- 1School of Life and Environmental Sciences, University of Sydney, Sydney, NSW, Australia
- 2The ithree institute, University of Technology Sydney, Sydney, NSW, Australia
- 3University of the Sunshine Coast, Maroochydore, QLD, Australia
- 4Comvita NZ Limited, Te Puke, New Zealand
Medicinal honey research is undergoing a substantial renaissance. From a folklore remedy largely dismissed by mainstream medicine as “alternative”, we now see increased interest by scientists, clinical practitioners and the general public in the therapeutic uses of honey. There are a number of drivers of this interest: first, the rise in antibiotic resistance by many bacterial pathogens has prompted interest in developing and using novel antibacterials; second, an increasing number of reliable studies and case reports have demonstrated that certain honeys are very effective wound treatments; third, therapeutic honey commands a premium price, and the honey industry is actively promoting studies that will allow it to capitalize on this; and finally, the very complex and rather unpredictable nature of honey provides an attractive challenge for laboratory scientists. In this paper we review manuka honey research, from observational studies on its antimicrobial effects through to current experimental and mechanistic work that aims to take honey into mainstream medicine. We outline current gaps and remaining controversies in our knowledge of how honey acts, and suggest new studies that could make honey a no longer “alternative” alternative.
Introduction
Honey has been used as a medicine throughout the history of the human race. One of the most common and persistent therapeutic uses of honey has been as a wound dressing, almost certainly due to its antimicrobial properties. With the advent of highly active antibiotics in the 1960s, honey was dismissed as a “worthless but harmless substance” (Soffer, 1976). However, the current and growing crisis of antibiotic resistance has revived interest in the use of honey, both as an effective agent in its own right and as a therapeutic lead to develop new methods of treatment. Honey is usually derived from the nectar of flowers and produced by bees, most commonly the European honey bee Apis mellifera, and is a complex mix of sugars, amino acids, phenolics, and other substances. Honey types derived from different flowering plants vary substantially in their ability to kill bacteria, and this has complicated the literature on honey and made it sometimes difficult to reproduce results across different studies (Allen et al., 1991; Irish et al., 2011). The majority of recent studies investigating the mechanism of action of honey have focused on well-characterized, standardized active manuka honey produced by certain Leptospermum species native to New Zealand and Australia, which has been registered as a wound care product with appropriate medical regulatory bodies. Thus, unless otherwise specified, this review will focus on manuka honey.
Chemical Analyses of Active Manuka Honey
Professor Peter Molan of Waikato University, New Zealand, was the first to report the unusual activity of manuka honey and began testing its action against a wide range of different bacterial species in the mid 1980s. However, while it was clear that even low concentrations of manuka honey killed bacterial pathogens, the specific active ingredient responsible for this remained elusive for many years. High sugar and low pH make honey inhibitory to microbial growth, but activity remains when these are diluted to negligible levels. Many different types of honey also produce hydrogen peroxide when glucose oxidase, which is derived from the honey bee, reacts with glucose and water. However, in manuka honey hydrogen peroxide production is relatively low and can be neutralized by catalase, yet activity still remains. The cause of this remaining activity, dubbed “non-peroxide activity” or NPA, was finally revealed in 2008, when two laboratories independently identified methyl glyoxal (MGO) in manuka honey (Adams et al., 2008; Mavric et al., 2008). MGO results from the spontaneous dehydration of its precursor dihydroxyacetone (DHA), a naturally occurring phytochemical found in the nectar of flowers of Leptospermum scoparium, Leptospermum polygalifolium, and some related Leptospermum species native to New Zealand and Australia (Adams et al., 2009; Williams et al., 2014; Norton et al., 2015). MGO can react relatively non-specifically with macromolecules such as DNA, RNA and proteins (Adams et al., 2008; Mavric et al., 2008; Majtan et al., 2014b), and could theoretically be toxic to mammalian cells (Kalapos, 2008). However, there is no evidence of damage to host cells when manuka honey is either consumed orally or used as a wound dressing; indeed honey appears to stimulate healing and reduce scarring when applied to wounds (Biglari et al., 2013; Majtan, 2014; Dart et al., 2015). How it exerts this apparently selective toxicity to bacterial cells is not known.
High levels of MGO or hydrogen peroxide usually produce the most active honey, however, the correlation is not always perfect suggesting other components of honey may modulate activity (Molan, 2008; Kwakman et al., 2011; Chen et al., 2012; Lu et al., 2013). Bee defensin-1, an antimicrobial bee-derived peptide is responsible for activity in Revamil honey, an active honey produced from an undisclosed source, but this appears to be structurally modified and inactive in manuka honey (Kwakman et al., 2011; Majtan et al., 2012). The level of leptosin, a glycoside found exclusively in Leptospermum honey, correlates with potency and may modulate the antimicrobial activity of manuka honey (Kato et al., 2012). Similarly, various phenolic compounds with potential antimicrobial activity can be present, particularly in darker colored honeys, and although these occur at levels that are unlikely to be inhibitory on their own they may synergize with one another or other components of honey to produce or alter activity (Estevinho et al., 2008; Stephens et al., 2010). Phenolics can also act as antioxidants and may be responsible for anti-inflammatory and wound-healing properties of honey (Stephens et al., 2010). It should be noted that not all Leptospermum species produce active honey, and even within L. scoparium and L. polygalifolium honey MGO levels can range from ∼100 to >1200 ppm (Windsor et al., 2012). A survey of Australian honey activity found honey sourced from Leptospermum plants growing around the New South Wales–Queensland border was particularly active, but whether this is due to plant, soil, climate or other factors is not known (Irish et al., 2011).
