- 1Department of Plant Physiology and Biotechnology, Tomsk State University, Tomsk, Russia
- 2Federal Research Centre (FRC) Biotechnology, Institute of Bioengineering, Moscow, Russia
- 3Federal Research Centre (FRC) Biotechnology, Winogradsky Institute of Microbiology, Research Center of Biotechnology, Russian Academy of Sciences (RAS), Moscow, Russia
- 4Glasgow and Holymoor Consultancy Ltd., Glasgow University, Chesterfield, UK
The goal of this work was to study the diversity of microorganisms inhabiting a deep subsurface aquifer system in order to understand their functional roles and interspecies relations formed in the course of buried organic matter degradation. A microbial community of a deep subsurface thermal aquifer in the Tomsk Region, Western Siberia was monitored over the course of 5 years via a 2.7 km deep borehole 3P, drilled down to a Palaeozoic basement. The borehole water discharges with a temperature of ca. 50°C. Its chemical composition varies, but it steadily contains acetate, propionate, and traces of hydrocarbons and gives rise to microbial mats along the surface flow. Community analysis by PCR-DGGE 16S rRNA genes profiling, repeatedly performed within 5 years, revealed several dominating phylotypes consistently found in the borehole water, and highly variable diversity of prokaryotes, brought to the surface with the borehole outflow. The major planktonic components of the microbial community were Desulfovirgula thermocuniculi and Methanothermobacter spp. The composition of the minor part of the community was unstable, and molecular analysis did not reveal any regularity in its variations, except some predominance of uncultured Firmicutes. Batch cultures with complex organic substrates inoculated with water samples were set in order to enrich prokaryotes from the variable part of the community. PCR-DGGE analysis of these enrichments yielded uncultured Firmicutes, Chloroflexi, and Ignavibacteriae. A continuous-flow microaerophilic enrichment culture with a water sample amended with acetate contained Hydrogenophilus thermoluteolus, which was previously detected in the microbial mat developing at the outflow of the borehole. Cultivation results allowed us to assume that variable components of the 3P well community are hydrolytic organotrophs, degrading buried biopolymers, while the constant planktonic components of the community degrade dissolved fermentation products to methane and CO2, possibly via interspecies hydrogen transfer. Occasional washout of minor community components capable of oxygen respiration leads to the development of microbial mats at the outflow of the borehole where residual dissolved fermentation products are aerobically oxidized. Long-term community analysis with the combination of molecular and cultivation techniques allowed us to characterize stable and variable parts of the community and propose their environmental roles.
Introduction
The deep subsurface is one of the largest habitats for prokaryotes, and the total biomass of subsurface microbes probably exceeds the numbers in the rest of the biosphere (Whitman et al., 1998; McMahon and Parnell, 2014). A number of studies have demonstrated sizable and metabolically active subsurface microbial communities in the deep sub-seafloor (Parkes et al., 2000; Kimura et al., 2003; Takai et al., 2004; Teske, 2005; Batzke et al., 2007; Edwards et al., 2011; Lomstein et al., 2012). Abundant and diverse microbial communities have been revealed in terrestrial deep subsurface habitats all over the world (Fredrickson and Hicks, 1987; Ghiorse and Wilson, 1988; Jiménez, 1990; Takai et al., 2001; Itävaara et al., 2011; Bomberg et al., 2015; Frank et al., 2016). Some studies have suggested that microbes in the deep subsurface have extremely slow in-situ growth rates because of the lack of detrital energy inputs over thousands to millions of years (Onstott et al., 2014; Lever et al., 2015). While uncultured and poorly studied microorganisms are speculated to play major roles in geochemical cycles in these environments (Wrighton et al., 2012; Castelle et al., 2013), a great deal of uncertainties exists, stimulating a search for novel microorganisms from subsurface ecosystems. Characterization of microbial and metabolic diversity in the deep terrestrial subsurface is in incipient stages and very far from complete (Hug et al., 2016).
Deep terrestrial subsurface environments may represent extreme habitats with high pressure, temperature, and/or salinity (Ollivier et al., 2007). Depending on the energy source, deep subsurface microbial communities could be “lithoautotrophic” and “organotrophic” (Fredrickson and Hicks, 1987). In the former ones, molecular hydrogen of abiotic origin is considered to be the main source of energy (Takai et al., 2004; Nealson et al., 2005; Pedersen, 2012), while, in the latter, the buried and completely or partly altered organic matter (kerogen or crude oil) provides energy substrates that fuel deep subsurface microbial communities. These communities have been sampled through drilled boreholes penetrating deep strata. In majority, the drilling activity was related to hydrocarbon exploration. Subsurface microbial communities have been sampled from the high-temperature hydrocarbon reservoirs of Western Siberia, Kazakhstan (Nazina et al., 1995; Bonch-Osmolovskaya et al., 2003; Frank et al., 2016), California (Orphan et al., 2003), North Sea (Dahle et al., 2008), China (Li et al., 2006; Nazina et al., 2006), etcetera. Microbes from borehole water samples represent the planktonic community, while microorganisms on mineral surfaces and in biofilms in subsurface environments remain elusive as they only appear in the borehole fluid sporadically (Edwards et al., 2005; Wanger et al., 2006). In some cases, this sporadic distribution of the immobilized microorganisms could lead to erroneous interpretation of subsurface community compositions judged by singular or irregularly repeated molecular fingerprints. To date, only a few research projects have concentrated on the long-term dynamics of deep subsurface communities (e.g., Wu et al., 2016).
Our work is part of a long-term study of microbial communities inhabiting deep thermal aquifers in the Mesozoic and Palaeozoic sedimentary sequences of the Western Siberian megabasin. During the 1950s, many hydrocarbon exploration wells drilled in this region penetrated deep aquifers rather than significant hydrocarbon reservoirs. The borehole depths typically varied from 500 to 4000 m, with water temperatures ranging from 20 to 140°C. In this study, we have characterized the microbial community of the borehole 3P (alternative designations in other publications: P3, P-3, 3-R or “Goryachii Istochnik”) situated on the west bank of the River Ob', between the ports of Parabel' and Narym, and in the Parabel' District, Tomsk Region of the Russian Federation. The borehole was drilled in 1957–1958 and was left unsealed. The thermal water overflowing from the borehole has subsequently been tapped for a spa resort: it discharges uncontaminated to the surface of a wooden conduit above the ground level. On the conduit, non-photosynthetic microbial mats develop downstream of the outflow (Figure S1). The goal of this work was to study the composition of microbial community in water during several years in order to find how stable it is and what the functional roles of its components are. Both borehole water and microbial mats were sampled in this study over a five-year period, from August 2009 to August 2013. Changes in the composition of planktonic microbial communities were traced by PCR-DGGE analysis of the 16S rRNA gene fragments. High-throughput sequencing of variable 16S rRNA gene fragments was additionally performed for a comprehensive analysis of the prokaryotic communities. Previous studies of deep subsurface aquifers showed that only 0.022–1% of all cells are present in water in an unattached state (McMahon and Parnell, 2014). We assumed that hydrolytic microorganisms attached to the organic-rich ore would be present in water as minor components and could be revealed in specific enrichment cultures. Thus, an attempt was made to study the hydrolytic part of a deep subsurface population by batch enrichment cultures further analyzed by PCR-DGGE of 16S rRNA gene fragments. A continuous microaerophilic acetate-utilizing enrichment from a water sample was also established. It was supposed to reproduce the microbial mat developing at the outflow of the 3P well. Therefore, the combination of different approaches allowed us to reveal stable planktonic and variable immobilized parts of the deep subsurface microbial community as well as provide insight into the origin and diversity of microbial mats consuming organic molecules present in deep subsurface water.
Materials and Methods
Site Description and Samples Collection
Borehole 3P is located on the west bank of the River Ob' (N58°50′, E81°30′) in the Tomsk Region, Western Siberia, Russia. According to different sources, the depth of the borehole is 2609 or 2775 m. It was drilled as an oil exploration well in 1957–1958 and then abandoned and harnessed by the local population as a source of thermal water. The borehole penetrated almost 30 m of Quaternary sediments, followed by 182 m of Palaeogene sediments. This was succeeded by a Cretaceous sedimentary sequence down to 2250 m and a Jurassic sequence to 2600 m. At 2600 m, the borehole reached the Palaeozoic basement, thus comprising the monzonite and granite (Ulmishek, 2003; Banks et al., 2011, 2014). Water discharges at approximately 9 m3 day−1, has a moderate mineralization (total dissolved solids 13–14 g l−1) and contains dissolved H2S and CH4. Methane exsolves as bubbles on the surface. The stratigraphic location from which the water is derived is not known with certainty. Given a water temperature of around 50°C and a typical geothermal gradient in tectonically stable areas of 20–30°C per km, and allowing for the fact that the water may cool during its ascent along the borehole, it seems likely that the water is derived from near the base of the borehole.
Samples of water were collected seven times: in August 2009, February 2010, March 2010, June 2011, July 2012, September 2012, and August 2013. Temperature, pH, and redox potential of water were measured on-site (HI 8314 Hanna pH/redox-meter and corresponding electrodes). Samples for chemical, molecular, and microbiological analyses were taken from the wellhead into sterile 500 ml serum bottles sealed with rubber stoppers. Stoppers of sample containers for analysis of organic compounds were covered with Teflon films. All samples were kept at +4°C for 24 h before analysis. Gas samples were collected four times: in August 2009, March 2010, June 2011, and July 2012. For the samples with the highest hydrocarbon content (2011), the carbon isotopic composition of the gas phase was determined. Gas bubbles exsolving from thermal water were collected by a method modified from those described by Giggenbach and Goguel (1989). We used a glass gas sampler (Savannah River National Laboratory) connected with a sterile 60 ml bottle by a Norprene tubing and submerged into the tank with thermal water inflow. The gas accumulating in the sampler was collected into a bottle by displacement of 50 ml thermal water from it, and 10 ml of water were left as a hydroseal. The filled bottles, being held under water, were sealed with butyl rubber stoppers and then capped and transferred to the laboratory upside down to prevent post-sampling atmospheric contamination. Samples of microbial mats growing along the water flow were collected three times (in August 2009, February 2010, and March 2010) in 50 ml Falcon tubes and were stored at 4°C until used.
