- 1Unitat Mixta d’Investigació en Genòmica i Salut, Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (FISABIO)-Salud Pública/Institut Cavanilles de Biodiversitat i Biologia Evolutiva, Universitat de València, València, Spain
- 2Consorcio de Investigación Biomédica en Red de Epidemiología y Salud Pública, Madrid, Spain
The human microbiome is overly exposed to antibiotics, due, not only to their medical use, but also to their utilization in farm animals and crops. Microbiome composition can be rapidly altered by exposure to antibiotics, with potential immediate effects on health, for instance through the selection of resistant opportunistic pathogens that can cause acute disease. Microbiome alterations induced by antibiotics can also indirectly affect health in the long-term. The mutualistic microbes in the human body interact with many physiological processes, and participate in the regulation of immune and metabolic homeostasis. Therefore, antibiotic exposure can alter many basic physiological equilibria, promoting long-term disease. In addition, excessive antibiotic use fosters bacterial resistance, and the overly exposed human microbiome has become a significant reservoir of resistance genes, contributing to the increasing difficulty in controlling bacterial infections. Here, the complex relationships between antibiotics and the human microbiome are reviewed, with focus on the intestinal microbiota, addressing (1) the effects of antibiotic use on the composition and function of the gut microbiota, (2) the impact of antibiotic-induced microbiota alterations on immunity, metabolism, and health, and (3) the role of the gut microbiota as a reservoir of antibiotic resistances.
Effects of Antibiotics on the Gut Microbiota
Several lines of evidence confirm that antibiotic administration can result in gut microbiota dysbiosis, i.e., disturbance in composition and function. Broad-spectrum antibiotics can affect the abundances of 30% of the bacteria in the gut community, causing rapid and significant drops in taxonomic richness, diversity and evenness (Dethlefsen et al., 2008; Dethlefsen and Relman, 2011). Once antibiotic treatment has stopped, the microbiota may present a certain degree of resilience, being capable of returning to a composition similar to the original one, but the initial state is often not totally recovered. In fact, antibiotic-induced microbiota alterations can remain after long periods of time, spanning months and even years (De La Cochetiere et al., 2005; Jernberg et al., 2007; Dethlefsen et al., 2008; Dethlefsen and Relman, 2011). In infants, few studies have gaged the extent to which gut microbiota development is affected by early exposure to antibiotics, in spite of the major impact of this process on life-long health. Tanaka et al. (2009) and Fouhy et al. (2012) studied the microbiota of infants treated with antibiotics in the first days of life, and reported effects within 1 week and within 2 months after birth. Early antibiotic exposure both reduced the diversity of the infants’ microbiota and altered its composition, with an attenuation of Bifidobacterium and marked increases of Proteobacteria. Moreover, the microbiota of those infants who were not treated, but whose mothers received antibiotics prior to delivery, showed the same alterations seen in the microbiota of the treated infants (Tanaka et al., 2009).
The impact of antibiotics on the gut microbiota has more recently been investigated through the variety of “omic” techniques available today for microbial community analyses (reviewed in Franzosa et al., 2015). These works have shown that, beyond altering the composition of taxa, antibiotics also affect the gene expression, protein activity and overall metabolism of the gut microbiota. These changes can occur at a much faster pace than those involving replacement of taxa in the community (Perez-Cobas et al., 2012). Moreover, the induced changes can drive the functionality of the microbiota toward states similar to those observed under disease conditions. In this direction, the microbiota of individuals treated with β-lactams has a repertoire of enzymatic activities for carbohydrate degradation that results in an unbalanced sugar metabolism, similar to that observed in obese individuals (Hernandez et al., 2013). Experimental approaches have also confirmed that antibiotics rapidly alter the physiological state and activity of the gut microbiota. In ex vivo incubations of fecal samples with different antibiotics, there was an increase in the proportion of gut microbiota cells with damaged membranes, the active populations of the microbiota changed, and genes involved in antibiotic resistance, stress response and phage induction augmented in expression (Maurice et al., 2013). In addition, expression also increased for genes related to genetic information processing (e.g., transcription and translation) in the case of antibiotics that inhibit translation, such as tetracycline and the macrolides. The substantial effects documented for antibiotics on the functioning of the gut microbiota stress the likely impact that antibiotic exposure will have on the physiological processes that depend on the activities performed by the microbes in this community.
Effects of Antibiotic-Induced Microbiota Alterations on Immune and Metabolic Health
Increased Susceptibility to Infections
One of the most imminent threats of gut microbiota alterations is the increased susceptibility to intestinal infections, which can stem from newly acquired pathogens or from the sudden overgrowth and pathogenic behavior of opportunistic organisms already present in the microbiota. In particular, antibiotic-associated diarrheas (AAD) due to nosocomial pathogens are a frequent occurrence. These are often associated with organisms such as Klebsiella pneumoniae, Staphylococcus aureus and, of most concern, Clostridium difficile, which can cause intractable, long-term recurrent infections and even a potentially lethal pseudomembranous colitis (Wilcox, 2003; Young and Schmidt, 2004; Song et al., 2008; Rupnik et al., 2009; Sekirov et al., 2010; Chen et al., 2013). A mouse model has provided evidence that the substantial losses of diversity caused by antibiotics in the small and large intestinal microbiota can result in the establishment of a chronic infection with C. difficile (Lawley et al., 2009; Buffie et al., 2012).
In addition, bloodstream infection in immunocompromised individuals is another life-threatening condition that increases in risk due to antibiotic treatment. In the clinical setting, intestinal domination by vancomycin-resistant Enterococcus has been shown to precede bloodstream infection by this pathogen, and experimental work in mice has established that antibiotic treatment sets the stage for the intestinal outgrowth of this bacterium (Ubeda et al., 2010). In premature infants, who are heavily treated with broad-spectrum antibiotics, the risk of sepsis has also been related to gut microbiota composition and length of antibiotic treatment (Madan et al., 2012; Mai et al., 2013).
Compromised Immune Homeostasis and Tolerance
The microbiota alterations caused by antibiotics, beyond increasing the immediate risk for infection, can also affect basic immune homeostasis with body-wide and long-term repercussions. Atopic, inflammatory and autoimmune diseases have been linked to gut microbiota dysbiosis, and, in some cases, significant associations have been established between these diseases and the intake of antibiotics during early life. Clearly, the effects of antibiotic-induced dysbiosis will be even more relevant if they occur early in life, a critical period for maturation of the immune system and establishment of immunological tolerance (Francino, 2014).
In the case of atopic diseases, numerous studies have demonstrated links to the composition of the gut microbiota during infancy and early childhood (Kuvaeva et al., 1984; Wold, 1998; Penders et al., 2006; Wang et al., 2008; Bisgaard et al., 2011; Abrahamsson et al., 2012). Most recurrently, a significant association has been detected with bifidobacteria deficiency (Sepp et al., 1997, 2005; Bjorksten et al., 1999; Kalliomaki et al., 2001; Mah et al., 2006; Sjogren et al., 2009). However, this link could not be upheld in two large prospective case-control studies (Murray et al., 2005; Penders et al., 2006). As not all Bifidobacterium species appear to have a protective role (He et al., 2001; Ouwehand et al., 2001; Sjogren et al., 2009), these discrepancies among studies could be due to the presence of different bifidobacteria in different geographical regions, in addition to the likely contribution of genetic variation among human populations. On the other hand, high abundances of organisms such as Clostridium coccoides and Escherichia coli and a microbiota of low diversity have also been repeatedly associated to the presence of different atopic diseases (Bjorksten et al., 2001; Sepp et al., 2005; Mah et al., 2006; Wang et al., 2008; Bisgaard et al., 2011; Thompson-Chagoyan et al., 2011; Abrahamsson et al., 2012). These associations suggest that early antibiotic use would likely increase the risk for atopic disease, but the existence of such link has been controversial (Bedford Russell and Murch, 2006; Kuo et al., 2013). Retrospective epidemiological studies have generally supported the association (Alm et al., 1999; von Mutius et al., 1999; Wickens et al., 1999; Droste et al., 2000; Wjst et al., 2001; Thomas et al., 2006; Foliaki et al., 2009), but most prospective analyses have failed to do so (Illi et al., 2001; Celedon et al., 2002, 2004; Harris et al., 2007; Wickens et al., 2008; Mai et al., 2010; Su et al., 2010). Nevertheless, the application of techniques aimed at reducing potential biases and confounding effects has resulted in the detection of dose-dependent associations between asthma and early life exposure to antibiotics in several prospective studies (McKeever et al., 2002; Kozyrskyj et al., 2007; Marra et al., 2009). Moreover, broad-spectrum antibiotics show a stronger association with asthma, indicating that a reduction of bacterial diversity in the microbiota is likely to contribute to the effect of antibiotics on asthma development (McKeever et al., 2002; Kozyrskyj et al., 2007). In addition to asthma, other allergic outcomes have also recently been associated with early intake of antibiotics (Risnes et al., 2011). Similarly, the risk for several atopic diseases is increased by maternal intake of antibiotics during pregnancy, in a dose-dependent manner (Jedrychowski et al., 2006).
