Introduction
Trillions of microorganisms form the “natural flora” of the human body. These microorganisms outnumber the host cells 10 to 1, essentially making humans a microbial ecosystem of sorts. Before the advent of the high-throughput sequencing technologies such elusive microorganisms were uncultivable and could not be studied using traditional microbiological techniques. However, since the past decade, with the establishment of the Human Microbiome Project reference database in 2012, research in this area has grown logarithmically (Grogan, 2015). The human microbiota is introduced from mother to the fetus and even infants, and remains with the growing individual for the rest of his/her life, being effected by and also affecting the individual's lifestyle, food habits, and metabolism. Friendly microbes have been shown to protect from several diseases and a dysbiosis in the microbiota has been linked to infections by pathogens, autoimmunity and lifestyle disorders. The establishment of the brain-gut axis (reviewed in Carabotti et al., 2015) in the past few years has even shown that gut microbiota and brain affect each other (Carabotti et al., 2015). A very recent study established the missing link between the central nervous system (CNS) and the immune system i.e., the CNS lymphatic vessels, thus completing the microbiota-neuronal-immune system triangle (Louveau et al., 2015). Even stress, and behavioral changes can result in an altered microbiota and vice–versa (Schmidt, 2015). This has brought microbiologists and neuroscientists together to look into the probable mechanisms in which dysbiosis of gut microbiota may affect development of neurodegenerative diseases and even neuronal autoimmunity.
Autoimmune diseases of both the central and the peripheral nervous system (PNS) have been linked to microbiota, although the extent to which the link has been established is very different. While some like multiple sclerosis (MS) has working mice-models, others like Guillain–Barré syndrome (GBS), a rare autoimmune disorder of the PNS has no defined models, although experimental autoimmune neuritis (EAN) may be used to study some aspects of GBS, especially macrophage mediated demyelination (Kieseier et al., 2012). Even so, we have been warned against the fallacies of studying such models in isolation, as there is no strict one model-one disorder kind of relationship between human autoimmunity and mouse/rat model (Gold et al., 2000). Since probiotics have been found to be useful in the treatment of autoimmune diseases like Inflammatory Bowel Disease (IBD) (Sheil et al., 2007), type1 diabetes (Calcinaro et al., 2005) and even extra-intestinal neuronal and systemic-autoimmune disorders like MS (Lavasani et al., 2010) and systemic lupus erythematosus (SLE) (Manirarora et al., 2008; Alard et al., 2009), testing the efficacy of probiotics in the treatment of GBS might offer a less invasive and more acceptable and economical treatment alternative.
Probiotics Modulate the Gut Microbiota to Affect the Host Immune System
Altered gut microbiota or “gut dysbiosis” has been linked to autoimmune responses both intra-intestinally as well as extra-intestinally. Recently concluded and active research has helped detail the mechanisms of interaction between the gut microbiota and the immune system (Maynard et al., 2012). Attempts to understand the mechanisms of restoration of healthy gut microbiota (eubiosis) by probiotics has started to reveal the complex interactions and signaling involved between the host cells including components of the immune system, the probiotic strain and the other commensal or pathogen residing in the host system (Wagner et al., 2009; Wine et al., 2009; Maynard et al., 2012; Bollrath and Powrie, 2013). It is generally accepted that adaptive immunity to microbes may be mediated by Toll-like receptors (TLRs), C-type lectin receptors (CLRs), and NOD-like receptors (NLRs), which are present on the epithelium, endothelium, and lymphoid tissue of the host. These receptors might signal downstream depending upon the situation of eubiosis (homeostasis)/dysbiosis (Walker, 2008; Maynard et al., 2012). Traditionally, two T-cell fates have dominated the immune system related signaling viz. T-helper cell (TH1) and TH2. TH1 type response is elicited on exposure to pathogens and leads to their phagocytosis, causing inflammation to the surrounding host tissue and even autoimmune responses. TH2 type of response is elicited mainly on exposure to environmental cues and also some pathogenic proteins, leading to the production of IgE and release of histamines that counter TH1 response (Berger, 2000). Later studies have, however, revealed a much more complex picture of autoimmunity and has introduced new types of CD4+ T-cell viz. TH17 (Damsker et al., 2010) and CD25+ FoxP3+ regulatory T-cells (Treg) (Sakaguchi et al., 2009). During immune homeostasis the concentration of Interleukin (IL-10) (also secreted by TH2 cells) secreting Treg cells dominate the intestinal mucosa. In most of the autoimmune conditions, a common feature seems to be diminished presence of Treg cells in the intestinal mucosa of the host resulting in a shift toward IL-17 secreting TH17 cells and this shift in the T-cell population seems to be dependent on the intestinal cytokine Transforming Growth Factor (TGF-β). Interestingly, in the face of pathogenic infection or an inflammation caused by the resident microbiota, the IL-10 secreting Treg cells are also converted to IL-17, Retinoic acid-related Orphan Receptor (RORγT), and Interferon (IFN-γ) producing TH17 cells (Xu et al., 2007) and vice–versa (Gagliani et al., 2015). Research exploring the gut-brain axis has lead to an understanding that altered gut microbiota plays a role not only in gastrointestinal autoimmunity but also extra-intestinal autoimmunity. Systemic and neuro-autoimmunities like SLE and MS have been linked to gut dysbiosis, making a strong case for linking GBS to altered microbiota.
