- 1Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
- 2Department of Microbiology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Zika virus (ZIKV) has now become a global public health concern. The vectors for ZIKV are Aedes aegypti and A. albopictus. Both these mosquitoes are predominant in Southeast Asia and are also responsible for the spread of other arboviral diseases like dengue virus and chikungunya virus. The incidence of dengue has been increasing over the years and this is of concern to public health workers. Simple laboratory tools for the detection of ZIKV is also lacking. In the absence of drugs and vaccine for these arboviral diseases, vector control is the main option for surveillance and control. Aedes larval surveys have been the hallmark of dengue control along with larviciding and fogging when cases are reported. However, we need new paradigms and options for control of these vectors. The current situation in Southeast Asia clearly proves that effective strategies for vector control need to be proactive and not reactive. This will be the way forward to control epidemics of these diseases inclusive of ZIKV until a vaccine becomes available.
Introduction
Zika virus (ZIKV) which was first discovered from the Rhesus monkey in the Zika forest of Uganda in 1947 (Dick et al., 1952) has now become a global public health concern (Fauci and Morens, 2016; Focosi et al., 2016). ZIKV is a flavivirus and is maintained in a sylvatic cycle which involves non-human primates and the Aedes mosquitoes as vectors (Aedes africanus, A. aegypti) (Haddow et al., 1964; Marchette et al., 1969). ZIKV was only known to cause infection in Africa and Southeast Asia (Haddow et al., 2012). However, in 2007 for the first time ZIKV was reported outside of Africa and Southeast Asia in Yap Island (Hayes, 2009). In Yap Island out of the 185 suspected cases 49 of them were confirmed to be ZIKV and majority of the cases occurred in the older age group (50–55 years) (Duffy et al., 2009). However, during that outbreak there was no death or haemorrhagic complications and the patients only suffered from symptoms like rash, fever, arthritis or arthralgia, conjunctivitis, myalgia, headache, retro-orbital pain, edema, and vomiting (Duffy et al., 2009).
In recent years 2012–2014 there were outbreaks of ZIKV in the Pacific Islands namely Cook Island, Easter Island, French Polynesia, and New Caledonia (Cao-Lormeau and Musso, 2014; Roth et al., 2014). In some of the Pacific Islands especially in 2014 all three viruses ZIKV, chikungunya virus (CHIKV), and dengue virus (DENV) were circulating (Cao-Lormeau and Musso, 2014). These viruses showed a gradual spread over the years starting in 2007 and becoming more widespread in 2014. It is of great concern to learn that in French Polynesia when 1505 asymptomatic blood donors were screened for ZIKV by RT-PCR, 42 of them were positive (Musso et al., 2014). This seems to implicate how travel by humans can help to spread the viruses to new areas.
From February to April 2015, north eastern states of Brazil reported almost 7000 cases of people having rash and minor illness; of which only a small percentage of them were positive for dengue while tests for other viruses (but not for ZIKV) were all negative (Kindhauser et al., 2016). It was only by early May 2015 it was confirmed that it was ZIKV by RT-PCR and was reported for the first time in the Americas (Kindhauser et al., 2016). By July 12 states in Brazil had confirmed ZIKV cases and by the end of 2015 Colombia, Suriname, El Salvador, Mexico, Guatemala, Paraguay, Venezuela, Honduras, and Panama had reported locally acquired ZIKV (Kindhauser et al., 2016). This implies that the ZIKV will go on spreading to many more countries unless concerted effort is taken on a global scale.
Zika virus which was thought to be just a mild viral disease was later found to cause neurologic symptoms and microcephaly (Oliveira Melo et al., 2016). ZIKV was also found in other body fluids and was also shown to be sexually transmitted (Musso et al., 2015; Mansuy et al., 2016; Venturi et al., 2016). The current situation seems to portray that ZIKV could lead to serious public health concerns on a global scale. In the Americas ZIKV has been circulating along with DENV and CHIKV.
In Southeast Asia it is known that arbovirus diseases like DENV, CHIKV, Japanese encephalitis are serious public health concerns (Dash et al., 2013). In recent years (2014–2015) in Indonesia, a positive case of ZIKV was detected during a dengue outbreak in Jambi province Sumatra (Perkasa et al., 2016). Similarly in Cambodia a confirmed case of ZIKV was reported in 2010 (Heang et al., 2012) and in 2012 in Cebu, Philippines a 15 year old boy was confirmed to be suffering from ZIKV by real time RT-PCR and virus isolation (Alera et al., 2015). Travelers to Thailand were found to be infected with ZIKV on return to their country (Tappe et al., 2014). The Thai Ministry of Health then reviewed cases and found ZIKV infection circulating in Thailand between 2012 and 2014 (Buathong et al., 2015). Due to large outbreaks of dengue and CHIKV in Southeast Asia, which cause similar symptoms, ZIKV may be overlooked in Malaysia (Sam et al., 2016). However, there have been no reports of other neurologic symptoms.
This review will delve into the methods available for the detection of ZIKV, the vectors involved, current tools used for the control of the vectors and finally on the recommendations of new paradigms for surveillance and control of these vectors.
Diagnostic Tools of ZIKV
Dengue virus and CHIKV share the same mosquito vectors (A. aegypti and A. albopictus) and potential distribution as ZIKV, and indeed co-circulation is described in the Americas (Rodriguez-Morales et al., 2016). It is difficult to clinically differentiate between these infections as there is much overlap in symptoms and signs. Laboratory diagnosis takes on added importance as the long-term consequences of these infections are quite different and require specific approaches, for example the follow-up of ZIKV-infected pregnant women. There has been a flurry of new diagnostic assays described recently to complement existing conventional techniques such as cell culture [reviewed by Waggoner and Pinsky (2016)]. To date (July 20, 2016), several PCR and IgM assays for ZIKV have been submitted to the WHO Emergency Use Assessment and Listing Procedure, which assesses and expedites the availability of in vitro diagnostics during public health emergencies (World Health Organization, 2016a).
The current gold standard for diagnosis is PCR, which should be carried out on serum samples (within 7 days of illness) or urine (within 14 days) (CDCP, 2016). ZIKV RNA can also be detected in saliva (Bingham, 2016) and semen (Reusken et al., 2016) (the latter for up to 62 days), and there is some evidence that the non-serum specimens urine, saliva, and semen may be more likely to yield positive results than serum (Bingham, 2016; Reusken et al., 2016). Serum samples should also be tested for co-circulating arboviruses such as DENV and CHIKV (Waggoner et al., 2016).
Detection of serum IgM from day five of illness onward is a mainstay for arboviral diagnosis in most diagnostic laboratories in developing countries, as culture and PCR facilities are not widely available. ZIKV IgM can also be detected in the CSF of babies with microcephaly suspected to be due to congenital ZIKV infection (Cordeiro et al., 2016). However, the utility of IgM is much reduced by the extensive cross-reactions seen with past infections of or vaccinations against other flaviviruses, notably DENV, Japanese encephalitis, and yellow fever viruses (Calisher et al., 1989), necessitating the use of the highly specific plaque reduction neutralization test for confirmation (Lindsey et al., 1976; Rabe, 2016). This assay is beyond the scope of most laboratories. The antibodies that cross-react to ZIKV or DENV are mainly targeted to envelope protein domains EDI/II, and can cause antibody-dependent enhancement of infection with either virus (Stettler et al., 2016). In contrast, antibodies to non-structural protein 1 (NS1) are ZIKV-specific and could be used to develop a serological assay that can distinguish DENV from ZIKV infections (Huzly et al., 2016; Stettler et al., 2016). However, negative test results by culture, PCR or serology can never fully rule out ZIKV infection.
