- 1Department of Biochemistry and Molecular Biology, University of Georgia, Athens, GA, USA
- 2Department of Chemical and Biomolecular Engineering, North Carolina State University, Raleigh, NC, USA
Carbon monoxide (CO) is an important intermediate in anaerobic carbon fixation pathways in acetogenesis and methanogenesis. In addition, some anaerobes can utilize CO as an energy source. In the hyperthermophilic archaeon Thermococcus onnurineus, which grows optimally at 80°C, CO oxidation and energy conservation is accomplished by a respiratory complex encoded by a 16-gene cluster containing a CO dehydrogenase, a membrane-bound [NiFe]-hydrogenase and a Na+/H+ antiporter module. This complex oxidizes CO, evolves CO2 and H2, and generates a Na+ motive force that is used to conserve energy by a Na+-dependent ATP synthase. Herein we used a bacterial artificial chromosome to insert the 13.2 kb gene cluster encoding the CO-oxidizing respiratory complex of T. onnurineus into the genome of the heterotrophic archaeon, Pyrococcus furiosus, which grows optimally at 100°C. P. furiosus is normally unable to utilize CO, however, the recombinant strain readily oxidized CO and generated H2 at 80°C. Moreover, CO also served as an energy source and allowed the P. furiosus strain to grow with a limiting concentration of sugar or with peptides as the carbon source. Moreover, CO oxidation by P. furiosus was also coupled to the re-utilization, presumably for biosynthesis, of acetate generated by fermentation. The functional transfer of CO utilization between Thermococcus and Pyrococcus species demonstrated herein is representative of the horizontal gene transfer of an environmentally relevant metabolic capability. The transfer of CO utilizing, hydrogen-producing genetic modules also has applications for biohydrogen production and a CO-based industrial platform for various thermophilic organisms.
Introduction
Carbon monoxide (CO) is a high energy compound with a very low reduction potential for the CO/CO2 couple [E0′ = -524 mV: (Thauer, 1990)], see Equation 1.
Anaerobic microorganisms that directly use CO as a carbon source do so via the Wood-Ljungdahl pathway. This is present in acetogenic bacteria and methanogenic archaea (Wood, 1991; Costa and Leigh, 2014) and has been studied for more than 80 years (Drake and Daniel, 2004). The key enzyme is a bifunctional and bimodular CO dehydrogenase/acetyl-CoA synthase (Codh/Acs) complex. This enzyme combines CO with a methyl group, donated by a corrinoid containing carrier protein, with coenzyme A to generate acetyl-CoA (Ragsdale et al., 1996). The Wood–Ljungdahl pathway functions to fix CO2 into the cellular material via acetyl-CoA where hydrogen gas is commonly used as the source of reductant (Ragsdale et al., 1996). The pathway consists of two branches. One reduces CO2 to the level of the methyl group in acetyl-CoA while the other reduces CO2 to CO, which forms the carbonyl group of acetyl-CoA (Schuchmann and Muller, 2014).
A diverse group of microorganism are able to use CO as a sole energy source independent of the Wood–Ljungdahl pathway (Oelgeschlager and Rother, 2008). Some anaerobes can utilize CO in the absence of an electron acceptor by coupling CO oxidation to the reduction of protons (H2/H+, E0′ = -414 mV, pH 7.0) thereby releasing a significant amount of energy (ΔG0’ = -27 kJ/mol). The thermophilic Firmicute Carboxydothermus hydrogenoformans grows very well using CO as a sole source of both energy and carbon (Uffen, 1976; Svetlichny et al., 1991; Wu et al., 2005) and has become the model system for studying CO-dependent growth by biochemical, physiological and evolutionary approaches (Soboh et al., 2002; Henstra and Stams, 2011; Techtmann et al., 2012). The membrane-bound H2-evolving Codh complex of C. hydrogenoformans contains eight subunits. Two of these represent the Codh module while the other six are homologous to the energy converting hydrogenase (Ech) first characterized in the methanogen Methanosarcina barkeri (Meuer et al., 1999). Hence the C. hydrogenoformans Codh complex is a bimodular system in which electrons from CO oxidation are directed to an ion-translocating hydrogenase (Svetlitchnyi et al., 2001; Wu et al., 2005). The Codh module of C. hydrogenoformans appears to be similar to the Codh module of the Codh/Acs complex found in acetogens and methanogens and contains a similar [Ni-4Fe-5S] cluster as part of its catalytic site (Dobbek et al., 2001; Techtmann et al., 2012).
The CO utilization pathways of acetogenic, methanogenic, and hydrogenogenic carboxydotrophs exemplify how CO can be metabolized under anaerobic conditions without an external electron acceptor (besides CO2). In such cases acetogens generate CO2 and acetate, methanogens produce CO2 and CH4, and the hydrogenotrophic carboxydotrophs convert CO to CO2 and H2 (Oelgeschlager and Rother, 2008; Sokolova et al., 2009). It should be noted that C. hydrogenoformans is an autotroph and uses the Wood–Ljungdahl pathway for carbon fixation. Thus this organism contains a bimodular Codh/Acs complex in addition to a bimodular Codh/Ech complex (Svetlitchnyi et al., 2004; Henstra and Stams, 2011).
The heterotrophic hyperthermophilic archaeon Thermococcus onnurineus grows optimally at 80°C and was recently shown to gain energy for growth by coupling CO oxidation to H2 production (Rittmann et al., 2015). T. onnurineus has been extensively used as a model organism for the production of biohydrogen from various one-carbon compounds such as formate and CO. However, T. onnurineus is not an autotroph and requires a source of organic carbon in the form of peptides for growth on CO (Bae et al., 2012). T. onnurineus is a member of the archaeal order Thermococcales, members of which grow optimally in the temperature range from 75 to 100°C (Schut et al., 2014). A hallmark of this group is the presence of a respiratory membrane-bound hydrogenase complex (MBH) that is responsible for evolving H2 as a means for disposing of the reductant generated from fermentation. MBH consists of two modules. One is a five-subunit respiratory hydrogenase (Mbh) that evolves H2 and generates a proton gradient (Sapra et al., 2003). The other is a nine-subunit Na+/H+ antiporter (Mrp) that converts the proton gradient into a Na+ ion gradient. How exactly the Na+ gradient is made is not clear; however, in a homologous formate-dependent system it was proposed that a proton gradient is formed first and is then converted to a Na+ gradient (Lim et al., 2014). It is clear that ultimately a Na+ gradient needs to be established in order for energy to be conserved by a Na+-dependent ATP synthase (Pisa et al., 2007).
