- 1Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing, China
- 2College of Life Sciences, University of Chinese Academy of Sciences, Beijing, China
The limitations of chemical fungicides for the control of postharvest diseases have recently become more apparent. The utilization of antagonistic microorganisms is a promising alternative to that of fungicides to control postharvest decay. In previous studies, the antagonistic yeast Cryptococcus laurentii has shown excellent effects of biocontrol and great potential for practical application. Adverse conditions, such as oxidative stress, limit the practical application of antagonistic yeast. In this study, we investigated the oxidative stress tolerance of C. laurentii and the associated mechanisms. The results indicated that exogenous oxidative stress has a significant effect on the viability and biocontrol efficiency of C. laurentii. H2O2-induced oxidative stress led to the accumulation of reactive oxygen species. The results of flow cytometric analysis suggested that apoptosis is responsible for the reduced survival rate of C. laurentii under oxidative stress. Using tests of antioxidant activity, we found that C. laurentii could employ enzymatic systems to resist exogenous oxidative stress. The addition of exogenous glutathione, a non-enzymatic antioxidant, to the media can significantly enhance oxidative tolerance and biocontrol efficiency of C. laurentii.
Introduction
Postharvest diseases of fruits and vegetables cause considerable economic losses worldwide, and account for more than 25% of total production in developed countries and more than 50% in developing countries (Nunes, 2012). The application of chemical fungicides is currently the primary means of controlling postharvest disease. Nevertheless, the excessive use of fungicides has led to several negative effects, e.g., drug resistance of pathogens, environmental pollution, and the subsequent harm to human health (Janisiewicz and Korsten, 2002; Droby et al., 2009; Jamalizadeh et al., 2011). Therefore, the quest for safe and effective alternatives to fungicides is crucial. Antagonistic yeasts, such as Cryptococcus laurentii, Rhodotorula glutinis, and Pichia membranifaciens, have been exploited as promising alternatives to synthetic fungicides, and have been gradually receiving considerable attention (Fan and Tian, 2000; Qin et al., 2003, 2004; Li and Tian, 2006).
During their application, biocontrol agents are subjected to many adverse stresses that affect their survival and performance (Sui et al., 2015). Yeasts are commonly subjected to oxidative stress (Li and Tian, 2007; Macarisin et al., 2010). After pathogenic attack in the host, an oxidative burst, which is associated with increased levels of H2O2 and O2-, can be generated in the area surrounding the infection site, and serves as an early resistance response to pathogenic invasion (Segal, 2005; Temme and Tudzynski, 2009). Furthermore, antagonistic yeasts could also act as an elicitor that triggers ROS signaling in host tissue and thereby activates host defenses (Chan and Tian, 2006; Xu et al., 2008; Macarisin et al., 2010). Excessive ROS can affect the viability and biocontrol efficacy of antagonistic yeasts. However, the ability of antagonistic yeasts to withstand oxidative stress varies among different species. Liu et al. (2011a,b, 2012) examined the responses of Metschnikowia fructicola, Candida oleophila, and Cystofilobasidium infirmominiatum to oxidative stress. They found that C. infirmominiatum was sensitive and M. fructicola was relatively tolerant to oxidative stress. The antagonistic yeast C. laurentii has been widely studied and has shown excellent biocontrol efficacy against many postharvest diseases of apples, strawberries, mangoes, and sweet cherries (Tian et al., 2004; Bautista-Rosales et al., 2014; Navarta et al., 2014; Zhang et al., 2015). A previous study has indicated that oxidative stress tolerance of an antagonistic yeast species is closely associated with its biocontrol performance in postharvest application (Castoria et al., 2003). Although many studies have reported on oxidative stress resistance of antagonistic yeasts, further discovery regarding the mechanisms of action by which oxidative stress regulates their viability and biocontrol efficacy remain unknown.
The present study aimed to evaluate the tolerance of C. laurentii to oxidative stress and elucidate the antioxidative mechanism. Moreover, the mechanisms by which oxidative stress is used to regulate survival and biocontrol efficacy of C. laurentii were investigated, using flow cytometric analysis. Methods to improve oxidative stress resistance and biocontrol performance were also exploited.
Materials and Methods
Yeast and Pathogens
Cryptococcus laurentii was isolated from the surfaces of apple fruits in a previous experiment (Qin et al., 2004) and grown in YPD broth (10 g yeast extract, 20 g peptone, and 20 g dextrose in 1 L water). Yeast cultures with an initial concentration of 1 × 105 cells/mL were incubated at 26°C on a rotary shaker at 200 rpm for 17 h to reach the mid-log phase. Penicillium expansum was isolated from naturally infected apple fruits. It was routinely cultured on potato dextrose agar plates for 14 days at 25°C. Fungal spores were harvested by flooding the surface of the culture with sterile distilled water, followed by filtration through four layers of sterile cheesecloth. The number of spores in the resulting suspension was calculated using a hemocytometer. Before inoculation, the spore concentration in sterile distilled water was adjusted to 1 × 104/mL.
Fruit
Peach fruits (Prunus persica L. Batsch) at commercial maturity were harvested from an orchard in Beijing and immediately transported to the laboratory. Fruits without blemishes or rot were selected based on uniformity of size. Selected fruits were surface-disinfected with 2% (v/v) sodium hypochlorite for 2 min, rinsed with tap water, and air-dried prior to further use.
Oxidative Stress Tolerance Assays
The median lethal concentration of H2O2 for C. laurentii was determined according to the methods of Chen et al. (2015). Cells in the mid-log phase were obtained by centrifugation. After being washed twice with sterile distilled water, yeast cells were resuspended in fresh YPD medium to a final concentration of 5 × 107 cells/mL. H2O2 was added to each yeast culture to final concentrations of 0, 100, 200, 300, and 400 mM. Following incubation for 90 min (150 rpm, 26°C), yeast cells of each sample were collected and adjusted to 1 × 106 cells/mL. To analyze survival rates, a 50 μL yeast sample was spread on a YPD solid plate. The plates were subsequently observed under a light microscope (Carl Zeiss, Oberkochen, Germany). The effects of treatment time with H2O2 on yeast viability were determined using a plate assay according to the methods of Liu et al. (2011b). Yeast cell viability was expressed as a percentage of the colony number following H2O2 treatment, relative to that without treatment. For each treatment, there were three replicates and the experiment was performed twice.
Detection of Intracellular ROS
Intracellular ROS was detected using a 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) oxidant-sensitive probe (Molecular Probes, Eugene, OR, USA). DCFH-DA was added to the yeast suspension and incubated in the dark at 37°C for 30 min. After being washed twice with PBS, yeast cells were examined under a microscope (Zeiss Axioskop, Oberkochen, Germany) using a 485-nm excitation and 530-nm emission filter combination. Three independent experiments were performed.
The fluorescence intensity of C. laurentii cells was determined using a fluorescence microplate reader (Synergy H4, BioTek, Winooski, VT, USA). Yeast samples were washed twice with N-2-hydroxyethylpiperazine-N-2’-ethanesulfonic acid (HEPES) buffer (pH 7.0) and incubated with 10 μM DCFH-DA at 37°C for 30 min. The samples were then washed twice with HEPES buffer and diluted to an optical density (OD) at 600 nm of 1.4. Yeast samples (200 μL/well) were then added to a 96-well dark microplate, and fluorescence was analyzed using a fluorescence microplate reader with an excitation wavelength of 492 nm and an emission wavelength of 527 nm. Three replicate wells were analyzed for each treatment, and the experiment was performed twice.
Biocontrol Analysis of C. laurentii
Biocontrol performance of C. laurentii against P. expansum was determined on peach fruits. C. laurentii cells at mid-log phase were either treated with 300 mM H2O2 for 90 min as described above or left untreated. Peach fruits were punctured at the equatorial line (three wounds per fruit) using a sterile nail, and first inoculated with 10 μL C. laurentii cell suspension (5 × 107 cells/mL) and then with 10 μL P. expansum spore suspension (1 × 104 spores/mL). Treated fruits were placed in plastic boxes. Each tray was enclosed with a polyethylene bag to maintain high humidity (about 95% relative humidity), and stored at 25°C. Disease incidence and lesion diameters of the fruits were recorded after 3, 4, and 5 days. Each treatment comprised three replicates with ten fruits per replicate, and the experiment was performed twice.
Analysis of Apoptosis of C. laurentii Cells under Oxidative Stress
To discriminate between viable, necrotic, and apoptotic cells, flow cytometric measurements of Hoechst 33342/PI double stained yeast cells were carried out using a MoFlo XDP Cell Sorter (Beckman Coulter, Brea, CA, USA). Stained cells were analyzed using laser-based flow cytometry systems. Yeast cells at the mid-log phase were collected and treated with 300 mM H2O2 for 90 min. After being washed twice with PBS, the treated and untreated yeast cells were incubated with 10 μg/mL PI and 5 μg/mL Hoechst 33342 for 20 min in the dark. PI-positive yeast cells indicated damaged plasma membranes and the presence of necrotic cells. PI-negative and Hoechst 33342-positive yeast cells were considered apoptotic (Hong et al., 2007). The cell density of each sample was maintained at approximately 1 × 106 cells/mL. The sample flow rate was 700 cells/s. A total of 20,000 cells were measured for each sample.
