
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 07 June 2017
Sec. Microbiotechnology
Volume 8 - 2017 | https://doi.org/10.3389/fmicb.2017.00920
This article is part of the Research Topic Electrochemically active microorganisms View all 20 articles
The performance of microbial electrochemical cells depends upon microbial community structure and metabolic activity of the electrode biofilms. Iron as a signal affects biofilm development and enrichment of exoelectrogenic bacteria. In this study, the effect of ferrous iron on microbial communities of the electrode biofilms in microbial fuel cells (MFCs) was investigated. Voltage production showed that ferrous iron of 100 μM facilitated MFC start-up compared to 150 μM, 200 μM, and without supplement of ferrous iron. However, higher concentration of ferrous iron had an inhibitive influence on current generation after 30 days of operation. Illumina Hiseq sequencing of 16S rRNA gene amplicons indicated that ferrous iron substantially changed microbial community structures of both anode and cathode biofilms. Principal component analysis showed that the response of microbial communities of the anode biofilms to higher concentration of ferrous iron was more sensitive. The majority of predominant populations of the anode biofilms in MFCs belonged to Geobacter, which was different from the populations of the cathode biofilms. An obvious shift of community structures of the cathode biofilms occurred after ferrous iron addition. This study implied that ferrous iron influenced the power output and microbial community of MFCs.
Microbial electrochemical cell (MEC) has been admired as a versatile device that can be used for alternative energy generation, electrosynthesis, biosensor, and waste treatment (Hou et al., 2016; Liu et al., 2016a; Huang et al., 2017). However, practical implementation of microbial fuel cells (MFCs) remains restricted by reasons of low electron transfer efficiency and high material costs (Logan et al., 2006). For the past few years, researchers studied electrode materials, exoelectrogenic bacteria, reactor configuration and operational conditions of MFCs (Watson and Logan, 2010; Yong et al., 2011; Janicek, 2015), and pointed out that microbial biofilm was the most direct and key element that affect current generation (Mohan et al., 2008). However, microbial biofilm and its community structure of MFCs can be influenced by temperature, pH, carbon source, inoculum, and metal ion (Lu et al., 2011, 2012; Patil et al., 2011; Wu et al., 2013). The diverse populations developed in the biofilms in MECs have been widely analyzed (Mei et al., 2015). Geobacter as a typical dissimilatory metal-reducing bacterium (DMRB) is commonly identified in MFCs (Mohan et al., 2014; Zhu et al., 2014; Kumar et al., 2016). Hence, to understand and optimize ecological conditions that facilitate exoelectrogens enrichment and electron transfer are essential for MEC application.
Iron plays a central role in the development and maintenance of biofilm of Pseudomonas (Hunter et al., 2013). Although ferric iron has been identified as an important parameter affecting the biofilm formation (Banin et al., 2005), the impact of ferrous iron on the biofilm is less known. Metal ions are essential minerals to composite microorganisms and biological molecules, including metalloproteins which play key roles in most biological processes (iron for respiration; Cvetkovic et al., 2010). The reactive metal ions may have the phenomenon of redox reaction, catalysis, or precipitation, etc. and thus directly affect the performance of MECs by influencing the metabolism of microorganisms or the activity of enzymes (Lu et al., 2015). Due to its high redox activity, the Fe2+ is able to be oxidized at the anode in an air-cathode fuel cells which are capable of abiotic electricity generation (Cheng et al., 2007). The addition of ferrous sulfate to the anode medium has improved the power densities of MFCs during start-up period (Wei et al., 2013). However, there are less literatures concerning the response of exoelectrogenic community in the electrode biofilms to ferrous iron.
Ferrous iron used in catholyte of dual-chambered MFC enhanced power output by increasing salt concentration or improving cathode potential (Ter Heijne et al., 2007). A comparison of results with and without ferrous iron as a cathodic reactant also revealed that the addition of ferrous iron enhanced power generation in batch MFC (Wang et al., 2011). However, the knowledge related to the effects of ferrous iron on performances of MFCs and microbial communities of electrode biofilms is less known. To reveal the response of microbial community of the electrode biofilm to ferrous iron, in this study, electrochemical performances of MFCs supplemented with different concentrations of ferrous iron were investigated. Meanwhile, microbial community structures of the anodes and cathodes biofilms in MFCs were analyzed using Illumina Hiseq sequencing of 16S rRNA gene amplicons.
Single-chamber MFCs with volume of 14 mL were constructed as previously described (Xing et al., 2008). Anodes were made of carbon paper (Toray TGP-H-090, Japan), while cathodes were stainless steel mesh by rolling activated carbon and polytetrafluoroethylene (PTFE) (Dong et al., 2012) (the area of anode and cathode were both 7 cm2). Domestic wastewater was used as inoculum in the first 5 days. Nutrient solutions were consisted of 1 g/L sodium acetate, 5 mL/L vitamins, 12.5 mL/L minerals, 100 mM phosphate buffer saline (PBS, pH of 6) and FeSO4 with different concentrations. The final pH value of nutrient solution was 6.2 ± 0.1. The final concentrations of FeSO4 in MFCs were 32 (control), 100, 150, and 200 μM.
Voltages across the external resistor (1000 Ω) of MFCs were measured using Keithley 2700 multimeter/data acquisition system. All MFCs were operated at 35°C and each Fe2+ concentration have three replicates. Cyclic voltammetry (CV) measurements of MFCs at the 15th day were performed on Autolab potentiostat (Metrohm, Netherlands) with scan rate of 0.01 V/s.
After MFCs were operated for 2 months, the anode and cathode biofilms of MFCs (control, fed with 100 and 200 μM Fe2+) were sampled for genomic DNA extraction by using PowerSoil DNA Isolation Kit according the manufacturer’s instructions. DNA concentration and purity were determined by NanoPhotometer P-Class (Implen, GmbH). Prior to polymerase chain reaction (PCR) amplification, DNA of anode and cathode biofilms from three duplicated bioreactors were mixed. The V4 region (length of ∼373 bp) of bacterial 16S rRNA gene was amplified by using a set of bacterial primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). After integrated with barcode, PCR amplification was implemented by using ABI GeneAmp® 9700 PCR system.
