- 1School of Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK
- 2Microbiology Department, Glasgow Royal Infirmary, Glasgow, UK
- 3Public Health England, Southwest Laboratory, Bristol, UK
- 4Microbiology Department, NHS Lothian, Edinburgh, UK
- 5University of the West of Scotland, Glasgow, UK
- 6Aberdeen Fungal Group, University of Aberdeen, Aberdeen, UK
This study provide an up-to-date overview of the epidemiology and risk factors for Candida bloodstream infection in Scotland in 2012/2013, and the antifungal susceptibility of isolates from blood cultures from 11 National Health Service boards within Scotland. Candida isolates were identified by chromogenic agar and confirmed by MALDI–TOF methods. Survival and associated risk factors for patients stratified as albicans and non-albicans cases were assessed. Information on the spectrum of antifungals used was collected and summarized. The isolates sensitivity to different antifungals was tested by broth microdilution method and interpreted according to CLSI/EUCAST guidelines. Forty one percent of candidaemia cases were associated with Candida albicans, followed by C. glabrata (35%), C. parapsilosis (11.5%), and remainder with other Candida spp. C. albicans and C. glabrata infections were associated with 20.9 and 16.3% mortality, respectively. Survival of patients with C. albicans was significantly lower compared to non-C. albicans and catheter line removal in C. albicans patients significantly increases the survival days. Predisposing factors such as total parenteral nutrition, and number of days on mechanical ventilation or in intensive care, were significantly associated with C. albicans infections. Fluconazole was used extensively (64.5%) for treating candidaemia cases followed by echinocandins (33.8%). Based on CLSI breakpoints, MIC test found no resistance to any antifungals tested except 5.26% fluconazole resistance among C. glabrata isolates. Moreover, by comparing to EUCAST breakpoints we found 3.95% of C. glabrata isolates were resistant to anidulafungin. We have observed a shift in Candida spp. with an increasing isolation of C. glabrata. Delay and choice of antifungal treatment are associated with poor clinical outcomes.
Introduction
Candida species remain a significant cause of nosocomial bloodstream infections (BSIs), associated with prolonged hospital stay in the ICU and high healthcare cost (Rentz et al., 1998; Gudlaugsson et al., 2003). The global prevalence of candidemia was reported to be highly variable among different countries ranging between 1.1 and 14.4 cases per 105 population (Odds et al., 2007; Arendrup, 2010; Gamaletsou et al., 2013; Quindos, 2014; Cleveland et al., 2015; Osmanov and Denning, 2015). The maximum of 14.4 cases per 105 population was reported in USA (Cleveland et al., 2015), though in Scotland was recently reported to be 4.8 (Odds et al., 2007).
Reports in recent years have established various risk factors for Candida BSIs. The risk factors including broad-spectrum antibiotics, parenteral nutrition, immuno-suppression due to chemotherapy and radiotherapy, disruption of mucosal barriers due to surgery, ICU admission and indwelling medical devices such as central venous catheters (CVCs), are among the most important predisposing factors for Candida BSI (Dimopoulos et al., 2008; Leroy et al., 2009). Indeed, biofilm formation by Candida spp. and choice of antifungal has been shown to be risk factors for morbidity and mortality in candidaemia cases (Tumbarello et al., 2007, 2012; Rajendran et al., 2015). Though Candida albicans is the most prevalent, pathogenic and robust biofilm former of the Candida species, there are recent studies reporting non-C. albicans species (NCAS) as frequent causes of candidemia (Nguyen et al., 1996; Dimopoulos et al., 2008; Hachem et al., 2008). Prior patient exposure to antifungals, particularly to azoles, appears to be a predictor for NCAS associated candidaemia (Nguyen et al., 1996).
Catheters are commonly used in ICU patients (∼90%), and represent an easy entry route for Candida spp (Almirante et al., 2005). Biofilm formation by Candida spp. including C. albicans in indwelling lines is considerably difficult to treat with antifungals due to their intrinsic drug resistance (Ramage et al., 2012). Appropriate antifungal medication and catheter removal are critical in preventing patient mortality with line infections (Mermel et al., 2009; Cornely et al., 2012; Koehler et al., 2014). Delaying antifungal therapy appears to be a common practice, and the impact on mortality may be significant with catheter related infections. Fluconazole is a mainstay of treatment for candida BSI, however, they are not effective against C. glabrata or preformed biofilms (Lee et al., 2009; Ramage et al., 2012; Castanheira et al., 2016; Goncalves et al., 2016). In appropriate antifungal usage was reported to be associated with high morbidity and excess hospital cost (Zilberberg et al., 2010).
National epidemiological surveillance and antifungal sensitivity testing of Candida BSIs is vital to stratify high-risk patients and to identify the pattern of causative Candida spp., which will in turn formulate guidelines for management of candidaemia. Therefore the purpose of this study was to investigate this in Scotland.
Materials and Methods
Patients and Variables
A prospective study of all cases of candidaemia was carried out within Scotland under NHS Caldicott Guardian approval from March 2012 to February 2013. This work formed an audit and as such ethics approval and patient consent are not required in the NHS. This was mandated for the NHS by Health Service Circular: HSC 1999/012. Candida BSI was reported in 217 patients from 11 different health boards; clinical data was obtained from 150 patients. The complete data sets of patient demographics, ICU admissions, underlying medical conditions, information on immunosuppression, and details of antifungal therapy were collected through a review of the medical case notes from 8 health boards. Where available, patient outcomes were followed prospectively for 30 days or until death from first blood culture positive, and clinical details including the presence of indwelling medical devices in the 30 days prior to the occurrence of candidemia were also collected.
Isolates and Antifungal Testing
Blood cultures were performed according to routine standard operating procedures in each of the referring laboratories. All clinical isolates were obtained at the beginning of episode 1 and independently identified using ColorexCandida chromogenic plates (E&O Laboratories Ltd, Bonnybridge, UK) and were stored in Microbank® vials (Pro-Lab Diagnostics, Cheshire, UK) at -80°C until further use. Isolates identification were further confirmed from sub-culture on glucose-peptone plates by MALDI-TOF MS analysis using a Bruker Microflex to produce the spectra which were then analyzed using the Bruker database. MIC test for different antifungals were performed centrally at Mycology Reference Laboratory, Public Health England, Bristol, according to standard Clinical and Laboratory Standards Institute (CLSI) broth microdilution methodology (CLSI, 2008). Interpretive criteria used for fluconazole (FLZ), voriconazole (VRZ), and anidulafungin (ANID) were performed in accordance with the current CLSI guidelines (CLSI, 2012).
