- 1Key Laboratory of Human Disease Comparative Medicine, Ministry of Health, Beijing, China
- 2Institution of Laboratory Animal Sciences, Centre for Tuberculosis, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Beijing Key Laboratory for Animal Models of Emerging and Reemerging Infectious, Beijing, China
- 4Beijing Engineering Research Center for Experimental Animal Models of Human Critical Diseases, Beijing, China
- 5Key Laboratory of Human Diseases Animal Model, State Administration of Traditional Chinese Medicine, Beijing, China
Tuberculosis (TB) is a health threat to the global population. Anti-TB drugs and vaccines are key approaches for TB prevention and control. TB animal models are basic tools for developing biomarkers of diagnosis, drugs for therapy, vaccines for prevention and researching pathogenic mechanisms for identification of targets; thus, they serve as the cornerstone of comparative medicine, translational medicine, and precision medicine. In this review, we discuss the current use of TB animal models and their problems, as well as offering perspectives on the future of these models.
Introduction
The number of tuberculosis (TB) patients has been increasing, and in recent years TB became the leading cause of death around the world. In 2015, 10.4 million people were diagnosed with TB, of which 480,000 cases were drug-resistant according to the WHO TB report. Anti-TB drugs, vaccines, and diagnostic reagents are essential tools for TB prevention and control, TB animal models have been widely used for development of these tools, as well as for research on pathogenic mechanisms of TB, constituting 35% TB-related research found in the NCBI database. Animal models of TB play a key role in translational medicine, basic medicine, and biology of TB (Moskovic et al., 1993; Coquard et al., 1997; El Husseiny et al., 2002; Naaman et al., 2011; Durnali et al., 2012).
In this paper, we review the current use of TB animal models in medical research, as well as problems, progress, and perspectives associated with them.
Current Use of TB Animal Models
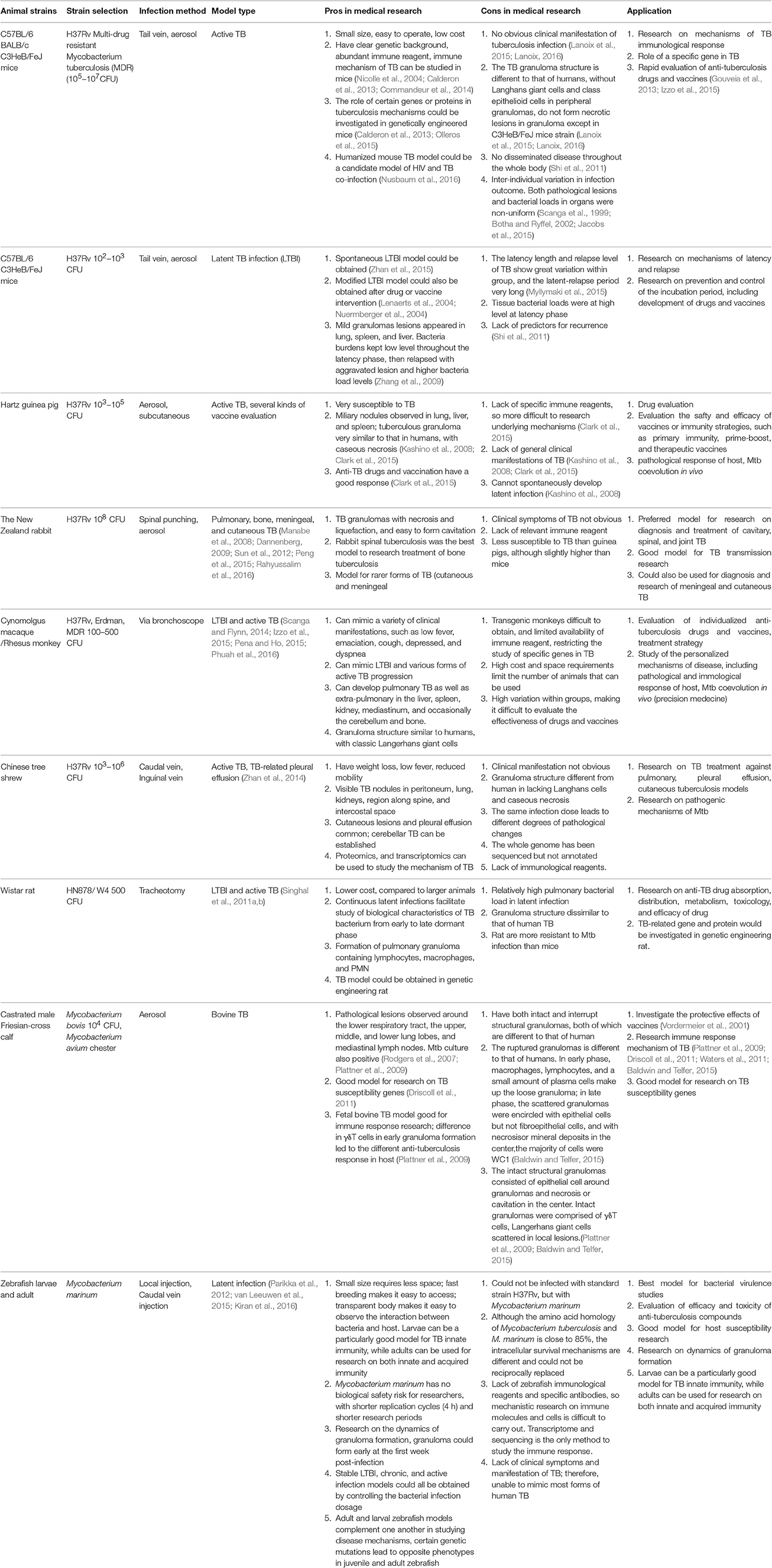
Many animal species have been used for TB models, with mice, guinea pigs, rabbits, and non-human primates among the most commonly used. Each model mimics one or more features of human TB, including clinical signs, pathological changes, bacteria loads in organs, disease progressions or immunological parameters (Rodgers et al., 2007; Waters et al., 2011; Clark et al., 2015; Myllymaki et al., 2015; Peng et al., 2015; Provan and Newland, 2015; Kramnik and Beamer, 2016; Phuah et al., 2016). Characteristics, as well as pros and cons, of current TB animal models are summarized, the utility of each model according to the pros and cons was also speculated. For instance, mouse active TB model is suitable for rapid anti-TB chemical drugs evaluation, due to the homogeneous pathological change and bacteria burden; guinea pig presents sensitive immune response when infection, thus it is a good model for anti-TB vaccine evaluation; monkey TB model has similar clinical signs and classic granulomas structure to that of patient, therefore it is priority for mechanisms of disease research. See in Table 1 and Figure 1.
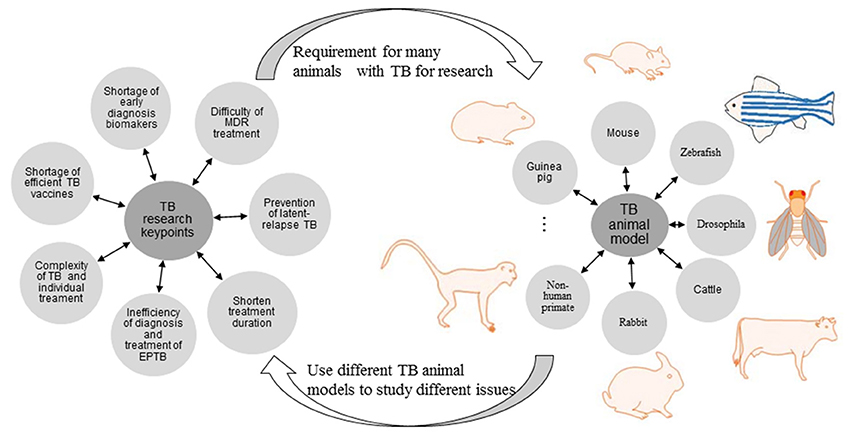
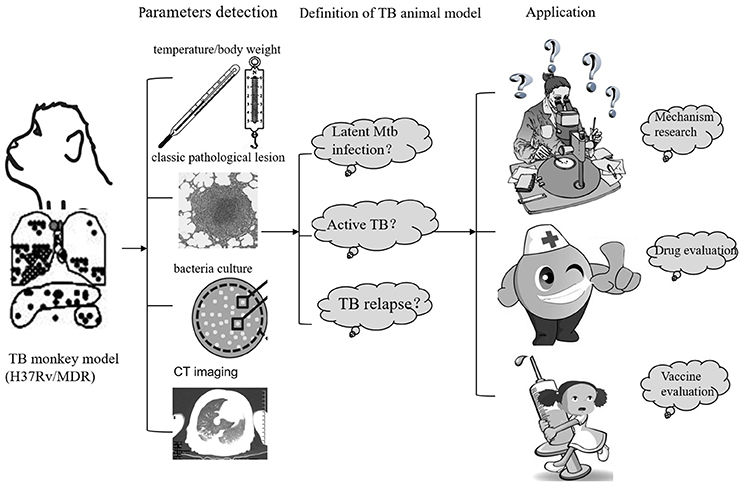
Figure 1. Current problems in diagnosis, treatment, and prevention of TB and the role of TB animal models.
