
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 28 March 2017
Sec. Microbial Immunology
Volume 8 - 2017 | https://doi.org/10.3389/fmicb.2017.00495
Since the development of antibody-production techniques, a number of immunoglobulins have been developed on a large scale using conventional methods. Hybridoma technology opened a new horizon in the production of antibodies against target antigens of infectious pathogens, malignant diseases including autoimmune disorders, and numerous potent toxins. However, these clinical humanized or chimeric murine antibodies have several limitations and complexities. Therefore, to overcome these difficulties, recent advances in genetic engineering techniques and phage display technique have allowed the production of highly specific recombinant antibodies. These engineered antibodies have been constructed in the hunt for novel therapeutic drugs equipped with enhanced immunoprotective abilities, such as engaging immune effector functions, effective development of fusion proteins, efficient tumor and tissue penetration, and high-affinity antibodies directed against conserved targets. Advanced antibody engineering techniques have extensive applications in the fields of immunology, biotechnology, diagnostics, and therapeutic medicines. However, there is limited knowledge regarding dynamic antibody development approaches. Therefore, this review extends beyond our understanding of conventional polyclonal and monoclonal antibodies. Furthermore, recent advances in antibody engineering techniques together with antibody fragments, display technologies, immunomodulation, and broad applications of antibodies are discussed to enhance innovative antibody production in pursuit of a healthier future for humans.
In recent years, the development of polyclonal and monoclonal antibody by means of laboratory animals has become a vital approach to protect against a number of pathogenic contagions (Marasco and Sui, 2007). These immunoprotective molecules provide defense against transmissible diseases and can eliminate the infection. Their prophylactic and therapeutic protection ability was first discovered in the late nineteenth century by the passive transmission of antibodies from a diseased animal that provided immunity against diphtheria. Subsequently, immune sera from various herbivores and humans were obtained, pooled, and used as therapeutics. Since then, the management of infectious diseases such as diphtheria, tetanus, pneumococcal pneumonia, meningococcal meningitis, and toxin-mediated diseases has considerably improved patient survival (Casadevall, 1999).
Antibodies consist of two heavy chains [variable (VH), joining (JH), diversity (D), and constant (C) region] and two light chains [variable (VH), joining (JH), and constant (C) region], that are linked by non-covalent bonding and disulfide (s-s) bridges (Hamers-Casterman et al., 1993). Antibodies bind antigen with the help of a VHH fragment that can identify specific and unique conformational epitopes by the presence of its long complementary determining regions (CDR3). Escherichia coli expression systems are unique for the validation of the correct functioning of antibody fragments in the periplasmic space or cytoplasm. Conversely, periplasmic expression systems help VH and VL pairing by providing optimal conditions to allow the production of functional molecules (Sonoda et al., 2011).
Polyclonal antibodies contain large and diverse concentrations of different antibodies with unknown specificities. They are broadly used for the detection of different antigens in research and diagnostics. However, non-human polyclonal antibodies induce immune responses in humans that impede their clinical use such as treating snake bites (Wilde et al., 1996). Monoclonal antibodies have revolutionized scientific research. Production of these molecules is based on the fusion of antibody generating spleen cells from immunized mice, rats, or rabbits with immortal myeloma cell lines. These monoclonal antibodies are a highly specific class of biological reagents that facilitate enhanced clinical diagnostics in the medical arena. Subsequently, various antibodies are used clinically as prophylactic or therapeutic agents. The first monoclonal antibody developed by hybridoma technology was reported in 1975 and subsequently licensed in 1986 (Köhler and Milstein, 1975; Nelson, 2010). This development technique signifies a novel way to target specific mutations in nucleic acids and provide extensive expression in disease and other conditions (Nelson et al., 2010).
Antibody production was primarily dependent on animal immunization until the late 1980s by using experimental mice, rabbits and other related laboratory animals (Wang et al., 2010). The main difficulty in the production and application of monoclonal antibodies is the incompetent immune response to highly toxic or conserved antigens. Furthermore, most clinical antibodies are of human origin or are at least humanized in some aspect to avoid immunogenicity (Reichert, 2013). Therefore, transgenic mice and rabbits with human antibody genes have been developed to solve this immunogenicity problem but not the necessity of an effective immune response after immunization. Finally, to overcome this problem, human antibodies were generated in vitro by antibody engineering technologies such as phage display, construction of antibody fragments, immunomodulatory antibodies, and cell-free systems (Edwards and He, 2012).
Expression of recombinant antibodies in vitro experienced a boost with the advent of new molecular tools using various model organism such as yeast, bacteria etc., and new techniques for the selection of genetically engineered recombinant libraries using phage display technology. The phage display technique was first established by George P. Smith, when he validated the display of exogenous proteins on filamentous phage by fusing the peptide of interest to gene III of the phage. The first recombinant antibody fragments were constructed in bacteria 17 years ago (Roque et al., 2004). The goal of antibody production technology is to achieve high-titers of highly specific, and high-affinity antisera. Antigen preparation and animal immunizations are carried out following the guidelines of production techniques via hybridoma technology and recombinant technology (Smith, 1985). Moreover, therapeutic antibodies have been developed by modulation to the fragment crystallizable (Fc) receptor function and contribution of Fc glycan to immunoglobins, and the regulation of the antibody glycosylation in relation to immunoglobins-based therapeutics (Shade and Anthony, 2013).
Human diseases have been known for ages. The comfort of global travel and better interdependence have supplemented layers of intricacy to comprehend infectious diseases. These life threatening contagions effect human health in relation to unpredicted illnesses, deaths, and interfere many other normal life activities. Moreover, the diseases take a significant human toll as well as cause public fear (Morens and Fauci, 2013). To date, limited knowledge is available on extended aspects of the production of antibodies by hybridoma technology, antibody engineering techniques, construction of antibody fragments, display technologies, and their extended applications (Fauci and Morens, 2012). Therefore, to cope these health threats and limitations, extraordinary advances in hybridoma technology and antibody engineering techniques for the development of countermeasures (diagnostics, and treatment by therapeutic antibodies) have been discussed in the present review. Additionally, widespread antibody applications have been described in detail for pursuing a healthier future for humans, and to live a happy life.
Antigen interactions are essential for the normal functions of antibodies that are widely used in research or therapeutics. The antigen-specific and membrane-associated receptor antibody response is mediated by T and/or B cells. Consequently, upon binding with a suitable antigen, B lymphocytes are induced to proliferate, and divide by a number of activating signals, thus increasing the numbers of B cells. These B cells are then differentiated into specific antibody producing plasma cell clones that recognize specific antigen epitopes via the antigen receptor. B cells are activated after recognizing their specific antigen (Figure 1A; Andersen et al., 2006). Some antigens are highly multifarious and exhibit abundant epitopes recognized by several lymphocytes. Consequently, lymphocytes multiply and differentiate by activation of these multifarious antigens into plasma cells that produce polyclonal antibody responses (McCullough and Summerfield, 2005).
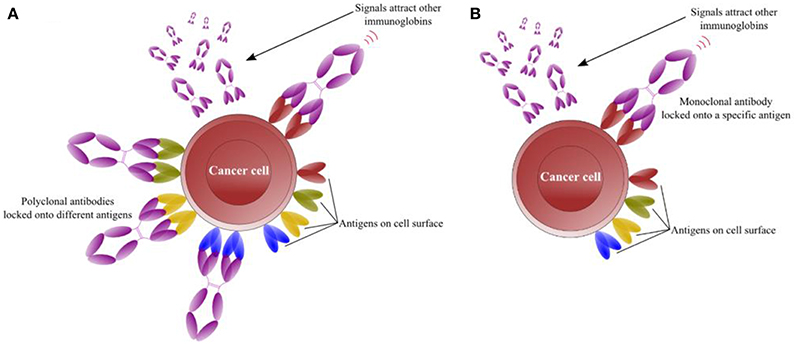
Figure 1. Interaction of antibodies with numerous antigens present on the surface of target cell. (A) Interaction of polyclonal antibodies with specific surface antigen activates B lymphocytes to divide and differentiate into plasma cell clones producing more antibodies that recognize antigens. (B) Interaction of monoclonal antibodies with specific surface antigen activates B lymphocytes to divide and differentiate into plasma cell clones that further recruit homogeneous and mono-specific antibodies.
Polyclonal antibodies (pAbs) can be produced rapidly in several months (compared to monoclonal antibodies), with low cost, low technical skill, and these are stable over a broad range of pH and salt concentrations. In addition, they have applications as therapeutic immunoglobulins (Lipman et al., 2005). PAbs recognize multiple linear epitopes with minimal conformational changes and contain numerous antibodies of varying affinities, which are useful for the immunoprecipitation of composite antigens to form a large precipitating lattice. Mice are used often to produce pAbs because of their small size and blood volume. As an alternative, pAbs are produced as ascites in mice. Moreover, these antibodies have superior specificity compared with monoclonal antibodies because they are generated by a large number of B-cell clones each producing antibodies to a specific epitope (Hudson et al., 2012; Zhuang et al., 2014).
Monoclonal antibodies (mAbs) are clinically significant homogeneous and mono-specific scientific biomolecules produced from hybridoma cells by hybridoma technology (Zhang, 2012). mAbs arise from single cell clone compared to multiple cell clones for pAbs (Figure 1B; Andersen et al., 2006). Since their discovery, these molecules have been used as research tools and have revolutionized the fields of biotechnology, immunology, diagnostics, and medicine. The technology was described for the first time by Köhler and Milstein (1975) in the mid-1970s in the journal Nature, and they were later awarded the Nobel Prize (Saeed and Awan, 2016).
Currently, mAb products approved by the US Food and Drug Administration (FDA) are increasing worldwide i.e., about four new products per year. Currently, 47 mAb products in the US, Europe and global markets have been approved for the treatment of a variety of diseases (Table 1; Ecker et al., 2015). At the current rate, about 70 mAb products will be on the market by 2020, and collective global trade will be approximately $125 billion (Ecker et al., 2015). Improvements in hybridoma technology are based on research demand, cost effectiveness, human labor, and reduced development time. Similarly, the production of mAbs requires multiple phases, long duration, and high cost. Currently, mAbs have been produced against a number of mycotoxins such as fumonisin B1 (Yuan et al., 2012; Ling et al., 2014, 2015b), citreoviridin (Jin et al., 2014), marine toxins (Saeed and Wang, 2016), and other exo- and endo-antigens. Similarly, mAbs against transmembrane enzymes have been produced (Yuan et al., 2012).
Hybridoma technology has been a significant and essential platform for producing high-quality mAbs (Zhang, 2012). It permits generation of therapeutic antibodies in a native form. However, technical difficulties in hybridoma production have updated the mainstream antibody production into new ways like display and transgenic mice techniques. Nevertheless, hybridoma technology is a classical and established route of generating specific antibodies all around the globe (Glukhova et al., 2016). The technology begins with immunization of test animals with an antigen of interest and serum antibody titer is determined by enzyme linked immunosorbent assay (ELISA). Subsequently, the spleen is aseptically removed and splenocytes are fused with myeloma cells to produce hybridoma cells. Hybridoma cells are then cultured in 96-well plates in the presence of hypoxanthine-aminopterin-thymidine (HAT) selection medium for high throughput screening. Later, hybridoma cells producing desired antibodies are screened by conventional ELISA and novel nanoparticle-probed immunoassay (colloidal gold or silver nanoparticles; Figure 2). Cell culture systems in vitro with specific mAb cell lines were then subjected to mass generation by media selection, shaker flasks, and bench-scale bioreactors (Ling et al., 2014).
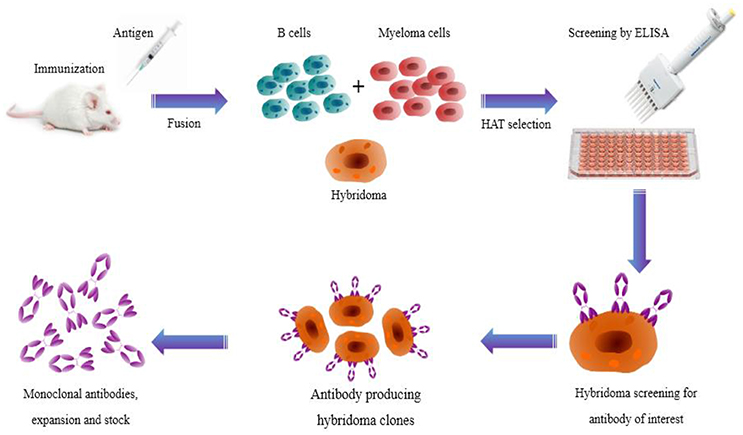
Figure 2. Illustration showing the production route of hybridoma technology. Monoclonal antibodies are generated by immunizing laboratory animals with a target antigen. B cells and myeloma cells are fused and then selected in HAT medium. Finally, hybridoma cells producing the desired antibodies are screened.
The optimal conditions such as temperature, percent carbon dioxide, and humidity for cell cultures should be determined (Sen and Roychoudhury, 2013), and then transferred to a pilot scale for scalability and toxicology studies. In addition, clinical materials should be produced on a large scale under the existing good manufacturing practice (cGMP) regulations. After production on a small scale, products that are already cultured in a laboratory are then transferred to pilot scale commercialization. The process then performs characterization, scaling, technology transfer, and validation. Commercial cell culture for the production of a biological product is completed by pilot scale laboratory methods (Li et al., 2010). Recently, commercialization is initiated by process characterization, scale-up, technology transfer, and validation of the manufacturing process (Li et al., 2006).
ELISA is enzyme-based colorimetric assay, requires large sample volumes, several incubation steps and has low detection sensitivity (Tang and Hewlett, 2010). Conversely, nanotechnology and nanoparticles (NPs) use nanomaterials with length scale of 1–100 nanometers (nm). Nanomaterials have unique biological properties such as small size, large surface-to-volume ratio, sharp melting temperature, magnetic properties, unusual target binding properties, and size based multi-coloring (Qi and Wang, 2004). NPs such as gold particles have been used to improve assay sensitivity and specificity of antibodies, low limit of detection (LOD), dose response over 10,000-fold and the detection sensitivity by 1,000-fold compared to ELISA (Tang and Hewlett, 2010).
Similarly, an essential factor of NPs selection is that nanometer-sized (colloidal gold or silver) particles can be conjugated with targeting ligands, including mAbs, peptides, or small molecules with functional and structural properties that are not available from either discrete molecules or bulk materials. Nanoparticles are probed with mAbs that can be used to target any antigen of interest (Ling et al., 2015a). The use of colloidal gold is a rapid, less time consuming, and cost effective technique. This technology has been used in the development of immunochromatic strip assays based on mAb. The technique has widespread applications for the detection of a number of antigens (exogenous antigens, endogenous antigens, autoantigens, neoantigens, viral antigens, and tumor antigens) with high specificity and binding affinity (Schumacher and Schreiber, 2015).
Antigen preparation including quality and quantity is essential for the production of antibodies. The purification of antigens is difficult and time consuming but antigen purity is vital for adequate immune responses (Leenaars and Hendriksen, 2005). Insignificant impurities (<1%) can be immunodominant in the case of various microbial antigens. They show non-specific activity against the antigens of interest and specific activity against impurities. Higher concentrations of specific antibodies are obtained after purification and the exclusion of impurities by extensive absorption procedures such as antibody affinity chromatography (Leenaars et al., 1997; Mazzer et al., 2015).
Before scheduling immunization, antigen contamination should be considered by the researcher. The antigen and diluents should be free of endotoxins such as lipopolysaccharide, or chemical residues that have been utilized to neutralize the microorganism. Additionally, the pH must be adjusted to prevent undesirable effects in the animal to be immunized (Hendriksen and Hau, 2003). Moreover, sterile working conditions, antigen concentration, animal preparation, and injection quality must be confirmed. These conditions are necessary to avoid suppression, sensitization, tolerance, or other superfluous immunomodulation and to induce effective immune responses. The required antigen concentration (μg to mg) is based on the intrinsic properties of the antigen. Usual doses of antigen conjugated with Freund's adjuvant for rabbits are in the range of 50–1,000 μg; for mice, 10–200 μg; and for goats and sheep, 250–5,000 μg (Leenaars et al., 1997). The inherent properties of antigen quantity include purification, animals to be immunized, type, and quality of the adjuvant (to elicit high-titer serum responses), route and the immunization (injection) frequency (Hanly et al., 1995).
Antibody production involves the immunization or injection of laboratory animals with an immunogenic protein and test sampling of antiserum. The immunization is performed in specific animal species and the adjuvant is processed to form an immunogenic emulsion that is insoluble in water (Leenaars and Hendriksen, 2005). Conjugation of smaller or less immunogenic antigens i.e., haptens (numerous myco- and marine toxins) to carrier proteins is essential before animal injection. After immunization, the animal is monitored daily or three times a week for side effects (clinical and pathological examination). Severe pathological changes such as tissue reactivity, infection and anaphylactic reactions in case of booster injections have been reported in the absence of visual clinical or behavioral changes (Leenaars et al., 1997).
The immunization route is based on the choice of animal species, adjuvant, concentration and quality parameters of the antigen (Apostolico Jde et al., 2016). Suitable immunization routes include subcutaneous (s.c.), intradermal (i.d.), intramuscular (i.m.), intraperitoneal (i.p.), and intravenous (i.v.). Blood sample handling should not interfere with the immunization site to avoid pain and distress to the animal while taking blood sample (Leenaars et al., 1997). The i.v. route for water-in-oil emulsions such as Freund's complete and incomplete adjuvants (FCA and FIA) may be fatal as embolisms caused by large particulates or viscous gel adjuvants (e.g., aluminum salts) can occur (Hanly et al., 1995). The s.c. administration of FCA is immunologically effective (0.1 mL in mice and 0.25 mL in rabbits) with some reports of pathological changes. The i.p. administration of FCA is not suggested for antibody development because it induces infection, peritonitis, and social variations. There is high risk of anaphylactic shock for booster injections by the i.v. or i.p. route (Leenaars et al., 1997).
The volume of the immunogenic mixture depends on the quality of the antigen and the degree of the lesions formed. Therefore, smaller volumes of mixtures are injected to induce antibody responses except when increased concentrations of antigen are required (Leenaars and Hendriksen, 2005). The immunization volume also depends on the animal species, route, and chemistry of the injection mixture. The volume of FCA has lethal effects on animal physiology and the extent of lesions produced is associated with high volumes of FCA (Ramos-Vara and Miller, 2014).
