- 1Food Microbiology Research Laboratory, Department of Microbiology, Faculty of Science, Lagos State University, Lagos, Nigeria
- 2Department of Biotechnology, Federal Institute of Industrial Research, Lagos, Nigeria
- 3Department of Microbiology, Faculty of Science, University of Lagos, Lagos, Nigeria
Legumes and oil bean seeds used for the production of condiments in Africa are inedible in their natural state; they contain some anti-nutritional factors especially undigestible oligosaccharides and phytate. Fermentation impact desirable changes by reducing anti-nutritional factors and increasing digestibility. Ugba is an alkaline fermented African oil bean cotyledon (Pentaclethra macrophylla) produced by the Ibos and other ethnic groups in southern Nigeria. Seen as a family business in many homes, its preparation is in accordance with handed-down tradition from previous generations and serves as a cheap source of plant protein. Its consumption as a native salad is made possible by fermentation of the cotyledon for 2–5 days, but could also serve as a soup flavoring agent when fermentation last for 6–10 days. The fermentation process involved is usually natural with an attendant issue of product safety, quality and inconsistency. The production of this condiment is on a small scale and the equipment used are very rudimentary, devoid of good manufacturing procedures that call to question the issue of microbial safety. This paper therefore reviews the production process and the spectrum of microbial composition involved during fermentation. In addition, potential spoilage agents, nutritional and biochemical changes during production are examined. Furthermore, information that can support development of starter cultures for controlled fermentation process in order to guarantee microbiological safety, quality and improved shelf life are also discussed.
Introduction
Ugba, a product of alkaline fermentation of oil bean seeds (Pentaclethra macrophylla) is very popular among the Ibos and other ethnic groups in southern Nigeria. The product serves both as a delicacy and a food flavoring agent. As an important nutritional item, ugba is very rich in protein. It similarly plays an economic, social and cultural role among the Ibos in the eastern part of Nigeria. The production of ugba is usually pursued as a family business that has become an art that is handed over from one generation to another.
The processing of these large brown glossy seeds of the African oil bean (Figure 1) to obtain ugba is usually by natural fermentation, a process that involves microbiological and biochemical changes, caused by hydrolysis and desirable changes. This process is usually influenced by the raw materials and the processing method with variations observed from one production batch or producer to another (Steinkraus, 1983).

Figure 1. African oil bean seed (A), Dehulled seeds of African oil bean (B) and Processed slices of the African oil bean cotyledon (C). (Okorie and Olasupo, 2013a).
Most studies on African fermented foods have focused on isolation and identification of desirable microorganisms involved in the fermentation process. The general consensus from these studies is that fermented African oil bean seeds during ugba fermentation is predominantly brought about by bacteria identified as Bacillus species (Odunfa, 1981; Obeta, 1983; Isu and Ofuya, 2000; Okorie and Olasupo, 2013a; Eze et al., 2014). Other groups of bacteria have also been implicated in the fermentation of this product and they include species of Escherichia, Proteus, Micrococcus, Staphylococcus, Streptococcus, Alcaligenes, Pseudomonas, Corynebacterium, and Enterococcus (Oyeyiola, 1981; Odunfa, 1986; Sanni et al., 2002; Okorie and Olasupo, 2013a). No fungi or yeast species have been implicated in the fermentation of ugba.
There is very little information on the occurrence and growth of pathogens in African fermented foods. The natural fermentation process used routinely for ugba production allows participation of diverse microorganisms. The involvement of pathogenic and spoilage microorganisms during production cannot be totally ruled out, especially if fermentation takes place under very poor hygiene conditions and sanitation, which is a very common occurrence in West Africa. Product inconsistency as a result of mixed-culture processing and post-fermentation contamination constitutes a major challenge to microbial safety and quality of this product.
Production of ugba in Nigeria is still on a small scale industrial process involving production at the household level where there is little or no consideration for good manufacturing practices (GMP) and sanitation (Olasupo et al., 2002; Gadaga et al., 2004). Consequently, microbiota responsible for fermentation is often unpredictable and equipment used is rudimentary. Similarly poor hygiene of handlers, lack of portable water and other raw materials often introduce spoilage and pathogenic microorganisms. All these factors affect the quality of the final product and ultimately the health of the consumers. Fermentation period is chosen according to human judgment and varies from one manufacturer to the other. The lack of standardization in the production process often results in product inconsistency and quality variation.
Lactic fermentation is noted to be a major mode of food processing used to achieve preservation and improve shelf life of foods especially in the West African sub-region, where cereals and tubers are processed to variety of foods. This practice has been very reliable in terms of maintaining quality and safety of food especially at the household level where many of the traditional foods are produced (Steinkraus, 1983). Unfortunately, alkaline fermentation of legumes is about hydrolysis of proteins and release of amino acids and ammonia responsible for the pungent smell as well as characteristic flavor. This preservative influence of condiments after fermentation appears to be limited; similar observation has been reported during the processing of fermented African oil bean seeds. The unfermented seeds are much more stable with longer shelf life than the fermented products. Fermentation thus leads to flavor enhancement, complex molecules reduction (oligosaccharides and proteins) but reduces the shelf life of the seeds and exposes the product to post fermentation contamination (Mbajunwa et al., 1998; Oguntoyinbo et al., 2007). Post processing techniques proposed for condiment production in Africa include drying and salting of final product (Achi, 2005; Eman, 2009). However, while these methods could increase shelf life considerably, it is characterized with inherent disadvantages such as loss of volatile compounds and vitamins. Also, the consumption of salt in diet has been identified as having deleterious effects on human health, responsible for cardiovascular diseases in the West African sub-region (Brown et al., 2009; He and MacGregor, 2009; Strazzullo et al., 2009).
Since fermentation of African oil bean seeds increases pH toward alkalinity (pH 8) (Odunfa, 1985a; Sanni and Oguntoyinbo, 2014), the anti-microbial effect often associated with most fermented food due to lowering of pH to acidity is lacking in this product. It is therefore possible that some organisms that are of public health concern could survive the fermentation process. Whether the presence of these organisms is as a result of post-fermentation contamination or they survive the fermentation process, their presence in the product portends great danger to the consuming public. The risk is particularly high also because the product can be eaten without pre-heating. The alkaline pH selects and encourages the dominance of Bacillus species. This has been consistently reported to be due to production of peptides, amino acids and ammonia during the hydrolysis of the cotyledons.
Recently, Oguntoyinbo (2014) reported that very little attention is placed on the type of packaging used for many traditional foods in West Africa. Unhygienic and substandard packaging materials can engender easy contamination by hazardous materials, including biological, physical, and chemical hazard of well-prepared foods during preservation. Ugba is usually wrapped in leaves (in most cases banana leaf), and nylon bags and sold to the public. These packaging materials could be the source of contamination of the product.
