
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 21 November 2016
Sec. Microbiotechnology
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.01836
 Panagiotis Gkorezis1*
Panagiotis Gkorezis1* Matteo Daghio2,3
Matteo Daghio2,3 Andrea Franzetti2
Andrea Franzetti2 Jonathan D. Van Hamme3
Jonathan D. Van Hamme3 Wouter Sillen1
Wouter Sillen1 Jaco Vangronsveld1*
Jaco Vangronsveld1*Widespread pollution of terrestrial ecosystems with petroleum hydrocarbons (PHCs) has generated a need for remediation and, given that many PHCs are biodegradable, bio- and phyto-remediation are often viable approaches for active and passive remediation. This review focuses on phytoremediation with particular interest on the interactions between and use of plant-associated bacteria to restore PHC polluted sites. Plant-associated bacteria include endophytic, phyllospheric, and rhizospheric bacteria, and cooperation between these bacteria and their host plants allows for greater plant survivability and treatment outcomes in contaminated sites. Bacterially driven PHC bioremediation is attributed to the presence of diverse suites of metabolic genes for aliphatic and aromatic hydrocarbons, along with a broader suite of physiological properties including biosurfactant production, biofilm formation, chemotaxis to hydrocarbons, and flexibility in cell-surface hydrophobicity. In soils impacted by PHC contamination, microbial bioremediation generally relies on the addition of high-energy electron acceptors (e.g., oxygen) and fertilization to supply limiting nutrients (e.g., nitrogen, phosphorous, potassium) in the face of excess PHC carbon. As an alternative, the addition of plants can greatly improve bioremediation rates and outcomes as plants provide microbial habitats, improve soil porosity (thereby increasing mass transfer of substrates and electron acceptors), and exchange limiting nutrients with their microbial counterparts. In return, plant-associated microorganisms improve plant growth by reducing soil toxicity through contaminant removal, producing plant growth promoting metabolites, liberating sequestered plant nutrients from soil, fixing nitrogen, and more generally establishing the foundations of soil nutrient cycling. In a practical and applied sense, the collective action of plants and their associated microorganisms is advantageous for remediation of PHC contaminated soil in terms of overall cost and success rates for in situ implementation in a diversity of environments. Mechanistically, there remain biological unknowns that present challenges for applying bio- and phyto-remediation technologies without having a deep prior understanding of individual target sites. In this review, evidence from traditional and modern omics technologies is discussed to provide a framework for plant–microbe interactions during PHC remediation. The potential for integrating multiple molecular and computational techniques to evaluate linkages between microbial communities, plant communities and ecosystem processes is explored with an eye on improving phytoremediation of PHC contaminated sites.
Petroleum hydrocarbons (PHCs) are organic compounds comprised of carbon and hydrogen atoms arranged in varying structural configurations with physical and chemical characteristics that vary over orders of magnitude; they are broadly classified in two categories namely, gasoline range organics (GROs) and diesel range organics (DROs). GROs include mono-aromatic hydrocarbons such as benzene, toluene, ethylbenzene, and xylenes (BTEX), and short chain alkanes (C6–C10) with low boiling points (60–170°C) such as isopentane, 2,3-dimethyl butane, n-butane, and pentane. DROs include longer chain alkanes (C10–C40) and hydrophobic chemicals such as polycyclic aromatic hydrocarbons (PAH) (Kamath et al., 2004). The industrialization of modern societies and the increasing demand for energy generation to heat our domestic and working areas, to fuel our transportation networks as well as to power fabricating processes has resulted in the extensive exploitation of PHCs, which are the most widespread class of organic contaminants worldwide (Brassington et al., 2007).
Prolonged exposure to PHCs can initiate detrimental damages to the central nervous system in humans and animals, can result in respiratory system dysfunction, disrupt the endocrine system and, as a result, considerably increase the probability of lung, skin, bladder, liver, and kidney cancers (Costello, 1979; Hutcheson et al., 1996; Boffetta et al., 1997; Singh et al., 2004; Locksley, 2010). Hence, the need to remediate PHC contaminated environments is of great importance.
Generally, conventional physical and chemical in situ and ex situ clean-up technologies for PHC remediation involve excavation, air sparging, removal and off-site treatment in biopiles, pump and treat, incineration, slurry- and solid phase reactors, soil washing, soil vapor extraction, asphalt batching, thermal desorption, chemical oxidation, hydrolysis and photolysis (Amatya et al., 2002; Khan et al., 2004; Zhou et al., 2005; Do et al., 2009). However, experience has demonstrated that these strategies are expensive, and often only result in incomplete decomposition of the pollutants of the concern.
Thus, research over the last two decades has focused on offering remediation schemes that are moving away from the conventional ones and are mainly based on biological methods with emphasis to the convergent action of plants and their related microorganisms to remove and degrade PHCs. However, there are still numerous aspects about the mechanisms involved that remain the subject of research and debate among members of the scientific community.
This review tries to provide one more piece of information in this complicated puzzle of plant–microbe partnerships with emphasis on the remediation of PHC contaminated sites mediated by plant–bacteria associations.
Bioremediation is defined as the use of biologically mediated processes to detoxify, degrade or transform pollutants to an innocuous state (Azubuike et al., 2016). Bioremediation is a useful tool for the treatment of PHC contaminated terrestrial and marine ecosystems (Atlas, 1995; Atlas and Cerniglia, 1995; Almeida et al., 2013; Xue et al., 2015; Scoma et al., 2016; Wang et al., 2016). Accession to PHC substrates while regulating toxic effects is the first hurdle that must be overcome for a microorganism to exploit these energy-rich molecules for growth and energy production. The pivotal parameters that dictate the degree of PHC “susceptibility” to biodegradation can be widely classified into three inter-related categories (Figure 1): (a) microbial properties (genetic complement, gene regulation and expression, surface hydrophobicity, metabolic diversity and flexibility, substrate uptake or adherence mechanisms, tolerance to metals and other toxic xenobiotics, chemotaxis, biofilm formation); (b) environmental factors (presence of terminal electron acceptors, nutrient availability, salinity, pressure, temperature, pH, water availability, and osmotic stress); and (c) properties of the hydrocarbon substrate (solubility, concentration, hydrophobicity, volatility, molecular mass) (Sikkema et al., 1995; Hino et al., 1997; Marquez-Rocha et al., 2001; Bressler and Gray, 2003; Martinez-Checa et al., 2007; Bordoloi and Konwar, 2009; Botalova et al., 2009; Calvo et al., 2009; Banat et al., 2010; Couling et al., 2010).
Generally, once a bacterial community begins to remove PHCs from a contaminated environment, bioavailability (here defined as “the quantity of a contaminant which is freely available to cross the cellular membrane of an organism from the surrounding medium”), and bioaccessibility [here defined as the “quantity of the contaminant which has the potential to cross an organism’s (cellular) membrane from the environment it inhabits”], determine the degree and rate at which the contaminant can be taken up by the microorganism (Semple et al., 2007; Dandie et al., 2010). Moreover, bioavailability may be assessed in two complementary ways: (i) by chemical methods (e.g., selective extraction methods), which determine the available fraction of a well-defined class of contaminants, and (ii) by biological methods, which expose organisms to contaminated media (Harmsen, 2007). Although plethora of reports supports the concept that bioremediation efficiency is normally limited by PHC bioavailability (Schwartz and Scow, 2001; Wick et al., 2001; Liste and Alexander, 2002; Shor et al., 2003; Tabak et al., 2003; Hamdi et al., 2007b), such generalizations should not be applied to all cases (Huesemann et al., 2004) given the diversity of the biological world.
In a classical experiment conducted by Rosenberg et al. (1980), the microbial adhesion to hydrocarbon (MATH) assay was established as a method to quantify microbial cell surface hydrophobicity via their attachment to hydrocarbon droplets. Other quantitative measures of cell hydrophobicity include the measurement of water contact angles (Reid et al., 1992) and zeta potentials (Busscher et al., 1995).
Microbial adhesion to hydrophobic surfaces, usually defined as the process of transferring unbound, suspended cells from the aqueous phase to an interface (pure or mixed, liquid or solid hydrocarbons in a water-immiscible phase), is one mechanism used by microorganisms to counteract the limited bioavailability of insoluble and poorly soluble PHCs (Bouchez-Naitali et al., 1999; Hermansson, 1999). The significance of adhesion in the biodegradation of aliphatic hydrocarbon non-aqueous phase liquids (NAPLs) has been reported by Volkering et al. (1997); however, adherence to PHCs does not necessarily correlate with utilization (Grimaud, 2010).
Depending on the physiology of the organism involved, microbial adhesion to hydrophobic surfaces may benefit growth on, and biodegradation of, very poorly water-soluble PHCs such as n-alkanes and large PAHs dissolved in a non-aqueous phase (Abbasnezhad et al., 2011). In other cases, the addition of cationic surfactants such as cetylpyridinium chloride (CPC), poly-L-lysine and chlorhexidine gluconate (CHX), or long chain alcohols such as 1-dodecanol and farnesol, may promote the growth of a hydrophilic bacterium, such as Pseudomonas fluorescens strain LP6a, at oil–water interfaces (Abbasnezhad et al., 2008).
Biosurfactants, either microbially derived or plant derived, can also be involved in hydrocarbon accession by regulating cell envelope hydrophobicity and, thus, the attachment and detachment to and from PHC droplets. This can be facilitated by exposing the hydrophilic or hydrophobic moieties of cell-bound biosurfactants external to the cell (Rosenberg et al., 1988). Microorganisms with degradation capabilities may also alter their cell hydrophobicity during growth on PHCs (Franzetti et al., 2008a; Tzintzun-Camacho et al., 2012).
Interestingly, it has been found that the qualitative and quantitative composition of bacterial outer surfaces are affected in a dose-dependent manner by biosurfactants such as rhamnolipids (Zhong et al., 2007; Sotirova et al., 2008), fatty acids (Chang et al., 2009), and chemical surfactants (Mohanty and Mukherji, 2012).
Bacteria, yeast and filamentous fungi can synthesize a structurally diverse array of organic compounds with surface activity. These amphiphilic compounds generally comprise a hydrophilic acid, peptide cations or anions, mono-, di- or polysaccharides, and a hydrophobic moiety of unsaturated or saturated hydrocarbon chains, fatty acids, or lipids (Banat et al., 2010). Surface active compounds in biological systems can be broadly classified as: (a) low-molecular-weight compounds called biosurfactants, such as lipopeptides, glycolipids, and proteins (e.g., glycolipids such as rhamnolipids, trehalose lipids, sophorolipids, mannosylerythritol lipids, and lipopeptides such as surfactin and fungicin (Franzetti et al., 2008a, 2010; Cameotra and Singh, 2009; Nguyen and Sabatini, 2011; Banat et al., 2014; Dobler et al., 2016; Santos et al., 2016); and (b) bioemulsifiers, high-molecular-weight polymers of lipopolysaccharides, polysaccharides, proteins or lipoproteins (e.g., such as the lipopolysaccharide emulsan and the polysaccharide and protein complex alasan) (Neu, 1996; Uzoigwe et al., 2015). Biosurfactants reduce surface and interfacial tensions, while bioemulsifiers stabilize oil-in-water emulsions and have less capacity to lower surface tension than biosurfactants (Smyth et al., 2010a,b).
Microbial surfactants can promote bacterial growth on PHCs by increasing the surface area between oil and water through emulsification, and by increasing pseudosolubility through partitioning into micelles (Volkering et al., 1997). In certain cases, this results in an increase in contaminant bioavailability to degrading microorganisms. Recent reviews provide paradigms of successful biosurfactant applications in bioremediation processes (Mulligan, 2009; Pacwa-Plociniczak et al., 2011; Lawniczak et al., 2013). For example, production of lipopeptides by Bacillus circulans (Das et al., 2008), as well as lipopeptides and protein-starch-lipids by two strains of Pseudomonas aeruginosa (Bordoloi and Konwar, 2009) have been shown to enhance PAH biodegradation.
Relatively recently, a comparative study between Triton X-100 and the commercial rhamnolipid JBR-515 (Jeneil Biosurfactant Company, USA), was conducted to explore the factors affecting the process of surfactant enhanced biodegradation of model NAPLs by a naphthalene degrader, Burkholderia multivorans (NG1). Briefly, Triton X-100 enhanced bioavailability through emulsification and supported direct interfacial uptake, while the rhamnolipid mixture JBR-515 did not substantially emulsify hydrocarbons, enhancing bioavailability instead through micellar solubilization (Mohanty and Mukherji, 2013).
In P. aeruginosa, it has been observed that the uptake of rhamolipid-coated hexadecane droplets occurred through a mechanism very similar to pinocytosis (Cameotra and Singh, 2009); the latter can be tentatively defined here as “internalization of biosurfactant layered hydrocarbon droplet.” Depending on the physiology of the organism with respect to its preferred hydrocarbon accession mode (direct contact with sparingly soluble hydrocarbons, direct attachment to insoluble hydrocarbon droplets, micellization of hydrocarbons with biosurfactants), the presence of biologically derived and synthetic surfactants may inhibit biodegradation. Micelle cores can trap organic contaminants, creating a hydrophilic barrier between “hydrophobic microorganisms” and organic molecules, the result of which is the potential substrate becoming less available (Colores et al., 2000). Crucially, some microorganisms can emulsify hydrocarbons even in the absence of cell growth or uptake of hydrocarbons. That suggests that emulsification may be associated with the surface properties of the cells, because of attachment to the oil–water interface by general hydrophobic interactions rather than specific recognition of the substrate. Therefore, microbial cells may behave as fine solid particles at interfaces. Having knowledge of that, prompt the hypothesis that intact, stationary-phase microorganisms, referred previously as “hydrophobic” can stabilize oil–water emulsions by adhering to the oil–water interface a property related to cell surface hydrophobicity.
In mixed microbial communities, in situ production of microbial- or plant-derived biosurfactants, or exogenously added (bio)surfactants, may serve as a preferred substrate for a normally hydrocarbonoclastic species, limiting remediation outcomes (Franzetti et al., 2008b). Endogenous and exogenous biosurfactants may also prove toxic to some organisms by disrupting membrane permeability, interfering with chemotaxis-driven motility, and disrupting or limiting biofilm formation.
Biofilms, bacterial communities surrounded by self-produced polymeric matrices reversibly attached to an inert or a biotic surface (Costerton et al., 1995), are an adaptive mechanism for microorganisms to better cope with harsh physical and chemical conditions, to facilitate catabolite exchange, to increase horizontal gene transfer, and to regulate the redox state of their environment (Gorbushina and Broughton, 2009; Shemesh et al., 2010). Biofilm matrices may consist of extracellular polysaccharides (EPSs), proteins and DNA (Sutherland, 2001; Branda et al., 2005; Rinaudi and Gonzalez, 2009), with EPS affecting the porosity, density, water content, charge, hydrophobicity, and mechanical stability of biofilms (Flemming and Wingender, 2010). Biofilms may also enhance PHC bioremediation processes by increasing pollutant availability (Wick et al., 2002; Johnsen and Karlson, 2004). The secretion of polymers is often correlated with establishment of the biofilm growth mode; thus, in case that secretion of polymers by microorganisms is followed by formation of biofilms on the surface of insoluble hydrocarbons, renders those microorganisms especially well-suited for the treatment of recalcitrant compounds because of their high microbial biomass within biofilm compared to the cells grown in dispersed culture along with their ability to immobilize compounds by biosorption. Moreover, the biofilm lifestyle facilitates degradation processes by maintaining optimal conditions of pH, localized solute concentrations and redox potential in the vicinity of the cells (Singh et al., 2006).
In addition to the production of biosurfactants and biofilm formation, chemotaxis, the targeted movement of microorganisms in response to chemical gradients with the aim of finding ideal conditions for growth and survival (Eisenbach and Caplan, 1998; Wadhams and Armitage, 2004; Baker et al., 2006a,b; Paul et al., 2006; Rao et al., 2008; Hazelbauer and Lai, 2010; Krell et al., 2011), has been shown to be important for microbial exploitation of PHCs in soil and water (Marx and Aitken, 2000; Pandey and Jain, 2002; Parales and Haddock, 2004; Ford and Harvey, 2007; Strobel et al., 2011). For example, the capability of bacteria to sense and swim toward n-hexadecane (Nisenbaum et al., 2013), gas oil (D’Ippolito et al., 2011), as well as various monocyclic and PAHs and their nitro-, amino-, or chloro-substituted relatives has been demonstrated to stimulate degradation of the corresponding PHCs (Grimm and Harwood, 1997; Parales et al., 2000; Samanta and Jain, 2000; Pandey et al., 2002; Lanfranconi et al., 2003; Law and Aitken, 2003; Ortega-Calvo et al., 2003; Vardar et al., 2005; Cunliffe et al., 2006; Gordillo et al., 2007; Iwaki et al., 2007; Peng et al., 2008; Bisht et al., 2010; Tremaroli et al., 2010; Fernandez-Luqueno et al., 2011), presumably by allowing the microorganism to balance access to substrate and substrate toxicity (Olson et al., 2004; Jeong et al., 2010).
In fact, the chemotactic behavior of bacteria can be either toward (positive chemotaxis) or away (negative) from the chemical gradient. Thus, chemotaxis presumably acts like a balance mechanism that helps the bacteria to perform in an ideal way if it increases bioavailability of pollutants whilst, at the same time protects them in case of toxicity. For example, this balance may explain why the naphthalene degrading Pseudomonas putida PpG7 was repelled by vapor-phase naphthalene at steady state gaseous concentrations that were significantly lower than the aqueous concentrations that resulted in positive chemotaxis (Hanzel et al., 2010).
In some cases, the chemotaxis mechanisms for PHC degrading microorganisms are well-characterized, and it has been observed that, in some cases, PHC catabolic genes are co-located with chemotaxis genes on plasmids (Grimm and Harwood, 1999). It has been shown in bacteria of the genus Pseudomonas that the chemotactic response is mediated by the McpT chemoreceptor encoded by the pGRT1 megaplasmid. Two alleles of mcpT are borne on this plasmid and inactivation of either one results in a loss of the chemotactic phenotype, while cloning of mcpT into a plasmid complemented not only the mcpT mutants, but also made it possible to transfer chemotactic response to other Pseudomonas strains for high PAH concentrations, indicating that chemotaxis toward toxic PAHs is gene-dose dependent (Lacal et al., 2011). Overall, increased expression of motility and chemotaxis genes suggest that microbial communities are able to ramp up metabolic pathways that will allow for direct contact with hydrocarbon compounds (Smith et al., 2013).
Historically, both ex situ and in situ bioremediation approaches have been used for the restoration of PHC-polluted environments (Stroud et al., 2007). However, in situ approaches have become more prevalent as costs compared to ex situ are generally lower with fewer disruptions to the natural landscape (Romantschuk et al., 2000; Jorgensen, 2007). The different approaches used for assessment of the ecological sustainability of in situ bioremediation processes have been thoroughly reviewed (Pandey et al., 2009), with natural attenuation (Smets and Pritchard, 2003; Scow and Hicks, 2005) and biostimulation/bioaugmentation being discussed below.
A growing body of studies, including modeling and field experimentation provide evidence that natural attenuation is a promising remediation option for soils, estuarine sediments and groundwater contaminated by PHCs (Khan and Husain, 2003; Suarez and Rifai, 2004; Verginelli and Baciocchi, 2013). In the same context, several other reports have underlined the significant role of subsurface natural attenuation processes in bioremediation (Pasteris et al., 2002; Devaull, 2007; Lundegard et al., 2008; Abreu et al., 2009). Natural attenuation has been shown as an effective bioremediation option for a chronically diesel-oil-polluted site over a long period of time under unfavorably cold conditions (Margesin and Schinner, 2001).
The recovery of the Gulf of Mexico after the Deepwater Horizon blowout testifies to the fact that in situ bioremediation based on natural attenuation can be successful after large scale spills. Indeed, quick adaptation of the native microflora of the deep sea ecosystem to oil contamination resulted in dominance of bacteria of the order Oceanospirillales in the γ-Proteobacteria, a group which includes known psychrophilic hydrocarbon degraders and microorganisms from hydrocarbon-dominated environments (Hazen et al., 2010).
The principle behind biostimulation as a method to increase PHC degradation relies on the establishment of a propitious environment for hydrocarbonclastic bacterial communities through the addition of nutrients (e.g., nitrogen and phosphorus, horse manure, poultry litter, domestic sewage, rice straw biochar, crop residues), and other supplementary components such as biosurfactants and electron acceptors [e.g., O2, chelated Fe (III), nitrates, sulfate] (Gallego et al., 2001; Molina-Barahona et al., 2004; Coles et al., 2009; Lai et al., 2009; Qin et al., 2013; Zhao et al., 2015; Ladino-Orjuela et al., 2016). The adjuvant role of these factors is related either to the metabolic activity of the naturally occurring degrading bacteria or to the bioavailability of PHCs. Among these biostimulants, addition of nutrients has been demonstrated to improve the degradation potential of native microbial communities (Thomassin-Lacroix et al., 2002; Delille et al., 2004; Garcia-Blanco et al., 2007). Studies at both laboratory and field scales have revealed enhanced degradation of PHCs (diesel oil, pyrene, phenanthrene) based on the addition of biosolids, inorganic fertilizers (rich in N and P) and organic fertilizers (Braddock et al., 1995; Carmichael and Pfaender, 1997; Margesin et al., 2003; Xu and Obbard, 2003; Sarkar et al., 2005).
Moreover, it has been observed that the higher the initial PHC contamination, the more marked was the effect of fertilization on PHC removal (Margesin et al., 2007). Similar results have been observed in aquatic environments, however, caution is required given that high nutrient levels can be the causative agent of ecological impairments such as eutrophication (Nikolopoulou and Kalogerakis, 2009).
Approximately, 1–5% N by weight of oil with a ratio of N:P between 5 and 10:1 is applicable for oil spill remediation (Swannell et al., 1996). Furthermore, based on a theoretical calculation the conversion of 1 g of hydrocarbon to cell materials requires the utilization of 150 mg of nitrogen and 30 mg of phosphorus (Rosenberg and Ron, 1996).
A number of comparative studies have reported different C:N:P ratios as the most suitable prior to the commencement of in situ bioremediation. In this sense, it has been proposed that optimal C:N:P mole-ratios to enhance hydrocarbon removal in soil are at the levels of 100:9:2, 100:10:1, 100:10:5, or 250:10:3 (Zawierucha and Malina, 2011).
Given that most energetically favorable terminal electron acceptor is O2, it is assumed that adequate aeration through mechanical tillage, forced aeration and addition of alternative oxygen sources, such as oxygen-releasing compounds (ORCs), or agents such as potassium permanganate (KMnO4), hydrogen peroxide (H2O2), or ozone (O3) should stimulate microbial activity and enhance aerobic biodegradation rates (Brown et al., 2003; Saito and Magara, 2003; Goi et al., 2006; Menendez-Vega et al., 2007; Tsai and Kao, 2009).
Furthermore, the rate of hydrocarbon removal has also been stimulated by generating optimal conditions for other physical factors such as temperature (Horel and Schiewer, 2009) and moisture (Zawierucha and Malina, 2011). Recently, the application of non-conventional biostimulation methods has been reported. For example, incorporating modified Fenton’s reagent as a pre-treatment in combination with inorganic fertilizers has improved the bioremediation of diesel polluted soil (Andrea Silva-Castro et al., 2013).
