- 1Key Laboratory of Zoonosis Research, Ministry of Education, Institute of Zoonosis, Jilin University, Changchun, China
- 2Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, China
Trichinellosis, caused by Trichinella, is an emerging or re-emerging zoonotic parasitic disease, which is distributed worldwide with major socio-economic importance in some developing countries. In particular, it has been calculated that more than 40 million people are at risk of Trichinella infection in China. This review summarizes the current information on the epidemiology, laboratory diagnosis and vaccines of trichinellosis in China. Moreover, study of the treatment potential of using Trichinella for immune-related diseases and cancer, as well as the transcription and post-transcription modification of Trichinella were also collected, providing viewpoints for future investigations. Current advances in research will help us to develop new strategies for the prevention and control of trichinellosis and may potentially yield biological agents for treating other diseases.
Introduction
Trichinellosis is a worldwide food-borne parasitic disease caused by eating raw or undercooked meat containing the infective larvae of Trichinella nematodes (Rainova et al., 2016). Pork and its products are the main sources of infection (Sofronic-Milosavljevic et al., 2017). Trichinella has a wide range of hosts and can infect more than 150 species of animals, including humans. It is evaluated that around 11 million people may be infected by Trichinella (Dupouy-Camet, 2000). The International Commission on Trichinellosis (ICT) reported total about 65818 cases of human trichinellosis from 1986 to 2009 (Murrell and Pozio, 2011). In 2014, the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) composed a list of 24 parasites ranked according to nine global criteria, Trichinella spiralis ranked the first in international trade (FAO/WHO, 2012).
At present, China is one of a few of countries with the highest number of cases of trichinellosis in the world. According to notice No.1149 announced by the Ministry of Agriculture in 2009, trichinellosis was included in the “list containing 26 kinds of the most hazardous zoonoses”. Trichinellosis also have important influence on animal production, food safety and trade in China (Dorny et al., 2009). The cost of prevention and control of Trichinella remains high. According to preliminary statistics, China spends 2.2 billion CNY on the inspection and control of Trichinella per year (Jen and Chen, 2017). Therefore, controlling trichinellosis is of great significance to the meat industry and human health. In this review, we systematically introduce the recent progress in trichinellosis research.
Epidemiology
Nematodes of the genus Trichinella are one of the most worldwide zoonotic pathogens (Knopp et al., 2012). Today, nine species and three genotypes are recognized in this genus (Pozio and Zarlenga, 2013; Korhonen et al., 2016). At present, out of the 16 isolates obtained from mainland China, 13 have been identified as T. spiralis, and these specimens were collected exclusively from pigs from all over the country, including six provinces (Heilongjiang, Liaoning, Henan, Shaanxi, Hubei, and Yunnan) and a municipality (Tianjin). The remaining two isolates from dogs and one from cat were identified as T. nativa, and were collected from two provinces (Heilongjiang and Jilin) in northeast of China (Takahashi et al., 2000). Aside from T. spiralis and T. nativa, T. pseudospiralis, and T. papuae infections have also been reported in Chinese Taiwan as a result of ingesting raw soft-shelled turtles (Lo et al., 2009). To date, Trichinella has been found in 15 species of animals, such as pig, dog, cat, rat, cow, fox, and bear et al., which are distributed throughout China, except in the Hainan province (Liu and Boireau, 2002).
From 2001 to 2004, the Ministry of Health surveyed the prevalence of parasitic diseases across China. The survey revealed an increasing occurrence of foodborne parasitic diseases where trichinellosis is ranked as one of the top three, with an increase of 69.44% and an estimated increase in infections of approximately 20 million people compared to the first national survey (CDC, 2005). During 1964–2011, more than 600 outbreaks of human trichinellosis were documented in mainland of China, affecting 38,797 people and causing 336 deaths (Wang and Cui, 2001; Wang et al., 2006; Cui et al., 2011; Zheng et al., 2011). In recent years, trichinellosis outbreaks have mainly occurred in Yunnan Province, such as the outbreaks in Lanping and Lancang County that occurred in 2009 and 2013, respectively (Jen and Chen, 2017). The high prevalence of trichinellosis in China is related to pig breeding and eating habits (Figure 1). For example, some inhabitants consume wild animals, raw meat and under-cooked foods such as dumplings or scalded dog meat as delicacies, however, there has not been mandatory test for Trichinella larvae in meats except pork in China at present (Wang et al., 2007; Li et al., 2010).
