
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 14 February 2017
Sec. Microbial Immunology
Volume 8 - 2017 | https://doi.org/10.3389/fmicb.2017.00223
This article is part of the Research Topic Spirochetes and immune evasion: infection, persistence and clearance View all 15 articles
Ticks transmit a variety of human pathogens, including Borrelia burgdorferi, the etiological agent of Lyme disease. Multiple pathogens that are transmitted simultaneously, termed “coinfections,” are of increasing importance and can affect disease outcome in a host. Arthropod immunity is central to pathogen acquisition and transmission by the tick. Pattern recognition receptors recognize pathogen-associated molecular patterns and induce humoral responses through the Toll and Immune Deficiency (IMD) pathways. Comparative analyses between insects and ticks reveal that while the Toll pathway is conserved, the IMD network exhibits a high degree of variability. This indicates that major differences in humoral immunity exist between insects and ticks. While many variables can affect immunity, one of the major forces that shape immune outcomes is the microbiota. In light of this, we discuss how the presence of commensal bacteria, symbionts and/or coinfections can lead to altered immune responses in the tick that impact pathogen persistence and subsequent transmission. By investigating non-insect arthropod immunity, we will not only better comprehend tick biology, but also unravel the intricate effects that pathogen coinfections have on vector competence and tick-borne disease transmission.
Ticks are increasingly important disease vectors that transmit a variety of pathogens relevant to public and veterinary health (de la Fuente et al., 2008; Stromdahl and Hickling, 2012; Hartemink and Takken, 2016; Kernif et al., 2016). The most prevalent vector-borne illness in the Northern hemisphere, Lyme disease, is transmitted by Ixodes spp. ticks and is caused by the spirochete Borrelia spp. (Mather and Mather, 1990). Ticks are first colonized by pathogens when they take a bloodmeal from an infected host. The microbes will then lie dormant throughout digestion and molting. Subsequent transmission to a new vertebrate host occurs during the second bloodmeal, where pathogens migrate to the salivary glands and are injected along with saliva. Multiple obstacles within the vector can impact pathogen survival and persistence (Liu and Bonnet, 2014), including the arthropod’s immune system. This is the foremost defense against invading microbes and largely impacts the ability of an arthropod to be a competent vector for pathogens (Hillyer et al., 2003; Garver et al., 2009; Blumberg et al., 2013).
Arthropod immunity lacks adaptive components and is limited to innate processes, which can be categorized as either cellular or humoral (Ganesan et al., 2011; Buchon et al., 2014; Myllymaki et al., 2014). Humoral immunity involves innate signaling cascades, such as the Toll and Immune Deficiency (IMD) pathways. Immune defenses are triggered by pathogen-associated molecular patterns (PAMPs), which are sensed by pattern recognition receptors (PRRs) (Hillyer, 2016). Both pathogenic and commensal bacteria can elicit immune responses in arthropods, which makes the composition of the microbiota a significant force in determining vector competence as well (Cirimotich et al., 2011). For the purposes of this article, the microbiome/microbiota will be defined as all microorganisms present in the arthropod including symbionts, commensals and pathogens.
Although insect immunity has been heavily studied and is well understood, owing to the model organism Drosophila melanogaster, recent data demonstrates that non-insect arthropods, such as ticks, are significantly different (Palmer and Jiggins, 2015; Gulia-Nuss et al., 2016; Rosa et al., 2016; Shaw et al., 2017). Genome sequencing data shows that ticks lack several genes involved in innate immunity when compared to insects including some PRRs, pathway signaling molecules and antimicrobial peptides (AMPs) (Severo et al., 2013; Smith and Pal, 2014; Palmer and Jiggins, 2015; Bechsgaard et al., 2016; Gulia-Nuss et al., 2016; Rosa et al., 2016). Nevertheless, immune pathways within ticks remain functional, suggesting that there are undiscovered principles governing non-insect arthropod immunity (Kopacek et al., 1999; Sonenshine et al., 2002; Simser et al., 2004; Pelc et al., 2014). Herein, we will discuss the current understanding of tick humoral signaling pathways in the context of disease transmission both with and without confounding factors, such as coinfections and the microbiota.
Two of the best studied immune signaling cascades in arthropod immunity are the Toll and IMD pathways. Both are initiated by distinct PAMPs and orchestrate the production of microbiocidal AMPs (Hillyer, 2016). The Toll pathway responds primarily to Gram-positive bacteria and fungi whereas the IMD pathway recognizes Gram-negative bacteria (Hillyer, 2016). Herein, we will describe our current understanding of tick humoral immunity in comparison to insects.
In Drosophila, Lysine-type peptidoglycan from the cell wall of Gram-positive bacteria is recognized by peptidoglycan recognition receptor proteins (PGRPs)-SA. β1-3-glucan from fungi is sensed by Gram-negative binding proteins (GNBPs) (Michel et al., 2001; Kanagawa et al., 2011) (Figure 1). Most of the components that comprise the Toll pathway in insects are conserved in the tick genome, although there are a few deviations (Figure 1; Table 1) (Palmer and Jiggins, 2015; Bechsgaard et al., 2016). For example, there are eight Toll receptors found in Drosophila, whereas only four have been identified in the Ixodes scapularis genome (Palmer and Jiggins, 2015). I. scapularis ticks also lack genes encoding GNBPs (Palmer and Jiggins, 2015; Gulia-Nuss et al., 2016). Despite the reduction in receptor repertoire, evidence for functional Toll signaling in ticks exists. In vitro challenge of Rhipicephalus microplus with Enterobacter cloacae, Micrococcus luteus and Saccharomyces cerevisiae lead to upregulation of toll, myD88, tube, pelle, and cactus suggesting pathway functionality (Rosa et al., 2016).