Gut microbiota composition has also been linked to numerous disorders involving processes of inflammation and autoimmunity. This is the case of necrotizing enterocolitis (NEC), a devastating inflammatory disease for newborns. A low abundance of Bifidobacterium, accompanied by a generally low bacterial diversity, has been detected before NEC onset (Mai et al., 2011, 2013). Morover, populations exposed to antibiotics, such as preterm infants (Deshpande et al., 2010) and infants whose mothers receive antibiotics in order to defer labor (Kenyon et al., 2001), present an increased incidence of NEC. Chron’s disease (CD), another inflammatory bowel disease (IBD), also increases in those children who receive antibiotics before 5 years of age (Hildebrand et al., 2008). This disease was one of the first for which an association with the human gut microbiota was clearly established through metagenomic analyses, with a reduction in Firmicutes (particularly C. leptum) and an increase of some Gram-negative bacteria (Porfiromonadaceae) often responsible for inflammatory processes (Manichanh et al., 2006; Vanderploeg et al., 2010). In the case of Irritable Bowel Syndrome (IBS), which is the most common functional gastrointestinal disorder in western countries, alterations in the gut microbiota have also been detected (Vanner, 2008; Yamini and Pimentel, 2010; Durban et al., 2012). Although no consensus has been reached regarding the association between specific bacteria and IBS, the gut microbiota of IBS patients has a reduced diversity. Moreover, IBS often follows bouts of gastrointestinal infection and there is evidence to suggest that antibiotics may play a role in the pathogenesis of the disorder (Mendall and Kumar, 1998).
Deregulated Metabolism
Increasingly, the gut microbiota is being established as an important factor in the regulation of host metabolism, in particular as it relates to energy homeostasis and adiposity. Several metabolic disorders have been linked with gut microbiota dysbiosis. In particular, obesity has been associated with phylum-level changes in the gut microbiota, reduced bacterial diversity, and altered representation of bacterial genes and metabolic pathways, differences that endow the obesity-associated microbiota with an increased capacity to harvest energy from the diet (Backhed et al., 2004; Turnbaugh et al., 2006, 2009). This is in line with the fact that long-term exposure to antibiotics is associated with increased body mass index, both in humans (Thuny et al., 2010; Ajslev et al., 2011; Angelakis et al., 2012) and in farm animals, where low-dose antibiotics have long been used to promote weight gain (Burch, 1996). Moreover, recent work in mice has shown that early antibiotic exposure can cause obesity even with normal dietary intake (Cho et al., 2012). Antibiotic use is therefore emerging as an important risk factor for the development of obesity. In addition, it may also contribute to the onset of metabolic syndrome in obese individuals. The metabolic syndrome is a cluster of metabolic conditions that increase the risk for cardiovascular disease, fatty liver disease, steatohepatitis and type 2 diabetes. The advancement from obesity to metabolic syndrome appears to involve the establishment of a state of chronic low-grade inflammation, which could be exacerbated by antibiotic use (Emanuela et al., 2012; Francino and Moya, 2013).
On the other hand, antibiotics have also recently been implicated in increasing the risk for type 1, insulin-dependent diabetes, an autoimmune disease whose incidence has been steadily going up in industrialized countries in the last decades. In an epidemiological study involving a large UK population, the repeated use of penicillin, cephalosporins, macrolides, or quinolones was associated with increase in diabetic risk (Boursi et al., 2015). In a mouse model of type I diabetes, different antibiotic treatments that altered gut microbiota composition were also shown to significantly increase the incidence of the disease (Candon et al., 2015).
Through What Mechanisms Do Gut Microbiota Alterations Affect Immunity and Metabolism?
Besides their direct ecological effects on the composition of the gut microbiota, antibiotics affect the manner in which this community interacts with the host and regulates basic physiological processes. Regarding the immune system’s capacity to fight infections, antibiotics indirectly alter the effectiveness of both innate and adaptive immune responses. As microbiota composition changes, not only non-resistant organisms capable of outcompeting potential pathogens are lost, but the altered community will present a substantially different repertoire of microbial-associated molecular patterns (MAMPS) to the receptors located in immune and epithelial cells. This will result in an altered stimulation of receptors such as NOD1 and the Toll-like receptors (TLRs), which can cascade down through a variety of immune processes, including lymphoid tissue development, T cell differentiation, neutrophil priming, production of antibacterials, and cytokine release (Ubeda and Pamer, 2012). A series of experiments in mice have shown that antibiotic treatment can reduce the capacity to fight infections by Gram-positive organisms by decreasing the expression of bactericidal compounds and diminishing neutrophil-mediated killing (Brandl et al., 2008; Vaishnava et al., 2008; Clarke et al., 2010; Ubeda et al., 2010). In the case of the adaptive immune system, both the expression of Major Histocompatibility Complex genes in the small and large intestine and the levels of immunoglobulin G (IgG) in serum have been shown to decrease in reponse to amoxicillin-induced gut microbiota changes (Dufour et al., 2005).
On the other hand, the cellular and molecular mechanisms by which gut microbiota alterations impact immunotolerance have long been debated (Rautava et al., 2004; Romagnani, 2004; Penders et al., 2007a; Sjogren et al., 2009; Jutel and Akdis, 2011). The balance between the Th1 and Th2 helper cell subsets of the adaptive immune system was, until recently, thought to be the main condition required for maintaining immune homeostasis. In support of this notion, chronic inflammatory/autoimmune and allergic diseases are known to associate with excessive Th1 or Th2 activation, respectively (Abbas, 1996; Oboki et al., 2008). Nevertheless, important roles for Th17 cells and regulatory T cells (Tregs) have been demonstrated in diseases that had classically been defined as Th1 or Th2-mediated (Nakae et al., 2002; Murphy, 2003; Oboki et al., 2008; Akdis and Akdis, 2009). In the current view, it is a new cellular balance that is considered critical for immune homeostasis: that between the Tregs and their effector cells, the different Th subsets. Alterations of the gut microbiota disrupt this balance, resulting in the deregulation of immune responses that can promote a variety of disease outcomes (Wills-Karp et al., 2001; Yazdanbakhsh et al., 2002; Rautava et al., 2004; Rook and Brunet, 2005; Penders et al., 2007b; Shen et al., 2014).
The generation of Tregs has indeed been shown to depend on the crosstalk between the gut microbiota and the immune system (Strauch et al., 2005). Experiments in mice or in in vitro cell cultures have revealed different specific bacteria and bacterial products that are capable of Treg cell induction (Kline, 2007; Baba et al., 2008). For instance, Bacteroides fragilis (Round and Mazmanian, 2010) and Clostridium species belonging to phylogenetic groups IV and XIV (Atarashi et al., 2011) promote the differentiation of T cells into Tregs in mice. In contrast, the Segmented Filamentous Bacteria (SFB) rather promote the differentiation of pro-inflammatory Th17 cells (Ivanov et al., 2009). This highlights the basic concept of different microbes driving the differentiation of naïve T cells into different subtypes. In humans, however, SFB are not commonly encountered in the gut microbiota, and these bacteria probably do not play any relevant role.
The routes through which antibiotic-induced microbiota alterations disrupt the balance among T cells and disturb immune homeostasis are being investigated in experimental mice models. Vancomycin, which kills Gram-positive bacteria, has been shown to cause a reduction of the number of Tregs in the lamina propia of the colon and to impair the induction of Th17 cells (Atarashi et al., 2011). On the other hand, a cocktail of antibiotics administered to two-week-old mice resulted in a reduced expression of TLRs and cytokine profiles promoting a Th2 response (Dimmitt et al., 2010). Similarly, kanamycin administered to three-week-old mice resulted in reduction of Peyer’s patch cellularity and in immune responses skewed toward Th2. Importantly, subsequent colonization with different bacterial species had very different effects: Enterococcus faecalis and Lactobacillus acidophilus reversed or attenuated the changes, respectively, whereas Bacteroides vulgatus actually caused their exacerbation. This underscores again the very different roles that specific types of bacteria play in relation to immune balance (Sudo et al., 2002).