Statistical Analysis
Initially, all data were numerically coded and labeled for each variable, which was analyzed using SPSS software (SPSS Inc., Chicago, IL, USA). Categorical variables were compared between groups using the two-tailed χ2 test or Fisher’s exact test, as appropriate. Two groups of any continuous variables were compared using Student’s t-test or Mann–Whitney U-test as appropriate. Variables showing a significant association with survival found by Student’s t test or χ2 test (Supplementary Table S2) were included into subsequent multivariate Cox’s regression analysis, to generate the survival curves.
Results and Discussion
We undertook a retrospective analysis of candidaemia patients in Scotland as means of understanding the national epidemiology of Candida species, associated risk factors and isolates sensitivity to antifungal agents. Data from the most recent 2011 census list the population of Scotland at 5,295,4031 Therefore the population-based incidence of candidaemia in Scotland was calculated as 4.1 per 100,000 population for the year 2012/13. This data is comparable to those of 2005/06 (4.8 cases per 100,000 of population per year) (Odds et al., 2007). The minor discrepancy between these two studies could be explained by a growing population and from reporting of the false positive cases, and is therefore likely to represent a minimum estimate of the incidence of candidaemia in Scotland. There were approximately equal numbers of male and female patients with candidaemia and at a mean age of 63 years (standard deviation ± 20.3 years). During the study period, 33 out of 141 candidemia patients were admitted to the ICU (23.4%).
Prevalence and Mortality Associated with Candida spp
The prevalence of the different Candida species isolated from the BS of candidaemia patients was assessed. Out of the 280 isolates collected in this study C. albicans predominated (41%), followed by C. glabrata (35%), C. parapsilosis (11.5%), C. tropicalis (3.6%), C. lusitaniae (3.6%), and other species (5.3%). Our previous Scottish candidaemia study (2005/06)(Odds et al., 2007) reported a prevalence of C. albicans and C. glabrata as 50 and 21%, respectively (Supplementary Table S1). This shows a changing trend in the prevalence of Candida spp in causing BSI, with a considerable decrease of C. albicans by 9% followed by a reciprocal increase of C. glabrata by 14%. C. parapsilosis did not show any clear change (∼12% in both studies). These fluctuations are in accordance with the global epidemiological changes, where in Denmark it has been shown that the overall prevalence of C. albicans has declined by 5% co-incidentally with an increase in C. glabrata by 6.9% (year 2010–2011 compared to 2004–2009) (Eggimann et al., 2011; Arendrup et al., 2013b). The reason for this is unclear; however, studies suggesting an increase in the use of FLZ have an impact on selection of C. glabrata (Pfaller and Diekema, 2007; Arendrup et al., 2013b). Of the 129 cases where patient mortality data was available, overall mortality was 41% (n = 53), which was primarily associated with C. albicans (49% [n = 26]), followed by C. glabrata (32% [n = 17]), C. parapsilosis (11.3% [n = 6]), and other species (7.7% [n = 4]).
Therapy
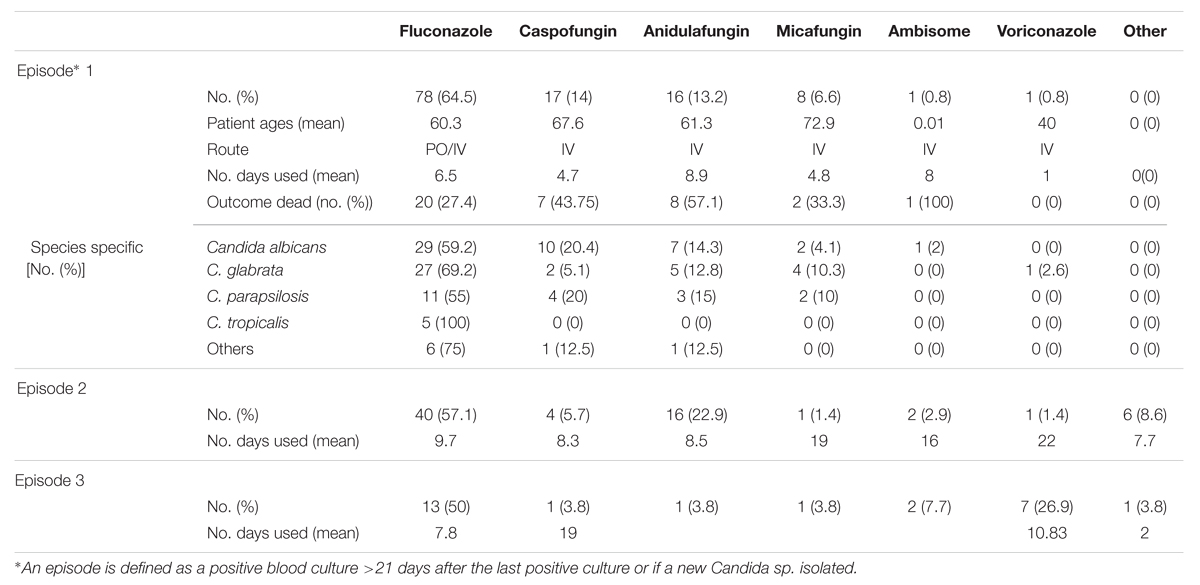
Fluconazole therapy was the predominant antifungal treatment, out of 121 patients for which there was available antifungal usage data. We define the episode as a positive blood culture >21 days after the last positive culture or if a new Candida spp. isolated. In this study we have reported maximum three episodes from the isolate collection date up to 30 days time period unless they discharged early or died. In episode 1, 78 were treated with FLZ (64.5%), 17 with CAS (14%), 16 with ANID (13.2%), 8 with micafungin (6.6%), 1 with AmBisome (0.8%), and 1 with VRZ (1%) (Table 1). In episode 2 there was a 7.4% reduction in the use of FLZ, 8.3% reduction with CAS, 5.2% reduction with micafungin and 9.7% increase in the use of ANID compared to previous episode (Table 1). Out of 78 FLZ treated patients in episode 1 20 (25.6%) were switched to echinocandins in episode 2, due to resistance, line infection, no improvement or microbiological advice. Patients with C. glabrata showed the greatest treatment changes 37% (n = 10), though 50% (n = 5) of these patients’ clinical outcome was unsuccessful (dead). For C. albicans 20.7% (n = 6) of the patients’ treatment was changed, and only one was not successful. A previous study reported an association of inappropriate initial therapy such as FLZ to treat C. glabrata, which resulted in an increased length of hospital stay and excess hospital cost (Zilberberg et al., 2010). In addition, appropriate selection of initial antifungals by empirical use of echinocandins was reported to be even more cost effective than the use of FLZ (Zilberberg et al., 2009).
