic compounds benzene and naphthalene was demonstrated here. Naphthalene degradation rates were higher than those of benzene, which agrees with previous study showing that naphthalene has a higher biodegradation rate than other major hydrocarbons (Li and Goel, 2011). However, both rates are somewhat lower than previously measured rates in other oil-contaminated natural environments (Alvarez et al., 1991; Eriksson et al., 1999; Nicholson and Fathepure, 2005; Chang et al., 2014), probably because of the complexity of the hydrocarbon mixture in OSPW and the resultant low concentration of individual components. For example, the total PAH cocktail in this pond was previously estimated as 2600 ng L−1, but the naphthalene level only 101 ng L−1 (Madill et al., 1999). Although we used benzene and naphthalene as model compounds because of availability, these are only two components of a diverse mixture of aromatic compounds in situ.
SIP studies using 13C-benzene revealed the activity of the aerobic methylotroph genus Methyloversatilis. The genome of the type strain FAM5T of Methyloversatilis universalis has a predicted membrane protein involved in aromatic hydrocarbon degradation through the metacleavage pathway (Kittichotirat et al., 2011). A recent study also discovered an uncultured bacterium related to M. universalis strain FAM5 (98% similarity) that can degrade benzene (Satija and Gore, 2014). Previous studies have also reported this bacterium in various petroleum related environments, but its relative abundance never reached more than 18% and its role in hydrocarbon degradation was not deemed crucial (Golby et al., 2012; Lenchi et al., 2013; Noguchi et al., 2014). Another prevalent genus identified in the heavy fractions of the 13C-benzene SIP experiment was related to Zavarzinia (Willems and De Vos, 2006). Zavarzinia is an aerobic carboxidotrophic bacterium (Meyer et al., 2015), and has also been detected in various hydrocarbon-contaminated sites (Nies and Schlegel, 1982; Al-Mailem et al., 2014, 2015). An isolate obtained in this study, named Zavarzinia sp. OSPW2, was shown to degrade benzene in pure culture (Supplementary Figure S7).
A different bacterial community was implicated in naphthalene degradation: predominantly Gammaproteobacteria of the family Chromatiaceae, Gammaproteobacteria of the genus Pseudomonas, and Betaproteobacteria of the genus Thauera. Chromatiaceae are abundant in OSPW surface water (2.6–8.0% relative abundance) (Saidi-Mehrabad et al., 2013). Some members of the family Chromatiaceae possess homogentisate 1,2-dioxygenase (HGD), a unique aromatic ring-cleavage enzyme, which cleaves an aromatic ring between ortho carbon atoms substituted with carboxyl and hydroxyl groups (Titus et al., 2000; Pérez-Pantoja et al., 2010). Thauera phenylacetica is a well-known facultative anaerobe that can degrade aromatic substrates under aerobic and denitrifying conditions (Mechichi et al., 2002). Similarly, many strains of Pseudomonas stutzeri have been studied biochemically and genetically for aerobic naphthalene degradation (Rosselló-Mora et al., 1994; Huang et al., 2015). Both a Thauera strain (OSPW4) and a Pseudomonas strain (OSPW3) were successfully cultivated from our WIP-OSPW sample supplemented with naphthalene, supporting the conclusions of the SIP study.
The use of metagenomics allowed us to identify genes encoding dioxygenases and monooxygenases for the aerobic degradation of aromatic and polyaromatic hydrocarbons in the OSPW community (Figures 2–4). At some level the metagenome data support the SIP experiments in identifying important bacterial groups. Five key taxa were identified as important in the SIP studies of benzene and naphthalene degradation: Thauera, Pseudomonas, Methyloversatilis, Chromatiaceae and Zavarzinia. Three of these: Thauera, Pseudomonas, and Rhodocyclaceae (which contains Methyloversatilis) were predominant in the analysis of metagenome genes encoding key enzymes of aromatic hydrocarbon degradation- both when assessing the number of reads mapping to these taxa and when assessing the percentage of genes in the gene set mapping to these taxa (Figure 3). That is, these taxa were predominant numerically, and contained most genes to encode the degradation pathways. A few genes related to catechol 2,3-dioxygenase, benzene/toluene dioxygenase, and phenol hydroxylase, and homogentisate 1,2-dioxygenase (HGD) in the metagenome were also mapped by BLAST to members of the Chromatiaceae. There are no genomes available for Zavarzinia, so the failure to identify genes belonging to it in the metagenome is unsurprising, although only a very few matches to its higher-level taxa occurred (Rhodospiralles, Alphaproteobacteria). This organism may be a rare member of the community that grew rapidly upon benzene amendment.
