
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 03 February 2016
Sec. Microbiological Chemistry and Geomicrobiology
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.00060
With knowledge on microbial composition and diversity, investigation of within-community interactions is a further step to elucidate microbial ecological functions, such as the biodegradation of hazardous contaminants. In this work, microbial functional molecular ecological networks were studied in both contaminated and uncontaminated soils to determine the possible influences of oil contamination on microbial interactions and potential functions. Soil samples were obtained from an oil-exploring site located in South China, and the microbial functional genes were analyzed with GeoChip, a high-throughput functional microarray. By building random networks based on null model, we demonstrated that overall network structures and properties were significantly different between contaminated and uncontaminated soils (P < 0.001). Network connectivity, module numbers, and modularity were all reduced with contamination. Moreover, the topological roles of the genes (module hub and connectors) were altered with oil contamination. Subnetworks of genes involved in alkane and polycyclic aromatic hydrocarbon degradation were also constructed. Negative co-occurrence patterns prevailed among functional genes, thereby indicating probable competition relationships. The potential “keystone” genes, defined as either “hubs” or genes with highest connectivities in the network, were further identified. The network constructed in this study predicted the potential effects of anthropogenic contamination on microbial community co-occurrence interactions.
Increased exploration and exploitation of oil resources have resulted in severe contamination worldwide (Kvenvolden and Cooper, 2003). Hundreds of micrograms of oil per gram of soil were detected in several heavily contaminated sites (Liang et al., 2012). Oil contamination alters the biotic taxonomic composition and physical and chemical properties of an environment, thus posing considerable threat to the ecological systems (Kingston, 2002). Oil contamination considerably affects the structures and functional diversity of microbial communities, including bacteria, fungi, and archaea (Liang et al., 2011; Lu et al., 2012; Bell et al., 2014). The overall microbial population diversity in oil-contaminated sites usually declines (Van Hamme et al., 2003), particularly, of those microbes participating in carbon and nitrogen cycling. The loss of microbial diversity and changes in community composition alter their functional processes. Although soil respiration can be stimulated as an enrichment of the biodegradable carbon source after new oil spills, an inhibitory effect on the hydrolase activities involved in nitrogen, phosphorus, or carbon cycles is observed (Labud et al., 2007). Although many microbial species can degrade oil contaminants (Atlas, 1981; Das and Chandran, 2011), effective oil removal is critically dependent on community-level functions. For example, the biodegradation of refractory components of oil contaminants, such as high molecular-weight polycyclic aromatic hydrocarbons (PAHs), requires the co-metabolism of several species (Nzila, 2013).
The microbial functional genes involved in the degradation of specific compounds in hydrocarbon-contaminated environments can be used as indicators of the biodegradation potential of the corresponding compounds and their bioavailability and transport in the environment (Stapleton and Sayler, 2000; Marlowe et al., 2002; Mesarch et al., 2004; Tuomi et al., 2004). The abundance of contaminant-degrading genes highly correlates with the concentrations of contaminant, as well as the efficiency at which the hydrocarbons are mineralized (Fleming et al., 1993; Park and Crowley, 2006; Salminen et al., 2008). In some PAH-contaminated soils and sediments, the abundance or expression of naphthalene-degrading genes is correlated with naphthalene concentrations (Dionisi et al., 2004; Cébron et al., 2008). Functional genes are also useful in monitoring the dynamics of contaminant-degrading bacteria in microcosms (Ringelberg et al., 2001; Sei et al., 2003) and evaluating hydrocarbon biodegradability (Cavalca et al., 2004; Baldwin et al., 2008).
The rapid methodological development of high-throughput metagenomic sequencing (Simon and Daniel, 2011) and microarray techniques (He et al., 2012) has dramatically advanced our understanding of the diverse and complex microbial functional communities in recent years. The sequencing technology uses Roche 454 or Illumina platforms to capture sequences for both targeted genes with available primers and metagenomics. As such, the approach provides novel insights into the phylogenetic and functional diversity as well as structure and composition of microbial communities. Shotgun metagenomic and metatranscriptomic sequencing revealed that genes for aliphatic hydrocarbon degradation are significantly enriched and expressed in hydrocarbon plume samples compared with uncontaminated seawater (Mason et al., 2012). Penton et al. (2013) evaluated new primers that target the dioxin- and dibenzofuran- degrading genes dxnA1, dbfA1, and carAa, found that the majority of the obtained environmental sequences were classified into novel sequence clusters in polychlorinated biphenyl-contaminated rhizosphere soil. However, in gene-targeted metagenomics analysis, each unique gene requires individual considerations in terms of primer design and sequence processing and classification. Microarray techniques, such as GeoChip, provide another approach for profiling the functional composition of known microbial populations by targeting hundreds to thousands of different gene families that play important roles in various biogeochemical processes at a time (He et al., 2010; Tu et al., 2014). In comparison with genome-resolved-metagenomic shotgun sequencing, GeoChip is required for primer matches that previously unsequenced organisms with divergent gene sequences will not be detected, and connection of organism with function is less clear. Nevertheless, GeoChip avoids the oversampling of dominant populations and is less challenging to employ in sequence assembly and data analysis than other approaches (Zhou et al., 2015). GeoChip 3.0 contains approximately 28000 50-mer oligonucleotide probes specific to the target genes, covering 292 functional gene families involved in carbon, nitrogen, phosphorus, and sulfur cycles, as well as energy metabolism, antibiotic resistance, metal resistance, and organic contaminant degradation (He et al., 2010). For organic contaminant degradation, a gene from each step of a contaminant degradation pathway is selected for probe design to monitor various degradation pathways. A total of 173 genes/enzymes are selected to detect the potential for degradation of 86 organic contaminants commonly found in the environment. These genes/enzymes mainly include 38 enzymes involved in the aromatic carboxylic acid (for example, benzoate, phenylpropionate, and phthalate) degradation, 18 for BTEX (benzene, toluene, ethylbenzene, and xylene), 10 for chlorinated aromatics (for example, 2-, 3-, and 4-chlorobenzoate, 2,4,5-trichlorophenoxyacetic acid), nine for heterocyclic aromatics (for example, carbazole and dibenzothiophene), nine for nitroaromatics (for example, nitrobenzene and nitrophenol), 18 for polycyclic aromatics (for example, biphenyl, fluorene, and naphthalene), 22 for other aromatics (for example, aniline, catechol, phenol), 15 for other hydrocarbons (for example, alkanes, cyclohexane, and tetrahydrofuran; He et al., 2010). The organic contaminant degradation gene probes on GeoChip are usually derived from the genes with known biological functions and microbial populations. Therefore, linking microbial diversity to ecosystem processes and functions is easily achieved in oil-contaminated sites through GeoChip. Several studies indicated that oil contamination significantly changes the composition and diversity of microbial functional genes by using GeoChip (Yergeau et al., 2007; Hazen et al., 2010; Liang et al., 2011; Lu et al., 2012). In our recent study on microbial functional gene diversity across five oil-contaminated sites, long-term oil contamination significantly decreases microbial alpha- (gene number, richness and the Shannon index) and beta-diversity (distance decay relationship; Liang et al., 2015). Moreover, the diversity changes along with the increase in deterministic assembly processes in soil microbes. However, little could be inferred quantitatively on the interactions among different microbial species/populations, which is of critical importance in maintaining the stability and functions of the community.