Sequencing was performed on Illumina Hiseq platforms according to the standard protocols. Raw Tags were overlapped by using the Fast Length Adjustment of SHort reads (FLASH; V1.2.7)1 software (Magoc and Salzberg, 2011) and filtered following pipelines of Quantitative Insights Into Microbial Ecology (QIIME, V1.7.0; Caporaso et al., 2010). Effective tags were obtained by removing chimeric sequences after aligned using Gold database2. Operational taxonomic units (OTUs) were determined based on the threshold of 97% similarity using UPARSE software (Uparse V7.0.1001). A representative sequence of each OTU was aligned for taxonomic identification using the GreenGene database3 and Ribosomal Database Project (RDP) classifier (version 2.2)4 with the threshold of 80–100% (DeSantis et al., 2006; Wang et al., 2007). The raw Illumina sequencing data were deposited in the Sequence Read Archive (SRA) of National Center for Biotechnology Information (NCBI) under the accession Nos. SRR5266191–SRR5266196.
Cyclic voltammetry curves showed that MFCs supplemented with 100 μM ferrous ion (Fe2+) obtained the highest current peak on the 15th day (Figure 1). The results suggested that low concentration of Fe2+ could obviously improve electrochemical activity of MFCs in the start-up period. During another 15 days of operation, MFCs with 100 μM ferrous ion showed the best electrochemical characteristics compared to MFCs with 150 and 200 μM Fe2+, and MFCs without additional Fe2+ supplement (Figure 2). The maximum voltage of 0.55 V was monitored in MFCs fed with 100 μM Fe2+, and then following the order control (0.54 V), 150 μM Fe2+ (0.52 V) and 200 μM Fe2+ (0.47 V). After all MFCs were operated for 30 days, MFCs of control groups maintained the steady voltage output, while other MFCs with Fe2+ addition performed a weaken efficiency.
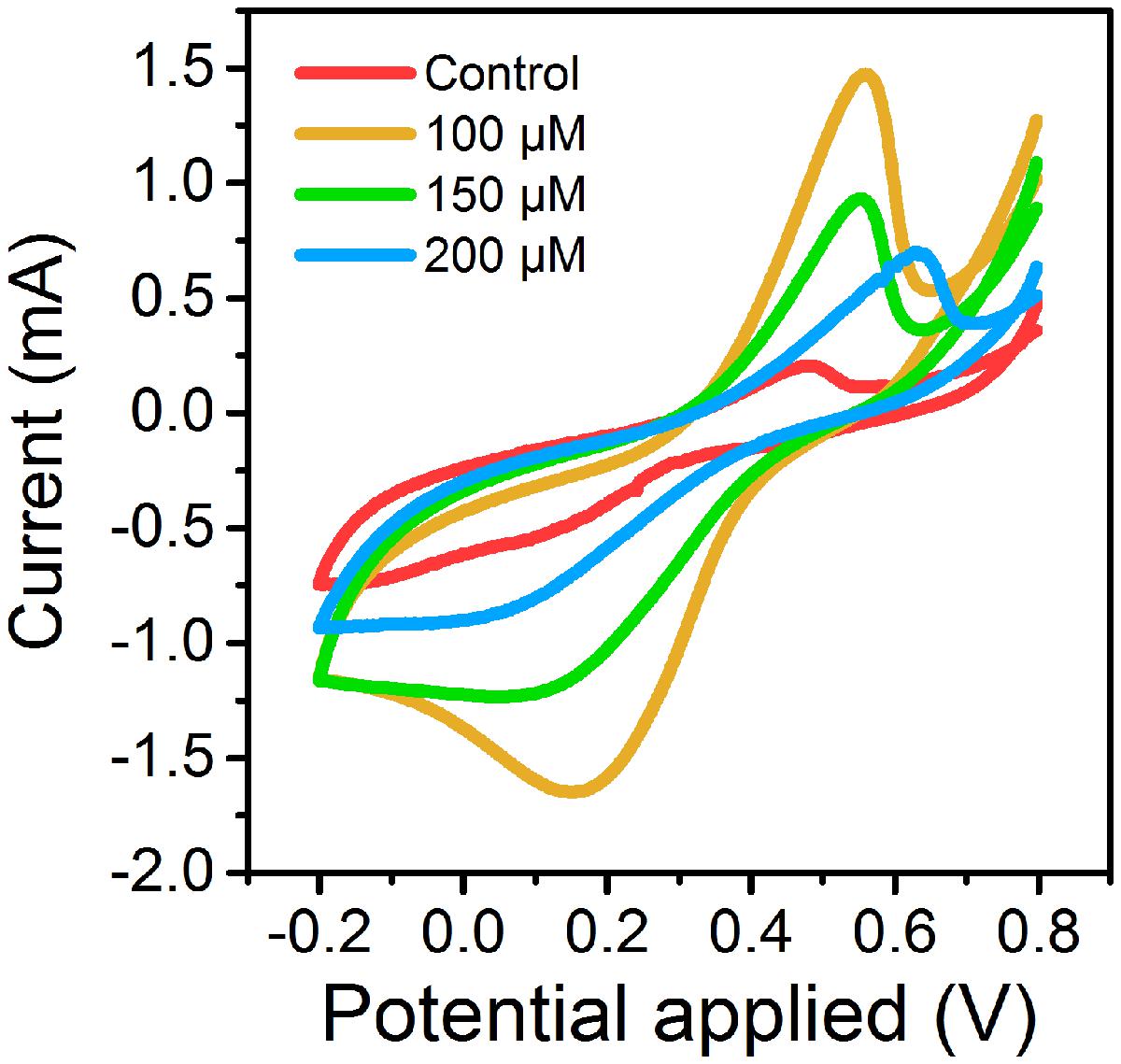
FIGURE 1. Cyclic voltammetry curves of MFCs supplemented with different concentrations of ferrous iron on 15th day.
Since the power outputs of MFCs with 150 and 200 μM were similar, and the CV result of 200 μM adequately represented the decrease of electrochemical activity of electrode biofilms, the biofilm samples of MFCs with ferrous iron of 150 μM were not used for microbial community analysis. After quality filtering the raw tags, 50,373 to 54,932 effective tags were obtained per sample, with average length of 373 bp. Total OTUs at the 97% similarity were ranged from 630 to 824 per sample with an average of 710 OTUs (Table 1). The anode biofilms in MFCs supplemented with ferrous iron showed slightly lower population diversity than that in control MFCs without ferrous iron supplement. Shannon indices were 3.72, 4.71, and 5.21 for the anodes biofilms with 100, 200 μM Fe2+, and without Fe2+, respectively. By contrast, Fe2+ increased the population diversities of the cathode biofilms, Shannon indices increased from 4.3 (control) to 5.02 (100 μM Fe2+) and 5.54 (200 μM Fe2+), suggesting that Fe2+ affected microbial community structure of the electrode biofilms in MFCs. Principal component analysis based on OTUs showed three clusters, the anode biofilms of MFC without Fe2+ was separated from the anode biofilms of MFC supplemented with Fe2+ of 100 and 200 μM Fe2+ and the cathode biofilms (control, 100, and 200 μM Fe2+; Figure 3).
