- 1College of Life Science, Jilin Agricultural University, Changchun, China
- 2State Key Laboratory of Veterinary Etiological Biology, Key Laboratory of Veterinary Parasitology of Gansu Province, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China
- 3Key Laboratory of Jilin Province for Zoonosis Prevention and Control, Military Veterinary Institute, Academy of Military Medical Sciences, Changchun, China
- 4Department of Emergency Medicine, Inner Mongolia General Forestry Hospital, Yakeshi, China
- 5Medical Library of the Chinese people's Liberation Army, Beijing, China
- 6Center for Prevention and Control of Animal Diseases of Banan District in Chongqing, Chongqing, China
- 7Key Laboratory of Zoonoses, Ministry of Education, Changchun, China
Toxoplasma gondii has been suggested as an important opportunistic pathogen in immunocompromised patients. We conducted a global meta-analysis to assess the prevalence and odds ratios (ORs) of T. gondii infection in immunocompromised individuals. Electronic databases were reviewed for T. gondii infection in HIV/AIDS patients, cancer patients, and transplant recipients, and meta-analyses were conducted to calculate overall estimated prevalence and ORs using random or fixed-effects models. Totally, 72 eligible studies were included. The estimated pooled prevalence of T. gondii infection in immunocompromised patients and the control was 35.9 and 24.7% (p < 0.001), with an OR of 2.24, i.e., 42.1 and 32.0% for HIV/AIDS patients and the control (p < 0.05), 26.0 and 12.1% for cancer patients and the control (p < 0.001), and 42.1 and 34.5% for transplant recipients and the control (p > 0.05), whose estimated pooled ORs were 1.92 (95% CI, 1.44–2.55), 2.89 (95% CI, 2.36–3.55), and 1.51 (95% CI, 1.16–1.95), respectively. This study is the first to demonstrate that the immunocompromised patients are associated with higher odds of T. gondii infection, and appropriate prevention and control measures are highly recommended for these susceptible populations.
Introduction
The protozoan parasite Toxoplasma gondii can infect nearly all warm-blooded animals, including humans (Robert-Gangneux and Darde, 2012; Liu et al., 2015). Approximately 30% of the world's population is estimated to be infected with T. gondii (Montoya and Liesenfeld, 2004). Humans become primarily infected by ingesting raw or undercooked meat containing viable tissue cysts, or by ingesting water or food contaminated with oocysts from infected cat feces (Baldursson and Karanis, 2011; Meireles et al., 2015). In healthy humans, the infection with T. gondii is usually asymptomatic, but it can be fatal in the immunocompromised individuals, such as HIV/AIDS patients, cancer patients, and organ transplant recipients (Da Cunha et al., 1994; Pott and Castelo, 2013; Agrawal et al., 2014; Lu et al., 2015).
Toxoplasmosis of immunosuppressed individuals is most often the result of reactivation of latent infection, which presents neurological signs, including headache, disorientation, drowsiness, hemiparesis, reflex changes, and convulsions (Barratt et al., 2010; Robert-Gangneux and Darde, 2012). Acute acquired T. gondii infection in immunocompromised patients may also occur and involve multiple organs. Pneumonia, retinochoroiditis, and other disseminated systemic diseases, can also be seen, but are not as common as encephalitis in immunocompromised patients (Machala et al., 2015).
An increased frequency of Toxoplasma encephalitis has been reported in AIDS patients, especially those with significant immunosuppression when CD4 T lymphocyte cell counts is <200 cells/μL, and T. gondii infection is regarded as an important opportunistic pathogen that lead to the death of AIDS patients (Luft et al., 1993; Jones et al., 1996). The cancer can also reactivate latent T. gondii infection during antitumor treatment process (Frenkel et al., 1978). A variety of malignancies, including lymphoma, leukemia, and myeloma, can reactivate toxoplasmosis (Maciel et al., 2000; Kojima et al., 2010). Transplantation of an organ from seropositive donor can activate latent infection in a seronegative recipient receiving immunotherapy (Chehrazi-Raffle et al., 2015). Transplantation of an organ from seronegative donor can also initiate fatal infection by activation of the latent infection in a seropositive recipient receiving immunosuppressive therapy. It seems that danger of transplanting an infected organ into a seronegative recipient is greater than that of transplanting a non-infected organ into a seropositive recipient (Chehrazi-Raffle et al., 2015). Fatal toxoplasmosis has been reported in heart, liver and bone marrow, haematopoietic stem cell transplant recipients (Castagnini et al., 2007; Caner et al., 2008; Stajner et al., 2013; Gajurel et al., 2015).
Toxoplasmosis can be complicated and is considered a serious disease in immunocompromised patients, in which the reactivation of a latent infection can be fatal. The incidence of reactivated toxoplasmosis may rely on the prevalence and concentration of IgG antibodies (Robert-Gangneux and Darde, 2012). It is necessary to obtain information concerning the prevalence of T. gondii infection in different special populations worldwide. We conducted a global meta-analysis to assess the seroprevalence and odds ratios (ORs) of T. gondii infection in immunocompromised patients compared with those in control individuals.
Materials and Methods
Search Strategy and Selection Criteria
We reported this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Moher et al., 2009). We searched PubMed, Embase, Google scholar, ScienceDirect, Chinese Web of Knowledge, Wanfang, and Chongqing VIP databases from inception to February 29, 2016, for all reports that possibly contained data for T. gondii prevalence in different immunocompromised populations. The databases were searched using the keywords “Toxoplasma gondii” and “toxoplasmosis” cross-referenced with “HIV,” “AIDS,” “acquired immune deficiency syndrome”, “cancer,” “tumor,” “malignancy,” “carcinoma,” “transplantation,” “organ grafting,” “immunodeficiency,” and “immune deficiency.” We included studies without language limitation.
We systematically searched the scientific literatures for case-control, cohort, and cross-sectional studies that reported T. gondii infection in immunocompromised individuals, stratified by one of the following criteria: population with HIV/AIDS or without HIV/AIDS; population with cancer or without cancer; transplant or non-transplant population. Studies were excluded if they were reviews, repeated studies, or animal studies. Studies were excluded if they provided the final result without raw data. Studies were excluded if the sample size from one of the two groups was <30.
All identified titles and abstracts were carefully examined by two independent reviewers (HHL and HYM). The full text of articles considered as potentially relevant based on title and abstract were independently examined by the same two reviewers. Any disagreements with the selected studies were resolved by discussion and the involvement of another two authors (ZDW and QL).
Data Extraction and Quality Assessment
The following information was extracted from each study: first author, publication year, country of the study, the number of patients and control, diagnostic methods, and demographic characteristics. Two reviewers (ZDW and YZL) independently extracted the data and reached a consensus after a discussion on the controversial literatures.
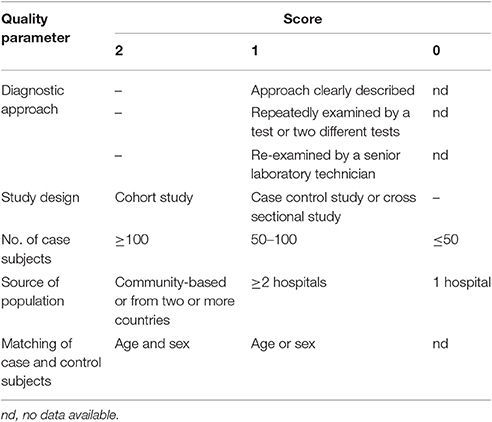
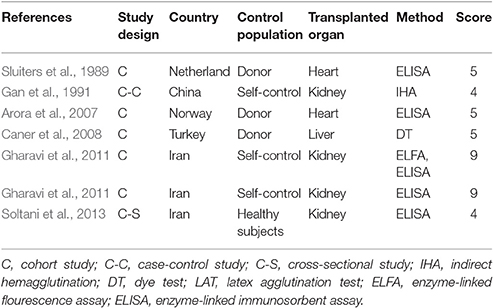
The quality of the included publications was assessed based on the criteria (Liu et al., 2009; Speich et al., 2016). These criteria were created based on the Grading of Recommendations Assessment, Development and Evaluation method (Atkins et al., 2004), and including the diagnostic approach of T. gondii infection and matching of case and control subjects (Table 1). A scoring approach was used for grading, and up to 11 points assigned to each study. Studies that were awarded 6–11 points were considered to be of high quality, 4–5 points were moderate quality, whereas lower scores indicated low quality.