Microbial communities are highly complex, diverse, evolving systems. Therefore, the behavior and characteristics of these communities are difficult to predict compared with macro-ecological systems. Beyond basic survey on microbial community composition and diversity, investigating large environmental datasets to determine potential interactions between microbial species and functions remains a challenge (Raes et al., 2007). Barberan et al. (2012) assumed that microbial relationships can be depicted under the following principle. When two species (or any operational taxonomic units) co-occur or show similar abundance patterns over multiple samples, a positive relationship is assumed (Faust and Raes, 2012). Conversely, a negative relationship is considered when two species show mutual exclusion or anticorrelation (Faust and Raes, 2012). Given the criteria, a novel random matrix theory (RMT)-based network approach has been recently developed to delineate and characterize functional molecular ecological networks (fMENs) that involve microbial functional genes (Zhou et al., 2010). This approach provides a robust method to decipher the potential interactions of complex microbial communities. A general framework for fMENs is as follows (Zhou et al., 2010): (i) functional gene abundance data are collected on array hybridization; (ii) a pairwise Pearson correlation between any two genes is estimated on the basis of gene abundance data, and the absolute values of pairwise correlation coefficients are used to measure similarities; (iii) the similarity matrix is then transformed into an adjacency matrix by applying a threshold to the correlation values based on an RMT approach, which measures the strengths of connections between nodes; (iv) the strengths of the connections of each gene with all of the other connected genes are summed, yielding a connectivity of a single network. The connectivity represents the strength of the connection of a gene to all of the other genes in the network. In this network, a node represents a functional gene. Meanwhile, the edge linking two nodes represents the relationship between these two genes or potential functions (positive or negative). The edge weights represent the relationship strength, whereas the node size represents the abundance of genes or the node properties. The ecological networks determined via this method should reflect the co-occurrence among different microbial populations carrying the functional genes of interest rather than the individual “species” in a microbial community. Currently, little is known about whether and how oil contamination changes interactions among different microbial functional groups. This alternation may be affected by the introduction of contamination because increasing disturbance promotes interspecies competition (Simon and Daniel, 2011).
In a previous study, we found that an significant increase (68%) in the deterministic selection processes that shape the community composition and structure in contaminated soils with respect to that in uncontaminated soils in the Baise site (an oil-exploring site located in South China), which is highest than those in all the other sites (Liang et al., 2015), indicating that oil contamination may alter microbial assembly networks most in the Baise site. In this paper, we have re-analyzed the GeoChip data from the Baise site soils presented in Liang et al. (2015) using the fMEN approach in order to examine the interaction between specific groups of functional genes involved in carbon, nitrogen, phosphorus cycling, metal resistance, organic contaminant degradation. The following research questions were addressed: (i) whether oil contamination affects the functional network structure of soil microbial functional genes, (ii) what the potential “keystone genes” in the network are and how they change in response to contamination, and (iii) what the relationships are among genes functioning in the degradation of different oil components.
Twenty soil samples were collected from Baise district (BS; 23°43′N, 107°04′E) in South China in May 2008 for network analyses of microbial functional genes. BS has a subtropical humid monsoon climate, with a mean annual rainfall of 1115 mm. Of these samples, 10 contaminated were collected from an oil-contaminated site in Baise Oilfield. Contaminated soils were collected adjacent to the crude oil pumping wells within a 2 km2 area where contamination occurred during oil extraction in 2004 according to record. The other 10 uncontaminated samples were taken simultaneously from undisturbed pristine soils about 5 km away from the contaminated site. At each sampling point, five soil cores (2.5 cm in diameter) within 0.015 m2 of the upper 10 cm soil were obtained and mixed thoroughly. The 20 soil samples were then individually used for microbial and chemical analyze. The collected soils were sealed in sterile sampling bags without air and transported to the laboratory on ice.
The oil contamination in BS oil field ranged from 12.1 mg/g to 168 mg/g. The contents of the four components of oil, namely, aliphatic hydrocarbons, aromatic hydrocarbons, polar fraction with heteroatoms of nitrogen, sulfur, and oxygen (NSO fraction), and asphaltenes, ranged from 28.7% to 53.6%, 8.9% to 25.7%, 10.0% to 23.9%, and 0.9% to 9.6%, respectively (Liang et al., 2012). The soil physical and chemical parameters in the contaminated soils were as follows: pH, 5.8 ± 0.52; water content, 17.3% ± 6.9%; total nitrogen (nitrogen in all organic and inorganic forms), 1176 ± 244 mg/kg; available nitrogen (NO3-, NO2-, and NH4+), 51.9 ± 30.3 mg/kg; total phosphorus (phosphorus in all organic and inorganic forms), 884 ± 403 mg/kg; available phosphorus (PO43-), 22.9 ± 12.6 mg/kg; total organic carbon, 5.7% ± 7.4%; and soluble salts, 0.17% ± 0.07%. In comparison, the physical and chemical parameters in the uncontaminated soils were as follows: pH, 6.2 ± 0.59; water content, 20.1% ± 4.9%; total nitrogen, 952 ± 275 mg/kg; available nitrogen, 43.5 ± 25.1 mg/kg; total phosphorus, 854 ± 149 mg/kg; available phosphorus, 10.2 ± 13.1 mg/kg; total organic carbon, 3.4% ± 3.8%; and soluble salts, 0.06% ± 0.05% (Liang et al., 2012).
Biolog EcoPlateTM (Biolog, Inc., Hayward, CA, USA), which contains three replicated wells of 31 carbon substrates, was used to investigate the carbon metabolic activity among the aerobic and heterotrophic bacterial communities in all the soil samples. Plates were incubated at room temperature (20°C). The optical density (λ = 590 nm) of each well was determined immediately (0 h) and every 24 h thereafter up to 180 h with a BioTek plate reader (ELX800; BioTek Inc., Winooski, VT, USA). Average well color development method (Garland and Mills, 1991) was used for Biolog data analysis.
Microbial genomic DNA was extracted from 20 soil samples individually. Particularly, 5 g of well-mixed soil from each sample was subjected to DNA extraction and purification (Zhou et al., 1996; Moore and Dowhan, 2002). An aliquot of 2 μg of DNA from each sample was directly labeled and hybridized with GeoChip 3.0 in triplicates as described previously (Liang et al., 2010, 2015). Microarray data processing was performed in the Microarray Data Manager system of the Institute for Environmental Genomics (IEG)1
To elucidate the effect of oil contamination on the overall microbial ecological network, we constructed two fMENs for the contaminated and uncontaminated soils, respectively. The GeoChip hybridization intensity data were log-transformed prior to the construction of a Pearson correlation matrix (Horvath and Dong, 2008) and converted to a similarity matrix. This similarity matrix measures the degree of concordance between the abundance profiles of genes across various samples by obtaining the absolute values of the correlation matrix (Horvath et al., 2006). Using RMT, ecological communities can be predicted by two universal extreme distributions of the nearest-neighbor spacing distribution of eigenvalues. These two distributions are the Gaussian orthogonal ensemble (GOE) statistics, which reflects the random properties of a complex system, and the Poisson distribution, which is related to the system-specific, non-random properties of a complex system (Luo et al., 2007). A threshold st can be defined as the transition of the nearest-neighbor spacing distribution of eigenvalues from GOE to Poisson distribution (Zhou et al., 2010). Subsequently, an adjacency matrix, which encodes the connection strength between each pair of nodes, was derived from the similarity matrix by applying the threshold (Luo et al., 2006, 2007). In this study, clear transitions of the nearest-neighbor spacing distribution of eigenvalues from GOE to Poisson distribution were observed for soil microbial communities with and without contamination. These transitions are indicated by the existence of a similarity threshold (Table 1). The topological indices of the network, such as the average degree (connectivity), average path length, and average clustering coefficient, were calculated to describe the properties of the two networks. The definitions and calculations of these indices were described previously (Zhou et al., 2010). Hartwell et al. (1999) defined a module in a biological system as “a discrete unit whose function is separable from those of other modules.” They suggested that the functional modules comprise a “critical level of biological organization.” In fMENs, a module is defined as a group of functional genes that are highly connected among themselves but exhibit few connections with the functional genes under other modules (Zhou et al., 2010). Modularity (M) measures the extent to which nodes attain more links within their own modules than expected if the linkage was random. The modularity was calculated as follows:
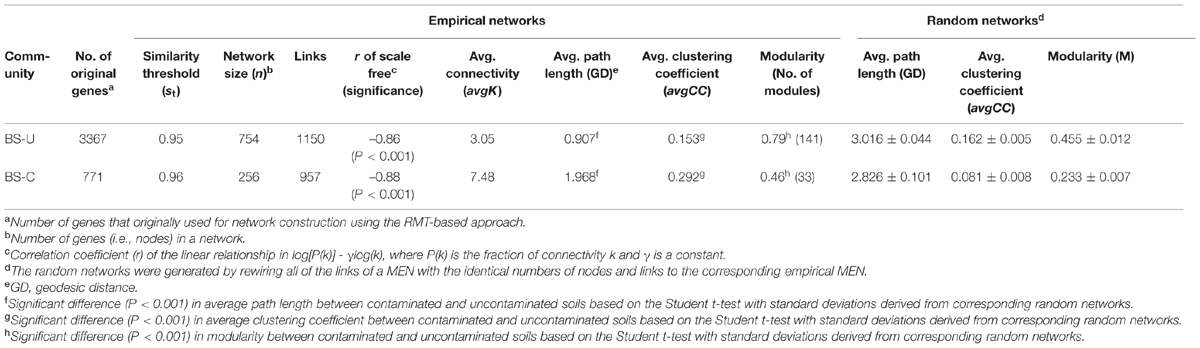
TABLE 1. Topological properties of the empirical molecular ecological networks (MENs) of microbial communities in uncontaminated (U) and contaminated (C) soils of the oil-contaminated site and their associated random MENs.