Assays of CAT and SOD Activity
Rhodotorula glutinis, an oxidative stress-sensitive yeast, was used as a positive control in the analysis of antioxidant systems. For the enzyme activity assay, yeast cells were collected by centrifugation at specific intervals (0, 0.5, 1, 2, and 3 h) after being treated with a moderately lethal concentration of H2O2 and washed twice with PBS. The yeast cells were disintegrated with glass beads through vibration on a vortex mixer. SOD and CAT were extracted with 50 mM PBS (pH 7.0, 1 mM EDTA, and 2 mM phenylmethanesulfonyl fluoride). The reaction mixture (3 mL) with SOD contained 50 mM PBS, 13 mM methionine, 75 μM nitroblue tetrazolium, 10 μM EDTA, 2 μM riboflavin, and 50 μL enzyme extract. The mixtures were illuminated by light (4000 lx) for 20 min, and the absorbance was determined at 560 nm. Identical solutions held in the dark served as blanks. One unit of SOD activity was defined as the amount of enzyme causing 50% inhibition in the nitroblue tetrazolium reduction. The reaction mixture (1.5 mL) with CAT consisted of 1.4 mL H2O2 (40 mM) and 100 μL enzyme extract. The decomposition of H2O2 was determined based on the decline in absorbance at 240 nm. One unit of CAT activity was defined as the decomposition of 1 μM H2O2 per min. The activity of both enzymes was expressed as U/mg protein.
Assays of Total Glutathione
Extracts for the total glutathione assay were prepared according to the methods of Ellman (1959), with minor modifications. Cells of R. glutinis and C. laurentii at the mid-log phase were collected and treated with 30 and 300 mM H2O2 for 90 min, respectively. The cells were then harvested by centrifugation at 8000 × g for 3 min, washed three times with sterile distilled water, resuspended in PBS (pH 7.0), and extracted by vortexing with glass beads. The extracts were used for the total glutathione assay. Total glutathione content was determined using the GSH and GSSG Assay Kit S0053 (Beyotime, Shanghai, China) and 5, 5′-dithio-bis-nitrobenzoic acid. GSSG was reduced to GSH by glutathione reductase and NADPH. The absorbance was monitored at 412 nm and the results were expressed as mM/g.
Exogenous GSH Treatment
The effects of exogenous GSH on yeast cell viability following H2O2 treatment were determined according to the methods of Liu et al. (2011b), with slight modifications. Yeast cells with an initial concentration of 1 × 105 cells/mL were supplemented with GSH to yield final concentrations of 1 and 10 mM. After overnight cultivation, yeast cells at the mid-log phase were harvested by centrifugation at 8000 × g for 3 min and washed three times with fresh YPD to remove any residual GSH. Yeast cells were then resuspended in fresh YPD medium to a final concentration of 5 × 107 cells/mL, and C. laurentii cells were treated with 300 mM H2O2 for 90 min. Cell viability was evaluated by the aforementioned methods.
Statistical Analysis
All statistical analyses were performed using the SPSS version 13 software (SPSS Inc., Chicago, IL, USA). Data were analyzed using one-way ANOVA, and comparisons between means were performed using the Duncan’s multiple range test. Differences at P < 0.05 were considered significant.
Results
Survival of C. laurentii under H2O2-Induced Oxidative Stress
Cell viability was measured following exposure of C. laurentii to increasing doses of H2O2 (ranging from 0 to 400 mM) and treatment intervals in YPD liquid media. Oxidative stress induced by H2O2 significantly inhibited cell viability in a dose- and time-dependent manner, and exposure to 300 mM H2O2 over 90 min was moderately lethal to cells (about 50% inhibitory) (Figures 1 and 2). Based on these results, 300 mM H2O2 over 90 min was selected as the appropriate concentration and interval to promote oxidative stress for subsequent studies.
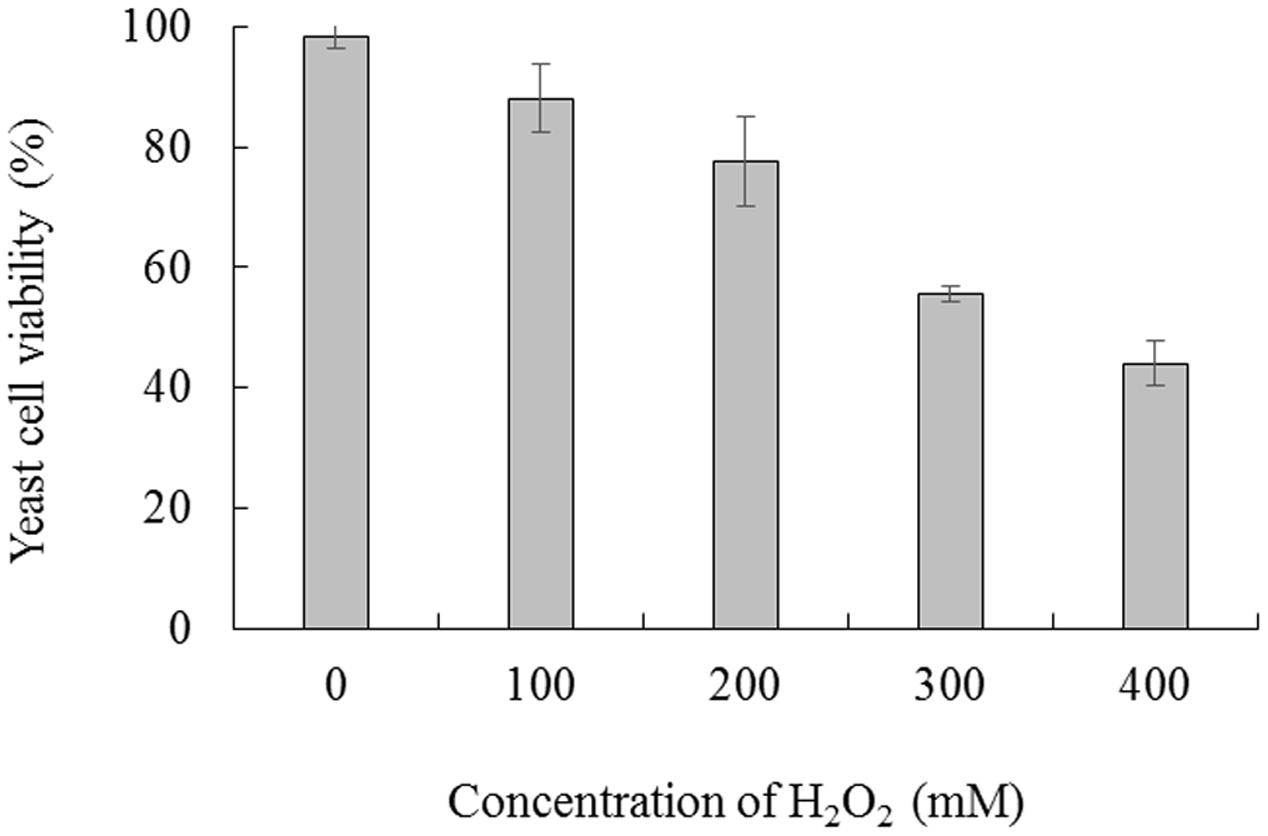
FIGURE 1. Viability of Cryptococcus laurentii under oxidative stress. C. laurentii cells were treated with a series of concentrations of H2O2 over 90 min. Vertical bars represent standard errors of the mean.