Many of the agricultural raw materials used for the preparation of traditional W. African food products contain endogenous toxins (Kar and Okechukwu, 1978; Okorie and Olasupo, 2014). However, studies have shown that fermentation drastically reduces anti-nutritional factors in many fermented legumes-based foods (Oboh et al., 1998; Khan et al., 2012; Okorie and Olasupo, 2014). It is well known that these foods contain naturally occurring toxins and anti-nutritional compounds. The removal of anti-nutrients from Nigerian fermented food is an important step in ensuring toxicological safety and quality. Fermentation plays significant roles in detoxification of substrates; for instance, removal of toxins during kawal production, through the fermentation of the leaves of Cassia obtusitfolia in Sudan has been shown to improve safety quality and acceptability (Egwim et al., 2013; Taylor and Duodu, 2015).
Most of the legumes and oil seeds used for the production of condiments are inedible in their unfermented state because they suffer from one drawback or the other. For instance, legumes are a particularly rich source of natural toxicants, including proteinase inhibitors, amylase inhibitors, metal chelates, flatus factors, hemagglutinins, saponins, cyanogens, lathyrogens, tannins, allergens, acetylenic furans, and isoflavonoid phytoalexins (Issoufou et al., 2013; Oguntoyinbo, 2014). The unfermented African oil bean seeds contain a number of anti-nutritional and /or toxic factors including saponins, alkaloids (alkaloid paucine), sterols, glycosides, and growth depressant caffeolyputrescine, but no hemagglutinins (Kar and Okechukwu, 1978).
Understanding the biotechnological principles during fermentation of African oil bean seeds is a crucial strategy for the process optimization of fermented condiments in West Africa. The understanding of the microbiological dynamics, biochemical kinetics and toxicology during fermentation will significantly impact product quality, safety and acceptability. The foregoing has been a review of the different scientific literatures relevant to biotechnology of ugba production in Nigeria and highlighted relevant strategies toward process improvement. In addition, current condiment food safety issues are discussed.
Production Process
The production process of ugba is shown in Figure 2. It has been previously described as alkaline fermentation of the seeds of the African oil bean tree (Ikenebomeh et al., 1986; Sanni et al., 2002; Ogueke et al., 2010). Although the production method varies from one community to the other and from one processor to another, a similar end-product, which usually comes with pungent ammonia-like smell is commonly produced across South Eastern Nigeria (Nwokeleme and Ugwuanyi, 2015). There is variation in boiling time and the procedure that aided dehulling of the seeds. Obeta (1983) reported 16–18 h of boiling, Odunfa and Oyeyiola (1985) and Odunfa (1986), reported initial 12 h boiling time, while Njoku and Okemadu (1989), boiled the seeds for 5–8 h. However, Sokari and Wachukwu (1997) used toasting of the bean seeds in hot (ca. 100°C) sand and holding for a further 30 min at 100°C to dehull the seeds. After dehulling, cotyledons are either sliced or cooked for 30 min or longer. Odunfa and Oyeyiola (1985) reported overnight boiling before soaking and slicing. In the fermentation process, varied methods are used. Odunfa and Oyeyiola (1985) reported that the cotyledons are mixed with salt (sodium chloride ca.1–2 w/w), put in a clean pot, covered and fermented for up to 5 days at room temperature, with or without salt. On the other hand, Sokari and Wachukwu (1997) reported that sliced cotyledons were washed and allowed to drain for ½-1 h, in a basket lined with banana leaves (Musa sapientum Linn.) and later wrapped (about 40–50 g of slices per wrap) using another leaf (Mallotus oppositifolius) and incubated for 72 h at room temperature.
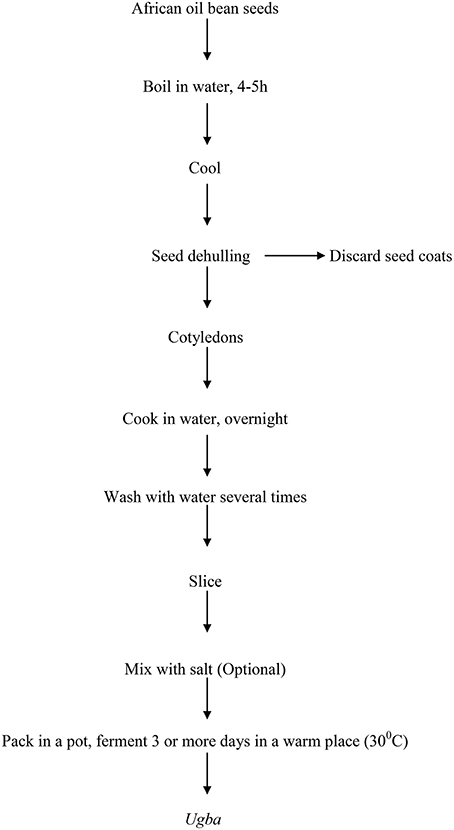
Figure 2. Flow chart for the preparation of ugba (Odunfa and Oyeyiola, 1985).
However, the essential steps in the production of this product are similar and as shown in Figure 2. The differences in the various processing methods described could be responsible for the variations in the products quality observed from one community to the other. The fermented bean slices at the end of the fermentation process are kept near smoldering firewood to develop the characteristic ugba flavor and the product is consumed as native salad. However, fermentation for a longer period of time (6–10 days) produces very soft ugba which is used as soup flavoring (Odunfa and Oyeyiola, 1985; Sanni et al., 2002). Irrespective of which method is employed in the processing, one major drawback observed is the drudgery involved in the slicing process.
Microbiological Changes During Fermentation
The microbiota in fermenting food matrix is a function of the hygienic status of the production environment, the utensil and raw materials used and the handlers. The traditional fermentation method employed in the processing of ugba is by chanced inoculation. The microbial interaction in its production is therefore determined by the microbiological status of the raw materials, utensils, handlers and the production environment. Daeschel et al. (1987) and Ling et al. (2013) noted that the dynamics of fermentation in any food matrix is a complex microbiological process involving interactions between different microorganisms. During fermentation of African oil bean seeds, dominant microorganisms capable of enzymatic hydrolysis are responsible for the biochemical and nutritional changes which constitute the observable changes especially in the chemical composition and taste of the final product.
Several works have been carried out on the microbiological changes during fermentation of African oil bean seed for ugba production (Obeta, 1983; Odunfa and Oyeyiola, 1985; Ejiofor et al., 1987; Ogueke and Aririatu, 2004; Enujiugha and Akanbi, 2008; Nwagu et al., 2010; Okorie and Olasupo, 2013a). The major fermenting microorganisms involved in the fermentation process have been identified to be proteolytic Bacillus species identified as B. subtilis (which is the most predominant), B. licheniformis, B. megaterium, B. macerans, and B. circulans (Obeta, 1983; Sanni, 1993; Isu and Ofuya, 2000; Sanni et al., 2002). The endospores of these bacilli must have been associated with the cotyledons from the beginning of the fermentation. Due to high level of hydrolytic enzyme production by Bacillus species, all the species have been reported to have one or more enzymatic hydrolytic properties during legume fermentation (Aderibigbe et al., 1990; Sanni et al., 1999; Oguntoyinbo et al., 2007). However, it appears that B. subtilis is the most adapted and dominant with properties such as higher protease and amylase production, production of poly glutamic acid (responsible for mucilage production), pyrazine and antimicrobial such as subtiliosin production (Oguntoyinbo et al., 2007).