Current Problems in Diagnosis, Treatment, and Prevention of TB and The Role of Animal Models
The current key problems of TB research refer to difficulty of MDR treatment, prevention of latent-relapse TB, treatment duration shorten, inefficiency of diagnosis, and treatment of extra-pulmonary tuberculosis (EPTB), complexity of TB and individual treatment, shortage of efficient TB vaccine and early diagnosis biomarkers. To investigate the above issues, lots of specific TB animal models are required. Vice versa, different TB animal model is used to research different issue (Figures 1, 2).
Lack of Specific Biomarkers for Early Diagnosis of Active Tuberculosis
Although diagnosis, treatment, and prevention are equally important to halt the TB epidemic, accurate early diagnosis is the headstone of TB control (Abubakar et al., 2016). Currently, diagnosis of active TB is mainly based on sputum Mycobacterium tuberculosis (Mtb) culture, X-ray/Computed Tomography (CT) radiography, and tuberculin skin test (TST), along with clinical signs and patient history. The gold standard for TB diagnosis is sputum Mtb culture, which requires the presence of necrotic infection foci in proximity to the airways. This test also results in a 3–4-week delay before providing definitive results. Furthermore, current diagostic tests commonly require Mtb-positive sputum. However, many active TB patients, including HIV-TB co-infected individuals, those with extra-pulmonary TB, diabetes patients, and children, do not present with Mtb-positive sputum. Therefore, sputum Mtb culture is not very helpful for rapid and specific diagnosis of all TB types.
Active TB can also be diagnosed using other specimens or indexes, such as: Mtb DNA, Mtb Ag85 complex, or Mtb cell wall component lipoarabinomannan (LAM) in blood or urine; cytokines such as IP10\VEGF\HO-1 in serum; transcriptomic or metabolomic signatures; and phenotypes of PBMC detected by Fluorescence Activated Cell Sorting (FACS), Enzyme-Linked Immuno Sorbent Assay (ELISA), and Enzyme-Linked Immunospot Assay (ELISPOT). However, urine LAM detection has low sensitivity, blood and urine Ag85 show highly variable performance in different studies, and transcriptional profiles are complex and expensive to use as routine diagnostic tests, especially in TB epidemic areas (Goletti et al., 2016). A rapid detection method with high specificity but low cost is highly desirable, especially for TB epidemic areas where access to experimental facilities is usually very limited (Ray et al., 2013; Goletti et al., 2016).
Biomarkers are identified by comparison between latent TB infection and active TB in animal models. Among them, mouse and monkey model are the most commonly used TB models. In the monkey model, sputum or bronchial alveolar lavage fluid culture do not show definitive results and Erythrocyte Sedimentation Rate (ESR)>15 until 2 months post-infection, and the tuberculous granulomas are not seen before the 3rd week post-infection with positron emission tomography-computed tomography (PET-CT) (Ankrah et al., 2016). Immunologic changes cannot be generally detected until 3rd–4th week after infection in the mouse TB model.
It is urgent to establish a more stable active-latent-relapse TB model, which is essential for developing biomarkers for earlier and simpler detection of TB. Biomarkers for each stage of TB and for both Mtb and host are particularly needed and can be cytokines, chemokines, and/or immunologic cells in blood and urine (Phuah et al., 2016).
Lack of Efficient and Reliable Evaluation System for Vaccine Development
At present, BCG is the only vaccine approved for TB. Unfortunately, BCG is only effective in preventing tuberculous meningitis in children, not for adult TB (Ottenhoff and Kaufmann, 2012). Thus, it is necessary to develop new vaccines for TB. However, there is no efficient and reliable system to evaluate the efficacy of candidate vaccines. The new candidate vaccine MVA85A was once regarded as the most promising boosting vaccine after BCG vaccine since 1990s when tested in animal models, but the results of phase III clinical study were disappointing (Checkley and McShane, 2011; Ottenhoff and Kaufmann, 2012). The problem might be rooted in lack of efficient and reliable evaluation systems for long-term protective immunity. The desirable immunological biomarkers should be capable of predicting TB relapse, helpful in formulating immunizations, and speed up vaccine research and “bench to bed” translation (Thakur et al., 2012). Ideal TB animal models and evaluation systems are efficient and reliable tools to evaluate vaccines (Iwenofu et al., 2008), immune molecules, and cells as biomarkers in different TB stages and can thus be essential for early diagnosis and vaccine evaluation (Agranoff et al., 2006; Walzl et al., 2008; Zanini et al., 2008; Thakur et al., 2012).
Side Effects and Duration of Active TB Treatment Must Be Reduced
The standard chemotherapy for TB consists of 6 months of initial treatment and 8 months of retreatment. Treatment of drug-resistant TB types may last as long as 2 years (Stagg et al., 2016). It was reported that the duration of treatment could be reduced from 6 to 4 months with equal outcomes, animal models can be used to determine whether treatment time can be further shortened with better efficacy and fewer side effects (Nahid et al., 2016). For example, chemotherapeutic drugs can be evaluated using the acute active TB model (Swanson et al., 2016), while drugs such as immune agents that target the host can be evaluated using chronic or latent TB infection models. Pretreatment with Vitamin D or lucid ganoderma reveals no obvious anti-TB effect in active TB models but clear effects in latent TB animal models. The selection of animal models for drug evaluation depends on the type of drugs (Zhang et al., 2009; Gouveia et al., 2013; Swanson et al., 2016).
Difficulty in Multi-Drug Resistant (MDR) TB treatment
Treatment of MDR TB usually lasts 24 months, with 50% recovery worldwide, resulting in severe threatens to public health and economic losses (Stagg et al., 2016). It is necessary to employ methods of quick diagnosis and regimens of shorter and cheaper treatment to achieve fast diagnosis and improve disease prognosis (van Cutsem et al., 2016). Such methods and regimens can be developed and/or optimized based on MDR TB animal models. However, there is no active or latent infection MDR TB animal model in any species. It is imperative to establish MDR TB animal models (Harris et al., 2016; Stagg et al., 2016; van Cutsem et al., 2016; Zumla et al., 2016).