Therefore most of the key players identified in the SIP experiments appear to be well represented in the metagenome, although only Thauera and Pseudomonas would have been predicted from an analysis of the metagenome alone. An analysis of the metagenome independent of the SIP experiments would have proposed a large variety of other candidates as well, especially members of the Burkholderiales, which are very abundant in OSPW (An et al., 2013; Saidi-Mehrabad et al., 2013). In this study, Burkholderiales were predominantly associated with most of the genes for catechol meta-cleavage and other key functions of aromatics degradation found in the metagenome (Figure 3). Members of the Rhizobiales (Alphaproteobacteria) also appear to encode monooxygenases for degradation of aromatic compounds in the metagenome (Figures 3,4). Nevertheless, these two groups (Burkholderiales and Rhizobiales) did not appear as major active groups in either 13C-benzene or 13C-naphthalene SIPs. This finding indicates either that these genes cannot be properly mapped taxonomically with BLAST, or more likely that the detected genes are involved primarily in degradation of other abundant PAHs in the OSPW, rather than of the model aromatic compounds used in our experiments.
Therefore cultivation and SIP studies were key in filtering the huge mass of metagenomic data. Metagenomes alone have limited value for identifying bacterial species involved in particular processes, because: (i) mapping of key genes can usually only be inferred from BLAST identities, which is not always accurate due to incomplete pure-culture databases, as well as lateral gene transfer, (ii) some species may have the genetic systems required for a process but be only weakly active in situ, and (iii) a large number of genes detected (in our case ring-activating and ring-cleaving oxygenases) are not closely homologous to studied enzymes and their exact substrates are therefore uncertain. For example, benzene/toluene dioxygenase and phenol hydroxylase gene scaffolds in the metagenome were mapped to Pseudomonas sp. based on BLAST, but this bacterium was identified by SIP to be important in naphthalene but not benzene degradation. Naphthalene degradation genes from the metagenome were not mapped to Chromatiaceae, although this was identified by SIP as the most predominant organism in naphthalene degradation. There are clear limitations of the homology-based metagenome analysis.
The coupling of metagenomic sequencing technology, SIP, and traditional microbiological methods allowed us to identify organisms with important roles in degrading model aromatic compounds within the OSPW. Utilizing the metagenome alone was not very useful, since it predicted a huge number of potential hydrocarbon degraders (mostly Burkholderiales and Rhizobiales), but only a few of these were shown to be active in the actual SIP experiments using 13C-benzene and 13C-naphthalene. The others presumably utilize other substrates or are active under other conditions. Some predominant bacteria found to be important in our benzene and naphthalene amended environments, such as Methyloversatilis and Chromatiaceae, were not predominant in other related studies (Pérez-Pantoja et al., 2010; Patel et al., 2012; Jechalke et al., 2013; Ortega-González et al., 2013; Hassanshahian and Boroujeni, 2016), demonstrating the uniqueness of this environment.