Booster injections have a significant effect on the outcomes of immunization and induction of B memory cells. Class switching of B cells depends on the time interval between two consecutive injections. A small volume of antigen can be used for a booster injection without adjuvant (Leenaars et al., 1997). A booster injection is used when the antibody titer has reached a plateau or begins to decline. Antibody titers typically peak at 2–3 weeks after immunization in the absence of the depot-forming adjuvant used in the first immunization. A booster injection is used after 4 weeks in the presence of depot-forming adjuvant. FCA should be used in the first s.c. injection to avoid subsequent (Mycobacteria proteins) severe tissue reactions (Hendriksen and Hau, 2003).
Hybridoma technology is essential for the production of high-quality mAbs as well as research and diagnostic reagents, and it is currently the most rapidly growing class of therapeutics (Hnasko and Stanker, 2015). MAbs can be effectively screened by a number of techniques such as ELISA, phage display, and other related technologies. Screening identifies and picks only specific antibody producing hybridomas. In hybridoma technology, laboratory animals are immunized with an antigen of interest and specific antibody-producing B cells are isolated from the spleen and then fused with immortal myeloma cells (such as sp2/0; Bradbury et al., 2011). Subsequently, expansions of clonal populations are produced from serially diluted sub-cloned individual hybridoma cells in a microtiter plate. Next, the hybridoma supernatant is checked by ELISA or other related immunoassays for desired antibody activity (El Debs et al., 2012).
After screening, hybridoma cells are expanded in bigger wells or in culture flasks to maintain the hybridoma and provide sufficient cells for cryopreservation and supernatants for later investigations (Nelson et al., 2010; El Debs et al., 2012). However, this technique restricts the number of clones that can be screened to no more than a few thousand. Consequently, insufficient numbers of cells remain available for clonal expansion and cell immortalization. Therefore, improved techniques such as antigen-based microarrays have been used for the screening of >105 clones in <12 h, or lithographically fabricated microwells for individual cell compartmentalization. Nevertheless, these techniques only screen for binding activity and do not allow functional assays (El Debs et al., 2012).
MAb characterization is based on a determination of its physicochemical and immunochemical character, heterogeneity, purity, impurities, biological activity, potency, and concentration (Berkowitz et al., 2012). For physicochemical properties, the isotype (class, subclass, light chain composition) and primary structure of the mAb are determined. Immunological properties include binding assay, affinity, avidity, immunoreactivity (cross-reactivity with other structurally homologous proteins), analysis of CDR, epitope characterization, and determination of effector functions (CHMP, 2008).
Heterogeneity (including chromatographic and electrophoretic methods) and quantity are determined for the characterization of mAb purity, impurity, contaminants, and concentration. Specificity of mAbs is also checked. Peptide map, anti-idiotype immunoassay, potency, and other appropriate methods are used to determine its identity and biological activity (CHMP, 2008).
MAb-based products exhibit superior specificity for a particular antigen. This characteristic feature of the immunoglobins makes them an ideal tool for many applications including disease diagnosis and therapy (Table 2; Modjtahedi et al., 2012; Redman et al., 2015; Steplewski et al., 2015). Diagnostic applications include biochemical analysis and imaging. It involves a number of immunoassays for the detection of hormonal, tissue, and cell products. Imaging is carried out using radiolabeled mAbs for diagnostics of infectious diseases. Therapeutic mAbs have a wide range of applications. They are used in the treatment of cancer, transplantation of bone marrow and organs, autoimmune diseases, cardiovascular diseases, and various infectious diseases. Therapy can be carried out by direct use of mAbs as therapeutic agents and as targeting agents respectively (Modjtahedi et al., 2012).
Computational and bioinformatics approaches play an essential role for antibody selection and epitope prediction. It is an interdisciplinary science and the term can be defined as “the application of computer tools to handle biological information.” Several computational and bioinformatics tools for prediction of antibody binders include RANKPEP, nHLAPred, NetMHC, kernel-based inter-allele peptide binding prediction system (KISS) with support vector machine (SVM; Bhasin and Raghava, 2007; Lundegaard et al., 2008). Moreover, these tools use databases that contain known epitopes from SYFPEITHI, MHCBN, LANL, and IEDB for protein epitope prediction (Soria-Guerra et al., 2015).
Furthermore, other tools include position specific scoring matrices (PSSM) used for sequence alignment, IEDB analysis resource database uses NetMHCpan for peptide affinity, quantitative matrices (QM), whole antigen processing pathway (WAPP), Matthews correlation coefficient (MCC), and spatial epitope prediction of protein antigens (SEPPA) (Soria-Guerra et al., 2015). Recently, a simulation tool C-ImmSim was developed for the study of a number of different immunological processes. The processes include simulations of immune response by representing pathogens, as well as lymphocytes receptors, amino acid sequences and T and B cell epitope prediction (Rapin et al., 2010).
The first fully human mAb was developed over 25 years ago by phage display and a selection of antigen-specific binders from blood lymphocyte libraries (Gavilondo and Larrick, 2000). This technique employed transgenic animals such as mice and rabbits with integrated human immunoglobulin (Ig) loci. Germline-configured chimeric constructs confirmed that human, mouse, and all mammalian Ig loci function in very similar ways (Neuberger and Bruggemann, 1997). Antibody development has progressed from hybridoma technology to a recombinant deoxyribonucleic acid (DNA) approach. In the last few years, a number of engineered antibody drugs have been approved or investigated in phase II or III clinical trials (Table 3; Nelson et al., 2010; Dantas-Barbosa et al., 2012; Nixon et al., 2014).
MAb immunoglobulins (IgG) are the starting material for the generation of smaller antibody fragments in lymphoid or non-lymphoid cells (Klimka et al., 2000). Traditional hybridoma technology has several limitations such as being exclusively murine based, time consuming, and exhibiting low-affinity in conventional assays. Therefore, antibody engineering, display system, and immunomodulation methods are now used to produce efficient therapeutic antibodies (Klimka et al., 2000).
The first study of recombinant antibodies in bacteria was difficult because of interference from disoriented proteins in the bacterial cytoplasm. A new antibody expression technique was developed to produce smaller antibody molecules (Fab or Fv fragments; Okamoto et al., 2004) where numerous types of vectors (phagemid) are used with competent E. coli. This technique involves the expression of antibody fragments for recombinant antibody construction. In this way, a number of genetically engineered antibodies have been constructed, such as Fab fragments, Fv fragments, single-chain variable fragments, bivalent antibodies, and bispecific antibodies (Little et al., 2000).
Different antibody fragments are used in phage display technology: scFv (single chain fragment variable), Fv (fragment variable), Fab (antigen binding fragment) and their derivatives, V-gene domain, bispecific or bivalent antibodies, and other oligomers (Figure 3; Nelson, 2010). Fab fragments are the linkage of VH–CH and VL–CL by disulfide bridges, and radiolabeled Fabs are used in tumor imaging. Fv is used for the construction of VL and VH domains or their modifications such as scFv, which is the most popular fragment. A (Gly4Ser)3 linker is used for the stabilization of VL–VH and proper antigen binding site formation without the loss of antibody affinity. Chelating recombinant antibodies (CRAbs) are scFv segments with a high binding affinity. These constructs consist of two scFv fragments specific to the identical antigen and adjacent epitopes. These fragments are connected by a short linker (up to 10 amino acids) for the dimerization and formation of diabodies (Wright and Deonarain, 2007; Nelson, 2010).
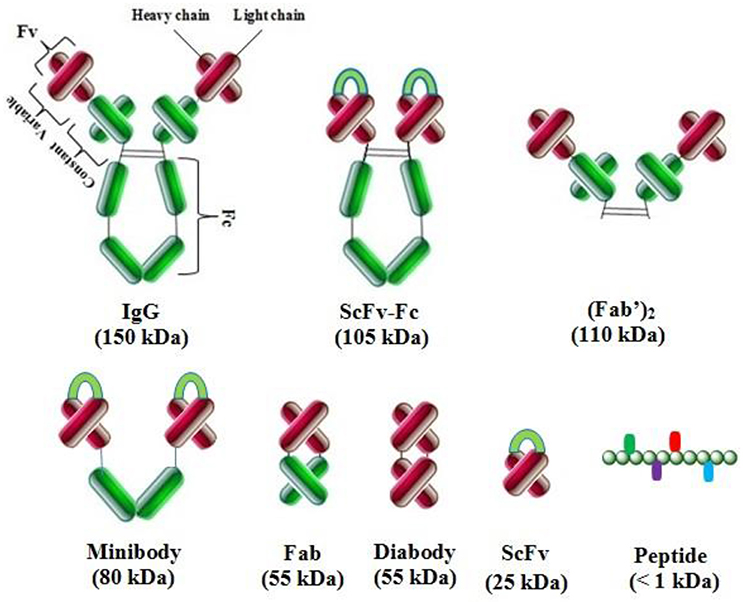
Figure 3. Complete antibody and various types of antibody fragments. These fragments are constructed by antibody engineering techniques for enhanced therapeutic applications.
ScFv has high affinity, highly solubility, multi-domains, and high binding specificity with their target antigen and they are used for antibody engineering, biotechnology, cancer research, and biomedical applications. ScFv have been engineered to improve the effector functions of full-length antibodies, carrying toxins to kill cells, or cytokines to activate the immune system. Furthermore, bispecific antibodies have been constructed to target multiple receptors (AlDeghaither et al., 2015). Recombinant antibody engineering and recombinant DNA technology has facilitated successful expression and cloning of widespread antibody fragments in bacteria (E. coli), as well as mammalian (Chinese hamster ovary (CHO), or myeloma cell lines e.g., Sp2/0), yeast (Pichia pastoris), plant (Arabidopsis), and insect cells (Drosophila melanogaster) (Frenzel et al., 2013). These smaller fragments have several advantages over full-length antibodies such as tumor and tissue penetration, blood clearance, short retention times, and reduced immunogenicity. Likewise, they have better fusion in bacteria, and display on a filamentous phage. These fragments permit the production of homogenous proteins for diagnostic and therapeutic purposes as well as structural studies (Frenzel et al., 2013; Wu et al., 2016).
ScFv fusion proteins are constructed by the association of heavy (VH) and light chains (VL) of immunoglobulins via a short peptide linker. Antigen specific scFv can be easily generated by phage display. These fusion proteins have extensive applications in cancer therapeutics such as in lymphatic invasion vessels, colon cancer, hepatocarcinoma, and diagnostics of human disease (Tonelli et al., 2012). Moreover, scFv have been widely used with phage display panning i.e., an affinity selection technique, to construct ligands to detect toxins produced by various pathogenic entities in vitro or in vivo. Similarly, co-expression vector systems have been used to prevent and cure diseases by scFv. Currently, various scFv fragments have been constructed against toxins and virulence factors of pathogens (Tonelli et al., 2012; Wang et al., 2013, 2016). Additionally, a method for rapid and effective high-affinity GFP-based antibody production corresponding to scFv was developed. It was demonstrated by inserting CDR3 into green fluorescence protein (GFP) loops for improved disease diagnosis and therapy (Wang et al., 2014b). Skp co-expressing scFv has high solubility and binding activity to antigen thermolabile hemolysin (TLH) (a pathogenic factor of Vibrio parahaemolyticus) and was developed by using pACYC-Duet-skp co-expression vector. This scFv was constructed to detect TLH directly in real samples. Furthermore, an antibody was developed against TLH that showed strong neutralizing effects on TLH-induced cell toxicity (Wang et al., 2006, 2007, 2012, 2013, 2014a; Chen et al., 2015).
Phage-displayed antibody libraries have been widely used for the construction of high-affinity target-specific antibodies (Chen et al., 2008). ScFv is a non-covalent heterodimer that consists of VH and VL domains. Moreover, antibody repertoires from phage-displayed libraries are constructed by harvesting messenger ribonucleic acids (mRNAs) from peripheral blood lymphocytes, hybridoma, spleen, bone marrow, tonsil, and similar other sources (Chen et al., 2008). Large libraries with a diverse range of antibodies and genes are created using reverse transcribed (RT) process into cDNA to function as a template for antibody gene amplification (PCR) (Lim et al., 2010).
Libraries are also created by PCR assembly, phagemid, and sequential cloning or combinatorial infection. The VH and VL chains are combined (linker orientation dependent) and cloned to construct a combinatorial scFv library for antigen selection (Ahmad et al., 2012). Another technique for recombinant antibody production is the utilization of phage recombinants displaying antibodies at their tips, and which undergo biopanning for the in vitro selection of scFv from large libraries of variable domains circumventing the traditional hybridoma method. Numerous scFv fragments have been constructed against haptens (Wang et al., 2006, 2014a), proteins, carbohydrates, receptors, and tumor antigens for medical therapies and diagnostic applications (Wang et al., 2012, 2013).
Phage display is a powerful biological technique for screening specific peptides or proteins. Screening of antibody libraries by phage display permits the rapid selection of scFvs to isolate VH and VL chains for mAb transformation. Thus, therapeutic antibodies against noxious or highly conserved antigens, plasma membrane proteins, and receptors can be obtained in their native conformation while avoiding animal immunization (Rader and Barbas, 1997). The peptide libraries are incubated on a plate coated with the antigen of interest. Next, unbound phages are washed away. Then, the phages are amplified using host bacteria for high-affinity elution and binding/amplification cycles to expand the pool in favor of binding sequences. Finally, individual clones are characterized after 3–5 rounds by DNA sequencing and ELISA to achieve the resultant structural and functional details (Catanese et al., 2007).
Numerous expression systems such as E. coli, yeast, mammalian, insect cell, wheat germ cell-free expression system, and plant-based expression system have been used for the successful isolation of scFv and display as fragments (Wang et al., 2013). The expression and activation of scFv is performed by appropriate folding and in vitro refolding for aggregation. The expression systems for the production of active scFv antibody are selected, designed, and constructed based on hosts. The bacterial expression system is the most suitable and widely used method for the production of scFv antibody fragments compared to other available expression strategies (Frenzel et al., 2013).
E. coli is a valuable tool for expression systems in the fields of genetics and biochemistry. The system has numerous advantages including enhanced folding, low cost, high throughput, well-studied physiology and genetics, rapid growth, high yields up to 10–30% of total cellular protein, and simple handling. Additionally, it can be used for multi-plexed cloning, expression, and purification of proteins for structural genomics (Rosano and Ceccarelli, 2014).
Antibody characterization involves peptide mapping, glycan characterization analysis, purification, fragmentation of antibody pharmacokinetics, and quality assurance for many applications in basic research (Roth et al., 2012). The scFv antibody is usually characterized by affinity, isotype, cross binding, phage-ELISA, and immunoblot. Biochemical characterization includes the expression of scFv antibody in a soluble form in infected E. coli cells. In addition, further characterization is carried out by immunofluorescence antibody test (IFAT), mass spectrometry, sequencing and indirect immunofluorescence (IFI) assays, cytotoxicity analysis, surface plasmon resonance, and NMR spectroscopy (Wang et al., 2012; Yuasa et al., 2014; Levenhagen et al., 2015).
Antibody engineering has become an aesthete discipline covering a wide range of production technologies. Moreover, techniques include the modification of clinically significant therapeutic drugs by antibody fragments especially for clinical imaging and to target multiple disease associated antigens (Spiess et al., 2015).
Fab antibody fragments are smaller with better tissue and tumor penetration than intact mAbs. Fab lack a constant region and therefore, antibody effector functions (Nelson, 2010). Moreover, Fab bind to specific antigens and are used in non-clinical studies (e.g., staining experiments) and clinical therapeutics such as anti-TNFα PEGylated fab fragment. This fragment has a 14 day serum half-life and is used to improve anti-tumor activity and to reduce immunogenicity (Chames et al., 2009).
The tumor necrosis factor (TNF) and interleukins 1 and 6 (IL-1 and IL-6) proinflammatory cytokines cause multifactorial disease such as cancer and systemic inflammations. Moreover, these factors are involved in redundancy of disease-mediation and crosstalk between signal cascades (Arango Duque and Descoteaux, 2014). Similarly, upregulation of alternative receptors and pathway switching is often related to resistance to therapy (Dong et al., 2011). The obstruction of several targets or multiple sites on one target is associated with improved therapeutic efficacy. Over the past decade, dual targeting with bispecific antibodies has gradually switched to combinatorial or cocktail therapy. This targeting technique is based on the targeting of multiple disease-modifying reagents with one drug. Several bispecific molecules such as diabodies, IgG-like tetravalent Di-diabodies, IgG-scFv fusion proteins, and bispecific Adnectins™ have been developed to target tumor mediated receptors such as members of the epidermal growth factor (EGF) receptor family, i.e., EGFR, HER2, HER3, and HER4,45 and the insulin-like growth factor 1 receptor (IGF-1R; Tao et al., 2007; Kontermann and Brinkmann, 2015). The application of a single, bispecific molecule is advantageous because it is less complicated to administer to patients, requires reduced preclinical and clinical testing, and has cost effective manufacturing (Kontermann and Brinkmann, 2015).
Smaller antibody fragments permit in depth tissue penetration associated with the affinity of the antibody fragments. Moreover, a high concentration of complete antibody restricts its ability to infiltrate tumors (Weiner and Adams, 2000). Additional engineered antibody fragments include CDRs, Fab, F(ab')2, monospecific Fab2, bispecific Fab2, trispecific Fab3, monovalent IgG, bispecific diabody, trispecific triabody, scFv-Fc, minibody, new antigen receptor (IgNAR), variable new antigen receptor (V-NAR) domains in sharks, camelid heavy chain IgG (hcIgG), and VhH (Nelson, 2010; Rodrigo et al., 2015).
Phage display is a selection technique for fusion proteins and phage coat proteins that are expressed on the phage surface. The library is developed by careful genetic manipulation (Figure 4; Chan et al., 2014). Peptide or protein coding genes are inserted into a vector fused to the 5′-terminal of pIII or pVIII that are phage coat proteins. The bacteria are transformed with phagemid libraries, and then infected with a helper phage to assemble phage particles that express fusion proteins on their surface. Subsequently, the displayed proteins/antibody fragments are rooted to the surface of the coat protein, and permit affinity purification with its analogous genes (Barbas et al., 1991; Chan et al., 2014).
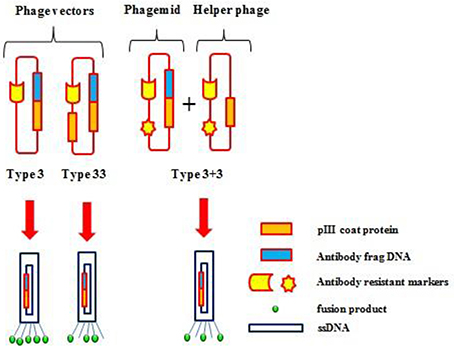
Figure 4. Various phage display systems. Gene pIII is represented as an orange box, the foreign DNA insert as a blue box, and the fusion products as a green circle.