The Inhibition of Pathogens by Honey
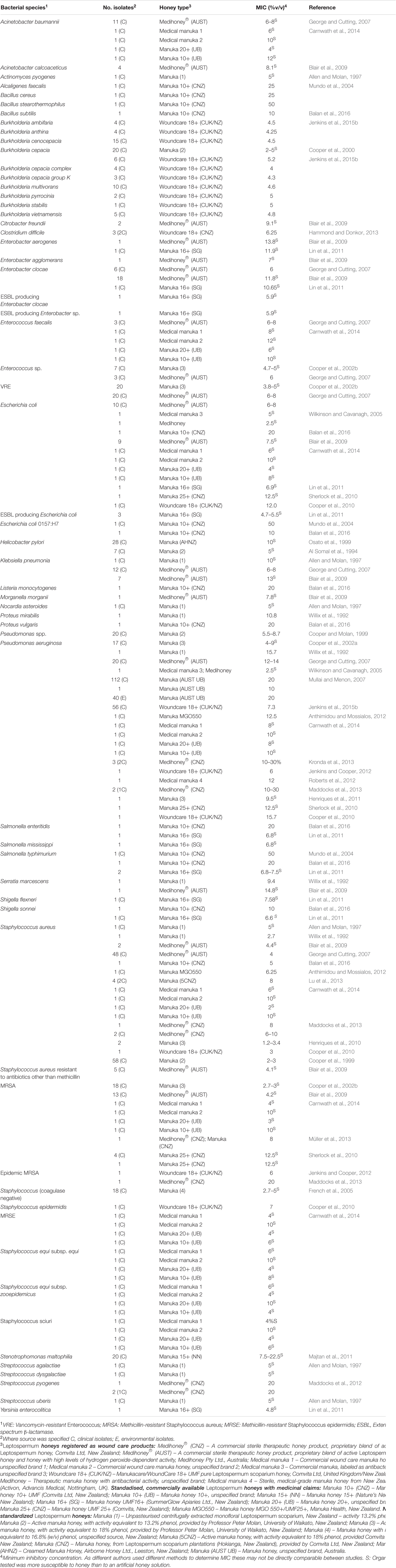
Honey has been tested in vitro on a diverse range of pathogens, particularly those that can colonize the skin, wounds and mucosal membranes, where topical honey treatment is possible. To date, in vitro assays have found manuka honey can effectively inhibit all problematic bacterial pathogens tested (summarized in Table 1). Of particular interest is that clinical isolates with multiple drug resistance (MDR) phenotypes have no reduction in their sensitivity to honey, indicating a broad spectrum of action that is unlike any known antimicrobial (Willix et al., 1992; Blair and Carter, 2005; George and Cutting, 2007; Tan et al., 2009). In addition, attempts to generate honey-resistant strains in the laboratory have not been successful and there have been no reports of clinical isolate with acquired resistance to honey (Blair et al., 2009; Cooper et al., 2010).
As well as inhibiting planktonic cells, honey can disperse and kill bacteria living in biofilms. Biofilms are communities of cells that are generally enclosed in a self-produced extracellular matrix and found adhering to surfaces, including wounds, teeth, mucosal surfaces, and implanted devices. Microbes resident in biofilms are protected from antimicrobial agents and they can cause persistent, non-resolving infections. Manuka honey disrupts cellular aggregates (Maddocks et al., 2012; Roberts et al., 2012) and prevents the formation of biofilms by a wide range of problematic pathogens, including Streptococcus and Staphylococcus species, Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, Enterobacter cloacae, Acinetobacter baumannii, and Klebsiella pneumonia (Maddocks et al., 2012, 2013; Lu et al., 2014; Majtan et al., 2014a; Halstead et al., 2016) Importantly, honey can also disrupt established biofilms and kill resident cells, although a higher concentration is required than for planktonic cells (Okhiria et al., 2009; Maddocks et al., 2013; Lu et al., 2014; Majtan et al., 2014a). Very recently, manuka honey was tested on a multispecies biofilm containing Staphylococcus aureus, Streptococcus agalactiae, Pseudomonas aeruginosa, and Enterococcus faecalis and was found to reduce viability of all species but E. faecalis, which could not be eradicated (Sojka et al., 2016). This has clear clinical implications for using honey on wounds containing biofilms, and understanding how the biofilm enables E. faecalis, to survive when it is normally killed by honey is an important and interesting area of future study. MGO appears to be mostly but not fully responsible for the inhibition of biofilms by manuka honey, again highlighting the importance of additional components that modulate activity (Kilty et al., 2011; Lu et al., 2014).
The spectrum of activity of honey toward non-bacterial pathogens is yet to be well established. Recent studies examining the antiviral effect of manuka honey have suggested it has potential for treatment of varicella-zoster virus (the cause of chicken pox and shingles) (Shahzad and Cohrs, 2012) and influenza (Watanabe et al., 2014). Fungal pathogens of the skin, including Candida albicans and dermatophyte species are substantially less susceptible than bacteria to manuka honey, but are inhibited by honey with high levels of hydrogen peroxide production (Brady et al., 1996; Irish et al., 2006). Manuka and non-manuka honey have been found to reduce the viability of spores of the microsporidian Nosema apis, an important pathogen of bees, but honey could not cure bee infection once this was underway (Malone et al., 2001). There have been very few studies on the use of honey for protozoan or helminth parasites and these have not used honey with well-characterized activity, making it difficult to assess the significance of their findings (Bassam et al., 1997; Nilforoushzadeh et al., 2007; Sajid and Azim, 2012).
