- 1Xinjiang Key Laboratory of Environmental Pollution and Bioremediation, Xinjiang Institute of Ecology and Geography – Chinese Academy of Sciences, Urumqi, China
- 2Xinjiang Academy of Environmental Protection Sciences, Urumqi, China
- 3State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry – Chinese Academy of Sciences, Guiyang, China
The effects of UV-B radiation (UVBR) on photosynthetic activity (Fv/Fm) of aquatic Synechocystis sp. and desert Chroococcus minutus and effects on composition and fluorescence property of extracellular polymeric substances (EPSs) from Synechocystis sp. and C. minutus were comparatively investigated. The desert cyanobacterium species C. minutus showed higher tolerance of PSII activity (Fv/Fm) to UVBR than the aquatic Synechocystis sp., and the inhibited PSII activity of C. minutus could be fully recovered while that of Synechocystis sp. could be partly recovered. UVBR had significant effect on the yield and biochemical composition of EPS of both species. Protein-like and humic acid-like substances were detected in EPS from Synechocystis sp., and protein-like and phenol-like fluorescent compounds were detected in EPS from C. minutus. Proteins in EPS of desert and aquatic species were significantly decomposed under UVBR, and the latter was more easily decomposed. The polysaccharides were much more resistant to UVBR than the proteins for both species. Polysaccharides of Synechocystis sp. was degraded slightly but those of C. minutus was little decomposed. The higher tolerance to UVBR of the desert cyanobacterium can be attributed to the higher resistance of its EPS to photodegradation induced by UVBR in comparison with the aquatic species.
Introduction
Ultraviolet-B radiation (UVBR) at 280–315 nm is one of the major detrimental environmental factors for photosynthetic organisms in arid regions (Bowker et al., 2002). Depletion of the ozone layer leads to increasing levels of UVBR reaching the earth’s surface (Madronich et al., 1995).
Cyanobacteria are important photosynthetic microorganisms that live widely in aquatic and terrestrial environments. Exposure to UVBR can be potentially harmful for cyanobacteria in case the provoked damage exceeds the capacity of defense and repair mechanism (Sutherland, 2001). Some studies have shown that UVBR could induce physiological changes, including inhibition and even damage to photosystem, and enhance the content of reactive oxygen species (ROS) in cyanobacterial cell (Kumar et al., 2004). The high level of ROS is potentially harmful to the normal metabolism and results in oxidative injury such as protein degradation and DNA damage (He and Häder, 2002). Cyanobacteria have developed many mechanisms to against UVBR (Sinha et al., 1998). For example, cyanobacteria can synthesize UV-B absorbing compounds to scavenge ROS, or escape from UV-B damage by migration (Quesada and Vincent, 1997).
As ubiquitous species in aquatic and terrestrial habitats, most cyanobacteria are capable of secreting large quantities of extracellular polymeric substances (EPSs), which are mainly composed of polysaccharides, proteins, humic substances, and nucleic acids (Liu and Fang, 2002; Zhu et al., 2012). EPS has important cellular functions, including accumulation of nutrients, cell motility formation of barrier for toxins, attachment to surfaces and contribution for biofilms (Nichols et al., 2005; Ozturk et al., 2009; Cosenza et al., 2013). Synthesis of EPS in cyanobacteria leads to a better survival capacity compared to many other phototrophic microorganisms under UV radiation (Helm et al., 2000). EPS of cyanobacteria form a buffer zone between living cell and environment to counteract the adverse effects of UV radiation. The free radicals-scavenging ability of EPS plays a major role in the tolerance of bacteria to UV radiation (Wang et al., 2007). However, the composition and structure of EPS vary with cyanobacteria species and environmental conditions (Stal and Krumbein, 1985). Some studies showed that the monosaccharides component, molecular weight, and proteins content of EPS may affect their activities to counteract with UVBR (Calazans et al., 2000).
As the most important protective barrier, little data on the response of photosynthetic activity of cyanobacteria and the protective function of their EPS to UVBR are available. In the present study, the freshwater Synechocystis sp. and the desert Chroococcus minutus are chosen as the model cyanobacteria to study the decomposability of EPS and its effect on growth and photosynthetical activity of cyanobacteria under UVBR.
Materials and Methods
Organisms and Culture Conditions
One freshwater Synechocystis sp. and one desert C. minutus were selected in this study. The Synechocystis sp. (FACHB-898) was obtained from the Institute of Hydrobiology – Chinese Academy of Sciences. The C. minutus was isolated and purified from desert soil. Cyanobacterial cells were grown in BG-11 medium (Stanier et al., 1971) at 30°C and an irradiance of 55 μmol photons/m2⋅s PAR with a 12-h light: dark photoperiod.
EPS Extraction
Extracellular polymeric substance were extracted by high-speed centrifugation (Zhang et al., 2010; Pan et al., 2012). The culture was centrifuged at 5500 rpm for 10 min at 4°C. The cyanobacterial cells were re-suspended in deionized water. Then the cell suspension was centrifuged at 12300 rpm for 20 min at 4°C. The supernatant was filtrated through 0.45 μm acetate cellulose membrane and purified with dialysis membrane (3500 Da) for 24 h at 4°C.