Filamentous bacteriophages used in phage display techniques are viruses that belong to the Inoviridae family. There are fewer of these filamentous phages in this genus compared with tailed phages. Inovirus virions are 7 mm in diameter, contain circular DNA enclosed in a protein capsid, and infect both Gram negative and positive bacteria. They do not lyse host cells, instead, they are packed and extrude at the surface (Marvin et al., 2014).
The genomes of these viruses consist of double strand DNA (dsDNA), single stranded DNA (ssDNA), double strand RNA (dsRNA), and single strand RNA (ssRNA). The viruses enter host cells via pilli and are involved in genome replication, and virion structure, assembly and regulation (Stassen et al., 1994). These viruses undergo extensive recombination, act as vectors (phagemid) for gene transfer and are closely related phenotypically and genotypically. They can integrate into the host genome by phage-encoded transposases and host-encoded XerC/D (Hassan et al., 2010). Filamentous phages have three distinct classes, including M13, f1, fd, and M13 that infects E. coli. Most proteins are displayed at phage proteins pIII (fusion) and pVIII (preserving functional coupling with 6–7 residues; Hess et al., 2012).
This phage can be easily manipulated due to its small genome. Therefore, these are the best-studied viruses and are extensively used in phage display technology. Large phage particles can be produced by the insertion of DNA into non-essential regions, are stable in extreme conditions, and can be produced in high amounts (Jończyk et al., 2011).
A number of in vitro techniques have been established for the development of antibodies in comparison with in vivo methods that involve animal immunization. PDT is a powerful tool for screening of specific recombinant protein binders against a large number of target antigens, including peptides, glycoproteins, glycolipids, saccharides, nucleic acids, and haptens. PDT is the most commonly used in vitro technology (Bradbury et al., 2003a). A feature of this technology is a direct physical link between genotype and phenotype of the displayed protein/variable antibody fragments. Screening of the displayed protein by antigen in vitro is analogous to the selection of protein fragments in natural immunity (Petrenko and Vodyanoy, 2003).
Phage display technology has facilitated the production of protein libraries, which are formed with large numbers of phage particles displaying different molecules (106–1011 different ligands in a population of >1012 phage molecules). Specific binder screening with biopanning allows the enrichment of the desired molecule (Bazan et al., 2012). The first step is the incubation of the display library with an immobilized surface (for example, microplate, magnetic beads, column matrix, PVDF membrane, or immunotubes) of the entire cell. The non-binding phages are then removed by extensive washing and the binders are eluted by acid or salt buffer. Then, binders are amplified using an appropriate bacterial host cell such as E. coli. To obtain high-affinity targets, up to five rounds of biopanning are performed (Figure 5). Finally, DNA sequencing of the primary structure is carried out to produce individual clones of the target protein (Bazan et al., 2012; Wu et al., 2016).
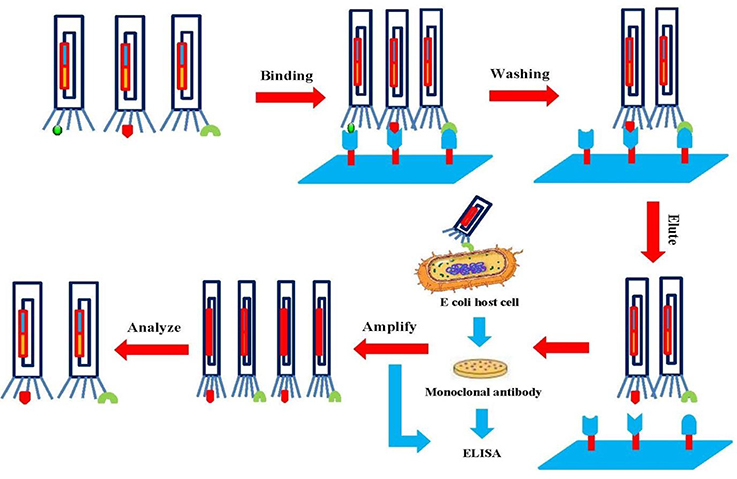
Figure 5. Schematic illustration of the biopanning technique. The target is attached to a phage library that is immobilized on a solid surface. Unbound phages are washed out, and specific phages are eluted and amplified. After several rounds of biopanning, the phages are analyzed to obtain diagnostic and therapeutic agents.
Many factors including proper biopanning design, type of immobilized surface, binding time, washing, and antigen concentration affect the level of selection and the screening of antibodies to unique epitopes (Bazan et al., 2012). Numerous screening cycles are essential in biopanning to attain the preferred binding activity of the acquired monoclonal phage antibodies. Several tests including ELISA, fluorometric microvolume assay technology (FMAT), and chromophore-assisted laser inactivation (CALI) are used to analyze this activity (Tonelli et al., 2012).
Various types of phage libraries are used for the screening of specific recombinant proteins/antibodies in relation to antigen specificity: (1) construction of specific high-affinity antibody library for specific antigens by the use of immunized animals with a dissociation constant in the nanomolar range, (2) a single-pot (general) non-specific library produced against antigens, and (3) construction of secondary mutant antibody phage libraries for the screening of antibodies with high specificity (Hust and Dubel, 2004). The development of an antibody library from immunized animals has become obsolete because of the construction of diverse universal single-pot libraries for the isolation of numerous antigens. PDT has many applications in biotechnology, production of recombinant multifunctional antibodies, cancer, immunotherapeutics, and the enhanced validity of protein fragments (Yau et al., 2003).
The study of epitopes and mimotopes in the interactions of antigen-antibody binding was the earliest application of PDT (Wu et al., 2016). Mimotopes are miniscule peptides that mimic linear, intermittent, or non-peptide epitopes. It was noted that scFv, Fab fragment, and VHH domains could be displayed on the phage successfully (Tonelli et al., 2012). This laid the foundation of new molecular recognition techniques to determine protein folding, stability, structure-to-function relationships, and other related protein-protein interactions. The fusion of many ligands with phage particles has enrich phage displayed cDNA libraries significantly (Vithayathil et al., 2011).
Several novel molecular techniques have been established for screening functional molecules. These techniques include the identification of peptide agonists, receptor antagonists, the determination of binding specificity of domains, mapping of simple carbohydrates and functional epitopes, the identification of tumor inhibitor targets, and molecular imaging by fluorescently labeled phages (Fukuda, 2012). Recently, PDT has been widely used in medical sciences for the production of a large number of humanized antibodies and the production of new therapeutics. These antibodies have preclinical and clinical applications (Rothe et al., 2006).
A large number of antibody reagents are being developed for hematological applications such as cell subpopulation identification, directed therapeutics, and in vivo imaging. Anti-ABO, anti-Rh, and anti-Kell hemagglutination antibodies have been developed against red blood antigens (Marks et al., 1993). Anti-Rh (D) and anti-HPA-Ia bispecific diabodies developed by PDT that are useful for hemagglutination assays. These diabodies are being used for the treatment of neonatal alloimmune thrombocytopenia (Watkins et al., 1999).
Moreover, various antibody reagents have been raised against fetal red blood cells (Huie et al., 2001). Additionally, this technique has helped the production of antibodies against dendritic cells, white blood cells (WBC) (Fitting et al., 2011), hairy cell leukemia (Kreitman et al., 2012), myeloma protein (paraproteins) (O'Nuallain et al., 2007), B and T cells (Maeda et al., 2009), clotting factors, AITP, GPIa, and GPIIIa antigens, CD antigens (Chu et al., 2006), and 11-dehydro-thromboxane B2 (11D-TX) antigens (Siegel et al., 2003).
Human immune libraries developed by PDT facilitate the study of autoimmune and neurological disorder physiology, clinical diagnostics, and the treatment of AITP (platelet disorder caused by anti-platelet autoantibodies), MS, myasthenia gravis (MG) [antibodies against nicotinic acetylcholine receptor (AcChoR)], thrombotic thrombocytopenic purpura (TTP), Cogan's syndrome (CS) caused by systemic vasculitis, acute anterior uveitis (AAU), ocular inflammation, insulin dependent diabetes mellitus (IDDM) caused by the destruction of pancreatic beta cells, Wegener's granulomatosis (Finnern et al., 1997), autoimmune thyroid disease (Latrofa et al., 2003), primary biliary cirrhosis (PBC), and Sjögren's syndrome (SS). Additionally, the technique has therapeutic uses in blistering skin diseases, pemphigus vulgaris (PV) (Payne et al., 2005), pemphigus foliaceus (PF) (Ishii et al., 2008), autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), mixed cryoglobulinemia (CryoII), systemic sclerosis (SSc), autoimmune cholangitis (AIC), antiphospholipid syndrome (APS), vitiligo rheumatoid arthritis, Crohn's disease, Graves' disease (GD), and celiac disease (genetic inflammatory disorder) (Zohreh and Hossein, 2008).
Neurological disorders are treated by intracellular antibody fragments (intrabodies), which are potentially therapeutic. Intrabodies select abnormal intracellular proteins. However, there are several limitations in the extracellular binding and internalization of DNA transfected by viral based vectors, lipofection or electroporation (Jazi et al., 2012). These are not efficient in vivo techniques and can alter cell viability. This problem can be overcome by fusing protein transduction domains (PTD) to antibodies (Langedijk et al., 2004). Phage display libraries have been utilized for novel immunotherapeutic strategies for the treatment of neurotoxins, Creutzfeldt–Jakob disease (CJD), and Gerstmann-Sträussler-Scheinker syndrome (GSS). They are also used for kuru disease, familial fatal insomnia by the accumulation of abnormal prion protein (PrPSc) (Thanongsaksrikul and Chaicumpa, 2011), Huntington's disease, and Parkinson's disease. Moreover, they have been employed in inhibitory studies of β-amyloid formation, and enzyme therapy of the brain vasculature and brain parenchyma (Chen et al., 2009).
Biopanning in vivo with phage display libraries has facilitated the isolation of peptides homing to all types of organs in the human body. Phage display is applied to stem cells for cell based regenerative medicine (Gothard et al., 2011). Moreover, this technique has assisted in the guided delivery of various peptides/drugs such as proapoptotic peptides, cytotoxic drugs, metalloprotease inhibitors, and cytokines to specific targets (Nixon et al., 2014). The binding of peptides with the extracellular domain of the LOX-1 receptor is associated with hypertension and atherogenesis (Nixon et al., 2014). Other studies have reported that the homing of an RGD-motif-containing peptide to angiogenic vasculature was linked to a proapoptotic peptide and was successfully used for the treatment of collagen-induced arthritis in mice. Phage libraries have also been used for anti-obesity, microparticle (MP), avb3 integrin angiogenesis therapy, and in targeting vascular endothelial growth factor (VEGF) (Cooke et al., 2001).
Similarly, phage displays are used for tumor targeting agents e.g., the scFv (MFE-23) molecule is specific for carcino embryonic antigen (CEA) (Edwards et al., 2008). This technique has replaced radiolabeled antibodies that have multiple disadvantages including reduced natural immunity (Adachi et al., 2011). Furthermore, PDT has been used to isolate a number of peptides for molecular imaging. Its advantages are small size, rapid blood clearance, lack of immunogenicity, tissue penetration, and increased diffusion. Numerous peptides for tumor targeting were isolated using human B cell lymphoma (McGuire et al., 2006), cervical (Robinson et al., 2005), colon (Rasmussen et al., 2002), gastric (Liang et al., 2006), breast (Askoxylakis et al., 2005), lung (Chang et al., 2009), glioblastoma (Wu et al., 2008), hepatic (Du et al., 2006), prostate (Zitzmann et al., 2005), neuroblastoma (Askoxylakis et al., 2006), and thyroid (Zitzmann et al., 2007) carcinoma cell cultures. However, about 80% of these peptides have not been reported to function in vivo. This inactivity was observed in peptides that recognized mouse double minute 2 homolog-p53 protein (MDM2/p53) (Pazgier et al., 2009), IL-11 receptor (Zurita et al., 2004), prostate specific antigen (PSA) (Pakkala et al., 2004), heat shock protein 90 (Kim et al., 2006), and growth factors (Hetian et al., 2002).
Hybridoma technology is a well-established method for the generation of murine mAb cell lines by the fusion of splenocytes (harvested from immunized mice) with myeloma cells. The technology remains a feasible method for laboratories that implement basic cell biological research. Hybridoma technology is a comparatively simple procedure with minimal cost for the steady production of native whole immunoglobulins (Tomita and Tsumoto, 2011). Nevertheless, this technology has various limitations such as antibodies produced by the hybridoma technique are strictly murine proteins that limits their therapeutic use in humans. In addition, they also trigger human anti-mouse antibody (HAMA) responses (Tjandra et al., 1990). Moreover, indefinite production costs, low fusion efficiency, limited number of mAbs, difficulty in developing mAbs against strictly conserved and toxin antigens and time consumption are other disadvantages (Hnasko and Stanker, 2015).
The production and amplification of antibodies in vitro using bacteria by PDT has a low turnaround time compared with other methods. Additionally, the library comprises of diverse variants up to 1013, which can be selected against a varied range of biological and inorganic targets (Sblattero and Bradbury, 2000). The experimental conditions can be controlled, and the required equipment and libraries are available commercially. Disadvantages include a difficult procedure and lack of antibodies displayed on the surface of each bacteriophage yielding a small number of mAbs (Willats, 2002).
Antibody engineering is a remarkable modification technique for production of highly specific and efficient antibody products. However, antibodies are bulky macromolecules that encompass challenges in construction, optimal pharmacokinetics, manufacturing, stability, and process development. Nonetheless, progress in antibody engineering technologies such as phage display, yeast display, bacterial display, and ribosomal or cell-free display continue to advance our capacity to rapidly screen and refine stable binding immunoglobins. These engineering techniques further improve biological properties significantly in the effector domains of the mAbs (Filpula, 2007).
Immunomodulatory techniques are persistently progressing to expand the clinical efficacy of therapeutic antibodies. Cell surface antigens exhibit a wide array of targets that are overexpressed, mutated or selectively expressed, and selected for modulated antibody-based therapeutics. The technology functions through engineering alterations in antigen or receptor function, the immune system i.e., altering Fc function and T cell activation and antibody conjugated drug delivery system (DDS) targeting a specific antigen. Immunomodulatory antibodies have gained significant clinical success (Scott et al., 2012).
The Fc region is modulated by engineering the effector function, for example to increase or lessen binding to Fc gamma receptors (FcγRs) or complement factors and the half-life of IgG. The half-life can be extended by improving affinity of Fc for Fc neonatal receptor (FcRn). Moreover, it can be prolonged by engineering pH-dependent antigen binding to enhance recycling of IgG via FcRn, and effective binding to the target molecule. Engineering the Fc region permits the development of molecules that are better suited to the pharmacology activity required of them (Vincent and Zurini, 2012; Rath et al., 2015). Recently, a study investigates engineering the pH-dependent interaction between IgG and FcRn. It involves modulation of constant Fc part of monoclonal human IgG1 (hIgG1) antibodies to improve effector functions and clinical efficacy of next-generation IgG1-based therapeutics (Grevys et al., 2015).
Similarly, new opportunities have been created by the development of antibody-drug conjugates (ADCs) to treat the infectious diseases or target cancer cells. ADCs are being developed by progressing in antibody generation, selection of exceedingly cytotoxic molecules, and construction of stable linkers that can be investigated in clinical trials (Vincent and Zurini, 2012). Cytotoxic therapeutic mAbs often help target cell-killing by eliciting immune effector functions. These include antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) mediated by innate immune effector cells, and complement-dependent cytotoxicity (CDC) mediated by humoral components (Figure 6). in vitro studies, Fc engineering methods have been specifically designed to modulate ADCC, ADCP, and CDC envisioned for therapeutic mediation (Kinder et al., 2015).
Natural killer (NK) cells exhibit essential role in immunity in the context of mAb treatment by exerting direct cytotoxicity toward infected or tumor cells and contributing in modeling the adaptive response (Cheng et al., 2013). Several T- or NK-cell modulators such as ipilimumab and nivolumab were approved for the treatment of metastatic melanoma (Berman et al., 2015).
Fc-engineered antibodies improve the ADCC/ADCP potential and target CD19, CD20, CD40, and Her2. Consequently, they enhance the therapeutic potential of mAbs. NK cells are exclusive in exhibiting low-affinity activating FcγRIIIa (CD16), and no inhibitory antibody receptors, featuring a substantial role in ADCC. Several studies have established a link between activating Fc receptors and the efficacy of mAb therapy using mouse tumor models (Romain et al., 2014).
Recently, glyco-engineering technique has been used to produce recombinant therapeutic proteins with optimized efficacy, half-life, specificity, and antigenicity. Glyco-engineering of expression platforms is progressively documented as an essential approach to advance biopharmaceuticals (Ferrer-Miralles et al., 2009; Dicker and Strasser, 2015). The technique has been applied to in vivo expression systems that include mammalian cells, insect cells, yeast, and plants for the production of recombinant proteins. The underlined approaches aim at developing glycoproteins with homogeneous N- and O-linked glycans of defined composition (Dicker and Strasser, 2015). Moreover, multi-level glyco-engineering techniques have been investigated to generate IgG with defined Fc-glycans in eukaryotic cells (Dekkers et al., 2016). Additionally, E. coli expression have been successfully employed to produce recombinant human interleukin-2 (IL-2) (Kamionka, 2011).
Antibodies are engineered with superior properties such as binding affinity, stability, and catalytic activity by several other display tools (for example, yeast and bacterial display) for broad spectrum of biotechnology, medicine, and biomedical applications. Yeast surface display exhibit development of recombinant antibodies by displaying on the surface of Saccharomyces cerevisiae via genetic fusion to an abundant cell wall protein (Cherf and Cochran, 2015).
Yeast display technique has been used for engineering protein affinity, stability, and enzymatic activity. Moreover, it is extensively applied in protein epitope mapping, identification of protein-protein interactions, and uses of displayed proteins in industry and medicine (Cherf and Cochran, 2015). Several recombinant antibodies have been generated by yeast display for lethal infections such as highly pathogenic H5N1 avian influenza virus (Lei et al., 2016), cell tumor (Li et al., 2017) and human tumor endothelial marker 1 (TEM1) (Yuan et al., 2017).