Protein has been identified as one of the major components of African oil bean cotyledon (Obeta, 1983). Metabolic and enzymatic hydrolysis of Bacillus species serves to break down the protein into amino acids (Isu and Njoku, 1997). Odunfa and Oyewole (1986) and Ghosh et al. (2013) observed that all the Bacillus species that have been associated with the fermentation of the oil bean seeds are mainly proteolytic, and 97.3% of these Bacillus species are also lipolytic. Proteolysis is therefore the major biochemical activity during the fermentation and has been found to increase constantly during the fermentation of ugba and the other food condiments (Odunfa, 1986; Wang and Fung, 1996; Oguntoyinbo et al., 2007). Also, a corresponding increase in the population of Bacillus species is reported from the beginning of the fermentation process till the end (Ogueke and Aririatu, 2004).
Other groups of organisms that have been found to be associated with the fermentation of this condiment include Escherichia species, Proteus, Pediococcus, Micrococcus, Staphylococcus, Streptococcus, Alcaligenes, Pseudomonas, Corynebacterium, Enterococcus (Odunfa, 1981; Antai and Ibrahim, 1986; Ogbadu and Okagbue, 1988; Njoku and Okemadu, 1989; Suberu and Akinyanju, 1996; Ogbonna et al., 2001; Okorie and Olasupo, 2013a).
Staphylococcus spp. and Micrococcus spp. are very active at the early stage of the fermentation process. They rapidly multiply within 24 h of fermentation and then decrease as fermentation progresses. Escherichia species, Proteus and Pediococcus are generally observed to play a minor role in the fermentation process (Odunfa, 1985a) while Staphylococcus sp. and Micrococcus sp. play a subsidiary role in the production process (Obeta, 1983; Odunfa and Komolafe, 1989).
Apart from proteolysis, other important biochemical changes mediated by microorganisms during the production of this condiment include production of flavor enhancing compounds, production of vitamins and essential fatty acids, and degradation of indigestible oligosaccharides responsible for flatus factors. A reduction in the contents of stachyose, raffinose, and melibiose in fermented soy bean cotyledon during kinema production was previously reported (Sarker et al., 1997). Significant increases in thiamine and riboflavin have been observed in ugba, and these have been ascribed to riboflavin synthase associated with Bacillus subtilis (Odunfa, 1986). These reductions are ascribed to sucrase activities of the Bacillus group Aderibigbe and Odunfa (1990) and possibly by the alpha galactosidase activities of the other microorganisms in the fermenting mash, especially Staphylococcus sp. and LAB among which alpha galactosidase activities are common (Odunfa and Oyewole, 1998).
Members of the Enterobacteriaceae have also been associated with the ecology of fermenting plant proteins (ugba inclusive) especially at the early stages of production (Mulyowidarso et al., 1989; Achi, 1992; Okorie and Olasupo, 2013a). These species do not survive until the end of the fermentation, presumably because of the modified environment. It is evident that production of this fermented condiment is initially mediated by a diverse microbial flora, which eventually becomes Gram-positive flora (a reflection of many African fermented foods; Odunfa, 1985b).
Nutritional Changes Associated with Fermentation of African Oil Bean Seed
Fermentation has been generally observed to improve the nutritional quality of the products obtained. The protein content, essential amino acids, vitamins and mineral contents of most fermented foods have been shown to increase during fermentation.
Fermented foods and beverages harbor diverse microorganisms from the environment, including mycelia molds, yeasts, and bacteria, mostly lactic acid bacteria and micrococci. These microorganisms transform the chemical constituents of raw materials during fermentation and enhance the nutritional value of the products. The activities of these microorganisms are noted to enrich bland diets with improved flavor and texture; preserve perishable foods; fortify products with essential amino acids, bioactive compounds, vitamins, and minerals for healthy living. They also bring about degradation of undesirable compounds and anti- nutritive factors; imparts antioxidant and antimicrobial properties; improve digestibility; and stimulate probiotic functions. While fermentation results in a lower proportion of dry matter in the food product, the concentration of the vitamins, minerals, and protein appear to increase when measured on dry weight basis (Adams, 1990; Chung et al., 2010; Shil et al., 2010; Savadogo et al., 2011; Makanjuola and Ajayi, 2012; Okechukwu et al., 2012; Olakunle and Adebayo, 2012; Tofalo et al., 2012).
African oil bean seeds support diet and improve nutritional availability. Proximate analysis of raw oil bean seed reveals that it is mainly composed of proteins (36–42%), lipids (43–47%) and carbohydrates (4–17%; Odunfa and Oyeyiola, 1985; Njoku and Okemadu, 1989; Ogueke and Aririatu, 2004).
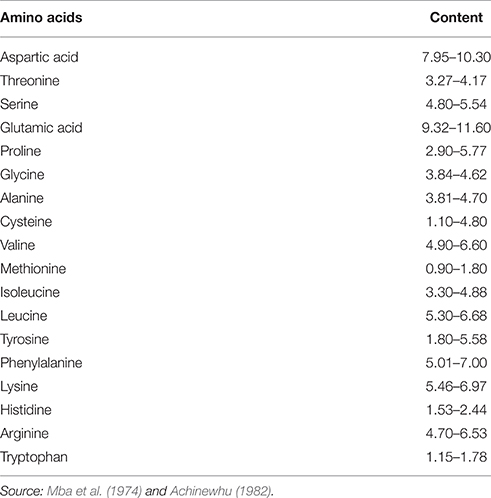
Slight increases in the crude protein and ash contents of the fermented beans have been reported. Enujiugha (2003) reported a steady increase in the level of amino nitrogen from 1.23 mg/Ng-1 DM at the start of fermentation to 13.68 mg/Ng-1 DM after 72 h. The amino acid component of the fermented seed has been shown to contain the 20 essential amino acids (Table 1). The high content of the essential amino acids makes the seed a potential source of protein (Achinewhu, 1982).
