- 1Fujian Provincial Key Laboratory of Agroecological Processing and Safety Monitoring, Fujian Agriculture and Forestry University, Fuzhou, China
- 2Key Laboratory of Biopesticide and Chemical Biology, Ministry of Education, Fujian Agriculture and Forestry University, Fuzhou, China
- 3Key Laboratory of Crop Ecology and Molecular Physiology, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, China
Radix pseudostellariae L. is a common and popular Chinese medication. However, continuous monoculture has increased its susceptibility to severe diseases. We identified two pathogenic microorganisms, Talaromyces helicus M. (KU355274) and Kosakonia sacchari W. (KU324465), and their antagonistic bacterium, Bacillus pumilus Z. in rhizosphere soil of continuously monocultured R. pseudostellariae. Nine types of phenolic acids were identified both in the rhizosphere soil and in culture medium under sterile conditions. A syringic acid and phenolic acid mixture significantly promoted the growth of T. helicus and K. sacchari. T. helicus could utilize eight types of phenolic acids, whereas K. sacchari could only use four phenolic acids. K. sacchari produced protocatechuic acid when consuming vanillin. Protocatechuic acid negatively affected the growth of B. pumilus. The 3A-DON toxin produced by T. helicus promoted the growth of K. sacchari and inhibited growth of B. pumilus at low concentrations. These data help explain why phenolic exudates mediate a microflora shift and structure disorder in the rhizosphere soil of continuously monocultured R. pseudostellariae and lead to increased replanting disease incidence.
Introduction
Radix pseudostellariae L. (Caryophyllaceae) is a common and popular Chinese medicine. It contains polysaccharides, ginseng saponins, flavonoids, cyclic peptides, amino acids, and trace elements (Hang and Wang, 2010). High-quality R. pseudostellariae is mainly produced in the ZheRong region of Fujian province in southern China, where soil and climate conditions are favorable for its growth. However, continuously monocultured R. pseudostellariae is prone to severe diseases, which result in reduced biomass of the plant tuberous roots (Lin et al., 2012a,b; Zhao et al., 2015). This phenomenon is known as replanting disease or soil sickness. It has been reported that more than 70% of medicinal plants, especially those with tuberous roots, such as R. pseudostellariae, have been attacked by replanting diseases (Zhang and Lin, 2009). Therefore, it has become the priority to study and overcome the consecutive monoculture problems, especially exhibited in medicinal plant production.
Replanting disease (Wu et al., 2009; Zhang and Wang, 2010), or “Sick Soil Syndrome,” is a problem related to replanting in soils where the same species was previously grown. The sick soil syndrome refers to a combination of plant growth dysplasia, pests, and disease problems, which result in reduced yield and quality. It is a consequence of continuous monocropping for many years. Autotoxicity is an intraspecific allelopathy phenomenon, common in monocropping systems, where the plants inhibit their own growth through release of autotoxic chemicals into the soil (Singh et al., 1999; Wu et al., 2015). Terpenoids, phenolics, steroids, alkaloids, and cyanogenic glycosidic exudates are secreted from monocultured crop plant roots (Kato-Noguchi et al., 2002; Kato-Noguchi and Ino, 2003; Kulmatiski et al., 2008). Phenolic acids are important in allelopathy and replanting disease incidence (Bais et al., 2004; Kulmatiski et al., 2008; Wu et al., 2015; Zhao et al., 2015). Phenolic compounds are autotoxins of the plants in monocropping system, such as Rehmannia glutinosa, cucumber, and tobacco (Fang et al., 2010, 2013; Lin et al., 2010; Li et al., 2012; Zhou and Wu, 2012b; Wu et al., 2015). Some studies indicate that phenolic acids change the soil microbial community with possible influences on plant performance (Zhou et al., 2012, 2014). The allelochemicals released by plants might also promote growth of soil-borne pathogens and inhibit beneficial microbes (Kong et al., 2008; Pollock et al., 2011). The details of these phenomena are poorly known, especially in medicinal plants under monoculture regimes.
Most research in crop monocultures has focused on the effects of phenolic acids on the soil microbial community. There is less information on the dynamic changes of phenolics in soil and their effects on specific microbes. Therefore, we identified the types of phenolic acids in soil and their effects on the potential pathogens (Talaromyces helices M. and Kosakonia sacchari W.) and a beneficial microbe (Bacillus pumilus Z.) under laboratory conditions. It was also determined whether the pathogenic microorganisms were able to cause replanting disease in moncultured R. pseudostellariae, and the changes were also examined in the soil microbial community structure and its relationship to replanting disease of R. pseudostellariae under long term continuous monoculture conditions.
Materials and Methods
The Isolation and Identification of Pathogenic Microorganisms
The pathogenic microorganisms were isolated using a tissue isolation method (Fang et al., 2011). After initial cleaning of the tuber roots of R. pseudostellariae sampled from the monoculture plots, we washed them for 30 min under running water, then used sterile water to wash them once on a sterile operation platform. A clean knife was used to cut the pathogen-infected tuber roots into slices. These slices were soaked in 75% ethanol for 30–40 s, washed twice with sterile water, dipped in 0.1% aqueous mercuric chloride solution for 7–10 min and washed again using sterile water (five times, for 2–3 min each time). The sterilized tissue slices of the infected roots were then cultured on MS medium at 32°C, after which the pure strains were separated from the mixed bacterial or fungal community in the culture.
PCR for Bacterial 16S rRNA Genes and Fungal its Regions
PCR amplification of partial bacterial 16S rRNA gene sequences was performed using the primer set 1405f and 456r (Zeng and Zheng, 2011). PCR assays were conducted in a 25-μL volume mix containing 12.5 μL of Taq PCR Master Mix (2 ×) (Transgen, Beijing, China), 1 μL of each primer, and 20 ng of purified DNA extracts. PCR conditions were: 94°C for 5 min; 94°C for 1 min; 61°C for 1 min; 72°C for 90 s, 35 cycles in total, with a final elongation at 72°C for 10 min. The fungal community sizes were estimated using the primer sets ITS4 and ITS86 (Ferrer et al., 2001). The PCR conditions for the fungal community were: 94°C for 5 min; 94°C for 45 s; 51°C for 45 s; and 7°C for 1 min. PCR products were separated by electrophoresis on a 1.2% agarose gel and the bands were purified using the Universal DNA Purification Kit (TIANGEN, Beijing, China). The DNA sequences were analyzed using BLAST tools and the NCBI database to determine the species.
Validation of Microbial Pathogenicity
Based on the tissue culture of R. pseudostellariae, we added 200 μL of activator fungi, Kosakonia and B. pumilus (isolated from the rhizosphere of R. pseudostellariae) to the tissue culture. The control received equivalent amounts of PDA broth and LB medium instead. Then, the growth of the tissue culture seedlings co-cultured with the specific pathogenic bacteria was observed.
Validation of B. pumilus Antagonists against T. helicus
The amended agar disk diffusion method (Bauer et al., 1966) was used to qualitatively screen the antagonists. Briefly, two strains of B. pumilus were streaked onto a potato dextrose agar (PDA) plate (square plate), and the Talaromyces helicus removed from an actively growing colony margin of T. helicus, was placed in the center of the plate. Plates were incubated for 8 d at 30°C.
Quantitative Real-Time PCR (qPCR) of T. helicus, K. sacchari, and B. pumilus Isolated from the R. pseudostellariae Rhizosphere Soil
According to the sequences of T. helicus, K. sacchari, and B. pumilus, taxon-specific primers including TH1F/TH1R, saesu-F/saesu-R, and BP-F/BP-R were set (Table S1). The identity and specificity of the primers (the amplifying gene) were confirmed (see Figure S5). Then we used the primers to amplify specific genes through the total DNA extracted from the R. pseudostellariae rhizosphere soil. And we also found these primers could amplify the special fragments corresponding to the T. helicus, K. sacchari, and B. pumilus.