Lack of Systematic Shorter and Stable Tuberculosis Latent-Relapse Infection Animal Models
Research on early diagnosis and relapse prevention of latent tuberculosis infection (LTBI) in human populations is difficult but could be facilitated by using animal models, such as latent-relapse TB mouse, monkey, rat, or rabbit models (Kashino et al., 2008; Klinkenberg et al., 2008; Manabe et al., 2008; Elwood et al., 2009; Lin et al., 2009; Zhang et al., 2009; Singhal et al., 2011a; Subbian et al., 2012), among them, mice and monkey models are the most commonly used. The mouse model was established by spontaneous infection and intervened with anti-TB drugs and BCG. The defects of the mouse models include varied length of latent period, high bacterial burdens during the latent stage, and varied starting time-points and levels of relapse (Klinkenberg et al., 2008; Zhang et al., 2009; Shi et al., 2011; Murawski et al., 2014; Han et al., 2016; Kupz et al., 2016). The monkey LTBI models might be the second most common, but the LTBI in monkey can be defined only when the infection relapses; it cannot be predicted at the time of inoculation. Moreover, the latent phase lasts several years with big variation within groups (Lin et al., 2009; Pena and Ho, 2015). Thus, the existing models are not suitable for utility in research, and models with shorter and stable latent-relapse stages should be generated.
Inefficient Diagnosis and Treatment of Extra-Pulmonary Tuberculosis (EPTB)
Latent TB infection can progress to active TB, including EPTB, when the immunity of host decreases due to aging, stress, overuse of immunosuppressants, or co-infection with HIV. EPTB, in sites such as bone, lymph nodes, and meninges, often has high morbidity. Diagnosis and treatment of EPTB is difficult, posing a major threat to public health (Ray et al., 2013; Nahid et al., 2016). The clinical symptom of EPTB are usually obscure, so diagnosis is difficult. Sputum Mtb culture is not feasible in EPTB, and examination of tissues from lesion sites and liquid samples would help to make a definite diagnosis. Tissue examination of EPTB with biomarkers of Mtb and host is not easily implemented in patients; however it is feasible in EPTB animal models, which may facilitate development of definite diagnosis methods. It is urgent to develop simpler, cheaper, and specific diagnostic tools (Ray et al., 2013).
Standard chemotherapy has limited efficacy on EPTB. Surgery combined with standard chemotherapy is usually essential for bone and lymph node TB (Harris et al., 2016; Rahyussalim et al., 2016). Treatment of EPTB is difficult and treatment duration is always longer than that of standard chemotherapy (Reichardt, 2012; Jullien et al., 2016).
Complexity of TB Features
There are multiple TB types, including pulmonary and extra-pulmonary TB, LTBI, and various active TB formats. Research on the pathogenic mechanisms and evaluation of drugs and vaccines for a specific TB type must be based on animal models with individualized and precise target TB type (Peng et al., 2015; Abubakar et al., 2016; Kupz et al., 2016; Phuah et al., 2016; Zumla et al., 2016). Most TB features can be found in the TB monkey model, but results may vary within groups. TB monkey models can be used for research in precision medicine (Lin et al., 2009; Pena and Ho, 2015). Cross-collaborative (CC) mice or specific gene knockout mice TB models are the basic tools in TB's precision medicine research (Rogala et al., 2014; Elbahesh and Schughart, 2016).
Progress in TB Animal Model Preparation
An ideal TB animal model is one not only with Mtb infection but also from which viable Mtb can be isolated from tissues with standard parameters and which consistently mimics TB clinical signs, characteristic pathological lesions, bacterial loads in organs, immunological indexes, radiographic changes, and hematological changes (Shi et al., 2011; Waters et al., 2011; Subbian et al., 2012; Clark et al., 2015; Myllymaki et al., 2015; Pena and Ho, 2015; Kramnik and Beamer, 2016). A TB animal model can always be improved with classic disease characteristic, shorter disease progression and better practical utility. (Figure 2)
Duration and Biological Safety of Active TB Animal Models
Active TB models can be prepared in a variety of experimental animals for drug and vaccine evaluation, but it takes at least 2 months to complete in vivo research and evaluation (Gouveia et al., 2013; Izzo et al., 2015; Zhan et al., 2015; Conde et al., 2016; Swanson et al., 2016). This lengthy experimental time hinders the development of new drugs and vaccines. The main method for model evaluation comprises: detection of characteristic pathological lesions, determination of bacterial loads in target organs, observation of related clinical manifestations, tests for immunological changes in blood, and assessment of radiological changes. Although these methods are established and useful, they are time-consuming, inefficient, and have bio-safety risks. By increasing the dose of inoculation to induce more severe disease or by using more susceptible candidate strains, such as C3HeB/FeJ (Lanoix et al., 2015; Henao-Tamayo et al., 2015; Li et al., 2015), TNF-α or IFN-γ knockout mice (Ehlers et al., 2001; Manca et al., 2001; Turner and Orme, 2004; Green et al., 2013; Dorhoi et al., 2014; Francisco et al., 2015; Olleros et al., 2015), the onset of TB and evaluation process in mice could be accelerated above that of C57BL/6 or BALB/c. In addition, further applications of in vivo tracer techniques could facilitate detection of the abundance and distribution of the bacillus, as well as continuous monitoring of lesion progress in target organs; using these approaches could improve the accuracy of pre-clinical research, have higher detection sensitivity and safety, and optimize the use of laboratory animals (Sugawara et al., 2009; Kong and Cirillo, 2010; Kong et al., 2010, 2016; Zhang et al., 2010; Ozeki et al., 2015; Kato et al., 2016).
The Latent-Relapse Tuberculosis Animal Models
At present, preparation of mouse and monkey latency-recurrence infection models requires a long time and exhibits poor consistency in replication. Meanwhile, lack of biomarkers to predict long-term recurrence of TB remains a main disadvantage for evaluation of vaccine and drug candidates. Various immune agents and drugs have been used to achieve latency infection and relapse with variation among animals within group (Scanga et al., 1999). These models can be significantly improved by reducing the latency period and increasing the stability of the progress of disease latency and recurrence (Lenaerts et al., 2004; Nuermberger et al., 2004; Elwood et al., 2009; Lin et al., 2009; Shi et al., 2011; Subbian et al., 2012).
It is possible to acquire relatively shorter and more stable cycles of latency-relapse after anti-TB treatment or immunization using more susceptible strains such as C3HeB/FeJ, and TNF-α or IFN-γ-knockout mice (Turner and Orme, 2004; Calderon et al., 2013; Henao-Tamayo et al., 2015; Olleros et al., 2015; Reeme and Robinson, 2016) or other sensitive strains screened from collaborative cross (CC) mice (Rogala et al., 2014; Elbahesh and Schughart, 2016). On the other hand, rapid and uniform relapse with moderate bacterial loads and lesion severity in target organs can be attained using certain hormones, new specific immunosuppressants such as TNF-α antibodies, or gamma irradiation; this approach could contribute to the statistical analysis in experiment due to the little variation within group (Scanga et al., 1999; Botha and Ryffel, 2002; Parikka et al., 2012; Goletti et al., 2016). Imaging detection can also help to shorten the latency course monitoring and allow earlier determination of the relapse phase (Botha and Ryffel, 2002; Zhang et al., 2010, 2012; Murawski et al., 2014; Kramnik and Beamer, 2016).
Drug-Resistant (DR) TB Animal Models
A qualified drug-resistant animal model for efficacious evaluation of new drugs or drug combinations should possess common resistance phenotypes, typical drug-resistant mutations, and representative genotype background DR Mtb strains (van Cutsem et al., 2016). Strong virulent strains with these features could be applied in rapid assessment of drug efficacy, and moderate virulent strains could be used in the research of immune agents and immunological mechanism (HaiRong et al., 2014). Unfortunately, although we screened standardized drug-resistant strains to inoculate animal models, there remain no specialized drug-resistant latent and active TB models or evaluation system reported (D'Ambrosio et al., 2015; Olmo et al., 2016) and no latency-relapse animal drug-resistant TB model reported till now, either.