The ideal diagnostic test for ZIKV should be affordable, sensitive, specific, user-friendly, rapid and robust, particularly for the developing countries where the vectors exist. One of the WHO’s top priorities for ZIKV medical products are multiplex tests for the three arboviruses (ZIKV, DENV, and CHIKV) which share the same mosquito vectors (World Health Organization, 2016b). The ideal test should detect RNA or antigen. The development of NS1 antigen detection assays (including rapid tests) was a major advance for dengue diagnosis. NS1 is secreted by flavivirus-infected cells and is involved in immune evasion and pathogenesis. ZIKV NS1 shares conserved features with DENV and West Nile virus, but has different electrostatic potential at the loop surface, which interacts with host factors and antibodies (Song H.et al., 2016). Unlike IgM assays, DENV NS1 assays do not seem to demonstrate cross-reactivity with ZIKV (Matheus et al., 2016), apart from a single case report using a particular kit (Gyurech et al., 2016). A ZIKV NS1 assay would theoretically be feasible as an accessible test to reliably differentiate DENV and ZIKV, and several candidate assays are in the pipeline (World Health Organization, 2016b).
Several alternative diagnostic field tools for resource-poor settings have been described (Meagher et al., 2016). These include a synthetic biology approach, whereby isothermal RNA amplification is carried out, and toehold switch RNA sensors induce a color change, with all reagents embedded into a paper-based sensor (Pardee et al., 2016). A point-of-care loop-mediated, isothermal amplification assay with colorimetric detection has also been described (Song J.et al., 2016).
The detection of arboviruses in wild mosquitoes is useful for surveillance or for identifying the vectors of a relatively understudied pathogen (such as ZIKV) (Samuel and Tyagi, 2006). However, there are specific challenges which reduce sensitivity of testing methods. For example, mosquitoes may not be collected from traps for some time, which will lead to drying, rapid loss of viability for culture, and RNA degradation. Pools of triturated mosquitoes may also contain PCR inhibitors and other microorganisms, which may contaminate cultures. The traditional culture techniques for arbovirus diagnosis in mosquitoes, such as inoculation in cells, suckling mice or mosquitoes, and immunofluorescence assay, are in any case too labor-intensive for routine surveillance. Next-generation sequencing is useful for mosquitoes which potentially carry more than one pathogen or during an outbreak with an unknown arbovirus, but it is expensive and requires complex bioinformatics analysis (Bishop-Lilly et al., 2010).
For DENV, the rapid commercial NS1 assays developed for human diagnosis are excellent tools for testing mosquitoes, with the benefits of similar sensitivity to PCR (Tan et al., 2011; Voge et al., 2013), simplicity, and the potential for field use with a hand-held battery-operated homogenizer (Muller et al., 2012). Antigen-capture enzyme immunoassays have been described for detection of other flaviviruses in desiccated mosquitoes kept at ambient temperatures, including DENV (Thenmozhi et al., 2005; Chao et al., 2015) and Japanese encephalitis virus (Tewari et al., 1999). The surveillance of mosquitoes is a potential additional application for future ZIKV antigen assays.
Vectors of ZIKV
Aedes aegypti and A. albopictus are known to be the vectors of ZIKV (Li et al., 2012; Wong et al., 2013) and these two mosquitoes are also responsible for transmission of DENV and CHIKV. These are container breeding mosquitoes and it is known that the eggs of these mosquitoes can withstand desiccation. Thus Aedes mosquitoes are easily dispersed to many areas. It is also known that A. aegypti exhibits skip oviposition where it deposit its eggs in many containers (Reiter, 2007).
Zika virus was first isolated from A. aegypti from the rural area of Bentong in Pahang, Malaysia in 1965 (Marchette et al., 1969). Recent studies carried out in Singapore demonstrated that A. aegypti was susceptible to ZIKV and by day five almost 60% of the mosquito’s salivary glands were positive and on day six 100% were positive (Li et al., 2012). Studies conducted by the same group also demonstrated that A. albopictus could transmit ZIKV and by day 10 100% transmission was obtained in mosquito’s saliva (Wong et al., 2013).
Zika virus was found naturally infected in A. aegypti in 1965 (Marchette et al., 1969) and seropositivity of ZKIV was also reported in 1960s (Dash et al., 2013). Thus, is it possible that the ZIKV has been in Southeast Asia all the time and people have developed immunity to this virus? It has been postulated that ZIKV originated in East Africa and spread to West Africa and Asia thus forming three different genotypes; the Asian genotype further spread to Pacific Islands and the Americas (Lanciotti et al., 2016). Also a case of ZIKV was confirmed in a traveler who visited Sabah, Malaysian Borneo on his return to Germany (Tappe et al., 2015). Thus, there must be other cases that have not been reported, perhaps people would only have suffered mild symptoms and it would not have been detected.
In Gabon there was an outbreak of CHIKV and DENV in 2007 and 2010 (Grard et al., 2014). The predominant vector found was A. albopictus and 91 pools of them were screened of which four pools were positive for CHIKV, three pools for DENV and two pools had mixed infection of CHIKV and ZIKV (Grard et al., 2014). When sera samples from humans were screened five were found to be positive for ZIKV (Grard et al., 2014). Here it clearly showed that ZIKV was circulating along with CHIKV and DENV. By screening both human and mosquito pools concrete evidence has been established that ZIKV can be transmitted alongside CHIKV and DENV. This clearly indicates that the trapping of adult mosquitoes and detection of viruses in them is the way forward to prevent epidemics.
It has been estimated that 440,000 to 1,300,000 ZIKV cases have occurred in Brazil (Bogoch et al., 2016), and the virus has finally been isolated from A. aegypti in that country (Gretchen, 2016). Now that A. aegypti can be easily trapped using the sticky gravid trap, this should be carried out and the vector should be confirmed in all localities. With such a large number of cases one would expect that it would be fairly easy to obtain infected mosquitoes. For example in a dengue prone area in Selangor, Malaysia, we obtained a minimum field infection rate (MIR) of 38.02 per 1000 using the NS1 antigen test kit (Lau et al., 2015). Since it is more difficult to get blood from people living in urban areas and it involves ethical clearance the best way to move forward is to detect the virus in the mosquitoes and to start proper control measures when results are positive.
The same vectors A. aegypti and A. albopictus are responsible for the spread of DENV and CHIKV and these vectors know no borders. If control measures can be instituted for these vectors the incidence of all these arboviral diseases will also be decreased. It seems like ZIKV is taking the same route as CHIKV (Musso and Gubler, 2015). If that is the case Southeast Asia could be in the forefront for ZIKV outbreak in the very near future. Perhaps the people of Southeast Asia are already immune to the disease, but visitors to the region may get infected and help to spread the disease globally.
