
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 27 May 2016
Sec. Food Microbiology
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.00798
This article is part of the Research Topic Emerging Technologies for Food Preservation and Safety View all 35 articles
Allura Red AC (E129) is an azo dye that widely used in drinks, juices, bakery, meat, and sweets products. High consumption of Allura Red has claimed an adverse effects of human health including allergies, food intolerance, cancer, multiple sclerosis, attention deficit hyperactivity disorder, brain damage, nausea, cardiac disease and asthma due to the reaction of aromatic azo compounds (R = R′ = aromatic). Several countries have banned and strictly controlled the uses of Allura Red in food and beverage products. This review paper is critically summarized on the available analytical and advanced methods for determination of Allura Red and also concisely discussed on the acceptable daily intake, toxicology and extraction methods.
The additives used in food processing may be divided in two groups: (i) naturally occurring compounds or additives isolated from natural sources and (ii) synthetic chemicals that are widely applied in foods industry from many years ago. Natural color additives contain lower tinctorial strength as compared to synthetic colors because of more sensitive to light, temperature, oxygen, pH, color uniformity, low microbiological contamination, and relatively low production costs. Coloring used in food industry to improve the food appearance, flavor, taste, color, texture, nutritive value and conservation. Hence, synthetic food dyes stand out as one of the essential additive class for food industry in the conquest of markets (Ashfaq and Masud, 2002; Salem et al., 2009).
Synthetic dyes are classified into azo dyes, triphenylmethane dyes, xanthene dyes, indigotine dyes, and quinoline dyes. Azo dyes contain azo group (-N = N-) as the chromophore in the molecular structure, which is largest group of color accounting more than half of global dyes production. One of the mostly used synthetic dyes in food industry is Allura Red (Figure 1), which could be found in many commercial foodstuffs, for example soft drinks, candies, ice cream and bakery products. Allura Red is an electrochemically active with irreversible reaction (Zhang et al., 2010). Previously, several researches have been reported regarding Allura Red toxicity and carcinogenic effects (Dinç et al., 2002; Kiseleva et al., 2003; Amate et al., 2010). Allura Red has potential behavioral effects on humans and animals; especially increase hyperactivity in children. Moreover, some studies have showed the presence of aromatic amine or amide functionalities in the chemical structures of the degradation products of Allura Red. Allura Red has absorbed to gastrointestinal and entered the bloodstream to associates with proteins during its transport and metabolism process (Combes and Haveland, 1982; Lok et al., 2006; McCann et al., 2007; Rebane et al., 2010; Gosetti et al., 2013). The excess usages of Allura Red in food and beverage products must be controlled.
In many countries, the uses of several food dyes including Allura Red has controlled or banned due to it toxicity. The lists of permitted synthetic dyes have different from each country, for examples, azorubine, quinoline yellow, and patent blue V are permitted in EU countries, but considered forbidden in Japan and USA. For the safety assessment, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and EU Scientific Committee for Food (SCF) established an acceptable daily intake (ADI) of Allura Red is 0–7 mg/kg/bw/day (European Food Safety Authority [EFSA], 2009). Due to the concern of human health, several analytical and advanced methods are developed for analyzing and quantifying of Allura Red. Thus, this review paper is emphasized the available of analytical and advanced methods for detection of Allura Red in food products, and also discussed on the ADI, toxicology and extraction methods.
Natural and synthetic dyes are classified into soluble colorants. Natural colors are obtained from various food or natural materials, for example riboflavin (E 101), chlorophylls (E 140), carotenes (E 160a), betalain (E 162) or anthocyans (E 163). Natural colors are not precise stable, so it could be characterized by their specific physiological activity. Synthetic colors are originally manufactured from coal tar or purified oil products (Amchova et al., 2015). Synthetic food colors have high stability to light, oxygen, pH changes and relatively low cost as compared to natural color (Gómez, 2012). Synthetic food dyes are chemically synthesized which found wide compounds structures on their structural characteristics (Carmen, 2008). Azo dyes have found more than 3000 compounds in worldwide uses and accounted about 65% of the commercial dye in the market (Ahlström et al., 2005).
Codex Committee on Food Additives and Contaminants (CCFAC) formed an International Numbering System (INS) for food colorant to be identifying on the list of ingredients by a three-digit number. These given numbers have replaced by the specific name of the colorants that are so long due to complex chemical structure. Based on EU, a system of E numbers has implemented in order to identify all food additives. E number is composed of the letter E represented for Europe, followed by the INS three-digit number, for example Allura Red is E 129 (Amchova et al., 2015). Allura Red has been approved by European Union (EU) Register and listed in Annex I of Directive 94/36/EC. Allura Red most commonly used synonyms of Food Red No. 40 and Food, Drug and Cosmetics Red No. 40 (FD&C Red No. 40). Allura Red consisted of disodium 2-hydroxy-1-(2-methoxy-5-methyl-4-sulphonato-phenylazo)naphthalene-6-sulphonate and subsidiary coloring agents, with sodium chloride and sodium sulfate as the principal uncolored components (European Food Safety Authority [EFSA], 2012). Allura Red manufactured by coupling diazotized 5-amino-4-methoxy-2-toluenesulphonic acid with 6-hydroxy-2-naphthalene sulphonic acid. The molecular formula of Allura Red is C18H14N2Na2O8S2 (MW: 496.42 g/mol) and structural formula is shown in Figure 1. It is dark red in color and water-soluble powder or granules, but slightly soluble in 50% ethanol. The maximum absorption in water is 504 nm, at pH 7 (E1 cm1% = 540). In order to replace Amaranth (E123), Allura Red AC was first time introduced in the US since 1980s and it had synthesized by the classical process of diazotization (Carmen, 2008). It has permitted to be used as a food additive in food products. However, it is not acceptable for use in animal feed because of the genotoxic effects (Ceyhan et al., 2013). USA Food and Drug Administration (FDA) have approved the uses of Allura Red in cosmetics, drugs, and food. Besides, Allura Red can be used in some tattoo inks. In US, Allura Red is commonly replacement used to Amaranth (Red 2) and Erythrosine (Red 3).
The ADI is estimated of daily total intake of food colorants without any adverse effect on health. ADI is expressed as mg per kg of body weight (Duffus et al., 2009). To prevent excessive uses of Allura Red, some countries have legislated laws and regulations to limit the amounts permitted of Allura Red in food and drinks. Allura Red has been evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1980 and the EU SCF in 1984 and 1989. JECFA and SCF have established an ADI of Allura Red of 0–7 mg/kg/bw/day in food and beverages products (European Food Safety Authority [EFSA], 2009). Several countries such as EU, China, Japan, Australia, Brazil, and New Zealand have permitted the uses of Allura Red in food, drugs and cosmetics products less than ADI values. However, it has banned in USA, Denmark, Belgium, France, Switzerland, and India (European Food Safety Authority [EFSA], 2009). Accordingly, the uses of synthetic dyes in foodstuffs are strictly controlled by legislation throughout the world (EC, 1994 and GB2760-2011, 2011). Food industries have required to be listed on the package label to avoid the excess consumption of synthetic dyes. Food Safety Law of the People’s Republic of China has required the application of synthetic color additives to maintain in surveillance by the China Food and Drug Administration (CFDA) and listed in Direct GB 2760-2011 of the Ministry of Health because of legally used in food markets. According to the Direct GB 2760-2011, eleven synthetic colors are listed including Allura Red as certifiable food color additives that can be added in food products. The maximum amount has allowed the most synthetic food colors but not more than 100 mg kg-1 of colorants (GB2760-2007, 2007). The maximum permitted levels of the uses of Allura Red AC in beverages and foodstuffs are summarized in Table 1 (European Food Safety Authority [EFSA], 2009).