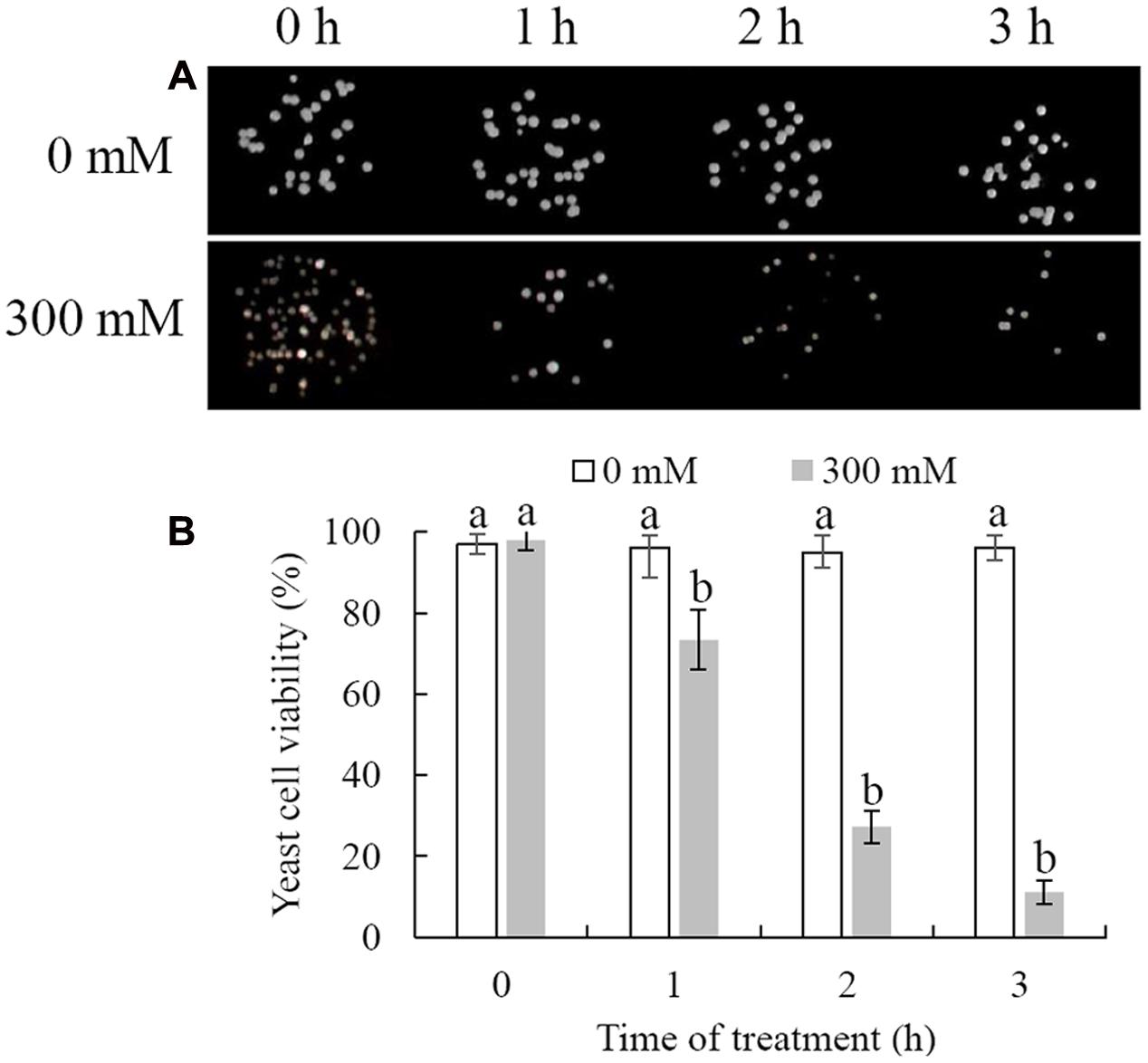
FIGURE 2. Survival of Cryptococcus laurentii following treatment with 300 mM H2O2. (A) Spotting assay of viability of C. laurentii following treatment with 300 mM H2O2. (B) Survival rate of C. laurentii following treatment with 300 mM H2O2. Vertical bars represent standard errors of the mean. Columns with different letters indicate significant differences (P < 0.05).
Measurement of ROS Production
Intracellular ROS production was measured by detection of fluorescence by DCFH-DA, which could be converted to highly fluorescent dichlorofluorescein in the presence of intracellular ROS. The results showed that H2O2 treatment could significantly induce the accumulation of intracellular ROS in C. laurentii (Figure 3A). Under conditions of oxidative stress induced by 300 mM H2O2, the percentage of ROS-positive cells was 46.6%. In contrast, only 5.6% of C. laurentii cells that were not subjected to exogenous oxidative stress showed visible ROS accumulation (Figure 3B). The results obtained in the analysis of fluorescence intensity were consistent with these findings. Treatment with 300 mM H2O2 significantly increased the intensity of dichlorofluorescein fluorescence in C. laurentii cells (Figure 3C).
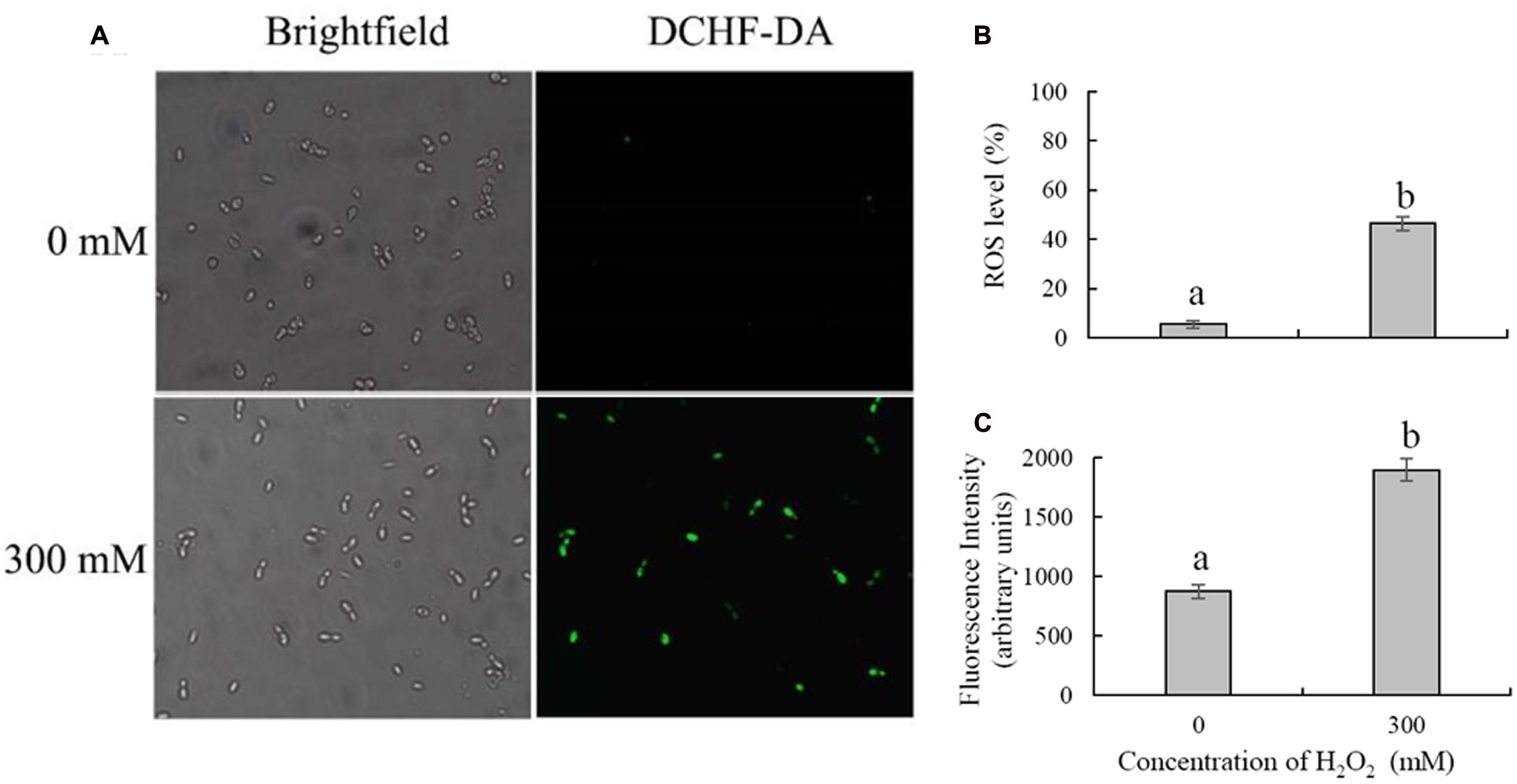
FIGURE 3. Detection of intracellular ROS in Cryptococcus laurentii following treatment with and without exogenous H2O2. (A) Microscopic images of C. laurentii cells stained with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). (B) Percentage of C. laurentii cells exhibiting visible ROS accumulation. (C) Statistical analysis of the fluorescence intensity of C. laurentii cells. Vertical bars represent standard errors of the mean of three independent experiments. Columns with different letters indicate significant differences (P < 0.05).
Biocontrol Efficacy of C. laurentii
In comparison with the control, non-H2O2-treated C. laurentii cells effectively inhibited postharvest decay caused by P. expansum on peach fruits (Figure 4A). Notably, the addition of 300 mM H2O2 significantly reduced biocontrol activity of C. laurentii. After 4 days, disease incidence in peach fruits treated with C. laurentii cells that had not been subjected to oxidative stress was 30%; whereas disease incidence in H2O2-treated samples was as high as 76% (Figure 4B). Moreover, the efficiency of C. laurentii cells in inhibiting lesion expansion was also significantly suppressed under exogenous oxidative stress (Figure 4C). These results suggest that exogenous oxidative stress could exert significant influence on biocontrol efficiency of C. laurentii. Thus, improving oxidative stress tolerance in C. laurentii is essential to enhancing its biocontrol efficiency.