Hydrochemical Analyses
Water samples were filtered (0.22 μm) using Millex-GS filter units (Merck Millipore) before analysis. Organic compounds were extracted with chloroform from the water samples. Liquid chromatography–mass spectrometry (LC-MS) was performed in the Institute of Petroleum Chemistry, Siberian branch of Russian Academy of Sciences (Tomsk, Russia). Determination of selected organic compounds was carried out using a Thermo Scientific DFS mass spectrometer. Major ions and chemical oxygen demand (COD) were determined by the Scientific Educational Production Center Voda of Tomsk Polytechnic University by titration (bicarbonate and chloride) or spectrophotometry (sulfate), and H2S was measured colorimetrically with N,N-dimethyl-p-phenylenediamine (dihydrochloride salt) as the chromophore (Cline, 1969). For COD measurements, the sample was refluxed with potassium dichromate in the sulfuric acid medium and the excess potassium dichromate was determined by titration against ferrous ammonium sulfate using ferroin as an indicator. Other ions were determined at the Chemical Analytical Center Plasma (Tomsk) using an ICP mass-spectrometer ELAN model DRC-e (PerkinElmer Instruments).
In 2010, parallel samples (0.45 μm filtration) were analyzed by ICP-AES and ion chromatography at the Geological Survey of Norway (Banks et al., 2011). In 2013, parallel samples (0.45 μm filtration) were analyzed by ICP-MS and ion chromatography at the British Geological Survey.
Gas samples were analyzed using the Crystal 5000.1 gas chromatograph (Chromatek) equipped with a HayeSep N 80–100 mesh Supelco column (Sigma-Aldrich), methanator, FID, and TCD detectors, which were conditioned at 40°, 350°, 200°, and 150°C, respectively. The carrier gas was argon (25 ml min−1). Volatile fatty acids in the water samples were analyzed using the same equipment with a FID detector and Porapak column conditioned at 180°C. The isotopic composition of methane and ethane (δ13C) was determined on a Thermo Electron gas chromatograph coupled with a Delta plus mass spectrometer (Thermo Fisher Scientific).
DNA Extraction
DNA was extracted from water and mat samples, enrichment cultures, and isolates. Cells from 1.5 l aliquots of borehole groundwater were retained on cellulose nitrate filters (0.2 μm) using a Sartorius filtration unit. Filters were pestled in liquid nitrogen at −70°C and then used for DNA extraction with a Power Soil DNA isolation kit (MO BIO Laboratories) according to the manufacturer's instructions. Cells from enrichment culture broths and pure cultures were collected by centrifugation (9000 g for 15 min). Extraction of DNA from approximately 5 ml of enrichments or pure cultures was performed as described by Tsai and Olson (1991), with minor modifications (i.e., proteinase K (10 μg ml−1) was added after three cycles of freezing the sample in liquid nitrogen at −70°C and thawing in a 65°C water bath).
PCR Amplification
Nested PCR (Vissers et al., 2009) was used for the amplification of bacterial and archaeal 16S rRNA genes with the primers listed in Table S2. Domain-specific primers were used separately for Bacteria and Archaea for both the first and second rounds of PCR. The products obtained in the reaction with outer primers were diluted with nuclease-free water (Fermentas) to a concentration of ca. 100 mg l−1 and then used as a template for the reaction with inner primers. 16S rRNA gene fragments of Bacteria were first amplified using outer primer pair 27F–1492R (DeLong, 1992) and then with Bacteria-specific inner primer pair GC-Bacv3f and 907r (Lane, 1991; Muyzer et al., 1996). The first round of amplification of the 16S rRNA gene fragments of Archaea was conducted with the primer pair 21F-958R (Weisburg et al., 1991; DeLong, 1992) and the second round with the Archaea-specific pair GC-Arch915r and Parch519f (Coolen et al., 2004). We have successfully used these primer sets in our previous studies to amplify bacterial and archaeal 16S rRNA gene fragments separately (Karnachuk et al., 2009; Frank et al., 2016).
Table S2 shows the details of the PCR conditions used in this study. The amounts of MgCl2, dNTPs, primers, and Taq DNA-polymerase were altered depending on the primer set. The reagents used were 25 mM MgCl2, 2 M dNTPs, 5 U μl−1 Taq DNA polymerase, 10x Taq buffer, and nuclease-free water (Fermentas). 100 μM stock solutions of oligonucleotide primers synthesized by Syntol (Moscow) were applied. 400 mg l−1 of BSA (Fermentas) were added to the reaction mixture for archaeal 16S rRNA gene amplification. Reactions with outer primers were conducted in a volume of 50 μl. PCR with inner primers was performed in a volume of 100 μl. Each mixture contained 50–100 mg l−1 of the DNA matrix. All PCRs were conducted in the MyCycler thermal cycler (Bio-Rad Laboratories).
Denaturing Gradient Gel Electrophoresis (DGGE)
The DGGE used for the separation of amplified fragments (Muyzer et al., 1993) was performed with the DCode System (Bio-Rad). Polyacrylamide gel (8%) with a 30–70% denaturing gradient was used for 16S rRNA fragments separation (100% denaturing solution contained 7 M urea and 40% formamide). Electrophoresis was performed for 17 h at 60°C and 120 V. For separation of archaeal 16S rRNA gene fragments, 8% polyacrylamide gel with 20 to 80% denaturing gradient was used. Electrophoresis was performed for 19.5 h at 60°C and 100 V, as described by Vissers et al. (2009). DGGE gel was stained with 0.5 mg l−1 ethidium bromide (Bio-Rad) in a TAE buffer for 15 min and then washed in TAE for 20 min. The stained gel was visualized in UV light (320 nm) using a GelDoc-It imaging system (UVP, UK). Separate bands were cut from the gel in UV (320 nm) using transilluminator ECX-26MX (Vilber Lourmat). Then DNA was extracted to 20 μl of nuclease-free water (Fermentas) for 12 h at 4°C. Amplification of 16S rRNA gene fragments was performed as described above, but the primers did not contain GC-clamps. DGGE analysis of each PCR product was performed in duplicate.
Phylogenetic Analysis
Commercial sequencing of 16S rRNA (585 bp and ca. 400 bp) gene fragments was performed by Syntol. The sequences were analyzed against the GenBank nucleotide collection database (http://www.ncbi.nlm.nih.gov/blast) by the BLASTN algorithm with standard parameters (Altschul et al., 1997). The sequences were aligned using BioEdit sequence alignment editor (Hall, 1999), Version 7.2.5. Maximum likelihood and neighbor-joining phylogenetic trees based on the comparison of 16S rRNA gene sequences were constructed using MEGA 6 (Tamura et al., 2013). All the sequences were uploaded to the GenBank nucleotide collection database under the accession numbers KT897597 – 651, KY010808, and KY010809.
Pyrosequencing of 16S rRNA Gene Fragments
Pyrosequencing analyses were performed for water samples obtained from the borehole in June 2011. Cells from 30 L of water were collected on 0.2 μm nitrate cellulose filters. The filters were frozen in liquid nitrogen and then ground and melted with TE buffer in a water bath at 37°C. The total DNA was extracted by the CTAB/NaCl method (Wilson, 2001).
Universal primers were used for amplification of the V3–V4 variable regions of the 16S rRNA gene, U341F (5′-CCT ACG GGR SGC AGC AG-3′), and PRK806R (5′-GGA CTA CYV GGG TAT CTA AT-3′). The primer check with SILVA TestPrime confirmed that they target most of Archaea and Bacteria except only a few lineages, notably Planctomycetes and some Crenarchaeota. The PCR fragment was pyrosequenced on GS FLX (Roche) using titanium chemistry. A total of 39,276 sequence reads were obtained. Most of the reads covered the full length of the PCR fragment. The reads with mismatches to primer sequences, those containing ambiguous nucleotides, and those shorter than 400 bp were excluded from the analysis. Then 16S rRNA data were analyzed by the RDP Classifier program package (Cole et al., 2009). Initially, the sequences obtained with universal primers were distributed between Bacteria and Archaea using the online RDP Naive Bayesian rRNA Classifier Version 2.0 (http://rdp.cme.msu.edu/classifier/classifier.jsp). Subsequently, bacterial and archaeal 16S rRNA gene datasets were analyzed separately and subjected to additional filters. First, AmpliconNoise (Quince et al., 2011) was used to account for homopolymer-derived and PCR errors. Then all remaining singletons (unique sequences occurring only once) were removed, as suggested by Behnke et al. (2011). The final datasets consisted of 21,608 bacterial and 6965 archaeal 16S reads. Complete linkage clustering and selection of representative sequences for operational taxonomic units (OTUs) were performed using the RDP Classifier (Cole et al., 2009). OTUs were assigned to taxonomic groups (i.e., bacterial and archaeal divisions) on the basis of BLASTN sequence similarity searches against the NCBI database. Taxonomic assignments were refined following the construction of phylogenetic trees consisting of representative sequences of the clusters and a set of 16S rRNA gene sequences from the representatives of different archaeal and bacterial lineages. The sequences were aligned using CLUSTALX (Thompson et al., 1997), and the neighbor-joining tree was computed by TREECON (Van de Peer and De Wachter, 1994). Shannon and equitability indices based on 16S rRNA gene sequence data were calculated at the 97 and 95% cutoff levels using the RDP Pipeline (Cole et al., 2009). Pyrosequencing read data has been deposited in the NCBI SRA database under accession SRR4450631.
Enrichment of Deep Subsurface Microorganisms in Batch and Continuous Cultures and Isolation of Pure Cultures
Microorganisms of different metabolic groups were enriched using aerobic and anaerobic conditions as previously described (Podosokorskaya et al., 2013a). Microcrystalline cellulose (Avicel; 2 g l−1), cellobiose (2 g l−1), lactate (Na salt; 20 mM), peptone (Sigma; 10 g l−1), gelatin (Sigma; 20 g l−1), and H2 (80 or 100%) were used as the energy substrates. Sulfate (Na salt, 10 mM), arsenate (Na salt, 5 mM), and glauconite (final Fe(III) concentration ca. 20 mM) were employed as the electron acceptors. Yeast extract (0.05–0.1 g l−1) was added as the source of growth factors. For batch enrichments, 10 ml portions of the media were dispensed in 18 ml Hungate tubes, and the head space was filled with an oxygen-free N2(100%) or N2/CO2 mixture (80:20%). When the medium was prepared aerobically or H2 was used as the substrate, the volume of the medium was 5 ml, and the head space was filled with either air or H2 (100%) or H2/CO2 mixture (80:20%), respectively. Incubation temperatures were 47, 50, 54, or 70°C. The growth of all cultures was monitored by direct cell counting under a phase contrast microscope at 1000 × magnification.