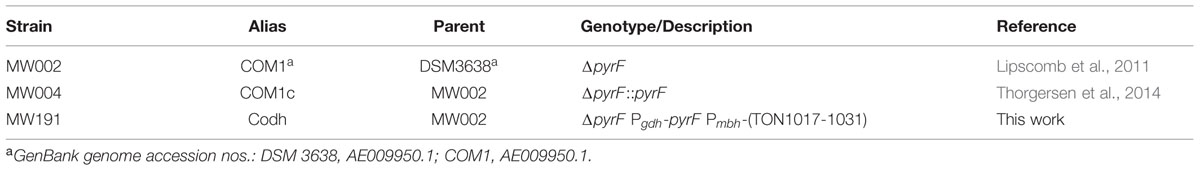
The CO-oxidizing Codh complex of T. onnurineus is therefore trimodular in nature (Mrp-Mbh-Codh) (Kim et al., 2013). It consists of a 12-subunit hydrogenase-antiporter complex (Mrp-Mbh) together with the two subunits of CO dehydrogenase with proposed structure shown in Figure 1A. By analogy with the known structure of the cytoplasmic CO dehydrogenase of acetogens (Kung et al., 2009), the Codh module of the T. onnurineus complex is proposed to contain two CooS subunits and one CooF subunit. T. onnurineus contains a 16-gene putative operon that encodes the complete Mrp-Mbh-Codh complex (Figure 1B) (Lim et al., 2010). This also includes two genes encoding a maturation factor for the Codh module and a protein with unknown function.
FIGURE 1. (A) Schematic representation of the CO oxidizing Codh complex of Thermococcus onnurineus, which conserves energy through an ion gradient. The naming of the Mrp and Mbh subunits is matched to those of the Mrp and Mbh subunits of Pyrococcus furiosus (Schut et al., 2013). (B) Operon structure of the Mrp-Mbh-Codh encoding genes (TON_1017-TON_1031). The gene encoding subunit N is depicted here as we have sequence verified that there is no frameshift in this gene, contrary to the published genome sequence.
The heterotrophic archaeon, Pyrococcus furiosus grows in the range of 70–103°C (Fiala and Stetter, 1986) and is also a member of the Thermococcales. Like T. onnurineus. P. furiosus is a strict heterotroph and does not fix CO2, but, unlike T. onnurineus, it cannot utilize CO as a source of energy. P. furiosus ferments a range of sugars and uses a respiratory MBH complex (Mrp-Mbh) to dispose of the excess reductant as H2 (Sapra et al., 2003; McTernan et al., 2014). It has many desirable features as a metabolic engineering host (Lipscomb et al., 2011; Hawkins et al., 2013; Keller et al., 2013). In this study, we engineered P. furiosus to produce the entire T. onnurineus Codh complex (Mrp-Mbh-Codh) and show that the resulting strain not only oxidizes CO to produce H2, it also grows using CO as a source of energy.
Materials and Methods
Strain Construction
The 22.2 kb BAC vector, pGL058 (Basen et al., 2014), containing the Codh gene cluster expression construct for homologous recombination into the P. furiosus chromosome at genome region five between convergent genes PF1232 and PF1233 was linearized using the unique XhoI restriction site (within repE in the BAC vector backbone) and used to transform P. furiosus COM1 according to previously published methods (Lipscomb et al., 2011). Transformant colonies were cultured in defined cellobiose (DC) medium (Lipscomb et al., 2011), and gDNA was isolated using the ZymoBeadTM Genomic DNA Kit (Zymo Research) for PCR screening. PCR screens were performed using the SpeedSTAR HS DNA polymerase (Takara) for amplification of the ∼16 kb product with primers ∼100 b outside the chromosomal homologous flanking regions. PCR-verified isolates were further purified twice on solid DC medium prior to final PCR screening and saving of glycerol stocks. One of the purified pGL058 transformants, designated MW191, was selected for phenotypic analyses. This strain is referred to herein as the Codh strain.
Growth and Cell Protein Quantitation
Pyrococcus furiosus strains (Table 1) were cultured in artificial seawater medium containing per liter: 1× base salts, 1× trace minerals, 1 μM sodium tungstate, 0.25 μg resazurin, 0.5 g cysteine, 0.5 g, 1 g sodium bicarbonate, and 1 mM potassium phosphate buffer, with pH adjusted to 6.8 prior to bottling (Adams et al., 2001). Media was aliquoted into serum bottles (50 mL per 150 mL bottle), and the headspace was replaced with argon after three cycles of vacuum and argon. For growth curves, this medium was supplemented with 1 g L-1 yeast extract (Difco) with or without 0.5 g L-1 maltose. CO was added as a 100% headspace with a light over pressure (1.2 atm). Medium was inoculated to ∼3 × 106 cells mL-1, and cultures were incubated at 80°C with shaking.
For extract preparation, growth medium was supplemented with 2 g maltooligosaccharides and 1 g yeast extract per liter, and cultures were incubated at 80°C with stirring. Cell growth was assessed by monitoring total cell protein using a Bradford protein assay kit (Bio-Rad). Briefly, cells were harvested by centrifugation from 1 mL culture samples and lysed by osmotic shock in an equal volume of water, with vortexing and one freeze-thaw cycle. Lysate was centrifuged at 10,000 g for 1 min to pellet insoluble cell debris prior to quantitation of soluble cell protein.
Cell Free Extract Preparation and Enzyme Assays
To obtain cell-free extracts, P. furiosus cell pellets were suspended in 50 mM EPPS pH 8.4, with 2 mM sodium dithionite (3 mL buffer per gram of cells) under strict anaerobic conditions. The cells were lysed by osmotic shock and sonication (Qsonica model Q55, 1 min at amplitude 30). In order to prepare membrane extracts, the cell-free extract was centrifuged at 100,000 g for 1 h. The resulting pellet was suspended in 8 mL buffer, centrifuged again, and the pellet was suspended in 0.8 mL buffer followed by storage at 4°C in stoppered glass vials until assayed. CO oxidizing:H2 formation activity was measured by the production of H2 from CO. Glass vials (8 mL volume) fitted with butyl rubber stoppers and containing 1 mL buffer (50 mM EPPS, pH 8.4) with a 100% CO headspace were preheated to 80°C, and the reaction was initiated by the addition of extracts (∼1 mg mL-1). Gas samples were analyzed by GC (Shimadzu GC8A with TCD detector, oven 70°C, injector/detector 120°C, Alltech Molecular Sieve column 5A 80/100). Benzyl viologen-dependent Codh activity was measured in 3 mL stoppered glass cuvettes containing 2 mL of anaerobic 50 mM EPPS, pH 8.4, and a 100% CO headspace. Reactions were started after preheating at 75°C with extract (∼2 μg ml-1), benzyl viologen reduction was monitored at 600 nm (𝜀 = 7400 M-1 cm-1).
H2 Quantitation
H2 was determined by sampling the headspace of closed bottle cultures with a pressure-lock syringe and analyzing H2 content by GC (see above). H2 production is expressed as molar amount produced per liquid phase.
Organic Acid Quantitation
Organic acids were measured using an Agilent 7890A GC equipped with a carbowax/20 m column and an FID detector. Culture samples of 1 mL were centrifuged at 10,000 g for 10 min, and the supernatant was acidified with formic acid (100 mM) before analysis.