Around 70% of candidemia patients do not receive empirical antifungal therapy within 24 h of the time the blood sample is drawn for culture. Previous studies have established the negative impact of delayed antifungal treatment on the clinical outcome of candidaemia cases (Morrell et al., 2005; Garey et al., 2006; Kollef et al., 2012). In our study, we found 9 (8.3%) patients received antifungal treatment before a positive sample (BPS) was collected, 21 (19.4%) patients received treatment between 0 and 24 h, and 78 (72%) patients after 24 h from the time at first positive blood sample for culture was drawn. The relationship between hospital mortality and the timing of the administration of antifungal treatment is shown in Figure 1. Patients receiving antifungal treatment BPS was drawn had a lower percentage mortality than patients begun on antifungal treatment between 1–24 h or >24 h (11% versus 19 or 33.1%). This is in line with a previous study that reported an association of delayed treatment by >12 h with increased hospital mortality (p < 0.05) (Morrell et al., 2005; Kollef et al., 2012). Of the patients with a BSI, we stratified groups according to the presence of C. albicans or NCAS, as previously described (Tumbarello et al., 2007; Dimopoulos et al., 2008). The data shows that delayed treatment has a considerable impact on NCAS associated mortality compared to C. albicans. Limitations of the present study include differences in clinical practice patterns across the different ward and different health boards, incomplete data sets and as well as information on antifungal dosing practices. Evading delays in treatment of candidemia patients is challenging due to use of culture based diagnosis methods.
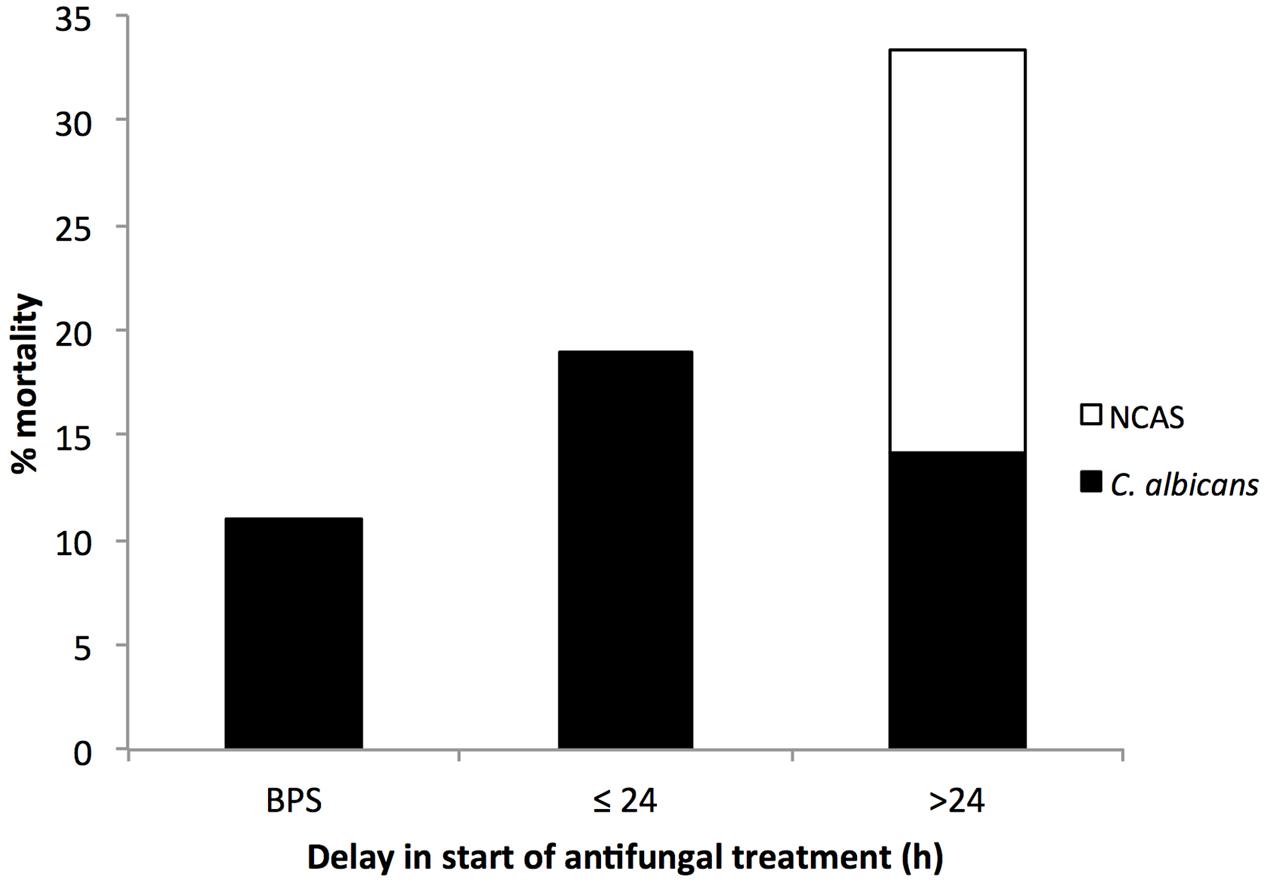
FIGURE 1. Association between patient mortality and the timing of antifungal treatment. The timing of antifungal therapy was determined to be before (BPS) or from the time when the first blood sample for different Candida culture positive was collected to the time when antifungal treatment was first administered to the patient. Number above each bar represents the overall % mortality over different antifungal timings and the black portion represent mortality associated with Candida albicans and white portion for NCAS infections.
