
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 08 December 2016
Sec. Microbial Symbioses
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.01936
This article is part of the Research TopicExperimental models in animal-associated microbiotaView all 36 articles
A commentary has been posted on this article:
Commentary: Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique?
The human vaginal microbiome is dominated by bacteria from the genus Lactobacillus, which create an acidic environment thought to protect women against sexually transmitted pathogens and opportunistic infections. Strikingly, lactobacilli dominance appears to be unique to humans; while the relative abundance of lactobacilli in the human vagina is typically >70%, in other mammals lactobacilli rarely comprise more than 1% of vaginal microbiota. Several hypotheses have been proposed to explain humans' unique vaginal microbiota, including humans' distinct reproductive physiology, high risk of STDs, and high risk of microbial complications linked to pregnancy and birth. Here, we test these hypotheses using comparative data on vaginal pH and the relative abundance of lactobacilli in 26 mammalian species and 50 studies (N = 21 mammals for pH and 14 mammals for lactobacilli relative abundance). We found that non-human mammals, like humans, exhibit the lowest vaginal pH during the period of highest estrogen. However, the vaginal pH of non-human mammals is never as low as is typical for humans (median vaginal pH in humans = 4.5; range of pH across all 21 non-human mammals = 5.4–7.8). Contrary to disease and obstetric risk hypotheses, we found no significant relationship between vaginal pH or lactobacilli relative abundance and multiple metrics of STD or birth injury risk (P-values ranged from 0.13 to 0.99). Given the lack of evidence for these hypotheses, we discuss two alternative explanations: the common function hypothesis and a novel hypothesis related to the diet of agricultural humans. Specifically, with regard to diet we propose that high levels of starch in human diets have led to increased levels of glycogen in the vaginal tract, which, in turn, promotes the proliferation of lactobacilli. If true, human diet may have paved the way for a novel, protective microbiome in human vaginal tracts. Overall, our results highlight the need for continuing research on non-human vaginal microbial communities and the importance of investigating both the physiological mechanisms and the broad evolutionary processes underlying human lactobacilli dominance.
Comparative research on mammalian microbiomes is critical to understanding the basic evolutionary and ecological principles guiding microbiome structure and function across mammalian hosts (Ley et al., 2008b; Delsuc et al., 2014; Yildirim et al., 2014; Moeller et al., 2016). To date, most comparative studies in mammals find that hosts with similar lifestyles and evolutionary histories harbor similar microbiomes at a given body site, both in the bacterial taxa they contain and the functions they provide to hosts (Ley et al., 2008a; Delsuc et al., 2014). One important exception to this pattern is the vaginal microbiome, where humans exhibit striking differences in community composition compared to other mammals (Spear et al., 2012; Swartz et al., 2014; Yildirim et al., 2014). Specifically, the human vaginal microbiome is dominated by Lactobacillus spp., which typically comprise >70% of resident bacteria in women, compared to <1% in other mammals. These lactobacilli process glycogen and its breakdown products to produce lactic acid, leading to an exceptionally low vaginal pH of ≤4.5 (Boskey et al., 1999, 2001; Mirmonsef et al., 2014; Spear et al., 2014). Lactobacilli-dominance and low pH of the human vaginal microbiome are hypothesized to benefit women by reducing disease risk (reviewed in Brotman, 2011; Graver and Wade, 2011; O'Hanlon et al., 2011; Gong et al., 2014; Nunn et al., 2015). Furthermore, the loss of lactobacilli-dominance is linked to bacterial vaginosis (BV), which is associated with an overgrowth of anaerobic bacteria, relatively high vaginal pH (>4.5; Aldunate et al., 2015), infertility, preterm birth, maternal infections, and increased risk of sexually transmitted diseases (Cherpes et al., 2003; Leitich et al., 2003; Atashili et al., 2008; Brotman et al., 2010; van Oostrum et al., 2013; DiGiulio et al., 2015; Redelinghuys et al., 2016). The fact that the human vaginal microbiome appears to be unique among mammals raises key questions about why humans are different: Do humans have a distinct reproductive physiology or experience different or stronger forces of selection compared to other mammals? Or are the selective pressures experienced by humans common among mammals, but humans have found a unique microbial solution to these evolutionary forces?
To date, four non-mutually exclusive hypotheses have been proposed to explain the uniqueness of the human vaginal microbiome relative to other primates, and, by extension, to other mammals (Stumpf et al., 2013). Two of these hypotheses focus on proximate, mechanistic explanations, and two propose ultimate or evolutionary explanations. The first proximate explanation, the “reproductive phase hypothesis,” proposes that the differences between human and non-human mammal vaginal microbiomes are due to between-species differences in reproductive physiology—especially the fact that humans exhibit continuous ovarian cycling, while many other mammals do not. Humans experience continuous, 28-day ovarian cycles between menarche and menopause, governed by fluctuations in reproductive steroids. In humans, estrogen levels are closely linked to lactobacilli abundance and vaginal pH, with an increase in estrogen promoting the thickening of the vaginal epithelium and intracellular production of glycogen (Ayre, 1951; Nauth and Haas, 1985; Patton et al., 2000; but see Mirmonsef et al., 2016). Consequently, lactobacilli are most abundant and vaginal pH is lowest when estrogen levels peak just before ovulation (Drake et al., 1980; Wagner and Ottesen, 1982; Eschenbach et al., 2000). In many non-human primates, and indeed in many mammals, females do not cycle continuously and are often only sexually receptive during a distinct breeding season (Hayssen et al., 1993; Noakes et al., 2009; Dixson, 2012). While estrogen levels follow similar patterns during the ovarian cycles of non-human mammals (Noakes et al., 2009; Dixson, 2012), many species may only experience high estrogen, high lactobacilli abundance, and low vaginal pH during a brief period of time that is commonly undetected by researchers (Stumpf et al., 2013). Thus, the reproductive phase hypothesis predicts that human uniqueness may be, in part, an artifact of sampling other mammals at the wrong time—outside of high estrogen cycle phases.
The second proximate explanation, called “the common function hypothesis,” proposes that, in non-human mammals, other bacteria may protect hosts via mechanisms other than lactic acid and low vaginal pH, such as production of bacteriocins and other antimicrobial compounds, competitive exclusion, or interactions with the host immune system (Klaenhammer, 1988; Abt and Artis, 2013; Stumpf et al., 2013; Aldunate et al., 2015). Thus, the presence of lactobacilli may not be a requirement for a healthy vaginal environment. This explanation may also be relevant to human health as some women consistently have low abundance of lactobacilli and a vaginal pH > 4.5, but they do not experience negative symptoms associated with BV (Ravel et al., 2011).
In addition to these mechanistic explanations, two evolutionary hypotheses have been proposed to explain why the human vaginal microbiome appears to be unique among mammals. The first evolutionary hypothesis, referred to as the “disease risk hypothesis,” proposes that humans face higher sexually transmitted disease (STD) risk than non-human mammals. Species with promiscuous mating strategies are predicted to have higher STD risk than those with only a single, brief reproductive episode per breeding season (Thrall et al., 1997, 2000; Nunn et al., 2000, 2003; Nunn, 2002). Humans, in particular, may experience relatively high STD risk due to prolonged intromission and continuous sexual receptivity throughout the menstrual cycle, pregnancy, and the postpartum period. Since STDs may have a significant impact on host and host offspring fitness (Lockhart et al., 1996), selective pressure for a protective, lactobacilli-dominated community may be stronger in humans compared to mammals with less frequent sexual contact (Stumpf et al., 2013).
