- 1Department of Applied Prosthodontics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan
- 2Division of Molecular and Regenerative Prosthodontics, Tohoku University Graduate School of Dentistry, Sendai, Japan
- 3Department of Fixed Prosthodontics, Osaka University Graduate School of Dentistry, Suita, Japan
Candida species have emerged as important and common opportunistic human pathogens, particularly in immunocompromised individuals. The current antifungal therapies either have toxic side effects or are insufficiently effect. The aim of this study is develop new small-molecule antifungal compounds by library screening methods using Candida albicans, and to evaluate their antifungal effects on Candida biofilms and cytotoxic effects on human cells. Wild-type C. albicans strain SC5314 was used in library screening. To identify antifungal compounds, we screened a small-molecule library of 1,280 pharmacologically active compounds (LOPAC1280TM) using an antifungal susceptibility test (AST). To investigate the antifungal effects of the hit compounds, ASTs were conducted using Candida strains in various growth modes, including biofilms. We tested the cytotoxicity of the hit compounds using human gingival fibroblast (hGF) cells to evaluate their clinical safety. Only 35 compounds were identified by screening, which inhibited the metabolic activity of C. albicans by >50%. Of these, 26 compounds had fungistatic effects and nine compounds had fungicidal effects on C. albicans. Five compounds, BAY11-7082, BAY11-7085, sanguinarine chloride hydrate, ellipticine and CV-3988, had strong fungicidal effects and could inhibit the metabolic activity of Candida biofilms. However, BAY11-7082, BAY11-7085, sanguinarine chloride hydrate and ellipticine were cytotoxic to hGF cells at low concentrations. CV-3988 showed no cytotoxicity at a fungicidal concentration. Four of the compounds identified, BAY11-7082, BAY11-7085, sanguinarine chloride hydrate and ellipticine, had toxic effects on Candida strains and hGF cells. In contrast, CV-3988 had fungicidal effects on Candida strains, but low cytotoxic effects on hGF cells. Therefore, this screening reveals agent, CV-3988 that was previously unknown to be antifungal agent, which could be a novel therapies for superficial mucosal candidiasis.
Introduction
Candida species have emerged as important and common opportunistic human pathogens, particularly in immunocompromised individuals, such as patients with HIV/AIDS, patients with cancer undergoing chemotherapy, organ transplant recipients receiving immunosuppressive drugs and patients with advanced diabetes (Richardson, 2005; Aperis et al., 2006). Candida sp. are responsible for a spectrum of diseases, which range from local mucosal infections to life-threatening invasive systemic candidiasis (Wisplinghoff et al., 2004).
A key feature of the virulence of Candida sp. is their ability to adhere to surfaces, before developing into distinct surface-attached communities called biofilms. Biofilms may develop on biological and inert surfaces, such as intravascular catheters, stents, shunts, prostheses and implants (Raad, 1998; Ramage et al., 2006). Candida biofilms are intrinsically more resistant to commercially available antifungal agents than their planktonic counterparts (Hawser and Douglas, 1995; Chandra et al., 2001; LaFleur et al., 2006; Seneviratne et al., 2008). Thus, the biofilms that form on medical device can resist the host immune defenses and antifungal treatments, thereby causing chronic infections and failure of implanted medical devices (Ramage et al., 2005). The increasing number of immunocompromised patients and advances in medical technology has led to an increase in biofilm-related infectious diseases, where Candida albicans is the major fungal pathogen. Recently, the frequency of these candidiasis caused by the non C. albicans species of Candida, such as C. glabrata, C. parapsilosis, C. dubliniensis, and C. tropicalis, has increased due to the indiscriminate use of antifungal drugs (Cuellar-Cruz et al., 2012; Pfaller, 2012).
In addition, C. glabrata, C. parapsilosis, and C. krusei exhibit intrinsic resistance to most azole-based antifungal drugs (Lee et al., 2009a; Kothavade et al., 2010; Pfaller et al., 2011) and the emergence of acquired drug resistance to most commercial antifungals has been reported.(Sanglard and Odds, 2002; Pfaller et al., 2010). Despite the urgent requirement for efficient antifungal therapies of systemic infections, the available antifungal drugs, such as novel polyene formulations, new azoles and echinocandins, are few and expensive and have side effects (Rex et al., 2000; Francois et al., 2005; Cornely et al., 2007; Pasqualotto and Denning, 2008). Furthermore, common non-life-threatening superficial infections, such as recurrent vulvovaginal candidiasis, impose significant restrictions on patients and result in a reduced quality of life. Thus, it is necessary to develop new antifungal agents that are effective against Candida biofilms. These agents should overwhelm biofilm-related candidiasis and lead to more effective antifungal treatments.
In recent studies, library screening methods have been used to identify new antifungal agents, which have focused on growth retardation or killing the pathogens (LaFleur et al., 2011; Siles et al., 2013; Stylianou et al., 2014). This type of screening method can identify candidate antifungal agents from large numbers of small-molecule compounds. Small-molecule compounds have many advantages, such as simple synthesis, high chemical stability and low costs compared with organic compounds. Therefore, the aim of the present study was to develop new small-molecule antifungal compounds by library screening methods using C. albicans. Moreover, we evaluated the antifungal effects of the small molecules detected by the library screening method using Candida biofilms as well as their cytotoxic effects on human cells.
Materials and Methods
Drugs and Fungal Strains
The in vitro susceptibility of well-characterized wild-type C. albicans strain SC5314, which was provided by Prof. N.A.R. Gow (University of Aberdeen, Aberdeen, UK) was tested against 1280 compounds from the Library of Pharmacologically Active Compounds (LOPAC1280TM, Sigma–Aldrich, USA). The screen was performed with C. albicans SC5314, and hits were further confirmed with the type strains C. dubliniensis MYA 577, C. glabrata ATCC 2001, C. kusei ATCC 6258, C. palapsilosis ATCC 22019, and C. tropicalis ATCC13803.