The DNA sequences of T. helicus, K. sacchari, and B. pumilus were amplified using TH1F/TH1R, saesu-F/saesu-R, and BP-F/BP-R from the plasmid and were purified using a TIAN pure Mini Plasmid Kit [TIANGENBIOTECH (BEIJING) CO., LTD]. The plasmids contained the target DNA which can be represented the different microorganisms. We used these plasmids as standard curves to calculate the contents of T. helices, K. sacchari, and B. pumilus, respectively. The concentration of the target DNA was determined using a spectrophotometer (NanoDrop2000c, Thermo Fisher, USA), and was diluted to 1, 0.5, 0.1, 0.05, 0.01, 0.005, and 0.001 ng/μL. qPCR was monitored using a Mastercycler ep realplex (Eppendorf, Germany). Standard curve plotting and melting curve analysis was performed following the qPCR amplification instructions. A standard curve was created by plotting the target DNA concentration against the threshold cycle (Ct)-value exported from the Mastercycler ep realplex. The primer sets TH1F/TH1R, saesu-F/saesu-R, and BP-F/BP-R were evaluated using the established standard curve, and melting curves were determined using qPCR amplification of four replicates with a serial dilution of the target DNA template. The qPCR reactions were performed in 15-μL reaction mixtures (7.5 mL 2 × SYBR Green PCR Master Mix, 0.6 μL TH1F/TH1R, 0.6 μL saesu-F/saesu-R, 0.6 μL BP-F/BP-R, and 40 ng DNA made up to a final volume of 15 μL with ddH2O). The PCR parameters for T. helicus were as follows: 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 56°C for 45 s, and 72°C for 45 s. The K. sacchari PCR parameters were 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 55°C for 30 s, and 72°C for 30 s, and the B. pumilus parameters were 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 60°C for 45 s, and 72°C for 45 s. After the qPCR run, melting curve analysis was performed to verify the specificity of the amplified product under the following conditions: 95°C for 15 s, 60°C for 15 s, followed by an increase to 95°C over 10 min, and then maintained at 95°C for 15 s.
Phenolics Extraction and Determination
Soil Phenolics Extraction
Soil samples were collected from the pots containing R. pseudostellaria at different growth stages for each treatment, including consecutive monoculture, newly planted, and control (no plant). Fresh soil was collected on April 22, May 5, June 6, and June 26 in 2014, and the sampled soil was sieved (2-mm mesh) to remove stones and plant residues. Soil phenolic acids of each sample were extracted using previously described methods (Dalton et al., 1987; Zhou and Wu, 2012a). Briefly, 5 g of each soil sample was added to 25 mL of 1 M NaOH and agitated for 24 h on a reciprocal shaker at 30°C, then spun in a vortex generator for 30 min at maximum speed. The suspension was centrifuged at 10,000 rpm for 10 min, and the liquid supernatant was collected. The filtrate was adjusted to pH 2.5 using 9 M HCl, and extracted five times with ethyl acetate. The resultant extracts were pooled and evaporated to dryness at 35°C. The residue was dissolved in 5 mL methanol using ultrasound for 5 min and maintained in the dark at 4°C.
Phenolics Extraction from the Tissue Culture Medium of R. pseudostellariae
The tissue cultures of R. pseudostellariae were incubated for 150, 215, 279, 305, and 355 d. Each treatment contained 60 mL of medium. Then, the used medium from each treatment was collected for extraction of the phenolic acids. Each sample was added to 40 mL of 1 M NaOH and agitated for 90 min in an ultrasonic generator. The other conditions were the same as those mentioned above.
Phenolics Determination
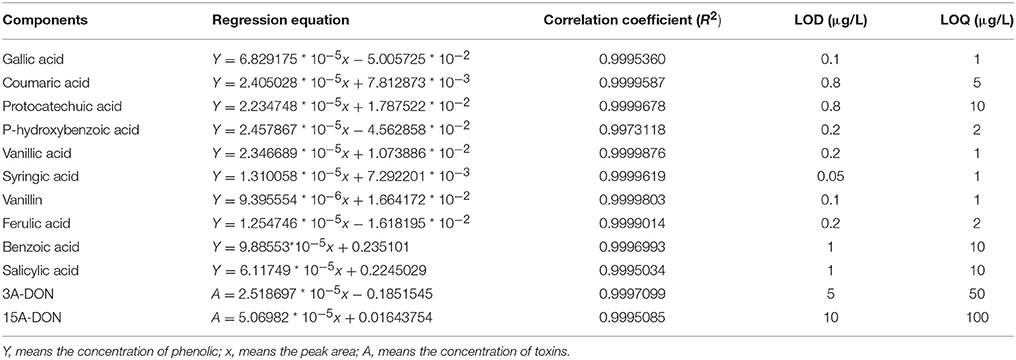
The methanol solution of the extracts was filtered through 0.22-μm filter membrane for HPLC analysis. The phenolic acids in each sample were determined using a Waters HPLC system (C18 column: Inertsil ODS-SP, 4.6 × 250 mm, 5 μm). The mobile phase A was methanol and mobile phase B was 2% glacial acetic acid. The flow rate was kept constant at 0.7 mL/min. Detection was performed at 280 nm. The injection volume was 20 μL and the column temperature was maintained at 30°C. Identification and quantification of phenolic compounds were confirmed by comparing retention times and areas with pure standards.
The Influence of Phenolic Acids on R. pseudostellariae
Based on the measurement results of the phenolic acids content and the ratio of the mixed phenolic acids detected in R. pseudostellariae rhizosphere soil, phenolic acids standards were used to form a “mixed phenolic acid solution (P1)” that simulated the average ratio of various phenolic acids detected in R. pseudostellariae rhizosphere soil (FY, SF, TF; i.e., gallic acid: coumaric acid: p-hydroxybenzoic acid: vanillic acid: syringic acid: vanillin: ferulic acid: benzoic acid = 3:11:26: 119:14:14:25:28).
In addition, a series of concentration gradients of the mixed phenolic acids were set at 0, 160, 320, 960, and 1280 μmol/L for the tissue culture of R. pseudostellariae. Each treatment had three replicates. Then, the tissue culture was incubated for 50 d. Plant dry mass was measured after oven drying at 70°C to a constant mass.
The Influence of Phenolic Acids on the Physiological Characteristics of T. helices
The Impact of Single and the Mixed Phenolic Acids on the Diameter Growth of the R. pseudostellariae Biotype T. helicus
Soil extract-medium (SEM) preparation: 1 kg of soil and 1 L of double distilled water were shaken for 30 min on the shaker, then sterilized at 121°C for 15 min. The leachate was filtered through talc in a double suction filter. The filtrate was collected and adjusted to neutral pH and the mother liquor retained for further use. According to method requirements, double distilled water was used to dilute the soil solution with appropriately diluted replicates, and then an appropriate amount of agar (15 g/L) was added to the solution, which was sterilized before use.
Based on the measurements of phenolic acid content in the R. pseudostellariae rhizosphere soil, a series of phenolic acid concentration gradients were set. The concentration gradients were 0, 40, 80, 160, 320, and 960 μmol/L. When the 10-fold dilution of the SEM medium was cooled (to about 40–50°C) after sterilization, appropriate amounts of various stock phenolic solutions were added (dissolved using methanol), after filtration through a 0.22-μm membrane, then immediately poured into the plates. The control received only double distilled water and an equal volume of methanol in order to exclude the effects of methanol on the growth of T. helices. Each treatment was replicated three times. After preparation of the phenolic acid-SEM plates, the activated T. helices spores were cultured in the center of the plate, then placed in a 30°C constant temperature incubator for 9 d, after which the mycelium diameter was measured.
Based on the phenolic acid content and the ratio of the mixed phenolic acids (P1), a series of phenolic concentrations were established, i.e., 0, 40, 80, 160, 320, and 960 μmol/L. The other conditions were the same as those mentioned above.
Effect of the Mixed Phenolic Acids on the Toxin Production of the R. pseudostellariae Biotype T. helicus
The dilution ratio of the soil filtrate and its preparation were the same as mentioned above. The concentrations of the mixed phenolic acids were 0, 80, 160, and 960 μmol/L. An equal amount of T. helices spores filtrate was added to the SEM liquid medium in the bottle. Then, the bottle was maintained at 30°C on a 180-rpm thermostatic shaker for 8 d. After culture, HPLC techniques were used to determine the content of two common toxins (3A-DON: 3-Acetyldeoxynivalenol and 15A-DON: 15-O-Acetyl-4-deoxynivalenol). To draw a standard curve, the concentrations of the two types of toxin standards used were 0.5, 1, 2, 5, and 10 ppm. The chromatographic conditions were as follows: chromatographic column: C18 column (Inertsil ODS-SP, 4.6 × 250 mm, 5 μm); mobile phase A: acetonitrile; mobile phase B: 0.005% phosphoric acid solution; elution gradient: mobile phase B 95% (0 min) → 30% (9 min) → 0% (18 min) → 0% (23 min) → 95% (23.01 min) → 95% (45 min); oven temperature: 35°C; detection wavelength: 224 nm; velocity: 0.7 mL/min.