Along a similar line of reasoning, the “obstetric protection hypothesis” suggests that selection for lactobacilli in the human vagina is due to the high risk of microbial complications associated with pregnancy and childbirth. Problems associated with gestation and parturition are not uncommon in mammals (e.g., Aksel and Abee, 1983; Sheldon et al., 2006), but humans may experience particularly difficult pregnancies and births (Rosenberg and Trevathan, 2002). For example, since the human maternal pelvic outlet is smaller than the neonatal head, there is substantial risk of trauma to uterine and vaginal walls, which increases the likelihood of microbial infection (Rosenberg and Trevathan, 2002; Chaim and Burstein, 2003; Dajani and Magann, 2014). Thus, lactobacilli and low vaginal pH may serve a protective function during human birth, and these traits are unnecessary in mammals with less risky pregnancies and birth (Stumpf et al., 2013).
Our objective was to test these hypotheses by comparing vaginal microbiomes and vaginal pH across mammalian species with a range of reproductive physiologies, mating systems, and obstetric risks. Recently, a comparative analysis of the vaginal microbiome across non-human primates found that interspecies differences were predominantly explained by host species, with additional evidence that host socio-ecological factors, including host neonatal birth weight, may contribute to interspecies variation (Yildirim et al., 2014). However, no studies have yet directly correlated specific host risk factors with lactobacilli abundance and vaginal pH, the aspects of the vaginal microbiome thought to be protective in humans. Furthermore, comparative data on vaginal microbial composition—especially interspecies and inter-individual variation in the vaginal microbiome as a function of female reproductive state and ovarian cycle phase—are rare. Here, we test three of these hypotheses—the reproductive phase, disease risk, and obstetric protection hypotheses—using comparative data on vaginal pH from 21 species of mammals and lactobacilli relative abundance from 14 species of mammals, as a function of female reproductive physiology, STD risk, and obstetric risk. To test the reproductive phase hypothesis, we predicted that: (i) non-human mammals would exhibit the lowest vaginal pH during the phase of their cycle with the highest estrogen, and (ii) at peak estrogen, non-human vaginal pH would be indistinguishable from humans. For the disease risk hypothesis, we predicted that species with high STD risk would possess lower vaginal pH and higher lactobacilli relative abundance than those species with low STD risk. Similarly, for the obstetric protection hypothesis, we predicted that mammals with high obstetric risk would have lower vaginal pH and higher lactobacilli relative abundance than species with low obstetric risk. Overall, this work represents one of the first attempts to explain interspecies variation in the vaginal microbiome, particularly with regard to human uniqueness (but see Yildirim et al., 2014).
We searched for data on mammalian vaginal lactobacilli and pH using appropriate search terms in Web of Science, Google Scholar, PubMed, and in the references of other publications. For information on lactobacilli prevalence and relative abundance, we focused on culture-independent studies (i.e., lactobacilli identification based on Illumina or 454 sequencing) because cultivation-based methods can under-represent or overlook some species (Zhou et al., 2004; Lamont et al., 2011). For data on vaginal pH, all pH measurements were collected using standard methods with pH meters or paper. Of particular note, human pH measurements from O'Hanlon et al. (2013) were not included because they collected data under hypoxic conditions and therefore were not comparable to measurements on non-human mammals. In addition, because the human vaginal microbiome changes in response to puberty and menopause and between reproductive states (e.g., Cauci et al., 2002; Thoma et al., 2011a; MacIntyre et al., 2015), we confined our data set to pH values collected during the ovarian cycle (i.e., follicular and luteal phases, anestrus) of sexually mature subjects. Furthermore, humans or non-human animals who had recently mated were not included as semen is alkaline and can temporarily neutralize vaginal tract pH (Tevi-Bénissan et al., 1997). Finally, because testing the reproductive phase hypothesis required data on how natural fluctuations in estrogen impact vaginal pH, we excluded subjects who were sterilized or supplemented with exogenous hormones. However, we did include studies of humans where women were taking hormonal birth control because past work has found no relationship between vaginal pH and birth control (Drake et al., 1980; Wagner and Ottesen, 1982). In total, we found 50 studies with data on vaginal pH and/or lactobacilli relative abundance across 26 mammalian species. Specifically, we found information on vaginal pH for 21 different species of non-human mammals from 44 studies (Figure 1; Table S1) and data on relative abundance of lactobacilli for 14 mammals, 8 of which also had information on vaginal pH (Table 2).
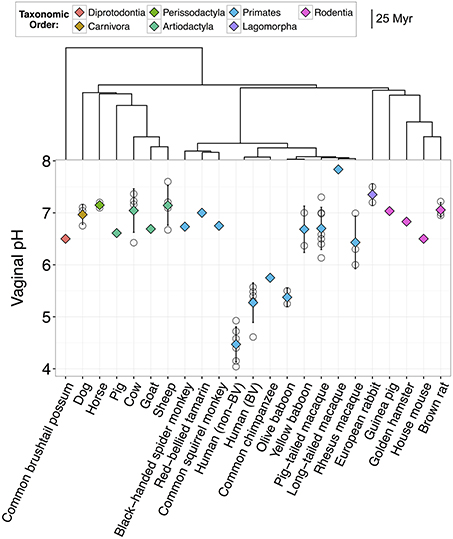
Figure 1. Vaginal pH across 22 species of mammals including humans. Open circles represent mean pH from individual studies and diamonds represent the overall mean for that species. Diamonds are color-coded based on taxonomic order. Error bars represent the standard deviation from the mean. Humans are divided into two groups, one with bacterial vaginosis (BV) and one without BV. Dendrogram indicates the evolutionary distance between species in millions of years (Myr).
For each mammalian species, we calculated the average vaginal pH across all available studies (Table S1). Because some studies measured vaginal pH at only a few cycle phases, the average pH of a species reflects the available data and not necessarily all cycle phases. If a study reported a pH range instead of a single value, we calculated the midrange (i.e., added the minimum and maximum values and divided by 2). For studies that represented their data graphically, but did not give numerical values, we used WebPlotDigitizer (Rohatgi, 2015) to extract pH values from the relevant plots. To test the reproductive phase hypothesis, we used vaginal pH values from the phases of the ovarian cycle when estrogen was highest and lowest (Table S1). For the majority of mammalian species, peak estrogen occurs during late proestrus or estrus, and the lowest level of estrogen occurs during the luteal phase or menses (Figure S1). However, for several species, including cows and dogs, peak estrogen occurs at both the end of proestrus and beginning of estrus. In these instances, we used the pH value from estrus to represent the high estrogen time point because proestrus can span multiple days and can encompass considerable variation in estrogen levels (Figure S1).
To test the disease risk and obstetric protection hypotheses, we required information on interspecies risk associated with STDs and reproduction. We calculated proxies of both STD and obstetric risk using species-specific trait data compiled from primary literature, edited books, and the life history database, PanTHERIA (Jones et al., 2009; Table S2). Whenever possible, we used data from wild, feral, or free-ranging populations over data from captive or domesticated individuals. In 13 instances, we could not find a particular life history trait for a species. In these cases, we used data from the closest related species with available data. For example, there was no information on the age of sexual maturity or life expectancy for the red-bellied tamarin (Sanginus labiatus), so we used data from the closely related cotton-top tamarin (S. oedipus). All proxies and their descriptions are listed in Table 1. Specifically, to test the disease risk hypothesis, we used five proxies: testes mass relative to body size, baseline white blood cell (WBC) count, annual sexual receptivity, total lifetime reproductive events, and intromission pattern (Table 1). For the obstetric protection hypothesis, we used gestation length, relative neonatal mass, and relative maternal pelvic area (Table 1).
All statistical tests were implemented in the R statistical environment (R Core Team, 2015). Before testing our primary hypotheses, we first tested for a phylogenetic signature on vaginal pH such that mammals with more similar evolutionary histories had more similar vaginal pH values. We obtained divergence times between the species in our data set from Bininda-Emonds et al. (2007), with an updated primate phylogeny by Perelman et al. (2011) and Papio spp. values from Purvis (1995). We constructed a distance matrix and phylogenetic tree using the R package, ape (Paradis et al., 2004), along with a distance matrix of vaginal pH values between species. We correlated phylogenetic distance with differences in vaginal pH using a Mantel test, which calculates a P-value based on comparison of the observed Pearson correlation coefficient to the distribution of coefficients from 10,000 permutations.
