
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 09 September 2015
Sec. Microbial Physiology and Metabolism
Volume 6 - 2015 | https://doi.org/10.3389/fmicb.2015.00906
This article is part of the Research TopicSecondary Metabolism: An Unlimited Foundation for Synthetic BiologyView all 7 articles
 Tiago Beites1,2†
Tiago Beites1,2† Marta V. Mendes1,2*
Marta V. Mendes1,2*The increased number of bacterial genome sequencing projects has generated over the last years a large reservoir of genomic information. In silico analysis of this genomic data has renewed the interest in bacterial bioprospecting for bioactive compounds by unveiling novel biosynthetic gene clusters of unknown or uncharacterized metabolites. However, only a small fraction of those metabolites is produced under laboratory-controlled conditions; the remaining clusters represent a pool of novel metabolites that are waiting to be “awaken”. Activation of the biosynthetic gene clusters that present reduced or no expression (known as cryptic or silent clusters) by heterologous expression has emerged as a strategy for the identification and production of novel bioactive molecules. Synthetic biology, with engineering principles at its core, provides an excellent framework for the development of efficient heterologous systems for the expression of biosynthetic gene clusters. However, a common problem in its application is the host-interference problem, i.e., the unpredictable interactions between the device and the host that can hamper the desired output. Although an effort has been made to develop orthogonal devices, the most proficient way to overcome the host-interference problem is through genome simplification. In this review we present an overview on the strategies and tools used in the development of hosts/chassis for the heterologous expression of specialized metabolites biosynthetic gene clusters. Finally, we introduce the concept of specialized host as the next step of development of expression hosts.
Over the last century, specialized metabolites derived from microbial secondary metabolic pathways have been used by the pharmaceutical industry as a source of lead compounds to feed the drug discovery pipeline. However, in the past years there has been a decrease in the number of new drugs approved for clinical use, which is a reflection of a depleted drug discovery pipeline. In addition, natural products have been gradually dismissed due to defective bioprospecting programs that allocate a high number of resources but reach a very limited number of new compounds facing the so-called “dereplication” problem.
The development of high-throughput DNA sequencing technologies has rediscovered bacterial secondary metabolism as a reservoir of new bioactive compounds. The proliferation of genome sequencing projects (Harrison and Studholme, 2014) and the development of genome mining computational tools such as antiSMASH (Weber et al., 2015) have exposed bacterial genomes as the hosts of multiple secondary metabolites biosynthetic gene clusters (Nett et al., 2009). Presently there is a large pool of genomic information regarding biosynthetic gene clusters whose associated metabolites are unknown or uncharacterized, however, only a small fraction of those metabolites is produced under laboratory-controlled conditions. Activation of the biosynthetic gene clusters that present reduced or no expression (known as cryptic or silent clusters), has emerged as a key strategy for the identification and production of novel bioactive molecules (Ochi and Hosaka, 2012).
Among the several bacteria used as natural product factories (Gross and Loper, 2009; Weissman and Muller, 2010; Sansinenea and Ortiz, 2011; Mondol et al., 2013; Brito et al., 2015) the actinomycetes, in particular streptomycetes, stand out as the most prolific source of bioactive microbial metabolites (Berdy, 2005). Moreover, the vast (meta)genomic data collected during the post-genomic era and the identification of multiple cryptic secondary metabolite biosynthetic gene clusters in the genomes, has identified actinobacteria as a renewed source of novel natural products (Baltz, 2008). Due to Streptomyces known proficiency as natural producers, these bacteria have been widely used for the activation of cryptic gene clusters, namely by modulating the expression of genes coding for the cluster-situated regulators and/or biosynthetic proteins (Laureti et al., 2011; Olano et al., 2014). However, this strategy poses some technical challenges for many strains that have proven to be recalcitrant to genetic manipulation. To circumvent the resistance to genetic manipulation of natural producers, heterologous expression of secondary metabolite biosynthetic gene clusters has emerged as an alternative strategy for the activation of silent clusters. A paradigmatic example was the identification and characterization of 13 novel terpenes through the heterologous expression in Streptomyces avermitilis SUKA22 strain of 7 Streptomyces-derived genes annotated as terpene synthases (Yamada et al., 2015). Moreover, the continuous efforts to develop an extended Streptomyces molecular toolbox based on synthetic biology principles (Rudolph et al., 2013; Luo et al., 2015) has potentiated even more cryptic gene clusters as an alternative for the discovery of novel bioactive compounds (Shao et al., 2013).
In the context of microbial specialized metabolite heterologous production, the development of suitable expression host strains, or as it is called in the synthetic biology jargon, the chassis, is key for the use of synthetic biological systems to the production and characterization of new bioactive metabolites. In this review we will focus on the strategies for development of Actinobacteria cell platforms devoted to the production of small molecules derived from microbial secondary metabolism and review the current status on natural compound heterologous production.
The increasingly competitive and efficient new generation sequencing techniques have completely revolutionized biological sciences. The applications are diverse and impacted the way how biological systems are approached, i.e., instead of a gene-based approach it is now possible to face the cell as a whole through a genome-based approach. And it is even possible to go one level up and study a community of organisms through meta-genomics. However, the massive amount of data currently available provided a clearer picture on the extreme complexity that a single cell encloses, exposing our lack of understanding on how the different network components are wired. Furthermore, the sequencing of genomes revealed a high percentage of predicted proteins with no discernible localization and/or function (Ijaq et al., 2015).
Cell complexity poses many challenges to synthetic biology, since one should not expect that the synthetic device would not interact with endogenous components of the host cell. In fact, the complexity of the system exponentially increases possibilities of unpredictable interactions that may hamper the output. As a response to this limitation, an effort has been made towards the generation of orthologous systems (Channon et al., 2008). Orthogonality is a concept borrowed from mathematics and computer science and it refers to synthetic devices that work independently from the host, meaning that the interference will be minimal. The engineering of ribosomes that recognize a different genetic code is a good example on the efforts that have been made toward orthogonal systems (Wang et al., 2007). However, even in this case the activity of the ribosomes is still very dependent on the cellular machinery and prone to be modulated by different factors (de las Heras et al., 2008).
The rationale behind genome streamlining aims at solving this host interference problem by reducing the complexity of the chassis genome (Leprince et al., 2012b). However, although genome simplification is a very logical step as a general concept, still there are many questions to answer. What strains will be used? What regions should be deleted? How should genome streamlining be performed? Where is the threshold beyond which genome simplification does not bring any additional advantages? The answer to these questions is highly dependent on the downstream application. In fact, as it will be discussed in the next sections, there is a vast multitude of genome streamlining workflows that can be used to meet the different aims.
Nevertheless, there is a primal question to all genome streamlining projects: what makes a good chassis? In spite of the unavoidable specificities, one could point out four main characteristics that must be taken into account: genetic manageability, growth robustness, genetic stability and the ability to accurately predict interactions between the synthetic device and the chassis. In the particular case of chassis development for the production of microbial specialized metabolites, one could add that it should also possess a minimal extracellular metabolome profile that would simplify the purification of the desirable molecule (Gomez-Escribano and Bibb, 2011).