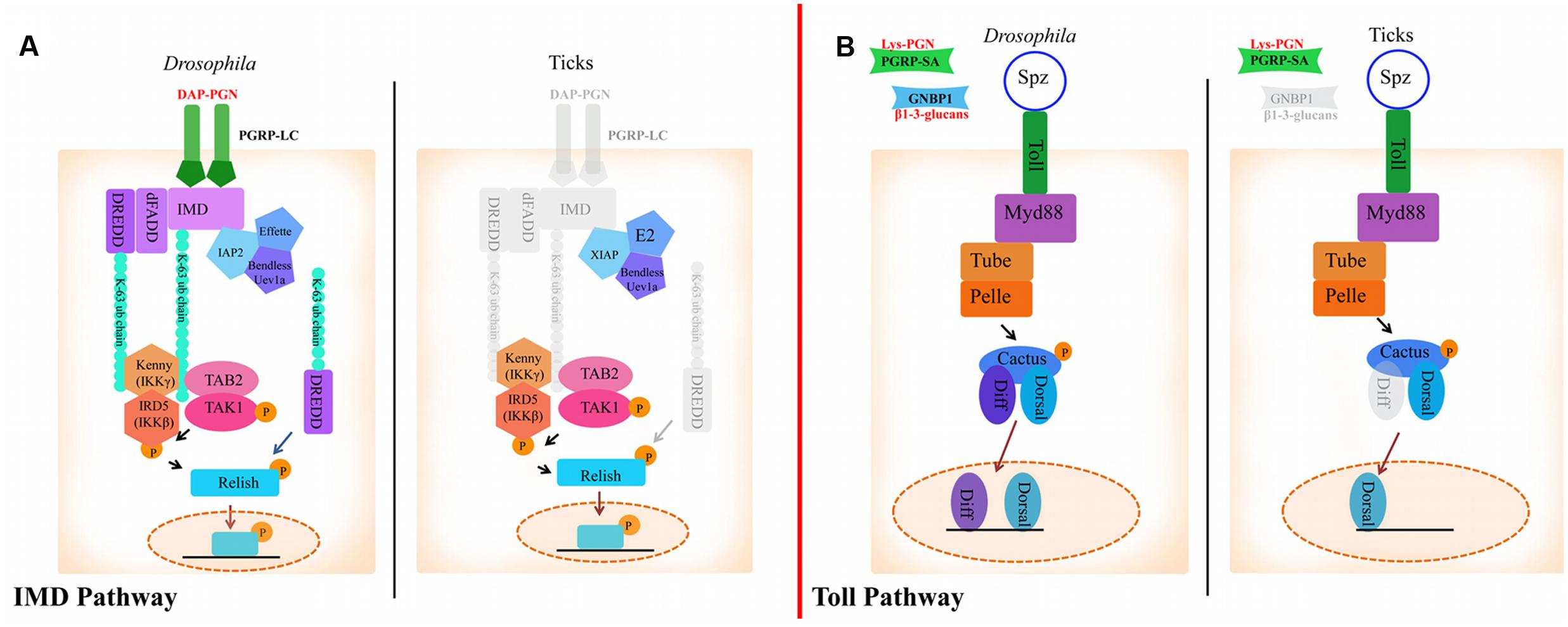
FIGURE 1. The Immune Deficiency (IMD) and Toll pathways in Drosophila and ticks. (A) Activation of the IMD pathway in Drosophila is initiated by PGRP-LC binding to diaminopimelic acid (DAP)-type peptidoglycan. This leads to IMD, dFADD, and DREDD recruitment. IMD is cleaved by DREDD, exposing an ubiquitylation site and is polyubiquitylated by IAP2, Effete, Uev1a and Bendless in a K63-dependent manner. K-63 polyubiquitin chains are believed to serve as the recruiters for the proteins TAB2, TAK1, and IKK (IKKγ and IKKβ), which transfer a phosphate group to Relish. Relish is then cleaved by DREDD, removing the C-terminal ankyrin repeats. The N-terminal portion of Relish is translocated to the nucleus where it induces the transcription of AMPs (Vandenabeele and Bertrand, 2012). In ticks, transmembrane PGRPs, IMD, dFADD and possibly DREDD are missing (shaded gray). XIAP is suggested to regulate the IMD pathway in ticks through direct interaction with Bendless (Shaw et al., 2017). (B) In Drosophila, the Toll pathway is activated by PGRPs and GNBPs binding to Lysine-type peptidoglycan or β1-3-glucan, respectively. PAMP binding to PRRs leads to activation of ModSP (Modular Serine Protease) and Grass in the extracellular milieu. Spz is then cleaved and binds to Toll receptors. Following Spz binding, MyD88 dimers interface with the Toll receptor and recruit Tube, an adaptor molecule that interacts with the protein kinase Pelle. Cactus is then phosphorylated and degraded, which leads to translocation of Dif (Dorsal-related immunity factor) and/or Dorsal to the nucleus and AMP upregulation (Lindsay and Wasserman, 2014). The tick genome encodes all components of the Toll pathway, with the exception of GNBPs and dif.
Drosophila transcriptional regulators controlled by the Toll pathway, Dif and Dorsal, regulate the expression of defensin and other AMPs (Meng et al., 1999). Interestingly, instances of cooperation between transcription factors have been described (Meng et al., 1999). Optimal induction of defensin was reported when the IMD pathway-regulated transcription factor, Relish, formed heterodimers with Dif or Dorsal (Han and Ip, 1999). These experiments were performed in vitro with stably transfected cell lines and thus the in vivo relevance is unclear, but suggests interesting potential for defenses orchestrated by multiple immune pathways. Ticks also produce several Defensin-like AMPs (Johns et al., 2001b; Sonenshine et al., 2002; Ceraul et al., 2003, 2007; Lai et al., 2004; Hynes et al., 2005; Zhou et al., 2007; Wang and Zhu, 2011; Chrudimska et al., 2014; Pelc et al., 2014). Although the mechanism of defensin regulation in ticks is not characterized, the highly conserved nature of the Toll pathway suggests that it may act similarly to insects. Moreover, tick Defensins are secreted in response to both Gram-positive and negative bacteria, suggesting that there may be a similar mechanism of cross-talk in non-insect arthropods (Sonenshine et al., 2002).
Diaminopimelic acid (DAP)-type peptidoglycan from Gram-negative bacteria stimulates the IMD pathway in Drosophila, which is recognized by both transmembrane and soluble PGRPs (Boutros et al., 2002; Hillyer, 2016). Ticks lack several key components of the IMD pathway such as transmembrane PGRPs, imd, dFADD, and IMD pathway-specific AMPs (Table 1; Figure 1) (Severo et al., 2013; Palmer and Jiggins, 2015; Gulia-Nuss et al., 2016; Rosa et al., 2016). Despite lacking key components, the IMD pathway is functional in ticks (Shaw et al., 2017). The I. scapularis Relish is activated in response to Anaplasma phagocytophilum infection and knocking down regulatory components from the IMD pathway (relish, capsar, uev1a, and bendless) lead to altered pathogen burden levels with both A. phagocytophilum and Borrelia burgdorferi (Shaw et al., 2017). A separate study also showed that bacterial infection of R. microplus lead to transcriptional upregulation of IMD signaling components (tak1, tab2, ikkβ, ikkγ, and relish) (Rosa et al., 2016). Taken together, these studies provide evidence for a functional IMD pathway in ticks.