Taking Honey Into Mainstream Medicine: Recent Experimental and Mechanistic Studies Shed Light on How Honey Works
Active manuka honey is widely available as a therapeutic agent and functional food, and most consumers accept it as a holistic, somewhat mysterious product. However, a lack of understanding on how honey kills bacteria and promotes healing limits its acceptance by mainstream medicine where it is still considered “alternative” or “complementary”. The vast majority of research studies on honey to date have been descriptive, however, recent studies are attempting to unravel how honey works and are using mechanistic approaches to determine how it acts at the cellular and the molecular level.
Ultrastructural Studies of Bacterial Cells and Communities Treated by Honey
Honey can profoundly alter the size and shape of bacterial cells, although the extent of this varies in different bacterial species. Using transmission electron microscopy (TEM), S. aureus cultures treated with manuka honey had more cells with completed septa compared to those treated with artificial honey, suggesting cells entered but failed to complete the division stage of the cell cycle, although externally these cells appeared normal by scanning electron microscopy (SEM) (Henriques et al., 2010). More recently, phase-contrast imaging following treatment with a sub-lethal dose of manuka honey found cells of S. aureus and Bacillus subtilis were significantly smaller and were more likely to have condensed DNA than those growing without honey (Lu et al., 2013). It is difficult to directly compare these studies as they used different amounts of honey and treatment times, but overall the results suggest an uncoupling of growth and cell division, which is often seen in response to nutritional and environmental stresses (Silva-Rocha and de Lorenzo, 2010).
Honey treatment has been reported to cause cultures of the Gram negative species E. coli and P. aeruginosa to have both abnormally shorter and longer cells (Lu et al., 2013). Interestingly, while P. aeruginosa appears to be less susceptible to inhibition by honey than other species, profound cellular changes were seen using TEM and SEM, including furrows and blebs (protrusions of cellular plasma membranes) on the cell surface and a substantial amount of extracellular debris indicative of cell lysis (Henriques et al., 2011). This was verified in a subsequent study using BacLight live-dead fluorescence staining and confocal microscopy, although this also demonstrated that a relatively large number of live cells remained. These studies used 20% (w/v) honey, which was higher that the MBC for their strain of P. aeruginosa and substantial inhibition and death would be expected. However, atomic force microscopy (AFM) using sub-bactericidal levels still found substantial cell distortion and blebbing in cells treated with MIC (12%) and half MIC (6%) concentrations, along with substantial cell lysis (Roberts et al., 2012). This apparent degeneration of the P. aeruginosa cell was supported by quantitative PCR analysis that showed a 10-fold down-regulation in honey-treated cells of oprF, which encodes an outer-membrane porin that is important for structural stability (Jenkins et al., 2015a).
‘Omics Analyses Assess the Whole-Cell Response to Inhibition by Honey
The ability to assess whole cell outputs has revolutionized the study of drug-pathogen interactions and has particular value for complex natural products like honey where effects on multiple processes are likely. Microarray and proteomic studies of bacteria exposed to honey suggested an induction of stress-related processes and suppression of protein synthesis (Blair et al., 2009; Jenkins et al., 2011; Packer et al., 2012). While overall this is fairly typical of a response to inhibitory agents, honey produced a unique “signature” of differential expression that included many proteins with hypothetical or unknown functions, suggesting a novel mode of action. Specific genes or proteins found to be down-regulated in ‘omics analyses of S. aureus and E. coli O157/H7 have functions relating to virulence, quorum sensing and biofilm formation (Lee et al., 2011; Jenkins et al., 2013), and in P. aeruginosa there was a down-regulation of proteins involved in flagellation (Roberts et al., 2015). These phenotypes are critical for pathogens to establish and produce invasive infection and indicate that as well as inhibiting growth, honey can reduce the pathogenic potential of infecting bacteria.
Although still relatively limited in number and scope, the ‘omics analyses conducted to date suggest a complex cellular response to honey with considerable variation in different bacterial species. Advanced systems biology approaches that allow contextualization of the data, and validation studies using quantitative PCR and gene deletion strains, are now required to unravel this complexity, and these may reveal new approaches for drug therapies aimed at inhibiting bacterial growth (Hudson et al., 2012).
Interactions Between Honey and Conventional Antibiotics
As well as use as a sole agent, there is scope for using honey to augment treatment with conventional antibiotics. This may have particular value when combined with systemic agents that can be delivered to a wound bed via blood circulation while honey is applied topically. Combined treatments can also lower the therapeutic dose of antimicrobial agents and prevent the development of resistance, and in some cases can result in drug synergy, where the combined activity is greater than the sum of the individual activities of each drug partner.
In vitro studies combining therapeutically approved manuka honey with antibiotic agents have found a synergistic effect with oxacillin, tetracycline, imipenem and mupirocin against the growth of an MRSA strain (Jenkins and Cooper, 2012). Furthermore, the presence of a sub-inhibitory concentration of honey in combination with oxacillin restored the MRSA strain to oxacillin susceptibility. The authors found down-regulation of mecR1, which encodes an MRSA-specific penicillin-binding protein (PBP2A) and suggested this as a mechanism of honey synergy. Strong synergistic activity between manuka honey and rifampicin against multiple S. aureus strains, including clinical isolates and MRSA strains, has also been found, and the presence of honey prevented the emergence of rifampicin resistance in vitro (Müller et al., 2013). This is of clinical significance as rifampicin penetrates well into tissues and abscesses and is commonly used to treat superficial staphylococcal infections, but rapidly induces resistance and must therefore be used in combination with another agent. An additional finding from this study was that synergy was not due to MGO, as a synthetic honey spiked with MGO was not synergistic with rifampicin.