Several authors have investigated the impacts of in situ biostimulation treatments on bacterial diversity aiming to understand the relationships between the dominance, physiology and function of specific genera able to degrade contaminants of concern (Iwamoto et al., 2000; Evans et al., 2004). These observations suggest that identifying the key players that drive community structure is a prerequisite to comprehend, model, forecast, monitor, and control biostimulation processes (Hazen, 2010).
Another variant of bioremediation, bioaugmentation, involves the introduction in adequate numbers of bacterial populations with the necessary catabolic potential to mediate PHC degradation (Vogel, 1996; Paliwal et al., 2012). Therefore, selection and addition of (a) a pre-adapted bacterial strain, (b) a pre-adapted consortium, (c) genetically engineered bacteria, or (d) catabolic genes packaged in a vector to be transferred by conjugation into indigenous microorganisms, is of paramount importance for any bioaugmentation process (El Fantroussi and Agathos, 2005; Singer et al., 2005; Thompson et al., 2005). When considering bioaugmentation, it is important to consult local regulations and decide if: (1) a single strain or a known mixed microbial consortium can be introduced, (2) an autochthonous, defined as an indigenous bacterial consortium previously enriched from the polluted soil and cultivated with hydrocarbons as the carbon source can be re-inoculated or (3) an allochthonous, defined as a foreign consortium previously drawn from another PHCs polluted site, can be used (Ueno et al., 2007). In fact, the bioremediation of soils freshly contaminated with petroleum constituents could benefit from the addition of biota primed for PHCs biodegradation (Greenwood et al., 2009). Interestingly, based on the use of selected native strains, bioaugmentation has been shown to accelerate the bioremediation of soils co-contaminated with diesel oil and various heavy metals (Alisi et al., 2009). A study conducted to evaluate the potential of indigenous and exogenous microorganisms for bioremediation of clayey and silty soils polluted with diesel oil revealed that a native consortium was the best option for remediating the silty soil, while a combination of native and exogenous consortia was more effective for remediating the clayey soil (Moliterni et al., 2012). Most recently, the introduction of an exogenous PHCs-degrading consortium consisting of Rhodococcus equi, Enterobacter sp., Acinetobacter calcoaceticus, Comamonas sp., and Pseudomonas alcaligenes, increased the production of high erucic acid rapeseed (Brassica napus) biomass in soils treated with diesel oil ranging from 6,000 to 24,000 mg kg-1 dry soil (Graj et al., 2013). Despite the satisfactory nature of these experiments, the Achilles’ heel of traditional bioaugmentation remains if foreign bacteria are able to establish stable communities in competitive environments. In more detail, the exogenous introduction (bioaugmentation) of efficient PHCs degraders is actually a rational re-arrangement of the microbial richness aiming to the dominance of bacterial group(s) with specific catabolic traits necessary for the clean-up.
Thus, the diverse natural life forms that live in communities within the biotope inoculated with an exogenous inoculum, represents a major obstacle in the successful remediation performance of such an inoculum. Overviewing the literature, there is a consensus that the decline in population size of active exogenously inoculated bacteria is attributed to various factors of which competition with autochthonous bacteria for nutrients and electron acceptors seems to be paramount. Therefore, the long term efficacy of such inoculum requisites a successful initial establishment (Goldstein et al., 1985; van Veen et al., 1997; Bouchez et al., 2000; El Fantroussi and Agathos, 2005; Thompson et al., 2005).
Numerous studies have concluded that bioaugmentation through isolation and reintroduction of hydrocarbon degrading bacteria from a contaminated site is more effective than in situ biostimulation and natural attenuation when applied to sites contaminated with various PHCs (Bento et al., 2005; Smith et al., 2005; Liu et al., 2008; Couto et al., 2010).
However, it is often found that biostimulation with a commercial fertilizer is more effective than bioaugmentation (Demque et al., 1997), or that fertilizer effects of foreign inoculants are more important than the inoculants themselves.
While it may be possible to adjust the makeup of a microbial community, as was done with bioaugmentation of a bench scale biobarrier (Daghio et al., 2015) and nutrient addition to a diesel-contaminated boreal forest soil (Kauppi et al., 2011), PHC removal efficiencies may not be increased, although outcomes are site specific (Yergeau et al., 2009).
Bioaugmentation with endophytic bacteria with biodegradative capabilities may have benefits compared to conventional bioaugmentation with free-living bacteria, as endophytes may have greater potential to find a suitable niche in an established community due to their association with a plant host. Further benefits can be achieved if the endophyte transfers metabolic genes for biodegradation to native endophytes (Taghavi et al., 2005).
For example, in situ bioaugmentation by P. putida W619 decreased trichloroethylene evapotranspiration up to 90% under field conditions (Weyens et al., 2009a). This result was achieved after the establishment and enrichment of P. putida W619-TCE as a poplar root endophyte followed by further horizontal gene transfer of TCE metabolic activity to members of the poplar’s endogenous endophytic community (Weyens et al., 2009b).
For more information about the different techniques developed for bioaugmenting environmental sites (Figure 2), with emphasis on PHC spills, the reader is referred to the reviews of Gentry et al. (2004), Hosokawa et al. (2009), and Tyagi et al. (2011).
In addition, both bioaugmentation and biostimulation appear to be effective for enhancing PHC biodegradation in soil and, in some cases, the simultaneous application of these techniques results in additional improvement (Hamdi et al., 2007a; Mrozik and Piotrowska-Seget, 2010; Xu and Lu, 2010; Sun et al., 2012; Taccari et al., 2012). For example, it has been demonstrated that the highest pyrene removal (84%) was obtained through a combined bioaugmentation-biostimulation process, followed by bioaugmentation (57%), biostimulation (50%), and control (37%) processes (Ghaly et al., 2013).
Overall, site conditions, composition of the indigenous microbial community, and the type, quantity and toxicity of the pollutant present demand a case by case approach to deal with contamination challenges.
In addition to promoting bioavailability (e.g., by addition or production of biosurfactants), and stimulating microbial activity (e.g., by biostimulation or bioaugmentation), PHC bioremediation can be further optimized by involving assiduously characterized bacterial strains carrying the necessary metabolic pathways for the complete degradation (mineralization) of components in petroleum mixtures.
In general, even though the biodegradation of PHCs can occur under anaerobic conditions, the majority of them are more efficiently metabolized under aerobic conditions. Figure 3 illustrates the basic principle of aerobic catabolism of PHCs. PHC biodegradability tends to decrease in the following order: n-alkanes > branched-chain alkanes > branched alkenes > low-molecular-weight n-alkyl aromatics > monoaromatics > cyclic alkanes > PAHs > asphaltenes (Atlas, 1981; Van Hamme et al., 2003; Tyagi et al., 2011). Despite the chemical stability of alkane molecules, in the presence of O2 they can be activated by oxygenases and completely oxidized to carbon dioxide and water.
The expression of genes involved in alkane degradation is strictly controlled (Wang and Shao, 2013), and microorganisms have multiple alkane degradation systems that target alkanes of different chain lengths (Table 1). Specific regulation mechanisms ensure that the genes involved in alkane degradation are expressed only under certain conditions, in the presence of the appropriate alkanes when other preferred substrates are not available (Rojo, 2009).
Generally, alkane-degradation by bacteria begins with an oxidative attack at the terminal methyl group with the formation of a fatty alcohol, aldehyde, and fatty acid. The carboxylic acid can then be combined with CoA and, via ß-oxidation, yield acetyl-CoA that enters the tricarboxylic acid (TCA) cycle. For short-chain length (C1–C4) n-alkanes, methane monooxygenases (MMO) are the first enzymes involved in the process. The MMO enzyme family consists of two distinct forms: a soluble di-iron methane monooxygenase (sMMO) and a membrane-bound copper-containing methane monooxygenase (pMMO); the alpha subunits of these enzymes are encoded by mmoX and pmoA genes, respectively. Notably, sMMO performs the co-oxidation of saturated, unsaturated, linear, branched and cyclic hydrocarbons, whereas pMMO has a much narrower substrate range, being mostly active against alkanes and alkenes with lengths up to five carbons (Berthe-Corti and Bruns, 2001 ; Steinkamp et al., 2001; Baik et al., 2003; Lieberman et al., 2003; Hua et al., 2011; Jiang et al., 2011). Gaseous alkanes are metabolized by strains expressing propane or butane monooxygenases (BMOs) that are related to pMMO or sMMO, respectively. For example, Gordonia sp. TY-5 has been reported to be able to use propane as the sole carbon source, but no other gaseous alkanes. A complete operon encoding for PmA, which is similar to the α subunit of sMMO, an NADH-dependent reductase and a regulatory protein, was cloned and sequenced from this strain. Upon deletion of one of the subunits, the ability of the organism to grow on propane was nullified, corroborating its role in propane oxidation (Kotani et al., 2003). The hydroxylase subunits of propane monooxygenase show relatively high sequence similarity with butane monooxygenase (sBMO) isolated from Pseudomonas butanovora, an organism which oxidizes butane to 1-butanol. This BMO has been cloned and is similar to sMMO: the hydroxylase subunits α and ß, and the regulatory protein B show more than 60%, 50% amino acid sequence identity, respectively, to the corresponding subunits of sMMOs (Sluis et al., 2002). The differential regulation of multiple alkane hydroxylases has been described in P. aeruginosa RR1 and in P. aeruginosa PAO1. These strains contain the alkane hydroxylases AlkB1 (which oxidizes C16–C24 n-alkanes) and AlkB2 (which oxidizes C12–C20 n-alkanes). When C10–C22 alkanes are present, both genes are expressed but the expression of alkB1 is double that of alkB2. Furthermore, alkB2 is preferentially induced at the beginning of the exponential phase, and alkB1 is preferentially induced during the late exponential phase, with expression of both genes decreasing during the stationary phase (Marin et al., 2003).
A more complex system has been described in Alcanivorax borkumensis, an organism with two alkane hydroxylases (AlkB1, active on C5–C12 n-alkanes and AklB2, active on C8–C16 n-alkanes) and three cytochrome P450s involved in alkane oxidation (P450-1, P450-2, and P450-3) (van Beilen et al., 2004; Schneiker et al., 2006). The expression of alkB1 and alkB2 genes is induced when C10–C16 alkanes are provided and decreases when the cells enter the stationary phase (van Beilen et al., 2004; Sabirova et al., 2006; Schneiker et al., 2006). An AlkS-like activator seems to be involved in the activation of alkB1 in response to the presence of alkanes. Higher levels of AlkS have been detected when hexadecane was provided instead of pyruvate, and the alkB1 promoter in A. borkumensis has an AlkS-binding site immediately upstream (van Beilen et al., 2004; Sabirova et al., 2006). A regulator of the AraC family is located close to P450-1, however, its role in the regulation of the expression of P450-1 still has to be investigated (Schneiker et al., 2006). It was recently suggested that a potential AraC family regulator (CypR) is involved in CYP153 gene activation, a gene that encodes an alkane hydroxylase that belongs to the cytochrome P450 superfamily (Funhoff et al., 2006) in the Gram-positive bacterium Dietzia sp. strain DQ12-45-1b (Liang et al., 2016). As in A. borkumensis, Alcanivorax hongdengensis degrades alkanes by using alkB1, alkB2, p450-1, p450-2, and p450-3. In A. hongdengensis a gene that encodes for a protein homologous to TetR family regulators is located downstream of alkB1. Furthermore, the presence of a regulator of the GntR family has been observed upstream of alkB2 but its role in the regulation of the degradation pathways is still not known (Wang and Shao, 2012). Acinetobacter sp. M-1 has two alkane hydroxylases, AlkMa and AlkMb. AlkMa is induced by AlkRa in the presence of >C22 n-alkanes, and the alkMb gene is induced by AlkRb when C16–C22 n-alkanes are provided (Tani et al., 2001).
Other important mechanisms regulating alkane metabolism are product repression and catabolite repression control (Rojo, 2009). For example, expression of BMO in P. butanovora is repressed by propionate, a downstream metabolite of propane oxidation (Doughty et al., 2006). Moreover, propionate acts as a repressor of alkane degradation in P. butanovora by competitive inhibition for the BMO catalytic site (Doughty et al., 2007). It has been shown that expression of BMO-encoding genes is activated by the putative sigma (54)-transcriptional regulator BmoR. This peptide recognizes alcohols and aldehydes produced during alkane degradation (Kurth et al., 2008).
In microorganisms that are versatile with respect to PHC metabolism can be repressed in the presence of other carbon sources that are used as preferred substrates via catabolite repression (Rojo, 2010). As an example, the most thoroughly characterized alkane degradation pathway, encoded by the OCT plasmid carried by P. putida GPo1 (van Beilen et al., 2001), will be described. In this system, the alkBFGHJKL operon encodes the enzymes necessary for converting alkanes into acetyl-coenzyme A (CoA), while alkST encodes a rubredoxin reductase (AlkT) and the positive regulator for the alkBFGHJKL operon (AlkS). These two operons are located end to end, separated by 9.7 kb of DNA, within which lies alkN, a gene coding for a methyl accepting transducer protein that may be involved in alkane chemotaxis. When alkanes are provided, the transcriptional regulator AlkS activates alkST gene expression by using the PalkS2 promoter (Canosa et al., 2000). Increased AlkS levels activate expression of alkBFGHJKL via the PalkB promoter (Canosa et al., 1999; Panke et al., 1999). However, when the cells are growing in a rich medium, the activation of both PalkB and PalkS2 is negatively affected even if alkanes are provided (Yuste et al., 1998; Staijen et al., 1999; Canosa et al., 2000). In a rich medium the global regulatory protein Crc (catabolite repression control) inhibits translation of alkS mRNA (Moreno et al., 2007). It was suggested that Crc and the protein Hfq form a stable complex with RNA resulting in the inhibition of translation initiation (Moreno et al., 2015). It has been demonstrated that in P. putida, Crc also limits the translation of mRNAs coding for enzymes involved in the first steps of alkane degradation (Hernandez-Arranz et al., 2013). Another regulation system involves the cytochrome o ubiquinol oxidase (Cyo), a component of the electron transport chain (Dinamarca et al., 2002). The expression of cyo depends on the oxygen concentration and the presence of the carbon source, with Cyo levels being correlated with repression of alkane degradation (Dinamarca et al., 2002, 2003). The role of Cyo during the degradation of long chain alkanes in Alcanivorax dieselolei has been reported (Wang and Shao, 2014). In the presence of long chain alkanes and pristane, Cyo was expressed resulting in decreased AlmR production. AlmR is a negative regulatory protein of almA, a gene which encodes for the AlmA hydroxylase that is active against both long chain and branched alkanes (Wang and Shao, 2014). Noteworthy, at this point is that of all the genes mentioned, the function of alkL remains unknown, although, it is suspected to be involved in transport (Figure 4).
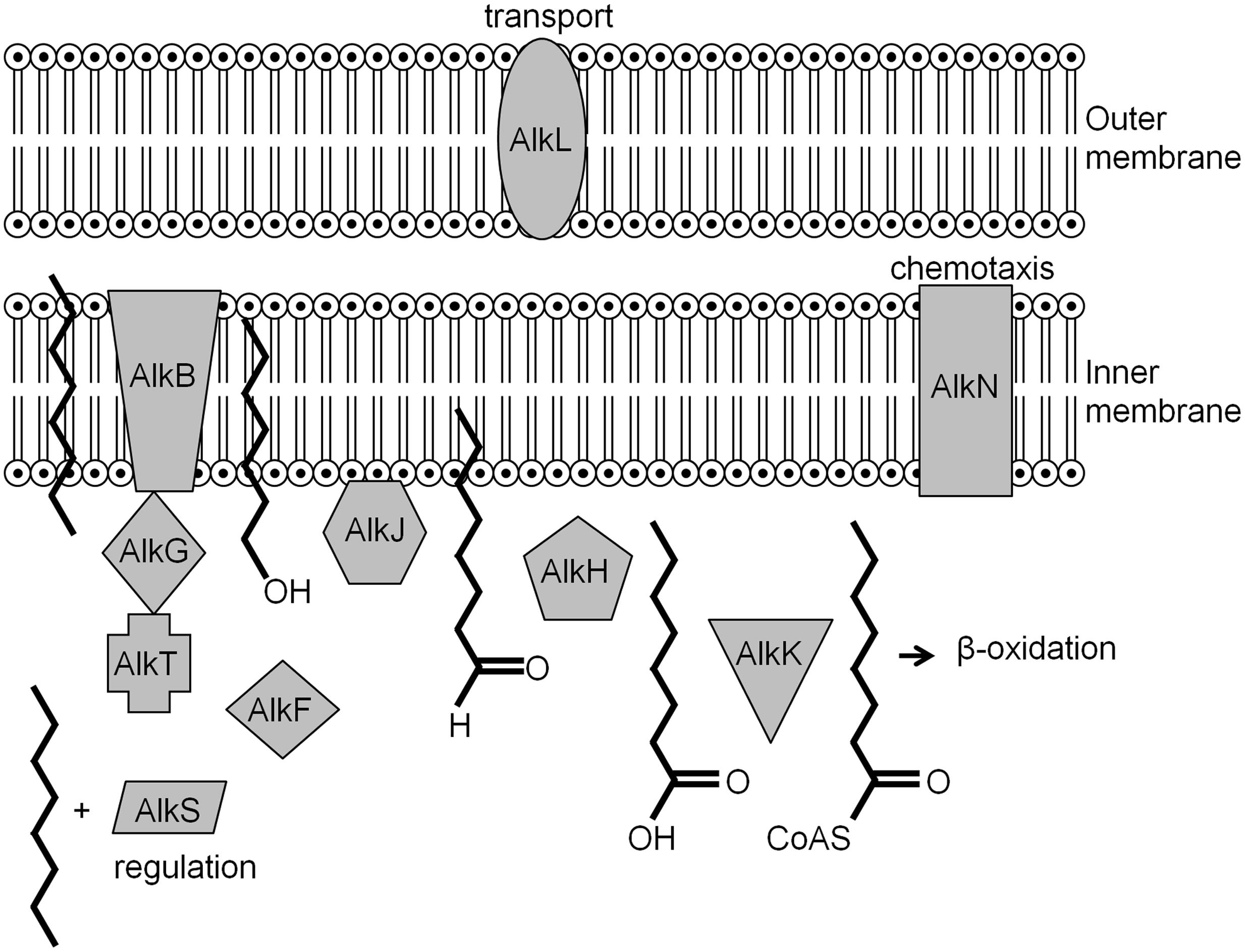
FIGURE 4. Locations and functions of the alk gene products in the inner and outer membrane of gram negative bacteria (Van Hamme et al., 2003).
Another class of hydroxylases, facilitating the terminal hydroxylation of medium-chain n-alkanes, includes enzymes related to the soluble cytochrome P450 CYP153 from Acinetobacter sp. EB104 (Maier et al., 2001). Since that enzyme was characterized, several researchers have reported that bacteria belonging to Mycobacterium, Rhodococcus, and Alcanivorax isolated from various environments such as contaminated soil, groundwater and surface water, use that enzymatic machinery to degrade medium-chain alkanes (Kubota et al., 2005; Schneiker et al., 2006; Sekine et al., 2006; Wang et al., 2010; Liu et al., 2011). Even though assimilation of alkanes up to C20 is reported for bacteria containing AlkB family and cytochrome P450 alkane hydroxylases, there is a scarcity of information on metabolic pathways and enzyme systems that degrade >C20 alkanes (Rojo, 2009).
Usually, the alkane hydroxylases present in bacteria able to degrade alkanes longer than C20 are not evolutionary related to known AlkB and P450-like proteins and include AlmA (a flavin binding monooxygenase involved in the degradation of long-chain n-alkanes of C32 and longer) from Acinetobacter strain DSM 17874 (Throne-Holst et al., 2007), and LadA from Geobacillus thermodenitrificans NG80-2 (Feng et al., 2007), able to generate primary alcohols from C15 to C36 alkanes. Acinetobacter sp. M-1 (Sakai et al., 1994), and Acinetobacter baylyi ADP1 (Vaneechoutte et al., 2006), have been also found to grow with C32 and C36, respectively.
In addition, long chain n-alkane degrading bacterial species such as: Marinobacter aquaeolei VT8, Oceanobacter sp. RED65, Ralstonia spp., Mycobacterium spp., Photorhabdus sp., Psychrobacter spp., and Nocardia farcinica IFM10152, has been reported (Wentzel et al., 2007). Lately, a unique functional AlkB-type alkane hydroxylase system has been described that allows growth on long-chain liquid and solid n-alkanes in the Gram-positive Gordonia strain SoCg (Lo Piccolo et al., 2011). In contrast to alkanes, the general mode of monoaromatic and PAH biodegradation requires the presence of bacteria that harbor catabolic genes coding for dioxygenases. Generally, catabolism of PAHs is triggered by a dioxygenase reaction that adds hydroxyl groups (OH) to one ring.
Thereafter, the hydroxylated ring is subjected to ring fission, producing a substituted PAH with one ring less than the parent molecule. Subsequent oxygenase reactions are utilized to ultimately mineralize the PAH (Olson et al., 2003). Ring-hydroxylating dioxygenases related to polycyclic aromatic hydrocarbon oxidation (PAH-RHD), such as those encoded by the nah, nod, and phn genes in Gram-negative bacteria, and the evolutionarily correlated nid, nir, and nar genes in Gram-positive bacteria, catalyze the first step of the PAH degradation pathway (Larkin et al., 1999; Saito et al., 2000; Khan et al., 2001). In this step, dioxygenase-catalyzed oxidation of arenes yields vicinal cis-dihydrodiols as the early bioproducts of a multicomponent enzyme system.
Furthermore, these di-hydroxylated intermediates may then be cleaved by intradiol or extradiol ring-cleaving dioxygenases through either an ortho-cleavage pathway or a meta-cleavage pathway, leading to central intermediates such as protocatechuates and catechols that are further converted to TCA cycle intermediates (Peng et al., 2008). The catalytic component with hydroxylase activity is composed of an alpha subunit of about 50 kDa and a beta subunit of 20 kDa, which assemble in a α3ß3 heterohexamer.
Each alpha subunit consists of two domains, the N-terminal Rieske domain, which contains a [2Fe-2S] cluster, and the C-terminal catalytic domain, which contains a mononuclear ferrous ion close to the substrate-binding site. The catalytic component requires electrons to activate oxygen at each cycle of hydroxylation of the substrate. Two auxiliary proteins, a ferredoxin and a flavin-containing oxidoreductase, often provide the necessary reductant at the expense of NAD(P)H oxidation (Jouanneau et al., 2011). Genes coding for the catalytic domain of PAH-RHDs (α-subunit) have been broadly used as biomarkers of PAH-degrading potential in various environments, making this subunit a valuable tool for studying RHD biodiversity (Flocco et al., 2009; Ding et al., 2010).
Based on amino acid sequence comparisons of the catalytic oxygenase α subunits, four discernible classes have been reported. These are: (a) the naphthalene family which includes Gram-negative bacterial enzymes responsible for the degradation of naphthalene and phenanthrene; (b) the benzoate family encompassing enzymes for the oxidation of aromatic acids; (c) the phthalate class that includes the diverse mono- and dioxygenases (interestingly the majority of the members of this family lack the ß subunits and possess only the reductase component in the electron transport chain); and (d) the toluene/biphenyl class that contains enzymes from both Gram-negative and Gram-positive microbes capable of transforming toluene, benzene, and chlorobenzenes (Gibson and Parales, 2000).