The extraction method for T. helices toxins was as follows. The liquid fermented for 10 d was first filtered using double filter paper and then filtered using a 0.22-μm ultrafiltration membrane. The filtrate (15 mL) was added to a bottle containing the same volume of ethyl acetate, and placed on the shaker for 8 h at 28°C, 160 rpm for oscillation extraction. This was followed by centrifugation at 10,000 rpm for 3 min at 4°C. The upper layer organic phase was transferred into a new 50-mL centrifuge tube. After concentration by vacuum and drying, 500 μL of double evaporated water was added to dissolve the solid residue, then subjected to ultrasound and analyzed immediately using HPLC. The sample toxins were quantitatively analyzed by comparison to the standard curve.
Detecting the Utilization of Various Phenolic Acids by the R. pseudostellariae Biotype T. helices
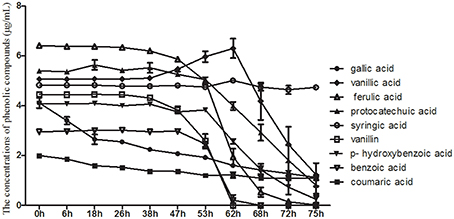
The soil leachate mother liquid (48 mL) was diluted three times and placed into a 250-mL triangle flask. After sterilization and precooling, 0.3 mL of the phenolic acid mixture (coumaric acid, protocatechuic acid, gallic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, vanillin, ferulic acid, benzoic acid, 4800 μmol/L) was added to the flask. These phenolic acids had been detected in the rhizosphere soil of R. pseudostellariae. Then, 250 μL of T. helices spore filtrate was added to the flask. All flasks containing the spores were placed in the constant temperature oscillation shaker at 30°C, 180 rpm. A 1 mL sample of bacterial liquid was collected at 0, 6, 18, 26, 38, 47, 53, 68, 72, and 75 h, filtered through a 0.22-μm ultrafiltration membrane, and then loaded into an HPLC bottle. These samples were analyzed by HPLC using the same chromatographic conditions as previously described.
Affects of Phenolic Acids on the Physiological Characteristics of K. sacchari
The Affects of Single and Mixed Phenolic Acids on the Growth of K. sacchari
The LB liquid culture medium was diluted six times, placed into glass tubes, and subjected to 121°C and high pressure sterilization for 20 min. When the culture medium had cooled, an appropriate amount of each phenolic acid stock solution (such as gallic acid, coumaric acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, vanillin, ferulic acid, benzoic acid), which had passed through a 0.22-μm ultrafiltration membrane and K. sacchari liquid (30 μL) that had already been activated were added to each tube. Then, all the tubes, which were maintained at 37°C, were placed on a thermostatic shaker at 200 rpm for 8–10 h. Finally, 200 μL of bacterial liquid was transferred to a 96-well enzyme-labeled plate, and a standard enzyme instrument (Thermo Scientific Multiskan MK3, Shanghai, China) was used to determinate the absorbance values at OD 600 nm.
The LB liquid culture medium was diluted six times and added to an appropriate amount of phenolic acid stock solution P1 previously filtered through a 0.22-μm filtration membrane. The final concentration of each phenolic acid was: 0, 30, 60, 120, 240, 480, and 960 μmol/L. The other conditions were the same as those mentioned above.
Detection Conditions for Phenolic Acids used by K. sacchari
Soil leachate mother liquor (48 mL) was diluted three times and placed into a 250-mL triangle flask. After sterilization and cooling, eight types of phenolic acids (coumaric acid, gallic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, vanillin, ferulic acid, and benzoic acid, 4800 μmol/L; 0.3 mL each) were added to the liquor. These phenolic acids were detected in the rhizosphere soil of R. pseudostellariae. Then, 50 μL of K. sacchari fluid which had been activated, was added to the flask. All the flasks were placed on the oscillation table at a constant 37°C, 180 rpm. From then on, At 0, 3, 6, 9, 12, 15, 26, 29, 32, 35, 38, 41, 48 h, 1 mL samples of bacteria liquid were taken, filtered through 0.22 μm ultrafiltration membrane, and loaded into HPLC bottles. These samples were immediately stored at 4°C or analyzed using HPLC with the same chromatographic conditions as noted earlier.
Detection Conditions for Each Single of Phenolic Acids used by K. sacchari
Every triangle flask contained a single phenolic acid, and the culture time was set at 0, 15, 28, 37, and 47 h. The other conditions were the same as those mentioned above.
Toxin Production and Autotoxicity Bioassay of K. sacchari and B. pumilus
The LB liquid culture medium was diluted four times and added to an appropriate amount of single toxin (e.g., 3A-DON, 15A-DON), which had been filtered through a 0.22-μm filtration membrane. The final concentrations of each toxin were: 0, 0.005, 0.01, 0.02, 0.04, 0.08, 0.16, and 0.32 mg/L. Bacterial liquid (60 μL), previously activated, was added to each tube. All tubes were maintained at 37°C on a thermostatic shaker at 200 rpm for 7 h. Finally, bacterial liquid (200 μL) was placed in a 96-well enzyme-labeled plate, and a standard enzyme instrument was used to determinate the absorbance values at OD 600 nm.
The Effect of the Reaction Intermediate, Protocatechuic Acid, on the Beneficial Microorganism B. pumilus
The concentrations of protocatechuic acid were: 0, 30, 60, 120, 240, 480, and 960 μmol/L. B. pumilus liquid (60 μL), previously activated, was added to each tube. The other conditions were the same as those mentioned above.
Statistical Analysis
Differences among the treatments were calculated and statistically analyzed using the analysis of variance (ANOVA) and the LSD multiple range test (p < 0.05). The Statistical Package for the GraphPad Prism version 5.1 and the Data Processing System (DPS) version 7.05 were used for statistical analysis.
Results
Identification of Microorganisms and Validation of Their Pathogenicity
The DNA sequences were analyzed by means of BLAST tools and the NCBI database. The detected microorganisms were K. sacchari (KU324465) and T. helices (KU355274) which were highly pathogenic to the tissue culture plantlets of R. pseudostellariae (Figures S1, S2). However, B. pumilus Z. was not pathogenic to the tissue culture plantlets (Figure S3) and suppressed the mycelial growth of T. helicus when it was co-cultured with the pathogen (Figure S4).
Dynamics of T. helicus, K. sacchari, and B. pumilus in the Rhizosphere Soil of R. pseudostellariae
The qPCR analysis showed a significant increase in the amount of pathogenic T. helicus and K. sacchari in the rhizosphere of R. pseudostellariae as the number of monoculture years increased, especially around the site of infected R. pseudostellariae (SS). This is consistent with the observation that soil-borne diseases become more severe with an increase in the number of monoculture years. However, B. pumilus showed the opposite trend, which tended to increase in 2-year monoculture soil and then significantly decrease in the 3-year monoculture soil (Figure 1).
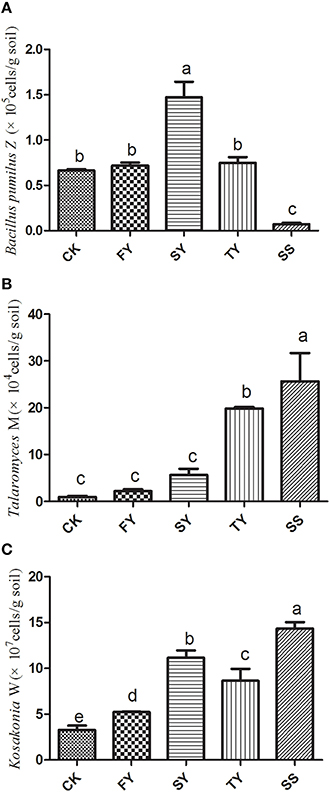
Figure 1. The content of Bacillus pumilus Z (A), Talaromyces M (B), and Kosakonia W (C) in R. pseudostellariae rhizosphere soil after a different number of years of monoculture. FY, SY, TY represent the first, second, and third cropping of R. pseudostellariae, respectively, grown in pots with the soil from the same plot; SS represents the soil around the pathogenic site of R. pseudostellariae. Columns with different letters are statistically different (LSD-test, p < 0.05).