Understanding how honey affects the action of antimicrobials with well-characterized modes of action may also further our understanding of how honey affects bacterial pathogens. Liu et al. (2014) extended the analysis of synergy to include additional antibiotics and different S. aureus and MRSA strains. They suggested that an increased susceptibility to clindamycin and gentamicin might result from the combined effect of down-regulated protein synthesis by honey with inhibition of ribosomes by the antibiotics, while synergy with β-lactam antibiotics could be due to increased oxidative stress caused by both partners. As S. aureus and MRSA strains were equally susceptible to the oxacillin-honey combination it appeared that synergy was unlikely to be due to PBP2A down-regulation. In one clinical MRSA isolate, however, there was no increase in sensitivity to clindamycin or gentamicin when honey was present, which is notable as it is the first reported case of a difference in response to honey by MRSA versus S. aureus. Investigating this strain-specific difference using transcriptomic or proteomic analyses would be an interesting avenue for future research (Liu et al., 2014).
Evidence of Efficacy From Animal Studies, Case Reports, and Clinical Trials
Companies that produce and market manuka honey promote high ethical standards and discourage the use of animal models to study infections and wound healing. Manuka honey has, however, been used to treat animals with surgical or accidental wounds, particularly horses, with positive outcomes (Dart et al., 2015; Bischofberger et al., 2016). Case reports using honey for non-healing wounds and ulcers have noted significant improvement with resolution of infection where conventional antibiotics had failed (Regulski, 2008; Smith et al., 2009). However, despite this and the evidence from numerous in vitro and in vivo models that honey kills problematic wound pathogens, there is a paucity of robust clinical data for manuka honey. There are various reasons for this, including technical difficulties in performing a double-blind placebo-controlled trial on a distinctive substance like honey, ethical considerations, lack of interest by clinical practitioners and cost-versus-benefit to honey companies, whose focus is on natural products and over-the-counter sales where manuka honey and associated dressings already command a premium price. These may change as antibiotic resistance erodes current treatment options and ongoing research highlighting the potential of honey brings it to the attention of medical practitioners.
Gaps and Emerging Opportunities in the Study of Honey
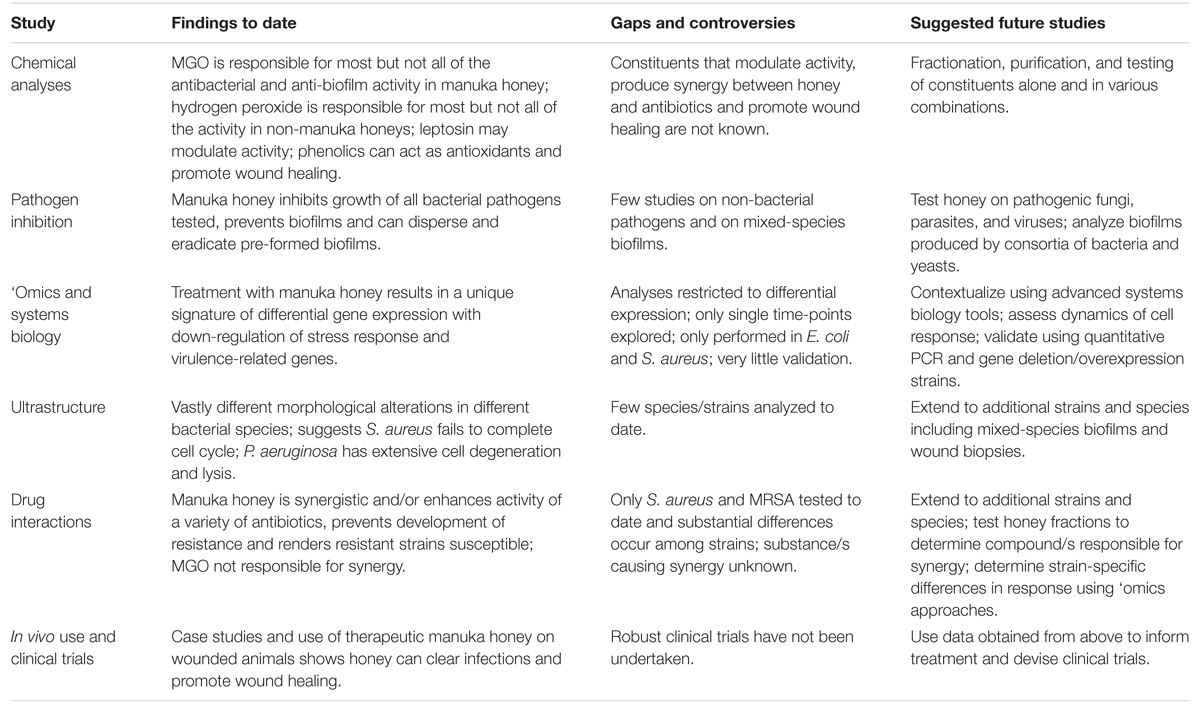
Great progress has been made recently in our understanding of therapeutic honey, yet its use in clinical medicine remains limited, even when conventional antibiotics are starting to fail. The complexity in honey, which is arguably its greatest strength in killing diverse pathogens and preventing resistance, complicates its study as many factors working together are likely to affect activity. We advocate further mechanistic studies using appropriately registered therapeutic manuka honey, in particular studies that use non-reductionist systems biology approaches, along with detailed chemical and microbiological analyses to elucidate how honey acts at the molecular, cellular and population level, how this can differ in different strains and species of microbial pathogens, and how the host cell responds (Table 2). Information gained from these studies can then inform therapy and produce the clinical data required to take honey into mainstream medicine; no longer the alternative therapy used only when all else has failed.