To test the reproductive phase hypothesis, we compared the vaginal pH during high- and low-estrogen phases of the female ovarian cycle using a paired t-test in R. To test the disease risk and obstetric protection hypotheses, we correlated vaginal pH and lactobacilli relative abundance with each proxy of STD or obstetric risk using linear regressions and ANOVAs. To calculate the minimum sample size required to detect a significant relationship, we used the R package, pwr (Champely, 2015), with a power value of 0.80. We further constructed multivariate linear regression models to test whether any combination of proxies predicted vaginal pH. Predictor variables in each model included relative testes mass, baseline WBC count, annual sexual receptivity, total lifetime reproductive events, intromission pattern, gestation length, and relative neonatal mass. Model selection was performed using stepwise backward regression with the stepAIC function from the R package, MASS (Venables and Ripley, 2002) and a 5% significance level was used as a threshold for inclusion in the final model.
Of the 26 mammalian species for which we found data on vaginal pH and/or lactobacilli relative abundance, most were captive primates used for medical studies, domesticated ungulates (e.g., horse, cow, pig), and common laboratory rodents (e.g., mouse, rat, and guinea pig). Of note, only one study measured vaginal pH in a wild population (yellow baboons; Miller et al., in review).
Together, our results confirm that humans are distinct from other mammals in dominance of lactobacilli and the acidity of their vaginal tract. For instance, across 10 studies of healthy human women, median vaginal pH was 4.5 (range = 4.0–4.9), while median vaginal pH across non-human mammals was 6.8, with no species falling in the range of healthy human pH, and only two species with a pH below 6.0 (Figure 1). Furthermore, while 13 of 14 mammals had detectable levels of Lactobacillus spp., the average relative abundance of lactobacilli was only 1.1% (±0.39% SEM) in non-human mammals compared to 69.6% in human women (±0.046% SEM) (Figure 2). This disparity is unlikely to be due to differences in the species of Lactobacillus in non-human mammals as compared to humans as the same four species that dominate the vaginal tract of women (L. cripsatus, L. gasseri, L. iners, and L. jensenii), were also frequently found in other mammals, albeit in low relative abundance. However, non-human mammals also harbored other lactobacilli generally not found in humans, including L. animalis, L. fornicalis, L. amylovorus, and L. johnsonii. Other members of the phylum Firmicutes were also common in non-human mammals, particularly species of two lactic acid-producing genera Aerococcus and Facklamia. Among primates, there was also high relative abundance of multiple genera linked to bacterial vaginosis (BV) in women, including Gardnerella, Sneathia, and Prevotella (Onderdonk et al., 2016).
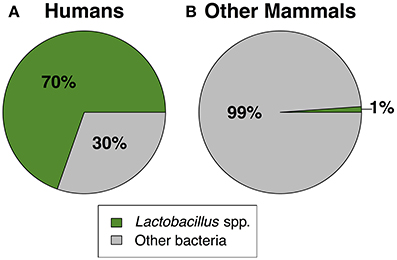
Figure 2. The mean relative abundance of Lactobacillus spp. vs. other bacteria in (A) humans and (B) non-human mammals. For non-human mammals, lactobacilli relative abundance was calculated as the mean across all species (N = 14). The standard error of the mean for lactobacilli was ±0.046% in humans and ±0.39% in other mammals. See Table 2 for the list of studies used to generate this figure.
Importantly, as has been reported previously, human women with BV had significantly higher vaginal pH than healthy women (N = 6 studies; two-sample t-test: t(8.14) = 3.65, P = 0.0063; Figure 1). Women with BV also exhibited compositional similarities to healthy baboons and macaques, including moderately high relative abundances of Gardnerella, Mobiluncus, Sneathia, and Prevotella (e.g., Miller et al., in review; Spear et al., 2010, 2012; Uchihashi et al., 2015; Onderdonk et al., 2016). However, of the five macaque and baboon species in our data set, all but the olive baboon had significantly higher vaginal pH than BV women (ANOVA: F(5, 14) = 12.84, P = 8.12e-05; Figure 1), which suggests that similar microbial composition may not always translate into similar ecological functions and consequences (e.g., Mirmonsef et al., 2012).
Before testing our main hypotheses, we first investigated whether shared evolutionary history among mammals explained variation in vaginal pH among mammal species. Figure 1 indicates that primates exhibited the widest variation in vaginal pH, with species-specific averages in non-human primates ranging from 5.4 to 7.8, and chimpanzees and olive baboons exhibiting pH values most similar to humans. Indeed, more variation in vaginal pH existed among primate species than among all other mammal lineages, which, despite encompassing many more years (and units of branch length) of evolutionary history, had vaginal pH values that ranged from just 6.5–7.4 (Figure 1). However, evolutionary distance between mammalian species did not predict similarity in vaginal pH, either among humans and non-human primates (Mantel test: N = 10, Mantel statistic r = −0.01, P = 0.47), or among all mammals (N = 22, Mantel statistic r = −0.2, P = 0.93). Hence, shared evolutionary history is unlikely to play a dominant role in shaping interspecies variation in vaginal pH.
The reproductive phase hypothesis proposes that differences between human and non-human primate vaginal microbiota are due to between-species variation in reproductive physiology—especially the continuous nature of human ovarian cycling. This hypothesis predicts that non-human mammals will exhibit lactobacilli dominance and a vaginal pH similar to humans during the period in their ovarian cycle when they experience highest estrogen levels. To test this idea, we compared vaginal pH during high- and low-estrogen phases of the female ovarian cycle in 10 non-human mammals where we had enough information on pH throughout the ovarian cycle. Notably, some of the mammal species we considered exhibit continuous, year-round reproductive cycling (i.e., spider monkey, cow, rat, pig-tailed macaque, common brushtail possum, and olive and yellow baboons), allowing us to make a strong comparison with humans. We found that, similar to humans, non-human mammals exhibit the lowest vaginal pH during high-estrogen phases (paired t-test: t(9.00) = −3.16, P = 0.012; Figure 3). However, even during peak estrogen, the pH of non-human mammals never declined to a level comparable to humans (Figure 3). Data on the relative abundance of Lactobacillus spp. as a function of ovarian cycle phase are rare in non-human mammals, but one study on wild baboons found that the order Lactobacillales, which comprises all lactic acid-producing bacteria, including lactobacilli, was more abundant in individuals experiencing ovulation compared to other ovarian cycle phases (Miller et al., in review). However, the mean relative abundance of Lactobacillus spp. in these ovulating baboons was only 0.0058%, which is considerably lower than the level typical in healthy human women (Miller et al., in review).
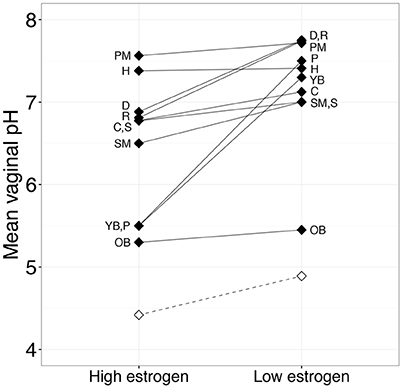
Figure 3. Mean vaginal pH between periods of high and low estrogen of 11 mammalian species during the ovarian cycle. Paired black diamonds represent the overall mean for each species at both estrogen levels. The open diamonds and dashed line show the mean human vaginal pHs during the high estrogen phases (follicular phase and ovulation) and low estrogen phases (luteal phase and menstruation) of the ovarian cycle. Letters next to diamonds identify the mammalian species. See Table S1 for the list of studies used to generate this figure. Abbreviations: C, Cow; D, Dog; H, Horse; OB, Olive baboon; P, Common brushtail possum; PM, Pig-tailed macaque; R, Brown rat; S, Sheep; SM, Black-handed spider monkey; YB, Yellow baboon.