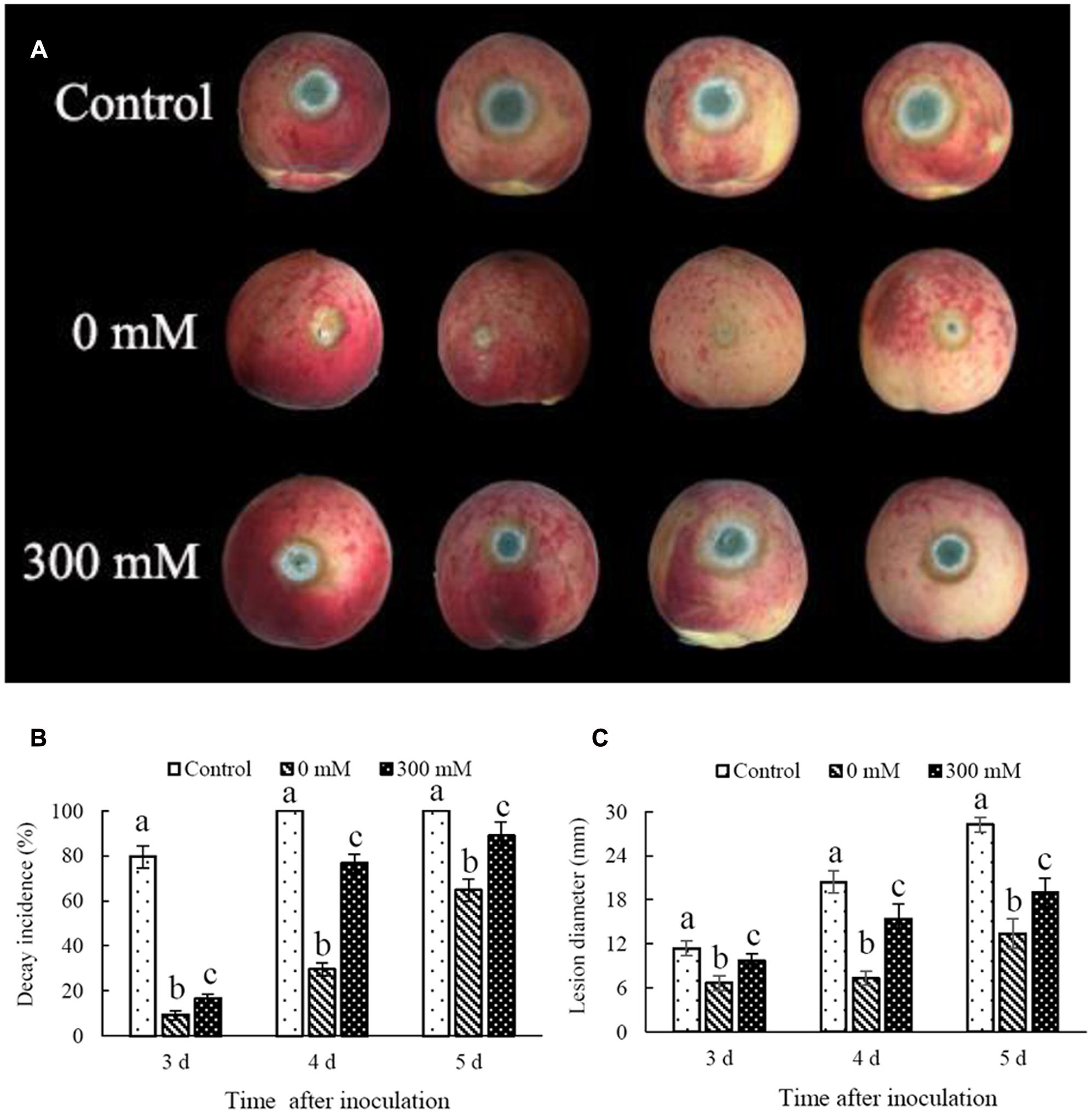
FIGURE 4. Effect of oxidative stress on biocontrol efficiency of Cryptococcus laurentii against Penicillium expansum. (A) Biocontrol performance of H2O2-treated C. laurentii cells and non-treated C. laurentii cells against P. expansum on peach fruits (5 days post inoculation). (B) Statistical analysis of decay incidence on peach fruits 3, 4, and 5 days post inoculation. (C) Statistical analysis of lesion diameters on peach fruits 3, 4, and 5 days post inoculation. Vertical bars represent standard errors of the mean. Columns with different letters indicate significant differences (P < 0.05).
Cell Membrane Integrity and Apoptosis
Necrosis and apoptosis of C. laurentii cells were demonstrated using Hoechst 33342 and PI staining. Necrotic yeast cells with a damaged plasma membrane were detectable, based on their high red fluorescence due to PI uptake (Figure 5A). Following exposure to 300 mM H2O2 for 90 min, the integrity of the plasma membrane of C. laurentii cells was reduced to 75%, and the number of stained apoptotic yeast cells was increased to 29% (Figure 5B). However, damage to the plasma membrane and apoptosis were not significant in C. laurentii cells that had not been subjected to H2O2 treatment (Figures 5A,B).
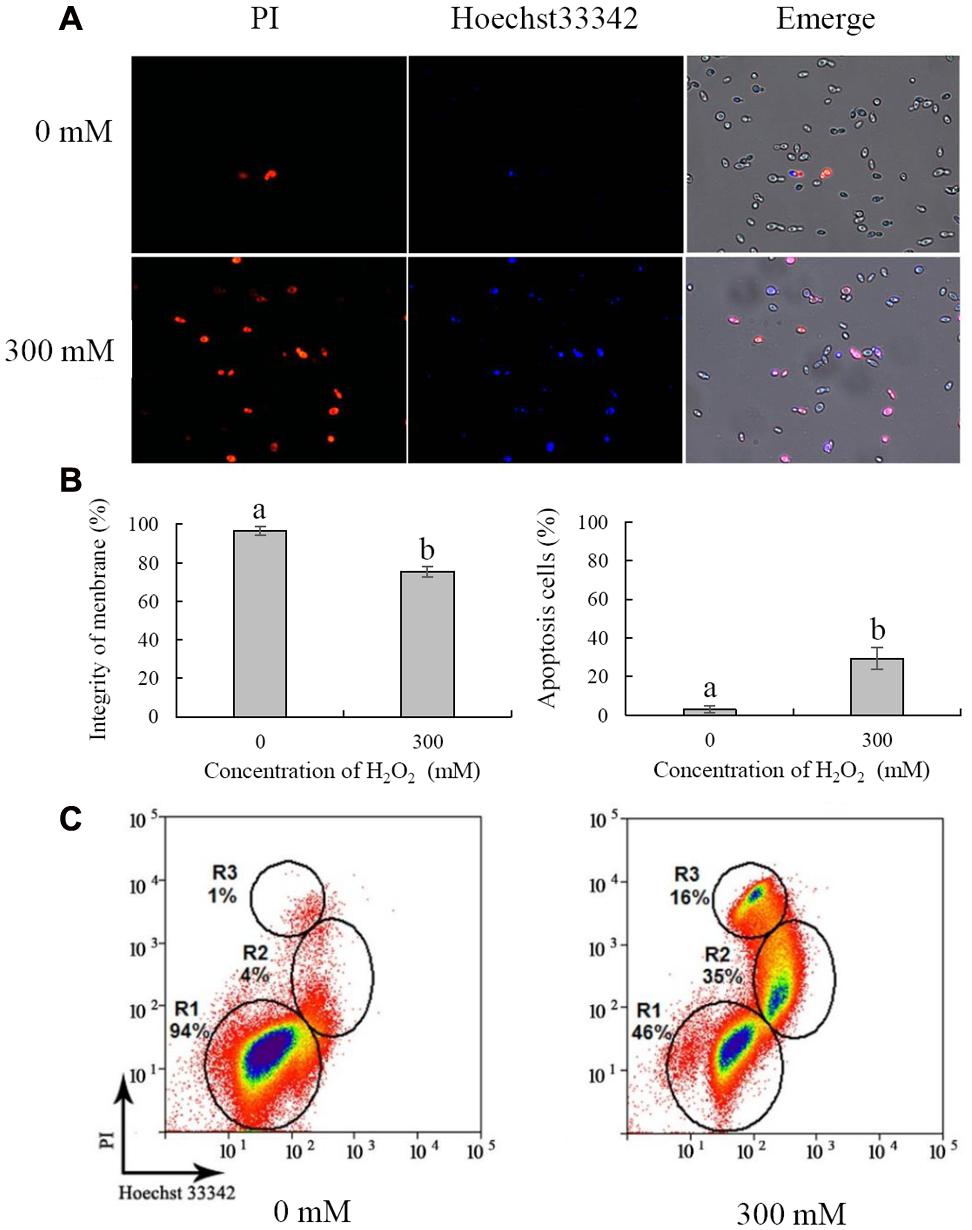
FIGURE 5. Necrosis and apoptosis analysis of Cryptococcus laurentii cells under oxidative stress. (A) Fluorescence microscopic image of C. laurentii cells treated with and without exogenous H2O2 and double stained with PI/Hoechst 33342. (B) Percentage of C. laurentii cells with plasma membrane integrity and percentage of apoptotic cells. (C) Flow cytometric analysis. R1 represents viable cells, R2 represents apoptotic cells, and R3 represents necrotic cells. Vertical bars represent standard errors of the means of three independent experiments. Columns with different letters indicate significant differences (P < 0.05).
For further analysis, necrosis and apoptosis in C. laurentii cells exposed to H2O2 were examined by flow cytometric analysis. On the dot plots, each cell is represented by a single dot (Figure 5C). When cells were stained with a combination of Hoechst 33342 and PI, three cell populations were observed: viable (R1), apoptotic (R2), and necrotic (R3) (Figure 5C). In the absence of H2O2-induced oxidative stress, the vast majority of C. laurentii cells was viable; however, following treatment with 300 mM H2O2, the percentage of viable cells was reduced to 46%, and the percentage of apoptotic and necrotic cells was 35 and 16%, respectively. These results are consistent with those of the survival analysis of C. laurentii under oxidative stress. These findings suggest that exogenous oxidative stress induced by 300 mM H2O2 could lead to apoptosis of C. laurentii cells that is primarily responsible for the decline in viability under oxidative stress.