Results
Insertion of the T. onnurineus Gene Cluster Encoding the Codh Complex into the P. furiosus Chromosome
The complete Codh complex (Mrp-Mbh-Codh) encoded by gene cluster TON_1017-TON_1031 is 13.2 kb in size and is assumed to consist of one transcriptional unit. The published genome sequence lacks the annotation for the N subunit gene (between TON_1023 and TON_1024) due to a sequencing error that introduced a base deletion and frameshift in this gene. This mistake has been reported by Lim et al. (2010), and we also verified the sequence for this work. We depict the correct operon structure including the N subunit gene in Figure 1. This TON_1017-TON_1031 gene cluster was inserted into the P. furiosus genome under the control of the promoter for the Mbh operon of P. furiosus (Pmbh). A bacterial artificial chromosome (BAC) vector was used to facilitate construction of the 15.3 kb genome insertion. This contained 0.5 kb flanking regions for targeted homologous recombination, the Pgdh-pyrF selectable marker cassette, and the Mrp-Mbh-Codh Codh gene cluster expression construct with Pmbh. To avoid potential deleterious effects on the host, the expression construct was inserted into a chromosomal region of low transcriptional activity as determined from analysis of tiling array data (Yoon et al., 2011). The genetically competent strain of P. furiosus, termed COM1 (Lipscomb et al., 2011), was transformed with the expression construct containing the Codh gene cluster of T. onnurineus, generating the strain MW191, referred to herein as the Codh strain of P. furiosus.
In Vitro Evaluation of Codh Complex Activity
To evaluate the activity of the engineered Codh strain of P. furiosus, it was grown in the presence of CO at 80°C, near the optimal growth temperature of T. onnurineus. Cell-free extracts were prepared for in vitro activity measurements. The T. onnurineus Codh complex converts CO to equimolar amounts of H2 and CO2, according to Equation 2.
In order to distinguish Codh activity from that of the native MBH activity in P. furiosus, Codh activity was measured in T. onnurineus cell extracts grown on CO as a control. Cell-free extracts of the P. furiosus parental control strain COM1c had no detectable CO-oxidizing activity using either the H2 production or benzyl viologen reduction assay. However, cell-free extracts of the P. furiosus Codh strain and of T. onnurineus had similar CO-dependent H2 formation activities (∼0.3 units mg-1; Table 2). After ultracentrifugation to separate the membrane fraction, this activity remained associated with the membrane, although in both cases more than half of the total activity was lost in the procedure (Table 2). As expected, the cytosolic fractions of the two strains did not contain any detectable CO-dependent H2 formation activity. However, they did contain high CO-oxidizing activity using benzyl viologen as the electron acceptor, and this represented virtually all (80–97%, Table 2) of the CO-oxidizing activity in the cell-free extract. These data suggest that the Codh module of the membrane-bound Codh complex (Mrp-Mbh-Codh) is at best loosely associated with the other two membrane-bound modules (Mrp and Mbh).
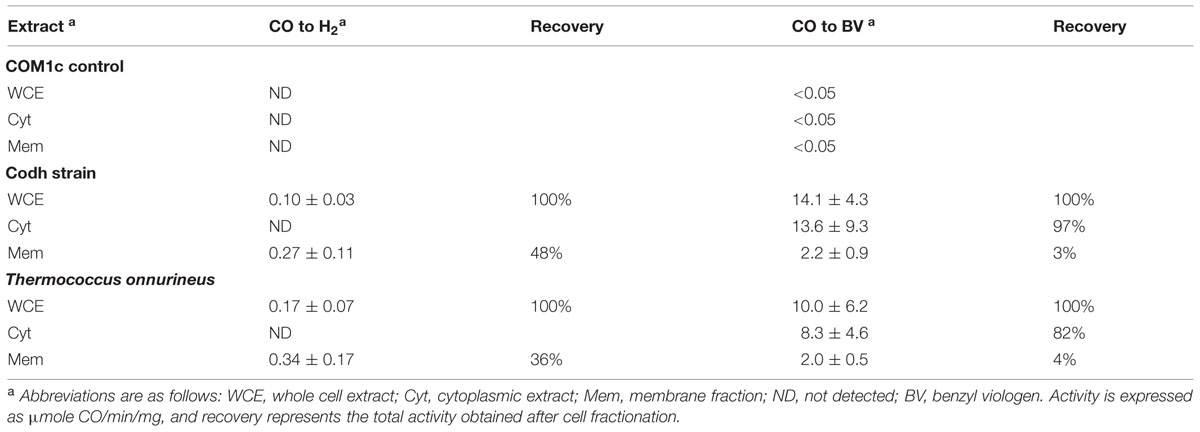
TABLE 2. CO-dependent activity in cellular extracts of P. furiosus strains Codh and COM1c and in Thermococcus onnurineus.
Growth of P. furiosus in the Presence of CO
To determine the phenotypic effect of the T. onnurineus Codh gene cluster on P. furiosus, the Codh strain and the COM1c control strain were grown with and without CO. The presence of 1.2 atm of CO did not seem to inhibit growth of either strain (Figure 2, Supplementary Figure S1). To ensure that the T. onnurineus Codh complex would be active, strains were grown at 80°C, near the optimal growth temperature of T. onnurineus. T. onnurineus has been shown to obtain energy for growth from CO oxidation (Yun et al., 2011). Consequently, in order to assess the effect of CO on the P. furiosus Codh strain, the amount of available organic carbon was limited in order to maximize the potential growth stimulating effect of CO oxidation. When a limiting amount of maltose was added as the carbon source, the P. furiosus Codh strain grew well, reaching ∼75 mg cell protein L-1 in the absence of CO, but the presence of CO in the headspace significantly increased growth to ∼120 mg cell protein L-1 (Figure 2). In addition, there was almost an order of magnitude increase in the amount of H2 produced compared to when CO was not present (Figure 2). At the end of growth, about 90% of the CO that was oxidized could be accounted for by the amount of H2 produced (after correcting for the H2 produced by the native P. furiosus Mbh in the absence of CO). This is close to the expected 1:1 ratio of CO oxidized to H2 produced.