UV-B Treatments
The glass plates containing the cultures and the glass plates containing only EPS were placed in a sterilized cabinet at room temperature under both photosynthetically active radiation (PAR) and UVBR. The radiation of 55 μmol photons/m2⋅s PAR was supplied by white fluorescent light, and the UVBR of 610 μW/cm2 (6.1 J/m2⋅s) was supplied by a ultraviolet radiation lamp (EB-160C/FE, USA) with the peak emission at 312 nm. The UV-B lamp was turned on 30 min prior to use to allow for stabilization of the UV output. The UV-B exposure time of cyanobacteria cells is 0, 1, 2, 5, 10, 15, and 20 min. The exposure time of EPS was 0, 1, 2, 3, 6, 9, and 12 h. In order to prevent the evaporation of solutions during radiation, cellulose acetate membranes were covered on the plates. After exposure to UVBR, the plates containing cyanobacteria cells were placed in artificial climate box at room temperature under an irradiance of 55 μmol photons/m2⋅s PAR with a 12-h light: dark photoperiod for recovery.
Determination of Chlorophyll a Fluorescence and Biomass
The chlorophyll a fluorescence was measured with a double-modulation fluorometer (FL3500, PSI, Brno, Czech). Cyanobacteria cells were dark-adapted for 5 min before measuring the fluorescence parameter Fv/Fm. Fv/Fm is the maximum photochemical efficiency of photosystem II and it is a most commonly used photosynthetic function parameter for photosynthetic microorganisms (Zhou et al., 2006).
The living cell biomass was represented by the optical density of cell suspension at 680 nm. The value of OD680 was measured with an ultraviolet-visible spectrophotometer (UNIC2800, Shanghai, China).
Determination of Biochemical Composition of EPS
Content of polysaccharides was measured by phenol sulfuric acid method, using glucose as the standard (Dubois et al., 1956). The content of proteins was measured by Bradford’ method, with bovine serum albumin as the standard (Bradford, 1976). Content of total organic carbon (TOC) in EPS solution was measured with a TOC analyzer (TOC-VCTH, Shimadzu, Japan). The molecular weight distribution was measured by size exclusion high performance liquid chromatography (HPLC-SEC; HPLC, Hitachi, Japan). EPS were subjected to gel permeation chromatography on column with Superdex TM 20010/300GL (9 mM NaCl+0.9 mM Na2HPO4, flow rate 0.4 ml/min). The standard substance with molecular weight in the range from 14.4 to 94 kDa from Polymer Standards Service was used to calibrate the retention time. The results were expressed as the relative (sum of all pigment classes by mass = 100%) amounts of molecular weight.
EEM Fluorescence Spectroscopy
The excitation emission matrix (EEM) spectra of EPS were measured by a fluorescence spectrophotometer (F-7000, Hitachi, Japan) equipped with 1.0 cm quartz cell and a thermostat bath (Pan et al., 2010, 2012; Song et al., 2010). EEM spectra were collected with subsequent scanning emission wavelength from 200 to 550 nm at 2 nm increments and excitation wavelength from 200 to 400 nm at 5 nm increments, with an excitation/emission slit width of 5.0 nm and a scanning speed of 1200 nm/min. The fluorometer’s response to a Milli-Q water blank solution was subtracted from the fluorescence spectra of EPS. Three-dimension EEM data were processed using the software SigmaPlot 10.0 (Systat, USA).
Staining of EPS
Extracellular polymeric substance of cyanobacteria cells were observed by alcian blue staining method (Wardi and Allen, 1972), the cells were washed six times with sterilized water and then fixed on a slide by adding a few drops of 4% formaldehyde for 12 h. Cells on slides were then stained with alcian blue (10% w/v in 0.5 M acetic acid). After 12 h of staining the slides were rinsed with distilled water. The cells and stained EPS were observed using a light microscope (XSZ-HYZ, COIC, China) equipped with a camera.
Results and Discussion
Effect of UVBR on PSII Activity
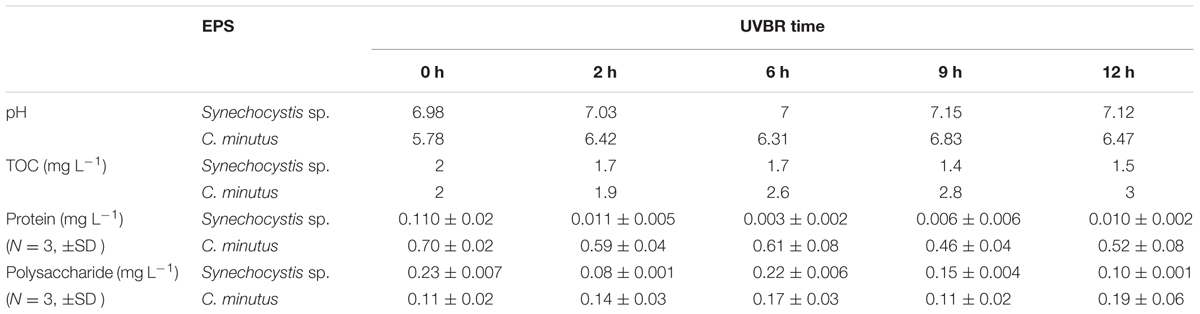
Figure 1 showed Fv/Fm of C. minutus and Synechocystis sp. as a function of UVBR time. It was found that 1 and 2 min of UVBR increased Fv/Fm of Synechocystis sp. compared with the control. However, longer UVBR time reduced Fv/Fm and the reduction increased with radiation time. In the case of C. minutus, Fv/Fm was reduced under all periods of UVBR in comparison with the control and its reduction correlated with radiation time. After radiation treatments, the recoverability of growth activity of both cyanobacteria species was assessed by continuous determination on Fv/Fm for 4 days. It was interesting that Fv/Fm increased to higher levels than their controls for both Synechocystis sp. and C. minutus irradiated with UV-B for 1 and 2 min, suggesting that short term UVBR can improve their PSII activities. In the case of Synechocystis sp. Fv/Fm decreased from 0.43 to 0.1 irradiated after 10 min of UVBR and continued to decrease significantly during first recovering 24 h and then recovered gradually to about 0.2 at 48 h. In 15 and 20 min of UVBR treatments reduced Fv/Fm to 0.2 and 0.1, respectively. After removal of UVBR, Fv/Fm for these two treatments continued to decrease to near zero and no recovery was observed. This means that PSII was completely destroyed by 15 or 20 min of UVBR. For C. minutus exposed to UVBR for 10 min or longer, Fv/Fm recovered somewhat to values below the control (Figure 1B), indicating that PSII function was injured and could be recovered partly. These results showed that C. minutus was more tolerant to UVBR than Synechocystis sp. and the former had higher ability to recover from depression in Fv/Fm induced by long-term (5–20 min) UVBR than the latter.
