
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 02 September 2015
Sec. Infectious Agents and Disease
Volume 6 - 2015 | https://doi.org/10.3389/fmicb.2015.00893
This article is part of the Research Topic A Multidisciplinary Look at Stenotrophomonas maltophilia: An Emerging Multi-Drug-Resistant Global Opportunistic Pathogen View all 12 articles
Stenotrophomonas maltophilia is a Gram-negative, biofilm-forming bacterium. Although generally regarded as an organism of low virulence, S. maltophilia is an emerging multi-drug resistant opportunistic pathogen in hospital and community settings, especially among immunocompromised hosts. Risk factors associated with S. maltophilia infection include underlying malignancy, cystic fibrosis, corticosteroid or immunosuppressant therapy, the presence of an indwelling central venous catheter and exposure to broad spectrum antibiotics. In this review, we provide a synthesis of information on current global trends in S. maltophilia pathogenicity as well as updated information on the molecular mechanisms contributing to its resistance to an array of antimicrobial agents. The prevalence of S. maltophilia infection in the general population increased from 0.8–1.4% during 1997–2003 to 1.3–1.68% during 2007–2012. The most important molecular mechanisms contributing to its resistance to antibiotics include β-lactamase production, the expression of Qnr genes, and the presence of class 1 integrons and efflux pumps. Trimethoprim/sulfamethoxazole (TMP/SMX) is the antimicrobial drug of choice. Although a few studies have reported increased resistance to TMP/SMX, the majority of studies worldwide show that S. maltophilia continues to be highly susceptible. Drugs with historically good susceptibility results include ceftazidime, ticarcillin-clavulanate, and fluoroquinolones; however, a number of studies show an alarming trend in resistance to those agents. Tetracyclines such as tigecycline, minocycline, and doxycycline are also effective agents and consistently display good activity against S. maltophilia in various geographic regions and across different time periods. Combination therapies, novel agents, and aerosolized forms of antimicrobial drugs are currently being tested for their ability to treat infections caused by this multi-drug resistant organism.
Stenotrophomonas maltophilia is a Gram-negative, aerobic, glucose non-fermenting, motile bacillus. S. maltophilia was first isolated from pleural effusion in 1943 and initially named Bacterium bookeri. The organism was reclassified as a member of the genus Pseudomonas in 1961, Xanthomonas in 1983, and then Stenotrophomonas in 1993 (Al-Anazi and Al-Jasser, 2014). It survives on almost any humid surface and has been isolated from a wide variety of aquatic sources, such as suction tubing, nebulizers, endoscopes, hemodialysis dialysate samples, plant rhizosphere, faucets, sink drains, and shower heads (Brooke, 2012). S. maltophilia is characterized by its ability to form biofilms on various abiotic and biotic surfaces, including lung cells (de Oliveira-Garcia et al., 2003; Pompilio et al., 2010), and by its resistance to a broad array of antimicrobial agents. The World Health Organization recently classified S. maltophilia as one of the leading multidrug resistant organisms (MDROs) in hospital settings (Brooke, 2014).
S. maltophilia is generally regarded as an organism of low virulence and therefore an opportunistic pathogen, especially in immunocompromised hosts. The risk factors associated with acquiring S. maltophilia infections are well-known and include underlying malignancy (especially hematologic malignancy), organ transplantation, human immunodeficiency virus (HIV) infection, cystic fibrosis, prolonged hospitalization, intensive care unit (ICU) admission, mechanical ventilation, indwelling catheters (vascular, urinary, biliary), corticosteroid or immunosuppressant therapy, and recent antibiotics treatment (Al-Anazi and Al-Jasser, 2014). These risk factors reflect specific features of S. maltophilia, such as its ability to survive on almost any humid surface, its propensity to form biofilm and colonize humid surfaces, and its employment of several mechanisms that confer resistance to a number of antimicrobial agents.
S. maltophilia causes a wide range of infections including respiratory tract infections (RTI), blood stream infections (BSI) and, less commonly, skin and soft tissue infections (SSTI), bone and joint infections, biliary tract infections, urinary tract infections, endophthalmitis, endocarditis, and meningitis (Falagas et al., 2009a; Looney et al., 2009). The correlations between S. maltophilia infection and structural abnormalities with or without obstruction or procedural manipulation are well documented. Biliary tract infections caused by obstruction due to hepatobiliary neoplasms (Papadakis et al., 1995; Chang et al., 2014) or post-operative anastomotic strictures of the gastrointestinal tract (Perez et al., 2014) have been reported in patients with biliary S. maltophilia sepsis. Pleural infections caused by post-surgical/tube thoracostomy or fistula (broncho-/esophageal-/bilio-) (Lee et al., 2014), post-neurosurgical meningitis (Sood et al., 2013; Lai et al., 2014b), complicated urinary tract infections (Vartivarian et al., 1996), and obstructive lung cancer (Fujita et al., 1996; Vartivarian et al., 2000) have all been reported to create a milieu for S. maltophilia infection. In addition, although commonly perceived as nosocomial pathogens, community-acquired infections appear to be on the rise (Falagas et al., 2009a; Chang et al., 2014).
There were few data before 1970 regarding the prevalence or clinical characteristics of S. maltophilia (previously Pseudomonas maltophilia or Xanthomonas maltophilia) because of its rarity and relative clinical insignificance. It was in the 1980s when S. maltophilia became more frequently reported as an emerging nosocomial pathogen (Jang et al., 1992; Victor et al., 1994), especially in patients with post-chemotherapy neutropenia (Kerr et al., 1990; Labarca et al., 2000) and in those with indwelling central venous catheters (CVC) (Victor et al., 1994; Lai et al., 2006; Chen et al., 2014). Beginning in the late 1990s worldwide surveillance programs and multi-center studies began to provide more comprehensive information about the pathogenicity of S. maltophilia. Of the global surveillance programs, the SENTRY Antimicrobial Surveillance Program initiated in 1997 and the Study for Monitoring Antimicrobial Resistance Trends (SMART) initiated in 2002 are the most well-known (Jean et al., 2015). A number of nationwide and antimicrobial agent-targeted projects were also launched during the late 1990s, including the Canadian Ward Surveillance Study (CANWARD), the Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) study, the British Society for Antimicrobial Chemotherapy (BSAC) Resistance Surveillance Project, the Taiwan Surveillance of Antimicrobial Resistance (TSAR) study, and the Tigecycline Evaluation Surveillance Trial (TEST).
Despite the massive scale of these surveillance studies, there are still limited integrated data on the prevalence and susceptibility patterns of S. maltophilia. The heterogeneity among the studies stems from the diverse patient demographics, geographic differences, and the ratio of the isolates collected from different sources, making inter-literature comparison difficult. To add to the complexity, there are no worldwide guidelines on susceptibility testing methodology and breakpoints for S. maltophilia (Nicodemo et al., 2004; Hombach et al., 2012), which results in different or absence of susceptibility breakpoints for some antibiotics. The lack of universal references for evaluating resistance of S. maltophilia to antimicrobial agents leads to confusion and complications when interpreting clinical data.
Table 1 shows the prevalence rates of infection due to S. maltophilia, categorized by sources of infection, reported by worldwide and nationwide surveillance projects as well as multi-center studies. Specific patient groups such as the critically ill in intensive care units (ICUs) and the pediatric population are presented separately in Table 1. By comparing data gathered by large surveillance studies over time we can estimate longitudinal change in prevalence of S. maltophilia infection in the general population. The frequency of occurrence among isolates from all sources ranged from 0.8 to 1.4% in five SENTRY studies during 1997~2003 (Fluit et al., 2001a; Gales et al., 2001a; Sader et al., 2004; Sader and Jones, 2005; Fedler et al., 2006b). During 2007–2012, the CANWARD surveillance study (Zhanel et al., 2011, 2013; Walkty et al., 2014) and the SENTRY antimicrobial surveillance program (Farrell et al., 2010b; Sader et al., 2013) reported prevalence rates ranging from 1.3 to 1.68%. These data indicate that there is an increasing trend in infections due to S. maltophilia in the general population.
It has been observed in the general population (Gales et al., 2001a) and in ICUs (Fluit et al., 2001b) alike that S. maltophilia is most frequently associated with respiratory tract infections (RTIs), followed by bloodstream infections (BSIs), and, rarely, skin and soft tissue infections (SSTIs) and urinary tract infections (UTI) (Gales et al., 2001a). The prevalence of RTIs due to S. maltophilia is generally higher than that of other infections caused by that pathogen, but varies widely among countries and continents, ranging from 1.6 to 6.3% during the period 1997–2012 (Sader et al., 1998, 2004, 2014a; Jones et al., 2000; Fluit et al., 2001a; Gales et al., 2001a, 2002; Mathai et al., 2001; Hoban et al., 2003; Jones, 2010; Zhanel et al., 2010; Farrell et al., 2014). The United States has the most consecutive records regarding RTI isolates collected by the SENTRY program. Based on data from four SENTRY studies (Gales et al., 2001a; Hoban et al., 2003; Jones, 2010; Sader et al., 2014a), the prevalence rates increased from 3.3–3.5% during 1997–2004 to 4.4% during 2009–2012. During that 15-year period, S. maltophilia went from being the eighth to the sixth most common cause of RTI. In a large study on 2968 RTI isolates collected from 59 medical centers in the USA and 15 centers in European countries in 2012, 6.3% of the pathogens were S. maltophilia (Farrell et al., 2014). These observations suggest an increasing frequency of occurrence of respiratory tract infections due to S. maltophilia.
S. maltophilia is less frequently isolated from patients with BSIs, UTIs, or SSTIs than from patients with RTIs, with reported isolation rates ranging from 0.7 to 1.1% for BSIs (Jones et al., 1997; Pfaller et al., 1998; Diekema et al., 1999; Fluit et al., 2001a; Gales et al., 2001a; Sader et al., 2004, 2005b), 0–0.3% for UTIs (Pfaller et al., 1998; Jones et al., 1999b; Fluit et al., 2001a; Gales et al., 2001a; Sader et al., 2004, 2005b), and 0.39–0.96% for SSTIs (Diekema et al., 1999; Fluit et al., 2001a; Gales et al., 2001a; Sader et al., 2004). SMART studies have also shown that isolation of S. maltophilia from intra-abdominal infections (IAIs) is also fairly uncommon, with rates ranging from 1 to 1.7% (2002–2010) (Guembe et al., 2008; Yang et al., 2010; Lee et al., 2012; Liu et al., 2012). However, data from African and Middle Eastern countries collected as part of the Tigecycline Evaluation Surveillance Trial during 2007–2012 (Renteria et al., 2014) revealed an uncommonly high rate of isolation (6.3%) of S. maltophilia from patients with IAIs. In addition, the results from a SMART study surveying UTIs in the Asian-Pacific region during 2009–2010 disclosed higher rates of S. maltophilia isolated from patients with UTIs in China (1.3%) and Thailand (3.3%) than in other countries (Lu et al., 2012), although the rates were not as high as those in certain countries in Africa and the Middle East.
The worldwide rate of isolation of S. maltophilia among GNB pathogens ranges from 2.29 to 2.7% according to a SENTRY study (2001–2004) (Gales et al., 2006) and a CANWARD surveillance study (2007–2009) (Zhanel et al., 2011). In the US state of Texas, however, a study at the M. D. Anderson Cancer Center revealed an increasing trend in the ratio of S. maltophilia among GNB isolates obtained from cancer patients during 1986–2002 (from 2% in 1986 to 7% in 2002) (Safdar and Rolston, 2007).