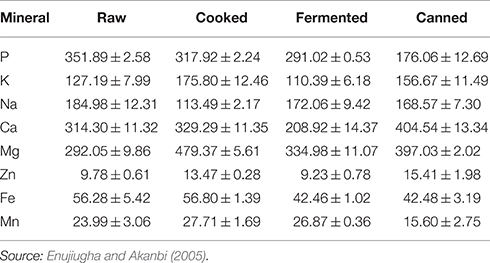
Glutamic acid appears to be the largest amino acid contained in the seed and its fermented product. This may be responsible for its use as a flavoring agent for soups in south eastern Nigeria. Aspartic acid, lysine and phenylalanine are also present in appreciable amounts in the fermented seeds. In their study of compositional changes in oil bean seeds observed during thermal treatment, Enujiugha and Akanbi (2005) reported a reduction of the protein content from 22.32% dry wt. in the raw seeds to 19.00% dry wt. in the canned product (Table 2). Each processing step brought about a decrease in levels of anti-nutritional factors analyzed. Oxalates, tannins and phytic acid were reduced from 2.79 mg/g, 0.38 g/100 g, and 2.11 g/100 g in the raw seeds to 0.81 mg/g, 0.22 g/100 g, and 1.16 g/100 g in the canned product, respectively.
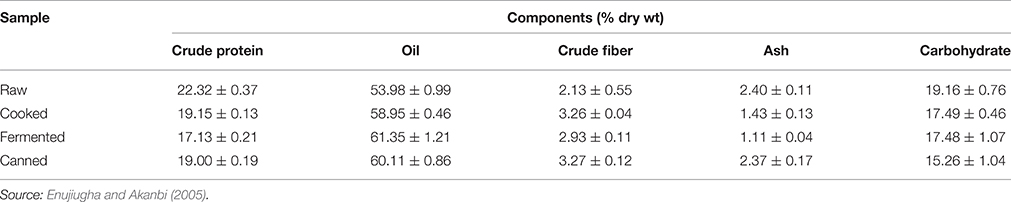
Table 2. Effect of processing on the proximate chemical composition of African oil bean seeds (mean ± s.d.).
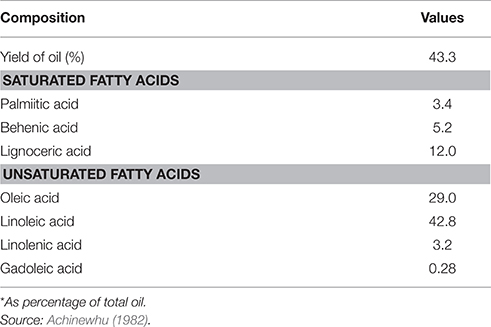
The oil component of the seed contains about 75% of saturated fatty acids and 25% of unsaturated fatty acids (Kar and Okechukwu, 1978; Table 3). For the saturated fatty acids, lignoceric acid appears to be present in the largest amount constituting about 12% of the total fatty acid concentration, while palmitic acid is the least with 3.4%. The major unsaturated fatty acid in the seeds is linoleic acid constituting 42.8%. Oleic acid is also present in appreciable amounts (29.0%). Linolenic and gadoleic acids are present in very small amounts (3.2 and 0.28%, respectively). The presence of appreciable amounts of behenic and lignoceric acids is not desirable for edible oils (Odunfa, 1986). However, Odoemelam (2005) noted that the high degree of unsaturation makes it suitable for cooking purposes and for use as a drying oil for cosmetics, paints and varnishes.
Fermentation has been found to have minimal effect on the fatty acid content of the oil bean seed. (Onwuliri et al., 2004) reported that fatty acid concentrations did not change appreciably with processing and fermentation. Enujiugha and Akanbi (2005) however observed an increase in the oil content from 53.98 to 60.11%. Information available shows that fatty acid content of the oil bean seeds is not qualitatively affected by fermentation. The principal fatty acid linoleic acid however has been shown to increase from 60.68 to 67.57% of the total fatty acids while oleic acid decreased from 26.95 to 22.59% during fermentation. Palmitic acid and other saturated fatty acids in the seed oil are also slightly affected by fermentation.
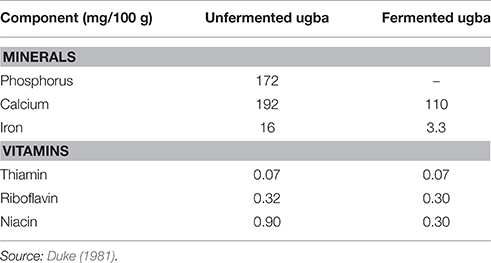
Available information shows that the vitamin content of the seeds is low while they are a poor source of calcium and phosphorus (Duke, 1981). The mineral and vitamin contents are observed to decrease during fermentation (Table 4). The niacin and riboflavin of the seeds have been found to decrease during fermentation. Enujiugha and Akanbi (2005) noted that fermentation and canning significantly (P < 0.05) reduced the phosphorus and iron contents of the seeds while processing generally raised the calcium and magnesium contents (Table 5).
Chemical and Biochemical Changes Associated with Fermentation of African Oil Bean Seeds
The major biochemical changes that take place during the fermentation of African oil bean seeds have been shown to be proteolysis. During the process, the protein component of the cotyledons is hydrolyzed to amino acids. Bacillus species are the predominant bacteria during fermentation. Protease activity has been shown to rapidly increase from the start of the fermentation period till the end (Odunfa, 1985a).
Another biochemical change that has been shown to occur during the fermentation of oil bean seeds is lipid hydrolysis. Lipids are usually hydrolyzed to fatty acids by lipases. However, though lipids are one of the major components of the oil bean seeds (43–47%), lipolytic activity is reported to be low during the fermentation of the oil bean seeds (Achinewhu, 1986; Njoku and Okemadu, 1989; Onwuliri et al., 2004). Enujiugha (2003) found out that the principal fatty acid of the seeds, linoleic acid, increased from 60.68 to 67.57% of the total fatty acids while oleic acid decreased from 26.95 to 22.59% during fermentation.
Carbohydrates constitute about 4–17% of the total components of the oil bean seed and the major sugars identified in the bean are oligosaccharides hydrolyzed by amylases (Achinewhu, 1982). These are oligosaccharides that are hydrolyzed by amylases to simple sugars during the fermentation process. Monago et al. (2004) observed that the content of this carbohydrate decreased significantly as fermentation time increased.
Obeta (1983) found out that pH increased from 6.5 at 0 h to 9.0 at 48 h and declined to 7.1 at 72 h. The rise in pH has been attributed to the abundant production of ammonia during the fermentation due to protein hydrolysis and deaminase activity.
Also, moisture content has been found to increase throughout the period of fermentation (52–56.90% to 71.20–73%; Odunfa and Oyeyiola, 1985; Njoku and Okemadu, 1989; Ogueke and Aririatu, 2004). The increase in moisture is believed to be due to the hydrolytic activities of the microorganisms. However, Odunfa and Oyeyiola (1985) and Ogueke and Aririatu (2004) believe that the high moisture level brought about by fermentation predisposes the product to rapid spoilage.