The clarification of the minimal genome and reduced genome concepts is also of great importance when it comes to genome streamlining. The quest for the minimal genome envisioned the determination of the minimal set of genes necessary to sustain life (de Lorenzo, 2011). The versions of the minimal genome have been swinging in number and components with no apparent consensus, mainly due to environmental constraints, i.e., different environments equal different requirements (Danchin, 2012). Nevertheless, these studies are dealing with the limits of life and thereby of paramount relevance to basic biological questions such as the identification of the “core” genome. The “core” genome defined as the set of common essential genes, is a concept intimately linked to the development of minimal or reduced genomes. For instance, comparative genome analysis of 17 genomes led to the definition of Streptomyces core genome composed of 2018 orthologous genes that corresponded to 24–38% of the analyzed genomes (Kim et al., 2015). However, if one intends to develop a chassis for biotechnological downstream applications, the suitability and applicability of such minimal platforms is not clear. In particular, if we consider the heterologous expression of specialized metabolites, the efficient supply of precursor units requires genetic features that go beyond the core genome. Therefore, concerning the genome streamlining for the heterologous expression of microbial specialized metabolites the reduced genome concept seems more suitable than the minimal genome.
Homologous recombination has long been used to genetically manipulate strains, getting advantage of the natural recombination systems. However, relying solely on the endogenous machinery may constitute a low efficient approach in some cases (Komatsu et al., 2010). Systems like the bacteriophage-derived -Red recombinase were shown to greatly increase the efficiency of homologous recombination and thereby very useful for genome editing (Murphy, 1998). This system only requires a minimum of 30–50 bp overlapping flanking regions, which attests its efficiency (Karlinsey, 2007). Recently, a system based on the meganuclease I-SecI from Sacharomyces cereviseae was developed for the genetic manipulation of actinomycetes (Fernandez-Martinez and Bibb, 2014). The strategy relies on the introduction of a DNA break by the endonuclease at an unique 18 bp recognition sequence that can only be repaired by homologous recombination. The double recombinants recovery efficiency reported (27–52%) validates this new system as a valuable tool for genome editing, particularly if we consider the traditional low-number of double recombinants obtained in the Streptomyces field (Kieser et al., 2000).
Interestingly, recombineering without the action of a recombinase was shown to be possible in some Gram-negative bacteria. In this case the transformation of cells with synthetic single-stranded DNA (ssDNA) oligonucleotides was shown to be able to recombine with genomic DNA – a process denominated by oligonucleotide recombineering (Swingle et al., 2010). All homologous recombination reactions rely on a ssDNA intermediate that will pair with the complementary strand in the target double-stranded DNA (dsDNA). The work of Li et al. (2013) has shown that the ssDNA is incorporated during DNA replication as an Okazaki fragment, demonstrating the importance of DNA polymerase in this process.
In a more high-throughput manner, Wang et al. (2009) developed a system that aims at editing multiple locus applying the oligonucleotide recombineering concept – Multiplex Automated Genome Engineering (MAGE). The MAGE technology is an automated process that transforms multiple oligonucleotides into bacteria (Escherichia coli was used as the working model) in an iterative way. The application of multiple cycles of transformation allows the formation of a heterogeneous population, which enables the usage of directed evolution to develop strains with interesting characteristics (Wang et al., 2009).
Site-specific recombinases catalyze the recombination between two specific DNA sequences, performing all the reactions needed namely DNA excision, inversion, integration, and translocation. Genome editing based on these enzymes has proven to be a very efficient tool (Branda and Dymecki, 2004). Most systems currently in use are based on the Cre/loxP from the P1 phage and Flp/FRT from yeast (Schweizer, 2003; Branda and Dymecki, 2004). Both Cre and Flp are tyrosine recombinases that recognize 34 bp target sites – loxP and FRT, respectively – and catalyze the site-specific recombination event. Depending on the orientation of the target sites, Cre and Flp can either promote excision (direct repeats) or inversion (inverted repeats; for a detailed review on recombinases see Siegl and Luzhetskyy, 2012). Interestingly, the excision ability of these enzymes allow the generation of marker-less mutant strains, which contributes to a more predictable phenotype and facilitates further genetic manipulations (Khodakaramian et al., 2006).
The fact that none of these two recombinases need a co-factor provided by the host, gives this system a wide applicability (Kuhn and Torres, 2002). However, some limitations may be encountered, such as codon usage. This is a common problem, for example, in heterologous gene expression in high GC content organisms such as Actinobacteria. To circumvent codon usage issues, Herrmann et al. (2012) synthesized Cre and Flp versions with codons compatible with Actinobacteria usage and demonstrated its high efficiency and accuracy.
The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs)/CRISPR associated (Cas) proteins belong to the so-called bacterial adaptive immune system (Haft et al., 2005). The different CRISPR systems can be grouped in three different systems. While type I and type III are characterized by requiring multiple Cas proteins to induce the cleavage of their target DNA, type II systems only require the Cas9 endonuclease (Haft et al., 2005). The versatility of Cas9 allows it to introduce double strand breaks in a target genomic sequence through the co-expression of customized single guide RNA (Jinek et al., 2012). This characteristic makes CRISPR-Cas9 a very attractive tool for genome editing. Putting a focus on secondary metabolism, this system was already successfully applied in the deletion of two genes from the actinorhodin biosynthetic gene cluster in S. coelicolor (Tong et al., 2015).
From a biotechnological point of view, top–down strategies are the most attractive, because they consist in the reduction of existing genomes. By deleting parts that are predicted to be non-essential to the microorganisms, or parts that may contribute negatively to the pretended outcome, it is expected to obtain more efficient and tractable chassis. Indeed, genome reduction by the deletion of non-essential genes has resulted in increased genome stability, growth robustness, simplification of the secreted metabolome and increased availability of precursor units (Gomez-Escribano and Bibb, 2011; Zhou et al., 2012; Komatsu et al., 2013). Genome reduction as the first step in the heterologous expression workflow is expected to render strains that will not only be capable of producing cryptic specialized metabolites, but also to over-produce the final product (Figure 1).