Monkey Tuberculosis Models
Notably, monkey TB models exhibit inter-individual differences, probably due to genetic variation in their populations. This is an advantage in terms of similarity to human population but a challenge in terms of uniform responses. These characteristics are similar for MDR TB in monkeys. The disadvantage might be reduced by improving the inoculation dose\the Mtb strain and application of intervention (Scanga and Flynn, 2014; Pena and Ho, 2015; Phuah et al., 2016). For preparation of an active tuberculosis model, inoculation dose could be increased to make disease progression more consistent (Zhang et al., 2014). This is in contrast to preparation of latent infection mouse or guinea pig models, where inoculation can be optimized with moderate doses and intervention with drugs or immunosuppressive agents (Lenaerts et al., 2004; Nuermberger et al., 2004; Lu et al., 2015, 2016) can improve the stability of latent status and reduce deviation of recurrence. In addition, to replicate primary syndrome and single granulomatous lesions in monkeys, local inoculation of moderate virulent strains should be tried in small doses (Cluver et al., 2013).
In vivo Monitoring of Animal TB Models
At present, TB animal models can be continuously evaluated through sampling sacrificed animals at multiple time points. This requires a huge number of experimental animals and increases bio-safety risks. There is also inter-individual variation within experimental groups (Zhan et al., 2015). These problems can be solved by in vivo monitoring technology. 18F-FDG PET/CT was commonly used in early diagnosis of infectious inflammation. FDG imaging was highly dependent on the strength of immune response by host cells; thus, the image was quite blurry in immunity-compromised hosts. Technologies such as FDG PET/CT should be improved to be sensitive enough for detection of pathogen, including bacterial localization and quantification (Murawski et al., 2014; Weinstein et al., 2014; Kato et al., 2016). FDS PET/CT is also a promising method for in vivo detection of bacterial infection (Weinstein et al., 2014; Ordonez et al., 2016; Yao et al., 2016). Fluorescence-labeled (GFP, reporter enzyme fluorescence) bacillus could be detected in vivo, including the location and quantity of the pathogen. However, the current detection sensitivity is above 100 CFU, which is not sensitive enough to diagnose latent infection. The structure or features of fluorescent proteins should be modified to achieve a lower in vivo detection limit (Sugawara et al., 2009; Kong et al., 2010; Kong and Cirillo, 2010; Ozeki et al., 2015).
Perspectives On Animal TB Models
The difficulty of research with TB animal models is that the pathogenesis and progression of TB are complex (Ottenhoff, 2012). Infection type can vary by lesion location, with manifestations as both pulmonary and extra-pulmonary (e.g., bone, lymphatic, enterophthisis, meningeal) TB (Myllymaki et al., 2015). The disease progression has diversified forms: latent and active infection. Active TB includes primary tuberculosis, blood disseminated tuberculosis, and secondary tuberculosis (Lin et al., 2009; Waters et al., 2011; Subbian et al., 2012; Clark et al., 2015; Kramnik and Beamer, 2016). A single TB animal model can only mimic one or several aspects of TB, not all forms. To reveal the overall picture of human TB, all features of TB should be replicated in various TB models for different research purposes (Rodgers et al., 2007; Kashino et al., 2008; Manabe et al., 2008; Dannenberg, 2009; Zhang et al., 2009; Waters et al., 2011; Parikka et al., 2012; Subbian et al., 2012; Baldwin and Telfer, 2015; Rahyussalim et al., 2016; Swanson et al., 2016).
Animal TB Models for Rapid Drug and Vaccine Assessment
It is urgent to develop animal models with rapidly-progressing TB for drug and vaccine evaluation. It may be faster to evaluate drug efficacy for survival using IFN-γ or TNF-α knockout active TB mice than wild-type TB mice. TNF-α KO mice might be useful in preparation of a latency-recurrence TB model for evaluation of drugs and vaccines. After 8 weeks of anti-tuberculosis chemotherapy, TNF-α KO mice can experience natural recurrence and death (Turner and Orme, 2004; Francisco et al., 2015; Olleros et al., 2015). Relapse can be induced in monkeys with TNF-α antibody, generating a homogenous rapid latent-relapse TB monkey model (Lin et al., 2010; Fillmore et al., 2012).
Application of New Detecting Technologies on Animal TB models
New technology should be exploited to improve sensitivity and specificity of detection, shorten the course of experiments, decrease systematic error by monitoring the same individuals continuously instead of sampling different dissected animals at different time points, increase reliability, and reduce bio-safety risk for personnel. For example, bacteria imaging and PET-CT were promising methods for bacillus and lesions imaging (Weinstein et al., 2014; Ankrah et al., 2016; Lin et al., 2016; Ordonez et al., 2016).
Application of New Gene-Modified Technologies on Animal TB Models
Specific gene-editing technology, including gene knock-in, knock-out, and knock-down with CRISPR-Cas9 or other new techniques, has been applied in mice, rats, and even monkeys in research of pathogenesis. This approach has enabled interpretation of precise roles of genes and proteins (Commandeur et al., 2014; Gengenbacher et al., 2014; Armstrong et al., 2016; Cheong et al., 2016; Jung et al., 2016; Lambert et al., 2016; Ma et al., 2016; Renaud et al., 2016) and elaboration of mechanisms for treatment and vaccine strategies.
Personalized Animal TB Models
Multiple TB animal models should be established for specific purposes. For example, different immunization models are needed, for prime immunization, prime-boost immunization, and therapeutic vaccine evaluation. These models have been established in guinea pigs (Lu et al., 2015, 2016). A gene-knockout mouse TB model with virulent strains can be used for fast evaluation of chemical drug efficacy (Ehlers et al., 2001), while standard and attenuated strains can be used in evaluation of in vivo immune responses and phenotype of immune agents. A monkey model is suitable for personalized medical research by in vivo monitoring methodologies and can be used in systematic research of early diagnosis and treatment of TB recurrence (Capuano et al., 2003).
Extra-Pulmonary TB Models
Models for extra-pulmonary TB are especially useful in research on pathogenesis and treatment. New technologies such as FDG-PET/CT and fluorescent-labeled imaging can also be used in EPTB models to improve sensitivity of diagnosis and rapid evaluation of drug efficacy, such that new biomarkers for diagnosis and evaluation systems can be explored.
Summary
TB animal models have been playing essential roles in the translational and precision medicine, not only in areas of early diagnosis, recurrence prevention, treatment optimization of MDR-TB, and EPTB, with the thinking of personalized medicine, but also for the new vaccine and drug development. Each TB animal model has its own advantages and disadvantages, thus lots of specific TB animal models are required for the above research. It is imperative to improve existing TB animal models to obtain more stable, more accurate, and more efficient models with shorter experimental cycles and lower bio-safety risks using new technologies and strategies.
Author Contributions
CQ and LZ conceived the idea, LZ wrote the manuscript, JT and MS revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research has been supported by the grant “Major innovation engineering project of Chinese Academy of Medical Sciences (2016-I2M-1-013)” and “Central public welfare institution basic research fund of Chinese Academy of Medical Science (2016ZX310183-2), pumc youth fund (No. 3332015153).”