Drosophila PGRP-LC and PGRP-LE are IMD pathway receptors (Kaneko et al., 2006) and PGRP-SD is an IMD co-receptor (Iatsenko et al., 2016). Transmembrane PGRP-LC and soluble PGRP-LE multimerize after binding to DAP-type peptidoglycan and initiate signaling by recruiting IMD to the RIP Homotypic Interaction Motif (RHIM) (Figure 1) (Kaneko et al., 2006). PGRP-SD, initially thought to activate Toll signaling (Bischoff et al., 2004), elicits the IMD pathway by interacting with PGRP-LC (Iatsenko et al., 2016) and DAP-type peptidoglycan (Leone et al., 2008). Although there are four encoded PGRPs in the I. scapularis genome, none are predicted to be transmembrane proteins or to have the IMD-interacting RHIM domain (Palmer and Jiggins, 2015). This is consistent with the lack of imd in the genome, suggesting an alternative mode of pathway activation (Figure 1) (Palmer and Jiggins, 2015; Bechsgaard et al., 2016). The role of secreted PGRPs in ticks is unknown, although a recent study showed that silencing the soluble I. scapularis PGRPs did not have a significant effect on A. phagocytophilum colonization (Shaw et al., 2017).
K63-dependent polyubiquitylation of IMD and dDREDD (Death related ced-3/Nedd2-like caspase) by the E3 ubiquitin ligase, inhibitor of apoptosis protein 2 (IAP2), is necessary for signal transduction in Drosophila (Paquette et al., 2010; Meinander et al., 2012). A different E3 ubiquitin ligase in ticks, X-linked inhibitor of apoptosis (XIAP), has been shown to influence A. phagocytophilum burden (Severo et al., 2013) by interfacing with the IMD pathway (Severo et al., 2013; Shaw et al., 2017). XIAP physically interacts with the IMD pathway E2 ubiquitin conjugating enzyme, Bendless, and carries out K63-dependent polyubiquitylation together with Uev1a (Shaw et al., 2017). Moreover, double knockdown of bendless-uev1a heterodimers and xiap lead to increased colonization by both A. phagocytophilum and B. burgdorferi, suggesting a defect in pathogen control (Shaw et al., 2017).
In addition to alternative signaling modes, there is evidence that PAMPs other than DAP-type peptidoglycan can trigger the IMD signaling cascade. Reports of virus and parasite-induced IMD pathway activation in insects lend support to this hypothesis (Baton et al., 2009; Costa et al., 2009). In ticks, the IMD circuitry senses infection-derived lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-34 phosphoglycerol (POPG) and 1-palmitoyl-2-oleoyl diacylglycerol (PODAG), and leads to Relish activation (Shaw et al., 2017). Moreover, priming ticks with these lipids induced protection against A. phagocytophilum and A. marginale infection both in vitro and in vivo, respectively (Shaw et al., 2017). These findings coupled with the lack of transmembrane PGRPs and key signaling molecules suggest that a non-canonical IMD pathway exists in ticks.
The Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway is not part of the humoral innate response in insects, but does have a role in immunity through crosstalk with IMD and Toll signaling (Myllymaki and Ramet, 2014). The JAK/STAT pathway is activated by the receptor Dome through recognition of the cytokine signaling molecule, Unpaired (Upd) (Harrison et al., 1998; Brown et al., 2001). This interaction results in phosphorylation of Hop proteins and translocation of Stat92E to the nucleus, which stimulates expression of cytokines and members of the tot family (Harrison et al., 1995; Agaisse et al., 2003; Bach et al., 2003; Lamiable et al., 2016). The I. scapularis JAK/STAT pathway is important for the control of A. phagocytophilum and regulates the expression of a gene family that encodes 5.3 kDa antimicrobial peptides (Liu et al., 2012). Comparative analysis demonstrates that the JAK/STAT pathway is well conserved between ticks and Drosophila (Palmer and Jiggins, 2015; Bechsgaard et al., 2016), with the exception of upd (Liu et al., 2012; Rosa et al., 2016).
Beyond pathogen control, the JAK/STAT pathway has an important role in physiological maintenance. Drosophila midgut homeostasis is influenced by the microbiota, which is regulated by phagocytic cells and the IMD pathway. This in turn impacts JAK/STAT signaling (Guillou et al., 2016). Mutation of either the CD36 phagocytic receptor, Croquemort, or Relish causes overexpression of Upd3 and dysregulated gut integrity, leading to increased mortality (Guillou et al., 2016). The mechanistic details involved in JAK/STAT activation in ticks are currently unknown, although the absence of upd is intriguing. A recent study showed that mammalian-derived interferon (IFN)-γ, present in the bloodmeal, stimulated the tick JAK/STAT pathway (Smith et al., 2016). This cytokine cross-talk upregulated the tick Rho-like GTPase (IGTPase) and induced expression of domesticated amidase effector (DAE2), an AMP homologous to eukaryotic effectors that hydrolyzes bacterial peptidoglycans (Chou et al., 2015; Smith et al., 2016). This is an interesting example of cross-species cytokine signaling and could indicate that midgut homeostasis in ticks and the microbiota are influenced by mammalian-derived signaling molecules.
The microbiome is comprised of commensal bacteria in the gut and other endosymbionts (Narasimhan and Fikrig, 2015). Ticks harbor less complex microbial communities, likely due to their blood-only diet, than other vectors that are not exclusively hematophagous, such as mosquitoes (Hawlena et al., 2013; Clayton et al., 2015; Rynkiewicz et al., 2015). Proteobacteria, Actinobacteria, Enterobacter, Sphingobacterium, Firmicutes, Pseudomonas, and Bacteroidetes have all been associated with ticks, although bacterial composition varies depending on geographic region and sex (Van Treuren et al., 2015). Interestingly, there is evidence that the microbiota impacts the arthropod through involvement with the immune system (Hawlena et al., 2013). Commensal bacteria stimulate gut epithelium renewal through JAK/STAT signaling in Drosophila (Buchon et al., 2009). Similarly, the tick microbiota also impacts midgut epithelium and peritrophic membrane integrity (Narasimhan et al., 2014; Narasimhan and Fikrig, 2015).
Although blood is a nutrient-rich source, it lacks some metabolites that are essential for survival. Endosymbiotic relationships can provide these nutrients and have been observed in many hematophagous arthropods including tsetse flies, bed bugs, lice, reduviid bugs and ticks (Rio et al., 2016). For example, a Coxiella-like endosymbiont provides vitamins and co-factors to Amblyomma americanum ticks and is required for adequate fecundity (Smith et al., 2015). A combination of mechanisms is likely used to ensure balance between the arthropod and endosymbiont. The arthropod host must control endosymbiont numbers to avoid over stimulation of immune responses and/or nutrient deprivation. In contrast, endosymbiotic bacteria must evade or suppress immune recognition to avoid clearance (Herren and Lemaitre, 2011; Masson et al., 2015; Shokal et al., 2016). Limited information is known about these relationships, owing to the difficult nature of in vitro symbiont cultivation, although a few studies have been reported (Kurtti et al., 2015, 2016). For example, the intracellular Dermacentor andersoni endosymbiont, R. peacockii, is 150-fold more resistant to AMPs than extracellular bacteria, illustrating a mechanism of immune tolerance (Baldridge et al., 2005). Avoidance mechanisms remain largely understudied, but likely vary depending on the endosymbiont and tick host species.