Antibiotic-induced dysbioses are also likely to influence numerous immune and metabolic outcomes through routes that affect the intestinal milieu’s overall inflammatory tone. In this regard, microbiota alterations can result in a decrease of IgA, a non-inflammatory immunoglobulin involved in pathogen and allergen exclusion (Rautava et al., 2004; Penders et al., 2007a; Cerutti and Rescigno, 2008). In addition, metronidazole has been shown to cause a decrease in the expression of Muc2, the major component of the mucin layer (Wlodarska et al., 2011); thinning of this layer would result in a more direct contact between gut microbiota and epithelium, with likely increases in innate immune stimulation and inflammation. Recent work in mice has demonstrated that antibiotics can promote inflammation by increasing translocation of native colonic bacteria across the intestinal epithelium. Such translocation requires the participation of both immune dendritic cells and colonic goblet cells, and translocation enhancement results from the decrease in microbial signals received by the goblet cells (Knoop et al., 2015).
Inflammation-enhancing alterations in the gut microbiota, such as can be produced by antibiotic exposure, are likely to play a predominant role in the case of metabolic disorders such as obesity, metabolic syndrome and diabetes. The microbiota has been shown to contribute to the chronic low-grade inflammation that is associated with an excess of adiposity and that likely promotes the progression from obesity toward the metabolic syndrome. In this respect, the microbiota alterations induced by High Fat Diets (HFD) involve an increase of bacteria containing lipopolysacharides (LPS) in the cell wall, resulting in higher serum levels of this pro-inflammatory molecule, and experiments mimicking the HFD state through continuous subcutaneous infusion of LPS result in the induction of some aspects of metabolic syndrome (Cani et al., 2007). A deficiency in TLR5 also results in microbiota alterations that induce metabolic syndrome conditions such as obesity, insulin resistance and dyslipidemia. Moreover, the dysbiotic microbiota in itself is capable of inducing these disorders, as they could be reproduced in experiments in which the microbiota of TLR5-deficient mice was transplanted into germ-free recipients (Vijay-Kumar et al., 2010). Inflammation was likely involved in the onset of the observed metabolic conditions, as the wild-type mice with the transplanted dysbiotic microbiota had higher intestinal levels of the pro-inflammatory molecules TNFα and IL-1β.
Another main route through which microbiota dysbioses will induce their effects on immunity and metabolism is likely to be the alteration of short-chain fatty acids (SCFA) production. Intestinal microbes consume non-digestible carbohydrates to produce SCFAs, particularly acetate, propionate, and butyrate, which are used locally by colonocytes or transported across the gut epithelium into the bloodstream. SCFAs are major players in the maintenance of gut physiology and integrity, promote immune and metabolic homeostasis and have important anti-inflammatory and antitumorigenic effects (Bindels et al., 2012; Tan et al., 2014). In particular, SCFAs interact with G-protein-coupled receptors (GPCRs) to regulate fat deposition (Samuel et al., 2008), and improve insulin secretion through modulation of the levels of the GLP1 hormone (Tolhurst et al., 2012). The specific composition of the microbiota alters the types and levels of SCFA that can be formed, impinging on numerous physiologic processes that are differentially affected by acetate, propionate, and butyrate (Macfarlane and Macfarlane, 2011). In addition to producing SCFAs, the gut microbiota is responsible for converting the primary bile acids synthesized in the human liver into secondary bile acids, also involved in promoting glucose homeostasis through GPCR binding (Thomas et al., 2009). Antibiotic-induced microbiota alterations have been shown to alter bile acid metabolism and insulin sensitivity in both humans and mice (Vrieze et al., 2014).
The Gut Microbiota as Reservoir of Antibiotic Resistances
The dysbioses brought about by antibiotics bear the added disadvantage of enriching the microbiota in resistant organisms. The human gut microbiota has been established as a significant reservoir of antibiotic resistances (Salyers et al., 2004; Seville et al., 2009; Sommer et al., 2009; Ghosh et al., 2013; Moore et al., 2013; Pehrsson et al., 2013; Card et al., 2014; Fouhy et al., 2014a,b; Hu et al., 2014; Lu et al., 2014; von Wintersdorff et al., 2014; Clemente et al., 2015; Field and Hershberg, 2015). The scale of the problem can be gaged by the fact that an analysis of 252 fecal metagenomes from different countries identified resistance genes for 50 out of the 68 antibiotic classes and subclasses that were being screened for, with an average of 21 per sample (Forslund et al., 2013). This study, the largest population-level analysis of the intestinal resistome to date, also showed that the abundance of antibiotic resistance genes (ARGs) is highest for antibiotics that have been longer in the market and for those approved for animal use, such as tetracycline, bacitracin and the cephalosporins. Also, European samples showed enrichment in resistances to vancomycin in comparison to samples from the US, where an analog of vancomycin used to treat animals in Europe was never employed. Moreover, the abundance of ARGs was higher in samples from Southern Europe than in those from Northern Europe, and correlated with measures of total outpatient antibiotic use in the different countries. This suite of observations confirms the notion that a higher exposure to antibiotics increases the likelihood of resistance acquisition by the gut microbiota.
Alarmingly, not only the microbiota of adults constitutes a resistance reservoir, but children and infants also harbor a variety of ARGs (Gueimonde et al., 2006; Mitsou et al., 2010; de Vries et al., 2011; Zhang et al., 2011; Alicea-Serrano et al., 2013; Ghosh et al., 2013; Field and Hershberg, 2015; Moore et al., 2015). Recent analyses have shown that, in fact, numerous ARGs can already be identified in feces of 1-week-old babies and even in meconium, the first deposition of newborns, which is formed by material accumulated in the gastrointestinal tract during fetal life (Gosalbes et al., 2015). Remarkably, ARGs are detected not only in adults and children that have undergone antibiotic treatments, but also in infants (Zhang et al., 2011; Fouhy et al., 2014a; Field and Hershberg, 2015; Gosalbes et al., 2015) and in isolated human populations (Pallecchi et al., 2007; Clemente et al., 2015) that have never been administered antibiotics. This indicates that ARGs can be stably maintained in the human gut microbiome in the absence of direct antibiotic selection, and is consistent with the fact that ARGs can be detected in a large range of natural environments, including those expected to have little exposure to antibiotics derived from human usage (Field and Hershberg, 2015).
In the case of infants, resistances may be vertically inherited, as maternal gut microbes can be transmitted to the offspring (Vaishampayan et al., 2010), with several lines of evidence indicating that such transfer actually starts before birth (Jimenez et al., 2005, 2008; Steel et al., 2005; DiGiulio et al., 2008; Gosalbes et al., 2013, 2015; Aagaard et al., 2014). Several studies have demonstrated shared ARG pools between mother and infant fecal samples, and, in some cases, the presence of the shared ARGs in meconium, colostrum or breast milk (de Vries et al., 2011; Zhang et al., 2011; Gosalbes et al., 2015). Nevertheless, these studies have also detected ARGs in infants that were not present in the mothers and were most probably acquired from other sources. Regarding remote human populations (Pallecchi et al., 2007; Clemente et al., 2015), the presence of ARGs in their microbiotas suggests two possibilities: (i) either their ARGs are ancestral and were present before the rampant spread of resistance due to human antibiotic use, presumably due to selective pressures imposed by naturally occurring antibiotics, or (ii) they have been acquired recently by dispersion of antibiotic resistant strains from other areas and/or by horizontal transfer of genes from such strains to their local bacterial populations. Phylogenetic and population genetic analyses should be able to discern between these alternatives.
Importantly, the human gut, given its enormous density of bacterial cells and species richness, is likely to be especially prone to horizontal gene exchange and to contribute to the spread and reassortment of ARGs among bacterial taxa. Transfer of ARGs between gut microbiota isolates of the genus Bacteroides, as well as between Bacteroides and Gram-positive bacteria, has been documented (Shoemaker et al., 2001). Identical ARG sequences have actually been identified in bacteria coexisting in the gut of a single individual, including different strains of E. coli (Karami et al., 2007) as well as distantly related organisms (de Vries et al., 2011). Experimental work has confirmed that ARG-carrying transposons can be transferred between bacterial species in the guts of rats and mice (Doucet-Populaire et al., 1991; Alpert et al., 2003; Bahl et al., 2004). Furthermore, the transfer of conjugative transposons can be stimulated 100- to 1000-fold by low concentrations of antibiotic (Whittle et al., 2002). Of most concern, the ARGs present in the gut microbiota can also be horizontally transferred from and to incoming pathogenic species, as indicated by the fact that many of the resistance genes identified in human gut isolates are identical at the nucleotide level to resistance genes from human pathogenic isolates (Sommer et al., 2009). Therefore, the human gut can be considered, not only a site of accumulation of ARGs, but also an environment where these genes can spread across species boundaries.