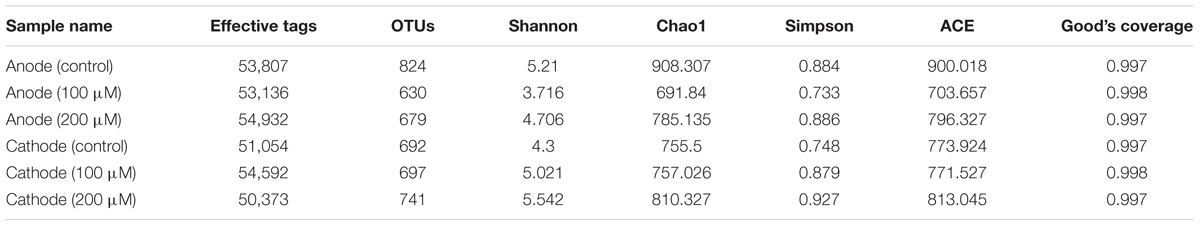
TABLE 1. Qualities of reads identified by Illumina Hiseq sequencing and bacterial diversity estimates based on OTUs (97% similarity).
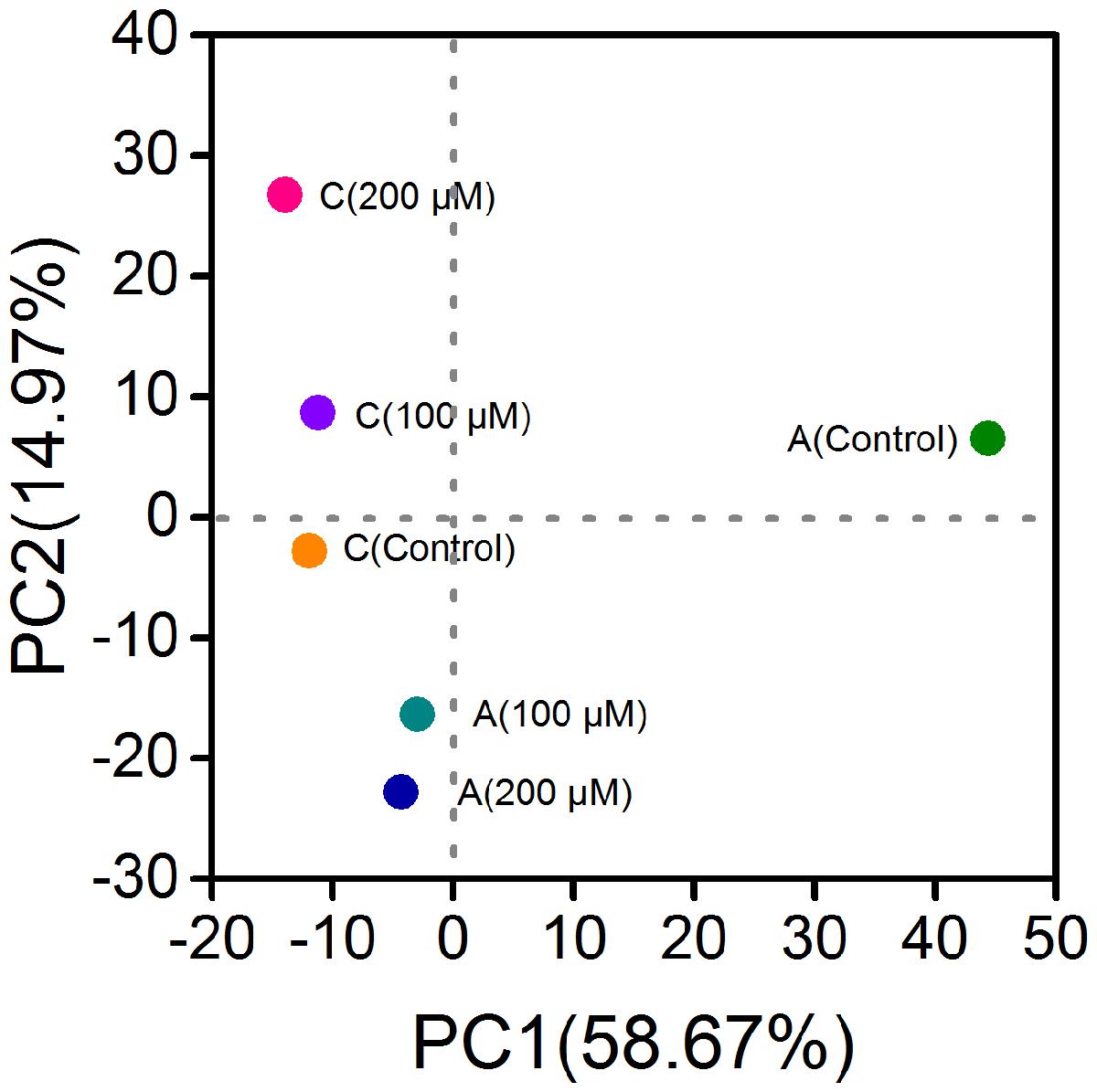
FIGURE 3. Principal component analysis based on operational taxonomic units of the anode and cathode biofilms of MFCs.
The bacterial communities of the anode biofilms were substantially shifted when additional Fe2+ was supplemented in MFCs. Proteobacteria were the most dominant phylum observed both in the anode (71–75%, relative abundance) and cathode biofilms (41–78%) (Figure 4A). Chlorobi (11–14%) and Bacteroidetes (4–8%) were also predominant phyla in the anode biofilms. The relative abundances of Lentisphaerae in the cathode biofilms, were much higher than that in the anode biofilms, reached to 31% (100 μM Fe2+), 22% (200 μM Fe2+), and 4% (control). Deltaproteobacteria, Ignavibacteria, and Betaproteobacteria were the most predominant classes in the anode biofilms and accounted for 75% more or less, of which, the abundance of Deltaproteobacteria in the anode of MFCs with 100 μM reached to 50%, speculating that Deltaproteobacteria were the dominant class since MFC start-up period (Figure 4B). By contrast, microbial community structures of cathodes were different from anodes. Alphaproteobacteria, Gammaproteobacteria, Bacteroidia, and Lentisphaeria were the predominant classes on the cathodes. Cathodes of MFCs with additional Fe2+ had similar communities that were much different with control group.