Simultaneous colonization by multiple pathogens is termed “coinfection” and is becoming a major health concern worldwide (Steiner et al., 2008; Schulze et al., 2013). In Europe, over half of all surveyed I. ricinus ticks are coinfected (Moutailler et al., 2016), with the most prevalent instances occurring in areas that are forested and endemic for Lyme disease (Swanson et al., 2006). Coinfections can increase the severity of illness, as demonstrated with babesiosis and Lyme disease (Diuk-Wasser et al., 2016). Moreover, simultaneous infection of Peromyscus leucopus mice with the parasite, Babesia microti, and B. burgdorferi increased the number of parasites acquired by ticks during a bloodmeal. This was likely due to heightened parasitemia in the mouse during coinfection (Diuk-Wasser et al., 2016). Conflicting reports have been published about coinfections with B. burgdorferi and A. phagocytophilum. One study reported no observable differences in acquisition and transmission with I. scapularis ticks (Levin and Fish, 2000), whereas another demonstrated that B. burgdorferi burden in ticks increased when fed on mice coinfected with A. phagocytophilum (Thomas et al., 2001). E. ruminantium levels increased during in vitro coinfection with B. burgdorferi as well (Moniuszko et al., 2014). Importantly, coinfections are not a phenomenon limited to Ixodes ticks, as both Rhipicephalus sp. and Hyalomma rufipes ticks can harbor between two to four pathogens (Berggoetz et al., 2014). Taken together, this information suggests that coinfection is a previously unappreciated phenomenon that likely impacts tick-borne disease transmission and outcome.
Lyme disease is the most important vector-borne disease in the Northern hemisphere and approximately 30,000 cases are reported annually in the United States (Kugeler et al., 2015; Diuk-Wasser et al., 2016). B. burgdorferi colonizes ticks during a bloodmeal, where they will persist during digestion and molting (Radolf et al., 2012). Transmission subsequently occurs during a second bloodmeal when spirochetes are introduced into a new host with the saliva injected by a feeding tick (Radolf et al., 2012) (Figure 2).
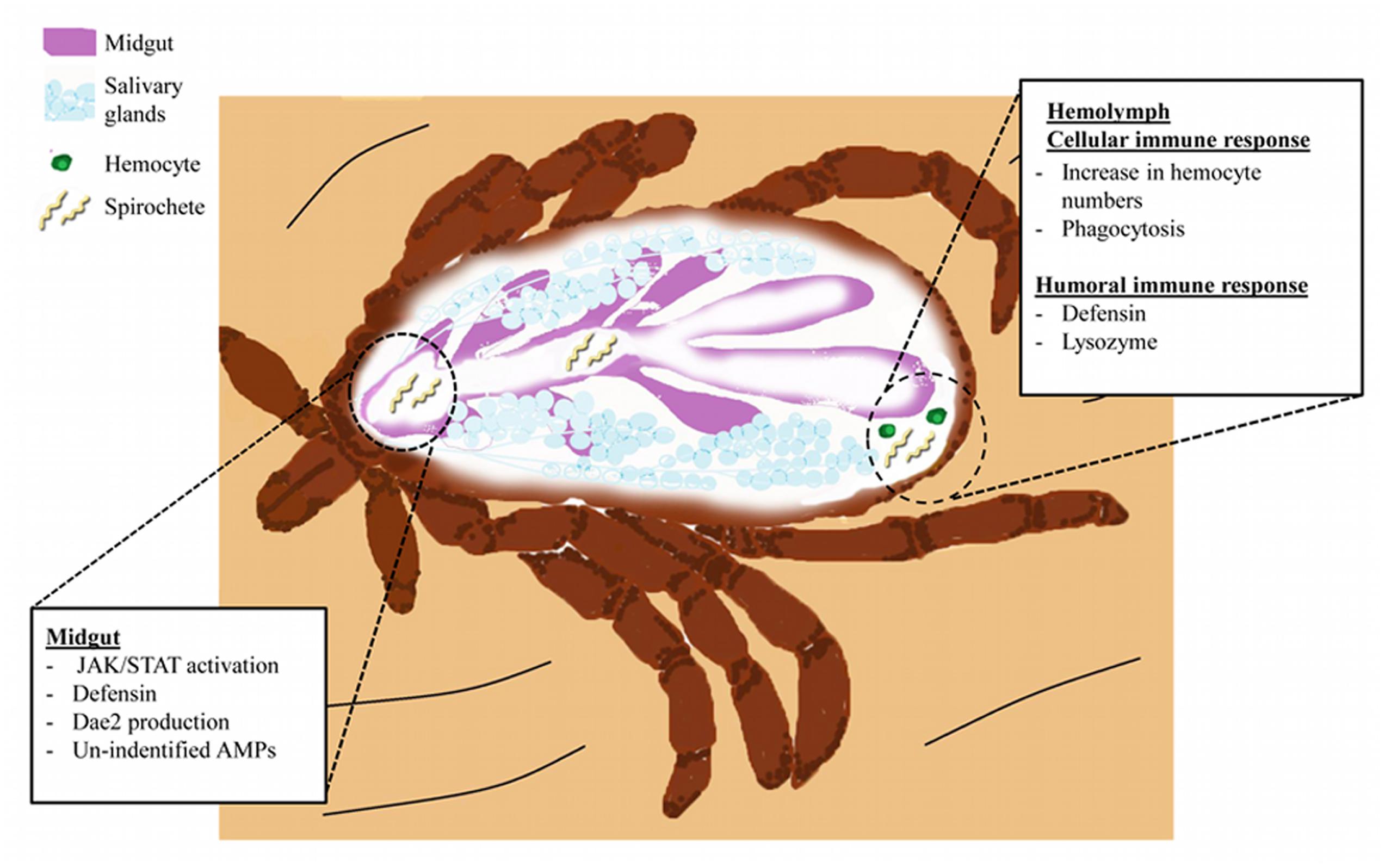
FIGURE 2. The I. scapularis response to B. burgdorferi infection. Spirochetes (light yellow) enter the tick midgut (purple) during blood feeding. Spirochetes interact with gut tissues and trigger activation of the JAK/STAT pathway. Induction of JAK/STAT signaling and possibly other pathways leads to AMP production (Defensins and DAE2). Spirochete migration into the hemolymph elicits cellular and humoral immunity. Cellular responses include increased prevalence of hemocytes (green) and initiation of phagocytosis. Humoral immunity results in the secretion of Defensins (originating from hemocytes and the fat body) and Lysozyme (hemocytes) into the hemolymph (Johns et al., 2001b; Ceraul et al., 2003, 2007). Niche-specific immune responses, such as those originating from the salivary glands (light blue structures), remain elusive.