Vector Control Measures
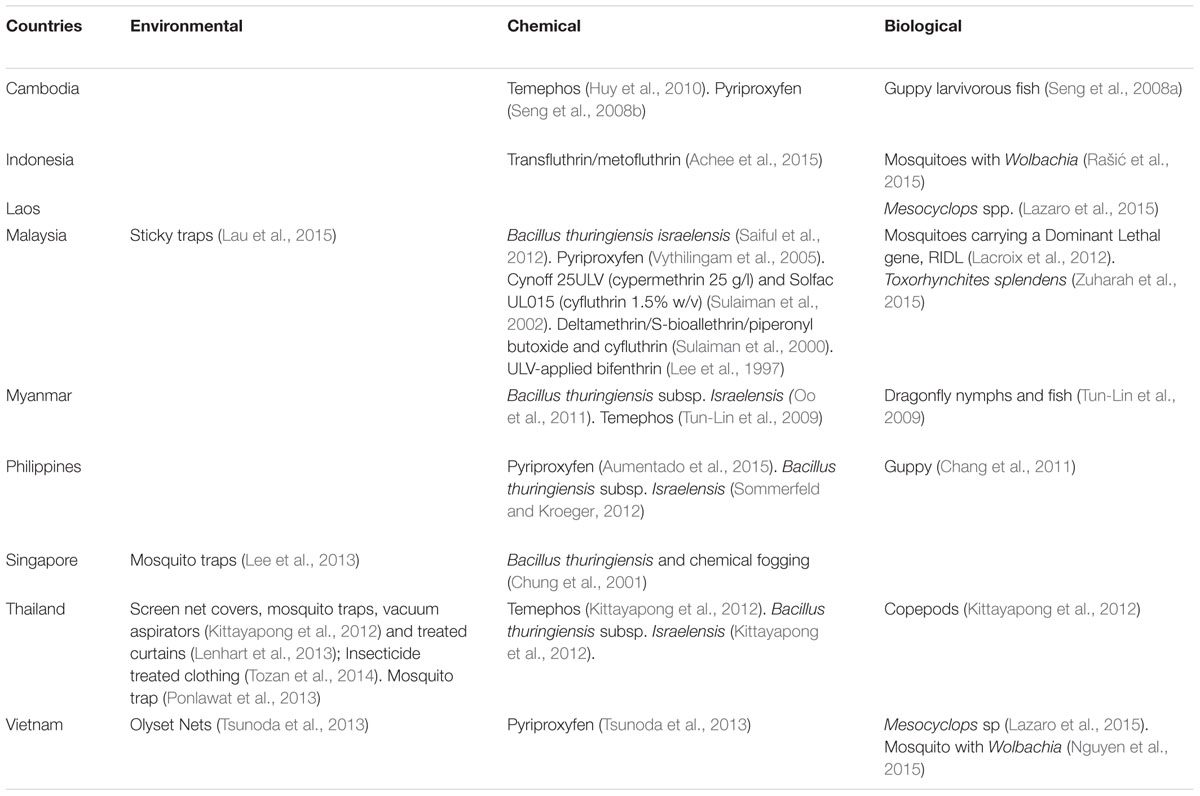
Vector control measures carried out in Southeast Asia for surveillance and control of A. aegypti are shown in Table 1. It can be seen that vector surveillance and control strategies are mainly targeting the larval breeding sites. This includes the use of chemicals (Lee et al., 1997; Sulaiman et al., 2000, 2002; Chung et al., 2001; Tun-Lin et al., 2009; Huy et al., 2010; Oo et al., 2011; Kittayapong et al., 2012; Saiful et al., 2012; Sommerfeld and Kroeger, 2012), biological agents (Seng et al., 2008a; Lacroix et al., 2012; Hugo et al., 2014; Lazaro et al., 2015; Zuharah et al., 2015), environmental management (Ooi et al., 2006; Tun-Lin et al., 2009; Lee et al., 2013; Lau et al., 2015), and community participation (Chang et al., 2011). We have become over-dependent on chemicals and now the Aedes mosquitoes are resistant to most pyrethroids (Ponlawat et al., 2005; Wan-Norafikah et al., 2010; Koou et al., 2014a,b; Ishak et al., 2015). Studies have also shown that space spraying has not conclusively been effective in reducing dengue transmission (Mount, 1998; Perich et al., 2000; Esu et al., 2010). Thus, when fogging/ULV is carried out impact on the vectors is minimal as shown in some studies (Vythilingam and Panart, 1991; Tanrang and Vythilingam, 2004). This could be one reason why cases of DENV are on the increase in countries in Southeast Asia.
Chang et al. (2011) have suggested that a positive move would be to include all three parameters of dengue transmission – vector density, human cases, and vector infection rate for prediction of early outbreaks. This seems to be the way forward in controlling these arboviral diseases. It has also been demonstrated that the gravid Aedes mosquito can be easily captured using sticky traps and the infected mosquito was obtained before human cases were reported (Lau et al., 2015). Studies conducted in different countries have demonstrated that the sticky traps are effective in collecting the Aedes mosquitoes when they come to oviposit (Ritchie et al., 2004; Gama et al., 2007; Honório et al., 2009; Chadee and Ritchie, 2010; de Santos et al., 2012; Resende et al., 2013). Thus a proactive approach is needed to test these mosquitoes for the different viruses so that a more positive control approach can be instituted. In Singapore too it has been shown that the sticky trap was able to trap the infected Aedes mosquito (Lee et al., 2013). It has also been documented that asymptomatic cases were infectious to Aedes mosquitoes and thus silent transmission was ongoing all the time (Duong et al., 2015).
House to house larval surveys have been the hallmark of dengue control program in many countries in Southeast Asia (Cheong, 1967; Ho and Vythilingam, 1980; Cheong et al., 1986; Chang et al., 2011; Mudin, 2015; Hapuarachchi et al., 2016). This method has also been used for the control of CHIKV vectors and can be used for ZIKV vectors. However, current studies have shown that although the Aedes house index has been reduced to as low as 0.07–0.14, yet epidemics of dengue have been explosive as reported in Singapore (Hapuarachchi et al., 2016). This has been due to the switch in serotypes as noted in 2007–2008 outbreaks (Lee et al., 2010) and also in 2013–2014 (Hapuarachchi et al., 2016). Singapore being a small and an affluent country can afford to carry out serotyping and sequencing in a timely manner but still face epidemics of dengue. It is agreed that a multi-pronged approach backed by the epidemiological, virological, and entomological understanding is necessary for the control of vector borne viral diseases. However, entomological activities have always been reactive and thus could be one of the reasons for the current epidemics in many countries.
Biological Control
The biological control approach traditionally reduce vector numbers by means of introducing their natural predators, such as larvivorous fish (Seng et al., 2008a) dragonfly nymphs (Tun-Lin et al., 2009), Mesocyclops sp (Lazaro et al., 2015), and Toxorhynchites splendens (Zuharah et al., 2015). While these approaches are environmentally friendly, they only affect the immature stages of the mosquito vector. In addition, they are effective only in containers that are constantly filled, such as wells and large containers (World Health Organization, 2012). Now that most breeding sites are cryptic it will be difficult to use biological control.
Insect Growth Regulator
Pyriproxyfen an insect growth regulator has been tested under field conditions and it has a unique mode of action where it inhibits the development of the adult mosquito and also when an adult mosquito comes in contact with pyriproxyfen it can help to transfer it to other containers (Invest and Lucas, 2008). Studies carried out in Southeast Asia have shown that low doses were required for the inhibition of adult Aedes and the residual activity can be maintained for 11–15 weeks (Vythilingam et al., 2005) in Malaysia, while in Cambodia using a slow release formulation residual activity was effective for 6 months (Seng et al., 2008b). In Philippines pyriproxyfen was successfully used to control the dengue outbreak after typhoon Haiyan (Aumentado et al., 2015).