Similarly, several bacterial display systems have been established for Gram-negative bacteria and Gram-positive bacteria (Lee et al., 2003). The display systems comprised of a carrier protein as an anchoring motif, a target protein, and a host strain. Proteins developed for use as anchoring motifs include outer membrane proteins, lipoproteins, autotransporters, subunits of surface appendages, and S-layer proteins (Han and Lee, 2015). Bacterial display has widespread applications including live vaccine development, screening-displayed peptide libraries, biosorbents, whole-cell biocatalysts, and biosensor development. Moreover, the promising technology is helping in the remediation of pollutants, biofuel production, and production of enantiomerically pure compounds (Han and Lee, 2015; Ramanan et al., 2016).
Ribosome display is a cell-free display system, and a technique to perform entirely in vitro selection of proteins or peptides to bind desired ligand. Ribosome display consists of both prokaryotic and eukaryotic display systems (Zahnd et al., 2007). It forms stable protein-ribosome-mRNA (PRM) complexes and links individual nascent proteins (phenotypes) to their analogous messenger RNA (mRNA) (genotypes). Ribosome display allows synchronized isolation of a functional nascent protein, through affinity for a ligand together with the encoding mRNA. The encoding mRNA is then transformed and amplified as DNA for further manipulation, including repeated cycles or protein expression. The advantages of ribosome display over other cell based methods include displaying very large libraries, generating toxic, proteolytically sensitive and unstable proteins, and incorporation of modified amino acids or mutations at distinct positions (He and Taussig, 2002; Zahnd et al., 2007). Ribosome display systems have been investigated to identify potential antigens of Clonorchis sinensis (Kasi et al., 2017), and human tumor necrosis factor α (hTNFα) for diagnosis and treatment (Zhao et al., 2016).
The large size of mAbs limits tumor penetration, and their long serum half-life is not suitable for therapy and imaging applications. Therefore, antibody fragments have been constructed in various formats as they are small, monovalent, penetrate tumor tissues efficiently, and are rapidly eliminated by renal clearance (Chames et al., 2009).
Similarly, recombinant antibodies have several advantages: (i) bacteria, yeast, plants, or animals can be used to produce antibodies, (ii) no need for immunization, and (iii) intrinsic properties (immunogenicity, binding affinity, pharmacokinetics, specificity, and stability of antibodies) can be modified easily using mutagenesis techniques. Genetically engineered antibodies have integral characteristics that suit various downstream applications or can be converted into functional whole immunoglobulins (Bradbury et al., 2003b). Antibodies exhibit strong immunity to defend against foreign antigens and non-self-agents. However, a variety of recombinant antibodies is needed to interact these hostile antigens. Over the last decade, the use of antibody engineering or recombinant antibody technology has shaped the genetic manipulation of a diverse range of antibody fragments for research, diagnosis, and therapy (Kontermann and Muller, 1999). This technology has resulted in better affinity and specificity of manipulated antibody fragments and has facilitated the replacement of hybridoma technology with various display systems for unlimited antibody production against any known antigen (Gram et al., 1992).
Conversely, engineered antibodies have various disadvantages such as they exhibit greater expense and complexity in manufacturing compared to antibodies developed by hybridoma technology (Spiess et al., 2015). Due to their foreign nature, engineered therapeutic antibodies lead to allotypic immune responses that results in rapid clearance from body by kidney, elicit on T-cell help, and have reduced antibody avidity. Moreover, engineered antibodies exhibit reduced half-life due to lack of an Fc domain and prevention of FcRn-mediated recycling. Likewise, antibody based therapies have more limitations based on the fact that many targets (sometimes in low level) have not yet been determined for various diseases (Chames et al., 2009; Attarwala, 2010).
In the 1890s, von Behring and Kitasato worked on tetanus antitoxin that lead to the development of a new discipline, immunology. They described antibodies for the first time and discovered that inactive toxins can elicit a protective immune response against active toxins in animals (Kantha, 1991). The transfusion of serum from these protected animals elicited an immune response in other animals. Therefore, antibodies were originally called “antitoxins.” Since then, antibodies have been shown to have a wider repertoire of antigen recognition. Antibodies are widely used in diagnostic tests referred as “immunoassays.” These are used to confirm diagnoses and for rapidly growing antibody based technologies (Mukherjee et al., 2012).
Similarly, antibodies have remarkable applications in the field of diagnostics, therapeutics and targeted DDS. They have been used to study various diseases such as cancer, metabolic and hormonal disorders, and infections caused by bacteria, viruses, fungi, algae, protozoa and other agents (Sundar and Prajapati, 2012). Moreover, these biomolecules have numerous application in the diagnosis of lymphoid and myeloid malignancies. Likewise, immunoglobins are used in tissue typing, ELISA, radio immunoassay, serotyping of microorganisms, immunological intervention with passive antibody, analyzing a patient's antibody profile, and antiidiotype inhibition (Siddiqui, 2010).
The detection of antibodies against infectious agents and the construction of rapid and sensitive antibody-based immunodiagnostic test kits is an important part of basic and clinical research. Assays have been developed against pathogens, toxins, and infectious proteins/peptides (Ahmad et al., 2012). The production of antibodies depends on specific protein titers, extent of immunity, and identification of B cell responses (Burbelo et al., 2010). Antibodies are routinely detected by ELISA and other immunoassays. Accessibility of full length DNA sequences is useful for the rapid and robust systematic identification of antigens by recombinant proteins using protein arrays for complete proteome analysis (Kierny et al., 2012). Furthermore, numerous novel high-throughput dominant immunoassays are used to detect antibody responses against antigens (Burbelo et al., 2010).
ELISA is the most common method for the quantitation of pathological antigens. In some cases, modified ELISA have been used in combination with various specific proteins/peptides. It is rapid, consistent, relatively easy to analyze, and adaptable to high-throughput screening (Alonso et al., 2015).
Principally, specific antibody is immobilized on high binding ELISA plates by incubation overnight at 4°C or for 1–2 h at 37°C, and then followed by 3–5 washes with PBST (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, and 0.5% Tween 20; Murayama et al., 1996). Then, plates are blocked with irrelevant protein e.g., albumin (for example, PBS containing 4–5% skimmed milk), and incubate overnight at 4°C or for 1–2 h at 37°C. After washes, samples and standard dilutions are added to the wells to be captured by bound protein, incubate for 1–2 h at 37°C and wash properly. Next, specific peroxidase-conjugated enzyme labeled detection antibody is added to the wells to enable detection of the captured protein and incubate for 1 h at 37°C. After appropriate wash, colorimetric substrate is added to the wells and incubated for 15–20 min at 37°C for the color development as catalyzed by the enzyme. For instance, addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate develops blue color that can be read by microplate plate reader at the wavelength of 562 nm. Additionally, addition of 0.16–2 M sulfuric acid (H2SO4) as a stop reaction solution develops yellow color that can be read at 405–450 nm correspondingly. In addition, the incubation conditions and reagent formulations should be preferably optimized based on the type of ELISA (Grange et al., 2014; Thiha and Ibrahim, 2015).
There are four ELISA methods. Direct ELISA (dELISA) uses competition between unwanted proteins for plastic binding spots. Antigen is attached to the solid phase followed by an enzyme-labeled antibody. This assay is used to detect various pathogenic antigens (Brasino et al., 2015).
Indirect ELISA (iELISA) uses an antigen attached to a solid phase followed by the addition of unlabeled primary antibody. Instead, a peroxidase enzyme-conjugated secondary antibody is added onto the first antibody. The iELISA is used to detect specific antibodies in sera (Peng et al., 2016).
Competitive ELISA (cELISA) is used for the detection of small molecules lacking multiple epitopes. Specific antibodies to the analyte of interest are immobilized on a micro-titer plate. Then, enzyme-conjugated antigen is incubated with capture antibody and the same antigen in its unconjugated form. This step yields a color following the addition of substrate. The signals produced are directly proportional to the quantity of conjugated enzyme bound and inversely proportional to the quantity of unconjugated antigen present (Dupont-Deshorgue et al., 2015).
Sandwich ELISA (sELISA) is used for larger proteins with multiple epitopes and two antibodies can be used consecutively. The capture antibody is immobilized on a microtiter plate. Then, unknown or known samples are added into the matrix to minimize attachment to the solid phase. Peroxidase-enzyme labeled antibody is then added for coloration, which is directly proportional to the amount of antigen present (Qu et al., 2016).
ELISA has been extensively used in the detection of various pathological antigens from viral, bacterial, fungal, protozoa, algal, and numerous other sources (Thavaselvam and Vijayaraghavan, 2010). An improved ELISA has been used for detecting anti-melanoma differentiation-associated gene 5 (MDA5) antibodies that are expressed in patients with dermatomyositis. Clinical study of this newly developed ELISA exhibited efficient detection of anti-MDA5 antibodies and showed promising potential to assist the routine clinical check of anti-MDA5 antibodies in patients who supposed to have DM (Sato et al., 2016).
Western blot assay (WBA) is also called immunoblotting. This technique is used for the determination of molecular weight and amount of relevant proteins present in a sample. Proteins in a sample are first separated by electrophoresis and then transferred to a nitrocellulose or polyvinylidene difluoride (PVDF) membrane for the detection of bound primary proteins with antibodies specific to the protein of interest (Ness et al., 2015).
Nonspecific sites in the membrane are then blocked with BSA, non-fat milk powder, or casein. Finally, a labeled secondary antibody is added for detection by chemiluminescence or fluorescence (Lewis et al., 2016). WBA has been used for the confirmation of presence of purified proteins produced against various pathological antigens such as the lethal toxin of Clostridium sordellii (Arya et al., 2013), shiga toxin Stx2f (Skinner et al., 2013), Staphylococcus aureus alpha-hemolysin (alpha-toxin) (Ladhani et al., 2001), Selenocosmia huwena huwentoxin-IV (HWTX-IV) (Yu et al., 2014), and V. parahaemolyticus thermolabile hemolysin (TLH) (Wang et al., 2015).
Dot Blot (DB) assays are used to measure protein concentrations semi-quantitatively. It is slightly different from the WBA. Proteins in the sample are not separated by electrophoresis but are spotted directly on a membrane and hybridized with an antibody probe (Emmerich and Cohen, 2015). This technique is cost effective and uses avidin-biotin technology with diaminobenzidine as a chromogen. It is used for the analysis and quantitation of 14-3-3 protein in cerebrospinal fluid (CSF) samples from cases of Creutzfeldt-Jakob disease (CJD), and for disease control of other neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) (Subramanian et al., 2016).
Immunohistochemistry (IHC) is used for the detection and identification of proteins and their localization in tissues. It is essential to retain the morphology of tissues, cells and the availability of antigen sites. Fresh, rapidly frozen tissue sections are preferably used for IHC by chemically fixing tissues in formalin and embedding in wax (Zhu et al., 2015). Additionally, fixing crosslinks amino acids in the tissue that block access to the epitope sites and prevent the action of any protein specific antibodies. The exposure of hidden epitope regions is performed by digestion with an enzyme or by heat treatment, which removes endogenous peroxidase activity and non-specific sites are blocked. A labeled antibody or an unlabeled primary antibody specific to the protein of interest is used, followed by the addition of a secondary labeled antibody specific to the primary antibody (Diorio et al., 2016).
IHC has recently been used for determination and identification of expressed proteins such as lysosome-associated protein transmembrane-4 beta (LAPTM4B) associated with the prognosis of several human malignancies (Xiao et al., 2017), glucagon-like peptide-1 (GLP-1) against renal tubular injury (Guo et al., 2017), and CD147 trans-membrane protein that induces expression and activity of matrix metalloproteinases (MMP) (Dana et al., 2016).
Immunoprecipitation (IP) is used for the study of protein-protein interactions, specific enzyme activity, posttranslational modifications, protein quantification, and determination of molecular weight of protein antigens. This technique includes antibody/antigen purification complexes at conditions that specifically bind antibodies. Rare proteins can be accumulated up to 10,000-fold by IP (Dwane and Kiely, 2011). Luciferase immunoprecipitation systems (LIPS) have been developed for the rapid detection of antibodies against peste des petits ruminants virus (PPRV) (Berguido et al., 2016), varicella-zoster virus (VZV) (Cohen et al., 2014), zinc transporter (ZnT8) autoantibodies (Ustinova et al., 2014), and pancreatic and duodenal homeobox 1 autoantibodies (PAA) (Donelan et al., 2013).
Flow cytometry (FC) is used to study antibodies on the cell surface and their related physiochemical properties. This technique was developed over 40 years ago (Robinson et al., 2012), and allows the rapid detection of various proteins on cells. Proteins produce fluorescence or scattered light when passed through the machine sensing point to generate quantitative data on a large number of cells. Labeled antibodies are used for the detection of target protein molecules or antigens on the surface of cells (Álvarez-Barrientos et al., 2000). FC has been used for the visualization of pulmonary clearance (Zhou et al., 2015), fluorescence decay lifetimes, control sorting (Houston et al., 2010), quantification of DNA-end resection in mammalian cells (Forment et al., 2012), microsphere-based protease assays, high-throughput screening (Saunders et al., 2010a), and botulinum neurotoxin type A light chain protease inhibitors (Saunders et al., 2010b).
Fluorescence Activated Cell Sorting (FACS) is used for the detection of specific cells from a mixed population of cells based on their distinctive fluorescence or light scattering characteristics (Yilmaz et al., 2010). The technique allows the isolation of cells by flow cytometry. FACS has been used to isolate high-lipid strains of Tetraselmis suecica (Montero et al., 2011), high-lipid mutants of Nannochloropsis (Doan and Obbard, 2011), E. coli O157, (Ozawa et al., 2016), high-lipid Chlamydomonas mutants (Terashima et al., 2015), and Chlorella (Manandhar-Shrestha and Hildebrand, 2013). In addition, the technology has been used for quantification of the cellular uptake of cell-penetrating peptides and mRNA (Date et al., 2014).
Enzyme linked immunospot (Elispot) assay is used for monitoring cellular immune responses in humans and other animals (Whiteside et al., 2003). It involves a polyvinylidene difluoride (PVDF) assisted microtiter plate pre-coated with antibodies specific to the antigen of interest. A capture antibody binds to the analyte of interest under precise conditions. Then, a biotinylated antibody specific to the analyte of interest is added to detect the original antibody after a wash to remove cell debris. Finally, an enzyme labeled conjugate is added after a second wash to remove unbound antibody and to visualize a colored product. The product is typically a black spot representing a single cell that produces the antigen of interest (Janetzki et al., 2005). This technique was used in the development of a coxsackievirus A16 neutralization test (Hou et al., 2015) and the determination of rotavirus infectivity (Li et al., 2014). Diagnostic applications include diagnosing sensitization to house dust mites (Chang et al., 2016), pulmonary tuberculosis (Pang et al., 2016), pleural tuberculosis (Adilistya et al., 2016), smear-negative tuberculosis (Li et al., 2015), spinal tuberculosis (Yuan et al., 2015), and cytomegalovirus infection (Nesher et al., 2016).
The lateral flow immunochromatographic test (LFT) is a simple and cost effective device used to detect the presence or absence of a target antigen. It is widely used for medical diagnostics at home (pregnancy test) or in a laboratory testing. The technology involves the transportation of fluids (e.g., urine) through capillary beds, fragments of porous paper, microstructured polymers, or sintered polymers (Hansson et al., 2016).
The technique comprises various components and steps. A sample pad acts as a sponge and absorbs the fluids. Then, fluids migrate to a conjugate pad containing protein conjugates immobilized on the surface of bio-active particles in a salt-sugar matrix that reacts with the antigen. Next, sample fluids dissolve the conjugate salt-sugar matrix and antibody-particles. The fluid mix flows through the porous structure causing the analyte to bind with particles while migrating further through the third capillary bed. Finally, there is a third capture molecule in striped areas that binds to the fluid mix containing analytes and the particle consequently changes color (Yu et al., 2012). LFTs can be used as a competitive or sandwich assay. Latex (blue color), nanometer-sized particles of gold (red color), or fluorescent or magnetic labeled particles are also used (Seydack, 2005). The technique is qualitative, nevertheless, the quantity of analyte in a sample can be measured by the intensity of the test line color. It is usually carried out by optical and non-optical lateral flow readers or biosensors (LFBs) such as complementary metal-oxide semiconductor (CMOS) or a charge-coupled device (CCD) and magnetic immunoassay (MIA) (Gui et al., 2014).
Progress in hybridoma technology and the production of highly specific mAbs has revolutionized the therapeutic use of antibodies for the diagnosis and cure of infections, the development of vaccines, antigenic characterization, and genetic manipulation. Antibodies have widespread applications in diagnostics, therapeutics and targeted DDS against potent pathogens, cancer, and physiological disorders (Tiwari et al., 2012).
They are used for the diagnosis of lymphoid and myeloid malignancies, tissue characterization, ELISA, radiolabeled immunoassay, and serotyping of microbes. In addition, they are used in the diagnosis of immunological interpolation with passive antibodies, anti-idiotype suppression, or magic bullet treatments with cytotoxic agents coupled to anti-mouse specific antibodies (Keller and Stiehm, 2000). Similarly, recombinant DNA technology (rDNA) has revolutionized the reconstruction of mAbs by genetic engineering using chimeric antibodies, humanized antibodies, CDR grafted antibodies for therapeutic use (Dimitrov and Marks, 2009; Shen et al., 2014; Fitting et al., 2015).
Molecular imaging provides a sensitive, non-invasive method for the molecular characterization of a cell surface phenotype for disease diagnosis and treatment. It is a rapidly growing multidiscipline that involves molecular biology, immunology, and medicine (Massoud and Gambhir, 2003). The therapeutic applications of antibodies include clinical diagnosis and treatment in hematology, cardiology, immunology, autoimmunity, infectious diseases, and oncology (Neves and Kwok, 2015).
MAbs can be used as molecular imaging probes for investigating cell surfaces in vivo. Coupling of cell surface targets with advances in antibody technology have facilitated the production of antibodies optimized for non-invasive imaging (Manning et al., 2008). They have numerous applications such as radioimmunoscintigraphy, antibody-based positron emission tomography (immuno-PET), and single-photon emission computed tomography (SPECT or SPET; Knowles and Wu, 2012).
Phage libraries are useful for mapping antibody epitopes, and display millions of peptides/proteins with unique random sequences. Antibodies select peptides according to the affinity of paratopes (their combining sites) from the libraries (Forsstrom et al., 2014).
Antibodies protect a host against invading microbes. These natural vaccines neutralize toxins and induce microbicidal effector functions. Antibody-mediated identification of pathogens during infection is essential for revealing immunoprotective responses in the host (Sharon et al., 2014). B cell epitope (variable region of protective antibodies in contact with infectious antigens) mapping is important for the development of effective vaccines in support of sero-diagnostics. This technique identifies protective epitopes for vaccine development and the estimation of conventional vaccines (killed or attenuated pathogens; Malito et al., 2015).