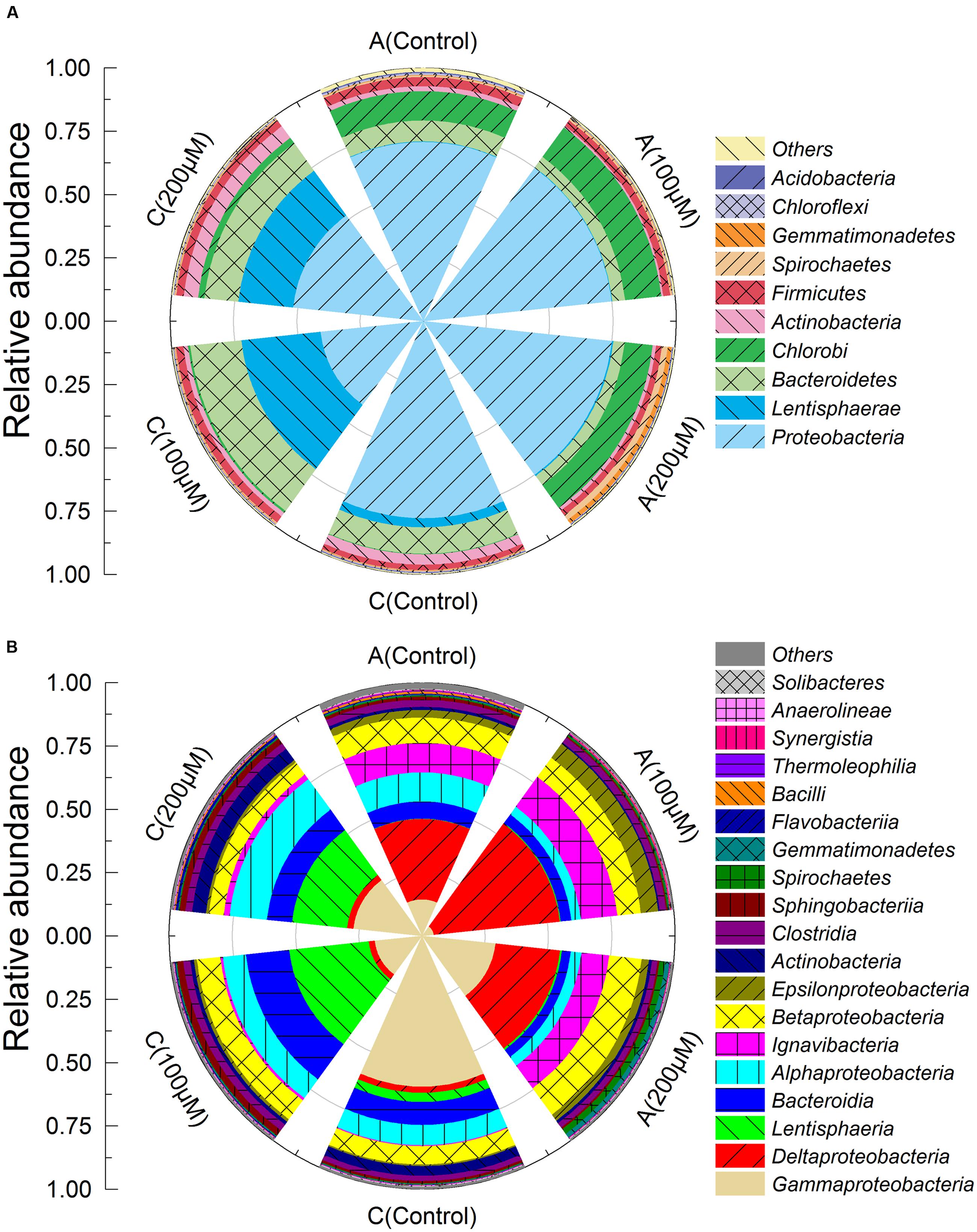
FIGURE 4. Microbial community taxonomic wind-rose plots based on relative abundance of 16S rRNA sequences of the anode and cathode biofilms in MFCs at the phylum (A) and class levels (B).
The predominant genera varied significantly among all anodes and cathodes biofilms (Figure 5). The majority of predominant populations in the control MFCs were affiliated with Geobacter spp. (30.7%) and Legionella spp. (50.3%). Geobacter was also the predominant genus in the anode of MFC supplemented with 100 and 200 μM Fe2+, the relative abundance of which population reached up to 49.3 and 24.4%. Another predominant genus in the anode biofilms of MFC (200 μM Fe2+) was affiliated to Rhodanobacter (19%). In the cathode biofilms of MFCs with 100 and 200 μM Fe2+, higher relative abundance of predominant genera belonged to Legionella spp. (2 and 6%), and no absolutely predominant populations were present. Hierarchical cluster analysis of microbial communities based on genus taxonomy revealed that the relative abundance of Sphaerochaeta, Dechloromonas, Paracoccus, Thermomonas, and Rhodanobacter increased in the anode biofilms of MFCs supplemented with 200 μM Fe2+ (Figure 6). Meanwhile, the relative abundance of Gordonia, Sphingopyxis, Hydrogenophaga, and Janthinobacterium in the cathode biofilms of MFCs with 200 μM Fe2+ were relatively higher than that in the cathodes biofilms of MFCs without Fe2+ and with 100 μM Fe2+, but higher proportion of Thauera, Dokdonella, Fusibacter, Devosia, and Desulfovibrio were observed in the cathode biofilms of MFCs with 100 μM Fe2+.
