- 1Genome Pharmaceuticals Institute Co. Ltd., Tokyo, Japan
- 2Laboratory of Microbiology, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan
- 3Department of Microbiology and Immunology, Teikyo University School of Medicine, Tokyo, Japan
- 4Teikyo University Institute of Medical Mycology, Tokyo, Japan
Lactic acid bacteria (LAB) have high immune system-stimulating activity and are considered beneficial for human health as probiotics in the gut. The innate immune system is highly conserved between mammals and insects. Microbe-associated molecular patterns (e.g., peptidoglycan and β-glucan) induce cytokine maturation, which, in silkworm larvae, leads to muscle contraction. The purpose of this study is to find a novel probiotic by using silkworm muscle contraction assay. In the present study, we isolated LAB derived from rice bran pickles. We selected highly active LAB to activate the innate immune system of the silkworm, which was assayed based on silkworm muscle contraction. Of various LAB, L. paraplantarum 11-1 strongly stimulated innate immunity in the silkworm, leading to stronger silkworm contraction than a dairy-based LAB. Silkworms fed a diet containing L. paraplantarum 11-1 exhibited tolerance against the pathogenicity of Pseudomonas aeruginosa. These findings suggest that L. paraplantarum 11-1 could be a useful probiotic for activating innate immunity.
Introduction
Innate immunity is highly conserved between invertebrates and vertebrates. In mammals, dendritic cells and macrophages produce cytokines in response to microbial pathogens (Janeway and Medzhitov, 2002), whereas in insects, hemocytes and fat bodies recognize microbial pathogens and induce an anti-microbial response (Brennan and Anderson, 2004). Especially in silkworms, immune cells produce reactive oxygen species to activate proteases, resulting in cytokine release. Our group discovered an active cytokine, paralytic peptide, that induces muscle contraction in silkworms (Ishii et al., 2008). We used silkworm muscle specimens to screen for innate immunity-activating substances and found that lactic acid bacteria (LAB) strongly induce silkworm muscle contraction (Dhital et al., 2011; Fujiyuki et al., 2012; Nishida et al., 2016). This screening method has several advantages compared with conventional screening using mammalian innate immune cells such as macrophages. First, the silkworm does not respond to lipopolysaccharides, which often produces a false-positive response in mammalian macrophages. Second, insect whole body assays reflect the absorption, distribution, metabolism, excretion, and toxicity factors that govern the therapeutic effects of medicines. Therefore, substances with less effective pharmacokinetics/pharmacodynamics and/or are toxic in the silkworm muscle contraction assay would be excluded by these tests.
LAB are traditionally used for fermenting foods, dairy products, and probiotics. LAB are Gram-positive, catalase-negative, form no spores, and are immotile. Foods fermented with LAB could be beneficial for human health by activating innate immunity (Ichikawa et al., 2012; Kawashima et al., 2013). In our previous report, we isolated a dairy-based LAB to activate the innate immune system in the silkworm (Nishida et al., 2016). LAB isolated from dairy products have been characterized well, whereas LAB from fermented pickles have not. The purpose of this study is to find a novel probiotic to activate the innate immune system in the silkworm by screening LAB from non-dairy products, such as fermented pickles.
In this work, we isolated LAB from rice bran pickles and Korean pickles (kimchi). We evaluated the innate-immunity stimulating activity of LAB in silkworms. We selected a highly active LAB based on the results of the silkworm muscle contraction assay. Isolated L. paraplantarum 11-1 exhibited high activity in the silkworm contraction assay. Silkworms that ingested an artificial diet containing L. paraplantarum 11-1 exhibited tolerance against the pathogenicity of Pseudomonas aeruginosa. To the best of our knowledge, this is the first report of a probiotic effect of L. paraplantarum against P. aeruginosa infection. This LAB might be valuable as a probiotic for activating innate immunity. The infection model used in this study has the potential to be used to study a novel probiotic against P. aeruginosae.
Materials and Methods
Materials
Gifu Anaerobic Medium (GAM) broth and GAM agar were purchased from Nissui (Tokyo, Japan). MRS broth and MRS agar were purchased from Becton Dickinson (Franklin Lakes, NJ, USA). CaCO3-MRS agar was prepared by adding CaCO3 (final concentration: 1%, Wako, Osaka, Japan) to the MRS agar after autoclaving. An AnaeroPak (Mitsubishi Gas Chemicals, Tokyo, Japan) was used for anaerobic culturing on agar plates. Saline was prepared as 0.9% NaCl (Wako, Osaka, Japan). Lysogeny broth (LB) medium was prepared with 1% bacto tryptone (Becton Dickinson, Franklin Lakes, NJ, USA), 0.5% bacto yeast extract (Becton Dickinson, Franklin Lakes, NJ, USA), and 1% NaCl (Wako, Osaka, Japan). An LB agar plate was prepared with LB medium containing 1.5% (w/v) agar (Nacalai Tesque, Kyoto, Japan).