where NM is the number of modules, L is the number of links in the network, ls is the number of links between nodes in module s, and ds is the sum of the degrees of nodes in module s. The module identification algorithm aims to determine the partition with largest modularity (Clauset et al., 2004). After scanning all branches of the hierarchical tree of a graph, the level with the maximum modularity score was used to separate the graph into multiple dense subgraphs (Clauset et al., 2004).
Each node can be assigned a role based on its topological properties. The role of node i is characterized by two parameters. The first parameter is within module connectivity (Zi), which describes how well a node is connected to the other nodes within its own module (Guimera and Amaral, 2005). Zi is described as follows:
where κi is the number of links of node i to the other nodes within its module si, is the average of k over all the nodes in si, and σκs i is the standard deviation of κ in si.
The second parameter is the connectivity among modules (Pi), which reflects how well a node connects to various modules (Guimera and Amaral, 2005). Pi is given as follows:
where κis is the number of links of node i to nodes in module s, and ki is the total degree of node i.
Only one network was constructed by combining 10 samples under each condition; hence, we cannot statistically compare the network indices between contaminated samples and the control. Thus, random networks were generated to assess the statistical significance of network indices by using the Maslov and Sneppen (2002) procedure based on a null model. This model keeps the numbers of nodes and links unchanged but rewires all of the links’ positions in the “null model” network (Maslov and Sneppen, 2002). Consequently, the network sizes are the same, and the random rewired networks are comparable to the original ones. For each network identified in this study, 100 randomly rewired networks were generated, and all of the network indices were calculated individually. The average and standard deviation for each index of all of the random networks were obtained (Zhou et al., 2010). For comparison of the network indices under different conditions, the Student t-test was employed using the standard deviations derived from corresponding random networks.
All of the above calculations were carried out in the IEG website2 The present study is focused on the interactions of oil contaminant degradation-related functional genes. Thus, the fMENs were also constructed and visualized using the Cytoscape 2.6.0 [26] software for two functional categories, particularly, alkaline and PAH degradations.
Gene significance was calculated on the basis of a sample geochemical trait (Zhou et al., 2010) across 10 replicate samples under oil contamination and non-contamination. Given that the measurement units for different soil variables vary, all of the trait data were standardized before statistical analysis. Correlations between the gene significance and the connectivity of individual genes were calculated. The statistical significances of these correlations were estimated on the basis of P-values.
fMENs were constructed for both contaminated and uncontaminated soils to determine the effect of oil contamination on microbial functional gene co-occurrence (Table 1). The network sizes, links, connectivities, and module numbers were calculated for microbial functional genes in the contaminated and uncontaminated soils. Random networks were generated to test the statistical significance of the network indices. Results indicated that the network indices, such as average path length, average clustering coefficient, and modularity, were significantly different between the contaminated and uncontaminated soils (P < 0.001). The overall network structures of the soil microbial communities were distinctly different under oil contamination.
In a network developed from gene abundance data to represent the ecological co-occurrence (links) of different gene markers (nodes) in a microbial community, different nodes play distinct roles (Guimera et al., 2007; Fuhrman and Steele, 2008). Within-module connectivities (Zi) and among-module connectivities (Pi) of both contaminated and uncontaminated soils were calculated and visualized in Figure 1 to understand the effect of oil contamination on the topological roles of individual nodes. In this study, we followed the simplified classification as follows: (i) peripheral nodes (Zi ≤ 2.5, Pi ≤ 0.62), which possess only a few links that are almost always to nodes within their modules; (ii) connectors (Zi ≤ 2.5, Pi > 0.62), which are highly linked to several modules; (iii) module hubs (Zi > 2.5, Pi ≤ 0.62), which are highly connected to numerous genes in their own modules; and (iv) network hubs (Zi > 2.5, Pi > 0.62), which act as both module hubs and connectors. The threshold value of Zi was determined by the density landscape of the nodes; a clear boundary at Zi = 2.5 was observed, and Zi > 2.5 was relatively “washed” down by the background of the non-hub region as described previously (Guimera and Amaral, 2005). Similarly, the Pi parameter space could be partitioned into different regions with a boundary at Pi = 0.62 by identifying the basins of attraction for the different node density plots (Guimera and Amaral, 2005). Tables 2 and 3 provide detailed information on the module hub genes and connectors. The majority of the genes, particularly, about 98.4 and 90.2% of uncontaminated and contaminated soils, respectively, were peripherals, having most of their links inside their modules. Fewer module hub genes were present in contaminated soils (two genes) compared with the control (12 genes). By contrast, more genes playing as connectors were found in contaminated soils (23 genes), and none in the control. None of the module hub genes, and connectors was overlapped in the contaminated and uncontaminated samples. Furthermore, no network hub genes were noted in the two conditions.
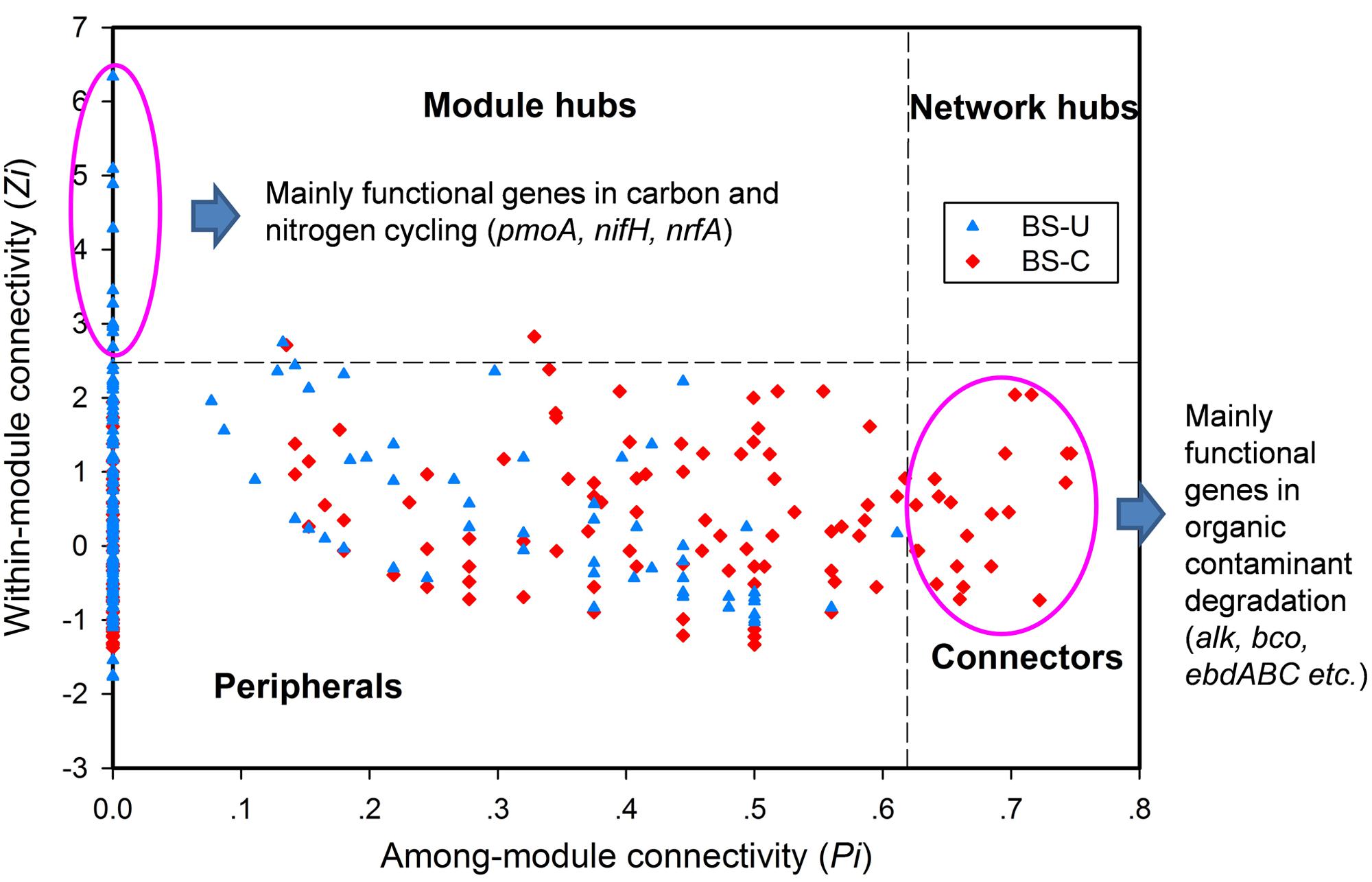
FIGURE 1. Z–P plot showing the distribution of genes based on their topological roles from the BS oil-exploring site. U, uncontaminated soils (blue), C, contaminated soils (red).