Among NFGNB, S. maltophilia has been reported to be the third most commonly isolated pathogen after Pseudomonas aeruginosa and Acinetobacter baumannii. In a large survey conducted as a part of the SENTRY program, 221,084 GNB isolates were collected worldwide, including 25,305 (11.5%) NFGNB isolates, of which Acinetobacter spp. and P. aeruginosa accounted for the vast majority (87.7%). The remaining 3509 isolates were deemed unusual NFGNB species. Of them, S. maltophilia was the most frequently isolated (n = 2076, 59.16%) (Sader and Jones, 2005). A similar finding was reported in a prospective multi-center study involving nine teaching hospitals in France, in which S. maltophilia was the most commonly isolated NFGNB among all unusual NFGNB species (39%) (Fihman et al., 2012). Other surveillance studies, namely SCOPE (Jones et al., 1997), SENTRY (Jones et al., 2003; Gales et al., 2006), and SMART (Liu et al., 2012) showed a steady increase in isolation of S. maltophilia among all NFGNB pathogens during the period 1995–2010 (6.7% in 1995–1996, 8.0% in 1997–2001, and 9.1% in 2001–2010). These findings show that S. maltophilia is not an insignificant pathogen among disease-causing GNB and NFGNB species.
As expected, the prevalence of infections due to S. maltophilia is higher in intensive care units (1.4–3.0%) than in the general population (Fluit et al., 2001b; Sader et al., 2004; Streit et al., 2004; Sader et al., 2005a; Meyer et al., 2006; Zhanel et al., 2008; Magret et al., 2011; Kim et al., 2014).
There is limited information on the worldwide prevalence of S. maltophilia infections in the general pediatric population. SENTRY studies conducted during 1998–2003 (Fedler et al., 2006b) and in 2004 (Fedler et al., 2006a) showed that the prevalence of infections due to S. maltophilia was 1.2% among children ≤ 7 years and 1.4% among children ≤ 18 years old. The rates are similar to those in the adult population. A comparison of two single-center studies in China and the USA revealed markedly different incidence rates of ventilator-associated pneumonia due to S. maltophilia among pediatric patients in ICUs. Ning et al. reported a rate of 20.3% among patients aged 2 months to 16 years in a pediatric ICU in China (Ning et al., 2013) whereas Arthur et al. found that the rate of infection due to S. maltophilia among infants aged 0–6 months in a cardiac ICU in the USA was only 0.8% (Arthur et al., 2015).
Several recent studies have shown that S. maltophilia is also an emerging opportunistic pathogen in community settings (Falagas et al., 2009a; Chang et al., 2014). Results of a worldwide SENTRY study in 1997 (Diekema et al., 1999) and the British Society for Antimicrobial Chemotherapy Resistance surveillance project conducted during 2001–2006 (Livermore et al., 2008) showed that 33.3 and 32%, respectively, of S. maltophilia isolates were collected within 48 h after admission (defined as community-acquired in these studies) from patients with bloodstream infections. The results from two recent SMART studies revealed that 14.3–17.2% of isolates from patients with community-acquired IAI (also defined by a 48-h time frame within admission) during 2003–2010 were S. maltophilia (Lee et al., 2012; Liu et al., 2012). Another recent study on the prevalence of community-acquired S. maltophilia BSI in Taiwan, which specifically divided the patients into three categories based on whether they had community-acquired (excluding patients hospitalized within 90 days before admission, cared in a nursing facility, etc.), healthcare-associated or hospital-acquired infections, reported that 17.6% of all community-acquired bloodstream infections were due to S. maltophilia (Chang et al., 2014). A similar study in France revealed that 23.7% of all community-acquired BSIs were due to S. maltophilia (Fihman et al., 2012). These studies show that community-acquired S. maltophilia infections are far less rare than previously thought.
A number of risk factors for death due to S. maltophilia infections have been reported. Paez et al. (Paez and Costa, 2008) reviewed the literature from 1985 to 2008 and found that BSI and pneumonia, shock, thrombocytopenia, and Acute Physiological Assessment and Chronic Health Evaluation (APACHE) score >15 are independent risk factors associated with outcome. In addition, underlying hematological malignancy and admission to ICU are independent risk factors for cancer patients. The impact of appropriate antimicrobial treatment and removal of CVC on mortality were concluded to require further clinical studies (Paez and Costa, 2008). The conclusion of the review corresponds to the aforementioned studies. Falagas et al. analyzed 15 articles for attributable mortality of S. maltophilia infections. Only four studies provided relevant data regarding inappropriate antibiotic treatment, and three out of the four studies found significantly higher mortality when compared with initial appropriate therapy (Falagas et al., 2009b).
There are limited antimicrobial options for infections due to S. maltophilia because of its extensive resistance to most antibiotics, including β-lactam antibiotics, cephalosporins, macrolides, aminoglycosides, and carbapenems. Interpretive breakpoints for susceptibility are available only for ticarcillin/clavulanate, ceftazidime, minocycline, levofloxacin, trimethoprim/sulfamethoxazole (TMP/SMX), and chloramphenicol (CLSI, 2015). Table 2 shows the rates of susceptibility of S. maltophilia to antimicrobial agents reported in the studies presented in Table 1. TMP/SMX is recognized as the drug of choice (Wang et al., 2014a). Resistance rates vary geographically but are generally less than 10% (Chung et al., 2013). However, high and various rates of resistance to TMP/SMX have been reported in patients with cancer (Vartivarian et al., 1994; Micozzi et al., 2000), cystic fibrosis (Saiman et al., 2002; Cantón et al., 2003; San Gabriel et al., 2004; Valenza et al., 2008), and in several countries, including Taiwan, Japan, Korea, Thailand, Spain, Mexico, Saudi Arabia, Turkey, and Canada (16–78.8%) (Valdezate et al., 2001; del Toro et al., 2002; Lai et al., 2004; Gülmez and Hasçelik, 2005; Memish et al., 2012; Wu et al., 2012; Rattanaumpawan et al., 2013; Rhee et al., 2013; Zhanel et al., 2013; Flores-Treviño et al., 2014; Hotta et al., 2014; Walkty et al., 2014; Wang et al., 2014a). In the present review, global surveillance data for the period 1997–2012 show that S. maltophilia continues to be highly susceptible to TMP/SMX (Table 2). Over that 15-year period, the susceptibility rates reported in worldwide SENRTY studies (Gales et al., 2001a; Jones et al., 2003; Gales et al., 2006; Sader et al., 2013, 2014a), a BSAC surveillance study (Livermore et al., 2008), and three large-scale multi-national studies (Sader et al., 2005b; Farrell et al., 2010a, 2014) ranged from 90 to 100%.
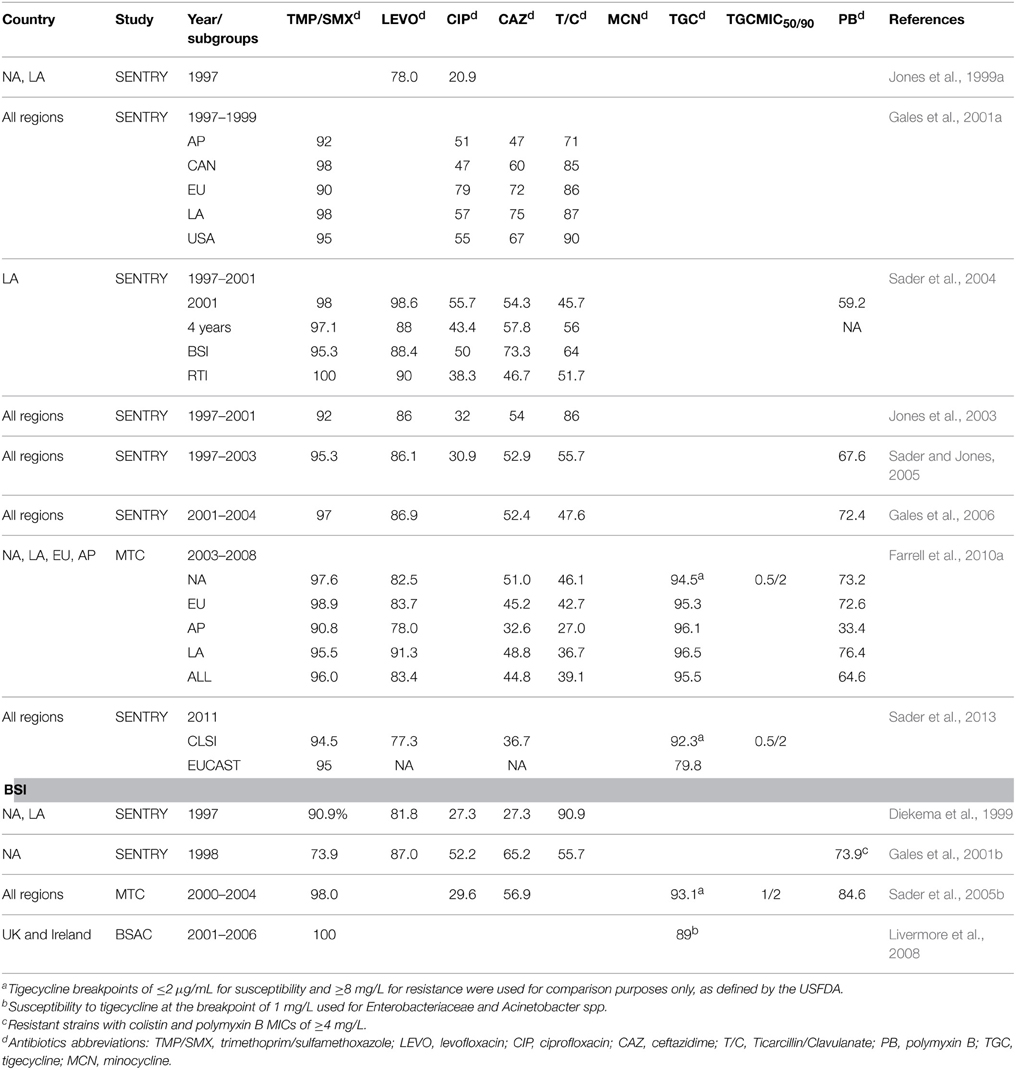
Table 2. Susceptibility of S. maltophilia to various antimicrobial agents in worldwide surveillance and multicenter studies.
Ceftazidime and ticarcillin/clavulanate used to be the most effective among β-lactam drugs against S. maltophilia. However, recent studies have demonstrated resistance rates of more than 30% and a trend in decreasing susceptibility with ceftazidime (47–75% during 1997–1999 to 30.5–36.8% during 2009–2012) (Table 2) (Gales et al., 2001a; Farrell et al., 2010a; Sader et al., 2014b). The same is true for ticarcillin/clavulanate. During 1997–1998, the rates of susceptibility of S. maltophilia to that combination ranged from 71–90% but dropped to 27–46.1% during 2003–2008.
New fluoroquinolones exhibit better potency against S. maltophilia than ceftazidime or ticarcillin/clavulanate and have become reasonable alternatives. Nonetheless, a comparison of data from worldwide SENTRY studies reveals a decrease in sensitivity of S. maltophilia to levofloxacin, from 83.4% during the period 2003–2008 (Farrell et al., 2010a) to 77.3% in 2011 (Sader et al., 2013). Low susceptibility rates ranging from 64–69.6% have also been reported in Canada (Zhanel et al., 2013), China (Yang et al., 2010; Tan et al., 2014), and Korea (Chung et al., 2013). Few multi-center studies have investigated the efficacy of fluoroquinolones against S. maltophilia in patients with UTIs. In a SMART study conducted in the Asia-Pacific region, isolates of S. maltophilia from patients with UTIs showed exceptionally high rates of resistance to levofloxacin (33.3%) (Lu et al., 2012). Two recent reports showed low MIC50 (minimum inhibitory concentration)values (0.5 mg/L and 0.5 mg/L) and low MIC90 values (8 and 4 mg/L) for moxifloxacin against S. maltophilia (Zhanel et al., 2008; Chung et al., 2013), indicating that moxifloxacin could be considered an effective alternative. Data from a number of studies demonstrate that ciprofloxacin has poor activity against S. maltophilia, with susceptibility rates averaging lower than 50% (Table 2).