DNA Sequencing
Fragments containing 16S rDNA were amplified with polymerase chain reaction using KOD FX Neo (Toyobo, Tokyo, Japan) with primers 9F and 1541R (Hashimoto et al., 2007). The DNA sequences were determined with direct sequencing, BigDye Terminator v3.1 Cycle Sequencing Kit and ABI PRISM 3100 Genetic Analyzer (ThermoFisher Applied Biosystems, Foster City, CA, USA). Sequences were analyzed with the NCBI BLASTN 2.2.27+ (Zhang et al., 2000), 16S ribosomal RNA sequences database (Bacteria and Archaea 7545 sequences). DNA sequences are currently in preparation for submission to the GenBank.
Characterization of LAB
Fluid from the pickles was spread on MRS agar. After incubation at 30°C for 2 days, white colonies appeared on each plate. Isolated bacteria were Gram-stained with Gram-color (Merck, Kenilworth, NJ, USA). For scanning electron microscopy (SEM), bacterial cells were pre-fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2), post-fixed with 1% osmium tetroxide in the same buffer, and freeze-dried in t-butyl alcohol. The sample was examined with a field-emission SEM (JSM- 7500F, JEOL, Japan). The bacterial colony was suspended in 3% H2O2 for a catalase test. Staphylococcus aureus RN4220 and Escherichia coli JM109 were used as controls. Other identification kits, Api Zym and Api 50 CHL, were purchased from bioMérieux (Marcy l'Etoile, France), and the data were analyzed using the Api web v5.1 database (bioMérieux, Marcy l'Etoile, France).
Silkworm Muscle Contraction Assay
Silkworm muscle contraction was measured as previously reported (Ishii et al., 2008). Briefly, autoclaved bacterial suspension (50 μl) was injected into a silkworm muscle specimen. The contraction value was determined as (prelength-postlength)/prelength. The sample amount (mg) that induced a contraction value of 0.15 was defined as 1 unit.
Silkworm Infection Model
The silkworm infection model was described previously (Hamamoto et al., 2004). Pathogens used in the infection model were P. aeruginosa PAO1 (Stover et al., 2000) and methicillin-sensitive S. aureus 1 (MSSA1) (Akimitsu et al., 1999) from our laboratory stock. Pathogenic bacteria grown in LB medium overnight were diluted with saline (0.9% NaCl) and injected into fifth instar larva (n = 7) fed overnight. Survival of silkworm larva was counted for 5 days.
Statistical Analysis
Statistical analysis was performed with Microsoft Excel 2007 for Windows (Redmond, WA, USA) and Excel Statistics 2008 (Social Survey Research Information, Tokyo, Japan). Survival plots were generated by the Kaplan-Meier method and analyzed by the log-rank test. Activity was compared with the Mann-Whitney U-test. Differences having a P < 0.05 were considered to be statistically significant. Survival curve was plotted using Kaleidagraph 4.1.4 (Synergy Software, Reading, PA, USA) and LD50 values were determined from fitting curve of the logistic equation, y = a + (b − a)/(1 + (x/c)∧d), y is the fraction of larva killed, x is the number of viable cells injected, c is LD50, a, b and d is the constant of fitting curve. Curve fitting was applied with Levenberg-Marquardt algolism.
Results
Isolation and Characterization of LAB
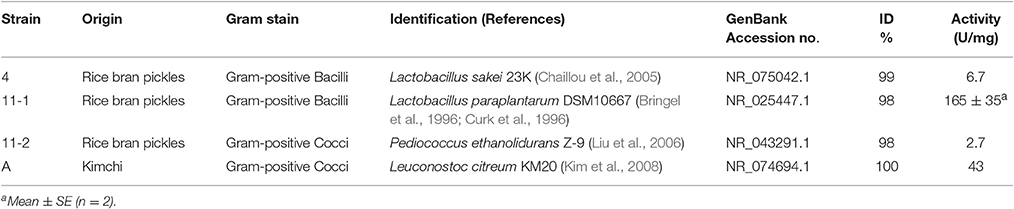
We selected Gram-positive bacteria on MRS agar, as LAB generally recognized as safe are Gram-positive bacteria. Colonies were re-streaked on CaCO3-MRS medium to confirm lactic-acid fermentation. Lactate-fermenting and Gram-positive bacteria were subjected to the silkworm contraction assay. LAB tested for the contraction assay was sequenced for 16S rDNA (Table 1).
In order to investigate morphological and ultrastructural appearance of isolated bacteria, we performed Gram staining and SEM. Gram stains of LAB displayed a Gram-positive bacillus with homogeneous morphology (Figure 1). SEM revealed that LAB was characterized as a rod shaped bacterium (Figure 2).