Summary and Outlook
It is clear that the excessively widespread use of antibiotics has created many threats. These include the increasing resistance of bacterial pathogens to antibiotics, which has become a global challenge for infection control. But the effects of excessive antibiotic exposure can be seen, not only in pathogenic bacteria, but also in the symbiotic microbiotas of the human body (Francino and Moya, 2013). As a result, the microbiota imbalances caused by antibiotics can negatively affect health in numerous manners and for long periods of time. The range of problems potentially generated by antibiotic-induced microbiota dysbioses, as reviewed in this paper, is summarized in Figure 1. In light of this knowledge, and given that bacterial infections remain a major public health concern, strategies are needed to minimize the negative consequences of antibiotics when their administration is required. Use of probiotic bacteria aimed at impeding dysbiosis or at reestablishing the gut microbiota after antibiotic treatment is a promising approach. On the other hand, strategies could also be aimed at reestablishing the interactions altered by antibiotic treatment through the targeted use of bacterial molecules that bind specific innate immune receptors (Ubeda and Pamer, 2012). Much further research is needed to delineate the best manners in which bacteria and bacterial products can be employed to counteract the deleterious effects of antibiotics on the gut microbiota and its multiple interactions with immunity and metabolism.
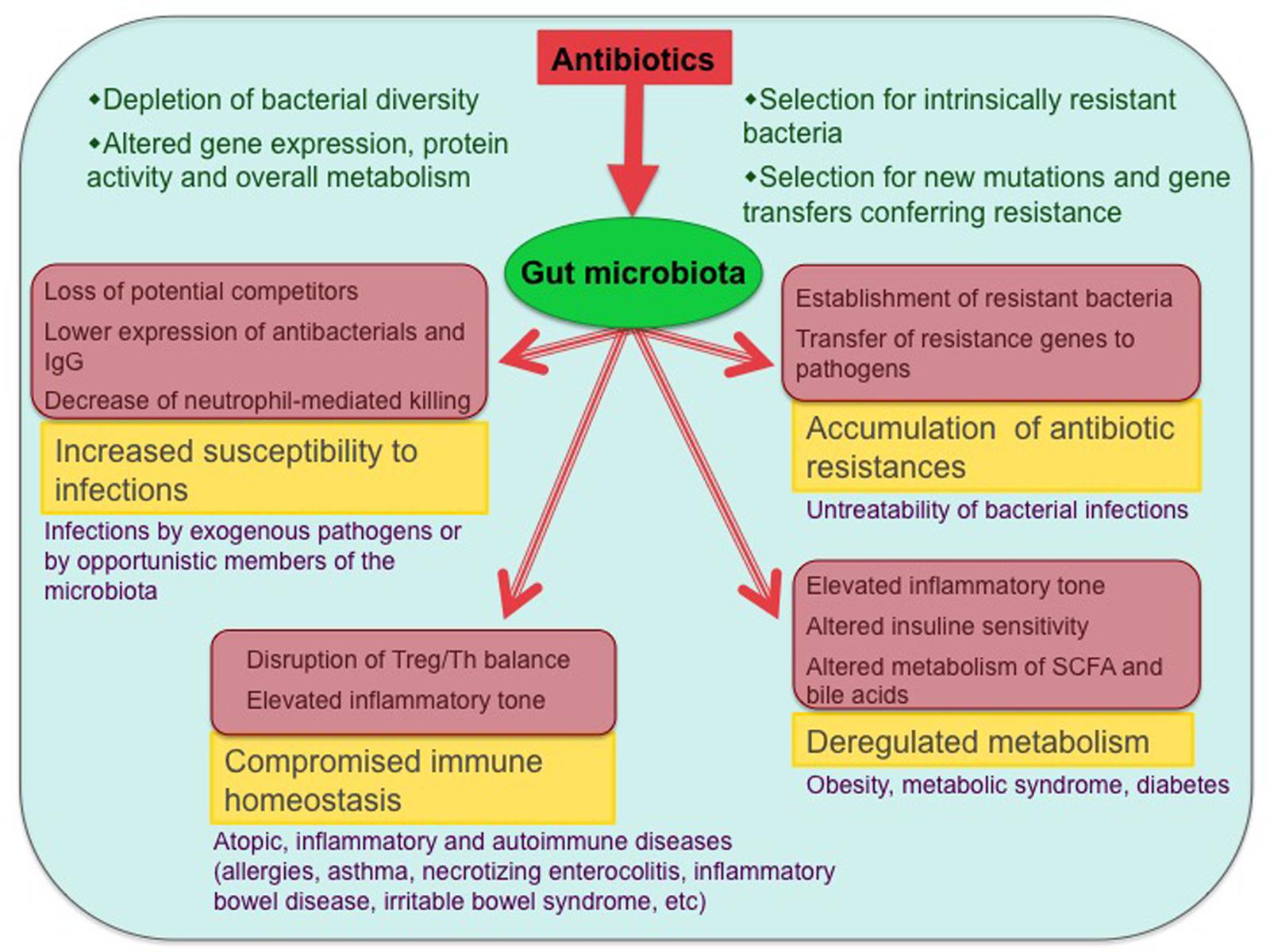
FIGURE 1. Antibiotic effects on the gut microbiota and associated health problems. The main biological consequences of antibiotic-induced dysbioses and the potential diseases that can ensue from them are shown (only diseases with published evidence of association with antibiotic exposure are included). Involved mechanisms are shown in pink-shaded boxes.
An equally important line of research should aim at understanding the patterns of dispersion and expansion of antibiotic resistant strains in the human gut microbiome, as well as the routes of gene exchange that may distribute resistances across different gut taxa. Virome and mobilome analyses should enable us to establish the associations of ARGs with specific genetic elements, providing clues to the paths through which they can be disseminated within and across gut microbial communities. Understanding the flow of resistances within the gut microbiome will contribute an important piece to the puzzle of antibiotic resistance epidemiology, which needs to integrate information from human and environmental microbiomes to the analysis of resistance spread in pathogenic isolates.
Funding
The author was supported by grants SAF2012-31187 (MINECO, Ministry of Economics and Competitiveness, Spain) and UGP-14-167 (FISABIO, Spain) during the preparation of this manuscript.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aagaard, K., Ma, J., Antony, K. M., Ganu, R., Petrosino, J., and Versalovic, J. (2014). The placenta harbors a unique microbiome. Sci. Transl. Med. 6:237ra265. doi: 10.1126/scitranslmed.3008599
Abbas, A. K. (1996). Die and let live: eliminating dangerous lymphocytes. Cell 84, 655–657. doi: 10.1016/S0092-8674(00)81042-9
Abrahamsson, T. R., Jakobsson, H. E., Andersson, A. F., Bjorksten, B., Engstrand, L., and Jenmalm, M. C. (2012). Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 129, 434–440, 440.e1-2 doi: 10.1016/j.jaci.2011.10.025
Ajslev, T. A., Andersen, C. S., Gamborg, M., Sorensen, T. I., and Jess, T. (2011). Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int. J. Obes. (Lond.) 35, 522–529. doi: 10.1038/ijo.2011.27
Akdis, C. A., and Akdis, M. (2009). Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J. Allergy Clin. Immunol. 123, 735–746. doi: 10.1016/j.jaci.2009.02.030
Alicea-Serrano, A. M., Contreras, M., Magris, M., Hidalgo, G., and Dominguez-Bello, M. G. (2013). Tetracycline resistance genes acquired at birth. Arch. Microbiol. 195, 447–451. doi: 10.1007/s00203-012-0864-4
Alm, J. S., Swartz, J., Lilja, G., Scheynius, A., and Pershagen, G. (1999). Atopy in children of families with an anthroposophic lifestyle. Lancet 353, 1485–1488. doi: 10.1016/S0140-6736(98)09344-1
Alpert, C. A., Mater, D. D., Muller, M. C., Ouriet, M. F., Duval-Iflah, Y., and Corthier, G. (2003). Worst-case scenarios for horizontal gene transfer from Lactococcus lactis carrying heterologous genes to Enterococcus faecalis in the digestive tract of gnotobiotic mice. Environ. Biosaf. Res. 2, 173–180. doi: 10.1051/ebr/2003010e
Angelakis, E., Armougom, F., Million, M., and Raoult, D. (2012). The relationship between gut microbiota and weight gain in humans. Future Microbiol. 7, 91–109. doi: 10.2217/fmb.11.142
Atarashi, K., Tanoue, T., Shima, T., Imaoka, A., Kuwahara, T., Momose, Y., et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. doi: 10.1126/science.1198469
Baba, N., Samson, S., Bourdet-Sicard, R., Rubio, M., and Sarfati, M. (2008). Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J. Leukoc. Biol. 84, 468–476. doi: 10.1189/jlb.0108017
Backhed, F., Ding, H., Wang, T., Hooper, L. V., Koh, G. Y., Nagy, A., et al. (2004). The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A. 101, 15718–15723. doi: 10.1073/pnas.0407076101
Bahl, M. I., Sorensen, S. J., Hansen, L. H., and Licht, T. R. (2004). Effect of tetracycline on transfer and establishment of the tetracycline-inducible conjugative transposon Tn916 in the guts of gnotobiotic rats. Appl. Environ. Microbiol. 70, 758–764. doi: 10.1128/AEM.70.2.758-764.2004
Bedford Russell, A. R., and Murch, S. H. (2006). Could peripartum antibiotics have delayed health consequences for the infant? BJOG 113, 758–765. doi: 10.1111/j.1471-0528.2006.00952.x
Bindels, L. B., Porporato, P., Dewulf, E. M., Verrax, J., Neyrinck, A. M., Martin, J. C., et al. (2012). Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 107, 1337–1344. doi: 10.1038/bjc.2012.409
Bisgaard, H., Li, N., Bonnelykke, K., Chawes, B. L., Skov, T., Paludan-Muller, G., et al. (2011). Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 128, e641–e645. doi: 10.1016/j.jaci.2011.04.060
Bjorksten, B., Naaber, P., Sepp, E., and Mikelsaar, M. (1999). The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin. Exp. Allergy 29, 342–346. doi: 10.1016/j.jaci.2011.04.060
Bjorksten, B., Sepp, E., Julge, K., Voor, T., and Mikelsaar, M. (2001). Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108, 516–520. doi: 10.1067/mai.2001.118130
Boursi, B., Mamtani, R., Haynes, K., and Yang, Y. X. (2015). The effect of past antibiotic exposure on diabetes risk. Eur. J. Endocrinol. 172, 639–648. doi: 10.1530/EJE-14-1163
Brandl, K., Plitas, G., Mihu, C. N., Ubeda, C., Jia, T., Fleisher, M., et al. (2008). Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807. doi: 10.1038/nature07250
Buffie, C. G., Jarchum, I., Equinda, M., Lipuma, L., Gobourne, A., Viale, A., et al. (2012). Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect. Immun. 80, 62–73. doi: 10.1128/IAI.05496-11
Burch, D. G. (1996). Is it time to ban all antibiotics as animal growth-promoting agents? Lancet 348, 1455–1456. doi: 10.1016/S0140-6736(04)70104-X
Candon, S., Perez-Arroyo, A., Marquet, C., Valette, F., Foray, A. P., Pelletier, B., et al. (2015). Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 10:e0125448. doi: 10.1371/journal.pone.0125448
Cani, P. D., Amar, J., Iglesias, M. A., Poggi, M., Knauf, C., Bastelica, D., et al. (2007). Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772.