Together, these results suggest that estrogen plays a similar role in shaping vaginal microbial composition and pH in humans and in other mammals. Specifically, rising estrogen levels increase available glycogen in the vaginal epithelium, which in turn, provides an energy source for lactobacilli to produce lactic acid. Indeed, like humans, many non-human mammals exhibit a thickening of the vaginal epithelium and increasing glycogen content in response to rising estrogen levels during the ovarian cycle (e.g., Gregoire and Guinness, 1968; Gregoire and Parakkal, 1972; Williams et al., 1992; Nyachieo et al., 2009). However, direct correlations between estrogen, glycogen, lactic acid, lactobacilli, and vaginal pH have yet to be explored in non-human mammals to the extent they have been in humans (e.g., Boskey et al., 1999, 2001; Mirmonsef et al., 2014; but see Mirmonsef et al., 2012). In summary, in terms of the reproductive phase hypothesis, we find that differences in reproductive cycling between humans and other mammals are not sufficient to explain why the human vaginal pH and lactobacilli abundance are such outliers relative to other mammals. Hence the uniqueness of the human vaginal microbiome is unlikely to be a consequence of sampling non-human mammals during the incorrect reproductive state.
The disease risk hypothesis proposes that high risk of STD exposure in humans, compared to other mammals, has selected for a protective, lactobacilli-dominated community. This hypothesis predicts that, in general, mammals, such as humans, with greater STD risk will have higher abundance of lactobacilli and lower vaginal pH compared to species with minimal STD risk. To test this hypothesis, we correlated the vaginal pH and lactobacilli relative abundance of mammals with species-specific proxies of STD risk (Table 1). Overall, we found no significant relationships between vaginal pH and any of the five STD risk proxies, including relative testes mass (linear regression: N = 21, F(1, 19) = 0.16, P = 0.70; Figure 4A), baseline WBC count (N = 21, F(1, 19) = 0.90, P = 0.36; Figure 4B), annual sexual receptivity (N = 21, F(1, 19) = 0.00030, P = 0.99; Figure 4C), number of lifetime reproductive events (N = 21, F(1, 19) = 2.56, P = 0.13; Figure 4D), and intromission pattern (ANOVA: N = 21, F(2, 18) = 1.07, P = 0.36; Figure 4E). Additionally, there was no significant relationship between lactobacilli relative abundance and any of the same STD risk proxies (N = 14; relative testes mass: F(1, 12) = 0.0050, P = 0.95; baseline WBC count: F(1, 12)= 0.072, P = 0.79; annual sexual receptivity: F(1, 12) = 0.79, P = 0.39; number of lifetime reproductive events: F(1, 12) = 2.66, P = 0.13; copulation pattern: F(2, 11) = 2.52 P = 0.13).
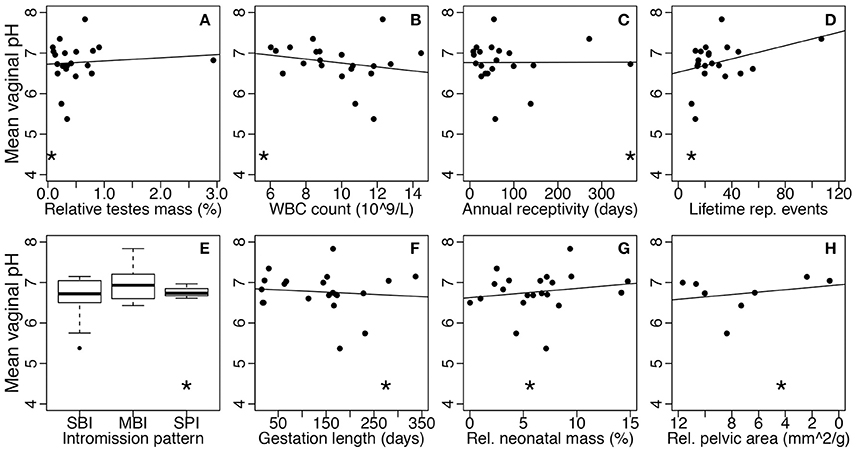
Figure 4. Mean vaginal pH as a function of STD or obstetric risk across mammals. Each point represents one species. Asterisks show where humans fall within each comparison. STD risk proxies are (A) relative testes mass, (B) baseline white blood cell count, (C) annual sexual receptivity, (D) maximum lifetime reproductive events, and (E) intromission pattern (SBI, single brief intromission; MBI, multiple brief intromissions; SPI, single prolonged intromission). Obstetric risk proxies are (F) gestation length, (G) relative neonatal mass, and (H) relative maternal pelvic area. Risk level increases moving left to right on all plots (note the reversed x-axis in plot H). The solid lines represent the best-fit linear models without humans.
Parallel to the disease risk hypothesis, the obstetric protection hypothesis proposes that the unique nature of the human vaginal microbiome is due to risk of infection during human pregnancy and birth. By extension, across mammals, species experiencing higher risk during gestation and parturition should have higher abundance of lactobacilli and lower vaginal pH. We correlated vaginal pH and lactobacilli relative abundance with three common proxies of obstetric risk across mammals (Table 1). Again, there was no relationship between vaginal pH and any obstetric risk proxy, including gestation length (linear regression: N = 21, F(1, 19) = 0.18, P = 0.67; Figure 4F), relative neonatal mass (N = 21, F(1, 19) = 0.54, P = 0.47; Figure 4G), and relative maternal pelvic area (N = 11, F(1, 6) = 0.42, P = 0.54; Figure 4H). There was also no relationship between lactobacilli relative abundance and any proxy (gestation length: N = 14, F(1, 12) = 0.69, P = 0.42; relative neonatal mass: N = 14, F(1, 12) = 0.20, P = 0.66; relative maternal pelvic area: N = 5, F(1, 3) = 0.0054, P = 0.95).
Finally, because disease and obstetric risk could be acting as simultaneous selection pressures on the vaginal microbiome, we also tested both hypotheses together using combinations of STD and obstetric risk proxies as factors in multivariate linear regressions predicting vaginal pH and lactobacilli relative abundance. Consistent with univariate results, no combination of proxies was significantly correlated with either vaginal pH or lactobacilli relative abundance (P-value range = 0.89–0.13). While the sample sizes for these correlations were small, a power analysis suggests that we still would need a sample size at least 2 times our current sample size and often more than 10 times the current sample size to detect significant effects. Hence, STD and obstetric risk are, at best, minor forces shaping mammalian vaginal pH and the relative abundance of lactobacilli.
Our results suggest that, contrary to the assumptions underlying the disease risk and obstetric protection hypotheses, humans do not have considerably higher disease or obstetric risk compared to other mammals (see asterisks on Figure 4). For example, many mammals have STDs and may experience high STD risk due to sexually promiscuous behavior (Smith and Dobson, 1992; Lockhart et al., 1996; Altizer et al., 2003). Additionally, a number of mammals have longer gestation duration than humans, and may experience extreme complications associated with parturition (Aksel and Abee, 1983; Frank and Glickman, 1994; Rosenberg and Trevathan, 2002). For instance, squirrel monkeys give birth to exceptionally large neonates for the diameter of the maternal pelvis, which can lead to an almost 50% perinatal mortality in some captive populations, yet vaginal pH in squirrel monkeys is around 6.75 (Aksel and Abee, 1983). Thus, the human STD and obstetric risk selective pressures do not appear to be particularly strong compared to selective pressures experienced by other mammals. Together, these results do not support the hypotheses that STD or obstetric risks have shaped the mammalian vaginal microbiome.