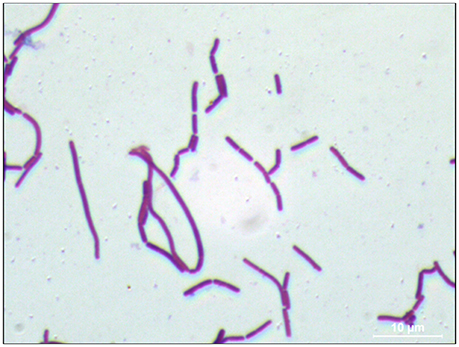
Figure 1. Gram staining of L. paraplantarum 11-1. An L. paraplantarum 11-1 colony on CaCO3-MRS agar was Gram-stained (Merck). The image was captured with a charge-coupled device camera (Hamamatsu Photonics) using an Olympus phase-contrast microscope at 1,000× magnification.
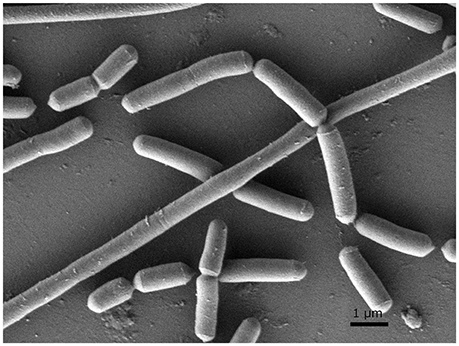
Figure 2. Scanning electron micrograph (SEM) of L. paraplantarum 11-1. The sample was examined with a field-emission SEM (JSM- 7500F, JEOL, Japan). The sample was depicted at 10,000× magnification.
The silkworm muscle contraction activity of LAB is shown in Table 1. LAB isolated from pickles showed diverse activity. L. paraplantarum 11-1 exhibited the highest activity, 165 U/mg, which was higher than that of the LAB in a previous report from our laboratory (Nishida et al., 2016). The activity of the other isolated LABs was as follows: Lactobacillus sakei 4, 6.7 U/mg; Pediococcus ethanolidurans 11-2, 2.7 U/mg; and Leuconostoc citreum A, 43 U/mg.
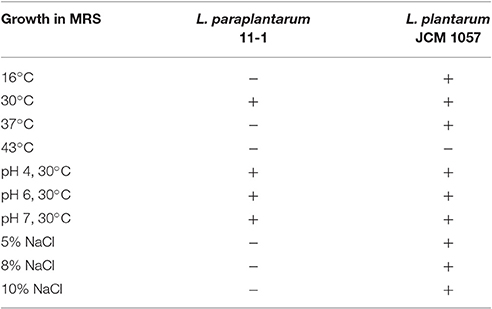
BLAST analysis of the 16S rDNA sequence of the 11-1 strain revealed that the 11-1 strain had 98% homology with L. paraplantarum DSM10667 (NR_025447.1) and L. plantarum WCFS1 (NR_075041.1). Strain 11-1 was more similar to L. paraplantarum DSM10667 than L. plantarum WCFS1. Therefore, we identified the 11-1 strain as L. paraplantarum. We then determined the growth characteristics of L. paraplantarum 11-1. L. paraplantarum 11-1 had strict temperature sensitivity as it grew in MRS medium at 30°C, but not at 16° or 37°C (Table 2). Growth of L. paraplantarum 11-1 in MRS medium was sensitive to salt higher than 5% NaCl in the medium and resistant to acidic conditions (pH 4.0). In contrast, L. plantarum JCM1057 grew at a wide-range of temperatures (16°, 30°, and 37°C) and its growth was resistant to salt (5, 8, and 10% NaCl).