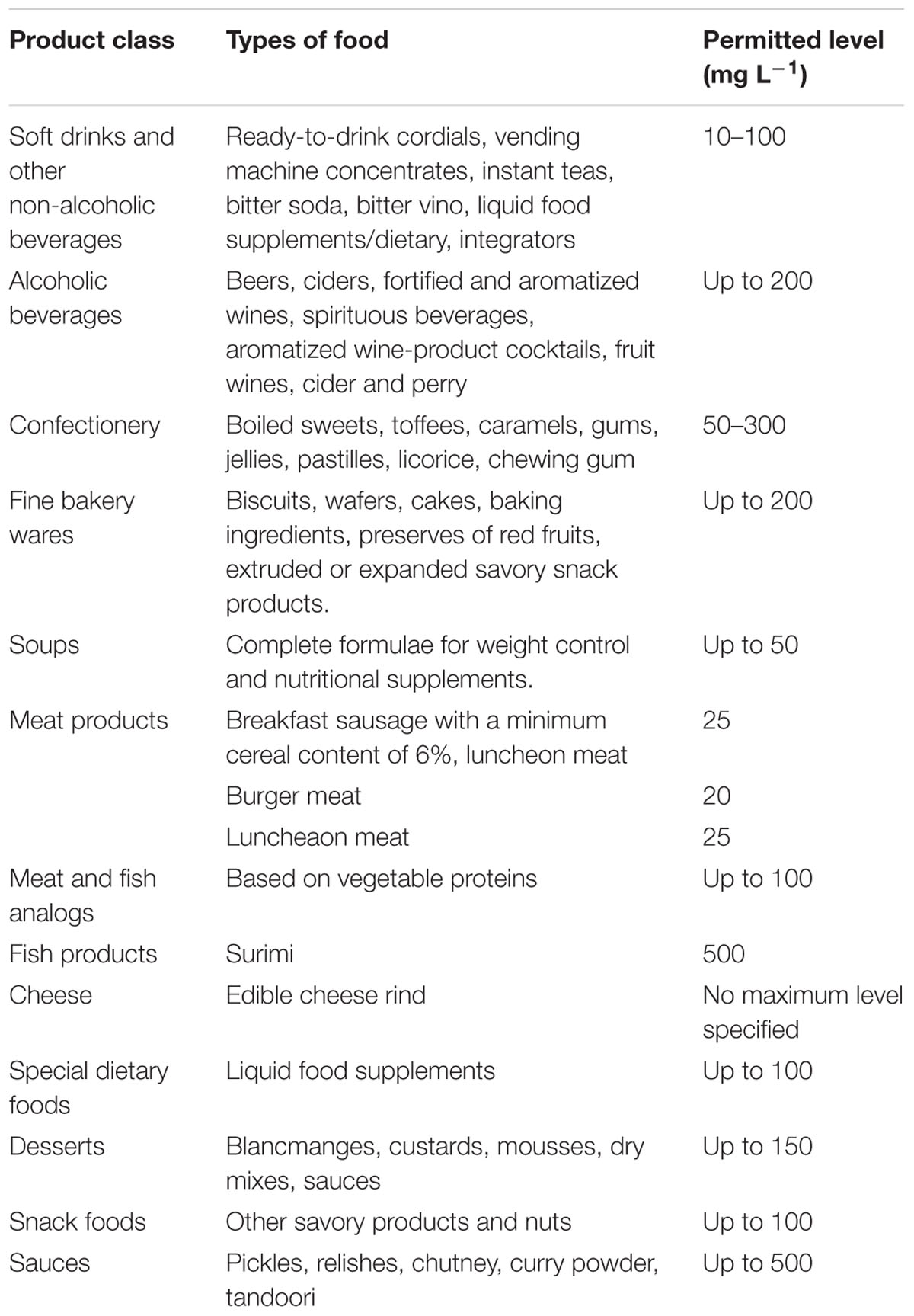
TABLE 1. The Maximum Permitted Levels of Allura Red AC in beverages and foodstuffs (European Food Safety Authority [EFSA], 2009).
Food adulterants are chemical or biological substances that are added into foods with intentionally reducing the manufacturing cost and artificially inflate food quality that caused illness to consumers (Eunice et al., 2010). Some synthetic food colorants can be toxic to aquatic organisms and carcinogenic effects on humans, particularly when they are extremely consumed (Ceyhan et al., 2013). Toxicological study of Allura Red was assessed by JECFA in 1980 and SCF in 1984 and 1989. Allura Red is able to reduce by azoreductase enzymes in intestinal bacteria and in liver cells with the release of aromatic amines to the organism that caused frequent headaches in adults while children often become distracted and hyperactive (Hawley and Buckley, 1976; Rafii et al., 1997; Huang et al., 2003). Feingold (1975) has initially claimed the detrimental effect of Allura Red to childhood behavior more than 30 years ago. Hyperactive is a pattern of behavior that showed substantial individual differences in the general population. Children probably found the above behavior pattern a large degree diagnosed with ADHD (Feingold, 1975; Overmeyer and Taylor, 1999; McCann et al., 2007). Recently, few studies have been published on the issue of Allura Red binding to human serum albumin (HSA) (Wang et al., 2014; Masone and Chanforan, 2015; Wu et al., 2015).
Scientific Opinion of the EFSA Panel has concluded that Allura Red may cause allergic reactions such as urticaria and asthma, especially when mixed with other synthetic color in food products. However, EFSA panel reported the ADI value was not changed due to the fact that current levels of use in the European population is far from reaching the official limit of 0.4–0.6 mg daily in the 95th percentile of the population (European Food Safety Authority [EFSA], 2009; Fallico et al., 2011). Several researchers have reported regarding its toxicity and carcinogenic effects on human health (Borzelleca et al., 1989; Jung et al., 1992). Sasaki et al. (2002) have studied the toxicity of several food additives, including Allura Red AC in mice. They found that there is no death observed at 2000 mg kg-1 Allura Red on four to five mice. So, it can be concluded that Allura Red has lower acute oral toxicity.
Food colors first extracted from the food matrix and purified for the removal of the potential interfering coextractives for the analysis and quantitation. Some samples pretreatment are often required including defatting of meat products, dilution of sugars and gums in confectionery products, and then can be proceed for extraction procedure. Most extraction procedures are followed a common path involving in the release of desired analytes from their matrices, followed by removal of extraneous matter and a suitable extraction method (Thompson and Trenerry, 1995; Wu et al., 2013).
The supercritical fluid extraction (SFE) technology has advanced tremendously since its inception and is a good method in many food processing industries. Past two decades, SFE has been well received as a clean and environmentally friendly “green” processing technique and in some cases, an alternative to organic solvent-based extraction. The most recent advances of SFE applications in food science (Allura Red), natural products, by-product recovery, pharmaceutical and environmental sciences have been published in extensive reviews (Herrero et al., 2010). Supercritical fluid solvents are of interest in chemical processes both for their involvement in chemical reactions as well as their solvent effects that are influenced by pressure and temperature.
Solvent extraction known as liquid-liquid extraction (LLE) which has involved the separation of compounds based on their relative solubility with two different immiscible liquids (organic phase and water). The extraction of Allura Red is most common solvents used as like as water, ethanol, methanol, isopropyl alcohol, ammoniacal ethanol, ethyl acetate, ammonia, cyclohexane and tetra-n-butyl ammonium phosphate. Yoshioka and Ichihashi (2008) have used different solvents for the simultaneous extraction among forty food dyes in drinks and candies. They mentioned that the mixture of ammonia and ethanol (1:1, v/v) solutions have showed good extraction efficiency after ultra-sonication and evaporation of the sample. Similarly, Zou et al. (2013) have addressed the tri-mixtures of ethanol, ammonia and water (80:1:19, v/v/v), and found better extraction recoveries for seven dyes in animal feed and meat samples. Harp et al. (2013) have analyzed seven certified food colors in forty-four food products by liquid chromatography method using the ammonium hydroxide and methanol as extraction solvents. Khanavi et al. (2012) have established a green extraction procedure using non-organic solvents, which are ammonia (0.25%, v/v) and water for Allura Red extraction from food products and medicines.