While we found little support for three of the four hypotheses proposed to explain the unique nature of the human vaginal microbiome (Stumpf et al., 2013), the fourth hypothesis (i.e., the common function hypothesis) could not be tested with available data. This hypothesis remains a promising avenue for future work. Specifically, it suggests that while all mammals may experience similar selective pressures, humans have found a unique microbial solution in lactobacilli-dominance. Further, in other host species, the protective role of lactobacilli may be fulfilled by other microbes that achieve similar functions without low vaginal pH. For example, vaginal bacteria, including some microbes commonly found at high abundances in the vaginal tract of non-human mammals (e.g., Streptococcus, Prevotella, and Corynebacterium), may produce high quantities of antimicrobial proteins, called bacteriocins (Zheng et al., 2015). Additionally, the microbes of non-human mammals may provide a protective function via interactions with the host immunity. Indeed, in humans, lactobacilli and lactic acid are thought to mediate host immune responses in vaginal mucosa, while BV-associated bacteria tend to promote pro-inflammatory immune responses (Mirmonsef et al., 2011; Aldunate et al., 2015). In non-human mammals, the interaction between commensal vaginal bacteria and host immunity have yet to be extensively investigated and it remains to be seen whether those interactions, particularly with regard to lactic acid and BV-associated bacteria, are similar to what is observed in humans or are species-specific.
While the common function hypothesis may clarify how humans and other mammals differ in their protective microbial communities, it does not explain why humans possess a unique microbial solution to relatively similar selective pressures. Here we propose a new proximate explanation for the uniqueness of human vaginal microbiome, which posits that exceptionally high levels of glycogen in the human vaginal tract create “lactobacilli-friendly” conditions, leading to lactobacilli dominance. While data on glycogen levels in non-human mammals are rare, the limited data that exist indicate that humans have considerably higher concentrations of glycogen present in vaginal epithelial tissue and in genital fluid compared to other mammals (Table 3; Mirmonsef et al., 2012). Because glycogen and its breakdown products (e.g., maltose) are a main energy source for lactobacilli, low glycogen levels in the vaginal tracts of non-human mammals may prevent lactobacilli dominance (Mirmonsef et al., 2014). Furthermore, recent work suggests that, in addition to glycogen, the mammalian enzyme α-amylase must also be present in the vaginal tract to break down glycogen into a form usable by lactobacilli strains that cannot metabolize glycogen directly (Spear et al., 2014, 2015; Nasioudis et al., 2015). It is currently unknown whether α-amylase is present in the vaginal tract of mammals other than humans, but a lack of this enzyme in non-human mammals might also contribute to the absence of lactobacilli dominance in non-human mammals. A comparison of α-amylase gene copy number to vaginal pH may provide further evidence for the function of this enzyme in the vaginal tract. Additionally, quantification of glycogen and α-amylase levels in mammalian vaginal tracts, including humans, would enable researchers to identify species with “lactobacilli-friendly” vaginal tracts.
Assuming that human vaginal tracts do have higher levels of glycogen than other mammals, the next question is: why do humans exhibit such high levels of glycogen? From an evolutionary perspective, high glycogen may be the result of selection for a protective microbial community, as suggested by the disease risk and obstetric risk hypotheses. However, given the lack of evidence for these hypotheses, we suggest that glycogen abundance is a byproduct of some other aspect of human physiology or behavior. In particular, we propose a “diet hypothesis,” which centers on the high starch content of human diets. Humans ingest relatively large quantities of starch, facilitated by the origins of agriculture, cooking food, and our ability to efficiently break carbohydrates down with high levels of amylase in saliva (Englyst et al., 1992; Diamond, 2002; Perry et al., 2007; Carmody and Wrangham, 2009; Hardy et al., 2015). Because glycogen is the major storage molecule of glucose, high starch diets may have led to high levels of glycogen in the vaginal tract, which, in turn, might create a favorable environment for lactobacilli proliferation. Although the mechanism by which high starch diets might lead to high levels of glycogen in the vaginal tract is unknown, it is well established carbohydrate ingestion increases glycogen in the liver and skeletal muscle (McGarry et al., 1987; Jeukendrup, 2003). In addition, there is some evidence that differences in women's diets predict differences in glycogen levels in the vagina and BV risk. For instance in one study, having a BMI > 30 was linked to increased free glycogen in vaginal fluid, although this relationship was only marginally significant (Mirmonsef et al., 2014). Further, diet may play a role in vaginal microbial composition, particularly with regard to risk of BV (Neggers et al., 2007; Tohill et al., 2007; Thoma et al., 2011b). A priori, we might hypothesize that a shift to diets rich in starch transformed vaginal microbial communities (and pH), and that evidence of this history might be found in comparisons among women with different ancestries. For example, it has been shown that individuals from human lineages that consumed more starch (e.g., agrarian societies as opposed to hunter gatherers) are more likely to have more copies of salivary amylase genes and, in turn, produce more amylase (Perry et al., 2007). Interestingly, there is variation in vaginal pH and community composition among human populations (Ravel et al., 2011; MacIntyre et al., 2015). However, because this variation is typically matched to race rather than to genotype, it is difficult to know whether this variation is in line with our prediction or not. To date, only one study has investigated the affect of a high starch diet on vaginal glycogen levels in humans (Willson and Goforth, 1942). Although the researchers did not detect a change in glycogen after the implementation of an abnormally high carbohydrate diet, we do not believe this finding represents an adequate test of this hypothesis as subjects were postmenopausal and glycogen concentrations were not assessed quantitatively.
Of the four hypotheses currently proposed to explain the unique nature of the human vaginal microbiome, our results provide little to no evidence for the reproductive phase, disease risk, and obstetric protection hypotheses. The fourth, common function hypothesis and our newly proposed diet-based hypothesis are as yet untested and represent two promising areas for future research. Overall, future work on the vaginal microbiome, particularly with regard to mechanistic and evolutionary explanations for human uniqueness, will require additional comparative data. Specifically, it would be useful to have more data on the vaginal microbiome of wild populations of mammals, especially from different stages of the ovarian cycle. It would also be useful to characterize vaginal microbiomes and vaginal pH in species of mammals with especially high obstetric risk, such as the hyena (Frank and Glickman, 1994), or similar patterns of socio-sexual behavior to humans, such as the bonobo or bottlenose dolphin (Dixson, 2012; Furuichi et al., 2014). Furthermore, there is an urgent need for more information on both glycogen and α-amylase content of the vaginal tract in both humans and other mammals. This type of data would be particularly useful for understanding the mechanistic origins of low vaginal pH in humans and exploring the relationship between the human vaginal tract and diet. To fully test the human diet hypothesis, however, both experimental and comparative work will be required, including the comparison of vaginal microbiota across human populations with differing diets, such as hunter-gatherers and agricultural societies.
Study concept and design was a collaboration between all authors. Literature searches and data collection were carried out by EM and DB. All statistical analyses were performed by EM. The manuscript was written by EM and EA with input from DB and RD.
This work was supported by the National Science Foundation (DGE-1313583 to EM, IOS-1053461 to EA, and MSP-1319293 to DB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank D. Jansen and S. Sanders for help with statistical analyses.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01936/full#supplementary-material
Abt, M. C., and Artis, D. (2013). The dynamic influence of commensal bacteria on the immune response to pathogens. Curr. Opin. Microbiol. 16, 4–9. doi: 10.1016/J.Mib.2012.12.002
Aksel, S., and Abee, C. R. (1983). A pelvimetry method for predicting perinatal mortality in pregnant squirrel monkeys (Saimiri sciuresus). Lab. Anim. Sci. 33, 165–167.
Aldunate, M., Srbinovski, D., Hearps, A. C., Latham, C. F., Ramsland, P. A., Gugasyan, R., et al. (2015). Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 6:164. doi: 10.3389/fphys.2015.00164
Alsammani, M. A., and Ahmed, S. R. (2012). Fetal and maternal outcomes in pregnancies complicated with fetal macrosomia. N. Am. J. Med. Sci. 4, 283–286. doi: 10.4103/1947-2714.97212
Altizer, S. M., Nunn, C. L., Thrall, P. H., Gittleman, J. L., Antonovics, J., Cunningham, A. A., et al. (2003). Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 34, 517–547. doi: 10.1146/annurev.ecolsys.34.030102.151725
Atashili, J., Poole, C., Ndumbe, P. M., Adimora, A. A., and Smith, J. S. (2008). Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22, 1493–1501. doi: 10.1097/QAD.0b013e3283021a37
Ayre, W. B. (1951). The Glycogen-Estrogen Relationship in the Vaginal Tract. J. Clin. Endocrinol. 11, 103–110.