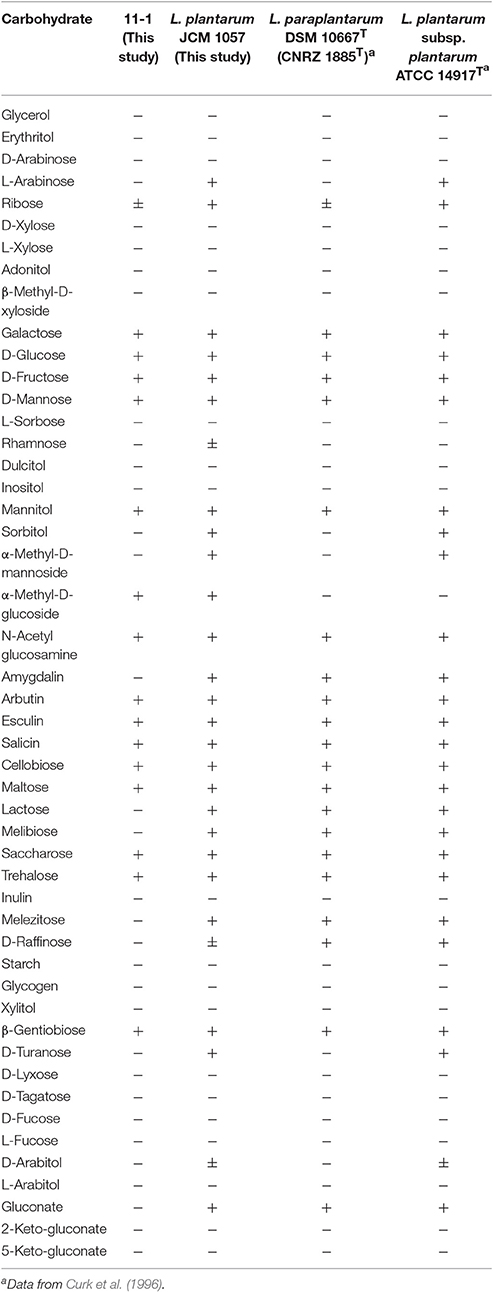
We next examined the ability of L. paraplantarum 11-1 to ferment carbohydrates using Api 50 CHL (Table 3). L. paraplantarum 11-1 exhibited different characteristics from L. paraplantarum DSM10667 (CNRZ 1885T) in 5 of 49 sugars and derivatives utilized. Amygdalin, lactose, melibiose, melezitose, and gluconate were not utilized by L. paraplantarum 11-1, whereas α-methyl-D-glucoside was utilized. On the other hand, L. plantarum JCM1057 utilized three different sugars than plant-derived L. plantarum ATCC14917T (Bringel et al., 1996; Curk et al., 1996). The carbohydrate fermentation scores of L. paraplantarum 11-1 matched 90.5% of those of Carnobacterium maltaromaticum in the Api web v5.1 database, whereas those of L. plantarum JCM1057 matched 99.2% of those of L. plantarum 1. The possibility that strain 11-1 was C. maltaromaticum was excluded due to the low similarity of the 16S rDNA sequences. The 16S rDNA sequence of strain 11-1 exhibited 98% similarity with L. paraplantarum DSM10667 (NR_025447.1) and 91% similarity with C. maltaromaticum DSM 20342 (NR_044710.2).
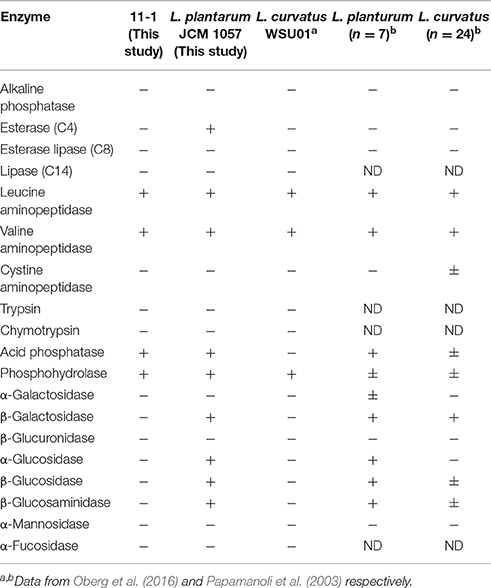
We also examined enzymatic characteristics of strain 11-1 using the Api Zym test and compared them with those of other related strains (Curk et al., 1996; Oberg et al., 2016) (Table 4). The enzymatic characteristics data of L. paraplantarum are unavailable, and therefore we compared the data of strain 11-1 and control strain L. plantarum JCM 1057, and previously reported data of L. plantarum and L. curvatus. L. paraplantarum 11-1 exhibited different activities (acid phosphatase) of 19 enzymes compared with L. curvatus WSU01. In contrast, L. paraplantarum 11-1 exhibited 5 different activities (esterase (C4), β-galactosidase, α-glucosidase, β-glucosidase, β-glucosaminidase) of 19 enzymes compared with L. plantarum JCM 1057. The enzymatic characteristics of L. paraplantarum 11-1 were more similar to those of L. curvatus WSU01 than L. plantarum JCM 1057.
Probiotic Effect of L. paraplantarum 11-1
To evaluate the probiotic effect of L. paraplantarum 11-1, we used the silkworm infection model (Figure 3). Injection of P. aeruginosa into silkworm larvae had time-dependent and dose-dependent silkworm killing effects. Silkworms were fed a diet containing L. paraplantarum 11-1 viable cells without apparent problems. Feeding silkworms a diet containing L. paraplantarum 11-1 viable cells increased the number of animals that survived after injection of P. aeruginosa, resulting in a ~100-fold higher LD50. We then tested the infection model with the Gram-positive bacteria S. aureus (Figure 4). Feeding the silkworm a diet containing L. paraplantarum 11-1 viable cells increased the number of animals that survived after injection of MSSA, resulting in a ~2-fold higher LD50.