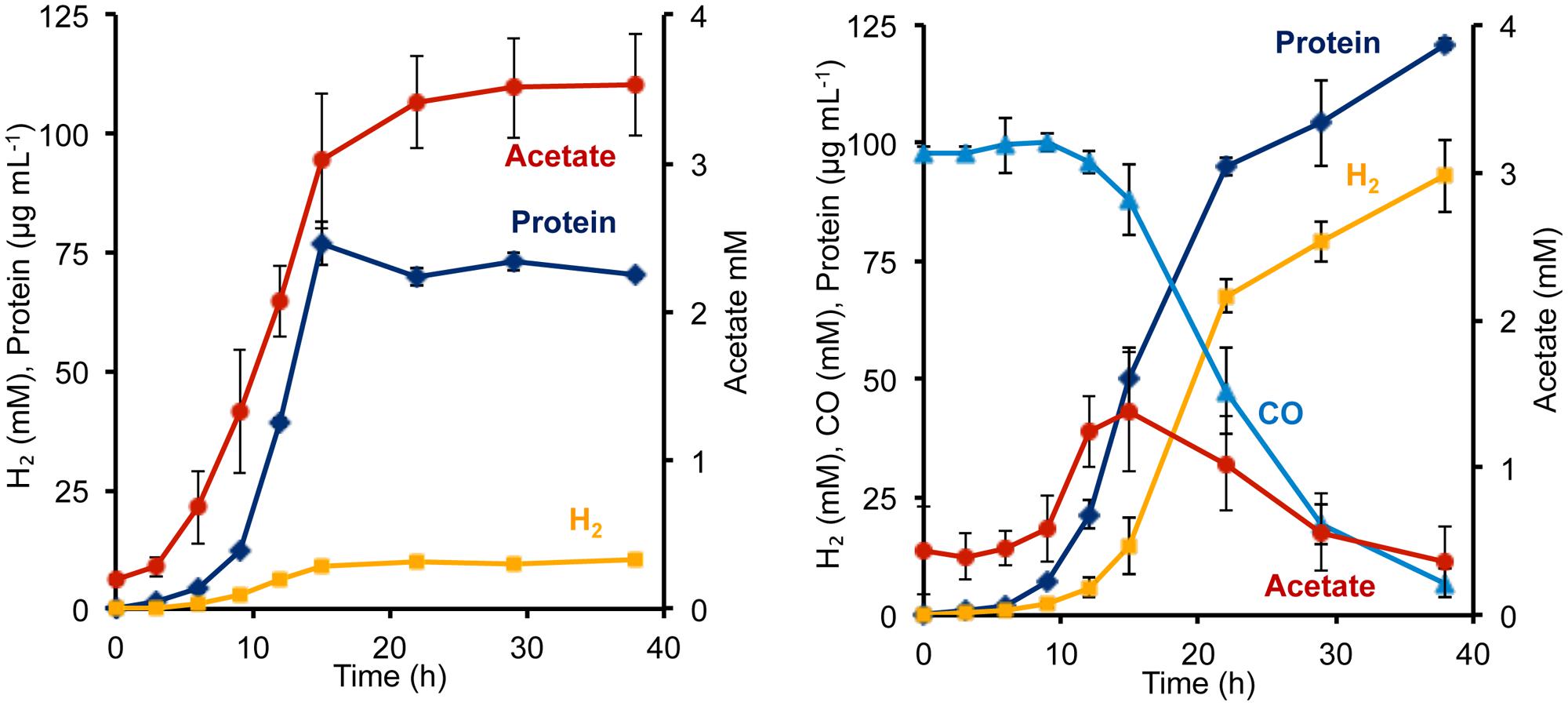
FIGURE 2. Growth characteristics of P. furiosus strain Codh in the absence (left) and presence (right) of CO with limiting maltose (0.5 g L-1) and yeast extract (1 g L-1). Compounds are represented as follows: blue triangles, CO; dark blue diamonds, cell protein; orange squares, H2; red circles, acetate. Error bars represent SD, n = 3.
Surprisingly, in the presence of CO, the Codh strain of P. furiosus was able to metabolize the acetate that it produced from the initial fermentation of the added sugar. We presume that this acetate is used for biosynthesis. As shown in Figure 2, in the absence of CO, the Codh strain produced approximately 3.5 mM acetate, but when CO was present, acetate production peaked at 1.3 mM at mid-log phase, just after CO began to be utilized. The acetate concentration then decreased to <0.5 mM as the CO concentration decreased to almost zero. Hence, it appears that CO oxidation provides energy, not only for growth, but also for acetate utilization as a source of carbon for biosynthesis. The kinetics of CO utilization and H2 production in the P. furiosus Codh strain are similar to that seen with T. onnurineus (Supplementary Figure S1), although T. onnurineus does not seem to be able to reutilize the acetate that is produced in the early growth phase. The P. furiosus parental strain COM1c did not utilize CO during growth, nor was growth inhibited by the presence of CO (Supplementary Figure S1).
During growth on sugar (maltose), P. furiosus generates reduced ferredoxin from sugar fermentation, and this is oxidized by the ferredoxin-dependent MBH to produce H2. During growth on peptides (1 g L-1 yeast extract), fermentation also generates NADPH, but its oxidation cannot be coupled directly to H2 production since MBH is specific for ferredoxin as the electron donor (Adams et al., 2001). Hence, in the absence of an external electron acceptor such as elemental sulfur, P. furiosus showed very poor growth on peptides, reaching <20 μg cell protein L-1, and only a low concentration of H2 was produced (3.6 mM; Figure 3). However, the addition of CO had a dramatic effect on the Codh strain of P. furiosus by significantly stimulating both growth and H2 production when peptides were used as the carbon source (Figure 3). Thus it is clear that the P. furiosus Codh strain obtains a significant amount of energy from the oxidation of CO. The contaminating glycans present in the yeast extract are assumed to be the source of the acetate that accumulated, and again, in the presence of CO, this was taken up and presumably assimilated into biomass (Figure 3). In addition to acetate, low concentrations of isobutyrate (up to 100 μM) and isovalerate (up to 200 μM) could also be detected in the medium at mid-log phase growth, and these are the products of amino acid fermentation. In the presence of CO, these organic acids were also consumed (data not shown).