Historically, the critical point for the analysis of PAH degradation by aerobic bacteria started with the discovery, in P. putida strain G7, of naphthalene catabolic genes (nah) located on the plasmid NAH7 (Simon et al., 1993). After that discovery, work mainly on Pseudomonas species made evident that naphthalene biodegradation occurs via the formation of salicylate as an intermediate.
Upon examination of the diversity of dioxygenases involved in the degradation of low molecular weight (LMW) and high molecular weight (HMW) PAH compounds (e.g., naphthalene, phenanthrene, anthracene, pyrene, benzo[a]pyrene, benzo[a]anthracene), it is noticeable that both Gram-negative genera like Pseudomonas, Ochrobactrum, Polaromonas, Sphingomonas, Novosphingobium, Acidovorax and Burkholderia, and Gram-positive genera like Mycobacterium, Gordonia, Bacillus, Nocardia, and Rhodococcus, are exploiting these enzymes for the degradation of the aforementioned compounds (Table 2). Overall, the oxidation of naphthalene follows either the gentisic acid (Grund et al., 1992), or catechol (ortho and/or meta) degradation pathways (Eaton and Chapman, 1992) in order to generate compounds for integration in the TCA cycle, and there is a good body of evidence linking stimulated microbial PHC biodegradation to the presence of plant metabolites in the rhizophere as discussed in the next sections.
Phytoremediation, defined as the use of plants and their associated microorganisms to assimilate, transform, metabolize, detoxify and degrade various toxic inorganic and organic compounds (e.g., PHCs, pesticides, dyes, solvents) found in soil, water, groundwater, and air is generally considered as an environmentally friendly, cost effective, and socially accepted remediation approach (Salt et al., 1995, 1998; Alkorta and Garbisu, 2001; Pilon-Smits, 2005; Sandhu et al., 2007; Reichenauer and Germida, 2008;, Wenzel, 2009; Prasad et al., 2010; Kabra et al., 2012). For more information about the advantages and disadvantages of phytoremediation we refer to the following reviews (Susarla et al., 2002; Kuiper et al., 2004; Arthur et al., 2005; Pandey et al., 2009 ; Vangronsveld et al., 2009).
Plant-associated bacteria include endophytic, phyllospheric and rhizospheric bacteria, and they have a variety of interactions with plants, ranging from being active pathogens, opportunistic pathogens, and bacteria that dwell within the plant and merit some physical protection, to bacteria actively interacting with the host plant generating mutually beneficial association for both organisms (Newman and Reynolds, 2004; Weyens et al., 2009c). The ability of bacteria to degrade PHCs is attributed to the presence of catabolic genes and enzymes, which allow them to utilize the complex chemicals found in petroleum mixtures as vital energy sources (Rojo, 2009; Das and Chandran, 2010). Many bacterial strains have been reported to encompass the metabolic pathways required for the degradation of the relevant hydrocarbons. Species of Pseudomonas, Acinetobacter, Mycobacterium, Haemophilus, Rhodococcus, Paenibacillus, and Ralstonia belong to the most extensively studied bacteria (Tyagi et al., 2011). On the other hand, though a substantial number of hydrocarbons can be metabolized by bacteria, in the absence of plants this process is not always efficient due to the relatively low number of these microorganisms in bulk soil. Indeed, in the rhizosphere 10–1000 times higher microbial activity has been reported. Hence, the role of plants in the ongoing process is equally important (Palmroth et al., 2002; Gaskin et al., 2008).
In another context, PHCs are giving rise as serious threat not only to soil but also to estuarine sediments (Chapman and Wang, 2001; Daane et al., 2001). The ecological importance of these ecosystems, along with their susceptibility to pollutants such as PHCs (Andrade et al., 2004), have fostered various research groups to investigate, whether plant–microorganisms associations may actively contribute to PHC degradation in estuarine environments. In fact, a number of recent studies have evaluated the influence of different salt marsh plant–bacteria associations on PHC fate and concluded that such symbiosis enhances significantly the degradation pattern via alteration of the functional diversity of the PHC degrading bacterial community (Oliveira et al., 2014, 2015).
Phytoremediation encompasses four distinct mechanisms namely phytostabilization, phytodegradation, phytovol-atilization, and rhizodegradation (Germida et al., 2002). Briefly, the term phytostabilization includes immobilization of the contaminants in soil, either simply by preventing erosion, leaching, or dispersion, or by transforming them through precipitation in the rhizosphere to less bioavailable forms. In an integrated approach phyto- and rhizodegradation can be approached as a mutually beneficial form of phytoremediation, where both plants and microorganisms mediate the breakdown of the contaminants via the use of their enzymatic machinery. Next phytovolatilization, due to the complete removal of the pollutant from the site as a gas, without further need for plant harvesting and disposal, holds promise as an attractive technology (Pilon-Smits, 2005; Lim et al., 2016).
In addition to the these concepts, a number of studies have shown that phyllosphere bacteria possess the ability to utilize gaseous and deposited PHCs (Waight et al., 2007; Yutthammo et al., 2010; Al-Awadhi et al., 2012; Ali et al., 2012); the latter holds great potential in air clean-up by opening up the new direction of air phyllo-remediation, which is actually the exploitation of air remediation capabilities based on the cooperation between plants and their associated phyllo-sphere microorganisms (Weyens et al., 2015).
Despite the fact of continuous exchange with airborne populations (Whipps et al., 2008), after recruitment phyllospheric bacteria are able to form real communities, prompting the hypothesis that they endure specific selection processes (Rastogi et al., 2012; Vorholt, 2012). The driving forces thought to govern community structure include plant species, leaf age, season, geographical location, and various environmental factors (Vokou et al., 2012; Muller and Ruppel, 2014). Thus, because of the high variability of phyllospheric community structure, further research about the bacterial communities hosted by different plant species in different environments is needed in order to evaluate their potential contribution to air bioremediation. Generally, in these very close plant–bacteria interactions, plants provide nutrients and residency for bacteria, which in exchange can improve applicability and efficiency of phytoremediation in case of sites contaminated by PHCs.
In a recent review (Thijs et al., 2016), it has been suggested that considering meta-organisms in their natural contexts (that is, the host and its microbiome together), will increase our knowledge of plant–microbial interactions and therefore facilitate translation to more effective, and predictable phytoremediation approaches. In the following sections, selected paradigms will be described to shed light to the field of PHC degradation via plants, bacteria, and their intimate interactions.
In order to survive and thrive in PHC contaminated environments, plants must exhibit: (i) a tolerance to one or more components of petroleum mixtures, (ii) high competitiveness, (iii) fast growth, and (iv) the ability to produce and secrete hydrocarbon degrading enzymes. In this context, plants may be positively influenced by the presence of bacteria that are able to: synthesize plant hormones, such as, indole-3-acetic acid (IAA), gibberellins (GAs), and cytokinins (CKs); suppress ethylene production via 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity; fix nitrogen; mobilize nutrients such as phosphorus and other minerals important in plant growth and development (Hardoim et al., 2008; Glick and Stearns, 2011); and metabolize a broach range of PHCs (Reed and Glick, 2005).
In situ implementation of phytoremediation strategies to restore contaminated sites has several drawbacks compared to traditional technologies such as pump and treat of contaminated groundwater, soil excavation and above-ground treatment. For example, if a plant has a shallow root zone and slow growth rates long periods of time may pass before contact with the target pollutant is made, if it is reached at all. The toxicity of the pollutants to native or introduced vegetation may result in inhibition of seed germination, reduced photosynthetic pigment production, compacted growth of tissues (root, aerial parts), slackening of nutrient assimilation and disruption of root architecture (Smith et al., 2006; Meudec et al., 2007; Euliss et al., 2008). Hence, selection of plants with increased pollutant tolerance, production of sufficient root and shoot biomass, suitability for various soil types, effective pollutant uptake mechanisms, and appropriate metabolic capabilities to degrade organic pollutants are prerequisites for successful remediation (Wenzel, 2009).
The initial physiological response of plants to PHCs in soil includes PHC uptake, translocation, and accumulation in organs such as roots and shoots. The rates of these processes are generally related to PHC concentration (Wild et al., 2005; Lu et al., 2010), lipophilicity, solubility, and volatility. Compound lipophilicity, expressed as an octanol-water partition coefficient (Kow), gives some indication about the tendency of a molecule to move through lipid bilayers, with log Kow values between 0.5 and 3 reflecting compounds with sufficient hydrophobicity to move through membrane lipid bilayers while exhibiting sufficient water solubility to dissolve in cellular fluids (Cherian and Oliveira, 2005). Compounds with a log Kow < 0,5 are characterized by high water-solubility, and plant roots do generally not translocate them at a rate surpassing passive influx (Cunningham and Berti, 1993), whereas compounds with a log Kow> 3.5 cannot be taken up and translocated into the plant due to tight sorption onto the soil and root surfaces (Meng et al., 2011).
After being transported inside the plant, PHCs can be either sequestered in root tissue, or transported into shoots and leaves, where they can be stored in vacuoles or volatilized into the atmosphere (Reichenauer and Germida, 2008).
Increasingly compelling evidence has accumulated about the use of plants for the remediation of environments polluted by PHCs (Liste and Alexander, 2000; van der Lelie et al., 2001; Newman and Reynolds, 2004; Pena-Castro et al., 2006; Euliss et al., 2008; Gerhardt et al., 2009; Peng et al., 2009; Zhang et al., 2012). Numerous studies focusing on plant species suitable for phytoremediation of PHC-contaminated soils have recognized that among others, Italian ryegrass (Lolium perenne), sorghum (Sorghum bicolor), maize (Zea mays), tall fescue (Festuca arundinacea), alfalfa (Medicago sativa var. Harpe), elephant grass (Pennisetum purpureum), bermuda grass (Cynodon dactylon), birdsfoot trefoil (Lotus corniculatus var. Leo), sunflower (Helianthus annuus), southern crabgrass (Digitaria sanguinalis), red clover (Trifolium pratense), beggar ticks (Bidens cernua), and sedge species (Cyperus rotundus) may be effective (Radwan et al., 1995; Wiltse et al., 1998; Chaineau et al., 2000; Huang et al., 2004; Parrish et al., 2004; Rutherford et al., 2005; Kaimi et al., 2007; Muratova et al., 2008; Shirdam et al., 2008; Ayotamuno et al., 2010; Tang et al., 2010; Yousaf et al., 2010; Hall et al., 2011; Basumatary et al., 2012, 2013). In general, the positive influence of leguminous plants is attributed in part to their ability to increase soil nitrogen concentrations in soils with high C:N ratio, whereas the positive contributions provided by grasses are correlated with their fibrous root systems, large root surface and deeper penetration into the soil matrix (Gaskin et al., 2008; Rezek et al., 2008). Taking into account the interplay between plants and their associate microorganisms in phytoremediaton, various research groups have investigated the role of fertilizers in this process and concluded that both the choice of, as well as the level of, added fertilizer is linked with the plant species present on site and the level of contamination (Cartmill et al., 2014; Jagtap et al., 2014; Ribeiro et al., 2014). It has been reported that the application of an ornamental plant (Mirabilis jalapa), characterized by non-trivial tolerance to petroleum contamination, strongly promoted PHC degradation when the concentration of PHC in soil was equal to or lower than 10,000 mg kg-1 (Peng et al., 2009).
Planting trees such as willows (Salix spp.) and hybrid poplars (Populus spp.) have been effective for remediating sites with contaminated groundwater (Cook et al., 2010) because they are easy to propagate, exhibit fast and perennial growth, generate phreatophytic roots that extend to the groundwater table, exhibit high water uptake rates, possess highly absorptive surface tissues, and are able to tolerate both a variety of contaminants and site flooding (Jordahl et al., 1997; Newman and Reynolds, 2004; Widdowson et al., 2005; Euliss et al., 2008; Barac et al., 2009).
The effects of varying concentrations of PHCs and nutrients on the spatial and temporal patterns of fine root production of hybrid poplar (P. deltoides × P. petrowskyana C. V. Griffin) has been investigated (Gunderson et al., 2008). It was observed that fine root production increased linearly up to approximately 500 mg kg-1 PHC, and then remained constant, and the working hypothesis is that the extensive fine root network may lead to enhanced contaminant degradation because of stimulated microbial activity due to a strong rhizosphere effect. A recent review compared the effectiveness of trees and grasses for remediation of PHCs and concluded that only minor differences are observed between trees and grasses with respect to average reduction of PHC concentrations (Cook and Hesterberg, 2013). Phytoremediation is a site-specific remediation method, explaining why contradictory results regarding the efficiency of this technology in removing contaminants from soil have been reported (Joner et al., 2004). Gaining knowledge about the molecular effects of PHCs on a range of plant species might contribute to better management of contaminated sites by providing physiological information to guide plant selection. In a recent study, aimed at unraveling PHC effects on plants, the global gene expression of 10-day-old A. thaliana seedlings exposed to the water-soluble fraction of a PHC mixture (WSF-MF380) was evaluated over time using whole genome microarray analysis. Results showed that the formation of an obstructive film covering the plant surface triggered gene expression responses similar to abiotic stresses such as heat, hypoxia, oxidative and osmotic stresses (Nardeli et al., 2016). Experiments with seedlings of Amorpha fruticosa exposed to PHC contaminated soil (≤15 g kg-1), demonstrated that the enzymes glutathione reductase (GR), superoxide dismutase (SOD) and catalase (CAT), effectively hampered reactive oxygen species (ROS) accumulation (Cui et al., 2016). The latter finding suggest the possibility of using the behavior of the antioxidant defense system and the growth reaction of seedlings under exposure to various PHCs concentrations as a valuable criterion for selection of the appropriate species for phytoremediation sites.
The photoautotrophic nature of plants, together with the fact that petroleum mixtures are poorly soluble in water means that for efficient PHC degradation the biocatalytic activities of rhizospheric microorganisms are essential. Generally, vegetated soils favor higher microbial numbers and diversity compared to bulk soil (Smalla et al., 2001; Haichar et al., 2008; Glick, 2010; Uroz et al., 2010). This effect is due to the release of organic compounds by plants commonly referred to as “rhizodeposits”; these compounds can be categorized as exudates, secretions, plant mucilages, mucigel, and root lysates (Olson et al., 2003) that are utilized by microorganisms as sources of carbon and energy (Chaudhry et al., 2005). Research has shown that plants, by releasing these organic compounds, change the physicochemical and biological properties of the soil most likely facilitating the attraction of chemotactic bacteria with desired metabolic activities (Hartmann et al., 2009). Plants release others organic compounds including terpenes, flavonoids and some lignin-derived components with chemical structures similar to those of PHCs, chemicals which may induce expression of PHC-degrading genes in rhizospheric microorganisms (Sun et al., 2010). Once attracted, PHC-degrading rhizosphere bacteria may ameliorate plant tolerance to PHCs and result in faster soil health recovery (Escalante-Espinosa et al., 2005; Barrutia et al., 2011). As an example, an increase of phenolic compounds found in root exudates has been associated with a higher degree of degradation of benzo[a]pyrene in the rhizosphere of Phragmites australis (Toyama et al., 2011).
More recently it has been demonstrated that PHC mineralization patterns by rhizosphere bacteria was substantially affected by root exudate composition. Specifically, certain compounds (e.g., acetate, alanine) were found to be associated with increased mineralization capacity, whilst others (e.g., malonate, trehalose, sucrose, glucose, xylose, mannose) resulted in decreased mineralization (Phillips et al., 2012).
A negative correlation in the degradation of PHCs (phenanthrene) and the presence of rhizodeposits (e.g., fumarate, mannitol, trehalose, sucrose, glucose, xylose, mannose, and fructose) in the rhizosphere of Lolium multiflorum has been demonstrated (Thomas and Cébron, 2016). Despite the divergent nature of these results, a vast body of literature confirms the beneficial association of bacteria and their host plants in the remediation PHCs at the level of the rhizosphere (Table 3). Root exudates may enhance microbial PHC metabolism in a number of ways: (i) PHC co-metabolism via plant secreted enzymes; (ii) increasing PHC bioavailability through the production of LMW carboxylates that may enhance PHC desorption and compete for soil adsorption sites (An et al., 2010; Gao et al., 2010), or through production of lipophilic or biosurfactant-like root exudates which may increase PHC solubility (Read et al., 2003); (iii) stimulation of microbial biomass and activity through excretion of labile C and N sources and by increasing nutrient availability due to the action of plant released enzymes (e.g., acid phosphatases) and organic chelators (Rohrbacher and St-Arnaud, 2016).
Bacteria dwelling the internal tissues of plants (roots, stems, leaves) overcome some competition for nutrients and space experienced by rhizosphere bacteria, and are physically protected from unfavorable environmental conditions (Schulz et al., 2006).
Cultivable endophytic bacteria have been isolated from various plants species ranging from herbaceous crop plants such as sugar cane (Loiret et al., 2004), wheat (Larran et al., 2002), maize (Gutierrez-Zamora and Martınez-Romero, 2001), the metal hyperaccumulating alpine pennycress (Thlaspi caerulescens) (Lodewyckx et al., 2002), tall fescue (Malinowski et al., 2000), Arabidopsis seeds (Truyens et al., 2015a,b), different grass species (Dalton et al., 2004; Thijs et al., 2014b), woody tree species such as oak and ash (Weyens et al., 2009a), sycamore (Thijs et al., 2014a), poplar (Porteous Moore et al., 2006; Van der Lelie et al., 2009), Mimosa pudica (Pandey et al., 2005), pine seeds (Cankar et al., 2005), and other forest trees (Pirttilä and Frank, 2011).
Endophytic root colonization follows a general model where initially bacteria move toward the plant roots either passively via soil water fluxes, or actively via specific induction of flagellar activity by plant-released compounds. Subsequently, non-specific adsorption of bacteria to roots occurs, followed by anchoring that result in firm attachment to the root surface. Specific or complex interactions between the bacterium and the host plant, such as the secretion of root exudates, may arise resulting in changes in bacterial gene expression. Microscopic studies using gfp-labeled bacterial strains have illustrated this model in poplar trees (Germaine et al., 2004; Taghavi et al., 2009), and it has been observed that the phyllosphere may be a source of endophytic bacteria (Quadt-Hallmann et al., 1997).
In a pioneering study, it was shown that the enrichment of bacteria with the appropriate catabolic genes in the endophytic root compartment is correlated with the type and amount of contaminant and the genotype of the plant (Siciliano et al., 2001). Since then, a number of reports have confirmed that endophytic bacteria, have a better capacity to enhance PHC phytoremediation than rhizosphere or soil bacteria (Barac et al., 2004; Doty, 2008; Ryan et al., 2008; Weyens et al., 2010; Yousaf et al., 2011). This may be due to the fact that some endophytic bacteria have the potential to mineralize PHCs in trees, herbaceous plants and grasses (Barac et al., 2004; Phillips et al., 2008; Afzal et al., 2011). In a field experiment with four plant species, Achillea millefolium, Solidago canadensis, Trifolium aureum, and Dactylis glomerata, the presence of bacterial endophytes with PHC degradation capacity was observed (Lumactud et al., 2016). With the microbial communities, the class Actinobacteria was identified as the dominant group in three of the plant species examined, with Gammaproteobacteria being more abundant in S. canadensis.
Despite of the selective pressure of PHCs, the plant species remains the key factor shaping endophytic bacterial community structures. Ascertaining the specific interaction between plants and observed microbial phylotypes could generate critical information for the selection of optimized microbiomes with desirable host performance traits such as survival, growth, and fitness (Mueller and Sachs, 2015; Yergeau et al., 2015). Analysis of the microbiomes of two willow cultivars (Salix purpurea cv. Fish Creek, and Salix miyabeana cv. SX67) growing at different PHC concentrations demonstrated that increased concentrations of PHCs favored the abundance of root endophytes belonging to the Proteobacteria, particularly the classes Gammaproteobacteria and Alphaproteobacteria, while the Betaproteobacteria were predominant in the stems (Tardif et al., 2016). The Protoebacteria are a diverse group of organisms that include hydrocarbonoclasts and plant-growth promoting bacterial (PGPB) species (Bruto et al., 2014). It is not unlikely that some intrinsice host plant genotype-microbe signaling can favor the prevalence of these groups (Bulgarelli et al., 2012; Sessitsch et al., 2012).
Another contribution of endophytic bacteria to the overall PHC dissipation refers to their plant growth promoting traits, which facilitate the host’s performance by alleviating the stress encountered upon exposure to PHCs (Afzal et al., 2014). Genome sequence analysis of 56 endophytic/symbiotic Proteobacteria has provided useful insights about the molecular mechanisms that plant growth promoting endophytes exert on their hosts (Bruto et al., 2014). For example among the various direct and indirect mechanisms used by endophytic bacteria to aid their hosts in overcoming the toxic nature of PHCs, ACC – deaminase activity holds a pivotal role (Arshad et al., 2007; Afzal et al., 2013; Khan et al., 2013; Fatima et al., 2015).
With respect to the application of plant growth-promoting and PHC - degrading endophytes, a number of recent studies has identified bacterial isolates that may be useful inoculants to stimulate phytoremediation of PHC contaminated sites (Kukla et al., 2014; Tara et al., 2014; Zhang et al., 2014; Pawlik and Piotrowska-Seget, 2015; Balseiro-Romero et al., 2016).
The use of PHCs has allowed for the development of privileged modern societies, with the associated cost of contaminated soil, seawater, freshwater and groundwater ecosystems. Given this, it is important to continue developing bio- and phyto-remediation approaches to deal with PHCs that are recalcitrant to metabolism because of their physico-chemical characteristics. Understanding plant-associated bacteria (endophytic, phyllospheric, and rhizospheric) and their varied interactions with plants (ranging from parasitism to mutualism) allows for an appreciation of the associations that have evolved between plants and bacteria to overcome constraints commonly found at contaminated sites.
The ability of bacteria to degrade PHCs is attributed to the presence of catabolic genes and enzymes, which allow them to utilize the complex chemicals found in petroleum mixtures for carbon and energy, an ability that can be enhanced by the presence of plants. Similarly, plants can be positively affected, directly or indirectly, by the presence of bacteria able to elicit drastic modifications in the health status of the plant via the synthesis of plant hormones, suppression of ethylene production, and the mobilization of otherwise unavailable nutrients.
While laboratory and field studies have indicated that bio- and phyto-remediation can be good treatment strategies for PHC polluted environments, more information is required to build accurate models for predicting treatment outcomes. Metagenomic, metatranscriptomic, metaproteomic, and metabolomic analyses of complex communities are allowing for a deeper understanding of how microbial communities interact with each other, the environment and the organisms around them (Villas-Boas and Bruheim, 2007; Bell et al., 2014; Kaul et al., 2016). It is easy to envision implementing metagenomic tools in the field of PHC remediation in order to: pre-assess the biodegradative capacity of an environment, monitor in situ biodegradation performance, assist with the selection of inoculants, identify new biodegradative pathways, and eventually to guide efforts in synthetic biology to develop new enzymatic activities (Baek et al., 2007; Yergeau et al., 2012; Uhlik et al., 2013; Dellagnezze et al., 2014; Sierra-Garcia et al., 2014). Having said this, there are still that need to be faced as the technologies mature and, for more information, the reader is referred to the following reviews (Desai et al., 2010; Hazen et al., 2013; Techtmann and Hazen, 2016).
The modern tools of microbial ecology promise to improve our understanding of plant–bacteria synergies and will hopefully lead to better models for designing and deploying effective biological remediation schemes across diverse environmental landscapes.