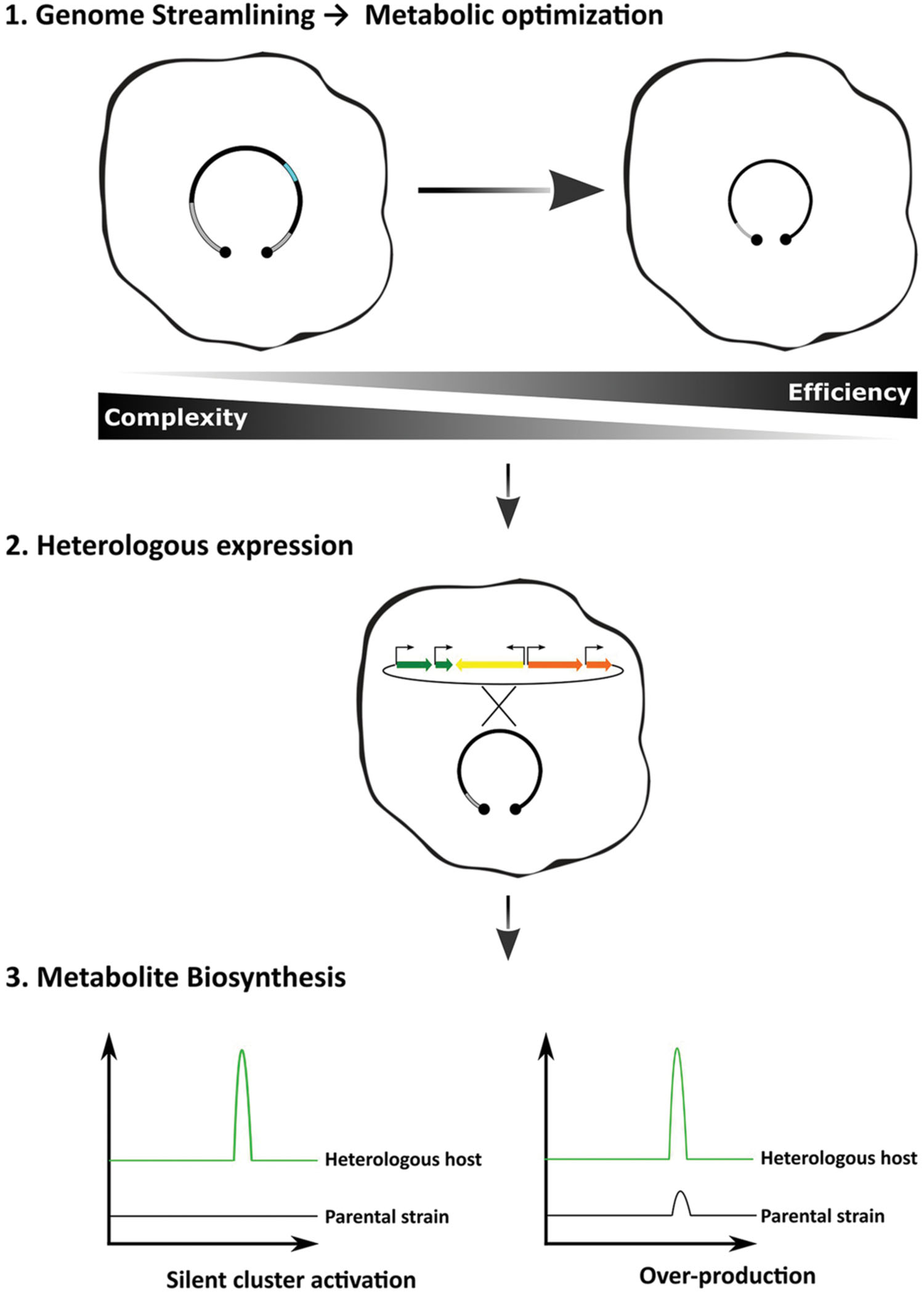
FIGURE 1. Optimized workflow for the heterologous expression of biosynthetic gene clusters. Applying the concept of genome simplification, one should expect that the host-interference problem would be minimized rendering more efficient strains. The methods of gene delivery and cluster refactoring can also improve the desired outcome, i.e., strains combining the ability to produce heterologous cryptic compounds and to behave as over-producers.
Efforts to streamline genomes have been particularly incident in Bacillus subtilis and in the molecular biology “workhorse” E. coli, mainly using directed mutagenesis strategies, such as site-specific recombination (Juhas et al., 2014). Multiple reports on genome reduction of industrial relevant organisms show a maximum reduction level below 25% of the total genome: E. coli, 15.3% (Posfai et al., 2006); B. subtillis, 20.7% (Morimoto et al., 2008); S. avermitilis, 16.9–18.6% (Komatsu et al., 2010) and Corynebacterium glutamicum 22% (Unthan et al., 2015). These low reduction percentages highlight how we are still very far from knowing how the networks that compose life are wired. In spite of these limitations, the application of directed mutagenesis to genome streamlining rendered some strains with interesting properties for biotechnological applications such as Streptomyces-derived strains, where genome reduction allowed the heterologous expression and production of small molecules (Table 1).
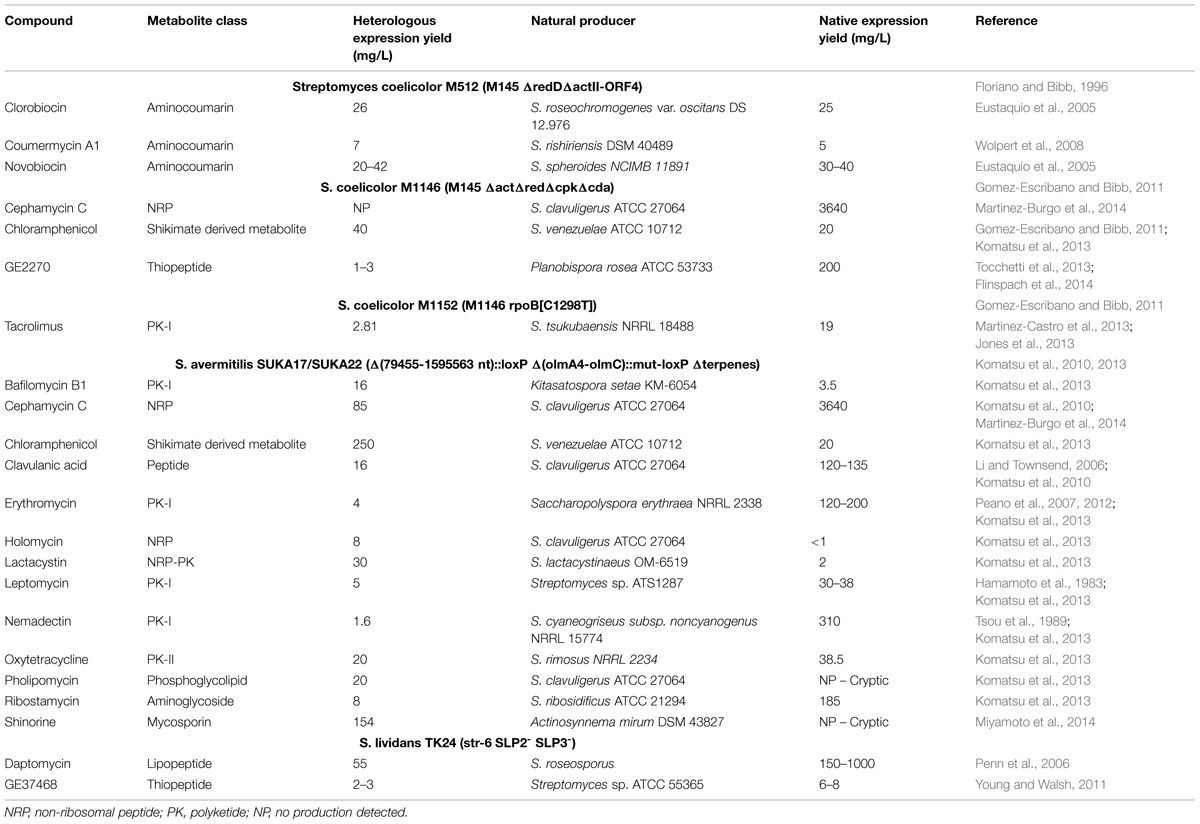
TABLE 1. Production yields of specialized metabolites heterologously expressed in Streptomyces-based chassis and in the native producers.