High-Throughput Screening (HTS) with Antifungal Susceptibility Tests (ASTs)
High-Throughput Screening was conducted using ASTs, according to the standard Clinical and Laboratory Standard Institute (CLSI) method (Watamoto et al., 2009). Inocula from 24-h yeast cultures on Sabouraud’s dextrose agar (SDA) (Gibco, UK) were adjusted to a turbidity equivalent to a 0.5 McFarland standard at 520 nm using a spectrophotometer. The suspension was diluted further with RPMI 1640 medium (Gibco, UK) to yield an inoculum concentration of 0.5 × 103 to 2.5 × 103 cells/mL. C. albicans was incubated with small-molecule compounds (10 μM) from LOPAC1280TM, which total volume was 150 μL, in 96-well plates at 37°C for 24 h to evaluate the antifungal effects. After incubation, the viability of the fungal cells was determined using the CellTiter-Glo luminescent cell viability kit (Promega, USA). The CellTiter-Glo reagent (150 μL) was added to the medium and incubated for 15 min at room temperature with shaking at 900 rpm. The luminescent signals were detected using a luminometer (GloMax Discover System, Promega, USA). The resulting signal intensity corresponds to ATP amounts and thus to the number of viable microbial cells upon drug exposure (Stylianou et al., 2014). In all 96-well plates, 100 and 0% growth controls were included as microbes plus dimethyl sulfoxide (0.1%) and microbes plus amphotericin B (100 μM), respectively. All assays were performed at least as two biological replicates in triplicate. The ATP level of C. albicans cells, which corresponded to the cell metabolic activity and viability, was calculated for each compound using the following equation (Figure 1A).
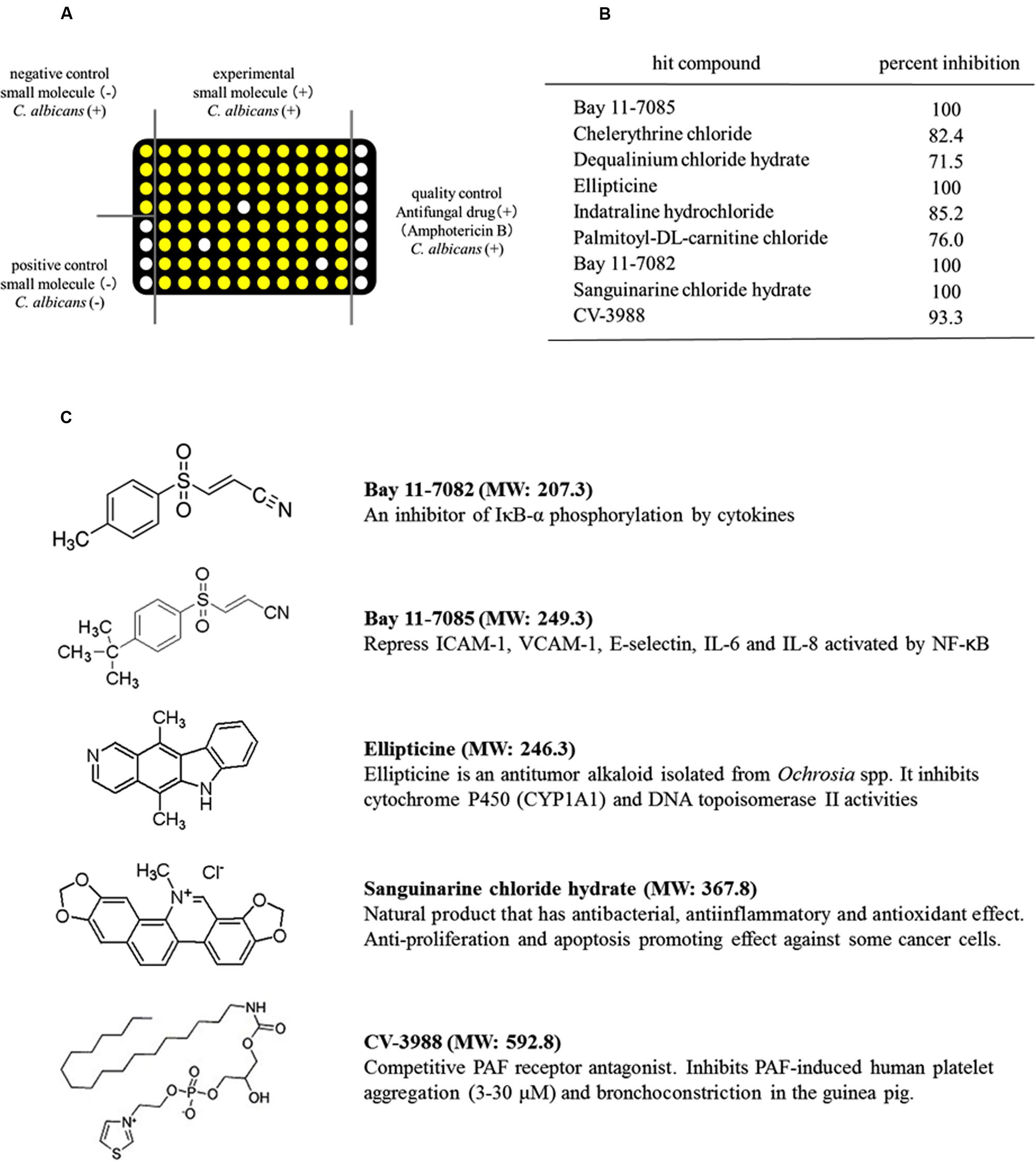
FIGURE 1. Identification of small-molecule compounds that inhibited the metabolic activity of Candida albicans using high-throughput screening (HTS). (A) Schematic showing the HTS procedure. White circles: low ATP level and no metabolic activity in C. albicans. Yellow circles: high ATP level and high metabolic activity in C. albicans. (B) Compounds that inhibited the metabolic activity of C. albicans. (C) Structures of the five compounds that inhibited the metabolic activity of C. albicans by >90%. MW, molecular weight.