All authors contributed extensively to the work presented in this review. MD and AF provided substantial knowledge on the mechanisms underlying regulation of alkane degradation, whilst JVH and JV contributed with their profound knowledge concerning petroleum microbiology and the role of plant–microbe interactions during phytoremediation, respectively. WS helped in editing the manuscript. PG coordinated and wrote this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Hasselt University BOF project 06G02 and the Methusalem project 08M03VGRJ.
Abbasnezhad, H., Gray, M., and Foght, J. M. (2011). Influence of adhesion on aerobic biodegradation and bioremediation of liquid hydrocarbons. Appl. Microbiol. Biotechnol. 92, 653–675. doi: 10.1007/s00253-011-3589-4
Abbasnezhad, H., Gray, M. R., and Foght, J. M. (2008). Two different mechanisms for adhesion of Gram-negative bacterium, Pseudomonas fluorescens LP6a, to an oil-water interface. Colloids Surf B-Biointer. 62, 36–41. doi: 10.1016/j.colsurfb.2007.09.023
Abreu, L. D. V., Ettinger, R., and McAlary, T. (2009). Simulated soil vapor intrusion attenuation factors including biodegradation for petroleum hydrocarbons. Ground Water Monitor. Remed. 29, 105–117. doi: 10.1111/j.1745-6592.2008.01219.x
Afzal, M., Khan, Q. M., and Sessitsch, A. (2014). Endophytic bacteria: prospects and applications for the phytoremediation of organic pollutants. Chemosphere 117, 232–242. doi: 10.1016/j.chemosphere.2014.06.078
Afzal, M., Khan, S., Iqbal, S., Mirza, M. S., and Khan, Q. M. (2013). Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int. Biodeterior. Biodegr. 85, 331–336. doi: 10.1016/j.ibiod.2013.08.022
Afzal, M., Yousaf, S., Reichenauer, T. G., Kuffner, M., and Sessitsch, A. (2011). Soil type affects plant colonization, activity and catabolic gene expression of inoculated bacterial strains during phytoremediation of diesel. J. Hazard. Mater. 186, 1568–1575. doi: 10.1016/j.jhazmat.2010.12.040
Afzal, M., Yousaf, S., Reichenauer, T. G., and Sessitsch, A. (2012). The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int. J. Phytoremediation 14, 35–47. doi: 10.1080/15226514.2011.552928
Al-Awadhi, H., Al-Mailem, D., Dashti, N., Hakam, L., Eliyas, M., and Radwan, S. (2012). The abundant occurrence of hydrocarbon-utilizing bacteria in the phyllospheres of cultivated and wild plants in Kuwait. Int. Biodeterior. Biodegr. 73, 73–79. doi: 10.1016/j.ibiod.2012.05.016
Ali, N., Sorkhoh, N., Salamah, S., Eliyas, M., and Radwan, S. (2012). The potential of epiphytic hydrocarbon-utilizing bacteria on legume leaves for attenuation of atmospheric hydrocarbon pollutants. J. Environ. Manag. 93, 113–120. doi: 10.1016/j.jenvman.2011.08.014
Alisi, C., Musella, R., Tasso, F., Ubaldi, C., Manzo, S., Cremisini, C., et al. (2009). Bioremediation of diesel oil in a co-contaminated soil by bioaugmentation with a microbial formula tailored with native strains selected for heavy metals resistance. Sci. Total Environ. 407, 3024–3032. doi: 10.1016/j.scitotenv.2009.01.011
Alkorta, I., and Garbisu, C. (2001). Phytoremediation of organic contaminants in soils. Bioresour. Technol. 79, 273–276. doi: 10.1016/S0960-8524(01)00016-5
Almeida, R., Mucha, A. P., Teixeira, C., Bordalo, A. A., and Almeida, C. M. R. (2013). Biodegradation of petroleum hydrocarbons in estuarine sediments: metal influence. Biodegradation 24, 111–123. doi: 10.1007/s10532-012-9562-9
Amatya, P. L., Hettiaratchi, J. P. A., and Joshi, R. C. (2002). Biotreatment of flare pit waste. J. Can. Pet. Technol. 41, 30–36. doi: 10.2118/02-09-02
An, C. J., Huang, G. H., Yu, H., Wei, J., Chen, W., and Li, G. C. (2010). Effect of short-chain organic acids and pH on the behaviors of pyrene in soil-water system. Chemosphere 81, 1423–1429. doi: 10.1016/j.chemosphere.2010.09.012
Andrade, M. L., Covelo, E. F., Vega, F. A., and Marcet, P. (2004). Effect of the prestige oil spill on salt marsh soils on the coast of Galicia (northwestern Spain). J. Environ. Q. 33, 2103–2110. doi: 10.2134/jeq2004.2103
Andrea Silva-Castro, G., Rodelas, B., Perucha, C., Laguna, J., Gonzalez-Lopez, J., and Calvo, C. (2013). Bioremediation of diesel-polluted soil using biostimulation as post-treatment after oxidation with Fenton-like reagents: assays in a pilot plant. Sci. Total Environ. 445, 347–355. doi: 10.1016/j.scitotenv.2012.12.081
Andria, V., Reichenauer, T. G., and Sessitsch, A. (2009). Expression of alkane monooxygenase (alkB) genes by plant-associated bacteria in the rhizosphere and endosphere of Italian ryegrass (Lolium multiflorum L.) grown in diesel contaminated soil. Environ. Pollut. 157, 3347–3350. doi: 10.1016/j.envpol.2009.08.023
Anokhina, T. O., Kochetkov, V. V., Zelenkova, N. F., Balakshina, V. V., and Boronin, A. M. (2004). Biodegradation of phenanthrene by pseudomonas bacteria bearing rhizospheric plasmids in model plant-microbial associations. Appl. Biochem. Microbiol. 40, 568–572. doi: 10.1023/B:ABIM.0000046992.01220.35
Arshad, M., Saleem, M., and Hussain, S. (2007). Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol. 25, 356–362. doi: 10.1016/j.tibtech.2007.05.005
Arthur, E. L., Rice, P. J., Rice, P. J., Anderson, T. A., Baladi, S. M., Henderson, K. L. D., et al. (2005). Phytoremediation - an overview. Critic. Rev. Plant Sci. 24, 109–122. doi: 10.1080/07352680590952496
Atlas, R. M. (1981). Microbial-degradation of petroleum-hydrocarbons - an environmental perspective. Microbiol. Rev. 45, 180–209.
Atlas, R. M. (1995). Bioremediation of petroleum pollutants. Int. Biodeterior. Biodegr. 35, 317–327. doi: 10.1016/0964-8305(95)00030-9
Atlas, R. M., and Cerniglia, C. E. (1995). Bioremediation of petroleum pollutants - diversity and environmental aspects of hydrocarbon biodegradation. Bioscience 45, 332–338. doi: 10.2307/1312494
Auffret, M., Labbe, D., Thouand, G., Greer, C. W., and Fayolle-Guichard, F. (2009). Degradation of a mixture of hydrocarbons, gasoline, and diesel oil additives by Rhodococcus aetherivorans and Rhodococcus wratislaviensis. Appl. Environ. Microbiol. 75, 7774–7782. doi: 10.1128/AEM.01117-09
Ayotamuno, J. M., Kogbara, R. B., Agele, E. A., and Agoro, O. S. (2010). Composting and phytoremediation treatment of petroleum sludge. Soil Sediment Contamination 19, 686–695. doi: 10.1080/15320383.2010.515627
Azubuike, C. C., Chikere, C. B., and Okpokwasili, G. C. (2016). Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 32, 1–18. doi: 10.1007/s11274-016-2137-x
Baek, K. H., Yoon, B. D., Kim, B. H., Cho, D. H., Lee, I. S., Oh, H. M., et al. (2007). Monitoring of microbial diversity and activity during bioremediation of crude OH-contaminated soil with different treatments. J. Microbiol. Biotechnol. 17, 67–73.
Baik, M. H., Newcomb, M., Friesner, R. A., and Lippard, S. J. (2003). Mechanistic studies on the hydroxylation of methane by methane monooxygenase. Chem. Rev. 103, 2385–2419. doi: 10.1021/cr950244f
Baker, M. D., Wolanin, P. M., and Stock, J. B. (2006a). Signal transduction in bacterial chemotaxis. Bioessays 28, 9–22. doi: 10.1002/bies.20343
Baker, M. D., Wolanin, P. M., and Stock, J. B. (2006b). Systems biology of bacterial chemotaxis. Curr. Opin. Microbiol. 9, 187–192. doi: 10.1016/j.mib.2006.02.007
Balseiro-Romero, M., Gkorezis, P., Kidd, P. S., Vangronsveld, J., and Monterroso, C. (2016). Enhanced degradation of diesel in the rhizosphere of Lupinus luteus after inoculation with diesel-degrading and plant growth-promoting bacterial strains. J. Environ. Q. 45, 924–932. doi: 10.2134/jeq2015.09.0465
Banat, I. M., Franzetti, A., Gandolfi, I., Bestetti, G., Martinotti, M. G., Fracchia, L., et al. (2010). Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 87, 427–444. doi: 10.1007/s00253-010-2589-0
Banat, I. M., Satpute, S. K., Cameotra, S. S., Patil, R., and Nyayanit, N. V. (2014). Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 5:697. doi: 10.3389/fmicb.2014.00697
Barac, T., Taghavi, S., Borremans, B., Provoost, A., Oeyen, L., Colpaert, J. V., et al. (2004). Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat. Biotechnol. 22, 583–588. doi: 10.1038/nbt960
Barac, T., Weyens, N., Oeyen, L., Taghavi, S., van der Lelie, D., Dubin, D., et al. (2009). Field note: hydraulic containment of a btex plume using poplar treeS. Int. J. Phytoremediation 11, 416–424. doi: 10.1080/15226510802655880
Barrutia, O., Garbisu, C., Epelde, L., Sampedro, M. C., Goicolea, M. A., and Becerril, J. M. (2011). Plant tolerance to diesel minimizes its impact on soil microbial characteristics during rhizoremediation of diesel-contaminated soils. Sci. Total Environ. 409, 4087–4093. doi: 10.1016/j.scitotenv.2011.06.025
Basumatary, B., Bordoloi, S., and Sarma, H. P. (2012). Crude oil-contaminated soil phytoremediation by using Cyperus brevifolius (Rottb.) Hassk. Water Air Soil Pollut. 223, 3373–3383. doi: 10.1007/s11270-012-1116-6
Basumatary, B., Saikia, R., Das, H. C., and Bordoloi, S. (2013). Field note: phytoremediation of petroleum sludge contaminated field using sedge species, cyperus rotundus (Linn.) and Cyperus Brevifolius (Rottb.) Hassk. Int. J. Phytoremed. 15, 877–888. doi: 10.1080/15226514.2012.760520
Bell, T. H., Joly, S., Pitre, F. E., and Yergeau, E. (2014). Increasing phytoremediation efficiency and reliability using novel omics approaches. Trends Biotechnol. 32, 271–280. doi: 10.1016/j.tibtech.2014.02.008
Bento, F. M., Camargo, F. A. O., Okeke, B. C., and Frankenberger, W. T. (2005). Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour. Technol. 96, 1049–1055. doi: 10.1016/j.biortech.2004.09.008
Berthe-Corti, L., and Bruns, A. (2001). Composition and activity of marine alkane-degrading bacterial communities in the transition from suboxic to anoxic conditions. Microb. Ecol. 42, 46–55.
Bisht, S., Pandey, P., Sood, A., Sharma, S., and Bisht, N. S. (2010). biodegradation of naphthalene and anthracene by chemo-tactically active rhizobacteria of populus deltoides. Br. J. Microbiol. 41, 922–930. doi: 10.1590/S1517-838220100004000011
Boffetta, P., Jourenkova, N., and Gustavsson, P. (1997). Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 8, 444–472. doi: 10.1023/A:1018465507029
Bordoloi, N. K., and Konwar, B. K. (2009). Bacterial biosurfactant in enhancing solubility and metabolism of petroleum hydrocarbons. J. Hazard. Mater. 170, 495–505. doi: 10.1016/j.jhazmat.2009.04.136
Bosch, R., Garcia-Valdes, E., and Moore, E. R. B. (2000). Complete nucleotide sequence and evolutionary significance of a chromosomally encoded naphthalene-degradation lower pathway from Pseudomonas stutzeri AN10. Gene 245, 65–74. doi: 10.1016/S0378-1119(00)00038-X
Botalova, O., Schwarzbauer, J., Frauenrath, T., and Dsikowitzky, L. (2009). Identification and chemical characterization of specific organic constituents of petrochemical effluents. Water Res. 43, 3797–3812. doi: 10.1016/j.watres.2009.06.006
Bouchez, T., Patureau, D., Dabert, P., Juretschko, S., Dore, J., Delgenes, P., et al. (2000). Ecological study of a bioaugmentation failure. Environ. Microbiol. 2, 179–190. doi: 10.1046/j.1462-2920.2000.00091.x
Bouchez-Naitali, M., Rakatozafy, H., Marchal, R., Leveau, J. Y., and Vandecasteele, J. P. (1999). Diversity of bacterial strains degrading hexadecane in relation to the mode of substrate uptake. J. Appl. Microbiol. 86, 421–428. doi: 10.1046/j.1365-2672.1999.00678.x
Braddock, J. F., Lindstrom, J. E., and Brown, E. J. (1995). Distribution of hydrocarbon-degrading microorganisms in sediments from prince-william-sound, alaska, following the exxon-valdez oil-spill. Mar. Pollut. Bull. 30, 125–132. doi: 10.1016/0025-326X(94)00110-U
Branda, S. S., Vik, A., Friedman, L., and Kolter, R. (2005). Biofilms: the matrix revisited. Trends Microbiol. 13, 20–26. doi: 10.1016/j.tim.2004.11.006
Brassington, K. J., Hough, R. L., Paton, G. I., Semple, K. T., Risdon, G. C., Crossley, J., et al. (2007). Weathered hydrocarbon wastes: a risk management primer. Crit. Rev. Environ. Sci. Technol. 37, 199–232. doi: 10.1080/10643380600819625
Bressler, D. C., and Gray, M. R. (2003). Transport and reaction processes in bioremediation of organic contaminants. 1. Review of bacterial degradation and transport. Int. J. Chem. React. Eng. 1, 1–16. doi: 10.2202/1542-6580.1027
Brezna, B., Khan, A. A., and Cerniglia, C. E. (2003). Molecular characterization of dioxygenases from polycyclic aromatic hydrocarbon-degrading Mycobacterium spp. Fems Microbiol. Lett. 223, 177–183. doi: 10.1016/S0378-1097(03)00328-8
Brown, G. S., Barton, L. L., and Thomson, B. M. (2003). Permanganate oxidation of sorbed polycyclic aromatic hydrocarbons. Waste Manag. 23, 737–740. doi: 10.1016/S0956-053X(02)00119-8
Bruto, M., Prigent-Combaret, C., Muller, D., and Moenne-Loccoz, Y. (2014). Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 4:6261. doi: 10.1038/srep06261
Bulgarelli, D., Rott, M., Schlaeppi, K., van Themaat, E. V. L., Ahmadinejad, N., Assenza, F., et al. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488, 91–95. doi: 10.1038/nature11336
Busscher, H. J., Vandebeltgritter, B., and Vandermei, H. C. (1995). Implications of microbial adhesion to hydrocarbons for evaluating cell-surface hydrophobicity.1. zeta-potentials of hydrocarbon droplets. Colloids Sur. B-Biointer. 5, 111–116. doi: 10.1016/0927-7765(95)01224-7
Calvo, C., Manzanera, M., Silva-Castro, G. A., Uad, I., and Gonzalez-Lopez, J. (2009). Application of bioemulsifiers in soil oil bioremediation processes. Future prospects. Sci. Total Environ. 407, 3634–3640. doi: 10.1016/j.scitotenv.2008.07.008
Cameotra, S. S., and Singh, P. (2009). Synthesis of rhamnolipid biosurfactant and mode of hexadecane uptake by Pseudomonas species. Microb. Cell Fact. 8:16. doi: 10.1186/1475-2859-8-16
Cankar, K., Kraigher, H., Ravnikar, M., and Rupnik, M. (2005). Bacterial endophytes from seeds of Norway spruce (Picea abies L. Karst). FEMS Microbiol. Lett. 244, 341–345. doi: 10.1016/j.femsle.2005.02.008
Canosa, I., Sanchez-Romero, J. M., Yuste, L., and Rojo, F. (2000). A positive feedback mechanism controls expression of AlkS, the transcriptional regulator of the Pseudomonas oleovorans alkane degradation pathway. Mol. Microbiol. 35, 791–799. doi: 10.1046/j.1365-2958.2000.01751.x
Canosa, I., Yuste, L., and Rojo, F. (1999). Role of the alternative sigma factor sigma(S) in expression of the AlkS regulator of the Pseudomonas oleovorans alkane degradation pathway. J. Bacteriol. 181, 1748–1754.
Carmichael, L. M., and Pfaender, F. K. (1997). The effect of inorganic and organic supplements on the microbial degradation of phenanthrene and pyrene in soils. Biodegradation 8, 1–13. doi: 10.1023/A:1008258720649
Cartmill, A. D., Cartmill, D. L., and Alarcon, A. (2014). Controlled release fertilizer increased phytoremediation of petroleum-contaminated sandy soil. Int. J. Phytoremed. 16, 285–301. doi: 10.1080/15226514.2013.773280
Chaineau, C. H., Morel, J. L., and Oudot, J. (2000). Biodegradation of fuel oil hydrocarbons in the rhizosphere of maize. J. Environ. Q. 29, 569–578. doi: 10.2134/jeq2000.00472425002900020027x
Chang, W.-N., Liu, C.-W., and Liu, H.-S. (2009). Hydrophobic cell surface and bioflocculation behavior of Rhodococcus erythropolis. Process. Biochem. 44, 955–962. doi: 10.1016/j.procbio.2009.04.014
Chapman, P. M., and Wang, F. Y. (2001). Assessing sediment contamination in estuaries. Environ. Toxicol. Chem. 20, 3–22. doi: 10.1002/etc.5620200102
Chaudhry, Q., Blom-Zandstra, M., Gupta, S., and Joner, E. J. (2005). Utilising the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ. Sci. Pollut. Res. 12, 34–48. doi: 10.1065/espr2004.08.213
Cherian, S., and Oliveira, M. M. (2005). Transgenic plants in phytoremediation: recent advances and new possibilities. Environ. Sci. Technol. 39, 9377–9390. doi: 10.1021/es051134l
Child, R., Miller, C. D., Liang, Y., Narasimham, G., Chatterton, J., Harrison, P., et al. (2007). Polycyclic aromatic hydrocarbon-degrading Mycobacterium isolates: their association with plant roots. Appl. Microbiol. Biotechnol. 75, 655–663. doi: 10.1007/s00253-007-0840-0
Child, R., Miller, C. D., Liang, Y., Sims, R. C., Anderson, A. J. (2007). Pyrene mineralization by Mycobacterium sp. strain KMS in a Barley rhizosphere. J. Environ. Qual. 36, 1260–1265. doi: 10.2134/jeq2007.0008
Chouychai, W., Thongkukiatkul, A., Upatham, S., Lee, H., Pokethitiyook, P., and Kruatrachue, M. (2009). Plant-enhanced phenanthrene and pyrene biodegradation in acidic soil. J. Environ. Biol. 30, 139–144.