Aliphatic and aromatic hydrocarbons are major components of crude oil contaminants in oil-contaminated sites (Liang et al., 2012). To understand the co-occurrence levels of microbial functional genes involved in aliphatic and aromatic hydrocarbons degradation, we further constructed the networks of alk and PAH degradation genes in contaminated soils (Figures 2 and 3). Supplementary Tables S1 and S2 show detailed information on node degrees (links), gene identifications, names, and derived microorganisms.
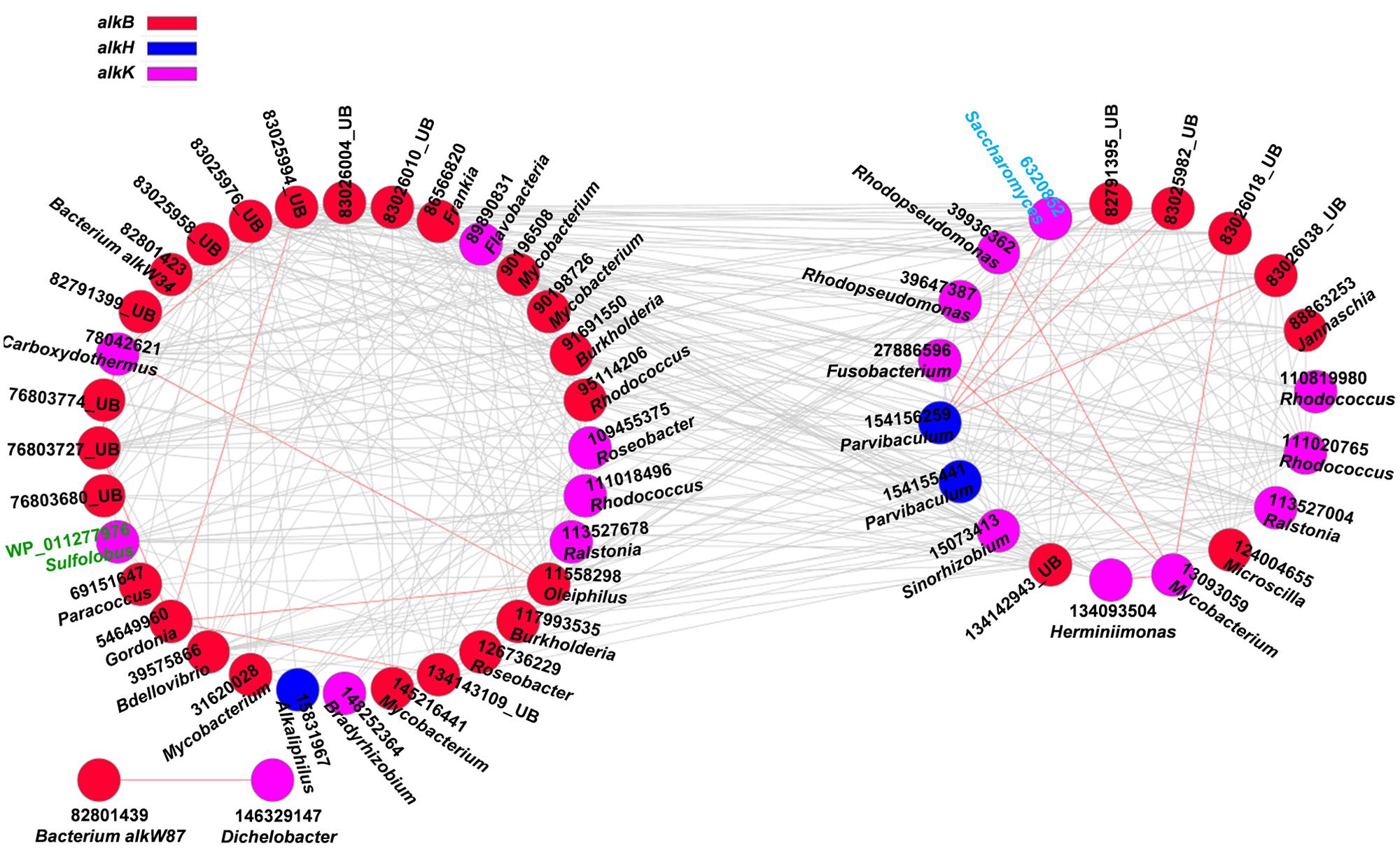
FIGURE 2. Network interactions of the alk genes in the oil-contaminated soils. The genes include alkB (alkane monooxygenase), alkH (aldehyde dehydrogenase), alkJ (alcohol dehydrogenase), and alkK (acyl-CoA synthetase). Numbers correspond to protein identification (National Center for Biotechnology Information, NCBI) and source genera. Black font indicates for bacteria (UB represents uncultured bacteria), green font for archaea, and blue font for fungi. Detailed information is listed in Supplementary Table S1. The two circles of the nodes represent different modules.