The Way Forward
If we learn lessons from malaria control with regards to vectors, it was always targeted toward adult mosquitoes and not so much against the larvae. One reason could be that because it was difficult to find the breeding sites of Anopheles mosquitoes and some sites were inaccessible. However, for dengue, the vectors breed in containers and thus control of larvae and source reduction were the initial strategies for dengue control. This has obviously worked since Aedes index has been reduced to low levels (Mudin, 2015; Hapuarachchi et al., 2016) yet the cases of DENV infection have increased. Thus, it is timely now to focus on interventions based on the adult population to reduce and prevent epidemics of DENV. If we control the Aedes adults, we will automatically reduce outbreaks of ZIKV and CHIKV.
Besides monitoring the adult Aedes population it is similarly important to detect the pathogen in the mosquitoes. Currently for dengue the NS1 antigen test kit can be used and the procedure is very simple for use by public health workers. Thus what is needed for CHIKV, ZIKV, and other common arthropod borne viruses are very simple tools that can be used by the public health workers. Molecular tools like PCR and real-time PCR are available but these are expensive and need experienced staff and expensive equipment which is not feasible for a control program.
Although it has been stated that in Americas the success of A. aegypti eradication was due to perifocal spraying and source reduction (Achee et al., 2015), this will not work in present times because currently it is difficult to persuade people in urban areas to allow indoor residual spraying to be carried out. Besides in Southeast Asia it is also known that the A. aegypti like to rest on temporary surfaces like clothes and curtains (Pant and Yasuno, 1973).
Field studies have been carried out on use of insecticide treated curtains and jar covers and these have shown reduction in mosquito population (Kroeger et al., 2006; Lenhart et al., 2013; Tsunoda et al., 2013), however, its efficacy in reducing dengue cases have not been deciphered. Thus although a number of studies were conducted on various methods to monitor adult population (Lee et al., 2013; Ponlawat et al., 2013; Tozan et al., 2014; Lau et al., 2015) and show promise, the end result of reduction of cases has not been established.
Studies are also on going on genetically modified mosquitoes and one showing promise is the release of insects with dominant lethality (RIDL). In the laboratory these mosquito larvae are bred in water containing tetracycline, however, in the absence of tetracycline these larvae and pupae will not be able to survive. Field studies have been conducted in Cayman Islands, where there was a suppression of 80% of the natural population (Harris et al., 2012) and in Brazil there was a suppression of 85% of the natural population (Achee et al., 2015). Although this method has reduced mosquito populations, it should be noted that only male mosquitoes were released. However, a fool proof method is needed to ensure that females are not released. RIDL females are equally susceptible to dengue virus compared to the wild A. aegypti. Besides, evidence is required to show that with the reduction of the A. aegypti population it is possible to reduce dengue cases. Each country will also need to obtain approval from regulatory bodies before they can release these mosquitoes. Insectaries will also have to be maintained if these RIDL mosquitoes are to be used. All these come with a cost and countries should be able to afford these expensive methods before they embark on such a program.
Another similar approach is the release of A. aegypti with the bacteria of Wolbachia sp. Wolbachia is naturally found in many arthropods and nematodes but is not found in A. aegypti (Werren, 1997). Currently, one of the novel approaches for bio-control is the introduction of Wolbachia from naturally infected arthropods into A. aegypti to reduce dengue transmission (Moreira et al., 2009; Walker et al., 2011). When an uninfected female A. aegypti mates with a Wolbachia-infected male, the female will produce eggs but no progeny will develop due to cytoplasmic incompatibility. However, when a Wolbachia infected female mates with either infected or uninfected male, all progeny will carry Wolbachia (Caragata et al., 2016). Field trials have been conducted in Australia with the release of A. aegypti with wMel Wolbachia and the frequency has remained at more than 90% for 3 years (Hoffmann et al., 2014). However, field release of A. aegypti with wMelPop Wolbachia in Vietnam and Australia failed to become successfully established (Nguyen et al., 2015). Studies are also ongoing in Indonesia (Rašić et al., 2015). Thus it will take time for more studies to be conducted before Wolbachia infected A. aegypti can be used in dengue control program.
Now with ZIKV becoming a huge public health global problem, it is timely that randomized control trials (RCT) need to be carried out in Southeast Asia and prove that some of these paradigms will be able to control and prevent epidemics caused by these Aedes mosquitoes. For a start RCT studies should show that the cases of dengue can be reduced (Reiner et al., 2016). If a particular paradigm proves to be successful, it would also work for all the other arboviruses transmitted by A. aegypti and A. albopictus.
Conclusion
There are several options for ZIKV diagnosis building on existing technologies, which can be used in both humans and mosquitoes. However, most are not available in developing countries, and there remains an urgent need for an accessible RNA/antigen assay, as well as an IgM assay with acceptable specificity against other flaviviruses. While extensive work is ongoing to develop a vaccine, diagnostic kits, and to study the epidemiology of ZIKV, it is equally important to develop new paradigms to control the vectors. We need to learn from the past and thus a more proactive approach is needed to control the vectors and not a reactive one. In the early years besides Africa, ZIKV was known to be circulating Southeast Asia. Thus it is imperative to ensure that Southeast Asia don’t become a hub for transmitting the ZIKV to other countries. We need to work together and carry out multi-country RCT for vector control to show that the way forward is to monitor the adult Aedes population along with infectious status.
Author Contributions
IV, JS, YC, LK, and WW all played a role in the preparation of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
JS and YC received funding from the University of Malaya (HIR grant E000013-20001).
References
Achee, N. L., Gould, F., Perkins, T. A., Reiner, R. C. Jr., Morrison, A. C., Ritchie, S. A., et al. (2015). A critical assessment of vector control for dengue prevention. PLoS Negl. Trop. Dis. 9:e0003655. doi: 10.1371/journal.pntd.0003655
Alera, M. T., Hermann, L., Tac-An, I. A., Klungthong, C., Rutvisuttinunt, W., Manasatienkij, W., et al. (2015). Zika virus infection, Philippines, 2012. Emerg. Infect. Dis. 21, 722–724. doi: 10.3201/eid2104.141707
Aumentado, C., Cerro, B. R., Olobia, L., Suy, L. L., Reyes, A., Kusumawathie, P. H., et al. (2015). The prevention and control of dengue after Typhoon Haiyan. Western Pac. Surveill. Response J. 6(Suppl. 1), 60–65. doi: 10.5365/wpsar.2015.6.4.HYN_026
Bingham, A. M. (2016). Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease—Florida, 2016. MMWR Morb. Mortal. Wkly. Rep. 65, 475–478. doi: 10.15585/mmwr.mm6518e2
Bishop-Lilly, K. A., Turell, M. J., Willner, K. M., Butani, A., Nolan, N. M., Lentz, S. M., et al. (2010). Arbovirus detection in insect vectors by rapid, high-throughput pyrosequencing. PLoS Negl. Trop. Dis. 4:e878. doi: 10.1371/journal.pntd.0000878
Bogoch, I. I., Brady, O. J., Kraemer, M., German, M., Creatore, M. I., Kulkarni, M. A., et al. (2016). Anticipating the international spread of Zika virus from Brazil. Lancet 387, 335–336. doi: 10.1016/S0140-6736(16)00080-5
Buathong, R., Hermann, L., Thaisomboonsuk, B., Rutvisuttinunt, W., Klungthong, C., Chinnawirotpisan, P., et al. (2015). Detection of Zika virus infection in Thailand, 2012–2014. Am. J. Trop. Med. Hyg. 93, 380–383. doi: 10.4269/ajtmh.15-0022
Calisher, C. H., Karabatsos, N., Dalrymple, J. M., Shope, R. E., Porterfield, J. S., Westaway, E. G., et al. (1989). Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 70, 37–43. doi: 10.1099/0022-1317-70-1-37
Cao-Lormeau, V.-M., and Musso, D. (2014). Emerging arboviruses in the Pacific. Lancet 384, 1571–1572. doi: 10.1016/S0140-6736(14)61977-2
Caragata, E. P., Dutra, H. L., and Moreira, L. A. (2016). Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol. 32, 207–218. doi: 10.1016/j.pt.2015.10.011
CDCP (2016). Zika Virus: Diagnostic Testing. Available: http://www.cdc.gov/zika/hc-providers/diagnostic.html [accessed 21 July, 2016].