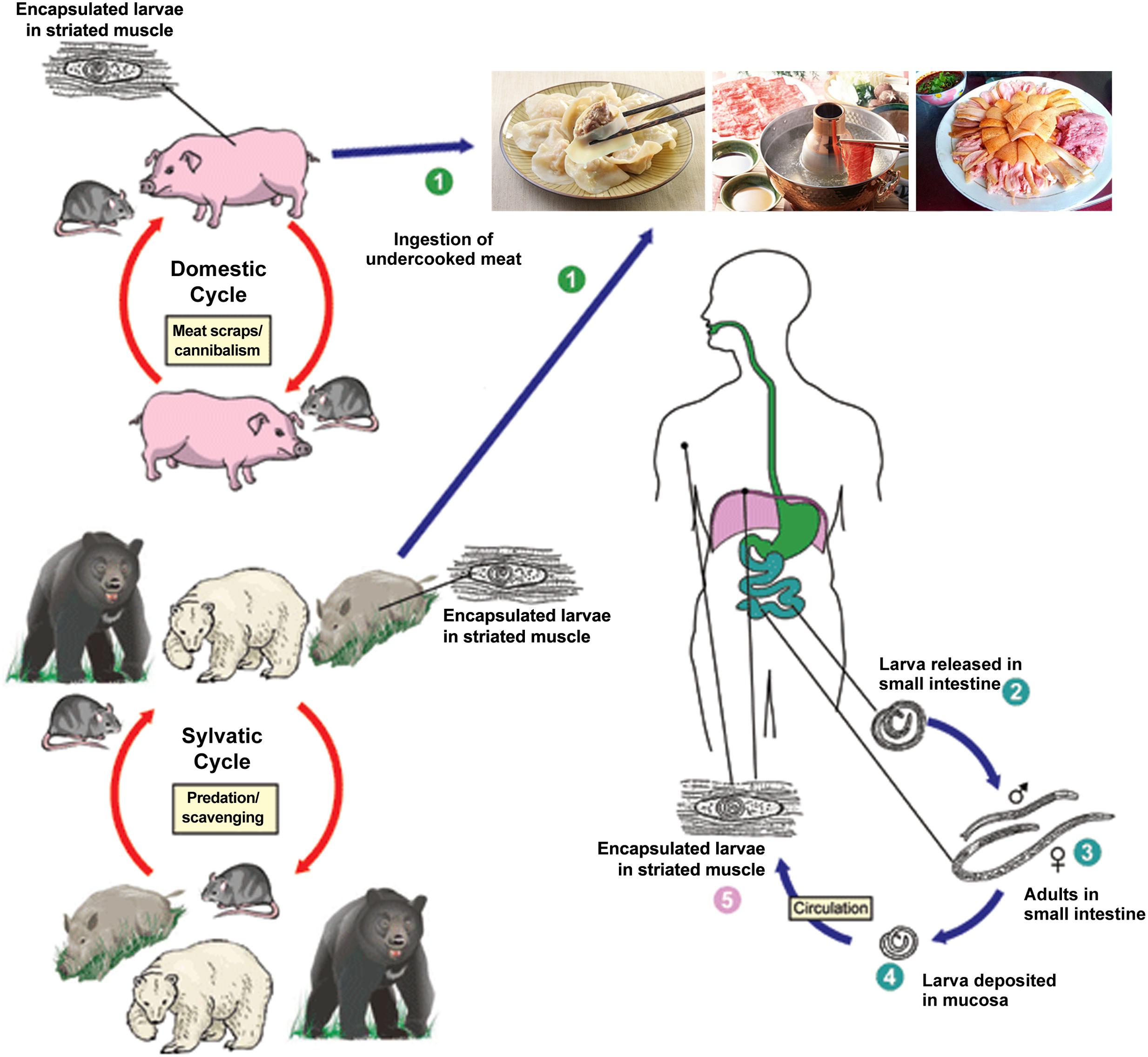
FIGURE 1. Schematic representation of the main sources of infection for trichinellosis and life cycle of Trichinella spiralis in host (cite by https://www.cdc.gov/parasites/trichinellosis/biology.html). ➀ Humans are infected by ingesting encapsulated larvae of Trichinella when eating improperly processed meat; ➁ After exposure to gastric acid and pepsin, the larvae are released from the capsule and invade the small bowel mucosa; ➂ Larvae develop into adult worms and copulate in the small intestine; ➃ Larvae are released and migrate into the vessels and lymphatics; ➄ Larvae reach and settle in the striated muscles where they are encapsulated.
Diagnosis
In 2016, the World Organization for Animal Health (OIE) reported that digestion method is the best testing method for diagnosing trichinellosis (OIE, 2016). This method involves routine examination of Trichinella larvae in muscle tissues for either food safety or disease surveillance and shows good sensitivity and effectiveness in preventing clinical trichinellosis (OIE, 2016). Currently, there are three national standards for the detection of trichinellosis in China: Diagnostic techniques for T. spiralis in swine (GB/T 18642-2002) by the General Administration of Quality Supervision, Inspection and Quarantine; Diagnosis of trichinellosis (WS 369-2012) by the Ministry of Health; and Technical standard for management of trichinellosis outbreak (WS 470-2015) by the National Health and Family Planning Commission (Xu et al., 2002; Wang et al., 2012, 2015). Otherwise, in order to better detect Trichinella, serology and molecularbiologic methods have been developed.
Immunoassays
Animals can be tested for the presence of antibodies against Trichinella in serum or meat juice in antemortem or postmortem examinations (Nöckler et al., 2005). A variety of immunological assays have been developed for the detection of Trichinella infection in domestic and wild animals. Among these tests, ELISA is the most common method for detecting Trichinella infection, and ELISA based on excretory/secretory antigens from ML is the only immunological assay endorsed for surveillance and epidemiological investigations of infections and outbreaks in domestic animals and wildlife by ICT (Gottstein et al., 2009). The disadvantage of using ML-ES ELISA is the high rate of false negative results when animals are in the early stage of infection (Gamble et al., 2004; Yang et al., 2016b). ELISA based on adult worm (AW) ES antigens showed a promising potential for the early and specific serodiagnosis of trichinellosis (Sun et al., 2015b). In addition, a sandwich ELISA based on IgY polyclonal antibodies and IgM monoclonal antibodies was established to detect CAg (Liu L.N. et al., 2013). This method was successfully employed for early detection of T. spiralis in mice and may provide an alternative and more reliable assay.
To improve the ES-ELISA, cDNA libraries of different developmental stages of Trichinella were screened using the serum of pigs at different days post-infection (dpi), and some immunodominant antigens of T. spiralis were evaluated to detect Trichinella infection, showing a promising diagnostic potential (Zhu et al., 2005; Liu et al., 2007; Wu et al., 2009; Liu P. et al., 2013). Interestingly, ELISA based on antigenic molecules (T668, Ts-CLP and 31 kDa antigens) could detect Trichinella infection earlier than ES antigens (Cui et al., 2015; Tang et al., 2015). In addition to these efforts, identification of immunodominant linear epitopes on antigen by monoclonal antibodies and sera from different host infected Trichinella will also greatly improve detection of T. spiralis using ELISA (Yang et al., 2016c).