Antioxidant Enzyme and Total Glutathione Assays
To resist the effects of oxidative stress and maintain cellular homeostasis, cells have evolved two sophisticated antioxidant systems, the enzymatic (e.g., CAT and SOD) and non-enzymatic antioxidant (e.g., glutathione and vitamins) defense systems. CAT and SOD are two major antioxidant enzymes involved in the enzymatic antioxidant defense system. Previous studies have shown that treatment with 30 mM H2O2 for 90 min has a moderately lethal effect on R. glutinis cells (about 50% inhibitory). In contrast, the same concentration of H2O2 has no significant effects on the viability of C. laurentii (data not shown), indicating that in comparison to R. glutinis, C. laurentii shows greater resistance to exogenous oxidative stress. To determine the reasons for this higher oxidative tolerance of C. laurentii, the enzyme activities of CAT, SODe and total glutathione of R. glutinis and C. laurentii under moderately lethal oxidative stress (R. glutinis: 30 mM H2O2; C. laurentii: 300 mM H2O2), were assessed. CAT activity in C. laurentii was strongly induced by H2O2 and maintained at a higher level than it was in R. glutinis. The activity level in C. laurentii was approximately eightfold that in R. glutinis (Figure 6A). Similarly, H2O2 treatment was able to induce SOD activity in C. laurentii. In contrast, exogenous oxidative stress showed slight inhibitory effects on SOD activity in R. glutinis (Figure 6B). These data indicate that enzymatic antioxidants are major contributors to the overall antioxidant capacity of C. laurentii under exogenous oxidative stress.
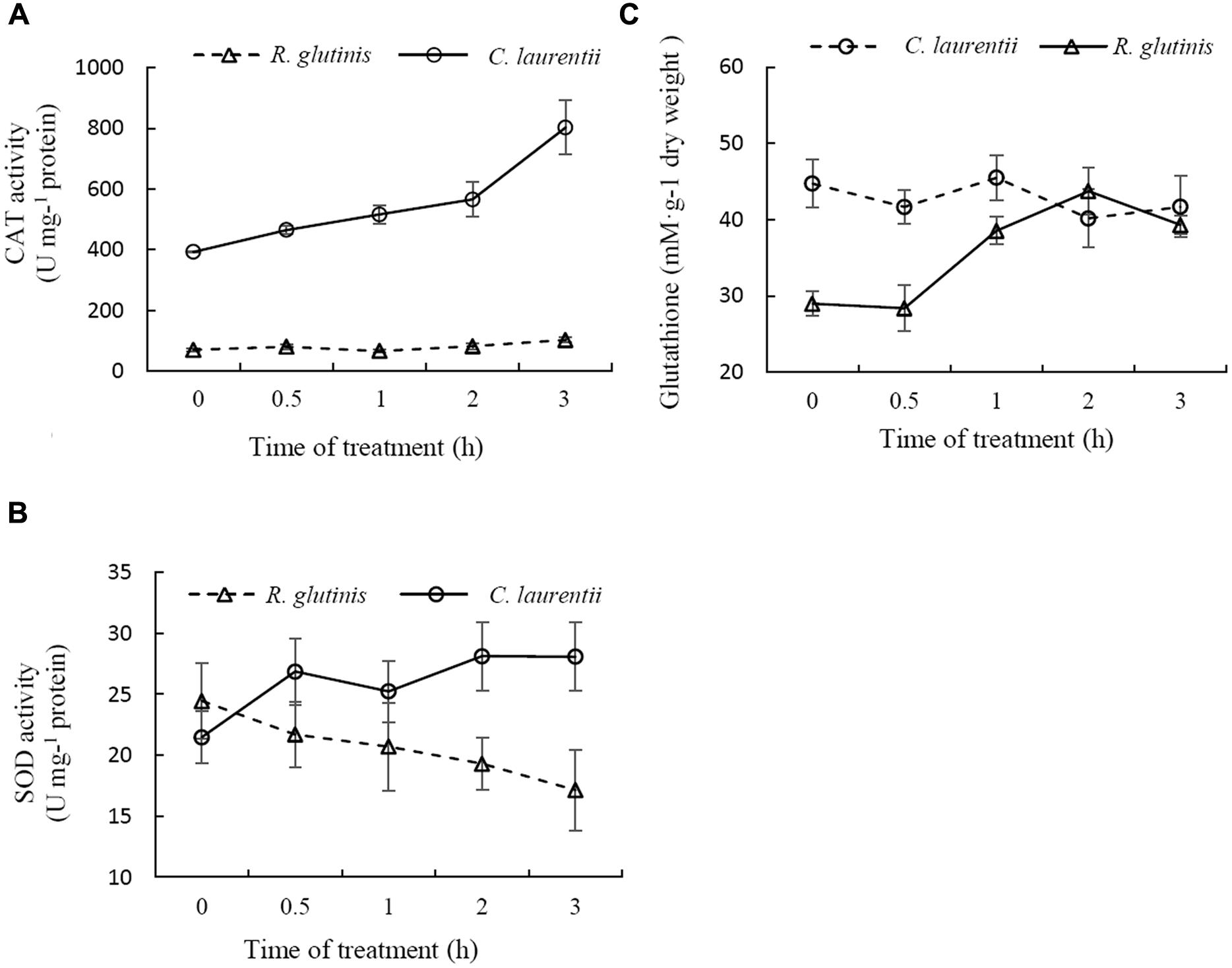
FIGURE 6. Comparison between antioxidative reactions of Cryptococcus laurentii and Rhodotorula glutinis following treatment with moderately lethal concentrations of H2O2. (A) Determination of CAT activities of C. laurentii and R. glutinis cells following treatment with 300 mM and 30 mM H2O2, respectively. (B) Determination of SOD activities of C. laurentii and R. glutinis cells following treatment with 300 mM and 30 mM H2O2, respectively. (C) Determination of glutathione activity of C. laurentii and R. glutinis cells following treatment with 300 mM and 30 mM H2O2, respectively. Vertical bars represent standard errors of the means of three independent experiments.
Glutathione also plays an important role in cellular antioxidant defenses. More than 90% of the total glutathione pool was in the reduced form (GSH) and the remainder was in the oxidized form (glutathione disulfide, GSSG). As shown in Figure 6C, total glutathione levels in C. laurentii cells remained relatively stable under H2O2-induced oxidative stress. However, this form of oxidative stress showed an obvious inductive effect on total glutathione levels in R. glutinis. In terms of resistance to oxidative stress, the difference between C. laurentii and R. glutinis might be dependent on the various antioxidant systems.
GSH Treatment Improves the Viability and Biocontrol Efficiency of C. laurentii
Glutathione is considered the main ROS scavenger in cells (Schafer and Buettner, 2001). We evaluated the effects of exogenous GSH on the cell viability and biocontrol performance of C. laurentii under exogenous oxidative stress. Adding GSH to the culture medium could suppress the accumulation of intracellular ROS and enhance the tolerance of C. laurentii to H2O2-induced oxidative stress. When treated with 10 mM GSH, the percentage of C. laurentii cells exhibiting visible ROS staining was reduced by 23% (Figures 7A,B). When 10 mM GSH was added to YPD medium, the cell viability of C. laurentii was improved by 19% under moderately lethal oxidative stress (Figure 7C). Furthermore, we validated the beneficial effects of exogenous GSH on the biocontrol efficiency of C. laurentii against blue mold on peach fruits. Treatment with 1 mM GSH improved biocontrol efficiency of C. laurentii at the earlier intervals following inoculation (Figures 8A,B), whereas 10 mM GSH improved biocontrol efficacy of C. laurentii throughout the entire experiment. On days 4 and 5 post inoculation, the lesion diameters were reduced by 24 and 13%, respectively, following treatment with 10 mM GSH (Figures 8A,B).