Our current understanding of the link between gut eubiosis and immune homeostasis, and the effect of probiotics on the former have been reviewed elsewhere (Hemarajata and Versalovic, 2013; Kueffel et al., 2013). Various studies have already tipped the balance toward the use of probiotics for providing relief in a number of diseases and disorders including extra-intestinal autoimmunities. As briefly mentioned above, a study has pointed toward the therapeutic effect of probiotic consisting of a mixture of lactobacilli on experimental autoimmune encephalomyelitis (EAE; an experimental model of MS) through IL-10 secreting Treg cells (Lavasani et al., 2010). In a later study, it has been shown that pro-inflammatory T-cell response consisting of IL-17A and IFN-γ producing TH17 cells caused EAE in germ-free mice upon infection from Segmented Filamentous Bactria (SFB) (Lee et al., 2011), further linking gut dysbiosis and neuronal autoimmunity. Immunopathology of GBS too is complicated by TH17 cells and the corresponding cytokines, thus making it a balance between TH1/TH17 and TH2, although mechanistic details are yet to be elucidated (Liang et al., 2012; Li et al., 2012, 2014). Functional aspects of TH17 cells and associated cytokines and their rational drug targeting with respect to GBS is discussed in some details elsewhere (Wang et al., 2013; Wu et al., 2015). Studies on the lines of drug trials have shown that in most stomach related ailments; probiotics do confer a health benefit (Mack, 2005). Some small studies in model organisms show that probiotics confer prophylactic or curative benefit against diabetes, certain cancers, Human Immunodeficiency Virus (HIV), rheumatoid arthritis, and MS among several other conditions (Erickson and Hubbard, 2000; Matsuzaki et al., 2007).
Guillain–Barré Syndrome: Prototype of an Immune Mediated Peripheral Neuropathy
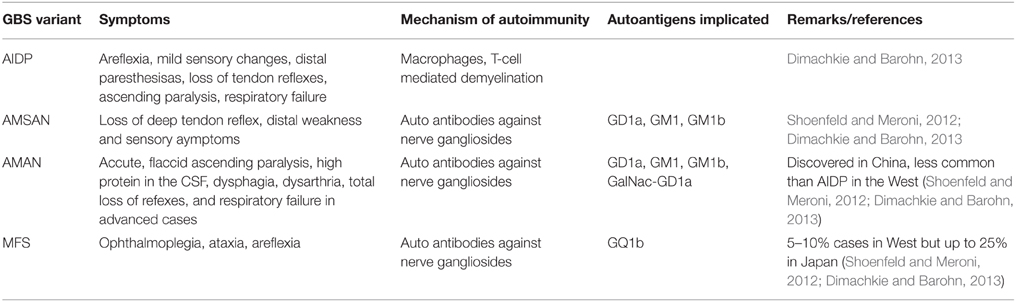
GBS is a post-infection autoimmune monophasic disorder of the PNS and is characterized by acute flaccid paralysis. The syndrome has variants categorized depending upon the kind of neuropathy viz. demyelinating or axon degenerating. These have been described in detail by Dimachkie and Barohn (2013) and have been summarized in the form of a table in this article (Table 1). The immunopathology of the GBS is not well understood however it is known that the syndrome occurs post-infection by Campylobacter jejuni (Kaldor and Speed, 1984; Allos, 1997), Mycoplasma pneumonia (Goldschmidt et al., 1980), certain Herpesviridae (Jacobs et al., 1998), HIV (Pontali et al., 2011) and flu viruses (Sivadon-Tardy et al., 2009) or in some cases after the administration of flu vaccine (Geier et al., 2003), most probably due to an imbalance in the various T-helper cell populations described above. Proinflammatory, TH1 type cytokines like Tumor Necrosis Factor (TNF-α), IFN-γ, and IL-1 have implicated in triggering mechanism of EAN/GBS. Other proinflammatory cytokines like IL-12, TNF-α/β, and IL-2 are also detected during the course of the disease progression. TH17 cytokines IL-17 and