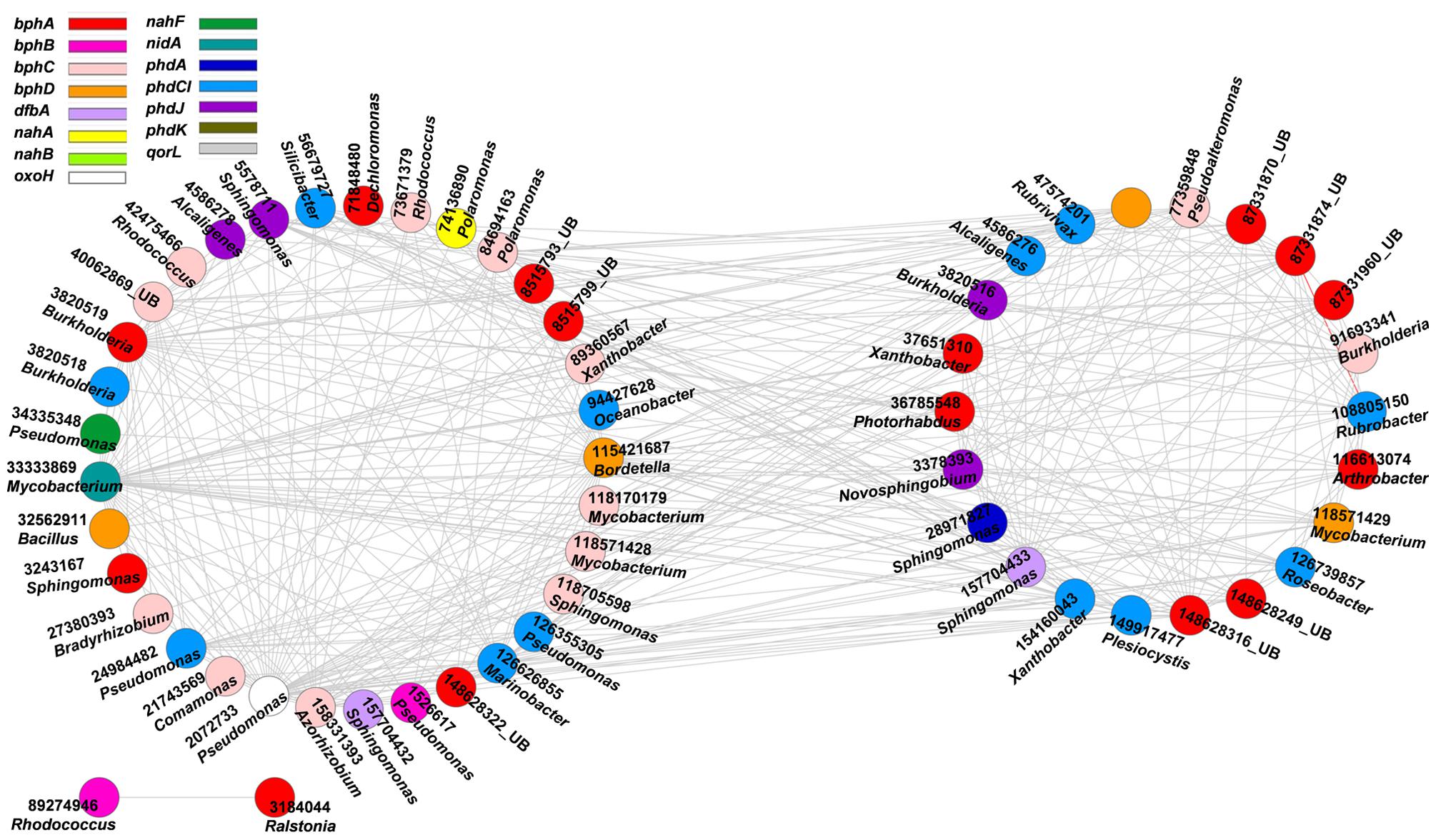
FIGURE 3. Network interactions of the PAH genes in the oil-contaminated soils. The genes are bphA (biphenyl 2,3-dioxygenase), bphB (cis-2,3-dihydrobiphenyl-2,3-diol dehydrogenase), bphC (biphenyl-2,3-diol 1,2-dioxygenase), bphD (2,6-dioxo-6-phenylhexa-3-enoate hydrolase), nahA (naphthalene 1,2-dioxygenase), nahB (cis-1,2-dihydro-1,2-dihydroxynaphthalene/dibenzothiophene dihydrodiol dehydrogenase), nahF (salicylaldehyde dehydrogenase), phdA (pyruvate dehydrogenase), phdCI (carboxylate isomerase), phdG (hydratase–aldolase), phdJ [4-(2-carboxyphenyl)-2-oxobut-3-enoate aldolase], phdK (2-carboxybenzaldehyde dehydrogenase), aorL (quinoline 2-oxidoreductase), nidA (putative ring-hydroxylating dioxygenase), oxoH (putative hydrolase). Numbers correspond to protein identification (NCBI) and source genera, all from bacteria (UB represents uncultured bacteria). Detailed information is listed in Supplementary Table S2. The two circles of the nodes represent different modules.
Several functional genes, namely, alkB, alkH, and alkK, were detected in oil-contaminated soils. These genes were responsible for degrading aliphatic hydrocarbons. Overall, 95.4% of the total interactions of alkane-degrading genes were negative (Figure 2, Supplementary Table S1), which may reflect the competitive behavior of microbial functional communities in alkane degradation. The functional gene with the highest connectivities was alkB (83025976, uncultured bacterium) with 31 connections. All were negative, and the strength degree was 0.284 (clustering coefficient).
High concentrations of PAHs, such as naphthalene, phenanthrene, pyrene, chrysene, benzo(e)pyrene, and their alkylated derivatives, were detected in oil-contaminated sites (Cline et al., 2007). Thus, we further explored the co-occurrence of microbial functional gene involved in PAH degradation (Figure 3, Supplementary Table S2). All interactions of the PAH genes were negative. The functional gene with the highest connectivity was nidA (33333869, Mycobacterium sp.), with 47 connections and strength degree of 0.205.
Pearson correlation analysis was performed between gene degrees and environmental factors to determine the relationships among microbial network interactions, oil contamination and soil geochemical variables, (Table 4). Gene degree was calculated by summing the strengths of the connections (i.e., links) of each gene (i.e., node) with all of the other connected genes in the network. Gene degree represents how strong a gene is connected to other genes; this degree is one of the most commonly used network indices. Negative correlations (P < 0.01) were observed between gene degrees and oil concentration, and total nitrogen and total phosphorus in contaminated soils. This result indicates that these factors may reduce the co-occurrence of microbial functional genes in the community network because of the potential competitive relationships among several microbial groups for available carbon and nutrient sources. Some of the correlations were significant but with low correlation coefficient levels, thus indicating the weak effect of these factors to the gene degrees.
To understand the influence of oil contamination on microbial interactions further, we investigated the changes of microbial molecular ecological networks in response to oil contamination. Network properties considerably changed in the contaminated samples when compared with the control. For example, connectivity, which provides information on how strong a node is connected to other nodes and is a commonly used network index (West, 1996), was reduced by 16.8% in response to oil contamination. Modularity measures the extent to which nodes possess more links within their own modules than expected if linkage is random. In this study, both module numbers and modularity decreased under oil contamination, with reductions of 76.6 and 41.8%, respectively. Small network sizes were also observed in contaminated soils than in the control at 256 and 754 functional genes (nodes) in the two networks, respectively. Although hydrocarbon contamination is known to exert profound effects on soil microbial community composition, diversity, and functional processes, the effects on the microbial ecological networks were first explored in this study. Findings revealed that the overall functional network structures were altered, thereby indicating a potential change in the organization of microbial communities (Faust and Raes, 2012). Mougi and Kondoh (2012) proposed that increasing complexity leads to increased stability in a community with mixed interaction types. Thus, the stability of microbial functional community may be reduced with the stress of oil contamination. Carbon input (as elevated CO2) has been reported to increase the complexity of soil microbial networks and produce a more stable community structure than usual (Zhou et al., 2010). Although hydrocarbons may have increased carbon source input and stimulated certain microbes that can utilize the carbons, degradable carbon was consumed and caused C:N imbalance. This imbalance may have resulted in the microbial functional instability. These findings can be partially confirmed by the decrease in microbial carbon substrate utilization accompanied by diversity loss in the culture-based Biolog analysis in the present study (Supplementary Figure S1). The insignificance of the distance-decay relationship (community similarity vs. geographic distance) in contaminated sites suggests that oil contamination significantly influences microbial communities to decrease in endemism, especially for the groups functioning in hydrocarbon degradation (Liang et al., 2015). Given that oil contamination cause a loss in the overall microbial diversity and alternation of community structures, many researchers concluded that anthropogenically induced oil contamination changed the microbial ecosystem (Hazen et al., 2010; Lu et al., 2012; Bell et al., 2013b). This change results in the potential switching of roles of microbial species and ecological functions of communities.
Identifying key populations/genes in a community is a challenge, because of the high diversity and uncultured status of microbes (Faust and Raes, 2012). In this study, fMEN analysis provided information on candidate genes/populations that are most important to microbial ecosystem structures and functions in oil-contaminated sites. We defined the two types of keystone genes. The first type refers to the genes that play key roles in the overall network based on network topology and their module memberships, such as module hubs (those highly connected to numerous genes in their own modules), connectors (those highly linked to several modules), and network hubs (acting as both module hubs and connectors). In this study, oil contamination changed the key genes in the ecological network. Two module hub genes (derived from Nitrococcus and Nitrosomonas, respectively) were present in the contaminated soils and 12 genes (mainly derived from Rhodobacter, Geobacter, Xylella, Sphingopyxis, etc.) in the uncontaminated soils with no overlap between the two conditions. Most of the module hub genes are functioning in carbon and nitrogen cycling, such as pmoA, nifH, and nrfA. The hub genes were derived from different organisms in the two conditions; hence, the changes of key module hubs by contamination may be due to the responses of microorganisms to the environmental stimulus. By contrast, 23 genes played as connectors (connecting modules) in the contaminated soils and none in the control. These genes mainly function in organic contaminant degradation, such as alk, bco, and edbABC (mainly derived from Sphingomonas, Geobacillus, Novosphingobium, Trichoderma, and Deinococcus), as well as nifH in nitrogen fixation. This result was expected because an increased number of genes and organisms functioning in hydrocarbon degradation were observed in oil-contaminated sites (Horvath, 1972; Baldwin et al., 2008; Bell et al., 2013b). We also observed that several genes played as module hub or connector function in other biogeochemical cycles, such as metal and antibiotic resistance. The actual roles of these hub or connector genes must be elucidated by real biological replicates of networks or co-culture experiments in the further work.