Author Contributions
FR, IT, JK, LG, and PD designed the experiments. FR conducted the majority of the experimental work including SIP, DNA processing, and biochemical work, assisted by IT, JK, LG and ASM. Bioinformatics analysis were performed by AS, IT, XD, and CS. FR and AS designed the figures. FR and PD wrote the manuscript. All authors discussed the findings and provided input on the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was made possible through financial assistance from Genome Canada, Genome Alberta, Genome BC and the Government of Alberta (GC Grant 1203), as well as by an NSERC (Natural Sciences and Engineering Research Council of Canada) Collaborative Research and Development Grant (NSERC grant CRDPJ478071-14). We acknowledge the assistance of Syncrude Canada, Ltd., particularly Tara Penner.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.01845/full#supplementary-material
References
Aguilar, M., Richardson, E., Tan, B., Walker, G., Dunfield, P., Bass, D., et al. (2016). Next-generation sequencing assessment of eukaryotic diversity in oil sands tailings ponds sediments and surface water. J. Eukaryot. Microbiol. 63, 732–743. doi: 10.1111/jeu.12320
Allen, E. W. (2008). Process water treatment in Canada's oil sands industry: I. Target pollutants and treatment objectives. J. Environ. Eng. Sci. 7, 123–138. doi: 10.1139/S07-038
Al-Mailem, D., Kansour, M., and Radwan, S. (2014). Bioremediation of hydrocarbons contaminating sewage effluent using man-made biofilms: effects of some variables. Appl. Biochem. Biotechnol. 174, 1736–1751. doi: 10.1007/s12010-014-1067-z
Al-Mailem, D., Kansour, M., and Radwan, S. (2015). Bacterial communities associated with biofouling materials used in bench-scale hydrocarbon bioremediation. Environ. Sci. Pollut. Res. 22, 3570–3585. doi: 10.1007/s11356-014-3593-1
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. doi: 10.1093/nar/25.17.3389
Alvarez, P. J. J., Anid, P. J., and Vogel, T. M. (1991). Kinetics of aerobic biodegradation of benzene and toluene in sandy aquifer material. Biodegradation 2, 43–51. doi: 10.1007/BF00122424
An, D., Caffrey, S. M., Soh, J., Agrawal, A., Brown, D., Budwill, K., et al. (2013). Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environ. Sci. Technol. 47, 10708–10717. doi: 10.1021/es4020184
Atlas, R. M. (1981). Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol. Rev. 45, 180–209.
Atlas, R. M. (1991). Microbial hydrocarbon degradation—bioremediation of oil spills. J. Chem. Technol. Biotechnol. 52, 149–156. doi: 10.1002/jctb.280520202
Berdugo-Clavijo, C., Dong, X., Soh, J., Sensen, C. W., and Gieg, L. M. (2012). Methanogenic biodegradation of two-ringed polycyclic aromatic hydrocarbons. FEMS Microbiol. Ecol. 81, 124–133. doi: 10.1111/j.1574-6941.2012.01328.x
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chang, Y.-I., Cheng, H.-P., Lai, S.-H., and Ning, H. (2014). Biodegradation of naphthalene in the oil refinery wastewater by enriched activated sludge. Int. Biodeterior. Biodegrad. 86, 272–277. doi: 10.1016/j.ibiod.2013.09.018
Coordinators, N. R. (2013). Database resources of the national center for biotechnology information. Nucleic Acids Res. 41, D8–D20. doi: 10.1093/nar/gks1189
COSIA, Canada's Oil Sands Innovation Alliance. (2016). Pit Lake Research [Online]. Available online at: http://www.cosia.ca/pit-lake-research (Accessed January 01, 2016).
Das, N., and Chandran, P. (2011). Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol. Res. Int. 2011:941810. doi: 10.4061/2011/941810
DeLaune, R. D., Hambrick, G. A., and Patrick, W. H. Jr. (1980). Degradation of hydrocarbons in oxidized and reduced sediments. Mar. Pollut. Bull. 11, 103–106.
Eriksson, M., Dalhammar, G., and Borg-Karlson, A.-K. (1999). Aerobic degradation of a hydrocarbon mixture in natural uncontaminated potting soil by indigenous microorganisms at 20°C and 6°C. Appl. Microbiol. Biotechnol. 51, 532–535. doi: 10.1007/s002530051429
Fine Tailings Fundamentals Consortium (FTFC) (1995). “Fine tails and process water reclamation,” in Advances in Oil Sands Tailings Research, Vol. II, eds T. Lord and L. R. Nelson (Edmonton, AB: Alberta Department of Energy, Oil Sands and Research Division), II-1–II-50.