IL-22 have also been detected in the serum of GBS patients thus confirming the role of TH17 mediated pro-inflammatory response (Li et al., 2012). Interestingly, proinflammatory cytokines IL-4, IL-5, and IL-6, may be produced by cells expressing the rival TH2 phenotype, contributing to GBS. However, TH2 type cytokines IL-10 and TGF-β are associated with receding EAN/GBS symptoms (Zhu et al., 1998). Both T-cell mediated and humoral immunity seems to be playing a role in GBS. In Acute Inflammatory Demyelinating Polyneuropathy (AIDP) variant, activated T-cells, upon antigen presentation by macrophages, cross the blood-nerve barrier (BNB) and release cytokines that activate endoneural macrophages, damaging the compact myelin. Alternatively either by directly recognizing a pathogenic epitope or elicited by T-cell, B-cells produce antibodies to the cross-reactive axolemmal autoantigens and by fixing a complement leads to the damage of Schwann cells and/or axons, leading to Acute Motor Axonal Neuropathy (AMAN), the more severe Acute Motor and Sensory Axonal Neuropathy (AMSAN), or Miller Fisher Syndrome (MFS) (Hughes and Cornblath, 2005). The most common autoantigens are GM1, GM1a, and GD1a (Dimachkie and Barohn, 2013). Due to these variants and their diverse pathology and pathogenesis, GBS is now considered a group of heterogeneous conditions with similar clinical phenotypes (Kieseier et al., 2012). GBS has been considered a unique autoimmune disorder due to the fact that unlike most other neuronal autoimmune disorders, it is monophasic and that its occurrence has been found to correspond to immunosuppression in the patient (Steiner et al., 2010). The antecedent causative organisms mostly linked with GBS include C. jejuni (≈38%) (Kaldor and Speed, 1984), M. pneumonia (≈21%) (Sharma et al., 2011). Infections by most or all of these agents are characterized by immunosuppression either as a primary result of the infection or as a secondary effect.
Eubiotic Gut to Cure Guillain-Barré Syndrome
Infection by C. jejuni, M. pneumonia, and viruses mentioned above also shows a differential effect on Treg and TH1 cell populations with infected individuals showing a reduced Treg cell presence (Steiner et al., 2010). Besides, there is evidence of C. jejuni colonization being exacerbated by a shift in the microbiota (characterized by increased Escherichia coli load in the gut) upon infection by Toxolasma gondii (Haag et al., 2012) and its alleviation by probiotic strain Lactobacillus helveticus strain R0052 (Wine et al., 2009). With studies pointing toward probiotic assisted increase in the population of anti-inflammatory Treg cells (Smits et al., 2005; Kwon et al., 2010), and probiotics modulating TH1(and TH17)/TH2 balance (Torii et al., 2007; Tanabe, 2013) it may be worth testing whether this increase in Treg cells reduces post infection autoimmunity in any way. It may not be wrong to expect a Treg cell mediated immune homeostasis of both the humoral (Wing and Sakaguchi, 2014) and cellular kind (Shevach, 2009), produced as a result of probiotic induced gut eubiosis.