ES proteins released by Trichinella induce a strong and specific humoral immune, and molecules containing ES are ideal as diagnostic antigens. Two-dimensional electrophoresis (2D) combined with western blot and mass spectrometry was used to screen the early diagnostic antigen from ML ES, identifying five proteins (Wang L. et al., 2013, 2014). Furthermore, several proteins (deoxyribonuclease II and serine protease family protein, et al.) were identified from the intestinal infective larvae (IIL) and adult worms as ES antigens, and these may also serve as potential early diagnostic antigens for trichinellosis (Sun et al., 2015a; Liu et al., 2016; Wang Z.Q. et al., 2017).
An emerging rapid and easy alternative to ELISA is immunochromatographic strips, which detect Trichinella antibody using colloidal gold labeling ES antigens. Zhang et al. prepared an immunochromatographic strip for rapid diagnosis and successfully detected serological trichinellosis in swine. The strips could serve as a substitute for diagnosis and surveillance of trichinellosis when lacking equipment (Zhang et al., 2006). Early diagnosis of trichinellosis is still facing serious challenges, identification of the antigens at different stages using molecular biology and immunology methods will provide a solid base for the further development of serological tools.
DNA Methods
PCR-based methods are most commonly used in live animal slaughter for meat products. LAMP is a novel nucleic acid detection method that can be performed within 1 h under isothermal conditions (Notomi et al., 2000). The LAMP assay also was developed for detection of T. spiralis larvae infection, and showed high sensitivity with detecting T. spiralis in all mouse muscle samples infected with 10 larvae on 20 dpi, demonstrating a valuable means to directly detect larvae during meat inspection (Li et al., 2012). A duplex PCR based on liquid gene chip technique was also developed for detecting T. spiralis in foods using primers designed from the T. spiralis 18S rDNA gene sequences, and the detection limit of this method is 8-fold more sensitive than using agarose gel (Yang P. et al., 2010).
Vaccines
Benzimidazole derivatives are principal anthelmintic drugs which are safe, cheap and effective for the treatment of human trichinellosis (Dupouy-Camet et al., 2002). Some new drug targets are being screened, e.g., Cathepsin F of T. spiralis is a major virulence factor shown to interact with more than ten kinds of drugs, indicating potential drug target for treatment. Although the control strategy of parasites primarily relies on drugs against a broad spectrum of parasites, the emergence of drug-resistant parasites has threatened their sustained use (Roberts, 2005; Schellenberg et al., 2006; Vercruysse et al., 2007). In this circumstance, the development of effective vaccines against Trichinella infection in livestock and humans is a promising strategy to control this parasite (Jacob et al., 2013). However, no effective vaccines are currently available to fully protect against Trichinella infections, except for some protective effects observed only in rat or pig models (Hotez et al., 2008).
Recombinant Protein Vaccine
Researchers have used different antigens to construct recombinant protein vaccines, most of which show some protection against Trichinella. A recombinant vaccine using combined sequences of the T. spiralis serine protease (rTs-Adsp) and Nudix hydrolase (TsNd) can limit the invasion of T. spiralis in mice (Feng et al., 2013; Long et al., 2014). The T. spiralis adult somatic protein Ts14-3-3 is an immunodominant protein identified by early infection sera, and immunization with Ts14-3-3 have shown promising results for preventing swine trichinellosis propagation (Yang et al., 2015, 2016a).
Although these vaccines appear promising, the immunoprotective effects still depends on the type of antigen, adjuvants and the delivery route used to trigger robust immune response (Mohsen et al., 2017). In addition to a variety of traditional adjuvants, new adjuvants consisting of cytokines, nanoadjuvants and toll-like receptor agonists have made great progress in experimental model (Qi and Fang, 2011). Compared to the Montanide ISA201 and Freund’s adjuvant formulated vaccines, the Montanide IMS 1313 NPR VG plus rTs-serpin mixture showed higher humoural and cellular immunity as well as a protective immune response against Trichinella infection in mice (Xu et al., 2017b).
DNA Vaccine
DNA vaccines can induce intense long-term immune responses and do not require booster immunization such as live vaccines. Additionally, DNA vaccines are usually well-tolerated by the animal and thereby safe for use with little risk. In addition, the DNA molecule itself can enhance the immune response as an adjuvant (Heppell and Davis, 2000).
DNA vaccines can contain some antigenic molecules, such as TsNd mentioned above and Ts-NBLsp (the serine protease of T. spiralis new-born larvae) (Liu et al., 2015a; Xu et al., 2017a). Vaccination of mice with pcDNA3.1-TsNd and Ts-NBLsp displayed 53.9 and 77.93% reductions in larval burden, respectively, which are higher protective levels than recombinant protein vaccine.
Attenuated Salmonella typhimurium is an effective carrier for oral delivery of heterologous antigens to induce the immune response. S. typhimurium has been investigated as a vaccine carrier for viruses, bacteria, gene therapy and parasites, inducing long-lasting systemic and mucosal humoral immune responses, and providing a rational design for efficient vaccine (Cazorla et al., 2015). DNA vaccines using TsPmy, TsNd, Ts87, and Ts-cystatin were made and delivered orally using attenuated live Salmonella typhimurium to provide partial protection against T. spiralis infection in mice, suggesting that this may be a promising approach for controlling trichinellosis in human and domestic animals (Yang Y. et al., 2010; Liu et al., 2014, 2015b; Wang et al., 2016).