Continuous enrichments were initiated in a 1.3 l BioFlo 110 bioreactor (New Brunswick Scientific) under microaerobic conditions (4.5% O2 in the gas phase) with Na-acetate (10 mM) as the substrate. The mineral salts solution was modified according to the chemical analysis results of the borehole water as follows. The mineral salts solution contained (per liter) 7.0 g NaCl, 0.19 g MgCl2, 0.25 g NH4Cl, 0.5 g KCl, 0.2 g KH2PO4, 0.15 g NaHCO3, 0.013 g Na2S, and 1.0 g CaCl2. The medium was prepared as described previously (Podosokorskaya et al., 2013a). Trace element solutions A (10 ml l−1) and B (1 ml l−1) (Table S3) were used to provide for minor and trace elements corresponding to the elemental composition of the borehole water. Spherical glass beads (av. diameter 2 mm) were spread at the bottom of the reactor before autoclaving to model the mineral surface of the wallrock in the aquifer. The medium was autoclaved in the bioreactor and feed bottles at 121°C for 20 min. The pH (7.0–7.5), temperature (50–70°C), and dissolved O2 of the medium were adjusted before inoculation and automatically sustained during the incubation. The bioreactor was inoculated with 0.5 l of borehole water sampled in February 2010 and started in a batch mode. At the commencement of the exponential growth phase, the bioreactor was switched to continuous mode at a gradually increasing dilution rate of 0.5–1.17 ml min−1. Mixing was adjusted with Rushton impellers to 160 ml min−1 (coefficient of turbulent diffusion 178.2 cm2 s−1), which ensured a perfect mixing model. The composition of enrichment cultures was studied using PCR-DGGE analyses as described above.
Results
Inorganic Hydrochemistry of the Borehole Water
The chemical analysis results of the borehole water, sampled at three different time points in 2009–2010, have been reviewed by Banks et al. (2011), and the sampling in August 2013 has also been summarized (Banks et al., 2014). Briefly, the water is of Na-(Ca)-Cl composition. The molar ratio of Na/Cl is between 0.78 and 0.86, which is almost identical to modern sea water (0.86). Bromide occurs at 28–33 mg l−1 in the water, while the Cl−/Br− mass ratio of around 262–275 is very close to 288 of standard seawater. The water contains ca. 1.1 g l−1 of Ca but only about 5 mg l−1 of Mg. It is supersaturated with respect to calcite and saturated with respect to dolomite; the Sr concentration is high at ca. 86 mg l−1.
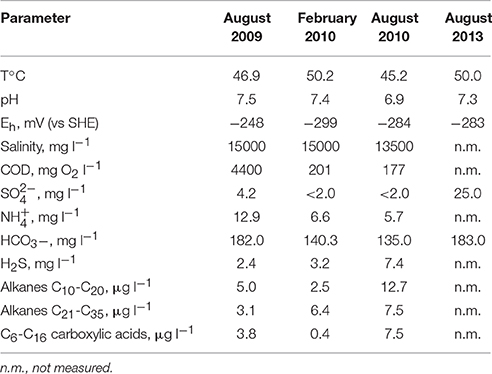
A significant variation in temperature was observed, from 50.2°C in February 2010 to 45.2°C in August 2010 (Table 1), while the temperature was around 50°C in August 2013. The water pH was circumneutral and also varied somewhat from 7.52 in August 2009 to 6.91 in August 2010. The redox potential of about −250 to −300 mV (vs. standard hydrogen electrode [SHE]) was indicative of strongly reducing conditions, also evidenced by the presence of dissolved H2S, CH4, near absence of sulfate, and elevated barium (17 mg l−1 in 2013) due to the lack of a barite solubility ceiling. The H2S concentrations varied from 2.46 mg l−1 in August 2009 to 7.42 mg l−1 in August 2010. Concentrations of most heavy metals (except Ba) measured in 2013 were low and were presumed to be suppressed by the high pH and sulfide concentrations. Arsenic concentrations were ca. 130 μg l−1 (Table S1).
Organic Hydrochemistry of the Borehole Water
The concentration of total carbon, including organics (as judged by COD measurements), varied significantly from ca. 103 mg l−1 in August 2009 to ca. 102 mg l−1 in 2010 sampling rounds. Modest concentrations of alkanes and non-volatile C6–C16 carboxylic acids were detected in all samples (Table 1). Acetate (160 mg l−1) and propionate (7.5 mg l−1) were present in significant concentrations, which underwent minor changes in the scope of 18% from August 2009 to March 2010. No other volatile fatty acids were detected in water samples.
Gas Composition of the Borehole Water
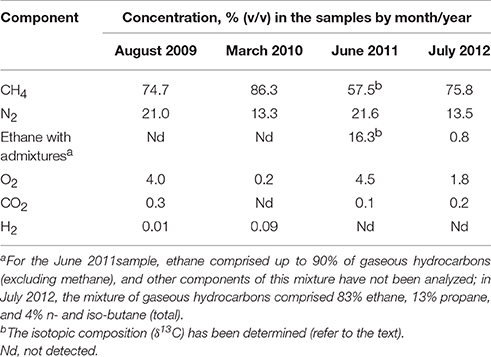
The composition of the exsolved gas bubbles collected from the water varied with time. CH4 was the main constituent, varying from 57.5% (v/v) (June 2011) to 86.3% (March 2010). The N2 content reached up to 21.6% of the gas in June 2011 (Table 2). O2 was detected in almost all gas samples, probably originating from nonequilibrium degassing of admixed meteoric recharge upon contact with deep hot sedimentary water. The minor components detected in almost all the samples were CO2 and H2 with concentrations not exceeding 0.3%. An ethane concentration of 16.3% was recorded in the June 2011 sample when methane was at its lowest fraction, but it decreased to 0.8% in the July 2012 sample (Table 2). The isotopic composition (δ13C) of CH4 and CH3CH3 in the June 2011 sample was −53%0 (indicative of thermogenic with minor admixture of biogenic CH4 as described by Schoell, 1984) and −40%0, respectively.
Microbial Cell Numbers and Morphotypes in the Borehole Water
Cell numbers assessed by direct cell counting varied significantly from one sample to another, being maximal in the first water sample taken in August 2009 (ca. 108 cells ml−1) and minimal in the February 2010 sample (3 × 104 cells ml−1). In other water samples, the cell content analyzed on-site varied from 105 to 107 cells ml−1. In the sample, taken in June 2011 for 16S rRNA-profiling with the pyrosequencing technique, the cell number comprised ca. 2 × 105 cells ml−1. In all the samples, various short rod-shaped morphotypes (less than 1 μm in length) prevailed and two different coccoid morphotypes were also detected.
PCR-DGGE Analysis of Archaeal and Bacterial Diversity in the Borehole Water
Some members of the prokaryotic community matched with bacterial genera (95–100% similarity), namely Delftia, Desulfovirgula, Thermoacetogenium, Desulfotomaculum, Symbiobacterium, Ignavibacterium, and archaea in the genus Methanothermobacter. Many phylotypes in the borehole water were uncultured Firmicutes (88–89% 16S rRNA gene similarity with closest cultured relatives). The majority of phylotypes were detected only once or, occasionally, twice. Among these, there were phylotypes matched with Delftia tsuruhatensis (only August 2009) and Ignavibacterium album (March 2010 and August 2013). Three phylotypes from the August 2009 sample were consistent with Thermoacetogenium phaeum (99% similarity), Symbiobacterium turbinis (97%), and Desulfotomaculum salinum (99%). Two Firmicutes were present in four out of the five DNA samples: a sulfate-reducing thermophile Desulfovirgula thermocuniculi (98–99%) and an uncultured bacterium that had 86% similarity with Thermanaerovibrio acidaminovorans (Table 3, Figure 1).
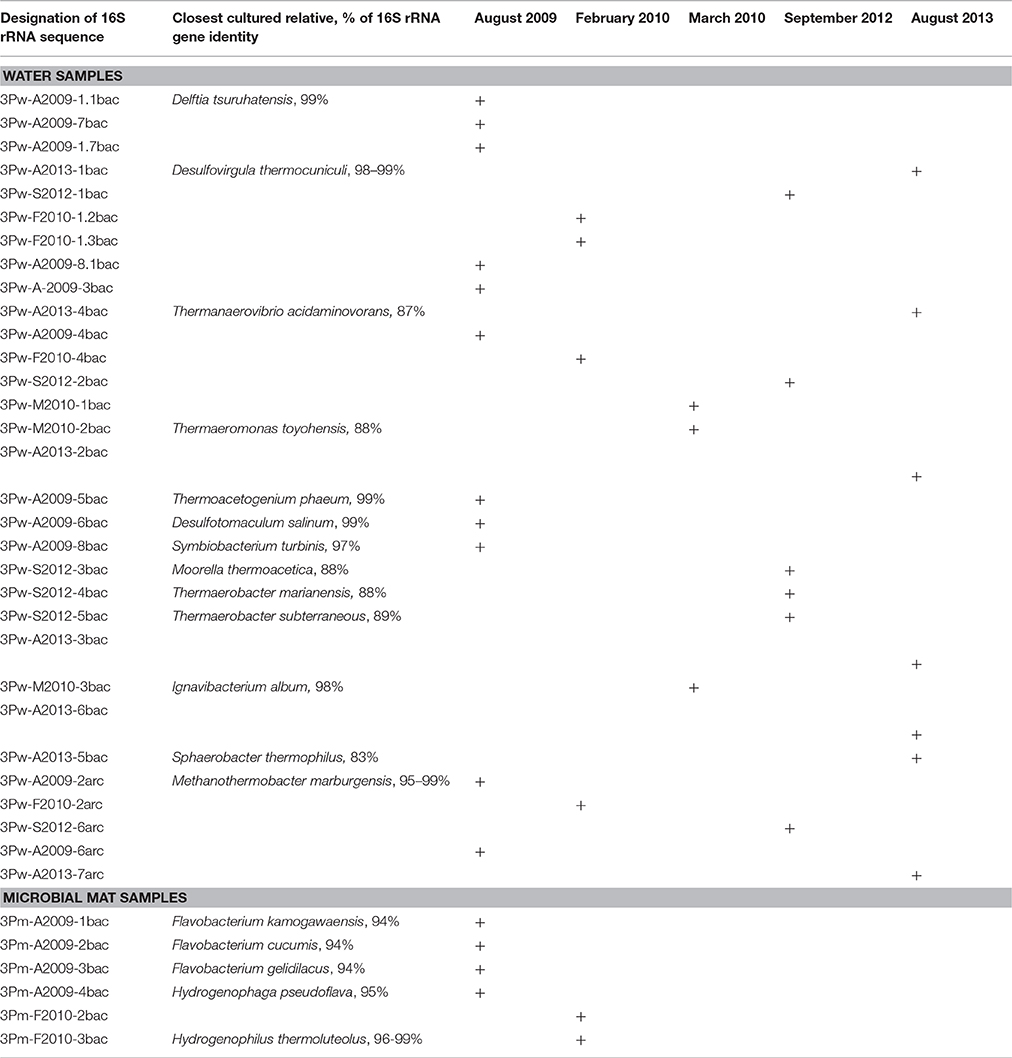
Table 3. Prokaryotic diversity in 3P borehole water and the microbial mat developing at the borehole outflow according to PCR-DGGE analyses of 16S rRNA genes.