Conclusion
GBS is a rare autoimmune disease effecting 2–4 people per 100,000. Being a rare condition it is neglected by the big pharmaceutical companies, as well as academic researchers searching for better treatments. Although the treatments are available in the form of intravenous immunoglobulin (IVIg) administration and plasma exchange, they become quite expensive, especially in the developing countries. Added to it the poor prognosis of the disease, there is a need for cheaper alternatives to the currently available treatments. Probiotics have been shown to be effective in treatment of several intestinal and extra-intestinal autoimmunities, as they act by replenishing the Treg cells in the immune system that have been shown to promote the immune system homeostasis. There is ample reason to believe that GBS also is a result of altered immune homeostasis caused by infection due to an antecedent infection. Thus, promoting the production of Treg cells may help cure GBS by acting against both T- cell mediated and humoral autoimmunity of the PNS.
Author Contributions
The author AS claims the sole responsibility of researching and writing this article.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The article is dedicated to Ms. Priyanka Saxena, my cousin, diagnosed with GBS and went through a painful recovery using conventional methods. The idea to write this article was conceived while visiting her at the hospital and was developed further while attending a symposium on Probiotics - From Bench to Community, organised by Yakult India Microbiota and Probiotic Science Foundation. No funds were utilized for the research that went into writing this article. The publication of this article has been facilitated by the grant of a full waiver of publishing fees by Frontiers.
References
Alard, P., Parnell, S., Manirarora, J., Kosiewicz, M., and Atay, S. (2009). Probiotics control lupus progression via induction of regulatory cells and IL-10 production. J. Immunol. 182, 50.30.
Allos, B. M. (1997). Association between Campylobacter infection and Guillain-Barré syndrome. J. Infect. Dis. 176(Suppl. 2), S125–S128. doi: 10.1086/513783
Berger, A. (2000). Th1 and Th2 responses: what are they? Br. Med. J. 321:424. doi: 10.1136/bmj.321.7258.424
Bollrath, J., and Powrie, F. (2013). Feed your tregs more fiber. Science 341, 463–464. doi: 10.1126/science.1242674
Calcinaro, F., Dionisi, S., Marinaro, M., Candeloro, P., Bonato, V., Marzotti, S., et al. (2005). Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia 48, 1565–1575. doi: 10.1007/s00125-005-1831-2
Carabotti, M., Scirocco, A., Maselli, M. A., and Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209.
Damsker, J. M., Hansen, A. M., and Caspi, R. R. (2010). Th1 and Th17 cells: adversaries and collaborators. Ann. N.Y. Acad. Sci. 1183, 211–221. doi: 10.1111/j.1749-6632.2009.05133.x
Dimachkie, M. M., and Barohn, R. J. (2013). Guillain-Barré syndrome and variants. Neurol. Clin. 31, 491–510. doi: 10.1016/j.ncl.2013.01.005
Erickson, K. L., and Hubbard, N. E. (2000). Probiotic immunomodulation in health and disease. J. Nutr. 130, 403S–409S.
Gagliani, N., Vesely, M. C. A., Iseppon, A., Brockmann, L., Xu, H., Palm, N. W., et al. (2015). Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225. doi: 10.1038/nature14452
Geier, M. R., Geier, D. A., and Zahalsky, A. C. (2003). Influenza vaccination and Guillain Barre syndrome? Clin. Immunol. 107, 116–121. doi: 10.1016/S1521-6616(03)00046-9
Gold, R., Hartung, H. P., and Toyka, K. V. (2000). Animal models for autoimmune demyelinating disorders of the nervous system. Mol. Med. Today 6, 88–91. doi: 10.1016/S1357-4310(99)01639-1
Goldschmidt, B., Menonna, J., Fortunato, J., Dowling, P., and Cook, S. (1980). Mycoplasma antibody in guillain-barre syndrome and other neurological disorders. Ann. Neurol. 7, 108–112. doi: 10.1002/ana.410070203
Haag, L.-M., Fischer, A., Otto, B., Plickert, R., Kühl, A. A., Göbel, U. B., et al. (2012). Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS ONE 7:e35988. doi: 10.1371/journal.pone.0035988
Hemarajata, P., and Versalovic, J. (2013). Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 6, 39–51. doi: 10.1177/1756283X12459294
Hughes, R. A., and Cornblath, D. R. (2005). Guillain-Barré syndrome. Lancet 366, 1653–1666. doi: 10.1016/S0140-6736(05)67665-9
Jacobs, B. C., Rothbarth, P. H., van der Meché, F. G., Herbrink, P., Schmitz, P. I., de Klerk, M. A., et al. (1998). The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology 51, 1110–1115. doi: 10.1212/WNL.51.4.1110
Kaldor, J., and Speed, B. R. (1984). Guillain-Barré syndrome and Campylobacter jejuni: a serological study. Br. Med. J. (Clin. Res. Ed.) 288, 1867–1870. doi: 10.1136/bmj.288.6434.1867
Kieseier, B. C., Lehmann, H. C., and Hörste, G. M. Z. (2012). Autoimmune diseases of the peripheral nervous system. Autoimmun. Rev. 11, 191–195. doi: 10.1016/j.autrev.2011.05.011
Kueffel, N., Ziolkowski, J., and Haslberger, A. (2013). Microbiota and diseases of the nervous system. J. Ernahrungsmedizin 15, 18–20.