Minocycline, doxycycline, and tigecycline have consistently displayed good potency against S. maltophilia in studies with various time periods, sources of specimens, and geographic regions (Sader et al., 2005b, 2013, 2014b; Gales et al., 2008; Chen et al., 2012; Wu et al., 2012; Chung et al., 2013). A TSAR surveillance study conducted in Taiwan tested 377 isolates of S. maltophilia obtained over a 10-year period (1998–2008) and revealed low MIC50 (0.25 mg/L) and MIC90 values (1 mg/L) for tigecycline (Wu et al., 2012). Similar results were demonstrated in several large-scale worldwide surveillance studies as well. A recent SENTRY study conducted during 2009–2012 (494 isolates) (Sader et al., 2014a) revealed a susceptibility of 96% and a recent TEST study conducted during 2007–2012 (2245 isolates) (Renteria et al., 2014) demonstrated low MIC50 (0.25 mg/L) and MIC90 (1 mg/L) values.
S. maltophilia has several molecular mechanisms contributing to its extensive antimicrobial resistance. The mechanisms are summarized in Table 3. Detailed descriptions of the major mechanisms are elaborated as follows.
S. maltophilia has two chromosomal-mediated inducible β-lactamases, namely L1 and L2. L1 is a molecular class B Zn2+-dependent metallo-β-lactamase and L2 is a molecular class A clavulanic acid-sensitive cephalosporinase. The L1 and L2 β-lactamases are simultaneously regulated by AmpR, a transcriptional regulator in the L2 upstream region (Okazaki and Avison, 2008). The ampR-L2 module is homologous to the ampR-ampC systems, which are widely distributed in some members of the family Enterobacteriaceae and in P. aeruginosa (Lodge et al., 1990). The regulation of chromosomal ampR-ampC systems has been well studied in Citrobacter freundii, where the AmpC β-lactamase induction is linked to peptidoglycan recycling and involves several regulatory genes, such as as ampR, ampG, and ampD (Lindberg et al., 1985). A similar induction mechanism was proposed for the ampR-ampC and the ampR-L2 modules (Okazaki and Avison, 2008). But unlike P. aeruginosa, the permease system in S. maltophilia requires an intact ampN-ampG operon for the induction of β-lactamase (Huang et al., 2010). Two ampD homologs, ampDI and ampDII, were found in S. maltophilia, but only ampDIappears to be relevant to the regulation of β-lactamase (Yang et al., 2009).
Penicillin-binding proteins (PBPs) participate in peptidoglycan biosynthesis and the inactivation of PBP4 in P. aeruginosa has been shown to confer AmpC overexpression and β-lactam resistance (Moya et al., 2009). The inactivation of a putative PBP1a gene, mrcA, recently was found to cause basal-level L1/L2 β-lactamase hyperproduction in S. maltophilia KJ. The inactivation of mrcA only affects basal L1/L2 production β-lactamase, which is ampR- and ampN-ampG-dependent, and does not augment their induction (Lin et al., 2011). The universality of disruption of ampDI or mrcA in β-lactamase-hyperproducing S. maltophilia mutants and clinical isolates has been proved by the existence of wild-type ampDI and mrcA genes. The result implicates mutation of at least one additional gene in this phenotype (Talfan et al., 2013).
Efflux pumps in microorganisms mediate the extrusion of drugs and are classified into five families, namely the resistance-nodulation-cell-division (RND) family, the major facilitator superfamily (MFS), the small multidrug resistance (SMR) family, the ATP binding cassette (ABC) family, and the multidrug and toxic compound extrusion (MATE) family (Putman et al., 2000). Two ABC-type (SmrA, MacABCsm), one MFS-type (EmrCABsm), a fusaric acid extrusion efflux pump (FuaABC), and six out of the eight postulated RND-type efflux systems have been characterized in S. maltophilia (Alonso and Martinez, 2000; Li et al., 2002; Crossman et al., 2008; Al-Hamad et al., 2009; Chen et al., 2011; Hu et al., 2012; Gould et al., 2013; Huang et al., 2013a; Lin et al., 2014a,b). The six characterized RND-type efflux pumps in the S. maltophilia genome are SmeABC, SmeEF, SmeIJK, SmeOP, SmeVWX, and SmeYZ (including SmeGH and SmeMN). Table 4 provides a summary of antimicrobial resistance associated with the abovementioned efflux pumps.
The overexpression of smeABC genes confers resistance to aminoglycosides, β-lactams, and fluoroquinolones. SmeC was identified to function independently of SmeAB, while deletions in smeC but not smeB compromised the antimicrobial resistance (Li et al., 2002).
SmeDEF is a complex formed by an efflux pump located on the inner membrane (SmeE), an outer membrane protein (SmeF) and a periplasmic membrane fusion protein (SmeD). It is involved in resistance to quinolones, tetracyclines, macrolides, chloramphenicol and novobiocin (Alonso and Martinez, 2000). Expression of the smeDEF operon is regulated by the SmeT repressor (Hernandez et al., 2009). A recent study showed that the SmeDEF efflux pump is associated with plant root colonization by S. maltophilia, and that deletion of the smeE gene impairs this function (García-León et al., 2014a).
The SmeVWX pump, encoded by a five-gene operon (smeU1, smeV, smeW, smeU2, and smeX), was identified and characterized in a multidrug-resistant mutant of S. maltophilia KJ. Overexpression of the SmeVWX pump resulted in increased resistance to chloramphenicol, quinolones, and tetracyclines but increased aminoglycoside susceptibility (Chen et al., 2011).
The smeZ, smeJ, and smeK genes were identified in S. maltophilia K M5, a selected mutant derivative. SmeZ contributes to elevated aminoglycoside MICs. SmeJ and SmeK jointly elevate tetracycline, minocycline, and ciprofloxacin MICs and confer resistance to levofloxacin (Gould et al., 2013). In addition to drug extrusion, the SmeIJK pump has been reported to play a physiologic role in the maintenance of cell membrane integrity (Huang et al., 2014). A recent study further elucidated the physiologic significance of the SmeYZ pump and demonstrated its correlation with virulence-related functions, including swimming, flagella formation, oxidative stress susceptibility, biofilm formation, and protease secretion (Lin et al., 2015).
A pcm-tolCsm operon was recently verified in S. maltophilia KJ2. The tolCsm gene is involved in the resistance of several antimicrobial agents, including aminoglycosides, macrolides, β-lactams, chloramphenicol, nalidixic acid, doxycycline and TMP/SMX. The deletion of pcm was shown to result in decreased expression of tolCsm, which compromised the pathogen's resistance to amikacin and gentamicin (Huang et al., 2013b). A very recent study characterized a five-gene cluster efflux pump (tolCSm-pcm-smeRo-smeO-smeP) in S. maltophilia. The study showed that SmeOP requires TolCSm for efflux pump function and suggested that TolCSm is the cognate outer membrane protein (OMP) for the SmeOP pump. The SmeOP-TolCSm efflux pump was shown to be associated with resistance to nalidixic acid, doxycycline, amikacin, gentamicin, erythromycin, and leucomycin (Lin et al., 2014a).
SmrA, the first ABC-type efflux pump identified in S. maltophilia, has been shown to confer resistance to fluoroquinolones and tetracycline (Al-Hamad et al., 2009). The MacABCsm efflux pump in S. maltophilia was recently shown to confer intrinsic resistance to antimicrobials (aminoglycosides, macrolides, and polymyxins) and to play an important role in regulating oxidative and envelope stress tolerance and biofilm formation (Lin et al., 2014b).
Only one MFS-type efflux pump, EmrCABsm, has been characterized so far. It is involved in the extrusion of hydrophobic compounds, including the antibiotics nalidixic acid and erythromycin (Huang et al., 2013a).
A novel tripartite fusaric acid efflux pump was found in S. maltophilia, namely FuaABC, which may constitute a new subfamily of the tripartite efflux pump. The fuaABC operon was demonstrated to be induced by fusaric acid and to contribute to fusaric acid resistance when overexpressed (Hu et al., 2012).
The sul1 gene carried by class 1 integrons and the sul2 gene, which is linked to insertion sequence common region (ISCR) elements, are known to be responsible for resistance to TMP/SMX in S. maltophilia (Barbolla et al., 2004; Toleman et al., 2007; Chung et al., 2015). The dfrA gene cassettes, which are located in class 1 integrons and encode for the dihydrofolate reductase enzyme, have also been reported to confer high-level resistance to TMP/SMX (Hu et al., 2011). Moreover, SmeDEF, TolCsm, and SmeYZ efflux pumps have recently been reported to be associated with TMP/SMX resistance (Huang et al., 2013b; Lin et al., 2015; Sánchez and Martínez, 2015).
Two mechanisms are associated with resistance of S. maltophilia to quinolones: efflux pumps and a chromosomally encoded qnr gene (Smqnr) that protects both gyrase and topoisomerase IV from quinolones (Sanchez et al., 2009). Unlike other bacteria, clinical isolates of quinolone-resistant S. maltophilia do not present mutations in topoisomerases (Valdezate et al., 2005). To date, the characterized genetic determinants involving resistance to quinolones are smeDEF, smeIJK, smeABC, smeVWX, and Smqnr genes, among which smeDEF and Smqnr are the best-described. Smqnr belongs to the qnr family. It confers low-level resistance and contributes to intrinsic resistance to quinolones in S. maltophilia (Sánchez et al., 2008; Shimizu et al., 2008; Sánchez and Martínez, 2010). García-León et al. further elucidated the interplay between intrinsic and acquired resistance to quinolones in S. maltophilia. Their study demonstrated that the capacity to develop mutation-driven antibiotic resistance is highly dependent on the intrinsic resistome. Their findings indicate that the most prevalent cause of acquired quinolone resistance in S. maltophilia is the overproduction of multidrug efflux pumps, among which SmeDEF efflux pump plays the most important role (García-León et al., 2014b). In addition, a more recent report by García-León et al. confirmed that overexpression of smeVWX in clinical isolates of S. maltophilia is associated with high-level quinolone resistance (García-León et al., 2015).
The aminoglycoside-resistant mechanisms in S. maltophilia primarily involve aminoglycoside-modifying enzymes and efflux pumps. The reported enzymes to date include AAC(6′)-Iz (an aminoglycoside acetyltransferase) (Li et al., 2003), APH(3′)-IIc (an aminoglycoside phosphotransferase) (Okazaki and Avison, 2007) and a novel AAC(6′)-Iak, which was recently identified in a MDR strain from Nepal (Tada et al., 2014). Efflux pumps including SmeABC, SmeYZ, SmeOP-TolCsm, and MacABCsm are associated with resistance as described above.
Trimethoprim/sulfamethoxazole (TMP/SMX) remains the most effective antimicrobial agent against S. maltophilia, with an overall susceptibility rate higher than 90% (Falagas et al., 2008). A recent study investigated the efficacy of sulfametrole/trimethoprim, an alternative sulphonamide/trimethoprim combination available in several Europe countries against non-fermenters (40 S. maltophilia included) and found that the activity of the alternative combination was similar to that of TMP/SMX (Livermore et al., 2014). Other common options include ceftazidime, ticarcillin-clavulanate, fluoroquinolones, and tetracyclines such as tigecycline, minocycline, and doxycyclines. As previously mentioned, resistance rates to ceftazidime and ticarcillin-clavulanate are high and rising and are, therefore, unreliable choices. Fluoroquinolones are now popular alternatives because of their less prominent side effects compared to TMP/SMX and their greater potency compared to β-lactams.