Chadee, D. D., and Ritchie, S. A. (2010). Efficacy of sticky and standard ovitraps for Aedes aegypti in Trinidad, West Indies. J. Vector Ecol. 35, 395–400. doi: 10.1111/j.1948-7134.2010.00098.x
Chang, M. S., Christophel, E. M., Gopinath, D., and Abdur, R. M. (2011). Challenges and future perspective for dengue vector control in the Western Pacific Region. Western Pac. Surveill. Response J. 2, 9–16. doi: 10.5365/wpsar.2010.1.1.012
Chao, D.-Y., Liu, Y.-J., Shen, W.-F., Tu, W.-C., Galula, J. U., and Wu, H.-C. (2015). Comparison of E and NS1 antigens capture ELISA to detect dengue viral antigens from mosquitoes. J. Vector Borne Dis. 52, 134–141.
Cheong, W. (1967). Preferred Aedes aegypti larval habitats in urban areas. Bull. World Health Organ. 36, 586–589.
Cheong, W., Rudnick, A., and Lin, T. (1986). The vectors of dengue and dengue hemorrhagic fevers in Malaysia. Dengue Fever Studies in Malaysia. Inst. Med. Res. Bull. 23, 155–167.
Chung, Y., Lam-Phua, S., Chua, Y., and Yatiman, R. (2001). Evaluation of biological and chemical insecticide mixture against Aedes aegypti larvae and adults by thermal fogging in Singapore. Med. Vet. Entomol. 15, 321–327. doi: 10.1046/j.0269-283x.2001.00311.x
Cordeiro, M. T., Pena, L. J., Brito, C. A., Gil, L. H., and Marques, E. T. (2016). Positive IgM for Zika virus in the cerebrospinal fluid of 30 neonates with microcephaly in Brazil. Lancet 387, 1811–1812. doi: 10.1016/S0140-6736(16)30253-7
Dash, A., Bhatia, R., Sunyoto, T., and Mourya, D. (2013). Emerging and re-emerging arboviral diseases in Southeast Asia. J. Vector Borne Dis. 50, 77–84.
de Santos, E., de Melo-Santos, M., de Oliveira, C., Correia, J. C., and de Albuquerque, C. (2012). Evaluation of a sticky trap (AedesTraP), made from disposable plastic bottles, as a monitoring tool for Aedes aegypti populations. Parasit. Vectors 5:195. doi: 10.1186/1756-3305-5-195
Dick, G., Kitchen, S., and Haddow, A. (1952). Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 46, 509–520. doi: 10.1016/0035-9203(52)90042-4
Duffy, M. R., Chen, T.-H., Hancock, W. T., Powers, A. M., Kool, J. L., Lanciotti, R. S., et al. (2009). Zika virus outbreak on Yap Island, federated states of Micronesia. N. Engl. J. Med. 360, 2536–2543. doi: 10.1056/NEJMoa0805715
Duong, V., Lambrechts, L., Paul, R. E., Ly, S., Lay, R. S., Long, K. C., et al. (2015). Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 112, 14688–14693. doi: 10.1073/pnas.1508114112
Esu, E., Lenhart, A., Smith, L., and Horstick, O. (2010). Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop. Med. Int. Health 15, 619–631. doi: 10.1111/j.1365-156.2010.02489.x
Fauci, A. S., and Morens, D. M. (2016). Zika virus in the Americas—yet another arbovirus threat. N. Engl. J. Med. 374, 601–604. doi: 10.1056/NEJMp1600297
Focosi, D., Maggi, F., and Pistello, M. (2016). Zika virus: implications for public health. Clin. Infect. Dis. 63, 227–233. doi: 10.1093/cid/ciw210
Gama, R. A., Silva, E. M., Silva, I. M., Resende, M. C., and Eiras, Á. E. (2007). Evaluation of the sticky MosquiTRAPTM for detecting Aedes (Stegomyia) aegypti (L.)(Diptera: Culicidae) during the dry season in Belo Horizonte, Minas Gerais, Brazil. Neotrop. Enomol. 36, 294–302. doi: 10.1590/S1519-566X2007000200018
Grard, G., Caron, M., Mombo, I. M., Nkoghe, D., Ondo, S. M., Jiolle, D., et al. (2014). Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 8:e2681. doi: 10.1371/journal.pntd.0002681
Gretchen, V. (2016). Top Mosquito Suspect Found Infected with Zika. Available: http://www.sciencemag.org/news/2016/05/top-mosquito-suspect-found-infected-zika [Accessed 25 July, 2016]
Gyurech, D., Schilling, J., Schmidt-Chanasit, J., Cassinotti, P., Kaeppeli, F., and Dobec, M. (2016). False positive dengue NS1 antigen test in a traveller with an acute Zika virus infection imported into Switzerland. Swiss Med. Wkly. 146:w14296. doi: 10.4414/smw.2016.14296
Haddow, A., Williams, M., Woodall, J., Simpson, D., and Goma, L. (1964). Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull. World Health Organ. 31, 57–69.
Haddow, A. D., Schuh, A. J., Yasuda, C. Y., Kasper, M. R., Heang, V., Huy, R., et al. (2012). Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 6:e1477. doi: 10.1371/journal.pntd.0001477
Hapuarachchi, H. C., Koo, C., Rajarethinam, J., Chong, C.-S., Lin, C., Yap, G., et al. (2016). Epidemic resurgence of dengue fever in Singapore in 2013–2014: a virological and entomological perspective. BMC Infect. Dis. 16:1. doi: 10.1186/s12879-016-1606-z
Harris, A. F., McKemey, A. R., Nimmo, D., Curtis, Z., Black, I., Morgan, S. A., et al. (2012). Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30, 828–830. doi: 10.1038/nbt.2350
Hayes, E. B. (2009). Zika virus outside Africa. Emerg. Infect. Dis. 15, 1347–1350. doi: 10.3201/eid1509.090442
Heang, V., Yasuda, C. Y., Sovann, L., Haddow, A. D., Travassos da Rosa, A. P., Tesh, R. B., et al. (2012). Zika virus infection, Cambodia, 2010. Emerg. Infect. Dis. 18, 349–351. doi: 10.3201/eid1802.111224
Ho, T., and Vythilingam, I. (1980). A preliminary survey of Ae. aegypti in Selangor Peninsular Malaysia. Med. J. Malaysia 24, 409–414.