Kwon, H.-K., Lee, C.-G., So, J.-S., Chae, C.-S., Hwang, J.-S., Sahoo, A., et al. (2010). Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. U.S.A. 107, 2159–2164. doi: 10.1073/pnas.0904055107
Lavasani, S., Dzhambazov, B., Nouri, M., Fåk, F., Buske, S., Molin, G., et al. (2010). A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 5:e9009. doi: 10.1371/journal.pone.0009009
Lee, Y. K., Menezes, J. S., Umesaki, Y., and Mazmanian, S. K. (2011). Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1), 4615–4622. doi: 10.1073/pnas.1000082107
Li, S., Jin, T., Zhang, H.-L., Yu, H., Meng, F., Concha Quezada, H., et al. (2014). Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barré syndrome and downregulated by IVIg treatments. Mediators Inflamm. 2014, 1–10. doi: 10.1155/2014/740947
Li, S., Yu, M., Li, H., Zhang, H., and Jiang, Y. (2012). IL-17 and IL-22 in cerebrospinal fluid and plasma are elevated in Guillain-Barré Syndrome. Mediators Inflamm. 2012, 1–7. doi: 10.1155/2012/260473
Liang, S. L., Wang, W. Z., Huang, S., Wang, X. K., Zhang, S., and Wu, Y. (2012). Th17 helper cell and T-cell immunoglobulin and mucin domain 3 involvement in Guillain–Barré syndrome. Immunopharmacol. Immunotoxicol. 34, 1039–1046. doi: 10.3109/08923973.2012.697469
Louveau, A., Smirnov, I., Keyes, T. J., Eccles, J. D., Rouhani, S. J., Peske, J. D., et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. doi: 10.1038/nature14432
Manirarora, J. N., Parnell, S. A., Atay, S., Kosiewicz, M. M., and Alard, P. (2008). Probiotics protect (NZBxNZW)F1 mice against lupus by a mechanism involving IL-10 production by dendritic cells and regulatory cells. FASEB J. 22.
Matsuzaki, T., Takagi, A., Ikemura, H., Matsuguchi, T., and Yokokura, T. (2007). Intestinal microflora: probiotics and autoimmunity. J. Nutr. 137, 798S–802S.
Maynard, C. L., Elson, C. O., Hatton, R. D., and Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231–241. doi: 10.1038/nature11551
Pontali, E., Feasi, M., Crisalli, M. P., and Cassola, G. (2011). Guillain-Barré Syndrome with fatal outcome during HIV-1-Seroconversion: a case report. Case Rep. Infect. Dis. 2011, 1–4. doi: 10.1155/2011/972096
Sakaguchi, S., Wing, K., Onishi, Y., Prieto-Martin, P., and Yamaguchi, T. (2009). Regulatory T cells: how do they suppress immune responses? Int. Immunol. 21, 1105–1111. doi: 10.1093/intimm/dxp095
Sharma, M. B., Chaudhry, R., Tabassum, I., Ahmed, N. H., Sahu, J. K., Dhawan, B., et al. (2011). The presence of Mycoplasma pneumoniae infection and GM1 ganglioside antibodies in Guillain-Barré syndrome. J. Infect. Dev. Ctries. 5, 459–464. doi: 10.3855/jidc.1847
Sheil, B., Shanahan, F., and O'Mahony, L. (2007). Probiotic effects on inflammatory bowel disease. J. Nutr. 137, 819S–824S.