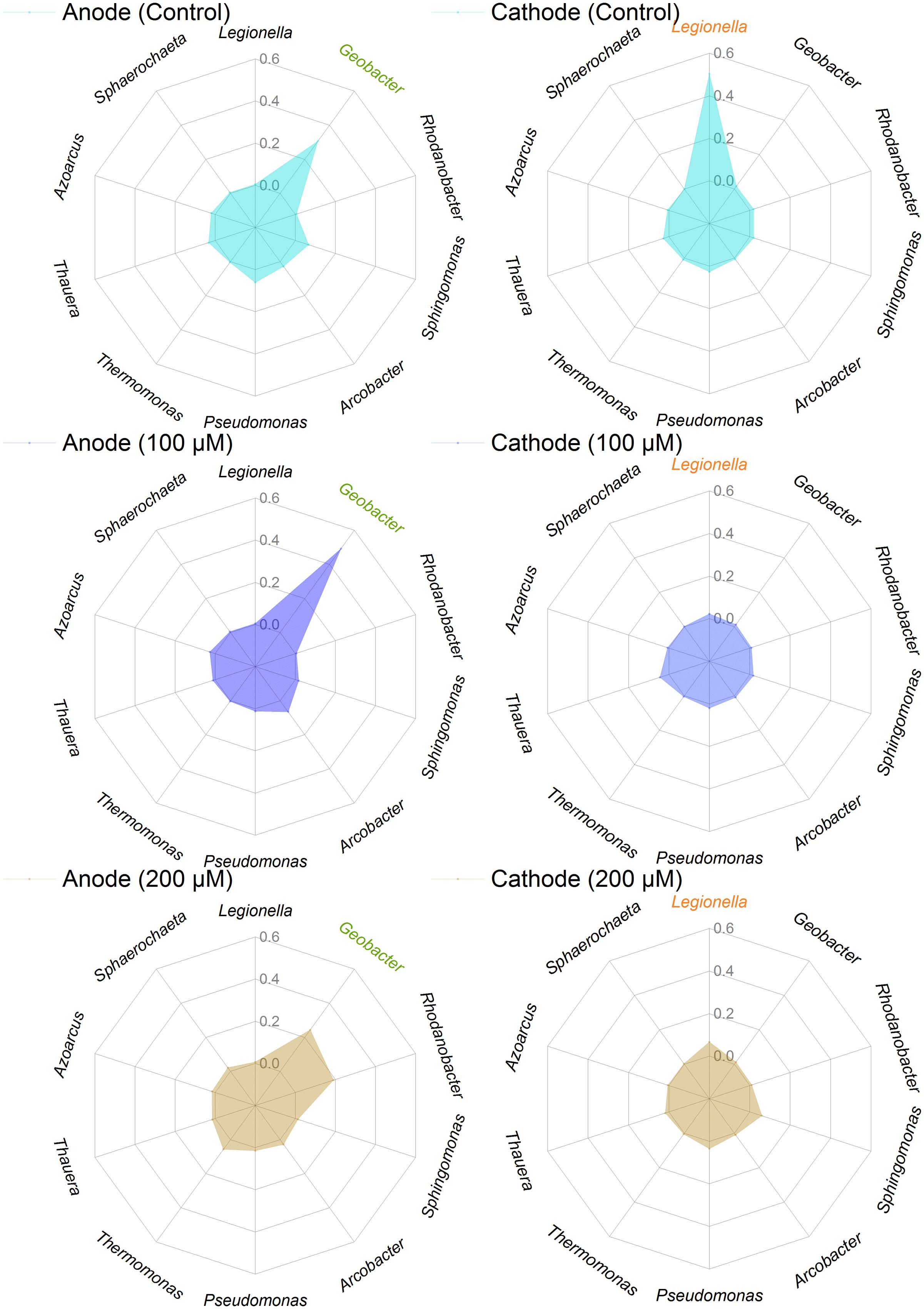
FIGURE 5. Relative abundance of predominant genera in the anode and cathode biofilms in MFCs supplemented with different concentrations of ferrous iron.
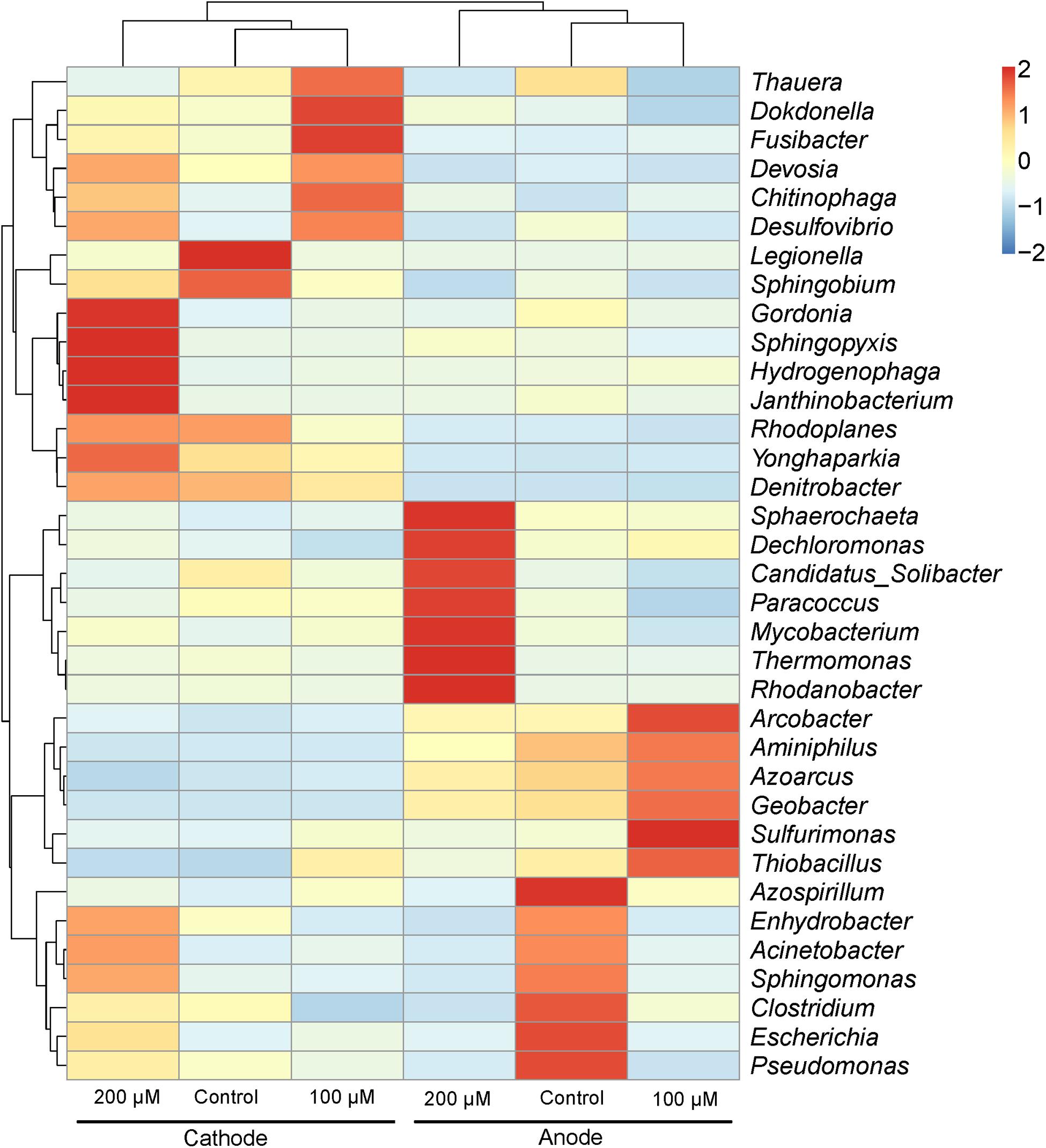
FIGURE 6. Hierarchical cluster analysis of predominant populations in the anode and cathode biofilms in MFCs. The genera with the relative abundance of the top 35 are shown. The species clustering tree is on the left and the sample clustering tree is on the top. Each box of the heatmap represents a Z-score, a positive score indicates a datum above the mean, while a negative score indicates a datum below the mean.