Wells were scored as hits if the percentage inhibition was >50%. Hit compounds were evaluated further to assess their antifungal effects.
ASTs of Hit Compound in Various Growth Modes Against Candida Strains
To investigate the antifungal effects of the hit compounds, ASTs were conducted using broth microdilution assays with high cell densities of the planktonic mode, adhesion phase and biofilm mode against Candida strains (C. albicans, C. dubliniensis, C. glabrata, C. kusei, C. palapsilosis, and C. tropicalis). First, high density cell (1 × 107 cells/mL) suspensions were added to the RPMI medium containing each hit compound (10–1000 μM) in 96-well plates and incubated at 37°C for 24 h. Next, the 50% minimum inhibitory concentrations (MICs) of high-density Candida planktonic cultures were determined using the CellTiter-Glo luminescent cell viability kit, as described above. The antifungal effects of the hit compounds were also evaluated in the adhesion phase and the biofilm mode, in the same manner as the planktonic mode. Candida biofilms were produced as described previously (Jin et al., 2004). In brief, Candida cells were grown on SDA at 37°C for 18 h. A loopful of the yeast culture was then inoculated into yeast nitrogen base (YNB) (Difco, USA) medium supplemented with 50 mM glucose. After overnight broth culture in a rotary shaker at 75 rpm, the cells were washed twice with 20 mL of PBS (pH 7.2, 0.1 M). The yeast cells were re-suspended in YNB supplemented with 100 mM glucose and adjusted to an optical density of 0.38 (1 × 107 cells/mL) at 520 nm. This standardized cell suspension was used immediately to form biofilms in the wells of 96-well polystyrene culture plates (Iwaki, Tokyo, Japan). First, the cells were incubated for 90 min at 37°C in a shaker at 75 rpm to allow yeast adherence to the well surface (adhesion phase), before the medium was aspirated and each well was washed once with PBS to remove non-adherent cells. YNB containing 100 mM glucose was then pipetted into each well and the plate was incubated at 37°C in a shaker at 75 rpm for 24 h. Non-adherent cells were removed by pipetting and the biofilms were washed twice with PBS. Following this biofilm growth phase, microscopic examination of the cultures was performed to exclude contamination. These ASTs were repeated on three different occasions.
Cytotoxicity
Primary human gingival fibroblast (hGF) cultures were established from discarded healthy gingival tissues after surgery with the informed consent of the donors (Nikawa et al., 2006). In brief, the gingival tissue specimens were treated overnight with 0.025% trypsin and 0.02% EDTA at 4°C. After trypsin neutralization, the lamina propria mucosae were separated from the epithelial layer and minced into pieces in a plastic tissue culture dish, and then maintained in Dulbecco’s modified Eagle medium (Nacalai Tesque, Kyoto, Japan) supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin and 250 ng/mL amphotericin B (Nacalai Tesque, Kyoto, Japan). After the fibroblasts had migrated out of the tissue, the tissues were removed and the cells were cultured until they reached confluence. The cells were then seeded onto 96-well tissue culture plates (500 cells per well) and the culture medium was exchanged with fresh growth medium containing the hit compounds (0.98–1000 μM). The cells were cultured continuously and the culture medium containing the hit compounds was renewed every other day. The number of cells was evaluated using the WST-1 cell counting assay (Dojindo Laboratories, Kumamoto, Japan), as described previously (Hamada et al., 2007). The highest concentration of each compound that caused greater than 50% reduction in the number of cell compare to that of compound free control cell was reported as the cytotoxic concentration. All the experiments were performed using three samples for each condition in triplicate.
Results
High-Throughput Screening (HTS) Results
We screened 1280 compounds using antifungal susceptibility tests (ASTs) in 96-well plates to identify antifungal agents. Only 35 compounds were identified, which inhibited the metabolic activity of C. albicans by >50%. Thus, the overall hit rate for HTS was approximately 3.9%. Among the hit compounds, 26 compounds had fungistatic effects and nine compounds had fungicidal effects on C. albicans (Figure 1B). Five compounds, BAY11-7082, BAY11-7085, sanguinarine chloride hydrate, ellipticine and CV-3988, had strong fungicidal effects and inhibited the metabolic activity of C. albicans by >90% (Figure 1B). The structures of these five compounds are shown in Figure 1C. The antifungal effects of these five compounds were evaluated using Candida strains (C. albicans, C. dubliniensis, C. glabrata, C. kusei, C. palapsilosis, and C. tropicalis) in high density planktonic, adhesion and biofilm modes.
ASTs of Hit Compounds Using Candida Strains in Various Growth Modes
The HTS results showed that C. albicans was susceptible to all the hit compounds when a low inoculum size (1 × 103 cells/mL) was used, according to the CLSI methodology (MIC < 1 μM). When the cell density increased to 1 × 107 cells/mL, Candida strains were slightly resistant to four of the compounds, but not sanguinarine chloride hydrate. However, all five compounds inhibited the metabolic activity of Candida strains at <31.3 μM and they had fungicidal effects on the high cell density planktonic mode (Table 1). As a control, amphotericin B inhibited the metabolic activity of C. albicans at <3.9 μM.
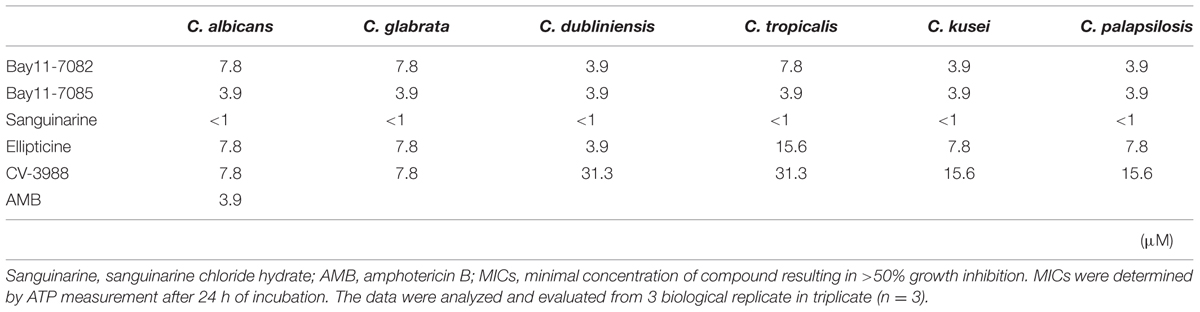
TABLE 1. Minimum inhibitory concentrations (MICs) of five candidate compounds against planktonic mode of Candida strains.