Foght, J. M., Gieg, L. M., and Siddique, T. (2017). The microbiology of oil sands tailings: past, present, future. FEMS Microbiol. Ecol. 93, 1–22. doi: 10.1093/femsec/fix034
Fowler, S. J., Dong, X., Sensen, C. W., Suflita, J. M., and Gieg, L. M. (2012). Methanogenic toluene metabolism: community structure and intermediates. Environ. Microbiol. 14, 754–764. doi: 10.1111/j.1462-2920.2011.02631.x
Golby, S., Ceri, H., Gieg, L. M., Chatterjee, I., Marques, L. L., and Turner, R. J. (2012). Evaluation of microbial biofilm communities from an Alberta oil sands tailings pond. FEMS Microbiol. Ecol. 79, 240–250. doi: 10.1111/j.1574-6941.2011.01212.x
Government of Alberta (2009). Environmental Management of Alberta's Oil Sands: Resourceful and Responsible. Edmonton, AB: Government of Alberta.
Grewer, D. M., Young, R. F., Whittal, R. M., and Fedorak, P. M. (2010). Naphthenic acids and other acid-extractables in water samples from Alberta: what is being measured? Sci. Total Environ. 408, 5997–6010. doi: 10.1016/j.scitotenv.2010.08.013
Hassanshahian, M., and Boroujeni, N. A. (2016). Enrichment and identification of naphthalene-degrading bacteria from the Persian Gulf. Mar. Poll Bull. 107, 59–65. doi: 10.1016/j.marpolbul.2016.04.020
Heyer, J., Galchenko, V. F., and Dunfield, P. F. (2002). Molecular phylogeny of type II methane-oxidizing bacteria isolated from various environments. Microbiology 148, 2831–2846. doi: 10.1099/00221287-148-9-2831
Hoefman, S., Heylen, K., and De Vos, P. (2014). Methylomonas lenta sp. nov., a methanotroph isolated from manure and a denitrification tank. Int. J. Sys.t Evol. Microbiol. 64, 1210–1217. doi: 10.1099/ijs.0.057794-0
Holowenko, F. M., MacKinnon, M. D., and Fedorak, P. M. (2002). Characterization of naphthenic acids in oil sands wastewaters by gas chromatography-mass spectrometry. Water Res. 36, 2843–2855. doi: 10.1016/S0043-1354(01)00492-4
Huang, T., Guo, L., Zhang, H., Su, J., Wen, G., and Zhang, K. (2015). Nitrogen-removal efficiency of a novel aerobic denitrifying bacterium, strain ZF31, isolated from a drinking-water reservoir. Bioresour. Technol. 196, 209–216. doi: 10.1016/j.biortech.2015.07.059
Huson, D. H., Auch, A. F., Qi, J., and Schuster, S. C. (2007). MEGAN analysis of metagenomic data. Genome Res. 17, 377–386. doi: 10.1101/gr.5969107
Jechalke, S., Franchini, A. G., Bastida, F., Bombach, P., Rosell, M., Seifert, J., et al. (2013). Analysis of structure, function, and activity of a benzene-degrading microbial community. FEMS Microbiol. Ecol. 85, 14–26. doi: 10.1111/1574-6941.12090
Jeon, C. O., Park, W., Ghiorse, W. C., and Madsen, E. L. (2004). Polaromonas naphthalenivorans sp. nov., a naphthalene-degrading bacterium from naphthalene-contaminated sediment. Int. J. Syst. Evol. Microbiol. 54, 93–97. doi: 10.1099/ijs.0.02636-0