Card, R. M., Warburton, P. J., MacLaren, N., Mullany, P., Allan, E., and Anjum, M. F. (2014). Application of microarray and functional-based screening methods for the detection of antimicrobial resistance genes in the microbiomes of healthy humans. PLoS ONE 9:e86428. doi: 10.1371/journal.pone.0086428
Celedon, J. C., Fuhlbrigge, A., Rifas-Shiman, S., Weiss, S. T., and Finkelstein, J. A. (2004). Antibiotic use in the first year of life and asthma in early childhood. Clin. Exp. Allergy 34, 1011–1016. doi: 10.1111/j.1365-2222.2004.01994.xCEA1994
Celedon, J. C., Litonjua, A. A., Ryan, L., Weiss, S. T., and Gold, D. R. (2002). Lack of association between antibiotic use in the first year of life and asthma, allergic rhinitis, or eczema at age 5 years. Am. J. Respir. Crit. Care Med. 166, 72–75. doi: 10.1164/rccm.2109074
Cerutti, A., and Rescigno, M. (2008). The biology of intestinal immunoglobulin A responses. Immunity 28, 740–750. doi: 10.1016/j.immuni.2008.05.001
Chen, J. W., Scaria, J., Mao, C., Sobral, B., Zhang, S., Lawley, T., et al. (2013) Proteomic comparison of historic and recently emerged hypervirulent Clostridium difficile strains. J. Proteome Res. 12, 1151–1161. doi: 10.1021/pr3007528
Cho, I., Yamanishi, S., Cox, L., Methe, B. A., Zavadil, J., Li, K., et al. (2012). Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626. doi: 10.1038/nature11400
Clarke, T. B., Davis, K. M., Lysenko, E. S., Zhou, A. Y., Yu, Y., and Weiser, J. N. (2010). Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231. doi: 10.1038/nm.2087
Clemente, J. C., Pehrsson, E. C., Blaser, M. J., Sandhu, K., Gao, Z., Wang, B., et al. (2015). The microbiome of uncontacted Amerindians. Sci. Adv. 348:e1500183.
De La Cochetiere, M. F., Durand, T., Lepage, P., Bourreille, A., Galmiche, J. P., and Dore, J. (2005). Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J. Clin. Microbiol. 43, 5588–5592. doi: 10.1128/JCM.43.11.5588-5592.2005
de Vries, L. E., Valles, Y., Agerso, Y., Vaishampayan, P. A., Garcia-Montaner, A., Kuehl, J. V., et al. (2011). The gut as reservoir of antibiotic resistance: microbial diversity of tetracycline resistance in mother and infant. PLoS ONE 6:e21644. doi: 10.1371/journal.pone.0021644
Deshpande, G., Rao, S., Patole, S., and Bulsara, M. (2010). Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 125, 921–930. doi: 10.1542/peds.2009-1301
Dethlefsen, L., Huse, S., Sogin, M. L., and Relman, D. A. (2008). The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6:e280. doi: 10.1371/journal.pbio.0060280
Dethlefsen, L., and Relman, D. A. (2011). Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1), 4554–4561. doi: 10.1073/pnas.1000087107
DiGiulio, D. B., Romero, R., Amogan, H. P., Kusanovic, J. P., Bik, E. M., Gotsch, F., et al. (2008). Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS ONE 3:e3056. doi: 10.1371/journal.pone.0003056
Dimmitt, R. A., Staley, E. M., Chuang, G., Tanner, S. M., Soltau, T. D., and Lorenz, R. G. (2010). Role of postnatal acquisition of the intestinal microbiome in the early development of immune function. J. Pediatr. Gastroenterol. Nutr. 51, 262–273. doi: 10.1097/MPG.0b013e3181e1a114
Doucet-Populaire, F., Trieu-Cuot, P., Dosbaa, I., Andremont, A., and Courvalin, P. (1991). Inducible transfer of conjugative transposon Tn1545 from Enterococcus faecalis to Listeria monocytogenes in the digestive tracts of gnotobiotic mice. Antimicrob. Agents Chemother. 35, 185–187. doi: 10.1128/AAC.35.1.185
Droste, J. H., Wieringa, M. H., Weyler, J. J., Nelen, V. J., Vermeire, P. A., and Van Bever, H. P. (2000). Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin. Exp. Allergy 30, 1547–1553. doi: 10.1046/j.1365-2222.2000.00939.x
Dufour, V., Millon, L., Faucher, J. F., Bard, E., Robinet, E., Piarroux, R., et al. (2005). Effects of a short-course of amoxicillin/clavulanic acid on systemic and mucosal immunity in healthy adult humans. Int. Immunopharmacol. 5, 917–928. doi: 10.1016/j.intimp.2005.01.007
Durban, A., Abellan, J. J., Jimenez-Hernandez, N., Salgado, P., Ponce, M., Ponce, J., et al. (2012). Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ. Microbiol. Rep. 4, 242–247. doi: 10.1111/j.1758-2229.2012.00327.x
Emanuela, F., Grazia, M., Marco de, R., Maria Paola, L., Giorgio, F., and Marco, B. (2012). Inflammation as a link between obesity and metabolic syndrome. J. Nutr. Metab. 2012:476380. doi: 10.1155/2012/476380
Field, W., and Hershberg, R. (2015). Alarmingly high segregation frequencies of quinolone resistance alleles within human and animal microbiomes are not explained by direct clinical antibiotic exposure. Genome Biol. Evol. 7, 1743–1757. doi: 10.1093/gbe/evv102
Foliaki, S., Pearce, N., Bjorksten, B., Mallol, J., Montefort, S., and von Mutius, E. (2009). Antibiotic use in infancy and symptoms of asthma, rhinoconjunctivitis, and eczema in children 6 and 7 years old: international study of asthma and allergies in childhood phase III. J. Allergy Clin. Immunol. 124, 982–989. doi: 10.1016/j.jaci.2009.08.017
Forslund, K., Sunagawa, S., Kultima, J. R., Mende, D. R., Arumugam, M., Typas, A., et al. (2013). Country-specific antibiotic use practices impact the human gut resistome. Genome Res. 23, 1163–1169. doi: 10.1101/gr.155465.113
Fouhy, F., Guinane, C. M., Hussey, S., Wall, R., Ryan, C. A., Dempsey, E. M., et al. (2012). High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 56, 5811–5820. doi: 10.1128/AAC.00789-12
Fouhy, F., Ogilvie, L. A., Jones, B. V., Ross, R. P., Ryan, A. C., Dempsey, E. M., et al. (2014a). Identification of aminoglycoside and beta-lactam resistance genes from within an infant gut functional metagenomic library. PLoS ONE 9:e108016. doi: 10.1371/journal.pone.0108016
Fouhy, F., Ross, R. P., Fitzgerald, G. F., Stanton, C., and Cotter, P. D. (2014b). A degenerate PCR-based strategy as a means of identifying homologues of aminoglycoside and beta-lactam resistance genes in the gut microbiota. BMC Microbiol. 14:25. doi: 10.1186/1471-2180-14-25
Francino, M. P. (2014). Early development of the gut microbiota and immune health. Pathogens 3, 769–790. doi: 10.3390/pathogens3030769
Francino, M. P., and Moya, A. (2013). Effects of antibiotic use on the microbiota of the gut and associated alterations of immunity and metabolism. EMJ Gastroenterol. 1, 74–80.