Balmain, J. H., Biggers, J. D., and Claringbold, P. J. (1956). Glycogen, wet weight, and dry weight changes in the vagina of the mouse. Aust. J. Biol. Sci. 9, 147–158. doi: 10.1071/BI9560147
Basarab, J. A., Rutter, L. M., and Day, P. A. (1993). The efficacy of predicting dystocia in yearling beef heifers.1. using ratios of pelvic area to birth-weight or pelvic area to heifer weight. J. Animal Sci. 71, 1359–1371.
Bininda-Emonds, O. R. P., Cardillo, M., Jones, K. E., MacPhee, R. D. E., Beck, R. M. D., Grenyer, R., et al. (2007). The delayed rise of present-day mammals. Nature 446, 507–512. doi: 10.1038/nature05634
Boskey, E. R., Cone, R. A., Whaley, K. J., and Moench, T. R. (2001). Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reproduct. 16, 1809–1813. doi: 10.1093/Humrep/16.9.1809
Boskey, E. R., Telsch, K. M., Whaley, K. J., Moench, T. R., and Cone, R. A. (1999). Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 67, 5170–5175.
Brotman, R. M. (2011). Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J. Clin. Invest. 121, 4610–4617. doi: 10.1172/JCI57172
Brotman, R. M., Klebanoff, M. A., Nansel, T. R., Yu, K. F., Andrews, W. W., Zhang, J., et al. (2010). Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J. Infect. Dis. 202, 1907–1915. doi: 10.1086/657320
Carmody, R. N., and Wrangham, R. W. (2009). The energetic significance of cooking. J. Hum. Evol. 57, 379–391. doi: 10.1016/j.jhevol.2009.02.011
Cauci, S., Driussi, S., De Santo, D., Penacchioni, P., Iannicelli, T., Lanzafame, P., et al. (2002). Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 40, 2147–2152. doi: 10.1128/Jcm.40.6.2147-2152.2002
Chaim, W., and Burstein, E. (2003). Postpartum infection treatments: a review. Expert Opin. Pharmacother. 4, 1297–1313. doi: 10.1517/14656566.4.8.1297
Champely, S. (2015). pwr: Basic Functions for Power Analysis. R package version 1.1-3 ed. Available online at: http://CRAN.R-project.org/package=pwr
Cherpes, T. L., Meyn, L. A., Krohn, M. A., Lurie, J. G., and Hillier, S. L. (2003). Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin. Infect. Dis. 37, 319–325. doi: 10.1086/375819
Dajani, N. K., and Magann, E. F. (2014). Complications of shoulder dystocia. Semin. Perinatol. 38, 201–204. doi: 10.1053/j.semperi.2014.04.005
Delsuc, F., Metcalf, J. L., Wegener Parfrey, L., Song, S. J., Gonzalez, A., and Knight, R. (2014). Convergence of gut microbiomes in myrmecophagous mammals. Mol. Ecol. 23, 1301–1317. doi: 10.1111/mec.12501
Deutscher, G. H. (1985). Using pelvic measurements to reduce dystocia in heifers. Mod. Vet. Pract. 66, 751–755.
Diamond, J. (2002). Evolution, consequences and future of plant and animal domestication. Nature 418, 700–707. doi: 10.1038/nature01019
DiGiulio, D. B., Callahan, B. J., McMurdie, P. J., Costello, E. K., Lyell, D. J., Robaczewska, A., et al. (2015). Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. U.S.A. 112, 11060–11065. doi: 10.1073/pnas.1502875112
Dixson, A. F. (2012). Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Human Beings. Oxford: Oxford University Press.
Drake, S. M., Evans, B. A., and Gerken, A. (1980). Vaginal Ph and Microflora related to yeast infections and treatment. Br. J. Venereal Dis. 56, 107–110.
Englyst, H. N., Kingman, S. M., and Cummings, J. H. (1992). Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 46, S33–S50.
Eschenbach, D. A., Thwin, S. S., Patton, D. L., Hooton, T. M., Stapleton, A. E., Agnew, K., et al. (2000). Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 30, 901–907. doi: 10.1086/313818
Frank, L. G., and Glickman, S. E. (1994). Giving birth through a penile clitoris: parturition and dystocia in the spotted hyaena (Crocuta crocuta). J. Zool. 234, 659–665.
Furuichi, T., Connor, R., and Hashimoto, C. (2014). “Non-conceptive conceptive sexual interactions in Monkeys, Apes, and Dolphins,” in Primates and Cetaceans: Field Research and Conservation of Complex Mammalian Societies, eds. J. Yamagiwa and L. Karczmarski (Tokyo: Springer), 385–408.
Gajer, P., Brotman, R. M., Bai, G. Y., Sakamoto, J., Schüette, U. M. E., Zhong, X., et al. (2012). Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4:132ra52. doi: 10.1126/scitranslmed.3003605
Gittleman, J. L., and Thompson, S. D. (1988). Energy allocation in mammalian reproduction. Am. Zool. 28, 863–875.
Gong, Z., Luna, Y., Yu, P. S., and Fan, H. (2014). Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS ONE 9:107758. doi: 10.1371/journal.pone.0107758
Graver, M. A., and Wade, J. J. (2011). The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 10:8. doi: 10.1186/1476-0711-10-8
Gregoire, A. T., and Guinness, B. J. (1968). Cyclic and pre-ovulatory changes in the glycogen content of the female hamster genital tract. J. Reprod. Fertil. 17, 427–432.
Gregoire, A. T., and Hafs, H. D. (1971). Glycogen content of rabbit tubular genitalia from mating through implantation. Anat. Rec. 169, 253–258.
Gregoire, A. T., Kandil, O., and Ledger, W. J. (1971). The glycogen content of human vaginal epithelial tissue. Fertil. Steril. 22, 64–68.
Gregoire, A. T., and Parakkal, P. F. (1972). Glycogen content in the vaginal tissue of normally cycling and estrogen and progesterone-treated rhesus monkeys. Biol. Reprod. 7, 9–14.
Harcourt, A. H., Harvey, P. H., Larson, S. G., and Short, R. V. (1981). Testis weight, body-weight and breeding system in primates. Nature 293, 55–57. doi: 10.1038/293055a0
Hardy, K., Brand-Miller, J., Brown, K. D., Thomas, M. G., and Copeland, L. (2015). The importance of dietary carbohydrate in human evolution. Quart. Rev. Biol. 90, 251–268. doi: 10.1086/682587
Hashway, S. A., Bergin, I. L., Bassis, C. M., Uchihashi, M., Schmidt, K. C., Young, V. B., et al. (2014). Impact of a hormone-releasing intrauterine system on the vaginal microbiome: a prospective baboon model. J. Med. Primatol. 43, 89–99. doi: 10.1111/jmp.12090
Hayssen, V., van Tienhoven, A., and van Tienhoven, A. (1993). Asdell's Patterns of Mammalian Reproduction: A Compendium of Species-Specific Data. Ithaca: Cornell University Press.