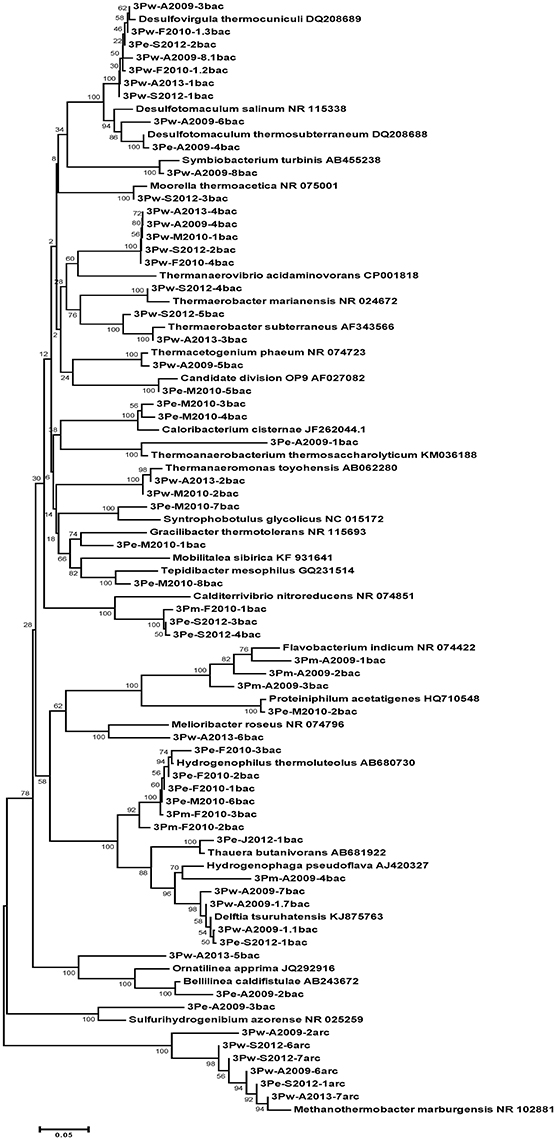
Figure 1. Phylogenetic position of Bacteria and Archaea detected in water, mats, and enrichments of 3P well by PCR-DGGE analysis. 3P, general designation; w, water; m, mat; e, enrichment; M2010, month and year of sampling; N, number of sequence; bac, bacteria; arc, archaea. Bootstrap values based on 1000 replications are shown at branch nodes.
Pyrosequencing Analysis of 16S rRNA Gene Fragments in the Borehole Water
Pyrosequencing analysis of 16S rRNA gene fragments was performed for the total DNA isolated from the borehole water sample of June 2011. Bacteria accounted for 76% of all 16S rRNA reads and were mostly represented by Firmicutes (Figure 2). Bacteria closely related to the sulfate-reducing thermophile Desulfovirgula thermocuniculi were the dominant component of the community (46.9% of the total). The second most abundant bacterial group (16.7%) comprised several lineages related to the genus Thermoacetogenium. Sulfate-reducer Desulfotomaculum kuznetsovii (Nazina et al., 1988) accounted for 1.3% of prokaryotes. Other Firmicutes of uncultured genera comprised 6.9% of the whole community. Members of the candidate division OP9 (Hugenholtz et al., 1998) accounted for 2.4% of the community. This group includes organotrophic bacteria proposed to ferment polysaccharides (Dodsworth et al., 2013). Other bacteria were about 1.3% of the total. Among them were Bellilinea caldifistulae from the phylum Chloroflexi (0.15%) and Ignavibacterium album (0.13%) from the phylum Ignavibacteriae.
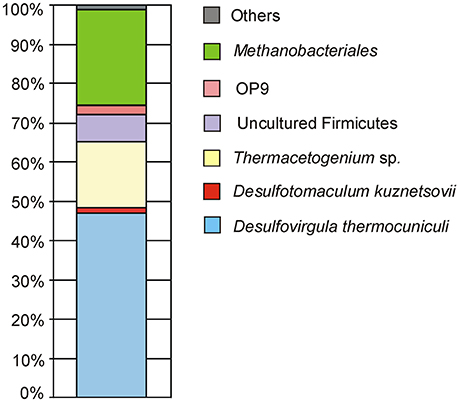
Figure 2. Composition of 3P well microbial community as characterized by the pyrosequencing of 16S rRNA gene variable fragments.
Archaeal sequences comprised about 24% of all 16S rRNA gene fragments. Most of them could be assigned to the hydrogenotrophic Methanothermobacter thermautotrophicus frequently found in aquifers. Among the other archaeal 16S rRNA sequences, members of the genera Methanobacterium and Methanosaeta were identified in minor amounts (<0.3%).
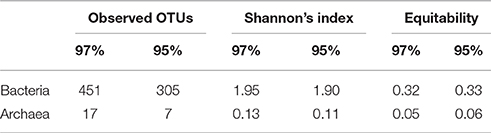
Overall, the microbial community diversity and richness, characterized by Shannon and equitability indices, is rather low (Table 4) in comparison with values reported for subsurface sediments and oil reservoirs, where Shannon indexes from 2 to 4 are typically reported (for example, Biddle et al., 2011; Gao et al., 2016). This is especially evident for the archaeal part of the community characterized by the presence of just a few species. Thus, in the pyrosequenced DNA sample, the two groups represented by the highest number of sequences—Desulfovirgula and Methanothermobacter spp.—were those consistently detected by DGGE as the components of the deep subsurface prokaryotic community.
PCR-DGGE Analysis of Bacterial Diversity in Microbial Mats
Microbial mats on the wooden conduit construction on the outflow pathway of the borehole water were about 1–2 mm thick and gelatinous and had a colorless, light pink, or pink-grayish hue (Figure S1). Based on the PCR-DGGE results (Table 3), completely different bacteria dominated mat communities in the two samplings in August 2009 and February 2010. Various
Bacteroidetes (Flavobacterium and Hydrogenophaga spp.) were present in August 2009 (colorless and light pink mats), while Hydrogenophilus spp. and uncultured Deferribacteres were detected in February 2010 (mainly grayish mats).
Batch and Continuous Enrichments from Borehole Water and Microbial Mats
Batch enrichment cultures were obtained on the media, containing diverse organic substrates in either the presence or absence of external electron acceptors, which were inoculated with borehole water samples (Table 5). PCR-DGGE analyses of the enrichment cultures showed the presence of phylogenetically diverse prokaryotes, most of them belonging to Bacteria.
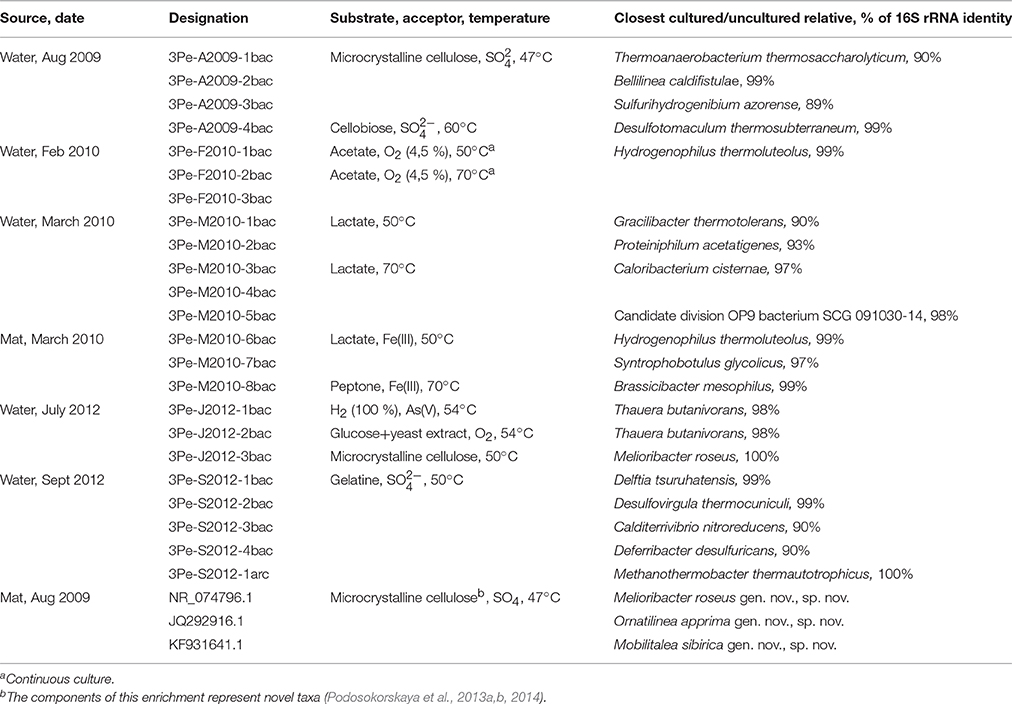
Table 5. Prokaryotic diversity in enrichment cultures obtained from 3P samples according to PCR-DGGE analyses of 16S rRNA genes.
Phylum Firmicutes was represented by numerous phylotypes, including both cultured and uncultured members of this group. Among other phylotypes, Bellilinea caldifistulae (99% similarity) and Melioribacter roseus (100% similarity with the type strain; Podosokorskaya et al., 2013a), representing Chloroflexi and Ignavibacteriae phyla, respectively, should be mentioned as well as Hydrogenophilus thermoluteolous (99% identity), a member of Betaproteobacteria and a representative of the candidate division OP9 (98% similarity) (Hugenholtz et al., 1998).
Several enrichment cultures were obtained in the presence of insoluble Fe(III) forms and As(V) as the electron acceptors.
The only enrichment culture that contained prevailing phylotypes of the consortium, which has also been identified directly by PCR-DGGE and pyrosequencing of the borehole water, was that incubated at 50°C with gelatin and sulfate. It comprised of Desulfovirgula thermocuniculi (99% similarity) and Methanothermobacter thermautotrophicus (100% similarity), accompanied by Delftia tsuruhatensis (99% similarity) and two uncultured Deferribacteres.
The enrichment culture obtained from a 3P microbial mat sample on the medium with microcrystalline cellulose contained three novel genera of hydrolytic bacteria that were subsequently isolated and described as Melioribacter roseus gen. nov., sp. nov., Ornatilinea apprima gen. nov., sp. nov., and Mobilitalea sibirica gen. nov., sp. nov. (Podosokorskaya et al., 2013a,b, 2014). They were able to grow on various polysaccharides, including cellulose, as well as on sugars and peptides.