Different species of ticks vary in their ability to transmit Borrelia spp. (Mather and Mather, 1990; Dolan et al., 1998). Dermacentor ticks, for instance, are not able to acquire or transmit B. burgdorferi (Mather and Mather, 1990). Spirochetes injected into D. variabilis are cleared from the hemocoel, whereas artificially infected I. scapularis retain the pathogen (Johns et al., 2001a). Inoculation of B. burgdorferi results in a rapid increase of hemocytes, lysozyme, and AMPs in D. variabilis (Johns et al., 2000; Sonenshine et al., 2002), which are likely major factors influencing this species’ competence. How and why these responses are not also induced in Ixodes ticks remains unknown, but is an intriguing topic.
The microbiome also influences vector competence. Ticks with a modified microbiota, termed “dysbiosed”, maintain lower B. burgdorferi numbers as compared to normal ticks (Narasimhan et al., 2014). Interestingly, this reduction in spirochetes appears to be related to midgut homeostasis and epithelial renewal controlled by JAK/STAT pathway-regulated expression of peritrophin-1 (Narasimhan et al., 2014). A graphic representation of the humoral and cellular responses of ticks during B. burgdorferi infection can be found in Figure 2.
Although ticks are of increasingly importance, little is known about what dictates their competence as disease vectors. It is known that immune networks heavily influence insect vector competence. However, there are fundamental differences in tick immunity when compared to insects. For example, the repertoire of Toll receptors found in ticks is reduced when compared to Drosophila (Palmer and Jiggins, 2015) and the IMD pathway has a significant amount of gene loss, yet both remain active (Severo et al., 2013; Smith and Pal, 2014; Palmer and Jiggins, 2015; Bechsgaard et al., 2016; Gulia-Nuss et al., 2016; Rosa et al., 2016). Unknown immune networks are likely present in ticks that facilitate the recognition of invading pathogens. Exploiting the long co-evolutionary history between ticks and the pathogens they can transmit, such as Borrelia, Anaplasma, Ehrlichia, and/or Rickettsia, is one avenue for approaching this gap in knowledge. For example, a non-canonical IMD network in ticks has recently been identified using both A. phagocytophilum and B. burgdorferi (Shaw et al., 2017).
Other confounding factors influencing pathogen transmission are coinfections and/or interactions with the microbiota. For instance, simultaneous infection of ticks with A. phagocytophilum and B. burgdorferi leads to higher spirochete burdens (Thomas et al., 2001). It is tempting to speculate that A. phagocytophilum virulence proteins exert an immunosuppressive effect on the tick that inadvertently confers a survival advantage for B. burgdorferi. Another point of interest is the recent evidence that mammalian-derived cytokines can cross-react with the tick immune system (Smith et al., 2016). This discovery sheds new light on what we know about vector competence because coinfection in the mammal will inevitably skew the cytokine profile of the host and thus the bloodmeal taken by a tick. Investigating alternative immune circuitry and agonists will not only lead to better understanding of tick biology and pathogen transmission, but will also illuminate how coinfections are maintained.
AO wrote this review. JP, UM, and DS contributed to intellectual discussions and editing of the article.
This work was supported by National Institute of Health grant R01AI116523 and the University of Maryland School of Medicine to JP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with one of the authors JP and states that the process nevertheless met the standards of a fair and objective review.
Agaisse, H., Petersen, U. M., Boutros, M., Mathey-Prevot, B., and Perrimon, N. (2003). Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 5, 441–450. doi: 10.1016/S1534-5807(03)00244-2
Bach, E. A., Vincent, S., Zeidler, M. P., and Perrimon, N. (2003). A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics 165, 1149–1166.
Baldridge, G. D., Kurtti, T. J., and Munderloh, U. G. (2005). Susceptibility of Rickettsia monacensis and Rickettsia peacockii to Cecropin A, Ceratotoxin A, and lysozyme. Curr. Microbiol. 51, 233–238. doi: 10.1007/s00284-005-4532-7
Baton, L. A., Robertson, A., Warr, E., Strand, M. R., and Dimopoulos, G. (2009). Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genomics 10:257. doi: 10.1186/1471-2164-10-257
Bechsgaard, J., Vanthournout, B., Funch, P., Vestbo, S., Gibbs, R. A., Richards, S., et al. (2016). Comparative genomic study of arachnid immune systems indicates loss of beta-1,3-glucanase-related proteins and the immune deficiency pathway. J. Evol. Biol. 29, 277–291. doi: 10.1111/jeb.12780
Berggoetz, M., Schmid, M., Ston, D., Wyss, V., Chevillon, C., Pretorius, A. M., et al. (2014). Protozoan and bacterial pathogens in tick salivary glands in wild and domestic animal environments in South Africa. Ticks Tick Borne Dis. 5, 176–185. doi: 10.1016/j.ttbdis.2013.10.003
Bischoff, V., Vignal, C., Boneca, I. G., Michel, T., Hoffmann, J. A., and Royet, J. (2004). Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat. Immunol. 5, 1175–1180. doi: 10.1038/ni1123
Blumberg, B. J., Trop, S., Das, S., and Dimopoulos, G. (2013). Bacteria- and IMD pathway-independent immune defenses against Plasmodium falciparum in Anopheles gambiae. PLoS ONE 8:e72130. doi: 10.1371/journal.pone.0072130
Boutros, M., Agaisse, H., and Perrimon, N. (2002). Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev. Cell 3, 711–722. doi: 10.1016/S1534-5807(02)00325-8
Brown, S., Hu, N., and Hombria, J. C. (2001). Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr. Biol. 11, 1700–1705. doi: 10.1016/S0960-9822(01)00524-3