Kelly, E. N., Schindler, D. W., Hodson, P. V., Short, J. W., Radmanovich, R., and Nielsen, C. C. (2010). Oil sands development contributes elements toxic at low concentrations to the Athabasca River and its tributaries. Proc. Natl. Acad. Sci. U.S.A. 107, 16178–16183. doi: 10.1073/pnas.1008754107
Kelly, E. N., Short, J. W., Schindler, D. W., Hodson, P. V., Ma, M., Kwan, A. K., et al. (2009). Oil sands development contributes polycyclic aromatic compounds to the Athabasca River and its tributaries. Proc. Natl. Acad. Sci. U.S.A. 106, 22346–22351. doi: 10.1073/pnas.0912050106
Kim, O.-S., Cho, Y.-J., Lee, K., Yoon, S.-H., Kim, M., Na, H., et al. (2012). Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 62, 716–721. doi: 10.1099/ijs.0.038075-0
Kittichotirat, W., Good, N. M., Hall, R., Bringel, F., Lajus, A., Médigue, C., et al. (2011). Genome sequence of Methyloversatilis universalis FAM5T, a methylotrophic representative of the order Rhodocyclales. J. Bacteriol. 193, 4541–4542. doi: 10.1128/JB.05331-11
Lenchi, N., İnceoǧlu, Ö., Kebbouche-Gana, S., Gana, M. L., Llirós, M., Servais, P., et al. (2013). Diversity of microbial communities in production and injection waters of Algerian oilfields revealed by 16S rRNA gene amplicon 454 pyrosequencing. PLoS ONE 8:e66588. doi: 10.1371/journal.pone.0066588
Li, L., and Goel, R. (2011). Biodegradation of naphthalene, benzene, toluene, ethyl benzene, and xylene in batch and membrane bioreactors. Environ. Eng. Sci. 29, 42–51. doi: 10.1089/ees.2010.0362
Madill, R. E. A., Brownlee, B. G., Josephy, P. D., and Bunce, N. J. (1999). Comparison of the ames Salmonella assay and mutatox genotoxicity assay for assessing the mutagenicity of polycyclic aromatic compounds in porewater from Athabasca oil sands mature fine tailings. Environ. Sci. Technol. 33, 2510–2516. doi: 10.1021/es981343o
Markowitz, V. M., Chen, I.-M. A., Palaniappan, K., Chu, K., Szeto, E., Grechkin, Y., et al. (2012). IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Res. 40, D115–D122. doi: 10.1093/nar/gkr1044
Mechichi, T., Stackebrandt, E., Gad'on, N., and Fuchs, G. (2002). Phylogenetic and metabolic diversity of bacteria degrading aromatic compounds under denitrifying conditions, and description of Thauera phenylacetica sp. nov., Thauera aminoaromatica sp. nov., and Azoarcus buckelii sp. nov. Arch. Microbiol. 178, 26–35. doi: 10.1007/s00203-002-0422-6
Meyer, O., Stackebrandt, E., and Auling, G. (2015). “Zavarzinia”, in Bergey's Manual of Systematics of Archaea and Bacteria, ed W. B. Whitman (John Wiley & Sons, Inc., in Association with Bergey's Manual Trust), 1–2.
Nakagawa, Y., Sakane, T., and Yokota, A. (1996). Transfer of “Pseudomonas riboflavina” (Foster 1944), a gram-negative, motile rod with long-chain 3-hydroxy fatty acids, to Devosia riboflavina gen. nov., sp. nov., nom. rev. Int. J. Sys. Evol. Microbiol. 46, 16–22.
National Energy Board (2015). Estimated Production of Canadian Crude Oil and Equivalent. Calgary, AB: Government of Canada.