Solid-phase extraction (SPE) known as absorption technique to separate food colorants by utilizing a variety of adsorption materials such as wool, powdered leather, cellulose, alumina, and polyamide powder. SPE commonly used because of simple procedure, rapid and able to treat large volume of samples free from contaminants with high recoveries. Recently, semi-micro adsorption cartridges containing reverse-phase bonded silica materials have widespread used. Typical sorbent for SPE include C18, while amino-functionalized low degrees of cross-linking magnetic polymer (NH2-LDC-MP), polyamide, gel permeation chromatography (GPC) and styrene-divinylbenzene polymer has good retention toward Allura Red (Soylak et al., 2011; Bonan et al., 2013; Chen et al., 2014; Tang et al., 2014). Different organic solvents have used in the analysis of Allura Red resulting in difficulty for selection of an appropriate solvent. The structure of analytical matrix and its components have played important role while selecting an appropriate solvent for extraction. Usually several solvents such as methanol, acetic acid, ethanol, acetone, ethyl acetate, tetra-n-butyl ammonium phosphate and others are more appropriately extracted of Allura Red. Tang et al. (2014) have used SPE for extraction among sixteen synthetic colorants in complex hotpot condiment with high oil content. The combination of methanol, acetone (1:1, v/v) and 2 mol L-1 carbamide solution containing 5% of ammonia in methanol showed good extraction efficiency while purified by a GPC column. Besides, Chen et al. (2014) have investigated the use of NH2-LDC-MP as a sorbent in SPE under magnetic field to enhance the extraction recoveries among seven synthetic food dyes by using water as an extraction solvent.
Enzymatic digestion of food samples are highly bound or associated with the food matrix. The combinations of enzyme-substrates are widely used including papain (protein digestion), lipase (lipids), phospholipase (phospholipid), amyloglucosidase (starch), pectinase (pectin), and cellulase (cellulose) (Rodriguez-Saona et al., 2001; Soltan and Shehata, 2012). It is one of most common method for extraction of Allura Red that included one-step extraction with membrane filter using water as diluents (Minioti et al., 2007; Gosetti et al., 2012). Other extraction methods such as dialysis, microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE) are eco-friendly methods that frequently applied in food samples. Shen et al. (2014) have established new extraction method using two-phase solvent (methanol and acetone) and UAE that improved the extraction recovery of both hydrophilic and hydrophobic pigments for Allura Red extraction. Sun et al. (2013) have developed MAE extraction method for isolation of 21 synthetic colorants including Allura Red in meat by using methanol-acetic acid (95:5, v/v) as a solvent. In contrast, there are a few methods available using without extraction procedure before analyzing the level of Allura Red (Sorouraddin et al., 2011). The extraction methods are summarized in Table 2.
Food coloring is one of the food adulterants which chemicals substances that intentionally added to food in order to improve customer’s perceptions of food. The presences of Allura Red in potentially interfering compounds are difficulty to identify by using analytical methods. For Allura Red, several analytical methods have developed such as voltammetry, polarography, spectrophotometry, mass spectrometry, capillary electrophoresis (CE), ion chromatography, thin layer chromatography, high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and liquid chromatography-tandem mass spectrometry (LC-MS/MS).
High-performance liquid chromatography becomes the major analytical method for determination of synthetic coloring materials in foodstuffs. The most widely used separation modes are ion exchange and reverse-phase. Other method used for separation, qualitative and quantitative determination of synthetic food dyes based on high performance liquid chromatography. The basis of separation has two phases; stationary phase and mobile phase. Dyes have different adsorption affinity to stationary phase. It has appeared from differences of their mass, structural space and presence of functional groups in each dye’s molecule. A wide range of liquid chromatography based techniques have analyzed for the detection of azo dyes, most of them are coupled with UV-Vis, PDA or MS detectors. The HPLC technique has reversed phase high performance liquid chromatography (RP-HPLC) and ion-pair high performance liquid chromatography (HPLC-IP).
In RP-HPLC system, the mobile phase has stronger polarity such as tetrahydrofuran, acetonitrile, methanol and water, while stationary phase is slightly polar or non-polar (Witkiewicz, 2000). Appropriate conditions are allowed for analyzing the most of food dyes. Ionized samples must have possibility to form neutral molecules. The most important characteristics into consideration during selection of hydrophobic properties are tested and presence the molecules with acidic groups. Hydrophobicity of azo dyes is the largest group as compare to other. Ion pair reverse-phase chromatography (IP-RP-HPLC) consisted in adding hydrophobic ionic substance to the mobile phase. It could be quaternary ammonium cation, alkilo- or arylsulfoniumanion. As a result of the reaction between sample and eluent neutral ionic pairs are formed and separated chromatographically in the reversed phase system. Another way is preparing of sample, which enables the conducting of analysis on ionic exchanger or modification of mobile phase that provides to obtain the ion-exchanger (Witkiewicz, 2000).
In contrast, HPLC combined with diode array detection (HPLC-DAD) is very popular for qualitative and quantitative determination with excellent precision, accuracy and lower cost, which can be more practical and economical in detecting non-illicit additives such as food colorants. Qi et al. (2015) developed an efficient, fast and sensitive method for determination of 11 synthetic dyes including Allura red, in flour and meat foodstuffs using HPLC coupled with DAD and MS/MS. The color additives are extracted with ammonia-methanol for further purified with SPE procedure using Strata-AW column in order to reduce matrix interference. The proposed method is intended for a comprehensive survey of color additives in foods. HPLC-MS/MS method is used for further confirmation of the results. Validation data showed good recoveries in the range of 75.2–113.8%, with relative standard deviations less than 15%. The proposed method has proved more suitable for the routine monitoring of eleven synthetic color additives due to its sensitivity, fast and low cost. Li et al. (2015) developed HPLC-DAD combined with ESI-IT-TOF/MS in positive and negative ion modes for identification and quantification among 34 water-soluble synthetic dyes in foodstuff. Under optimal condition, the averages LOD of dyes were found between 0.01 and 0.05 μg mL-1. The recoveries and RSD range between 76.1–105.0% and 1.4–6.4%, respectively. Karanikolopoulos et al. (2015) developed the protocol based on RP-HPLC/DAD for the analysis of Allura Red in complex food matrices presenting high protein and fat content. The issue of high fat content matrices addressed; it was needed an additional defatting step in the procedure. The proposed method showed high precision and accuracy of detection in other complex food matrices. Other method developed by Kong et al. (2015) based on freeze method for deproteinization coupling with the chitosan purification process in protein-rich samples. Chitosan used for the purification after deproteinization as compared with the traditional technique. Under optimum conditions, the method showed good linearity between 0.6 and 10 mg kg-1, with LOD between 0.1 and 0.4 mg kg-1.
Bazregar et al. (2015) established a method based on the electro-kinetic migration of ionized compounds by the application of an electrical potential difference. Efficient extraction technique is used with a sub-microliter organic solvent consumption termed as in-tube electro-membrane extraction (IEME). The result showed high extraction yield recoveries and the consumption of the organic solvents are less. IEME-HPLC-UV showed a good linearity in the range of 1.00–800 ng mL-1, with LOD of 0.3-1.0 ng mL-1. Tsai et al. (2015) have simultaneously determined among 20 synthetic dyes including Allura Red by using LC-MS/MS method. The linearity and recoveries are observed at the concentration range of 0.10–200 μg kg-1 and more than 90% for all dyes. Chen et al. (2014) developed a sensitive method based on the use of magnetic dispersive solid-phase extraction (M-dSPE) procedure combine with ultra-fast liquid chromatography-tandem quadrupole mass spectrometry (UFLC-MS/MS). The obtained results showed higher extraction capacity of NH2-LDC-MP with recoveries between 84.0 and 116.2%, with limit of quantification (LOQs) for the seven synthetic pigments are of 1.51 for wines and 5.0 μg L-1 for soft drinks. The developed M-dSPE UFLC-MS/MS confirmed that the NH2-LDC-MP is a kind of high effective M-dSPE materials for the pigments analyses.
Jurcovan and Diacu (2014) developed a simple method for the simultaneous measurement of Allura Red and Ponceau 4R in soft drinks by employing water and acetonitrile as a mobile phase. Bonan et al. (2013) proposed the simultaneous analysis of red and yellow dyes by using HPLC-DAD in solid food matrices and beverages. A water-alcohol mixture, cleaned up on a polyamide SPE cartridge and eluted with basic methanol solution, extracts the food samples. The method is successfully validated according to Regulation (2004/882/CE) and could be applied to a concentration range between 5 and 300 mg kg-1 (5–100 mg l-1 for drinks) depending on the dyes. Tang et al. (2014) have determined among 16 synthetic colorants in hotpot condiment by HPLC. Based on results, a good linear relationship between peak areas and the concentrations of the synthetic colorants are obtained with LOD of 1–3 μg kg-1. The proposed method is more sensitive and reliable that can be used for simultaneously determined among eight lipid-soluble and eight water-soluble colorants in hotpot condiment.