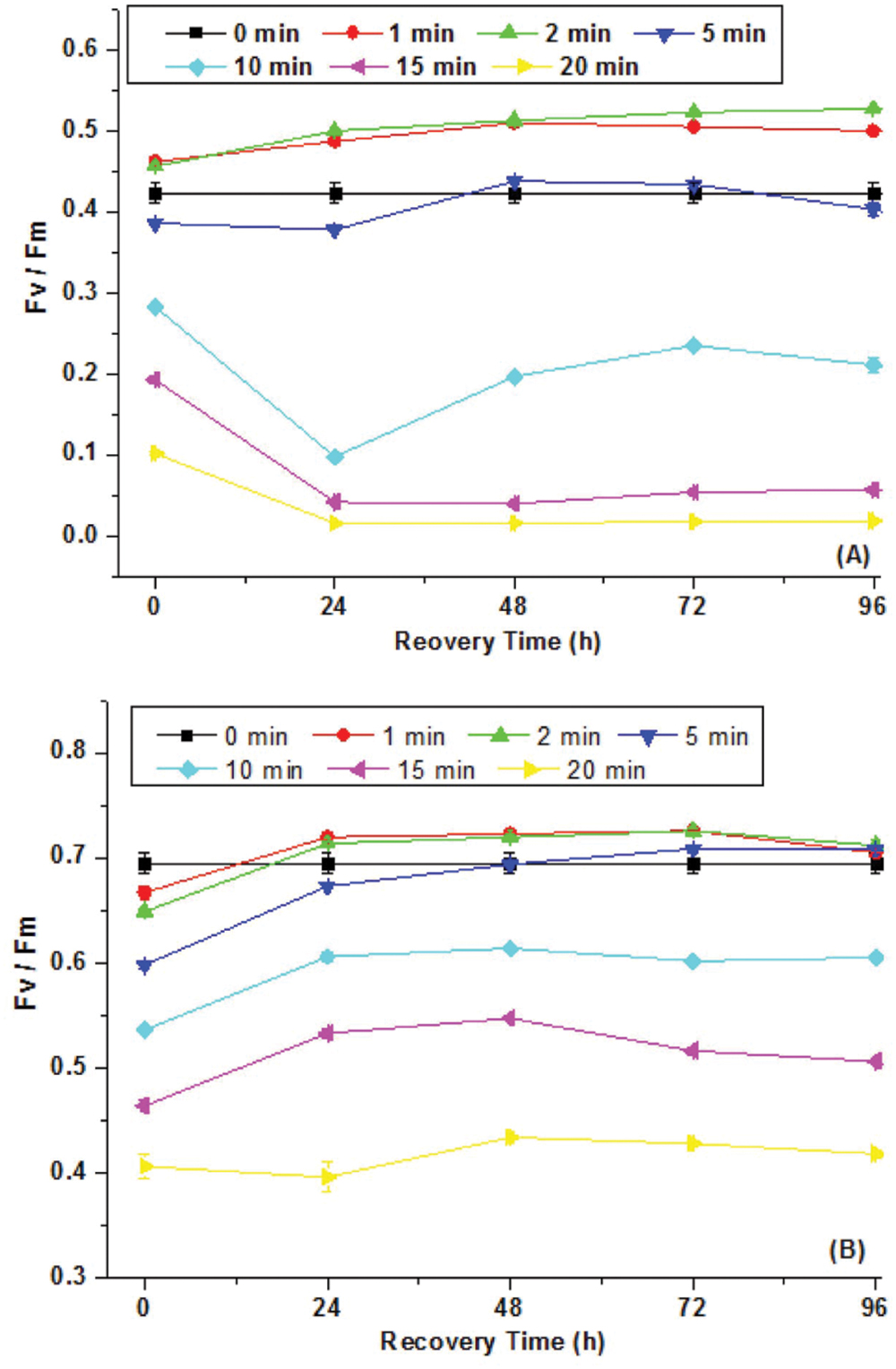
FIGURE 1. The maximum photochemical efficiency of PSII, Fv/Fm (N = 3, ±SD), of Synechocystis sp. (A) and Chroococcus minutus (B) cells exposed to UVBR for various time and its recovery curves up to 96 h.
A number of studies also showed that strong UVBR can damage PSII and cause a reduction in the maximal quantum yield (Fv/Fm; Karsten et al., 2007). Limited studies showed that PSII activity for UVBR stressed terrestrial and aquatic algal species showed contrasting recovery potential. Karsten et al. (2007) showed that Fv/Fm of three terrestrial algal species Stichococcus sp., C. luteoviridis, and M. incise and one aquatic algae D. subspicatus significantly declined under 3 days of radiation of 50 μmol photons m-2 s-1 PAR and 40 μW cm-2 UVB. Fv/Fm fully recovered for all the three terrestrial algal species while the aquatic algae recovered about 80% during12 days of recovery. Similarly, our study also indicates that the aquatic cyanobacterium is more susceptible to UVBR than the terrestrial species.