Chouychai, W., Thongkukiatkul, A., Upatham, S., Pokethitiyook, P., Kruatrachue, M., Lee, H. (2012). Effect of corn plant on survival and phenanthrene degradation capacity of Pseudomonas sp. UG14LR in two soils. Int. J. Phytoremediation. 14, 585–595. doi: 10.1080/15226514.2011.587478
Coles, C. A., Patel, T. R., Akinnola, A. P., and Helleur, R. J. (2009). Influence of bulking agents, fertilizers and bacteria on the removal of diesel from a newfoundland soil. Soil Sediment Contam. 18, 383–396. doi: 10.1080/15320380902772687
Colores, G. M., Macur, R. E., Ward, D. M., and Inskeep, W. P. (2000). Molecular analysis of surfactant-driven microbial population shifts in hydrocarbon-contaminated soil. Appl. Environ. Microbiol. 66, 2959–2964. doi: 10.1128/AEM.66.7.2959-2964.2000
Cook, R. L., and Hesterberg, D. (2013). Comparison of trees and grasses for rhizoremediation of petroleum hydrocarbons. Int. J. Phytoremed. 15, 844–860. doi: 10.1080/15226514.2012.760518
Cook, R. L., Landmeyer, J. E., Atkinson, B., Messier, J.-P., and Nichols, E. G. (2010). Field note: successful establishment of a phytoremediation system at a petroleum hydrocarbon contaminated shallow aquifer: trends, trials, and tribulations. Int. J. Phytoremed. 12, 716–732. doi: 10.1080/15226510903390395
Costello, J. (1979). Morbidity and mortality study of shale oil workers in the united-states. Environ. Health Perspect. 30, 205–208. doi: 10.1289/ehp.7930205
Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R., and Lappinscott, H. M. (1995). Microbial biofilms. Annu. Rev. Microbiol. 49, 711–745. doi: 10.1146/annurev.mi.49.100195.003431
Couling, N. R., Towell, M. G., and Semple, K. T. (2010). Biodegradation of PAHs in soil: influence of chemical structure, concentration and multiple amendment. Environ. Pollut. 158, 3411–3420. doi: 10.1016/j.envpol.2010.07.034
Couto, M. N. P. F. S., Monteiro, E., and Vasconcelos, M. T. S. D. (2010). Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: comparison of natural attenuation, biostimulation and bioaugmentation. Environ. Sci. Pollut. Res. 17, 1339–1346. doi: 10.1007/s11356-010-0318-y
Cui, B. X., Zhang, X. X., Han, G., and Li, K. R. (2016). antioxidant defense response and growth reaction of amorpha fruticosa seedlings in petroleum-contaminated soil. Water Air Soil Pollut. 227, 121. doi: 10.1007/s11270-016-2821-3
Cunliffe, M., Kawasaki, A., Fellows, E., and Kertesz, M. A. (2006). Effect of inoculum pretreatment on survival, activity and catabolic gene expression of Sphingobium yanoikuyae B1 in an aged polycyclic aromatic hydrocarbon-contaminated soil. FEMS Microbiol. Ecol. 58, 364–372. doi: 10.1111/j.1574-6941.2006.00167.x
Cunningham, S. D., and Berti, W. R. (1993). Remediation of contaminated soils with green plants - an overview. In Vitro Cell. Dev. Biol. Plant 29P, 207–212. doi: 10.1007/BF02632036
Daane, L. L., Harjono, I., Zylstra, G. J., and Haggblom, M. M. (2001). Isolation and characterization of polycyclic aromatic hydrocarbon-degrading bacteria associated with the rhizosphere of salt marsh plants. Appl. Environ. Microbiol. 67, 2683–2691. doi: 10.1128/AEM.67.6.2683-2691.2001
Daghio, M., Tatangelo, V., Franzetti, A., Gandolfi, I., Papacchini, M., Careghini, A., et al. (2015). Hydrocarbon degrading microbial communities in bench scale aerobic biobarriers for gasoline contaminated groundwater treatment. Chemosphere 130, 34–39. doi: 10.1016/j.chemosphere.2015.02.022
Dalton, D. A., Kramer, S., Azios, N., Fusaro, S., Cahill, E., and Kennedy, C. (2004). Endophytic nitrogen fixation in dune grasses (Ammophila arenaria and Elymus mollis) from Oregon. FEMS Microbiol Ecol. 49, 469–479. doi: 10.1016/j.femsec.2004.04.010
Dandie, C. E., Weber, J., Aleer, S., Adetutu, E. M., Ball, A. S., and Juhasz, A. L. (2010). Assessment of five bioaccessibility assays for predicting the efficacy of petroleum hydrocarbon biodegradation in aged contaminated soils. Chemosphere 81, 1061–1068. doi: 10.1016/j.chemosphere.2010.09.059
Das, N., and Chandran, P. (2010). Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol. Res. Int. 2011:941810. doi: 10.4061/2011/941810
Das, P., Mukherjee, S., and Sen, R. (2008). Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. Chemosphere 72, 1229–1234. doi: 10.1016/j.chemosphere.2008.05.015
Delille, D., Coulon, F., and Pelletier, E. (2004). Effects of temperature warming during a bioremediation study of natural and nutrient-amended hydrocarbon-contaminated sub-Antarctic soils. Cold Reg. Sci. Technol. 40, 61–70. doi: 10.1016/j.coldregions.2004.05.005
Dellagnezze, B. M., de Sousa, G. V., Martins, L. L., Domingos, D. F., Limache, E. E. G., de Vasconcellos, S. P., et al. (2014). Bioremediation potential of microorganisms derived from petroleum reservoirs. Mar. Pollut. Bull. 89, 191–200. doi: 10.1016/j.marpolbul.2014.10.003
Demque, D. E., Biggar, K. W., and Heroux, J. A. (1997). Land treatment of diesel contaminated sand. Can. Geotech. J. 34, 421–431. doi: 10.1139/t97-008
Desai, C., Pathak, H., and Madamwar, D. (2010). Advances in molecular and “-omics” technologies to gauge microbial communities and bioremediation at xenobiotic/anthropogen contaminated sites. Bioresour. Technol. 101, 1558–1569. doi: 10.1016/j.biortech.2009.10.080
Devaull, G. E. (2007). Indoor vapor intrusion with oxygen-limited biodegradation for a subsurface gasoline source. Environ. Sci. Technol. 41, 3241–3248. doi: 10.1021/es060672a
Dinamarca, M. A., Aranda-Olmedo, I., Puyet, A., and Rojo, F. (2003). Expression of the Pseudomonas putida OCT plasmid alkane degradation pathway is modulated by two different global control signals: evidence from continuous cultures. J. Bacteriol. 185, 4772–4778. doi: 10.1128/JB.185.16.4772-4778.2003
Dinamarca, M. A., Ruiz-Manzano, A., and Rojo, F. (2002). Inactivation of cytochrome o ubiquinol oxidase relieves catabolic repression of the Pseudomonas putida GPo1 alkane degradation pathway. J. Bacteriol. 184, 3785–3793. doi: 10.1128/JB.184.14.3785-3793.2002
Ding, G.-C., Heuer, H., Zuehlke, S., Spiteller, M., Pronk, G. J., Heister, K., et al. (2010). Soil Type-dependent responses to phenanthrene as revealed by determining the diversity and abundance of polycyclic aromatic hydrocarbon Ring-Hydroxylating dioxygenase genes by using a novel PCR detection system. Appl. Environ. Microbiol. 76, 4765–4771. doi: 10.1128/AEM.00047-10
D’Ippolito, S., De Castro, R. E., and Herrera Seitz, K. (2011). Chemotactic responses to gas oil of Halomonas spp. strains isolated from saline environments in Argentina. Rev. Arg. Microbiol. 43, 107–110. doi: 10.1590/S0325-75412011000200007
Do, S. H., Jo, J. H., Jo, Y. H., Lee, H. K., and Kong, S. H. (2009). Application of a peroxymonosulfate/cobalt (PMS/Co(II)) system to treat diesel-contaminated soil. Chemosphere 77, 1127–1131. doi: 10.1016/j.chemosphere.2009.08.061
Dobler, L., Vilela, L. F., Almeida, R. V., and Neves, B. C. (2016). Rhamnolipids in perspective: gene regulatory pathways, metabolic engineering, production and technological forecasting. New Biotechnol. 33, 123–135. doi: 10.1016/j.nbt.2015.09.005
Doty, S. L. (2008). Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 179, 318–333. doi: 10.1111/j.1469-8137.2008.02446.x
Doughty, D. M., Halsey, K. H., Vieville, C. J., Sayavedra-Soto, L. A., Arp, D. J., and Bottomley, P. J. (2007). Propionate inactivation of butane monooxygenase activity in ‘Pseudomonas butanovora’: biochemical and physiological implications. Microbiology 153, 3722–3729. doi: 10.1099/mic.0.2007/008441-0
Doughty, D. M., Sayavedra-Soto, L. A., Arp, D. J., and Bottomley, P. J. (2006). Product repression of alkane monooxygenase expression in Pseudomonas butanovora. J. Bacteriol. 188, 2586–2592. doi: 10.1128/JB.188.7.2586-2592.2006
Eaton, R. W., and Chapman, P. J. (1992). Bacterial metabolism of naphthalene - construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J. Bacteriol. 174, 7542–7554.
Eisenbach, M., and Caplan, S. R. (1998). Bacterial chemotaxis: unsolved mystery of the flagellar switch. Curr. Biol. 8, R444–R446. doi: 10.1016/S0960-9822(98)70288-X
El Fantroussi, S., and Agathos, S. N. (2005). Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr. Opin. Microbiol. 8, 268–275. doi: 10.1016/j.mib.2005.04.011
Escalante-Espinosa, E., Gallegos-Martinez, M. E., Favela-Torres, E., and Gutierrez-Rojas, M. (2005). Improvement of the hydrocarbon phytoremediation rate by Cyperus laxus Lam. inoculated with a microbial consortium in a model system. Chemosphere 59, 405–413. doi: 10.1016/j.chemosphere.2004.10.034
Euliss, K., Ho, C.-H., Schwab, A. P., Rock, S., and Banks, A. K. (2008). Greenhouse and field assessment of phytoremediation for petroleum contaminants in a riparian zone. Bioresour. Technol. 99, 1961–1971. doi: 10.1016/j.biortech.2007.03.055
Evans, F. F., Rosado, A. S., Sebastian, G. V., Casella, R., Machado, P., Holmstrom, C., et al. (2004). Impact of oil contamination and biostimulation on the diversity of indigenous bacterial communities in soil microcosms. FEMS Microbiol. Ecol. 49, 295–305. doi: 10.1016/j.femsec.2004.04.007
Fatima, K., Afzal, M., Imran, A., and Khan, Q. M. (2015). Bacterial rhizosphere and endosphere populations associated with grasses and trees to be used for phytoremediation of crude oil contaminated soil. Bull. Environ. Contam. Toxicol. 94, 314–320. doi: 10.1007/s00128-015-1489-5
Feng, L., Wang, W., Cheng, J., Ren, Y., Zhao, G., Gao, C., et al. (2007). Genome and proteome of long-chain alkane degrading Geobacillus thermodenitrificans NG80-2 isolated from a deep-subsurface oil reservoir. Proc. Natl. Acad. Sci. U.S.A. 104, 5602–5607. doi: 10.1073/pnas.0609650104
Fernandez-Luqueno, F., Valenzuela-Encinas, C., Marsch, R., Martinez-Suarez, C., Vazquez-Nunez, E., and Dendooven, L. (2011). Microbial communities to mitigate contamination of PAHs in soil-possibilities and challenges: a review. Environ. Sci. Pollut. Res. 18, 12–30. doi: 10.1007/s11356-010-0371-6
Flemming, H.-C., and Wingender, J. (2010). The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. doi: 10.1038/nrmicro2415
Flocco, C. G., Gomes, N. C. M., Mac Cormack, W., and Smalla, K. (2009). Occurrence and diversity of naphthalene dioxygenase genes in soil microbial communities from the Maritime Antarctic. Environ. Microbiol. 11, 700–714. doi: 10.1111/j.1462-2920.2008.01858.x
Ford, R. M., and Harvey, R. W. (2007). Role of chemotaxis in the transport of bacteria through saturated porous media. Advan. Water Resour. 30, 1608–1617. doi: 10.1021/es5056484
Franzetti, A., Bestetti, G., Caredda, P., La Colla, P., and Tamburini, E. (2008a). Surface-active compounds and their role in the access to hydrocarbons in Gordonia strains. FEMS Microbiol. Ecol. 63, 238–248. doi: 10.1111/j.1574-6941.2007.00406.x
Franzetti, A., Di Gennaro, P., Bestetti, G., Lasagni, A., Pitea, D., and Collina, E. (2008b). Selection of surfactants for enhancing diesel hydrocarbons-contaminated media bioremediation. J. Hazard. Mater. 152, 1309–1316. doi: 10.1016/j.jhazmat.2007.08.005
Franzetti, A., Tamburini, E., and Banat, I. M. (2010). Applications of biological surface active compounds in remediation technologies. Biosurfactants 672, 121–134. doi: 10.1007/978-1-4419-5979-9_9
Funhoff, E. G., Bauer, U., Garcia-Rubio, I., Witholt, B., and van Beilen, J. B. (2006). CYP153A6, a soluble P450 oxygenase catalyzing terminal-alkane hydroxylation. J. Bacteriol. 188, 5220–5227. doi: 10.1128/JB.00286-06
Gallego, J. L. R., Loredo, J., Llamas, J. F., Vazquez, F., and Sanchez, J. (2001). Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation 12, 325–335. doi: 10.1023/A:1014397732435
Gao, Y. Z., Ren, L. L., Ling, W. T., Gong, S. S., Sun, B. Q., and Zhang, Y. (2010). Desorption of phenanthrene and pyrene in soils by root exudates. Bioresour. Technol. 101, 1159–1165. doi: 10.1016/j.biortech.2009.09.062
Garcia-Blanco, S., Venosa, A. D., Suidan, M. T., Lee, K., Cobanli, S., and Haines, J. R. (2007). Biostimulation for the treatment of an oil-contaminated coastal salt marsh. Biodegradation 18, 1–15. doi: 10.1007/s10532-005-9029-3
Gaskin, S., Soole, K., Bentham, R. (2008). Screening of Australian native grasses for rhizoremediation of aliphatic hydrocarbon-contaminated soil. Int. J. Phytoremed. 10, 378–389. doi: 10.1080/15226510802100465
Gentry, T. J., Rensing, C., and Pepper, I. L. (2004). New approaches for bioaugmentation as a remediation technology. Critic. Rev. Environ. Sci. Technol. 34, 447–494. doi: 10.1080/10643380490452362
Gerhardt, K. E., Huang, X. D., Glick, B. R., and Greenberg, B. M. (2009). Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci. 176, 20–30. doi: 10.1016/j.plantsci.2008.09.014
Germaine, K., Keogh, E., Garcia-Cabellos, G., Borremans, B., van der Lelie, D., Barac, T., et al. (2004). Colonisation of poplar trees by gfp expressing bacterial endophytes. FEMS Microbiol. Ecol. 48, 109–118. doi: 10.1016/j.femsec.2003.12.009
Germida, J. J., Frick, C. M., and Farrell, R. E. (2002). “Phytoremediation of oil-contaminated soils,” in Soil Mineral-Organic Matter-Microorganism Interactions and Ecosystem Health. Ecological Significance of the Interactions among Clay Minerals, Organic Matter and Soil Biota, Vol. 28b, eds A. Voilante, P. M. Huang, J. M. Bollag, and L. Gianfreda (Amsterdam: Elsevier), 169–186.
Ghaly, A. E., Yusran, A., and Dave, D. (2013). Effects of Bisotimulation and Bioaugmentation on the degradation of pyrene in soil. J. Bioremed. Biodegradation. 5, 1–13.
Gibson, D. T., and Parales, R. E. (2000). Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr. Opin. Biotechnol. 11, 236–243. doi: 10.1016/S0958-1669(00)00090-2
Glick, B. R. (2010). Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 28, 367–374. doi: 10.1016/j.biotechadv.2010.02.001
Glick, B. R., and Stearns, J. C. (2011). Making phytoremediation work better: maximizing a plant’s growth potential in the midst of adversity. Int. J. Phytoremed. 13, 4–16. doi: 10.1080/15226514.2011.568533
Goi, A., Kulik, N., and Trapido, M. (2006). Combined chemical and biological treatment of oil contaminated soil. Chemosphere 63, 1754–1763. doi: 10.1016/j.chemosphere.2005.09.023
Goldstein, R. M., Mallory, L. M., and Alexander, M. (1985). Reasons for possible failure of inoculation to enhance biodegradation. Appl. Environ. Microbiol. 50, 977–983.
Gorbushina, A. A., and Broughton, W. J. (2009). Microbiology of the atmosphere-rock interface: how biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 63, 431–450. doi: 10.1146/annurev.micro.091208.073349
Gordillo, F., Chavez, F. P., and Jerez, C. A. (2007). Motility and chemotaxis of Pseudomonas sp B4 towards polychlorobiphenyls and chlorobenzoates. FEMS Microbiol. Ecol. 60, 322–328. doi: 10.1111/j.1574-6941.2007.00293.x
Graj, W., Lisiecki, P., Szulc, A., Chrzanowski, L., and Wojtera-Kwiczor, J. (2013). Bioaugmentation with petroleum-degrading consortia has a selective growth-promoting impact on crop plants germinated in diesel oil-contaminated soil. Water Air Soil Pollut. 224:1676. doi: 10.1007/s11270-013-1676-0
Greenwood, P. F., Wibrow, S., George, S. J., and Tibbett, M. (2009). Hydrocarbon biodegradation and soil microbial community response to repeated oil exposure. Organ. Geochem. 40, 293–300. doi: 10.1016/j.orggeochem.2008.12.009
Grimaud, R. (2010). “Biofilm development at interfaces between hydrophobic organic compounds and water,” in Handbook of Hydrocarbon and Lipid Microbiology, eds K. N. Timmis, T. J. McGenity, J. R. van der Meer, and V. de Lorenzo (Berlin: Springer), 1491–1499. doi: 10.1007/978-3-540-77587-4_102
Grimm, A. C., and Harwood, C. S. (1997). Chemotaxis of Pseudomonas spp. to the polyaromatic hydrocarbon naphthalene. Appl. Environ. Microbiol. 63, 4111–4115.
Grimm, A. C., and Harwood, C. S. (1999). NahY, a catabolic plasmid-encoded receptor required for chemotaxis of Pseudomonas putida to the aromatic hydrocarbon naphthalene. J. Bacteriol. 181, 3310–3316.
Grund, E., Denecke, B., and Eichenlaub, R. (1992). Naphthalene degradation via salicylate and gentisate by rhodococcus sp strain b4. Appl. Environ. Microbiol. 58, 1874–1877.
Gunderson, J. J., Knight, J. D., and Van Rees, K. C. J. (2008). Relating hybrid poplar fine root production, soil nutrients, and hydrocarbon contamination. Bioremed. J. 12, 156–167. doi: 10.1080/10889860802261968
Gutierrez-Zamora, M., and Martınez-Romero, E. (2001). Natural endophytic association between Rhizobium etli and maize (Zea mays L.). J. Biotechnol. 91, 117–126. doi: 10.1016/S0168-1656(01)00332-7
Haichar, F. Z., Marol, C., Berge, O., Rangel-Castro, J. I., Prosser, J. I., et al. (2008). Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2, 1221–1230. doi: 10.1038/ismej.2008.80
Hall, J., Soole, K., and Bentham, R. (2011). Hydrocarbon phytoremediation in the family fabacea - review. Int. J. Phytoremed. 13, 317–332. doi: 10.1080/15226514.2010.495143
Hamdi, H., Benzarti, S., Manusadzianas, L., Aoyama, I., and Jedidi, N. (2007a). Bioaugmentation and blostimulation effects on PAH dissipation and soil ecotoxicity under controlled conditions. Soil Biol. Biochem. 39, 1926–1935. doi: 10.1016/j.soilbio.2007.02.008
Hamdi, H., Benzarti, S., Manusadzianas, L., Aoyama, I., and Jedidi, N. (2007b). Solid-phase bioassays and soil microbial activities to evaluate PAH-spiked soil ecotoxicity after a long-term bioremediation process simulating landfarming. Chemosphere 70, 135–143. doi: 10.1016/j.chemosphere.2007.06.043
Hanzel, J., Harms, H., and Wick, L. Y. (2010). Bacterial chemotaxis along vapor-phase gradients of naphthalene. Environ. Sci. Technol. 44, 9304–9310. doi: 10.1021/es100776h
Hardoim, P. R., van Overbeek, L. S., and van Elsas, J. D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 16, 463–471. doi: 10.1016/j.tim.2008.07.008
Harmsen, J. (2007). Measuring bioavailability: from a scientific approach to standard methods. J. Environ. Q. 36, 1420–1428. doi: 10.2134/jeq2006.0492
Hartmann, A., Schmid, M., van Tuinen, D., and Berg, G. (2009). Plant-driven selection of microbes. Plant Soil 321, 235–257. doi: 10.1007/s11104-008-9814-y
Hazelbauer, G. L., and Lai, W.-C. (2010). Bacterial chemoreceptors: providing enhanced features to two-component signaling. Curr. Opin. Microbiol. 13, 124–132. doi: 10.1016/j.mib.2009.12.014
Hazen, T. C. (2010). “Biostimulation,” in Handbook of Hydrocarbon and Lipid Microbiology, eds T. J. McGenity, K. N. Timmis, and B. Nogales (Berlin: Springer), 4518–4527.
Hazen, T. C., Dubinsky, E. A., DeSantis, T. Z., Andersen, G. L., Piceno, Y. M., Singh, N., et al. (2010). Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330, 204–208. doi: 10.1126/science.1195979
Hazen, T. C., Rocha, A. M., and Techtmann, S. M. (2013). Advances in monitoring environmental microbes. Curr. Opin. Biotechnol. 24, 526–533. doi: 10.1016/j.copbio.2012.10.020
Hermansson, M. (1999). The DLVO theory in microbial adhesion. Colloids Sur. B-Biointer. 14, 105–119. doi: 10.1016/S0927-7765(99)00029-6
Hernandez-Arranz, S., Moreno, R., and Rojo, F. (2013). The translational repressor Crc controls the Pseudomonas putida benzoate and alkane catabolic pathways using a multi-tier regulation strategy. Environ. Microbiol. 15, 227–241. doi: 10.1111/j.1462-2920.2012.02863.x
Hino, S., Watanabe, K., and Takahashi, N. (1997). Isolation and characterization of slime-producing bacteria capable of utilizing petroleum hydrocarbons as a sole carbon source. J. Fermen. Bioeng. 84, 528–531. doi: 10.1016/S0922-338X(97)81906-X
Hong, S. H., Ryu, H., Kim, J., and Cho, K. S. (2011). Rhizoremediation of diesel-contaminated soil using the plant growth-promoting rhizobacterium Gordonia sp. S2RP-17. Biodegradation 22, 593–601. doi: 10.1007/s10532-010-9432-2
Horel, A., and Schiewer, S. (2009). Investigation of the physical and chemical parameters affecting biodegradation of diesel and synthetic diesel fuel contaminating Alaskan soils. Cold Reg. Sci. Technol. 58, 113–119. doi: 10.1016/j.coldregions.2009.04.004
Hosokawa, R., Nagai, M., Morikawa, M., and Okuyama, H. (2009). Autochthonous bioaugmentation and its possible application to oil spills. World J. Microbiol. Biotechnol. 25, 1519–1528. doi: 10.1007/s11274-009-0044-0
Hua, S. F., Song, Y. Y., Xia, C. G., and Li, S. B. (2011). Sequencing analysis of 16S rDNA and soluble methane monooxygenase genes from a methanotroph Methylosinus trichosporium IMV 3011. Annal. Microbiol. 61, 391–396. doi: 10.1007/s13213-010-0143-z
Huang, X. D., El-Alawi, Y., Penrose, D. M., Glick, B. R., and Greenberg, B. M. (2004). A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ. Pollut. 130, 465–476. doi: 10.1016/j.envpol.2003.09.031
Huesemann, M. H., Hausmann, T. S., and Fortman, T. J. (2004). Does bioavailability limit biodegradation? A comparison of hydrocarbon biodegradation and desorption rates in aged soils. Biodegradation 15, 261–274. doi: 10.1023/B:BIOD.0000042996.03551.f4
Hutcheson, M. S., Pedersen, D., Anastas, N. D., Fitzgerald, J., and Silverman, D. (1996). Beyond TPH: health-based evaluation of petroleum hydrocarbon exposures. Regul. Toxicol. Pharmacol. 24, 85–101. doi: 10.1006/rtph.1996.0066
Iwabuchi, T., and Harayama, S. (1997). Biochemical and genetic characterization of 2-carboxybenzaldehyde dehydrogenase, an enzyme involved in phenanthrene degradation by Nocardioides sp. strain KP7. J. Bacteriol. 179, 6488–6494.
Iwaki, H., Muraki, T., Ishihara, S., Hasegawa, Y., Rankin, K. N., Sulea, T., et al. (2007). Characterization of a pseudomonad 2-nitrobenzoate nitroreductase and its catabolic pathway-associated 2-hydroxylaminobenzoate mutase and a chemoreceptor involved in 2-nitrobenzoate chemotaxis. J. Bacteriol. 189, 3502–3514. doi: 10.1128/JB.01098-06
Iwamoto, T., Tani, K., Nakamura, K., Suzuki, Y., Kitagawa, M., Eguchi, M., et al. (2000). Monitoring impact of in situ biostimulation treatment on groundwater bacterial community by DGGE. FEMS Microbiol. Ecol. 32, 129–141. doi: 10.1111/j.1574-6941.2000.tb00707.x
Jagtap, S. S., Woo, S. M., Kim, T. S., Dhiman, S. S., Kim, D., and Lee, J. K. (2014). Phytoremediation of diesel-contaminated soil and saccharification of the resulting biomass. Fuel 116, 292–298. doi: 10.1016/j.fuel.2013.08.017
Jeong, H.-H., Lee, S.-H., Kim, J.-M., Kim, H.-E., Kim, Y.-G., Yoo, J. Y., et al. (2010). Microfluidic monitoring of Pseudomonas aeruginosa chemotaxis under the continuous chemical gradient. Biosens. Bioelectron. 26, 351–356. doi: 10.1016/j.bios.2010.08.006
Jiang, H., Chen, Y., Murrell, J. C., Jiang, P., Zhang, C., Xing, X. H., et al. (2011). “Methanotrophs: multifunctional bacteria with promising applications in environmental bioengineering,” in Comprehensive Biotechnology: Environmental Biotechnology and Safety, 2nd Edn, Vol. 6, (Amsterdam: Elsevier), 249–262.
Johnsen, A. R., and Karlson, U. (2004). Evaluation of bacterial strategies to promote the bioavailability of polycyclic aromatic hydrocarbons. Appl. Microbiol. Biotechnol. 63, 452–459. doi: 10.1007/s00253-003-1265-z
Johnson, D. L., Maguire, K. L., Anderson, D. R., and McGrath, S. P. (2004). Enhanced dissipation of chrysene in planted soil: the impact of a rhizobial inoculum. Soil Biol. Biochem. 36, 33–38. doi: 10.1016/j.soilbio.2003.07.004
Joner, E. J., Hirmann, D., Szolar, O. H., Todorovic, D., Leyval, C., and Loibner, A. P. (2004). Priming effects on PAH degradation and ecotoxicity during a phytoremediation experiment. Environ. Pollut. 128, 429–435. doi: 10.1016/j.envpol.2003.09.005
Jordahl, J. L., Foster, L., Schnoor, J. L., and Alvarez, P. J. J. (1997). Effect of hybrid poplar trees on microbial populations important to hazardous waste bioremediation. Environ. Toxicol. Chem. 16, 1318–1321. doi: 10.1002/etc.5620160630
Jorgensen, K. S. (2007). In situ bioremediation. Adv. Appl. Microbiol. 61, 285–305. doi: 10.1016/S0065-2164(06)61008-3
Jouanneau, Y., Martin, F., Krivobok, S., and Willison, J. (2011). “Ringhydroxylating dioxygenases involved in PAH biodegradation: structure, function and biodiversity,” in Microbial Bioremediation of Non Metals: Current Research, ed. A.-I. Koukkou (Norflok: Caister Academic Press), 149–175.