Component Identification and Analysis of Phenolic Acid Content in R. pseudostellariae Rhizosphere Soil and Tissue Culture Medium
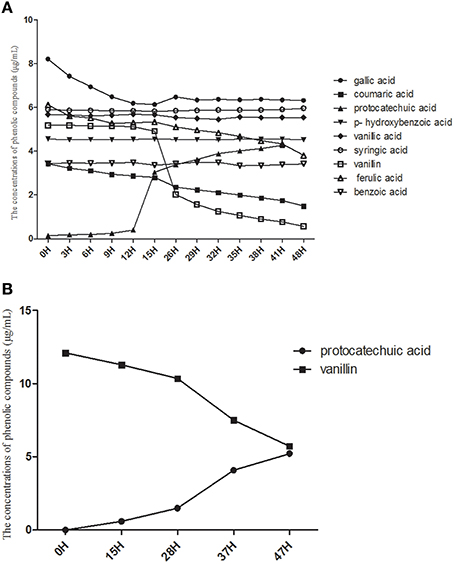
HPLC was used to determine phenolic acids in the rhizosphere soil of R. pseudostellariae at different growth stages in different years of monoculture. We identified nine types of phenolic acids in soil. These were gallic acid, coumaric acid, protocatechuic acid, p-hydroxy benzoic acid, vanillic acid, syringic acid, vanillin, ferulic acid, and benzoic acid as shown in Figure 2, Figure S6, and Table 1. We also found these phenolic acids in the tissue culture medium of R. pseudostellariae (Figure 3).
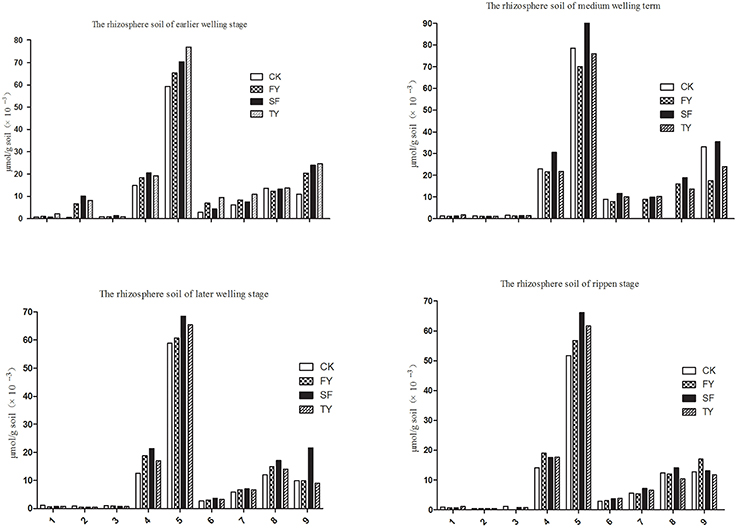
Figure 2. Changes in the levels of phenolic compounds in the rhizosphere soil of R. pseudostellariae in a continuous cropping system sampled at different growth stages. 1, Represents gallic acid; 2, represents coumaric acid; 3, represents protocatechuic acid; 4, represents p-hydroxybenzoic acid; 5, represents vanillic acid; 6, represents syringic acid; 7, represents vanillin; 8, represents ferulic acid; 9, represents benzoic acid; FY, SY, TY: represent the first, second, and third cropping of R. pseudostellariae, respectively, grown in pots with the soil from the same plot.
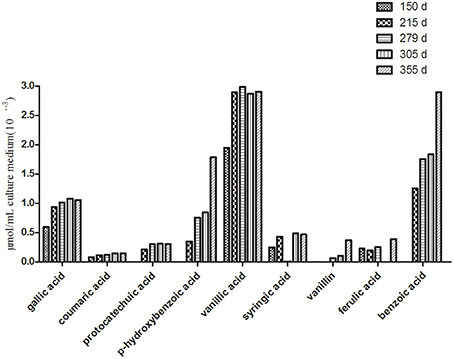
Figure 3. Changes in the contents of the phenolic compounds in the tissue culture medium of R. pseudostellariae on different growth days.
HPLC analysis showed that the phenolic acid levels in the monocultured rhizosphere soil varied with different plant growth stages. For example, most of the phenolic acids fluctuated. They increased initially and then declined (Table 2 and Figure S7). However, phenolic acid levels tended to increase in the culture medium as the growth of the tissue culture plantlets increased. Gallic acid, P-hydroxybenzoic acid vanillic acid, and benzoic acid significantly accumulated in the culture medium (Figure 3). These phenolic acids did not show autotoxicity toward the culture plantlets of R. pseudostellariae (Figure S8). This implies that microorganisms might be involved in the variability of phenolic acid levels in monocultured rhizosphere soil.
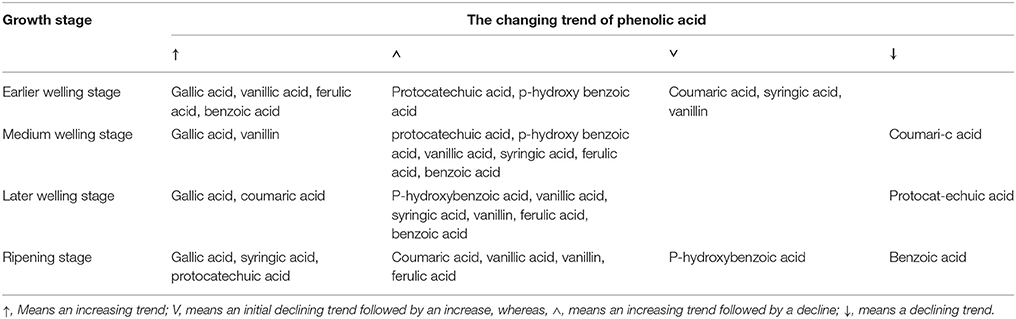
Table 2. The dynamic changes of phenolic acids in the rhizosphere soil of R. pseudostellariae at different growth stages in a continuous cropping system.
The Influence of Phenolic Acids on the Physiological Characteristics of the R. pseudostellariae Biotype T. helices
Mixed phenolic acids significantly promoted the mycelial growth of T. helices. The greatest increase occurred when exposed to the dosage at 160 μmol/L of the mixed phenolic acids (Figure 4). All treatment concentrations of phenolic acids in the mixture had a positive effect on mycelial growth of T. helicus. The various single phenolic acids had different influences on the mycelial growth. Syringic acid had the greatest positive effect on the T. helicus at 40 μmol/L, whereas vanillin and gallic acid showed variable effects, with a growth promotion effect at a low concentration and a suppressive effect at a high concentration. Hydroxybenzoic acid, ferulic acid, protocatechuic acid, coumaric acid, vanillic acid, and benzoic acid had no significant effect on T. helicus at a low concentration, but they had an inhibitory effect at high concentrations (Figure 4). We suggest that not all of the individual phenolic acids can boost the growth of T. helices. Some phenolic acids showed an inhibitory effect. However, the phenolic acids mixed in the same ratio as the result of HPLC analysis for monocultured R. pseudostellariae rhizosphere soil significantly promoted the growth of the pathogenic T. helicus strains.