Buchon, N., Broderick, N. A., Chakrabarti, S., and Lemaitre, B. (2009). Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344. doi: 10.1101/gad.1827009
Buchon, N., Silverman, N., and Cherry, S. (2014). Immunity in Drosophila melanogaster–from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 14, 796–810. doi: 10.1038/nri3763
Ceraul, S. M., Dreher-Lesnick, S. M., Gillespie, J. J., Rahman, M. S., and Azad, A. F. (2007). New tick defensin isoform and antimicrobial gene expression in response to Rickettsia montanensis challenge. Infect. Immun. 75, 1973–1983. doi: 10.1128/IAI.01815-06
Ceraul, S. M., Sonenshine, D. E., Ratzlaff, R. E., and Hynes, W. L. (2003). An arthropod defensin expressed by the hemocytes of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). Insect Biochem. Mol. Biol. 33, 1099–1103. doi: 10.1016/S0965-1748(03)00122-X
Chou, S., Daugherty, M. D., Peterson, S. B., Biboy, J., Yang, Y., Jutras, B. L., et al. (2015). Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature 518, 98–101. doi: 10.1038/nature13965
Chrudimska, T., Cerovsky, V., Slaninova, J., Rego, R. O., and Grubhoffer, L. (2014). Defensin from the ornate sheep tick Dermacentor marginatus and its effect on Lyme borreliosis spirochetes. Dev. Comp. Immunol. 46, 165–170. doi: 10.1016/j.dci.2014.04.005
Cirimotich, C. M., Ramirez, J. L., and Dimopoulos, G. (2011). Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 10, 307–310. doi: 10.1016/j.chom.2011.09.006
Clayton, K. A., Gall, C. A., Mason, K. L., Scoles, G. A., and Brayton, K. A. (2015). The characterization and manipulation of the bacterial microbiome of the Rocky Mountain wood tick, Dermacentor andersoni. Parasit. Vectors 8, 632. doi: 10.1186/s13071-015-1245-z
Costa, A., Jan, E., Sarnow, P., and Schneider, D. (2009). The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 4:e7436. doi: 10.1371/journal.pone.0007436
de la Fuente, J., Estrada-Pena, A., Venzal, J. M., Kocan, K. M., and Sonenshine, D. E. (2008). Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13:6938–6946. doi: 10.2741/3200
Diuk-Wasser, M. A., Vannier, E., and Krause, P. J. (2016). Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 32, 30–42. doi: 10.1016/j.pt.2015.09.008
Dolan, M. C., Piesman, J., Mbow, M. L., Maupin, G. O., Peter, O., Brossard, M., et al. (1998). Vector competence of Ixodes scapularis and Ixodes ricinus (Acari: Ixodidae) for three genospecies of Borrelia burgdorferi. J. Med. Entomol. 35, 465–470. doi: 10.1093/jmedent/35.4.465
Ganesan, S., Aggarwal, K., Paquette, N., and Silverman, N. (2011). NF-kappaB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr. Top. Microbiol. Immunol. 349, 25–60. doi: 10.1007/82_2010_107
Garver, L. S., Dong, Y., and Dimopoulos, G. (2009). Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 5:e1000335. doi: 10.1371/journal.ppat.1000335
Gerardo, N. M., Altincicek, B., Anselme, C., Atamian, H., Barribeau, S. M., De Vos, M., et al. (2010). Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol. 11:R21. doi: 10.1186/gb-2010-11-2-r21
Guillou, A., Troha, K., Wang, H., Franc, N. C., and Buchon, N. (2016). The Drosophila CD36 homologue croquemort is required to maintain immune and gut homeostasis during development and aging. PLoS Pathog. 12:e1005961. doi: 10.1371/journal.ppat.1005961
Gulia-Nuss, M., Nuss, A. B., Meyer, J. M., Sonenshine, D. E., Roe, R. M., Waterhouse, R. M., et al. (2016). Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 7:10507. doi: 10.1038/ncomms10507
Han, Z. S., and Ip, Y. T. (1999). Interaction and specificity of Rel-related proteins in regulating Drosophila immunity gene expression. J. Biol. Chem. 274, 21355–21361. doi: 10.1074/jbc.274.30.21355
Harrison, D. A., Binari, R., Nahreini, T. S., Gilman, M., and Perrimon, N. (1995). Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14, 2857–2865.
Harrison, D. A., Mccoon, P. E., Binari, R., Gilman, M., and Perrimon, N. (1998). Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 12, 3252–3263. doi: 10.1101/gad.12.20.3252
Hartemink, N., and Takken, W. (2016). Trends in tick population dynamics and pathogen transmission in emerging tick-borne pathogens in Europe: an introduction. Exp. Appl. Acarol. 68, 269–278. doi: 10.1007/s10493-015-0003-4
Hawlena, H., Rynkiewicz, E., Toh, E., Alfred, A., Durden, L. A., Hastriter, M. W., et al. (2013). The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J. 7, 221–223. doi: 10.1038/ismej.2012.71
Herren, J. K., and Lemaitre, B. (2011). Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol. 13, 1385–1396. doi: 10.1111/j.1462-5822.2011.01627.x
Hillyer, J. F. (2016). Insect immunology and hematopoiesis. Dev. Comp. Immunol. 58, 102–118. doi: 10.1016/j.dci.2015.12.006
Hillyer, J. F., Schmidt, S. L., and Christensen, B. M. (2003). Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 313, 117–127. doi: 10.1007/s00441-003-0744-y
Hynes, W. L., Ceraul, S. M., Todd, S. M., Seguin, K. C., and Sonenshine, D. E. (2005). A defensin-like gene expressed in the black-legged tick, Ixodes scapularis. Med. Vet. Entomol. 19, 339–344. doi: 10.1111/j.1365-2915.2005.00579.x
Iatsenko, I., Kondo, S., Mengin-Lecreulx, D., and Lemaitre, B. (2016). PGRP-SD, an extracellular pattern-recognition receptor, enhances peptidoglycan-mediated activation of the Drosophila Imd Pathway. Immunity 45, 1013–1023. doi: 10.1016/j.immuni.2016.10.029
Johns, R., Ohnishi, J., Broadwater, A., Sonenshine, D. E., De Silva, A. M., and Hynes, W. L. (2001a). Contrasts in tick innate immune responses to Borrelia burgdorferi challenge: immunotolerance in Ixodes scapularis versus immunocompetence in Dermacentor variabilis (Acari: Ixodidae). J. Med. Entomol. 38, 99–107. doi: 10.1603/0022-2585-38.1.99
Johns, R., Sonenshine, D. E., and Hynes, W. L. (2000). Response of the tick Dermacentor variabilis (Acari: Ixodidae) to hemocoelic inoculation of Borrelia burgdorferi (Spirochetales). J. Med. Entomol. 37, 265–270.