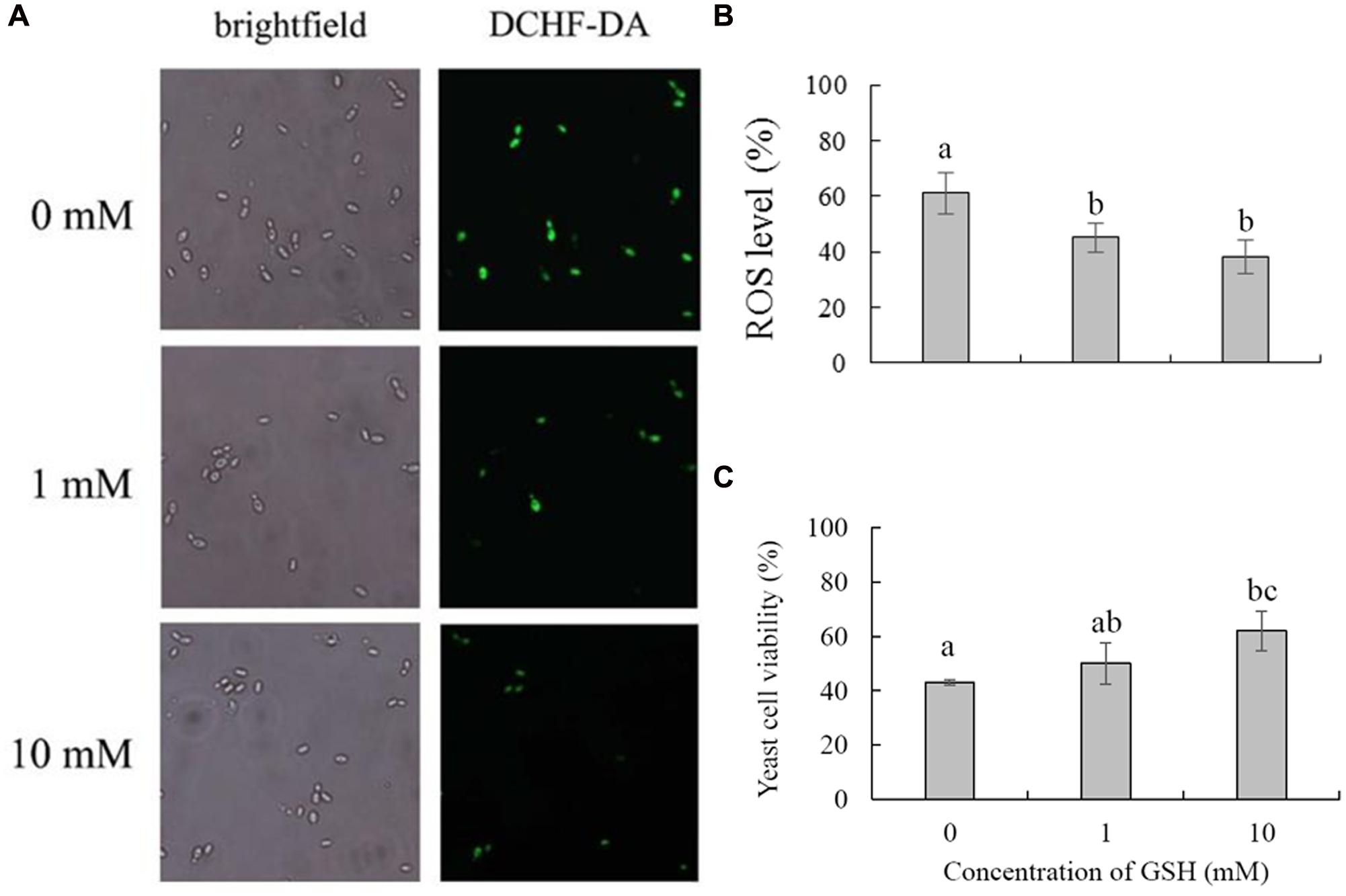
FIGURE 7. Effects of GSH treatment on oxidative stress tolerance of Cryptococcus laurentii. (A) ROS accumulation in C. laurentii cells under oxidative stress following treatment with different concentrations of GSH. Detection of intracellular ROS was facilitated by staining with DCHF2-DA. (B) Percentage of C. laurentii cells exhibiting visible ROS accumulation under oxidative stress following treatment with GSH. (C) Viability of C. laurentii cells under oxidative stress following treatment with GSH. Vertical bars represent standard errors of the means of three independent experiments. Columns with different letters indicate significant differences (P < 0.05).
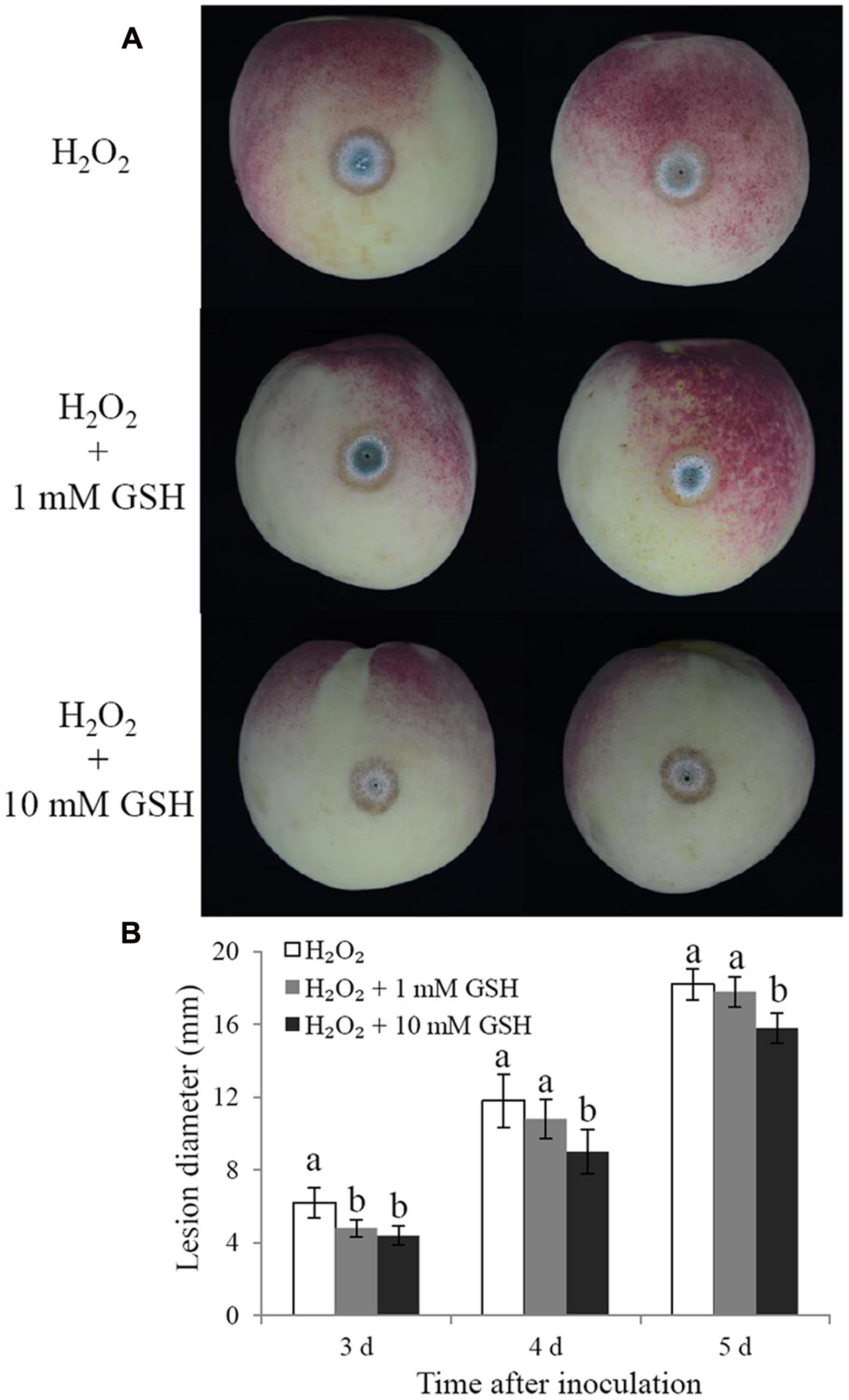
FIGURE 8. Effects of GSH treatment on the biocontrol efficiency of C. laurentii. (A) Signs of disease on peach fruits at 5 days post inoculation. (B) Statistical analysis of lesion diameters on peach fruits at 3, 4, and 5 days post inoculation. Vertical bars represent standard errors of the mean. Columns with different letters indicate significant differences (P < 0.05).
Discussion
In this study, we investigated the influence of exogenous oxidative stress on the viability and biocontrol efficiency of C. laurentii and the putative mechanisms of action. Furthermore, the methods by which the oxidative resistance of C. laurentii can be improved were explored.
Over the past few decades, numerous laboratory studies have been conducted to evaluate the biocontrol performance of antagonistic yeasts (Droby et al., 2009; Sharma et al., 2009; Nunes, 2012; Liu et al., 2013). However, only a few yeast-based biocontrol products are presently available commercially. Various stresses in the natural environment that have significant effects on yeast viability and product stability make the use of commercial antagonistic yeasts as biocontrol agents particularly challenging (Chen et al., 2015; Sui et al., 2015). Oxidative stress is one of the major challenges posed to antagonistic yeasts in the control of pre- and postharvest diseases (Li and Tian, 2007; Macarisin et al., 2010). Enhancing the tolerance of antagonistic yeasts to oxidative stress is an effective way to improve their biocontrol ability.
The results of the present study indicate that C. laurentii possesses higher levels of adaptability to oxidative stress in comparison with other antagonistic yeasts, such as R. glutinis and C. infirmominiatum. Treating C. laurentii with 300 mM H2O2 for 90 min was moderately lethal to the cells (Figure 1), whereas the median lethal concentration of H2O2 for R. glutinis was 30 mM (Chen et al., 2015). After 20 min of incubation, the survival of C. infirmominiatum in 20 mM H2O2 was 23% (Liu et al., 2011b). Previous studies have indicated that competition for space and nutrients is a major factor that affects the resistance of antagonistic yeasts to postharvest fungal pathogens (Chan and Tian, 2005; Bautista-Rosales et al., 2014). These fungal pathogens infect the host tissue mainly through wounds inflicted during harvest, transportation, packinghouse operations, and storage processes (Barkai-Goland, 2001). Therefore, wound competence of yeasts is important in their mechanisms of antagonism against pathogens, even as they compete for space and nutrients. Castoria et al. (2003) suggested that resistance to oxidative stress represents a pivotal mechanism of action involved in wound competence of antagonistic yeasts, which is closely associated with their biocontrol activity. The C. laurentii isolate LS-28 showed higher biocontrol activity, compared with the R. glutinis isolate LS-11, a finding that could be attributed to the higher tolerance of C. laurentii to oxidative stress, as compared with R. glutinis (Castoria et al., 2003). In the present study, we also found that the biocontrol efficiency of C. laurentii was significantly suppressed under oxidative stress (Figure 4).