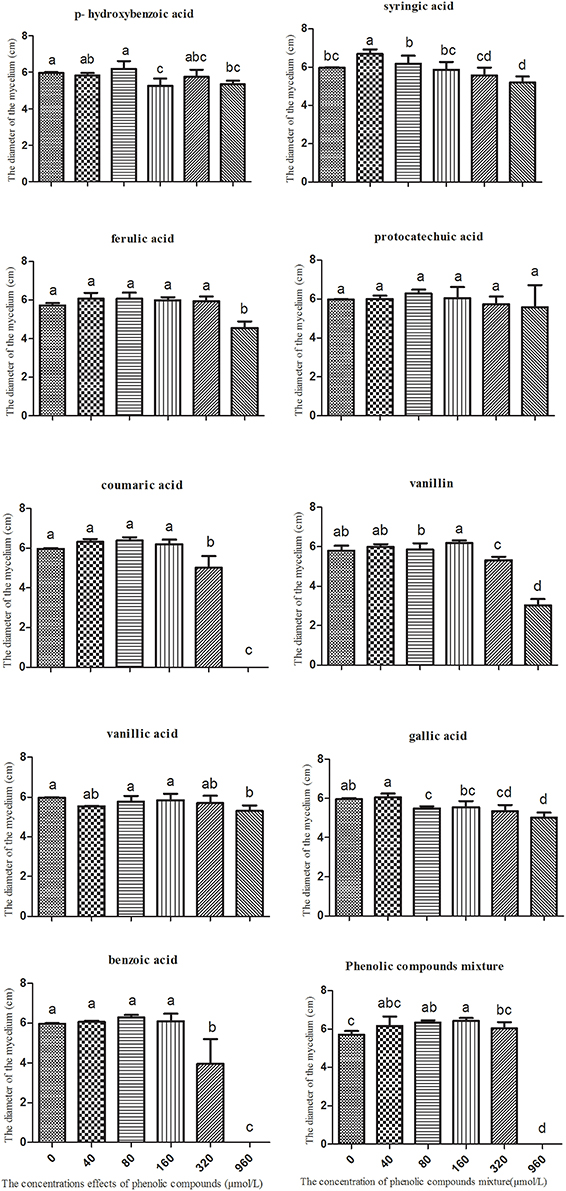
Figure 4. The effects of single phenolic compounds and their mixture on the mycelial growth of Talaromyces helices M. Columns with different letters are statistically different (LSD-test, p < 0.05).
The soil extracts, which were diluted 10 times and used as the culture medium for T. helicus, contained two types of toxins, 3A-DON and 15A-DON. The concentration of 3A-DON was significantly higher than that of 15A-DON (Table 1 and Figure S9). However, the production of the 15A-DON toxin can be promoted by T. helicus when exposed to increasing dosages of the mixed phenolic acids, and the toxin content increased sharply, then reached its highest value at 160 μmol/L. The 15A-DON toxin levels were also greater than those in the control group (0 μmol/L). The production of 3A-DON also increased with an increase in the treatment concentration of the mixed phenolic acids. This suggests that the mixed phenolic acids were favorable for the mycelial growth and toxin production of the pathogenic bacteria, such as T. helicus (Figure 5).
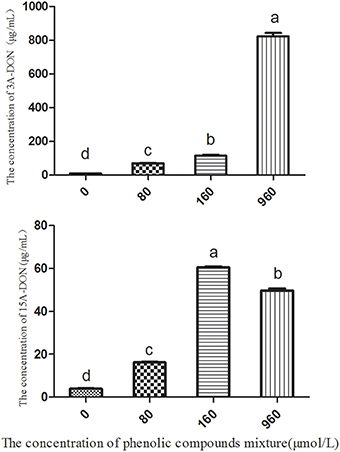
Figure 5. The effects of the phenolic compound mixture on the toxin production of T. helicus. Columns with different letters are statistically different (LSD-test, p < 0.05).
HPLC was used to detect the use of nine phenolic acids by the pathogenic fungus, T. helices, which was detected in the rhizosphere soil of the monocultured R. pseudostellariae. The results showed that T. helicus can use eight types of phenolic acids, i.e., gallic acid, coumaric acid, protocatechuic acid, p-hydroxy benzoic acid, vanillic acid, vanillin, ferulic acid, and benzoic acid (Figure 6). However, syringic acid, which had a stimulatory effect on the pathogenic fungus at a low treatment concentration but an inhibitory effect at a high concentration, could not be used although a stimulatory effect was observed on the mycelial growth of T. helicus at treatment concentrations of more than 40 μmol/L.
The Influence of Phenolic Acids on the Physiological Characteristics of K. sacchari
Based on the molar proportions of the phenolic acids in the soil, we made a series of mixtures with eight types of phenolic acids at concentrations of 0, 30, 60, 120, 240, 480, and 960 μmol/L. These were used to analyze the impact on the growth of K. sacchari. Different concentrations of the mixed phenolic acids had different effects on K. sacchari compared with T. helices. Low concentrations of the phenolic acid mixture had a stimulatory effect on specific pathogens, whereas the reverse was true at high treatment concentrations. Mixed phenolic acids promote the growth of K. sacchari, but only within a specific range of concentrations. Each phenolic acid had distinct effects on the growth of K. sacchari. Syringic acid had the greatest effect on the pathogenic bacterium at a treatment concentration of 60 μmol/L. Gallic acid, p-hydroxybenzoic acid, vanillic acid, and benzoic acid promote growth at low concentrations but suppress it at high concentrations. Coumaric acid, protocatechuic acid, vanillin, and ferulic acid had no significant effect at low concentrations (Figure 7). Thus, the main allelochemicals of R. pseudostellariae root exudates could have distinctly positive or negative effects on the growth of pathogenic fungi and bacteria, implying that root exudates can exert either positive or negative selection on specific microbes.
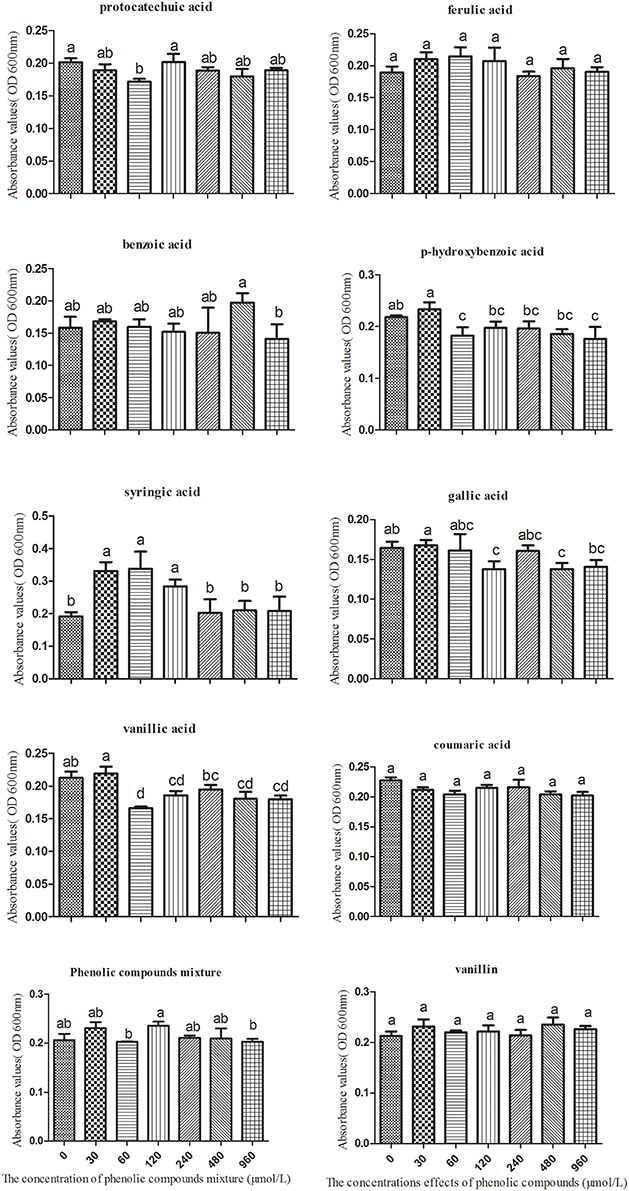
Figure 7. The effects of single phenolic compounds and the phenolic compound mixture on the growth of Kosakonia sacchari. Columns with different letters are statistically different (LSD-test, p < 0.05).
Based on the consumption of eight phenolic acids detected in the soil, we found that K. sacchari could only use four types, i.e., gallic acid, coumaric acid, vanillin, and ferulic acid (Figure 8). The utilization efficiency of vanillin was the highest of the four compounds. K. sacchari is able to produce protocatechuic acid from consumption of vanillin (Figure 8).
The Influence of Toxin Production and Protocatechuic Acid on the Growth of K. sacchari and B. pumilus
The 3A-DON toxin, at a low concentration promoted the growth of K. sacchari and inhibited the growth of B. pumilus. In contrast, the 15A-DON toxin had no significant effect on their growth at a low treatment concentration (Figures S10, S11). Protocatechuic acid, at 60 μmol/L, had a negative effect on the growth of the beneficial B. pumilus Z. (Figure 9).