Although successful at some extent, directed mutagenesis still has major limitations when it comes to streamlining genomes, mainly due to the lack of knowledge regarding cell components and, more importantly, their interactions. As counterintuitive as it may seems, a random mutation approach may be a good alternative for genome streamlining. The creation of libraries of mutants with random deletions and the selection of strains with desirable traits gives a “Darwinian evolution” twist to the process, by combining unbiased mutations with a selective pressure toward a certain outcome.
This approach has been successfully applied to Pseudomanas putida (Leprince et al., 2012a). Two mini-transposons – mini-Tn5 KpF and mini-Tn5 TF – were modified in order to contain selective markers and a FRT site (recognized by Flt recombinase). The isolation of mutants containing both transposon insertions in random genome locations and the usage of the recombinase Flt allowed the deletion of certain segments of the chromosome and the selection of derived strains with unaffected fitness. Single segment deleted mutants reached a genome reduction of 4.1%. But perhaps the most interesting feature of this study is the capacity of iteration. Indeed a second cycle of deletion allowed the isolation of strains with a maximum of 7.4% genome reduction (Leprince et al., 2012a). In a short-term perspective this kind of approaches may constitute the more efficient way to streamline a genome. Furthermore, the creation of mutant libraries is also interesting due to the possibility of choosing different strains for different purposes, which might be very advantageous to biotechnology.
The usage of MAGE applied to the optimization of the production of 1-deoxy-D-xylulose-5-phosphate (DXP) – an industrially important isoprenoid lycopene – in E. coli was proven to be successful (Wang et al., 2009). The authors targeted 24 genes involved in the biosynthesis of DXP and obtained a very heterogeneous mutant population, from which some isolated strains revealed a fivefold increase in DXP yields (Wang et al., 2009). Although the oligonucleotides targeted pre-defined genes, this technique should be regarded as random mutagenesis due to the unpredictable combinatorial mutations that render the final heterogeneous population. This way, one can envision MAGE technology as a powerful technique to streamline genomes toward the optimization of a chassis.
The usage of random mutagenesis and isolation of mutants with interesting phenotypes is also at the core of strain development in the pharmaceutical industry. This actually means that genome streamlining has been practiced for decades now, although with different purposes than obtaining a suitable chassis for expressing synthetic pathways. Nevertheless, the metabolic network of these strains is highly optimized for the production of a certain product and may actually be useful for the heterologous expression of secondary metabolite biosynthetic gene clusters. S. ambofaciens is a natural producer of the important polyketide spiramycin. BES2074 strain derived from the spiramycin over-producer 111–59 was isolated and shown to have a blockage in the production of spiramycin (Richardson et al., 1990). The introduction of a BAC vector with the entire cyclic lipopeptide A54145 biosynthetic gene cluster rendered a final yield that was 285% higher than its natural producer S. fradiae (Alexander et al., 2010).
The natural ability of Streptomyces bacteria to produce a wide range of specialized metabolites grants them, in principle, the metabolic background needed for the heterologous expression of biosynthetic gene clusters. Thus, it is not surprising that Streptomyces bacteria have been frequently used for the development of optimized expression hosts.
Streptomyces avermitilis is a producer of the anti-infective avermectin with a linear chromosome of 9.02 Mb (Omura et al., 2001). Taking advantage of the typical Streptomyces genome organization, a region from the left subtelomeric region (∼2 Mb) that corresponds to the more variable genome regions was deleted using general homologous recombination or site-specific recombination (Cre-loxP) techniques. A series of genome-reduced S. avermitilis mutants strains were obtained of which we should highlight SUKA5 and SUKA22 (isogenic to SUKA17) strains that had a genome reduction of 17.9 and 18.5% respectively, and a 78% reduction of the total transposase genes when compared to the wild-type (Komatsu et al., 2010). S. avermitilis SUKA5 strain had the oligomycin biosynthetic gene cluster deleted in addition to the left subtelomeric region that included avermectin and filipin biosynthetic gene clusters; SUKA22 strain was a derivative of SUKA5 that had the terpene biosynthetic encoding genes deleted (Komatsu et al., 2010). In both strains, growth was not significantly affected and no production of endogenous metabolites was observed (Komatsu et al., 2013). The generated strains presented advantages for the heterologous expression of biosynthetic gene clusters, presumably due to the lack of endogenous metabolic pathways that would compete for cell resources and to a decrease in genome instability. The authors were able to express heterologously several biosynthetic gene clusters (Table 1) (Komatsu et al., 2010, 2013) including the cryptic biosynthetic clusters of pholipomycin from S. clavuligerus ATCC 27064 and shironine from Actinosynnema mirum DSM 43827. In most of the cases, the streamlined strains produced higher titers of the compounds in comparison with the natural producers (Table 1).
Although not originally generated for the heterologous expression of specialized metabolites, S. coelicolor M512 (Floriano and Bibb, 1996) that lacks the cluster-situated regulators of the actinorhodin and undecylprodigiosin biosynthetic gene clusters, was used successfully in the expression of clorobiocin, coumermycin, and novobiocin among others (Table 1). Few years later, S. coelicolor was subjected to a rational genome reduction. The streamlining project of S. coelicolor M145 strain (derivative of S. coelicolor A3(2) that lacks the two natural plasmids SCP1 and SCP2) was not as drastic as the S. avermitilis project, since it only targeted the native secondary metabolite biosynthetic gene clusters (Gomez-Escribano and Bibb, 2011). In this case the biosynthetic gene clusters of the secondary metabolites majorly produced by this strain (actinorhodin, prodiginine, CPK and CDA) were sequentially deleted by homologous double-recombination, generating a plethora of streamlined strains characterized by a low percentage of genome reduction (2%). Interestingly, the authors did not solely proceed with genome reduction. In fact, a strain optimization was putted in place through the introduction of point mutations into rpoB and rpsL with the expectation of pleiotropically increasing the production of secondary metabolites. The authors succeeded to heterologously express the chloramphenicol and congocidine gene clusters from S. venezuelae ATCC 10712 and S. ambofaciens ATCC 23877 (Gomez-Escribano and Bibb, 2011).