Two continuous enrichments from the borehole water developed in the bioreactor under microaerobic conditions (4% O2). The enrichments were obtained with an incremental temperature increase from 50 to 70°C, with acetate as the sole carbon source and electron donor. The cell density in the bioreactor started at 1 × 104 cells ml−1 at 50°C and reached 3 × 107 cells ml−1 after 144 h of batch cultivation, thus indicating the beginning of the exponential growth phase. The exponential phase was sustained for 50 h of continuous cultivation under a perfect mixing regimen. Subsequently, the cultivation mode was switched back to batch culture, and the cell density reached its maximum of 7 × 109 cells ml−1 within 96 h. The high cell density was accompanied by the formation of biofilms on the interior glass walls, rotating impellers of the bioreactor, and the glass beads used to model the wallrock. When the cells started to lyse, the temperature was increased to 70°C, and the bioreactor was switched again to continuous mode. The growth ensued for about 100 h but to a lesser cell density (2·108 cells ml−1). Acetate consumption correlated well with the growth curve of the culture (Figure S2). DGGE analysis throughout the enrichments at 50 and 70°C showed only one phylotype, which is a close relative of Hydrogenophylus thermoluteolus (99% similarity).
Discussion
The oil exploration well 3P near Parabel' in Tomsk Region was drilled to the recorded depth of 2775 m 60 years ago and has never been an oil production well. For this study, the continuous outflow of deep saline thermal water provided an easy and reliable access to the deep subsurface biosphere. Assuming an annual average surface temperature close to 0°C, the 50°C temperature of water emerging from the borehole is compatible or slightly lower than calculated according to the typical geothermal gradient of 2–3°C/100 m (Banks, 2012; Slobodkin and Slobodkina, 2014). Considering the fact that the water has potentially cooled during passage up the borehole, it seems likely that the majority of the overflowing water is derived from close to the base of the borehole. Analyses of water performed three times during one year (2009–2010) showed that the borehole water is neutral and highly reduced, with salinity around 1.4% and a moderate concentration of dissolved organic carbon. The Na content of 4.1–4.3 g l−1 and the Cl− content of 7.5–8.6 g l−1 (2013 data) suggest that the water could represent a mixture of fresh meteoric recharge water with around 40% marine water, which could be original connate marine sedimentary water or be derived from a subsequent marine inundation of the terrain. Extremely low Mg/Sr mass ratios (~ 0.06) suggest that Sr has accumulated strongly during a prolonged residence time, while Mg has been depleted, possibly by dolomitization. Water temperature and chemical composition were not completely constant. These changes could not be explained as seasonal, as the samples from February and August 2010 were more similar than those of August 2009 and 2010. One hypothesis is that atmospheric pressure or tidal effects may affect the flow rate and, thus, the temperature and the dissolved gases (the slower the upflow rate in the borehole, the more heat is lost to the surrounding rocks). The possibility of mixing with variable fractions of shallow groundwater (e.g., via leaks in the borehole casing) also exists. However, our previous data show that stable isotope composition of the borehole water, when plotted on an 18O vs. 2H plot, falls to the right of the global meteoric water line (GMWL). This suggests that the water may partly be derived from meteoric recharge but with the isotopic signature, modified by 18O exchange with the aquifer matrix in a mildly geothermal environment, i.e., with a greater component of connate marine water in the 3P well (Banks et al., 2014).
The 5 years monitoring the same site with the same molecular technique reliably demonstrates that the groundwater planktonic microbial community consists of permanent major part and variable minor components. The revealed constant components are sulfate-reducers of the genus Desulfovirgula and methanogens of the family Methanobacteraceae. Pyrosequencing of 16S rRNA gene fragments showed that these two groups of prokaryotes comprise the majority of sequences (71% of the total amount) in the samples. This fact is in good correlation with repeated PCR-DGGE results. Regarding bacterial part of the community, these results are in contrast with some studies of deep subsurface bedrock-related terrestrial environments where the representatives of Comamonadaceae and Acholeplasmataceae (Nyyssönen et al., 2014) or Alicyclobacillaceae (Miettinen et al., 2015) dominated. However the representatives of the family Methanobacteraceae are fairly common for deep subsurface microbial communities in oil and gas bearing environments formed in sedimentary sequences (Ng et al., 1989; Nazina et al., 1995, 2006; Bonch-Osmolovskaya et al., 2003; Mochimaru et al., 2007; Yamane et al., 2011; Frank et al., 2016).
The minor components of the studied community appeared to be highly variable. It is plausible that some of the variability in the community composition could be related to variations in the water chemistry. PCR-DGGE analyses of the August 2009 water samples showed the highest diversity of bacteria. The initial presence of the representatives of the genus Delftia has not been observed in subsequent samples. Delftia spp. are mesophilic, organotrophic facultative anaerobes capable of degrading organic substrates, including hydrocarbons via aerobic or nitrate respiration. The presence of Delftia spp. corresponds to the lowest temperature, the highest ammonia concentration, and significant amounts of organics in August 2009 (Table 1). It was recently found that Delftia isolates originating from a deep subsurface aquifer are able to grow anaerobically with insoluble electron acceptors mimicked by electrodes (Jangir et al., 2016). It could be assumed that bacteria of this genus are present in the attached part of the microbial community growing organotrophically with minerals as the electron acceptors and are spread in the water at favorable conditions, as in August 2009, when an easily accessible electron acceptor nitrate was abundant. Noteworthily, sequences related to Delftia comprised the majority of RNA library from a borehole in Pyhäsalmi mine (Finland) representing the main active part of the microbial community (Miettinen et al., 2015). Similar sequences have been recovered from saline hydrothermal water in a Mexican mine (Ragon et al., 2013). Another minor component of the 3P well community—bacteria of the genus Desulfotomaculum—appeared in August 2009, both in water samples and in the enrichment culture with cellobiose and sulfate; they were also detected in June 2011 by 16S rRNA gene fragments pyrosequencing analysis. Their presence in the water could be attributed to the elevated concentration of sulfate (4.2 mg l−1 in August 2009 in comparison with that of <2.0 mg l−1 in February and August 2010). However, most of the detected Firmicutes had a very low level of 16S rRNA gene similarity with cultured taxa (83–89%; Table 3). Thus, their presence is unlikely to be directly connected with the water characteristics and chemical composition.
Both the planktonic community and the batch enrichment cultures contained many previously uncultured prokaryotes that belong in majority to the phylum Firmicutes. Many authors consider Firmicutes an important component of subsurface microbial communities (Davidson et al., 2011; Itävaara et al., 2011; Miettinen et al., 2015; Frank et al., 2016). Enrichments with microcrystalline cellulose and sulfate at 47°C contained three types of anaerobic organisms: (i) unidentified Clostridia (90% similarity with Thermoanaerobacterium thermosaccharolyticum), (ii) unidentified Aquificae (89% similarity with Sulfurihydrogenibium azorense), and (iii) Bellilinea caldifistulae (99%). Many Clostridia are known to hydrolyze cellulose and ferment glucose to volatile fatty acids. B. caldifistulae can utilize some short chain volatile fatty acids and a range of carbohydrates and was also identified in the planktonic community by pyrosequencing.
Another culture under comparable enrichment conditions but from microbial mat samples yielded three hydrolytic bacteria that were subsequently isolated in pure cultures and described as (i) Melioribacter roseus gen. nov., sp. nov., (ii) Ornatilinea apprima gen. nov., sp. nov., and (iii) Mobilitalea sibirica gen. nov., sp. nov. (Podosokorskaya et al., 2013a,b, 2014). Their combined spectrum of substrates includes cellulose as well as various di- and polysaccharides and proteinaceous substrates. M. roseus is a facultative anaerobe and can use arsenate, insoluble Fe(III) oxide ferrihydrite, and nitrite as external electron acceptors. This bacterium was also found in the enrichment culture from a borehole water sample (Table 5). It belongs to the new phylum Ignavibacteriae (Podosokorskaya et al., 2013a); another representative of which was also detected by PCR-DGGE analysis in the borehole water sample of March 2010 (Table 3). Thus, M. roseus and two other polysaccharide-degrading isolates represent, most probably, immobilized organotrophic bacteria that perform anaerobic degradation of buried organic matter in the 3P well community. Moreover, M. roseus is capable of oxygen respiration, and, being brought to the surface with the borehole outflow, it could proliferate in periodically aerated microbial mats fed with organic-rich thermal water.
The gelatin-utilizing enrichment culture contained all components of the planktonic prokaryotic consortium: Delftia, Desulfovirgula and Methanothermobacter spp., together with uncultured representatives of Deferribacteres. This suggests that gelatin may successfully substitute for natural polymeric substrates for cultural recoveries of prokaryotes from the subsurface.
Fe(III), in the form of glauconite (a mica group phyllosilicate), was added as an external electron acceptor in some enrichment cultures. It is an insoluble electron acceptor candidate in geothermal systems (others include ferrihydrite, Fe(III)-oxyhydroxides, and iron-containing aluminosilicates), as it is widely present in marine sedimentary depositional sequences of the deep West Siberian basin (Nikitenko, 2009). Previous studies have demonstrated a broad ability for ferric iron reduction of microorganisms inhabiting the deep subsurface biosphere and oil wells in particular (Slobodkin et al., 1999; Li et al., 2006). Direct enrichments using glauconite as an electron acceptor were obtained only from the mat samples and contained bacteria of Brassicibacter, Hydrogenophilus, and Syntrophobotulus genera. Representatives of these taxa have not been previously shown to reduce Fe(III), although Hydrogenophilus can grow chemolithoautotrophically with molecular H2 as the electron donor, CO2 as the carbon source, and oxygen or nitrate as external electron acceptors. As the reduction of nitrate may involve multiheme c-type cytochromes, which are also the major determinants of Fe(III) reduction (Sharma et al., 2010), one might expect the iron-reducing activity in a Hydrogenophilus strain enriched with glauconite in our experiments.