Jurelevicius, D., Alvarez, V. M., Peixoto, R., Rosado, A. S., and Seldin, L. (2012). Bacterial polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenases (PAH-RHD) encoding genes in different soils from King George Bay, Antarctic Peninsula. Appl. Soil Ecol. 55, 1–9. doi: 10.1016/j.apsoil.2011.12.008
Kabra, A. N., Khandare, R. V., Waghmode, T. R., and Govindwar, S. P. (2012). Phytoremediation of textile effluent and mixture of structurally different dyes by Glandularia pulchella (Sweet) Tronc. Chemosphere 87, 265–272. doi: 10.1016/j.chemosphere.2011.12.052
Kaimi, E., Mukaidani, T., and Tamaki, M. (2007). Screening of twelve plant species for phytoremediation of petroleum hydrocarbon-contaminated soil. Plant Prod. Sci. 10, 211–218. doi: 10.1626/pps.10.211
Kamath, R., Rentz, J. A., Schnoor, J. L., and Alvarez, P. J. J. (2004). Phytoremediation of hydrocarbon-contaminated soils: principles and applications. Pet. Biotechnol. Dev. Perspect. 151, 447–478.
Kaul, S., Sharma, T., and Dhar, M. K. (2016). “Omics” tools for better understanding the plant-endophyte interactions. Front. Plant Sci. 7:955. doi: 10.3389/fpls.2016.00955
Kauppi, S., Sinkkonen, A., and Romantschuk, M. (2011). Enhancing bioremediation of diesel-fuel-contaminated soil in a boreal climate: comparison of biostimulation and bioaugmentation. Int. Biodeterior. Biodegr. 65, 359–368. doi: 10.1016/j.ibiod.2010.10.011
Khan, A. A., Wang, R. F., Cao, W. W., Doerge, D. R., Wennerstrom, D., and Cerniglia, C. E. (2001). Molecular cloning, nucleotide sequence, and expression of genes encoding a polcyclic aromatic ring dioxygenase from Mycobacterium sp strain PYR-1. Appl. Environ. Microbiol. 67, 3577–3585. doi: 10.1128/AEM.67.8.3577-3585.2001
Khan, F. I., and Husain, T. (2003). Evaluation of a petroleum hydrocarbon contaminated site for natural attenuation using ‘RBMNA’ methodology. Environ. Modell. Softw. 18, 179–194. doi: 10.1016/S1364-8152(02)00034-8
Khan, F. I., Husain, T., and Hejazi, R. (2004). An overview and analysis of site remediation technologies. J. Environ. Manag. 71, 95–122. doi: 10.1016/j.jenvman.2004.02.003
Khan, S., Afzal, M., Iqbal, S., and Khan, Q. M. (2013). Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90, 1317–1332. doi: 10.1016/j.chemosphere.2012.09.045
Kotani, T., Yamamoto, T., Yurimoto, H., Sakai, Y., and Kato, N. (2003). Propane monooxygenase and NAD(+)-dependent secondary alcohol dehydrogenase in propane metabolism by Gordonia sp strain TY-5. J. Bacteriol. 185, 7120–7128. doi: 10.1128/JB.185.24.7120-7128.2003
Krell, T., Lacal, J., Munoz-Martinez, F., Antonio Reyes-Darias, J., Hilal Cadirci, B., Garcia-Fontana, C., et al. (2011). Diversity at its best: bacterial taxis. Environ. Microbiol. 13, 1115–1124. doi: 10.1111/j.1462-2920.2010.02383.x
Kubota, M., Nodate, M., Yasumoto-Hirose, M., Uchiyama, T., Kagami, O., Shizuri, Y., et al. (2005). Isolation and functional analysis of cytochrome p450 CYP153A genes from various environments. Biosci. Biotechnol. Biochem. 69, 2421–2430. doi: 10.1271/bbb.69.2421
Kuiper, I., Bloemberg, G. V., and Lugtenberg, B. J. (2001). Selection of a plant-bacterium pair as a novel tool for rhizostimulation of polycyclic aromatic hydrocarbon-degrading bacteria. Mol. Plant-Microbe Interact. 14, 1197–1205. doi: 10.1094/MPMI.2001.14.10.1197
Kuiper, I., Lagendijk, E. L., Bloemberg, G. V., and Lugtenberg, B. J. J. (2004). Rhizoremediation: a beneficial plant-microbe interaction. Mol. Plant-Microbe Interact. 17, 6–15. doi: 10.1094/MPMI.2004.17.1.6
Kukla, M., Plociniczak, T., and Piotrowska-Seget, Z. (2014). Diversity of endophytic bacteria in Lolium perenne and their potential to degrade petroleum hydrocarbons and promote plant growth. Chemosphere 117, 40–46. doi: 10.1016/j.chemosphere.2014.05.055
Kulakov, L. A., Allen, C. C. R., Lipscomb, D. A., and Larkin, M. J. (2000). Cloning and characterization of a novel cis-naphthalene dihydrodiol dehydrogenase gene (narB) from Rhodococcus sp NCIMB12038. FEMS Microbiol. Lett. 182, 327–331. doi: 10.1111/j.1574-6968.2000.tb08916.x
Kurth, E. G., Doughty, D. M., Bottomley, P. J., Arpi, D. J., and Sayavedra-Sotol, L. A. (2008). Involvement of BmoR and BmoG in n-alkane metabolism in ‘Pseudomonas butanovora’. Microbiology 154, 139–147. doi: 10.1099/mic.0.2007/012724-0
Lacal, J., Munoz-Martinez, F., Reyes-Darias, J.-A., Duque, E., Matilla, M., Segura, A., et al. (2011). Bacterial chemotaxis towards aromatic hydrocarbons in Pseudomonas. Environ. Microbiol. 13, 1733–1744. doi: 10.1111/j.1462-2920.2011.02493.x
Ladino-Orjuela, G., Gomes, E., Silva, R., Salt, C., and Parsons, J. R. (2016). “Metabolic pathways for degradation of aromatic hydrocarbons by bacteria,” in Reviews of Environmental Contamination and Toxicology, Vol. 237, ed. W. P. de Vooge (Cham: Springer), 105–121.
Lai, C.-C., Huang, Y.-C., Wei, Y.-H., and Chang, J.-S. (2009). Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 167, 609–614. doi: 10.1016/j.jhazmat.2009.01.017
Lanfranconi, M. P., Alvarex, H. M., and Studdert, C. A. (2003). A strain isolated from gas oil-contaminated soil displays chemotaxis towards gas oil and hexadecane. Environ. Microbiol. 5, 1002–1008. doi: 10.1046/j.1462-2920.2003.00507.x
Larkin, M. J., Allen, C. C. R., Kulakov, L. A., and Lipscomb, D. A. (1999). Purification and characterization of a novel naphthalene dioxygenase from Rhodococcus sp strain NCIMB12038. J. Bacteriol. 181, 6200–6204.
Larran, S., Perello, A., Simon, M., and Moreno, V. (2002). Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J. Microbiol. Biotechnol. 18, 683–686. doi: 10.1023/A:1016857917950
Law, A. M. J., and Aitken, M. D. (2003). Bacterial chemotaxis to naphthalene desorbing from a nonaqueous liquid. Appl. Environ. Microbiol. 69, 5968–5973. doi: 10.1128/AEM.69.10.5968-5973.2003
Lawniczak, L., Marecik, R., and Chrzanowski, L. (2013). Contributions of biosurfactants to natural or induced bioremediation. Appl. Microbiol. Biotechnol. 97, 2327–2339. doi: 10.1007/s00253-013-4740-1
Lee, H. J., Kim, J. M., Lee, S. H., Park, M., Lee, K., Madsen, E. L., et al. (2011). Gentisate 1,2-dioxygenase, in the third naphthalene catabolic gene cluster of Polaromonas naphthalenivorans CJ2, has a role in naphthalene degradation. Microbiology 157, 2891–2903. doi: 10.1099/mic.0.049387-0
Liang, J.-L., JiangYang, J.-H., Nie, Y., and Wu, X.-L. (2016). Regulation of the alkane hydroxylase CYP153 gene in a gram-positive alkane-degrading bacterium, Dietzia sp Strain DQ12-45-1b. Appl. Environ. Microbiol. 82, 608–619. doi: 10.1128/AEM.02811-15
Lieberman, R. L., Shrestha, D. B., Doan, P. E., Hoffman, B. M., Stemmler, T. L., and Rosenzweig, A. C. (2003). Purified particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a dimer with both mononuclear copper and a copper-containing cluster. Proc. Natl. Acad. Sci. U.S.A. 100, 3820–3825. doi: 10.1073/pnas.0536703100
Lim, M. W., Von Lau, E., and Poh, P. E. (2016). A comprehensive guide of remediation technologies for oil contaminated soil - present works and future directions. Mar. Pollut. Bull. 109, 14–45. doi: 10.1016/j.marpolbul.2016.04.023
Liste, H. H., and Alexander, M. (2000). Plant-promoted pyrene degradation in soil. Chemosphere 40, 7–10. doi: 10.1016/S0045-6535(99)00216-7
Liste, H. H., and Alexander, M. (2002). Butanol extraction to predict bioavailability of PAHs in soil. Chemosphere 46, 1011–1017. doi: 10.1016/S0045-6535(01)00165-5
Liu, C., Wang, W., Wu, Y., Zhou, Z., Lai, Q., and Shao, Z. (2011). Multiple alkane hydroxylase systems in a marine alkane degrader, Alcanivorax dieselolei B-5. Environ. Microbiol. 13, 1168–1178. doi: 10.1111/j.1462-2920.2010.02416.x
Liu, P.-W. G., Whang, L.-M., Yang, M.-C., and Cheng, S.-S. (2008). Biodegradation of diesel-contaminated soil: a soil column study. J. Chin. Inst. Chem. Eng. 39, 419–428. doi: 10.1016/j.jcice.2008.03.006
Lo Piccolo, L., De Pasquale, C., Fodale, R., Puglia, A. M., and Quatrini, P. (2011). Involvement of an alkane hydroxylase system of Gordonia sp strain SoCg in degradation of Solid n-Alkanes. Appl. Environ. Microbiol. 77, 1204–1213. doi: 10.1128/AEM.02180-10
Locksley, R. M. (2010). Asthma and allergic inflammation. Cell 140, 777–783. doi: 10.1016/j.cell.2010.03.004
Lodewyckx, C., Mergeay, M., Vangronsveld, J., Clijsters, H., and Van Der Lelie, D. (2002). Isolation, Characterization, and identification of bacteria associated with the zinc hyperaccumulator Thlaspi caerulescens subsp, Calaminaria. Int. J. Phytoremed. 4, 101–115. doi: 10.1080/15226510208500076
Loiret, F., Ortega, E., Kleiner, D., Ortega-Rodés, P., Rodes, R., and Dong, Z. (2004). A putative new endophytic nitrogen-fixing bacterium Pantoea sp. from sugarcane. J. Appl. Microbiol. 97, 504–511. doi: 10.1111/j.1365-2672.2004.02329.x
Lu, S., Teng, Y., Wang, J., and Sun, Z. (2010). Enhancement of pyrene removed from contaminated soils by Bidens maximowicziana. Chemosphere 81, 645–650. doi: 10.1016/j.chemosphere.2010.08.022
Lumactud, R., Shen, S. Y., Lau, M., and Fulthorpe, R. (2016). Bacterial endophytes isolated from plants in natural oil seep soils with chronic hydrocarbon contamination. Front. Microbiol. 7:755. doi: 10.3389/fmicb.2016.00755
Lundegard, P. D., Johnson, P. C., and Dahlen, P. (2008). Oxygen transport from the atmosphere to soil gas beneath a slab-on-grade foundation overlying petroleum-impacted soil. Environ. Sci. Technol. 42, 5534–5540. doi: 10.1021/es070607g
Ma, Y., and Herson, D. S. (2000). The catechol 2,3-dioxygenase gene and toluene monooxygenase genes from Burkholderia sp AA1, an isolate capable of degrading aliphatic hydrocarbons and toluene. J. Ind. Microbiol. Biotechnol. 25, 127–131. doi: 10.1038/sj.jim.7000042
Maier, T., Forster, H. H., Asperger, O., and Hahn, U. (2001). Molecular characterization of the 56-kDa CYP153 from Acinetobacter sp EB104. Biochem. Biophys. Res. Commun. 286, 652–658. doi: 10.1006/bbrc.2001.5449
Malinowski, D. P., Alloush, G. A., and Belesky, D. P. (2000). Leaf endophyte Neotyphodium coenophialum modifies mineral uptake in tall fescue. Plant Soil 227, 115–126. doi: 10.1023/A:1026518828237
Margesin, R., Haemmerle, M., and Tscherko, D. (2007). Microbial activity and community composition during bioremediation of diesel-oil-contaminated soil: effects of hydrocarbon concentration, fertilizers, and incubation time. Microb. Ecol. 53, 259–269. doi: 10.1007/s00248-006-9136-7
Margesin, R., Labbe, D., Schinner, F., Greer, C. W., and Whyte, L. G. (2003). Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine alpine soils. Appl. Environ. Microbiol. 69, 3085–3092. doi: 10.1128/AEM.69.6.3085-3092.2003
Margesin, R., and Schinner, F. (2001). Bioremediation (natural attenuation and biostimulation) of diesel-oil-contaminated soil in an alpine glacier skiing area. Appl. Environ. Microbiol. 67, 3127–3133. doi: 10.1128/AEM.67.7.3127-3133.2001
Marin, M. M., Yuste, L., and Rojo, F. (2003). Differential expression of the components of the two alkane hydroxylases from Pseudomonas aeruginosa. J. Bacteriol. 185, 3232–3237. doi: 10.1128/JB.185.10.3232-3237.2003
Marquez-Rocha, F. J., Hernandez-Rodri, V., and Lamela, M. T. (2001). Biodegradation of diesel oil in soil by a microbial consortium. Water Air Soil Pollut. 128, 313–320. doi: 10.1023/A:1010392821353
Martinez-Checa, F., Toledo, F. L., El Mabrouki, K., Quesada, E., and Calvo, C. (2007). Characteristics of bioemulsifier V2-7 synthesized in culture media added of hydrocarbons: chemical composition, emulsifying activity and rheological properties. Bioresour. Technol. 98, 3130–3135. doi: 10.1016/j.biortech.2006.10.026
Marx, R. B., and Aitken, M. D. (2000). Bacterial chemotaxis enhances naphthalene degradation in a heterogeneous aqueous system. Environ. Sci. Technol. 34, 3379–3383. doi: 10.1021/es000904k
Mattes, T. E., Alexander, A. K., Richardson, P. M., Munk, A. C., Han, C. S., Stothard, P., et al. (2008). The genome of Polaromonas sp strain JS666: insights into the evolution of a hydrocarbon- and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Appl. Environ. Microbiol. 74, 6405–6416. doi: 10.1128/AEM.00197-08
Menendez-Vega, D., Gallego, J. L. R., Isabel Pelaez, A., de Cordoba, G. F., Moreno, J., Munoz, D., et al. (2007). Engineered in situ bioremediation of soil and groundwater polluted with weathered hydrocarbons. Eur. J. Soil Biol. 43, 310–321. doi: 10.1016/j.ejsobi.2007.03.005
Meng, L., Qiao, M., and Arp, H. P. H. (2011). Phytoremediation efficiency of a PAH-contaminated industrial soil using ryegrass, white clover, and celery as mono- and mixed cultures. J. Soils Sediments 11, 482–490. doi: 10.1007/s11368-010-0319-y
Meudec, A., Poupart, N., Dussauze, J., and Deslandes, E. (2007). Relationship between heavy fuel oil phytotoxicity and polycyclic aromatic hydrocarbon contamination in Salicornia fragilis. Sci. Total Environ. 381, 146–156. doi: 10.1016/j.scitotenv.2007.04.005
Mohanty, S., and Mukherji, S. (2012). Alteration in cell surface properties of Burkholderia spp. during surfactant-aided biodegradation of petroleum hydrocarbons. Appl. Microbiol. Biotechnol. 94, 193–204. doi: 10.1007/s00253-011-3703-7
Mohanty, S., and Mukherji, S. (2013). Surfactant aided biodegradation of NAPLs by Burkholderia multivorans: comparison between Triton X-100 and rhamnolipid JBR-515. Colloids Sur. B-Biointer. 102, 644–652. doi: 10.1016/j.colsurfb.2012.08.064
Molina-Barahona, L., Rodriguez-Vazquez, R., Hernandez-Velasco, M., Vega-Jarquin, C., Zapata-Perez, O., Mendoza-Cantu, A., et al. (2004). Diesel removal from contaminated soils by biostimulation and supplementation with crop residues. Appl. Soil Ecol. 27, 165–175. doi: 10.1016/j.apsoil.2004.04.002
Moliterni, E., Rodriguez, L., Fernandez, F. J., and Villasenor, J. (2012). Feasibility of different bioremediation strategies for treatment of clayey and silty soils recently polluted with diesel hydrocarbons. Water Air Soil Pollut. 223, 2473–2482. doi: 10.1007/s11270-011-1040-1
Moreno, R., Hernandez-Arranz, S., La Rosa, R., Yuste, L., Madhushani, A., Shingler, V., et al. (2015). The Crc and Hfq proteins of Pseudomonas putida cooperate in catabolite repression and formation of ribonucleic acid complexes with specific target motifs. Environ. Microbiol. 17, 105–118. doi: 10.1111/1462-2920.12499
Moreno, R., Ruiz-Manzano, A., Yuste, L., and Rojo, F. (2007). The Pseudomonas putida Crc global regulator is an RNA binding protein that inhibits translation of the AlkS transcriptional regulator. Mol. Microbiol. 64, 665–675. doi: 10.1111/j.1365-2958.2007.05685.x
Mrozik, A., and Piotrowska-Seget, Z. (2010). Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 165, 363–375. doi: 10.1016/j.micres.2009.08.001
Mueller, U. G., and Sachs, J. L. (2015). Engineering microbiomes to improve plant and animal health. Trends Microbiol. 23, 606–617. doi: 10.1016/j.tim.2015.07.009
Muller, T., and Ruppel, S. (2014). Progress in cultivation-independent phyllosphere microbiology. FEMS Microbiol. Ecol. 87, 2–17. doi: 10.1111/1574-6941.12198
Mulligan, C. N. (2009). Recent advances in the environmental applications of biosurfactants. Curr. Opin. Colloid Inter. Sci. 14, 372–378. doi: 10.1016/j.cocis.2009.06.005
Muratova, A. Y., Bondarenkova, A. D., Panchenko, L. V., and Turkovskaya, O. V. (2010). Use of integrated phytoremediation for cleaning-up of oil-sludge-contaminated Soil. Appl. Biochem. Microbiol. 46, 789–794. doi: 10.1134/S0003683810080090
Muratova, A. Y., Dmitrieva, T. V., Panchenko, L. V., and Turkovskaya, O. V. (2008). Phytoremediation of oil-sludge-contaminated soil. Int. J. Phytoremed. 10, 486–502. doi: 10.1080/15226510802114920
Muratova, A. Y., Golubev, S. N., Merbach, W., Turkovskaya, O. V. (2009). Biochemical and physiological peculiarities of the interactions between Sinorhizobium meliloti and Sorghum bicolor in the presence of phenanthrene. Microbiology 78, 308–314. doi: 10.1134/S0026261709030084
Nardeli, S. M., Saad, C. F., Rossetto, P. D., Caetano, V. S., Ribeiro-Alves, M., Paes, J. E. S., et al. (2016). Transcriptional responses of Arabidopsis thaliana to oil contamination. Environ. Exp. Bot. 127, 63–72. doi: 10.1016/j.envexpbot.2016.03.007
Neu, T. R. (1996). Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol. Rev. 60, 151–166.
Newman, L. A., and Reynolds, C. M. (2004). Phytodegradation of organic compounds. Curr. Opin. Biotechnol. 15, 225–230. doi: 10.1016/j.copbio.2004.04.006
Nguyen, T. T., and Sabatini, D. A. (2011). Characterization and emulsification properties of rhamnolipid and sophorolipid biosurfactants and their applications. Int. J. Mol. Sci. 12, 1232–1244. doi: 10.3390/ijms12021232
Nikolopoulou, M., and Kalogerakis, N. (2009). Biostimulation strategies for fresh and chronically polluted marine environments with petroleum hydrocarbons. J. Chem. Technol. Biotechnol. 84, 802–807. doi: 10.1002/jctb.2182
Nisenbaum, M., Hernan Sendra, G., Cerda Gilbert, G. A., Scagliola, M., Froilan Gonzalez, J., and Elena Murialdo, S. (2013). Hydrocarbon biodegradation and dynamic laser speckle for detecting chemotactic responses at low bacterial concentration. J. Environ. Sci. China 25, 613–625. doi: 10.1016/S1001-0742(12)60020-5
Oliveira, V., Gomes, N. C. M., Almeida, A., Silva, A. M. S., Silva, H., and Cunha, A. (2015). Microbe-assisted phytoremediation of hydrocarbons in estuarine environments. Microb. Ecol. 69, 1–12. doi: 10.1007/s00248-014-0455-9
Oliveira, V., Gomes, N. C. M., Cleary, D. F. R., Almeida, A., Silva, A. M. S., Simoes, M. M. Q., et al. (2014). Halophyte plant colonization as a driver of the composition of bacterial communities in salt marshes chronically exposed to oil hydrocarbons. FEMS Microbiol. Ecol. 90, 647–662. doi: 10.1111/1574-6941.12425
Olson, M. S., Ford, R. M., Smith, J. A., and Fernandev, E. J. (2004). Ouantification of bacterial chemotaxis in porous media using magnetic resonance imaging. Environ. Sci. Technol. 38, 3864–3870. doi: 10.1021/es035236s
Olson, P. E., Reardon, K. F., and Pilon-Smits, E. A. H. (2003). “Ecology of rhizosphere bioremediation,” in Phytoremediation: Transformation and Control of Contaminants, eds S. C. McCutcheon and J. L. Schnoor (Hoboken, NJ: Wiley), 317–353.