References
Abubakar, I., Lipman, M., McHugh, T. D., and Fletcher, H. (2016). Uniting to end the TB epidemic: advances in disease control from prevention to better diagnosis and treatment. BMC Med. 14:47. doi: 10.1186/s12916-016-0599-1
Agranoff, D., Fernandez-Reyes, D., Papadopoulos, M. C., Rojas, S. A., Herbster, M., Loosemore, A., et al. (2006). Identification of diagnostic markers for tuberculosis by proteomic fingerprinting of serum. Lancet 368, 1012–1021. doi: 10.1016/S0140-6736(06)69342-2
Ankrah, A. O., van der Werf, T. S., de Vries, E. F., Dierckx, R. A., Sathekge, M. M., and Glaudemans, A. W. (2016). PET/CT imaging of Mycobacterium tuberculosis infection. Clin. Transl. Imaging 4, 131–144. doi: 10.1007/s40336-016-0164-0
Armstrong, G. A., Liao, M., You, Z., Lissouba, A., Chen, B. E., and Drapeau, P. (2016). Homology directed knockin of point mutations in the Zebrafish tardbp and fus genes in ALS using the CRISPR/Cas9 System. PLoS ONE 11:e0150188. doi: 10.1371/journal.pone.0150188
Baldwin, C. L., and Telfer, J. C. (2015). The bovine model for elucidating the role of gammadelta T cells in controlling infectious diseases of importance to cattle and humans. Mol. Immunol. 66, 35–47. doi: 10.1016/j.molimm.2014.10.024
Botha, T., and Ryffel, B. (2002). Reactivation of latent tuberculosis by an inhibitor of inducible nitric oxide synthase in an aerosol murine model. Immunology 107, 350–357. doi: 10.1046/j.1365-2567.2002.01511.x
Calderon, V. E., Valbuena, G., Goez, Y., Judy, B. M., Huante, M. B., Sutjita, P., et al. (2013). A humanized mouse model of tuberculosis. PLoS ONE 8:e63331. doi: 10.1371/journal.pone.0063331
Capuano, S. V., Croix, D. A., Pawar, S., Zinovik, A., Myers, A., Lin, P. L., et al. (2003). Experimental Mycobacterium tuberculosis Infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71, 5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003
Checkley, A. M., and McShane, H. (2011). Tuberculosis vaccines: progress and challenges. Trends Pharmacol. Sci. 32, 601–606. doi: 10.1016/j.tips.2011.06.003
Cheong, T. C., Compagno, M., and Chiarle, R. (2016). Editing of mouse and human immunoglobulin genes by CRISPR-Cas9 system. Nat. Commun. 7:10934. doi: 10.1038/ncomms10934
Clark, S., Hall, Y., and Williams, A. (2015). Animal models of tuberculosis: guinea pigs. Cold Spring Harb. Perspect. Med. 5:a018572. doi: 10.1101/cshperspect.a018572
Cluver, L., Orkin, M., Moshabela, M., Kuo, C., and Boyes, M. (2013). The hidden harm of home-based care: pulmonary tuberculosis symptoms among children providing home medical care to HIV/AIDS-affected adults in South Africa. AIDS Care 25, 748–755. doi: 10.1080/09540121.2013.772281
Commandeur, S., van den Eeden, S. J., Dijkman, K., Clark, S. O., van Meijgaarden, K. E., Wilson, L., et al. (2014). The in vivo expressed Mycobacterium tuberculosis (IVE-TB) antigen Rv2034 induces CD4(+) T-cells that protect against pulmonary infection in HLA-DR transgenic mice and guinea pigs. Vaccine 32, 3580–3588. doi: 10.1016/j.vaccine.2014.05.005
Conde, M. B., Mello, F. C., Duarte, R. S., Cavalcante, S. C., Rolla, V., Dalcolmo, M., et al. (2016). A phase 2 randomized trial of a rifapentine plus moxifloxacin-based regimen for treatment of pulmonary tuberculosis. PLoS ONE 11:e0154778. doi: 10.1371/journal.pone.0154778
Coquard, R., Romestaing, P., Ardiet, J. M., Mornex, F., Sentenac, I., and Gerard, J. P. (1997). Uterine sarcoma treated by surgery and postoperative radiation therapy. Patterns of relapse, prognostic factors and role of radiation therapy. Bull. Cancer 84, 625–629.
D'Ambrosio, L., Centis, R., Sotgiu, G., Pontali, E., Spanevello, A., and Migliori, G. B. (2015). New anti-tuberculosis drugs and regimens: 2015 update. ERJ Open Res. 1:00010-2015. doi: 10.1183/23120541.00010-2015
Dannenberg, A. M. Jr. (2009). Liquefaction and cavity formation in pulmonary TB: a simple method in rabbit skin to test inhibitors. Tuberculosis 89, 243–247. doi: 10.1016/j.tube.2009.05.006
Dorhoi, A., Yeremeev, V., Nouailles, G., Weiner, J. III., Jorg, S., Heinemann, E., et al. (2014). Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur. J. Immunol. 44, 2380–2393. doi: 10.1002/eji.201344219
Driscoll, E. E., Hoffman, J. I., Green, L. E., Medley, G. F., and Amos, W. (2011). A preliminary study of genetic factors that influence susceptibility to bovine tuberculosis in the British cattle herd. PLoS ONE 6:e18806. doi: 10.1371/journal.pone.0018806
Durnali, A., Tokluoglu, S., Ozdemir, N., Inanc, M., Alkis, N., Zengin, N., et al. (2012). Prognostic factors and treatment outcomes in 93 patients with uterine sarcoma from 4 centers in Turkey. Asian Pac. J. Cancer Prev. 13, 1935–1941. doi: 10.7314/APJCP.2012.13.5.1935
Ehlers, S., Benini, J., Held, H. D., Roeck, C., Alber, G., and Uhlig, S. (2001). Alphabeta T cell receptor-positive cells and interferon-gamma, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J. Exp. Med. 194, 1847–1859. doi: 10.1084/jem.194.12.1847
El Husseiny, G., Al Bareedy, N., Mourad, W. A., Mohamed, G., Shoukri, M., Subhi, J., et al. (2002). Prognostic factors and treatment modalities in uterine sarcoma. Am. J. Clin. Oncol. 25, 256–260. doi: 10.1097/00000421-200206000-00010
Elbahesh, H., and Schughart, K. (2016). Genetically diverse CC-founder mouse strains replicate the human influenza gene expression signature. Sci. Rep. 6:26437. doi: 10.1038/srep26437
Elwood, R. L., Rajnik, M., Wilson, S., Yim, K., Blanco, J. C., Nikonenko, B., et al. (2009). Characterization of late tuberculosis infection in Sigmodon hispidus. Tuberculosis 89, 183–188. doi: 10.1016/j.tube.2009.01.003
Fillmore, D., Frye, L. J., Rutledge, T., DiFazio, R. M., Janssen, C., Klein, E., et al. (2012). Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc. Natl. Acad. Sci. U.S.A. 109, 14188–14193. doi: 10.1073/pnas.1121497109
Francisco, N. M., Hsu, N. J., Keeton, R., Randall, P., Sebesho, B., Allie, N., et al. (2015). TNF-dependent regulation and activation of innate immune cells are essential for host protection against cerebral tuberculosis. J. Neuroinflammation 12:125. doi: 10.1186/s12974-015-0345-1
Gengenbacher, M., Vogelzang, A., Schuerer, S., Lazar, D., Kaiser, P., and Kaufmann, S. H. (2014). Dietary pyridoxine controls efficacy of vitamin B6-auxotrophic tuberculosis vaccine bacillus Calmette-Guerin DeltaureC::hly Deltapdx1 in mice. MBio 5, e01262–e01214. doi: 10.1128/mBio.01262-14
Goletti, D., Petruccioli, E., Joosten, S. A., and Ottenhoff, T. H. (2016). Tuberculosis biomarkers: from diagnosis to protection. Infect. Dis. Rep. 8:6568. doi: 10.4081/idr.2016.6568
Gouveia, A. C., Brugiolo, A. S., Alves, C. C., Silva, F. M., Mesquita, F. P., Gameiro, J., et al. (2013). Th2 responses in OVA-sensitized BALB/c mice are down-modulated by Mycobacterium bovis BCG treatment. J. Clin. Immunol. 33, 235–245. doi: 10.1007/s10875-012-9746-4
Green, A. M., Difazio, R., and Flynn, J. L. (2013). IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 190, 270–277. doi: 10.4049/jimmunol.1200061
HaiRong, D. L. S., JinBiao, L. V., HaiQing, D., GuoYi, Y., Chuan, Q., and LingJun, Z. (2014). Screening of drug-resistant Mycobacterium tuberculosis isolates for candidate standard strains for in vivo assays. J. Microbes Infect. 9, 83–88.