B and T cell epitope conformation is determined by pepscan technique, which has led to the development of antibody therapeutics, vaccine design, and recognition of protective antibody responses (Ahmad et al., 2016). The technique also targets pathogens with novel antimicrobials. Examples include the V-shaped Ab52 glycan epitope in the O-antigen of Francisella tularensis, the CR6261 peptidic epitope in influenza virus H1N1, and the PG16 glycopeptidic epitope in the gp120 V1/V2 loop of HIV type 1 (Sharon et al., 2014). Peptides are screened by biopanning with antibodies from the sera of various human diseases, including severe acute respiratory syndrome (SARS), human papillomavirus (HPV), and avian influenza viruses (AIV). Moreover, peptide-based antigens are also used for serological diagnosis and development of vaccines (Wu et al., 2016).
The surface plasmon resonance (SPR) technique was first used in the year 2000, for the epitope mapping of polysaccharides in L. pneumophila and the epitope mapping of various low affinity peptide/protein interactions. SPR is analogous to the working principles of the ELISA technique, but it is label free (Säfsten, 2009). This technique screens molecular interactions in real-time with complex physical principles. Binding of recombinant peptide/protein can be analyzed against natural ligands and mAbs (Säfsten, 2009). SPR was used for the epitope mapping of vitamin B12 (holo-transcobalamin), hemophilia disease, ricin toxin and the manganese transport protein C (MntC) of Staphylococcus sp. It also identified Goodpasture auto-immune disease epitopes in combination with PDT (Nguyen et al., 2014).
Phage display panning methods have failed to yield specific results because of the presence of parasitic phage clones that are often not removed by washing. To overcome these limitations, next generation sequencing (NGS) technology is used to sequence sub-libraries in biopanning experiments (Naqid et al., 2016). Next generation phage display (NGPD) can sequence 3,000 up to a million reads per panning round. This method is more rapid compared with traditional ELISA screening. NGPD has been used to identify target specific ligands by a single panning round using a library or ligand motif (Ravn et al., 2013). This technique has widespread applications for the identification of thousands of ligands and mapping antibodies in response to infectious particles. Clinical targets and antigen-related ligands have been discovered with the help of genetically encoded peptide libraries. Correspondingly, ligands are selected using NGPD by the detection of low abundant copy clones without numerous rounds of selection. Subsequently, NGS has facilitated the quantification of gene expression, genome assembly, and metagenome analysis. Additionally, it is used for PDT with the ion torrent technique (Matochko and Derda, 2015).
Antibodies are critical for immunity against infectious diseases, and extensively used for prevention and treatment of infection caused by bacteria, virus, and other infectious agents to improve public health. Emil von Behring was awarded the first Nobel Prize in Physiology or Medicine in 1901 for his discovery of serum therapy for diphtheria. This led to the term antitoxin and later named as “antikörper” translated “antibody” by Paul Erlich in a 1891 paper (Graham and Ambrosino, 2015). The emergence of recombinant technologies has revolutionized the selection, humanization, and development of therapeutic antibodies and permitted the strategic design of antibody-based elements for specificity and diversity. A number of genetically engineered mAbs have been used for the treatment of numerous infectious diseases (Hudson and Souriau, 2003).
Ebola virus disease (EVD) is a severe, often fatal, zoonotic infection documented with most recent widespread outbreak in west Africa. It is caused by a virus of the Filoviridae family (genus Ebolavirus). The disease is transmitted from human to human via contact with body fluids of the patients, the incubation period is 1–21 days and case fatality rates range from 30 to 90% (Beeching et al., 2014). A mAb named ZMapp is manufactured in the tobacco plant Nicotiana benthamiana to treat EVD patients and to improve public health. It contains a cocktail of different mAbs (MB-003, ZMab, c2G4, and c4G7) that work to avert the spread of the disease within the body (Qiu et al., 2014). A recent study investigated the treatment of EVD patient with ZMapp, a buffy coat transfusion from an Ebola survivor, and the broad-spectrum antiviral GS-5734. The patient is a first neonate documented to have successfully survived EVD (Dörnemann et al., 2017).
Therapeutic antibodies have been generated to fight off several infectious cancers. Cancer is a group of diseases that involve abnormal cell growth and proliferation caused by alterations, or mutations, in the genetic material of the cells. The cell outgrowths or lumps may be benign or metastatic (Marusyk and Polyak, 2010). Cancer outpaces the immune system, avoids detection, or blocks immune system activity. Thus, numerous therapeutic antibodies such as necitumumab, ramucirumab, GRN1005, ganitumab, cixutumumab MM-121, BIIB 033, mapatumumab, trebananib, BHQ880, and carlumab have been investigated for the successful treatment of human cancers. The malignancies include NSCLC, metastatic gastric adenocarcinoma, brain metastasis, breast, ovarian, peritoneal, fallopian tube, prostate, pancreatic, and colorectal cancers, melanoma of the eye and liver, non-Hodgkin lymphoma, and multiple myeloma (Dantas-Barbosa et al., 2012; Nixon et al., 2014).
Similarly, engineered antibodies have been used for the treatment of various types of arthritis. Arthritis (from Greek word “joint”) is a chronic multifactorial disease in which immune system attacks and starts degrading the body's joints (Persidis, 1999). Therapeutic antibodies such as adalimumab, mavrilimumab, and GSK3196165 have been developed for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis (Dantas-Barbosa et al., 2012; Nixon et al., 2014).
Therapeutic antibodies developed for the treatment of other infectious diseases include prophylaxis, anthrax, autoantibody-positive, lupus, angioedema, immune thrombocytopenic purpura, macular degeneration, hemophilia A, psoriasis, alzheimer, muscle loss and weakness, optic neuritis, ulcerative colitis, pulmonary fibrosis, and asthma (Dantas-Barbosa et al., 2012; Nixon et al., 2014).
Prognosis of a disease is a complex process that involve deduction from several observable symptoms and the choice of treatment based on therapeutic effectiveness. Antibodies, because of their exquisite specificity, and mAbs in general exhibit greater specificity. Therefore, they are used widely in a variety of assay formats, in the diagnosis and treatment of infectious diseases (Zola and Thomson, 2005). Moreover, they show rapid identification of new or rare infectious agents that is an important public health measure. Shortly, development of novel mAbs are helping to monitor and lessen the likelihoods of epidemics and other disease threats imposed on human health by prevalent infectious agents (Zola et al., 2013).
In the past few decades, antibodies have been developed by using conventional techniques such as hybridomas with significant applications in therapeutics. However, more recently, there have been advancements in recombinant technologies that have enhanced the generation of efficient immunoglobulins and their fragments. Antibody engineering offers rapid, cost-effective, and efficient biomedical tools for the discovery of high-affinity peptides/proteins, investigation of protein-protein interactions, receptor binding, and epitope identification. This powerful technology is used in a variety of systems to approach different questions from a background of cell biology and biotechnology. Moreover, it is used for the production of different types of engineered antibodies against any target molecule or highly unique conserved antigens. Additionally, it is facilitating in diagnosis and treatment of infections to improve human health. Antibody engineering possesses broad spectrum of biological, biotechnological, medical, and antibody applications for the development of novel therapeutics in various disease fields. Numerous novel therapeutic drugs have been developed by in vitro screening and selection techniques based on several high throughput immune effector functions, engineered antibodies, and high-affinity antibody fragments. This review has comprehensively described hybridoma technology, advances in antibody engineering techniques, engineered antibodies, antibody fragments, display technologies, and applications of antibodies. In conclusion, the study will help in understanding the significance of antibody fabrication approaches with extensive uses in molecular, immunological, diagnostic, imaging, biomedical, and biotechnological fields, pursuing a healthier future for humans.
AS, RW, SL, and SW designed the study. AS and SW wrote the paper and all the authors approved its submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Program from Key Scientific and Technology Project of Fujian Province of China (No. 2014YZ0001-1, 2014Y4001), and Fujian Provincial Department of Ocean and Fisheries (Fujian Ocean Hi-tech [2015] 26).
Adachi, S., Yoshimura, T., Matsuoka, T., Okada, K., Yasuda, T., and Kamei, K. (2011). Appearance of skin and nail toxicity in patients with breast cancer who underwent trastuzumab-containing chemotherapy. Gan To Kagaku Ryoho 38, 1453–1456.
Adilistya, T., Astrawinata, D. A., and Nasir, U. Z. (2016). Use of pleural fluid interferon-gamma enzyme-linked immunospot assay in the diagnosis of pleural tuberculosis. Acta Med. Indones. 48, 41–47.
Ahmad, T. A., Eweida, A. E., and Sheweita, S. A. (2016). B-cell epitope mapping for the design of vaccines and effective diagnostics. Trials Vaccinol. 5, 71–83. doi: 10.1016/j.trivac.2016.04.003
Ahmad, Z. A., Yeap, S. K., Ali, A. M., Ho, W. Y., Alitheen, N. B., and Hamid, M. (2012). scFv antibody: principles and clinical application. Clin. Dev. Immunol. 2012:980250. doi: 10.1155/2012/980250
AlDeghaither, D., Smaglo, B. G., and Weiner, L. M. (2015). Beyond peptides and mAbs–current status and future perspectives for biotherapeutics with novel constructs. J. Clin. Pharmacol. 55(Suppl. 3), S4–S20. doi: 10.1002/jcph.407
Alonso, N., Guillen, R., Chambers, J. W., and Leng, F. (2015). A rapid and sensitive high-throughput screening method to identify compounds targeting protein-nucleic acids interactions. Nucleic Acids Res. 43:e52. doi: 10.1093/nar/gkv069
Álvarez-Barrientos, A., Arroyo, J., Cantón, R., Nombela, C., and Sánchez-Pérez, M. (2000). Applications of flow cytometry to clinical microbiology. Clin. Microbiol. Rev. 13, 167–195. doi: 10.1128/CMR.13.2.167-195.2000
Andersen, M. H., Schrama, D., Thor Straten, P., and Becker, J. C. (2006). Cytotoxic T cells. J. Invest. Dermatol. 126, 32–41. doi: 10.1038/sj.jid.5700001
Apostolico Jde, S., Lunardelli, V. A., Coirada, F. C., Boscardin, S. B., and Rosa, D. S. (2016). Adjuvants: classification, modus operandi, and licensing. J. Immunol. Res. 2016:1459394. doi: 10.1155/2016/1459394
Arango Duque, G., and Descoteaux, A. (2014). Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5:491. doi: 10.3389/fimmu.2014.00491
Arya, P., Ponmariappan, S., Singh, L., and Kumar, O. (2013). Development of ELISA based detection system for lethal toxin of Clostridium sordellii. Indian J. Med. Res. 137, 1180–1187.
Askoxylakis, V., Mier, W., Zitzmann, S., Ehemann, V., Zhang, J., Kramer, S., et al. (2006). Characterization and development of a peptide (p160) with affinity for neuroblastoma cells. J. Nucl. Med. 47, 981–988.
Askoxylakis, V., Zitzmann, S., Mier, W., Graham, K., Kramer, S., von Wegner, F., et al. (2005). Preclinical evaluation of the breast cancer cell-binding peptide, p160. Clin. Cancer Res. 11, 6705–6712. doi: 10.1158/1078-0432.CCR-05-0432
Attarwala, H. (2010). Role of antibodies in cancer targeting. J. Nat. Sci. Biol. Med. 1, 53–56. doi: 10.4103/0976-9668.71675
Barbas, C. F. III., Kang, A. S., Lerner, R. A., and Benkovic, S. J. (1991). Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. U.S.A. 88, 7978–7982. doi: 10.1073/pnas.88.18.7978
Bazan, J., Calkosinski, I., and Gamian, A. (2012). Phage display–a powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum. Vaccin. Immunother. 8, 1817–1828. doi: 10.4161/hv.21703
Beeching, N. J., Fenech, M., and Houlihan, C. F. (2014). Ebola virus disease. BMJ 349:g7348. doi: 10.1136/bmj.g7348
Berguido, F. J., Bodjo, S. C., Loitsch, A., and Diallo, A. (2016). Specific detection of peste des petits ruminants virus antibodies in sheep and goat sera by the luciferase immunoprecipitation system. J. Virol. Methods 227, 40–46. doi: 10.1016/j.jviromet.2015.10.008
Berkowitz, S. A., Engen, J. R., Mazzeo, J. R., and Jones, G. B. (2012). Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat. Rev. Drug Discov. 11, 527–540. doi: 10.1038/nrd3746
Berman, D., Korman, A., Peck, R., Feltquate, D., Lonberg, N., and Canetta, R. (2015). The development of immunomodulatory monoclonal antibodies as a new therapeutic modality for cancer: the Bristol-Myers Squibb experience. Pharmacol. Ther. 148, 132–153. doi: 10.1016/j.pharmthera.2014.11.017
Bhasin, M., and Raghava, G. P. (2007). A hybrid approach for predicting promiscuous MHC class I restricted T cell epitopes. J. Biosci. 32, 31–42. doi: 10.1007/s12038-007-0004-5
Bradbury, A., Velappan, N., Verzillo, V., Ovecka, M., Chasteen, L., Sblattero, D., et al. (2003a). Antibody in proteomics I. Trends Biotechnol. 21, 275–281. doi: 10.1016/S0167-7799(03)00112-4
Bradbury, A., Velappan, N., Verzillo, V., Ovecka, M., Chasteen, L., Sblattero, D., et al. (2003b). Antibodies in proteomics II: screening, high-throughput characterization and downstream applications. Trends Biotechnol. 21, 312–317. doi: 10.1016/S0167-7799(03)00117-3
Bradbury, A. R., Sidhu, S., Dubel, S., and McCafferty, J. (2011). Beyond natural antibodies: the power of in vitro display technologies. Nat. Biotechnol. 29, 245–254. doi: 10.1038/nbt.1791
Brasino, M., Lee, J. H., and Cha, J. N. (2015). Creating highly amplified enzyme-linked immunosorbent assay signals from genetically engineered bacteriophage. Anal. Biochem. 470, 7–13. doi: 10.1016/j.ab.2014.10.006
Burbelo, P. D., Ching, K. H., Bush, E. R., Han, B. L., and Iadarola, M. J. (2010). Antibody-profiling technologies for studying humoral responses to infectious agents. Expert Rev. Vaccines 9, 567–578. doi: 10.1586/erv.10.50
Casadevall, A. (1999). Passive antibody therapies: progress and continuing challenges. Clin. Immunol. 93, 5–15. doi: 10.1006/clim.1999.4768
Catanese, M. T., Graziani, R., von Hahn, T., Moreau, M., Huby, T., Paonessa, G., et al. (2007). High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J. Virol. 81, 8063–8071. doi: 10.1128/JVI.00193-07
Chames, P., Van Regenmortel, M., Weiss, E., and Baty, D. (2009). Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharmacol. 157, 220–233. doi: 10.1111/j.1476-5381.2009.00190.x
Chan, C. E., Lim, A. P., MacAry, P. A., and Hanson, B. J. (2014). The role of phage display in therapeutic antibody discovery. Int. Immunol. 26, 649–657. doi: 10.1093/intimm/dxu082
Chang, D. K., Lin, C. T., Wu, C. H., and Wu, H. C. (2009). A novel peptide enhances therapeutic efficacy of liposomal anti-cancer drugs in mice models of human lung cancer. PLoS ONE 4:e4171. doi: 10.1371/journal.pone.0004171
Chang, D. Y., Lee, J., Choi, S. W., Lee, H. J., Kang, H., Yeo, S. C., et al. (2016). Interleukin-4 enzyme-linked immunospot assay may be useful for diagnosing sensitization to house dust mite. Int. Forum Allergy Rhinol. 6, 1007–1012. doi: 10.1002/alr.21786
Chen, W., Zhu, Z., Feng, Y., Xiao, X., and Dimitrov, D. S. (2008). Construction of a large phage-displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain. J. Mol. Biol. 382, 779–789. doi: 10.1016/j.jmb.2008.07.054
Chen, Y., Huang, X., Wang, R., Wang, S., and Shi, N. (2015). The structure of a GFP-based antibody (fluorobody) to TLH, a toxin from Vibrio parahaemolyticus. Acta Crystallogr. F Struct. Biol. Commun. 71(Pt 7), 913–918. doi: 10.1107/S2053230X15008845
Chen, Y. H., Chang, M., and Davidson, B. L. (2009). Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat. Med. 15, 1215–1218. doi: 10.1038/nm.2025
Cheng, M., Chen, Y., Xiao, W., Sun, R., and Tian, Z. (2013). NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 10, 230–252. doi: 10.1038/cmi.2013.10
Cherf, G. M., and Cochran, J. R. (2015). Applications of yeast surface display for protein engineering. Methods Mol. Biol. 1319, 155–175. doi: 10.1007/978-1-4939-2748-7_8
Committee for Medicinal Products for Human Use (CHMP) (2008). Guideline on Development, Production, Characterisation and Specification for Monoclonal Antibodies and Related Products. EMA/CHMP/BWP/532517.
Chu, X. X., Hou, M., Peng, J., Zhu, Y. Y., Ji, X. B., and Wang, L. (2006). Effects of IgG and its F(ab')2 fragments of some patients with idiopathic thrombocytopenic purpura on platelet aggregation. Eur. J. Haematol. 76, 153–159. doi: 10.1111/j.1600-0609.2005.00557.x
Cohen, J. I., Ali, M. A., Bayat, A., Steinberg, S. P., Park, H., Gershon, A. A., et al. (2014). Detection of antibodies to varicella-zoster virus in recipients of the varicella vaccine by using a luciferase immunoprecipitation system assay. Clin. Vaccine Immunol. 21, 1288–1291. doi: 10.1128/CVI.00250-14
Cooke, S. P., Boxer, G. M., Lawrence, L., Pedley, R. B., Spencer, D. I., Begent, R. H., et al. (2001). A strategy for antitumor vascular therapy by targeting the vascular endothelial growth factor: receptor complex. Cancer Res. 61, 3653–3659.
Dana, P., Kariya, R., Vaeteewoottacharn, K., Sawanyawisuth, K., Seubwai, W., Matsuda, K., et al. (2016). Upregulation of CD147 promotes metastasis of cholangiocarcinoma by modulating the epithelial-to-mesenchymal transitional process. Oncol. Res. doi: 10.3727/096504016X14813899000565. [Epub ahead of print].