Immune-Related Diseases and Cancer
Trichinella infection or its derived antigens can induce various immunity-related diseases, including experimental colitis and airway allergic inflammation (Wang M. et al., 2017). One study demonstrated the intervening effect of T. spiralis infection in the mouse TNBS-IBD model (Zhao et al., 2013). In IBD therapy using Trichinella or ES products (ESP), negative regulation of TLR signaling is critical for reducing the expression of genes involved in inflammation and pro-inflammatory cytokine production (Sun et al., 2011). ESP induced macrophage towards the alternatively activated macrophage, suggesting that ES products have the ability to affect macrophages, thereby influencing the host’s immune response and therapeutic potential (Bai et al., 2012). ESP also exhibits anti-inflammatory properties in the septic mouse model, improving survival, reducing organ damage and enhancing bacterial clearance (Du et al., 2014; Chen et al., 2016; Li et al., 2016). In addition to inducing anti-inflammatory immune response, Trichinella and its ESP also have the ability to reduce immune rejection. Mice that were infected with T. spiralis showed higher survival rates after solid organ transplantations, suggesting that the ESP released by T. spiralis may provide an anti-allograft rejection immune response (Deng et al., 2016).
The immunoregulation effect of some immunomodulatory molecules has also been demonstrated, such as the recombinant 53-kDa protein of T. spiralis (rTs-p53) in the TNBS-IBD and septic mouse models (Du et al., 2011; Chen et al., 2016). The effects of T. spiralis cathepsin B-like protein (rTs-CPB) on intestinal ischaemia/reperfusion injury through altering macrophage phenotypes were also investigated, and the results showed that rTs-CPB significantly relieve intestinal injury and protect intestinal function (Liu W.F. et al., 2015).
Trichinella spiralis infection can inhibit tumor growth by cytokines released by activated immune cell. In addition, molecules from T. spiralis can induce tumor or cancer cell apoptosis by inducing apoptosis-related genes, mitochondrial pathways or the death receptor pathway (Wang et al., 2009). In a screen for anti-tumor genes using a T7 phage display cDNA library with organic phase multi-cells, the protein named A200711 showed the potential to induce H7402 cells apoptosis (Duan et al., 2013; Wang X.L. et al., 2013). These studies suggest that T. spiralis should be considered as a potential source of an anti-tumor protein that may have therapeutic applications.
Transcription Small RNA and Post Transcription Modification
Currently, there are stage-specific gene expression results using various immunological and cDNA cloning method; however, genome-wide transcriptome and expression patterns of T. spiralis remain largely unknown. Based on the draft genome of T. spiralis, the global gene expression profile in the three different developmental stages of T. spiralis was analyzed using digital gene expression (DGE) analysis in our group. The transcriptomic analysis of T. spiralis revealed that many genes related to metabolic and biological pathways in the genome were developmentally regulated (Liu et al., 2012). Small non-coding RNAs (sncRNAs) are involved in gene silencing through transcriptional destabilization or translational repression (Mokhtarzadeh et al., 2017). In our previous study, we identified 21 conserved miRNAs related to 13 previously identified metazoan miRNA families as well as 213 miRNAs unique to T. spiralis in three developmental stages, with some miRNAs showing clear stage-specific expression patterns (Liu et al., 2011). These data provide a basis for further understanding molecular mechanisms of parasite biology and functional evolution of miRNAs in parasitic nematodes.
DNA methylation plays a crucial role in modulating gene expression under various conditions, and is suggested to be related with transitions between life cycle stages in parasitic nematodes (Hewezi et al., 2017). Gao et al. (2012) presented the first study to confirm the existence of DNA methylation in T. spiralis using MethylC-seq, and they observed a drastic increase in DNA methylation during the transition from the new-born to mature stage and found parasitism-related genes that show changes in DNA methylation status between life cycle stages. Based on these results, authors suggested that interference DNA methylation processes may be a beneficial strategy in developing therapeutics to control parasite infection.
Conclusion
Although some trichinellosis control programs have been implemented and advances have been made to better understand T. spiralis at the molecular level, trichinellosis remains prevalent in China due to the absence of systematic interventions. The wide distribution of Trichinella, dietary habits, the lack of meat safety regulation, and without developed techniques for detection and treatment are contributing to the prevalence of trichinellosis. Importantly, new strategies of combining non-polluted domestic animal breeding with the use of vaccines may represent a viable alternative to block the transmission of Trichinella and ensure meat safety. By the end of 2015, the OIE set up a total of 12 reference laboratories and 3 collaborating centers in China. Among them, a center for foodborne parasites in the Asian-Pacific region center was set up in Jilin University to provide comprehensive monitoring and detection of foodborne parasitic diseases, including trichinellosis. New methods for effective diagnosis and prevention of trichinellosis are being developed in cooperation with domestic and international research institutions.
Moreover, the rapid development in Trichinella-omics research has provided a new opinion for understanding the biology of Trichinella and screening target molecules to develop new anti-parasitic agents. In addition, identified Trichinella molecules also serve as protective agents for immune-related disease and cancer in humans.
Author Contributions
XB and XH wrote the initial draft of the paper. XL organized and proofread the paper. BT helped to draft the figure. ML approved the version to be published. All authors read and approved the final manuscript.
Funding
This study was supported by the National Nature Science Foundation of China (NSFC31520103916, NSFC 31402185) and Guangdong Innovative and Entrepreneurial Research Team Program (no. 2014ZT05S123).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bai, X., Wu, X., Wang, X., Guan, Z., Gao, F., Yu, J., et al. (2012). Regulation of cytokine expression in murine macrophages stimulated by excretory/secretory products from Trichinella spiralis in vitro. Mol.Cell. Biochem. 360, 79–88. doi: 10.1007/s11010-011-1046-4
Cazorla, S. I., Matos, M. N., Cerny, N., Ramirez, C., Alberti, A. S., Bivona, A. E., et al. (2015). Oral multicomponent DNA vaccine delivered by attenuated Salmonella elicited immunoprotection against American trypanosomiasis. J. Infect. Dis. 211, 698–707. doi: 10.1093/infdis/jiu480
CDC (2005). A national survey on current status of the important parasitic diseases in human population. Chin. J. Parasitol. Paras. Dis. 23(Suppl.), 332–340.