The second keystone genes are defined as those highly connected nodes (genes) involved in the degradation of the main components of oil contaminant. In the fMENs, patterns with a few highly connected nodes render the network more robust to change (Albert et al., 2000; Montoya et al., 2006). If highly connected nodes are lost, the network changes dramatically. Thus, these highly connected nodes may be analogous to microbial “keystone genes.” The top six keystone alk genes are alkB (83025976, 31 connections), alkK (89890831, 29 connections), alkK (111018496, 26 connections), alkK (39647387, 24 connections), alkB (134142943, 24 connections), and alkH (154155441, 24 connections). Many keystone genes in the alk gene network were derived from some species belonging to Flavobacteria, Rhodococcus, Rhodopseudomonas, and Parvibaculum. These bacteria are widely reported in alkane degradation (Naïtali et al., 2003; Mohanty and Mukherji, 2008). The top six keystone PAH genes are nidA (33333869, 47 connections), oxoH (2072733, 45 connections), bphD (115421687, 43 connections), bphA (3820519, 29 connections), phdCI (126626855, 26 connections), and bphC (84694163, 25 connections). These genes are mainly derived from Mycobacterium, Pseudomonas, Bordetella, Burkholderia, Marinobacter, and Polaromonas, which have been found to be capable of naphthalene and phenanthrene utilization (Chaillana et al., 2004), as well as the degradation of other petroleum hydrocarbons (Atlas, 1981; Hamamura et al., 2013; Meyneta et al., 2014). Our study further indicated that these functional groups carrying the keynote functional genes may play important roles in maintaining the stability of the biological network.
Microorganisms do not exist in isolation but form complex ecological interaction webs with several interaction types (Faust and Raes, 2012). Detecting and investigating various types of interactions in microbial ecosystems are difficult to accomplish (Raes et al., 2007), specifically in an environment disturbed by anthropogenic activities. A previous study experimentally demonstrated that increasing disturbance promotes microbial interspecies competition (Violle et al., 2010). Competition can constrain the specific functions of a community in several cases because of limited resources and habitat available to the most productive species (Bell et al., 2013a). In oil-contaminated soils, negative co-occurrence patterns prevailed among functional genes involved in alkane and PAH degradation. This finding suggests the competition for carbon compound and/or nutrient under oil contamination. For example, Sphingomonas is more competitive in nutrient acquisition than other genera in hydrocarbon-contaminated sites (Bell et al., 2011). In our study, we also observed that gene xylJ (28971837) derived from Alphaproteobacteria (Sphingomonas sp.) with the highest links in BS showed negative interactions with several other genes derived from Gammaproteobacteria (Pseudomonas sp. and Aeromonas sp.), Actinobacteria (Mycobacterium sp., Nocardia sp., and Corynebacterium sp.), Spirochaetes (Spirochaeta sp.), and Firmicutes (Streptococcus sp.). A small proportion of positive interactions, particularly, 4.5% of the total interactions in the alk functional gene network, were also observed. These interactions include those between alkK (6320852, Saccharomyces) and alkH (154156259, Parvibaculum); alkB (11558298, Oleiphilus) and the other two genes alkB (54649960, Gordonia) and alkK (78042621, Carboxydothermus); alkK (13093059, Mycobacterium) and the other four genes alkK (27886596, Fusobacterium), alkK (39936362, Rhodopseudomonas), alkB (83026018, uncultured bacterium), and alkK (134093504, Herminiimonas); and alkB (54649960, Gordonia) and the other three alkB genes (76803727, 83025994 and 134143109), which are all derived from uncultured bacterium. The positive interactions may reflect the commonly preferred environmental conditions or cooperative behaviors, such as cross feeding, syntrophic interactions, and mutualistic interactions (Raes and Bork, 2008; Steele et al., 2011).
Interactions between domains (bacteria, fungi, and archaea) were reported previously (Rousk et al., 2008; Steele et al., 2011; Bell et al., 2013b). In the current study, the network of functional genes involved in contaminant degradation showed that bacteria, fungi, and/or archaea were connected. Bacteria and fungi are generally described as antagonists for substrate competition in the soil environment (Mille-Lindblom et al., 2006; Meidute et al., 2008; Rousk et al., 2008). In oil-contaminated soils, the negative interactions among functional genes (alk and PAH degrading) derived from different microorganisms may be inferred as competition among microbial groups for degradable carbon sources, limited supply of nitrogen, and phosphorus.
Comprehensive information on microbial species or taxonomic units across relatively large numbers of samples is essential in detecting the co-occurrence relationships among microbial communities using network analysis (Barberan et al., 2012). Sample sets should ideally include spatial or temporal gradients in environmental conditions to achieve sufficient variability in taxon abundances to resolve co-occurrence patterns (Barberan et al., 2012). In the RMT-based molecular ecological network approach (Zhou et al., 2010), 10 samples are required to construct a network of microbial communities to ensure that the co-occurrence patterns are statistically significant rather than a random process. Thus, in this study, 20 soil samples (10 contaminated and 10 uncontaminated soils) were selected to infer the possible co-occurrence relationship between microbial functional genes under long-term oil contamination. Although we could not scale the results to all the situations with only a few samples, constructing a co-occurrence network is important to determine the potential interactions among different microorganisms. The results would provide better understanding of the responses of biological communities to severe environmental contamination. Additional sampling efforts combined with laboratory experiments are required to further obtain fundamental insight into microbial ecological networks in complex environmental habitats.
All authors contributed intellectual input and assistance to this study and manuscript preparation. GL, JZ, BS, and YL developed the original framework. YL and HZ contributed reagents and data analysis. YL, BS, and JZ wrote the paper.
This study was supported by National Natural Scientific Foundation of China (No. 41371256), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant # XDB15010100), Foundation for Distinguished Young Talents in State Key Laboratory of Soil and Sustainable Agriculture (Y412010008), the Youth Innovation Promotion Association of Chinese Academy of Sciences, and Foundation of Jiangsu Educational committee (13KJB610001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00060
Albert, R., Jeong, H., and Barabasi, A. L. (2000). Error and attack tolerance of complex networks. Nature 406, 378–382. doi: 10.1038/35019019
Atlas, R. (1981). Microbial-degradation of petroleumhydrocarbons: an environmental perspective. Microbiol. Rev. 45, 180–209.