Ferrous iron with appropriate concentration (100 μM) stimulated electrochemical activity of MFCs during the start-up period, but Fe2+ cannot enhance power output after 30 days of operation and higher concentration of Fe2+ had the negative effect (Wei et al., 2013), presumably the Fe2+ facilitated biofilm formation at the early stage. The metal ions may act as redox active sites in the enzymes which catalyze the electron transfer and redox reaction to affect the performance of bio-electrochemical systems (BESs) (Lu et al., 2015). In mature anode biofilms, pH decreased through different growth phases, showing that the pH is not always a limiting factor in a biofilm. Meanwhile, increasing redox potential at the biofilm electrode was associated only with the biofilm, demonstrating that microbial biofilms respire in a unique internal environment (Babauta et al., 2012). Oxidation of ferrous ion by microbes is an important component of iron geochemical cycle (Croal et al., 2004). Recent studies also confirmed that Fe2+ oxidation provides an energetic benefit for some microbes’ growth when using Fe2+ and acetate as the co-substrate (Muehe et al., 2009; Chakraborty et al., 2011). Illumina Hiseq sequencing of 16S rRNA gene indicated that Fe2+ shifted bacterial community and influenced enrichment of exoelectrogenic bacteria in the anode biofilms.
An excessive amount of metal salts may result in negative effects on the performance of BESs by inhibiting the activity of microorganisms (Jiang et al., 2011). The relative abundance of Geobacter increased from 30.7 to 49.3% in MFCs with 100 μM Fe2+ but decreased to 24.4% in MFCs with 200 μM Fe2+, implying higher Fe2+ concentration could not further enrich Geobacter. As a result, the power output of MFC with higher Fe2+ concentration (200 μM) was lower than control and 100 μM Fe2+ during MFC steady operation. Rhodanobacter accounted for a large proportion (19%) in MFCs with Fe2+ concentration of 200 μM. To date, the function of Rhodanobacter was mostly investigated on denitrifying (Green et al., 2012) and thiosulfate-oxidizing (Lee et al., 2007), but little is reported about Fe2+ oxidation especially mediated by C-type cytochromes (Croal et al., 2007; Bird et al., 2011). Whether it participates in interspecies interaction with Geobacter should be further proved. Other exoelectrogenic bacteria also formed a certain proportion in different anode biofilms, such as Pseudomonas (1–6%) and Arcobacter (3–7%) (Fedorovich et al., 2009; Yong et al., 2011). Pseudomonas has a positive role to benefit other exoelectrogens in anode biofilm under a high concentration of salt addition (Liu et al., 2016b). Arcobacter can be selectively enriched in an acetate-fed MFC and rapidly generates a strong electronegative potential (Fedorovich et al., 2009). It indicated that additional ions, like Fe2+, will take part in biofilm metabolism or microbial communication, which resulted in community structure changes.
The microbial communities on the cathodes clearly differed from the anodes biofilms in all MFCs. The most predominant genera in the cathode biofilms of MFCs without additional ferrous iron came from Legionella spp. (50.3% of relative abundance). However, the relative abundance of Legionella on the cathode biofilms declined to 2–6% with Fe2+ addition, suggesting that Legionella was inhibited by high concentration of Fe2+. The abundance of Fe(II)-oxidizing bacteria, Janthinobacterium (Geissler et al., 2011), in the cathode biofilms of MFC with 200 μM Fe2+ were relatively higher than other groups (Figure 6). Hierarchical cluster analysis based on genus taxonomy demonstrated that the response of predominant populations in the electrode biofilms to ferrous iron occurred, indicating the effect of ferrous iron on microbial community in MFCs.
Some environmental factors, such as nutrients, pH, and temperature, influence the performances of MFCs by changing microbial activity and community structure. Our study indicated that ferrous iron changed microbial community structures of electrode biofilms of MFCs. Other metals (e.g., Ca, Mg, Pt, Au, Pd, Fe, V, Mn) and metal-nanomaterials affected current generation of MECs by changing the metabolism and enzyme activity of microorganisms (Lu et al., 2015). These studies have analyzed effect of single metal on electricity generation by MFCs, however, the effect of combined metals on microbial community structure and performance of MFCs should be further investigated.
Neutral pH is considered as the optimal condition for current generation by MFCs (Gil et al., 2003; Jadhav and Ghangrekar, 2009). However, a higher pH has been demonstrated to enhance the electrochemical activity of riboflavin which is a metabolite responsible for extracellular electron transfer in some species (Yuan et al., 2011; Yong et al., 2013). By contrast, MFCs have also been operated at pH less than 4.0 and produced high current densities by acidophilic bacterium (Malki et al., 2008; Winfield et al., 2016). Previous studies proved that temperate substantially affected the performances of MECs or MFCs by shaping microbial community (Lu et al., 2011, 2012). Synergistic effect of metals, pH and temperature on performances of MECs and correlation analysis of these environmental factors should be further investigated in the future.
DX designed the experiment. QL performed specific experiments. QL, BL, and DX contributed to analyze the experiment data. QL, WL, WZ, XZ, and DX wrote the manuscript. All authors were involved in revision of the manuscript and approved its final version.