Ortega-Calvo, J. J., Marchenko, A. I., Vorobyov, A. V., and Borovick, R. V. (2003). Chemotaxis in polycyclic aromatic hydrocarbon-degrading bacteria isolated from coal-tar- and oil-polluted rhizospheres. FEMS Microbiol. Ecol. 44, 373–381. doi: 10.1016/S0168-6496(03)00092-8
Pacwa-Plociniczak, M., Plaza, G. A., Piotrowska-Seget, Z., and Cameotra, S. S. (2011). Environmental applications of biosurfactants: recent advances. Int. J. Mol. Sci. 12, 633–654. doi: 10.3390/ijms12010633
Paliwal, V., Puranik, S., and Purohit, H. J. (2012). Integrated perspective for effective bioremediation. Appl. Biochem. Biotechnol. 166, 903–924. doi: 10.1007/s12010-011-9479-5
Palmroth, M. R. T., Pichtel, J., and Puhakka, J. A. (2002). Phytoremediation of subarctic soil contaminated with diesel fuel. Bior. Technol. 84, 221–228. doi: 10.1016/S0960-8524(02)00055-X
Pandey, G., Chauhan, A., Samanta, S. K., and Jain, R. K. (2002). Chemotaxis of a ralstonia sp SJ98 toward co-metabolizable nitroaromatic compounds. Biochem. Biophys. Res. Commun. 299, 404–409. doi: 10.1016/S0006-291X(02)02601-3
Pandey, G., and Jain, R. K. (2002). Bacterial chemotaxis toward environmental pollutants: role in bioremediation. Appl. Environ. Microbiol. 68, 5789–5795. doi: 10.1128/AEM.68.12.5789-5795.2002
Pandey, J., Chauhan, A., and Jain, R. K. (2009). Integrative approaches for assessing the ecological sustainability of in situ bioremediation. FEMS Microbiol. Rev. 33, 324–375. doi: 10.1111/j.1574-6976.2008.00133.x
Pandey, P., Kang, S., and Maheshwari, D. (2005). Isolation of endophytic plant growth promoting Burkholderia sp. MSSP from root nodules of Mimosa pudica. Curr. Sci. 89, 177–180.
Panke, S., Meyer, A., Huber, C. M., Witholt, B., and Wubbolts, M. G. (1999). An alkane-responsive expression system for the production of fine chemicals. Appl. Environ. Microbiol. 65, 2324–2332.
Parales, R. E., Ditty, J. L., and Harwood, C. S. (2000). Toluene-degrading bacteria are chemotactic towards the environmental pollutants benzene, toluene, and trichloroethylene. Appl. Environ. Microbiol. 66, 4098–4104. doi: 10.1128/AEM.66.9.4098-4104.2000
Parales, R. E., and Haddock, J. D. (2004). Biocatalytic degradation of pollutants. Curr. Opin. Biotechnol. 15, 374–379. doi: 10.1016/j.copbio.2004.06.003
Parrish, Z. D., Banks, M. K., and Schwab, A. P. (2004). Effectiveness of phytoremediation as a secondary treatment for polycyclic aromatic hydrocarbons (PAHs) in composted soil. Int. J. Phytoremed. 6, 119–137. doi: 10.1080/16226510490454803
Pasteris, G., Werner, D., Kaufmann, K., and Hohener, P. (2002). Vapor phase transport and biodegradation of volatile fuel compounds in the unsaturated zone: a large scale lysimeter experiment. Environ. Sci. Technol. 36, 30–39. doi: 10.1021/es0100423
Paul, D., Singh, R., and Jain, R. K. (2006). Chemotaxis of Ralstonia sp SJ98 towards p-nitrophenol in soil. Environ. Microbiol. 8, 1797–1804. doi: 10.1111/j.1462-2920.2006.01064.x
Pawlik, M., and Piotrowska-Seget, Z. (2015). Endophytic bacteria associated with hieracium piloselloides: their potential for hydrocarbon-utilizing and plant growth-promotion. J. Toxicol. Environ. Health-Part A-Curr. Issues 78, 860–870. doi: 10.1080/15287394.2015.1051200
Pena-Castro, J. M., Barrera-Figueroa, B. E., Fernandez-Linares, L., Ruiz-Medrano, R., and Xoconostle-Cazares, B. (2006). Isolation and identitication of up-regulated genes in bermudagrass roots (Cynodon dactylon L.) grown under petroleum hydrocarbon stress. Plant Sci. 170, 724–731. doi: 10.1016/j.plantsci.2005.11.004
Peng, R.-H., Xiong, A.-S., Xue, Y., Fu, X.-Y., Gao, F., Zhao, W., et al. (2008). Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol. Rev. 32, 927–955. doi: 10.1111/j.1574-6976.2008.00127.x
Peng, S., Zhou, Q., Cai, Z., and Zhang, Z. (2009). Phytoremediation of petroleum contaminated soils by Mirabilis Jalapa L. in a greenhouse plot experiment. J. Hazard. Mater. 168, 1490–1496. doi: 10.1016/j.jhazmat.2009.03.036
Phillips, L. A., Germida, J. J., Farrell, R. E., and Greer, C. W. (2008). Hydrocarbon degradation potential and activity of endophytic bacteria associated with prairie plants. Soil Biol. Biochem. 40, 3054–3064. doi: 10.1016/j.soilbio.2008.09.006
Phillips, L. A., Greer, C. W., Farrell, R. E., and Germida, J. J. (2012). Plant root exudates impact the hydrocarbon degradation potential of a weathered-hydrocarbon contaminated soil. Appl. Soil Ecol. 52, 56–64. doi: 10.1016/j.apsoil.2011.10.009
Pilon-Smits, E. (2005). Phytoremediation. Annu. Rev. Plant Biol. 56, 15–39. doi: 10.1146/annurev.arplant.56.032604.144214
Pirttilä, A. M., and Frank, A. C. (eds) (2011). Endophytes of Forest Trees. Amsterdam: Springer. doi: 10.1007/978-94-007-1599-8
Porteous Moore, F., Barac, T., Borremans, B., Oeyen, L., Vangronsveld, J., van der Lelie, D., et al. (2006). Endophytic bacterial diversity in poplar trees growing on a BTEX-contaminated site: the characterisation of isolates with potential to enhance phytoremediation. Syst. Appl. Microbiol. 29, 539–556. doi: 10.1016/j.syapm.2005.11.012
Prasad, M. N. V., Freitas, H., Fraenzle, S., Wuenschmann, S., and Markert, B. (2010). Knowledge explosion in phytotechnologies for environmental solutions. Environ. Pollut. 158, 18–23. doi: 10.1016/j.envpol.2009.07.038
Qin, G., Gong, D., and Fan, M.-Y. (2013). Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegr. 85, 150–155. doi: 10.1016/j.ibiod.2013.07.004
Quadt-Hallmann, A., Hallmann, J., and Kloepper, J. W. (1997). Bacterial endophytes in cotton: location and interaction with other plant associated bacteria. Can. J. Microbiol. 43, 254–259. doi: 10.1139/m97-035
Radwan, S., Sorkhoh, N., and Elnemr, I. (1995). Oil biodegradation around roots. Nature 376, 302–302. doi: 10.1038/376302a0
Rao, C. V., Glekas, G. D., and Ordal, G. W. (2008). The three adaptation systems of Bacillus subtilis chemotaxis. Trends Microbiol. 16, 480–487. doi: 10.1016/j.tim.2008.07.003
Rastogi, G., Sbodio, A., Tech, J. J., Suslow, T. V., Coaker, G. L., and Leveau, J. H. J. (2012). Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 6, 1812–1822. doi: 10.1038/ismej.2012.32
Read, D. B., Bengough, A. G., Gregory, P. J., Crawford, J. W., Robinson, D., Scrimgeour, C. M., et al. (2003). Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol. 157, 315–326. doi: 10.1046/j.1469-8137.2003.00665.x
Reed, M. L. E., and Glick, B. R. (2005). Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can. J. Microbiol. 51, 1061–1069. doi: 10.1139/w05-094
Reichenauer, T. G., and Germida, J. J. (2008). Phytoremediation of organic contaminants in soil and groundwater. Chemsuschem 1, 708–717. doi: 10.1002/cssc.200800125
Reid, G., Cuperus, P. L., Bruce, A. W., Vandermei, H. C., Tomeczek, L., Khoury, A. H., et al. (1992). Comparison of contact angles and adhesion to hexadecane of urogenital, dairy, and poultry lactobacilli - effect of serial culture passageS. Appl. Environ. Microbiol. 58, 1549–1553.
Rezek, J., Wiesche Cid, Mackova, M., Zadrazil, F., and Macek, T. (2008). The effect of ryegrass (Lolium perenne) on decrease of PAH content in long term contaminated soil. Chemosphere 70, 1603–1608. doi: 10.1016/j.chemosphere.2007.08.003
Ribeiro, H., Mucha, A. P., Almeida, C. M. R., and Bordalo, A. A. (2014). Potential of phytoremediation for the removal of petroleum hydrocarbons in contaminated salt marsh sediments. J. Environ. Manag. 137, 10–15. doi: 10.1016/j.jenvman.2014.01.047
Rinaudi, L. V., and Gonzalez, J. E. (2009). The low-molecular-weight fraction of exopolysaccharide II from Sinorhizobium meliloti is a crucial determinant of biofilm formation. J. Bacteriol. 191, 7216–7224. doi: 10.1128/JB.01063-09
Rohrbacher, F., and St-Arnaud, M. (2016). Root exudation: the ecological driver of hydrocarbon rhizoremediation. Agronomy 6:19. doi: 10.3390/agronomy6010019
Rojo, F. (2009). Degradation of alkanes by bacteria. Environ. Microbiol. 11, 2477–2490. doi: 10.1111/j.1462-2920.2009.01948.x
Rojo, F. (2010). Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev. 34, 658–684. doi: 10.1111/j.1574-6976.2010.00218.x
Romantschuk, M., Sarand, I., Petanen, T., Peltola, R., Jonsson-Vihanne, M., Koivula, T., et al. (2000). Means to improve the effect of in situ bioremediation of contaminated soil: an overview of novel approaches. Environ. Pollut. 107, 179–185. doi: 10.1016/S0269-7491(99)00136-0
Rosenberg, E., and Ron, E. Z. (1996). “Bioremediation of petroleum contamination,” in Bioremediation: Principles and Applications, eds R. L. Crawford and D. G. Crawford (Cambridge: Cambridge University Press), 100–125. doi: 10.1017/CBO9780511608414.006
Rosenberg, E., Rubinovitz, C., Legmann, R., and Ron, E. Z. (1988). Purification and chemical-properties of acinetobacter-calcoaceticus a2 biodispersan. Appl. Environ. Microbiol. 54, 323–326.
Rosenberg, M., Gutnick, D., and Rosenberg, E. (1980). Adherence of bacteria to hydrocarbons - a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9, 29–33. doi: 10.1111/j.1574-6968.1980.tb05599.x
Rutherford, P. M., Dickinson, S. J., and Arocena, J. M. (2005). Emergence, survival and growth of selected plant species in petroleum-impacted flare pit soils. Can. J. Soil Sci. 85, 139–148. doi: 10.4141/S03-088
Ryan, R. P., Germaine, K., Franks, A., Ryan, D. J., and Dowling, D. N. (2008). Bacterial endophytes: recent developments and applications. FEMS Microbiol. Lett. 278, 1–9. doi: 10.1111/j.1574-6968.2007.00918.x
Sabirova, J. S., Ferrer, M., Regenhardt, D., Timmis, K. N., and Golyshin, P. N. (2006). Proteomic insights into metabolic adaptations in Alcanivorax borkumensis induced by alkane utilization. J. Bacteriol. 188, 3763–3773. doi: 10.1128/JB.00072-06
Saito, A., Iwabuchi, T., and Harayama, S. (2000). A novel phenanthrene dioxygenase from Nocardioides sp strain KP7: expression in Escherichia coli. J. Bacteriol. 182, 2134–2141. doi: 10.1128/JB.182.8.2134-2141.2000
Saito, M., and Magara, Y. (2003). Removal of organic pollutants and metabolic adaptation of microorganisms by micro-aeration. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 38, 991–1005. doi: 10.1081/ESE-120019858
Sakai, Y., Maeng, J. H., Tani, Y., and Kato, N. (1994). Use of long-chain n-alkanes (C-13-C-44) by an isolate, acinetobacter sp M-1. Biosci. Biotechnol. Biochem. 58, 2128–2130. doi: 10.1271/bbb.58.2128
Salt, D. E., Blaylock, M., Kumar, N., Dushenkov, V., Ensley, B. D., Chet, I., et al. (1995). Phytoremediation - a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13, 468–474. doi: 10.1038/nbt0595-468
Salt, D. E., Smith, R. D., and Raskin, I. (1998). Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 643–668. doi: 10.1146/annurev.arplant.49.1.643
Samanta, S. K., and Jain, R. K. (2000). Evidence for plasmid-mediated chemotaxis of Pseudomonas putida towards naphthalene and salicylate. Can. J. Microbiol. 46, 1–6. doi: 10.1139/cjm-46-1-1
Sandhu, A., Halverson, L. J., and Beattie, G. A. (2007). Bacterial degradation of airborne phenol in the phyllosphere. Environ. Microbiol. 9, 383–392. doi: 10.1111/j.1462-2920.2006.01149.x
Santos, D. K. F., Rufino, R. D., Luna, J. M., Santos, V. A., and Sarubbo, L. A. (2016). Biosurfactants: multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 17, 401. doi: 10.3390/ijms17030401
Sarkar, D., Ferguson, M., Datta, R., and Birnbaum, S. (2005). Bioremediation of petroleum hydrocarbons in contaminated soils: comparison of biosolids addition, carbon supplementation, and monitored natural attenuation. Environ. Pollut. 136, 187–195. doi: 10.1016/j.envpol.2004.09.025
Schneiker, S., dos Santos, V. M., Bartels, D., Bekel, T., Brecht, M., Buhrmester, J., et al. (2006). Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat. Biotechnol. 24, 997–1004. doi: 10.1038/nbt1232
Schuler, L., Jouanneau, Y., Chadhain, S. M. N., Meyer, C., Pouli, M., Zylstra, G. J., et al. (2009). Characterization of a ring-hydroxylating dioxygenase from phenanthrene-degrading Sphingomonas sp strain LH128 able to oxidize benz a anthracene. Appl. Microbiol. Biotechnol. 83, 465–475. doi: 10.1007/s00253-009-1858-2
Schulz, B. J. E., Boyle, C. J. C., and Sieber, T. N. (eds) (2006). Microbial Root Endophytes. Berlin: Springer-Verlag. doi: 10.1007/3-540-33526-9
Schwartz, E., and Scow, K. M. (2001). Repeated inoculation as a strategy for the remediation of low concentrations of phenanthrene in soil. Biodegradation 12, 201–207. doi: 10.1023/A:1013136524377
Scoma, A., Yakimov, M. M., and Boon, N. (2016). Challenging oil bioremediation at deep-sea hydrostatic pressure. Front. Microbiol. 7:1203. doi: 10.3389/fmicb.2016.01203
Scow, K. M., and Hicks, K. A. (2005). Natural attenuation and enhanced bioremediation of organic contaminants in groundwater. Curr. Opin. Biotechnol. 16, 246–253. doi: 10.1016/j.copbio.2005.03.009
Sekine, M., Tanikawa, S., Omata, S., Saito, M., Fujisawa, T., Tsukatani, N., et al. (2006). Sequence analysis of three plasmids harboured in Rhodococcus erythropolis strain PR4. Environ. Microbiol. 8, 334–346. doi: 10.1111/j.1462-2920.2005.00899.x
Semple, K. T., Doick, K. J., Wick, L. Y., and Harms, H. (2007). Microbial interactions with organic contaminants in soil: definitions, processes and measurement. Environ. Pollut. 150, 166–176. doi: 10.1016/j.envpol.2007.07.023
Sessitsch, A., Hardoim, P., Doring, J., Weilharter, A., Krause, A., Woyke, T., et al. (2012). Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant Microbe Interact. 25, 28–36. doi: 10.1094/MPMI-08-11-0204
Shemesh, M., Kolter, R., and Losick, R. (2010). The biocide chlorine dioxide stimulates biofilm formation in Bacillus subtilis by activation of the histidine kinase KinC. J. Bacteriol. 192, 6352–6356. doi: 10.1128/JB.01025-10
Sheng, X. F., and Gong, J. X. (2006). Increased degradation of phenanthrene in soil by Pseudomonas sp GF3 in the presence of wheat. Soil Biol. Biochem. 38, 2587–2592.
Shirdam, R., Zand, A. D., Bidhendi, G. N., and Mehrdadi, N. (2008). Phytoremediation of hydrocarbon-contaminated soils with emphasis on the effect of petroleum hydrocarbons on the growth of plant species. Phytoprotection 89, 21–29. doi: 10.7202/000379ar
Shor, L. M., Rockne, K. J., Taghon, G. L., Young, L. Y., and Kosson, D. S. (2003). Desorption kinetics for field-aged polycyclic aromatic hydrocarbons from sediments. Environ. Sci. Technol. 37, 1535–1544. doi: 10.1021/es025734l
Siciliano, S. D., Fortin, N., Mihoc, A., Wisse, G., Labelle, S., Beaumier, D., et al. (2001). Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl. Environ. Microbiol. 67, 2469–2475. doi: 10.1128/AEM.67.6.2469-2475.2001
Sierra-Garcia, I. N., Alvarez, J. C., de Vasconcellos, S. P., de Souza, A. P., dos Santos, E. V., and de Oliveira, V. M. (2014). New hydrocarbon degradation pathways in the microbial metagenome from brazilian petroleum reservoirs. PLoS ONE 9:e90087. doi: 10.1371/journal.pone.0090087
Sikkema, J., Debont, J. A. M., and Poolman, B. (1995). Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59, 201–222.
Silva, A. S., de Oliveira Camargo, F. A., Andreazza, R., Seminoti Jacques, R. J., Baldoni, D. B., and Bento, F. M. (2012). Enzymatic activity of catechol 1,2-dioxygenase and catechol 2,3-dioxygenase produced BY Gordonia polyisoprenivorans. Quimica Nova 35, 1587–1592. doi: 10.1590/S0100-40422012000800018
Simon, M. J., Osslund, T. D., Saunders, R., Ensley, B. D., Suggs, S., Harcourt, A., et al. (1993). Sequences of genes encoding naphthalene dioxygenase in Pseudomonas-putida strains g7 and ncib-9816-4. Gene 127, 31–37. doi: 10.1016/0378-1119(93)90613-8
Singer, A. C., van der Gast, C. J., and Thompson, I. P. (2005). Perspectives and vision for strain selection in bioaugmentation. Trends Biotechnol. 23, 74–77. doi: 10.1016/j.tibtech.2004.12.012
Singh, P., DeMarini, D. M., Dick, C. A. J., Tabor, D. G., Ryan, J. V., Linak, W. P., et al. (2004). Sample characterization of automobile and forklift diesel exhaust particles and comparative pulmonary toxicity in mice. Environ. Health Perspect. 112, 820–825. doi: 10.1289/ehp.6579
Singh, R., Paul, D., and Jain, R. K. (2006). Biofilms: implications in bioremediation. Trends Microbiol. 14, 389–397. doi: 10.1016/j.tim.2006.07.001
Singh, S. N., Kumari, B., Upadhyay, S. K., Mishra, S., and Kumar, D. (2013). Bacterial degradation of pyrene in minimal salt medium mediated by catechol dioxygenases: enzyme purification and molecular size determination. Bioresour. Technol. 133, 293–300. doi: 10.1016/j.biortech.2013.01.068
Singleton, D. R., Ramirez, L. G., and Aitken, M. D. (2009). Characterization of a polycyclic aromatic hydrocarbon degradation gene cluster in a phenanthrene-degrading acidovorax strain. Appl. Environ. Microbiol. 75, 2613–2620. doi: 10.1128/AEM.01955-08
Sluis, M. K., Sayavedra-Soto, L. A., and Arp, D. J. (2002). Molecular analysis of the soluble butane monooxygenase from ‘Pseudomonas butanovora’. Microbiology 148, 3617–3629. doi: 10.1099/00221287-148-11-3617
Smalla, K., Wieland, G., Buchner, A., Zock, A., Parzy, J., Kaiser, S., et al. (2001). Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 67, 4742–4751. doi: 10.1128/AEM.67.10.4742-4751.2001
Smets, B. F., and Pritchard, P. H. (2003). Elucidating the microbial component of natural attenuation. Curr. Opin. Biotechnol. 14, 283–288. doi: 10.1016/S0958-1669(03)00062-4
Smith, A. E., Hristova, K., Wood, I., Mackay, D. M., Lory, E., Lorenzana, D., et al. (2005). Comparison of biostimulation versus bioaugmentation with bacterial strain PM1 for treatment of groundwater contaminated with methyl tertiary butyl ether (MTBE). Environ. Health Perspect. 113, 317–322. doi: 10.1289/ehp.6939
Smith, M. J., Flowers, T. H., Duncan, H. J., and Alder, J. (2006). Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environ. Pollut. 141, 519–525. doi: 10.1016/j.envpol.2005.08.061
Smith, R. J., Jeffries, T. C., Adetutu, E. M., Fairweather, P. G., and Mitchell, J. G. (2013). Determining the metabolic footprints of hydrocarbon degradation using multivariate analysis. PLoS ONE 8:e81910. doi: 10.1371/journal.pone.0081910
Smyth, T. J. P., Perfumo, A., Marchant, R., and Banat, I. M. (2010a). “Isolation and analysis of low molecular weight microbial glycolipids,” in Handbook of Hydrocarbon and Lipid Microbiology, ed. K. N. Timmis (Berlin: Springer-Verlag), 3705–3723. doi: 10.1007/978-3-540-77587-4_291
Smyth, T. J. P., Perfumo, A., McClean, S., Marchant, R., and Banat, I. M. (2010b). “Isolation and analysis of lipopeptides and high molecular weight biosurfactants,” in Handbook of Hydrocarbon and Lipid Microbiology, ed. K. N. Timmis (Berlin: Springer-Verlag), 3689–3704.
Sotirova, A. V., Spasova, D. I., Galabova, D. N., Karpenko, E., and Shulga, A. (2008). Rhamnolipid-biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Curr. Microbiol. 56, 639–644. doi: 10.1007/s00284-008-9139-3
Staijen, I. E., Marcionelli, R., and Witholt, B. (1999). The P-alkBFGHJKL promoter is under carbon catabolite repression control in Pseudomonas oleovorans but not in Escherichia coli alk(+) recombinants. J. Bacteriol. 181, 1610–1616.
Steinkamp, R., Zimmer, W., and Papen, H. (2001). Improved method for detection of methanotrophic bacteria in forest soils by PCR. Curr. Microbiol. 42, 316–322. doi: 10.1007/s002840010223
Strobel, K. L., McGowan, S., Bauer, R. D., Griebler, C., Liu, J., and Ford, R. M. (2011). Chemotaxis increases vertical migration and apparent transverse dispersion of bacteria in a bench-scale microcosm. Biotechnol. Bioeng. 108, 2070–2077. doi: 10.1002/bit.23159
Stroud, J. L., Paton, G. I., and Semple, K. T. (2007). Microbe-aliphatic hydrocarbon interactions in soil: implications for biodegradation and bioremediation. J. Appl. Microbiol. 102, 1239–1253. doi: 10.1111/j.1365-2672.2007.03401.x
Suarez, M. P., and Rifai, H. S. (2004). Modeling natural attenuation of total BTEX and benzene plumes with different kinetics. Ground Water Monitor. Remed. 24, 53–68. doi: 10.1111/j.1745-6592.2004.tb01292.x
Sun, G.-D., Xu, Y., Jin, J.-H., Zhong, Z.-P., Liu, Y., Luo, M., et al. (2012). Pilot scale ex-situ bioremediation of heavily PAHs-contaminated soil by indigenous microorganisms and bioaugmentation by a PAHs-degrading and bioemulsifier-producing strain. J. Hazard. Mater. 233, 72–78. doi: 10.1016/j.jhazmat.2012.06.060
Sun, T.-R., Cang, L., Wang, Q.-Y., Zhou, D.-M., Cheng, J.-M., and Xu, H. (2010). Roles of abiotic losses, microbes, plant roots, and root exudates on phytoremediation of PAHs in a barren soil. J. Hazard. Mater. 176, 919–925. doi: 10.1016/j.jhazmat.2009.11.124
Susarla, S., Medina, V. F., and McCutcheon, S. C. (2002). Phytoremediation: an ecological solution to organic chemical contamination. Ecol. Eng. 18, 647–658. doi: 10.1016/S0925-8574(02)00026-5
Sutherland, I. W. (2001). Exopolysaccharides in biofilms, flocs and related structures. Water Sci. Technol. 43, 77–86.