Statistical Analysis
We estimated prevalence of T. gondii infection by pooling of data from each study. Data were pooled with a DerSimonian-Laird random-effects model (DerSimonian and Laird, 1986; Borenstein et al., 2010), whose difference was compared using Wilcoxon two-sample test or t-test. The risk of T. gondii infection in patient and control groups was estimated by odds ratio (OR). It was considered statistically significant when p < 0.05. In the forest plots, OR > 1 showed a risk effect and OR < 1 showed a protective effect. Statistical heterogeneity of results was appraised using a x2-based Q-test and I2 statistic. The heterogeneity was considered not significant only when p > 0.1 and I2 < 50%. The fixed-effects model was used when literature heterogeneity not existed; otherwise, the random-effects model was employed. Sensitivity analysis was performed by modification of the inclusion criteria of this meta-analysis. The analysis was conducted using Stata software version 12.0 (Stata Corporation, College Station, TX, USA). The publication bias was considered significant when p-value of Begg's test and Egger's test was <0.05.
Results
Literature Search
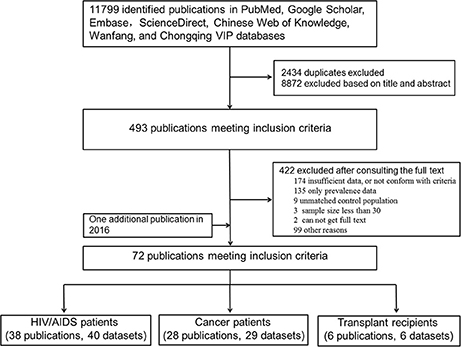
As shown in Figure 1, the literature search yielded 11,799 relevant studies, which included 2,434 duplicates. After a careful examination of each article's title and abstract, 493 were considered as having potential value, and the full texts were retrieved for detailed evaluation. A total of 422 potentially relevant articles were excluded from this meta-analysis after consulting the full text. Of these, 273 articles did not present sufficient data that required, or not conform with the included criteria; 135 had prevalence without raw data; 9 had unmatched control populations; the sample size in three articles was <30; there were two publications whose full texts were not retrieved. One additional publication regarding T. gondii infection in HIV patients was identified through the second search on Feb, 29, 2016. Finally, a total of 72 publications were included for our meta-analyses.
Characteristics of Included Studies
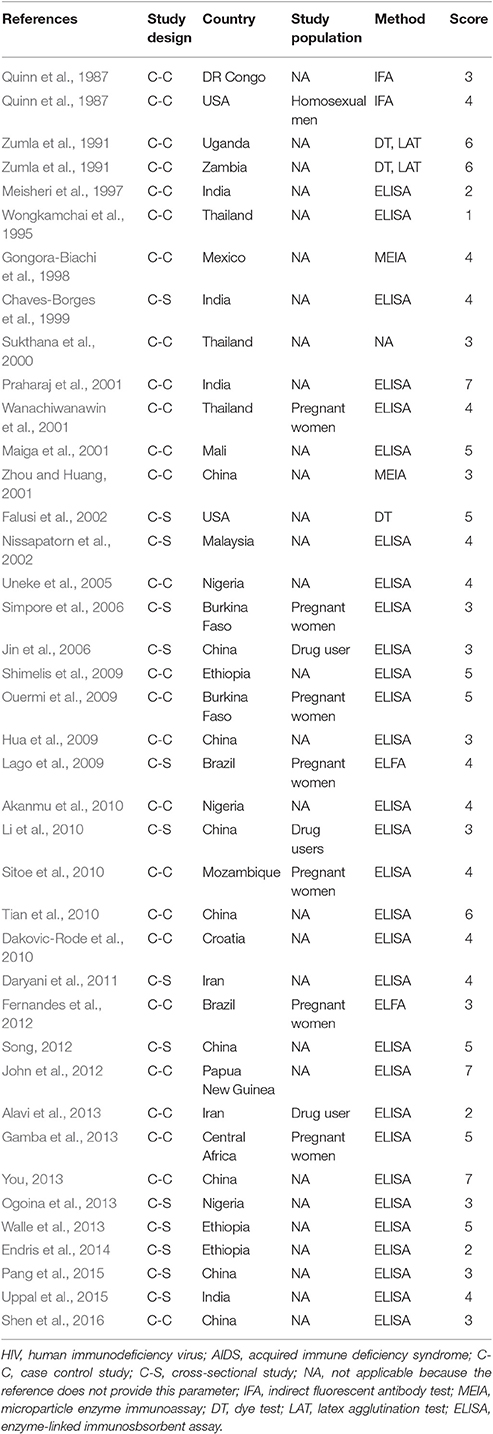
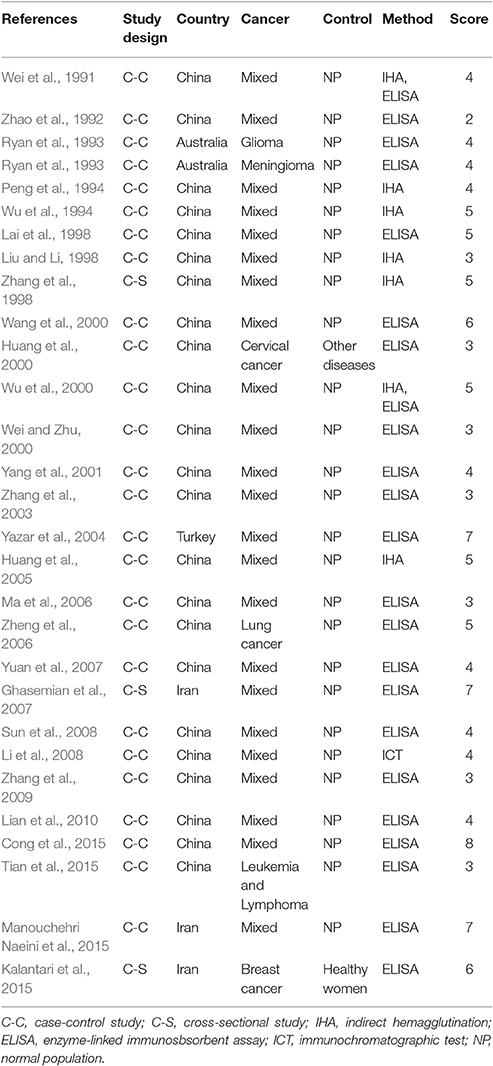
Characteristics of the included publications are listed in Tables 2–4. In brief, 38 publications described T. gondii infection in HIV/AIDS patients, 28 articles investigated T. gondii infection in cancer patients, whereas 6 studies reported T. gondii infection in transplant patients. The identified studies were conducted worldwide (Figure 2). In terms of epidemiological design, 51 of the included publications were case-control studies, 17 were cross-sectional studies, and four were cohort studies. Thirty-nine papers were written in English, 29 were in Chinese, two in French (Maiga et al., 2001; Gamba et al., 2013), and one each in Spanish (Gongora-Biachi et al., 1998) and in Croatian (Dakovic-Rode et al., 2010). There were 6 papers whose raw data were extracted from the abstract (Ryan et al., 1993; Gongora-Biachi et al., 1998; Sukthana et al., 2000; Uneke et al., 2005; Akanmu et al., 2010; Manouchehri Naeini et al., 2015). The oldest study was conducted in 1987 (Quinn et al., 1987). Totally, 40 datasets investigated T. gondii infection in HIV/AIDS patients, and 29 datasets examined T. gondii infection in cancer patients, whereas only six datasets studied T. gondii infection in transplant recipients.