Other group also streamlined S. coelicolor M145 genome by sequential deletion of all the gene clusters containing polyketide synthases (PKS) and non-ribosomal protein synthases (NRPS), as well as a 900 kb fragment from a sub-telomeric region (Zhou et al., 2012). A double homologous recombination strategy was followed in this work. To attest the usefulness of the streamlined strains, the authors overexpressed the actinorhodin biosynthetic gene cluster. The results have shown that the strains with a reduced genome produced higher titers of actinorhodin. In this case the strategy was focused on the production of polyketides and the fact that the metabolism of S. coelicolor is naturally optimized to produce this kind of compounds, together with the lack of metabolic pathways competing for the same precursor molecules, accounts for the observed over-producing phenotypes.
These examples, especially in the case of S. avermitilis, foresee the ability to use these strains as universal chassis for the production of secondary metabolites. However, one should not forget that all these strains are still naturally constrained by their genomic background. In fact, the biological complexity that is still present in these strains and the lack of true orthogonal systems are likely to hamper their universality and application in the context of heterologous expression at the industrial scale. This is attested by the different production yields obtained for the same specialized metabolite (Table 1). Although the expression of the chloramphenicol gene cluster in S. coelicolor M1146 (Gomez-Escribano and Bibb, 2011) and S. avermitilis SUKA22 (Komatsu et al., 2013) led to higher production levels than the native producer S. venezuelae ATCC 10712, S. avermitilis SUKA22 was able to produce sixfold higher amounts than S. coelicolor M1146. Conversely, heterologous expression of the cephamycin C gene cluster in the same strains resulted in no production or lower yields than in the native producer S. clavuligerus (Komatsu et al., 2013; Martinez-Burgo et al., 2014) (Table 1). Thus, a more practical alternative to develop expression chassis would be to use strains that have been previously optimized to produce a certain class of compounds such as the strains already in use in the industry. These strains not only possess an optimized metabolism, but also have been adapted to the industrial process through the improvement of other characteristics, such as morphology (Nieminen et al., 2013). A posterior genome streamline process of these strains would further optimize their ability to perform as “specialized chassis.”
The bottom–up strategy deals with the design and development of strains from the scratch. Regarding chassis construction with biotechnological purposes the bottom–up strategy would really put engineering principles at the core of the technology as it is preconized in the synthetic biology field. In the future, cells designed to deliver a certain function could be developed through the wiring of synthetic metabolic pathways, opening endless possibilities. However, this is not a realistic scenario yet. In fact, the efforts made in this field are more related with understanding more basic questions, such as the origin of life (Leprince et al., 2012b).
The current vision on bottom–up approaches encompasses the assembly of DNA along with core protein machinery encapsulated on vesicles, allowing self-replication and energy generation (Kurihara et al., 2011). Some efforts done within the synthetic virology field can also be included in the bottom–up category. Due to their small genomes and low requirements for replication, the molecular networks enclosed in a virus are more discernible. Thus, the design or re-factoring of a virus is an achievable goal with our technology and with possible interesting applications, such as gene delivery (Guenther et al., 2014). Although it was still not possible to make functional “blueprint” for life, a landmark on bottom–up strategies was achieved when a total synthetic genome of Mycoplasma mycoides was assembled and successfully transplanted into a M. capricolum host devoid of its own genome (Gibson et al., 2010).
From a pragmatic point of view, one could consider using strains that went through a natural genome streamlining process and use them as a chassis. Although there might be a lack of knowledge in the genetic circuitries of these strains and thus being a sub-optimal strategy, it may be useful in some cases.
Regarding secondary metabolite biosynthesis, S. albus J1074 is a good example of a suitable chassis for heterologous expression of biosynthetic gene clusters with a naturally streamlined genome. This strain possesses the smallest genome in Streptomyces genus (∼6.8 Mb) and a low number of gene duplications (Zaburannyi et al., 2014). This highlights the tendency of redundancy reduction in this strain, which constitutes a good characteristic for a chassis. A transcriptomic analysis showed an early metabolic switch, which is coherent with the high growth rate presented by this strain (Zaburannyi et al., 2014). In addition it is known that this strain can be manipulated genetically in a very efficient way. Altogether these characteristics allowed its usage as an efficient chassis for heterologous biosynthetic gene cluster expression and, more importantly, a flexible one. The anti-tumor anthracycline steffimycin biosynthetic gene cluster from S. steffisburgensis (Gullon et al., 2006), fredericamycin from S. griseus (Wendt-Pienkowski et al., 2005), napyradiomycin from S. aculeolatus (Winter et al., 2007) and cyclooctatin from S. melanosporofaciens (Kim et al., 2009) were all cloned and successfully expressed in S. albus.
The identification of the so-called silent or cryptic clusters shows that under laboratory conditions bacteria are not able to present the metabolic flexibility needed for the production of all encoded metabolites. The development of “universal” Streptomyces heterologous expression hosts based on genetically modified strains of Streptomyces sp. (Komatsu et al., 2010, 2013; Gomez-Escribano and Bibb, 2011; Zhou et al., 2012; Ikeda et al., 2013) has validated the heterologous expression of secondary metabolite clusters particularly for the activation of silent clusters and characterization of new metabolites. However, the production yields are generally low and often lower than the original producers (Table 1) unveiling a deficient metabolic flux background and undermining this strategy for industrial applications that rely on high production yields.
For an industrial downstream application of the heterologous expression of specialized metabolites, there is the need of combining the stability features of the universal expression hosts with the metabolic fitness for producing high-added value products of the industrial strains. The vast knowledge regarding Streptomyces metabolic networks and their regulation (Liu et al., 2013) as well as a successful history on secondary metabolism engineering (Olano et al., 2008) turns Streptomyces, particularly those strains already optimized for industrial processes, in attractive subjects for the development of metabolite class specialized hosts through the synergic use of synthetic biology, system biology and metabolic engineering methodologies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by National funds through FCT – Fundação para a Ciência e Tecnologia/MEC – Ministério da Educação e Ciência and when applicable co-funded by FEDER funds within the partnership agreement PT2020 related with the research unit number 4293. TB was supported by a post-doctoral fellowship under the PEst-C/SAU/LA0002/2013 (FCOMP-01-0124-FEDER-037277) project and MVM was supported by the FCT fellowship SFRH/BPD/95683/2013.