Two major components of the planktonic community in the borehole water were consistently present: sulfate-reducers assigned to Desulfovirgula thermocuniculi and methanogens of the genus Methanothermobacter. Thermophilic sulfate-reducing bacteria in oil formation water have been documented in many studies (e.g., Magot et al., 2000; Slobodkin and Slobodkina, 2014) and are especially characteristic for submarine oil deposits where sulfates are easily available. Thermophilic sulfate-reducers of the genera Desulfotomaculum, Desulfacinum, Thermodesulfobacterium, and Thermodesulforhabdus, as well as hyperthermophilic archaea of the genus Archaeoglobus, have been either isolated or detected by molecular methods in oil wells of the North Sea (Beeder et al., 1994; Rees et al., 1995; Nilsen et al., 1996). In terrestrial high-temperature oil reservoirs where the concentration of sulfates is much lower, sulfate-reducers or their activity have also been detected (Nazina et al., 1995, 2006; Bonch-Osmolovskaya et al., 2003; Frank et al., 2016). The most commonly mentioned sulfate-reducers in high-temperature oil reservoirs are thermophilic members of the genus Desulfotomaculum. However, quantitative data on their presence in microbial communities of organic-rich deep subsurface habitats have not been presented to date. In this study, sulfate-reducing Desulfotomaculum spp. was only detected in borehole water as a minor component. Another sulfate-reducing Firmicute—Desulfovirgula thermocuniculi—was found to be a key constituent of the planktonic microbial community of the borehole water. The type strain of D. thermocuniculi was isolated from a geothermally influenced underground mine sample in Japan (Kaksonen et al., 2007). D. thermocuniculi is able to oxidize H2 and organic acids with sulfate as the electron acceptor or perform organic acid fermentation. Considering the extremely low concentration of sulfates in the borehole water (from <2 to 4.23 mg l−1), we assume that in the borehole community D. thermocuniculi grows by the oxidation of carboxylic acids present in the water, and the produced hydrogen is scavenged by hydrogenotrophic methanogens via interspecies hydrogen transfer, which takes place at the low availability of sulfates.
Methanogens of the family Methanobacteraceae are common members of deep subsurface microbial communities, especially of those associated with oil deposits (Ng et al., 1989; Nazina et al., 1995, 2006; Bonch-Osmolovskaya et al., 2003; Mochimaru et al., 2007; Yamane et al., 2011; Frank et al., 2016). Methanobacteraceae were found to be consistent components of the borehole water. According to pyrosequencing analysis, Methanobacteraceae comprised 24% of the total amount of sequences. In contrast, representatives of Methanosaeta were only detected in 2011 by 16S rRNA pyrosequencing and in only minor quantities. Methanosarcinales were not detected in the borehole water, while these methanogens were reported to dominate a high-temperature oil reservoir in California (Orphan et al., 2003).
A syntrophic mechanism of acetate conversion to methane occurring in high-temperature oil reservoirs has been proposed by Nazina et al. (2006), whereby Thermoacetogenium phaeum (Hattori et al., 2000) degrades acetate to hydrogen and CO2, and Methanothermobacter spp. converts these products to CH4. The presence of bacteria related to T. phaeum in the formation water of oil reservoirs has been previously noted in Japan (Yamane et al., 2011). Firmicutes related to T. phaeum were detected in the 3P borehole water by PCR-DGGE in 2009 and by pyrosequencing in 2011; in the latter sampling, they represented a significant part (16%) of the microbial community. Thus, acetate-utilizing syntrophs may participate in methanogenesis in the borehole microbial community. In the absence of T. phaeum, syntrophy with methanogens may involve other members of uncultured Firmicutes detected in five borehole water samples.
Another point is that the prevalence of sulfate-reducers and methanogens may indicate reverse methanogenesis by an anaerobic, methane-oxidizing consortium, with sulfate as the electron acceptor (Knittel and Boetius, 2009). The reversibility of methyl-coenzyme M reductase, the key enzyme of methanogenesis, is supported by thermodynamic and kinetic considerations (Thauer, 2011) and by the activity assays with purified enzymes from Methanothermobacter marburgensis (Scheller et al., 2010) and M. thermautotrophicus (Chen et al., 2012). The thermogenic nature of methane in the borehole water, as indicated by the carbon isotopic composition, suggests conditions favorable for methanotrophy coupled to sulfate reduction rather than methanogenesis.
When the reduced water containing energy-rich substrates, but lacking easily available electron acceptors, flows out of the well, microbial mats develop on the wooden conduit along the flow pathway of the borehole water. These were found to consist of either Hydrogenophilus thermoluteolus and uncultured Deferribacteres or Flavobacterium and Hydrogenophaga-related organisms (Table 3). None of these prokaryotes were detected in the borehole water by PCR-DGGE or pyrosequencing. H. thermoluteolus was present in lactate-utilizing enrichment cultures amended with glauconite from the microbial mat, and it appeared to be the only phylotype in the continuous-flow microaerophilic enrichment obtained from the borehole water. This continuous enrichment may be regarded as a laboratory model of a microbial mat fed with the thermal borehole water, which brings H. thermoluteolus from its subsurface habitats. The ability of the enriched H. thermoluteolus to form thick biofilms, even at turbulent mixing, indicates that this microorganism could thrive at intermediate layers of the borehole, where oxygen, putatively released from extrinsic shallow groundwater, is mixed with intensive connate hot borehole outflow. While H. thermoluteolus could be a minor component in the borehole water community, it becomes dominant in the microbial mats as a result of more favorable conditions.
It should be emphasized that the deep subsurface, we were able to access via borehole water sampling, has two (planktonic and immobilized) microbial components that are both supported by organic matter contained in the Mesozoic sedimentary deposits. Biopolymers from that source could be degraded by anaerobes able to attach to various surfaces and possessing multiple hydrolytic catabolic pathways, such as the representatives of the phylum Ignavibacteriae (Podosokorskaya et al., 2013a). Aqueous organic solutes produced by hydrolytic microorganisms can potentially be completely oxidized by sulfate-reducers in the planktonic community (e.g., Desulfovirgula and Desulfotomaculum spp.) or by syntrophic methanogenic associations, such as sulfate-reducers or Thermacetogenium and Methanothermobacter spp. Another option could be reverse methanogenesis in syntrophy with sulfate-reducers, recycling methane to the biomass or yielding CO2 and H2S. However, considering the thermogenic nature of methane (i.e., the mixture of biogenic and abiogenic CH4) in the borehole, neither methanogenesis nor methanotrophy prevalence can be reliably inferred from our data. At the artesian outflow of the borehole, aerobic or microaerobic processes can drive the oxidation of soluble organics supporting the growth of microbial mats formed by minor or immobilized components of the subsurface water ecosystem. The presence of many “uncultured” microorganisms in enrichment cultures provides hope for their successful cultivation and, thus, to the understanding of their metabolic function in the deep subsurface environment.
Author Contributions
YF, VK, SG, DB, AG, OP, and OK contributed to field work. YF, AG, OP, and SG obtained enrichment and pure cultures. YF, DB, and OK contributed to physical-chemical analysis. YF, AG, NC, AYM, and OK performed PCR-DGGE and phylogenetic analysis. VK, AVM, and NR performed pyrosequencing analysis. EB, OK, SG, and YF designed the work. EB, OK, SG, YF, NR, DB, AYM, and OP wrote the manuscript.
Funding
This work was supported by the Russian Science Foundation [14–24–00165] (studies on biogeochemical cycling of C1-compounds), Russian Federation Agency of Science and Innovations (field work), and Russian Foundation for Basic Research [13–04–40205]. Analysis of microbial communities by pyrosequencing of 16S rRNA genes was supported by the Russian Science Foundation [14–14–01016] to NR group. Molecular screening of sulfate-reducing enrichments was performed with support of the Russian Science Foundation [14–14–00427].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Alexander Adam, Sergey Vorobiov, and Evgeny Tan for their help with organization of sampling. Ekaterina Komleva and Vera Teplyashina are recognized for their technical assistance. Andrey Novikov from Gubkin University of Oil and Gas (Moscow) is acknowledged for analysis of the ethane mixture with gaseous hydrocarbons from 2012. The authors also thank Michael Watts of the British Geological Survey for inorganic analysis of water samples from 2010 and 2013.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.02101/full#supplementary-material
References
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nuclic Acids Res. 25, 389–402. doi: 10.1093/nar/25.17.3389
Banks, D. (2012). An Introduction to Thermogeology: Ground Source Heating and Cooling, 2nd Edn. Chichester: John Wiley and Sons.
Banks, D., Frank, Y. A., Kadnikov, V. V., Watts, M., Boyce, A., and Frengstad, B. S. (2014). Hydrochemical Data Report from Sampling of Two Deep Abandoned Hydrocarbon Exploration Wells: Byelii Yar and Parabel', Tomsk oblast', Western Siberia, Russian Federation, NGU Report 2014.034, Geological Survey of Norway, Trondheim.
Banks, D., Parnachev, V. P., Karnachuk, O. V., Arkhipov, A. L., Gundersen, P., and Davis, J. (2011). Hydrogeochemical Data Report: The Sampling of Selected Localities in Kemerovo oblast' and Tomsk oblast', Siberia, Russian Federation, NGU Report 2011.054, Geological Survey of Norway, Trondheim.
Batzke, A., Engelen, B., Sass, H., and Cypionka, H. (2007). Phylogenetic and physiological diversity of cultured deep-biosphere bacteria from equatorial Pacific ocean and Peru margin sediments. Geomicrobiol. J. 24, 261–273. doi: 10.1080/01490450701456453
Beeder, J., Nilsen, R. K., Rosnes, J. T., Torsvik, T., and Lien, T. (1994). Archaeoglobus fulgidus isolated from hot North Sea oil field waters. Appl. Environ. Microbiol. 60, 1227–1231.