Comparison of C. albicans with NCAS Associated Candidaemia
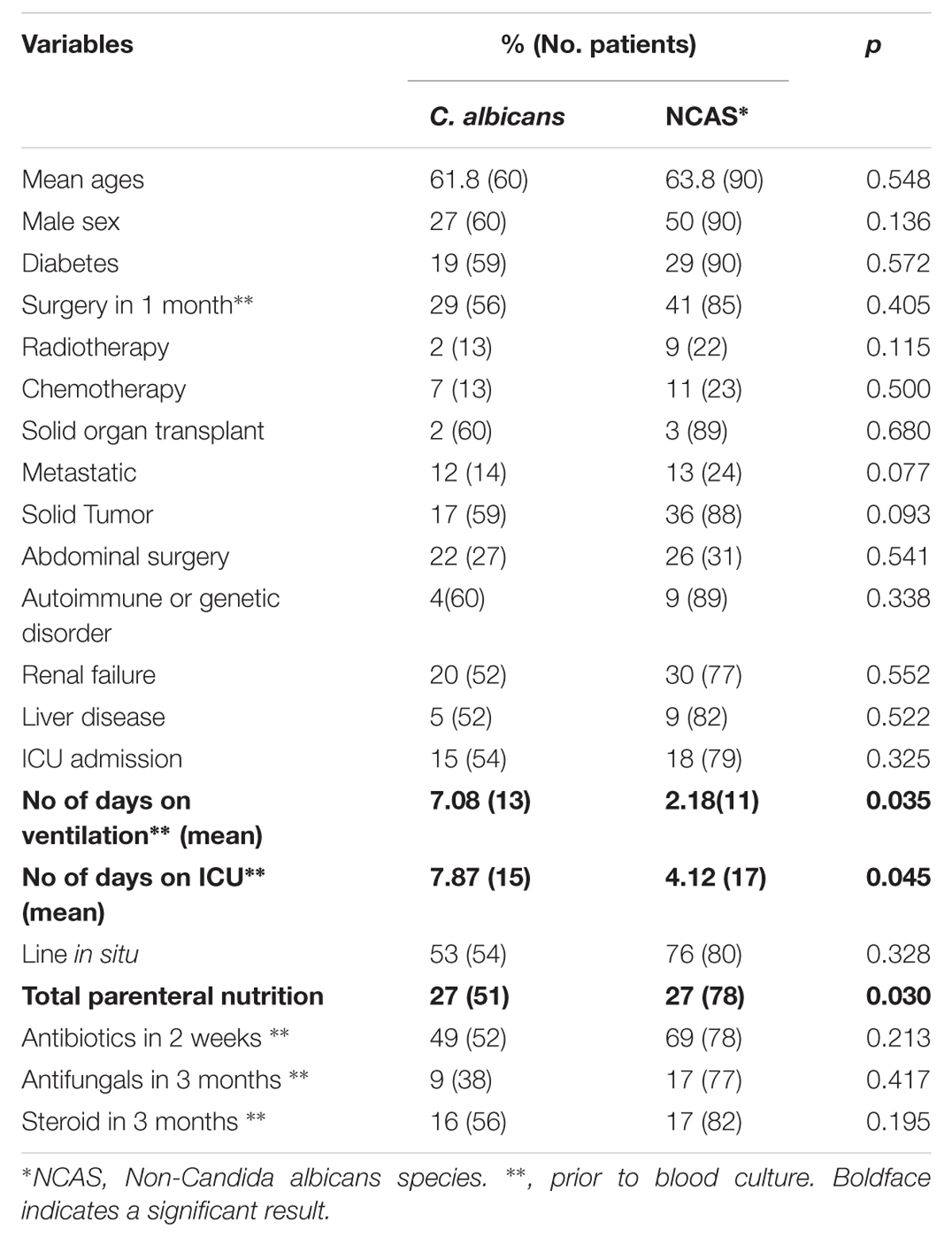
The proportion of C. albicans and NCAS associated infections was considerable among candidaemia cases from different health boards, i.e., Ayrshire and Arran ([n = 22] 50 and 50%), Fife ([n = 11] 27.3 and 72.7%), Forth valley ([n = 11] 27.3 and 72.7%), Grampian ([n = 14] 50 and 50%), Greater Glasgow and Clyde ([n = 39] 38.5 and 61.5), Lanarkshire ([n = 9] 33.3 and 66.7%), Lothian ([n = 26] 42.3 and 57.7%), and Tayside ([n = 18] 38.9 and 61.1%). Overall, survival analysis found a significantly higher mortality with patients infected with C. albicans compared to NCAS (p < 0.05) (Figure 2). Though previous studies from US, Greece and Taiwan, compared the crude mortality of C. albicans with NCAS and showed mixed results, here we showed a significant differences between these spp. in patient survival curve analysis (Dimopoulos et al., 2008; Moran et al., 2009; Chi et al., 2011). The various risk factors were analyzed to find its influence on causing BSI by either C. albicans or NCAS (Table 2). Patient demographics, including age and sex were not statistically significant association with species. Factors such as patients received total parenteral nutrition; no of days on ICU and number of days on mechanical ventilation prior to blood culture were independently related to infection by C. albicans (p = 0.030, p = 0.045, and p = 0.035, respectively). The average number of days on ICU or ventilation BPS for different Candida spp. is displayed in Figure 3. Though other variables have potential impact on infections caused by different Candida spp. we have not found any statistically significant correlation in this study. A similar study from Italy showed a significant relation between solid organ cancer, immunosuppressive therapy, indwelling urinary catheter and type of fungal isolate from candidemia patients (Tumbarello et al., 2007). Furthermore, other risk factors such as neutropenia, glucocorticosteroids, parenteral nutrition and CVCs were also reported to be associated with C. albicans and NCAS infections (Dimopoulos et al., 2008).