Alexander, D. C., Rock, J., He, X., Brian, P., Miao, V., and Baltz, R. H. (2010). Development of a genetic system for combinatorial biosynthesis of lipopeptides in Streptomyces fradiae and heterologous expression of the A54145 biosynthesis gene cluster. Appl. Environ. Microbiol. 76, 6877–6887. doi: 10.1128/aem.01248-10
Baltz, R. H. (2008). Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 8, 557–563. doi: 10.1016/j.coph.2008.04.008
Berdy, J. (2005). Bioactive microbial metabolites. J. Antibiot. (Tokyo) 58, 1–26. doi: 10.1038/ja.2005.1
Branda, C. S., and Dymecki, S. M. (2004). Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28. doi: 10.1016/S1534-5807(03)00399-X
Brito, Â., Gaifem, J., Ramos, V., Glukhov, E., Dorrestein, P. C., Gerwick, W. H., et al. (2015). Bioprospecting Portuguese Atlantic coast cyanobacteria for bioactive secondary metabolites reveals untapped chemodiversity. Algal Res. 9, 218–226. doi: 10.1016/j.algal.2015.03.016
Channon, K., Bromley, E. H., and Woolfson, D. N. (2008). Synthetic biology through biomolecular design and engineering. Curr. Opin. Struct. Biol. 18, 491–498. doi: 10.1016/j.sbi.2008.06.006
Danchin, A. (2012). Scaling up synthetic biology: do not forget the chassis. FEBS Lett. 586, 2129–2137. doi: 10.1016/j.febslet.2011.12.024
de las Heras, A., Carreno, C. A., and De Lorenzo, V. (2008). Stable implantation of orthogonal sensor circuits in Gram-negative bacteria for environmental release. Environ. Microbiol. 10, 3305–3316. doi: 10.1111/j.1462-2920.2008.01722.x
de Lorenzo, V. (2011). Beware of metaphors: chasses and orthogonality in synthetic biology. Bioeng. Bugs 2, 3–7. doi: 10.4161/bbug.2.1.13388
Eustaquio, A. S., Gust, B., Galm, U., Li, S. M., Chater, K. F., and Heide, L. (2005). Heterologous expression of novobiocin and clorobiocin biosynthetic gene clusters. Appl. Environ. Microbiol. 71, 2452–2459. doi: 10.1128/AEM.71.5.2452-2459.2005
Fernandez-Martinez, L. T., and Bibb, M. J. (2014). Use of the meganuclease I-SceI of Saccharomyces cerevisiae to select for gene deletions in actinomycetes. Sci. Rep. 4, 7100. doi: 10.1038/srep07100
Flinspach, K., Kapitzke, C., Tocchetti, A., Sosio, M., and Apel, A. K. (2014). Heterologous expression of the thiopeptide antibiotic GE2270 from Planobispora rosea ATCC 53733 in Streptomyces coelicolor requires deletion of ribosomal genes from the expression construct. PLoS ONE 9:e90499. doi: 10.1371/journal.pone.0090499
Floriano, B., and Bibb, M. (1996). afsR is a pleiotropic but conditionally required regulatory gene for antibiotic production in Streptomyces coelicolor A3(2). Mol. Microbiol. 21, 385–396. doi: 10.1046/j.1365-2958.1996.6491364.x
Gibson, D. G., Glass, J. I., Lartigue, C., Noskov, V. N., Chuang, R. Y., Algire, M. A., et al. (2010). Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56. doi: 10.1126/science.1190719
Gomez-Escribano, J. P., and Bibb, M. J. (2011). Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol. 4, 207–215. doi: 10.1111/j.1751-7915.2010.00219.x
Gross, H., and Loper, J. E. (2009). Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 26, 1408–1446. doi: 10.1039/b817075b
Guenther, C. M., Kuypers, B. E., Lam, M. T., Robinson, T. M., Zhao, J., and Suh, J. (2014). Synthetic virology: engineering viruses for gene delivery. WIREs Nanomed. Nanobiotechnol. 6, 548–558. doi: 10.1002/wnan.1287
Gullon, S., Olano, C., Abdelfattah, M. S., Brana, A. F., Rohr, J., Mendez, C., et al. (2006). Isolation, characterization, and heterologous expression of the biosynthesis gene cluster for the antitumor anthracycline steffimycin. Appl. Environ. Microbiol. 72, 4172–4183. doi: 10.1128/aem.00734-06
Haft, D. H., Selengut, J., Mongodin, E. F., and Nelson, K. E. (2005). A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 1:e60. doi: 10.1371/journal.pcbi.0010060
Hamamoto, T., Gunji, S., Tsuji, H., and Beppu, T. (1983). Leptomycins A and B, new antifungal antibiotics. I. Taxonomy of the producing strain and their fermentation, purification and characterization. J. Antibiot. (Tokyo) 36, 639–645.
Harrison, J., and Studholme, D. J. (2014). Recently published Streptomyces genome sequences. Microb. Biotechnol. 7, 373–380. doi: 10.1111/1751-7915.12143
Herrmann, S., Siegl, T., Luzhetska, M., Petzke, L., Jilg, C., Welle, E., et al. (2012). Site-specific recombination strategies for engineering actinomycete genomes. Appl. Environ. Microbiol. 78, 1804–1812. doi: 10.1128/aem.06054-11
Ijaq, J., Chandrasekharan, M., Poddar, R., Bethi, N., and Sundararajan, V. S. (2015). Annotation and curation of uncharacterized proteins- challenges. Front. Genet. 6:119. doi: 10.3389/fgene.2015.00119
Ikeda, H., Shin-Ya, K., and Omura, S. (2013). Genome mining of the Streptomyces avermitilis genome and development of genome-minimized hosts for heterologous expression of biosynthetic gene clusters. J. Ind. Microbiol. Biotechnol. 41, 233–250. doi: 10.1007/s10295-013-1327-x
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., and Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. doi: 10.1126/science.1225829
Jones, A. C., Gust, B., Kulik, A., Heide, L., Buttner, M. J., and Bibb, M. J. (2013). Phage p1-derived artificial chromosomes facilitate heterologous expression of the FK506 gene cluster. PLoS ONE 8:e69319. doi: 10.1371/journal.pone.0069319
Juhas, M., Reuss, D. R., Zhu, B., and Commichau, F. M. (2014). Bacillus subtilis and Escherichia coli essential genes and minimal cell factories after one decade of genome engineering. Microbiology 160, 2341–2351. doi: 10.1099/mic.0.079376-0
Karlinsey, J. E. (2007). Lambda-Red genetic engineering in Salmonella enterica serovar Typhimurium. Methods Enzymol. 421, 199–209. doi: 10.1016/s0076-6879(06)21016-4
Khodakaramian, G., Lissenden, S., Gust, B., Moir, L., Hoskisson, P. A., Chater, K. F., et al. (2006). Expression of Cre recombinase during transient phage infection permits efficient marker removal in Streptomyces. Nucleic Acids Res. 34, e20. doi: 10.1093/nar/gnj019
Kieser, T., Bibb, M. J., Buttner, M. J., Chater, K. F., and Hopwood, D. A. (2000). Practical Streptomyces Genetics. Norwich: The John Innes Foundation.
Kim, J. N., Kim, Y., Jeong, Y., Roe, J. H., Kim, B. G., and Cho, B. K. (2015). Comparative genomics reveals the core and accessory genomes of Streptomyces species. J. Microbiol. Biotechnol. doi: 10.4014/jmb.1504.04008 [Epub ahead of print].