Jeukendrup, A. E. (2003). Modulation of carbohydrate and fat utilization by diet, exercise and environment. Biochem. Soc. Trans. 31, 1270–1273. doi: 10.1042/bst0311270
Jones, K. E., Bielby, J., Cardillo, M., Fritz, S. A., O'Dell, J., Orme, C. D. L., et al. (2009). PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648. doi: 10.1890/08-1494.1
Kenagy, G. J., and Trombulak, S. C. (1986). Size and function of Mammalian testes in relation to body size. J. Mammal. 67, 1–22. doi: 10.2307/1380997
Klaenhammer, T. R. (1988). Bacteriocins of lactic acid bacteria. Biochimie 70, 337–349. doi: 10.1016/0300-9084(88)90206-4
Krishnan, L., Nguyen, T., and McComb, S. (2013). From mice to women: the conundrum of immunity to infection during pregnancy. J. Reprod. Immunol. 97, 62–73. doi: 10.1016/j.jri.2012.10.015
Lamont, R. F., Sobel, J. D., Akins, R. A., Hassan, S. S., Chaiworapongsa, T., Kusanovic, J. P., et al. (2011). The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG 118, 533–549. doi: 10.1111/J.1471-0528.2010.02840.X
Leitich, H., Bodner-Adler, B., Brunbauer, M., Kaider, A., Egarter, C., and Husslein, P. (2003). Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am. J. Obstet. Gynecol. 189, 139–147. doi: 10.1067/Mob.2003.339
Leutenegger, W. (1973). Maternal-fetal weight relationships in primates. Folia Primatol. 20, 280–293. doi: 10.1159/000155580
Ley, R. E., Hamady, M., Lozupone, C., Turnbaugh, P. J., Ramey, R. R., Bircher, J. S., et al. (2008a). Evolution of mammals and their gut microbes. Science 320, 1647–1651. doi: 10.1126/Science.1155725
Ley, R. E., Lozupone, C. A., Hamady, M., Knight, R., and Gordon, J. I. (2008b). Worlds within worlds: evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 6, 776–788. doi: 10.1038/Nrmicro1978
Lockhart, A. B., Thrall, P. H., and Antonovics, J. (1996). Sexually transmitted diseases in animals: ecological and evolutionary implications. Biol. Rev. Camb. Philos. Soc. 71, 415–471.
Loehle, C. (1995). Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335.
Lorenzen, E., Kudirkiene, E., Gutman, N., Grossi, A. B., Agerholm, J. S., Erneholm, K., et al. (2015). The vaginal microbiome is stable in prepubertal and sexually mature Ellegaard Göttingen Minipigs throughout an estrous cycle. Vet. Res. 46:125. doi: 10.1186/s13567-015-0274-0
MacIntyre, D. A., Chandiramani, M., Lee, Y. S., Kindinger, L., Smith, A., Angelopoulos, N., et al. (2015). The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 5:8988. doi: 10.1038/srep08988
McGarry, J. D., Kuwajima, M., Newgard, C. B., Foster, D. W., and Katz, J. (1987). From dietary glucose to liver-glycogen - the full circle round. Annu. Rev. Nutr. 7, 51–73.
Mirmonsef, P., Gilbert, D., Veazey, R. S., Wang, J., Kendrick, S. R., and Spear, G. T. (2012). A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility. AIDS Res. Hum. Retroviruses 28, 76–81. doi: 10.1089/Aid.2011.0071
Mirmonsef, P., Gilbert, D., Zariffard, M. R., Hamaker, B. R., Kaur, A., Landay, A. L., et al. (2011). The effects of commensal bacteria on innate immune responses in the female genital tract. Am. J. Reprod. Immunol. 65, 190–195. doi: 10.1111/J.1600-0897.2010.00943.X
Mirmonsef, P., Hotton, A. L., Gilbert, D., Burgad, D., Landay, A., Weber, K. M., et al. (2014). Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS ONE 9102467: doi: 10.1371/journal.pone.0102467
Mirmonsef, P., Hotton, A. L., Gilbert, D., Gioia, C. J., Maric, D., Hope, T. J., et al. (2016). Glycogen levels in undiluted genital fluid and their relationship to Vaginal pH, Estrogen, and Progesterone. PLoS ONE 11:e153553. doi: 10.1371/journal.pone.0153553
Moeller, A. H., Caro-Quintero, A., Mjungu, D., Georgiev, A. V., Lonsdorf, E. V., Muller, M. N., et al. (2016). Cospeciation of gut microbiota with hominids. Science 353, 380–382. doi: 10.1126/science.aaf3951
Nasioudis, D., Beghini, J., Bongiovanni, A. M., Giraldo, P. C., Linhares, I. M., and Witkin, S. S. (2015). a-Amylase in vaginal fluid: association with conditions favorable to dominance of Lactobacillus. Reproduct. Sci. 22, 1393–1398. doi: 10.1177/1933719115581000
Nauth, H. F., and Haas, M. (1985). Cytologic and histologic observations on the Sex-Hormone dependence of the Vulva. J. Reproduct. Med. 30, 667–674.
Neggers, Y. H., Nansel, T. R., Andrews, W. W., Schwebke, J. R., Yu, K. F., Goldenberg, R. L., et al. (2007). Dietary intake of selected nutrients affects bacterial vaginosis in women. J. Nutrit. 137, 2128–2133.
Neuendorf, E., Gajer, P., Bowlin, A. K., Marques, P. X., Ma, B., Yang, H., et al. (2015). Chlamydia caviae infection alters abundance but not composition of the guinea pig vaginal microbiota. FEMS Pathog. Dis. 73, 1–12. doi: 10.1093/femspd/ftv019
Noakes, D. E., Parkinson, T. J., and England, G. C. W. (eds.). (2009). Veterinary Reproduction and Obstetrics, 9th Edn. London: Saunders Ltd.
Nunn, C. L. (2002). A comparative study of leukocyte counts and disease risk in primates. Evolution 56, 177–190. doi: 10.1111/j.0014-3820.2002.tb00859.x
Nunn, C. L. (2003). Behavioural defenses against sexually transmitted diseases in primates. Anim. Behav. 66, 37–48. doi: 10.1006/Anbe.2003.2130
Nunn, C. L., Gittleman, J. L., and Antonovics, J. (2000). Promiscuity and the primate immune system. Science 290, 1168–1170. doi: 10.1126/science.290.5494.1168
Nunn, C. L., Gittleman, J. L., and Antonovics, J. (2003). A comparative study of white blood cell counts and disease risk in carnivores. Proc. R. Soc. B Biol. Sci. 270, 347–356. doi: 10.1098/rspb.2002.2249
Nunn, K. L., Wang, Y.-Y., Harit, D., Humphrys, M. S., Ma, B., Cone, R., et al. (2015). Enhanced trapping of HIV-1 by human cervicovaginal mucus is associated with Lactobacillus crispatus-dominant microbiota. MBio 6:e01084-15. doi: 10.1128/mBio.01084-15
Nyachieo, A., Kiulia, N. M., Arimi, M. M., Chai, D. C., and Mwenda, J. M. (2009). Vaginal histological changes of the baboon during the normal menstrual cycle and pregnancy. East Afr. Med. J. 86, 166–172. doi: 10.4314/eamj.v86i4.46946
O'Hanlon, D. E., Moench, T. R., and Cone, R. A. (2011). In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 11:200. doi: 10.1186/1471-2334-11-200
O'Hanlon, D. E., Moench, T. R., and Cone, R. A. (2013). Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 8:e80074. doi: 10.1371/journal.pone.0080074
Onderdonk, A. B., Delaney, M. L., and Fichorova, R. N. (2016). The human microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 29, 223–238. doi: 10.1128/Cmr.00075-15
Paradis, E., Claude, J., and Strimmer, K. (2004). APE: analyses of Phylogenetics and Evolution in R language. Bioinformatics 20, 289–290. doi: 10.1093/bioinformatics/btg412
Patton, D. L., Thwin, S. S., Meier, A., Hooton, T. M., Stapleton, A. E., and Eschenbach, D. A. (2000). Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am. J. Obstet. Gynecol. 183, 967–973. doi: 10.1067/Mob.2000.108857
Perelman, P., Johnson, W. E., Roos, C., Seuánez, H. N., Horvath, J. E., Moreira, M. A. M., et al. (2011). A molecular phylogeny of living primates. PLoS Genet. 7:1001342. doi: 10.1371/journal.pgen.1001342
Perry, G. H., Dominy, N. J., Claw, K. G., Lee, A. S., Fiegler, H., Redon, R., et al. (2007). Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 39, 1256–1260. doi: 10.1038/ng2123
Purvis, A. (1995). A composite estimate of primate phylogeny. Philos Trans. R. Soc. Lond. Ser. B Biol. Sci. 348, 405–421. doi: 10.1098/rstb.1995.0078
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S. K., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108, 4680–4687. doi: 10.1073/Pnas.1002611107
Redelinghuys, M. J., Ehlers, M. M., Dreyer, A. W., and Kock, M. M. (2016). Normal flora and bacterial vaginosis in pregnancy: an overview. Crit. Rev. Microbiol. 42, 352–363. doi: 10.3109/1040841X.2014.954522
Rohatgi, A. (2015). WebPlotDigitizer. 3.9 ed (Austin, TX). Available online at: http://arohatgi.info/WebPlotDigitizer/
Rosenberg, K., and Trevathan, W. (2002). Birth, obstetrics and human evolution. BJOG 109, 1199–1206. doi: 10.1046/j.1471-0528.2002.00010.x
Sheldon, I. M., Lewis, G. S., LeBlanc, S., and Gilbert, R. O. (2006). Defining postpartum uterine disease in cattle. Theriogenology 65, 1516–1530. doi: 10.1016/j.theriogenology.2005.08.021
Shukla, S., Mathur, R., and Prakash, A. O. (1989). Butanolic extract of Pueraria tuberosa DC.: physiological response in the genital tract of cyclic female rats. Phytother. Res. 3, 5–10. doi: 10.1002/ptr.2650030103
Smith, G., and Dobson, A. P. (1992). Sexually-transmitted diseases in animals. Parasitol. Today 8, 159–166.