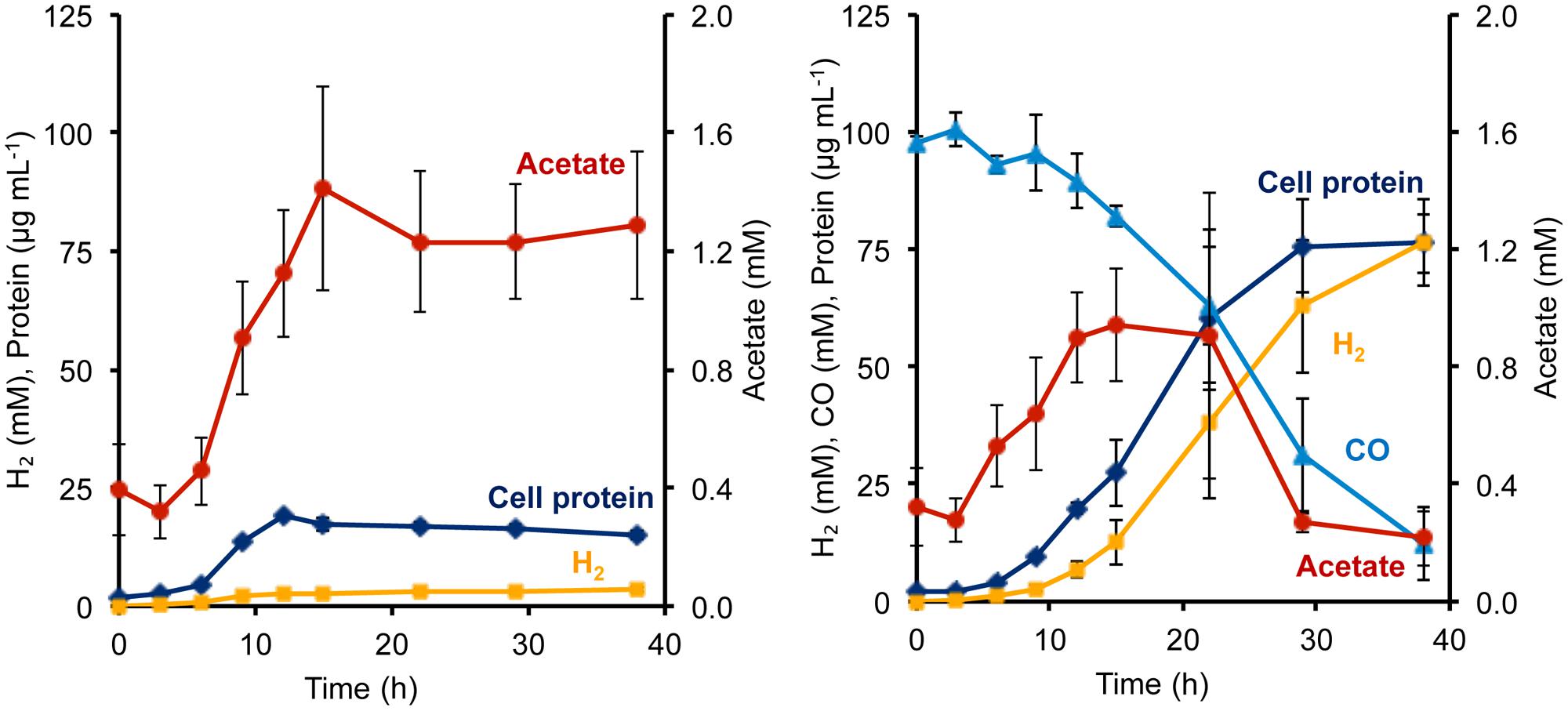
FIGURE 3. Growth characteristics of P. furiosus strain Codh in the absence (left) and presence (right) of CO with limiting yeast extract (1 g L-1). Compounds are represented as follows: blue triangles, CO; dark blue diamonds, cell protein; orange squares, H2; red circles, acetate. Error bars represent SD, n = 3.
Discussion
The ability to utilize CO in the form of syngas, which is a mixture of mainly CO and H2, is an attractive option for the microbial production of liquid biofuels such as alcohols (Bengelsdorf et al., 2013). Herein we show that the 16-gene cluster encoding the trimodular respiratory Codh complex (Mrp-Mbh-Codh) can be functionally transferred between Thermococcales species. With the acquisition of the T. onnurineus Mrp-Mbh-Codh gene cluster, the P. furiosus Codh strain can utilize CO as an energy source. P. furiosus is not capable of autotrophic growth (Fiala and Stetter, 1986; Sokolova et al., 2004), and so addition of organic carbon sources such as peptides (yeast extract) is required for growth under carboxydotrophic conditions. It is interesting that in the presence of CO, the Codh strain is capable of metabolizing exogenous acetate as well as organic acids derived from branched chain amino acids. Organic acid utilization is presumably accomplished via the reverse reaction of the ADP-dependent acetyl-CoA synthetase (ACS) group of enzymes. These catalyze the ATP-dependent conversion of organic acids to the corresponding CoA derivative, and these can then be converted to amino acids for biosynthesis (Mai and Adams, 1996). In the case of strain Codh, the ATP is supplied by P. furiosus ATP synthase using the Na+ gradient generated by Codh during CO oxidation. The genome of P. furiosus encodes 10 different ACS isozymes that together are capable of reversibly converting acyl and aryl-CoAs derived from almost all of the amino acids to their corresponding organic acids (Scott et al., 2014).
The Codh gene cluster therefore could provide a metabolic advantage for microorganisms in hydrothermal vent systems where CO can be generated by volcanic action, although measured concentrations of CO in these environments are rather low (1–2 μM) (Cadle, 1980; Charlou et al., 2002). The exchange of CO utilization capabilities between Thermococcales species has a distinct advantage for the organisms involved when CO becomes available. In addition, organisms containing an Mrp-Mbh-Codh complex can be utilized to produce biohydrogen from waste gasses that contain CO (Kim et al., 2013), and the Codh strain of P. furiosus now has application for this process as well. Previously we have engineered P. furiosus to convert organic acids to the corresponding alcohols. Here we also have shown that in the Codh complex can be utilized to provide low potential electrons directly from CO to drive reduction of organic acids to the corresponding alcohols rather than generating hydrogen gas (Basen et al., 2014).
Using the CO-dependent formation of H2 as the in vitro assay for the Codh complex, it was shown that this activity is, as expected, strictly associated with the membranes in P. furiosus and in T. onnurineus. The Mbh and Mrp modules must be membrane-associated if the free energy from CO oxidation is to be conserved via the generation of an ion gradient. Surprisingly, the Codh module of the Codh complex, as measured by CO oxidation coupled to the reduction of an artificial dye (benzyl viologen), was mainly located in the cytoplasmic fractions of cells from both species. It is not clear if the dissociation of the Codh module from the membrane is a result of cell breakage, if it is naturally loosely associated with the membrane, or even if it is naturally soluble and transfers electrons to the Mrp-Mbh modules via a cytoplasmic ferredoxin. The latter possibility is not unreasonable as ferredoxin is the natural electron donor to the bimodular MBH complex (Mrp-Mbh) of P. furiosus (Sapra et al., 2003). A soluble Codh module is also consistent with the observation of high dye-linked CO-oxidizing activity in the cytoplasmic fractions of other anaerobic microorganisms with analogous CO oxidizing systems, such as C. hydrogenoformans and Rhodospirillum rubrum (Svetlitchnyi et al., 2001; Singer et al., 2006). However, in the case of C. hydrogenoformans, an eight-subunit membrane bound Codh-Ech complex was successfully purified (Soboh et al., 2002).
The MBH complex consisting of two modules (Mrp-Mbh) is found in all known members of the Thermococcales order (Schut et al., 2013). The energy yield for P. furiosus MBH was estimated to be 0.3 ATP per mole of H2 produced (Sapra et al., 2003). Given that the Codh (Mrp-Mbh-Codh) complex contains the same two modules as MBH (Mrp-Mbh), we assume that the energy yields of the two systems are comparable and that 0.3 ATP is generated for every mole of CO oxidized. Based on the standard molar growth yield per mole of ATP (Stouthamer and Bettenhausen, 1973), we plotted the calculated cell protein yield based on the amount of CO oxidized using the data from Figure 3. As shown in Figure 4, during exponential growth, the calculated growth curve is reasonably close to that measured, so the production of 0.3 ATP per CO oxidized is a good representation of the energetics of CO metabolism. Previously, the ion translocating trimodular formate hydrogen lyase (FHL; Mrp-Mbh-Fdh) of T. onnurineus was heterologously expressed in P. furiosus (Kim et al., 2010; Lipscomb et al., 2014). However, in contrast to the situation reported here with Codh, the recombinant P. furiosus strain did not display formate-dependent growth on peptides or limiting amounts of sugar, even though it converted high concentrations of formate (>50 mM) to H2 (Lipscomb et al., 2014). In contrast to P. furiosus, T. onnurineus was reported to use formate oxidation coupled to H2 production as a source of energy (Kim et al., 2010). The Gibbs free energy for formate to hydrogen conversion at 80°C was calculated to be -2.7 kJ/mol and +1.3 kJ/mol at 25°C, and this was used as the rationale for why formate oxidation is energetically favorable at elevated temperatures (Kim et al., 2010). However, how T. onnurineus maintains local low concentrations of H2 during growth on formate in order to allow enough energy for ion translocation is not clear (Lim et al., 2012). In contrast, in the case of CO conversion to H2, more than enough energy is available for a minimum biological quantum of 20 kJ/mol to translocate ions over the membrane and allow ultimately for the synthesis of ATP (Schink, 1997).