Author Contributions
This review was written by DC, SB, NNC, DB, and PB and was critically reviewed by RS and EH.
Funding
NNC receives salary support from the Rural Industries Research and Development Corporation – Honey Bee Program (Grant PRJ-009186).
Conflict of Interest Statement
DC, PB, and EH report grant and non-financial support in the form of manuka honey from Comvita NZ Limited and Capilano Honey Limited; RS is employed by Comvita NZ Limited, which trades in medical grade manuka honey (Medihoney).
The rest of the authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Abbreviation
ESBL, extended spectrum β-lactamase; MBC, minimum bactericidal concentration; MGO, methyl glyoxal; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermis; NPA, non-peroxide activity; VRE, vancomycin-resistant Enterococcus.
References
Adams, C. J., Boult, C. H., Deadman, B. J., Farr, J. M., Grainger, M. N. C., Manley-Harris, M., et al. (2008). Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 343, 651–659. doi: 10.1016/j.carres.2007.12.011
Adams, C. J., Manley-Harris, M., and Molan, P. C. (2009). The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 344, 1050–1053. doi: 10.1016/j.carres.2009.03.020
Al Somal, N., Coley, K. E., Molan, P. C., and Hancock, B. M. (1994). Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J. R. Soc. Med. 87, 9–12.
Allen, K., Molan, P., and Reid, G. (1991). A survey of the antibacterial activity of some New Zealand honeys. J. Pharm. Pharmacol. 43, 817–822. doi: 10.1111/j.2042-7158.1991.tb03186.x
Allen, K. L., and Molan, P. C. (1997). The sensitivity of mastitis-causing bacteria to the antibacterial activity of honey. N. Z. J. Agric. Res. 40, 537–540. doi: 10.1080/00288233.1997.9513276
Anthimidou, E., and Mossialos, D. (2012). Antibacterial activity of Greek and Cypriot honeys against Staphylococcus aureus and Pseudomonas aeruginosa in comparison to manuka honey. J. Med. Food 16, 42–47. doi: 10.1089/jmf.2012.0042
Balan, P., Mal, G., Das, S., and Singh, H. (2016). Synergistic and additive antimicrobial activities of curcumin, manuka honey and whey proteins. J. Food Biochem. doi: 10.1111/jfbc.12249
Bassam, Z., Zohra, B. I., and Saada, A.-A. (1997). The effects of honey on Leishmania parasites: an in vitro study. Trop. Doctor 27, 36–38.
Biglari, B., Moghaddam, A., Santos, K., Blaser, G., Büchler, A., Jansen, G., et al. (2013). Multicentre prospective observational study on professional wound care using honey (Medihoney). Int. Wound J. 10, 252–259. doi: 10.1111/j.1742-481X.2012.00970.x
Bischofberger, A., Dart, C., Horadagoda, N., Perkins, N., Jeffcott, L., Little, C., et al. (2016). Effect of Manuka honey gel on the transforming growth factor β1 and β3 concentrations, bacterial counts and histomorphology of contaminated full-thickness skin wounds in equine distal limbs. Aust. Vet. J. 94, 27–34. doi: 10.1111/avj.12405
Blair, S., Cokcetin, N., Harry, E., and Carter, D. (2009). The unusual antibacterial activity of medical-grade Leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 28, 1199–1208. doi: 10.1007/s10096-009-0763-z
Blair, S. E., and Carter, D. A. (2005). The potential for honey in the management of wounds and infections. J. Austral. Infect. Control 10, 24–31.
Brady, N., Molan, P., and Harfoot, C. (1996). The sensitivity of dermatophytes to the antimicrobial activity of manuka honey and other honey. Pharm. Pharmacol. Commun. 2, 471–473.
Carnwath, R., Graham, E. M., Reynolds, K., and Pollock, P. J. (2014). The antimicrobial activity of honey against common equine wound bacterial isolates. Vet. J. 199, 110–114. doi: 10.1016/j.tvjl.2013.07.003
Chen, C., Campbell, L., Blair, S. E., and Carter, D. A. (2012). The effect of heat treatment on the antimicrobial properties of honey. Front. Microbiol. 3:265. doi: 10.3389/fmicb.2012.00265
Cooper, R., Jenkins, L., Henriques, A., Duggan, R., and Burton, N. (2010). Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 29, 1237–1241. doi: 10.1007/s10096-010-0992-1
Cooper, R. A., Halas, E., and Molan, P. C. (2002a). The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J. Burn Care Rehabil. 23, 366–370. doi: 10.1097/00004630-200211000-00002
Cooper, R. A., and Molan, P. C. (1999). The use of honey as an antiseptic in managing Pseudomonas infection. J. Wound Care 8, 161–164. doi: 10.12968/jowc.1999.8.4.25867
Cooper, R. A., Molan, P. C., and Harding, K. G. (1999). Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J. R. Soc. Med. 92, 283–285.