Shevach, E. M. (2009). Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645. doi: 10.1016/j.immuni.2009.04.010
Shoenfeld, Y., and Meroni, P.-L. (2012). The General Practice Guide to Autoimmune Diseases. Lengerich: Pabst Science Publishers.
Sivadon-Tardy, V., Orlikowski, D., Porcher, R., Sharshar, T., Durand, M. C., Enouf, V., et al. (2009). Guillain−Barré syndrome and influenza virus infection. Clin. Infect. Dis. 48, 48–56. doi: 10.1086/594124
Smits, H. H., Engering, A., van der Kleij, D., de Jong, E. C., Schipper, K., van Capel, T. M. M., et al. (2005). Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 115, 1260–1267. doi: 10.1016/j.jaci.2005.03.036
Steiner, I., Rosenberg, G., and Wirguin, I. (2010). Transient immunosuppression: a bridge between infection and the atypical autoimmunity of Guillain-Barré syndrome? Clin. Exp. Immunol. 162, 32–40. doi: 10.1111/j.1365-2249.2010.04223.x
Tanabe, S. (2013). The effect of probiotics and gut microbiota on Th17 Cells. Int. Rev. Immunol. 32, 511–525. doi: 10.3109/08830185.2013.839665
Torii, A., Torii, S., Fujiwara, S., Tanaka, H., Inagaki, N., and Nagai, H. (2007). Lactobacillus Acidophilus strain L-92 regulates the production of Th1 cytokine as well as Th2 cytokines. Allergol. Int. 56, 293–301. doi: 10.2332/allergolint.O-06-459
Wagner, R. D., Johnson, S. J., and Kurniasih Rubin, D. (2009). Probiotic bacteria are antagonistic to Salmonella enterica and Campylobacter jejuni and influence host lymphocyte responses in human microbiota-associated immunodeficient and immunocompetent mice. Mol. Nutr. Food Res. 53, 377–388. doi: 10.1002/mnfr.200800101
Walker, W. A. (2008). Mechanisms of action of probiotics. Clin. Infect. Dis. 46, S87–S91. doi: 10.1086/523335
Wang, X., Ma, C., Wu, J., and Zhu, J. (2013). Roles of T helper 17 cells and interleukin-17 in neuroautoimmune diseases with emphasis on multiple sclerosis and Guillain-Barré syndrome as well as their animal models. J. Neurosci. Res. 91, 871–881. doi: 10.1002/jnr.23233
Wine, E., Gareau, M. G., Johnson-Henry, K., and Sherman, P. M. (2009). Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 300, 146–152. doi: 10.1111/j.1574-6968.2009.01781.x
Wing, J. B., and Sakaguchi, S. (2014). Foxp3+ Treg cells in humoral immunity. Int. Immunol. 26, 61–69. doi: 10.1093/intimm/dxt060
Wu, X., Wang, J., Liu, K., Zhu, J., and Zhang, H.-L. (2015). Are Th17 cells and their cytokines a therapeutic target in Guillain-Barré syndrome? Expert Opin. Ther. Targets. doi: 10.1517/13543776.2016.1111337. [Epub ahead of print].
Xu, L., Kitani, A., Fuss, I., and Strober, W. (2007). Cutting edge: regulatory t cells induce CD4+CD25-Foxp3- T Cells or are self-induced to become Th17 Cells in the absence of exogenous TGF-beta. J. Immunol. 178, 6725–6729. doi: 10.4049/jimmunol.178.11.6725
Keywords: Guillain–Barré syndrome, peripheral neuropathies, neuronal autoimmunity, probiotics, microbiota, gut–brain axis
Citation: Saxena A (2016) Probiotics as a Potential Alternative for Relieving Peripheral Neuropathies: a Case for Guillain-Barré Syndrome. Front. Microbiol. 6:1497. doi: 10.3389/fmicb.2015.01497
Received: 05 October 2015; Accepted: 11 December 2015;
Published: 07 January 2016.
Edited by:
Ryo Inoue, Kyoto Prefectural University, JapanReviewed by:
Mayuko Osada-Oka, Kyoto Prefectural University, JapanCopyright © 2016 Saxena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhishek Saxena, YXNheGVuYTg3QGdtYWlsLmNvbQ==
 Abhishek Saxena
Abhishek Saxena