Néron, B., Ménager, H., Maufrais, C., Joly, N., Maupetit, J., Letort, S., et al. (2009). Mobyle: a new full web bioinformatics framework. Bioinformatics 25, 3005–3011. doi: 10.1093/bioinformatics/btp493
Nicholson, C. A., and Fathepure, B. Z. (2005). Aerobic biodegradation of benzene and toluene under hypersaline conditions at the Great Salt Plains, Oklahoma. FEMS Microbiol. Lett. 245, 257–262. doi: 10.1016/j.femsle.2005.03.014
Nies, D., and Schlegel, H. G. (1982). Catalase from Comamonas compransoris. J. Gen. Appl. Microbiol. 28, 311–319. doi: 10.2323/jgam.28.311
Noguchi, M., Kurisu, F., Kasuga, I., and Furumai, H. (2014). Time-resolved DNA stable isotope probing links Desulfobacterales- and Coriobacteriaceae-related bacteria to anaerobic degradation of benzene under methanogenic conditions. Microbes Environ. 29, 191–199. doi: 10.1264/jsme2.ME13104
Oller, I., Malato, S., and Sánchez-Pérez, J. (2011). Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci. Total Environ. 409, 4141–4166. doi: 10.1016/j.scitotenv.2010.08.061
Ortega-González, D. K., Zaragoza, D., Aguirre-Garrido, J., Ramírez-Saad, H., Hernández-Rodríguez, C., and Jan-Roblero, J. (2013). Degradation of benzene, toluene, and xylene isomers by a bacterial consortium obtained from rhizosphere soil of Cyperus sp. grown in a petroleum-contaminated area. Folia Microbiol. 58, 569–577. doi: 10.1007/s12223-013-0248-4
Patel, V., Jain, S., and Madamwar, D. (2012). Naphthalene degradation by bacterial consortium (DV-AL) developed from Alang-Sosiya ship breaking yard, Gujarat, India. Bioresour. Technol. 107, 122–130. doi: 10.1016/j.biortech.2011.12.056
Pérez-Pantoja, D., Donoso, R., Junca, H., González, B., and Pieper, H. D. (2010). “Phylogenomics of aerobic bacterial degradation of aromatics,” in Handbook of Hydrocarbon and Lipid Microbiology, ed K. N. Timmis (Berlin; Heidelberg: Springer Berlin Heidelberg), 1355–1397.
Quagraine, E., Peterson, H., and Headley, J. (2005). In situ bioremediation of naphthenic acids contaminated tailing pond waters in the Athabasca oil sands region—demonstrated field studies and plausible options: a review. J. Environ. Sci. Health 40, 685–722. doi: 10.1081/ESE-200046649
Rahnama, F., Marsh, R. A., and Philp, L. (2013). “The alberta oil sands: reserves and long-term supply outlook,” in Heavy-Oil and Oil-Sand Petroleum Systems in Alberta and Beyond, eds F. J. Hein, D. Leckie, S. Larter, and J. R. Suter (Tulsa, OK: American Association of Petroleum Geologists), 133–144.
Reasoner, D. J., and Geldreich, E. E. (1985). A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 49, 1–7.
Rogers, V. V., Liber, K., and MacKinnon, M. D. (2002). Isolation and characterization of naphthenic acids from Athabasca oil sands tailings pond water. Chemosphere 48, 519–527. doi: 10.1016/S0045-6535(02)00133-9
Rosselló-Mora, R., Lalucat, J., and García-Valdés, E. (1994). Comparative biochemical and genetic analysis of naphthalene degradation among Pseudomonas stutzeri strains. Appl. Environ. Microbiol. 60, 966–972.
Saidi-Mehrabad, A., He, Z., Tamas, I., Sharp, C. E., Brady, A. L., Rochman, F. F., et al. (2013). Methanotrophic bacteria in oil sands tailings ponds of northern Alberta. ISME J. 7, 908–921. doi: 10.1038/ismej.2012.163
Satija, S., and Gore, D. (2014). BLASTN based methodology to classify benzene degrading uncultured bacteria into bacterial species using 16S rRNA gene. J. Pharm. Res. 8, 1105–1112. Available online at: http://jprsolutions.info/article_detail.php?article_id=1590
Sharp, C. E., Brady, A. L., Sharp, G. H., Grasby, S. E., Stott, M. B., and Dunfield, P. F. (2014). Humboldt's spa: microbial diversity is controlled by temperature in geothermal environments. ISME J. 8, 1166–1174. doi: 10.1038/ismej.2013.237
Sharp, C. E., Stott, M. B., and Dunfield, P. F. (2012). Detection of autotrophic verrucomicrobial methanotrophs in a geothermal environment using stable isotope probing. Front. Microbiol. 3:303. doi: 10.3389/fmicb.2012.00303
Shuler, M. L., and Kargi, F. (2002). Bioprocess Engineering: Basic Concepts. Upper Saddle River, NJ: Prentice-Hall, Inc.