Various spectrometry techniques are available for the analysis of Allura Red including the measurements at ultraviolet and visible wavelengths. Spectrometry is suitable for quantitative analysis of food dyes in different food matrices. Spectrometry frequently applied for determination of Allura Red because of high values of molar absorption. Spectrometry shows low instrumentation cost and does not require any expert skill manpower. The distinguishing features of the spectra obtained for single color is significantly affected by the adjustment of pH of the solution with acid or alkali; characterized by shifts in absorption wavelength maxima and intensities. María et al. (2007) have used time flight mass spectrometry (TOF-MS) instruments that represent a valuable tool for screening of target and non-target compounds in food products. Accurate mass measurements along with specific retention times can be detected highly reliable target species, avoiding isobaric interferences in complex samples. Moreover, a mass spectrometry combine with an ESI (or APCI) source and an ion trap analyzer linked to a TOF mass analyzer (ESI/APCI-IT-TOF/MS) that able to provide multistage tandem spectra with accurate masses. This feature makes IT-TOF/MS useful for identifying target dyes and non-target dyes in foodstuffs. Holčapek et al. (2007) investigated various functional groups of synthetic dyes that could affect their fragmentation behavior in the sources of ESI and APCI. Currently, there are interested in the fragmentation mechanism of synthetic food dyes using ESI-IT-TOF/MSn in positive and negative ion modes.
Spectrophotometric method is simple, direct, rapid and versatile. Turak and Ozgur (2013) simultaneously determined Allura Red and Ponceau 4R in drinks with four derivative spectrophotometric methods as compared to the results with those of HPLC method. Soylak et al. (2011) developed a simple method with appreciable precision and low analytical cost the spectrophotometric determination of Allura Red in water samples by sensitive SPE procedure extraction on a glass column containing MCI GEL CHP20P resin. A new application of bulk liquid membrane (BLM) with second-order calibration based on the bilinear least squares/residual bilinearization (BLLS/RBL) algorithm as a novel method for simultaneous removal and quantification of Allura Red and Sunset Yellow which model compounds in soft drinks and food samples (Khani et al., 2015). The proposed method was validated by comparison with a reference method based on HPLC-UV and found no significant differences between the reference values and the obtained values. El-Sheikh and Al-Degs (2013) simultaneously quantified three common synthetic food color including Allura Red in powdered soft drinks by employing a combination of absorbance spectra-pH data matrices and multivariate processing of the generated second-order data. They used PARAFAC and bilinear least squares/residual bilinearization BLLS/RBL that applied for deconvolution of trilinear data to get spectral and concentration profiles of the dyes as a function of pH. The comparison of chemometric results with those obtained by standard chromatographic technique has proven that the former protocol is a reasonable accuracy with satisfied recoveries study.
Capillary electrophoresis has been widely used for the analysis of Allura Red. It is an electrophoretic method to perform in a capillary tube for analysis and efficient separation of both small and large molecules. The separations of Allura Red are influenced by buffer composition, pH, and additives such as cyclodextrins. CE analysis showed rapid and economic as compared to the conventional electrophoresis and chromatography. Modern CE is driven by the production of low cost narrow-bore capillaries for gas chromatography (GC) and high sensitive on-line detection systems for HPLC. Besides, CE has a wide range of separation modes which including capillary zone electrophoresis, micellar electrokinetic capillary chromatography (MEKC), and capillary isotachophoresis etc., to complete efficient separations using high voltage (Xu, 1996). Thompson and Trenerry (1995) developed a rapid and economical method for determination of ten commonly used azo dyes including Allura Red in confectionary and cordial by MEKC. Similarly, Huang et al. (2005) established a microemulsion electrokinetic chromatography (MEEKC) method for the analysis of eight food colorants using a microemulsion solution. Prado et al. (2006) analyzed eleven synthetic food dyes in alcoholic beverages without any sample pre-treatment using CE-UV/Vis with excellent result.
Thin-layer chromatography (TLC) is a simple, economic and most appropriate chromatographic technique for qualitative analysis of the mixtures of analytes. TLC systems for the separations of food dyes are fairly widespread; however, it is gradually being superseded by HPLC. Besides, one of the difficulties is facing an appropriate mobile phase and stationary phase, on which dyes are applied. A few TLC methods for the analysis of synthetic azo dyes have reported by Soponar et al. (2008). Kucharska and Grabka (2010) have reviewed various sample preparation techniques and chromatographic conditions for the analysis of synthetic dyes in different food samples by TLC and HPLC. de Andrade et al. (2014) have analyzed synthetic food dyes in soft drinks using SPE technique and analytes eluted by a mixture of isopropyl alcohol and ammonium hydroxide as the mobile phase.
Electrochemical sensors have been widely applied for the analysis of Allura Red in foods due to fast response, low cost, simple operation procedure, required small amount and high sensitivity (Ahammad et al., 2011; Shaikh et al., 2013). It is feasible to miniaturize instrument for on-site detection. Recently, Yu et al. (2016) fabricated a sensitive and facile electrochemical sensor based on composite of poly(diallyldimethy- lammonium chloride) functionalized graphene with nickel nanoparticles on glassy carbon electrode (PDDA-Gr-Ni/GCE) to determine Allura Red. PDDA-Gr-Ni/GCE showed excellent mechanical strength, large specific surface area and high thermal and electric conductivity. The peak current of Allura Red exhibit remarkably increased on PDDA-Gr-Ni/GCE because of synergistic effect on the large surface area and improved electron transfer efficiency of the nanomaterial. The electrochemcial process of Allura Red on surface of PDDA-Gr-Ni/GCE involves one electron and one proton transfer as illustrated in Figure 2, which revealing that the oxidation of Allura Red is irreversible. Under optimum conditions, the limit of detection (LOD) found of 8.0 nmol L-1. Wang and Zhao (2015) developed an electrochemical sensor based on the modification of GCE with multi-walled carbon nanotubes in ionic liquid-graphene oxides (IL-GO-MWCNT/CGE). Different concentration of Allura Red was detected in the ranges of 8.0 × 10-10 – 5.0 × 10-7 mol L-1, with LOD value of 5.0 × 10-10 mol L-1 (S/N = 3). Rodríguez et al. (2015) studied an antimony film electrode prepared on-line and installed as part of a sequential injection system for determination of azo dyes in food samples. The influence of several flow variables is evaluated using a central composite design. The LOD was found of 0.3 μM with relative standard deviation (RSD) more than 5.0%. Cheng et al. (2015) have prepared a series of porous carbon (PC) using CaCO3 nanoparticles as the hard template and starch as the carbon precursor to determine azo dyes including Allura Red. The LOD was determined on the range of 1.4–1.7 μg L-1.