Chen, Z. B., Tang, H., Liang, Y. B., Yang, W., Wu, J. G., Hu, X. C., et al. (2016). Recombinant Trichinella spiralis 53-kDa protein activates M2 macrophages and attenuates the LPS-induced damage of endotoxemia. Innate. Immun. 22, 419–432. doi: 10.1177/1753425916651984
Cui, J., Wang, L., Sun, G. G., Liu, L. N., Zhang, S. B., Liu, R. D., et al. (2015). Characterization of a Trichinella spiralis 31 kDa protein and its potential application for the serodiagnosis of trichinellosis. Acta Trop. 142, 57–63. doi: 10.1016/j.actatropica.2014.10.017
Cui, J., Wang, Z. Q., and Xu, B. L. (2011). The epidemiology of human trichinellosis in China during 2004–2009. Acta Trop. 118, 1–5. doi: 10.1016/j.actatropica.2011.02.005
Deng, G., Deng, R., Yao, J., Liao, B., Chen, Y., Wu, Z., et al. (2016). Trichinella spiralis infection changes immune response in mice performed abdominal heterotopic cardiac transplantation and prolongs cardiac allograft survival time. Parasitol. Res. 115, 407–414. doi: 10.1007/s00436-015-4762-y
Dorny, P., Praet, N., Deckers, N., and Gabriel, S. (2009). Emerging food-borne parasites. Vet. Parasitol. 163, 196–206. doi: 10.1016/j.vetpar.2009.05.026
Du, L., Liu, L., Yu, Y., Shan, H., and Li, L. (2014). Trichinella spiralis excretory-secretory products protect against polymicrobial sepsis by suppressing MyD88 via mannose receptor. Biomed. Res. Int. 2014:898646. doi: 10.1155/2014/898646
Du, L., Tang, H., Ma, Z., Xu, J., Gao, W., Chen, J., et al. (2011). The protective effect of the recombinant 53-kDa protein of Trichinella spiralis on experimental colitis in mice. Dig. Dis. Sci. 56, 2810–2817. doi: 10.1007/s10620-011-1689-1688
Duan, L., Li, J., Cheng, B., Lv, Q., Gong, P. T., Su, L. B., et al. (2013). Identification of a novel gene product expressed by Trichinella spiralis that binds antiserum to Sp2/0 myeloma cells. Vet. Parasitol. 194, 183–185. doi: 10.1016/j.vetpar.2013.01.051
Dupouy-Camet, J. (2000). Trichinellosis: a worldwide zoonosis. Vet. Parasitol. 93, 191–200. doi: 10.1016/S0304-4017(00)00341-1
Dupouy-Camet, J., Kociecka, W., Bruschi, F., Bolas-Fernandez, F., and Pozio, E. (2002). Opinion on the diagnosis and treatment of human trichinellosis. Expert Opin. Pharmacother. 3, 1117–1130. doi: 10.1517/14656566.3.8.1117
FAO/WHO (2012). Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites. Microbiological Risk Assessment Series No. 23. Report of a Joint FAO/WHO Expert Meeting. Rome: FAO/WHO.
Feng, S., Wu, X., Wang, X., Bai, X., Shi, H., Tang, B., et al. (2013). Vaccination of mice with an antigenic serine protease-like protein elicits a protective immune response against Trichinella spiralis infection. J. Parasitol. 99, 426–432. doi: 10.1645/12-46.1
Gamble, H. R., Pozio, E., Bruschi, F., Nockler, K., Kapel, C. M., and Gajadhar, A. A. (2004). International Commission on Trichinellosis: recommendations on the use of serological tests for the detection of Trichinella infection in animals and man. Parasite 11, 3–13. doi: 10.1051/parasite/20041113
Gao, F., Liu, X., Wu, X. P., Wang, X. L., Gong, D., Lu, H., et al. (2012). Differential DNA methylation in discrete developmental stages of the parasitic nematode Trichinella spiralis. Genome. Biol. 13:R100. doi: 10.1186/gb-2012-13-10-r100
Gottstein, B., Pozio, E., and Nockler, K. (2009). Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev 22, 127–145. doi: 10.1128/cmr.00026-08
Heppell, J., and Davis, H. L. (2000). Application of DNA vaccine technology to aquaculture. Adv. Drug Deliv. Rev. 43, 29–43. doi: 10.1016/s0169-409x(00)00075-2
Hewezi, T., Lane, T., Piya, S., Rambani, A., Rice, J. H., and Staton, M. (2017). Cyst nematode parasitism induces dynamic changes in the root epigenome. Plant Physiol. 174, 405–420. doi: 10.1104/pp.16.01948
Hotez, P. J., Brindley, P. J., Bethony, J. M., King, C. H., Pearce, E. J., and Jacobson, J. (2008). Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 118, 1311–1321. doi: 10.1172/jci34261
Jacob, S. S., Cherian, S., Sumithra, T. G., Raina, O. K., and Sankar, M. (2013). Edible vaccines against veterinary parasitic diseases-current status and future prospects. Vaccine 31, 1879–1885. doi: 10.1016/j.vaccine.2013.02.022
Jen, J. J.-S., and Chen, J. (2017). “Chapter 9: foodborne parasitic diseases in China,” in Food Safety in China, Hoboken, NJ: John Wiley & Sons.
Knopp, S., Steinmann, P., Keiser, J., and Utzinger, J. (2012). Nematode infections: soil-transmitted helminths and Trichinella. Infect. Dis. Clin. North. Am. 26, 341–358. doi: 10.1016/j.idc.2012.02.006
Korhonen, P. K., Pozio, E., La Rosa, G., Chang, B. C., Koehler, A. V., Hoberg, E. P., et al. (2016). Phylogenomic and biogeographic reconstruction of the Trichinella complex. Nat. Commun. 7:10513. doi: 10.1038/ncomms10513
Li, H. H., He, W. X., Song, D., Wu, Q., Li, N., Wan, Y. K., et al. (2016). Effect of Trichinella spiralis and its worm-derived proteins on CLP-induced sepsis in mice. Nan Fang Yi Ke Da Xue Xue Bao 36, 1048–1054.