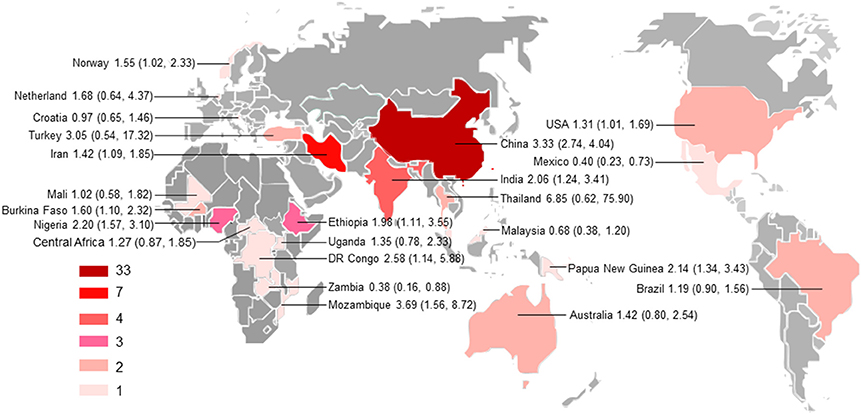
Figure 2. Geographical distribution of the included studies. The map was created using MapInfo Professional software version 9.5. Pooled odds ratio and 95% confidence interval are shown for each country.
According to our criteria, eight publications were of high quality (>6 points), 43 publications had quality scores of 4–6 points indicating moderate quality, whereas the remaining 21 publications were of low quality (<4 points).
Pooled Prevalence of T. gondii Infection (IgG) in Immunocomprised Patients
The estimated pooled prevalence of T. gondii infection in the HIV/AIDS patients and control population was 42.1% (95% CI, 34.0–50.2%) and 32.0% (95% CI, 24.0–40.1%), respectively (p < 0.05); the prevalence in cancer patients and control was 26.0% (95% CI, 20.5–31.5%) and 12.1% (95% CI, 9.5–14.8%), respectively (p < 0.001); and the prevalence in transplant recipients and its control was 42.1% (95% CI, 27.1–57.2%) and 34.5% (95% CI, 17.1–51.9%), respectively (p = 0.59). The results are shown in Supplementary Figures 1–6.
Association of Immunocomprised Patients with T. gondii Infection
Forest plots on the association of immunosuppressed populations with T. gondii infection are presented in Figures 3–5. The estimated pooled random effects ORs of HIV/AIDS, cancer, and transplant patients compared with their controls were 1.92 (95% CI, 1.44–2.55), 2.89 (95% CI, 2.36–3.55), and 1.51 (95% CI, 1.16–1.95) for infection with T. gondii. However, the heterogeneity analysis showed that there was a relatively high-level heterogeneity in our meta-analysis of HIV/AID patients (Q = 401.6, I2 = 90.3%, p = 0.000) and cancer individuals (Q = 76.4, I2 = 63.4%, p = 0.000), and no heterogeneity was found in transplant recipients (Q = 8.0, I2 = 37.3%, p = 0.157).
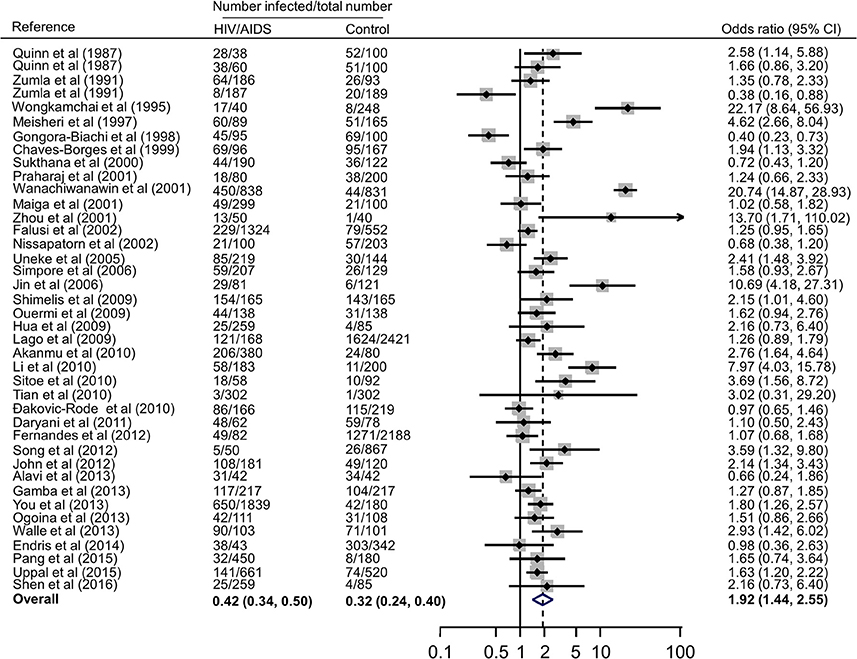
Figure 3. Meta-analysis of the association of HIV/AIDS patients and T. gondii infection (IgG) with random-effects analysis. CI, confidence interval; OR, odds ratio.
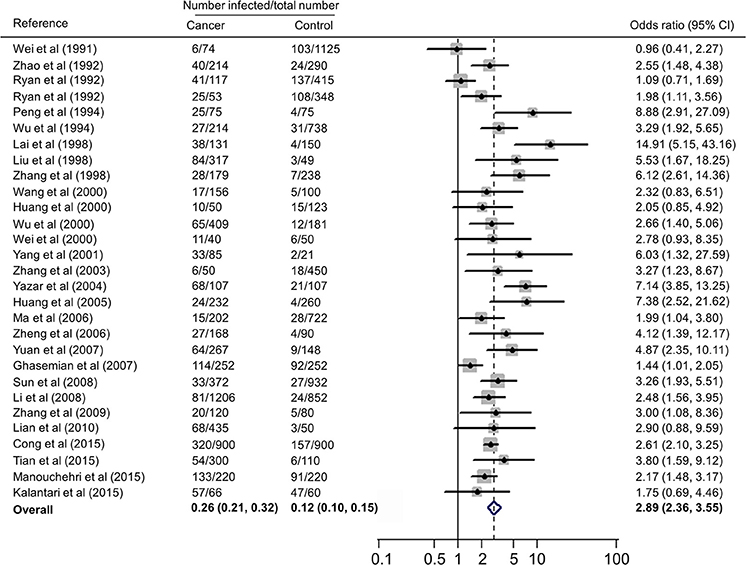
Figure 4. Meta-analysis of the association of cancer patients and T. gondii infection (IgG) with random-effects analysis. CI, confidence interval; OR, odds ratio.
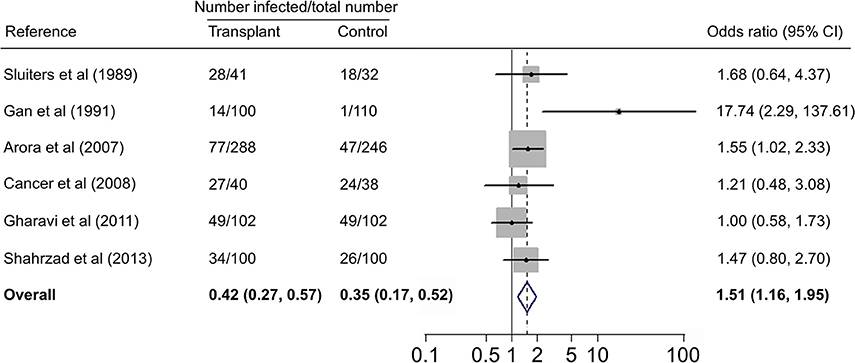
Figure 5. Meta-analysis of the association of transplant recipients and T. gondii infection (IgG) with fixed-effects analysis. CI, confidence interval; OR, odds ratio.
We also analyzed all the data, showing that the estimated pooled prevalence of T. gondii infection in immunocompromised patients and the controls was 35.9% (95% CI, 31.0–40.8%) and 24.7% (95% CI, 20.5–28.8%, p < 0.001), with an OR of 2.24 (95% CI, 1.87–2.69).
Subgroup Analysis
All subgroup analysis, including those of geographical distribution, country income, published years, sample size, detection methods, study design, and population, did not show any significant differences between the respective groups, as indicated by overlapping 95% CIs, with the exception of subgroups based on geographical distribution and income level (Supplementary Table 1). For example, the odd of T. gondii infection in HIV/AIDS patients in Asia (OR = 2.77, 95% CI, 1.58–4.87) was significantly higher as comparison with that in Latin America (OR = 1.19, 95% CI, 0.90–1.56) and in Europe (OR = 0.97, 95% CI, 0.65–1.46); the odd of T. gondii infection in cancer patients in Asia (OR = 3.07, 95% CI, 2.51–3.76) was significantly higher, compared with that in Oceania (OR = 1.42, 95% CI, 0.80–2.54). Additionally, higher odds of T. gondii infection in both HIV/AIDS and cancer patients were found in middle-income and low-income countries, compared with that of high-income countries.