Han, Y., Li, S., Holt, H. K., and Wu, L. (2016). Curative effect of bevacizumab combined with chemotherapy in advanced or recurrent uterine sarcoma. Mol. Clin. Oncol. 4, 245–248. doi: 10.3892/mco.2015.709
Harris, R. C., Khan, M. S., Martin, L. J., Allen, V., Moore, D. A., Fielding, K., et al. (2016). The effect of surgery on the outcome of treatment for multidrug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis. 16:262. doi: 10.1186/s12879-016-1585-0
Henao-Tamayo, M., Obregon-Henao, A., Creissen, E., Shanley, C., Orme, I., and Ordway, D. J. (2015). Differential Mycobacterium bovis BCG vaccine-derived efficacy in C3Heb/FeJ and C3H/HeOuJ mice exposed to a clinical strain of Mycobacterium tuberculosis. Clin. Vaccine Immunol. 22, 91–98. doi: 10.1128/CVI.00466-14
Iwenofu, O. H., Lackman, R. D., Staddon, A. P., Goodwin, D. G., Haupt, H. M., and Brooks, J. S. (2008). Phospho-S6 ribosomal protein: a potential new predictive sarcoma marker for targeted mTOR therapy. Mod. Pathol. 21, 231–237. doi: 10.1038/modpathol.3800995
Izzo, A. A., Leung-Theung-Long, S., Gouanvic, M., Coupet, C.-A., Ray, A., Tupin, E., et al. (2015). A novel MVA-based multiphasic vaccine for prevention or treatment of tuberculosis induces broad and multifunctional cell-mediated immunity in mice and primates. PLoS ONE 10:e0143552. doi: 10.1371/journal.pone.0143552
Jacobs, R. E., Gu, P., and Chachoua, A. (2015). Reactivation of pulmonary tuberculosis during cancer treatment. Int. J. Mycobacteriol. 4, 337–340. doi: 10.1016/j.ijmyco.2015.05.015
Jullien, S., Ryan, H., Modi, M., and Bhatia, R. (2016). Six months therapy for tuberculous meningitis. Cochrane Database Syst. Rev. 9:CD012091. doi: 10.1002/14651858.cd012091.pub2
Jung, C. J., Menoret, S., Brusselle, L., Tesson, L., Usal, C., Chenouard, V., et al. (2016). Comparative analysis of piggyBac, CRISPR/Cas9 and TALEN mediated BAC Transgenesis in the zygote for the generation of humanized SIRPA rats. Sci. Rep. 6:31455. doi: 10.1038/srep31455
Kashino, S. S., Napolitano, D. R., Skobe, Z., and Campos-Neto, A. (2008). Guinea pig model of Mycobacterium tuberculosis latent/dormant infection. Microbes Infect. 10, 1469–1476. doi: 10.1016/j.micinf.2008.08.010
Kato, A., Yamamoto, H., Ikeda, M., Tateishi, K., Ushiki, A., Yasuo, M., et al. (2016). A case of pulmonary Mycobacterium avium infection in an immunocompetent patient who showed a huge consolidation with a high FDG uptake on PET/CT. Respir. Med. Case Rep. 19, 49–52. doi: 10.1016/j.rmcr.2016.07.004
Kiran, D., Podell, B. K., Chambers, M., and Basaraba, R. J. (2016). Host-directed therapy targeting the Mycobacterium tuberculosis granuloma: a review. Semin. Immunopathol. 38, 167–183. doi: 10.1007/s00281-015-0537-x
Klinkenberg, L. G., Sutherland, L. A., Bishai, W. R., and Karakousis, P. C. (2008). Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J. Infect. Dis. 198, 275–283. doi: 10.1086/589515
Kong, Y., and Cirillo, J. D. (2010). Reporter enzyme fluorescence (REF) imaging and quantification of tuberculosis in live animals. Virulence 1, 558–562. doi: 10.4161/viru.1.6.13901
Kong, Y., Yang, D., Cirillo, S. L., Li, S., Akin, A., Francis, K. P., et al. (2016). Application of fluorescent protein expressing strains to evaluation of anti-tuberculosis therapeutic efficacy in vitro and in vivo. PLoS ONE 11:e0149972. doi: 10.1371/journal.pone.0149972
Kong, Y., Yao, H., Ren, H., Subbian, S., Cirillo, S. L., Sacchettini, J. C., et al. (2010). Imaging tuberculosis with endogenous beta-lactamase reporter enzyme fluorescence in live mice. Proc. Natl. Acad. Sci. U.S.A. 107, 12239–12244. doi: 10.1073/pnas.1000643107
Kramnik, I., and Beamer, G. (2016). Mouse models of human TB pathology: roles in the analysis of necrosis and the development of host-directed therapies. Semin. Immunopathol. 38, 221–237. doi: 10.1007/s00281-015-0538-9
Kupz, A., Zedler, U., Staber, M., and Kaufmann, S. H. (2016). A mouse model of latent tuberculosis infection to study intervention strategies to prevent reactivation. PLoS ONE 11:e0158849. doi: 10.1371/journal.pone.0158849
Lambert, L. J., Challa, A. K., Niu, A., Zhou, L., Tucholski, J., Johnson, M. S., et al. (2016). Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology. Dis. Model. Mech. 9, 1169–1179. doi: 10.1242/dmm.025247
Lanoix, J. P. (2016). Sterilizing activity of pyrazinamide in combination with first-line drugs in a C3HeB/FeJ mouse model of tuberculosis. J. Immunol. 60, 1091–1096. doi: 10.1128/aac.02637-15
Lanoix, J. P., Lenaerts, A. J., and Nuermberger, E. L. (2015). Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis. Model. Mech. 8, 603–610. doi: 10.1242/dmm.019513
Lenaerts, A. J., Chapman, P. L., and Orme, I. M. (2004). Statistical limitations to the Cornell model of latent tuberculosis infection for the study of relapse rates. Tuberculosis 84, 361–364. doi: 10.1016/j.tube.2004.03.002
Li, S. Y., Irwin, S. M., Converse, P. J., Mdluli, K. E., Lenaerts, A. J., and Nuermberger, E. L. (2015). Evaluation of moxifloxacin-containing regimens in pathologically distinct murine tuberculosis models. Antimicrob. Agents Chemother. 59, 4026–4030. doi: 10.1128/AAC.00105-15
Lin, P. L., Maiello, P., Gideon, H. P., and Coleman, M. T. (2016). PET CT identifies reactivation risk in cynomolgus macaques with latent M. tuberculosis. PLoS Pathog. 12:e1005739. doi: 10.1371/journal.ppat.1005739
Lin, P. L., Myers, A., Smith, L., Bigbee, C., Bigbee, M., Fuhrman, C., et al. (2010). Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62, 340–350. doi: 10.1002/art.27271
Lin, P. L., Rodgers, M., Smith, L., Bigbee, M., Myers, A., Bigbee, C., et al. (2009). Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 77, 4631–4642. doi: 10.1128/IAI.00592-09
Lu, J. B., Chen, B. W., Wang, G. Z., Fu, L. L., Shen, X. B., Su, C., et al. (2015). Recombinant tuberculosis vaccine AEC/BC02 induces antigen-specific cellular responses in mice and protects guinea pigs in a model of latent infection. J. Microbiol. Immunol. Infect. 48, 597–603. doi: 10.1016/j.jmii.2014.03.005
Lu, J. B., Cheng, B. W., Deng, H. Q., Su, C., Shen, X. B., Du, W. X., et al. (2016). [Analysis of Koch phenomenon of Mycobacterium tuberculosis-infected guinea pigs vaccinated with recombinant tuberculosis vaccine AEC/BC02]. Zhonghua Jie He He Hu Xi Za Zhi 39, 524–528. doi: 10.3760/cma.j.issn.1001-0939.2016.07.007
Ma, Y., Chen, W., Zhang, X., Yu, L., Dong, W., Pan, S., et al. (2016). Increasing the efficiency of CRISPR/Cas9-mediated precise genome editing in rats by inhibiting NHEJ and using Cas9 protein. Dis. Model. Mech. 13, 605–612. doi: 10.1080/15476286.2016.1185591
Manabe, Y. C., Kesavan, A. K., Lopez-Molina, J., Hatem, C. L., Brooks, M., Fujiwara, R., et al. (2008). The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis 88, 187–196. doi: 10.1016/j.tube.2007.10.006
Manca, C., Tsenova, L., Bergtold, A., Freeman, S., Tovey, M., Musser, J. M., et al. (2001). Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α /β. Proc. Natl. Acad. Sci. U.S.A. 98, 5752–5757. doi: 10.1073/pnas.091096998
Moskovic, E., MacSweeney, E., Law, M., and Price, A. (1993). Survival, patterns of spread and prognostic factors in uterine sarcoma: a study of 76 patients. Br. J. Radiol. 66, 1009–1015. doi: 10.1259/0007-1285-66-791-1009
Murawski, A. M., Gurbani, S., Harper, J. S., Klunk, M., Younes, L., Jain, S. K., et al. (2014). Imaging the evolution of reactivation pulmonary tuberculosis in mice using 18F-FDG PET. J. Nucl. Med. 55, 1726–1729. doi: 10.2967/jnumed.114.144634
Myllymaki, H., Niskanen, M., Oksanen, K. E., and Ramet, M. (2015). Animal models in tuberculosis research - where is the beef? Expert Opin. Drug Discov. 10, 871–883. doi: 10.1517/17460441.2015.1049529
Naaman, Y., Shveiky, D., Ben-Shachar, I., Shushan, A., Mejia-Gomez, J., and Benshushan, A. (2011). Uterine sarcoma: prognostic factors and treatment evaluation. Isr. Med. Assoc. J. 13, 76–79.