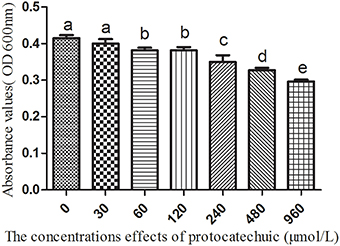
Figure 9. The effects of protocatechuic acid on the growth of Bacillus pumilus Z. Columns with different letters are statistically different (LSD-test, p < 0.05).
Discussion
Previous research related to plant allelopathy or the negative effects of continuous monoculture mainly focused on isolation, identification, and bioassay of allelochemicals, and the evaluation of the direct influence of allelochemicals on the growth of receptor plants (Li et al., 2012). However, the composition and effective concentration of allelochemicals remains controversial. Some believe that the effective concentration of phenolic allelochemicals that can inhibit weeds or stunt crop growth is much higher than that found under field conditions. It is now known that allelochemicals released into the soil can be catabolized, transformed, and processed by microorganisms (Eisenhauer et al., 2012). Allelopathy can operate indirectly between the donor and the recipient plant or between crop residue and new plantings (Kato, 2009; Lin, 2013). Positive or negative effects of plant-plant interactions, such as plant allelopathy, allelopathic autotoxicity, intercropping benefits, or exotic plant invasion, can result from interactions between plants and specific microorganisms, which are often mediated by root exudates (Wang et al., 2013). Plants can transport the carbon fixed by photosynthesis to below ground, where it is released into the soil and provides a source of carbon and energy for microbial growth. Simultaneously, the changes in microbial community structure will affect plant growth both below and above ground (Wardle et al., 2004). Lin et al. (2011) added major rice allelochemicals (p-hydroxy benzoic acid, ferulic acid, salicylic acid, vanillic acid, cinnamic acid) to the soil and found that these phenolic acids decreased by approximately 50–90% from 3 to 7 d. This may be related to microbial decomposition and utilization.
In the present study, we found that many of the phenolic acids produced by tissue culture plantlet roots of R. pseudostellariae accumulated in the culture medium and showed no autotoxicity over the period of plantlet growth (Figure S8). However, these phenolic acids did not increase over the years of monoculture and they did not accumulate in the rhizosphere soil. What is more, the levels of some phenolic acids detected in consecutively monocultured soil were lower than the levels in normal cropping soil, and most of the concerned phenolic acids increased initially and then declined after a several years of continuous cropping. In addition, the content of all the phenolic acids concerned was variable among the different growth stages due to secretions of root exudates, decomposition, synthesis, and transformation of allelochemicals by soil microbes in different monoculture systems. This findings suggest that the consecutive monoculture problems are not due to the direct effects of high phenolic acid concentrations on the receptor plants, but more likely related to indirect effects on plant growth caused by changing microbial flora as shown in Figure 10. Zhou and Wu (2012b) found that after 7 years of continuous cucumber cropping, the plant biomass had declined to the lowest level in the study. However, after 7th year of monocropping, the plant biomass gradually increased. The content of total soil phenolics and some phenolic acids (such as ferulic acid, p-hydroxybenzoic, p-coumaric acid, etc.) showed a similar trend, i.e., the content was lowest in the 7th year, after which it gradually increased. This may be the reason that the most serious disease problems result from the rhizosphere interaction involved in shifts of microbial community differentially mediated by root exudates in monocropping system.
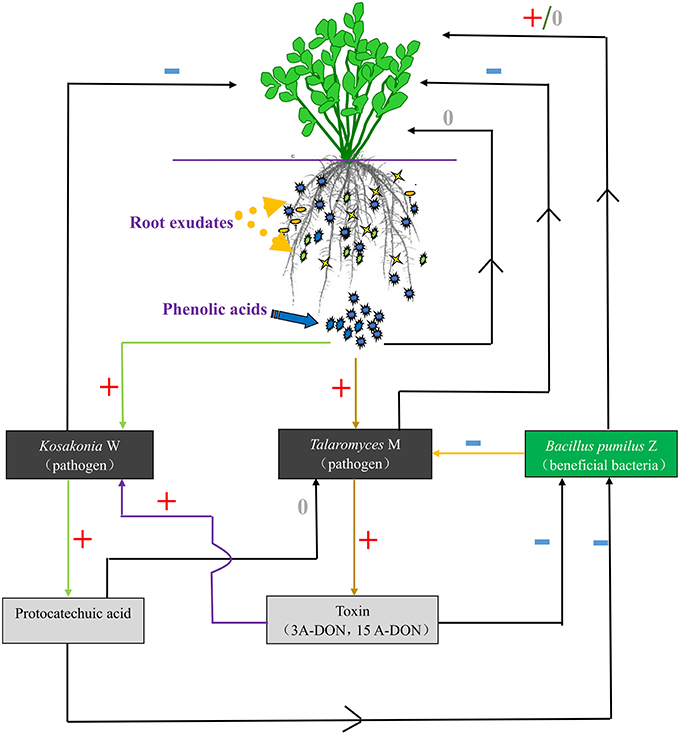
Figure 10. The communication between plants and microorganisms. +, Positive effect; −, negative effect; 0, no effect.
T. helices and K. sacchari used in this study were validated as important soil-borne pathogens. We found different phenolic acids differentially mediate the proliferation of the two targets. T. helices and K. sacchari cannot directly utilize syringic acid as carbon or nitrogen sources. But they may play an important role in promoting the growth of pathogenic microorganisms in the rhizosphere soil of R. pseudostellariae. Many studies (Zhang et al., 2008; Wei et al., 2010) have demonstrated that syringic acid functioning as a signal molecule, promotes cell proliferation and regulates the cell cycle. Some researchers also reported that the K. sacchari was isolated from infected stem, root or rhizosphere soil of sugar cane (Zhu et al., 2013; Gu et al., 2014). We have used the quantitative real-time PCR to detect it in the rhizosphere soil of R. pseudostellariae. The result showed a significant increase in the amount of pathogenic K. sacchari in the rhizosphere of R. pseudostellariae as the number of monoculture years increased, especially around the site of infected R. pseudostellariae (SS). K. sacchari was highly pathogenic to the tissue culture plantlets of R. pseudostellariae (Figure S1). And the pathogen could also affect other beneficial bacteria population such as B. pumilus used in this study via its toxin production and its metabolic intermediate. Our results demonstrated that, K. sacchari was able to utilize root exudate, vanillin to produce an intermediate product, protocatechuic acid (PA), which was considered as a better antibacterial substance in previous studies. We also found that PA did not suppress the growth of the pathogenic K. sacchari, but it did have negative effects on the growth of the beneficial, B. pumilus at a low concentration. B. pumilus is a PGPR, which has been successfully used for the biological control of damping-off diseases (Huang et al., 2012). Tadych et al. (2015) also proposed quinic acids could significantly influence virulent facctors of the fruit rot fungi. In our studies, the 3A-DON toxin production of the pathogenic, T. helices, can be promoted by some phenolic acids, especially by the phenolic acids mixed in the same ratio as detected in monocultured rhizosphere soil, then a cascade reaction was triggered off to promote the growth of the other pathogenic fungus, K. sacchari and inhibited the growth of its counterpart, the beneficial bacterium, B. pumilus, (Figure S4). An increase in populations of soil-borne pathogenic fungi (e.g., Fusarium oxysporum) is likely responsible for soil sickness (Qi et al., 2009), but the present study also confirmed that the two soil-borne pathogenic microorganisms were also responsible for the monoculture problems. Therefore, it is very complicated by the fact that monoculture problems are involved in an intricate rhizosphere interaction. The imbalanced population structure, with the higher population sizes of T. helices and K. sacchari but lower B. pumilus population influenced by the mixed phenolic acid exudates, may partially account for the soil sickness of R. pseudostellariae.