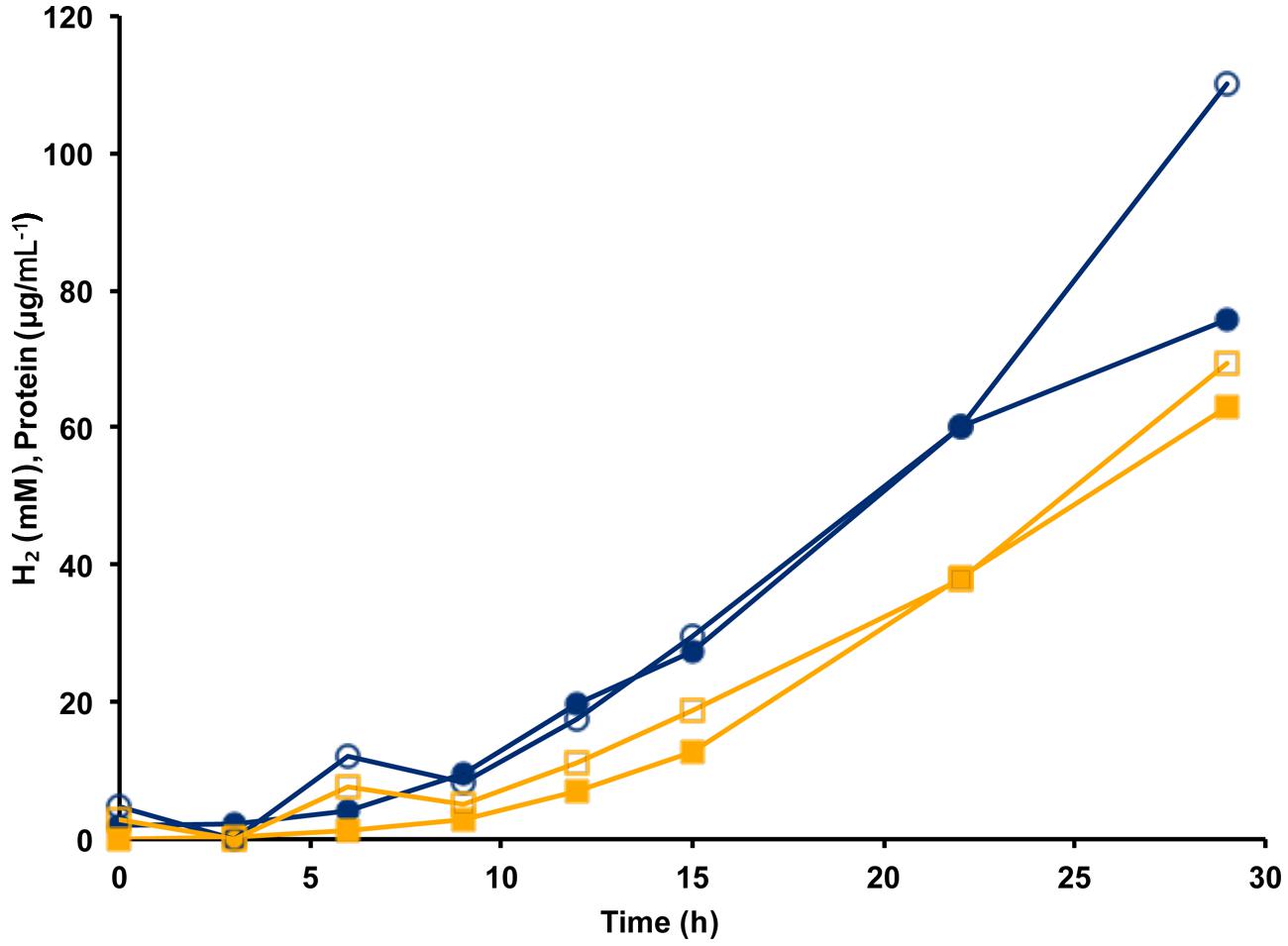
FIGURE 4. Calculation of cell yield from CO utilization in the presence of minimal fixed carbon (1 g L-1 yeast extract) using an estimated yield of 0.3 ATP per mol CO oxidized/H2 produced. Cell growth as represented by cell protein is indicated by blue lines with closed circles showing measured protein and open circles indicating calculated protein. Hydrogen formation is represented by orange lines with closed squares showing measured H2 (mM) and open squares indicating estimated H2 calculated from the amount of CO utilized.
Author Contributions
GL constructed the recombinant strains. GS and DN carried out the physiological, biochemical, and chemical analyses. RK and MA designed and oversaw the research. All authors contributed to writing and editing the manuscript.