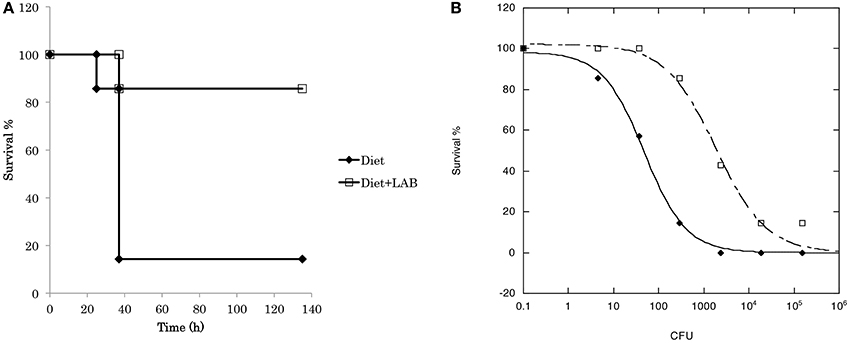
Figure 3. Probiotic effect of L. paraplantarum 11-1 on P. aeruginosa infection. (A) Time course of survival of silkworms fed a diet with or without L. paraplantarum 11-1 viable cells (1 × 107 cfu/larva) after P. aeruginosa PAO1 infection. Survival of silkworms fed a diet with L. paraplantarum 11-1 was significantly higher than that of silkworms fed a normal diet (p = 0.038). (B) Dose response of P. aeruginosa PAO1 on silkworm survival after 2 days. P. aeruginosa PAO1 was injected into 5th instar larva fed a diet with or without L. paraplantarum 11-1 viable cells.
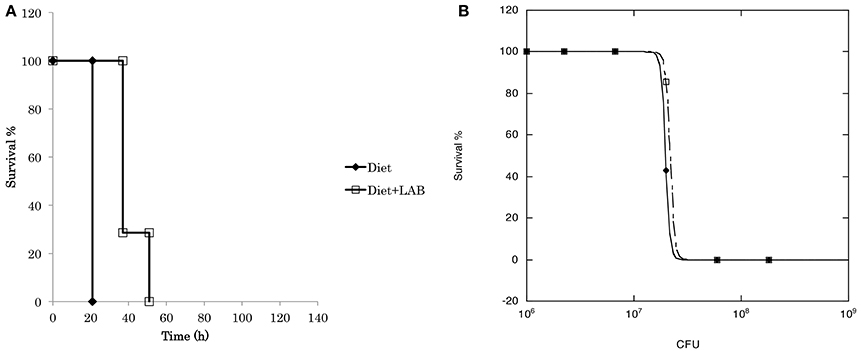
Figure 4. Probiotic effect of L. paraplantarum 11-1 on S. aureus infection. (A) Time course of survival of silkworms fed a diet with or without L. paraplantarum 11-1 viable cells (1 × 107 cfu/larva) after S. aureus MSSA1 infection. Survival of silkworms fed a diet with L. paraplantarum 11-1 was significantly higher than that of silkworms fed a normal diet (p = 0.030). (B) Dose response of S. aureus MSSA1 on silkworm survival after 2 days. S. aureus MSSA1 was injected into 5th instar larva fed a diet with or without L. paraplantarum 11-1 viable cells.
Discussion
Isolation of LAB with High Innate Immunity-Stimulating Activity
In general, LAB are thought to be beneficial for human health as probiotics in the gut. LAB were recently reported to have high immunity stimulating activity (Ichikawa et al., 2012; Kawashima et al., 2013). Pickles are a food that is fermented with LAB (Hammes, 2009). Fermented-vegetable foods such as pickles are potential sources of non-dairy LAB. In this study, we isolated LAB from pickles by streaking samples of pickle fluid on MRS agar, a selection plate for LAB (de Man et al., 1960). We confirmed the Gram-positive feature of the isolated bacteria and its lactic acid production on CaCO3-MRS agar, in which lactic acid solubilizes CaCO3 to form a transparent zone around the colony. Gram staining and SEM characterized LAB as a Gram-positive bacillus and a rod shaped bacterium respectively. (Figures 1, 2). Pleomorphism, defined as variation in size or shape of a bacterial cell, is described for different Lactobacillicae in response to the absence of deoxyribosides, vitamin B12, or divalent cation. Culture broth composition is correlated with the morphology of L. acidophilus NCFM (Senz et al., 2015). Different sizes in microscopy morphology might depend on nutrient composition in culture medium. Next, we determined the 16S rDNA sequences of each isolate. The identity of strain 11-1 as L. paraplantarum was based on 98% similarity between strain 11-1 and L. paraplantarum DSM10667 (NR_025447.1).
L. plantarum and L. paraplantarum are closely related and heterofermentive. L. paraplantarum is isolated from beer and human feces. L. plantarum is a nonpathogenic LAB colonizing in fermented foods and in the human mouth and gut. Therefore, L. plantarum is normal human gut microbiota (Bernardeau et al., 2008; Hammes, 2009). The L. plantarum WCFS1 genome was sequenced and a recombinant DNA technique was established to construct a strain expressing a specific antigen (Grangette et al., 2001; Seegers, 2002; Kleerebezem et al., 2003; Wells and Mercenier, 2008; Siezen et al., 2012).