Effect of UVBR on Cell Growth and EPS Production
The optical density of cell suspension at 680 nm (OD680) was used to indicate the living cell biomass. Synechocystis sp. and C. minutus showed different growth patterns during 4 days of continuous exposure to UV-B (Figure 2). The decreasing magnitude of the biomass of Synechocystis sp. was bigger than C. minutus during UVBR (Figure 2), implying that C. minutus was more tolerant to UVBR. Growth of C. minutus recovered at 24 h while Synechocystis sp. started its growth at 60 h, much later than C. minutus. This suggests that C. minutus can acclimate to UVBR more rapidly than Synechocystis sp.
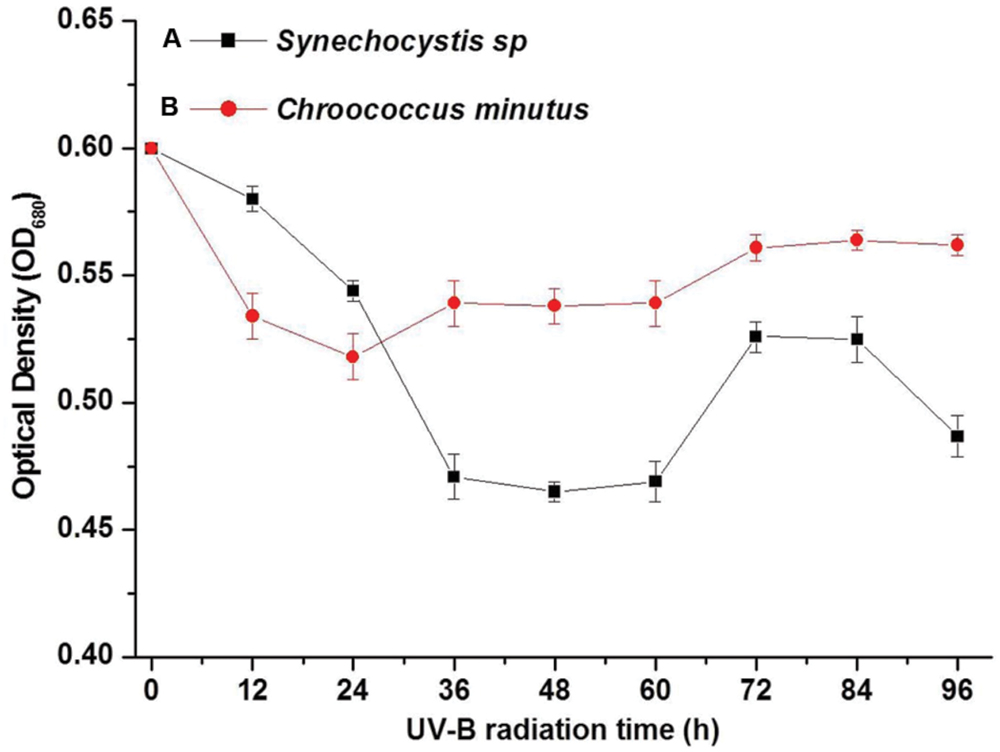
FIGURE 2. The mean optical densities (OD680) (N = 3, ±SD) of Synechocystis sp. cells (A) and C. minutus cells (B) with UVBR time.
During the treatments of UVBR and staining, no changes in cell shape were observed. When the EPS were stained with alcian blue, EPS exhibited a blue–green fluorescence. It was observed that most of EPS of Synechocystis sp. cells disappeared after UVBR (Supplementary Figures S1A,B). However, it seemed that UVBR increased production of EPS of C. minutus cells (Supplementary Figures S1C,D). The quantitative analysis confirmed the results of light microscopy. EPS yield of Synechocystis sp. decreased from 407.8 mg g-1 dry cell before UVBR to 70 mg g-1 dry cell after 12 h of UVBR whereas yield of EPS of C. minutus increased from 60.7 mg g-1 dry cell before UVBR to 198.2 mg g-1 dry cell after 12 h of exposure to UVBR. This indicates that EPS of Synechocystis sp. was decomposed under UVBR whilst production of EPS of C. minutus was stimulated by UVBR.
In previous studies, the cell growth received much more attention as it is considered to be an important physiological parameter that integrates all positive and negative stress effects in several biochemical processes within the cell (Karsten et al., 2007). The growth studies on macroalgae indicate that more cellular processes are impaired than just photosynthesis (Dring et al., 1996). The change of cellular metabolism could induce the secretion change of EPSs as it is regarded as one important response of microorganism to environmental stress. A few earlier studies reported responses of EPS of cyanobacteria to UV radiation. EPS production of Microcoleus vaginatus decreased significantly after radiation with UV-B (Chen et al., 2009). He and Häder’s (2002) study indicated that EPS of Microcoleus vaginatus had a significant protection against the oxidative stress and photosynthetic damage and thus reversed the negative effects of UV-B on survival. In previous studies, it has been observed similar result. For example, the production of extracellular glycan in Nostoc commune was stimulated threefold under UV-B and EPS had important effect on protection against UV-B (Ehling-Schulz et al., 1997).