The drug susceptibility of adhesion phase Candida strains to the five compounds was higher than that of the high density planktonic cultures (Table 2). In particular, sanguinarine chloride hydrate was effective against adhesion phase and it could inhibit the metabolic activity at <15.6 μM. Bay 11-7082 and Bay 11-7085 were also effective against the adhesion phase and could inhibit the metabolic activity at <31.3 μM. As a control, amphotericin B inhibited the metabolic activity of C. albicans adhesion phase at 15.6 μM.
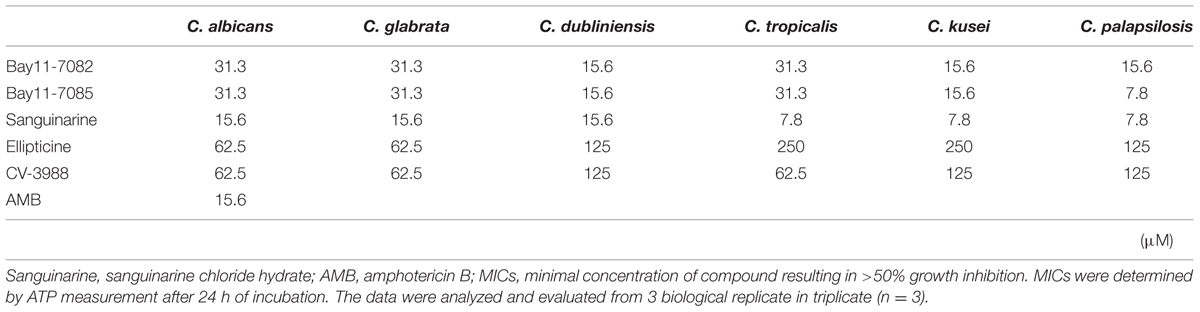
TABLE 2. Minimum inhibitory concentrations of five candidate compounds against adhesion phase of Candida strains.
Most Candida biofilms were more resistant to the five compounds than other growth mode. Especially, C. tropicalis biofilm was most resistant to the five compounds in all growth modes (Table 3). Bay 11-7082, Bay 11-7085, Ellipticine and CV-3988 could inhibit the metabolic activity of Candida biofilms at <62.5, 62.5, 500, and 125 μM, respectively. Sanguinarine chloride hydrate was the most effective antifungal agent in this study and it could inhibit the metabolic activity of Candida strains at <31.3 μM. As a control, amphotericin B inhibited the metabolic activity of C. albicans biofilm at 62.5 μM.
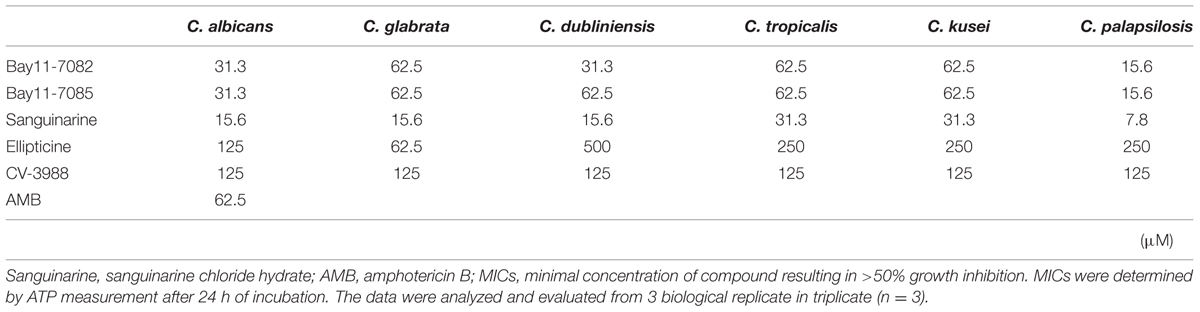
TABLE 3. Minimum inhibitory concentrations of five candidate compounds against biofilm mode of Candida strains.
Cytotoxicity
In addition to pharmacologically active compounds, small-molecule libraries often contain toxic molecules that do not make good drug candidates. To evaluate the safety for clinical use, we tested the cytotoxic effects of the hit compounds using human cell cultures. We used hGF cells because of their ubiquitous nature and their widespread use in cytotoxicity testing (Egusa et al., 2009; LaFleur et al., 2011). The hGF cells were grown in 96-well plates and exposed to increasing doses (two-fold increments) of each hit compound for 4 days. The hGF metabolic activity was measured every other day and used as an indicator of cell viability. After 4 days, Bay 11-7082, Bay 11-7085, ellipticine, sanguinarine chloride hydrate and CV-3988 inhibited cell proliferation no more than 50%, namely, did not kill cells at less than 7.81, 7.81, 1.95, 0.73, and 250 μM, respectively (Table 4).
Discussion
Candida species are the main fungal pathogen that causes infections in humans, ranging from superficial mucosal infection to systemic mycoses (Navarro-Garcia et al., 2001). Candida infections are intractable and recurrent diseases, which have increased due to the rise in the number of immunocompromised host populations (Beck-Sague and Jarvis, 1993; Wisplinghoff et al., 2004). Drug-resistant Candida strains have also increased dramatically because of the increased use of antifungal agents. Thus, the development of novel antifungal drugs and treatment strategies are essential for combating Candida infections. High-throughput screening (HTS) is an effective method for identifying candidate novel antifungal drugs. It is important to apply adequate screening methods to small-molecule compound libraries because appropriate selection procedures are the key to successful screening. In this study, LOPAC1280TM was used as the small-molecule library, which contained pharmacologically active compounds and all the compounds were commercially available. Thus, the main effects of these small molecules on human cells are already known and described in database of manufacture. Therefore, it may be easier to apply these compounds in clinical practice with fewer unexpected drug side effects.