Li, T., He, S., Zhao, H., Zhao, G., and Zhu, X. Q. (2010). Major trends in human parasitic diseases in China. Trends Parasitol. 26, 264–270. doi: 10.1016/j.pt.2010.02.007
Li, X., Liu, W., Wang, J., Zou, D., Wang, X., Yang, Z., et al. (2012). Rapid detection of Trichinella spiralis larvae in muscles by loop-mediated isothermal amplification. Int. J. Parasitol. 42, 1119–1126. doi: 10.1016/j.ijpara.2012.09.011
Liu, L. N., Jing, F. J., Cui, J., Fu, G. Y., and Wang, Z. Q. (2013). Detection of circulating antigen in serum of mice infected with Trichinella spiralis by an IgY-IgM mAb sandwich ELISA. Exp. Parasitol. 133, 150–155. doi: 10.1016/j.exppara.2012.11.001
Liu, P., Wu, X. P., Bai, X., Wang, X. L., Yu, L., Rosenthal, B., et al. (2013). Screening of early antigen genes of adult-stage Trichinella spiralis using pig serum from different stages of early infection. Vet. Parasitol. 194, 222–225. doi: 10.1016/j.vetpar.2013.02.001
Liu, M., and Boireau, P. (2002). Trichinellosis in China: epidemiology and control. Trends Parasitol. 18, 553–556. doi: 10.1016/s1471-4922(02)02401-7
Liu, M. Y., Wang, X. L., Fu, B. Q., Li, C. Y., Wu, X. P., Le Rhun, D., et al. (2007). Identification of stage-specifically expressed genes of Trichinella spiralis by suppression subtractive hybridization. Parasitology 134(Pt 10), 1443–1455. doi: 10.1017/s0031182007002855
Liu, P., Cui, J., Liu, R. D., Wang, M., Jiang, P., Liu, L. N., et al. (2015a). Protective immunity against Trichinella spiralis infection induced by TsNd vaccine in mice. Parasit. Vectors 8, 185. doi: 10.1186/s13071-015-0791-8
Liu, P., Wang, Z. Q., Liu, R. D., Jiang, P., Long, S. R., Liu, L. N., et al. (2015b). Oral vaccination of mice with Trichinella spiralis nudix hydrolase DNA vaccine delivered by attenuated Salmonella elicited protective immunity. Exp. Parasitol. 153, 29–38. doi: 10.1016/j.exppara.2015.02.008
Liu, W. F., Wen, S. H., Zhan, J. H., Li, Y. S., Shen, J. T., Yang, W. J., et al. (2015). Treatment with recombinant Trichinella spiralis cathepsin B-like protein ameliorates intestinal ischemia/reperfusion injury in mice by promoting a switch from M1 to M2 Macrophages. J. Immunol. 195, 317–328. doi: 10.4049/jimmunol.1401864
Liu, R. D., Jiang, P., Wen, H., Duan, J. Y., Wang, L. A., Li, J. F., et al. (2016). Screening and characterization of early diagnostic antigens in excretory-secretory proteins from Trichinella spiralis intestinal infective larvae by immunoproteomics. Parasitol. Res. 115, 615–622. doi: 10.1007/s00436-015-4779-2
Liu, X., Song, Y., Jiang, N., Wang, J., Tang, B., Lu, H., et al. (2012). Global gene expression analysis of the zoonotic parasite Trichinella spiralis revealed novel genes in host parasite interaction. PLoS Negl. Trop. Dis. 6:e1794. doi: 10.1371/journal.pntd.0001794
Liu, X., Song, Y., Lu, H., Tang, B., Piao, X., Hou, N., et al. (2011). Transcriptome of small regulatory RNAs in the development of the zoonotic parasite Trichinella spiralis. PLoS ONE 6:e26448. doi: 10.1371/journal.pone.0026448
Liu, X. D., Wang, X. L., Bai, X., Liu, X. L., Wu, X. P., Zhao, Y., et al. (2014). Oral administration with attenuated Salmonella encoding a Trichinella cystatin-like protein elicited host immunity. Exp. Parasitol. 141, 1–11. doi: 10.1016/j.exppara.2014.03.015
Lo, Y. C., Hung, C. C., Lai, C. S., Wu, Z., Nagano, I., Maeda, T., et al. (2009). Human trichinosis after consumption of soft-shelled turtles, Taiwan. Emerg. Infect. Dis. 15, 2056–2058. doi: 10.3201/eid1512.090619
Long, S. R., Wang, Z. Q., Liu, R. D., Liu, L. N., Li, L. G., Jiang, P., et al. (2014). Molecular identification of Trichinella spiralis nudix hydrolase and its induced protective immunity against trichinellosis in BALB/c mice. Parasit Vectors 7, 600. doi: 10.1186/s13071-014-0600-9
Mohsen, M. O., Gomes, A. C., Cabral-Miranda, G., Krueger, C. C., Leoratti, F. M., Stein, J. V., et al. (2017). Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J. Control. Release 251, 92–100. doi: 10.1016/j.jconrel.2017.02.031
Mokhtarzadeh, A., Alibakhshi, A., Hashemi, M., Hejazi, M., Hosseini, V., de la Guardia, M., et al. (2017). Biodegradable nano-polymers as delivery vehicles for therapeutic small non-coding ribonucleic acids. J. Control. Release 245, 116–126. doi: 10.1016/j.jconrel.2016.11.017
Murrell, K. D., and Pozio, E. (2011). Worldwide occurrence and impact of human trichinellosis, 1986-2009. Emerg. Infect. Dis. 17, 2194–2202. doi: 10.3201/eid1712.110896
Nöckler, K., Serrano, F., Boireau, P., Kapel, C. M., and Pozio, E. (2005). Experimental studies in pigs on Trichinella detection in different diagnostic matrices. Vet. Parasitol. 132, 85–90. doi: 10.1016/j.vetpar.2005.05.033
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., et al. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids. Res. 28:E63. doi: 10.1093/nar/28.12.e63
OIE (2016). OIE Terrestrial Manual. Part 2 OIE Listed Diseases and Other Diseases of Importance Section 2.1. Multiple Species (Chapter 2.1.20 TRICHINELLOSIS). Paris: OIE1–10.