Effect of UVBR on Molecular Weight Distribution of EPS
Three chromatographic fractions were separated from EPS of both cyanobacteria species untreated and treated with UVBR (Figure 3). Most of the molecular weights of both Synechocystis sp. and C. minutus fell into the range of retention time from 45 to 60 min. Two small peaks at 20 and 77 min were also observed for EPS of both cyanobacterial species. The area of peak at 20 min for EPS from Synechocystis sp. (Figure 3A) was a little bigger than that from C. minutus (Figure 3C), indicating the EPS of Synechocystis sp. contained more macromolecular substances than those of C. minutus. According to the organic carbon mode (Paul et al., 2012), the high-molecular weight matter with retention time less than 40 min was composed of mainly hydrophilic biopolymers, such as polysaccharides and proteins. The middle-molecular weight matter with retention time of 40–60 min could be humic acids, fulvic acids, and fractions of low-molecular weight acids. The minor peak at retention time >60 min represents the low-molecular weight neutral and amphiphilic compounds including sugars, alcohols and amino acids. The proportions of the substances in the three molecular weight ranges were shown in Table 1. EPS from Synechocystis sp. and C. minutus contained 11.5 and 7.7% of the MW > 94 kDa substances (mainly polysaccharides and proteins), respectively. After 12 h of UVBR, about 3.8 and 0.8% of the MW > 94 kDa substances in EPS from Synechocystis sp. and C. minutus were decomposed, respectively. Substances with MW in the range of 14.4–94 kDa (low-molecular weight acids) accounted for 62.9 and 53.1% in EPS from Synechocystis sp. and C. minutus, respectively. 9.8 and 3.6% of 14.4–94 kDa substances were decomposed due to 12 h of exposure to UV-B for Synechocystis sp. and C. minutus, respectively. In contrast to the decreases of proportion of substances with MW > 14 kDa, MW < 14 kDa substances (low-molecular weight neutral and amphiphilic compounds) increased from 25.5 and 20.2% before UVBR to 39.1 and 24.6% after 12 h of UVBR for Synechocystis sp. EPS and C. minutus EPS, respectively. The decreases of proportion of high MW substances and increases of low MW substances due to UVBR indicated that the high MW components were decomposed into low MW substances. 13.6% of MW > 14 kDa substances were decomposed into MW < 14 kDa molecules for Synechocystis sp. during UVBR, which was much more than the 4.4% for C. minutus, indicating that EPS of the aquatic Synechocystis sp. was more decomposable than the desert C. minutus.
FIGURE 3. High performance liquid chromatography (HPLC) chromatogram of EPS abstracted from Synechocystis sp. cells and C. minutus cells. (A) EPS from Synechocystis sp.; (B) EPS from Synechocystis sp., was UV-B irradiated for 12 h; (C) EPS from C. minutus; (D) EPS from C. minutus, was UV-B irradiated for 12 h.
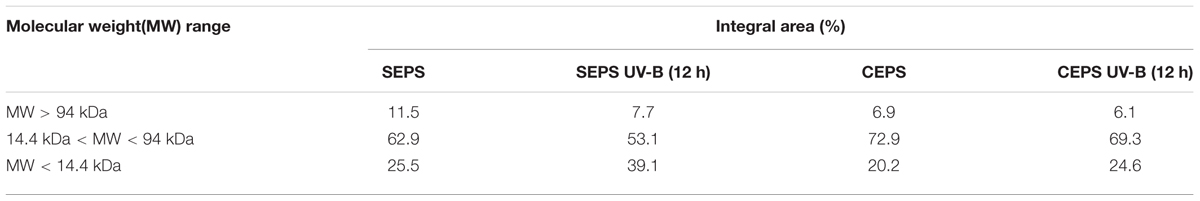
TABLE 1. The molecular weight distribution of EPS from Synechocystis sp. and Chroococcus minutus before and after 12 h of UVBR.
The decomposition of EPS under UVBR is similar to the decomposition of dissolved organic carbon (DOC) caused by UV radiation. In a few earlier studies, effects of UV-radiation on DOC were evaluated using chromatographic techniques (Frimmel, 1998; Paul et al., 2012). These studies showed that transformation of DOC induced by UV radiation was characterized by reduction of macromolecular matter and an increase of low-molecular weight matter (Paul et al., 2012). Some studies also reported that UV radiation caused decomposition of large natural organic matter molecules to small ones (Lehtola et al., 2003).
Effect of UVBR on Biochemical Characteristics of EPS
The pH, proteins content, and polysaccharides content of EPS from Synechocystis sp. and C. minutus during UVBR were listed in Table 2. The pH of Synechocystis sp. EPS was about 7 and pH of C. minutus was weakly acidic, 5.8. UVBR slightly increased pH of both EPS solutions. The change of pH might be attributed to the decomposition of EPS induced by UVBR (Stal and Krumbein, 1985). The TOC content showed contrary change patterns after exposure to UV-B. TOC content of Synechocystis sp. EPS decreased with increasing UVBR time, suggesting that EPS were decomposed due to UVBR. On the contrary, TOC content of C. minutus EPS showed an increasing trend after UVBR. This might be attributed to the incomplete oxidation of C. minutus EPS at 680°C (the combustion temperature of the TOC analyzer). With UVBR time, more EPS were decomposed into low MW components that could be more easily oxidized at 680°C by the TOC analyzer and this resulted in a small increase of TOC content. The contrasting response pattern of TOC content of Synechocystis sp. and C. minutus to UVBR suggests that EPS of C. minutus was more resistant to UV-B induced degradation than that of Synechocystis sp.
Extracellular polymeric substance of Synechocystis sp. had lower protein content and higher polysaccharides content compared to EPS of C. minutus. Protein concentration in EPS of Synechocystis sp. showed slightly fluctuating changes with increasing exposure time to UVBR, and finally decreased from 0.11 to 0.01 mg L-1 after 12 h of exposure to UVBR. Whereas protein concentration in EPS of C. minutus decreased significantly after UVBR and it had a good linear relation with the exposure time (r2 = 0.8133, p < 0.05). The decreasing of protein content in EPS after UVBR exposure indicated protein could be degraded by UVBR. The polysaccharides content in EPS of Synechocystis sp. decreased slightly after UVBR but that of C. minutus almost kept unchanged. This might be due to the differences in composition and structure of polysaccharides in EPS from contrasting environments. The degradation of proteins in EPS of both species could be attributed to ROS produced by UVBR (Kumar et al., 2004). The decomposition of EPS and the decreasing of protein content in EPS might explain the decreases of photosynthetic activity and biomass of Synechocystis sp. and C. minutus after UVBR.