Kim, S. Y., Zhao, P., Igarashi, M., Sawa, R., Tomita, T., Nishiyama, M., et al. (2009). Cloning and heterologous expression of the cyclooctatin biosynthetic gene cluster afford a diterpene cyclase and two p450 hydroxylases. Chem. Biol. 16, 736–743. doi: 10.1016/j.chembiol.2009.06.007
Komatsu, M., Komatsu, K., Koiwai, H., Yamada, Y., Kozone, I., Izumikawa, M., et al. (2013). Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth. Biol. 2, 384–396. doi: 10.1021/sb3001003
Komatsu, M., Uchiyama, T., Omura, S., Cane, D. E., and Ikeda, H. (2010). Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc. Natl. Acad. Sci. U.S.A. 107, 2646–2651. doi: 10.1073/pnas.0914833107
Kuhn, R., and Torres, R. M. (2002). Cre/loxP recombination system and gene targeting. Methods Mol. Biol. 180, 175–204. doi: 10.1385/1-59259-178-7:175
Kurihara, K., Tamura, M., Shohda, K., Toyota, T., Suzuki, K., and Sugawara, T. (2011). Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem. 3, 775–781. doi: 10.1038/nchem.1127
Laureti, L., Song, L., Huang, S., Corre, C., Leblond, P., Challis, G. L., et al. (2011). Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens. Proc. Natl. Acad. Sci. U.S.A. 108, 6258–6263. doi: 10.1073/pnas.1019077108
Leprince, A., De Lorenzo, V., Voller, P., Van Passel, M. W., and Martins Dos Santos, V. A. (2012a). Random and cyclical deletion of large DNA segments in the genome of Pseudomonas putida. Environ. Microbiol. 14, 1444–1453. doi: 10.1111/j.1462-2920.2012.02730.x
Leprince, A., Van Passel, M. W., and Dos Santos, V. A. (2012b). Streamlining genomes: toward the generation of simplified and stabilized microbial systems. Curr. Opin. Biotechnol. 23, 651–658. doi: 10.1016/j.copbio.2012.05.001
Li, R., and Townsend, C. A. (2006). Rational strain improvement for enhanced clavulanic acid production by genetic engineering of the glycolytic pathway in Streptomyces clavuligerus. Metab. Eng. 8, 240–252. doi: 10.1016/j.ymben.2006.01.003
Li, X. T., Thomason, L. C., Sawitzke, J. A., Costantino, N., and Court, D. L. (2013). Bacterial DNA polymerases participate in oligonucleotide recombination. Mol. Microbiol. 88, 906–920. doi: 10.1111/mmi.12231
Liu, G., Chater, K. F., Chandra, G., Niu, G., and Tan, H. (2013). Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 77, 112–143. doi: 10.1128/MMBR.00054-12
Luo, Y., Zhang, L., Barton, K. W., and Zhao, H. (2015). Systematic identification of a panel of strong constitutive promoters from Streptomyces albus. ACS Synth. Biol. doi: 10.1021/acssynbio.5b00016 [Epub ahead of print].
Martinez-Burgo, Y., Alvarez-Alvarez, R., Perez-Redondo, R., and Liras, P. (2014). Heterologous expression of Streptomyces clavuligerus ATCC 27064 cephamycin C gene cluster. J. Biotechnol. 186, 21–29. doi: 10.1016/j.jbiotec.2014.06.002
Martinez-Castro, M., Salehi-Najafabadi, Z., Romero, F., Perez-Sanchiz, R., Fernandez-Chimeno, R. I., Martin, J. F., et al. (2013). Taxonomy and chemically semi-defined media for the analysis of the tacrolimus producer Streptomyces tsukubaensis. Appl. Microbiol. Biotechnol. 97, 2139–2152. doi: 10.1007/s00253-012-4364-x
Miyamoto, K. T., Komatsu, M., and Ikeda, H. (2014). Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. Appl. Environ. Microbiol. 80, 5028–5036. doi: 10.1128/AEM.00727-14
Mondol, M. A., Shin, H. J., and Islam, M. T. (2013). Diversity of secondary metabolites from marine Bacillus species: chemistry and biological activity. Mar. Drugs 11, 2846–2872. doi: 10.3390/md11082846
Morimoto, T., Kadoya, R., Endo, K., Tohata, M., Sawada, K., Liu, S., et al. (2008). Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 15, 73–81. doi: 10.1093/dnares/dsn002
Murphy, K. C. (1998). Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180, 2063–2071.
Nett, M., Ikeda, H., and Moore, B. S. (2009). Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 26, 1362–1384. doi: 10.1039/b817069j
Nieminen, L., Webb, S., Smith, M. C., and Hoskisson, P. A. (2013). A flexible mathematical model platform for studying branching networks: experimentally validated using the model actinomycete, Streptomyces coelicolor. PLoS ONE 8:e54316. doi: 10.1371/journal.pone.0054316
Ochi, K., and Hosaka, T. (2012). New strategies for drug discovery: activation of silent or weakly expressed microbial gene clusters. Appl. Microbiol. Biotechnol. 97, 87–98. doi: 10.1007/s00253-012-4551-9
Olano, C., Garcia, I., Gonzalez, A., Rodriguez, M., Rozas, D., Rubio, J., et al. (2014). Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb. Biotechnol. 7, 242–256. doi: 10.1111/1751-7915.12116
Olano, C., Lombo, F., Mendez, C., and Salas, J. A. (2008). Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab. Eng. 10, 281–292. doi: 10.1016/j.ymben.2008.07.001
Omura, S., Ikeda, H., Ishikawa, J., Hanamoto, A., Takahashi, C., Shinose, M., et al. (2001). Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. U.S.A. 98, 12215–12220. doi: 10.1073/pnas.211433198
Peano, C., Bicciato, S., Corti, G., Ferrari, F., Rizzi, E., Bonnal, R. J., et al. (2007). Complete gene expression profiling of Saccharopolyspora erythraea using GeneChip DNA microarrays. Microb. Cell Fact. 6, 37. doi: 10.1186/1475-2859-6-37
Peano, C., Tala, A., Corti, G., Pasanisi, D., Durante, M., Mita, G., et al. (2012). Comparative genomics and transcriptional profiles of Saccharopolyspora erythraea NRRL 2338 and a classically improved erythromycin over-producing strain. Microb. Cell Fact. 11, 32. doi: 10.1186/1475-2859-11-32
Penn, J., Li, X., Whiting, A., Latif, M., Gibson, T., Silva, C. J., et al. (2006). Heterologous production of daptomycin in Streptomyces lividans. J. Ind. Microbiol. Biotechnol. 33, 121–128. doi: 10.1007/s10295-005-0033-8
Posfai, G., Plunkett, G. III, Feher, T., Frisch, D., Keil, G. M., Umenhoffer, K., et al. (2006). Emergent properties of reduced-genome Escherichia coli. Science 312, 1044–1046. doi: 10.1126/science.1126439
Richardson, M. A., Kuhstoss, S., Huber, M. L., Ford, L., Godfrey, O., Turner, J. R., et al. (1990). Cloning of spiramycin biosynthetic genes and their use in constructing Streptomyces ambofaciens mutants defective in spiramycin biosynthesis. J. Bacteriol. 172, 3790–3798.