Publication Bias and Sensitivity Analysis
Begg and Egger tests were used to evaluate the publication bias. No significant bias was revealed in HIV/AIDS- or transplant-related publications, but significant bias was observed in cancer-associated publications (p < 0.05, Supplementary Table 1, Supplementary Figure 7).
A sensitivity analysis was conducted by excluding one single study each time to find out whether modification of the inclusion criteria of this meta-analysis had an effect on the final results. All the results were not materially altered (data not shown).
Pooled Prevalence of T. gondii Infection (IgM) in Immunocomprised Patients
Our meta-analysis focused on T. gondii IgG antibodies, which are a marker of lifetime exposure to toxoplasmosis, whereas IgM antibodies are a marker of acute or recent infection, or also potentially persistent infection or reinfection with a different genotype (Sharma et al., 1983; Dzitko et al., 2006). During extraction of data in this study, the IgM antibodies against T. gondii in immunocompromised patients were also collected (Supplementary Tables 2–4). Due to insufficient data on HIV/AIDS and transplant patients, we only analyzed T. gondii IgM in cancer patients and the control, showing a prevalence of 11.4% (95% CI, 8.1–14.7%) in cancer patients and 2.7% (95% CI, 1.5–4.0%, p < 0.01) in its control group and OR of 2.65 (95% CI, 2.04–3.45, Supplementary Figures 8–10). The results also confirmed that the immunocompromised patients were associated with significantly higher odds of recently acquired T. gondii infection.
Discussion
T. gondii has been suggested as an important opportunistic pathogen in immunocompromised patients (Walker and Zunt, 2005). The infection in healthy (immunocompetent) people is usually self-limited and asymptomatic, resulting in chronic infection of tissue cysts that can lie dormant, probably for the entire lifetime of the hosts. However, immunocompromised individuals, such as HIV/ADIS patients, cancer patients with chemotherapy, and transplant recipients, are at risk of developing Toxoplasma encephalitis, myocarditis, or pneumonitis, due to reactivation of the chronic infection. For example, approximately 30–40% of HIV co-infected immunocompromised individuals with T. gondii develop encephalitis (Walker and Zunt, 2005).
The associations of HIV and seroprevalence of T. gondii infection are varied in the world (Grant et al., 1990). Some reports showed higher prevalence of T. gondii infection in HIV-infected patients compared to non-infected individuals, whereas others did not find any differences between the two groups (Sukthana et al., 2000; Galvan-Ramirez Mde et al., 2012). This global systematic review and meta-analyses were conducted to quantify the prevalence and ORs of T. gondii infection in immunocompromised individuals compared with those in control individuals.
Subgroup analyses comparing published year, sample size, detection method, study design, country income, and population revealed non-significant differences, but high odds were found for T. gondii infection in HIV/AIDS patients in Asia and Africa as comparison with that of America and Europe, and in cancer patients in Asia compared to that in Oceania (Supplementary Table 1). However, only one or two studies examined the association of immunocompromised patients with T. gondii infection in these regions. Thus, no meta-analysis could be done and no firm conclusion should be drawn. Most studies were conducted in the countries of Asia. Our analyses further demonstrated that the studies are geographically clustered, with few studies in Latin America, Europe, and Oceania (Figure 2).
The presence of heterogeneity was observed in HIV/AIDS and cancer patients, but subgroup analyses did not explain the specific causes of heterogeneity, which may come from various sources, including geographical distribution, published years, sample size, detection methods, study design, or populations. Without meta-regression or additional subgroup analysis that requires a large number of studies, it is difficult to investigate the causes of heterogeneity. The presence of heterogeneity shows that pooled results are averaging multiple related, but different effects (Strunz et al., 2014).
In fact, higher prevalence of T. gondii infection in HIV/AIDS patients has been reported in many countries, such as Nigeria, Mali, Ethopia, India, China, and Thailand (Maiga et al., 2001; Wanachiwanawin et al., 2001; Akanmu et al., 2010; Daryani et al., 2011; Uppal et al., 2015; Shen et al., 2016). The present study provided robust evidence that support the conclusion, and demonstrated that HIV/AIDS patients are associated risk factors (OR = 1.92, 95% CI 1.44–2.55) for T. gondii infection. The data were derived from 38 publications from 20 countries, which included 10,028 HIV/AIDS patients and 12,334 control people (Table 2).
A recent study reported T. gondii infection in Chinese cancer population, with a prevalence of 20.6% in cancer patients and 6.3% in the control (OR = 3.9) (Jiang et al., 2015). Our meta-analysis also included the data of other countries, such as Australia, Iran, and Turkey (Table 3), which involved 7,011 cancer patients and 9,254 control people, therefore, the results would be more reliable. However, of the included 28 publications, 21 were written in Chinese, resulting in significant publication bias.
There are many case reports of toxoplasmosis in transplant recipients, including haematopoietic cell (Barcan et al., 2002), heart (Gajurel et al., 2015), liver (Hamza et al., 2015), and kidney (Petty et al., 2015) transplant patients. A previous study reported a higher prevalence of T. gondii infection in renal transplant recipients (Soltani et al., 2013). In the present study, we analyzed T. gondii infection in 671 transplant patients (heart, kidney, and liver) and 628 control people from five countries (Table 4), revealing no significant difference of T. gondii infection between the two groups, but showing that transplant population is an risk factor (OR = 1.51, 95% CI, 1.16–1.95) for T. gondii infection. Interestingly, it was found that 14.3% of renal transplant recipients were detected positive for T. gondii infection in the first year of transplantation, and the prevalence increased to 85.7% in 1 year post-transplantation (Aufy et al., 2009).
There are several limitations in this meta-analysis, which may affect the results. First, a number of potentially relevant studies were identified through our systematic review, but not all the underlying data were available. Therefore, though most of these studies might not have relevant data, there is a certain risk to miss some eligible data.
Second, based on our scoring system, most studies were of moderate or even relatively low quality. This finding is mainly due to the epidemiological design of the studies; most were cross-sectional in nature. The differences between the study groups also included ages, lifestyles, and geographical conditions, which all contribute to the difference of T. gondii infection between the patient and control groups (Minbaeva et al., 2013; Walle et al., 2013; Wang et al., 2015). The cluster randomized controlled trial would provide high-quality data, but their implementation is more difficult. An alternative way to generate high-quality data would be using a time-series approach as a study design (Speich et al., 2016).
Third, diagnosis of T. gondii infection in immunocompromised patients is difficult. Though detection of the parasite by microscopy and bioassays is considered as the gold standard for diagnosis of toxoplasmosis, its clinical diagnosis more likely relies on serological methods (Liu et al., 2015). However, the serological methods may be unreliable in the immunocompromised individuals, whose immune system has been impaired, and cannot produce enough antibodies (Lewis et al., 2015). In the identified studies, all the detection methods were serological, including indirect hemagglutination (IHA), dye test (DT), immunochromatographic test (ICT), and enzyme-linked immunosorbent assay (ELISA) (Tables 2–4). Thus, by the reason of lack of specific antibody, the detected results would be lower than the actual prevalence in immunocompromised patients, including HIV/AIDS patients, cancer patients, and transplant recipients (Saadatnia and Golkar, 2012).
Fourth, insufficient data about further relevant factors on T. gondii infection (e.g., age, cancer type, transplanted organ) were available for subgroup analysis.
In summary, our global meta-analysis shows a higher prevalence of T. gondii infection in immunocompromised patients, and demonstrates that the immunocompromised individuals, including HIV/AIDS patients, cancer patients, and transplant recipients, were associated with higher odds of T. gondii infection. Therefore, a routine serological screening test for T. gondii infection is suggested to be conducted in immunocompromised patients in endemic area, or patients with no proper chemoprophylaxis and/or HAART. Patients with a positive result are at risk of reactivation of the infection, while patients with a negative result should be informed to prevent primary infection. Health education, particularly toward avoiding eating raw and undercooked meat, and avoiding contact with cats' feces should also be considered.