Hoffmann, A. A., Iturbe-Ormaetxe, I., Callahan, A. G., Phillips, B. L., Billington, K., Axford, J. K., et al. (2014). Stability of the w Mel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl. Trop. Dis. 8:e3115. doi: 10.1371/journal.pntd.0003115
Honório, N. A., Codeço, C. T., Alves, F. C., Magalhães, M. A., and Lourenço-de-Oliveira, R. (2009). Temporal distribution of Aedes aegypti in different districts of Rio de Janeiro, Brazil, measured by two types of traps. J. Med. Entomol. 46, 1001–1014. doi: 10.1603/033.046.0505
Hugo, L. E., Jeffery, J. A., Trewin, B. J., Wockner, L. F., Yen, N. T., Le, N. H., et al. (2014). Adult survivorship of the dengue mosquito Aedes aegypti varies seasonally in central Vietnam. PLoS Negl. Trop. Dis. 8:e2669. doi: 10.1371/journal.pntd.0002669
Huy, R., Buchy, P., Conan, A., Ngan, C., Ong, S., Ali, R., et al. (2010). National dengue surveillance in Cambodia 1980–2008: epidemiological and virological trends and the impact of vector control. Bull. World Health Organ. 88, 650–657. doi: 10.2471/BLT.09.073908
Huzly, D., Hanselmann, I., Schmidt-Chanasit, J., and Panning, M. (2016). High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro. Surveill. 21:30203. doi: 10.2807/1560-7917.ES.2016.21.16.30203
Invest, J., and Lucas, J. (2008). “Pyriproxyfen as a mosquito larvicide,” in Proceedings of the Sixth International Conference on Urban Pests Veszprem.
Ishak, I. H., Jaal, Z., Ranson, H., and Wondji, C. S. (2015). Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit. Vectors 8:181. doi: 10.1186/s13071-015-0797-2
Kindhauser, M. K., Allen, T., Frank, V., Santhana, R. S., and Dye, C. (2016). Zika: the origin and spread of a mosquito-borne virus. Bull. World Health Organ. 1–18. doi: 10.2471/BLT.16.171082
Kittayapong, P., Thongyuan, S., Olanratmanee, P., Aumchareoun, W., Koyadun, S., Kittayapong, R., et al. (2012). Application of eco-friendly tools and eco-bio-social strategies to control dengue vectors in urban and peri-urban settings in Thailand. Pathog. Glob. Health 106, 446–454. doi: 10.1179/2047773212Y.0000000059
Koou, S.-Y., Chong, C.-S., Vythilingam, I., Lee, C., and Ng, L.-C. (2014a). Insecticide resistance and its underlying mechanisms in field populations of Aedes aegypti adults (Diptera: Culicidae) in Singapore. Parasit. Vectors 7:471. doi: 10.1186/s13071-014-0471-0
Koou, S.-Y., Chong, C.-S., Vythilingam, I., Ng, L.-C., and Lee, C.-Y. (2014b). Pyrethroid resistance in Aedes aegypti larvae (Diptera: Culicidae) from Singapore. J. Med. Entomol. 51, 170–181. doi: 10.1603/ME13113
Kroeger, A., Lenhart, A., Ochoa, M., Villegas, E., Levy, M., Alexander, N., et al. (2006). Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. Br. Med. J. 332, 1247–1252. doi: 10.1136/bmj.332.7552.1247
Lacroix, R., McKemey, A. R., Raduan, N., Wee, L. K., Ming, W. H., Ney, T. G., et al. (2012). Open field release of genetically engineered sterile male Aedes aegypti in Malaysia. PLoS ONE 7:e42771. doi: 10.1371/journal.pone.0042771
Lanciotti, R. S., Lambert, A. J., Holodniy, M., Saavedra, S., and Signor, L. D. C. C. (2016). Phylogeny of Zika virus in western hemisphere, 2015. Emerg. Infect. Dis. 22, 933–935. doi: 10.3201/eid2205.160065
Lau, S. M., Vythilingam, I., Doss, J. I., Sekaran, S. D., Chua, T. H., Sulaiman, W., et al. (2015). Surveillance of adult Aedes mosquitoes in Selangor, Malaysia. Trop. Med. Int. Health 20, 1271–1280. doi: 10.1111/tmi.12555
Lazaro, A., Han, W., Manrique-Saide, P., George, L., Velayudhan, R., Toledo, J., et al. (2015). Community effectiveness of copepods for dengue vector control: systematic review. Trop. Med. Int. Health 20, 685–706. doi: 10.1111/tmi.12485
Lee, C., Vythilingam, I., Chong, C.-S., Razak, M. A. A., Tan, C.-H., Liew, C., et al. (2013). Gravitraps for management of dengue clusters in Singapore. Am. J. Trop. Med. Hyg. 88, 888–892. doi: 10.4269/ajtmh.12-0329
Lee, H., Khadri, M., and Chiang, Y. (1997). Preliminary field evaluation of the combined adulticidal, larvicidal, and wall residual activity of ULV-applied bifenthrin against mosquitoes. J. Vector Ecol. 22, 146–149.
Lee, K.-S., Lai, Y.-L., Lo, S., Barkham, T., Aw, P., Ooi, P.-L., et al. (2010). Dengue virus surveillance for early warning, Singapore. Emerg. Infect. Dis. 16, 847–849. doi: 10.3201/eid1605.091006
Lenhart, A., Trongtokit, Y., Alexander, N., Apiwathnasorn, C., Satimai, W., Vanlerberghe, V., et al. (2013). A cluster-randomized trial of insecticide-treated curtains for dengue vector control in Thailand. Am. J. Trop. Med. Hyg. 88, 254–259. doi: 10.4269/ajtmh.2012.12-0423
Li, M. I., Wong, P. S. J., Ng, L. C., and Tan, C. H. (2012). Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl. Trop. Dis. 6:e1792. doi: 10.1371/journal.pntd.0001792
Lindsey, H. S., Calisher, C. H., and Mathews, J. H. (1976). Serum dilution neutralization test for California group virus identification and serology. J. Clin. Microbiol. 4, 503–510.
Mansuy, J. M., Dutertre, M., Mengelle, C., Fourcade, C., Marchou, B., Delobel, P., et al. (2016). Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen. Lancet Infect. Dis. 16:405. doi: 10.1016/S1473-3099(16)00138-9.
Marchette, N., Garcia, R., and Rudnick, A. (1969). Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 18, 411–415.
Matheus, S., Boukhari, R., Labeau, B., Ernault, V., Bremand, L., Kazanji, M., et al. (2016). Specificity of dengue NS1 antigen in differential diagnosis of dengue and Zika virus infection. Emerg. Infect. Dis. 22, 1691–1693. doi: 10.3201/eid2209.160725
Meagher, R. J., Negrete, O. A., and Van Rompay, K. K. (2016). Engineering paper-based sensors for Zika virus. Trends Mol. Med. 22, 529–530. doi: 10.1016/j.molmed.2016.05.009
Moreira, L. A., Iturbe-Ormaetxe, I., Jeffery, J. A., Lu, G., Pyke, A. T., Hedges, L. M., et al. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278. doi: 10.1016/j.cell.2009.11.042
Mount, G. A. (1998). A critical review of ultralow-volume aerosols of insecticide applied with vehicle-mounted generators for adult mosquito control. J. Am. Mosq. Control Assoc. 14, 305–334.
Mudin, R. N. (2015). Dengue incidence and the prevention and control program in Malaysia. Int. Med. J. Malaysia 14, 05–10.
Muller, D. A., Frentiu, F. D., Rojas, A., Moreira, L. A., O’Neill, S. L., and Young, P. R. (2012). A portable approach for the surveillance of dengue virus-infected mosquitoes. J. Virol. Methods 183, 90–93. doi: 10.1016/j.jviromet.2012.03.033
Musso, D., and Gubler, D. J. (2015). Zika virus: following the path of dengue and chikungunya? Lancet 386, 243–244. doi: 10.1016/S0140-6736(15)61273-9
Musso, D., Nhan, T., Robin, E., Roche, C., Bierlaire, D., Zisou, K., et al. (2014). Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro. Surveill. 19, 1–3.