This study was supported by National Natural Science Foundation of China (Nos. 51422805, 31270004), the Science Fund for Distinguished Young Scholars of Heilongjiang Province (Grant No. JC201407), the State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (No. 2016DX10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Babauta, J. T., Nguyen, H. D., Harrington, T. D., Renslow, R., and Beyenal, H. (2012). pH, redox potential and local biofilm potential microenvironments within Geobacter sulfurreducens biofilms and their roles in electron transfer. Biotechnol. Bioeng. 109, 2651–2662. doi: 10.1002/bit.24538
Banin, E., Vasil, M. L., and Greenberg, E. P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. P. Natl. Acad. Sci. U.S.A. 102, 11076–11081. doi: 10.1073/pnas.0504266102
Bird, L. J., Bonnefoy, V., and Newman, D. K. (2011). Bioenergetic challenges of microbial iron metabolisms. Trends. Microbiol. 19, 330–340. doi: 10.1016/j.tim.2011.05.001
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chakraborty, A., Roden, E. E., Schieber, J., and Picardal, F. (2011). Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(II) oxidation in batch and continuous-flow systems. Appl. Environ. Microbiol. 77, 8548–8556. doi: 10.1128/aem.06214-11
Cheng, S., Dempsey, B. A., and Logan, B. E. (2007). Electricity generation from synthetic acid-mine drainage (AMD) water using fuel cell technologies. Environ. Sci. Technol. 41, 8149–8153. doi: 10.1021/es0712221
Croal, L. R., Gralnick, J. A., Malasarn, D., and Newman, D. K. (2004). The genetics of geochemistry. Annu. Rev. Genet. 38, 175–202. doi: 10.1146/annurev.genet.38.072902.091138
Croal, L. R., Jiao, Y., and Newman, D. K. (2007). The fox operon from Rhodobacter strain SW2 promotes phototrophic Fe(II) oxidation in Rhodobacter capsulatus SB1003. J. Bacteriol. 189, 1774–1782. doi: 10.1128/JB.01395-06
Cvetkovic, A., Menon, A. L., Thorgersen, M. P., Scott, J. W., Poole, F. L. III, and Jenney, F. E. Jr. (2010). Microbial metalloproteomes are largely uncharacterized. Nature 466, 779–782. doi: 10.1038/nature09265
DeSantis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072. doi: 10.1128/AEM.03006-05
Dong, H., Yu, H., Wang, X., Zhou, Q., and Feng, J. (2012). A novel structure of scalable air-cathode without Nafion and Pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells. Wat. Res. 46, 5777–5787. doi: 10.1016/j.watres.2012.08.005
Fedorovich, V., Knighton, M. C., Pagaling, E., Ward, F. B., Free, A., and Goryanin, I. (2009). Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell. Appl. Environ. Microbiol. 75, 7326–7334. doi: 10.1128/AEM.01345-09
Geissler, A., Law, G. T. W., Boothman, C., Morris, K., Burke, I. T., Livens, F. R., et al. (2011). Microbial communities associated with the oxidation of iron and technetium in bioreduced sediments. Geomicrobiol. J. 28, 507–518. doi: 10.1080/01490451.2010.515287
Gil, G.-C., Chang, I.-S., Kim, B. H., Kim, M., Jang, J.-K., Park, H. S., et al. (2003). Operational parameters affecting the performannce of a mediator-less microbial fuel cell. Biosens. Bioelectron. 18, 327–334. doi: 10.1016/s0956-5663(02)00110-0
Green, S. J., Prakash, O., Jasrotia, P., Overholt, W. A., Cardenas, E., Hubbard, D., et al. (2012). Denitrifying bacteria from the genus Rhodanobacter dominate bacterial communities in the highly contaminated subsurface of a nuclear legacy waste site. Appl. Environ. Microbiol. 78, 1039–1047. doi: 10.1128/AEM.06435-11
Hou, D., Lu, L., and Ren, Z. J. (2016). Microbial fuel cells and osmotic membrane bioreactors have mutual benefits for wastewater treatment and energy production. Wat. Res. 98, 183–189. doi: 10.1016/j.watres.2016.04.017
Huang, Z., Lu, L., Jiang, D., Xing, D., and Ren, Z. J. (2017). Electrochemical hythane production for renewable energy storage and biogas upgrading. Appl. Energ. 187, 595–600. doi: 10.1016/j.apenergy.2016.11.099
Hunter, R. C., Asfour, F., Dingemans, J., Osuna, B. L., Samad, T., Malfroot, A., et al. (2013). Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. mBio 4:e00557-13. doi: 10.1128/mBio.00557-13
Jadhav, G. S., and Ghangrekar, M. M. (2009). Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 100, 717–723. doi: 10.1016/j.biortech.2008.07.041
Janicek, A. M. (2015) Cathode Development and Reactor Design for Scaling-Up Microbial Fuel Cells. dissertation’s thesis, Oregon State University, Corvallis, OR.