Funding
Microbial strain construction was supported by the National Science Foundation (CBET-1264052/CBET-1264053). Biochemical and phenotypic analyses were carried out as part of the Biological and Electron Transfer and Catalysis (BETCy) Energy Frontiers Research Center funded by the U.S. Department of Energy (DE-SC0012518).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Sung Gyun Kang, Korea Institute of Ocean Science and Technology for providing us with the T. onnurineus NA1 strain.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00029
References
Adams, M. W. W., Holden, J. F., Menon, A. L., Schut, G. J., Grunden, A. M., Hou, C., et al. (2001). Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183, 716–724. doi: 10.1128/JB.183.2.716-724.2001
Bae, S. S., Kim, T. W., Lee, H. S., Kwon, K. K., Kim, Y. J., Kim, M. S., et al. (2012). H2 production from CO, formate or starch using the hyperthermophilic archaeon, Thermococcus onnurineus. Biotechnol. Lett. 34, 75–79. doi: 10.1007/s10529-011-0732-3
Basen, M., Schut, G. J., Nguyen, D. M., Lipscomb, G. L., Benn, R. A., Prybol, C. J., et al. (2014). Single gene insertion drives bioalcohol production by a thermophilic archaeon. Proc. Natl. Acad. Sci. U.S.A. 111, 17618–17623. doi: 10.1073/pnas.1413789111
Bengelsdorf, F. R., Straub, M., and Durre, P. (2013). Bacterial synthesis gas (syngas) fermentation. Environ. Technol. 34, 1639–1651. doi: 10.1080/09593330.2013.827747
Cadle, R. D. (1980). A comparison of volcanic with other fluxes of atmospheric trace gas constituents. Rev. Geophys. 18, 746–752. doi: 10.1029/Rg018i004p00746
Charlou, J. L., Donval, J. P., Fouquet, Y., Jean-Baptiste, P., and Holm, N. (2002). Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36 degrees 14 ’ N, MAR). Chem. Geol. 191, 345–359. doi: 10.1016/S0009-2541(02)00134-1
Costa, K. C., and Leigh, J. A. (2014). Metabolic versatility in methanogens. Curr. Opin. Biotechnol. 29C, 70–75. doi: 10.1016/j.copbio.2014.02.012
Dobbek, H., Svetlitchnyi, V., Gremer, L., Huber, R., and Meyer, O. (2001). Crystal structure of a carbon monoxide dehydrogenase reveals a [Ni-4Fe-5S] cluster. Science 293, 1281–1285. doi: 10.1126/science.1061500
Drake, H. L., and Daniel, S. L. (2004). Physiology of the thermophilic acetogen Moorella thermoacetica. Res. Microbiol. 155, 869–883. doi: 10.1016/j.resmic.2004.10.002
Fiala, G., and Stetter, K. O. (1986). Pyrococcus furiosus sp. nov., represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 145, 56–61. doi: 10.1007/BF00413027
Hawkins, A. S., Mcternan, P. M., Lian, H., Kelly, R. M., and Adams, M. W. (2013). Biological conversion of carbon dioxide and hydrogen into liquid fuels and industrial chemicals. Curr. Opin. Biotechnol. 24, 376–384. doi: 10.1016/j.copbio.2013.02.017
Henstra, A. M., and Stams, A. J. (2011). Deep conversion of carbon monoxide to hydrogen and formation of acetate by the anaerobic thermophile Carboxydothermus hydrogenoformans. Int. J. Microbiol. 2011, 641582. doi: 10.1155/2011/641582
Keller, M. W., Schut, G. J., Lipscomb, G. L., Menon, A. L., Iwuchukwu, I. J., Leuko, T. T., et al. (2013). Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc. Natl. Acad. Sci. U.S.A. 110, 5840–5845. doi: 10.1073/pnas.1222607110
Kim, M. S., Bae, S. S., Kim, Y. J., Kim, T. W., Lim, J. K., Lee, S. H., et al. (2013). CO-dependent H2 production by genetically engineered Thermococcus onnurineus NA1. Appl. Environ. Microbiol. 79, 2048–2053. doi: 10.1128/AEM.03298-12
Kim, Y. J., Lee, H. S., Kim, E. S., Bae, S. S., Lim, J. K., Matsumi, R., et al. (2010). Formate-driven growth coupled with H2 production. Nature 467, 352–355. doi: 10.1038/nature09375
Kung, Y., Doukov, T. I., Seravalli, J., Ragsdale, S. W., and Drennan, C. L. (2009). Crystallographic snapshots of cyanide– and water-bound C-clusters from bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Biochemistry 48, 7432–7440. doi: 10.1021/bi900574h
Lim, J. K., Bae, S. S., Kim, T. W., Lee, J. H., Lee, H. S., and Kang, S. G. (2012). Thermodynamics of formate-oxidizing metabolism and implications for H2 production. Appl. Environ. Microbiol. 78, 7393–7397. doi: 10.1128/AEM.01316-12
Lim, J. K., Kang, S. G., Lebedinsky, A. V., Lee, J. H., and Lee, H. S. (2010). Identification of a novel class of membrane-bound [NiFe]-hydrogenases in Thermococcus onnurineus NA1 by in silico analysis. Appl. Environ. Microbiol. 76, 6286–6289. doi: 10.1128/AEM.00123-10
Lim, J. K., Mayer, F., Kang, S. G., and Muller, V. (2014). Energy conservation by oxidation of formate to carbon dioxide and hydrogen via a sodium ion current in a hyperthermophilic archaeon. Proc. Natl. Acad. Sci. U.S.A. 111, 11497–11502. doi: 10.1073/pnas.1407056111
Lipscomb, G. L., Schut, G. J., Thorgersen, M. P., Nixon, W. J., Kelly, R. M., and Adams, M. W. W. (2014). Engineering hydrogen gas production from formate in a hyperthermophile by heterologous production of an 18-subunit membrane-bound complex. J. Biol. Chem. 289, 2873–2879. doi: 10.1074/jbc.M113.530725
Lipscomb, G. L., Stirrett, K., Schut, G. J., Yang, F., Jenney, F. E. Jr., Scott, R. A., et al. (2011). Natural competence in the hyperthermophilic archaeon Pyrococcus furiosus facilitates genetic manipulation: construction of markerless deletions of genes encoding the two cytoplasmic hydrogenases. Appl. Environ. Microbiol. 77, 2232–2238. doi: 10.1128/AEM.02624-10
Mai, X., and Adams, M. W. (1996). Purification and characterization of two reversible and ADP-dependent acetyl coenzyme A synthetases from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 178, 5897–5903. doi: 10.1111/j.1432-1033.1997.00561.x
McTernan, P. M., Chandrayan, S. K., Wu, C. H., Vaccaro, B. J., Lancaster, W. A., Yang, Q., et al. (2014). Intact functional fourteen-subunit respiratory membrane-bound [NiFe]-hydrogenase complex of the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 289, 19364–19372. doi: 10.1074/jbc.M114.567255
Meuer, J., Bartoschek, S., Koch, J., Kunkel, A., and Hedderich, R. (1999). Purification and catalytic properties of Ech hydrogenase from Methanosarcina barkeri. Eur. J. Biochem. 265, 325–335. doi: 10.1046/j.1432-1327.1999.00738.x
Oelgeschlager, E., and Rother, M. (2008). Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch. Microbiol. 190, 257–269. doi: 10.1007/s00203-008-0382-6
Pisa, K. Y., Huber, H., Thomm, M., and Muller, V. (2007). A sodium ion-dependent A1AO ATP synthase from the hyperthermophilic archaeon Pyrococcus furiosus. FEBS J. 274, 3928–3938. doi: 10.1111/j.1742-4658.2007.05925.x
Ragsdale, S. W., Kumar, M., Seravalli, J., Qiu, D., and Spiro, T. G. (1996). “Anaerobic carbon monoxide dehydrogenase,” in Microbial Growth on C1 Compounds, eds M. Lidstrom and F. R. Tabita (Dordrecht: Springer Netherlands), 191–196.
Rittmann, S. K., Lee, H. S., Lim, J. K., Kim, T. W., Lee, J. H., and Kang, S. G. (2015). One-carbon substrate-based biohydrogen production: microbes, mechanism, and productivity. Biotechnol. Adv. 33, 165–177. doi: 10.1016/j.biotechadv.2014.11.004
Sapra, R., Bagramyan, K., and Adams, M. W. (2003). A simple energy-conserving system: proton reduction coupled to proton translocation. Proc. Natl. Acad. Sci. U.S.A. 100, 7545–7550. doi: 10.1073/pnas.1331436100
Schink, B. (1997). Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61, 262–280.