Fluoroquinolones (FQs) are commonly used to treat infections due to S. maltophilia (Nicodemo and Paez, 2007). However, their overuse worldwide has resulted in higher resistance rates in many kinds of pathogenic bacteria, including S. maltophilia (Chang et al., 2014; Pien et al., 2015).
To evaluate the effectiveness of FQs in this era of high FQ resistance, a retrospective study published in 2014 compared the outcomes of patients with S. maltophilia infections treated with TMP/SMX and those of patients treated with FQs monotherapy (Wang et al., 2014b). A total of 38 adults received TMP/SMX and 63 adults received FQs (levofloxacin n = 48 and ciprofloxacin n = 15). The overall microbiological cure rate was 63% (65% in the TMP/SMX group and 62% in the FQ group), and the overall clinical success rate was 55% (61% in the TMP/SXT group and 52% in the FQ group). The antibiotic regimens were equally effective in both groups. Another retrospective study compared the effectiveness of TMP/SMX (n = 51) with that of levofloxacin (n = 35) in treating S. maltophilia bacteremia and revealed no significant differences in treatment outcome between the two groups, including 30-day mortality, length of hospital day, and antibiotic withdrawal (Cho et al., 2014). However, the rate of adverse events was significantly lower in the levofloxacin group (0%) than in the TMP/SXT group (23.5%, p = 0.001).
Several new quinolones have been developed and some of them have recently been approved for clinical application, including nemonoxacin (Huang et al., 2015) and delafloxacin (Bassetti et al., 2015). Oral nemonoxacin, a novel nonfluorinated quinolone antibiotic, has been shown to have good activity against Gram-positive bacilli, such as methicillin-resistant Staphylococcus aureus (Huang et al., 2015). However, in vitro susceptibility assays on 32 clinical isolates of S. maltophilia revealed high MIC90 (32 mg/L) and MIC50 (8 mg/L) values (Lai et al., 2014a). Regarding delafloxacin, a phase II study published in 2009 that compared two doses of delafloxacin to tigecycline in adults with complicated skin and skin structure infections found that only one patient was infected with S. maltophilia and was treated successfully with delafloxacin (O'Riordan et al., 2015). More in vivo studies are needed to better understand the effectiveness of these new quinolones in treating S. maltophilia infections.
Tetracyclines such as tigecycline, minocycline, and doxycycline are some of the most active antimicrobial agents against S. maltophilia other than TMP/SMX, even in the cystic fibrosis population (Cantón et al., 2003; San Gabriel et al., 2004; Gülmez et al., 2010; Milne and Gould, 2012; Castanheira et al., 2014). The results agree with our aforementioned observation that these antibiotics consistently exhibited good activity against S. maltophilia in global surveillance studies (Table 2).
Tigecycline, a derivative of minocycline, has broad-spectrum antimicrobial activity (Stein and Babinchak, 2013) and is an alternative agent against S. maltophilia infections. A recent study from Brazil showed that the MIC50 and MIC90 values of tigecyline for S. maltophilia isolates, including isolates resistant to levofloxacin and/or TMP/SMX harboring sul-1, sul-2, and qnrMR, were 1 and 4 μg/ml, respectively (Rizek et al., 2015). However, tigecycline has great bio-distribution after intravenous injection, which leads to lower serum drug levels. So, there are concerns about its efficacy for the treatment of bacteremia due to S. maltophilia (Stein and Babinchak, 2013). In a recent study, a high dose of tigecycline was effective at treating S. maltophilia bacteremia (Wu and Shao, 2014), although in another study, high-dose tigecycline treatment was associated with significant adverse effects (Falagas et al., 2014). In addition, a 3-year clinical therapeutic study that compared the effectiveness of TMP/SMX and tigecycline in treating nosocomial S. maltophilia infections revealed no significant differences in mortality or clinical response rates between the two regimens. Clinical improvement rates on the 14th day were 69.2% in the TMP/SMX group and 68.4% in the tigecycline group (P = 0.954) and mortality rates on the 30th day were 30.8% in the TMP/SMX group and 21.1% in the tigecycline group (P = 0.517) (Tekce et al., 2012). Therefore, tigecycline might be an alternative for patients who are unable to tolerate TMP/SMX. In addition to monotherapy, a combination regimen with tigecycline might be a better option for severe infections, especially for nosocomial infections (Samonis et al., 2012).
In a recent large collection of resistant organisms from the SENTRY program during 2007–2011 (1706 S. maltophilia included), minocycline was shown to be significantly more active than other tetracyclines against S. maltophilia. The rate of susceptibility of S. maltophilia to minocycline exceeded 97% across all geographic regions, and the potency was 2-fold higher than doxycycline (MIC50∕90: 0.5/2 μg/mL) (Castanheira et al., 2014). A study evaluated 53 multidrug resistant isolates of S. maltophilia, including 48 that were resistant to levofloxacin and/or TMP/SMX, and found that minocycline exhibited excellent activity against S. maltophilia. However, the clinical experience is still anecdotal (Rizek et al., 2015). A patient with pneumonia was reportedly successfully treated with minocycline (Irifune et al., 1994), and the combination of minocycline with TMP and ticarcillin/clavulanate has been suggested to be effective (Vartivarian et al., 1994).
Polymyxins and fosfomycin are being reconsidered as alternatives or “last-resort” options because of the increasing emergence of multidrug-resistant organisms. Unfortunately, interpreting the susceptibility rates of S. maltophilia to polymyxins is problematic because of the discordance between different testing methods (Nicodemo et al., 2004; Gülmez et al., 2010; Moskowitz et al., 2010; Betts et al., 2014). A SENTRY surveillance program study conducted during 2001–2004 assessed the antimicrobial activity of polymyxin B among 54731 isolates of GNB and 1256 isolates of S. maltophilia and found that 72.4% of the S. maltophilia isolates were susceptible to polymyxin B (MIC50 and MIC90 values, 1 and 8 mg/L, respectively) (Gales et al., 2006). Colistin (polymyxin E) appears to have a considerable in vitro activity against S. maltophilia (83–88%) (Falagas and Kasiakou, 2005). In addition, synergy with rifampin, TMP/SMX, and doxycycline has been shown (Giamarellos-Bourboulis et al., 2002; Gülmez et al., 2010). Two studies by the SENTRY program in the year 2011 (globally) and during 2009–2012 (USA and Europe) that collected 362 and 494 isolates of S. maltophilia, respectively, reported very different rates of susceptibility to colistin (98.5%, Sader et al., 2013 and 38.7–49.7%, Sader et al., 2014a). In a recent study that collected 641 isolates of S. maltophilia in a university hospital in Argentina (Rodríguez et al., 2014), Rodríguez et al. showed that colistin resistance increased from 8% in 1996 to 45% in 2013 and found that the increase correlated with a marked increase (11.4-fold) in colistin consumption during the study period. Fosfomycin has been shown in several reports to be an inappropriate treatment option because of its poor activity and high MICs against S. maltophilia (Macleod et al., 2009; Khan et al., 2014; Rizek et al., 2015).
Owing to the impressive array of antimicrobial resistance mechanisms of S. maltophilia, various combinations of antimicrobial agents have been surveyed in order to overcome resistance or to attain synergism (Table 5). Combinations of two or three agents with good susceptibility results such as TMP/SMX, ceftazidime, ticarcillin/clavulanate, and aminoglycosides have demonstrated synergistic effects to different degrees in prior studies. In more recent studies, combinations of TMP/SMX or β-lactam/β-lactam inhibitors with new or old antibiotics such as tigecycline, fluoroquinolones, televancin (Hornsey et al., 2012), rifampin (Betts et al., 2014), and aerosolized colistin have been investigated; they demonstrated various extents of synergism and the ability to maintain effectivity in biofilm. TMP/SMX plus ceftazidime plus levofloxacin has been shown to be effective in treatment of meningitis (Correia et al., 2014) and intrabiliary infusion of colistin plus parenteral fosfomycin with tigecycline was reported to be effective at treating complicated biliary tract infection (Perez et al., 2014). Several combinations of novel agents are currently under investigation, including a β-lactam and dual β-lactamase inhibitor combination (Page et al., 2011) and MD3 (a novel synthetic inhibitor of peptidases) plus colistin (Personne et al., 2014). It is important to mention that in vitro synergy attained by combination should be further correlated with clinical outcomes.
Nebulization of antimicrobials results in high concentrations in the respiratory tract and is associated with low toxicity because this method of delivery results in limited systemic absorption (Table 5). These characteristics are especially important for patients with cystic fibrosis, who are prone to frequent infection and colonization of multidrug resistant pathogens including S. maltophilia. Wood et al. reported a case of recurrent ventilator-associated pneumonia successfully treated with aerosolized colistin and doxycycline (Wood et al., 2010). King et al. surveyed the in vitro pharmacodynamics of aerosolized levofloxacin and suggested that the high concentrations of levofloxacin achieved in the lung by aerosol delivery may be useful for the treatment of patients with cystic fibrosis (King et al., 2010). When S. maltophilia isolates were grown as a biofilm, the top 3 most effective antibiotic combinations included high-dose levofloxacin or colistin delivered at doses achievable by aerosolization plus a β-lactam or TMP/SMX (Table 5) (Wu et al., 2013). The potentials of other antibiotics to be nebulized to achieve high drug levels in airway have been investigated in order to overcome the high MICs that cannot be conquered when the agents are administered systemically. A device (Podhaler device) that delivers new inhalational tobramycin (tobramycin inhalation powder, TIP) and attains high drug levels to the lung may be able to exceed current high MICs of tobramycin in S. maltophilia (Ratjen et al., 2015). Waters suggested a potential role of inhaled aztreonam lysine in the treatment of S. maltophilia pulmonary infection because of its resistance to the L1 β-lactamase produced by S. maltophilia and the ability to achieve high drug levels in respiratory secretions (approximately 1000-fold higher than the corresponding plasma concentration) (Waters, 2012). The antibacterial activity of a novel inhaled combination of fosfomycin and tobramycin (FTI) was investigated in patients with bronchiectasis. However, FTI demonstrated relatively poor activity against S. maltophilia (Macleod et al., 2009).