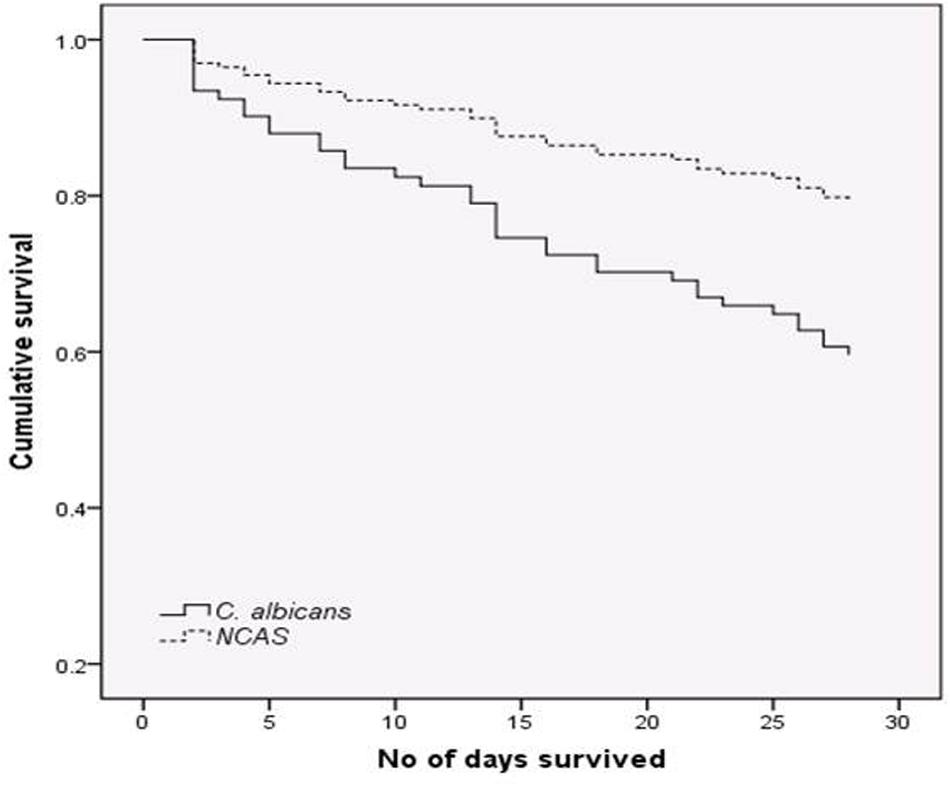
FIGURE 2. Survival analysis for candidaemia patients. Patients with bloodstream infections (BSIs) were grouped by C. albicans (n = 52) or non-C. albicans (NCAS) spp. (n = 72) and censored at death, or day 30. Cox-regression plots adjusted for patient age is shown. Comparison between these curves found a statistically significant difference in mortality rate (p < 0.05).
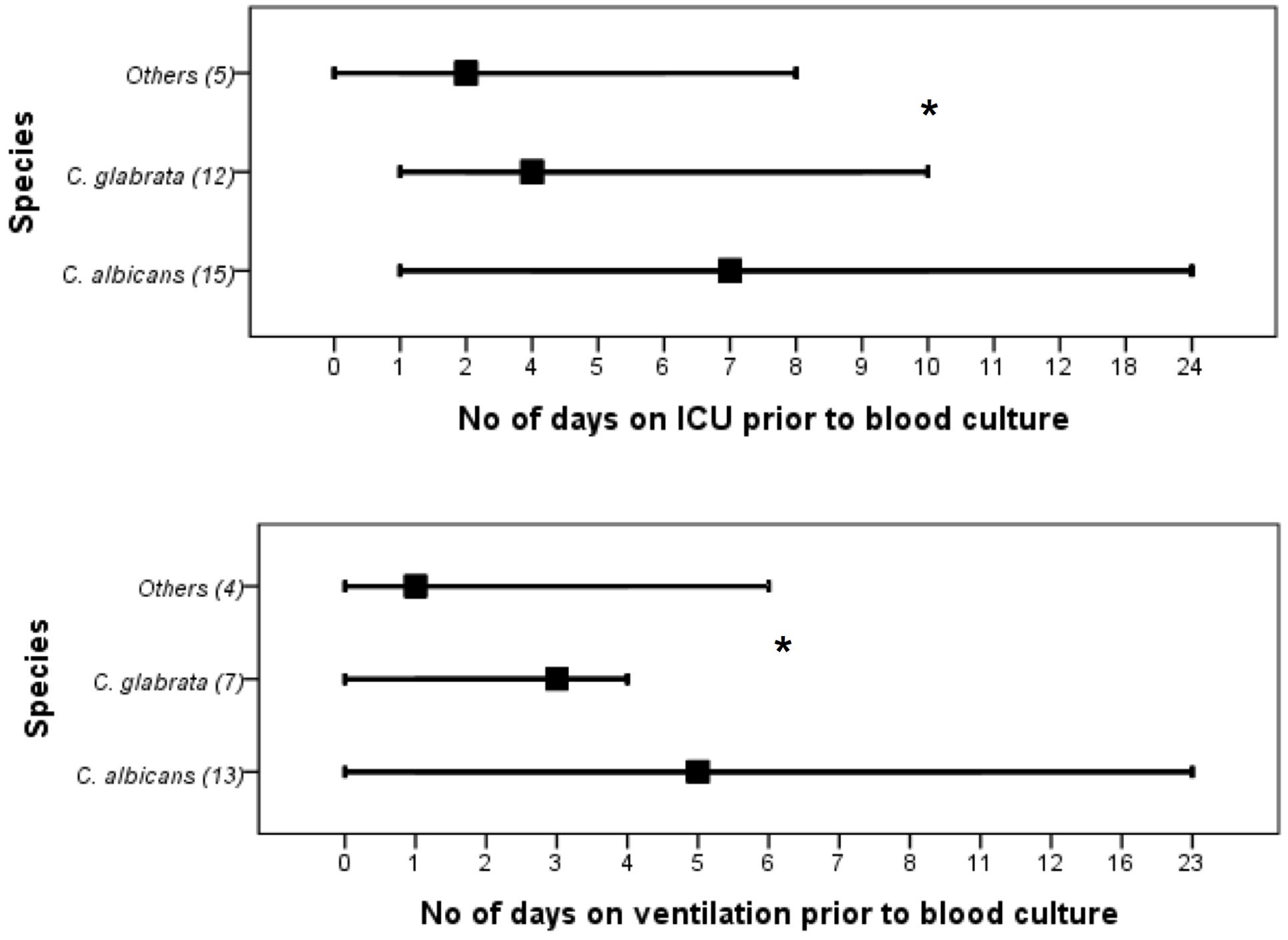
FIGURE 3. Days in the intensive care unit (ICU) or days in the ventilation before the extended prevalence of C. albicans, C. glabrata or other Candida spp. infection. Values in bracket indicate number of patients included in each group. ∗C. albicans vs NCAS (p < 0.05).