Dantas-Barbosa, C., de Macedo Brigido, M., and Maranhao, A. Q. (2012). Antibody phage display libraries: contributions to oncology. Int. J. Mol. Sci. 13, 5420–5440. doi: 10.3390/ijms13055420
Date, A., Maeda, T., Watanabe, M., Hidaka, Y., Iwatani, Y., and Takano, T. (2014). An improved protocol for mRNA quantification after fluorescence-activated cell sorting with an increased signal to noise ratio in flow cytometry. Mol. Biotechnol. 56, 591–598. doi: 10.1007/s12033-014-9733-5
Dekkers, G., Plomp, R., Koeleman, C. A., Visser, R., von Horsten, H. H., Sandig, V., et al. (2016). Multi-level glyco-engineering techniques to generate IgG with defined Fc-glycans. Sci. Rep. 6:36964. doi: 10.1038/srep36964
Dicker, M., and Strasser, R. (2015). Using glyco-engineering to produce therapeutic proteins. Expert Opin. Biol. Ther. 15, 1501–1516. doi: 10.1517/14712598.2015.1069271
Dimitrov, D. S., and Marks, J. D. (2009). Therapeutic antibodies: current state and future trends–is a paradigm change coming soon? Methods Mol. Biol. 525, 1–27. doi: 10.1007/978-1-59745-554-1_1
Diorio, C., Furrer, D., Michaud, A., Laberge, S., Popa, I., Jacob, S., et al. (2016). Validation of EP1 antibody clone for estrogen receptor immunohistochemistry for breast cancer. Anticancer Res. 36, 435–437.
Doan, T. T. Y., and Obbard, J. P. (2011). Enhanced lipid production in Nannochloropsis sp. using fluorescence-activated cell sorting. GCB Bioenergy 3, 264–270. doi: 10.1111/j.1757-1707.2010.01076.x
Donelan, W., Wang, H., Li, S. W., Pittman, D., Li, Y., Han, S., et al. (2013). Novel detection of pancreatic and duodenal homeobox 1 autoantibodies (PAA) in human sera using luciferase immunoprecipitation systems (LIPS) assay. Int. J. Clin. Exp. Pathol. 6, 1202–1210.
Dong, J., Sereno, A., Aivazian, D., Langley, E., Miller, B. R., Snyder, W. B., et al. (2011). A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs 3, 273–288. doi: 10.4161/mabs.3.3.15188
Dörnemann, J., Burzio, C., Ronsse, A., Sprecher, A., De Clerck, H., Van Herp, M., et al. (2017). First newborn baby to receive experimental therapies survives ebola virus disease. J. Infect. Dis. 215, 171–174. doi: 10.1093/infdis/jiw493
Du, B., Qian, M., Zhou, Z., Wang, P., Wang, L., Zhang, X., et al. (2006). in vitro panning of a targeting peptide to hepatocarcinoma from a phage display peptide library. Biochem. Biophys. Res. Commun. 342, 956–962. doi: 10.1016/j.bbrc.2006.02.050
Dupont-Deshorgue, A., Oudart, J. B., Brassart, B., Deslee, G., Perotin, J. M., Diebold, M. D., et al. (2015). A competitive enzyme-linked immunosorbent assay for quantification of tetrastatin in body fluids and tumor extracts. Anal. Biochem. 482, 16–21. doi: 10.1016/j.ab.2015.04.023
Dwane, S., and Kiely, P. A. (2011). Tools used to study how protein complexes are assembled in signaling cascades. Bioeng. Bugs 2, 247–259. doi: 10.4161/bbug.2.5.17844
Ecker, D. M., Jones, S. D., and Levine, H. L. (2015). The therapeutic monoclonal antibody market. MAbs 7, 9–14. doi: 10.4161/19420862.2015.989042
Edwards, B. M., and He, M. (2012). Evolution of antibodies in vitro by ribosome display. Methods Mol. Biol. 907, 281–292. doi: 10.1007/978-1-61779-974-7_16
Edwards, W. B., Xu, B., Akers, W., Cheney, P. P., Liang, K., Rogers, B. E., et al. (2008). Agonist-antagonist dilemma in molecular imaging: evaluation of a monomolecular multimodal imaging agent for the somatostatin receptor. Bioconjug. Chem. 19, 192–200. doi: 10.1021/bc700291m
El Debs, B., Utharala, R., Balyasnikova, I. V., Griffiths, A. D., and Merten, C. A. (2012). Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. U.S.A. 109, 11570–11575. doi: 10.1073/pnas.1204514109
Emmerich, C. H., and Cohen, P. (2015). Optimising methods for the preservation, capture and identification of ubiquitin chains and ubiquitylated proteins by immunoblotting. Biochem. Biophys. Res. Commun. 466, 1–14. doi: 10.1016/j.bbrc.2015.08.109
Fauci, A. S., and Morens, D. M. (2012). The perpetual challenge of infectious diseases. N. Engl. J. Med. 366, 454–461. doi: 10.1056/NEJMra1108296
Ferrer-Miralles, N., Domingo-Espin, J., Corchero, J. L., Vazquez, E., and Villaverde, A. (2009). Microbial factories for recombinant pharmaceuticals. Microb. Cell Fact. 8:17. doi: 10.1186/1475-2859-8-17
Filpula, D. (2007). Antibody engineering and modification technologies. Biomol. Eng. 24, 201–215. doi: 10.1016/j.bioeng.2007.03.004
Finnern, R., Pedrollo, E., Fisch, I., Wieslander, J., Marks, J. D., Lockwood, C. M., et al. (1997). Human autoimmune anti-proteinase 3 scFv from a phage display library. Clin. Exp. Immunol. 107, 269–281. doi: 10.1111/j.1365-2249.1997.254-ce1127.x
Fitting, J., Blume, T., Ten Haaf, A., Blau, W., Gattenlohner, S., Tur, M. K., et al. (2015). Phage display-based generation of novel internalizing antibody fragments for immunotoxin-based treatment of acute myeloid leukemia. MAbs 7, 390–402. doi: 10.1080/19420862.2015.1007818
Fitting, J., Killian, D., Junghanss, C., Willenbrock, S., Murua Escobar, H., and Lange, S. (2011). Generation of recombinant antibody fragments that target canine dendritic cells by phage display technology. Vet. Comp. Oncol. 9, 183–195. doi: 10.1111/j.1476-5829.2010.00246.x
Forment, J. V., Walker, R. V., and Jackson, S. P. (2012). A high-throughput, flow cytometry-based method to quantify DNA-end resection in mammalian cells. Cytometry A 81, 922–928. doi: 10.1002/cyto.a.22155
Forsstrom, B., Axnas, B. B., Stengele, K. P., Buhler, J., Albert, T. J., Richmond, T. A., et al. (2014). Proteome-wide epitope mapping of antibodies using ultra-dense peptide arrays. Mol. Cell. Proteomics 13, 1585–1597. doi: 10.1074/mcp.M113.033308
Frenzel, A., Hust, M., and Schirrmann, T. (2013). Expression of recombinant antibodies. Front. Immunol. 4:217. doi: 10.3389/fimmu.2013.00217
Fukuda, M. N. (2012). Peptide displaying phage technology in glycobiology. Glycobiology 22, 318–325. doi: 10.1093/glycob/cwr140
Gavilondo, J. V., and Larrick, J. W. (2000). Antibody engineering at the millennium. Biotechniques 29, 128–132.
Glukhova, X. A., Prusakova, O. V., Trizna, J. A., Zaripov, M. M., Afanas'eva, G. V., Glukhov, A. S., et al. (2016). Updates on the production of therapeutic antibodies using human hybridoma technique. Curr. Pharm. Des. 22, 870–878. doi: 10.2174/1381612822666151223102845
Gothard, D., Tare, R. S., Mitchell, P. D., Dawson, J. I., and Oreffo, R. O. (2011). In search of the skeletal stem cell: isolation and separation strategies at the macro/micro scale for skeletal regeneration. Lab Chip 11, 1206–1220. doi: 10.1039/c0lc00575d
Graham, B. S., and Ambrosino, D. M. (2015). History of passive antibody administration for prevention and treatment of infectious diseases. Curr. Opin. HIV AIDS 10, 129–134. doi: 10.1097/COH.0000000000000154
Gram, H., Marconi, L. A., Barbas, C. F. III., Collet, T. A., Lerner, R. A., and Kang, A. S. (1992). In vitro selection and affinity maturation of antibodies from a naive combinatorial immunoglobulin library. Proc. Natl. Acad. Sci. U.S.A. 89, 3576–3580. doi: 10.1073/pnas.89.8.3576
Grange, R. D., Thompson, J. P., and Lambert, D. G. (2014). Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 112, 213–216. doi: 10.1093/bja/aet293
Grevys, A., Bern, M., Foss, S., Bratlie, D. B., Moen, A., Gunnarsen, K. S., et al. (2015). Fc engineering of human IgG1 for altered binding to the neonatal Fc receptor affects Fc effector functions. J. Immunol. 194, 5497–5508. doi: 10.4049/jimmunol.1401218
Gui, C., Wang, K., Li, C., Dai, X., and Cui, D. (2014). A CCD-based reader combined with CdS quantum dot-labeled lateral flow strips for ultrasensitive quantitative detection of CagA. Nanoscale Res. Lett. 9:57. doi: 10.1186/1556-276X-9-57
Guo, H., Li, H., Wang, B., Ding, W., Ling, L., Yang, M., et al. (2017). Protective effects of glucagon-like peptide-1 analog on renal tubular injury in mice on high-fat diet. Cell. Physiol. Biochem. 41, 1113–1124. doi: 10.1159/000464118
Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hamers, C., Songa, E. B., et al. (1993). Naturally occurring antibodies devoid of light chains. Nature 363, 446–448. doi: 10.1038/363446a0
Han, M. J., and Lee, S. H. (2015). An efficient bacterial surface display system based on a novel outer membrane anchoring element from the Escherichia coli protein YiaT. FEMS Microbiol. Lett. 362, 1–7. doi: 10.1093/femsle/fnu002
Hanly, W. C., Artwohl, J. E., and Bennett, B. T. (1995). Review of polyclonal antibody production procedures in mammals and poultry. ILAR J. 37, 93–118. doi: 10.1093/ilar.37.3.93
Hansson, J., Yasuga, H., Haraldsson, T., and van der Wijngaart, W. (2016). Synthetic microfluidic paper: high surface area and high porosity polymer micropillar arrays. Lab Chip 16, 298–304. doi: 10.1039/C5LC01318F
Hassan, F., Kamruzzaman, M., Mekalanos, J. J., and Faruque, S. M. (2010). Satellite phage TLCφ enables toxigenic conversion by CTX phage through dif site alteration. Nature 467, 982–985. doi: 10.1038/nature09469
He, M., and Taussig, M. J. (2002). Ribosome display: cell-free protein display technology. Brief. Funct. Genomic. Proteomic. 1, 204–212. doi: 10.1093/bfgp/1.2.204
Hendriksen, C., and Hau, J. (2003). “Production of polyclonal and monoclonal antibodies,” in Handbook of Laboratory Animal Science, 2nd Edn., eds J. Hau and S. J. Schapiro (Boca Raton, FL: CRC Press LLC), 391–411.
Hess, G. T., Cragnolini, J. J., Popp, M. W., Allen, M. A., Dougan, S. K., and Spooner, E. (2012). M13 bacteriophage display framework that allows sortase-mediated modification of surface-accessible phage proteins. Bioconjug. Chem. 23, 1478–1487. doi: 10.1021/bc300130z
Hetian, L., Ping, A., Shumei, S., Xiaoying, L., Luowen, H., Jian, W., et al. (2002). A novel peptide isolated from a phage display library inhibits tumor growth and metastasis by blocking the binding of vascular endothelial growth factor to its kinase domain receptor. J. Biol. Chem. 277, 43137–43142. doi: 10.1074/jbc.M203103200
Hnasko, R. M., and Stanker, L. H. (2015). Hybridoma technology. Methods Mol. Biol. 1318, 15–28. doi: 10.1007/978-1-4939-2742-5_2
Hou, W., Yang, L., He, D., Zheng, J., Xu, L., Liu, J., et al. (2015). Development of a coxsackievirus A16 neutralization test based on the enzyme-linked immunospot assay. J. Virol. Methods 215–216, 56–60. doi: 10.1016/j.jviromet.2015.02.010
Houston, J. P., Naivar, M. A., and Freyer, J. P. (2010). Digital analysis and sorting of fluorescence lifetime by flow cytometry. Cytometry A 77, 861–872. doi: 10.1002/cyto.a.20930
Hudson, E. P., Uhlen, M., and Rockberg, J. (2012). Multiplex epitope mapping using bacterial surface display reveals both linear and conformational epitopes. Sci. Rep. 2:706. doi: 10.1038/srep00706
Hudson, P. J., and Souriau, C. (2003). Engineered antibodies. Nat. Med. 9, 129–134. doi: 10.1038/nm0103-129
Huie, M. A., Cheung, M. C., Muench, M. O., Becerril, B., Kan, Y. W., and Marks, J. D. (2001). Antibodies to human fetal erythroid cells from a nonimmune phage antibody library. Proc. Natl. Acad. Sci. U.S.A. 98, 2682–2687. doi: 10.1073/pnas.051631798
Hust, M., and Dubel, S. (2004). Mating antibody phage display with proteomics. Trends Biotechnol. 22, 8–14. doi: 10.1016/j.tibtech.2003.10.011
Ishii, K., Lin, C., Siegel, D. L., and Stanley, J. R. (2008). Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J. Invest. Dermatol. 128, 939–948. doi: 10.1038/sj.jid.5701132
Janetzki, S., Cox, J. H., Oden, N., and Ferrari, G. (2005). Standardization and validation issues of the ELISPOT assay. Methods Mol. Biol. 302, 51–86. doi: 10.1385/1-59259-903-6:051
Jazi, M. H., Dabaghian, M., Tebianian, M., Gharagozlou, M. J., and Ebrahimi, S. M. (2012). In vivo electroporation enhances immunogenicity and protection against influenza A virus challenge of an M2e-HSP70c DNA vaccine. Virus Res. 167, 219–225. doi: 10.1016/j.virusres.2012.05.002
Jin, N., Ling, S., Yang, C., and Wang, S. (2014). Preparation and identification of monoclonal antibody against Citreoviridin and development of detection by Ic-ELISA. Toxicon 90, 226–236. doi: 10.1016/j.toxicon.2014.08.057
Jończyk, E., Kłak, M., Międzybrodzki, R., and Górski, A. (2011). The influence of external factors on bacteriophages-review. Folia Microbiol. (Praha). 56, 191–200. doi: 10.1007/s12223-011-0039-8
Kamionka, M. (2011). Engineering of therapeutic proteins production in Escherichia coli. Curr. Pharm. Biotechnol. 12, 268–274. doi: 10.2174/138920111794295693
Kantha, S. S. (1991). A centennial review; the 1890 tetanus antitoxin paper of von Behring and Kitasato and the related developments. Keio J. Med. 40, 35–39. doi: 10.2302/kjm.40.35
Kasi, D., Catherine, C., Lee, S.-W., Lee, K.-H., Kim, Y. J., Ro Lee, M., et al. (2017). Cell-free translational screening of an expression sequence tag library of Clonorchis sinensis for novel antigen discovery. Biotechnol. Prog. doi: 10.1002/btpr.2440. [Epub ahead of print].
Keller, M. A., and Stiehm, E. R. (2000). Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 13, 602–614. doi: 10.1128/CMR.13.4.602-614.2000
Kierny, M. R., Cunningham, T. D., and Kay, B. K. (2012). Detection of biomarkers using recombinant antibodies coupled to nanostructured platforms. Nano Rev. 3:17240. doi: 10.3402/nano.v3i0.17240
Kim, Y., Lillo, A. M., Steiniger, S. C., Liu, Y., Ballatore, C., Anichini, A., et al. (2006). Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry 45, 9434–9444. doi: 10.1021/bi060264j
Kinder, M., Greenplate, A. R., Strohl, W. R., Jordan, R. E., and Brezski, R. J. (2015). An Fc engineering approach that modulates antibody-dependent cytokine release without altering cell-killing functions. MAbs 7, 494–504. doi: 10.1080/19420862.2015.1022692
Klimka, A., Matthey, B., Roovers, R. C., Barth, S., Arends, J. W., Engert, A., et al. (2000). Human anti-CD30 recombinant antibodies by guided phage antibody selection using cell panning. Br. J. Cancer 83:252. doi: 10.1054/bjoc.2000.1226
Knowles, S. M., and Wu, A. M. (2012). Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J. Clin. Oncol. 30, 3884–3892. doi: 10.1200/JCO.2012.42.4887
Köhler, G., and Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497. doi: 10.1038/256495a0
Kontermann, R. E., and Brinkmann, U. (2015). Bispecific antibodies. Drug Discov. Today 20, 838–847. doi: 10.1016/j.drudis.2015.02.008
Kontermann, R. E., and Muller, R. (1999). Intracellular and cell surface displayed single-chain diabodies. J. Immunol. Methods 226, 179–188. doi: 10.1016/S0022-1759(99)00062-9
Kreitman, R. J., Tallman, M. S., Robak, T., Coutre, S., Wilson, W. H., Stetler-Stevenson, M., et al. (2012). Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J. Clin. Oncol. 30, 1822–1828. doi: 10.1200/JCO.2011.38.1756
Ladhani, S., Robbie, S., Garratt, R. C., Chapple, D. S., Joannou, C. L., and Evans, R. W. (2001). Development and evaluation of detection systems for staphylococcal exfoliative toxin A responsible for scalded-skin syndrome. J. Clin. Microbiol. 39, 2050–2054. doi: 10.1128/JCM.39.6.2050-2054.2001
Langedijk, J. P., Olijhoek, T., Schut, D., Autar, R., and Meloen, R. H. (2004). New transport peptides broaden the horizon of applications for peptidic pharmaceuticals. Mol. Divers. 8, 101–111. doi: 10.1023/B:MODI.0000025653.26130.ce
Latrofa, F., Pichurin, P., Guo, J., Rapoport, B., and McLachlan, S. M. (2003). Thyroglobulin-thyroperoxidase autoantibodies are polyreactive, not bispecific: analysis using human monoclonal autoantibodies. J. Clin. Endocrinol. Metab. 88, 371–378. doi: 10.1210/jc.2002-021073
Lee, S. Y., Choi, J. H., and Xu, Z. (2003). Microbial cell-surface display. Trends Biotechnol. 21, 45–52. doi: 10.1016/S0167-7799(02)00006-9
Leenaars, M., and Hendriksen, C. F. (2005). Critical steps in the production of polyclonal and monoclonal antibodies: evaluation and recommendations. ILAR J. 46, 269–279. doi: 10.1093/ilar.46.3.269
Leenaars, P. P. A. M., Claassen, E., and Boersma, W. J. A. (1997). Antigens and Antigen Presentation. Immunology Methods Manual. London: Academic Press Ltd.