Franzosa, E. A., Hsu, T., Sirota-Madi, A., Shafquat, A., Abu-Ali, G., Morgan, X. C., et al. (2015). Sequencing and beyond: integrating molecular ‘omics’ for microbial community profiling. Nat. Rev. Microbiol. 13, 360–372. doi: 10.1038/nrmicro3451
Ghosh, T. S., Gupta, S. S., Nair, G. B., and Mande, S. S. (2013). In silico analysis of antibiotic resistance genes in the gut microflora of individuals from diverse geographies and age-groups. PLoS ONE 8:e83823. doi: 10.1371/journal.pone.0083823
Gosalbes, M. J., Llop, S., Valles, Y., Moya, A., Ballester, F., and Francino, M. P. (2013). Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 43, 198–211. doi: 10.1111/cea.12063
Gosalbes, M. J., Valles, Y., Jimenez-Hernandez, N., Balle, C., Riva, P., Miravet-Verde, S., et al. (2015). High frequencies of antibiotic resistance genes in infants’ meconium and early fecal samples. J. Dev. Orig. Health Dis. 1–10. [Epub ahead of print].
Gueimonde, M., Salminen, S., and Isolauri, E. (2006). Presence of specific antibiotic (tet) resistance genes in infant faecal microbiota. FEMS Immunol. Med. Microbiol. 48, 21–25. doi: 10.1111/j.1574-695X.2006.00112.x
Harris, J. M., Mills, P., White, C., Moffat, S., Newman Taylor, A. J., and Cullinan, P. (2007). Recorded infections and antibiotics in early life: associations with allergy in UK children and their parents. Thorax 62, 631–637. doi: 10.1136/thx.2006.072124
He, F., Ouwehand, A. C., Isolauri, E., Hosoda, M., Benno, Y., and Salminen, S. (2001). Differences in composition and mucosal adhesion of bifidobacteria isolated from healthy adults and healthy seniors. Curr. Microbiol. 43, 351–354. doi: 10.1007/s002840010315
Hernandez, E., Bargiela, R., Diez, M. S., Friedrichs, A., Perez-Cobas, A. E., Gosalbes, M. J., et al. (2013). Functional consequences of microbial shifts in the human gastrointestinal tract linked to antibiotic treatment and obesity. Gut Microbes 4, 306–315. doi: 10.4161/gmic.25321
Hildebrand, H., Malmborg, P., Askling, J., Ekbom, A., and Montgomery, S. M. (2008). Early-life exposures associated with antibiotic use and risk of subsequent Crohn’s disease. Scand. J. Gastroenterol. 43, 961–966. doi: 10.1080/00365520801971736
Hu, Y., Yang, X., Lu, N., and Zhu, B. (2014). The abundance of antibiotic resistance genes in human guts has correlation to the consumption of antibiotics in animal. Gut Microbes 5, 245–249. doi: 10.4161/gmic.27916
Illi, S., von Mutius, E., Lau, S., Bergmann, R., Niggemann, B., Sommerfeld, C., et al. (2001). Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ 322, 390–395. doi: 10.1136/bmj.322.7283.390
Ivanov, I. I., Atarashi, K., Manel, N., Brodie, E. L., Shima, T., Karaoz, U., et al. (2009). Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. doi: 10.1016/j.cell.2009.09.033
Jedrychowski, W., Galas, A., Whyatt, R., and Perera, F. (2006). The prenatal use of antibiotics and the development of allergic disease in one year old infants. Int. J. Occup. Med. Environ. Health 19, 70–76. doi: 10.2478/v10001-006-0010-0
Jernberg, C., Lofmark, S., Edlund, C., and Jansson, J. K. (2007). Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 1, 56–66. doi: 10.1038/ismej.2007.3
Jimenez, E., Fernandez, L., Marin, M. L., Martin, R., Odriozola, J. M., Nueno-Palop, C., et al. (2005). Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 51, 270–274. doi: 10.1007/s00284-005-0020-3
Jimenez, E., Marin, M. L., Martin, R., Odriozola, J. M., Olivares, M., Xaus, J., et al. (2008). Is meconium from healthy newborns actually sterile? Res. Microbiol. 159, 187–193. doi: 10.1016/j.resmic.2007.12.007
Jutel, M., and Akdis, C. A. (2011). T-cell subset regulation in atopy. Curr. Allergy Asthma Rep. 11, 139–145. doi: 10.1007/s11882-011-0178-7
Kalliomaki, M., Kirjavainen, P., Eerola, E., Kero, P., Salminen, S., and Isolauri, E. (2001). Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107, 129–134. doi: 10.1067/mai.2001.111237
Karami, N., Martner, A., Enne, V. I., Swerkersson, S., Adlerberth, I., and Wold, A. E. (2007). Transfer of an ampicillin resistance gene between two Escherichia coli strains in the bowel microbiota of an infant treated with antibiotics. J. Antimicrob. Chemother. 60, 1142–1145. doi: 10.1093/jac/dkm327
Kenyon, S. L., Taylor, D. J., and Tarnow-Mordi, W. (2001). Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 357, 979–988. doi: 10.1016/S0140-6736(00)04234-3
Kline, J. N. (2007). Eat dirt: CpG DNA and immunomodulation of asthma. Proc. Am. Thorac Soc. 4, 283–288. doi: 10.1513/pats.200701-019AW
Knoop, K. A., McDonald, K. G., Kulkarni, D. H., and Newberry, R. D. (2015). Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut [Epub ahead of print].