Spear, G. T., French, A. L., Gilbert, D., Zariffard, M. R., Mirmonsef, P., Sullivan, T. H., et al. (2014). Human alpha-amylase present in lower genital tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J. Infect. Dis. 210, 1019–1028. doi: 10.1093/infdis/jiu231
Spear, G. T., Gilbert, D., Sikaroodi, M., Doyle, L., Green, L., Gillevet, P. M., et al. (2010). Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res. Hum. Retroviruses 26, 193–200. doi: 10.1089/Aid.2009.0166
Spear, G. T., Kersh, E., Guenthner, P., Vishwanathan, S. A., Gilbert, D., Zariffard, M. R., et al. (2012). Longitudinal assessment of pigtailed macaque lower genital tract microbiota by 454-sequencing reveals dissimilarity to the genital microbiota of healthy humans. AIDS Res. Hum. Retroviruses 28, 1244–1249. doi: 10.1089/aid.2011.0382
Spear, G. T., McKenna, M., Landay, A. L., Makinde, H., Hamaker, B., French, A. L., et al. (2015). Effect of pH on cleavage of glycogen by vaginal enzymes. PLoS ONE 10:e132646. doi: 10.1371/journal.pone.0132646
Stumpf, R. M., Wilson, B. A., Rivera, A., Yildirim, S., Yeoman, C. J., Polk, J. D., et al. (2013). The primate vaginal microbiome: comparative context and implications for human health and disease. Am. J. Phys. Anthropol. 152, 119–134. doi: 10.1002/ajpa.22395
Swartz, J. D., Lachman, M., Westveer, K., O'Neill, T., Geary, T., Kott, R. W., et al. (2014). Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of lactobacilli and near-neutral pH. Front. Vet. Sci. 1:19. doi: 10.3389/fvets.2014.00019
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Tevi-Bénissan, C., Bélec, L., Lévy, M., Schneider-Fauveau, V., Mohamed, A. S., Hallouin, M. C., et al. (1997). In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 4, 367–374.
Thoma, M. E., Gray, R. H., Kiwanuka, N., Aluma, S., Wang, M. C., Sewankambo, N., et al. (2011a). Longitudinal changes in vaginal microbiota composition assessed by gram stain among never sexually active pre- and postmenarcheal adolescents in Rakai, Uganda. J. Pediatr. Adolesc. Gynecol. 24, 42–47. doi: 10.1016/j.jpag.2010.07.002
Thoma, M. E., Klebanoff, M. A., Rovner, A. J., Nanse, T. R., Neggers, Y., Andrews, W. W., et al. (2011b). Bacterial vaginosis is associated with variation in dietary indices. J. Nutr. 141, 1698–1704. doi: 10.3945/jn.111.140541
Thrall, P. H., Antonovics, J., and Bever, J. D. (1997). Sexual transmission of disease and host mating systems: within-season reproductive success. Am. Nat. 149, 485–506.
Thrall, P. H., Antonovics, J., and Dobson, A. P. (2000). Sexually transmitted diseases in polygynous mating systems: prevalence and impact on reproductive success. Proc. R. Soc. Lond. Ser. B Biol. Sci. 267, 1555–1563. doi: 10.1098/rspb.2000.1178
Tohill, B. C., Heilig, C. M., Klein, R. S., Rompalo, A., Cu-Uvin, S., Piwoz, E. G., et al. (2007). Nutritional biomarkers associated with gynecological conditions among US women with or at risk of HIV infection. Am. J. Clin. Nutr. 85, 1327–1334.
Uchihashi, M., Bergin, I. L., Bassis, C. M., Hashway, S. A., Chai, D., and Bell, J. D. (2015). Influence of age, reproductive cycling status, and menstruation on the vaginal microbiome in baboons (Papio anubis). Am. J. Primatol. 77, 563–578. doi: 10.1002/ajp.22378
van Oostrum, N., De Sutter, P., Meys, J., and Verstraelen, H. (2013). Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum. Reprod. 28, 1809–1815. doi: 10.1093/humrep/det096
van Schaik, C. P., van Noordwijk, M. A., and Nunn, C. L. (1999). “Sex and social evolution in primates,” in Comparative Primate Socioecology, ed P. C. Lee (Cambridge: Cambridge University Press), 204–241.
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S, 4th Edn. New York, NY: Springer.
Wagner, G., and Ottesen, B. (1982). Vaginal physiology during menstruation. Ann. Intern. Med. 96, 921–923.
Weissmann-Brenner, A., Simchen, M. J., Zilberberg, E., Kalter, A., Weisz, B., Achiron, R., et al. (2012). Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet. Gynecol. Scand. 91, 844–849. doi: 10.1111/j.1600-0412.2012.01412.x
Williams, E. S., Thorne, E. T., Kwiatkowski, D. R., Lutz, K., and Anderson, S. L. (1992). Comparative vaginal cytology of the estrous-cycle of Black-Footed Ferrets (Mustela-Nigripes), Siberian Polecats (M-Eversmanni), and Domestic Ferrets (Mustela-Putorius-Furo). J. Vet. Diagn. Invest. 4, 38–44.
Willson, J. R., and Goforth, M. L. (1942). Effect of an excess of ingested carbohydrate upon the glycogen content of vaginal epithelium. J. Clin. Endocrinol. 2, 223–225.
Yildirim, S., Yeoman, C. J., Janga, S. C., Thomas, S. M., Ho, M., Leigh, S. R., et al. (2014). Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. Int. Soc. Microbial. Ecol. 8, 2431–2444. doi: 10.1038/ismej.2014.90
Zheng, J., Gänzle, M. G., Lin, X. B., Ruan, L., and Sun, M. (2015). Diversity and dynamics of bacteriocins from human microbiome. Environ. Microbiol. 17, 2133–2143. doi: 10.1111/1462-2920.12662
Keywords: vaginal microbiome, lactobacilli, pH, estrogen, mammals, evolution
Citation: Miller EA, Beasley DE, Dunn RR and Archie EA (2016) Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 7:1936. doi: 10.3389/fmicb.2016.01936
Received: 24 August 2016; Accepted: 17 November 2016;
Published: 08 December 2016.
Edited by:
Robert Brucker, Rowland Institute at Harvard, USACopyright © 2016 Miller, Beasley, Dunn and Archie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth A. Miller, ZW1pbGxlMThAbmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.