Swannell, R. P. J., Lee, K., and McDonagh, M. (1996). Field evaluations of marine oil spill bioremediation. Microbiol. Rev. 60, 342–365.
Tabak, H. H., Lazorchak, J. M., Lei, L., Khodadoust, A. P., Antia, J. E., Bagchi, R., et al. (2003). Studies on bioremediation of polycyclic aromatic hydrocarbon-contaminated sediments: bioavailability, biodegradability, and toxicity issues. Environ. Toxicol. Chem. 22, 473–482. doi: 10.1002/etc.5620220303
Taccari, M., Milanovic, V., Comitini, F., Casucci, C., and Ciani, M. (2012). Effects of biostimulation and bioaugmentation on diesel removal and bacterial community. Int. Biodeterior. Biodegr. 66, 39–46. doi: 10.1016/j.ibiod.2011.09.012
Taghavi, S., Barac, T., Greenberg, B., Borremans, B., Vangronsveld, J., and van der Lelie, D. (2005). Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene. Appl. Environ. Microbiol. 71, 8500–8505. doi: 10.1128/AEM.71.12.8500-8505.2005
Taghavi, S., Garafola, C., Monchy, S., Newman, L., Hoffman, A., Weyens, N., et al. (2009). Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 75, 748–757. doi: 10.1128/AEM.02239-08
Tang, J., Wang, R., Niu, X., and Zhou, Q. (2010). Enhancement of soil petroleum remediation by using a combination of ryegrass (Lolium perenne) and different microorganisms. Soil Tillage Res. 110, 87–93. doi: 10.1016/j.still.2010.06.010
Tani, A., Ishige, T., Sakai, Y., and Kato, N. (2001). Gene structures and regulation of the alkane hydroxylase complex in Acinetobacter sp strain M-1. J. Bacteriol. 183, 1819–1823. doi: 10.1128/JB.183.5.1819-1823.2001
Tara, N., Afzal, M., Ansari, T. M., Tahseen, R., Iqbal, S., and Khan, K. M. (2014). Combined use of alkane-degrading and plant growth-promoting bacteria enhanced phytoremediation of diesel contaminated soil. Int. J. Phytoremed. 16, 1268–1277. doi: 10.1080/15226514.2013.828013
Tardif, S., Yergeau,É., Tremblay, J., Legendre, P., Whyte, L. G., and Greer, C. W. (2016). The willow microbiome is influenced by soil petroleum-hydrocarbon concentration with plant compartment-specific effects. Front. Microbiol. 7:1363. doi: 10.3389/fmicb.2016.01363
Techtmann, S. M., and Hazen, T. C. (2016). Metagenomic applications in environmental monitoring and bioremediation. J. Ind. Microbiol. Biotechnol. 43, 1345–1354. doi: 10.1007/s10295-016-1809-8
Teng, Y., Shen, Y., Luo, Y., Sun, X., Sun, M., Fu, D., et al. (2011). Influence of Rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. J. Hazard. Mater. 186, 1271–1276. doi: 10.1016/j.jhazmat.2010.11.126
Thijs, S., Sillen, W., Rineau, F., Weyens, N., and Vangronsveld, J. (2016). Towards an enhanced understanding of plant–microbiome interactions to improve phytoremediation: engineering the metaorganism. Front. Microbiol. 7:341. doi: 10.3389/fmicb.2016.00341
Thijs, S., Van Dillewijn, P., Sillen, W., Truyens, S., Holtappels, M., D’Haen, J., et al. (2014a). Exploring the rhizospheric and endophytic bacterial communities of Acer pseudoplatanus growing on a TNT-contaminated soil: towards the development of a rhizocompetent TNT-detoxifying plant growth promoting consortium. Plant Soil 385, 15–36. doi: 10.1007/s11104-014-2260-0
Thijs, S., Weyens, N., Sillen, W., Gkorezis, P., Carleer, R., and Vangronsveld, J. (2014b). Potential for plant growth promotion by a consortium of stress-tolerant 2,4-dinitrotoluene-degrading bacteria: isolation and characterization of a military soil. Microbiol. Biotechnol. 7, 294–306. doi: 10.1111/1751-7915.12111
Thomas, F., and Cébron, A. (2016). Short-term rhizosphere effect on available carbon sources, phenanthrene degradation, and active microbiome in an aged-contaminated industrial soil. Front. Microbiol. 7:92. doi: 10.3389/fmicb.2016.00092
Thomassin-Lacroix, E. J. M., Eriksson, M., Reimer, K. J., and Mohn, W. W. (2002). Biostimulation and bioaugmentation for on-site treatment of weathered diesel fuel in Arctic soil. Appl. Microbiol. Biotechnol. 59, 551–556. doi: 10.1007/s00253-002-1038-0
Thompson, I. P., van der Gast, C. J., Ciric, L., and Singer, A. C. (2005). Bioaugmentation for bioremediation: the challenge of strain selection. Environ. Microbiol. 7, 909–915. doi: 10.1111/j.1462-2920.2005.00804.x
Throne-Holst, M., Wentzel, A., Ellingsen, T. E., Kotlar, H.-K., and Zotchev, S. B. (2007). Identification of novel genes involved in long-chain n-alkane degradation by Acinetobacter sp strain DSM 17874. Appl. Environ. Microbiol. 73, 3327–3332. doi: 10.1128/AEM.00064-07
Toyama, T., Furukawa, T., Maeda, N., Inoue, D., Sei, K., Mori, K., et al. (2011). Accelerated biodegradation of pyrene and benzo a pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions. Water Res. 45, 1629–1638. doi: 10.1016/j.watres.2010.11.044
Tremaroli, V., Suzzi, C. V., Fedi, S., Ceri, H., Zannoni, D., and Turner, R. J. (2010). Tolerance of Pseudomonas pseudoalcaligenes KF707 to metals, polychlorobiphenyls and chlorobenzoates: effects on chemotaxis-, biofilm- and planktonic-grown cells. FEMS Microbiol. Ecol. 74, 291–301. doi: 10.1111/j.1574-6941.2010.00965.x
Truyens, S., Beckers, B., Thijs, S., Weyens, N., Cuypers, A., and Vangronsveld, J. (2015a). Cadmium-induced and transgenerational changes in the cultivable and total seed endophytic community of Arabidopsis thaliana. Plant Biol. 18, 376–381. doi: 10.1111/plb.12415
Truyens, S., Beckers, B., Thijs, S., Weyens, N., Cuypers, A., and Vangronsveld, J. (2015b). The effects of the growth substrate on cultivable and total endophytic assemblages of Arabidopsis thaliana. Plant Soil 45, 325–336.
Tsai, T. T., and Kao, C. M. (2009). Treatment of petroleum-hydrocarbon contaminated soils using hydrogen peroxide oxidation catalyzed by waste basic oxygen furnace slag. J. Hazard. Mater. 170, 466–472. doi: 10.1016/j.jhazmat.2009.04.073
Tyagi, M., da Fonseca, M. M. R., and de Carvalho, C. (2011). Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22, 231–241. doi: 10.1007/s10532-010-9394-4
Tzintzun-Camacho, O., Loera, O., Ramirez-Saad, H. C., and Gutierrez-Rojas, M. (2012). Comparison of mechanisms of hexadecane uptake among pure and mixed cultures derived from a bacterial consortium. Int. Biodeterior. Biodegr. 70, 1–7. doi: 10.1016/j.ibiod.2012.01.009
Ueno, A., Ito, Y., Yumoto, I., and Okuyama, H. (2007). Isolation and characterization of bacteria from soil contaminated with diesel oil and the possible use of these in autochthonous bioaugmentation. World J. Microbiol. Biotechnol. 23, 1739–1745. doi: 10.1007/s11274-007-9423-6
Uhlik, O., Leewis, M. C., Strejcek, M., Musilova, L., Mackova, M., Leigh, M. B., et al. (2013). Stable isotope probing in the metagenomics era: a bridge towards improved bioremediation. Biotechnol. Adv. 31, 154–165. doi: 10.1016/j.biotechadv.2012.09.003
Uroz, S., Buee, M., Murat, C., Frey-Klett, P., and Martin, F. (2010). Pyrosequencing reveals a contrasted bacterial diversity between oak rhizosphere and surrounding soil. Environ. Microbiol. Rep. 2, 281–288. doi: 10.1111/j.1758-2229.2009.00117.x
Uzoigwe, C., Burgess, J. G., Ennis, C. J., and Rahman, P. K. S. M. (2015). Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 6:245. doi: 10.3389/fmicb.2015.00245
van Beilen, J. B., Marin, M. M., Smits, T. H. M., Rothlisberger, M., Franchini, A. G., Witholt, B., et al. (2004). Characterization of two alkane hydroxylase genes from the marine hydrocarbonoclastic bacterium Alcanivorax borkumensis. Environ. Microbiol. 6, 264–273. doi: 10.1111/j.1462-2920.2004.00567.x
van Beilen, J. B., Panke, S., Lucchini, S., Franchini, A. G., Rothlisberger, M., and Witholt, B. (2001). Analysis of Pseudomonas putida alkane-degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk genes. Microbiology 147, 1621–1630. doi: 10.1099/00221287-147-6-1621
van der Lelie, D., Schwitzguebel, J. P., Glass, D. J., Vangronsveld, J., and Baker, A. (2001). Assessing phytoremediation’s progress in the United States and Europe. Environ. Sci. Technol. 35, 446A–452A. doi: 10.1021/es012543u
Van der Lelie, D., Taghavi, S., Monchy, S., Schwender, J., Miller, L., Ferrieri, R., et al. (2009). Poplar and its bacterial endophytes: coexistence and harmony. Crit. Rev. Plant Sci. 28, 346–358. doi: 10.1080/07352680903241204
Van Hamme, J. D., Singh, A., and Ward, O. P. (2003). Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 67, 503–549. doi: 10.1128/MMBR.67.4.503-549.2003
van Veen, J. A., van Overbeek, L. S., and van Elsas, J. D. (1997). Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 61, 121–135.
Vaneechoutte, M., Young, D. M., Ornston, L. N., De Baere, T., Nemec, A., Van Der Reijden, T., et al. (2006). Naturally transformable Acinetobacter sp strain ADP1 belongs to the newly described species Acinetobacter baylyi. Appl. Environ. Microbiol. 72, 932–936. doi: 10.1128/AEM.72.1.932-936.2006
Vangronsveld, J., Herzig, R., Weyens, N., Boulet, J., Adriaensen, K., Ruttens, A., et al. (2009). Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ. Sci. Pollut. Res. 16, 765–794. doi: 10.1007/s11356-009-0213-6
Vardar, G., Barbieri, P., and Wood, T. K. (2005). Chemotaxis of Pseudomonas stutzeri OX1 and Burkholderia cepacia G4 toward chlorinated ethenes. Appl. Microbiol. Biotechnol. 66, 696–701. doi: 10.1007/s00253-004-1685-4
Verginelli, I., and Baciocchi, R. (2013). Role of natural attenuation in modeling the leaching of contaminants in the risk analysis framework. J. Environ. Manag. 114, 395–403. doi: 10.1016/j.jenvman.2012.10.035
Villas-Boas, S. G., and Bruheim, P. (2007). The potential of metabolomics tools in Bioremediation studies. Omics 11, 305–313. doi: 10.1089/omi.2007.0005
Vogel, T. M. (1996). Bioaugmentation as a soil bioremediation approach. Curr. Opin. Biotechnol. 7, 311–316. doi: 10.1016/S0958-1669(96)80036-X
Vokou, D., Vareli, K., Zarali, E., Karamanoli, K., Constantinidou, H. I. A., Monokrousos, N., et al. (2012). Exploring biodiversity in the bacterial community of the mediterranean phyllosphere and its relationship with airborne bacteria. Microb. Ecol. 64, 714–724. doi: 10.1007/s00248-012-0053-7
Volkering, F., Breure, A. M., and Rulkens, W. H. (1997). Microbiological aspects of surfactant use for biological soil remediation. Biodegradation 8, 401–417. doi: 10.1023/A:1008291130109
Vorholt, J. A. (2012). Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840. doi: 10.1038/nrmicro2910
Wadhams, G. H., and Armitage, J. P. (2004). Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5, 1024–1037. doi: 10.1038/nrm1524
Waight, K., Pinyakong, O., and Luepromchai, E. (2007). Degradation of phenanthrene on plant leaves by phyllosphere bacteria. J. Gen. Appl. Microbiol. 53, 265–272. doi: 10.2323/jgam.53.265
Wang, L., Wang, W., Lai, Q., and Shao, Z. (2010). Gene diversity of CYP153A and AlkB alkane hydroxylases in oil-degrading bacteria isolated from the Atlantic Ocean. Environ. Microbiol. 12, 1230–1242. doi: 10.1111/j.1462-2920.2010.02165.x
Wang, S. Y., Kuo, Y. C., Hong, A., Chang, Y. M., and Kao, C. M. (2016). Bioremediation of diesel and lubricant oil-contaminated soils using enhanced landfarming system. Chemosphere 164, 558–567. doi: 10.1016/j.chemosphere.2016.08.128
Wang, W., and Shao, Z. (2012). Genes involved in alkane degradation in the Alcanivorax hongdengensis strain A-11-3. Appl. Microbiol. Biotechnol. 94, 437–448. doi: 10.1007/s00253-011-3818-x
Wang, W., and Shao, Z. (2013). Enzymes and genes involved in aerobic alkane degradation. Front. Microbiol. 4:116. doi: 10.3389/fmicb.2013.00116
Wang, W., and Shao, Z. (2014). The long-chain alkane metabolism network of Alcanivorax dieselolei. Nat. Commun. 5:5755. doi: 10.1038/ncomms6755
Wentzel, A., Ellingsen, T. E., Kotlar, H.-K., Zotchev, S. B., and Throne-Holst, M. (2007). Bacterial metabolism of long-chain n-alkanes. Appl. Microbiol. Biotechnol. 76, 1209–1221. doi: 10.1007/s00253-007-1119-1
Wenzel, W. W. (2009). Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 321, 385–408. doi: 10.1007/s11104-008-9686-1
Weyens, N., Croes, S., Dupae, J., Newman, L., van der Lelie, D., Carleer, R., et al. (2010). Endophytic bacteria improve phytoremediation of Ni and TCE co-contamination. Environ. Pollut. 158, 2422–2427. doi: 10.1016/j.envpol.2010.04.004
Weyens, N., Taghavi, S., Barac, T., van der Lelie, D., Boulet, J., Artois, T., et al. (2009a). Bacteria associated with oak and ash on a TCE-contaminated site: characterization of isolates with potential to avoid evapotranspiration of TCE. Environ. Sci. Pollut. Res. 16, 830–843. doi: 10.1007/s11356-009-0154-0
Weyens, N., Van Der Lelie, D., Artois, T., Smeets, K., Taghavi, S., Newman, L., et al. (2009b). Bioaugmentation with engineered endophytic bacteria improves contaminant fate in phytoremediation. Environ. Sci. Technol. 43, 9413–9418. doi: 10.1021/es901997z
Weyens, N., van der Lelie, D., Taghavi, S., Newman, L., and Vangronsveld, J. (2009c). Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 27, 591–598. doi: 10.1016/j.tibtech.2009.07.006
Weyens, N., Thijs, S., Popek, R., Witters, N., Przybysz, A., Espenshade, J., et al. (2015). The role of plant-microbe interactions and their exploitation for phytoremediation of air pollutants. Int. J. Mol. Sci. 16, 25576–25604. doi: 10.3390/ijms161025576
Whipps, J. M., Hand, P., Pink, D., and Bending, G. D. (2008). Phyllosphere microbiology with special reference to diversity and plant genotype. J. Appl. Microbiol. 105, 1744–1755. doi: 10.1111/j.1365-2672.2008.03906.x
Wick, L. Y., Colangelo, T., and Harms, H. (2001). Kinetics of mass transfer-limited bacterial growth on solid PAHs. Environ. Sci. Technol. 35, 354–361. doi: 10.1021/es001384w
Wick, L. Y., de Munain, A. R., Springael, D., and Harms, H. (2002). Responses of Mycobacterium sp LB501T to the low bioavailability of solid anthracene. Appl. Microbiol. Biotechnol. 58, 378–385. doi: 10.1007/s00253-001-0898-z
Widdowson, M. A., Shearer, S., Andersen, R. G., and Novak, J. T. (2005). Remediation of polycyclic aromatic hydrocarbon compounds in groundwater using poplar trees. Environ. Sci. Technol. 39, 1598–1605. doi: 10.1021/es0491681
Wild, E., Dent, J., Thomas, G. O., and Jones, K. C. (2005). Direct observation of organic contaminant uptake, storage, and metabolism within plant roots. Environ. Sci. Technol. 39, 3695–3702. doi: 10.1021/es048136a
Wiltse, C. C., Rooney, W. L., Chen, Z., Schwab, A. P., and Banks, M. K. (1998). Greenhouse evaluation of agronomic and crude oil phytoremediation potential among alfalfa genotypes. J. Environ. Q. 27, 169–173. doi: 10.2134/jeq1998.00472425002700010024x
Wongwongsee, W., Chareanpat, P., and Pinyakong, O. (2013). Abilities and genes for PAH biodegradation of bacteria isolated from mangrove sediments from the central of Thailand. Mar. Pollut. Bull. 74, 95–104. doi: 10.1016/j.marpolbul.2013.07.025
Xu, R., and Obbard, J. P. (2003). Effect of nutrient amendments on indigenous hydrocarbon biodegradation in oil-contaminated beach sediments. J. Environ. Q. 32, 1234–1243. doi: 10.2134/jeq2003.1234
Xu, Y., and Lu, M. (2010). Bioremediation of crude oil-contaminated soil: comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 183, 395–401. doi: 10.1016/j.jhazmat.2010.07.038
Xue, J. L., Yu, Y., Bai, Y., Wang, L. P., and Wu, Y. N. (2015). Marine oil-degrading microorganisms and biodegradation process of petroleum hydrocarbon in marine environments: a review. Curr. Microbiol. 71, 220–228. doi: 10.1007/s00284-015-0825-7
Yergeau, E., Arbour, M., Brousseau, R., Juck, D., Lawrence, J. R., Masson, L., et al. (2009). Microarray and real-time PCR analyses of the responses of high-arctic soil bacteria to hydrocarbon pollution and bioremediation treatments. Appl. Environ. Microbiol. 75, 6258–6267. doi: 10.1128/AEM.01029-09
Yergeau, E., Bell, T. H., Champagne, J., Maynard, C., Tardif, S., Tremblay, J., et al. (2015). Transplanting soil microbiomes leads to lasting effects on willow growth, but not on the rhizosphere microbiome. Front. Microbiol. 6:1436. doi: 10.3389/fmicb.2015.01436
Yergeau, E., Sanschagrin, S., Beaumier, D., and Greer, C. W. (2012). Metagenomic analysis of the bioremediation of diesel-contaminated canadian high arctic soils. PLoS ONE 7:e30058. doi: 10.1371/journal.pone.0030058
Yousaf, S., Afzal, M., Reichenauer, T. G., Brady, C. L., and Sessitsch, A. (2011). Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains. Environ. Pollut. 159, 2675–2683. doi: 10.1016/j.envpol.2011.05.031
Yousaf, S., Ripka, K., Reichenauer, T. G., Andria, V., Afzal, M., and Sessitsch, A. (2010). Hydrocarbon degradation and plant colonization by selected bacterial strains isolated from Italian ryegrass and birdsfoot trefoil. J. Appl. Microbiol. 109, 1389–1401. doi: 10.1111/j.1365-2672.2010.04768.x
Yu, X. Z., Wu, S. C., Wu, F. Y., and Wong, M. H. (2011). Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J. Hazard. Mater. 186, 1206–1217. doi: 10.1016/j.jhazmat.2010.11.116
Yuste, L., Canosa, I., and Rojo, F. (1998). Carbon-source-dependent expression of the PalkB promoter from the Pseudomonas oleovorans alkane degradation pathway. J. Bacteriol. 180, 5218–5226.
Yutthammo, C., Thongthammachat, N., Pinphanichakarn, P., and Luepromchai, E. (2010). Diversity and Activity of PAH-degrading bacteria in the phyllosphere of ornamental plants. Microb. Ecol. 59, 357–368. doi: 10.1007/s00248-009-9631-8
Zawierucha, I., and Malina, G. (2011). “Bioremediation of contaminated soils: effects of bioaugmentation and biostimulation on enhancing biodegradation of oil hydrocarbons,” in Bioaugmentation, Biostimulation and Biocontrol, Soil biology 28, Chap. 8, ed. A. Singh (Berlin: Springer-Verlag) 187–201. doi: 10.1007/978-3-642-19769-7_8
Zhang, X. Y., Chen, L. S., Liu, X. Y., Wang, C. H., Chen, X. P., Xu, G., et al. (2014). Synergic degradation of diesel by Scirpus triqueter and its endophytic bacteria. Environ. Sci. Pollut. Res. 21, 8198–8205. doi: 10.1007/s11356-014-2807-x
Zhang, Z., Rengel, Z., Chang, H., Meney, K., Pantelic, L., and Tomanovic, R. (2012). Phytoremediation potential of Juncus subsecundus in soils contaminated with cadmium and polynuclear aromatic hydrocarbons (PAHs). Geoderma 175, 1–8. doi: 10.1016/j.geoderma.2012.01.020
Zhao, Y., Qu, D., Hou, Z., and Zhou, R. (2015). Enhanced natural attenuation of BTEX in the nitrate-reducing environment by different electron acceptors. Environ. Technol. 36, 615–621. doi: 10.1080/09593330.2014.954006
Zhong, H., Zeng, G. M., Yuan, X. Z., Fu, H. Y., Huang, G. H., and Ren, F. Y. (2007). Adsorption of dirhamnolipid on four microorganisms and the effect on cell surface hydrophobicity. Appl. Microbiol. Biotechnol. 77, 447–455. doi: 10.1007/s00253-007-1154-y
Keywords: phytoremediation, bioremediation, remediation, petroleum hydrocarbons, plant–bacteria assisted remediation
Citation: Gkorezis P, Daghio M, Franzetti A, Van Hamme JD, Sillen W and Vangronsveld J (2016) The Interaction between Plants and Bacteria in the Remediation of Petroleum Hydrocarbons: An Environmental Perspective. Front. Microbiol. 7:1836. doi: 10.3389/fmicb.2016.01836
Received: 20 June 2016; Accepted: 01 November 2016;
Published: 21 November 2016.
Edited by:
Regina-Michaela Wittich, Spanish High Council for Scientific Research – Estación Experimental del Zaidín, SpainReviewed by:
Elizabeth Lucy Rylott, University of York, UKCopyright © 2016 Gkorezis, Daghio, Franzetti, Van Hamme, Sillen and Vangronsveld. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panagiotis Gkorezis, cGFub3MuZ2tvcmV6aXNAdWhhc3NlbHQuYmU= Jaco Vangronsveld, amFjby52YW5ncm9uc3ZlbGRAdWhhc3NlbHQuYmU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.