Anti-Nutritional Content of Ugba
The African oil bean seeds are inedible in its unfermented state because it suffers from some drawbacks. Little is known about anti-nutritional factors in the raw and fermented African oil bean seeds. Although, Kar and Okechukwu (1978) and Enujiugha and Agbede (2000) reported the presence of a number of anti-nutritional and /or toxic factors, our recent studies (Table 6), have revealed the detection of tannins, saponins, alkaloids, steroids, glycosides, flavonoids, and phytate in the unfermented African oil bean seed (Okorie and Olasupo, 2014). This study also showed that processing and fermentation drastically reduced the content of these toxic factors in the fermented product (Table 7) (Okorie and Olasupo, 2014), mainly due to soaking of the seeds overnight and washing in water before fermentation. This had a significant effect on all the phytochemicals/anti-nutritional factors identified. Tannin was reduced from 12.58 to 3. 65 mg/100 g, saponin from 52.00 to 22.00 mg/100 g, phytate from 25.63 to 14.47 mg/100 g, glycosides from 34.76 to 11.33 mg/100 g, alkaloids from 2.52 to 0.14 mg/100 g, flavonoids from 4.66 to 2.49 mg/100 g and steroids from 26.48 to 5.43 mg/100 g. Alkaloids and tannins were completely removed from the samples after 24 and 48 h of fermentation respectively.
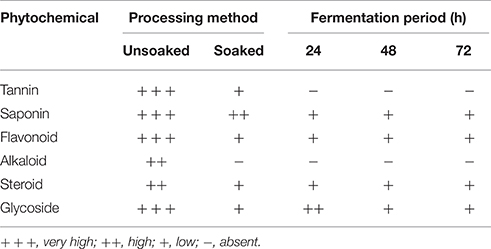
Table 6. Preliminary assay for anti-nutritional factors and phytochemicals in African oil bean seed (Okorie and Olasupo, 2014).
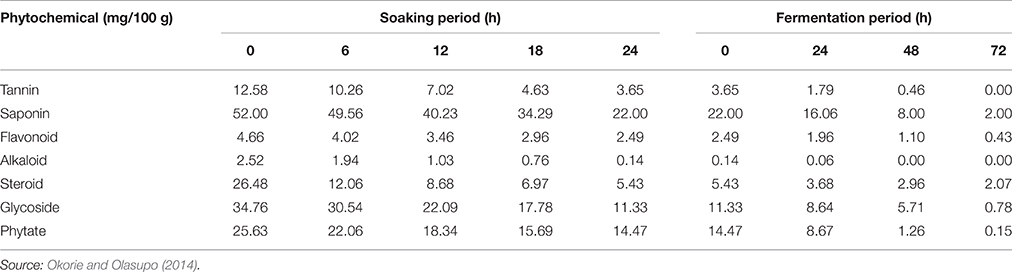
Table 7. Effect of soaking and fermentation period on the anti-nutritional/phytochemical contents of African oil bean seed.
Microbiological Safety of Fermented African Oil Bean Seeds
Most works on African fermented foods (ugba inclusive) have centered on the isolation and characterizations of organisms involved in the fermentation processes. Not much effort seems to have been made toward the occurrence and growth of possible pathogens in the product. However, Adewunmi et al. (2014) used a combination of genome-based culture dependent and independent techniques to examine iru microbiota and reported bacterial species with both spoilage and pathogenic history. In addition, genome typing of Bacillus species isolated from okpehe and soumbala identified species of Bacillus cereus with enterotoxin production potential (Ouaba et al., 2008; Oguntoyinbo et al., 2010). It is therefore very important to use genotypic method in combination with phenotypic data to assess microbial quality of fermenting ugba, in order to guarantee its microbial safety. Furthermore, because of the stress associated with the food processing, it would be important to use culture dependent and independent methods in order to find/detect non-culturable or not yet cultured microorganisms. Available information in literature shows that organisms such as E. coli, Staphylococcus aureus and other members of the Enterobacteriaceae have been isolated from condiments in West Africa (Isu and Njoku, 1997; Okorie and Olasupo, 2013a).
Selection of Starter Cultures for Controlled Fermentation of Ugba
The traditional method of production of ugba involves natural solid state fermentation of the African oil bean seeds. This chanced inoculation method has the inherent drawback of possible growth and occurrence of pathogens in the final product. Although, microbiota that best adapted brings about the final product, variation in final product due to fermentation time and unhygienic handling does affect the product and its consistency.
Selection and application of starter cultures in the production process has been identified as critical to the elimination of pathogens and spoilage microbes (Holzapfel, 2002). Several efforts have been made on the selection and application of starter cultures in a controlled fermentation of some fermented condiments including ugba in Nigeria. Oguntoyinbo et al. (2007) used a combination of highly proteolytic and bacteriocin producing starter cultures for the production of okpehe, a fermented Prosopis africana cotyledon. Isu and Ofuya (2000) studied the use of pure cultures of Bacillus subtilis attached to cowpea and maize granules in the fermentation process of ugba. They monitored changes in pH, amino-nitrogen and protease activity as fermentation indicators, carried out with the immobilized cells. Protease activity increased from 4.5 to 27.65 mg N/min for the immobilized cells with respect to 10.5 mg N/min produced by the natural fermentation, and there was a reduction in the fermentation time to 48 h as compared to 96 h for the natural fermentation process.
Okorie and Olasupo (2013b) developed controlled fermentation of ugba using B. subtilis and B. lichenformis singly and as mixed cultures fermentation. The process fermentation time was reduced from 96 to 48 h. Ugba produced with the starters were similar in terms of color, taste and nutritional content to those produced by natural fermentation.
Several other attempts have been made to control the fermentation of this product with similar results as stated above (Ogueke and Aririatu, 2004; Eze et al., 2014). There, however, still exists a need for more field application and extension of starter cultures to small and cottage processors of condiments in Nigeria.
Conclusion
Ugba is an important part of the diet of the Ibos and other ethnic groups in the eastern and southeastern parts of Nigeria. It is produced through a natural solid state fermentation of the oil bean seeds. The major microorganisms involved in the process are Bacillus species. These microorganisms metabolize the protein content of the seeds into free amino acids and ammonia, having undergone a biochemical reaction during the fermentation process known as proteolysis.
Fermentation of the oil bean seeds leads to increase in the nutritional values of the product. The natural process of its production, and the subsistent level at which the condiment is being produced leaves the safety of this product in doubt and makes its quality inconsistent. Efforts at controlled fermentation of the product have shown that some of these observed drawbacks could be overcome by the application of starter cultures in the production process. There is therefore a need to make the local processors of this product realize the potential benefits derivable from the application of starter cultures in their process line.