Pozio, E., and Zarlenga, D. S. (2013). New pieces of the Trichinella puzzle. Int. J. Parasitol. 43, 983–997. doi: 10.1016/j.ijpara.2013.05.010
Qi, W. J., and Fang, Q. (2011). Progress of research on DNA vaccines against parasitosis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zh 23, 340–344.
Rainova, I., Kaftandjiev, I., Harizanov, R., Tsvetkova, N., Jordanova, D., Marinova, I., et al. (2016). Outbreaks of human trichinellosis, still a challenge for the public health authorities in Bulgaria. J. Public Health 24, 291–297. doi: 10.1007/s10389-016-0724-9
Roberts, M. T. (2005). Current understandings on the immunology of leishmaniasis and recent developments in prevention and treatment. Br. Med. Bull. 7, 115–130. doi: 10.1093/bmb/ldl003
Schellenberg, D., Abdulla, S., and Roper, C. (2006). Current issues for anti-malarial drugs to control P. falciparum malaria. Curr. Mol. Med. 6, 253–260. doi: 10.2174/156652406776055168
Sofronic-Milosavljevic, L., Ilic, N., and Gruden-Movsesijan, A. (2017). “50. Trichinella,” in Laboratory Models for Foodborne Infections, ed. D. Liu (Boca Raton FL: CRC Press), 793–808.
Sun, G. G., Liu, R. D., Wang, Z. Q., Jiang, P., Wang, L., Liu, X. L., et al. (2015a). New diagnostic antigens for early trichinellosis: the excretory-secretory antigens of Trichinella spiralis intestinal infective larvae. Parasitol. Res. 114, 4637–4644. doi: 10.1007/s00436-015-4709-3
Sun, G. G., Wang, Z. Q., Liu, C. Y., Jiang, P., Liu, R. D., Wen, H., et al. (2015b). Early serodiagnosis of trichinellosis by ELISA using excretory-secretory antigens of Trichinella spiralis adult worms. Parasit. Vectors 8, 484. doi: 10.1186/s13071-015-1094-9
Sun, S., Wang, X., Wu, X., Zhao, Y., Wang, F., Liu, X., et al. (2011). Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit. Vectors 4:186. doi: 10.1186/1756-3305-4-186
Takahashi, Y., Mingyuan, L., and Waikagul, J. (2000). Epidemiology of trichinellosis in Asia and the Pacific Rim. Vet. Parasitol. 93, 227–239. doi: 10.1016/s0304-4017(00)00343-5
Tang, B., Liu, M., Wang, L., Yu, S., Shi, H., Boireau, P., et al. (2015). Characterisation of a high-frequency gene encoding a strongly antigenic cystatin-like protein from Trichinella spiralis at its early invasion stage. Parasit. Vectors 8, 78. doi: 10.1186/s13071-015-0689-5
Vercruysse, J., Schetters, T. P., Knox, D. P., Willadsen, P., and Claerebout, E. (2007). Control of parasitic disease using vaccines: an answer to drug resistance? Rev. Sci. Tech. 26, 105–115.
Wang, L., Cui, J., Hu, D. D., Liu, R. D., and Wang, Z. Q. (2014). Identification of early diagnostic antigens from major excretory-secretory proteins of Trichinella spiralis muscle larvae using immunoproteomics. Parasit. Vectors 7:40. doi: 10.1186/1756-3305-7-40
Wang, L., Wang, X., Bi, K., Sun, X., Yang, J., Gu, Y., et al. (2016). Oral vaccination with attenuated Salmonella typhimurium-Delivered Ts Pmy DNA vaccine elicits protective immunity against Trichinella spiralis in BALB/c Mice. PLoS Negl. Trop. Dis. 10:e0004952. doi: 10.1371/journal.pntd.0004952
Wang, L., Wang, Z. Q., Hu, D. D., and Cui, J. (2013). Proteomic analysis of Trichinella spiralis muscle larval excretory-secretory proteins recognized by early infection sera. Biomed. Res. Int. 2013:139745. doi: 10.1155/2013/139745
Wang, X. L., Liu, M. Y., Sun, S. M., Liu, X. L., Yu, L., Wang, X. R., et al. (2013). An anti-tumor protein produced by Trichinella spiralis induces apoptosis in human hepatoma H7402 cells. Vet. Parasitol. 194, 186–188. doi: 10.1016/j.vetpar.2013.01.052
Wang, M., Wu, L., Weng, R., Zheng, W., Wu, Z., and Lv, Z. (2017). Therapeutic potential of helminths in autoimmune diseases: helminth-derived immune-regulators and immune balance. Parasitol. Res. doi: 10.1007/s00436-017-5544-5 [Epub ahead of print].
Wang, X. L., Fu, B. Q., Yang, S. J., Wu, X. P., Cui, G. Z., Liu, M. F., et al. (2009). Trichinella spiralis–a potential anti-tumor agent. Vet. Parasitol. 159, 249–252. doi: 10.1016/j.vetpar.2008.10.052
Wang, Z., Cui, J., Xu, B., Chen, Y., Zhang, H., and Jiang, P. (2015). Technical Standard for Management of Trichinellosis Outbreak (WS 470-2015). Beijing: NHFPC, 1–8.
Wang, Z., Cui, J., Xu, B., Zhang, H., Guan, Y., and Tang, L. (2012). Diagnosis of Trichinellosis (WS 369-2012). Singapore: MOH, 1–18.