Nahid, P., Dorman, S. E., Alipanah, N., Barry, P. M., Brozek, J. L., Cattamanchi, A., et al. (2016). Executive summary: official american thoracic society/centers for disease control and prevention/infectious diseases society of america clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 63, 853–867. doi: 10.1093/cid/ciw566
Nicolle, D., Fremond, C., Pichon, X., Bouchot, A., Maillet, I., Ryffel, B., et al. (2004). Long-term control of Mycobacterium bovis BCG infection in the absence of Toll-like receptors (TLRs): investigation of TLR2-, TLR6-, or TLR2-TLR4-deficient mice. Infect. Immun. 72, 6994–7004. doi: 10.1128/IAI.72.12.6994-7004.2004
Nuermberger, E. L., Yoshimatsu, T., Tyagi, S., Bishai, W. R., and Grosset, J. H. (2004). Paucibacillary tuberculosis in mice after prior aerosol immunization with Mycobacterium bovis BCG. Infect. Immun. 72, 1065–1071. doi: 10.1128/IAI.72.2.1065-1071.2004
Nusbaum, R. J., Calderon, V. E., Huante, M. B., Sutjita, P., Vijayakumar, S., Lancaster, K. L., et al. (2016). Pulmonary tuberculosis in humanized mice infected with HIV-1. Sci. Rep. 6:21522. doi: 10.1038/srep21522
Olleros, M. L., Chavez-Galan, L., Segueni, N., Bourigault, M. L., Vesin, D., Kruglov, A. A., et al. (2015). Control of Mycobacterial infections in mice expressing human Tumor Necrosis Factor (TNF) but not mouse TNF. Infect. Immun. 83, 3612–3623. doi: 10.1128/IAI.00743-15
Olmo, E. D., Molina-Salinas, G. M., Bini, E. I., Gonzalez-Hernandez, S., Bustos, L. A., Escarcena, R., et al. (2016). Efficacious in vitro and in vivo effects of dihydrosphingosine-ethambutol analogues against susceptible and multi-drug-resistant Mycobacterium tuberculosis. Arch. Med. Res. 47:262–270. doi: 10.1016/j.arcmed.2016.07.004
Ordonez, A. A., Weinstein, E. A., Bambarger, L. E., Saini, V., Chang, Y. S., DeMarco, V. P., et al. (2016). A systematic approach for developing bacteria-specific imaging tracers. J. Nucl. Med. 58, 144–150. doi: 10.2967/jnumed.116.181792
Ottenhoff, T. H. (2012). New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol. 20, 419–428. doi: 10.1016/j.tim.2012.06.002
Ottenhoff, T. H., and Kaufmann, S. H. (2012). Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 8:e1002607. doi: 10.1371/journal.ppat.1002607
Ozeki, Y., Igarashi, M., Doe, M., Tamaru, A., Kinoshita, N., Ogura, Y., et al. (2015). A new screen for tuberculosis drug candidates utilizing a luciferase-expressing recombinant mycobacterium bovis bacillus calmette-gueren. PLoS ONE 10:e0141658. doi: 10.1371/journal.pone.0141658
Parikka, M., Hammaren, M. M., Harjula, S. K., Halfpenny, N. J., Oksanen, K. E., Lahtinen, M. J., et al. (2012). Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog. 8:e1002944. doi: 10.1371/journal.ppat.1002944
Pena, J. C., and Ho, W. Z. (2015). Monkey models of tuberculosis: lessons learned. Infect. Immun. 83, 852–862. doi: 10.1128/IAI.02850-14
Peng, X., Knouse, J. A., and Hernon, K. M. (2015). Rabbit models for studying human infectious diseases. Comp. Med. 65, 499–507.
Phuah, J., Wong, E. A., Gideon, H. P., Maiello, P., Coleman, M. T., Hendricks, M. R., et al. (2016). Effects of B cell depletion on early Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 84, 1301–1311. doi: 10.1128/IAI.00083-16
Plattner, B. L., Doyle, R. T., and Hostetter, J. M. (2009). Gamma-delta T cell subsets are differentially associated with granuloma development and organization in a bovine model of mycobacterial disease. Int. J. Exp. Pathol. 90, 587–597. doi: 10.1111/j.1365-2613.2009.00679.x
Provan, D., and Newland, A. C. (2015). Current management of primary immune thrombocytopenia. Adv. Ther. 32, 875–887. doi: 10.1007/s12325-015-0251-z
Rahyussalim, A. J., Kurniawati, T., Siregar, N. C., Syahrurachman, A., Dilogo, I. H., Iskandriati, D., et al. (2016). New Bone formation in tuberculous-infected vertebral body defect after administration of bone marrow stromal cells in rabbit model. Asian Spine J. 10, 1–5. doi: 10.4184/asj.2016.10.1.1
Ray, S., Talukdar, A., Kundu, S., Khanra, D., and Sonthalia, N. (2013). Diagnosis and management of miliary tuberculosis: current state and future perspectives. Ther. Clin. Risk Manag. 9, 9–26. doi: 10.2147/TCRM.S29179
Reeme, A. E., and Robinson, R. T. (2016). Dietary vitamin D3 suppresses pulmonary immunopathology associated with late-stage tuberculosis in C3HeB/FeJ mice. J. Immunol. 196, 1293–1304. doi: 10.4049/jimmunol.1500931
Reichardt, P. (2012). The treatment of uterine sarcomas. Ann. Oncol. 23(Suppl. 10), x151–x157. doi: 10.1093/annonc/mds359
Renaud, J. B., Boix, C., Charpentier, M., De Cian, A., Cochennec, J., Duvernois-Berthet, E., et al. (2016). Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR-Cas9 nucleases. Cell Rep. 14, 2263–2272. doi: 10.1016/j.celrep.2016.02.018
Rodgers, J. D., Connery, N. L., McNair, J., Welsh, M. D., Skuce, R. A., Bryson, D. G., et al. (2007). Experimental exposure of cattle to a precise aerosolised challenge of Mycobacterium bovis: a novel model to study bovine tuberculosis. Tuberculosis 87, 405–414. doi: 10.1016/j.tube.2007.04.003
Rogala, A. R., Morgan, A. P., Christensen, A. M., Gooch, T. J., Bell, T. A., Miller, D. R., et al. (2014). The Collaborative Cross as a resource for modeling human disease: CC011/Unc, a new mouse model for spontaneous colitis. Mamm. Genome 25, 95–108. doi: 10.1007/s00335-013-9499-2
Scanga, C. A., and Flynn, J. L. (2014). Modeling tuberculosis in nonhuman primates. Cold Spring Harb. Perspect. Med. 4:a018564. doi: 10.1101/cshperspect.a018564
Scanga, C. A., Mohan, V. P., Joseph, H., Yu, K., Chan, J., and Flynn, J. L. (1999). Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect. Immun. 67, 4531–4538.