Effect of UVBR on EEM Spectra of SEPS and CEPS
The fluorescence spectra of EPS were shown in Figure 4. Two protein-like peaks (peak A and peak B) and one humic-like peak (peak C) were identified from EEM spectra of Synechocystis sp. EPS (Figure 4A). One protein-like peak (peak D) and one phenol-like peak (peak E) were identified from the EEM spectra of C. minutus EPS (Figure 4C). According to the peak position (Table 3), fluorescence peaks A and B could be assigned to secondary tryptophan-like fluorescence and tryptophan-like fluorescence, respectively (Song et al., 2010; Zhu et al., 2012).
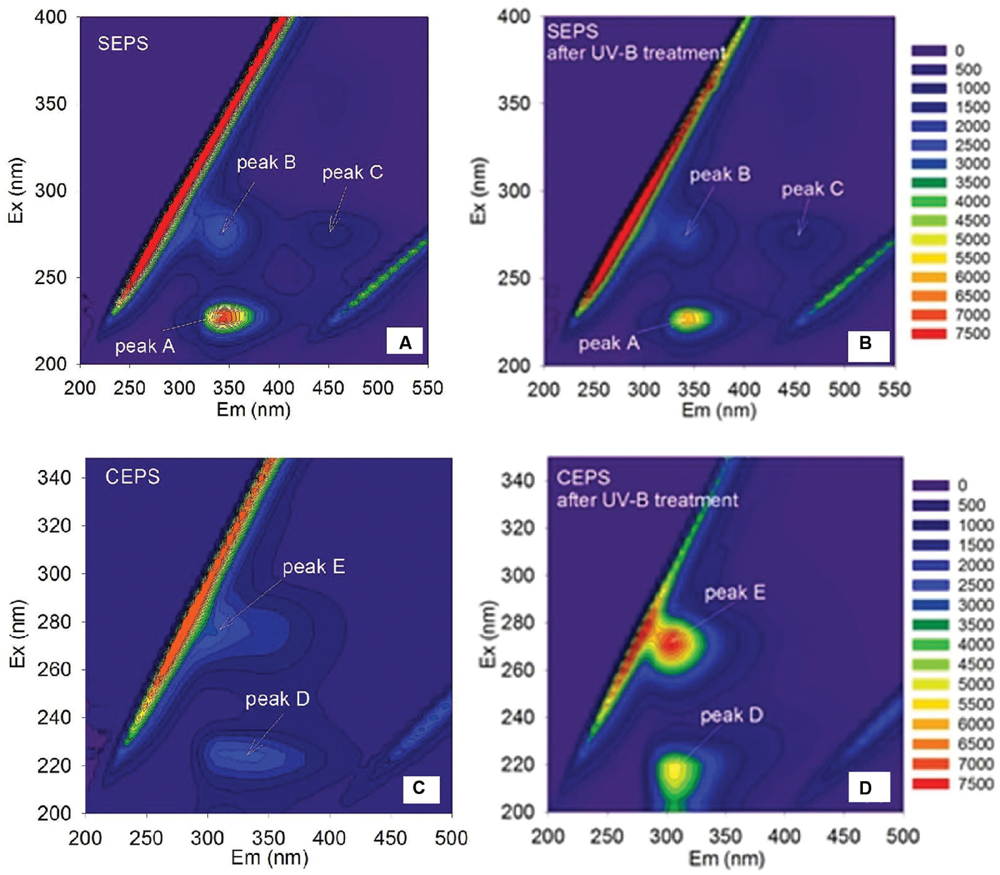
FIGURE 4. Excitation emission matrix (EEM) spectra of Synechocystis sp. EPS before (A) and after (B) 12 h of UVBR, and of C. minutus EPS before (C) and after (D) 12 h of UVBR.
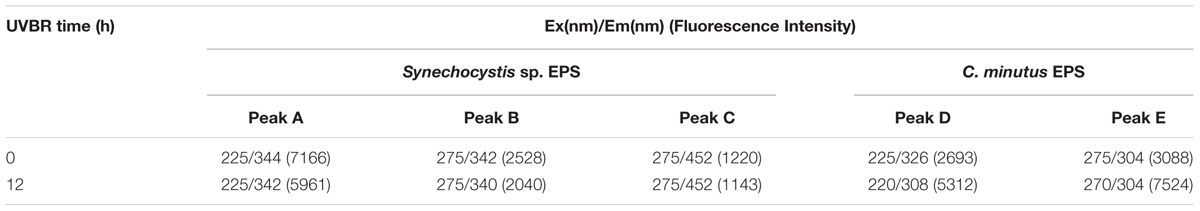
TABLE 3. Comparison between the effects of UVBR on fluorescence properties of EPS from Synechocystis sp. EPS and C. minutus EPS, respectively.
After 12 h of UVBR, the position of fluorescence peaks for Synechocystis sp. EPS changed little but the fluorescence intensity at the protein-like peak was reduced (Figures 4A,B and Table 3), indicating that some protein-like substances were degraded by UVBR. This is consistent with the result of molecular weight, indicating the proteins in Synechocystis sp. EPS were decomposed and this significantly reduced the proportion of the high molecular weight components. UV photodegradation favored larger molecules in NOM fragmentation (Frimmel, 1998). Some previous studies also show that photobleaching is due to the photodegradation of DOM and is often accompanied by a decrease in the molecular weight (Tremblay et al., 2007).