As signal molecules, ROS can regulate senescence, apoptosis, and the stress response (Perrone et al., 2008). Low concentrations of ROS can activate a variety of antioxidant systems in yeast cells, and delay cell division that relies on the transcription factors Yap1p and Msn2/4p, thereby enhancing the resistance of yeast cells to subsequent lethal stress (Collinson and Dawes, 1992; Temple et al., 2005). However, excessive oxidative stress can cause a series of injuries to cellular components, including the cell membrane, proteins, lipids, and nucleic acids, resulting in compromised cell function or loss of viability (Reverter-Branchat et al., 2004; Branduardi et al., 2007).
Apoptosis and necrosis are two common forms of cell death that are associated with the viability of yeast cells. Apoptosis is a highly regulated form of programmed cell death that is characterized by nuclear DNA fragmentation, condensed chromatin, and inversion of the plasma membrane (Madeo et al., 1999; Poljak et al., 2003). The difference between apoptosis and necrosis is mainly manifested in the integrity of the cell membrane. When apoptosis occurs, the cell membrane remains intact, whereas in the necrotic cell, the membrane is broken down. The cell dye Hoechst 33342 has strong cell membrane permeability. However, PI cannot permeate the intact cell membrane. Flow cytometric analysis combined with Hoechst 33342-PI double staining is usually used to distinguish between viable, apoptotic, and necrotic cells (Sriram et al., 1992; Vermes et al., 2000). Thus, we used this method to investigate the mechanisms whereby H2O2 causes cell death in C. laurentii. The results of flow cytometry suggested that exogenous oxidative stress primarily triggered apoptosis in C. laurentii cells, resulting in the eventual suppression of viability. This indicated that the main mechanism associated with exogenous oxidative stress on C. laurentii cells was both systematic and progressive.
Both enzymatic and non-enzymatic antioxidant defense systems exist in yeasts (Jamnik and Raspor, 2005). In enzymatic antioxidant defense systems, SOD and CAT are two important components (Scandalios, 1993; Lee and Lee, 2000). SOD catalyzes the superoxide radical to H2O2, and H2O2 is then converted to H2O and O2, via the action of CAT. GSH, a small antioxidant molecule that is ubiquitous in plants and animals, plays a vital role in maintaining the antioxidant status of organisms (Cnubben et al., 2001; Herouart et al., 2002). C. laurentii cells subjected to treatment with exogenous H2O2, mainly employ enzymatic antioxidant defense systems against oxidative stress. In contrast, the ROS-sensitive yeast strain, R. glutinis tends to use the non-enzymatic antioxidant glutathione to resist oxidative stress. This might explain the disparity observed between C. laurentii and R. glutinis in their tolerance to oxidative stress.
Methods for improving stress resistance of biocontrol yeasts include preadaptation to stress, physiological manipulation, and the addition of anti-stress compounds to the medium. Previous reports have shown that combining biocontrol yeasts with exogenous chemical compounds, such as calcium (Tian et al., 2002), salicylic acid (Qin et al., 2003), sodium bicarbonate (Yao et al., 2004), and trehalose (Li and Tian, 2006) are effective ways to enhance their biocontrol performance. GSH has strong antioxidant capacity, and can be easily absorbed by cells. In the present study, we first validated the application of exogenous GSH as an effective method to improve oxidative stress tolerance in C. laurentii. In addition, the protective effect of GSH on the biocontrol efficiency of C. laurentii was confirmed on altered peach fruits. These results provide us with a potential alternative to enhancing the environmental adaptability and biocontrol performance of antagonistic yeasts.
Conclusion
We found that oxidative stress could induce apoptosis in C. laurentii that further leads to a reduction in cell viability and biocontrol efficiency. The enzymatic defense system might play a significant role in the antioxidative effects of C. laurentii. The addition of the non-enzymatic compound GSH to the culture media is an effective method to improve the oxidative stress resistance and biocontrol efficiency of C. laurentii.
Author Contributions
ST conceived and designed the experiments. ZZ, JC, CH, and YC performed the experiments. ZZ analyzed the data. ZZ and BL drafted the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 31530057 and 31671910), the Chinese Ministry of Science and Technology (grant number 2016YFD0400902) and open fund of Key Laboratory of Postharvest Physiology and Storage of Agro-Products, Ministry of Agriculture, P. R. China.
Abbreviations
CAT, catalase; GSH, reduced glutathione; GSSG, oxidized glutathione; NADPH, nicotinamide adenine dinucleotide phosphate; PBS, phosphate buffered saline; PI, propidium iodide; ROS, reactive oxygen species; SOD, superoxide dismutase; YPD, yeast peptone dextrose.
References
Barkai-Goland, R. (2001). Postharvest Diseases of Fruit and Vegetables. Development and Control. Amsterdam: Elsevier.
Bautista-Rosales, P. U., Calderon-Santoyo, M., Servín-Villegas, R., Ochoa-Álvarez, N. A., Vázquez-Juárez, R., and Ragazzo-Sánchez, J. A. (2014). Biocontrol action mechanisms of Cryptococcus laurentii on Colletotrichum gloeosporioides of mango. Crop Prot. 65, 194–201. doi: 10.1016/j.cropro.2014.07.019
Branduardi, P., Fossati, T., Sauer, M., Pagani, R., Mattanovich, D., and Porro, D. (2007). Biosynthesis of vitamin C by yeast leads to increased stress resistance. PLoS ONE 2:e1092. doi: 10.1371/journal.pone.0001092
Castoria, R., Caputo, L., De Curtis, F., and De Cicco, V. (2003). Resistance of postharvest biocontrol yeasts to oxidative stress: a possible new mechanism of action. Phytopathology 93, 564–572. doi: 10.1094/PHYTO.2003.93.5.564
Chan, Z. L., and Tian, S. (2006). Induction of H2O2-metabolizing enzymes and total protein synthesis by antagonistic yeast and salicylic acid in harvested sweet cherry fruit. Postharvest Biol. Technol. 39, 314–320. doi: 10.1016/j.postharvbio.2005.10.009
Chan, Z. L., and Tian, S. P. (2005). Interaction of antagonistic yeasts against postharvest pathogens of apple fruit and possible mode of action. Postharvest Biol. Technol. 36, 215–223. doi: 10.1016/j.postharvbio.2005.01.001
Chen, J., Li, B. Q., Qin, G. Z., and Tian, S. P. (2015). Mechanism of H2O2-induced oxidative stress regulating viability and biocontrol ability of Rhodotorula glutinis. Int. J. Food Microbiol. 193, 152–158. doi: 10.1016/j.ijfoodmicro.2014.10.025
Cnubben, N. H., Rietjens, I. M. C. M., Wortelboer, H., van Zanden, J., and van Bladeren, P. J. (2001). The interplay of glutathione-related processes in antioxidant defense. Environ. Toxicol. Phar. 10, 141–152. doi: 10.1016/S1382-6689(01)00077-1
Collinson, L. P., and Dawes, I. W. (1992). Inducibility of the response of yeast cells to peroxide stress. J. Gen. Microbiol. 138, 329–335. doi: 10.1099/00221287-138-2-329
Droby, S., Wisniewski, M., Macarisin, D., and Wilson, C. (2009). Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 52, 137–145. doi: 10.1016/j.postharvbio.2008.11.009
Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77. doi: 10.1016/0003-9861(59)90090-6
Fan, Q., and Tian, S. P. (2000). Postharvest biological control of rhizopus rot of nectarine fruits by Pichia membranefaciens. Plant Dis. 84, 1212–1216. doi: 10.1094/PDIS.2000.84.11.1212
Herouart, D., Baudouin, E., Frendo, P., Harrison, J., Santos, R., Jamet, A., et al. (2002). Reactive oxygen species, nitric oxide and glutathione: a key role in the establishment of the legume-Rhizobium symbiosis? Plant Physiol. Biochem. 40, 619–624. doi: 10.1016/S0981-9428(02)01415-8
Hong, S., Lee, J. E., Kim, C. Y., and Seong, G. J. (2007). Agmatine protects retinal ganglion cells from hypoxia-induced apoptosis in transformed rat retinal ganglion cell line. BMC Neurosci. 8:81. doi: 10.1186/1471-2202-8-81