Author Contributions
NO: Participated in conception, literature search, design and write up. CO: Participate in literature search, design and write up. FO: Participated in literature search, design and write up.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Achi, O. K. (1992). Microorganisms associated with natural fermentation of Prosopis africana seeds for the production of okpehe. Plt. Foods Hum. Nutr. 42, 304–309. doi: 10.1007/BF02194090
Achi, O. K. (2005). The potential of upgrading of traditional fermented foods through biotechnology. Afr. J. Biotechnol. 4, 375–380.
Achinewhu, S. C. (1982). Chemical and nutrient composition of fermented products from plant foods. Nig. Food J. 1, 115–117.
Achinewhu, S. C. (1986). The effect of fermentation on carbohydrate and fatty acid Composition of the African oil bean (Pentaclethra macrophylla) seed. Food Chem. 19, 105–116. doi: 10.1016/0308-8146(86)90104-4
Adams, M. R. (1990). Topical aspect of fermented foods. Trends Food Sci. Technol. 1, 141–144. doi: 10.1016/0924-2244(90)90111-B
Aderibigbe, E. Y., and Odunfa, S. A. (1990). Growth and extracellular enzyme production by strains of Bacillus species isolated from fermenting African locust bean, iru. J. Appl. Bacteriol. 69, 662–671. doi: 10.1111/j.1365-2672.1990.tb01560.x
Aderibigbe, E. Y., Schink, B., and Odunfa, S. A. (1990). Extracellular proteinases of Bacillus sp isolated from African locust bean, iru. Food Microbiol. 7, 281–293. doi: 10.1016/0740-0020(90)90033-E
Adewunmi, A. R., Ajayi, J. O., and Omotoso, B. O. A. (2014). Assessment of the hygienic practices of food vendors and government intervention in selected secondary schools from Abeokuta south local government area of Ogun State, Nigeria. J. Sci. Multidiscipl. Res. 6, 2277–2285.
Antai, S. P., and Ibrahim, M. H. (1986). Microorganisms associated with African locust bean (Parkia filicoidea) fermentation for dawadawa production. J. Appl. Biotechnol. 61, 145–148.
Brown, I. J., Tzoulaki, I., Candeias, V., and Elliott, P. (2009). Salt intakes around the world: implications for public health. Int. J. Epidemiol. 38, 791–813. doi: 10.1093/ije/dyp139
Chung, S. K., Mee, S. L., Se, I. O., and Sang, C. P. (2010). Discovery of novel sources of vitamin B12 in: traditional korean foods from nutritional surveys of centenarian. Curr. Gerontol. Geriatr. Res. 2010:374897. doi: 10.1155/2010/374897
Daeschel, M. A., Anderson, R. E., and Fleming, H. P. (1987). Microbial ecology of fermenting plant materials. FEMS Microbiol. Rev. 46, 357–367. doi: 10.1111/j.1574-6968.1987.tb02472.x
Duke, J. A. (1981). Handbook of Legumes of World Economic Importance. 1st Edn. New York, NY: Plenum Press. doi: 10.1007/978-1-4684-8151-8
Egwim, E., Amanabo, M., and Yahaya, A. (2013). “Nigerian indigenous fermented foods; process and prospects,” in Mycotoxin and Food Safety in Developing Countries, ed A. A. Hussaini (Rijeka: Intech), 153–180.
Ejiofor, M. A. N., Oti, E., and Okafor, J. C. (1987). Studies on the fermentation of seeds of the African oil bean tree (Pentaclethra macrophylla). Int. Tree Crops J. 4, 135–144. doi: 10.1080/01435698.1987.9752818
Eman, H. E. A. (2009). Starter culture development for improving safety and quality of Domiati cheese. Food Microbiol. 26, 533–5410. doi: 10.1016/j.fm.2009.03.007
Enujiugha, V. N. (2003). Nutrient changes during the fermentation of African oil bean (Penttoclethra macrophylla Benth) seeds. Pakistan J. Nutr. 2, 320–323. doi: 10.3923/pjn.2003.320.323
Enujiugha, V. N., and Akanbi, C. T. (2008). Quality evaluation of canned fermented African oil bean seed slices during ambient storage. Afr. J. Food Sci. 2, 54–59.
Enujiugha, V. N., and Agbede, J. O. (2000). Nutritional and anti-nutritional characteristics of African oil bean (Pentaclethra macrophylla, Benth.) seeds. Appl. Trop. Agric. 5, 11–14.
Enujiugha, V. N., and Akanbi, C. T. (2005). Compositional changes in African oil bean (Pentaclethra macrophylla Benth) seeds during thermal processing. Pakistan J. Nutr. 4, 27–31. doi: 10.3923/pjn.2005.27.31
Eze, V. C., Onwuakor, C. E., and Ukeka, E. (2014). Proximate composition, biochemical and microbiological changes associated with fermenting African oil bean (Pentaclethra macrophylla Benth) seeds. Amer. J. Microbiol. 2, 674–681.
Gadaga, T. H., Nyanga, L. K., and Mutukumira, A. N. (2004). The occurrence, food, growth and control of pathogens in African fermented foods. Afr. J. Agric. Nutr. Dev. 4, 20–23. doi: 10.4314/ajfand.v4i1.19155
Ghosh, D., Chattora, D. K., and Chattopadhyay, P. (2013). Studies on changes in microstructure and proteolysis in cow and soy milk curd during fermentation using lactic cultures for improving protein bioavailability. J. Food Sci. Technol. 50, 979–985. doi: 10.1007/s13197-011-0421-1
He, F. J., and MacGregor, G. A. (2009). A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J. Hum. Hypertens. 23, 363–384. doi: 10.1038/jhh.2008.144
Holzapfel, W. H. (2002). Appropriate starter culture technologies for small- scale fermentation in developing countries. Int. Food Microbiol. 75, 197–212. doi: 10.1016/S0168-1605(01)00707-3
Ikenebomeh, M. J., Kok, R., and Ingram, J. M. (1986). Processing and fermentation of the African locust bean (Parkia folicodea Welw) to produce dawadawa. J. Sci. Food Agric. 37, 273–282. doi: 10.1002/jsfa.2740370312
Issoufou, A., Guo-Wei, L., Tidjani, A., Jin, S., and Yong-Hui, S. (2013). Purification and characterization of foxtail millet- derived peptides with antioxidant and antimicrobial activities. Food Res. Int. 51, 422–428. doi: 10.1016/j.foodres.2012.12.045
Isu, N. R., and Njoku, H. O. (1997). An evalution of the microflora associated with fermented African oil bean (Pentaclethra macrophylla Benth) seeds durin ugba production. Plt Foods Hum. Nutr. 51, 145–157. doi: 10.1023/A:1007906413195
Isu, N. R., and Ofuya, C. O. (2000). Improvement of the traditional processing and fermentation of African oil bean (Pentaclethra macrophylla Bentham) seeds into a food snack ugba. Int. J. Food Microbiol. 59, 235–239. doi: 10.1016/S0168-1605(00)00318-4
Kar, A., and Okechukwu, A. D. (1978). Chemical investigations on the edible seeds of Pentaclethra macrophylla Benth. Qual. Plt Foods Hum. Nutr. 38, 29–36. doi: 10.1007/BF01092998
Khan, H., Khan, M. A., and Dullah, A. (2012). Antibacterial, antioxidant and cytotoxic studies of total saponins, alkaloids and sterols contents of decoction of Joshanda: identification through thin layer chromatography. Toxicol. Indust. Health. 6, 528–535. doi: 10.1177/0748233712468023
Ling, J., Wu, Q., Xu, Y., and Fan, W. (2013). Interactions between Bacillus licheniformis and Saccharomyces cerevisiae in the fermentation of soy-sauce flavor liquor. Microbiol. China 40, 2014–2021.