Worldwide, multi-institutional studies confirm that S. maltophilia is an emerging multi-drug resistant opportunistic pathogen in hospital and community settings, especially among immunocompromised hosts. TMP/SMX remains the most effective antimicrobial agent in the general population. Drugs with historically good susceptibility results include ceftazidime, ticarcillin-clavulanate, and fluoroquinolones; however, a number of studies show an alarming trend in resistance to those agents. Tetracyclines such as tigecycline, minocycline, and doxycycline are also effective agents and consistently display good activity against S. maltophilia in various geographic regions and across different time periods. Combination therapies, novel agents, and aerosolized forms of antimicrobial drugs are currently being tested for their ability to treat infections caused by this multi-drug resistant organism. In addition, recent advances in molecular methods have identified various new mechanisms contributing to drug resistance, which hopefully will lead to future breakthroughs in the treatment of infections due to S. maltophilia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Al-Anazi, K. A., and Al-Jasser, A. M. (2014). Infections caused by Stenotrophomonas maltophilia in recipients of hematopoietic stem cell transplantation. Front. Oncol. 4:232. doi: 10.3389/fonc.2014.00232
Al-Hamad, A., Upton, M., and Burnie, J. (2009). Molecular cloning and characterization of SmrA, a novel ABC multidrug efflux pump from Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 64, 731–734. doi: 10.1093/jac/dkp271
Alonso, A., and Martínez, J. L. (2000). Cloning and characterization of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 44, 3079–3086. doi: 10.1128/AAC.44.11.3079-3086.2000
Arthur, C., Tang, X., Romero, J. R., Gossett, J. G., Harik, N., and Prodhan, P. (2015). Stenotrophomonas maltophilia infection among young children in a cardiac intensive care unit: a single institution experience. Pediatr. Cardiol. 36, 509–515. doi: 10.1007/s00246-014-1041-0
Barbolla, R., Catalano, M., Orman, B. E., Famiglietti, A., Vay, C., Smayevsky, J., et al. (2004). Class 1 integrons increase trimethoprim-sulfamethoxazole MICs against epidemiologically unrelated Stenotrophomonas maltophilia isolates. Antimicrob. Agents Chemother. 48, 666–669. doi: 10.1128/AAC.48.2.666-669.2004
Bassetti, M., Della Siega, P., Pecori, D., Scarparo, C., and Righi, E. (2015). Delafloxacin for the treatment of respiratory and skin infections. Expert Opin. Investig. Drugs 24, 433–442. doi: 10.1517/13543784.2015.1005205
Berenbaum, M. C., Yu, V. L., and Felegie, T. P. (1983). Synergy with double and triple antibiotic combinations compared. J. Antimicrob. Chemother. 12, 555–563. doi: 10.1093/jac/12.6.555
Betts, J. W., Phee, L. M., Woodford, N., and Wareham, D. W. (2014). Activity of colistin in combination with tigecycline or rifampicin against multidrug-resistant Stenotrophomonas maltophilia. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1565–1572. doi: 10.1007/s10096-014-2101-3
Bouchillon, S. K., Badal, R. E., Hoban, D. J., and Hawser, S. P. (2013). Antimicrobial susceptibility of inpatient urinary tract isolates of gram-negative bacilli in the United States: results from the study for monitoring antimicrobial resistance trends (SMART) program: 2009-2011. Clin. Ther. 35, 872–877. doi: 10.1016/j.clinthera.2013.03.022
Brooke, J. S. (2012). Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin. Microbiol. Rev. 25, 2–41. doi: 10.1128/CMR.00019-11
Brooke, J. S. (2014). New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert. Rev. Anti. Infect. Ther. 12, 1–4. doi: 10.1586/14787210.2014.864553
Cantón, R., Valdezate, S., Vindel, A., Sánchez Del Saz, B., Maíz, L., and Baquero, F. (2003). Antimicrobial susceptibility profile of molecular typed cystic fibrosis Stenotrophomonas maltophilia isolates and differences with noncystic fibrosis isolates. Pediatr. Pulmonol. 35, 99–107. doi: 10.1002/ppul.10216
Castanheira, M., Mendes, R. E., and Jones, R. N. (2014). Update on Acinetobacter species: mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clin. Infect. Dis. 59(Suppl. 6), S367–S373. doi: 10.1093/cid/ciu706
Chang, Y. T., Lin, C. Y., Lu, P. L., Lai, C. C., Chen, T. C., Chen, C. Y., et al. (2014). Stenotrophomonas maltophilia bloodstream infection: comparison between community-onset and hospital-acquired infections. J. Microbiol. Immunol. Infect. 47, 28–35. doi: 10.1016/j.jmii.2012.08.014
Chen, C. H., Huang, C. C., Chung, T. C., Hu, R. M., Huang, Y. W., and Yang, T. C. (2011). Contribution of resistance-nodulation-division efflux pump operon smeU1-V-W-U2-X to multidrug resistance of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 55, 5826–5833. doi: 10.1128/AAC.00317-11
Chen, Y. H., Lu, P. L., Huang, C. H., Liao, C. H., Lu, C. T., Chuang, Y. C., et al. (2012). Trends in the susceptibility of clinically important resistant bacteria to tigecycline: results from the tigecycline in vitro surveillance in Taiwan study, 2006 to 2010. Antimicrob. Agents Chemother. 56, 1452–1457. doi: 10.1128/AAC.06053-11
Chen, X. X., Lo, Y. C., Su, L. H., and Chang, C. L. (2014). Investigation of the case number of catheter-related bloodstream infection overestivated by the central line-associated bloodstream infection surveillance definition. J. Microbiol. Immunol. Infect. doi: 10.1016/j.jmii.2014.03.006. [Epub ahead of print].
Cho, S. Y., Kang, C. I., Kim, J., Ha, Y. E., Chung, D. R., Lee, N. Y., et al. (2014). Can levofloxacin be a useful alternative to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob. Agents Chemother. 58, 581–583. doi: 10.1128/AAC.01682-13
Chow, A. W., Wong, J., and Bartlett, K. H. (1988). Synergistic interactions of ciprofloxacin and extended-spectrum beta-lactams or aminoglycosides against multiply drug-resistant Pseudomonas maltophilia. Antimicrob. Agents Chemother. 32, 782–784. doi: 10.1128/AAC.32.5.782
Chung, H. S., Hong, S. G., Kim, Y. R., Shin, K. S., Whang, D. H., Ahn, J. Y., et al. (2013). Antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from Korea, and the activity of antimicrobial combinations against the isolates. J. Korean Med. Sci. 28, 62–66. doi: 10.3346/jkms.2013.28.1.62
Chung, H. S., Kim, K., Hong, S. S., Hong, S. G., Lee, K., and Chong, Y. (2015). The sul1 gene in Stenotrophomonas maltophilia with high-level resistance to trimethoprim/sulfamethoxazole. Ann. Lab. Med. 35, 246–249. doi: 10.3343/alm.2015.35.2.246
Church, D., Lloyd, T., Peirano, G., and Pitout, J. (2013). Antimicrobial susceptibility and combination testing of invasive Stenotrophomonas maltophilia isolates. Scand. J. Infect. Dis. 45, 265–270. doi: 10.3109/00365548.2012.732240
Clinical Laboratory Standards Institute (CLSI). (2015). Performance Standards for Antimicrobial Susceptibility Testing; 25th Informational Supplement. CLSI Document M100-S25. Wayne, PA: CLSI.
Correia, C. R., Ferreira, S. T., and Nunes, P. (2014). Stenotrophomonas maltophilia: rare cause of meningitis. Pediatr. Int. 56, e21–e22. doi: 10.1111/ped.12352
Crossman, L. C., Gould, V. C., Dow, J. M., Vernikos, G. S., Okazaki, A., Sebaihia, M., et al. (2008). The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 9:R74. doi: 10.1186/gb-2008-9-4-r74
de Oliveira-Garcia, D., Dall'agnol, M., Rosales, M., Azzuz, A. C., Alcantara, N., Martinez, M. B., et al. (2003). Fimbriae and adherence of Stenotrophomonas maltophilia to epithelial cells and to abiotic surfaces. Cell Microbiol. 5, 625–636. doi: 10.1046/j.1462-5822.2003.00306.x
del Toro, M. D., Rodríguez-Bano, J., Herrero, M., Rivero, A., García-Ordoñez, M. A., Corzo, J., et al. (2002). Clinical epidemiology of Stenotrophomonas maltophilia colonization and infection: a multicenter study. Medicine (Baltimore) 81, 228–239. doi: 10.1097/00005792-200205000-00006
Diekema, D. J., Pfaller, M. A., Jones, R. N., Doern, G. V., Winokur, P. L., Gales, A. C., et al. (1999). Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin. Infect. Dis. 29, 595–607. doi: 10.1086/598640
Doern, G. V., Jones, R. N., Pfaller, M. A., Kugler, K. C., and Beach, M. L. (1999). Bacterial pathogens isolated from patients with skin and soft tissue infections: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). SENTRY Study Group (North America). Diagn. Microbiol. Infect. Dis. 34, 65–72. doi: 10.1016/S0732-8893(98)00162-X
Entenza, J. M., and Moreillon, P. (2009). Tigecycline in combination with other antimicrobials: a review of in vitro, animal and case report studies. Int. J. Antimicrob. Agents 34, 8.e1–8.e9. doi: 10.1016/j.ijantimicag.2008.11.006
Falagas, M. E., and Kasiakou, S. K. (2005). Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40, 1333–1341. doi: 10.1086/429323
Falagas, M. E., Kastoris, A. C., Vouloumanou, E. K., and Dimopoulos, G. (2009a). Community-acquired Stenotrophomonas maltophilia infections: a systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 28, 719–730. doi: 10.1007/s10096-009-0709-5
Falagas, M. E., Kastoris, A. C., Vouloumanou, E. K., Rafailidis, P. I., Kapaskelis, A. M., and Dimopoulos, G. (2009b). Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol. 4, 1103–1109. doi: 10.2217/fmb.09.84
Falagas, M. E., Valkimadi, P. E., Huang, Y. T., Matthaiou, D. K., and Hsueh, P. R. (2008). Therapeutic options for Stenotrophomonas maltophilia infections beyond co-trimoxazole: a systematic review. J Antimicrob. Chemother. 62, 889–894. doi: 10.1093/jac/dkn301
Falagas, M. E., Vardakas, K. Z., Tsiveriotis, K. P., Triarides, N. A., and Tansarli, G. S. (2014). Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int. J. Antimicrob. Agents 44, 1–7. doi: 10.1016/j.ijantimicag.2014.01.006
Farrell, D. J., Sader, H. S., Flamm, R. K., and Jones, R. N. (2014). Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int. J. Antimicrob. Agents 43, 533–539. doi: 10.1016/j.ijantimicag.2014.01.032
Farrell, D. J., Sader, H. S., and Jones, R. N. (2010a). Antimicrobial susceptibilities of a worldwide collection of Stenotrophomonas maltophilia isolates tested against tigecycline and agents commonly used for S. maltophilia infections. Antimicrob. Agents Chemother. 54, 2735–2737. doi: 10.1128/AAC.01774-09
Farrell, D. J., Turnidge, J. D., Bell, J., Sader, H. S., and Jones, R. N. (2010b). The in vitro evaluation of tigecycline tested against pathogens isolated in eight countries in the Asia-Western Pacific region (2008). J. Infect. 60, 440–451. doi: 10.1016/j.jinf.2010.03.024
Fedler, K. A., Biedenbach, D. J., and Jones, R. N. (2006a). Assessment of pathogen frequency and resistance patterns among pediatric patient isolates: report from the 2004 SENTRY Antimicrobial Surveillance Program on 3 continents. Diagn. Microbiol. Infect. Dis. 56, 427–436. doi: 10.1016/j.diagmicrobio.2006.07.003
Fedler, K. A., Jones, R. N., Sader, H. S., and Fritsche, T. R. (2006b). Activity of gatifloxacin tested against isolates from pediatric patients: report from the SENTRY Antimicrobial Surveillance Program (North America, 1998-2003). Diagn. Microbiol. Infect. Dis. 55, 157–164. doi: 10.1016/j.diagmicrobio.2006.01.005
Felegie, T. P., Yu, V. L., Rumans, L. W., and Yee, R. B. (1979). Susceptibility of Pseudomonas maltophilia to antimicrobial agents, singly and in combination. Antimicrob. Agents Chemother. 16, 833–837. doi: 10.1128/AAC.16.6.833
Fihman, V., Le Monnier, A., Corvec, S., Jaureguy, F., Tankovic, J., Jacquier, H., et al. (2012). Stenotrophomonas maltophilia–the most worrisome threat among unusual non-fermentative gram-negative bacilli from hospitalized patients: a prospective multicenter study. J. Infect. 64, 391–398. doi: 10.1016/j.jinf.2012.01.001