Author Contributions
QL was responsible for the idea and concept of the paper. ZW and QL analyzed the results. HL, ZM, HM, ZL, FW, ZY, and BX collected and analyzed the data. QL and XZ wrote the manuscript. All authors contributed to the manuscript editing and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31672542, 31472183, 31372430 and 31230073) and the Special Fund for Agro-scientific Research in the Public Interest in China (201303042).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00389/full#supplementary-material
Supplementary Figure 1. Forest plot of estimated pooled prevalence (IgG) of T. gondii infection in HIV/AIDS patients.
Supplementary Figure 2. Forest plot of estimated pooled prevalence (IgG) of T. gondii infection in HIV/AIDS-negative population.
Supplementary Figure 3. Forest plot of estimated pooled prevalence (IgG) of T. gondii infection in cancer patients.
Supplementary Figure 4. Forest plot of estimated pooled prevalence (IgG) of T. gondii infection in cancer-negative patients.
Supplementary Figure 5. Forest plot of estimated pooled prevalence (IgG) of T. gondii infection in transplant patients.
Supplementary Figure 6. Forest plot of estimated pooled prevalence (IgG) of T. gondii infection in non-transplant population.
Supplementary Figure 7. Funnel plots to assess publication bias in the meta-analysis. (A) Funnel plot for experimental studies. (B) Funnel plot for observational studies.
Supplementary Figure 8. Meta-analysis of the association of cancer patients and T. gondii infection (IgM).
Supplementary Figure 9. Forest plot of estimated pooled prevalence (IgM) of T. gondii infection in cancer patients.
Supplementary Figure 10. Forest plot of estimated pooled prevalence (IgM) of T. gondii infection in cancer-negative population.
Supplementary Table 1. Results of the subgroup analyses examining the association of immunosuppressed individuals with T. gondii infection*.
Supplementary Table 2. Characteristics of the included studies for T. gondii infection (IgM) in HIV/AIDS patients.
Supplementary Table 3. Characteristics of the included studies for T. gondii infection (IgM) in cancer patients.
Supplementary Table 4. Characteristics of the included studies for T. gondii infection (IgM) in transplant patients.
References
Agrawal, S. R., Singh, V., Ingale, S., and Jain, A. P. (2014). Toxoplasmosis of spinal cord in acquired immunodeficiency syndrome patient presenting as paraparesis: a rare entity. J. Glob. Infect. Dis. 6, 178–181. doi: 10.4103/0974-777X.145248
Akanmu, A. S., Osunkalu, V. O., Ofomah, J. N., and Olowoselu, F. O. (2010). Pattern of demographic risk factors in the seroprevalence of anti-Toxoplasma gondii antibodies in HIV infected patients at the Lagos University Teaching Hospital. Nig. Q. J. Hosp. Med. 20, 1–4. doi: 10.4314/nqjhm.v20i1.57974
Alavi, S. M., Jamshidian, R., and Salmanzadeh, S. (2013). Comparative study on Toxoplasma serology among HIV positive and HIV negative illicit drug users in Ahvaz, Iran. Caspian J. Intern. Med. 4, 781–784.
Arora, S., Jenum, P. A., Aukrust, P., Rollag, H., Andreassen, A. K., Simonsen, S., et al. (2007). Pre-transplant Toxoplasma gondii seropositivity among heart transplant recipients is associated with an increased risk of all-cause and cardiac mortality. J. Am. Coll. Cardiol. 50, 1967–1972. doi: 10.1016/j.jacc.2007.07.068
Atkins, D., Best, D., Briss, P. A., Eccles, M., Falck-Ytter, Y., Flottorp, S., et al. (2004). Grading quality of evidence and strength of recommendations. BMJ 328:1490. doi: 10.1136/bmj.328.7454.1490
Aufy, S. M., Mahgoub, A. M., Saadi, M. G., and Adel Elmallawany, M. (2009). Serological detection of Toxoplasma gondii in chronic renal failure patients and renal transplant recipients. J. Egypt. Soc. Parasitol. 39, 943–950.
Baldursson, S., and Karanis, P. (2011). Waterborne transmission of protozoan parasites: review of worldwide outbreaks-an update 2004-2010. Water Res. 45, 6603–6614. doi: 10.1016/j.watres.2011.10.013
Barcan, L. A., Dallurzo, M. L., Clara, L. O., Valledor, A., Macias, S., Zorkin, E., et al. (2002). Toxoplasma gondii pneumonia in liver transplantation: survival after a severe case of reactivation. Transpl. Infect. Dis. 4, 93–96. doi: 10.1034/j.1399-3062.2002.t01-1-00006.x
Barratt, J. L., Harkness, J., Marriott, D., Ellis, J. T., and Stark, D. (2010). Importance of nonenteric protozoan infections in immunocompromised people. Clin. Microbiol. Rev. 23, 795–836. doi: 10.1128/CMR.00001-10
Borenstein, M., Hedges, L. V., Higgins, J. P., and Rothstein, H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1, 97–111. doi: 10.1002/jrsm.12
Caner, A., Doskaya, M., Karasu, Z., Degirmenci, A., Guy, E., Kilic, M., et al. (2008). Incidence and diagnosis of active Toxoplasma infection among liver transplant recipients in Western Turkey. Liver Transpl. 14, 1526–1532. doi: 10.1002/lt.21558
Castagnini, M., Bernazzali, S., Ginanneschi, C., Marchi, B., Maccherini, M., Tsioulpas, C., et al. (2007). Fatal disseminated toxoplasmosis in a cardiac transplantation with seropositive match for Toxoplasma: should prophylaxis be extended? Transpl. Immunol. 18, 193–197. doi: 10.1016/j.trim.2007.05.016
Chaves-Borges, F. A., Souza, M. A., Silva, D. A., Kasper, L. H., and Mineo, J. R. (1999). Detection of Toxoplasma gondii soluble antigen, SAG-1(p30), antibody and immune complex in the cerebrospinal fluid of HIV positive or negative individuals. Rev. Inst. Med. Trop. Sao. Paulo. 41, 329–338. doi: 10.1590/S0036-46651999000600001
Chehrazi-Raffle, A., Luu, M., Yu, Z., Liou, F., Kittleson, M., Hamilton, M., et al. (2015). Toxoplasma gondii serology and outcomes after heart transplantation: contention in the literature. Transplant. Proc. 47, 1949–1953. doi: 10.1016/j.transproceed.2015.06.022
Cong, W., Liu, G. H., Meng, Q. F., Dong, W., Qin, S. Y., Zhang, F. K., et al. (2015). Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Lett. 359, 307–313. doi: 10.1016/j.canlet.2015.01.036
Da Cunha, S., Ferreira, E., Ramos, I., Martins, R., De Freitas, L., Borges, J. L., et al. (1994). Cerebral toxoplasmosis after renal transplantation. Case report and review. Acta Med. Port. 7, S61–66.
Dakovic-Rode, O., Zidovec-Lepej, S., Martucci, M. V., Polanda, V. L., and Begovac, J. (2010). Prevalence of antibodies against Toxoplasma gondii in patients infected with human immunodeficiency virus in Croatia. Croatian J. Infect. 30, 5–10.
Daryani, A., Sharif, M., and Meigouni, M. (2011). Seroprevalence of IgG and IgM anti-Toxoplasma antibodies in HIV/AIDS patients, northern Iran. Asian Pac. J. Trop. Med. 4, 271–274. doi: 10.1016/S1995-7645(11)60084-9
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control Clin. Trials 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Dzitko, K., Staczek, P., Gatkowska, J., and Dlugonska, H. (2006). Toxoplasma gondii: serological recognition of reinfection. Exp. Parasitol. 112, 134–137. doi: 10.1016/j.exppara.2005.09.010
Endris, M., Belyhun, Y., Moges, F., Adefiris, M., Tekeste, Z., Mulu, A., et al. (2014). Seroprevalence and associated risk factors of Toxoplasma gondii in pregnant women attending in Northwest Ethiopia. Iran J. Parasitol. 9, 407–414.