Innate-Immunity Activation in Silkworms
Multiple studies demonstrate the validity of silkworm contraction assay for innate immune activation (Ishii et al., 2008; Dhital et al., 2011; Fujiyuki et al., 2012). We determined the activity of LAB to stimulate innate immunity in silkworms using a muscle contraction assay (Ishii et al., 2008). When L. paraplantarum 11-1 was injected into the silkworm body fluid, the insect cytokine paralytic peptide was activated upon innate immune stimulation, resulting in silkworm muscle contraction. Compared with a conventional method using macrophages, the muscle contraction assay does not require cell culture and is insensitive to lipopolysaccharides, which often cause a false-positive response in test samples. We isolated several LABs that exhibited a variety of muscle contraction activities (Nishida et al., 2016). Among them, L. paraplantarum 11-1 exhibited the highest activity (Supplemental Figure 1).
Silkworm Acquired Tolerance to Bacterial Infection by Ingesting L. paraplantarum 11-1
Use of silkworms as a surrogate animal for animal tests poses the fewer ethical, financial, and logistical problem than mammalian tests. In addition, silkworms are large enough to inject a precise amount of samples compared to other insect (Sekimizu et al., 2012). The infection model described here also has the potential to be used to study novel probiotics for in vivo activity against P. aeruginosae.
We have used the silkworm as a surrogate animal to test the probiotic effect of LAB (Nishida et al., 2016). Silkworms were fed a diet containing LAB. Silkworms fed LAB exhibited tolerance to the lethality of P. aeruginosa and S. aureus infections. Our previous results demonstrated that silkworms acquire tolerance to P. aeruginosa infection by ingesting a diet containing Lactococcus lactis or peptidoglycans of Lactobacillus plantarum (Miyashita et al., 2015; Nishida et al., 2016). In this study, we demonstrated that ingesting L. paraplantarum 11-1 extended the survival of silkworm after infection with P. aeruginosa. These findings suggest that activation of the innate immune system induced tolerance against microbial infection.
LAB in dairy and fermented products are expected to benefit human health. Reports on the probiotic effects of LAB in animal infection models, however, are limited. Oral administration of heat-killed L. casei protects against P. aeruginosa infection in mice (Miake et al., 1985; Setoyama et al., 1985). Bifidobacterium longum prevents P. aeruginosa gut-derived sepsis in a mouse model (Matsumoto et al., 2008). Bifidobacterium protects germ-free mice from E. coli O157 infection (Fukuda et al., 2011). Our data indicate that the silkworm is a useful model animal for evaluating the probiotic effects of LAB. P. aeruginosa is the most commonly isolated antibiotic-resistant Gram-negative bacteria in ventilator-assisted pneumonia. Oral administration of a probiotic delays respiratory tract colonization and infection by P. aeruginosa in human (Forestier et al., 2008). Searching novel probiotics would be matching potential medical needs. Further study on the prevention of P. aeruginosa in silkworm infection model would be required for the translation of probiotics to benefit human health.
Ethics Statement
This study was exempted by The University of Tokyo Life Science Research Ethics and Safety Committee, and Teikyo University Animal Ethics Committee.
Author Contributions
SN designed and conducted the experiments, and performed data analysis. MI and YN conducted the experiment of Gram stain and SEM. SN and KS wrote the manuscript. MI, YN, SA, and YO reviewed and edited the manuscript. SN, YO, and KS revised the manuscript.
Conflict of Interest Statement
KS is a consultant for Genome Pharmaceuticals Institute Co. Ltd.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a grant from Genome Pharmaceuticals Institute Co. Ltd. We thank other members of the Laboratory of Microbiology at the University of Tokyo for helpful discussions. We especially thank Dr. Kataoka, Mr. Yamashita, and Ms. Hashimoto at Genome Pharmaceuticals Institute Co. Ltd. for experimental help.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00436/full#supplementary-material
References
Akimitsu, N., Hamamoto, H., Inoue, R., Shoji, M., Akamine, A., Takemori, K., et al. (1999). Increase in resistance of methicillin-resistant Staphylococcus aureus to beta-lactams caused by mutations conferring resistance to benzalkonium chloride, a disinfectant widely used in hospitals. Antimicrob. Agents. Chemother. 43, 3042–3043.