Cooper, R. A., Molan, P. C., and Harding, K. G. (2002b). The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 93, 857–863. doi: 10.1046/j.1365-2672.2002.01761.x
Cooper, R. A., Wigley, P., and Burton, N. F. (2000). Susceptibility of multiresistant strains of Burkholderia cepacia to honey. Lett. Appl. Microbiol. 31, 20–24. doi: 10.1046/j.1472-765x.2000.00756.x
Dart, A., Bischofberger, A., Dart, C., and Jeffcott, L. (2015). A review of research into second intention equine wound healing using manuka honey: current recommendations and future applications. Equine Vet. Educ. 27, 658–664. doi: 10.1111/eve.12379
Estevinho, L., Pereira, A. P., Moreira, L., Dias, L. G., and Pereira, E. (2008). Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 46, 3774–3779. doi: 10.1016/j.fct.2008.09.062
French, V. M., Cooper, R. A., and Molan, P. C. (2005). The antibacterial activity of honey against coagulase-negative Staphylococci. J. Antimicrobial Chemother. 56, 228–231. doi: 10.1093/jac/dki193
George, N. M., and Cutting, K. F. (2007). Antibacterial honey (Medihoney): in-vitro activity against clinical isolates of MRSA, VRE, and other multiresistant gram-negative organisms including Pseudomonas aeruginosa. Wounds 19:231.
Halstead, F. D., Webber, M. A., Rauf, M., Burt, R., Dryden, M., and Oppenheim, B. A. (2016). In vitro activity of an engineered honey, medical-grade honeys, and antimicrobial wound dressings against biofilm-producing clinical bacterial isolates. J. Wound Care 25, 93–102. doi: 10.12968/jowc.2016.25.2.93
Hammond, E. N., and Donkor, E. S. (2013). Antibacterial effect of Manuka honey on Clostridium difficile. BMC Res. 6:188. doi: 10.1186/1756-0500-6-188
Henriques, A. F., Jenkins, R. E., Burton, N. F., and Cooper, R. A. (2010). The intracellular effects of manuka honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 29, 45–50. doi: 10.1007/s10096-009-0817-2
Henriques, A. F., Jenkins, R. E., Burton, N. F., and Cooper, R. A. (2011). The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 30, 167–171. doi: 10.1007/s10096-010-1065-1
Hudson, N. J., Dalrymple, B. P., and Reverter, A. (2012). Beyond differential expression: the quest for causal mutations and effector molecules. BMC Genomics 13:356. doi: 10.1186/1471-2164-13-356
Irish, J., Blair, S., and Carter, D. (2011). The antibacterial activity of honey derived from Australian flora. PLoS ONE 6:e18229. doi: 10.1371/journal.pone.0018229
Irish, J., Carter, D. A., Shokohi, T., and Blair, S. E. (2006). Honey has an antifungal effect against Candida species. Med. Mycol. 44, 289–291. doi: 10.1080/13693780600931986
Jenkins, R., Burton, N., and Cooper, R. (2011). Effect of manuka honey on the expression of universal stress protein A in methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 37, 373–376. doi: 10.1016/j.ijantimicag.2010.11.036
Jenkins, R., Burton, N., and Cooper, R. (2013). Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus (MRSA) exposed to manuka honey in vitro demonstrated down-regulation of virulence markers. J. Antimicrobial Chemother. 69, 603–615. doi: 10.1093/jac/dkt430
Jenkins, R., and Cooper, R. (2012). Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS ONE 7:e45600. doi: 10.1371/journal.pone.0045600
Jenkins, R., Roberts, A., and Brown, H. L. (2015a). On the antibacterial effects of manuka honey: mechanistic insights. Res. Rep. Biol. 6, 215–224. doi: 10.2147/RRB.S75754
Jenkins, R., Wootton, M., Howe, R., and Cooper, R. (2015b). A demonstration of the susceptibility of clinical isolates obtained from cystic fibrosis patients to manuka honey. Arch. Microbiol. 197, 597–601. doi: 10.1007/s00203-015-1091-6
Kalapos, M. P. (2008). The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 171, 251–271. doi: 10.1016/j.cbi.2007.11.009
Kato, Y., Umeda, N., Maeda, A., Matsumoto, D., Kitamoto, N., and Kikuzaki, H. (2012). Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J. Agric. Food Chem. 60, 3418–3423. doi: 10.1021/jf300068w
Kilty, S. J., Duval, M., Chan, F. T., Ferris, W., and Slinger, R. (2011). Methylglyoxal: (active agent of manuka honey) in vitro activity against bacterial biofilms. Int. Forum Allergy Rhinol. 1, 348–350. doi: 10.1002/alr.20073
Kronda, J. M., Cooper, R. A., and Maddocks, S. E. (2013). Manuka honey inhibits siderophore production in Pseudomonas aeruginosa. J. Appl. Microbiol. 115, 86–90. doi: 10.1111/jam.12222
Kwakman, P. H., te Velde, A. A., de Boer, L., Vandenbroucke-Grauls, C. M., and Zaat, S. A. (2011). Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS ONE 6:e17709. doi: 10.1371/journal.pone.0017709
Lee, J.-H., Park, J.-H., Kim, J.-A., Neupane, G. P., Cho, M. H., Lee, C.-S., et al. (2011). Low concentrations of honey reduce biofilm formation, quorum sensing, and virulence in Escherichia coli O157: H7. Biofouling 27, 1095–1104. doi: 10.1080/08927014.2011.633704