Jamalizadeh, M., Etebarian, H. R., Aminian, H., and Alizadeh, A. (2011). A review of mechanisms of action of biological control organisms against post-harvest fruit spoilage. EPPO Bull. 41, 65–71. doi: 10.1111/j.1365-2338.2011.02438.x
Jamnik, P., and Raspor, P. (2005). Methods for monitoring oxidative stress response in yeasts. J. Biochem. Mol. Toxicol. 19, 195–203. doi: 10.1002/jbt.20091
Janisiewicz, W. J., and Korsten, L. (2002). Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 40, 411–441. doi: 10.1146/annurev.phyto.40.120401.130158
Lee, D. H., and Lee, C. B. (2000). Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci. 159, 75–85. doi: 10.1016/S0168-9452(00)00326-5
Li, B. Q., and Tian, S. P. (2006). Effects of trehalose on stress tolerance and biocontrol efficacy of Cryptococcus laurentii. J. Appl. Microbiol. 100, 854–861. doi: 10.1111/j.1365-2672.2006.02852.x
Li, B. Q., and Tian, S. P. (2007). Effect of intracellular trehalose in Cryptococcus laurentii and exogenous lyoprotectants on its viability and biocontrol efficacy on Penicillium expansum in apple fruit. Lett. Appl. Microbiol. 44, 437–442. doi: 10.1111/j.1365-2672.2006.02852.x
Liu, J., Sui, Y., Wisniewski, M., Droby, S., and Liu, Y. (2013). Review: utilization of antagonistic yeasts to manage postharvest fungal disease of fruit. Int. J. Food Microbiol. 167, 153–160. doi: 10.1016/j.ijfoodmicro.2013.09.004
Liu, J., Wisniewski, M., Droby, S., Norelli, J., Hershkovitz, V., Tian, S., et al. (2012). Increase in antioxidant gene transcripts, stress tolerance and biocontrol efficacy of Candida oleophila following sublethal oxidative stress exposure. FEMS Microbiol. Ecol. 80, 578–590. doi: 10.1111/j.1574-6941.2012.01324.x
Liu, J., Wisniewski, M., Droby, S., Tian, S., Hershkovitz, V., and Tworkoski, T. (2011a). Effect of heat shock treatment on stress tolerance and biocontrol efficacy of Metschnikowia fructicola. FEMS Microbiol. Ecol. 76, 145–155. doi: 10.1111/j.1574-6941.2010.01037.x
Liu, J., Wisniewski, M., Droby, S., Vero, S., Tian, S., and Hershkovitz, V. (2011b). Glycine betaine improves oxidative stress tolerance and biocontrol efficacy of the antagonistic yeast Cystofilobasidium infirmominiatum. Int. J. Food Microbiol. 146, 76–83. doi: 10.1016/j.ijfoodmicro.2011.02.007
Macarisin, D., Droby, S., Bauchan, G., and Wisniewski, M. (2010). Superoxide anion and hydrogen peroxide in the yeast antagonist–fruit interaction: a new role for reactive oxygen species in postharvest biocontrol? Postharvest Biol. Technol. 58, 194–202. doi: 10.1016/j.postharvbio.2010.07.008
Madeo, F., Fröhlich, E., Ligr, M., Grey, M., Sigrist, S. J., Wolf, D. H., et al. (1999). Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145, 757–767. doi: 10.1083/jcb.145.4.757
Navarta, L. G., Calvo, J., Posetto, P., Cerutti, S., Raba, J., Benuzzi, D., et al. (2014). Postharvest control of grey mold in apples with lyophilized formulations of Cryptococcus laurentii: the effect of cold stress in the survival and effectiveness of the yeast. Food Bioprocess. Technol. 7, 2962–2968. doi: 10.1007/s11947-014-1303-0
Nunes, C. A. (2012). Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 133, 181–196. doi: 10.1007/s10658-011-9919-7
Perrone, G. G., Tan, S. X., and Dawes, I. W. (2008). Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta Mol. Cell Res. 1783, 1354–1368. doi: 10.1016/j.bbamcr.2008.01.023
Poljak, A., Dawes, I. W., Ingelse, B. A., Duncan, M. W., Smythe1, G. A., and Grant, C. M. (2003). Oxidative damage to proteins in yeast cells exposed to adaptive levels of H2O2. Redox Rep. 8, 371–377. doi: 10.1179/135100003225003401
Qin, G. Z., Tian, S. P., and Xu, Y. (2004). Biocontrol of postharvest diseases on sweet cherries by four antagonistic yeasts in different storage conditions. Postharvest Biol. Technol. 31, 51–58. doi: 10.1016/S0925-5214(03)00130-3
Qin, Q. Z., Tian, S. P., Xu, Y., and Wan, Y. K. (2003). Enhancement of biocontrol efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiol. Mol. Plant Pathol. 62, 147–154. doi: 10.1016/S0885-5765(03)00046-8
Reverter-Branchat, G., Cabiscol, E., Tamarit, J., and Ros, J. (2004). Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae. J. Biol. Chem. 279, 31983–31989. doi: 10.1074/jbc.M404849200
Scandalios, J. G. (1993). Oxygen stress and superoxide dismutase. Plant Physiol. 107, 7–12. doi: 10.1104/pp.101.1.7
Schafer, F. Q., and Buettner, G. R. (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30, 1191–1212. doi: 10.1016/S0891-5849(01)00480-4
Segal, A. W. (2005). How neutrophils kill microbes. Annu. Rev. Immunol. 23, 197–223. doi: 10.1146/annurev.immunol.23.021704.115653
Sharma, R. R., Singh, D., and Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol. Control 50, 205–221. doi: 10.1016/j.biocontrol.2009.05.001
Sriram, M., Vandermarel, G. A., Roelen, H. L. P. F., Vanboom, J. H., and Wang, A. H. J. (1992). Conformation of b-DNA containing O6-ethyl-g-c base-pairs stabilized by minor groove binding-drugs: molecular structure of d(CGC[e6G]AATTCGCG) complexed with Hoechst33258 or Hoechst33342. EMBO J. 11, 225–232.
Sui, Y., Wisniewski, M., Droby, S., and Liu, J. (2015). Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 81, 2968–2975. doi: 10.1128/AEM.04203-14
Temme, N., and Tudzynski, P. (2009). Does Botrytis cinerea ignore H2O2-induced oxidative stress during infection? Characterization of Botrytis activator protein 1. Mol. Plant Microbe Interact. 22, 987–998. doi: 10.1094/MPMI-22-8-0987
Temple, M. D., Perrone, G. G., and Dawes, I. W. (2005). Complex cellular responses to reactive oxygen species. Trends Cell Biol. 15, 319–326. doi: 10.1016/j.tcb.2005.04.003
Tian, S. P., Fan, Q., Xu, Y., and Jiang, A. L. (2002). Effects of calcium on biocontrol activity of yeast antagonists against postharvest fungal pathogen. Plant Pathol. 5, 352–358. doi: 10.1046/j.1365-3059.2002.00711.x
Tian, S. P., Qin, G. Z., and Xu, Y. (2004). Survival of antagonistic yeasts under field conditions and their biocontrol ability against postharvest diseases of sweet cherry. Postharvest Biol. Technol. 33, 327–331. doi: 10.1016/j.postharvbio.2004.03.010
Vermes, I., Haanen, C., and Reutelingsperger, C. (2000). Flow cytometry of apoptotic cell death. J. Immunol. Methods 243, 167–190. doi: 10.1016/S0022-1759(00)00233-7
Xu, X., Qin, G. Z., and Tian, S. P. (2008). Effect of microbial biocontrol agents on alleviating oxidative damage of peach fruit subjected to fungal pathogen. Int. J. Food Microbiol. 126, 153–158. doi: 10.1016/j.ijfoodmicro.2008.05.019
Yao, H. J., Tian, S. P., and Wang, Y. S. (2004). Sodium bicarbonate enhances biocontrol efficacy of yeasts on fungal spoilage of pears. Int. J. Food Microbiol. 93, 297–304. doi: 10.1016/j.ijfoodmicro.2003.11.011
Zhang, X. Y., Sun, Y., Yang, Q. Y., Chen, L. L., Li, W. H., and Zhang, H. Y. (2015). Control of postharvest black rot caused by Alternaria alternata in strawberries by the combination of Cryptococcus laurentii and Benzo-(1,2,3)-thiadiazole-7 -carbothioic acid S-methyl ester. Biol. Control 90, 96–101. doi: 10.1016/j.biocontrol.2015.05.018
Keywords: Cryptococcus laurentii, biocontrol, oxidative stress, apoptosis, antioxidant systems, glutathione
Citation: Zhang Z, Chen J, Li B, He C, Chen Y and Tian S (2017) Influence of Oxidative Stress on Biocontrol Activity of Cryptococcus laurentii against Blue Mold on Peach Fruit. Front. Microbiol. 8:151. doi: 10.3389/fmicb.2017.00151
Received: 27 October 2016; Accepted: 20 January 2017;
Published: 02 February 2017.
Edited by:
Aldo Corsetti, University of Teramo, ItalyReviewed by:
Odile Tresse, Oniris, FranceMichael Wisniewski, United States Department of Agriculture – Agricultural Research Service, USA
Copyright © 2017 Zhang, Chen, Li, He, Chen and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiping Tian, dHNwQGliY2FzLmFjLmNu
†These authors have contributed equally to this work.
 Zhanquan Zhang
Zhanquan Zhang Jian Chen
Jian Chen Boqiang Li
Boqiang Li Chang He
Chang He Yong Chen
Yong Chen Shiping Tian
Shiping Tian