In this study, out of 134 patient’s record, 129 were found to be inserted with either central or peripheral CVC. There was no significant difference in the rate of lines indwelling between C. albicans and NCAS groups. Within C. albicans and NCAS groups 18 and 29 patients, respectively, were line removed after diagnosis. Survival analysis shows a significantly higher survival of patients infected with C. albicans who have line removal compared to the non- removal (p < 0.05) (Figure 4A). Conversely, no significant difference was found with line removal in NCAS group (p > 0.05) (Figure 4B). The reason for this may be the high biofilm forming ability of C. albicans in catheters compared to NCAS such as C. glabrata. The biofilm phenotype as a significant clinical entity and this phenotype are highly recalcitrant to antifungal therapy (Rajendran et al., 2015). This data support the current guidelines for the management of catheter associated infection and their clinical management which indicate that where possible the catheter should be removed in non-neutropenic patients (Mermel et al., 2009; Cornely et al., 2012; Koehler et al., 2014). The caveat of this study includes the complicated nature of the patient population, frequency of concomitant bacterial infection and prior exposure to antimicrobials.
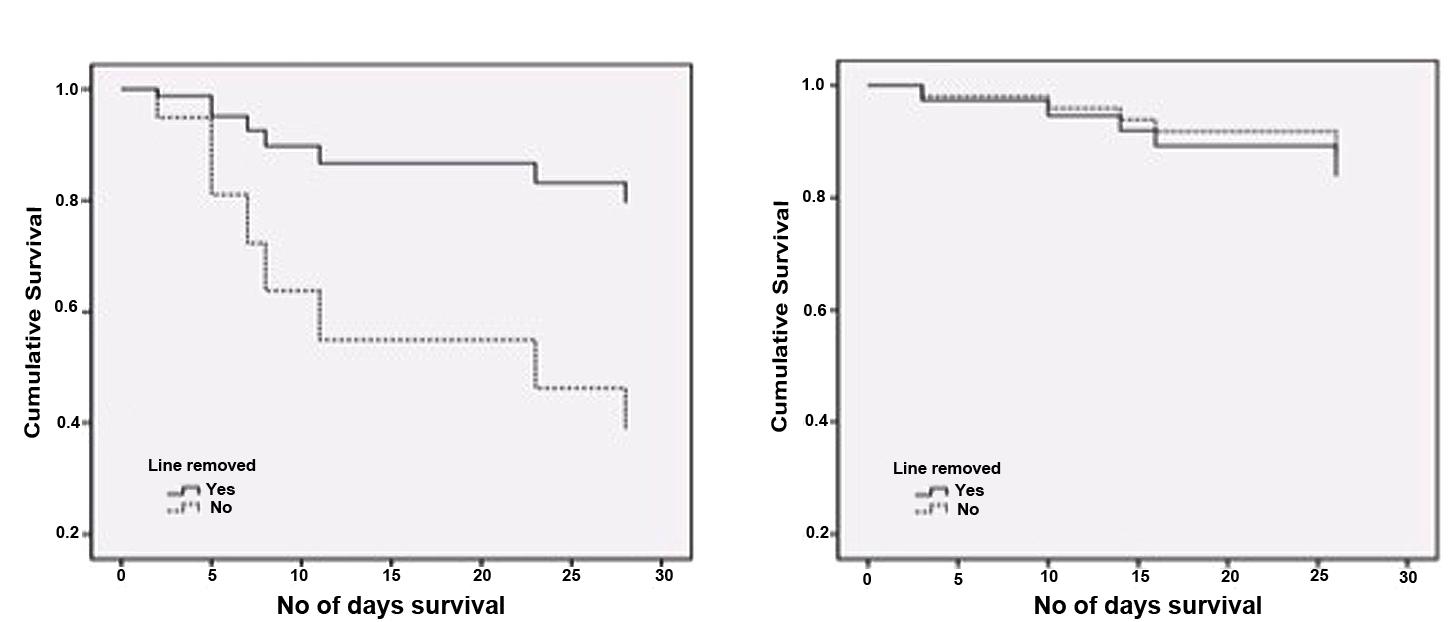
FIGURE 4. Survival analysis for candidaemia patients with and without line removal. Cox-regression plots adjusted for patient age is shown (A) Patients with C. albicans infection (n = 24); p < 0.05, (B) patients with non-C. albicans infections (NCAS [n = 37]). p > 0.05 for C. albicans vs NCAS.
Antifungal Sensitivity
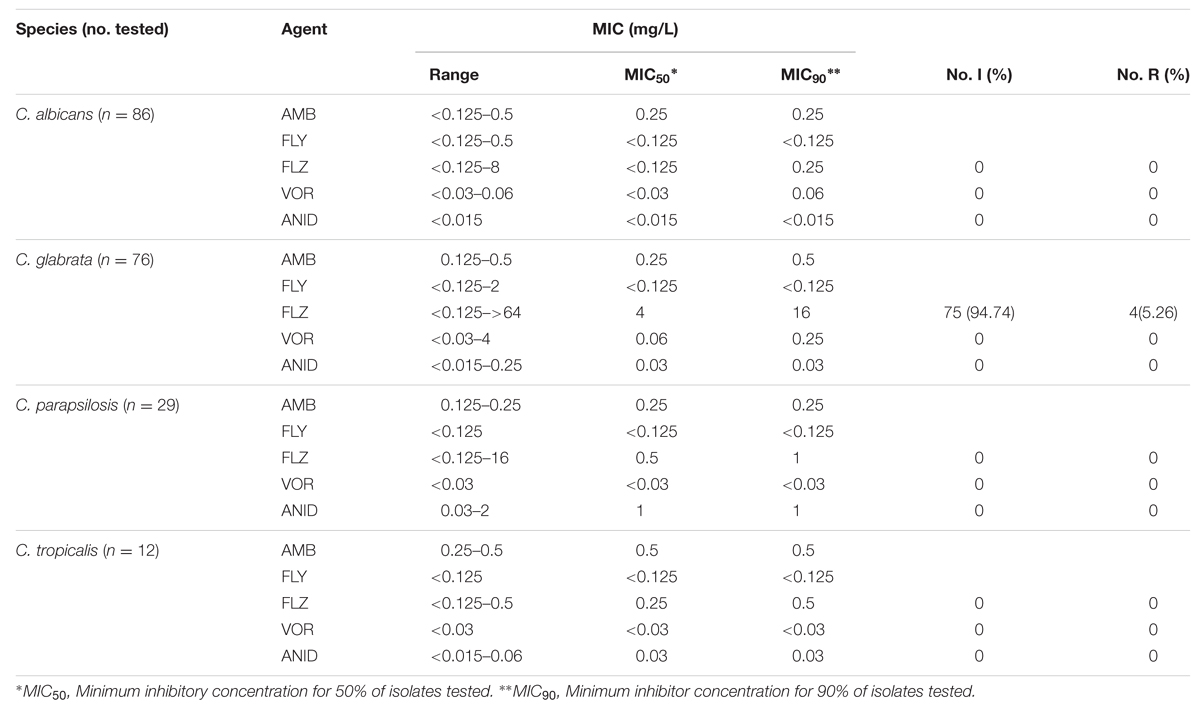
A total of 204 clinical isolates were assessed for planktonic MIC testing of five different antifungal drugs. Concentration range, MIC50 and MIC90 for each antifungal tested against different Candida spp. are presented in Table 2. We applied interpretive breakpoints defined by CLSI for FLZ, VRZ, and ANID. No resistance was observed for C. albicans, C. tropicalis, or C. parapsilosis for any antifungal tested. However, the results showed a considerable prevalence of low azole susceptibility among C. glabrata isolates, for which was either intermediate (94.74%) or resistant (5.26%) to FLZ. Currently, no interpretable breakpoints for amphotericin B (AMB) and flucytosine (FLY) exist, therefore MIC values and the range of the measured MICs are given and no statement concerning the clinical resistance could be made (Table 3). Furthermore, comparing to EUCAST breakpoints (As of 2013, Arendrup et al., 2013a) we found that 3 (3.95%) of C. glabrata isolates were resistant to anidulafungin (Table 3). Our susceptibility data are similar to those of other studies published in recent years, with limited or no resistance found among C. albicans, C. parapsilosis or C. tropicalis isolates (Odds et al., 2007). Conversely all isolates of C. glabrata were considered intermediate for fluconazole except four resistance based on recent CLSI guidelines (CLSI, 2012). The decreased susceptibility of C. glabrata to the azoles is a well-known concern. Logically, the implications would suggest that patients with C. glabrata were at the greatest risk, though our data shows that mortality in C. albicans infection was significantly greater than C. glabrata infection. This provides further evidence that the biofilm phenotype as a significant clinical entity and this phenotype are highly recalcitrant to antifungal therapy (Ramage et al., 2012). Though we not found any resistance with C. albicans isolates in their planktonic form, with our expanded knowledge on biofilm susceptibility to antifungals, the use of these MIC values to treat biofilm based infections is a matter of concern (Kuhn et al., 2002; Nett et al., 2011; Rajendran et al., 2015).
In summary, the data herein shows a changing trend in the prevalence of NCAS causing BSI in Scotland, emphasizing the need for early and appropriate antifungal treatment for patients at high risk for candidaemia. In addition, removal of infected catheter lines, especially with C. albicans, have been shown to be associated with positive clinical outcome. This suggests the necessity for the development of rapid diagnosis methods for catheter related biofilm infections, which in turn as an aid to improving antifungal therapy.