In general, polyenes, azoles, allylamines, morpholines, antimetabolites, and echinocandins are the six major antifungal drug categories to manage fungal infections (Khan and Jain, 2000; Ruhnke et al., 2008). Most of these antifungal drugs have fungistatic or fungicidal effects on exponentially growing planktonic cells, but Candida cells are resistant to these drugs after biofilm formation (Watamoto et al., 2009). Interestingly, we found that five small-molecule compounds (BAY11-7082, BAY11-7085, sanguinarine chloride hydrate, ellipticine and CV-3988) were antifungal drug candidates with inhibitory effects on various Candida biofilms at concentrations below 500 μM.
BAY11-7082 and BAY 11-7085 is known to be an inhibitor of nuclear factor κB (NF-κB) activation by the blockade of inhibitor κB (IκB) phosphorylation, which is a trigger of apoptosis (Pierce et al., 1997; Guzman and Jordan, 2005; Chopra et al., 2008; Lee et al., 2009b; Zanotto-Filho et al., 2010). Bay 11-7082 triggers cell membrane scrambling and cell shrinkage (Lang et al., 2008). BAY 11-7085 has been shown to activate c-jun N-terminal kinase and p38 mitogen-activated protein kinase (MAPK) (Pierce et al., 1997). BAY 11-7085 inhibits cell proliferation by inducing apoptosis and G0/G1 arrest of the cell cycle in human cells (Bockelmann et al., 2005). These actions have anti-inflammatory, anticancer and slight hemolytic effects (Ghashghaeinia et al., 2011).
Sanguinarine chloride hydrate is a phytoalexin and has been reported to suppress activation of the transcription factor NF-κB (Chaturvedi et al., 1997) and to modulate the functions of various enzymes, such as MAPK phosphatase-1 (Vogt et al., 2005), protein kinase C (Gopalakrishna et al., 1995) and phosphoinositide-dependent protein kinase 1 (Vrba et al., 2008). These actions of Sanguinarine have antimicrobial, antioxidant, anti-inflammatory, hemolytic and cytotoxic effects (Lenfeld et al., 1981; Godowski, 1989; Malikova et al., 2006; Babu et al., 2008; Matkar et al., 2008; Jang et al., 2009).
Ellipticine, an alkaloid isolated from Apocyanaceae plants, has been reported to mediate primarily DNA damage such as DNA intercalation (Auclair, 1987), inhibition of topoisomerase II (Auclair, 1987; Stiborova et al., 2006), inhibition of casein kinase 2 (Prudent et al., 2010) and the formation of covalent DNA adducts by cytochrome P450s and peroxidases (Stiborova et al., 2011). These actions of Ellipticine has anti-tumor, cytotoxic, hemolytic and mutagenic activities (Lee, 1976; Rouesse et al., 1985). Therefore, the known cell proliferation inhibitory effects of these four small-molecules agree with the findings of the present study. Furthermore, the antifungal and cytotoxic effects of these small molecules on Candida strains may involve the same mechanism because Candida strains are eukaryotes and possesses the same targets. Thus, these small molecules are toxic to human cells and Candida strains, and inappropriate for clinical use corroborated by the relatively low cytotoxic concentration on hGF.
On the other hand, platelet-activating factor (PAF), which is released almost immediately in response to inflammatory stimuli (Im et al., 1997) by various inflammatory cells, is a potent lipid messenger involved in cellular activation, fertilization, intracellular signaling, apoptosis and diverse inflammatory reactions (Braquet et al., 1987; Shukla, 1992; Buttke and Sandstrom, 1995; Fukuda and Breuel, 1996). CV-3988 (Terashita et al., 1983; Terashita et al., 1987) is a structural analog of PAF, which has been shown to specifically inhibit the in vitro and in vivo activities of PAF (Sultana et al., 1999) by competitive binding with the PAF receptor (PAF-R) (Terashita et al., 1983; Summers and Albert, 1995; Negro Alvarez et al., 1997). Therefore, CV-3988 is an antagonist of PAF-R, which inhibits the functions of leukocytes, including platelet aggregation, inflammation and anaphylaxis. We showed for the first time that CV-3988 had a fungicidal effect on various Candida biofilms and low cytotoxity effect on hGF cells. In past study, CV-3988 had slight hemolytic effect and can safely be administered to human (Arnout et al., 1988). These results demonstrate that CV-3988 has a novel and specific fungicidal effect on Candida strains and may become initial drug choice for the treatment of candidiasis. Furthermore, Candida sp. are common microbes in the oral cavity and vagina and causes mucotitis in immunocompromised and healthy hosts. Mouthwashes and ointments containing antifungal agents are primary treatment for oral and vaginal candidiasis. Therefore, CV-3988 may be suitable for use on oral mucosal surfaces to combat Candida biofilm infections such as thrush and denture-related stomatitis. Although CV-3988 may facilitate novel treatment strategies to combat Candida infections, further studies about fungicidal mechanism and pharmacokinetics are required before it can be applied in clinical practice.
Conclusion
We identified five small-molecule compounds (BAY11-7082, BAY11-7085, sanguinarine chloride hydrate, ellipticine and CV-3988) as novel antifungal drug candidates using HTS methods. BAY11-7082, BAY11-7085, sanguinarine chloride hydrate and ellipticine were toxic to Candida strains as well as hGF cells. In contrast, CV-3988 had fungicidal effects on Candida strains, but low cytotoxic effects on hGF cells. Therefore, in future, mouthwashes and ointments containing CV-3988 may be used as a novel treatment for superficial mucosal candidiasis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work was supported by a Grant-in-Aid for Young Scientists (B 24792078) from the Japan Society of the Promotion of Science.