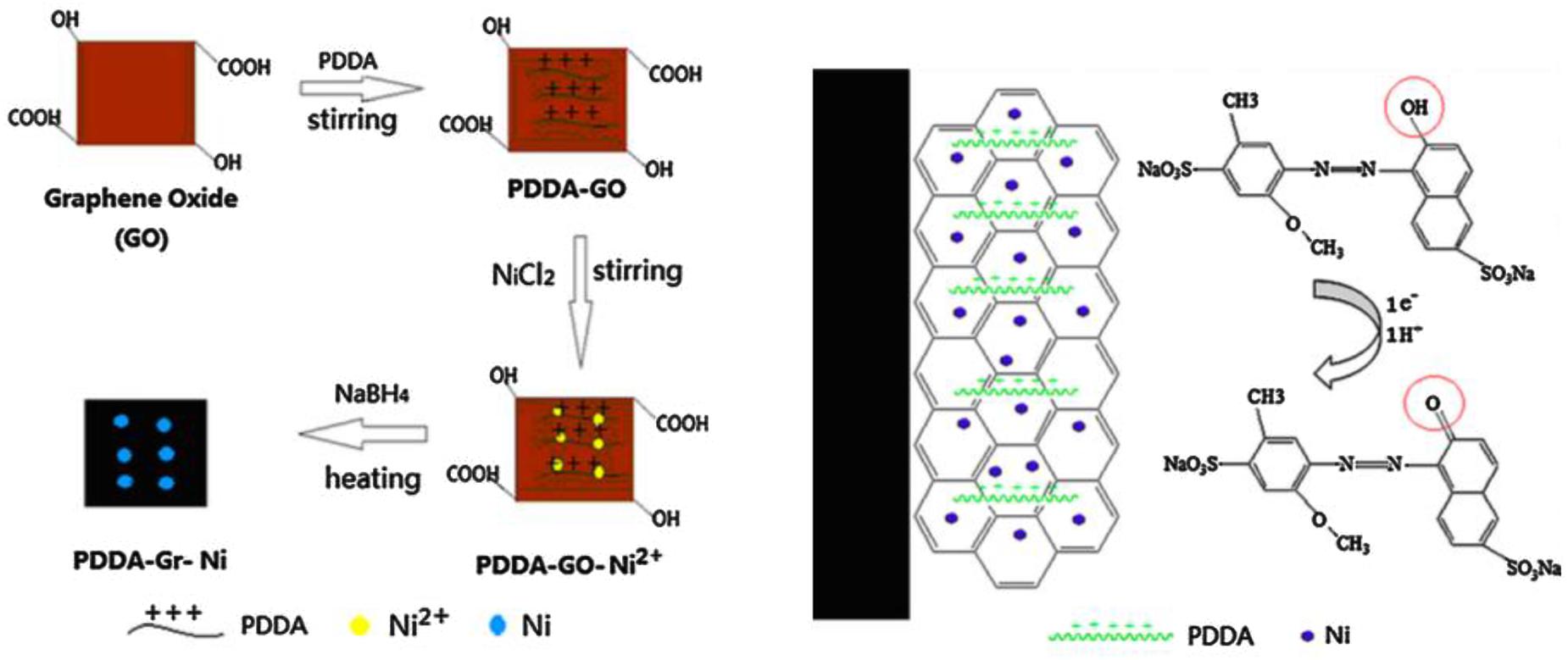
FIGURE 2. The proposed scheme of the formation of PDDA-Gr-Ni composite and mechanism for the electrochemical process of Allura Red (Yu et al., 2016).
Zhang et al. (2010) constructed multiwall carbon nanotubes (MWCNTs) as sensing film to modified the surface of GCE due to have large surface area and high accumulation efficiency. The MWCNTs/CGE has enhanced the oxidation signals of Allura Red and Ponceau 4R Red during electrochemical reaction process. The MWNTs film sensor possesses high sensitivity to Allura Red and Ponceau 4R, and LOD found as low as 15 and 25 μg L-1, respectively. The proposed method was successfully used to detect Allura Red and Ponceau 4R in different beverages with satisfactory results. Silva et al. (2007) developed a polyallylamine modified tubular GCE for determination of three type azo dyes including Allura Red in several food samples by using Square wave voltammetry (SWV). The LOD of Allura Red value was obtained 1.4 μmol L-1. Alghamdi (2005) also determined of Allura Red by using SWV technique with hanging mercury drop electrode (HMDE). Adsorptive voltammetric method is detected in the concentration ranges from 2.5 × 10-8 – 2.0 × 10-7 mol L-1 (r2 = 0.998), with LOD of 8.5 × 10-9 mol L-1. The proposed method has successfully applied for determination of Allura Red dye in commercially available candy and a soft drink. In conclusion, electrochemical techniques have several advantages such as high sensitivity, rapid, simplicity and promising the enhanced material for detection of Allura Red with food safety level.
Differential pulsed polarography and differential pulse adsorptive stripping voltammetry are used for the estimation with different concentrations in food dye matrices. The addition of gelatin has advantageous in the partial identification and determination of food colors due to its pronounced effects on the measured peak currents. Combeau et al. (2002) addressed DPP that possible to differentiate Azorubin, Ponceau 4R and Allura red from the natural dyes providing from fruits. They are used different electrolytes that are tested such as potassium chloride, which is a classical supporting electrolyte, citric acid which is one of the components of the soft beverages, sodium citrate and a phosphate buffer. Chanlon et al. (2005) developed a sensitive method that could be easily distinguished from the natural dyes using DPP in syrups, soda, and sweets. The influence of the pH on the intensities and the potentials of the peaks are found between pH 3 and 11, and acidic or strong basic media but seemed not convenient. This method is successfully applied for monitoring food dyes in the commercial soft drinks and sweets with satisfactory results.
Molecularly imprinted polymers (MIPs) are biomimetic synthetic receptors possessing specific cavities designed for target molecule. A templating process has produced at the molecular level by co-polymerization of functional and cross-linking monomers; MIPs are capable of recognizing and binding target molecules with specificities and affinities comparable to those of natural receptors (Alexander et al., 2006; Bui and Haupt, 2010). It is extensively used in many fields including the SPE and chemo-/bio-sensors due to easy preparation, good stability, and highly specificity. The molecular imprinting coupling to the SPE has become an ideal technique for the extraction and enrichment of the trace analysis from the complicated food matrixes (Wang et al., 2009; Tan et al., 2012). MIP-functionalized magnetic composites have become a hotspot issue owing to the bi-functional property of the target molecule and the rapid magnetic response (Hu et al., 2013). MIPs have commonly showed excellent recognition selectivity and higher adsorption ability for determination of synthetic food dyes.
Food additives are substances added to food to preserve flavor or enhance its taste, nutritional values and appearance. However, the various effects of food additives and preservatives on human as a result of the indiscriminate uses by food producers and food consumers. Many effects like food allergies, food intolerance, cancer, multiple sclerosis, attention deficit hyperactivity disorder (ADHD), brain damage, nausea, cardiac disease among others have been reported. European Food Safety Authority [EFSA] (2009) has revised ADI values and re-evaluated safety concerns of all synthetic dyes since 2008. The developments of analytical and advance methods with high sensitive and selective are needed to monitor of Allura Red in various food matrices. Sample preparation procedures have involved varying extraction techniques including leaching and SFE, solvent extraction, enzymatic digestion, membrane filtration and solid phase extraction techniques. Most of these analytical methods are actual complicated pre-concentration, time-consuming steps and high-cost instruments limit the application of existing methods. Therefore, these methods are unsuited for rapid and efficient or point-of-use foods dyes determination. In contrast, determination of Allura Red in foodstuffs based on the advanced methods (electrochemical sensor, differential pulsed polarography, MIPs) can offer various advantageous such as simple operation procedure, faster analysis, saving time, high sensitivity and selectivity, real time detection and low detection limit. Future study regarding the unknown pharmacological mechanisms of food colorants need to be highlighting. Moreover, systematic research should perform as like as elucidate pharmacological, neurodevelopmental and other effects that various colorants or their mixtures may have. We can suggest to the Government pass a law refusing permission for the food industries to add unauthorized toxic agents into our daily foods and beverages products. We need to protect the health of our population of young children, youths, adolescents and adults, as well as the health of our future generation because a healthy nation is a wealthy nation.
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PAP and handling Editor declared their shared affiliation and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
This work was supported by grants from the Ministry of Education Malaysia, Fundamental Research Grant Scheme (FRGS) (No. FRGS/1/2014/SG05/UMS/02/4).
Ahammad, A. J. S., Rahman, M. M., Xu, G. R., Kim, S., and Lee, J. J. (2011). Highly sensitive and simultaneous determination of hydroquinone and catechol at poly(thionine) modified glassy carbon electrode. Electrochem. Acta 56, 5266–5271. doi: 10.1016/j.electacta.2011.03.004
Ahlström, L. H., Eskilsson, C. S., and Björklund, E. (2005). Determination of banned azo dyes in consumer goods. Trends Anal. Chem. 24, 49–56. doi: 10.1016/j.trac.2004.09.004
Al-Degs, Y. S. (2009). Determination of three dyes in commercial soft drinks using HLA/GO and liquid chromatography. Food Chem. 117, 485–490. doi: 10.1016/j.foodchem.2009.04.097
Alexander, C., Andersson, H. S., Andersson, L. I., Ansell, R. J., Kirsch, N., Nicholls, I. A., et al. (2006). Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J. Mol. Recognit. 19, 106–180. doi: 10.1002/jmr.760
Alghamdi, A. H. (2005). Determination of allura red in some food samples by adsorptive stripping voltammetry. J. AOAC Int. 88, 1387–1393.