However, the position and intensity of fluorescence peak, especially protein-like peak, in spectra of CEPS changed significantly after UVBR (Figures 4C,D and Table 3). It was observed that there was one slight red shift of protein-like fluorescence peaks and fluorescence intensity at both protein-like peak and phenol-like peak increased significantly. This might be attributed to the change of structure and composition of fluorescence substances induced by UVBR, such as unfolds of some rigid fluorescent substances (Stal and Krumbein, 1985).
Extracellular polymeric substance play a key role in buffering against the adverse effects exerted by the environmental stressors such as UVBR because EPS physically serve as a barrier between the cell and the environment. However, the buffering capacity of EPS seems to be closely related to the habitat factors of the cyanobacteria live in. In the present study, the PSII activity of the desert species C. minutus showed much higher tolerance to UVBR than the aquatic Synechocystis sp., which can be explained by the different resistance of their EPS to UVBR. C. minutus inhabits the desert and it has been always exposed to high UV radiation while Synechocystis sp. lives in the surface water with less UV radiation. The long-term exposure to UV radiation results in more stable EPS that can effectively mitigate the adverse effects of UV radiation.
Conclusion
The desert cyanobacterium species C. minutus showed higher tolerance of PSII to UVBR than the aquatic Synechocystis sp., and the inhibited PSII activity of C. minutus could be fully recovered while that of Synechocystis sp. could be partly recovered. Proteins in EPS of desert and aquatic species were significantly decomposed under UVBR, with the latter being more easily decomposed. The polysaccharides were much more resistant to UVBR than the proteins for both species. Polysaccharides of Synechocystis sp. were degraded slightly but almost no C. minutus polysaccharides were decomposed. The higher tolerance to UVBR of the desert cyanobacterium can be partly attributed to the higher resistance of its EPS to photodegradation induced by UVBR in comparison with the aquatic species.
Author Contributions
WJS is the first author responsible for doing experiments and writing. XLP is the corresponding author. CXZ, DYZ, and SYM are coauthors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the West Light Foundation of Chinese Academy of Science (XBBS201304), National Natural Science Foundation of China (41203088, 31360027, U1120302, and 21177127) and Postdoctoral Science Foundation of China (131622). Partial funding for this research was also received from the Visiting Professor Program at King Saud University, Riyadh, Saudi Arabia.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01208
References
Bowker, M. A., Reed, S. C., Belnap, J., and Philips, S. L. (2002). Temporal variation in community composition, pigmentation and Fv/Fm of desert cyanobacterial soil crusts. Microb. Ecol. 43, 13–25. doi: 10.1007/s00248-001-1013-9
Bradford, M. (1976). A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. J. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Calazans, G. M. T., Lima, R. C., and Franca, F. P. (2000). Molecular weight and antitumor activity of Zymomonas mobilis levans. Int. J. Biol. Macromol. 27, 24–247. doi: 10.1016/S0141-8130(00)00125-2
Chen, L. Z., Wang, G. H., Song, H., Liu, A., Li, C., and Liu, Y. D. (2009). UV-B-induced oxidative damage and protective role of exopolysaccharides in desert cyanobacterium microcoleus vaginatus. J. Integr. Plant. Biol. 51, 194–200. doi: 10.1111/j.1744-7909.2008.00784.x
Cosenza, A., Bella, G. D., Mannina, G., and Torregrossa, M. (2013). The role of EPS in fouling and foaming phenomena for a membrane bioreactor. Bioresour. Technol. 147, 184–192. doi: 10.1016/j.biortech.2013.08.026
Dring, M. J., Makarov, V., Schoschina, E., Lorenz, M., and Luning, K. (1996). Infiuence of ultraviolet-radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta). Mar. Biol. 126, 183–191. doi: 10.1007/BF00347443
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. J. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Ehling-Schulz, M., Bilger, W., and Scherer, S. (1997). UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 179, 1940–1945.
Frimmel, F. H. (1998). Impact of light on the properties of aquatic natural organic matter. Environ. Int. 24, 559–571. doi: 10.1016/S0160-4120(98)00033-6
He, Y. Y., and Häder, D. P. (2002). UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: protective effects of ascorbic acid and N-acetyl-L-cysteine. J. Photochem. Photobiol. B. 66, 115–124. doi: 10.1016/S1011-1344(02)00231-2
Helm, R. F., Huang, Z., Edwards, D., Leeson, H., Peery, W., and Potts, M. (2000). Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc commune DRH-1. J. Bacteriol. 182, 974–982. doi: 10.1128/JB.182.4.974-982.2000
Karsten, U., Lembcke, S., and Schumann, R. (2007). The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta 225, 991–1000. doi: 10.1007/s00425-006-0406-x
Kumar, A., Tyagi, M. B., and Jha, P. N. (2004). Evidences showing ultraviolet-B radiation-induced damage of DNA in cyanobacteria and its detection by PCR assay. Biochem. Biophys. Res. Commun. 318, 1025–1030. doi: 10.1016/j.bbrc.2004.04.129
Lehtola, M. J., Miettinen, I. T., Vartiainen, T., Rantakokko, P., Hirvonen, A., and Martikaine, P. J. (2003). Impact of UV disinfection on microbially available phosphorus, organic carbon, and microbial growth in drinking water. Water. Res. 37, 1064–1070. doi: 10.1016/S0043-1354(02)00462-1
Liu, H., and Fang, H. P. (2002). Characterization of electrostatic binding sites of extra cellular polymers by linear programming analysis of titration data. Biotechnol. Bioeng. 80, 806–811. doi: 10.1002/bit.10432
Madronich, S., McKenzie, R. L., Caldwell, M. M., and Bjorn, L. O. (1995). Changes in ultraviolet radiation reaching the earth’s surface. Ambio 24, 143–152.