References
Aperis, G., Myriounis, N., Spanakis, E. K., and Mylonakis, E. (2006). Developments in the treatment of candidiasis: more choices and new challenges. Expert Opin. Investig. Drugs 15, 1319–1336. doi: 10.1517/13543784.15.11.1319
Arnout, J., Van Hecken, A., De Lepeleire, I., Miyamoto, Y., Holmes, I., De Schepper, P., et al. (1988). Effectiveness and tolerability of CV-3988, a selective PAF antagonist, after intravenous administration to man. Br. J. Clin. Pharmacol. 25, 445–451.
Auclair, C. (1987). Multimodal action of antitumor agents on DNA: the ellipticine series. Arch. Biochem. Biophys. 259, 1–14. doi: 10.1016/0003-9861(87)90463-2
Babu, C. K., Khanna, S. K., and Das, M. (2008). Antioxidant status of erythrocytes and their response to oxidative challenge in humans with argemone oil poisoning. Toxicol. Appl. Pharmacol. 230, 304–311. doi: 10.1016/j.taap.2008.02.017
Beck-Sague, C., and Jarvis, W. R. (1993). Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. National Nosocomial Infections Surveillance System. J. Infect. Dis. 167, 1247–1251. doi: 10.1093/infdis/167.5.1247
Bockelmann, R., Horn, T., Gollnick, H., and Bonnekoh, B. (2005). Interferon-gamma-dependent in vitro model for the putative keratin 17 autoimmune loop in psoriasis: exploration of pharmaco- and gene-therapeutic effects. Skin Pharmacol. Physiol. 18, 42–54. doi: 10.1159/000081685
Braquet, P., Touqui, L., Shen, T. Y., and Vargaftig, B. B. (1987). Perspectives in platelet-activating factor research. Pharmacol. Rev. 39, 97–145.
Buttke, T. M., and Sandstrom, P. A. (1995). Redox regulation of programmed cell death in lymphocytes. Free Radic. Res. 22, 389–397. doi: 10.3109/10715769509147548
Chandra, J., Kuhn, D. M., Mukherjee, P. K., Hoyer, L. L., Mccormick, T., and Ghannoum, M. A. (2001). Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183, 5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001
Chaturvedi, M. M., Kumar, A., Darnay, B. G., Chainy, G. B., Agarwal, S., and Aggarwal, B. B. (1997). Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-kappaB activation, IkappaBalpha phosphorylation, and degradation. J. Biol. Chem. 272, 30129–30134. doi: 10.1074/jbc.272.48.30129
Chopra, P., Bajpai, M., Dastidar, S. G., and Ray, A. (2008). Development of a cell death-based method for the screening of nuclear factor-kappaB inhibitors. J. Immunol. Methods 335, 126–131. doi: 10.1016/j.jim.2008.02.016
Cornely, O. A., Maertens, J., Winston, D. J., Perfect, J., Ullmann, A. J., Walsh, T. J., et al. (2007). Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356, 348–359. doi: 10.1056/NEJMoa061094
Cuellar-Cruz, M., Vega-Gonzalez, A., Mendoza-Novelo, B., Lopez-Romero, E., Ruiz-Baca, E., Quintanar-Escorza, M. A., et al. (2012). The effect of biomaterials and antifungals on biofilm formation by Candida species: a review. Eur. J. Clin. Microbiol. Infect. Dis. 31, 2513–2527. doi: 10.1007/s10096-012-1634-6
Egusa, H., Kaneda, Y., Akashi, Y., Hamada, Y., Matsumoto, T., Saeki, M., et al. (2009). Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 30, 4676-4686. doi: 10.1016/j.biomaterials.2009.05.032
Francois, I. E., Aerts, A. M., Cammue, B. P., and Thevissen, K. (2005). Currently used antimycotics: spectrum, mode of action and resistance occurrence. Curr. Drug Targets 6, 895–907. doi: 10.2174/138945005774912744
Fukuda, A. I., and Breuel, K. F. (1996). Effect of platelet activating factor on embryonic development and implantation in the mouse. Hum. Reprod. 11, 2746–2749. doi: 10.1093/oxfordjournals.humrep.a019202
Ghashghaeinia, M., Toulany, M., Saki, M., Bobbala, D., Fehrenbacher, B., Rupec, R., et al. (2011). The NFkB pathway inhibitors Bay 11-7082 and parthenolide induce programmed cell death in anucleated Erythrocytes. Cell Physiol. Biochem. 27, 45–54. doi: 10.1159/000325204
Gopalakrishna, R., Chen, Z. H., and Gundimeda, U. (1995). Modifications of cysteine-rich regions in protein kinase C induced by oxidant tumor promoters and enzyme-specific inhibitors. Methods Enzymol. 252, 132–146. doi: 10.1016/0076-6879(95)52016-3
Guzman, M. L., and Jordan, C. T. (2005). Feverfew: weeding out the root of leukaemia. Expert Opin. Biol. Ther. 5, 1147–1152. doi: 10.1517/14712598.5.9.1147
Hamada, Y., Egusa, H., Kaneda, Y., Hirata, I., Kawaguchi, N., Hirao, T., et al. (2007). Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dent. Mater. J. 26, 487–492. doi: 10.4012/dmj.26.487
Hawser, S. P., and Douglas, L. J. (1995). Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39, 2128–2131. doi: 10.1128/AAC.39.9.2128
Im, S. Y., Han, S. J., Ko, H. M., Choi, J. H., Chun, S. B., Lee, D. G., et al. (1997). Involvement of nuclear factor-kappa B in platelet-activating factor-mediated tumor necrosis factor-alpha expression. Eur. J. Immunol. 27, 2800–2804. doi: 10.1002/eji.1830271109
Jang, B. C., Park, J. G., Song, D. K., Baek, W. K., Yoo, S. K., Jung, K. H., et al. (2009). Sanguinarine induces apoptosis in A549 human lung cancer cells primarily via cellular glutathione depletion. Toxicol. In Vitro 23, 281–287. doi: 10.1016/j.tiv.2008.12.013
Jin, Y., Samaranayake, L. P., Samaranayake, Y., and Yip, H. K. (2004). Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch. Oral. Biol. 49, 789–798. doi: 10.1016/j.archoralbio.2004.04.011
Khan, Z. K., and Jain, P. (2000). Antifungal agents and immunomodulators in systemic mycoses. Indian J. Chest. Dis. Allied Sci. 42,345–355.