Lin, S. M., Molan, P. C., and Cursons, R. T. (2011). The controlled in vitro susceptibility of gastrointestinal pathogens to the antibacterial effect of manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 30, 569–574. doi: 10.1007/s10096-010-1121-x
Liu, M., Lu, J., Müller, P., Turnbull, L., Burke, C. M., Schlothauer, R. C., et al. (2014). Antibiotic-specific differences in the response of Staphylococcus aureus to treatment with antimicrobials combined with manuka honey. Front. Microbiol. 5:779. doi: 10.3389/fmicb.2014.00779
Lu, J., Carter, D. A., Turnbull, L., Rosendale, D., Hedderley, D., Stephens, J., et al. (2013). The effect of New Zealand kanuka, manuka and clover honeys on bacterial growth dynamics and cellular morphology varies according to the species. PLoS ONE 8:e55898. doi: 10.1371/journal.pone.0055898
Lu, J., Turnbull, L., Burke, C. M., Liu, M., Carter, D. A., Schlothauer, R. C., et al. (2014). Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ 2:e326. doi: 10.7717/peerj.326
Maddocks, S. E., Jenkins, R. E., Rowlands, R. S., Purdy, K. J., and Cooper, R. A. (2013). Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Fut. Microbiol. 8, 1523–1536. doi: 10.2217/fmb.13.126
Maddocks, S. E., Lopez, M. S., Rowlands, R. S., and Cooper, R. A. (2012). Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 158, 781–790. doi: 10.1099/mic.0.053959-0
Majtan, J. (2014). Honey: an immunomodulator in wound healing. Wound Repair Regenerat. 22, 187–192. doi: 10.1111/wrr.12117
Majtan, J., Bohova, J., Horniackova, M., Klaudiny, J., and Majtan, V. (2014a). Anti-biofilm effects of honey against wound pathogens Proteus mirabilis and Enterobacter cloacae. Phytother. Res. 28, 69–75. doi: 10.1002/ptr.4957
Majtan, J., Bohova, J., Prochazka, E., and Klaudiny, J. (2014b). Methylglyoxal may affect hydrogen peroxide accumulation in manuka honey through the inhibition of glucose oxidase. J. Med. Food 17, 290–293. doi: 10.1089/jmf.2012.0201
Majtan, J., Klaudiny, J., Bohova, J., Kohutova, L., Dzurova, M., Sediva, M., et al. (2012). Methylglyoxal-induced modifications of significant honeybee proteinous components in manuka honey: possible therapeutic implications. Fitoterapia 83, 671–677. doi: 10.1016/j.fitote.2012.02.002
Majtan, J., Majtanova, L., Bohova, J., and Majtan, V. (2011). Honeydew honey as a potent antibacterial agent in eradication of multi-drug resistant Stenotrophomonas maltophilia isolates from cancer patients. Phytother. Res. 25, 584–587. doi: 10.1002/ptr.3304
Malone, L. A., Gatehouse, H. S., and Tregidga, E. L. (2001). Effects of time, temperature, and honey on Nosema apis (Microsporidia: Nosematidae), a parasite of the honeybee, Apis mellifera (Hymenoptera: Apidae). J. Invertebrate Pathol. 77, 258–268. doi: 10.1006/jipa.2001.5028
Mavric, E., Wittmann, S., Barth, G., and Henle, T. (2008). Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutrit. Food Res. 52, 483–489. doi: 10.1002/mnfr.200700282
Molan, P. M. (2008). An explanation of why the MGO level in manuka honey does not show the antibacterial activity. New Zealand Beekeeper 16, 11–13.
Mullai, V., and Menon, T. (2007). Bactericidal activity of different types of honey against clinical and environmental isolates of Pseudomonas aeruginosa. J. Alternat. Complement. Med. 13, 439–442. doi: 10.1089/acm.2007.6366
Müller, P., Alber, D. G., Turnbull, L., Schlothauer, R. C., Carter, D. A., Whitchurch, C. B., et al. (2013). Synergism between Medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA). PLoS ONE 8:e57679. doi: 10.1371/journal.pone.0057679
Mundo, M. A., Padilla-Zakour, O. I., and Worobo, R. W. (2004). Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 97, 1–8. doi: 10.1016/j.ijfoodmicro.2004.03.025
Nilforoushzadeh, M. A., Jaffary, F., Moradi, S., Derakhshan, R., and Haftbaradaran, E. (2007). Effect of topical honey application along with intralesional injection of glucantime in the treatment of cutaneous leishmaniasis. BMC Complement Altern. Med. 7:1. doi: 10.1186/1472-6882-7-1
Norton, A. M., McKenzie, L. N., Brooks, P. R., and Pappalardo, L. J. (2015). Quantitation of dihydroxyacetone in Australian Leptospermum nectar via High-Performance Liquid Chromatography. J. Agric. Food Chem. 63, 6513–6517. doi: 10.1021/acs.jafc.5b01930
Okhiria, O., Henriques, A., Burton, N., Peters, A., and Cooper, R. (2009). Honey modulates biofilms of Pseudomonas aeruginosa in a time and dose dependent manner. J. ApiProduct. ApiMedical Sci. 1, 6–10. doi: 10.3896/IBRA.4.01.1.03
Osato, M. S., Reddy, S. G., and Graham, D. Y. (1999). Osmotic effect of honey on growth and viability of Helicobacter pylori. Dig. Dis. Sci. 44, 462–464. doi: 10.1023/A:1026676517213
Packer, J. M., Irish, J., Herbert, B. R., Hill, C., Padula, M., Blair, S. E., et al. (2012). Specific non-peroxide antibacterial effect of manuka honey on the Staphylococcus aureus proteome. Int. J. Antimicrob. Agents 40, 43–50. doi: 10.1016/j.ijantimicag.2012.03.012
Regulski, M. (2008). A novel wound care dressing for chronic leg ulcerations. Podiatry Manag. 27, 235–246.