Our results unveil the underlying mechanism of replanting diseases of R. pseudostellariae popularly in modern agriculture system, especially in nowadays China. The case study deeply illustrates about how root exudates play important roles in the formation of monoculture problems involved in rhizosphere interactions under monocropping regimes. As we know, root exudates are carbon sources for soil microbial growth and also signal substances that function as communication tools between plants and microorganisms in the rhizosphere (Mandal et al., 2010). Peters et al. (1986) found that expression of the nodulation gene (nodD) in nodule bacteria is directly related to legume root secretions of flavonoids and isoflavonoids. Venkatachalam et al. (2012) showed that tomatoes whose leaves were infected by pathogens were able to attract more beneficial bacteria such as B. subtilis to the rhizosphere by regulating root secretion. This was accomplished by increasing the synthesis and release of malic acid, leading to induced systemic resistance allowing plants to better defend against further pathogen infestation. Rousk et al. (2010) also found the pH gradients could change the diversity of the bacterial and fungal communities in an arable soil. Root exudates play an important role in the interaction between plants and microbes. They can have different effects on different microbes, which include both stimulatory and inhibitory influences. For example, corn root secretion of DIMBOA (DIMBOA, 2,4-dihydroxy-7-methoxy-2H-1, 4-benzoxazin-3 (4H)-one) and Ocimum basilicum L. secretion of rosmarinic acid resulted in strong antibacterial activity (Bais et al., 2006; Berendsen et al., 2012). Zhou et al. (2012) found that the allelopathic autotoxicity of coumaric acid from cucumber can significantly promote the growth of the soil pathogen F. oxysporum. Others have found that root exudates of maize, peanuts, watermelons, and American ginseng can significantly promote the growth of pathogens, leading to increased soil-borne disease (Ju et al., 2002; Li et al., 2009; Hao et al., 2010). Benizri et al. (2005) showed that the number of beneficial bacteria was reduced and the number of pathogenic bacteria was increased in continuously cropped peach soil. Our results show the same phenomenon, which is attributed to the changes in soil microbial composition and structure differentially mediated by phenolic acids of root exudates.
This findings provide a clue to open a new avenue for modulating the root microbiome to enhance medicinal plant production and sustainability. Such approaches might include the use of microbial fertilizer and organic amendment application to keep the balance of microbial community in rhizosphere soil in modern agriculture system. And the amendment of the root exudates in R. pseudostellariae can also be used to relieve the problems. Additional work is needed to further understand the intrinsic mechanism of these specific microbial functions in the future.
Funding
This work was supported by the National Science Foundation of China (Grant no. U1205021, 81573530, 81303170, 31401950, 31301858), the National Key Basic Research Program of China (Grant no. 2012CB126309), and the Health and Family planning Program of Fujian province (Grant no. WKJ-FJ-34).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank National Science Foundation of China and the National Key Basic Research Program of China for providing the funds used in this work. This work was also supported by Fujian-Taiwan Joint Innovative Center for Germplasm Resources and cultivation of crop [Fujian 2011 Program, (2015), 75].
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00335
References
Bais, H. P., Park, S. W., Weir, T. L., Callaway, R. M., and Vivanco, J. M. (2004). How plants communicate using the underground information superhighway. Trends Plant Sci. 9, 26–32. doi: 10.1016/j.tplants.2003.11.008
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi: 10.1146/annurev.arplant.57.032905.105159
Bauer, A. W., Kirby, W. M., Sherris, J. C., and Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. AM. J. Clin. Pathol. 45, 493–496.
Benizri, E., Piutti, S., Vergerb, S., Page‘s, L., Vercambre, G., Poessel, J. L., et al. (2005). Replant diseases: bacterial community structure and diversity in peach rhizosphere as determined by metabolic and genetic fingerprinting. Soil Biol. Biochem. 37, 1738–1746. doi: 10.1016/j.soilbio.2005.02.009
Berendsen, R. L., Pieterse, C. M., and Bakker, P. A. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486. doi: 10.1016/j.tplants.2012.04.001
Dalton, B. R., Weed, S. B., and Blum, U. (1987). Plant phenolic acids in soils:a comparison of extraction procedures. Soil Sci. Soc. Am. J. 51, 1515–1521. doi: 10.2136/sssaj1987.03615995005100060020x
Eisenhauer, N., Scheu, S., and Jousset, A. (2012). Bacterial diversity stabilizes community productivity. PLoS ONE 7:e34517. doi: 10.1371/journal.pone.0034517
Fang, C. X., He, H. B., and Lin, W. X. (2010). Genomic analysis of allelopatic response to low nitrogen and barnyardgrass competition in rice. Plant Growth Regul. 61, 277–286. doi: 10.1007/s10725-010-9475-8
Fang, C., Zhuang, Y., Xu, T., Li, Y., Li, Y., and Lin, W. (2013). Changes in rice allelopathy and rhizosphere microflora by inhibiting rice phenylalanine ammonia-lyase gene expression. J. Chem. Ecol. 39, 2014–2212. doi: 10.1007/s10886-013-0249-4
Fang, S. Q., Wu, F. A., and Chen, M. S. (2011). Isolation and primary identification of the pathogen causing root rot disease of adult mulberry trees (in Chinese). Sci. Sericulture 37, 785–791.
Ferrer, C., Colom, F., Frasés, S., Mulet, E., Abad, J. L., and Alió, J. L. (2001). Detection and Identification of Fungal Pathogens by PCR and by ITS2 and 5.8S Ribosomal DNA Typing in Ocular Infections. J. Clin. Microbiol. 39, 2873–2879. doi: 10.1128/JCM.39.8.2873-2879.2001
Gu, C. T., Li, C. Y., and Yang, L. J. (2014). Enterobacter xiangfangensis sp. nov., isolated from Chinese traditional sourdough, and reclassification of Enterobacter sacchari Zhu et al. 2013 as Kosakonia sacchari comb. nov. Int. J. Syst. Evol. Microbiol. 64, 2650–2656. doi: 10.1099/ijs.0.064709-0
Hang, D. S., and Wang, S. G. (2010). Study on genuineness cultivation environment of Pseudostellaria Fujian (in Chinese). Chin. Wild Plant Resour. 29, 12–14.
Hao, W. Y., Ren, L. X., Ran, W., and Shen, Q. S. (2010). Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporun. Plant Soil 336, 485–497. doi: 10.1007/s11104-010-0505-0
Huang, X., Zhang, N., Yong, X., Yang, X., and Shen, Q. (2012). Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol. Res. 167, 135–143. doi: 10.1016/j.micres.2011.06.002
Ju, H. Y., Han, L. M., Wang, S. Q., and Cong, D. L. (2002). Allelopathic effect of root exudates on pathogenic fungi of root rot in continuous cropping soybean (in Chinese). Chin. J. Appl. Ecol. 13, 723–727. doi: 10.13287/j.1001-9332.2002.0171
Kato, N. H. (2009). Stress-induced allelopathic activity and momilactone B in rice. Plant Growth Regul. 59, 153–158. doi: 10.1007/s10725-009-9398-4
Kato-Noguchi, H., and Ino, T. (2003). Rice seedlings release momilactone B into the environment. Phytochemistry 63, 551–554. doi: 10.1016/S0031-9422(03)00194-8
Kato-Noguchi, N. H., Ino, T., Sata, N., and Yamamura, S. (2002). Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol. Plant. 115, 401–405. doi: 10.1034/j.1399-3054.2002.1150310.x
Kong, C. H., Wang, P., Zhao, H., Xu, X. H., and Zhu, Y. D. (2008). Impact of allelochemical exuded from allelopathic rice on soil microbial community. Soil Biol. Biochem. 40, 1862–1869. doi: 10.1016/j.soilbio.2008.03.009
Kulmatiski, A., Beard, K. H., Stevens, J. R., and Cobbold, S. M. (2008). Plant-soil feedbacks: a meta-analytical review. Ecol. Lett. 11, 980–992. doi: 10.1111/j.1461-0248.2008.01209.x
Li, X. G., Liu, B., Heia, S., Liu, D. D., Han, Z. M., Zhou, K. X., et al. (2009). The effect of root exudates from two transgenic insect-resistant cotton lines on the growth of Fusarium oxysporun. Transgenic Res. 18, 757–767. doi: 10.1007/s11248-009-9264-1
Li, Z. F., Yang, Y. Q., and Lin, W. X. (2012). Identification of Autotoxic Compounds in Fibrous Roots of Rehmannia (Rehmannia glutinosa Libosch.). PLoS ONE 7:e28806. doi: 10.1371/journal.pone.0028806.s001
Lin, M. Z., Huang, S. H., Chen, Q. Q., and Lin, W. X. (2012a). Studies on continuous cropping obstacle of Pseudostelariae heterophylla and the Change of Fusarium oxysporum numbers in its rhizosphere (in Chinese). J. Yunnan Agric. Univ. 27, 716–721.