Kothavade, R. J., Kura, M. M., Valand, A. G., and Panthaki, M. H. (2010). Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J. Med. Microbiol. 59, 873–880. doi: 10.1099/jmm.0.013227-0
LaFleur, M. D., Kumamoto, C. A., and Lewis, K. (2006). Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50, 3839–3846. doi: 10.1128/AAC.00684-06
LaFleur, M. D., Lucumi, E., Napper, A. D., Diamond, S. L., and Lewis, K. (2011). Novel high-throughput screen against Candida albicans identifies antifungal potentiators and agents effective against biofilms. J. Antimicrob. Chemother. 66, 820–826. doi: 10.1093/jac/dkq530
Lang, F., Gulbins, E., Lerche, H., Huber, S. M., Kempe, D. S., and Foller, M. (2008). Eryptosis, a window to systemic disease. Cell Physiol. Biochem. 22, 373–380. doi: 10.1159/000185448
Lee, I., Fishman, N. O., Zaoutis, T. E., Morales, K. H., Weiner, M. G., Synnestvedt, M., et al. (2009a). Risk factors for fluconazole-resistant Candida glabrata bloodstream infections. Arch. Intern. Med. 169, 379–383. doi: 10.1001/archinte.169.4.379
Lee, S. J., Long, M., Adler, A. J., Mittler, R. S., and Vella, A. T. (2009b). The IKK-neutralizing compound Bay11 kills supereffector CD8 T cells by altering caspase-dependent activation-induced cell death. J. Leukoc. Biol. 85, 175–185. doi: 10.1189/jlb.0408248
Lee, I. P. (1976). A possible mechanism of ellipticine-induced hemolysis. J. Pharmacol. Exp. Ther. 196, 525–535.
Lenfeld, J., Kroutil, M., Marsalek, E., Slavik, J., Preininger, V., and Simanek, V. (1981). Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonium majus. Planta Med. 43, 161–165. doi: 10.1055/s-2007-971493
Malikova, J., Zdarilova, A., Hlobilkova, A., and Ulrichova, J. (2006). The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine. Cell Biol. Toxicol. 22, 439–453. doi: 10.1007/s10565-006-0109-x.
Matkar, S. S., Wrischnik, L. A., and Hellmann-Blumberg, U. (2008). Sanguinarine causes DNA damage and p53-independent cell death in human colon cancer cell lines. Chem. Biol. Interact. 172, 63–71. doi: 10.1016/j.cbi.2007.12.006
Navarro-Garcia, F., Sanchez, M., Nombela, C., and Pla, J. (2001). Virulence genes in the pathogenic yeast Candida albicans. FEMS Microbiol. Rev. 25, 245–268. doi: 10.1016/S0168-6445(00)00066-8
Negro Alvarez, J. M., Miralles Lopez, J. C., Ortiz Martinez, J. L., Abellan Aleman, A., and Rubio Del Barrio, R. (1997). Platelet-activating factor antagonists. Allergol Immunopathol (Madr) 25, 249–258.
Nikawa, H., Egusa, H., Makihira, S., Okamoto, T., Kurihara, H., Shiba, H., et al. (2006). An in vitro evaluation of the adhesion of Candida species to oral and lung tissue cells. Mycoses 49, 14–17. doi: 10.1111/j.1439-0507.2005.01176.x
Pasqualotto, A. C., and Denning, D. W. (2008). New and emerging treatments for fungal infections. J. Antimicrob. Chemother. 61(Suppl. 1), i19–i30. doi: 10.1093/jac/dkm428.
Pfaller, M. A. (2012). Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 125, S3–S13. doi: 10.1016/j.amjmed.2011.11.001
Pfaller, M. A., Diekema, D. J., Gibbs, D. L., Newell, V. A., Ellis, D., Tullio, V., et al. (2010). Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 48, 1366–1377. doi: 10.1128/JCM.02117-09
Pfaller, M. A., Messer, S. A., Moet, G. J., Jones, R. N., and Castanheira, M. (2011). Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int. J. Antimicrob. Agents 38, 65–69. doi: 10.1016/j.ijantimicag.2011.02.016
Pierce, J. W., Schoenleber, R., Jesmok, G., Best, J., Moore, S. A., Collins, T., et al. (1997). Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103. doi: 10.1074/jbc.272.34.21096
Prudent, R., Moucadel, V., Nguyen, C. H., Barette, C., Schmidt, F., Florent, J. C., et al. (2010). Antitumor activity of pyridocarbazole and benzopyridoindole derivatives that inhibit protein kinase CK2. Cancer. Res. 70, 9865–9874. doi: 10.1158/0008-5472.CAN-10-0917
Raad, I. (1998). Intravascular-catheter-related infections. Lancet 351, 893–898. doi: 10.1016/S0140-6736(97)10006-X.
Ramage, G., Martinez, J. P., and Lopez-Ribot, J. L. (2006). Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 6, 979–986. doi: 10.1111/j.1567-1364.2006.00117.x
Ramage, G., Saville, S. P., Thomas, D. P., and Lopez-Ribot, J. L. (2005). Candida biofilms: an update. Eukaryot. Cell 4, 633–638. doi: 10.1128/EC.4.4.633-638.2005
Rex, J. H., Walsh, T. J., Sobel, J. D., Filler, S. G., Pappas, P. G., Dismukes, W. E., et al. (2000). Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis 30, 662–678. doi: 10.1086/313749
Richardson, M. D. (2005). Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother 56(Suppl. 1), i5–i11. doi: 10.1093/jac/dki218
Rouesse, J. G., Le Chevalier, T., Caille, P., Mondesir, J. M., Sancho-Garnier, H., May-Levin, F., et al. (1985). Phase II study of elliptinium in advanced breast cancer. Cancer Treat Rep. 69, 707–708.