Johns, R., Sonenshine, D. E., and Hynes, W. L. (2001b). Identification of a defensin from the hemolymph of the American dog tick, Dermacentor variabilis. Insect Biochem. Mol. Biol. 31, 857–865. doi: 10.1603/0022-2585-37.2.265
Kanagawa, M., Satoh, T., Ikeda, A., Adachi, Y., Ohno, N., and Yamaguchi, Y. (2011). Structural insights into recognition of triple-helical beta-glucans by an insect fungal receptor. J. Biol. Chem. 286, 29158–29165. doi: 10.1074/jbc.M111.256701
Kaneko, T., Yano, T., Aggarwal, K., Lim, J. H., Ueda, K., Oshima, Y., et al. (2006). PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 7, 715–723. doi: 10.1038/ni1356
Kernif, T., Leulmi, H., Raoult, D., and Parola, P. (2016). Emerging tick-borne bacterial pathogens. Microbiol. Spectr. 4:EI10-0012-2016. doi: 10.1128/microbiolspec.EI10-0012-2016
Kopacek, P., Vogt, R., Jindrak, L., Weise, C., and Safarik, I. (1999). Purification and characterization of the lysozyme from the gut of the soft tick Ornithodoros moubata. Insect Biochem. Mol. Biol. 29, 989–997. doi: 10.1016/S0965-1748(99)00075-2
Kugeler, K. J., Farley, G. M., Forrester, J. D., and Mead, P. S. (2015). Geographic distribution and expansion of human Lyme Disease, United States. Emerg. Infect. Dis. 21, 1455–1457. doi: 10.3201/eid2108.141878
Kurtti, T. J., Burkhardt, N. Y., Heu, C. C., and Munderloh, U. G. (2016). Fluorescent protein expressing Rickettsia buchneri and Rickettsia peacockii for tracking symbiont-tick cell interactions. Vet. Sci. 3, 34. doi: 10.3390/vetsci3040034
Kurtti, T. J., Felsheim, R. F., Burkhardt, N. Y., Oliver, J. D., Heu, C. C., and Munderloh, U. G. (2015). Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int. J. Syst. Evol. Microbiol. 65, 965–970. doi: 10.1099/ijs.0.000047
Lai, R., Lomas, L. O., Jonczy, J., Turner, P. C., and Rees, H. H. (2004). Two novel non-cationic defensin-like antimicrobial peptides from haemolymph of the female tick, Amblyomma hebraeum. Biochem. J. 379, 681–685. doi: 10.1042/bj20031429
Lamiable, O., Kellenberger, C., Kemp, C., Troxler, L., Pelte, N., Boutros, M., et al. (2016). Cytokine diedel and a viral homologue suppress the IMD pathway in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 113, 698–703. doi: 10.1073/pnas.1516122113
Leone, P., Bischoff, V., Kellenberger, C., Hetru, C., Royet, J., and Roussel, A. (2008). Crystal structure of Drosophila PGRP-SD suggests binding to DAP-type but not lysine-type peptidoglycan. Mol. Immunol. 45, 2521–2530. doi: 10.1016/j.molimm.2008.01.015
Levin, M. L., and Fish, D. (2000). Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect. Immun. 68, 2183–2186. doi: 10.1128/IAI.68.4.2183-2186.2000
Li, F., and Xiang, J. (2013). Signaling pathways regulating innate immune responses in shrimp. Fish Shellfish Immunol. 34, 973–980. doi: 10.1016/j.fsi.2012.08.023
Lindsay, S. A., and Wasserman, S. A. (2014). Conventional and non-conventional Drosophila Toll signaling. Dev. Comp. Immunol. 42, 16–24. doi: 10.1016/j.dci.2013.04.011
Liu, F., Li, F., Dong, B., Wang, X., and Xiang, J. (2009). Molecular cloning and characterisation of a pattern recognition protein, lipopolysaccharide and beta-1,3-glucan binding protein (LGBP) from Chinese shrimp Fenneropenaeus chinensis. Mol. Biol. Rep. 36, 471–477. doi: 10.1007/s11033-007-9203-2
Liu, L., Dai, J., Zhao, Y. O., Narasimhan, S., Yang, Y., Zhang, L., et al. (2012). Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J. Infect. Dis. 206, 1233–1241. doi: 10.1093/infdis/jis484
Liu, X. Y., and Bonnet, S. I. (2014). Hard tick factors implicated in pathogen transmission. PLoS Negl. Trop. Dis. 8:e2566. doi: 10.1371/journal.pntd.0002566
Masson, F., Vallier, A., Vigneron, A., Balmand, S., Vincent-Monegat, C., Zaidman-Remy, A., et al. (2015). Systemic infection generates a local-like immune response of the bacteriome organ in insect symbiosis. J. Innate Immun. 7, 290–301. doi: 10.1159/000368928
Mather, T. N., and Mather, M. E. (1990). Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J. Med. Entomol. 27, 646–650. doi: 10.1093/jmedent/27.4.646
McTaggart, S. J., Conlon, C., Colbourne, J. K., Blaxter, M. L., and Little, T. J. (2009). The components of the Daphnia pulex immune system as revealed by complete genome sequencing. BMC Genomics 10:175. doi: 10.1186/1471-2164-10-175
Meinander, A., Runchel, C., Tenev, T., Chen, L., Kim, C. H., Ribeiro, P. S., et al. (2012). Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. EMBO J. 31, 2770–2783. doi: 10.1038/emboj.2012.121
Meng, X., Khanuja, B. S., and Ip, Y. T. (1999). Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev. 13, 792–797. doi: 10.1101/gad.13.7.792
Michel, T., Reichhart, J. M., Hoffmann, J. A., and Royet, J. (2001). Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414, 756–759. doi: 10.1038/414756a
Moniuszko, A., Ruckert, C., Alberdi, M. P., Barry, G., Stevenson, B., Fazakerley, J. K., et al. (2014). Coinfection of tick cell lines has variable effects on replication of intracellular bacterial and viral pathogens. Ticks Tick Borne Dis. 5, 415–422. doi: 10.1016/j.ttbdis.2014.01.010
Moutailler, S., Valiente Moro, C., Vaumourin, E., Michelet, L., Tran, F. H., Devillers, E., et al. (2016). Co-infection of ticks: the rule rather than the exception. PLoS Negl. Trop. Dis. 10:e0004539. doi: 10.1371/journal.pntd.0004539
Myllymaki, H., and Ramet, M. (2014). JAK/STAT pathway in Drosophila immunity. Scand. J. Immunol. 79, 377–385. doi: 10.1111/sji.12170
Myllymaki, H., Valanne, S., and Ramet, M. (2014). The Drosophila imd signaling pathway. J. Immunol. 192, 3455–3462. doi: 10.4049/jimmunol.1303309
Narasimhan, S., and Fikrig, E. (2015). Tick microbiome: the force within. Trends Parasitol. 31, 315–323. doi: 10.1016/j.pt.2015.03.010
Narasimhan, S., Rajeevan, N., Liu, L., Zhao, Y. O., Heisig, J., Pan, J., et al. (2014). Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 15, 58–71. doi: 10.1016/j.chom.2013.12.001
Palmer, W. J., and Jiggins, F. M. (2015). Comparative genomics reveals the origins and diversity of arthropod immune systems. Mol. Biol. Evol. 32, 2111–2129. doi: 10.1093/molbev/msv093
Paquette, N., Broemer, M., Aggarwal, K., Chen, L., Husson, M., Erturk-Hasdemir, D., et al. (2010). Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol. Cell 37, 172–182. doi: 10.1016/j.molcel.2009.12.036
Pelc, R. S., Mcclure, J. C., Sears, K. T., Chung, A., Rahman, M. S., and Ceraul, S. M. (2014). Defending the fort: a role for defensin-2 in limiting Rickettsia montanensis infection of Dermacentor variabilis. Insect Mol. Biol. 23, 457–465. doi: 10.1111/imb.12094
Radolf, J. D., Caimano, M. J., Stevenson, B., and Hu, L. T. (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99. doi: 10.1038/nrmicro2714
Rio, R. V., Attardo, G. M., and Weiss, B. L. (2016). Grandeur alliances: symbiont metabolic integration and obligate arthropod hematophagy. Trends Parasitol. 32, 739–749. doi: 10.1016/j.pt.2016.05.002
Rosa, R. D., Capelli-Peixoto, J., Mesquita, R. D., Kalil, S. P., Pohl, P. C., Braz, G. R., et al. (2016). Exploring the immune signalling pathway-related genes of the cattle tick Rhipicephalus microplus: from molecular characterization to transcriptional profile upon microbial challenge. Dev. Comp. Immunol. 59, 1–14. doi: 10.1016/j.dci.2015.12.018
Rynkiewicz, E. C., Hemmerich, C., Rusch, D. B., Fuqua, C., and Clay, K. (2015). Concordance of bacterial communities of two tick species and blood of their shared rodent host. Mol. Ecol. 24, 2566–2579. doi: 10.1111/mec.13187
Schulze, T. L., Jordan, R. A., Healy, S. P., and Roegner, V. E. (2013). Detection of Babesia microti and Borrelia burgdorferi in host-seeking Ixodes scapularis (Acari: Ixodidae) in Monmouth County, New Jersey. J. Med. Entomol. 50, 379–383. doi: 10.1603/ME12088
Severo, M. S., Choy, A., Stephens, K. D., Sakhon, O. S., Chen, G., Chung, D. W., et al. (2013). The E3 ubiquitin ligase XIAP restricts Anaplasma phagocytophilum colonization of Ixodes scapularis ticks. J. Infect. Dis. 208, 1830–1840. doi: 10.1093/infdis/jit380
Shaw, D., Wang, X., Brown, L., Oliva Chávez, A., Reif, K., Smith, A., et al. (2017). Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat. Commun.