Author Contributions
RR, LS, AD, EJ, and MH participated in isolate and clinical data collection, study design, and carried out the experimental studies, performed statistical analysis, and were responsible for the manuscript. CW and CM participated in study design, statistical analysis, and helped draft the manuscript. GR and BJ conceived the study, participated in study design, data analysis and were responsible for writing and submission of the final manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the Wellcome Trust Strategic Award for Medical Mycology and Fungal Immunology 097377/Z/11/Z. Data collection was supported by a grant from Pfizer. GR was also supported by a research fellowship grant from Gilead Sciences. The collection of the isolates was funded by a Gilead Fellowship to GR.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to microbiology colleagues throughout Scotland for submitting isolates. Antimicrobial sensitivity testing was performed by the Mycology Reference Laboratory, Public Health England, Bristol.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00915
Footnotes
References
Almirante, B., Rodriguez, D., Park, B. J., Cuenca-Estrella, M., Planes, A. M., Almela, M., et al. (2005). Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43, 1829–1835. doi: 10.1128/JCM.43.4.1829-1835.2005
Arendrup, M. C. (2010). Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 16, 445–452. doi: 10.1097/MCC.0b013e32833e84d2
Arendrup, M. C., Cuenca-Estrella, M., Lass-Florl, C., and Hope, W. W. (2013a). Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist. Updat. 16, 81–95. doi: 10.1016/j.drup.2014.01.001
Arendrup, M. C., Dzajic, E., Jensen, R. H., Johansen, H. K., Kjaeldgaard, P., Knudsen, J. D., et al. (2013b). Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. 19:E343–E353. doi: 10.1111/1469-0691.12212
Castanheira, M., Messer, S. A., Rhomberg, P. R., and Pfaller, M. A. (2016). Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY antifungal surveillance program (2013). Diagn. Microbiol. Infect. Dis. 85, 200–204. doi: 10.1016/j.diagmicrobio.2016.02.009
Chi, H. W., Yang, Y. S., Shang, S. T., Chen, K. H., Yeh, K. M., Chang, F. Y., et al. (2011). Candida albicans versus non-albicans bloodstream infections: the comparison of risk factors and outcome. J. Microbiol. Immunol. Infect. 44, 369–375. doi: 10.1016/j.jmii.2010.08.010
Cleveland, A. A., Harrison, L. H., Farley, M. M., Hollick, R., Stein, B., Chiller, T. M., et al. (2015). Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS ONE 10:e0120452. doi: 10.1371/journal.pone.0120452
CLSI (2008). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, Third Edition. CLSI document m27-a3. Wayne, PA: Clinical and laboratory Standards Institute.
CLSI (2012). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; 4th Informational Supplement. CLSI document m27-s4. Wayne, PA: Clinical and Laboratory Standards Institute.
Cornely, O. A., Bassetti, M., Calandra, T., Garbino, J., Kullberg, B. J., Lortholary, O., et al. (2012). ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin. Microbiol. Infect. 18(Suppl. 7), 19–37. doi: 10.1111/1469-0691.12039
Dimopoulos, G., Ntziora, F., Rachiotis, G., Armaganidis, A., and Falagas, M. E. (2008). Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth. Analg. 106, 523–529. doi: 10.1213/ane.0b013e3181607262
Eggimann, P., Bille, J., and Marchetti, O. (2011). Diagnosis of invasive candidiasis in the ICU. Ann. Intensive Care 1, 37. doi: 10.1186/2110-5820-1-37
Gamaletsou, M. N., Walsh, T. J., Zaoutis, T., Pagoni, M., Kotsopoulou, M., Voulgarelis, M., et al. (2013). A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin. Microbiol. Infect. 20, O50–O57. doi: 10.1111/1469-0691.12312
Garey, K. W., Rege, M., Pai, M. P., Mingo, D. E., Suda, K. J., Turpin, R. S., et al. (2006). Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43, 25–31. doi: 10.1086/504810
Goncalves, S. S., Souza, A. C., Chowdhary, A., Meis, J. F., and Colombo, A. L. (2016). Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses doi: 10.1111/myc.12469 [Epub ahead of print].