Ruhnke, M., Hartwig, K., and Kofla, G. (2008). New options for treatment of candidaemia in critically ill patients. Clin. Microbiol. Infect. 14(Suppl. 4), 46–54. doi: 10.1111/j.1469-0691.2008.01981.x
Sanglard, D., and Odds, F. C. (2002). Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2, 73–85. doi: 10.1016/S1473-3099(02)00181-0
Seneviratne, C. J., Jin, L. J., Samaranayake, Y. H., and Samaranayake, L. P. (2008). Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob. Agents Chemother. 52, 3259–3266. doi: 10.1128/AAC.00541-08
Shukla, S. D. (1992). Platelet-activating factor receptor and signal transduction mechanisms. FASEB J. 6, 2296–2301.
Siles, S. A., Srinivasan, A., Pierce, C. G., Lopez-Ribot, J. L., and Ramasubramanian, A. K. (2013). High-Throughput Screening of a Collection of Known Pharmacologically Active Small Compounds for Identification of Candida albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 57, 3681–3687. doi: 10.1128/AAC.00680-13
Stiborova, M., Borek-Dohalska, L., Aimova, D., Kotrbova, V., Kukackova, K., Janouchova, K., et al. (2006). Oxidation pattern of the anticancer drug ellipticine by hepatic microsomes - similarity between human and rat systems. Gen. Physiol. Biophys. 25, 245–261.
Stiborova, M., Rupertova, M., and Frei, E. (2011). Cytochrome P450- and peroxidase-mediated oxidation of anticancer alkaloid ellipticine dictates its anti-tumor efficiency. Biochim. Biophys. Acta 1814, 175–185. doi: 10.1016/j.bbapap.2010.05.016
Stylianou, M., Kulesskiy, E., Lopes, J. P., Granlund, M., Wennerberg, K., and Urban, C. F. (2014). Antifungal application of nonantifungal drugs. Antimicrob. Agents Chemother. 58, 1055–1062. doi: 10.1128/AAC.01087-13
Sultana, C., Shen, Y., Johnson, C., and Kalra, V. K. (1999). Cobalt chloride-induced signaling in endothelium leading to the augmented adherence of sickle red blood cells and transendothelial migration of monocyte-like HL-60 cells is blocked by PAF-receptor antagonist. J. Cell. Physiol. 179, 67–78. doi: 10.1002/(SICI)1097-4652(199904)179
Summers, J. B., and Albert, D. H. (1995). Platelet activating factor antagonists. Adv. Pharmacol. 32, 67–68. doi: 10.1016/S1054-3589(08)61012-1
Terashita, Z., Imura, Y., Takatani, M., Tsushima, S., and Nishikawa, K. (1987). CV-6209, a highly potent antagonist of platelet activating factor in vitro and in vivo. J. Pharmacol. Exp. Ther. 242, 263–268.
Terashita, Z., Tsushima, S., Yoshioka, Y., Nomura, H., Inada, Y., and Nishikawa, K. (1983). CV-3988 - a specific antagonist of platelet activating factor (PAF). Life Sci. 32, 1975–1982. doi: 10.1016/0024-3205(83)90049-8
Vogt, A., Tamewitz, A., Skoko, J., Sikorski, R. P., Giuliano, K. A., and Lazo, J. S. (2005). The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J. Biol. Chem. 280, 19078–19086. doi: 10.1074/jbc.M501467200
Vrba, J., Dvorak, Z., Ulrichova, J., and Modriansky, M. (2008). Conventional protein kinase C isoenzymes undergo dephosphorylation in neutrophil-like HL-60 cells treated by chelerythrine or sanguinarine. Cell Biol. Toxicol. 24, 39–53. doi: 10.1007/s10565-007-9014-1
Watamoto, T., Samaranayake, L. P., Jayatilake, J. A., Egusa, H., Yatani, H., and Seneviratne, C. J. (2009). Effect of filamentation and mode of growth on antifungal susceptibility of Candida albicans. Int. J. Antimicrob. Agents 34, 333–339. doi: 10.1016/j.ijantimicag.2009.03.008
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., and Edmond, M. B. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39, 309–317. doi: 10.1086/421946
Zanotto-Filho, A., Delgado-Canedo, A., Schroder, R., Becker, M., Klamt, F., and Moreira, J. C. (2010). The pharmacological NFkappaB inhibitors BAY117082 and MG132 induce cell arrest and apoptosis in leukemia cells through ROS-mitochondria pathway activation. Cancer Lett. 288, 192–203. doi: 10.1016/j.canlet.2009.06.038
Keywords: drug discovery, antifungal drug, biofilm, Small molecules, Candida albicans
Citation: Watamoto T, Egusa H, Sawase T and Yatani H (2015) Screening of Pharmacologically Active Small Molecule Compounds Identifies Antifungal Agents Against Candida Biofilms. Front. Microbiol. 6:1453. doi: 10.3389/fmicb.2015.01453
Received: 21 August 2015; Accepted: 04 December 2015;
Published: 22 December 2015.
Edited by:
Edvaldo Antonio Ribeiro Rosa, The Pontifical Catholic University of Paraná, BrazilReviewed by:
Sabine Fillinger, Institut National De La Recherche Agronomique, FranceJack Wong, The Chinese University of Hong Kong, Hong Kong
Copyright © 2015 Watamoto, Egusa, Sawase and Yatani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takao Watamoto, d2F0YW1vdG9AbmFnYXNha2ktdS5hYy5qcA==
 Takao Watamoto
Takao Watamoto Hiroshi Egusa
Hiroshi Egusa Takashi Sawase1
Takashi Sawase1