Wang, Z. Q., and Cui, J. (2001). The epidemiology of human trichinellosis in China during 1964–1999. Parasite 8, S63–S66. doi: 10.1051/parasite/200108s2063
Wang, Z. Q., Cui, J., and Shen, L. J. (2007). The epidemiology of animal trichinellosis in China. Vet. J. 173, 391–398. doi: 10.1016/j.tvjl.2005.08.002
Wang, Z. Q., Cui, J., and Xu, B. L. (2006). The epidemiology of human trichinellosis in China during 2000–2003. Acta Trop. 97, 247–251. doi: 10.1016/j.actatropica.2005.03.012
Wang, Z. Q., Liu, R. D., Sun, G. G., Song, Y. Y., Jiang, P., Zhang, X., et al. (2017). Proteomic analysis of Trichinella spiralis adult worm excretory-secretory proteins recognized by sera of patients with early Trichinellosis. Front. Microbiol. 8:986. doi: 10.3389/fmicb.2017.00986
Wu, X., Fu, B., Wang, X., Yu, L., Yu, S., Deng, H., et al. (2009). Identification of antigenic genes in Trichinella spiralis by immunoscreening of cDNA libraries. Vet. Parasitol. 159, 272–275. doi: 10.1016/j.vetpar.2008.10.035
Xu, J., Bai, X., Wang, L. B., Shi, H. N., van der Giessen, J. W., Boireau, P., et al. (2017a). Immune responses in mice vaccinated with a DNA vaccine expressing serine protease-like protein from the new-born larval stage of Trichinella spiralis. Parasitology 144, 712–719. doi: 10.1017/S0031182016002493
Xu, J., Bai, X., Wang, L. B., Shi, H. N., van der Giessen, J. W. B., Boireau, P., et al. (2017b). Influence of adjuvant formulation on inducing immune response in mice immunized with a recombinant serpin from Trichinella spiralis. Parasite Immunol. 39:e12437. doi: 10.1111/pim.12437
Xu, K., Yan, Y., Zheng, M., and Zhou, Z. (2002). Diagnostic Techniques for T. spiralis in Swine (GB/T 18642-2002). Beijing: AQSIQ, 1–5.
Yang, J., Pan, W., Sun, X., Zhao, X., Yuan, G., Sun, Q., et al. (2015). Immunoproteomic profile of Trichinella spiralis adult worm proteins recognized by early infection sera. Parasit. Vectors 8:20. doi: 10.1186/s13071-015-0641-8
Yang, J., Zhu, W., Huang, J., Wang, X., Sun, X., Zhan, B., et al. (2016a). Partially protective immunity induced by the 14-3-3 protein from Trichinella spiralis. Vet. Parasitol. 231, 63–68. doi: 10.1016/j.vetpar.2016.06.028
Yang, P., Zhang, Z., Lu, Y., Han, C., Li, X., and Song, M. (2010). Development of liquid gene chip technique for detecting Toxoplasma gondii and Trichinella spiralis in food. Prev. Vet. Med. 32, 777–780.
Yang, Y., Cai, Y. N., Tong, M. W., Sun, N., Xuan, Y. H., Kang, Y. J., et al. (2016b). Serological tools for detection of Trichinella infection in animals and humans. One Health 2, 25–30. doi: 10.1016/j.onehlt.2015.11.005
Yang, Y., Vallee, I., Lacour, S. A., Boireau, P., Cheng, S. P., and Liu, M. Y. (2016c). Identification and characterization of immunodominant linear epitopes on the antigenic region of a serine protease in newborn Trichinella larvae. J. Helminthol. 90, 232–237. doi: 10.1017/s0022149x15000267
Yang, Y., Zhang, Z., Yang, J., Chen, X., Cui, S., and Zhu, X. (2010). Oral vaccination with Ts87 DNA vaccine delivered by attenuated Salmonella typhimurium elicits a protective immune response against Trichinella spiralis larval challenge. Vaccine 28, 2735–2742. doi: 10.1016/j.vaccine.2010.01.026
Zhang, G. P., Guo, J. Q., Wang, X. N., Yang, J. X., Yang, Y. Y., Li, Q. M., et al. (2006). Development and evaluation of an immunochromatographic strip for trichinellosis detection. Vet. Parasitol. 137, 286–293. doi: 10.1016/j.vetpar.2006.01.026
Zhao, Y., Liu, M. Y., Wang, X. L., Liu, X. L., Yang, Y., Zou, H. B., et al. (2013). Modulation of inflammatory bowel disease in a mouse model following infection with Trichinella spiralis. Vet. Parasitol. 194, 211–216. doi: 10.1016/j.vetpar.2013.01.058
Zheng, D., Xiao, N., Feng, P., Xu, G., Ouyang, Q., Liao, L., et al. (2011). Epidemiological analysis of human trichinellosis in the mainland of China. Parasit. Infect. Dis. 9, 119–125.
Keywords: trichinellosis, diagnosis, vaccine, immune-related disease, China
Citation: Bai X, Hu X, Liu X, Tang B and Liu M (2017) Current Research of Trichinellosis in China. Front. Microbiol. 8:1472. doi: 10.3389/fmicb.2017.01472
Received: 25 May 2017; Accepted: 20 July 2017;
Published: 02 August 2017.
Edited by:
Bang Shen, Huazhong Agricultural University, ChinaReviewed by:
Jing Cui, Zhengzhou University, ChinaQuan Liu, Military Veterinary Institute, Academy of Military Medical Sciences, China
Longxian Zhang, Henan Agricultural University, China
Copyright © 2017 Bai, Hu, Liu, Tang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyuan Liu, bGl1bXlAamx1LmVkdS5jbg==;, bGl1bXkzNkAxNjMuY29t
†These authors have contributed equally to this work.
 Xue Bai
Xue Bai Xiaoxiang Hu
Xiaoxiang Hu Xiaolei Liu
Xiaolei Liu Bin Tang
Bin Tang Mingyuan Liu
Mingyuan Liu