Shi, C., Shi, J., and Xu, Z. (2011). A review of murine models of latent tuberculosis infection. Scand. J. Infect. Dis. 43, 848–856. doi: 10.3109/00365548.2011.603745
Singhal, A., Aliouat el, M., Herve, M., Mathys, V., Kiass, M., Creusy, C., et al. (2011a). Experimental tuberculosis in the Wistar rat: a model for protective immunity and control of infection. PLoS ONE 6:e18632. doi: 10.1371/journal.pone.0018632
Singhal, A., Mathys, V., Kiass, M., Creusy, C., Delaire, B., Aliouat el, M., et al. (2011b). BCG induces protection against Mycobacterium tuberculosis infection in the Wistar rat model. PLoS ONE 6:e28082. doi: 10.1371/journal.pone.0028082
Stagg, H. R., Harris, R. J., Hatherell, H. A., Obach, D., Zhao, H., Tsuchiya, N., et al. (2016). What are the most efficacious treatment regimens for isoniazid-resistant tuberculosis? A systematic review and network meta-analysis. Thorax 71, 940–949. doi: 10.1136/thoraxjnl-2015-208262
Subbian, S., Tsenova, L., O'Brien, P., Yang, G., Kushner, N. L., Parsons, S., et al. (2012). Spontaneous latency in a rabbit model of pulmonary tuberculosis. Am. J. Pathol. 181, 1711–1724. doi: 10.1016/j.ajpath.2012.07.019
Sugawara, I., Udagawa, T., Aoki, T., and Mizuno, S. (2009). Establishment of a guinea pig model of latent tuberculosis with GFP-introduced Mycobacterium Tuberculosis. Tohoku J. Exp. Med. 219, 257–262. doi: 10.1620/tjem.219.257
Sun, H., Ma, X., Zhang, G., Luo, Y., Tang, K., Lin, X., et al. (2012). Effects of immunomodulators on liquefaction and ulceration in the rabbit skin model of tuberculosis. Tuberculosis 92, 345–350. doi: 10.1016/j.tube.2012.03.005
Swanson, R. V., Ammerman, N. C., Ngcobo, B., Adamson, J., Moodley, C., Dorasamy, A., et al. (2016). Clofazimine contributes sustained antimicrobial activity after treatment cessation in a mouse model of tuberculosis chemotherapy. Antimicrob. Agents Chemother. 60, 2864–2869. doi: 10.1128/AAC.00177-16
Thakur, A., Pedersen, L. E., and Jungersen, G. (2012). Immune markers and correlates of protection for vaccine induced immune responses. Vaccine 30, 4907–4920. doi: 10.1016/j.vaccine.2012.05.049
Turner, J., and Orme, I. M. (2004). The expression of early resistance to an infection with Mycobacterium tuberculosis by old mice is dependent on IFN type II (IFN-gamma) but not IFN type I. Mech. Ageing Dev. 125, 1–9. doi: 10.1016/j.mad.2003.09.002
van Cutsem, G., Isaakidis, P., Farley, J., Nardell, E., Volchenkov, G., and Cox, H. (2016). Infection control for drug-resistant tuberculosis: early diagnosis and treatment is the key. Clin. Infect. Dis. 62(Suppl. 3), S238–S243. doi: 10.1093/cid/ciw012
van Leeuwen, L. M., van der Sar, A. M., and Bitter, W. (2015). Animal models of tuberculosis: Zebrafish. Cold Spring Harb. Perspect. Med. 5:a018580. doi: 10.1101/cshperspect.a018580
Vordermeier, H. M., Whelan, A., Cockle, P. J., Farrant, L., Palmer, N., and Hewinson, R. G. (2001). Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8, 571–578. doi: 10.1128/cdli.8.3.571-578.2001
Walzl, G., Ronacher, K., Djoba Siawaya, J. F., and Dockrell, H. M. (2008). Biomarkers for TB treatment response: challenges and future strategies. J. Infect. 57, 103–109. doi: 10.1016/j.jinf.2008.06.007
Waters, W. R., Palmer, M. V., Thacker, T. C., Davis, W. C., Sreevatsan, S., Coussens, P., et al. (2011). Tuberculosis immunity: opportunities from studies with cattle. Clin. Dev. Immunol. 2011:768542. doi: 10.1155/2011/768542
Weinstein, E. A., Ordonez, A. A., DeMarco, V. P., Murawski, A. M., Pokkali, S., MacDonald, E. M., et al. (2014). Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 6, 259ra146. doi: 10.1126/scitranslmed.3009815
Yao, S., Xing, H., Zhu, W., Wu, Z., Zhang, Y., Ma, Y., et al. (2016). Infection imaging With (18)F-FDS and first-in-human evaluation. Nucl. Med. Biol. 43, 206–214. doi: 10.1016/j.nucmedbio.2015.11.008
Zanini, C., Pulera, F., Carta, F., Giribaldi, G., Mandili, G., Maule, M. M., et al. (2008). Proteomic identification of heat shock protein 27 as a differentiation and prognostic marker in neuroblastoma but not in Ewing's sarcoma. Virchows Arch. 452, 157–167. doi: 10.1007/s00428-007-0549-6
Zhan, L., Ding, H., Lin, S., Tang, J., Deng, W., Xu, Y., et al. (2014). Experimental Mycobacterium tuberculosis infection in the Chinese tree shrew. FEMS Microbiol. Lett. 360, 23–32. doi: 10.1111/1574-6968.12524
Zhan, L., Tang, J., Lin, S., Xu, Y., Xu, Y., and Qin, C. (2015). Prophylactic use of ganoderma lucidum extract may inhibit Mycobacterium tuberculosis replication in a new mouse model of spontaneous latent tuberculosis infection. Front. Microbiol. 6:1490. doi: 10.3389/fmicb.2015.01490
Zhang, J., Xian, Q., Guo, M., Huang, Z., Rao, Y., Wang, Y., et al. (2014). Mycobacterium tuberculosis Erdman infection of rhesus macaques of Chinese origin. Tuberculosis 94, 634–643. doi: 10.1016/j.tube.2014.08.005
Zhang, T., Bishai, W. R., Grosset, J. H., and Nuermberger, E. L. (2010). Rapid assessment of antibacterial activity against Mycobacterium ulcerans by using recombinant luminescent strains. Antimicrob. Agents Chemother. 54, 2806–2813. doi: 10.1128/AAC.00400-10
Zhang, T., Li, S. Y., and Nuermberger, E. L. (2012). Autoluminescent Mycobacterium tuberculosis for rapid, real-time, non-invasive assessment of drug and vaccine efficacy. PLoS ONE 7:e29774. doi: 10.1371/journal.pone.0029774
Zhang, T., Zhang, M., Rosenthal, I. M., Grosset, J. H., and Nuermberger, E. L. (2009). Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 180, 1151–1157. doi: 10.1164/rccm.200905-0795OC
Keywords: tuberculosis, animal models, comparative medicine, translational medicine, precision medicine
Citation: Zhan L, Tang J, Sun M and Qin C (2017) Animal Models for Tuberculosis in Translational and Precision Medicine. Front. Microbiol. 8:717. doi: 10.3389/fmicb.2017.00717
Received: 22 December 2016; Accepted: 06 April 2017;
Published: 04 May 2017.
Edited by:
Dongsheng Zhou, Beijing Institute of Microbiology and Epidemiology, ChinaReviewed by:
Jinbiao Lu, National Institutes for Food and Drug Control, ChinaXiao-Lian Zhang, Wuhan University, China
Copyright © 2017 Zhan, Tang, Sun and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Qin, Y2h1YW5xaW5AdmlwLnNpbmEuY29t
 Lingjun Zhan
Lingjun Zhan Jun Tang1,2,3,4,5
Jun Tang1,2,3,4,5