Lei, H., Jin, S., Karlsson, E., Schultz-Cherry, S., and Ye, K. (2016). Yeast surface-displayed H5N1 avian influenza vaccines. J. Immunol. Res. 2016:4131324. doi: 10.1155/2016/4131324
Levenhagen, M. A., de Almeida Araujo Santos, F., Fujimura, P. T., Carneiro, A. P., Costa-Cruz, J. M., and Goulart, L. R. (2015). Structural and functional characterization of a novel scFv anti-HSP60 of Strongyloides sp. Sci. Rep. 5:10447. doi: 10.1038/srep10447
Lewis, C. W., Taylor, R. G., and Golsteyn, R. M. (2016). Measurement of Cdk1/Cyclin B kinase activity by specific antibodies and western blotting. Methods Mol. Biol. 1342, 337–348. doi: 10.1007/978-1-4939-2957-3_21
Li, D., Gong, R., Zheng, J., Chen, X., Dimitrov, D. S., and Zhao, Q. (2017). Engineered antibody CH2 domains binding to nucleolin: isolation, characterization and improvement of aggregation. Biochem. Biophys. Res. Commun. 485, 446–453. doi: 10.1016/j.bbrc.2017.02.058
Li, F., Hashimura, Y., Pendleton, R., Harms, J., Collins, E., and Lee, B. (2006). A systematic approach for scale-down model development and characterization of commercial cell culture processes. Biotechnol. Prog. 22, 696–703. doi: 10.1021/bp0504041
Li, F., Vijayasankaran, N., Shen, A. Y., Kiss, R., and Amanullah, A. (2010). Cell culture processes for monoclonal antibody production. MAbs 2, 466–479. doi: 10.4161/mabs.2.5.12720
Li, T., Lin, H., Yu, L., Xue, M., Ge, S., Zhao, Q., et al. (2014). Development of an enzyme-linked immunospot assay for determination of rotavirus infectivity. J. Virol. Methods 209, 7–14. doi: 10.1016/j.jviromet.2014.08.012
Li, Z., Qin, W., Li, L., Wu, Q., and Chen, X. (2015). Accuracy of bronchoalveolar lavage enzyme-linked immunospot assay to diagnose smear-negative tuberculosis: a meta-analysis. Int. J. Clin. Exp. Med. 8, 12637–12643.
Liang, S., Lin, T., Ding, J., Pan, Y., Dang, D., Guo, C., et al. (2006). Screening and identification of vascular-endothelial-cell-specific binding peptide in gastric cancer. J. Mol. Med. 84, 764–773. doi: 10.1007/s00109-006-0064-2
Lim, T. S., Mollova, S., Rubelt, F., Sievert, V., Dubel, S., Lehrach, H., et al. (2010). V-gene amplification revisited - an optimised procedure for amplification of rearranged human antibody genes of different isotypes. N. Biotechnol. 27, 108–117. doi: 10.1016/j.nbt.2010.01.001
Ling, S., Chen, Q. A., Zhang, Y., Wang, R., Jin, N., Pang, J., et al. (2015a). Development of ELISA and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody. Biosens. Bioelectron. 71, 256–260. doi: 10.1016/j.bios.2015.04.049
Ling, S., Pang, J., Yu, J., Wang, R., Liu, L., Ma, Y., et al. (2014). Preparation and identification of monoclonal antibody against fumonisin B(1) and development of detection by Ic-ELISA. Toxicon 80, 64–72. doi: 10.1016/j.toxicon.2013.12.008
Ling, S., Wang, R., Gu, X., Wen, C., Chen, L., Chen, Z., et al. (2015b). Rapid detection of fumonisin B1 using a colloidal gold immunoassay strip test in corn samples. Toxicon 108, 210–215. doi: 10.1016/j.toxicon.2015.10.014
Lipman, N. S., Jackson, L. R., Trudel, L. J., and Weis-Garcia, F. (2005). Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J. 46, 258–268. doi: 10.1093/ilar.46.3.258
Little, M., Kipriyanov, S. M., Le Gall, F., and Moldenhauer, G. (2000). Of mice and men: hybridoma and recombinant antibodies. Immunol. Today 21, 364–370. doi: 10.1016/S0167-5699(00)01668-6
Lundegaard, C., Lund, O., and Nielsen, M. (2008). Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics 24, 1397–1398. doi: 10.1093/bioinformatics/btn128
Maeda, M., Ito, Y., Hatanaka, T., Hashiguchi, S., Torikai, M., Nakashima, T., et al. (2009). Regulation of T cell response by blocking the ICOS signal with the B7RP-1-specific small antibody fragment isolated from human antibody phage library. MAbs 1, 453–461. doi: 10.4161/mabs.1.5.9633
Malito, E., Carfi, A., and Bottomley, M. J. (2015). Protein crystallography in vaccine research and development. Int. J. Mol. Sci. 16, 13106–13140. doi: 10.3390/ijms160613106
Manandhar-Shrestha, K., and Hildebrand, M. (2013). Development of flow cytometric procedures for the efficient isolation of improved lipid accumulation mutants in a Chlorella sp. microalga. J. Appl. Phycol. 25, 1643–1651. doi: 10.1007/s10811-013-0021-8
Manning, H. C., Lander, A., McKinley, E., and Mutic, N. J. (2008). Accelerating the development of novel molecular imaging probes: a role for high-throughput screening. J. Nucl. Med. 49, 1401–1404. doi: 10.2967/jnumed.108.053009
Marasco, W. A., and Sui, J. (2007). The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 25, 1421–1434. doi: 10.1038/nbt1363
Marks, J. D., Ouwehand, W. H., Bye, J. M., Finnern, R., Gorick, B. D., Voak, D., et al. (1993). Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology 11, 1145–1149. doi: 10.1038/nbt1093-1145
Marusyk, A., and Polyak, K. (2010). Tumor heterogeneity: causes and consequences. Biochim. Biophys. Acta 1805, 105–117. doi: 10.1016/j.bbcan.2009.11.002
Marvin, D. A., Symmons, M. F., and Straus, S. K. (2014). Structure and assembly of filamentous bacteriophages. Prog. Biophys. Mol. Biol. 114, 80–122. doi: 10.1016/j.pbiomolbio.2014.02.003
Massoud, T. F., and Gambhir, S. S. (2003). Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17, 545–580. doi: 10.1101/gad.1047403
Matochko, W. L., and Derda, R. (2015). Next-generation sequencing of phage-displayed peptide libraries. Methods Mol. Biol. 1248, 249–266. doi: 10.1007/978-1-4939-2020-4_17
Mazzer, A. R., Perraud, X., Halley, J., O'Hara, J., and Bracewell, D. G. (2015). Protein A chromatography increases monoclonal antibody aggregation rate during subsequent low pH virus inactivation hold. J. Chromatogr. A 1415, 83–90. doi: 10.1016/j.chroma.2015.08.068
McCullough, K. C., and Summerfield, A. (2005). Basic concepts of immune response and defense development. ILAR J. 46, 230–240. doi: 10.1093/ilar.46.3.230
McGuire, M. J., Samli, K. N., Chang, Y. C., and Brown, K. C. (2006). Novel ligands for cancer diagnosis: selection of peptide ligands for identification and isolation of B-cell lymphomas. Exp. Hematol. 34, 443–452. doi: 10.1016/j.exphem.2005.12.013
Modjtahedi, H., Ali, S., and Essapen, S. (2012). Therapeutic application of monoclonal antibodies in cancer: advances and challenges. Br. Med. Bull. 104, 41–59. doi: 10.1093/bmb/lds032
Montero, M., Aristizabal, M., and Reina, G. (2011). Isolation of high-lipid content strains of the marine microalga Tetraselmis suecica for biodiesel production by flow cytometry and sing-cell sorting. J. Appl. Phycol. 23, 1053–1057. doi: 10.1007/s10811-010-9623-6
Morens, D. M., and Fauci, A. S. (2013). Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 9:e1003467. doi: 10.1371/journal.ppat.1003467
Mukherjee, J., Tremblay, J. M., Leysath, C. E., Ofori, K., Baldwin, K., Feng, X., et al. (2012). A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS ONE 7:e29941. doi: 10.1371/journal.pone.0029941
Murayama, O., Nishida, H., and Sekiguchi, K. (1996). Novel peptide ligands for integrin α6β1 selected from a phage display library. J. Biochem. 120, 445–451. doi: 10.1093/oxfordjournals.jbchem.a021431
Naqid, I. A., Owen, J. P., Maddison, B. C., Spiliotopoulos, A., Emes, R. D., Warry, A., et al. (2016). Mapping polyclonal antibody responses to bacterial infection using next generation phage display. Sci. Rep. 6:24232. doi: 10.1038/srep24232
Nelson, A. L., Dhimolea, E., and Reichert, J. M. (2010). Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 9, 767–774. doi: 10.1038/nrd3229
Nesher, L., Shah, D. P., Ariza-Heredia, E. J., Azzi, J. M., Siddiqui, H. K., Ghantoji, S. S., et al. (2016). Utility of the enzyme-linked immunospot interferon-gamma-release assay to predict the risk of cytomegalovirus infection in hematopoietic cell transplant recipients. J. Infect. Dis. 213, 1701–1707. doi: 10.1093/infdis/jiw064
Ness, T. L., Robinson, R. L., Mojadedi, W., Peavy, L., and Weiland, M. H. (2015). A streamlined Western blot exercise: an efficient and greener approach in the laboratory classroom. Biochem. Mol. Biol. Educ. 43, 358–365. doi: 10.1002/bmb.20876
Neuberger, M., and Bruggemann, M. (1997). Monoclonal antibodies. Mice perform a human repertoire. Nature 386, 25–26. doi: 10.1038/386025a0
Neves, H., and Kwok, H. F. (2015). Recent advances in the field of anti-cancer immunotherapy. BBA Clin. 3, 280–288. doi: 10.1016/j.bbacli.2015.04.001
Nguyen, P. C., Lewis, K. B., Ettinger, R. A., Schuman, J. T., Lin, J. C., and Healey, J. F. (2014). High-resolution mapping of epitopes on the C2 domain of factor VIII by analysis of point mutants using surface plasmon resonance. Blood Coagul. Fibrinol. 123, 2732–2739. doi: 10.1182/blood-2013-09-527275
Nixon, A. E., Sexton, D. J., and Ladner, R. C. (2014). Drugs derived from phage display: from candidate identification to clinical practice. MAbs 6, 73–85. doi: 10.4161/mabs.27240
Okamoto, T., Mukai, Y., Yoshioka, Y., Shibata, H., Kawamura, M., Yamamoto, Y., et al. (2004). Optimal construction of non-immune scFv phage display libraries from mouse bone marrow and spleen established to select specific scFvs efficiently binding to antigen. Biochem. Biophys. Res. Commun. 323, 583–591. doi: 10.1016/j.bbrc.2004.08.131
O'Nuallain, B., Allen, A., Ataman, D., Weiss, D. T., Solomon, A., and Wall, J. S. (2007). Phage display and peptide mapping of an immunoglobulin light chain fibril-related conformational epitope. Biochem. Mol. Biol. Educ. 46, 13049–13058. doi: 10.1021/bi701255m
Ozawa, S., Okabe, S., and Ishii, S. (2016). Specific single-cell isolation of Escherichia coli O157 from environmental water samples by using flow cytometry and fluorescence-activated cell sorting. Foodborne Pathog. Dis. 13, 456–461. doi: 10.1089/fpd.2016.2125
Pakkala, M., Jylhäsalmi, A., Wu, P., Leinonen, J., Stenman, U. H., and Santa, H. (2004). Conformational and biochemical analysis of the cyclic peptides which modulate serine protease activity. J. Pept. Sci. 10, 439–447. doi: 10.1002/psc.557
Pang, C., Wu, Y., Wan, C., Shen, K., Hu, Y., Yang, T., et al. (2016). Accuracy of the bronchoalveolar lavage enzyme-linked immunospot assay for the diagnosis of pulmonary tuberculosis: a meta-analysis. Medicine (Baltimore). 95:e3183. doi: 10.1097/MD.0000000000003183
Payne, A. S., Ishii, K., Kacir, S., Lin, C., Li, H., Hanakawa, Y., et al. (2005). Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J. Clin. Invest. 115, 888–899. doi: 10.1172/JCI24185
Pazgier, M., Liu, M., Zou, G., Yuan, W., Li, C., Li, C., et al. (2009). Structural basis for high-affinity peptide inhibition of p53 interactions with MDM2 and MDMX. Proc. Natl. Acad. Sci. U.S.A. 106, 4665–4670. doi: 10.1073/pnas.0900947106
Peng, D., Yang, B., Pan, Y., Wang, Y., Chen, D., Liu, Z., et al. (2016). Development and validation of a sensitive monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for the determination of the aflatoxin M1 levels in milk. Toxicon 113, 18–24. doi: 10.1016/j.toxicon.2016.02.006
Petrenko, V. A., and Vodyanoy, V. J. (2003). Phage display for detection of biological threat agents. J. Microbiol. Methods 53, 253–262. doi: 10.1016/S0167-7012(03)00029-0
Qi, W. H., and Wang, M. P. (2004). Size and shape dependent melting temperature of metallic nanoparticles. Mater. Chem. Phys. 88, 280–284. doi: 10.1016/j.matchemphys.2004.04.026
Qiu, X., Wong, G., Audet, J., Bello, A., Fernando, L., Alimonti, J. B., et al. (2014). Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53. doi: 10.1038/nature13777
Qu, H., Wang, X., Qu, B., Kong, H., Zhang, Y., Shan, W., et al. (2016). Sandwich enzyme-linked immunosorbent assay for naringin. Anal. Chim. Acta 903, 149–155. doi: 10.1016/j.aca.2015.09.058
Rader, C., and Barbas, C. F. III. (1997). Phage display of combinatorial antibody libraries. Curr. Opin. Biotechnol. 8, 503–508. doi: 10.1016/S0958-1669(97)80075-4
Ramanan, R., Kim, B. H., Cho, D. H., Oh, H. M., and Kim, H. S. (2016). Algae-bacteria interactions: evolution, ecology and emerging applications. Biotechnol. Adv. 34, 14–29. doi: 10.1016/j.biotechadv.2015.12.003
Ramos-Vara, J. A., and Miller, M. A. (2014). When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry–the red, brown, and blue technique. Vet. Pathol. 51, 42–87. doi: 10.1177/0300985813505879
Rapin, N., Lund, O., Bernaschi, M., and Castiglione, F. (2010). Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE 5:e9862. doi: 10.1371/journal.pone.0009862
Rasmussen, U. B., Schreiber, V., Schultz, H., Mischler, F., and Schughart, K. (2002). Tumor cell-targeting by phage-displayed peptides. Cancer Gene Ther. 9, 606–612. doi: 10.1038/sj.cgt.7700476
Rath, T., Baker, K., Dumont, J. A., Peters, R. T., Jiang, H., Qiao, S. W., et al. (2015). Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit. Rev. Biotechnol. 35, 235–254. doi: 10.3109/07388551.2013.834293
Ravn, U., Didelot, G., Venet, S., Ng, K. T., Gueneau, F., Rousseau, F., et al. (2013). Deep sequencing of phage display libraries to support antibody discovery. Methods 60, 99–110. doi: 10.1016/j.ymeth.2013.03.001
Redman, J. M., Hill, E. M., AlDeghaither, D., and Weiner, L. M. (2015). Mechanisms of action of therapeutic antibodies for cancer. Mol. Immunol. 67(2 Pt A), 28–45. doi: 10.1016/j.molimm.2015.04.002
Reichert, J. M. (2013). Which are the antibodies to watch in 2013? MAbs 5, 1–4. doi: 10.4161/mabs.22976
Robinson, J. P., Rajwa, B., Patsekin, V., and Davisson, V. J. (2012). Computational analysis of high-throughput flow cytometry data. Expert Opin. Drug Discov. 7, 679–693. doi: 10.1517/17460441.2012.693475
Robinson, P., Stuber, D., Deryckère, F., Tedbury, P., Lagrange, M., and Orfanoudakis, G. (2005). Identification using phage display of peptides promoting targeting and internalization into HPV-transformed cell lines. J. Mol. Recogn. 18, 175–182. doi: 10.1002/jmr.723
Rodrigo, G., Gruvegård, M., and Van Alstine, J. M. (2015). Antibody fragments and their purification by protein l affinity chromatography. Antibodies 4, 259–277. doi: 10.3390/antib4030259
Romain, G., Senyukov, V., Rey-Villamizar, N., Merouane, A., Kelton, W., Liadi, I., et al. (2014). Antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood 124, 3241–3249. doi: 10.1182/blood-2014-04-569061
Roque, A. C., Lowe, C. R., and Taipa, M. A. (2004). Antibodies and genetically engineered related molecules: production and purification. Biotechnol. Prog. 20, 639–654. doi: 10.1021/bp030070k
Rosano, G. L., and Ceccarelli, E. A. (2014). Recombinant protein expression in Escherichia coli: advances and challenges. Front. Microbiol. 5:172. doi: 10.3389/fmicb.2014.00172
Roth, Z., Yehezkel, G., and Khalaila, I. (2012). Identification and quantification of protein glycosylation. Int. J. Carbohydr. Chem. 2012, 1–10. doi: 10.1155/2012/640923
Rothe, A., Hosse, R. J., and Power, B. E. (2006). in vitro display technologies reveal novel biopharmaceutics. FASEB J. 20, 1599–1610. doi: 10.1096/fj.05-5650rev
Saeed, A. F., and Wang, S. (2016). In vitro improved production of monoclonal antibody against domoic acid in supplemented cell culture media. Sci. Int. 28, 1197–1203.
Saeed, A. F. U. H., and Awan, S. A. (2016). Advances in monoclonal antibodies production and cancer therapy. MOJ Immunol. 3:00099.