Behnke, A., Engel, M., Christen, R., Nebel, M., Klein, R. R., and Stoeck, T. (2011). Depicting more accurate pictures of protistan community complexity using pyrosequencing of hypervariable SSU rRNA gene regions. Environ. Microbiol. 13, 340–349. doi: 10.1111/j.1462-2920.2010.02332.x
Biddle, J. F., White, J. R., Teske, A. P., and House, C. H. (2011). Metagenomics of the subsurface Brazos-Trinity Basin (IODP site 1320): comparison with other sediment and pyrosequenced metagenomes. ISME J. 5, 1038–1047. doi: 10.1038/ismej.2010.199
Bomberg, M., Nyyssönen, M., Pitkänen, P., Lehtinen, A., and Itävaara, M. (2015). Active microbial communities inhabit sulphate-methane interphase in deep bedrock fracture fluids in Olkiluoto, Finland. BioMed Res. Int. 2015:979530. doi: 10.1155/2015/979530
Bonch-Osmolovskaya, E. A., Miroshnichenko, M. L., Lebedinsky, A. V., Chernyh, N. A., Nazina, T. N., Ivoilov, V. S., et al. (2003). Radioisotopic, culture-based, and oligonucleotide microchip analyses of thermophilic microbial communities in a continental high-temperature petroleum reservoir. Appl. Environ. Microbiol. 69, 6143–6151. doi: 10.1128/AEM.69.10.6143-6151.2003
Castelle, C. J., Hug, L. A., Wrighton, K. C., Thomas, B. C., Williams, K. H., and Wu, D. (2013). Extraordinary phylogenetic diversity and metabolic versatility in aquifer sediment. Nat. Commun. 4:2120. doi: 10.1038/ncomms3120
Chen, S. L., Blomberg, M. R., and Siegbahn, P. E. (2012). How is methane formed and oxidized reversibly when catalyzed by Ni-containing methyl-coenzyme M reductase? Chemistry 18, 6309–6315. doi: 10.1002/chem.201200274
Cline, J. D. (1969). Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14, 454–458. doi: 10.4319/lo.1969.14.3.0454
Cole, J. R., Wang, Q., Cardenas, E., Fish, J., Chai, B., Farris, R. J., et al. (2009). The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, 141–145. doi: 10.1093/nar/gkn879
Coolen, M. J. L., Hopmans, E. C., Rijpstra, W. I. C., Muyzer, G., Schouten, S., Volkman, J. K., et al. (2004). Evolution of the methane cycle in Ace Lake (Antarctica) during the Holocene: response of methanogens and methanotrophs to environmental change. Org. Geochem. 35, 1151–1167. doi: 10.1016/j.orggeochem.2004.06.009
Dahle, H., Garshol, F., Madsen, M., and Birkeland, N. K. (2008). Microbial community structure analysis of produced water from a high-temperature North Sea oil field. Antonie Van Leeuwenhoek 93, 37–49. doi: 10.1007/s10482-007-9177-z
Davidson, M. M., Silver, B. J., Onstott, T. C., Moser, D. P., Gihring, T. M., Pratt, L. M., et al. (2011). Capture of planktonic microbial diversity in fractures by long-term monitoring of flowing boreholes, Evander Basin, South Africa. Geomicrobiol. J. 28, 275–300. doi: 10.1080/01490451.2010.499928
DeLong, E. F. (1992). Archaea in coastal marine environments. Proc. Natl. Acad. Sci. U.S.A. 89, 5685–5689. doi: 10.1073/pnas.89.12.5685
Dodsworth, J. A., Blainey, P. C., Murugapiran, S. K., Swingley, W. D., Ross, C. A., Tringe, S. G., et al. (2013). Single-cell and metagenomic analyses indicate a fermentative, saccharolytic lifestyle for members of the OP9 lineage. Nat. Commun. 4:1854. doi: 10.1038/ncomms2884
Edwards, K. J., Bach, W., and McCollom, T. M. (2005). Geomicrobiology in oceanography: microbe–mineral interactions at and below the seafloor. Trends Microbiol. 13, 449–456. doi: 10.1016/j.tim.2005.07.005
Edwards, K. J., Wheat, C. G., and Sylvan, J. B. (2011). Under the sea: microbial life in volcanic oceanic crust. Nat. Rev. Microbiol. 9, 703–712. doi: 10.1038/nrmicro2647
Frank, Y., Banks, D., Avakian, M., Antsiferov, D., Kadychagov, P., and Karnachuk, O. (2016). Firmicutes is an important component in water-injected and pristine oil reservoirs; Western Siberia, Russia. Geomicrobiol. J. 33, 387–400. doi: 10.1080/01490451.2015.1045635
Fredrickson, J. K., and Hicks, R. J. (1987). Probing reveals many microbes beneath Earth's surface. ASM News. 53, 78–79.
Gao, P., Tian, H., Wang, Y., Li, Y., Li, Y., Xie, J., et al. (2016). Spatial isolation and environmental factors drive distinct bacterial and archaeal communities in different types of petroleum reservoirs in China. Sci. Rep. 6:20174. doi: 10.1038/srep20174
Ghiorse, W. C., and Wilson, J. T. (1988). Microbial ecology of the terrestrial subsurface. Adv. Appl. Microbiol. 33, 107–173. doi: 10.1016/S0065-2164(08)70206-5
Giggenbach, W. F., and Goguel, R. L. (1989). Collection and Analysis of Geothermal and Volcanic Water and Gas Discharges, New Zealand Department of Scientific and Industrial Research, Report No. CD2401. 81.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Hattori, S., Kamagata, Y., Hanada, S., and Shoun, H. (2000). Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 50, 1601–1609. doi: 10.1099/00207713-50-4-1601
Hug, L. A., Thomas, B. C., Sharon, I., Brown, C. T., Sharma, R., Hettich, R. L., et al. (2016). Critical biogeochemical functions in the subsurface are associated with bacteria from new phyla and little studied lineages. Environ. Microbiol. 18, 159–173. doi: 10.1111/1462-2920.12930
Hugenholtz, P., Pitulle, C., Hershberger, K. L., and Pace, N. R. (1998). Novel division level bacterial diversity in a Yellowstone hot spring. J. Bacteriol. 180, 366–376.
Itävaara, M., Nyyssönen, M., Kapanen, A., Nousiainen, A., Ahonen, L., and Kukkonen, I. (2011). Characterization of bacterial diversity to a depth of 1500 m in the Outokumpu deep borehole, Fennoscandian Shield. FEMS Microbiol. Ecol. 77, 295–309. doi: 10.1111/j.1574-6941.2011.01111.x
Jangir, M., French, S., Momper, L. M., Moser, D. P., Amend, J. P., and El-Naggar, M. Y. (2016). Isolation and characterization of electrochemically active subsurface Delftia and Azonexus species. Front. Microbiol. 7:756. doi: 10.3389/fmicb.2016.00756
Jiménez, L. (1990). Molecular analysis of deep-subsurface Bacteria. Appl. Environ. Microbiol. 56, 2108–2113.
Kaksonen, A. H., Spring, S., Schumann, P., Kroppenstedt, R. M., and Puhakka, J. A. (2007). Desulfovirgula thermocuniculi gen. nov., sp. nov., a thermophilic sulfate-reducer isolated from a geothermal underground mine in Japan. Int. J. Syst. Evol. Microbiol. 57, 98–102. doi: 10.1099/ijs.0.64655-0
Karnachuk, O. V., Gerasimchuk, A. L., Banks, D., Frengstad, B., Stykon, G. A., Tikhonova, Z. L., et al. (2009). Bacteria of the sulfur cycle in the sediments of gold mine tailings, Kuznetsk Basin, Russia. Microbiology 78, 483–491. doi: 10.1134/S0026261709040122
Kimura, H., Asada, R., Masta, A., and Naganuma, T. (2003). Distribution of microorganisms in the subsurface of the Manus Basin hydrothermal vent field in Papua New Guinea. Appl. Environ. Microbiol. 69, 644–648. doi: 10.1128/AEM.69.1.644-648.2003
Knittel, K., and Boetius, A. (2009). Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63, 311–334. doi: 10.1146/annurev.micro.61.080706.093130
Lane, D. (1991). “16s/23s rRNA sequencing,” in Nucleic Acid Techniques in Bacterial Systematics, eds E. Stackebrandt and M. Goodfellow (Chichester: John Wiley and Sons), 115–175.
Lever, M. A., Rogers, K. L., Lloyd, K. G., Overmann, J., Schink, B., Thauer, R. K., et al. (2015). Life under extreme energy limitation: a synthesis of laboratory- and field-based investigations. FEMS Microbiol. Rev. 39, 688–728. doi: 10.1093/femsre/fuv020
Li, H., Yang, S. Z., Mu, B. Z., Rong, Z. F., and Zhang, J. (2006). Molecular analysis of the bacterial community in a continental high temperature and water-flooded petroleum reservoir. FEMS Microbiol. Lett. 257, 92–98. doi: 10.1111/j.1574-6968.2006.00149.x
Lomstein, B. A., Langerhuus, A. T., D'Hondt, S., Jørgensen, B. B., and Spivack, A. J. (2012). Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature 484, 101–104. doi: 10.1038/nature10905
Magot, M., Ollivier, B., and Patel, B. K. C. (2000). Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek 77, 103–116. doi: 10.1023/A:1002434330514
McMahon, S., and Parnell, J. (2014). Weighing the deep continental biosphere. FEMS Microbiol. Ecol. 87, 113–120. doi: 10.1111/1574-6941.12196
Miettinen, H., Kietäväinen, R., Sohlberg, E., Numminen, M., Ahonen, L., and Itävaara, M. (2015). Microbiome composition and geochemical characteristics of deep subsurface high-pressure environment, Pyhäsalmi mine Finland. Front. Microbiol. 6:1203. doi: 10.3389/fmicb.2015.01203
Mochimaru, H., Uchiyama, H., Yoshioka, H., Imachi, H., Hoaki, T., Tamaki, H., et al. (2007). Methanogen diversity in deep subsurface gas-associated water at the Minami-Kanto gas field in Japan. Geomicrobiol. J. 24, 93–100. doi: 10.1080/01490450701266571
Muyzer, G., de Waal, E. C., and Uitterlinden, A. G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59, 695–700.
Muyzer, G., Hottenträger, S., Teske, A., and Waver, C. (1996). “Denaturing gradient gel electrophoresis of PCR-amplified 16S rDNA – a new molecular approach to analyse the genetic diversity of mixed microbial communities,” in Molecular Microbial Ecology Manual, eds A. D. L. Akkermans, J. D. van Elsas, and F. De Bruijn (Dordrecht: Kluwer Academic Publishers), 1–23.
Nazina, T. N., Ivanova, A. E., Borzenkov, I. A., Belyaev, S. S., and Ivanov, M. V. (1995). Occurrence and geochemical activity of microorganisms in high-temperature, water-flooded oil fields of Kazakhstan and Western Siberia. Geomicrobiol. J. 13, 403–408. doi: 10.1080/01490459509378016
Nazina, T. N., Ivanova, A. E., Kanchaveli, L. P., and Rozanova, E. P. (1988). A new spore-forming thermophilic methylotrophic sulfate-reducing bacterium Desulfotomaculum kuznetsovii sp. nov. Microbiology 57, 823–827.
Nazina, T. N., Shestakova, N. M., Grigor'yan, A. A., Mikhailova, E. M., Tourova, T. P., Poltaraus, A. B., et al. (2006). Phylogenetic diversity and activity of anaerobic microorganisms of high-temperature horizons of the Dagang oil field (P.R. China). Microbiology 75, 55–65. doi: 10.1134/S0026261706010115
Nealson, K. H., Inagaki, F., and Takai, K. (2005). Hydrogen-driven subsurface lithoautotrophic microbial ecosystems (SLiMEs): do they exist and why should we care? Trends Microbiol. 13, 405–410. doi: 10.1016/j.tim.2005.07.010
Ng, T. K., Weimer, P., and Gawel, L. J. (1989). Possible nonantropogenic origin of two methanogenic isolates from oil-producing wells in San Miguelito field, Ventura County, California. Geomicrobiol. J. 7, 185–192. doi: 10.1080/01490458909377861
Nikitenko, B. L. (2009). Jurassic Stratigraphy, Palaeobiogeography and Biofacies of Siberia on Microfauna (Foraminifers and Ostracodes). [In Russian]. Novosibirsk: Parallel' Publishing House.