Amate, C. F., Unterluggauer, H., Fischer, R. J., Fernández-Alba, A. R., and Masselter, S. (2010). Development and validation of a LC–MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal. Bioanal. Chem. 397, 93–107. doi: 10.1007/s00216-010-3526-x
Amchova, P., Kotolova, H., and Ruda-Kucerova, J. (2015). Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 73, 914–922. doi: 10.1016/j.yrtph.2015.09.026
Ashfaq, N., and Masud, T. (2002). Surveillance on artificial colors in different ready to eat foods. Pak. J. Nutr. 1, 223–225 doi: 10.3923/pjn.2002.223.225
Bazregar, M., Rajabi, M., Yamini, Y., Asghari, A., and Abdossalami Asl, Y. (2015). In-tube electro-membrane extraction with a sub-microliter organic solvent consumption as an efficient technique for synthetic food dyes determination in foodstuff samples. J. Chromatogr. A 1410, 35–43. doi: 10.1016/j.chroma.2015.07.084
Bonan, S., Fedrizzi, G., Menotta, S., and Elisabetta, C. (2013). Simultaneous determination of synthetic dyes in foodstuffs and beverages by high-performance liquid chromatography coupled with diode-array detector. Dyes Pigm. 99, 36–40. doi: 10.1016/j.dyepig.2013.03.029
Borzelleca, J. F., Olson, J. W., and Reno, F. E. (1989). Lifetime toxicity/carcinogenicity study of FD & C Red No. 40 (allura red) in Sprague–Dawley rats. Food Chem. Toxicol. 27, 701–705. doi: 10.1016/0278-6915(89)90074-4
Bui, B. T. S., and Haupt, K. (2010). Molecularly imprinted polymers: synthetic receptors in bioanalysis. Anal. Bioanal. Chem. 398, 2481–2492 doi: 10.1007/s00216-010-4158-x
Ceyhan, B. M., Gultekin, F., Doguc, D. K., and Kulac, E. (2013). Effects of maternally exposed coloring food additives on receptor expressions related to learning and memory in rats. Food Chem. Toxicol. 56, 145–148. doi: 10.1016/j.fct.2013.02.016
Chanlon, S., Joly-Pottuz, L., Chatelut, M., Vittori, O., and Cretier, J. L. (2005). Determination of carmoisine, allura red and Ponceau 4R in sweets and soft drinks by differential pulse polarography. J. Food Compost. Anal. 18, 503–515. doi: 10.1016/j.jfca.2004.05.005
Chen, X. H., Zhao, Y. G., Shen, H. Y., Zhou, L. X., Pan, S. D., and Jin, M. C. (2014). Fast determination of seven synthetic pigments from wine and soft drinks using magnetic dispersive solid-phase extraction followed by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1346, 123–128. doi: 10.1016/j.chroma.2014.04.060
Cheng, Q., Xi, S., Tong, J., and Wu, K. (2015). Highly-sensitive electrochemical sensing platforms for food colourants based on the property-tuning of porous carbon. Anal. Chim. Acta 887, 75–81. doi: 10.1016/j.aca.2015.06.013
Combeau, S., Chatelut, M., and Vittori, O. (2002). Identification and simultaneous determination of Azorubin, Allura red and Ponceau 4R by differential pulse polarography: application to soft drinks. Talanta 56, 115–122. doi: 10.1016/S0039-9140(01)00540-9
Combes, R. D., and Haveland, S. R. B. (1982). A review of the genotoxicity of food, drug and cosmetic colours and other azo, triphenylmethane and xanthene dyes. Mutat. Res. 98, 101–248. doi: 10.1016/0165-1110(82)90015-X
de Andrade, F. I., Guedes, M. I. F., Vieira,Í. G. P., Mendes, F. N. P., Rodrigues, P. A. S., Costa Maia, C. S., et al. (2014). Determination of synthetic food dyes in commercial soft drinks by TLC and ion-pair HPLC. Food Chem. 157, 193–198. doi: 10.1016/j.foodchem.2014.01.100
Dinç, E., Baydan, E., Kanbur, M., and Onur, F. (2002). Spectrophotometric multicomponent determination of sunset yellow, tartrazine and allura red in soft drink powder by double divisor-ratio spectra derivative, inverse least-squares and principal component regression methods. Talanta 58, 579–594. doi: 10.1016/S0039-9140(02)00320-X
Duffus, J., Nordberg, M., and Templeton, D. (2009). Glossary of terms used in toxicology, 2nd edition (IUPAC Recommendations 2007). Pure Appl. Chem. 79, 1153–1344. doi: 10.1351/pac200779071153
EC (1994). Directive of the European Parliament and of the council 94/36/EC of June 30, 1994 on colours for use in foodstuffs. Official J. 237, 13.
El-Sheikh, A. H., and Al-Degs, Y. S. (2013). Spectrophotometric determination of food dyes in soft drinks by second order multivariate calibration of the absorbance spectra-pH data matrices. Dyes Pigm. 97, 330–339. doi: 10.1016/j.dyepig.2013.01.007
Eunice, L. C., Griffiths, P. R., and Chalmers, J. M. (2010). Applications of Vibrational Spectroscopy in Food Science. Hoboken, NJ: John Wiley & Sons, Ltd.
European Food Safety Authority [EFSA] (2009). Scientific Opinion on the re-evaluation of Allura Red (E 129) as a food additive. EFSA J. 7, 1327–1363.
European Food Safety Authority [EFSA] (2012). Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), scientific opinion on the safety and efficacy of Allura Red AC (E 129) in feed for cats and dogs. EFSA J. 10, 2675.
Fallico, B., Chiappara, E., Arena, E., and Ballistreri, G. (2011). Assessment of the exposure to Allura Red colour from the consumption of red juice-based and red soft drinks in Italy. Food Additive Contaminants; A Chemical Analysis Control Expo. Risk Assess. 28, 1501–1515. doi: 10.1080/19440049.2011.596166
Feingold, B. F. (1975). Hyperkinesis and learning disabilities linked to artificial food flavors and colours. Am. J. Nurs. 75, 797–803 doi: 10.1097/00000446-197505000-00021
GB2760-2007 (2007). Hygienic Standards for the Use of Food Additives. Beijing: Ministry of Health of the People’s Republic of China.
GB2760-2011 (2011). Hygienic Standards for the Use of Food Additives. Beijing: Ministry of Health of the People’s Republic of China.
Gómez, M. (2012). Adsorptive stripping voltammetric determination of Tartrazine and Sunset Yellow in gelatins and soft drink powder in the presence of cetylpyridinium bromide. Int. J. Electrochem. Sci. 7, 7493–7502.
González, M., Gallego, M., and Valcárcel, M. (2003). Liquid chromatographic determination of natural and synthetic colorants in lyophilized foods using an automatic solid-phase extraction system. J. Agric. Food Chem. 51, 2121–2129. doi: 10.1021/jf0261147
Gosetti, F., Chiuminatto, U., Mazzucco, E., Calabrese, G., Gennaro, M. C., and Marengo, E. (2012). Identification of photodegradation products of Allura Red AC (E129) in a beverage by ultra high performance liquid chromatography–quadrupole-time-of-flight mass spectrometry. Anal. Chim. Acta 746, 84–89. doi: 10.1016/j.aca.2012.08.020
Gosetti, F., Chiuminatto, U., Mazzucco, E., Calabrese, G., Gennaro, M. C., and Marengo, E. (2013). Non-target screening of Allura Red AC photodegradation products in a beverage through ultra high performance liquid chromatography coupled with hybrid triple quadrupole/linear ion trap mass spectrometry. Food Chem. 136, 617–623. doi: 10.1016/j.foodchem.2012.08.019
Harp, B. P., Miranda-Bermudez, E., Baron, C. I., and Richard, G. I. (2012). Qualitative identification of permitted and non-permitted colour additives in food products. Food Addit. Contam. Part A 29, 886–896. doi: 10.1080/19440049.2012.658526
Harp, B. P., Miranda-Bermudez, E., and Barrows, J. N. (2013). Determination of seven certified color additives in food products using liquid chromatography. J. Agric. Food Chem. 61, 3726–3736. doi: 10.1021/jf400029y
Hawley, C., and Buckley, R. E. (1976). Hyperkinesis and sensitivity to aniline food dyes. J. Orthomol. Psychiatry 5, 129–137.