Baldwin, B. R., Nakatsu, C. H., and Nies, L. (2008). Enumeration of aromatic oxygenase genes to evaluate monitored natural attenuation at gasoline-contaminated site. Water Res. 42, 723–731. doi: 10.1016/j.watres.2007.07.052
Barberan, A., Bates, S. T., Casamayor, E. O., and Fierer, N. (2012). Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 6, 343–351. doi: 10.1038/ismej.2011.119
Bell, T. H., Callender, K. L., Whyte, L. G., and Greer, C. W. (2013a). Microbial competition in polar soils: a review of an understudied but potentially important control on productivity. Biology 2, 533–554. doi: 10.3390/biology2020533
Bell, T. H., Yergeau, E., Maynard, C., Juck, D., Whyte, L. G., and Greer, C. W. (2013b). Predictable bacterial composition and hydrocarbon degradation in Arctic soils following diesel and nutrient disturbance. ISME J. 7, 1200–1210. doi: 10.1038/ismej.2013.1
Bell, T. H., Hassan, S. E., Lauron-Moreau, A. L., Al-Otaibi, F., Hijri, M., Yergeau, E., et al. (2014). Linkage between bacterial and fungal rhizosphere communities in hydrocarbon-contaminated soils is related to plant phylogeny. ISME J. 8, 331–343. doi: 10.1038/ismej.2013.149
Bell, T. H., Yergeau, E., Martineau, C., Juck, D., Whyte, L. G., and Greer, C. W. (2011). Identification of nitrogen-incorporating bacteria in petroleum-contaminated arctic soils by using [15N]DNA-based stable isotope probing and pyrosequencing. Appl. Environ. Microbiol. 77, 4163–4171. doi: 10.1128/AEM.00172-11
Cavalca, L., Dell Amico, E., and Andreoni, V. (2004). Intrinsic bioremediability of an aromatic hydrocarbon-polluted groundwater: diversity of bacterial populations and toluene monoxygenase genes. Appl. Microbiol. Biotechnol. 64, 576–587. doi: 10.1007/s00253-003-1449-6
Cébron, A., Norini, M. P., Beguiristain, T., and Leyval, C. (2008). Real-time PCR quantification of PAH-ring hydroxylating dioxygenase (PAH-RHDα) genes from Gram positive and Gram negative bacteria in soil and sediment samples. J. Microbiol. Methods 73, 148–159. doi: 10.1016/j.mimet.2008.01.009
Chaillana, F., Le Flècheb, A., Burya, E., Phantavonga, Y., Grimontb, P., Saliotc, A., et al. (2004). Identification and biodegradation potential of tropical aerobic hydrocarbon-degrading microorganisms. Res. Microbiol. 155, 587–595. doi: 10.1016/j.resmic.2004.04.006
Clauset, A., Newman, M., and Moore, C. (2004). Finding community structure in very large networks. Phys. Rev. E 70, 066111. doi: 10.1103/PhysRevE.70.066111
Cline, M. S., Smoot, M., Cerami, E., Kuchinsky, A., Landys, N., Workman, C., et al. (2007). Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2, 2366–2382. doi: 10.1038/nprot.2007.324
Das, N., and Chandran, P. (2011). Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol. Res. Int. 2011, 1–13. doi: 10.4061/2011/941810
Dionisi, H. M., Chewning, C. S., Morgan, K. H., Menn, F. M., Easter, J. P., and Sayler, G. S. (2004). Abundance of dioxygenase genes similar to Ralstonia sp. strain U2 nagAc is correlated with naphthalene concentrations in coal tar-contaminated freshwater sediments. Appl. Environ. Microbiol. 70, 3988–3995. doi: 10.1128/AEM.70.7.3988-3995.2004
Faust, K., and Raes, J. (2012). Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550. doi: 10.1038/nrmicro2832
Fleming, J. T., John Sanseverino, S., and Sayler, G. S. (1993). Quantitative relationship between naphthalene catabolic gene frequency and expression in predicting PAH degradation in soils at town gas manufacturing sites. Environ. Sci. Technol. 41, 1068–1074. doi: 10.1021/es00043a005
Fuhrman, J. A., and Steele, J. A. (2008). Community structure of marine bacterioplankton: patterns, networks, and relationships to function. Aquat. Microb. Ecol. 53, 69–81. doi: 10.3354/ame01222
Garland, J. L., and Mills, A. L. (1991). Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–2359.
Guimera, R., and Amaral, L. (2005). Functional cartography of complex metabolic networks. Nature 433, 895–900. doi: 10.1038/nature03288
Guimera, R., Sales-Pardo, M., and Amaral, L. A. N. (2007). Classes of complex networks defined by role-to-role connectivity profiles. Nat. Phys. 3, 63–69. doi: 10.1038/nphys489
Hamamura, N., Ward, D., and Inskeep, W. (2013). Effects of petroleum mixture types on soil bacterial population dynamics associated with the biodegradation of hydrocarbons in soil environments. FEMS Microbiol. Ecol. 85, 168–178. doi: 10.1111/1574-6941.12108
Hartwell, L. H., Hopfield, J. J., Leibler, S., and Murray, A. W. (1999). From molecular to modular cell biology. Nature 402(6761 Suppl), C47–C52. doi: 10.1038/35011540
Hazen, T. C., Dubinsky, E. A., Desantis, T. Z., Andersen, G. L., Piceno, Y. M., Singh, N., et al. (2010). Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330, 204–208. doi: 10.1126/science.1195979
He, Z., Van Nostrand, J. D., and Zhou, J. (2012). Applications of functional gene microarrays for profiling microbial communities. Curr. Opin. Biotechnol. 23, 460–466. doi: 10.1016/j.copbio.2011.12.021
He, Z. L., Deng, Y., Van Nostrand, J. D., Tu, Q. C., Xu, M. Y., Hemme, C. L., et al. (2010). GeoChip 3.0 as a high-throughput tool for analyzing microbial community composition, structure and functional activity. ISME J. 4, 1167–1179. doi: 10.1038/ismej.2010.46
Horvath, R. S. (1972). Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol. Rev. 36, 146–155.
Horvath, S., and Dong, J. (2008). Geometric interpretation of gene coexpression network analysis. PLoS Comput. Biol. 4:e1000117. doi: 10.1371/journal.pcbi.1000117
Horvath, S., Zhang, B., Carlson, M., Lu, K. V., Zhu, S., Felciano, R. M., et al. (2006). Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proc. Natl. Acad. Sci. U.S.A. 103, 17402–17407. doi: 10.1073/pnas.0608396103
Kingston, P. F. (2002). Long-term environmental impact of oil spills. Spill Sci. Technol. Bull. 7, 53–61. doi: 10.1016/S1353-2561(02)00051-8
Kvenvolden, K. A., and Cooper, C. K. (2003). Natural seepage of crude oil into the marine environment. Geo-Mar. Lett. 23, 140–146. doi: 10.1007/s00367-003-0135-0
Labud, V., Garcia, C., and Hernandez, T. (2007). Effect of hydrocarbon pollution on the microbial properties of a sandy and a clay soil. Chemosphere 66, 1863–1871. doi: 10.1016/j.chemosphere.2006.08.021
Liang, Y., Van Nostrand, J. D., Deng, Y., He, Z., Wu, L., Zhang, X., et al. (2011). Functional gene diversity of soil microbial communities from five oil-contaminated fields in China. ISME J. 5, 403–413. doi: 10.1038/ismej.2010.142
Liang, Y., Zhang, X., Wang, J., and Li, G. (2012). Spatial variations of hydrocarbon contamination and soil properties in oil exploring fields across China. J. Hazard. Mater. 241, 371–378. doi: 10.1016/j.jhazmat.2012.09.055
Liang, Y. T., Zhang, X., Zhou, J., and Li, G. (2010). Long-term oil contamination increases deterministic assembly processes in soil microbes. Appl. Environ. Microbiol. 76, 1088–1094. doi: 10.1128/AEM.02749-09
Liang, Y., Zhang, X., Zhou, J., and Li, G. (2015). Long-term oil contamination increases deterministic assembly processes in soil microbes. Ecol. Appl. 25, 1235–1243. doi: 10.1890/14-1672.1
Lu, Z., Deng, Y., Van Nostrand, J. D., He, Z., Voordeckers, J., Tom, M. L., et al. (2012). Microbial gene functions enriched in the Deepwater Horizon deep-sea oil plume. ISME J. 6, 451–460. doi: 10.1038/ismej.2011.91
Luo, F., Yang, Y., Zhong, J., Gao, H., Khan, L., Thompson, D. K., et al. (2007). Constructing gene co-expression networks and predicting functions of unknown genes by random matrix theory. BMC Bioinformatics 8:299. doi: 10.1186/1471-2105-8-299
Luo, F., Zhong, J., Yang, Y., Scheuermann, R. H., and Zhou, J. (2006). Application of random matrix theory to biological networks. Phys. Lett. A 357, 420–423. doi: 10.1016/j.physleta.2006.04.076
Marlowe, E. M., Wang, J. M., Pepper, I. L., and Maier, R. M. (2002). Application of reverse transcription-PCR assay to monitor regulation of the catabolic nahAc gene during phenanthrene degradation. Biodegradation 13, 251–260. doi: 10.1023/A:1021221104425
Maslov, S., and Sneppen, K. (2002). Specificity and stability in topology of protein networks. Science 296, 910–913. doi: 10.1126/science.1065103
Mason, O. U., Hazen, T. C., Borglin, S., Chain, P. S., Dubinsky, E. A., Fortney, J. L., et al. (2012). Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 6, 1715–1727. doi: 10.1038/ismej.2012.59
Meidute, S., Demoling, F., and Baath, E. (2008). Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol. Biochem. 40, 2334–2343. doi: 10.1016/j.soilbio.2008.05.011
Mesarch, M. B., Nakatsu, C. H., and Nies, L. (2004). Bench-scale and field-scale evaluation of catechol 2,3-dioxygenase specific primers for monitoring BTX bioremediation. Water Res. 38, 1281–1288. doi: 10.1016/j.watres.2003.10.052
Meyneta, P., Moliternic, E., Davenporta, R. J., Sloanb, W. T., Camachoc, J. V., and Werner, D. (2014). Predicting the effects of biochar on volatile petroleum hydrocarbon biodegradation and emanation from soil: a bacterial community finger-print analysis inferred modelling approach. Soil Biol. Biochem. 68, 20–30. doi: 10.1016/j.soilbio.2013.09.015
Mille-Lindblom, C., Fischer, H., and Tranvik, L. J. (2006). Antagonism between bacteria and fungi: substrate competition and a possible tradeoff between fungal growth and tolerance towards bacteria. Oikos 113, 233–242. doi: 10.1111/j.2006.0030-1299.14337.x
Mohanty, G., and Mukherji, S. (2008). Biodegradation rate of diesel range n-alkanes by bacterial cultures Exiguobacterium aurantiacum and Burkholderia cepacia. Int. Biodeter. Biodegradation 61, 240–250. doi: 10.1016/j.ibiod.2007.06.011
Montoya, J. M., Pimm, S. L., and Sole, R. V. (2006). Ecological networks and their fragility. Nature 442, 259–264. doi: 10.1038/nature04927
Moore, D., and Dowhan, D. (2002). Purification and concentration of DNA from aqueous solutions. Curr. Protoc. Mol. Biol. Chap. 2, Unit2.1A.