Schuchmann, K., and Muller, V. (2014). Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 12, 809–821. doi: 10.1038/nrmicro3365
Schut, G. J., Boyd, E. S., Peters, J. W., and Adams, M. W. (2013). The modular respiratory complexes involved in hydrogen and sulfur metabolism by heterotrophic hyperthermophilic archaea and their evolutionary implications. FEMS Microbiol. Rev. 37, 182–203. doi: 10.1111/j.1574-6976.2012.00346.x
Schut, G. J., Lipscomb, G., Han, Y., Notey, J. S., Kelly, R. M., and Adams, M. W. W. (2014). “The order thermococcales and the family thermococcaceae,” in The Prokaryotes: Other Major Lineages of Bacteria and the Archaea, 4th Edn, ed. E. Rosenberg (Heidelberg: Springer Berlin), 363–383.
Scott, J. W., Poole, F. L., and Adams, M. W. (2014). Characterization of ten heterotetrameric NDP-dependent acyl-CoA synthetases of the hyperthermophilic archaeon Pyrococcus furiosus. Archaea 2014, 176863. doi: 10.1155/2014/176863
Singer, S. W., Hirst, M. B., and Ludden, P. W. (2006). CO-dependent H2 evolution by Rhodospirillum rubrum: role of CODH : CooF complex. Biochim. Biophys. Acta 1757, 1582–1591. doi: 10.1016/j.bbabio.2006.10.003
Soboh, B., Linder, D., and Hedderich, R. (2002). Purification and catalytic properties of a CO-oxidizing: H2-evolving enzyme complex from Carboxydothermus hydrogenoformans. Eur. J. Biochem. 269, 5712–5721. doi: 10.1046/j.1432-1033.2002.03282.x
Sokolova, T. G., Henstra, A. M., Sipma, J., Parshina, S. N., Stams, A. J., and Lebedinsky, A. V. (2009). Diversity and ecophysiological features of thermophilic carboxydotrophic anaerobes. FEMS Microbiol. Ecol. 68, 131–141. doi: 10.1111/j.1574-6941.2009.00663.x
Sokolova, T. G., Jeanthon, C., Kostrikina, N. A., Chernyh, N. A., Lebedinsky, A. V., Stackebrandt, E., et al. (2004). The first evidence of anaerobic CO oxidation coupled with H2 production by a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Extremophiles 8, 317–323. doi: 10.1007/s00792-004-0389-0
Stouthamer, A., and Bettenhausen, C. (1973). Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms – re-evaluation of method for determination of ATP production by measuring molar growth yields. Biochim. Biophys. Acta 301, 53–70. doi: 10.1016/0304-4173(73)90012-8
Svetlichny, V. A., Sokolova, T. G., Gerhardt, M., Ringpfeil, M., Kostrikina, N. A., and Zavarzin, G. A. (1991). Carboxydothermus hydrogenoformans Gen-nov, Sp-nov, a CO-utilizing thermophilic anaerobic bacterium from hydrothermal environments of Kunashir island. Syst. Appl. Microbiol. 14, 254–260. doi: 10.1016/S0723-2020(11)80377-2
Svetlitchnyi, V., Dobbek, H., Meyer-Klaucke, W., Meins, T., Thiele, B., Romer, P., et al. (2004). A functional Ni-Ni-[4Fe-4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans. Proc. Natl. Acad. Sci. U.S.A. 101, 446–451. doi: 10.1073/pnas.0304262101
Svetlitchnyi, V., Peschel, C., Acker, G., and Meyer, O. (2001). Two membrane-associated NiFeS-carbon monoxide dehydrogenases from the anaerobic carbon-monoxide-utilizing eubacterium Carboxydothermus hydrogenoformans. J. Bacteriol. 183, 5134–5144. doi: 10.1128/JB.183.17.5134-5144.2001
Techtmann, S. M., Lebedinsky, A. V., Colman, A. S., Sokolova, T. G., Woyke, T., Goodwin, L., et al. (2012). Evidence for horizontal gene transfer of anaerobic carbon monoxide dehydrogenases. Front. Microbiol. 3:132. doi: 10.3389/fmicb.2012.00132
Thauer, R. K. (1990). Energy-metabolism of methanogenic bacteria. Biochim. Biophys. Acta 1018, 256–259. doi: 10.1016/0005-2728(90)90261-2
Thorgersen, M. P., Lipscomb, G. L., Schut, G. J., Kelly, R. M., and Adams, M. W. W. (2014). Deletion of acetyl-CoA synthetases I and II increases production of 3-hydroxypropionate by the metabolically-engineered hyperthermophile Pyrococcus furiosus. Metab. Eng. 22, 83–88. doi: 10.1016/j.ymben.2013.12.006
Uffen, R. L. (1976). Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc. Natl. Acad. Sci. U.S.A. 73, 3298–3302. doi: 10.1073/pnas.73.9.3298
Wood, H. G. (1991). Life with CO or CO2 and H2 as a source of carbon and energy. FASEB J. 5, 156–163.
Wu, M., Ren, Q., Durkin, A. S., Daugherty, S. C., Brinkac, L. M., Dodson, R. J., et al. (2005). Life in hot carbon monoxide: the complete genome sequence of Carboxydothermus hydrogenoformans Z-2901. PLoS Genet. 1:e65. doi: 10.1371/journal.pgen.0010065
Yoon, S. H., Reiss, D. J., Bare, J. C., Tenenbaum, D., Pan, M., Slagel, J., et al. (2011). Parallel evolution of transcriptome architecture during genome reorganization. Genome Res. 21, 1892–1904. doi: 10.1101/gr.122218.111
Keywords: hyperthermophile, archaea, hydrogen, carbon monoxide, energy, anaerobic respiration, Thermococcales
Citation: Schut GJ, Lipscomb GL, Nguyen DMN, Kelly RM and Adams MWW (2016) Heterologous Production of an Energy-Conserving Carbon Monoxide Dehydrogenase Complex in the Hyperthermophile Pyrococcus furiosus. Front. Microbiol. 7:29. doi: 10.3389/fmicb.2016.00029
Received: 17 November 2015; Accepted: 11 January 2016;
Published: 29 January 2016.
Edited by:
Philippe M. Oger, UMR CNRS 5276 Ecole Normale Supérieure de Lyon, FranceReviewed by:
James F. Holden, University of Massachusetts Amherst, USAAlexander V. Lebedinsky, Winogradsky Institute of Microbiology, Russia
Copyright © 2016 Schut, Lipscomb, Nguyen, Kelly and Adams. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael W. W. Adams, YWRhbXNtQHVnYS5lZHU=
 Gerrit J. Schut
Gerrit J. Schut Gina L. Lipscomb1
Gina L. Lipscomb1 Diep M. N. Nguyen
Diep M. N. Nguyen Robert M. Kelly
Robert M. Kelly Michael W. W. Adams
Michael W. W. Adams