Herrero, M., Mendiola, J. A., Cifuentes, A., and Ibáñez, E. (2010). Supercritical fluid extraction: recent advances and application. J. Chromatogr. A 1217, 2495–2511. doi: 10.1016/j.chroma.2009.12.019
Holčapek, M., Volna, K., and Vaněrková, D. (2007). Effects of functional groups on the fragmentation of dyes in electrospray and atmospheric pressure chemical ionization mass spectra. Dyes Pigm. 75, 156–165. doi: 10.1016/j.dyepig.2006.05.040
Hu, Y. L., Pan, J. L., Zhang, K. G., Lian, H. X., and Li, G. K. (2013). Novel applications of molecularly-imprinted polymers in sample preparation. Trends Anal. Chem. 43, 37–52. doi: 10.1016/j.trac.2012.08.014
Huang, H. Y., Chiu, C. W., Sue, S. L., and Cheng, C. F. (2003). Analysis of food colorants by capillary electrophoresis with large-volume sample stacking. J. Chromatogr. A 995, 29–36. doi: 10.1016/S0021-9673(03)00530-2
Huang, H. Y., Chuang, C. L., Chiu, C. W., and Chung, M. C. (2005). Determination of food colorants by microemulsion electrokinetic chromatography. Electrophoresis 26, 867–877. doi: 10.1002/elps.200410436
Jung, R., Steinle, D., and Anliker, R. (1992). A compilation of genotoxicity and carcinogenicity data on aromatic aminosulphonic acids. Food Chem. Toxicol. 30, 635–660. doi: 10.1016/0278-6915(92)90199-U
Jurcovan, M. M., and Diacu, E. (2014). Development of a reversed-phase high performance liquid chromatographic method for simultaneous determination of allura red ac and ponceau 4r in soft drinks. Rev. Chim. 65, 137–141.
Karanikolopoulos, G., Gerakis, A., Papadopoulou, K., and Mastrantoni, I. (2015). Determination of synthetic food colorants in fish products by an HPLC-DAD method. Food Chem. 177, 197–203. doi: 10.1016/j.foodchem.2015.01.026
Khanavi, M., Hajimahmoodi, M., Ranjbar, A. M., Oveisi, M. R., Ardekani, M. R. S., and Mogaddam, G. (2012). Development of a green chromatographic method for simultaneous determination of food colorants. Food Anal. Methods 5, 408–415. doi: 10.1007/s12161-011-9259-4
Khani, R., Ghasemi, J. B., Shemirani, F., and Rahmanian, R. (2015). Application of bilinear least squares/residual bilinearization in bulk liquid membrane system for simultaneous multicomponent quantification of two synthetic dyes. Chemometr. Intell. Lab. Syst. 144, 48–55. doi: 10.1016/j.chemolab.2015.03.012
Kirschbaum, J., Krause, C., and Brückner, H. (2006). Liquid chromatographic quantification of synthetic colorants in fish roe and caviar. Eur. Food Res. Technol. 222, 572–579. doi: 10.1007/s00217-005-0157-0
Kiseleva, M. G., Pimenova, V. V., and Eller, K. I. (2003). Optimization of conditions for the HPLC determination of synthetic dyes in food. J. Anal. Chem. URSS 58, 685–690. doi: 10.1016/j.chroma.2015.07.084
Kong, C., Fodjo, E. K., Li, D., Cai, Y., Huang, D., Wang, Y., et al. (2015). Chitosan-based adsorption and freeze deproteinization: improved extraction and purification of synthetic colorants from protein-rich food samples. Food Chem. 188, 240–247. doi: 10.1016/j.foodchem.2015.04.115
Kucharska, M., and Grabka, J. (2010). A review of chromatographic methods for determination of synthetic food dyes. Talanta 80, 1045–1051. doi: 10.1016/j.talanta.2009.09.032
Li, X. Q., Zhang, Q. H., Ma, K., Li, H. M., and Guo, Z. (2015). Identification and determination of 34 water-soluble synthetic dyes in foodstuff by high performance liquid chromatography–diode array detection–ion trap time-of-flight tandem mass spectrometry. Food Chem. 182, 316–326. doi: 10.1016/j.foodchem.2015.03.019
Lok, K. Y. W., Chung, W. Y., Benzie, I. F., and Woo, J. (2010). Colour additives in snack foods consumed by primary school children in Hong Kong. Food Addit. Contam. 3, 148–155. doi: 10.1080/19393210.2010.509815
Lok, K., Grimshaw, K., McCann, D. C., and Stevenson, J. E. (2006). Is an azo-free diet nutritionally superior than one containing azo-dyes? J. Hum. Nutr. Diet. 19, 465–466.
María, J. M. B., Agüera, A., Gómez, M. J., Hernando, M. D., García-Reyes, J. F., and Fernández-Alba, A. R. (2007). Application of liquid chromatography/quadrupole-linear ion trap mass spectrometry and time-of-flight mass spectrometry to the determination of pharmaceuticals and related contaminants in wastewater. Anal. Chem. 79, 9372–9384. doi: 10.1021/ac0715672
Masone, D., and Chanforan, C. (2015). Study on the interaction of artificial and natural food colorants with human serum albumin: a computational point of view. Comput. Biol. Chem. 56, 152–158. doi: 10.1016/j.compbiolchem.2015.04.006
McCann, D., Barrett, A., Cooper, A., Crumpler, D., Dalen, L., Grimshaw, K., et al. (2007). Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 370, 1560–1567. doi: 10.1016/S0140-6736(07)61306-3
Minioti, K. S., Sakellariou, C. F., and Thomaidis, N. S. (2007). Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 583, 103–110. doi: 10.1016/j.aca.2006.10.002
Oka, H., Ikai, Y., Ohno, T., Kawamura, N., Hayakawa, J., Harada, K., et al. (1994). Simple method for the analysis of food dyes on reversed-phase thin-layer plates. J. Chromatogr. A 674, 301–307. doi: 10.1016/0021-9673(94)85235-9
Overmeyer, S., and Taylor, E. (1999). Annotation: principles of treatment for hyperkinetic disorder: practice approaches for the UK. J. Child Psychol. Psychiatry 40, 1147–1157. doi: 10.1111/1469-7610.00532
Prado, M. A., Boas, L. F. V., Bronze, M. R., and Godoy, H. T. (2006). Validation of methodology for simultaneous determination of synthetic dyes in alcoholic beverages by capillary electrophoresis. J. Chromatogr. A 1136, 231–236. doi: 10.1016/j.chroma.2006.09.071
Qi, P., Lin, Z. H., Chen, G. Y., Xiao, J., Liang, Z. A., Luo, L. N., et al. (2015). Fast and simultaneous determination of eleven synthetic color additives in flour and meat products by liquid chromatography coupled with diode-array detector and tandem mass spectrometry. Food Chem. 181, 101–110. doi: 10.1016/j.foodchem.2015.02.075
Rafii, F., Hall, J. D., and Cerniglia, C. E. (1997). Mutagenicity of azo dyes used in foods, drugs and cosmetics before and after reduction by Clostridium species from the human intestinal tract. Food Chem. Toxicol. 35, 897–901. doi: 10.1016/S0278-6915(97)00060-4
Rebane, R., Leito, I., Yurchenko, S., and Herodes, K. (2010). A review of analytical techniques for determination of Sudan I–IV dyes in food matrixes. J. Chromatogr. A 1217, 2747–2757. doi: 10.1016/j.chroma.2010.02.038
Rodríguez, J. A., Juárez, M. G., Galán-Vidal, C. A., Miranda, J. M., and Barrado, E. (2015). Determination of allura red and tartrazine in food samples by sequential injection analysis combined with voltammetric detection at antimony film electrode. Electroanalysis 27, 2329–2334. doi: 10.1002/elan.201500295
Rodriguez-Saona, L. E., Giusti, M. M., Durst, R. W., and Wrolstad, R. E. (2001). Development and process optimization of red raddish concentrate extract as potential natural red colorant. J. Food Process. Preserv. 25, 165–182. doi: 10.1111/j.1745-4549.2001.tb00452.x