Bernardeau, M., Vernoux, J. P., Henri-Dubernet, S., and Guéguen, M. (2008). Safety assessment of dairy microorganisms: the Lactobacillus genus. Int. J. Food Microbiol. 126, 278–285. doi: 10.1016/j.ijfoodmicro.2007.08.015
Brennan, C. A., and Anderson, K. V. (2004). Drosophila: The genetics of innate immune recognition and response. Annu. Rev. Immunol. 22, 457–483. doi: 10.1146/annurev.immunol.22.012703.104626
Bringel, F., Curk, M. C., and Hubert, J. C. (1996). Characterization of lactobacilli by southern-type hybridization with a Lactobacillus plantarum pyrDFE probe. Int. J. Syst. Bacteriol. 46, 588–594. doi: 10.1099/00207713-46-2-588
Chaillou, S., Champomier-Vergès, M. C., Cornet, M., Crutz-Le Coq, A. M., Dudez, A. M., Martin, V., et al. (2005). The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 23, 1527–1533. doi: 10.1038/nbt1160
Curk, M. C., Hubert, J. C., and Bringel, F. (1996). Lactobacillus paraplantarum sp. nov., a new species related to Lactobacillus plantarum. Int. J. Syst. Bacteriol. 46, 595–598. doi: 10.1099/00207713-46-2-595
de Man, J. D., Rogosa, M., and Sharpe, M. E. (1960). A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 23, 130–135. doi: 10.1111/j.1365-2672.1960.tb00188.x
Dhital, S., Hamamoto, H., Urai, M., Ishii, K., and Sekimizu, K. (2011). Purification of innate immunostimulant from green tea using a silkworm muscle contraction assay. Drug Discov. Ther. 5, 18–25. doi: 10.5582/ddt.v5.1.18
Forestier, C., Guelon, D., Cluytens, V., Gillart, T., Sirot, J., and De Champs, C. (2008). Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit. Care. 12, R69. doi: 10.1186/cc6907
Fujiyuki, T., Hamamoto, H., Ishii, K., Urai, M., Kataoka, K., Takeda, T., et al. (2012). Evaluation of innate immune stimulating activity of polysaccharides using a silkworm (Bombyx mori) muscle contraction assay. Drug Discov. Ther. 6, 88–93. doi: 10.5582/ddt.2012.v6.2.88
Fukuda, S., Toh, H., Hase, K., Oshima, K., Nakanishi, Y., Yoshimura, K., et al. (2011). Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 469, 543–547. doi: 10.1038/nature09646
Grangette, C., Müller-Alouf, H., Goudercourt, D., Geoffroy, M. C., Turneer, M., and Mercenier, A. (2001). Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect. Immun. 69, 1547–1553. doi: 10.1128/IAI.69.3.1547-1553.2001
Hamamoto, H., Kurokawa, K., Kaito, C., Kamura, K., Manitra Razanajatovo, I., Kusuhara, H., et al. (2004). Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents Chemother. 48, 774–779. doi: 10.1128/AAC.48.3.774-779.2004
Hammes, W. P. (2009). “Lactobacillus,” in Bergey's Manual of Systematics of Archaea and Bacteria, eds W. B. Whitman, P. DeVos, J. Chun, S. Dedysh, B. Hedlund, P. Kämpfer, F. Rainey, and M. Trujillo (Hoboken, NJ: John Wiley and Sons, Inc.), 1–21. doi: 10.1002/9781118960608.gbm00604
Hashimoto, M., Taguchi, T., Nishida, S., Ueno, K., Koizumi, K., Aburada, M., et al. (2007). Isolation of 8′-phosphate ester derivatives of amicoumacins: structure-activity relationship of hydroxy amino acid moiety. J. Antibiot. 60, 752–756. doi: 10.1038/ja.2007.99
Ichikawa, S., Miyake, M., Fujii, R., and Konishi, Y. (2012). MyD88 associated ROS generation is crucial for Lactobacillus induced IL-12 production in macrophage. PLoS ONE 7:e35880. doi: 10.1371/journal.pone.0035880
Ishii, K., Hamamoto, H., Kamimura, M., and Sekimizu, K. (2008). Activation of the silkworm cytokine by bacterial and fungal cell wall components via a reactive oxygen species-triggered mechanism. J. Biol. Chem. 283, 2185–2191. doi: 10.1074/jbc.M705480200
Janeway, C. A. Jr., and Medzhitov, R. (2002). Innate immune recognition. Annu. Rev. Immunol. 20, 197–216. doi: 10.1146/annurev.immunol.20.083001.084359
Kawashima, T., Kosaka, A., Yan, H., Guo, Z., Uchiyama, R., Fukui, R., et al. (2013). Double-Stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity. 38, 1187–1197. doi: 10.1016/j.immuni.2013.02.024
Kim, J. F., Jeong, H., Lee, J. S., Choi, S. H., Ha, M., Hur, C. G., et al. (2008). Complete genome sequence of Leuconostoc citreum KM20. J. Bacteriol. 190, 3093–3094. doi: 10.1128/JB.01862-07
Kleerebezem, M., Boekhorst, J., van Kranenburg, R., Molenaar, D., Kuipers, O. P., Leer, R., et al. (2003). Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. U.S.A. 100, 1990–1995. doi: 10.1073/pnas.0337704100
Liu, L., Zhang, B., Tong, H., and Dong, X. (2006). Pediococcus ethanolidurans sp. nov., isolated from the walls of a distilled-spirit-fermenting cellar. Int. J. Syst. Evol. Microbiol. 56, 2405–2408. doi: 10.1099/ijs.0.64407-0
Matsumoto, T., Ishikawa, H., Tateda, K., Yaeshima, T., Ishibashi, N., and Yamaguchi, K. (2008). Oral administration of Bifidobacterium longum prevents gut-derived Pseudomonas aeruginosa sepsis in mice. J. Appl. Microbiol. 104, 672–680. doi: 10.1111/j.1365-2672.2007.03593.x
Miake, S., Nomoto, K., Yokokura, T., Yoshikai, Y., Mutai, M., and Nomoto, K. (1985). Protective effect of Lactobacillus casei on Pseudomonas aeruginosa infection in mice. Infect. Immun. 48, 480–485.