Roberts, A. E., Maddocks, S. E., and Cooper, R. A. (2012). Manuka honey is bactericidal against Pseudomonas aeruginosa and results in differential expression of oprF and algD. Microbiology 158, 3005–3013. doi: 10.1099/mic.0.062794-0
Roberts, A. E. L., Maddocks, S. E., and Cooper, R. A. (2015). Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J. Antimicrob. Chemother. 70, 716–725. doi: 10.1093/jac/dku448
Sajid, M., and Azim, M. K. (2012). Characterization of the nematicidal activity of natural honey. J. Agric. Food Chem. 60, 7428–7434. doi: 10.1021/jf301653n
Shahzad, A., and Cohrs, R. J. (2012). In vitro antiviral activity of honey against varicella zoster virus (VZV): a translational medicine study for potential remedy for shingles. Transl. Biomed. 3:2.
Sherlock, O., Dolan, A., Athman, R., Power, A., Gethin, G., Cowman, S., et al. (2010). Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement Altern. Med. 10:47. doi: 10.1186/1472-6882-10-47
Silva-Rocha, R., and de Lorenzo, V. (2010). Noise and robustness in prokaryotic regulatory networks. Annu. Rev. Microbiol. 64, 257–275. doi: 10.1146/annurev.micro.091208.073229
Smith, T., Hanft, J. R., and Legel, K. (2009). Topical Leptospermum honey in recalcitrant venous leg wounds: a preliminary case series. Adv. Skin Wound Care 22, 68–71. doi: 10.1097/01.ASW.0000345283.05532.9a
Soffer, A. (1976). Chihuahuas and laetrile, chelation therapy, and honey from Boulder, Colorado [editorial]. Arch. Intern. Med. 136, 865–866. doi: 10.1001/archinte.136.8.865
Sojka, M., Valachova, I., Bucekova, M., and Majtan, J. (2016). Antibiofilm efficacy of honey and bee-derived defensin-1 on multi-species wound biofilm. J. Med. Microbiol. doi: 10.1099/jmm.0.000227 [Epub ahead of print].
Stephens, J. M., Schlothauer, R. C., Morris, B. D., Yang, D., Fearnley, L., Greenwood, D. R., et al. (2010). Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 120, 78–86. doi: 10.1016/j.foodchem.2009.09.074
Tan, H. T., Rahman, R. A., Gan, S. H., Halim, A. S., Hassan, S. A., Sulaiman, S. A., et al. (2009). The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement Altern. Med. 9:34. doi: 10.1186/1472-6882-9-34
Watanabe, K., Rahmasari, R., Matsunaga, A., Haruyama, T., and Kobayashi, N. (2014). Anti-influenza viral effects of honey in vitro: potent high activity of manuka honey. Arch. Med. Res. 45, 359–365. doi: 10.1016/j.arcmed.2014.05.006
Wilkinson, J. M., and Cavanagh, H. M. A. (2005). Antibacterial activity of 13 honeys against Escherichia coli and Pseudomonas aeruginosa. J. Med. Food 8, 100–103. doi: 10.1089/jmf.2005.8.100
Williams, S., King, J., Revell, M., Manley-Harris, M., Balks, M., Janusch, F., et al. (2014). Regional, annual, and individual variations in the dihydroxyacetone content of the nectar of manuka (Leptospermum scoparium) in New Zealand. J. Agric. Food Chem. 62, 10332–10340. doi: 10.1021/jf5045958
Willix, D. J., Molan, P. C., and Harfoot, C. G. (1992). A comparison of the sensitivity of wound-infecting species of bacteria to the antibacterial activity of manuka honey and other honey. J. Appl. Bacteriol. 73, 388–394. doi: 10.1111/j.1365-2672.1992.tb04993.x
Keywords: manuka honey, antibacterial, Leptospermum, methyl glyoxal, natural product
Citation: Carter DA, Blair SE, Cokcetin NN, Bouzo D, Brooks P, Schothauer R and Harry EJ (2016) Therapeutic Manuka Honey: No Longer So Alternative. Front. Microbiol. 7:569. doi: 10.3389/fmicb.2016.00569
Received: 25 February 2016; Accepted: 05 April 2016;
Published: 20 April 2016.
Edited by:
Luis Cláudio Nascimento da Silva, University Centre of Maranhão, BrazilReviewed by:
Osmar Nascimento Silva, Dom Bosco Catholic University, BrazilJuraj Majtan, Slovak Academy of Sciences, Slovakia
Copyright © 2016 Carter, Blair, Cokcetin, Bouzo, Brooks, Schothauer and Harry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dee A. Carter, dee.carter@sydney.edu.au
 Dee A. Carter
Dee A. Carter Shona E. Blair2
Shona E. Blair2 Nural N. Cokcetin
Nural N. Cokcetin Daniel Bouzo
Daniel Bouzo Ralf Schothauer
Ralf Schothauer