Shokal, U., Yadav, S., Atri, J., Accetta, J., Kenney, E., Banks, K., et al. (2016). Effects of co-occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non-pathogenic bacteria. BMC Microbiol. 16:16. doi: 10.1186/s12866-016-0634-6
Simser, J. A., Macaluso, K. R., Mulenga, A., and Azad, A. F. (2004). Immune-responsive lysozymes from hemocytes of the American dog tick, Dermacentor variabilis and an embryonic cell line of the Rocky Mountain wood tick, D. andersoni. Insect Biochem. Mol. Biol. 34, 1235–1246. doi: 10.1016/j.ibmb.2004.07.003
Smith, A. A., Navasa, N., Yang, X., Wilder, C. N., Buyuktanir, O., Marques, A., et al. (2016). Cross-species interferon signaling boosts microbicidal activity within the tick vector. Cell Host Microbe 20, 91–98. doi: 10.1016/j.chom.2016.06.001
Smith, A. A., and Pal, U. (2014). Immunity-related genes in Ixodes scapularis–perspectives from genome information. Front. Cell Infect. Microbiol. 4:116. doi: 10.3389/fcimb.2014.00116
Smith, T. A., Driscoll, T., Gillespie, J. J., and Raghavan, R. (2015). A Coxiella-like endosymbiont is a potential vitamin source for the Lone Star tick. Genome Biol. Evol. 7, 831–838. doi: 10.1093/gbe/evv016
Sonenshine, D. E., Ceraul, S. M., Hynes, W. E., Macaluso, K. R., and Azad, A. F. (2002). Expression of defensin-like peptides in tick hemolymph and midgut in response to challenge with Borrelia burgdorferi, Escherichia coli and Bacillus subtilis. Exp. Appl. Acarol. 28, 127–134. doi: 10.1023/A:1025354326877
Steiner, F. E., Pinger, R. R., Vann, C. N., Grindle, N., Civitello, D., Clay, K., et al. (2008). Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 45, 289–297. doi: 10.1093/jmedent/45.2.289
Stromdahl, E. Y., and Hickling, G. J. (2012). Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the south-eastern United States. Zoonoses Public Health 59(Suppl. 2), 48–64. doi: 10.1111/j.1863-2378.2012.01475.x
Swanson, S. J., Neitzel, D., Reed, K. D., and Belongia, E. A. (2006). Coinfections acquired from ixodes ticks. Clin. Microbiol. Rev 19, 708–727. doi: 10.1128/CMR.00011-06
Thomas, V., Anguita, J., Barthold, S. W., and Fikrig, E. (2001). Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect. Immun. 69, 3359–3371. doi: 10.1128/IAI.69.5.3359-3371.2001
Udompetcharaporn, A., Junkunlo, K., Senapin, S., Roytrakul, S., Flegel, T. W., and Sritunyalucksana, K. (2014). Identification and characterization of a QM protein as a possible peptidoglycan recognition protein (PGRP) from the giant tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 46, 146–154. doi: 10.1016/j.dci.2014.04.003
Van Treuren, W., Ponnusamy, L., Brinkerhoff, R. J., Gonzalez, A., Parobek, C. M., Juliano, J. J., et al. (2015). Variation in the microbiota of Ixodes ticks with regard to geography, Species, and Sex. Appl. Environ. Microbiol. 81, 6200–6209. doi: 10.1128/AEM.01562-15
Vandenabeele, P., and Bertrand, M. J. (2012). The role of the IAP E3 ubiquitin ligases in regulating pattern-recognition receptor signalling. Nat. Rev. Immunol. 12, 833–844. doi: 10.1038/nri3325
Wang, Y., and Zhu, S. (2011). The defensin gene family expansion in the tick Ixodes scapularis. Dev. Comp. Immunol. 35, 1128–1134. doi: 10.1016/j.dci.2011.03.030
Waterhouse, R. M., Kriventseva, E. V., Meister, S., Xi, Z., Alvarez, K. S., Bartholomay, L. C., et al. (2007). Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316, 1738–1743. doi: 10.1126/science.1139862
Keywords: tick-borne diseases, Lyme disease, vector, ticks, humoral immunity
Citation: Oliva Chávez AS, Shaw DK, Munderloh UG and Pedra JHF (2017) Tick Humoral Responses: Marching to the Beat of a Different Drummer. Front. Microbiol. 8:223. doi: 10.3389/fmicb.2017.00223
Received: 27 October 2016; Accepted: 31 January 2017;
Published: 14 February 2017.
Edited by:
Melissa Jo Caimano, University of Connecticut Health Center, USAReviewed by:
Juan Anguita, CIC bioGUNE, SpainCopyright © 2017 Oliva Chávez, Shaw, Munderloh and Pedra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adela S. Oliva Chávez, YW9saXZhY2hhdmV6QHNvbS51bWFyeWxhbmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.