Falusi, O., French, A. L., Seaberg, E. C., Tien, P. C., Watts, D. H., Minkoff, H., et al. (2002). Prevalence and predictors of Toxoplasma seropositivity in women with and at risk for human immunodeficiency virus infection. Clin. Infect. Dis. 35, 1414–1417. doi: 10.1086/344462
Fernandes, M. A., Batista, G. I., Carlos Jda, C., Gomes, I. M., Azevedo, K. M., Setubal, S., et al. (2012). Toxoplasma gondii antibody profile in HIV-1-infected and uninfected pregnant women and the impact on congenital toxoplasmosis diagnosis in Rio de Janeiro, Brazil. Braz J. Infect. Dis. 16, 170–174. doi: 10.1016/S1413-8670(12)70300-8
Frenkel, J. K., Amare, M., and Larsen, W. (1978). Immune competence in a patient with Hodgkin's disease and relapsing toxoplasmosis. Infection 6, 84–91. doi: 10.1007/BF01642165
Gajurel, K., Dhakal, R., and Montoya, J. G. (2015). Toxoplasma prophylaxis in haematopoietic cell transplant recipients: a review of the literature and recommendations. Curr. Opin. Infect. Dis. 28, 283–292. doi: 10.1097/QCO.0000000000000169
Galvan-Ramirez Mde, L., Troyo, R., Roman, S., Calvillo-Sanchez, C., and Bernal-Redondo, R. (2012). A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population. Parasit. Vectors 5:271. doi: 10.1186/1756-3305-5-271
Gamba, E. P., Nambei, W. S., and Kamandji, L. (2013). Integrated screening for HIV, syphilis, and toxoplasmosis among pregnant women in the Central African Republic. Med. Sante Trop. 23, 421–426. doi: 10.1684/mst.2013.0256
Gan, S. B., Ni, S. G., Li, S., Fu, C. W., and Yu, Y. (1991). Toxoplasma gondii infection in immunosuppressed patients. Chin. J. Zoonosis 7, 61.
Gharavi, M. J., Jalali, S., Khademvatan, S., and Heydari, S. (2011). Detection of IgM and IgG anti-Toxoplasma antibodies in renal transplant recipients using ELFA, ELISA and ISAGA methods: comparison of pre- and post-transplantation status. Ann. Trop. Med. Parasitol. 105, 367–371. doi: 10.1179/1364859411Y.0000000022
Ghasemian, M., Maraghi, S. H., Saki, J., and Pedram, M. (2007). Determination of antibodies (IgG, IgM) against Toxoplasma gondii in patients with cancer. Iran J. Parasitol. 2, 1–6.
Gongora-Biachi, R. A., Gonzalez-Martinez, P., Castro-Sansores, C., Alvarez-Moguel, R., Pavia-Ruz, N., Lara-Perera, D., et al. (1998). Antibodies against Toxoplasma gondii in patients with HIV in Yucatan. Rev. Invest. Clin. 50, 419–422.
Grant, I. H., Gold, J. W., Rosenblum, M., Niedzwiecki, D., and Armstrong, D. (1990). Toxoplasma gondii serology in HIV-infected patients: the development of central nervous system toxoplasmosis in AIDS. AIDS 4, 519–521. doi: 10.1097/00002030-199006000-00004
Hamza, H., El-Taweel, H., Abou-Holw, S., Khalil, S., and Wagdy, E. (2015). Toxoplasma gondii seropositivity in renal patients: rate, pattern, predictors and related morbidity. J. Egypt. Soc. Parasitol. 45, 7–15. doi: 10.12816/0010844
Hua, H. Y., Huan, X. P., Dong, M. H., Zhang, Y., Zhu, J., Xu, X. X., et al. (2009). Survey on infection of Toxoplasma gondii in HIV/AIDS patients. Chin. Modern Med. J. 11, 47–49.
Huang, H., Yan, F. H., Li, C. J., Zhao, M. Y., Tang, J. W., and Li, X. R. (2000). Detection of Toxoplasma gondii infection in women with gynaecologic neoplasms using ELISA. Chin. J. Parasitol. Parasit Dis. 18, 165–166.
Huang, L., Li, Y. B., Zhao, R., and Wang, S. J. (2005). The application of dot immunogold filtration assay for the detection of antibody Toxoplasma gondii IgG in serum of the tumor patient. Acta Parasitol. Med. Entomol. Sin. 12, 17–19.
Jiang, C., Li, Z., Chen, P., and Chen, L. (2015). The Seroprevalence of Toxoplasma gondii in Chinese population with cancer: a systematic review and meta-analysis. Medicine 94:e2274. doi: 10.1097/md.0000000000002274
Jin, Z. S., Feng, Z. B., Dai, H. D., and Li, J. R. (2006). Study on condition of Toxoplasma gondii infection among intravenous drug users and affecting factors. Cent. Chin. Med. J. 30, 489–490.
John, N. L., McBride, W. J., Millan, J., and Wilsons, K. (2012). Seroprevalence of anti-Toxoplasma gondii antibodies in HIV/AIDS patients and healthy blood donors at the Port Moresby General Hospital, Papua New Guinea. PNG Med. J. 2012, 88–93.
Jones, J. L., Hanson, D. L., Chu, S. Y., Ciesielski, C. A., Kaplan, J. E., Ward, J. W., et al. (1996). Toxoplasmic encephalitis in HIV-infected persons: risk factors and trends. The Adult/Adolescent Spectrum of Disease Group. AIDS 10, 1393–1399. doi: 10.1097/00002030-199610000-00012
Kalantari, N., Ghaffari, S., Bayani, M., Elmi, M. M., Moslemi, D., Nikbakhsh, N., et al. (2015). Preliminary study on association between toxoplasmosis and breast cancer in Iran. Asian Pac. J. Trop. Biomed. 5, 44–47. doi: 10.1016/S2221-1691(15)30169-6
Kojima, M., Nakamura, N., Murayama, K., Igarashi, T., Matsumoto, M., Matsuda, H., et al. (2010). Reactive lymphoid hyperplasia with giant follicles associated with a posttherapeutic state of hematological malignancies. A report of eight cases. Tumori 96, 143–148.
Lago, E. G., Conrado, G. S., Piccoli, C. S., Carvalho, R. L., and Bender, A. L. (2009). Toxoplasma gondii antibody profile in HIV-infected pregnant women and the risk of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 28, 345–351. doi: 10.1007/s10096-008-0631-2
Lai, X. Q., Zhu, J. Y., and Wei, Q. D. (1998). Toxoplasma antibody in patients with malignant tumors in Yuexi area. Guangdong Med. J. 19, 281–282.
Lewis, J. M., Clifford, S., and Nsutebu, E. (2015). Toxoplasmosis in immunosuppressed patients. Rheumatology 54, 1939–1940. doi: 10.1093/rheumatology/kev115
Li, J. R., Gong, R. Y., Li, Y. P., Bai, Y., You, F., and Deng, S. (2010). Research on HIV/Toxoplasma gondii co-infection and cytokine levels among intravenous drug users. Parasite Immunol. 32, 161–164. doi: 10.1111/j.1365-3024.2009.01174.x
Li, K. S., Du, H. F., Lian, X. W., Yuan, M., Sun, Y. F., Zhang, L., et al. (2008). Investigation of anti-Toxoplasmas gondii antibodies in different tumor patients using gold immunochromatographic assay. J. Pathog. Biol. 3, 478–479.
Lian, X. W., Li, K. S., Du, H. F., Yuan, M., Ye, W. H., and Zhou, Y. Q. (2010). Contrast analysis of antibody IgG and IgM of patients suffering different malignant tumor in Lanzhou area. Health Vocational Educ. 28, 117–118.
Liu, A. Q., and Li, Y. J. (1998). Different clinical types of Toxoplasma infection in malignant tumor patients. Chin. J. Zoonoses 14, 73–74.
Liu, Q., Wang, Z. D., Huang, S. Y., and Zhu, X. Q. (2015). Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit. Vectors 8, 292. doi: 10.1186/s13071-015-0902-6
Liu, S., Zhang, H., Gu, C., Yin, J., He, Y., Xie, J., et al. (2009). Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J. Natl. Cancer Inst. 101, 1066–1082. doi: 10.1093/jnci/djp180
Lu, N., Liu, C., Wang, J., Ding, Y., and Ai, Q. (2015). Toxoplasmosis complicating lung cancer: a case report. Int. Med. Case Rep. J. 8, 37–40. doi: 10.2147/IMCRJ.S76488
Luft, B. J., Hafner, R., Korzun, A. H., Leport, C., Antoniskis, D., Bosler, E. M., et al. (1993). Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N.Engl. J. Med. 329, 995–1000. doi: 10.1056/NEJM199309303291403
Ma, X. B., Cai, L., Zhang, B. X., Fu, Y. H., Chen, J., Cao, L., et al. (2006). A survey on Toxoplasma gondii infection of human in Shanghai. Shanghai J. Prev. Med. 18, 483–486. doi: 10.3969/j.issn.1004-9231.2006.10.001
Machala, L., Kodym, P., Maly, M., Geleneky, M., Beran, O., and Jilich, D. (2015). Toxoplasmosis in immunocompromised patients. Epidemiol. Mikrobiol. Imunol. 64, 59–65.