Mougi, A., and Kondoh, M. (2012). Diversity of interaction types and ecological community stability. Science 337, 349–351. doi: 10.1126/science.1220529
Naïtali, M. B., Rakatozafy, H., Marchal, R., Leveau, J. Y., and Vandecasteele, J. P. (2003). Diversity of bacterial strains degrading hexadecane in relation to the mode of substrate uptake. J. Appl. Microbiol. 86, 421–428. doi: 10.1046/j.1365-2672.1999.00678.x
Nzila, A. (2013). Update on the cometabolism of organic pollutants by bacteria. Environ. Pollut. 178, 474–482. doi: 10.1016/j.envpol.2013.03.042
Park, J. W., and Crowley, D. E. (2006). Dynamic changes in nahAc gene copy numbers during degradation of naphthalene in PAH-contaminated soils. Appl. Microbiol. Biot. 72, 1322–1329. doi: 10.1007/s00253-006-0423-5
Penton, C. R., Johnson, T. A., Quensen, J. F., Iwai, S., Cole, J. R., and Tiedje, J. M. (2013). Functional genes to assess nitrogen cycling and aromatic hydrocarbon degradation: primers and processing matter. Front. Microbiol. 4:279. doi: 10.3389/fmicb.2013.00279
Raes, J., and Bork, P. (2008). Systems microbiology – Timeline – Molecular eco-systems biology: towards an understanding of community function. Nat. Rev. Microbiol. 6, 693–699. doi: 10.1038/nrmicro1935
Raes, J., Foerstner, K. U., and Bork, P. (2007). Get the most out of your metagenome: computational analysis of environmental sequence data. Curr. Opin. Microbiol. 10, 490–498. doi: 10.1016/j.mib.2007.09.001
Ringelberg, D. B., Talley, J. W., Perkins, E. J., Tucker, S. G., Luthy, R. G., Bouwer, E. J., et al. (2001). Succession of phenotypic, genotypic, and metabolic community characteristics during in vitro bioslurry treatment of polycyclic aromatic hydrocarbon-contaminated sediments. Appl. Environ. Microb. 67, 1542–1550. doi: 10.1128/AEM.67.4.1542-1550.2001
Rousk, J., Demoling, L. A., Bahr, A., and Baath, E. (2008). Examining the fungal and bacterial niche overlap using selective inhibitors in soil. FEMS Microbiol. Ecol. 63, 350–358. doi: 10.1111/j.1574-6941.2008.00440.x
Salminen, J. M., Tuomi, P. M., and Jørgensen, K. S. (2008). Functional gene abundances (nahAc, alkB, xylE) in the assessment of the efficacy of bioremediation. Appl. Biochem. Microbiol. 151, 638–652. doi: 10.1007/s12010-008-8275-3
Sei, K., Sugimoto, Y., Mori, K., Maki, H., and Kohno, T. (2003). Monitoring of alkane degrading bacteria in a sea-water microcosm during crude oil degradation by polymerase chain reaction based on alkane-catabolic genes. Environ. Microbiol. 5, 517–522. doi: 10.1046/j.1462-2920.2003.00447.x
Simon, C., and Daniel, R. (2011). Metagenomic analyses: past and future trends. Appl. Environ. Microbiol. 77, 1153–1161. doi: 10.1128/AEM.02345-10
Stapleton, R. D., and Sayler, G. S. (2000). Changes in subsurface catabolic gene frequences during natural attenuation of petroleum hydrocarbons. Environ. Sci. Technol. 69, 461–467.
Steele, J. A., Countway, P. D., Xia, L., Vigil, P. D., Beman, J. M., Kim, D. Y., et al. (2011). Marine bacterial, archaeal and protistan association networks reveal ecological linkages. ISME J. 5, 1414–1425. doi: 10.1038/ismej.2011.24
Tu, Q., Yu, H., He, Z., Deng, Y., Wu, L., Van Nostrand, J. D., et al. (2014). GeoChip 4: a functional gene-array-based high-throughput environmental technology for microbial community analysis. Mol. Ecol. Resour. 14, 914–928. doi: 10.1111/1755-0998.12239
Tuomi, M., Salminen, J. M., and Jorgensen, K. S. (2004). The abundance of nahAc genes correlates with the 14C-naphthalene mineralization potential in petroleum hydrocarbon-contaminated oxic soil layers. FEMS Microbiol. Ecol. 51, 99–107. doi: 10.1016/j.femsec.2004.07.011
Van Hamme, J. D., Singh, A., and Ward, O. P. (2003). Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 67, 503–549. doi: 10.1128/MMBR.67.4.503-549.2003
Violle, C., Pu, Z., and Jiang, L. (2010). Experimental demonstration of the importance of competition under disturbance. Proc. Natl. Acad. Sci. U.S.A. 107, 12925–12929. doi: 10.1073/pnas.1000699107
Yergeau, E., Kang, S., He, Z., Zhou, J., and Kowalchuk, G. A. (2007). Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. ISME J. 1, 163–179. doi: 10.1038/ismej.2007.24
Zhou, J. Z., Bruns, M. A., and Tiedje, J. M. (1996). DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62, 316–322.
Zhou, J., Deng, Y., Luo, F., He, Z., Tu, Q., and Zhi, X. (2010). Functional molecular ecological networks. MBio 1, e169–e110. doi: 10.1128/mBio.00169-10
Keywords: microbial interaction, oil contamination, molecular ecological network, functional genes, hydrocarbon degradation
Citation: Liang Y, Zhao H, Deng Y, Zhou J, Li G and Sun B (2016) Long-Term Oil Contamination Alters the Molecular Ecological Networks of Soil Microbial Functional Genes. Front. Microbiol. 7:60. doi: 10.3389/fmicb.2016.00060
Received: 22 September 2015; Accepted: 13 January 2016;
Published: 03 February 2016.
Edited by:
Jennifer Glass, Georgia Institute of Technology, USAReviewed by:
Laura Audrey Hug, University of California, Berkeley, USACopyright © 2016 Liang, Zhao, Deng, Zhou, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Sun, YnN1bkBpc3Nhcy5hYy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.