Musso, D., Roche, C., Robin, E., Nhan, T., Teissier, A., and Cao-Lormeau, V.-M. (2015). Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 21, 359–361. doi: 10.3201/eid2102.141363
Nguyen, T. H., Le Nguyen, H., Nguyen, T. Y., Vu, S. N., Tran, N. D., Le, T., et al. (2015). Field evaluation of the establishment potential of wMelpop Wolbachia in Australia and Vietnam for dengue control. Parasit. Vectors 8:563. doi: 10.1186/s13071-015-1174-x
Oliveira Melo, A., Malinger, G., Ximenes, R., Szejnfeld, P., Alves Sampaio, S., and Bispo de Filippis, A. (2016). Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet. Gynecol. 47, 6–7. doi: 10.1002/uog.15831
Oo, T., Storch, V., Madon, M., and Becker, N. (2011). Factors influencing the seasonal abundance of Aedes (Stegomyia) aegypti and the control strategy of dengue and dengue haemorrhagic fever in Thanlyin Township, Yangon City, Myanmar. Trop. Biomed. 28, 302–311.
Ooi, E.-E., Goh, K.-T., and Gubler, D. J. (2006). Dengue prevention and 35 years of vector control in Singapore. Emerg. Infect. Dis. 12, 887–893. doi: 10.3201/eid1206.051210
Pant, C., and Yasuno, M. (1973). Field studies on the gonotrophic cycle of Aedes aegypti in Bangkok, Thailand. J. Med. Entomol. 10, 219–223. doi: 10.1093/jmedent/10.2.219
Pardee, K., Green, A. A., Takahashi, M. K., Braff, D., Lambert, G., Lee, J. W., et al. (2016). Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 165, 1255–1266. doi: 10.1016/j.cell.2016.04.059
Perich, M., Davila, G., Turner, A., Garcia, A., and Nelson, M. (2000). Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. J. Med. Entomol. 37, 541–546. doi: 10.1603/0022-2585-37.4.541
Perkasa, A., Yudhaputri, F., Haryanto, S., Hayati, R. F., Ma’roef, C. N., Antonjaya, U., et al. (2016). Isolation of Zika virus from febrile patient, Indonesia. Emerg. Infect. Dis. 22, 924–925. doi: 10.3201/eid2205.151915
Ponlawat, A., Fansiri, T., Kurusarttra, S., Pongsiri, A., McCardle, P. W., Evans, B. P., et al. (2013). Development and evaluation of a pyriproxyfen-treated device to control the dengue vector, Aedes aegypti (L.)(Diptera: culicidae). Southeast Asian J. Trop. Med. Public Health 44, 167–178.
Ponlawat, A., Scott, J. G., and Harrington, L. C. (2005). Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J. Med. Entomol. 42, 821–825. doi: 10.1093/jmedent/42.5.821
Rabe, I. B. (2016). Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb. Mortal. Wkly. Rep. 65, 543–546. doi: 10.15585/mmwr.mm6521e1
Rašić, G., Endersby-Harshman, N., Tantowijoyo, W., Goundar, A., White, V., Yang, Q., et al. (2015). Aedes aegypti has spatially structured and seasonally stable populations in Yogyakarta, Indonesia. Parasit. Vectors 8:610. doi: 10.1186/s13071-015-1230-6
Reiner, R. C. Jr., Achee, N., Barrera, R., Burkot, T. R., Chadee, D. D., Devine, G. J., et al. (2016). Quantifying the epidemiological impact of vector control on dengue. PLoS Negl. Trop. Dis. 10:e0004588. doi: 10.1371/journal.pntd.0004588
Reiter, P. (2007). Oviposition, dispersal, and survival in Aedes aegypti: implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 7, 261–273. doi: 10.1089/vbz.2006.0630
Resende, M. C. D., Silva, I. M., Ellis, B. R., and Eiras, A. E. (2013). A comparison of larval, ovitrap and MosquiTRAP surveillance for Aedes (Stegomyia) aegypti. Mem. Inst. Oswaldo Cruz 108, 1024–1030. doi: 10.1590/0074-0276130128
Reusken, C., Pas, S., GeurtsvanKessel, C., Mögling, R., van Kampen, J., Langerak, T., et al. (2016). Longitudinal follow-up of Zika virus RNA in semen of a traveller returning from Barbados to the Netherlands with Zika virus disease, March 2016. Euro. Surveill. 21:30251. doi: 10.2807/1560-7917.ES.2016.21.23.30251
Ritchie, S. A., Long, S., Smith, G., Pyke, A., and Knox, T. B. (2004). Entomological investigations in a focus of dengue transmission in Cairns, Queensland, Australia, by using the sticky ovitraps. J. Med. Entomol. 41, 1–4. doi: 10.1603/0022-2585-41.1.1
Rodriguez-Morales, A. J., Villamil-Gómez, W. E., and Franco-Paredes, C. (2016). The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med. Infect. Dis. 14, 177–179. doi: 10.1016/j.tmaid.2016.05.004
Roth, A., Mercier, A., Lepers, C., Hoy, D., Duituturaga, S., Benyon, E., et al. (2014). Concurrent outbreaks of dengue, chikungunya and Zika virus infections-an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro. Surveill. 19:20929. doi: 10.2807/1560-7917.ES2014.19.41.20929
Saiful, A., Lau, M., Sulaiman, S., and Hidayatulfathi, O. (2012). Residual effects of TMOF–Bti formulations against 1 st instar Aedes aegypti Linnaeus larvae outside laboratory. Asian Pac. J. Trop. Biomed. 2, 315–319. doi: 10.1016/S2221-1691(12)60031-8
Sam, J. I., Chan, Y. F., Vythilingam, I., and Wan Sulaiman, W. Y. (2016). Zika virus and its potential re-emergence in Malaysia. Med. J. Malaysia 71, 66–68.
Samuel, P. P., and Tyagi, B. (2006). Diagnostic methods for detection & isolation of dengue viruses from vector mosquitoes. Indian J. Med. Res. 123, 615–628.
Seng, C. M., Setha, T., Nealon, J., Socheat, D., Chantha, N., and Nathan, M. B. (2008a). Community-based use of the larvivorous fish Poecilia reticulata to control the dengue vector Aedes aegypti in domestic water storage containers in rural Cambodia. J. Vector Ecol. 33, 139–144. doi: 10.3376/1081-1710
Seng, C. M., Setha, T., Nealon, J., Socheat, D., and Nathan, M. B. (2008b). Six months of Aedes aegypti control with a novel controlled-release formulation of pyriproxyfen in domestic water storage containers in Cambodia. Southeast Asian J. Trop. Med. Public Health 39, 822–826.
Sommerfeld, J., and Kroeger, A. (2012). Eco-bio-social research on dengue in Asia: a multicountry study on ecosystem and community-based approaches for the control of dengue vectors in urban and peri-urban Asia. Pathog. Glob. Health 106, 428–435. doi: 10.1179/2047773212Y.0000000055
Song, H., Qi, J., Haywood, J., Shi, Y., and Gao, G. F. (2016). Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat. Struct. Mol. Biol. 23, 456–458. doi: 10.1038/nsmb.3213
Song, J., Mauk, M. G., Hackett, B. A., Cherry, S., Bau, H. H., and Liu, C. (2016). Instrument-free point-of-care molecular detection of Zika virus. Anal. Chem. 88, 7289–7294. doi: 10.1021/acs.analchem.6b01632
Stettler, K., Beltramello, M., Espinosa, D. A., Graham, V., Cassotta, A., Bianchi, S., et al. (2016). Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science 353, 823–826. doi: 10.1126/science.aaf8505
Sulaiman, S., Pawanchee, Z., Othman, H., Shaari, N., Yahaya, S., Wahab, A., et al. (2002). Field evaluation of cypermethrin and cyfluthrin against dengue vectors in a housing estate in Malaysia. J. Vector Ecol. 27, 230–234.