Maciel, E., Siqueira, I., Queiroz, A. C., and Melo, A. (2000). Toxoplasma gondii myelitis in a patient with adult T-cell leukemia-lymphoma. Arq. Neuropsiquiatr. 58, 1107–1109. doi: 10.1590/S0004-282X2000000600019
Maiga, I., Kiemtore, P., and Tounkara, A. (2001). Prevalence of antitoxoplasma antibodies in patients with acquired immunodeficiency syndrome and blood donors in Bamako. Bull. Soc. Pathol. Exot. 94, 268–270.
Manouchehri Naeini, K., Hanifepoor, H., Kheiri, S., and Javanmardi, G. (2015). Seroprevalence and risk factors of Toxoplasma infection in patients with malignancy in central and south central areas of Iran compared with control group using enzyme-linked immunosorbent assay (ELISA). Trop. Med. Inter. Health 20, 211–212.
Meireles, L. R., Ekman, C. C., de Andrade, H. F. Jr., and Luna, E. J. (2015). Human toxoplasmosis outbreaks and the agent infecting form. Findings from a systematic review. Rev. Inst. Med. Trop. Sao. Paulo. 57, 369–376. doi: 10.1590/S0036-46652015000500001
Meisheri, Y. V., Mehta, S., and Patel, U. (1997). A prospective study of seroprevalence of toxoplasmosis in general population, and in HIV/AIDS patients in Bombay, India. J. Postgrad Med. 43, 93–97.
Minbaeva, G., Schweiger, A., Bodosheva, A., Kuttubaev, O., Hehl, A. B., Tanner, I., et al. (2013). Toxoplasma gondii infection in Kyrgyzstan: seroprevalence, risk factor analysis, and estimate of congenital and AIDS-related toxoplasmosis. PLoS Negl. Trop. Dis. 7:e2043. doi: 10.1371/journal.pntd.0002043
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Montoya, J. G., and Liesenfeld, O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. doi: 10.1016/S0140-6736(04)16412-X
Nissapatorn, V., Kamarulzaman, A., Init, I., Tan, L. H., Rohela, M., Norliza, A., et al. (2002). Seroepidemiology of toxoplasmosis among HIV-infected patients and healthy blood donors. Med. J. Malaysia 57, 304–310.
Ogoina, D., Onyemelukwe, G. C., Musa, B. O., and Obiako, R. O. (2013). Seroprevalence of IgM and IgG antibodies to Toxoplasma infection in healthy and HIV-positive adults from Northern Nigeria. J. Infect. Dev. Countr. 7, 398–403. doi: 10.3855/jidc.2797
Ouermi, D., Simpore, J., Belem, A. M., Sanou, D. S., Karou, D. S., Ilboudo, D., et al. (2009). Co-infection of Toxoplasma gondii with HBV in HIV-infected and uninfected pregnant women in Burkina Faso. Pak. J. Biol. Sci. 12, 1188–1193. doi: 10.3923/pjbs.2009.1188.1193
Pang, X. L., Chen, S. Y., Gao, K., Mai, H. X., Han, Z. G., Xu, H. F., et al. (2015). Serum epidemiological analysis of opportunistic infection of pathogenic protozoa in HIV/AIDS. J. Trop. Med. 15, 1425–1429.
Peng, L. J., Bao, Y. Y., Xu, L. F., Zhu, X. M., Chen, H. Y., Tang, D. S., et al. (1994). Study on malignant tumor accompanied with Toxoplasma gondii infection. J. Parasit. Dis. Control 23, 21–23.
Petty, L. A., Qamar, S., Ananthanarayanan, V., Husain, A. N., Murks, C., Potter, L., et al. (2015). Cardiac toxoplasmosis after heart transplantation diagnosed by endomyocardial biopsy. Transpl. Infect. Dis. 17, 719–722. doi: 10.1111/tid.12415
Pott, H. Jr., and Castelo, A. (2013). Isolated cerebellar toxoplasmosis as a complication of HIV infection. Int. J. STD AIDS 24, 70–72. doi: 10.1258/ijsa.2012.012189
Praharaj, A. K., Singh, S. P., Chander, Y., and Nagendra, A. (2001). Serological diagnosis of Toxoplasma gondii infection in various patient population in the armed forces. Med. J. Armed Forces India 57, 298–301. doi: 10.1016/S0377-1237(01)80007-1
Quinn, T. C., Piot, P., McCormick, J. B., Feinsod, F. M., Taelman, H., Kapita, B., et al. (1987). Serologic and immunologic studies in patients with AIDS in North America and Africa. The potential role of infectious agents as cofactors in human immunodeficiency virus infection. JAMA 257, 2617–2621. doi: 10.1001/jama.1987.03390190095027
Robert-Gangneux, F., and Darde, M. L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296. doi: 10.1128/CMR.05013-11
Ryan, P., Hurley, S. F., Johnson, A. M., Salzberg, M., Lee, M. W., North, J. B., et al. (1993). Tumours of the brain and presence of antibodies to Toxoplasma gondii. Int. J. Epidemiol. 22, 412–419. doi: 10.1093/ije/22.3.412
Saadatnia, G., and Golkar, M. (2012). A review on human toxoplasmosis. Scand. J. Infect. Dis. 44, 805–814. doi: 10.3109/00365548.2012.693197
Sharma, S. D., Mullenax, J., Araujo, F. G., Erlich, H. A., and Remington, J. S. (1983). Western Blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 131, 977–983.
Shen, G., Wang, X., Sun, H., and Gao, Y. (2016). Seroprevalence of Toxoplasma gondii infection among HIV/AIDS patients in Eastern China. Korean J. Parasitol. 54, 93–96. doi: 10.3347/kjp.2016.54.1.93
Shimelis, T., Tebeje, M., Tadesse, E., Tegbaru, B., and Terefe, A. (2009). Sero-prevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa, Ethiopia: a comparative cross-sectional study. BMC Res. Notes 2:213. doi: 10.1186/1756-0500-2-213
Simpore, J., Savadogo, A., Ilboudo, D., Nadambega, M. C., Esposito, M., Yara, J., et al. (2006). Toxoplasma gondii, HCV, and HBV seroprevalence and co-infection among HIV-positive and -negative pregnant women in Burkina Faso. J. Med. Virol. 78, 730–733. doi: 10.1002/jmv.20615
Sitoe, S. P., Rafael, B., Meireles, L. R., Andrade, H. F. Jr., and Thompson, R. (2010). Preliminary report of HIV and Toxoplasma gondii occurrence in pregnant women from Mozambique. Rev. Inst. Med. Trop. Sao. Paulo. 52, 291–295. doi: 10.1590/S0036-46652010000600002
Sluiters, J. F., Balk, A. H., Essed, C. E., Mochtar, B., Weimar, W., Simoons, M. L., et al. (1989). Indirect enzyme-linked immunosorbent assay for immunoglobulin G and four immunoassays for immunoglobulin M to Toxoplasma gondii in a series of heart transplant recipients. J. Clin. Microbiol. 27, 529–535.
Soltani, S., Khademvatan, S., Saki, J., and Shahbazian, H. (2013). Detection of toxoplasmosis in renal transplant recipients by ELISA and PCR methods in Ahvaz, South-West of Iran. Jundishapur J. Microbiol. 6, 1–5. doi: 10.5812/jjm.7642
Song, R. H. (2012). Relationship between Toxoplasma gondii IgG and routine screening items of infectious diseases of blood donors. Chin. J. Schisto. Control 24, 371–372.