Makanjuola, O. M., and Ajayi, A. (2012). Effect of natural fermentation on the nutritive value and mineral composition of African locust beans. Pakistan J. Nutr. 11, 11–13. doi: 10.3923/pjn.2012.11.13
Mba, A. V., Njike, M. C., and Oyenuga, V. A. (1974). Proximate chemical composition and amino acid content of Nigerian oil seeds. J. Sci. Food Agric. 25, 1547–1553. doi: 10.1002/jsfa.2740251216
Mbajunwa, O. K., Akingbala, J. O., Mulongoy, K., and Oguntimela, G. (1998). Starter culture evaluation for the production of ugba from African oil bean seed Pentaclethra macrophylla. J. Sci. Food Agric. 77, 127–132.
Monago, C. C., Ogbomeh, P. A., and Joshua, P. E. (2004). Effect of African oil bean seed (Pentaclethra macrophylla Benth) on blood cholesterol level in rats. Global J. Pure Appl. Sci. 10, 165–168.
Mulyowidarso, R. K., Fleet, G. H., and Buckle, K. A. (1989). The microbial ecology of soybean soaking for tempe production. Int. J. Food Microbiol. 8, 35–46. doi: 10.1016/0168-1605(89)90078-0
Njoku, H. O., and Okemadu, C. P. (1989). Biochemical changes during the natural fermentation of the African oil bean for the production of ugba. J. Sci. Food Agric. 49, 457–465. doi: 10.1002/jsfa.2740490408
Nwagu, T. N., Amadi, C., and Alaekwe, O. (2010). Role of Bacteria Isolates in the Spoilage of fermented African Oil Bean Seed Ugba. Pakistan J. Biol. Sci. 13, 497–503. doi: 10.3923/pjbs.2010.497.503
Nwokeleme, C. O., and Ugwuanyi, J. O. (2015). Evolution of volatile flavour compounds during fermentation of African oil bean (Pentaclethra macrophylla Benth) seed for “ugba” production. Int. J. Food Sci. 2015:706328. doi: 10.1155/2015/706328
Obeta, J. A. N. (1983). A note on the microorganisms associated with the fermentation of seeds of African oil bean (Pentaclethra macrophylla). J. Appl. Biotechnol. 54, 433–435.
Oboh, H. A., Muzquiz, M., Burbano, C., Cuadrado, C., Pedrosa, M. M., Ayet, G., et al. (1998). Anti-nutritional constituents of six underutilized legumes grown in Nigeria. Chromatogr. Analyt. 823, 307–312. doi: 10.1016/S0021-9673(98)00542-1
Odoemelam, S. A. (2005). Proximate composition and selected physicochemical properties of the seeds of African oil bean (Pentaclethra marcrophylla). Pakistan. J. Nutr. 4, 382–383. doi: 10.3923/pjn.2005.382.383
Odunfa, S. A. (1981). Microorganisms associated with fermentation of African locust bean (Parkia filicoidea) during iru preparation. J. Plant Foods 91, 219–223.
Odunfa, S. A. (1985a). Biochemical changes in fermenting African locust bean (Parkia biglobosa) during iru fermentation. J. Food Technol. 20, 295–303. doi: 10.1111/j.1365-2621.1985.tb00379.x
Odunfa, S. A. (1985b). “African fermented foods,” in Microbiology of Fermented Foods, Vol. 2, ed B. J. B. Wood (London; New York, NY: Elsevier Applied Science), 155–191.
Odunfa, S. A. (1986). “Dawadawa,” in Legume Based Fermented Foods, ed N. R. Raddy, M. D. Pierson, and D. K. Salunkhe (Boca Raton, FL: CRC Press), 173–189.
Odunfa, S. A., and Komolafe, O. B. (1989). Nutritional characteristics of Staphylococcus species from fermenting African locust bean(Parkia biglobosa). Die Nahrung 33, 607–615.
Odunfa, S. A., and Oyewole, O. B. (1986). Identification of Bacillus species from iru. a fermented African locust bean product. J. Basic Microbiol. 26, 101–108. doi: 10.1002/jobm.3620260212
Odunfa, S. A., and Oyeyiola, G. F. (1985). Microbiological study of the fermentation of ugba. A Nigerian indigenous fermented food flavor. J. Plt. Foods 6, 155–163.
Odunfa, S. A., and Oyewole, O. B. (1998). “African fermented foods,” in Microbiology of Fermented Foods. 2nd Edn. ed B. J. B. Woods (London: Blackie Academic and Professionals), 713–752. doi: 10.1007/978-1-4613-0309-1_23
Ogbadu, C. O., and Okagbue, R. N. (1988). Bacterial fermentation of soybean for dawadawa production. J. Appl. Bacteriol. 65, 353–356. doi: 10.1111/j.1365-2672.1988.tb01902.x
Ogbonna, D. N., Sokari, T. G., and Achinewhu, S. C. (2001). Development of owoh-type product from African yam bean (Sphenostylis stenocarpa) seeds by solid substrate fermentation. Plt. Foods Hum. Nutr. 56, 183–194. doi: 10.1023/A:1011185513717
Ogueke, C. C., and Aririatu, L. E. (2004). Microbial and organoleptic changes associated with ugba stored at ambient temperature. Nig. Food J. 22, 133–140.
Ogueke, C. C., Nwosu, J. N., Owuamanam, C. I., and Iwouno, J. N. (2010). Ugba, the fermented African oil bean seeds; its production, chemical composition, preservation, safety and health benefits. Pakistan J. Biol. Sci. 13, 489–496. doi: 10.3923/pjbs.2010.489.496
Oguntoyinbo, F. A. (2014). Safety challenges associated with traditional foods of West Africa. Food Rev. Int. 30, 338–358. doi: 10.1080/87559129.2014.940086
Oguntoyinbo, F. A., Huch, M., Cho, G. S., Schillinger, U., Holzapfel, W. H., Sanni, A. I., et al. (2010). Diversity of Bacillus species isolated from okpehe, a traditional fermented soup condiment from Nigeria. J. Food Protect. 73, 870–878.