Säfsten, P. (2009). Epitope mapping by surface plasmon resonance. Methods Mol. Biol. 524, 67–76. doi: 10.1007/978-1-59745-450-6_5
Sato, S., Murakami, A., Kuwajima, A., Takehara, K., Mimori, T., Kawakami, A., et al. (2016). Clinical utility of an enzyme-linked immunosorbent assay for detecting anti-melanoma differentiation-associated gene 5 autoantibodies. PLoS ONE 11:e0154285. doi: 10.1371/journal.pone.0154285
Saunders, M. J., Edwards, B. S., Zhu, J., Sklar, L. A., and Graves, S. W. (2010a). Microsphere-based flow cytometry protease assays for use in protease activity detection and high-throughput screening. Curr. Protoc. Cytom. Chapter 13, Unit 13.12. 1–17. doi: 10.1002/0471142956.cy1312s54
Saunders, M. J., Graves, S. W., Sklar, L. A., Oprea, T. I., and Edwards, B. S. (2010b). High-throughput multiplex flow cytometry screening for botulinum neurotoxin type a light chain protease inhibitors. Assay Drug Dev. Technol. 8, 37–46. doi: 10.1089/adt.2009.0219
Sblattero, D., and Bradbury, A. (2000). Exploiting recombination in single bacteria to make large phage antibody libraries. Nat. Biotechnol. 18, 75–80. doi: 10.1038/71958
Schumacher, T. N., and Schreiber, R. D. (2015). Neoantigens in cancer immunotherapy. Science 348, 69–74. doi: 10.1126/science.aaa4971
Scott, A. M., Wolchok, J. D., and Old, L. J. (2012). Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287. doi: 10.1038/nrc3236
Sen, S., and Roychoudhury, P. K. (2013). Development of optimal medium for production of commercially important monoclonal antibody 520C9 by hybridoma cell. Cytotechnology 65, 233–252. doi: 10.1007/s10616-012-9480-z
Seydack, M. (2005). Nanoparticle labels in immunosensing using optical detection methods. Biosens. Bioelectron. 20, 2454–2469. doi: 10.1016/j.bios.2004.11.003
Shade, K., and Anthony, R. (2013). Antibody glycosylation and inflammation. Antibodies 2:392. doi: 10.3390/antib2030392
Sharon, J., Rynkiewicz, M. J., Lu, Z., and Yang, C. Y. (2014). Discovery of protective B-cell epitopes for development of antimicrobial vaccines and antibody therapeutics. Immunology 142, 1–23. doi: 10.1111/imm.12213
Shen, D., Tang, Y., Li, S., Xu, W., and Zhang, L. (2014). Successful construction and massive expression of a novel Anti-CD19 human-mouse chimeric antibody Hm2E8b. Monoclon. Antib. Immunodiagn. Immunother. 33, 215–220. doi: 10.1089/mab.2013.0079
Siddiqui, M. Z. (2010). Monoclonal antibodies as diagnostics; an appraisal. Indian J. Pharm. Sci. 72, 12–17. doi: 10.4103/0250-474X.62229
Siegel, D., Loftu, J. C., and McMillan, R. (2003). Characterization of an ITP patient-derived anti-alpha(2b)beta(3) monoclonal antibody that inhibits platelet aggregation. Blood 102, 87A-A.
Skinner, C., Patfield, S., Stanker, L., and He, X. (2013). Development of monoclonal antibodies and immunoassays for sensitive and specific detection of Shiga toxin Stx2f. PLoS ONE 8:e76563. doi: 10.1371/journal.pone.0076563
Smith, G. P. (1985). Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228, 1315–1317. doi: 10.1126/science.4001944
Sonoda, H., Kumada, Y., Katsuda, T., and Yamaji, H. (2011). Effects of cytoplasmic and periplasmic chaperones on secretory production of single-chain Fv antibody in Escherichia coli. J. Biosci. Bioeng. 111, 465–470. doi: 10.1016/j.jbiosc.2010.12.015
Soria-Guerra, R. E., Nieto-Gomez, R., Govea-Alonso, D. O., and Rosales-Mendoza, S. (2015). An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J. Biomed. Inform. 53, 405–414. doi: 10.1016/j.jbi.2014.11.003
Spiess, C., Zhai, Q., and Carter, P. J. (2015). Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol. Immunol. 67(2 Pt A), 95–106. doi: 10.1016/j.molimm.2015.01.003
Stassen, A. P., Folmer, R. H., Hilbers, C. W., and Konings, R. N. (1994). Single-stranded DNA binding protein encoded by the filamentous bacteriophage M13: structural and functional characteristics. Mol. Biol. Rep. 20, 109–127. doi: 10.1007/BF00990543
Steplewski, Z., Thurin, M., and Kieber-Emmons, T. (2015). Antibodies: at the nexus of antigens and cancer vaccines. J. Infect. Dis. 212(Suppl. 1), S59–S66. doi: 10.1093/infdis/jiu638
Subramanian, S., Mahadevan, A., Satishchandra, P., and Shankar, S. K. (2016). Development of a dot blot assay with antibodies to recombinant “core” 14-3-3 protein: evaluation of its usefulness in diagnosis of Creutzfeldt-Jakob disease. Ann. Indian Acad. Neurol. 19, 205–210. doi: 10.4103/0972-2327.176867
Sundar, S., and Prajapati, V. K. (2012). Drug targeting to infectious diseases by nanoparticles surface functionalized with special biomolecules. Curr. Med. Chem. 19, 3196–3202. doi: 10.2174/092986712800784630
Tang, S., and Hewlett, I. (2010). Nanoparticle-based immunoassays for sensitive and early detection of HIV-1 capsid (p24) antigen. J. Infect. Dis. 201(Suppl. 1), S59–S64. doi: 10.1086/650386
Tao, Y., Pinzi, V., Bourhis, J., and Deutsch, E. (2007). Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway–therapeutic perspectives in cancer. Nat. Clin. Pract. Oncol. 4, 591–602. doi: 10.1038/ncponc0934
Terashima, M., Freeman, E. S., Jinkerson, R. E., and Jonikas, M. C. (2015). A fluorescence-activated cell sorting-based strategy for rapid isolation of high-lipid Chlamydomonas mutants. Plant J. 81, 147–159. doi: 10.1111/tpj.12682
Thanongsaksrikul, J., and Chaicumpa, W. (2011). Botulinum neurotoxins and botulism: a novel therapeutic approach. Toxins (Basel). 3, 469–488. doi: 10.3390/toxins3050469
Thavaselvam, D., and Vijayaraghavan, R. (2010). Biological warfare agents. J. Pharm. Bioallied Sci. 2, 179–188. doi: 10.4103/0975-7406.68499
Thiha, A., and Ibrahim, F. (2015). A colorimetric enzyme-linked immunosorbent assay (ELISA) detection platform for a point-of-care dengue detection system on a lab-on-compact-disc. Sensors (Basel). 15, 11431–11441. doi: 10.3390/s150511431
Tiwari, G., Tiwari, R., Sriwastawa, B., Bhati, L., Pandey, S., Pandey, P., et al. (2012). Drug delivery systems: an updated review. Int. J. Pharm. Investig. 2, 2–11. doi: 10.4103/2230-973X.96920
Tjandra, J. J., Ramadi, L., and McKenzie, I. F. (1990). Development of human anti-murine antibody (HAMA) response in patients. Immunol. Cell Biol. 68(Pt 6), 367–376. doi: 10.1038/icb.1990.50
Tomita, M., and Tsumoto, K. (2011). Hybridoma technologies for antibody production. Immunotherapy 3, 371–380. doi: 10.2217/imt.11.4
Tonelli, R. R., Colli, W., and Alves, M. J. (2012). Selection of binding targets in parasites using phage-display and aptamer libraries in vivo and in vitro. Front. Immunol. 3:419. doi: 10.3389/fimmu.2012.00419
Ustinova, J., Zusinaite, E., Utt, M., Metskula, K., Reimand, K., Huchaiah, V., et al. (2014). Development of a luciferase-based system for the detection of ZnT8 autoantibodies. J. Immunol. Methods 405, 67–73. doi: 10.1016/j.jim.2014.01.009
Vincent, K. J., and Zurini, M. (2012). Current strategies in antibody engineering: Fc engineering and pH-dependent antigen binding, bispecific antibodies and antibody drug conjugates. Biotechnol. J. 7, 1444–1450. doi: 10.1002/biot.201200250
Vithayathil, R., Hooy, R. M., Cocco, M. J., and Weiss, G. A. (2011). The scope of phage display for membrane proteins. J. Mol. Biol. 414, 499–510. doi: 10.1016/j.jmb.2011.10.021
Wang, R., Fang, S., Wu, D., Lian, J., Fan, J., Zhang, Y., et al. (2012). Screening for a single-chain variable-fragment antibody that can effectively neutralize the cytotoxicity of the Vibrio parahaemolyticus thermolabile hemolysin. Appl. Environ. Microbiol. 78, 4967–4975. doi: 10.1128/AEM.00435-12
Wang, R., Gu, X., Zhuang, Z., Zhong, Y., Yang, H., and Wang, S. (2016). Screening and molecular evolution of a single chain variable fragment antibody (scFv) against citreoviridin toxin. J. Agric. Food Chem. 64, 7640–7648. doi: 10.1021/acs.jafc.6b02637
Wang, R., Huang, A., Liu, L., Xiang, S., Li, X., Ling, S., et al. (2014a). Construction of a single chain variable fragment antibody (scFv) against tetrodotoxin (TTX) and its interaction with TTX. Toxicon 83, 22–34. doi: 10.1016/j.toxicon.2014.02.021
Wang, R., Xiang, S., Feng, Y., Srinivas, S., Zhang, Y., Lin, M., et al. (2013). Engineering production of functional scFv antibody in E. coli by co-expressing the molecule chaperone Skp. Front. Cell. Infect. Microbiol. 3:72. doi: 10.3389/fcimb.2013.00072
Wang, R., Xiang, S., Zhang, Y., Chen, Q., Zhong, Y., and Wang, S. (2014b). Development of a functional antibody by using a green fluorescent protein frame as the template. Appl. Environ. Microbiol. 80, 4126–4137. doi: 10.1128/AEM.00936-14
Wang, R., Zhong, Y., Gu, X., Yuan, J., Saeed, A. F., and Wang, S. (2015). The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front. Microbiol. 6:144. doi: 10.3389/fmicb.2015.00144
Wang, S. H., Du, X. Y., Huang, Y. M., Lin, D. S., Hart, P. L., and Wang, Z. H. (2007). Detection of deoxynivalenol based on a single chain fragment variable(scFv) of the antideoxynivalenol antibody. FEMS Microbiol. Lett. 272, 214–219. doi: 10.1111/j.1574-6968.2007.00765.x
Wang, S. H., Zhang, J. B., Zhang, Z. P., Zhou, Y. F., Yang, R. F., Chen, J., et al. (2006). Construction of single chain variable fragment (ScFv) and BiscFv-alkaline phosphatase fusion protein for detection of Bacillus anthracis. Anal. Chem. 78, 997–1004. doi: 10.1021/ac0512352
Wang, W., Xu, R., and Li, J. (2010). Production of native bispecific antibodies in rabbits. PLoS ONE 5:e10879. doi: 10.1371/journal.pone.0010879
Watkins, N. A., Armour, K. L., Smethurst, P. A., Metcalfe, P., Scott, M. L., and Hughes, D. L. (1999). Rapid phenotyping of HPA-1a using either diabody-based hemagglutination or recombinant IgG1-based assays. Transfusion 39, 781–789. doi: 10.1046/j.1537-2995.1999.39070781.x
Weiner, L. M., and Adams, G. P. (2000). New approaches to antibody therapy. Oncogene 19, 6144–6151. doi: 10.1038/sj.onc.1204000
Whiteside, T. L., Zhao, Y., Tsukishiro, T., Elder, E. M., Gooding, W., and Baar, J. (2003). Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin. Cancer Res. 9, 641–649.
Wilde, H., Thipkong, P., Sitprija, V., and Chaiyabutr, N. (1996). Heterologous antisera and antivenins are essential biologicals: perspectives on a worldwide crisis. Ann. Intern. Med. 125, 233–236. doi: 10.7326/0003-4819-125-3-199608010-00012
Willats, W. G. (2002). Phage display: practicalities and prospects. Plant Mol. Biol. 50, 837–854. doi: 10.1023/A:1021215516430
Wright, M. J., and Deonarain, M. P. (2007). Phage display of chelating recombinant antibody libraries. Mol. Immunol. 44, 2860–2869. doi: 10.1016/j.molimm.2007.01.026
Wu, C., Lo, S. L., Boulaire, J., Hong, M. L., Beh, H. M., and Leung, D. S. (2008). A peptide-based carrier for intracellular delivery of proteins into malignant glial cells in vitro. J. Control. Release 130, 140–145. doi: 10.1016/j.jconrel.2008.05.015
Wu, C. H., Liu, I. J., Lu, R. M., and Wu, H. C. (2016). Advancement and applications of peptide phage display technology in biomedical science. J. Biomed. Sci. 23:8. doi: 10.1186/s12929-016-0223-x
Xiao, M., Yang, S., Meng, F., Qin, Y., Yang, Y., Jia, S., et al. (2017). LAPTM4B predicts axillary lymph node metastasis in breast cancer and promotes breast cancer cell aggressiveness in vitro. Cell. Physiol. Biochem. 41, 1072–1082. doi: 10.1159/000464115
Yau, K. Y., Lee, H., and Hall, J. C. (2003). Emerging trends in the synthesis and improvement of hapten-specific recombinant antibodies. Biotechnol. Adv. 21, 599–637. doi: 10.1016/S0734-9750(03)00104-6
Yilmaz, S., Haroon, M. F., Rabkin, B. A., Tyson, G. W., and Hugenholtz, P. (2010). Fixation-free fluorescence in situ hybridization for targeted enrichment of microbial populations. ISME J. 4, 1352–1356. doi: 10.1038/ismej.2010.73
Yu, A., Shang, J., Cheng, F., Paik, B. A., Kaplan, J. M., Andrade, R. B., et al. (2012). Biofunctional paper via covalent modification of cellulose. Langmuir 28, 11265–11273. doi: 10.1021/la301661x
Yu, H., Liu, H., Yan, Y., Duan, Z., and Wang, X. (2014). Detection and identification of huwentoxin-IV interacting proteins by biotin-avidin chemistry combined with mass spectrometry. J. Venom. Anim. Toxins Incl. Trop. Dis. 20:18. doi: 10.1186/1678-9199-20-18
Yuan, J., Zhuang, C., Fang, S., Zhuang, Z., Ling, S., and Wang, S. (2012). A monoclonal antibody against F1-F0 ATP synthase beta subunit. Hybridoma 31, 352–357. doi: 10.1089/hyb.2012.0033
Yuan, K., Liang, D., Wu, X. Q., Yao, Z. S., Jin, D. X., Yang, Z. D., et al. (2015). Diagnostic value of enzyme-linked immunospot assay using CFP10/ESAT6 fusion protein as antigen in spinal tuberculosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 37, 44–49. doi: 10.3881/j.issn.1000-503X.2015.01.008
Yuan, X., Yang, M., Chen, X., Zhang, X., Sukhadia, S., Musolino, N., et al. (2017). Characterization of the first fully human anti-TEM1 scFv in models of solid tumor imaging and immunotoxin-based therapy. Cancer Immunol. Immunother. 66, 367–378. doi: 10.1007/s00262-016-1937-z
Yuasa, N., Koyama, T., and Fujita-Yamaguchi, Y. (2014). Purification and refolding of anti-T-antigen single chain antibodies (scFvs) expressed in Escherichia coli as inclusion bodies. Biosci. Trends 8, 24–31. doi: 10.5582/bst.8.24
Zahnd, C., Amstutz, P., and Pluckthun, A. (2007). Ribosome display: selecting and evolving proteins in vitro that specifically bind to a target. Nat. Methods 4, 269–279. doi: 10.1038/nmeth1003
Zhang, C. (2012). Hybridoma technology for the generation of monoclonal antibodies. Methods Mol. Biol. 901, 117–135. doi: 10.1007/978-1-61779-931-0_7
Zhao, X. L., Tian, L. F., Zhang, S. J., Li, J. M., Feng, H., Wang, L. M., et al. (2016). Novel human three-domain antibody fragments against sTNFalpha as well as tmTNFalpha with high affinity generated by the combination of ribosome display and E. coli expression system. Scand. J. Immunol. 83, 267–278. doi: 10.1111/sji.12417
Zhou, H., Gunsten, S. P., Zhegalova, N. G., Bloch, S., Achilefu, S., Christopher Holley, J., et al. (2015). Visualization of pulmonary clearance mechanisms via noninvasive optical imaging validated by near-infrared flow cytometry. Cytometry A 87, 419–427. doi: 10.1002/cyto.a.22658
Zhu, S., Schuerch, C., and Hunt, J. (2015). Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch. Pathol. Lab. Med. 139, 55–66. doi: 10.5858/arpa.2014-0167-RA
Zhuang, Z. H., Que, S. J., Gao, Y. M., Yuan, J., Ye, Z., Du, M., et al. (2014). Artificial antigen synthesis and the development of polyclonal antibody-based immunoassay for citreoviridin determination. World J. Microbiol. Biotechnol. 30, 343–349. doi: 10.1007/s11274-013-1431-0
Zitzmann, S., Kramer, S., Mier, W., Hebling, U., Altmann, A., Rother, A., et al. (2007). Identification and evaluation of a new tumor cell-binding peptide, FROP-1. J. Nucl. Med. 48, 965–972. doi: 10.2967/jnumed.106.036699
Zitzmann, S., Mier, W., Schad, A., Kinscherf, R., Askoxylakis, V., Kramer, S., et al. (2005). A new prostate carcinoma binding peptide (DUP-1) for tumor imaging and therapy. Clin. Cancer Res. 11, 139–146.
Zohreh, J., and Hossein, S. (2008). The autoimmune diseases manifested by production of autoantibodies: the autoantigens identified by random peptide library. Iran. J. Allergy Asthma Immunol. 7, 115–131. doi: 10.07.03/ijaai.115131
Zola, H., and Thomson, P. R. (2005). “Monoclonal antibodies: diagnostic uses,” in eLS (John Wiley & Sons, Ltd.). doi: 10.1038/npg.els.0004019
Zola, H., Mohandas, A. P., and Krumbiegel, D. (2013). “Monoclonal antibodies: diagnostic uses,” in eLS (Chichester: John Wiley & Sons Ltd.). doi: 10.1002/9780470015902.a0002177.pub3. Available online at: http://www.els.net
Keywords: antibody engineering, hybridoma technology, antibody fragments, scFv, phage display technology, immunomodulation, immunology
Citation: Saeed AFUH, Wang R, Ling S and Wang S (2017) Antibody Engineering for Pursuing a Healthier Future. Front. Microbiol. 8:495. doi: 10.3389/fmicb.2017.00495
Received: 26 January 2017; Accepted: 09 March 2017;
Published: 28 March 2017.
Edited by:
Kuldeep Dhama, Indian Veterinary Research Institute, IndiaReviewed by:
Maryam Dadar, Razi Vaccine and Serum Research Institute, IranCopyright © 2017 Saeed, Wang, Ling and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihua Wang, d3NoeXlsQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.