Flores-Treviño, S., Gutiérrez-Ferman, J. L., Morfín-Otero, R., Rodríguez-Noríega, E., Estrada-Rívadeneyra, D., Rivas-Morales, C., et al. (2014). Stenotrophomonas maltophilia in Mexico: antimicrobial resistance, biofilm formation and clonal diversity. J. Med. Microbiol. 63, 1524–1530. doi: 10.1099/jmm.0.074385-0
Fluit, A. C., Schmitz, F. J., Verhoef, J., and European, S. P. G. (2001a). Frequency of isolation of pathogens from bloodstream, nosocomial pneumonia, skin and soft tissue, and urinary tract infections occurring in European patients. Eur. J. Clin. Microbiol. Infect. Dis. 20, 188–191. doi: 10.1007/s100960100455
Fluit, A. C., Verhoef, J., Schmitz, F. J., and European, S. P. (2001b). Frequency of isolation and antimicrobial resistance of gram-negative and gram-positive bacteria from patients in intensive care units of 25 European university hospitals participating in the European arm of the SENTRY Antimicrobial Surveillance Program 1997-1998. Eur. J. Clin. Microbiol. Infect. Dis. 20, 617–625. doi: 10.1007/s10096-001-8078-8
Fujita, J., Yamadori, I., Xu, G., Hojo, S., Negayama, K., Miyawaki, H., et al. (1996). Clinical features of Stenotrophomonas maltophilia pneumonia in immunocompromised patients. Respir. Med. 90, 35–38. doi: 10.1016/S0954-6111(96)90242-5
Gales, A. C., Jones, R. N., Forward, K. R., Liñares, J., Sader, H. S., and Verhoef, J. (2001a). Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997-1999). Clin. Infect. Dis. 32(Suppl. 2), S104–S113. doi: 10.1086/320183
Gales, A. C., Jones, R. N., and Sader, H. S. (2006). Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001-2004). Clin. Microbiol. Infect. 12, 315–321. doi: 10.1111/j.1469-0691.2005.01351.x
Gales, A. C., Reis, A. O., and Jones, R. N. (2001b). Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 39, 183–190. doi: 10.1128/JCM.39.1.183-190.2001
Gales, A. C., Sader, H. H., and Jones, R. N. (2002). Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia in Latin America: frequency of occurrence and antimicrobial susceptibility profile: results from the SENTRY Antimicrobial Surveillance Program (1997-2000). Diagn. Microbiol. Infect. Dis. 44, 301–311. doi: 10.1016/S0732-8893(02)00499-6
Gales, A. C., Sader, H. S., and Fritsche, T. R. (2008). Tigecycline activity tested against 11808 bacterial pathogens recently collected from US medical centers. Diagn. Microbiol. Infect. Dis. 60, 421–427. doi: 10.1016/j.diagmicrobio.2007.10.017
García-León, G., Hernández, A., Hernando-Amado, S., Alavi, P., Berg, G., and Martínez, J. L. (2014a). A function of SmeDEF, the major quinolone resistance determinant of Stenotrophomonas maltophilia, is the colonization of plant roots. Appl. Environ. Microbiol. 80, 4559–4565. doi: 10.1128/AEM.01058-14
García-León, G., Ruiz de Alegría Puig, C., García de la Fuente, C., Martínez-Martínez, L., Martínez, J. L., and Sánchez, M. B. (2015). High-level quinolone resistance is associated with the overexpression of smeVWX in Stenotrophomonas maltophilia clinical isolates. Clin. Microbiol. Infect. 21, 464–467. doi: 10.1016/j.cmi.2015.01.007
García-León, G., Salgado, F., Oliveros, J. C., Sánchez, M. B., and Martínez, J. L. (2014b). Interplay between intrinsic and acquired resistance to quinolones in Stenotrophomonas maltophilia. Environ. Microbiol. 16, 1282–1296. doi: 10.1111/1462-2920.12408
Giamarellos-Bourboulis, E. J., Karnesis, L., and Giamarellou, H. (2002). Synergy of colistin with rifampin and trimethoprim/sulfamethoxazole on multidrug-resistant Stenotrophomonas maltophilia. Diagn. Microbiol. Infect. Dis. 44, 259–263. doi: 10.1016/S0732-8893(02)00443-1
Gould, V. C., Okazaki, A., and Avison, M. B. (2013). Coordinate hyperproduction of SmeZ and SmeJK efflux pumps extends drug resistance in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 57, 655–657. doi: 10.1128/AAC.01020-12
Guembe, M., Cercenado, E., Alcalá, L., Marin, M., Insa, R., and Bouza, E. (2008). Evolution of antimicrobial susceptibility patterns of aerobic and facultative gram-negative bacilli causing intra-abdominal infections: results from the SMART studies 2003-2007. Rev. Esp. Quimioter. 21, 166–173. Retrieved from http://seq.es/seq/0214-3429/21/3/guembe.pdf
Gülmez, D., Cakar, A., Sener, B., Karakaya, J., and Hasçelik, G. (2010). Comparison of different antimicrobial susceptibility testing methods for Stenotrophomonas maltophilia and results of synergy testing. J. Infect. Chemother. 16, 322–328. doi: 10.1007/s10156-010-0068-2
Gülmez, D., and Hasçelik, G. (2005). Stenotrophomonas maltophilia: antimicrobial resistance and molecular typing of an emerging pathogen in a Turkish University Hospital. Clin. Microbiol. Infect. 11, 880–886. doi: 10.1111/j.1469-0691.2005.01257.x
Harthan, A. A., and Heger, M. L. (2013). Stenotrophomonas infection in a patient with glucose-6-phosphate dehydrogenase deficiency. J. Pediatr. Pharmacol. Ther. 18, 137–141. doi: 10.5863/1551-6776-18.2.137
Hernández, A., Maté, M. J., Sánchez-Díaz, P. C., Romero, A., Rojo, F., and Martínez, J. L. (2009). Structural and functional analysis of SmeT, the repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. J. Biol. Chem. 284, 14428–14438. doi: 10.1074/jbc.M809221200
Hoban, D. J., Biedenbach, D. J., Mutnick, A. H., and Jones, R. N. (2003). Pathogen of occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY Antimicrobial Surveillance Study (2000). Diagn. Microbiol. Infect. Dis. 45, 279–285. doi: 10.1016/S0732-8893(02)00540-0
Hombach, M., Bloemberg, G. V., and Böttger, E. C. (2012). Effects of clinical breakpoint changes in CLSI guidelines 2010/2011 and EUCAST guidelines 2011 on antibiotic susceptibility test reporting of Gram-negative bacilli. J. Antimicrob. Chemother. 67, 622–632. doi: 10.1093/jac/dkr524
Hornsey, M., Longshaw, C., Phee, L., and Wareham, D. W. (2012). In vitro activity of telavancin in combination with colistin versus Gram-negative bacterial pathogens. Antimicrob. Agents Chemother. 56, 3080–3085. doi: 10.1128/AAC.05870-11
Hotta, G., Matsumura, Y., Kato, K., Nakano, S., Yunoki, T., Yamamoto, M., et al. (2014). Risk factors and outcomes of Stenotrophomonas maltophilia bacteraemia: a comparison with bacteraemia caused by Pseudomonas aeruginosa and Acinetobacter species. PLoS ONE 9:e112208. doi: 10.1371/journal.pone.0112208
Hu, L. F., Chang, X., Ye, Y., Wang, Z. X., Shao, Y. B., Shi, W., et al. (2011). Stenotrophomonas maltophilia resistance to trimethoprim/sulfamethoxazole mediated by acquisition of sul and dfrA genes in a plasmid-mediated class 1 integron. Int. J. Antimicrob. Agents 37, 230–234. doi: 10.1016/j.ijantimicag.2010.10.025
Hu, L. F., Gao, L. P., Ye, Y., Chen, X., Zhou, X. T., Yang, H. F., et al. (2014). Susceptibility of Stenotrophomonas maltophilia clinical strains in China to antimicrobial combinations. J. Chemother. 26, 282–286. doi: 10.1179/1973947814Y.0000000168
Hu, R. M., Liao, S. T., Huang, C. C., Huang, Y. W., and Yang, T. C. (2012). An inducible fusaric acid tripartite efflux pump contributes to the fusaric acid resistance in Stenotrophomonas maltophilia. PLoS ONE 7:e51053. doi: 10.1371/journal.pone.0051053
Huang, C. H., Lai, C. C., Chen, Y. H., and Hsueh, P. R. (2015). The potential role of nemonoxacin for treatment of common infections. Expert. Opin. Pharmacother. 16, 263–270. doi: 10.1517/14656566.2015.978288
Huang, Y. W., Hu, R. M., Chu, F. Y., Lin, H. R., and Yang, T. C. (2013a). Characterization of a major facilitator superfamily (MFS) tripartite efflux pump EmrCABsm from Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 68, 2498–2505. doi: 10.1093/jac/dkt250
Huang, Y. W., Hu, R. M., and Yang, T. C. (2013b). Role of the pcm-tolCsm operon in the multidrug resistance of Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 68, 1987–1993. doi: 10.1093/jac/dkt148
Huang, Y. W., Lin, C. W., Hu, R. M., Lin, Y. T., Chung, T. C., and Yang, T. C. (2010). AmpN-AmpG operon is essential for expression of L1 and L2 beta-lactamases in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 54, 2583–2589. doi: 10.1128/AAC.01283-09
Huang, Y. W., Liou, R. S., Lin, Y. T., Huang, H. H., and Yang, T. C. (2014). A linkage between SmeIJK efflux pump, cell envelope integrity, and sigmaE-mediated envelope stress response in Stenotrophomonas maltophilia. PLoS ONE 9:e111784. doi: 10.1371/journal.pone.0111784
Irifune, K., Ishida, T., Shimoguchi, K., Ohtake, J., Tanaka, T., Morikawa, N., et al. (1994). Pneumonia caused by Stenotrophomonas maltophilia with a mucoid phenotype. J. Clin. Microbiol. 32, 2856–2857.
Jang, T. N., Wang, F. D., Wang, L. S., Liu, C. Y., and Liu, I. M. (1992). Xanthomonas maltophilia bacteremia: an analysis of 32 cases. J. Formos. Med. Assoc. 91, 1170–1176.
Jean, S. S., Lee, W. S., Bai, K. J., Lam, C., Hsu, C. W., Yu, K. W., et al. (2015). Relationship between the distribution of cefepime minimum inhibitory concentrations and detection of extended-spectrum β-lactamase production among clinically important Enterobacteriaceae isolates obtained from patients in intensive care units in Taiwan: results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) in 2007. J. Microbiol. Immunol. Infect. 48, 85–91. doi: 10.1016/j.jmii.2013.07.002
Jones, R. N. (2010). Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin. Infect. Dis. 51(Suppl. 1), S81–S87. doi: 10.1086/653053
Jones, R. N., Croco, M. A., Kugler, K. C., Pfaller, M. A., and Beach, M. L. (2000). Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). Diagn. Microbiol. Infect. Dis. 37, 115–125. doi: 10.1016/S0732-8893(00)00115-2
Jones, R. N., Croco, M. A., Pfaller, M. A., Beach, M. L., Kugler, K. C., and SENTRY Antimicrobial Surveillance Program Participants. (1999a). Antimicrobial activity evaluations of gatifloxacin, a new fluoroquinolone: contemporary pathogen results from a global antimicrobial resistance surveillance program (SENTRY, 1997). Clin. Microbiol. Infect. 5, 540–546. doi: 10.1111/j.1469-0691.1999.tb00432.x
Jones, R. N., Kugler, K. C., Pfaller, M. A., and Winokur, P. L. (1999b). Characteristics of pathogens causing urinary tract infections in hospitals in North America: results from the SENTRY Antimicrobial Surveillance Program, 1997. Diagn. Microbiol. Infect. Dis. 35, 55–63. doi: 10.1016/S0732-8893(98)00158-8
Jones, R. N., Pfaller, M. A., Marshall, S. A., Hollis, R. J., and Wilke, W. W. (1997). Antimicrobial activity of 12 broad-spectrum agents tested against 270 nosocomial blood stream infection isolates caused by non-enteric gram-negative bacilli: occurrence of resistance, molecular epidemiology, and screening for metallo-enzymes. Diagn. Microbiol. Infect. Dis. 29, 187–192. doi: 10.1016/S0732-8893(97)81808-1
Jones, R. N., Sader, H. S., and Beach, M. L. (2003). Contemporary in vitro spectrum of activity summary for antimicrobial agents tested against 18569 strains non-fermentative Gram-negative bacilli isolated in the SENTRY Antimicrobial Surveillance Program (1997-2001). Int. J. Antimicrob. Agents 22, 551–556. doi: 10.1016/S0924-8579(03)00245-0
Kerr, K. G., Hawkey, P. M., Child, J. A., Norfolk, D. R., and Anderson, A. W. (1990). Pseudomonas maltophilia infections in neutropenic patients and the use of imipenem. Postgrad. Med. J. 66:1090. doi: 10.1136/pgmj.66.782.1090
Khan, I. U., Mirza, I. A., Ikram, A., Ali, S., Hussain, A., and Ghafoor, T. (2014). In vitro activity of fosfomycin tromethamine against extended spectrum beta-lactamase producing urinary tract bacteria. J. Coll. Physicians. Surg. Pak. 24, 914–917.