Salem, M. A., Al-Ghonemiy, A. F., and Zaki, A. B. (2009). Photocatalytic degradation of allura red and quinoline yellow with polyaniline/TiO2 nanocomposite. Appl. Catal. B Environ. 91, 59–66. doi: 10.1016/j.apcatb.2009.05.027
Sasaki, Y. F., Kawaguchi, S., Kamaya, A., Ohshita, M., Kabasawa, K., Iwama, K., et al. (2002). The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat. Res. 519, 103–119. doi: 10.1016/S1383-5718(02)00128-6
Shaikh, A. A., Saha, S. K., Bakshi, P. K., Hussain, A., and Ahammad, A. J. S. (2013). Poly(brilliant cresyl blue)-modified electrde for highly sensitive and simultaneous determination of hydroquinone and catecho. J. Electrochem. Soc. 160, 37–42. doi: 10.1149/2.008304jes
Shen, Y., Zhang, X., Prinyawiwatkul, W., and Xu, Z. (2014). Simultaneous determination of red and yellow artificial food colourants and carotenoid pigments in food products. Food Chem. 157, 553–558. doi: 10.1016/j.foodchem.2014.02.039
Silva, M. L. S., Garcia, M. B. Q., Lima, J. L. F. C., and Barrado, E. (2007). Voltammetric determination of food colorants using a polyallylamine modified tubular electrode in a multicommutated flow system. Talanta 72, 282–288. doi: 10.1016/j.talanta.2006.10.032
Soltan, S. S. A., and Shehata, M. M. E. M. (2012). The effects of using color foods of children on immunity properties and liver, kidney on rats. Food Nutr. Sci. 3, 897–904. doi: 10.4236/fns.2012.37119
Soponar, F., Mot, A. C., and Sarbu, C. (2008). Quantitative determination of some food dyes using digital processing of images obtained by thin-layer chromatography. J. Chromatogr. A 1188, 295–300. doi: 10.1016/j.chroma.2008.02.077
Sorouraddin, M. H., Rostami, A., and Saadati, M. (2011). A simple and portable multi-colour light emitting diode based photocolourimeter for the analysis of mixtures of five common food dyes. Food Chem. 127, 308–313. doi: 10.1016/j.foodchem.2010.12.124
Soylak, M., Unsal, Y. E., and Tuzen, M. (2011). Spectrophotometric determination of trace levels of allura red in water samples after separation and preconcentration. Food Chem. Toxicol. 49, 1183–1187. doi: 10.1016/j.fct.2011.02.013
Sun, H., Sun, N., Li, H., Zhang, J., and Yang, Y. (2013). Development of multiresidue analysis for 21 synthetic colorants in meat by microwave-assisted extraction–solid-phase extraction–reversed-phase ultrahigh performance liquid chromatography. Food Anal. Methods 6, 1291–1299. doi: 10.1007/s12161-012-9542-z
Suzuki, S., Shirao, M., Aizawa, M., Nakazawa, H., Sasa, K., and Sasagawa, H. (1994). Determination of synthetic food dyes by capillary electrophoresis. J. Chromatogr. A 680, 541–547. doi: 10.1016/0021-9673(94)85153-0
Tan, J., Jiang, Z. T., Li, R., and Yan, X. P. (2012). Molecularly-imprinted monoliths for sample treatment and separation. Trends Anal. Chem. 39, 207–217. doi: 10.1016/j.trac.2012.05.009
Tang, B., Xi, C., Zou, Y., Wang, G., Li, X., Zhang, L., et al. (2014). Simultaneous determination of 16 synthetic colorants in hotpot condiment by high performance liquid chromatography. J. Chromatogr. B 960, 87–91. doi: 10.1016/j.jchromb.2014.04.026
Thompson, C. O., and Trenerry, V. C. (1995). Determination of synthetic colours in confectionery and cordials by micellar electrokinetic capillary chromatography. J. Chromatogr. A 704, 195–201. doi: 10.1016/0021-9673(95)00165-J
Tsai, C. F., Kuo, C. H., and Shih, D. Y. C. (2015). Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J. Food Drug Anal. 23, 453–462. doi: 10.1016/j.jfda.2014.09.003
Turak, F., and Ozgur, M. U. (2013). Simultaneous determination of allura red and ponceau 4r in drinks with the use of four derivative spectrophotometric methods and comparison with high-performance liquid chromatography. J. AOAC Int. 96, 1377–1386. doi: 10.5740/jaoacint.12-393
Wang, H. F., He, Y., Ji, T. R., and Yan, X. P. (2009). Surface molecular imprinting on Mn-doped ZnS quantum dots for room-temperature phosphorescence optosensing of pentachlorophenol in water. Anal. Chem. 81, 1615–1621. doi: 10.1021/ac802375a
Wang, L., Zhang, G., and Wang, Y. (2014). Binding properties of food colorant Allura Red with human serum albumin in vitro. Mol. Biol. Rep. 41, 3381–3391. doi: 10.1007/s11033-014-3200-z
Wang, M. L., and Zhao, J. W. (2015). A facile method used for simultaneous determination of ponceau 4R, allura red and tartrazine in alcoholic beverages. J. Electrochem. Soc. 162, 321–327. doi: 10.1149/2.0111506jes
Wu, D., Yan, J., Wang, J., Wang, Q., and Li, H. (2015). Characterisation of interaction between food colourant Allura red AC and human serum albumin: multi- spectroscopic analyses and docking simulations. Food Chem. 170, 423–429. doi: 10.1016/j.foodchem.2014.08.088
Wu, H., Guo, J. B., Du, L. M., Tian, H., Hao, C. X., Wang, Z. F., et al. (2013). A rapid shaking-based ionic liquid dispersive liquid phase micro extraction for the simultaneous determination of six synthetic food colourants in soft drinks, sugar-and gelatin-based confectionery by high-performance liquid chromatography. Food Chem. 141, 182–186. doi: 10.1016/j.foodchem.2013.03.015
Xu, Y. (1996). Tutorial: capillary electrophoresis. Chem. Educ. 1, 11–14. doi: 10.1007/s00897960023a
Yoshioka, N., and Ichihashi, K. (2008). Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 74, 1408–1413. doi: 10.1016/j.talanta.2007.09.015
Yu, L. L., Shi, M. X., Yue, X., and Qu, L. B. (2016). Detection of allura red based on the composite of poly (diallyldimethylammonium chloride) functionalized graphene and nickel nanoparticles modified electrode. Sens. Actuators B Chem. 225, 398–404. doi: 10.1016/j.snb.2015.11.061
Zhang, Y., Zhang, X., Lu, X., Yang, J., and Wu, K. (2010). Multi-wall carbon nanotube film-based electrochemical sensor for rapid detection of Ponceau 4R and Allura Red. Food Chem. 122, 909–913. doi: 10.1016/j.foodchem.2010.035
Keywords: Allura Red, toxicological, spectrophotometry, capillary electrophoresis, thin-layer chromatography, high performance liquid chromatography, electrochemical sensor, molecularly imprinted polymers
Citation: Rovina K, Siddiquee S and Shaarani SM (2016) Extraction, Analytical and Advanced Methods for Detection of Allura Red AC (E129) in Food and Beverages Products. Front. Microbiol. 7:798. doi: 10.3389/fmicb.2016.00798
Received: 26 December 2015; Accepted: 11 May 2016;
Published: 27 May 2016.
Edited by:
Amit Kumar Tyagi, The University of Texas MD Anderson Cancer Center, USAReviewed by:
Eva-Guadalupe Lizárraga-Paulín, Instituto Tecnológico y de Estudios Superiores de Monterrey-Campus Estado de México, MexicoCopyright © 2016 Rovina, Siddiquee and Shaarani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shafiquzzaman Siddiquee, c2hhZmlxcGFiQHVtcy5lZHUubXk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.