Siddique, T., Fedorak, P. M., MacKinnon, M. D., and Foght, J. M. (2007). Metabolism of BTEX and naphtha compounds to methane in oil sands tailings. Environ. Sci. Technol. 41, 2350–2356. doi: 10.1021/es062852q
Suthersan, S. S., and McDonough, J. (1996). “In situ bioremediation,” in Remediation Engineering: Design Concepts, eds S. S. Suthersan, J. Horst, M. Schnobrich, N. Welty, and J. McDonough (Boca Raton, FL: CRC Press LLC), 126–131.
Syncrude. (2012). “Mildred lake extension project” in Public Disclosure Document (Fort McMurray: Syncrude Canada Ltd.). Available online at: http://www.syncrude.ca/assets/pdf/News-Room/MLX-Project-Public-Disclosure-Document.pdf
Tan, B., Dong, X., Sensen, C. W., and Foght, J. (2013). Metagenomic analysis of an anaerobic alkane-degrading microbial culture: potential hydrocarbon-activating pathways and inferred roles of community members. Genome 56, 599–611. doi: 10.1139/gen-2013-0069
Titus, G. P., Mueller, H. A., Burgner, J., Rodriguez de Cordoba, S., Penalva, M. A., and Timm, D. E. (2000). Crystal structure of human homogentisate dioxygenase. Nat. Struct. Mol. Biol. 7, 542–546. doi: 10.1038/76756
Tocchi, C., Federici, E., Fidati, L., Manzi, R., Vincigurerra, V., and Petruccioli, M. (2012). Aerobic treatment of dairy wastewater in an industrial three-reactor plant: effect of aeration regime on performances and on protozoan and bacterial communities. Water Res. 46, 3334–3344. doi: 10.1016/j.watres.2012.03.032
Wayland, M., Headley, J., Peru, K., Crosley, R., and Brownlee, B. (2008). Levels of polycyclic aromatic hydrocarbons and dibenzothiophenes in wetland sediments and aquatic insects in the oil sands area of Northeastern Alberta, Canada. Environ. Monit. Assess. 136, 167–182. doi: 10.1007/s10661-007-9673-7
Weisburg, W. G., Barns, S. M., Pelletier, D. A., and Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703. doi: 10.1128/jb.173.2.697-703.1991
Willems, A., and De Vos, P. (2006). “Comamonas,” in The Prokaryotes, eds M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (NewYork, NY: Springer), 723–736.
Keywords: oil sands, tailings pond, hydrocarbon degradation, benzene, naphthalene, metagenomics, stable isotope probing
Citation: Rochman FF, Sheremet A, Tamas I, Saidi-Mehrabad A, Kim J-J, Dong X, Sensen CW, Gieg LM and Dunfield PF (2017) Benzene and Naphthalene Degrading Bacterial Communities in an Oil Sands Tailings Pond. Front. Microbiol. 8:1845. doi: 10.3389/fmicb.2017.01845
Received: 09 April 2017; Accepted: 08 September 2017;
Published: 28 September 2017.
Edited by:
Sabine Kleinsteuber, Helmholtz-Zentrum für Umweltforschung (UFZ), GermanyReviewed by:
Martina Cappelletti, Università di Bologna, ItalyAlberto Scoma, Aarhus University, Denmark
Copyright © 2017 Rochman, Sheremet, Tamas, Saidi-Mehrabad, Kim, Dong, Sensen, Gieg and Dunfield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter F. Dunfield, cGZkdW5maWVAdWNhbGdhcnkuY2E=
 Fauziah F. Rochman
Fauziah F. Rochman Andriy Sheremet
Andriy Sheremet Ivica Tamas1,2
Ivica Tamas1,2 Alireza Saidi-Mehrabad
Alireza Saidi-Mehrabad Joong-Jae Kim
Joong-Jae Kim Xiaoli Dong
Xiaoli Dong Christoph W. Sensen
Christoph W. Sensen Lisa M. Gieg
Lisa M. Gieg Peter F. Dunfield
Peter F. Dunfield