Speich, B., Croll, D., Furst, T., Utzinger, J., and Keiser, J. (2016). Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect. Dis. 16, 87–99. doi: 10.1016/S1473-3099(15)00349-7
Stajner, T., Vasiljevic, Z., Vujic, D., Markovic, M., Ristic, G., Micic, D., et al. (2013). Atypical strain of Toxoplasma gondii causing fatal reactivation after hematopoietic stem cell transplantion in a patient with an underlying immunological deficiency. J. Clin. Microbiol. 51, 2686–2690. doi: 10.1128/JCM.01077-13
Strunz, E. C., Addiss, D. G., Stocks, M. E., Ogden, S., Utzinger, J., and Freeman, M. C. (2014). Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 11:e1001620. doi: 10.1371/journal.pmed.1001620
Sukthana, Y., Chintana, T., and Lekkla, A. (2000). Toxoplasma gondii antibody in HIV-infected persons. J. Med. Assoc. Thai. 83, 681–684.
Sun, Y. F., Jia, N., Du, H. F., Yuan, M., Lian, X. W., and Li, K. S. (2008). The analysis of anti-Toxoplasma gondii antibody IgG of patients suffering different kinds of malignant tumor in Lanzhou area tested by ELISA. Chin. Med. Herald 5, 120–121.
Tian, L. G., Cheng, G. J., Chen, J. X., Guo, J., Tong, X. M., Cai, Y. C., et al. (2010). Survey on coinfection with Toxoplasma gondii and HIV among rural people in China. Chin. J. Schisto. Control 22, 368–370.
Tian, M. Y., Huang, Y. H., Hu, Y. F., Peng, F. T., Zou, C. Y., and Li, Y. N. (2015). Toxoplasma gondi antibody profile in patients with leukemia or lymphoma. Chin. J. Parasitol. Parasit. Dis. 33, 153–155.
Uneke, C. J., Duhlinska, D. D., Njoku, M. O., and Ngwu, B. A. (2005). Seroprevalence of acquired toxoplasmosis in HIV-infected and apparently healthy individuals in Jos, Nigeria. Parassitologia 47, 233–236.
Uppal, B., Aggarwal, P., Perween, N., and Sud, A. (2015). Seroprevalence of Toxoplasma among HIV infected and HIV non-infected individuals in North India. Asian Pac. J. Trop. Dis. 5, S15–S18. doi: 10.1016/s2222-1808(15)60848-9
Walker, M., and Zunt, J. R. (2005). Parasitic central nervous system infections in immunocompromised hosts. Clin. Infect. Dis. 40, 1005–1015. doi: 10.1086/428621
Walle, F., Kebede, N., Tsegaye, A., and Kassa, T. (2013). Seroprevalence and risk factors for toxoplasmosis in HIV infected and non-infected individuals in Bahir Dar, Northwest Ethiopia. Parasit. Vectors 6:15. doi: 10.1186/1756-3305-6-15
Wanachiwanawin, D., Sutthent, R., Chokephaibulkit, K., Mahakittikun, V., Ongrotchanakun, J., and Monkong, N. (2001). Toxoplasma gondii antibodies in HIV and non-HIV infected Thai pregnant women. Asian Pac. J. Allergy Immunol. 19, 291–293.
Wang, B. L., Pan, X. Z., Yin, Y. K., and Weng, X. H. (2000). Investigation of immunodeficiency disorders in patients with Toxoplasma antibody. Chin. J. Parasitol. Parasit. Dis. 18, 34–36.
Wang, L., He, L. Y., Meng, D. D., Chen, Z. W., Wen, H., Fang, G. S., et al. (2015). Seroprevalence and genetic characterization of Toxoplasma gondii in cancer patients in Anhui Province, Eastern China. Parasit. Vectors 8, 162. doi: 10.1186/s13071-015-0778-5
Wei, Q. D., and Zhu, J. Y. (2000). Malignant tumor patients with serum levels of nitric oxide research of Toxoplasma gondii infection. Chin. Pub. Health 16, 58.
Wei, R. M., Ding, Z. Y., Xu, X. P., and Zeng, X. J. (1991). Special crowd of Toxoplasma goondii infection situation investigation in Jiangxi province. Chin. J. Zoonoses 7, 31–32.
Wongkamchai, S., Rungpitaransi, B., Wongbunnate, S., and Sittapairochana, C. (1995). Toxoplasma gondii infection in healthy persons and in patients with HIV or ocular disease. Southeast Asian J. Trop. Med. Public Health 26, 655–658.
Wu, L. D., Jiang, W. S., Yi, F. Y., Xiong, M. L., and Ding, Z. Y. (2000). Analysis of Toxoplasma gondii infection in patients with cancer. Chin. J. Zoonoses 16, 105–108.
Wu, Z. Q., Yang, S. S., Wu, S. P., Wang, P. S., and Zhang, A. L. (1994). Cancer and diabetes patients serum epidemiological analysis of Toxoplasma infection. Chin. J. Parasit. Dis. Control. 7, 74–75.
Yang, Z. J., Wang, C. B., and Hou, X. F. (2001). Analysis of Toxoplasma infection results in malignant cancer. Henan J. Ocol. 14, 135–136.
Yazar, S., Yaman, O., Eser, B., Altuntacs, F., Kurnaz, F., and Sahin, I. (2004). Investigation of anti-Toxoplasma gondii antibodies in patients with neoplasia. J. Med. Microbiol. 53, 1183–1186. doi: 10.1099/jmm.0.45587-0
You, Y. X. (2013). Seroprevalence and Infection Immune Function of Toxoplasma gondii of HIV Positive in Western Yunnan Province of China. Master degree thesis, Dali University.
Yuan, Z. G., Gao, S. Y., Liu, Q., Xia, X. Z., Liu, X. F., Liu, B., et al. (2007). Toxoplasma gondii antibodies in cancer patients. Cancer Lett. 254, 71–74. doi: 10.1016/j.canlet.2007.02.011
Zhang, L. X., Huang, W. G., and Yuan, J. F. (2003). Study on Toxoplasma gondii infections in Wuxi. Chin. J. Schistosomiasis Control 15, 466–467.
Zhang, Q. Q., Zhang, X. W., and Hui, Q. F. (2009). Results of sampling survey of malignant tumor patients co-infected with Toxoplasma gondii. Chin. Trop. Med. 9, 337–338.
Zhang, W. Z., Zhao, J. L., and Qu, M. (1998). Investigation of cancer patients serum antibodies to Toxoplasma gondii. Chin. J. Parasit. Dis. Control. 11, 122.
Zhao, H., Wu, S. P., and Yang, S. S. (1992). Antibody against Toxoplasma detection of cancer patients. Tianjin Med. J. 1, 46–47.
Zheng, S. L., Yang, Q. S., and Ma, X. H. (2006). The relationship between non small cell lung cancer and Toxoplasma infection. Chin. J. Pathophysiol. 5, 1031–1032.
Zhou, M., and Huang, X. (2001). Analysis on the co-infection of Toxoplasma gondii and AIDS in Xinjiang population. Chin. J. Zoonoses 17:127.
Keywords: Toxoplasma gondii, immunocompromised patients, HIV/AIDS patients, cancer patients, transplant recipients, prevalence, odds ratio
Citation: Wang Z-D, Liu H-H, Ma Z-X, Ma H-Y, Li Z-Y, Yang Z-B, Zhu X-Q, Xu B, Wei F and Liu Q (2017) Toxoplasma gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front. Microbiol. 8:389. doi: 10.3389/fmicb.2017.00389
Received: 25 October 2016; Accepted: 24 February 2017;
Published: 09 March 2017.
Edited by:
James H. McKerrow, University of California, San Diego, USAReviewed by:
Lars Eckmann, University of California, San Diego, USAVeeranoot Nissapatorn, University of Malaya, Malaysia
Ziguo Yuan, South China Agricultural University, China
Copyright © 2017 Wang, Liu, Ma, Ma, Li, Yang, Zhu, Xu, Wei and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Liu, bGl1cXVhbjE5NzNAaG90bWFpbC5jb20=
Feng Wei, amxjY3dmQDEyNi5jb20=
 Ze-Dong Wang
Ze-Dong Wang Huan-Huan Liu
Huan-Huan Liu Zhan-Xi Ma
Zhan-Xi Ma Hong-Yu Ma
Hong-Yu Ma Zhong-Yu Li
Zhong-Yu Li Zhi-Bin Yang
Zhi-Bin Yang Xing-Quan Zhu
Xing-Quan Zhu Bin Xu
Bin Xu Feng Wei
Feng Wei Quan Liu
Quan Liu