Kozyrskyj, A. L., Ernst, P., and Becker, A. B. (2007). Increased risk of childhood asthma from antibiotic use in early life. Chest 131, 1753–1759. doi: 10.1378/chest.06-3008
Kuo, C. H., Kuo, H. F., Huang, C. H., Yang, S. N., Lee, M. S., and Hung, C. H. (2013). Early life exposure to antibiotics and the risk of childhood allergic diseases: an update from the perspective of the hygiene hypothesis. J. Microbiol. Immunol. Infect. 46, 320–329. doi: 10.1016/j.jmii.2013.04.005
Kuvaeva, I. B., Orlova, N. G., Veselova, O. L., Kuznezova, G. G., and Borovik, T. E. (1984). Microecology of the gastrointestinal tract and the immunological status under food allergy. Nahrung 28, 689–693. doi: 10.1002/food.19840280645
Lawley, T. D., Clare, S., Walker, A. W., Goulding, D., Stabler, R. A., Croucher, N., et al. (2009). Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect. Immun. 77, 3661–3669. doi: 10.1128/IAI.00558-09
Lu, N., Hu, Y., Zhu, L., Yang, X., Yin, Y., Lei, F., et al. (2014). DNA microarray analysis reveals that antibiotic resistance-gene diversity in human gut microbiota is age related. Sci. Rep. 4:4302. doi: 10.1038/srep04302
Macfarlane, G. T., and Macfarlane, S. (2011). Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 45(Suppl.), S120–S127. doi: 10.1097/MCG.0b013e31822fecfe
Madan, J. C., Salari, R. C., Saxena, D., Davidson, L., O’Toole, G. A., Moore, J. H., et al. (2012). Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 97, F456–F462. doi: 10.1136/fetalneonatal-2011-301373
Mah, K. W., Bjorksten, B., Lee, B. W., van Bever, H. P., Shek, L. P., Tan, T. N., et al. (2006). Distinct pattern of commensal gut microbiota in toddlers with eczema. Int. Arch. Allergy Immunol. 140, 157–163. doi: 10.1159/000092555
Mai, V., Torrazza, R. M., Ukhanova, M., Wang, X., Sun, Y., Li, N., et al. (2013). Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE 8:e52876. doi: 10.1371/journal.pone.0052876
Mai, V., Young, C. M., Ukhanova, M., Wang, X., Sun, Y., Casella, G., et al. (2011). Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6:e20647. doi: 10.1371/journal.pone.0020647
Mai, X. M., Kull, I., Wickman, M., and Bergstrom, A. (2010). Antibiotic use in early life and development of allergic diseases: respiratory infection as the explanation. Clin. Exp. Allergy 40, 1230–1237. doi: 10.1111/j.1365-2222.2010.03532.x
Manichanh, C., Rigottier-Gois, L., Bonnaud, E., Gloux, K., Pelletier, E., Frangeul, L., et al. (2006). Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55, 205–211. doi: 10.1136/gut.2005.073817
Marra, F., Marra, C. A., Richardson, K., Lynd, L. D., Kozyrskyj, A., Patrick, D. M., et al. (2009). Antibiotic use in children is associated with increased risk of asthma. Pediatrics 123, 1003–1010. doi: 10.1542/peds.2008-1146
Maurice, C. F., Haiser, H. J., and Turnbaugh, P. J. (2013). Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 152, 39–50. doi: 10.1016/j.cell.2012.10.052
McKeever, T. M., Lewis, S. A., Smith, C., Collins, J., Heatlie, H., Frischer, M., et al. (2002). Early exposure to infections and antibiotics and the incidence of allergic disease: a birth cohort study with the West Midlands General Practice Research Database. J. Allergy Clin. Immunol. 109, 43–50. doi: 10.1067/mai.2002.121016
Mendall, M. A., and Kumar, D. (1998). Antibiotic use, childhood affluence and irritable bowel syndrome (IBS). Eur. J. Gastroenterol. Hepatol. 10, 59–62. doi: 10.1097/00042737-199801000-00011
Mitsou, E. K., Kirtzalidou, E., Pramateftaki, P., and Kyriacou, A. (2010). Antibiotic resistance in faecal microbiota of Greek healthy infants. Benef. Microbes. 1, 297–306. doi: 10.3920/BM2010.0007
Moore, A. M., Ahmadi, S., Patel, S., Gibson, M. K., Wang, B., Ndao, M. I., et al. (2015). Gut resistome development in healthy twin pairs in the first year of life. Microbiome 3:27. doi: 10.1186/s40168-015-0090-9
Moore, A. M., Patel, S., Forsberg, K. J., Wang, B., Bentley, G., Razia, Y., et al. (2013). Pediatric fecal microbiota harbor diverse and novel antibiotic resistance genes. PLoS ONE 8:e78822. doi: 10.1371/journal.pone.0078822
Murray, C. S., Tannock, G. W., Simon, M. A., Harmsen, H. J., Welling, G. W., Custovic, A., et al. (2005). Fecal microbiota in sensitized wheezy and non-sensitized non-wheezy children: a nested case-control study. Clin. Exp. Allergy 35, 741–745. doi: 10.1111/j.1365-2222.2005.02259.x
Nakae, S., Komiyama, Y., Nambu, A., Sudo, K., Iwase, M., Homma, I., et al. (2002). Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387. doi: 10.1016/S1074-7613(02)00391-6
Oboki, K., Ohno, T., Saito, H., and Nakae, S. (2008). Th17 and allergy. Allergol. Int. 57, 121–134. doi: 10.2332/allergolint.R-07-160
Ouwehand, A. C., Isolauri, E., He, F., Hashimoto, H., Benno, Y., and Salminen, S. (2001). Differences in Bifidobacterium flora composition in allergic and healthy infants. J. Allergy Clin. Immunol. 108, 144–145. doi: 10.1067/mai.2001.115754
Pallecchi, L., Lucchetti, C., Bartoloni, A., Bartalesi, F., Mantella, A., Gamboa, H., et al. (2007). Population structure and resistance genes in antibiotic-resistant bacteria from a remote community with minimal antibiotic exposure. Antimicrob. Agents Chemother. 51, 1179–1184. doi: 10.1128/AAC.01101-06
Pehrsson, E. C., Forsberg, K. J., Gibson, M. K., Ahmadi, S., and Dantas, G. (2013). Novel resistance functions uncovered using functional metagenomic investigations of resistance reservoirs. Front. Microbiol. 4:145. doi: 10.3389/fmicb.2013.00145
Penders, J., Stobberingh, E. E., Thijs, C., Adams, H., Vink, C., van Ree, R., et al. (2006). Molecular fingerprinting of the intestinal microbiota of infants in whom atopic eczema was or was not developing. Clin. Exp. Allergy 36, 1602–1608. doi: 10.1111/j.1365-2222.2006.02599.x
Penders, J., Stobberingh, E. E., van den Brandt, P. A., and Thijs, C. (2007a). The role of the intestinal microbiota in the development of atopic disorders. Allergy 62, 1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x
Penders, J., Thijs, C., van den Brandt, P. A., Kummeling, I., Snijders, B., Stelma, F., et al. (2007b). Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 56, 661–667. doi: 10.1136/gut.2006.100164
Perez-Cobas, A. E., Gosalbes, M. J., Friedrichs, A., Knecht, H., Artacho, A., Eismann, K., et al. (2012). Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62, 1591–1601. doi: 10.1136/gutjnl-2012-303184
Rautava, S., Ruuskanen, O., Ouwehand, A., Salminen, S., and Isolauri, E. (2004). The hygiene hypothesis of atopic disease–an extended version. J. Pediatr. Gastroenterol. Nutr. 38, 378–388. doi: 10.1097/00005176-200404000-00004
Risnes, K. R., Belanger, K., Murk, W., and Bracken, M. B. (2011). Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am. J. Epidemiol. 173, 310–318. doi: 10.1093/aje/kwq400
Romagnani, S. (2004). The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology 112, 352–363. doi: 10.1111/j.1365-2567.2004.01925.xIMM1925
Rook, G. A., and Brunet, L. R. (2005). Microbes, immunoregulation, and the gut. Gut 54, 317–320. doi: 10.1136/gut.2004.053785
Round, J. L., and Mazmanian, S. K. (2010). Inducible Foxp3++ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 107, 12204–12209. doi: 10.1073/pnas.0909122107
Rupnik, M., Wilcox, M. H., and Gerding, D. N. (2009) Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7, 526–536. doi: 10.1038/nrmicro2164
Salyers, A. A., Gupta, A., and Wang, Y. (2004). Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 12, 412–416. doi: 10.1016/j.tim.2004.07.004
Samuel, B. S., Shaito, A., Motoike, T., Rey, F. E., Backhed, F., Manchester, J. K., et al. (2008). Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. U.S.A. 105, 16767–16772. doi: 10.1073/pnas.0808567105
Sekirov, I., Russell, S. L., Antunes, L. C., and Finlay, B. B. (2010). Gut microbiota in health and disease. Physiol. Rev. 90, 859–904. doi: 10.1152/physrev.00045.2009
Sepp, E., Julge, K., Mikelsaar, M., and Bjorksten, B. (2005). Intestinal microbiota and immunoglobulin E responses in 5-year-old Estonian children. Clin. Exp. Allergy 35, 1141–1146. doi: 10.1111/j.1365-2222.2005.02315.x
Sepp, E., Julge, K., Vasar, M., Naaber, P., Bjorksten, B., and Mikelsaar, M. (1997). Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 86, 956–961. doi: 10.1111/j.1651-2227.1997.tb15178.x
Seville, L. A., Patterson, A. J., Scott, K. P., Mullany, P., Quail, M. A., Parkhill, J., et al. (2009). Distribution of tetracycline and erythromycin resistance genes among human oral and fecal metagenomic DNA. Microb. Drug Resist. 15, 159–166. doi: 10.1089/mdr.2009.0916