Kim, T., Chong, Y. P., Park, S. Y., Jeon, M. H., Choo, E. J., Chung, J. W., et al. (2014). Risk factors for hospital-acquired pneumonia caused by carbapenem-resistant Gram-negative bacteria in critically ill patients: a multicenter study in Korea. Diagn. Microbiol. Infect. Dis. 78, 457–461. doi: 10.1016/j.diagmicrobio.2013.08.011
King, P., Lomovskaya, O., Griffith, D. C., Burns, J. L., and Dudley, M. N. (2010). In vitro pharmacodynamics of levofloxacin and other aerosolized antibiotics under multiple conditions relevant to chronic pulmonary infection in cystic fibrosis. Antimicrob. Agents Chemother. 54, 143–148. doi: 10.1128/AAC.00248-09
Krueger, T. S., Clark, E. A., and Nix, D. E. (2001). In vitro susceptibility of Stenotrophomonas maltophilia to various antimicrobial combinations. Diagn. Microbiol. Infect. Dis. 41, 71–78. doi: 10.1016/S0732-8893(01)00281-4
Labarca, J. A., Leber, A. L., Kern, V. L., Territo, M. C., Brankovic, L. E., Bruckner, D. A., et al. (2000). Outbreak of Stenotrophomonas maltophilia bacteremia in allogenic bone marrow transplant patients: role of severe neutropenia and mucositis. Clin. Infect. Dis. 30, 195–197. doi: 10.1086/313591
Lai, C. C., Lee, K. Y., Lin, S. W., Chen, Y. H., Kuo, H. Y., Hung, C. C., et al. (2014a). Nemonoxacin (TG-873870) for treatment of community-acquired pneumonia. Expert Rev. Anti. Infect. Ther. 12, 401–417. doi: 10.1586/14787210.2014.894881
Lai, C. H., Chi, C. Y., Chen, H. P., Chen, T. L., Lai, C. J., Fung, C. P., et al. (2004). Clinical characteristics and prognostic factors of patients with Stenotrophomonas maltophilia bacteremia. J. Microbiol. Immunol. Infect. 37, 350–358.
Lai, C. H., Wong, W. W., Chin, C., Huang, C. K., Lin, H. H., Chen, W. F., et al. (2006). Central venous catheter-related Stenotrophomonas maltophilia bacteraemia and associated relapsing bacteraemia in haematology and oncology patients. Clin. Microbiol. Infect. 12, 986–991. doi: 10.1111/j.1469-0691.2006.01511.x
Lai, W. A., Chen, S. F., Tsai, N. W., Chang, W. N., Lu, C. H., Chuang, Y. C., et al. (2014b). Non-cephalosporin-susceptible, glucose non-fermentative Gram-negative bacilli meningitis in post-neurosurgical adults: clinical characteristics and therapeutic outcome. Clin. Neurol. Neurosurg. 116, 61–66. doi: 10.1016/j.clineuro.2013.10.020
Lee, M. R., Wang, H. C., Yang, C. Y., Lin, C. K., Kuo, H. Y., Ko, J. C., et al. (2014). Clinical characteristics and outcomes of patients with pleural infections due to Stenotrophomonas maltophilia at a medical center in Taiwan, 2004-2012. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1143–1148. doi: 10.1007/s10096-014-2060-8
Lee, Y. L., Chen, Y. S., Toh, H. S., Huang, C. C., Liu, Y. M., Ho, C. M., et al. (2012). Antimicrobial susceptibility of pathogens isolated from patients with complicated intra-abdominal infections at five medical centers in Taiwan that continuously participated in the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2006 to 2010. Int. J. Antimicrob. Agents 40(Suppl.), S29–S36. doi: 10.1016/s0924-8579(12)70007-9
Li, X. Z., Zhang, L., Mckay, G. A., and Poole, K. (2003). Role of the acetyltransferase AAC(6')-Iz modifying enzyme in aminoglycoside resistance in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 51, 803–811. doi: 10.1093/jac/dkg148
Li, X. Z., Zhang, L., and Poole, K. (2002). SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 46, 333–343. doi: 10.1128/AAC.46.2.333-343.2002
Lin, C. W., Huang, Y. W., Hu, R. M., and Yang, T. C. (2014a). SmeOP-TolCSm efflux pump contributes to the multidrug resistance of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 58, 2405–2408. doi: 10.1128/AAC.01974-13
Lin, C. W., Lin, H. C., Huang, Y. W., Chung, T. C., and Yang, T. C. (2011). Inactivation of mrcA gene derepresses the basal-level expression of L1 and L2 beta-lactamases in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 66, 2033–2037. doi: 10.1093/jac/dkr276
Lin, Y. T., Huang, Y. W., Chen, S. J., Chang, C. W., and Yang, T. C. (2015). SmeYZ efflux pump of Stenotrophomonas maltophilia contributes to drug resistance, virulence-related characteristics, and virulence to mice. Antimicrob. Agents Chemother. 59, 4067–4073. doi: 10.1128/AAC.00372-15
Lin, Y. T., Huang, Y. W., Liou, R. S., Chang, Y. C., and Yang, T. C. (2014b). MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. J. Antimicrob. Chemother. 69, 3221–3226. doi: 10.1093/jac/dku317
Lindberg, F., Westman, L., and Normark, S. (1985). Regulatory components in Citrobacter freundii ampC beta-lactamase induction. Proc. Natl. Acad. Sci. U.S.A. 82, 4620–4624. doi: 10.1073/pnas.82.14.4620
Liu, Y. M., Chen, Y. S., Toh, H. S., Huang, C. C., Lee, Y. L., Ho, C. M., et al. (2012). In vitro susceptibilities of non-Enterobacteriaceae isolates from patients with intra-abdominal infections in the Asia-Pacific region from 2003 to 2010: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 40(Suppl.), S11–S17. doi: 10.1016/s0924-8579(12)70004-3
Livermore, D. M., Hope, R., Brick, G., Lillie, M., Reynolds, R., and BSAC Working Parties on Resistance Surveillance. (2008). Non-susceptibility trends among Pseudomonas aeruginosa and other non-fermentative Gram-negative bacteria from bacteraemias in the UK and Ireland, 2001-06. J. Antimicrob. Chemother. 62(Suppl. 2), ii55–ii63. doi: 10.1093/jac/dkn352
Livermore, D. M., Mushtaq, S., Warner, M., and Woodford, N. (2014). Comparative in vitro activity of sulfametrole/trimethoprim and sulfamethoxazole/trimethoprim and other agents against multiresistant Gram-negative bacteria. J. Antimicrob. Chemother. 69, 1050–1056. doi: 10.1093/jac/dkt455
Lodge, J. M., Minchin, S. D., Piddock, L. J., and Busby, S. J. (1990). Cloning, sequencing and analysis of the structural gene and regulatory region of the Pseudomonas aeruginosa chromosomal ampC beta-lactamase. Biochem. J. 272, 627–631. doi: 10.1042/bj2720627
Looney, W. J., Narita, M., and Mühlemann, K. (2009). Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect. Dis. 9, 312–323. doi: 10.1016/S1473-3099(09)70083-0
Lu, P. L., Liu, Y. C., Toh, H. S., Lee, Y. L., Liu, Y. M., Ho, C. M., et al. (2012). Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009-2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 40(Suppl.), S37–S43. doi: 10.1016/s0924-8579(12)70008-0
Macleod, D. L., Barker, L. M., Sutherland, J. L., Moss, S. C., Gurgel, J. L., Kenney, T. F., et al. (2009). Antibacterial activities of a fosfomycin/tobramycin combination: a novel inhaled antibiotic for bronchiectasis. J. Antimicrob. Chemother. 64, 829–836. doi: 10.1093/jac/dkp282
Magret, M., Lisboa, T., Martin-Loeches, I., Máñez, R., Nauwynck, M., Wrigge, H., et al. (2011). Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: a prospective and observational multicenter study. Crit. Care 15, R62. doi: 10.1186/cc10036
Mathai, D., Lewis, M. T., Kugler, K. C., Pfaller, M. A., Jones, R. N., and SENTRY Participants Group (North America). (2001). Antibacterial activity of 41 antimicrobials tested against over 2773 bacterial isolates from hospitalized patients with pneumonia: I–results from the SENTRY Antimicrobial Surveillance Program (North America, 1998). Diagn. Microbiol. Infect. Dis. 39, 105–116. doi: 10.1016/S0732-8893(00)00234-0
Memish, Z. A., Shibl, A. M., Kambal, A. M., Ohaly, Y. A., Ishaq, A., and Livermore, D. M. (2012). Antimicrobial resistance among non-fermenting Gram-negative bacteria in Saudi Arabia. J. Antimicrob. Chemother. 67, 1701–1705. doi: 10.1093/jac/dks091
Meyer, E., Schwab, F., Gastmeier, P., Rueden, H., Daschner, F. D., and Jonas, D. (2006). Stenotrophomonas maltophilia and antibiotic use in German intensive care units: data from Project SARI (Surveillance of Antimicrobial Use and Antimicrobial Resistance in German Intensive Care Units). J. Hosp. Infect. 64, 238–243. doi: 10.1016/j.jhin.2006.07.006
Micozzi, A., Venditti, M., Monaco, M., Friedrich, A., Taglietti, F., Santilli, S., et al. (2000). Bacteremia due to Stenotrophomonas maltophilia in patients with hematologic malignancies. Clin. Infect. Dis. 31, 705–711. doi: 10.1086/314043
Milne, K. E., and Gould, I. M. (2012). Combination antimicrobial susceptibility testing of multidrug-resistant Stenotrophomonas maltophilia from cystic fibrosis patients. Antimicrob. Agents Chemother. 56, 4071–4077. doi: 10.1128/AAC.00072-12
Morrissey, I., Hackel, M., Badal, R., Bouchillon, S., Hawser, S., and Biedenbach, D. (2013). A review of ten years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 6, 1335–1346. doi: 10.3390/ph6111335
Moskowitz, S. M., Garber, E., Chen, Y., Clock, S. A., Tabibi, S., Miller, A. K., et al. (2010). Colistin susceptibility testing: evaluation of reliability for cystic fibrosis isolates of Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 65, 1416–1423. doi: 10.1093/jac/dkq131
Moya, B., Dötsch, A., Juan, C., Blázquez, J., Zamorano, L., Haussler, S., et al. (2009). Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5:e1000353. doi: 10.1371/journal.ppat.1000353
Nicodemo, A. C., Araujo, M. R., Ruiz, A. S., and Gales, A. C. (2004). In vitro susceptibility of Stenotrophomonas maltophilia isolates: comparison of disc diffusion, Etest and agar dilution methods. J. Antimicrob. Chemother. 53, 604–608. doi: 10.1093/jac/dkh128
Nicodemo, A. C., and Paez, J. I. (2007). Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur. J. Clin. Microbiol. Infect. Dis. 26, 229–237. doi: 10.1007/s10096-007-0279-3
Ning, B. T., Zhang, C. M., Liu, T., Ye, S., Yang, Z. H., and Chen, Z. J. (2013). Pathogenic analysis of sputum from ventilator-associated pneumonia in a pediatric intensive care unit. Exp. Ther. Med. 5, 367–371. doi: 10.3892/etm.2012.757
Nord, C. E., Wadström, T., and Wretlind, B. (1974). Synergistic effect of combinations of sulfamethoxazole, trimethoprim, and colistin against Pseudomonas maltophilia and Pseudomonas cepacia. Antimicrob. Agents Chemother. 6, 521–523. doi: 10.1128/AAC.6.4.521
O'Riordan, W., Mehra, P., Manos, P., Kingsley, J., Lawrence, L., and Cammarata, S. (2015). A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int. J. Infect. Dis. 30, 67–73. doi: 10.1016/j.ijid.2014.10.009
Okazaki, A., and Avison, M. B. (2007). Aph(3′)-IIc, an aminoglycoside resistance determinant from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 51, 359–360. doi: 10.1128/AAC.00795-06
Okazaki, A., and Avison, M. B. (2008). Induction of L1 and L2 beta-lactamase production in Stenotrophomonas maltophilia is dependent on an AmpR-type regulator. Antimicrob. Agents Chemother. 52, 1525–1528. doi: 10.1128/AAC.01485-07
Paez, J. I., and Costa, S. F. (2008). Risk factors associated with mortality of infections caused by Stenotrophomonas maltophilia: a systematic review. J. Hosp. Infect. 70, 101–108. doi: 10.1016/j.jhin.2008.05.020
Page, M. G., Dantier, C., Desarbre, E., Gaucher, B., Gebhardt, K., and Schmitt-Hoffmann, A. (2011). In vitro and in vivo properties of BAL30376, a beta-lactam and dual beta-lactamase inhibitor combination with enhanced activity against Gram-negative Bacilli that express multiple beta-lactamases. Antimicrob. Agents Chemother. 55, 1510–1519. doi: 10.1128/AAC.01370-10
Papadakis, K. A., Vartivarian, S. E., Vassilaki, M. E., and Anaissie, E. J. (1995). Stenotrophomonas maltophilia: an unusual cause of biliary sepsis. Clin. Infect. Dis. 21, 1032–1034. doi: 10.1093/clinids/21.4.1032
Perez, P. N., Ramirez, M. A., Fernandez, J. A., and De Guevara, L. L. (2014). A patient presenting with cholangitis due to Stenotrophomonas maltophilia and Pseudomonas aeruginosa successfully treated with intrabiliary colistin. Infect. Dis. Rep. 6:5147. doi: 10.4081/idr.2014.5147
Personne, Y., Curtis, M. A., Wareham, D. W., and Waite, R. D. (2014). Activity of the type I signal peptidase inhibitor MD3 against multidrug-resistant Gram-negative bacteria alone and in combination with colistin. J. Antimicrob. Chemother. 69, 3236–3243. doi: 10.1093/jac/dku309
Pien, C. J., Kuo, H. Y., Chang, S. W., Chen, P. R., Yeh, H. W., Liu, C. C., et al. (2015). Risk factors for levofloxacin resistance in Stenotrophomonas maltophilia from respiratory tract in a regional hospital. J. Microbiol. Immunol. Infect. 48, 291–295. doi: 10.1016/j.jmii.2013.09.005
Pfaller, M. A., Jones, R. N., Doern, G. V., and Kugler, K. (1998). Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997). Antimicrob. Agents Chemother. 42, 1762–1770.