Sulaiman, S., Pawanchee, Z., Othman, H. F., Jamal, J., Wahab, A., Sohadi, A., et al. (2000). Field evaluation of deltamethrin/S-bioallethrin/piperonyl butoxide and cyfluthrin against dengue vectors in Malaysia. J. Vector Ecol. 25, 94–97.
Tan, C.-H., Wong, P.-S. J., Li, M.-Z. I., Vythilingam, I., and Ng, L.-C. (2011). Evaluation of the dengue NS1 Ag Strip® for detection of dengue virus antigen in Aedes aegypti (Diptera: Culicidae). Vector Borne Zoonotic Dis. 11, 789–792. doi: 10.1089/vbz.2010.0028
Tanrang, Y., and Vythilingam, I. (2004). Field trial to determine the efficacy of pyrethroid Fendona 10 SC@ application using ultra- low- volume for the eontrol of Aedes mosquitoes. Trop. Biomed. 21, 57–65.
Tappe, D., Nachtigall, S., Kapaun, A., Schnitzler, P., Gunther, S., and Schmidt-Chanasit, J. (2015). Acute Zika virus infection after travel to Malaysian Borneo, September 2014. Emerg. Infect. Dis. 21, 911–913. doi: 10.3201/eid2105.141960
Tappe, D., Rissland, J., Gabriel, M., Emmerich, P., Günther, S., Held, G., et al. (2014). First Tappe et al., 2013 case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro. Surveill. 19:20685.
Tewari, S., Thenmozhi, V., Rajendran, R., Appavoo, N., and Gajanana, A. (1999). Detection of Japanese encephalitis virus antigen in desiccated mosquitoes: an improved surveillance system. Trans. R. Soc. Trop. Med. Hyg. 93, 525–526. doi: 10.1016/S0035-9203(99)90365-6
Thenmozhi, V., Kabilan, L., Philip Samuel, P., and Dash, A. (2005). Short Communication: detection of dengue virus antigens in desiccated mosquitoes: an improved tool for surveillance. Trop. Med. Int. Health 10, 187–189. doi: 10.1111/j.1365-3156.2004.01360.x
Tozan, Y., Ratanawong, P., Louis, V. R., Kittayapong, P., and Wilder-Smith, A. (2014). Use of insecticide-treated school uniforms for prevention of dengue in schoolchildren: a cost-effectiveness analysis. PLoS ONE 9:e108017. doi: 10.1371/journal.pone.0108017
Tsunoda, T., Kawada, H., Huynh, T. T., Le Luu, L., Tran, H. N., Vu, H. T. Q., et al. (2013). Field trial on a novel control method for the dengue vector, Aedes aegypti by the systematic use of Olyset® Net and pyriproxyfen in Southern Vietnam. Parasit. Vectors 6:6. doi: 10.1186/1756-3305-6-6
Tun-Lin, W., Lenhart, A., Nam, V. S., Rebollar-Téllez, E., Morrison, A., Barbazan, P., et al. (2009). Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop. Med. Int. Health 14, 1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x
Venturi, G., Zammarchi, L., Fortuna, C., Remoli, M., Benedetti, E., Fiorentini, C., et al. (2016). An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro. Surveill. 21:30148. doi: 10.2807/1560-7917.ES.2016.21.8.30148
Voge, N. V., Sánchez-Vargas, I., Blair, C. D., Eisen, L., and Beaty, B. J. (2013). Detection of dengue virus NS1 antigen in infected Aedes aegypti using a commercially available kit. Am. J. Trop. Med. Hyg. 88, 260–266. doi: 10.4269/ajtmh.2012.12-0477
Vythilingam, I., Luz, B. M., Hanni, R., Beng, T. S., and Huat, T. C. (2005). Laboratory and field evaluation of the insect growth regulator pyriproxyfen (Sumilarv 0.5G) against dengue vectors. J. Am. Mosq. Control Assoc. 21, 296–300. doi: 10.2987/8756-971X
Vythilingam, I., and Panart, P. (1991). A field trial on the comparative effectiveness of malathion and Resigen by ULV application on Aedes aegypti. Southeast Asian J. Trop. Med. Public Health 22, 102–107.
Waggoner, J. J., Gresh, L., Mohamed-Hadley, A., Ballesteros, G., Davila, M., Tellez, Y., et al. (2016). Single-reaction multiplex reverse transcription PCR for detection of Zika, chikungunya, and dengue viruses. Emerg. Infect. Dis. 22, 1295–1297. doi: 10.3201/eid2207.160326
Waggoner, J. J., and Pinsky, B. A. (2016). Zika virus: diagnostics for an emerging pandemic threat. J. Clin. Microbiol. 54, 860–867. doi: 10.1128/JCM.00279-16
Walker, T., Johnson, P., Moreira, L., Iturbe-Ormaetxe, I., Frentiu, F., McMeniman, C., et al. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453. doi: 10.1038/nature10355
Wan-Norafikah, O., Nazni, W. A., Lee, H. L., Zainol-Ariffin, P., and Sofian-Azirun, M. (2010). Permethrin resistance in Aedes aegypti (Linnaeus) collected from Kuala Lumpur, Malaysia. J. Asia Pac. Entomol. 13, 175–182. doi: 10.1016/j.aspen.2010.03.003
Werren, J. H. (1997). Biology of Wolbachia. Annu. Rev. Entomol. 42, 587–609. doi: 10.1146/annurev.ento.42.1.587
Wong, P.-S. J., Li, M.-Z. I., Chong, C.-S., Ng, L.-C., and Tan, C.-H. (2013). Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 7:e2348. doi: 10.1371/journal.pntd.0002348
World Health Organization (2012). Global Strategy for Dengue Prevention and Control 2012–2020. Geneva: WHO.
World Health Organization (2016a). Emergency USE ASSESSMENT AND LISTing (EUAL) Procedure for Zika Virus Disease (IVDs). Geneva: WHO, 2–3.
World Health Organization (2016b). WHO and experts prioritize vaccines, diagnostics and innovative vector control tools for Zika R&D. Saudi Med. J. 37, 471–472.
Keywords: Zika virus, vectors, diagnostic tools, new paradigms, control
Citation: Vythilingam I, Sam JI-C, Chan YF, Khaw LT and Wan Sulaiman WY (2016) New Paradigms for Virus Detection, Surveillance and Control of Zika Virus Vectors in the Settings of Southeast Asia. Front. Microbiol. 7:1452. doi: 10.3389/fmicb.2016.01452
Received: 27 July 2016; Accepted: 30 August 2016;
Published: 13 September 2016.
Edited by:
Rubén Bueno-Marí, University of Valencia, SpainReviewed by:
Andrew Jardine, Department of Health Western Australia, AustraliaCelio Geraldo Freire De Lima, Federal University of Rio de Janeiro, Brazil
Copyright © 2016 Vythilingam, Sam, Chan, Khaw and Wan Sulaiman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Indra Vythilingam, aW5kcmF2QHVtLmVkdS5teQ==
 Indra Vythilingam
Indra Vythilingam Jamal I-C. Sam
Jamal I-C. Sam Yoke F. Chan
Yoke F. Chan Loke T. Khaw
Loke T. Khaw Wan Y. Wan Sulaiman1
Wan Y. Wan Sulaiman1