Gudlaugsson, O., Gillespie, S., Lee, K., Vande Berg, J., Hu, J., Messer, S., et al. (2003). Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37, 1172–1177. doi: 10.1086/378745
Hachem, R., Hanna, H., Kontoyiannis, D., Jiang, Y., and Raad, I. (2008). The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112, 2493–2499. doi: 10.1002/cncr.23466
Koehler, P., Tacke, D., and Cornely, O. A. (2014). Our 2014 approach to candidaemia. Mycoses 57, 581–583. doi: 10.1111/myc.12207
Kollef, M., Micek, S., Hampton, N., Doherty, J. A., and Kumar, A. (2012). Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin. Infect. Dis. 54, 1739–1746. doi: 10.1093/cid/cis305
Kuhn, D. M., George, T., Chandra, J., Mukherjee, P. K., and Ghannoum, M. A. (2002). Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46, 1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002
Lee, I., Fishman, N. O., Zaoutis, T. E., Morales, K. H., Weiner, M. G., Synnestvedt, M., et al. (2009). Risk factors for fluconazole-resistant Candida glabrata bloodstream infections. Arch. Intern. Med. 169, 379–383. doi: 10.1001/archinte.169.4.379
Leroy, O., Gangneux, J. P., Montravers, P., Mira, J. P., Gouin, F., Sollet, J. P., et al. (2009). Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit. Care Med. 37, 1612–1618. doi: 10.1097/CCM.0b013e31819efac0
Mermel, L. A., Allon, M., Bouza, E., Craven, D. E., Flynn, P., O’Grady, N. P., et al. (2009). Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49, 1–45. doi: 10.1086/599376
Moran, C., Grussemeyer, C. A., Spalding, J. R., Benjamin, D. K. Jr., and Reed, S. D. (2009). Candida albicans and non-albicans bloodstream infections in adult and pediatric patients: comparison of mortality and costs. Pediatr. Infect. Dis. J. 28, 433–435. doi: 10.1097/INF.0b013e3181920ffd
Morrell, M., Fraser, V. J., and Kollef, M. H. (2005). Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49, 3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005
Nett, J. E., Cain, M. T., Crawford, K., and Andes, D. R. (2011). Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J. Clin. Microbiol. 49, 1426–1433. doi: 10.1128/JCM.02273-10
Nguyen, M. H., Peacock, J. E. Jr., Morris, A. J., Tanner, D. C., Nguyen, M. L., Snydman, D. R., et al. (1996). The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100, 617–623. doi: 10.1016/S0002-9343(95)00010-0
Odds, F. C., Hanson, M. F., Davidson, A. D., Jacobsen, M. D., Wright, P., Whyte, J. A., et al. (2007). One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 56, 1066–1075. doi: 10.1099/jmm.0.47239-0
Osmanov, A., and Denning, D. W. (2015). Burden of serious fungal infections in Ukraine. Mycoses. 58(Suppl. 5), 94–100. doi: 10.1111/myc.12409
Pfaller, M. A., and Diekema, D. J. (2007). Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163. doi: 10.1128/CMR.00029-06
Quindos, G. (2014). Epidemiology of candidaemia and invasive candidiasis. a changing face. Rev. Iberoam. Micol. 31, 42–48. doi: 10.1016/j.riam.2013.10.001
Rajendran, R, Sherry, L., Nile, C. J., Sherriff, A., Johnson, E., Hanson, M., et al. (2015). Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection – Scotland, 2012-2013. Clin. Microbiol. Infect. 22, 87–93. doi: 10.1016/j.cmi.2015.09.018
Ramage, G., Rajendran, R., Sherry, L., and Williams, C. (2012). Fungal biofilm resistance. Int. J. Microbiol. 2012, 528521. doi: 10.1155/2012/528521
Rentz, A. M., Halpern, M. T., and Bowden, R. (1998). The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin. Infect. Dis. 27, 781–788. doi: 10.1086/514955
Tumbarello, M., Fiori, B., Trecarichi, E. M., Posteraro, P., Losito, A. R., De Luca, A., et al. (2012). Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE 7:e33705. doi: 10.1371/journal.pone.0033705.
Tumbarello, M., Posteraro, B., Trecarichi, E. M., Fiori, B., Rossi, M., Porta, R., et al. (2007). Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45, 1843–1850. doi: 10.1128/JCM.00131-07
Zilberberg, M. D., Kollef, M. H., Arnold, H., Labelle, A., Micek, S. T., Kothari, S., et al. (2010). Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect. Dis. 10:150. doi: 10.1186/1471-2334-10-150
Keywords: Candida albicans, Candida glabrata, candidaemia, antifungals, drug resistance
Citation: Rajendran R, Sherry L, Deshpande A, Johnson EM, Hanson MF, Williams C, Munro CA, Jones BL and Ramage G (2016) A Prospective Surveillance Study of Candidaemia: Epidemiology, Risk Factors, Antifungal Treatment and Outcome in Hospitalized Patients. Front. Microbiol. 7:915. doi: 10.3389/fmicb.2016.00915
Received: 30 March 2016; Accepted: 27 May 2016;
Published: 16 June 2016.
Edited by:
Dominique Sanglard, University of Lausanne and University Hospital Center, SwitzerlandReviewed by:
Oliver Kurzai, Friedrich Schiller University Jena, GermanyMuriel Cornet, CHU Grenoble Alpes & Université Grenoble Alpes, France
Copyright © 2016 Rajendran, Sherry, Deshpande, Johnson, Hanson, Williams, Munro, Jones and Ramage. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gordon Ramage, Z29yZG9uLnJhbWFnZUBnbGFzZ293LmFjLnVr
 Ranjith Rajendran
Ranjith Rajendran Leighann Sherry
Leighann Sherry Ashutosh Deshpande
Ashutosh Deshpande Elizabeth M. Johnson
Elizabeth M. Johnson Mary F. Hanson
Mary F. Hanson Craig Williams
Craig Williams Carol A. Munro
Carol A. Munro Brian L. Jones2
Brian L. Jones2 Gordon Ramage
Gordon Ramage