Rudolph, M. M., Vockenhuber, M. P., and Suess, B. (2013). Synthetic riboswitches for the conditional control of gene expression in Streptomyces coelicolor. Microbiology 159, 1416–1422. doi: 10.1099/mic.0.067322-0
Sansinenea, E., and Ortiz, A. (2011). Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 33, 1523–1538. doi: 10.1007/s10529-011-0617-5
Schweizer, H. P. (2003). Applications of the Saccharomyces cerevisiae Flp-FRT system in bacterial genetics. J. Mol. Microbiol. Biotechnol. 5, 67–77. doi: 10.1159/000069976
Shao, Z., Rao, G., Li, C., Abil, Z., Luo, Y., and Zhao, H. (2013). Refactoring the silent spectinabilin gene cluster using a plug-and-play scaffold. ACS Synth. Biol. 2, 662–669. doi: 10.1021/sb400058n
Siegl, T., and Luzhetskyy, A. (2012). Actinomycetes genome engineering approaches. Antonie Van Leeuwenhoek 102, 503–516. doi: 10.1007/s10482-012-9795-y
Swingle, B., Markel, E., Costantino, N., Bubunenko, M. G., Cartinhour, S., and Court, D. L. (2010). Oligonucleotide recombination in Gram-negative bacteria. Mol. Microbiol. 75, 138–148. doi: 10.1111/j.1365-2958.2009.06976.x
Tocchetti, A., Maffioli, S., Iorio, M., Alt, S., Mazzei, E., Brunati, C., et al. (2013). Capturing linear intermediates and C-terminal variants during maturation of the thiopeptide GE2270. Chem. Biol. 20, 1067–1077. doi: 10.1016/j.chembiol.2013.07.005
Tong, Y., Charusanti, P., Zhang, L., Weber, T., and Lee, S. Y. (2015). CRISPR-Cas9 based engineering of actinomycetal genomes. ACS Synth. Biol. doi: 10.1021/acssynbio.5b00038 [Epub ahead of print].
Tsou, H. R., Ahmed, Z. H., Fiala, R. R., Bullock, M. W., Carter, G. T., Goodman, J. J., et al. (1989). Biosynthetic origin of the carbon skeleton and oxygen atoms of the LL-F28249 alpha, a potent antiparasitic macrolide. J. Antibiot. (Tokyo) 42, 398–406. doi: 10.7164/antibiotics.42.398
Unthan, S., Baumgart, M., Radek, A., Herbst, M., Siebert, D., Bruhl, N., et al. (2015). Chassis organism from Corynebacterium glutamicum – a top-down approach to identify and delete irrelevant gene clusters. Biotechnol. J. 10, 290–301. doi: 10.1002/biot.201400041
Wang, H. H., Isaacs, F. J., Carr, P. A., Sun, Z. Z., Xu, G., Forest, C. R., et al. (2009). Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898. doi: 10.1038/nature08187
Wang, K., Neumann, H., Peak-Chew, S. Y., and Chin, J. W. (2007). Evolved orthogonal ribosomes enhance the efficiency of synthetic genetic code expansion. Nat. Biotechnol. 25, 770–777. doi: 10.1038/nbt1314
Weber, T., Blin, K., Duddela, S., Krug, D., Kim, H. U., Bruccoleri, R., et al. (2015). antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 43, 1–7. doi: 10.1093/nar/gkv437
Weissman, K. J., and Muller, R. (2010). Myxobacterial secondary metabolites: bioactivities and modes-of-action. Nat. Prod. Rep. 27, 1276–1295. doi: 10.1039/c001260m
Wendt-Pienkowski, E., Huang, Y., Zhang, J., Li, B., Jiang, H., Kwon, H., et al. (2005). Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces griseus. J. Am. Chem. Soc. 127, 16442–16452. doi: 10.1021/ja054376u
Winter, J. M., Moffitt, M. C., Zazopoulos, E., Mcalpine, J. B., Dorrestein, P. C., and Moore, B. S. (2007). Molecular basis for chloronium-mediated meroterpene cyclization: cloning, sequencing, and heterologous expression of the napyradiomycin biosynthetic gene cluster. J. Biol. Chem. 282, 16362–16368. doi: 10.1074/jbc.M611046200
Wolpert, M., Heide, L., Kammerer, B., and Gust, B. (2008). Assembly and heterologous expression of the coumermycin A1 gene cluster and production of new derivatives by genetic engineering. Chembiochem 9, 603–612. doi: 10.1002/cbic.200700483
Yamada, Y., Arima, S., Nagamitsu, T., Johmoto, K., Uekusa, H., Eguchi, T., et al. (2015). Novel terpenes generated by heterologous expression of bacterial terpene synthase genes in an engineered Streptomyces host. J. Antibiot. (Tokyo) 68, 385–394. doi: 10.1038/ja.2014.171
Young, T. S., and Walsh, C. T. (2011). Identification of the thiazolyl peptide GE37468 gene cluster from Streptomyces ATCC 55365 and heterologous expression in Streptomyces lividans. Proc. Natl. Acad. Sci. U.S.A. 108, 13053–13058. doi: 10.1073/pnas.1110435108
Zaburannyi, N., Rabyk, M., Ostash, B., Fedorenko, V., and Luzhetskyy, A. (2014). Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genomics 15:97. doi: 10.1186/1471-2164-15-97
Zhou, M., Jing, X., Xie, P., Chen, W., Wang, T., Xia, H., et al. (2012). Sequential deletion of all the polyketide synthase and nonribosomal peptide synthetase biosynthetic gene clusters and a 900-kb subtelomeric sequence of the linear chromosome of Streptomyces coelicolor. FEMS Microbiol. Lett. 333, 169–179. doi: 10.1111/j.1574-6968.2012.02609.x
Keywords: chassis, heterologous expression, Streptomyces, specialized metabolites, genome streamlining, genome reduction
Citation: Beites T and Mendes MV (2015) Chassis optimization as a cornerstone for the application of synthetic biology based strategies in microbial secondary metabolism. Front. Microbiol. 6:906. doi: 10.3389/fmicb.2015.00906
Received: 30 June 2015; Accepted: 19 August 2015;
Published: 09 September 2015.
Edited by:
Ana Lúcia Leitão, Universidade Nova de Lisboa, PortugalReviewed by:
Paul Alan Hoskisson, University of Strathclyde, UKCopyright © 2015 Beites and Mendes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta V. Mendes, Instituto de Biologia Molecular e Celular, Universidade do Porto, Rua do Campo Alegre 823, 4150-180 Porto, Portugal,bXZtQGlibWMudXAucHQ=
†Present address: Tiago Beites, Department of Microbiology and Immunology, Weill Cornell Medical College, New York, NY, USA
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.