Lin, M. Z., Wang, H. B., and Lin, H. F. (2012b). Effects of Pseudostellariae heterophylla continuous cropping on rhizosphere soil microorganisms (in Chinese). Chin. J. Ecol. 31, 106–111. doi: 10.13292/j.1000-4890.2012.0010
Lin, R. Y., Wang, H. B., Guo, X. K., and Lin, W. X. (2011). Impact of applied phenolic acids on the microbes, enzymes and available nutrients in paddy soils. Allelopathy J. 28, 225–236.
Lin, W. X. (2013). Rhizobiological properties of allelopathic rice in suppression of weeds and its research prospect. Acta Agron. Sin. 39, 951–960. doi: 10.3724/SP.J.1006.2013.00951
Lin, W. X., Fang, C. X., and Chen, T. (2010). Rice allelopathy and its properties of molecular ecology. Front. Biol. 5:4. doi: 10.1007/s11515-010-0035-4
Mandal, S. M., Chakraborty, D., and Dey, S. (2010). Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 5, 359–368. doi: 10.4161/psb.5.4.10871
Peters, N. K., Frost, J. W., and Long, S. R. (1986). A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233, 977–980.
Pollock, J. L., Kogan, L. A., Thorpe, A. S., and Holben, W. E. (2011). (±)-Catechin, a root exudate of the invasive Centaurea Stoebe Lam. (Spotted Knapweed) exhibits bacteriostatic activity against multiple soil bacterial populations. J. Chem. Ecol. 37, 1044–1053. doi: 10.1007/s10886-011-0005-6
Qi, J. J., Yao, H. Y., and Ma, X. J. (2009). Soil microbial community composition and diversity in the rhizosphere of a Chinese medicinal plant. Commun. Soil Sci. Plan 40, 1462–1482. doi: 10.1080/00103620902818104
Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G., et al. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351. doi: 10.1038/ismej.2010.58
Singh, H. P., Batish, D. R., and Kohli, R. K. (1999). Autotoxicity: concept, organisms and ecological significance. Crit. Rev. Plant Sci. 18, 757–772. doi: 10.1080/07352689991309478
Tadych, M., Vorsa, N., Wang, Y., Bergen, M. S., Johnson-Cicalese, J., Polashock, J. J., et al. (2015). Interactions between cranberries and fungi: the proposed function of organic acids in virulence suppression of fruit rot fungi. Front. Microbiol. 6:835. doi: 10.3389/fmicb.2015.00835
Venkatachalam, L., Sherry, L. K., Jeffrey, L. C., Yi-Huang, H., Daniel, B. K., Wu, Y. S., et al. (2012). Microbe-associated molecular patterns-triggered root response mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 160, 1642–1661. doi: 10.1104/pp.112.200386
Wang, J. H., Chen, T., and Lin, W. X. (2013). Plant allelopathy types and their application in agriculture (in Chinese). Chin. J. Eco Agric. 21, 1173–1183. doi: 10.3724/SP.J.1011.2013.01173
Wardle, D. A., Bardgett, R. D., Klironomos, J. N., Setälä, H., Putten, W. H., and Wall, D. H. (2004). Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633. doi: 10.1126/science.1094875
Wei, X. Y., Ma, W. F., and Fang, H. (2010). The effect of syringic acid extracted from dendrobium on human lens epithelial cells in oxidation condition (in Chinese). Glob. Tradit. Chin. Med. 3, 187–189, 197.
Wu, F. Z., Wang, X. Z., and Xue, C. Y. (2009). Effect of cinnamic acid on soil microbial characteristics in the cucumber rhizosphere. Eur. J. Soil Biol. 45, 356–362. doi: 10.1016/j.ejsobi.2009.04.001
Wu, L. K., Wang, J. Y., Huang, W. M., Wu, H. M., Chen, J., Yang, Y. Q., et al. (2015). Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci. REP UK 5:15871. doi: 10.1038/srep15871
Zeng, Y. X., and Zheng, T. L. (2011). Relationships between two Pseudoalteromonas strains isolated from the Canada Basin and the Southern Ocean using a polyphasic approach. Adv. Polar Sci. 22, 25–34. doi: 10.3724/SP.J.1085.2011.00025
Zhang, X., Xu, J. K., and Wang, N. L. (2008). Studies on antioxidant activity of bibenzyls and phenolic components from dendrobium nobile (in Chinese). Chin. Pharm. J. 11, 829–832.
Zhang, Z. L., and Wang, W. Q. (2010). Progress on formation mechanism and control measurements of continuous cropping obstacles in plants. J. Biol. 27, 69–72.
Zhang, Z. Y., and Lin, W. X. (2009). Continuous cropping obstacle and allelopathic autotoxicity of medicinal plants (in Chinese). Chin. J. Eco Agric. 17, 189–196.
Zhao, Y., Wu, L., Chu, L., Yang, Y., Li, Z., Azeem, S., et al. (2015). Interaction of Pseudostellaria heterophylla with Fusarium oxysporum f. sp. heterophylla mediated by its root exudates in a consecutive monoculture system. Sci. REP UK 5:8197. doi: 10.1038/srep08197
Zhou, X. G., Gao, D. M., Liu, J., and Wu, F. Z. (2014). Changes in rhizosphere soil microbial communities in a continuously monocropped cucumber (Cucumis sativus L.) system. Eur. J. Soil Biol. 60, 1–8. doi: 10.1016/j.ejsobi.2013.10.005
Zhou, X., and Wu, F. (2012a). p-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of Fusarium oxysporum f.sp. Cucumerinum Owen. PLoS ONE 7:e48288. doi: 10.1371/journal.pone.0048288
Zhou, X., and Wu, F. (2012b). Soil phenolics in a continuously mono-cropped cucumber (Cucumis sativus L.) system and their effects on cucumber seedling growth and soil microbial communities. Eur. J. Soil Biol. 63, 332–340. doi: 10.1111/j.1365-2389.2012.01442.x
Zhou, X., Yu, G., and Wu, F. (2012). Responses of soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.) to exogenously applied p-Hydroxybenzoic acid. J. Chem. Ecol. 38, 975–983. doi: 10.1007/s10886-012-0156-0
Keywords: Radix pseudostellariae, monoculture cropping problem, phenolic acids, Talaromyces helicus, Kosakonia sacchari, Bacillus pumilus
Citation: Wu H, Wu L, Wang J, Zhu Q, Lin S, Xu J, Zheng C, Chen J, Qin X, Fang C, Zhang Z, Azeem S and Lin W (2016) Mixed Phenolic Acids Mediated Proliferation of Pathogens Talaromyces helicus and Kosakonia sacchari in Continuously Monocultured Radix pseudostellariae Rhizosphere Soil. Front. Microbiol. 7:335. doi: 10.3389/fmicb.2016.00335
Received: 20 June 2015; Accepted: 03 March 2016;
Published: 17 March 2016.
Edited by:
Kumar Krishnamurthy, Tamil Nadu Agricultural University, IndiaReviewed by:
Oswaldo Valdes-Lopez, National Autonomus University of Mexico, MexicoPratyoosh Shukla, Maharshi Dayanand University, India
Copyright © 2016 Wu, Wu, Wang, Zhu, Lin, Xu, Zheng, Chen, Qin, Fang, Zhang, Azeem and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiong Lin, d2VueGlvbmcxODFAMTYzLmNvbQ==
 Hongmiao Wu
Hongmiao Wu Linkun Wu
Linkun Wu Juanying Wang
Juanying Wang Quan Zhu
Quan Zhu Sheng Lin
Sheng Lin Jiahui Xu
Jiahui Xu Cailiang Zheng
Cailiang Zheng Jun Chen
Jun Chen Xianjin Qin
Xianjin Qin Changxun Fang
Changxun Fang Zhixing Zhang
Zhixing Zhang Saadia Azeem
Saadia Azeem Wenxiong Lin
Wenxiong Lin