Nilsen, R. K., Beeder, J., Thorstenson, T., and Torsvik, T. (1996). Distribution of thermophilic marine sulfate reducers in North Sea oil field waters and oil reservoirs. Appl. Environ. Microbiol. 62, 1793–1798.
Nyyssönen, M., Hultman, J., Ahonen, L., Kukkonen, I., Paulin, L., Laine, P., et al. (2014). Taxonomically and functionally diverse microbial communities in deep crystalline rocks of the Fennoscandian shield. ISME J. 8, 126–138. doi: 10.1038/ismej.2013.125
Ollivier, B., Cayol, J.-L., and Fauque, G. (2007). “Sulphate-reducing bacteria from oil field environments and deep-sea hydrothermal vents,” in Sulphate-Reducing Bacteria. Environmental and Engineered Systems, eds L. L. Barton and W. A. Hamilton (Cambridge: Cambridge University Press), 305–328.
Onstott, T. C., Magnabosco, C., Aubrey, A. D., Burton, A. S., Dworkin, J. P., Elsila, J. E., et al. (2014). Does aspartic acid racemization constrain the depth limit of the subsurface biosphere? Geobiology 12, 1–19. doi: 10.1111/gbi.12069
Orphan, V. J., Goffredi, S. K., and DeLong, E. F. (2003). Geochemical influence on diversity and microbial processes in high temperature oil reservoirs. Geomicrobiol. J. 20, 295–311. doi: 10.1080/01490450303898
Parkes, R. J., Cragg, B. A., and Wellsbury, P. (2000). Recent studies on bacterial populations and processes in subseafloor sediments: a review. Hydrogeol. J. 8, 11–28. doi: 10.1007/PL00010971
Pedersen, K. (2012). Subterranean microbial populations metabolize hydrogen and acetate under in situ conditions in granitic groundwater at 450 m depth in the Äspö Hard Rock Laboratory, Sweden. FEMS Microbiol. Ecol. 81, 217–229. doi: 10.1111/j.1574-6941.2012.01370.x
Podosokorskaya, O. A., Bonch-Osmolovskaya, E. A., Beskorovaynyy, A. V., Toshchakov, S. V., Kolganova, T. V., and Kublanov, I. V. (2014). Mobilitalea sibirica gen. nov., sp. nov., a halotolerant polysaccharide-degrading bacterium. Int. J. Syst. Evol. Microbiol. 64, 2657–2661. doi: 10.1099/ijs.0.057109-0
Podosokorskaya, O. A., Bonch-Osmolovskaya, E. A., Novikov, A. A., Kolganova, T. V., and Kublanov, I. V. (2013b). Ornatilinea apprima gen. nov., sp. nov., a novel cellulolytic representative of class Anaerolineae. Int. J. Syst. Evol. Microbiol. 63, 86–92. doi: 10.1099/ijs.0.041012-0
Podosokorskaya, O. A., Kadnikov, V. A., Gavrilov, S. N., Mardanov, A. V., Karnachuk, O. V., Ravin, N. A., et al. (2013a). Characterization of Melioribacter roseus gen. nov., sp. nov., a novel facultatively anaerobic thermophilic cellulolytic bacterium from the class Ignavibacteria, and a proposal of a novel bacterial phylum Ignavibacteriae. Environ. Microbiol. 15, 1759–1771. doi: 10.1111/1462-2920.12067
Quince, C., Lanzen, A., Davenport, R. J., and Turnbaugh, P. J. (2011). Removing noise from pyrosequenced amplicons. BMC Bioinformatics 12:38. doi: 10.1186/1471-2105-12-38
Ragon, M., Van Driessche, A. E. S., García-Ruíz, J. M., Moreira, D., and López-García, P. (2013). Microbial diversity in the deep-subsurface hydrothermal aquifer feeding the giant gypsum crystal-bearing Naica Mine, Mexico. Front. Microbiol. 4:37. doi: 10.3389/fmicb.2013.00037
Rees, G. N., Grassia, G. S., Sheehy, A. J., Dwivedi, P. P., and Patel, B. C. (1995). Desulfacinum infernum gen. nov., sp. nov., a thermophilic sulfate-reducing bacterium from a petroleum reservoir. Int. J. Syst. Evol. Microbiol. 45, 85–89. doi: 10.1099/00207713-45-1-85
Scheller, S., Goenrich, M., Boecher, R., Thauer, R. K., and Jaun, B. (2010). The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 465, 606–608. doi: 10.1038/nature09015
Schoell, M. (1984). Wasserstoff- und Kohlenstoffisotope in organischen Substanzen, Erdölen und Erdgasen. Geologisches Jahrbuch. Reihe D 11 Mineralogie, Petrographiet Geochemie, Lagerstatfenkunde. 164.
Sharma, S., Cavallaro, G., and Rosato, A. (2010). A systematic investigation of multiheme c-type cytochromes in prokaryotes. J. Biol. Inorg. Chem. 15, 559–571. doi: 10.1007/s00775-010-0623-4
Slobodkin, A. I., Jeanthon, C., L'Haridon, S., Nazina, T. N., Miroshnichenko, M. L., and Bonch-Osmolovskaya, E. A. (1999). Dissimilatory reduction of Fe(III) by thermophilic bacteria and archaea in deep subsurface petroleum reservoirs of Western Siberia. Curr. Microbiol. 39, 99–102. doi: 10.1007/s002849900426
Slobodkin, A. I., and Slobodkina, G. B. (2014). Thermophilic prokaryotes from deep subterranean habitats. Microbiology 83, 169–183. doi: 10.1134/S0026261714030151
Takai, K., Gamo, T., Tsunogai, U., Nakayama, N., Hirayama, H., and Nealson, K. N. (2004). Geochemical and microbiological evidence for a hydrogen-based, hyperthermophilic subsurfacelithoautotrophic microbial ecosystem (HyperSLiME) beneath an active deep-sea hydrothermal field. Extremophiles 8, 269–282. doi: 10.1007/s00792-004-0386-3
Takai, K., Moser, D. P., DeFlaun, M., Onstott, T. C., and Fredrickson, J. K. (2001). Archaeal diversity in waters from deep South African gold mines. Appl. Environ. Microbiol. 67, 5750–5760. doi: 10.1128/AEM.67.21.5750-5760.2001
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Teske, A. P. (2005). The deep subsurface biosphere is alive and well. Trends Microbiol. 13, 402–404. doi: 10.1016/j.tim.2005.07.004
Thauer, R. K. (2011). Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr. Opin. Microbiol. 14, 292–299. doi: 10.1016/j.mib.2011.03.003
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24, 4876–4882. doi: 10.1093/nar/25.24.4876
Tsai, Y. L., and Olson, B. H. (1991). Rapid method for direct extraction of DNA from soil and sediments. Appl. Environ. Microbiol. 57, 1070–1074.
Ulmishek, G. F. (2003). “Petroleum geology and resources of the West Siberian Basin, Russia,” in U.S. Geological Survey Bulletin 2201-G (Reston, VA: Geological Survey), 49.
Van de Peer, Y., and De Wachter, R. (1994). TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biol. Sci. 10:569–570. doi: 10.1093/bioinformatics/10.5.569
Vissers, E. W., Bodelier, P. L., Muyzer, G., and Laanbroek, H. J. (2009). A nested PCR approach for improved recovery of archaeal 16S rRNA gene fragments from freshwater samples. FEMS Microbiol. Lett. 298, 193–198. doi: 10.1111/j.1574-6968.2009.01718.x
Wanger, G., Southam, G., and Onstott, T. C. (2006). Structural and chemical characterization of a natural fracture surface from 2.8 kilometers below land surface: biofilms in the deep subsurface. Geomicrobiol. J. 23, 443–452. doi: 10.1080/01490450600875746
Weisburg, W. G., Barns, S. M., Pelletier, D. A., and Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703. doi: 10.1128/jb.173.2.697-703.1991
Whitman, W. B., Coleman, D. C., and Wiebe, J. W. (1998). Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. U.S.A. 95, 6578–6583. doi: 10.1073/pnas.95.12.6578
Wilson, K. (2001). Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 2:2.4. doi: 10.1002/0471142727.mb0204s56
Wrighton, K. C., Thomas, B. C., Sharon, I., Miller, C. S., Castelle, C. J., VerBerkmoes, N. C., et al. (2012). Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial phyla. Science 337, 1661–1665. doi: 10.1126/science.1224041
Wu, X., Holmfeldt, K., Hubalek, V., Lundin, D., Åström, M., Bertilsson, S., et al. (2016). Microbial metagenomes from three aquifers in the Fennoscandian shield terrestrial deep biosphere reveal metabolic portioning among populations. ISME J. 10, 1192–1203. doi: 10.1038/ismej.2015.185
Keywords: deep subsurface, thermophilic microbial communities, element cycles and biogeochemical processes, Western Siberia, phylogenetic analysis
Citation: Frank YA, Kadnikov VV, Gavrilov SN, Banks D, Gerasimchuk AL, Podosokorskaya OA, Merkel AY, Chernyh NA, Mardanov AV, Ravin NV, Karnachuk OV and Bonch-Osmolovskaya EA (2016) Stable and Variable Parts of Microbial Community in Siberian Deep Subsurface Thermal Aquifer System Revealed in a Long-Term Monitoring Study. Front. Microbiol. 7:2101. doi: 10.3389/fmicb.2016.02101
Received: 08 August 2016; Accepted: 12 December 2016;
Published: 27 December 2016.
Edited by:
Mark Alexander Lever, ETH Zurich, SwitzerlandReviewed by:
Jeremy Dodsworth, California State University, San Bernardino, USAMalin Bomberg, VTT Technical Research Centre of Finland, Finland
Copyright © 2016 Frank, Kadnikov, Gavrilov, Banks, Gerasimchuk, Podosokorskaya, Merkel, Chernyh, Mardanov, Ravin, Karnachuk and Bonch-Osmolovskyaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sergey N. Gavrilov, c25nYXZyaWxvdkBnbWFpbC5jb20=
 Yulia A. Frank
Yulia A. Frank Vitaly V. Kadnikov2
Vitaly V. Kadnikov2 Sergey N. Gavrilov
Sergey N. Gavrilov David Banks
David Banks Olga A. Podosokorskaya
Olga A. Podosokorskaya Alexander Y. Merkel
Alexander Y. Merkel Nikolai A. Chernyh
Nikolai A. Chernyh Andrey V. Mardanov
Andrey V. Mardanov Nikolai V. Ravin
Nikolai V. Ravin Olga V. Karnachuk
Olga V. Karnachuk Elizaveta A. Bonch-Osmolovskaya
Elizaveta A. Bonch-Osmolovskaya