Oguntoyinbo, F. A., Sanni, A. I., Franz, C. M. A. P., and Holzapfel, W. H. (2007). In-vitro fermentation studies for selection and evaluation of Bacillus strains as starter cultures for production of okpehe, a traditional African fermented condiment. Int. J. Food Microbiol. 113, 208–218. doi: 10.1016/j.ijfoodmicro.2006.07.006
Okechukwu, R. I., Ewelike, N., Ukaoma, A. A., Emejulu, A. A., and Azuwike, C. O. (2012). Changes in the nutrient composition of the African oil bean meal “ugba” (Pentaclethra macrophylla Benth) subjected to solid state natural fermentation. J. Appl. Biosci. 51, 3591–3595.
Okorie, P. C., and Olasupo, N. A. (2013a). Growth and extracellular enzyme production by microorganisms isolated from Ugba- an indigenous Nigerian fermented food. Afr. J. Biotechnol. 12, 4158–4167. doi: 10.5897/AJB11.2842
Okorie, P. C., and Olasupo, N. A. (2013b). Controlled fermentation and preservation of ugba –an indigenous Nigerian fermented food. Springerplus 2, 470–478. doi: 10.1186/2193-1801-2-470
Okorie, P. C., and Olasupo, N. A. (2014). Effect of processing method and fermentation on the antinutritional factors/phytochemical contents of African oil bean seed. Int. J. Sci. Eng. Res. 5, 1535–1553.
Olakunle, M. M., and Adebayo, A. (2012). Effect of natural fermentation on the nutritive value and mineral composition of African locust beans. Pakistan J. Nutr. 11, 11–13. doi: 10.3923/pjn.2012.11.13
Olasupo, N. A., Smith, S. I., and Akinsinde, K. A. (2002). Examination of microbial status of selected indigenous fermented foods in Nigeria. J. Food Safety 22, 85–93. doi: 10.1111/j.1745-4565.2002.tb00332.x
Onwuliri, V. A., Attah, I., and Nwankwo, J. O. (2004). Anti-nutritional factors, essential and non-essential fatty acids composition of ugba (Pantaclethra macrophylla) seed at different stages of processing and fermentation. J. Bio Sci. 4, 671–675. doi: 10.3923/jbs.2004.671.675
Ouaba, L. I. I., Parkouda, C., Diawara, B., Scotti, C., and Varman, A. H. (2008). Identification of Bacillus spp. from Bikalga, fermented seed of Hibiscus sabdariffa: phenotypic and genotypic characterization. J. Appl. Microbiol. 104, 122–131. doi: 10.1111/j.1365-2672.2007.03550.x
Oyeyiola, G. P. (1981). Studies on the Microorganisms Isolated from Ugba, a Nigerian Indigenous Food. Master's dissertation, University of Ibadan (Ibadan).
Sanni, A. I. (1993). Biochemical changes during production of Okpehe- a Nigerian fermented food condiment. Chem. Microbiol. Technol. Lebensm. 15, 97–100.
Sanni, A. I., Onilude, A. A., Fadahunsi, I. F., Ogubanwo, S. T., and Afolabi, R. O. (2002). Selection of starter cultures for the production of ugba, a fermented soup condiment. Eur. Food Res. Technol. 215, 176–180. doi: 10.1007/s00217-002-0520-3
Sanni, A. I., and Oguntoyinbo, F. A. (2014). “Ugba,” in Handbook of Indigenous Foods Involving Alkaline Fermentation, eds P. K. Sarker and M. J. Robert Nout (Boca Raton; London; New York: CRC Press).
Sanni, A. I., Onilude, A. A., and Oguntoyinbo, F. A. (1999). Optimization of process conditions for owoh, a fermented cotton seed condiment. Adv. Food Sci. 20, 163–167.
Sarker, P. K., Jones, I. J., Craven, G. S., and Somerset, S. M. (1997). Oligosaccharide profiles of soybeans during kinema production. Lett. Appl. Microbiol. 24, 337–339. doi: 10.1046/j.1472-765X.1997.00035.x
Savadogo, A., Tapi, A., Chollet, M., Wathelet, B., Traoré, A. S., and Jacques, P. H. (2011). Identification of surfactin producing strains in Soumbala and Bikalga fermented condiments using polymerase chain reaction and matrix assisted laser desorption/ionization-mass spectrometry methods. Int. J. Food Microbiol. 151, 299–306. doi: 10.1016/j.ijfoodmicro.2011.09.022
Shil, K., Mee, S. L., Se, I. O., and Sang, C. P. (2010). Discovery of novel sources of vitamin B12 in traditional Korean foods from nutritional surveys of centenarians. Curr. Gerontol. Geriatr. Res. 2010:374897.
Sokari, T. G., and Wachukwu, C. K. (1997). Simple rapid processing of African oil beans for ugba production. Int. J. Food Sci. Technol. 32, 77–79. doi: 10.1046/j.1365-2621.1997.00371.x
Strazzullo, P., D'Elia, L., Kandala, N. B., and Cappuccio, F. P. (2009). Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 339:b4567. doi: 10.1136/bmj.b4567
Suberu, H. A., and Akinyanju, J. A. (1996). Starter culture for the production of Soyiru. World J. Microbiol. Biotechnol. 12, 403–404. doi: 10.1007/BF00340220
Taylor, J. R. N., and Duodu, K. G. (2015). Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J. Sci. Food Agric. 95, 225–237. doi: 10.1002/jsfa.6713
Tofalo, R., Schirone, M., Perpetuini, G., Angelozzi, G., Suzzi, G., and Corsetti, A. (2012). Microbiological and chemical profiles of naturally fermented table olives and brines from different Italian cultivars. Anton. Leeuw. Int. J. 102, 121–131. doi: 10.1007/s10482-012-9719-x
Keywords: microbiology, Ugba, fermentation, condiment
Citation: Olasupo NA, Okorie CP and Oguntoyinbo FA (2016) The Biotechnology of Ugba, a Nigerian Traditional Fermented Food Condiment. Front. Microbiol. 7:1153. doi: 10.3389/fmicb.2016.01153
Received: 04 May 2016; Accepted: 11 July 2016;
Published: 04 August 2016.
Edited by:
Jyoti Prakash Tamang, Sikkim University, IndiaReviewed by:
Carmen Wacher, National Autonomous University of Mexico, MexicoChiara Montanari, Centro Interdipartimentale di Ricerca Industriale Agroalimentare, Italy
Copyright © 2016 Olasupo, Okorie and Oguntoyinbo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nurudeen A. Olasupo, bmFvbGFzdXBvQHlhaG9vLmNvbQ==
 Nurudeen A. Olasupo
Nurudeen A. Olasupo Chimezie P. Okorie2
Chimezie P. Okorie2