Shen, X., Du, J., Guan, W., and Zhao, Y. (2014). The balance of intestinal Foxp3+ regulatory T cells and Th17 cells and its biological significance. Exp. Rev. Clin. Immunol. 10, 353–362. doi: 10.1586/1744666X.2014.882232
Shoemaker, N. B., Vlamakis, H., Hayes, K., and Salyers, A. A. (2001). Evidence for extensive resistance gene transfer among Bacteroides spp. and among Bacteroides and other genera in the human colon. Appl. Environ. Microbiol. 67, 561–568. doi: 10.1128/AEM.67.2.561-568.2001
Sjogren, Y. M., Jenmalm, M. C., Bottcher, M. F., Bjorksten, B., and Sverremark-Ekstrom, E. (2009). Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy 39, 518–526. doi: 10.1111/j.1365-2222.2008.03156.x
Sommer, M. O., Dantas, G., and Church, G. M. (2009). Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 325, 1128–1131. doi: 10.1126/science.1176950
Song, H. J., Shim, K. N., Jung, S. A., Choi, H. J, Lee, M. A., Ryu, K. H., et al. (2008). Antibiotic-associated diarrhea: candidate organisms other than Clostridium difficile. Korean J. Intern. Med. 23, 9–15. doi: 10.3904/kjim.2008.23.1.9
Steel, J. H., Malatos, S., Kennea, N., Edwards, A. D., Miles, L., Duggan, P., et al. (2005). Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr. Res. 57, 404–411. doi: 10.1203/01.PDR.0000153869.96337.90
Strauch, U. G., Obermeier, F., Grunwald, N., Gurster, S., Dunger, N., Schultz, M., et al. (2005). Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut 54, 1546–1552. doi: 10.1136/gut.2004.059451
Su, Y., Rothers, J., Stern, D. A., Halonen, M., and Wright, A. L. (2010). Relation of early antibiotic use to childhood asthma: confounding by indication? Clin. Exp. Allergy 40, 1222–1229. doi: 10.1111/j.1365-2222.2010.03539.x
Sudo, N., Yu, X. N., Aiba, Y., Oyama, N., Sonoda, J., Koga, Y., et al. (2002). An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin. Exp. Allergy 32, 1112–1116. doi: 10.1046/j.1365-2222.2002.01430.x
Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., and Macia, L. (2014). The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119. doi: 10.1016/B978-0-12-800100-4.00003-9
Tanaka, S., Kobayashi, T., Songjinda, P., Tateyama, A., Tsubouchi, M., Kiyohara, C., et al. (2009). Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 56, 80–87. doi: 10.1111/j.1574-695X.2009.00553.x
Thomas, C., Gioiello, A., Noriega, L., Strehle, A., Oury, J., Rizzo, G., et al. (2009). TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10, 167–177. doi: 10.1016/j.cmet.2009.08.001
Thomas, M., Custovic, A., Woodcock, A., Morris, J., Simpson, A., and Murray, C. S. (2006). Atopic wheezing and early life antibiotic exposure: a nested case-control study. Pediatr. Allergy Immunol. 17, 184–188. doi: 10.1111/j.1399-3038.2006.00389.x
Thompson-Chagoyan, O. C., Fallani, M., Maldonado, J., Vieites, J. M., Khanna, S., Edwards, C., et al. (2011). Faecal microbiota and short-chain fatty acid levels in faeces from infants with cow’s milk protein allergy. Int. Arch. Allergy Immunol. 156, 325–332. doi: 10.1159/000323893
Thuny, F., Richet, H., Casalta, J. P., Angelakis, E., Habib, G., and Raoult, D. (2010). Vancomycin treatment of infective endocarditis is linked with recently acquired obesity. PLoS ONE 5:e9074. doi: 10.1371/journal.pone.0009074
Tolhurst, G., Heffron, H., Lam, Y. S., Parker, H. E., Habib, A. M., Diakogiannaki, E., et al. (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371. doi: 10.2337/db11-1019
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi: 10.1038/nature07540
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
Ubeda, C., and Pamer, E. G. (2012). Antibiotics, microbiota, and immune defense. Trends Immunol. 33, 459–466. doi: 10.1016/j.it.2012.05.003
Ubeda, C., Taur, Y., Jenq, R. R., Equinda, M. J., Son, T., Samstein, M., et al. (2010). Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Invest. 120, 4332–4341. doi: 10.1172/JCI43918
Vaishampayan, P. A., Kuehl, J. V., Froula, J. L., Morgan, J. L., Ochman, H., and Francino, M. P. (2010). Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol. Evol. 2, 53–66. doi: 10.1093/gbe/evp057
Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L., and Hooper, L. V. (2008). Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. U.S.A. 105, 20858–20863. doi: 10.1073/pnas.0808723105
Vanderploeg, R., Panaccione, R., Ghosh, S., and Rioux, K. (2010). Influences of intestinal bacteria in human inflammatory bowel disease. Infect. Dis. Clin. North Am. 24, 977–993. doi: 10.1016/j.idc.2010.07.008
Vanner, S. (2008). The small intestinal bacterial overgrowth. Irritable bowel syndrome hypothesis: implications for treatment. Gut 57, 1315–1321. doi: 10.1136/gut.2007.133629
Vijay-Kumar, M., Aitken, J. D., Carvalho, F. A., Cullender, T. C., Mwangi, S., Srinivasan, S., et al. (2010). Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231. doi: 10.1126/science.1179721
von Mutius, E., Illi, S., Hirsch, T., Leupold, W., Keil, U., and Weiland, S. K. (1999). Frequency of infections and risk of asthma, atopy and airway hyperresponsiveness in children. Eur. Respir. J. 14, 4–11. doi: 10.1034/j.1399-3003.1999.14a03.x
von Wintersdorff, C. J., Penders, J., Stobberingh, E. E., Oude Lashof, A. M., Hoebe, C. J., Savelkoul, P. H., et al. (2014). High rates of antimicrobial drug resistance gene acquisition after international travel, The Netherlands. Emerg. Infect. Dis. 20, 649–657. doi: 10.3201/eid.2004.131718
Vrieze, A., Out, C., Fuentes, S., Jonker, L., Reuling, I., Kootte, R. S., et al. (2014). Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 60, 824–831. doi: 10.1016/j.jhep.2013.11.034
Wang, M., Karlsson, C., Olsson, C., Adlerberth, I., Wold, A. E., Strachan, D. P., et al. (2008). Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 121, 129–134. doi: 10.1016/j.jaci.2007.09.011
Whittle, G., Shoemaker, N. B., and Salyers, A. A. (2002). The role of Bacteroides conjugative transposons in the dissemination of antibiotic resistance genes. Cell Mol. Life. Sci. 59, 2044–2054. doi: 10.1007/s000180200004
Wickens, K., Ingham, T., Epton, M., Pattemore, P., Town, I., Fishwick, D., et al. (2008). The association of early life exposure to antibiotics and the development of asthma, eczema and atopy in a birth cohort: confounding or causality? Clin. Exp. Allergy 38, 1318–1324. doi: 10.1111/j.1365-2222.2008.03024.x
Wickens, K. L., Crane, J., Kemp, T. J., Lewis, S. J., D’Souza, W. J., Sawyer, G. M., et al. (1999). Family size, infections, and asthma prevalence in New Zealand children. Epidemiology 10, 699–705. doi: 10.1097/00001648-199911000-00009
Wilcox, M. H. (2003). Gastrointestinal disorders and the critically ill. Clostridium difficile infection and pseudomembranous colitis. Best Pract. Res. Clin. Gastroenterol. 17, 475–493. doi: 10.1016/S1521-6918(03)00017-9
Wills-Karp, M., Santeliz, J., and Karp, C. L. (2001). The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1, 69–75. doi: 10.1038/35095579
Wjst, M., Hoelscher, B., Frye, C., Wichmann, H. E., Dold, S., and Heinrich, J. (2001). Early antibiotic treatment and later asthma. Eur. J. Med. Res. 6, 263–271.
Wlodarska, M., Willing, B., Keeney, K. M., Menendez, A., Bergstrom, K. S., Gill, N., et al. (2011). Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 79, 1536–1545. doi: 10.1128/IAI.01104-10
Wold, A. E. (1998). The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy 53, 20–25. doi: 10.1111/j.1398-9995.1998.tb04953.x
Yamini, D., and Pimentel, M. (2010). Irritable bowel syndrome and small intestinal bacterial overgrowth. J. Clin. Gastroenterol. 44, 672–675. doi: 10.1097/MCG.0b013e3181ef3476
Yazdanbakhsh, M., Kremsner, P. G., and van Ree, R. (2002). Allergy, parasites, and the hygiene hypothesis. Science 296, 490–494. doi: 10.1126/science.296.5567.490
Young, V. B., and Schmidt, T. M. (2004). Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J. Clin. Microbiol. 42, 1203–1206. doi: 10.1128/JCM.42.3.1203-1206.2004
Keywords: antibiotics, human gut microbiota, autoimmunity, immunotolerance, atopy, inflammation, dysbiosis, resistance reservoir
Citation: Francino MP (2016) Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 6:1543. doi: 10.3389/fmicb.2015.01543
Received: 07 August 2015; Accepted: 21 December 2015;
Published: 12 January 2016.
Edited by:
Peter Mullany, University College London, UKReviewed by:
Polpass Arul Jose, Council of Scientific and Industrial Research – Central Salt and Marine Chemicals Research Institute, IndiaAshima Kushwaha Bhardwaj, Indian Institute of Advanced Research, India
Manuela Caniça, National Institute of Health Doutor Ricardo Jorge, Portugal
Copyright © 2016 Francino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. P. Francino, bXBmcmFuY2lub0BnbWFpbC5jb20=
 M. P. Francino
M. P. Francino