Miyashita, A., Takahashi, S., Ishii, K., Sekimizu, K., and Kaito, C. (2015). Primed immune responses triggered by ingested bacteria lead to systemic infection tolerance in silkworms PLoS ONE 10:e0130486. doi: 10.1371/journal.pone.0130486
Nishida, S., Ono, Y., and Sekimizu, K. (2016). Lactic acid bacteria activating innate immunity improve survival in bacterial infection model of silkworm. Drug Discov. Ther. 10, 49–56. doi: 10.5582/ddt.2016.01022
Oberg, C. J., Oberg, T. S., Culumber, M. D., Ortakci, F., Broadbent, J. R., and McMahon, D. J. (2016). Lactobacillus wasatchensis sp. nov., a non-starter lactic acid bacteria isolated from aged Cheddar cheese. Int. J. Syst. Evol. Microbiol. 66, 158–164. doi: 10.1099/ijsem.0.000689
Papamanoli, E., Tzanetakis, N., Litopoulou-Tzanetaki, E., and Kotzekidou, P. (2003). Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 65, 859–867. doi: 10.1016/S0309-1740(02)00292-9
Seegers, J. F. (2002). Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol. 20, 508–515. doi: 10.1016/S0167-7799(02)02075-9
Sekimizu, N., Paudel, A., and Hamamoto, H. (2012). Animal welfare and use of silkworm as a model animal. Drug Discov. Ther. 6, 226–229. doi: 10.5582/ddt.2012.v6.4.226
Senz, M., van Lengerich, B., Bader, J., and Stahl, U. (2015). Control of cell morphology of probiotic Lactobacillus acidophilus for enhanced cell stability during industrial processing. Int. J. Food Microbiol. 192, 34–42. doi: 10.1016/j.ijfoodmicro.2014.09.015
Setoyama, T., Nomoto, K., Yokokura, T., and Mutai, M. (1985). Protective effect of lipoteichoic acid from Lactobacillus casei and Lactobacillus fermentum against Pseudomonas aeruginosa in mice. J. Gen. Microbiol. 131, 2501–2503. doi: 10.1099/00221287-131-9-2501
Siezen, R. J., Francke, C., Renckens, B., Boekhorst, J., Wels, M., Kleerebezem, M., et al. (2012). Complete resequencing and reannotation of the Lactobacillus plantarum WCFS1 genome. J. Bacteriol. 194, 195–196. doi: 10.1128/JB.06275-11
Stover, C. K., Pham, X. Q., Erwin, A. L., Mizoguchi, S. D., Warrener, P., Hickey, M. J., et al. (2000). Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 406, 959–964. doi: 10.1038/35023079
Wells, J. M., and Mercenier, A. (2008). Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 6, 349–362. doi: 10.1038/nrmicro1840
Keywords: Lactic acid bacteria, Lactobacillus sp., silkworm, innate immunity, infection, Pseudomonas aeruginosa
Citation: Nishida S, Ishii M, Nishiyama Y, Abe S, Ono Y and Sekimizu K (2017) Lactobacillus paraplantarum 11-1 Isolated from Rice Bran Pickles Activated Innate Immunity and Improved Survival in a Silkworm Bacterial Infection Model. Front. Microbiol. 8:436. doi: 10.3389/fmicb.2017.00436
Received: 05 December 2016; Accepted: 02 March 2017;
Published: 20 March 2017.
Edited by:
Rebeca Martin, Centre de Recherches de Jouy-en-Josas (INRA), FranceReviewed by:
Ariadnna Cruz-Córdova, Hospital Infantil de México Federico Gómez, MexicoJia Sun, Jiangnan University, China
Kenneth James Genovese, Agricultural Research Service (USDA), USA
Copyright © 2017 Nishida, Ishii, Nishiyama, Abe, Ono and Sekimizu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuhisa Sekimizu, c2VraW1penVAbWFpbi50ZWlreW8tdS5hYy5qcA==
 Satoshi Nishida
Satoshi Nishida Masaki Ishii
Masaki Ishii Yayoi Nishiyama4
Yayoi Nishiyama4