Jiang, D., Curtis, M., Troop, E., Scheible, K., McGrath, J., Hu, B., et al. (2011). A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCs) to enhance the power production in wastewater treatment. Int. J. Hydrogen. Energy 36, 876–884. doi: 10.1016/j.ijhydene.2010.08.074
Kumar, R., Singh, L., and Zularisam, A. W. (2016). Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 56, 1322–1336. doi: 10.1016/j.rser.2015.12.029
Lee, C. S., Kim, K. K., Aslam, Z., and Lee, S. T. (2007). Rhodanobacter thiooxydans sp. nov., isolated from a biofilm on sulfur particles used in an autotrophic denitrification process. Int. J. Syst. Evol. Microbiol. 57, 1775–1779. doi: 10.1099/ijs.0.65086-0
Liu, Q., Ren, Z. J., Huang, C., Liu, B., Ren, N., and Xing, D. (2016a). Multiple syntrophic interactions drive biohythane production from waste sludge in microbial electrolysis cells. Biotechnol. Biofuels. 9:162. doi: 10.1186/s13068-016-0579-x
Liu, W., He, Z., Yang, C., Zhou, A., Guo, Z., Liang, B., et al. (2016b). Microbial network for waste activated sludge cascade utilization in an integrated system of microbial electrolysis and anaerobic fermentation. Biotechnol. Biofuels. 9:83. doi: 10.1186/s13068-016-0493-2
Logan, B. E., Hamelers, B., Rozendal, R., Schroder, U., Keller, J., Freguia, S., et al. (2006). Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40, 5181–5192. doi: 10.1021/es0605016
Lu, L., Ren, N., Zhao, X., Wang, H., Wu, D., and Xing, D. (2011). Hydrogen production, methanogen inhibition and microbial community structures in psychrophilic single-chamber microbial electrolysis cells. Energy Environ. Sci. 4, 1329–1336. doi: 10.1039/C0EE00588F
Lu, L., Xing, D., and Ren, N. (2012). Bioreactor performance and quantitative analysis of methanogenic and bacterial community dynamics in microbial electrolysis cells during large temperature fluctuations. Environ. Sci. Technol. 46, 6874–6881. doi: 10.1021/es300860a
Lu, Z., Chang, D., Ma, J., Huang, G., Cai, L., and Zhang, L. (2015). Behavior of metal ions in bioelectrochemical systems: a review. J. Power. Sour. 275, 243–260. doi: 10.1016/j.jpowsour.2014.10.168
Magoc, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Malki, M., De Lacey, A. L., Rodríguez, N., Amils, R., and Fernandez, V. M. (2008). Preferential use of an anode as an electron acceptor by an acidophilic bacterium in the presence of oxygen. Appl. Environ. Microbiol. 74, 4472–4476. doi: 10.1128/AEM.00209-08
Mei, X., Guo, C., Liu, B., Tang, Y., and Xing, D. (2015). Shaping of bacterial community structure in microbial fuel cells by different inocula. RSC Adv. 5, 78136–78141. doi: 10.1039/C5RA16382J
Mohan, S. V., Raghavulu, S. V., and Sarma, P. (2008). Influence of anodic biofilm growth on bioelectricity production in single chambered mediatorless microbial fuel cell using mixed anaerobic consortia. Biosens. Bioelectron. 24, 41–47. doi: 10.1016/j.bios.2008.03.010
Mohan, S. V., Velvizhi, G., Modestra, J. A., and Srikanth, S. (2014). Microbial fuel cell: critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew. Sustain. Energy Rev. 40, 779–797. doi: 10.1016/j.rser.2014.07.109
Muehe, E. M., Gerhardt, S., Schink, B., and Kappler, A. (2009). Ecophysiology and the energetic benefit of mixotrophic Fe(II) oxidation by various strains of nitrate-reducing bacteria. FEMS. Microbiol. Ecol. 70, 335–343. doi: 10.1111/j.1574-6941.2009.00755.x
Patil, S. A., Harnisch, F., Koch, C., Hubschmann, T., Fetzer, I., Carmona-Martinez, A. A., et al. (2011). Electroactive mixed culture derived biofilms in microbial bioelectrochemical systems: the role of pH on biofilm formation, performance and composition. Bioresour. Technol. 102, 9683–9690. doi: 10.1016/j.biortech.2011.07.087
Ter Heijne, A., Hamelers, H. V., and Buisman, C. J. (2007). Microbial fuel cell operation with continuous biological ferrous iron oxidation of the catholyte. Environ. Sci. Technol. 41, 4130–4134. doi: 10.1021/es0702824
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Wang, Y., Niu, C.-G., Zeng, G.-M., Hu, W.-J., Huang, D.-W., and Ruan, M. (2011). Microbial fuel cell using ferrous ion activated persulfate as a cathodic reactant. Int. J. Hydrogen Energy 36, 15344–15351. doi: 10.1016/j.ijhydene.2011.08.071
Watson, V. J., and Logan, B. E. (2010). Power production in MFCs inoculated with Shewanella oneidensis MR-1 or mixed cultures. Biotechnol. Bioeng. 105, 489–498. doi: 10.1002/bit.22556
Wei, L., Han, H., and Shen, J. (2013). Effects of temperature and ferrous sulfate concentrations on the performance of microbial fuel cell. Int. J. Hydrogen Energy 38, 11110–11116. doi: 10.1016/j.ijhydene.2013.01.019
Winfield, J., Greenman, J., Dennis, J., and Ieropoulos, I. (2016). Analysis of microbial fuel cell operation in acidic conditions using the flocculating agent ferric chloride. J. Chem. Technol. Biotechnol. 91, 138–143. doi: 10.1002/jctb.4552
Wu, D., Xing, D., Lu, L., Wei, M., Liu, B., and Ren, N. (2013). Ferric iron enhances electricity generation by Shewanella oneidensis MR-1 in MFCs. Bioresour. Technol. 135, 630–634. doi: 10.1016/j.biortech.2012.09.106
Xing, D., Zuo, Y., Cheng, S., Regan, J. M., and Logan, B. E. (2008). Electricity generation by Rhodopseudomonas palustris DX-1. Environ. Sci. Technol. 42, 4146–4151. doi: 10.1021/es800312v
Yong, Y. C., Cai, Z., Yu, Y. Y., Chen, P., Jiang, R., Cao, B., et al. (2013). Increase of riboflavin biosynthesis underlies enhancement of extracellular electron transfer of Shewanella in alkaline microbial fuel cells. Bioresour. Technol. 130, 763–768. doi: 10.1016/j.biortech.2012.11.145
Yong, Y. C., Yu, Y. Y., Li, C. M., Zhong, J. J., and Song, H. (2011). Bioelectricity enhancement via overexpression of quorum sensing system in Pseudomonas aeruginosa-inoculated microbial fuel cells. Biosens. Bioelectron. 30, 87–92. doi: 10.1016/j.bios.2011.08.032
Yuan, Y., Zhao, B., Zhou, S., Zhong, S., and Zhuang, L. (2011). Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 102, 6887–6891. doi: 10.1016/j.biortech.2011.04.008
Keywords: microbial fuel cell, ferrous iron, electricity generation, microbial community, high throughput sequencing
Citation: Liu Q, Liu B, Li W, Zhao X, Zuo W and Xing D (2017) Impact of Ferrous Iron on Microbial Community of the Biofilm in Microbial Fuel Cells. Front. Microbiol. 8:920. doi: 10.3389/fmicb.2017.00920
Received: 08 January 2017; Accepted: 08 May 2017;
Published: 07 June 2017.
Edited by:
Haoyi Cheng, Research Center for Eco-Environmental Sciences (CAS), ChinaCopyright © 2017 Liu, Liu, Li, Zhao, Zuo and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Defeng Xing, ZHhpbmdAaGl0LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.