Nichols, C. A., Guezennec, J., and Bowman, J. P. (2005). Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar. Biotechnol. 7, 253–271. doi: 10.1007/s10126-004-5118-2
Ozturk, S., Aslim, B., and Suludere, Z. (2009). Evaluation of chromium (VI) removal behavior by two isolates of Synechocystis sp. in terms of exopolysaccharide (EPS) production and monomer composition. Bioresour. Technol. 100, 5588–5593. doi: 10.1016/j.biortech.2009.06.001
Pan, X. L., Liu, J., Song, W. J., and Zhang, D. Y. (2012). Biosorption of Cu(II) to extracellular polymeric substances (EPS) from Synechocystis sp.: a fluorescence quenching study. Front. Environ. Sci. Eng. 6:493–497. doi: 10.1007/s11783-012-0416-9
Pan, X. L., Liu, J., Zhang, D. Y., Chen, X., Li, L., Song, W. J., et al. (2010). A comparison of five extraction methods for extracellular polymeric substances (EPS) from biofilm by using three-dimensional excitation-emission matrix (3DEEM) fluorescence spectroscopy. Water SA 36, 111–116. doi: 10.4314/wsa.v36i1.50914
Paul, A., Dziallas, C., Zwirnmann, E., Gjessing, E. T., and Grossart, H. P. (2012). UV irradiation of natural organic matter (NOM):impact on organic carbon and bacteria. Aquat. Sci. 74, 443–454. doi: 10.1997/s00027-011-0239-y
Quesada, A., and Vincent, W. F. (1997). Strategies of adaptation by Antarctic cyanobacteria to ultraviolet radiation. Eur. J. Phycol. 32, 335–342. doi: 10.1080/09670269710001737269
Sinha, R. P., Klisch, M., Groniger, A., and Hader, D. (1998). Ultraviolet absorbing/screening in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B Biol. 47, 82–94. doi: 10.1016/S1011-1344(98)00198-5
Song, W. J., Mu, G. J., Zhang, D. Y., and Pan, X. L. (2010). Interaction of acetamiprid with extracellular polymeric substances (EPS) from activated sludge: a fluorescence study. Afr. J. Biotechnol. 9, 7667–7673. doi: 10.5897/AJB09.1539
Stal, L. J., and Krumbein, W. E. (1985). Isolation and characterization of cyanobacteria from a marine microbial mat. Bot. Mar. 28, 351–365. doi: 10.1515/botm.1985.28.8.351
Stanier, R. Y., Kunisawa, R., Mandel, M., and Cohen-Bazire, G. (1971). Purification and properties of unicellular blue-green alage (order Chroococcales). Bacteriol. Rev. 35, 171–205.
Sutherland, I. W. (2001). Microbial polysaccharides from gram-negative bacteria. Int. Dairy J. 11, 663–674. doi: 10.1016/S0958-6946(01)00112-1
Tremblay, L. B., Dittmar, T., Marshall, A. G., Cooper, W. J., and Cooper, W. T. (2007). Molecular characterization of dissolved organic matter in a North Brazilian mangrove porewater and mangrove-fringed estuaries by ultrahigh resolution fourier transformion cyclotron resonance mass spectrometry and excitation/emission spectroscopy. Mar. Chem. 105, 15–29. doi: 10.1016/j.marchem.2006.12.015
Wang, H. Y., Jiang, X. L., Mu, H. J., Liang, X. T., and Guan, H. S. (2007). Structure and protective effect exopolysaccharide from P.Agglomerans strain KFS-9 against UV radiation. Microbiol. Res. 162, 124–129. doi: 10.1016/j.micres.2006.01.011
Wardi, A. H., and Allen, W. S. (1972). Alcian blue staining of glycoproteins. Anal. Biochem. 48, 621–623. doi: 10.1016/0003-2697(72)90118-2
Zhang, D. Y., Pan, X. L., Mostofa, K. M. G., Chen, X., Mu, G. J., Wu, F. C., et al. (2010). Complexation between Hg(II) and biofilm extracellular polymeric substances: an application of fluorescence spectroscopy. J. Hazard. Mater. 175, 359–365. doi: 10.1016/j.jhazmat.2009.10.011
Zhou, W. B., Juneau, P., and Qiu, B. S. (2006). Growth and photosynthetic responses of the bloom-forming cyanobacterium Microcystis aeruginosa to elevated levels of cadmium. Chemosphere 65, 1738–1746. doi: 10.1016/j.chemosphere.2006.04.078
Keywords: chlorophyll fluorescence, decomposition, EEM fluorescence spectroscopy, EPS, photosynthesis
Citation: Song WJ, Zhao CX, Zhang DY, Mu SY and Pan XL (2016) Different Resistance to UV-B Radiation of Extracellular Polymeric Substances of Two Cyanobacteria from Contrasting Habitats. Front. Microbiol. 7:1208. doi: 10.3389/fmicb.2016.01208
Received: 17 November 2015; Accepted: 20 July 2016;
Published: 22 August 2016.
Edited by:
Maria Dittrich, University of Toronto Scarborough, CanadaReviewed by:
Marie J. Durand, University of Nantes, FranceDayananda Chandrappa, University of Exeter, UK
Antonio Flores-Moya, University of Málaga, Spain
Copyright © 2016 Song, Zhao, Zhang, Mu and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangliang Pan, eGxwYW5AbXMueGpiLmFjLmNu
 Wenjuan Song1
Wenjuan Song1 Chenxi Zhao
Chenxi Zhao Xiangliang Pan
Xiangliang Pan