Pompilio, A., Crocetta, V., Confalone, P., Nicoletti, M., Petrucca, A., Guarnieri, S., et al. (2010). Adhesion to and biofilm formation on IB3-1 bronchial cells by Stenotrophomonas maltophilia isolates from cystic fibrosis patients. BMC Microbiol. 10:102. doi: 10.1186/1471-2180-10-102
Poulos, C. D., Matsumura, S. O., Willey, B. M., Low, D. E., and McGeer, A. (1995). In vitro activities of antimicrobial combinations against Stenotrophomonas (Xanthomonas) maltophilia. Antimicrob. Agents Chemother. 39, 2220–2223. doi: 10.1128/AAC.39.10.2220
Putman, M., Van Veen, H. W., and Konings, W. N. (2000). Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64, 672–693. doi: 10.1128/MMBR.64.4.672-693.2000
Ratjen, A., Yau, Y., Wettlaufer, J., Matukas, L., Zlosnik, J. E., Speert, D. P., et al. (2015). In vitro efficacy of high-dose tobramycin against Burkholderia cepacia complex and Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 59, 711–713. doi: 10.1128/AAC.04123-14
Rattanaumpawan, P., Ussavasodhi, P., Kiratisin, P., and Aswapokee, N. (2013). Epidemiology of bacteremia caused by uncommon non-fermentative gram-negative bacteria. BMC Infect. Dis. 13:167. doi: 10.1186/1471-2334-13-167
Renteria, M. I., Biedenbach, D. J., Bouchillon, S. K., Hoban, D. J., Raghubir, N., Sajben, P., et al. (2014). In vitro activity of tigecycline against isolates collected from complicated skin and skin structure infections and intra-abdominal infections in Africa and Middle East countries: TEST 2007-2012. Diagn. Microbiol. Infect. Dis. 79, 54–59. doi: 10.1016/j.diagmicrobio.2014.01.017
Rhee, J. Y., Choi, J. Y., Choi, M. J., Song, J. H., Peck, K. R., and Ko, K. S. (2013). Distinct groups and antimicrobial resistance of clinical Stenotrophomonas maltophilia complex isolates from Korea. J. Med. Microbiol. 62, 748–753. doi: 10.1099/jmm.0.053355-0
Rizek, C., Ferraz, J. R., Van der Heijden, I. M., Giudice, M., Mostachio, A. K., Paez, J., et al. (2015). In vitro activity of potential old and new drugs against multidrug-resistant gram-negatives. J. Infect. Chemother. 21, 114–117. doi: 10.1016/j.jiac.2014.10.009
Rodríguez, C. H., Nastro, M., Calvo, J. L., Fariña, M. E., Dabos, L., and Famiglietti, A. (2014). In vitro activity of colistin against Stenotrophomonas maltophilia. J. Glob. Antimicrob. Resist. 2, 316–317. doi: 10.1016/j.jgar.2014.04.004
Sader, H. S., Farrell, D. J., Flamm, R. K., and Jones, R. N. (2014a). Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int. J. Antimicrob. Agents 43, 328–334. doi: 10.1016/j.ijantimicag.2014.01.007
Sader, H. S., Farrell, D. J., Flamm, R. K., and Jones, R. N. (2014b). Variation in potency and spectrum of tigecycline activity against bacterial strains from U.S. medical centers since its approval for clinical use (2006 to 2012). Antimicrob. Agents Chemother. 58, 2274–2280. doi: 10.1128/AAC.02684-13
Sader, H. S., Flamm, R. K., and Jones, R. N. (2013). Tigecycline activity tested against antimicrobial resistant surveillance subsets of clinical bacteria collected worldwide (2011). Diagn. Microbiol. Infect. Dis. 76, 217–221. doi: 10.1016/j.diagmicrobio.2013.02.009
Sader, H. S., and Jones, R. N. (2005). Antimicrobial susceptibility of uncommonly isolated non-enteric Gram-negative bacilli. Int. J. Antimicrob. Agents 25, 95–109. doi: 10.1016/j.ijantimicag.2004.10.002
Sader, H. S., Jones, R. N., Dowzicky, M. J., and Fritsche, T. R. (2005a). Antimicrobial activity of tigecycline tested against nosocomial bacterial pathogens from patients hospitalized in the intensive care unit. Diagn. Microbiol. Infect. Dis. 52, 203–208. doi: 10.1016/j.diagmicrobio.2005.05.002
Sader, H. S., Jones, R. N., Gales, A. C., Silva, J. B., Pignatari, A. C., and SENTRY Participants Group (Latin America). (2004). SENTRY antimicrobial surveillance program report: Latin American and Brazilian results for 1997 through 2001. Braz. J. Infect. Dis. 8, 25–79. doi: 10.1590/S1413-86702004000100004
Sader, H. S., Jones, R. N., Gales, A. C., Winokur, P., Kugler, K. C., Pfaller, M. A., et al. (1998). Antimicrobial susceptibility patterns for pathogens isolated from patients in Latin American medical centers with a diagnosis of pneumonia: analysis of results from the SENTRY Antimicrobial Surveillance Program (1997). SENTRY Latin America Study Group. Diagn. Microbiol. Infect. Dis. 32, 289–301. doi: 10.1016/S0732-8893(98)00124-2
Sader, H. S., Jones, R. N., Stilwell, M. G., Dowzicky, M. J., and Fritsche, T. R. (2005b). Tigecycline activity tested against 26,474 bloodstream infection isolates: a collection from 6 continents. Diagn. Microbiol. Infect. Dis. 52, 181–186. doi: 10.1016/j.diagmicrobio.2005.05.005
Safdar, A., and Rolston, K. V. (2007). Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clin. Infect. Dis. 45, 1602–1609. doi: 10.1086/522998
Saiman, L., Chen, Y., Gabriel, P. S., and Knirsch, C. (2002). Synergistic activities of macrolide antibiotics against Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. Antimicrob. Agents Chemother. 46, 1105–1107. doi: 10.1128/AAC.46.4.1105-1107.2002
Samonis, G., Karageorgopoulos, D. E., Maraki, S., Levis, P., Dimopoulou, D., Spernovasilis, N. A., et al. (2012). Stenotrophomonas maltophilia infections in a general hospital: patient characteristics, antimicrobial susceptibility, and treatment outcome. PLoS ONE 7:e37375. doi: 10.1371/journal.pone.0037375
San Gabriel, P., Zhou, J., Tabibi, S., Chen, Y., Trauzzi, M., and Saiman, L. (2004). Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 48, 168–171. doi: 10.1128/AAC.48.1.168-171.2004
Sanchez, M. B., Hernandez, A., and Martinez, J. L. (2009). Stenotrophomonas maltophilia drug resistance. Future Microbiol. 4, 655–660. doi: 10.2217/fmb.09.45
Sánchez, M. B., Hernández, A., Rodríguez-Martínez, J. M., Martínez-Martínez, L., and Martínez, J. L. (2008). Predictive analysis of transmissible quinolone resistance indicates Stenotrophomonas maltophilia as a potential source of a novel family of Qnr determinants. BMC Microbiol. 8:148. doi: 10.1186/1471-2180-8-148
Sanchez, M. B., and Martinez, J. L. (2010). SmQnr contributes to intrinsic resistance to quinolones in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 54, 580–581. doi: 10.1128/AAC.00496-09
Sánchez, M. B., and Martínez, L. (2015). The efflux pump SmeDEF contributes to trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 59, 4347–4348. doi: 10.1128/AAC.00714-15
Shimizu, K., Kikuchi, K., Sasaki, T., Takahashi, N., Ohtsuka, M., Ono, Y., et al. (2008). Smqnr, a new chromosome-carried quinolone resistance gene in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 52, 3823–3825. doi: 10.1128/AAC.00026-08
Sood, S., Vaid, V. K., and Bhartiya, H. (2013). Meningitis due to Stenotrophomonas maltophilia after a Neurosurgical Procedure. J. Clin. Diagn. Res. 7, 1696–1697. doi: 10.7860/jcdr/2013/5614.3248
Stein, G. E., and Babinchak, T. (2013). Tigecycline: an update. Diagn. Microbiol. Infect. Dis. 75, 331–336. doi: 10.1016/j.diagmicrobio.2012.12.004
Streit, J. M., Jones, R. N., Sader, H. S., and Fritsche, T. R. (2004). Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001). Int. J. Antimicrob. Agents 24, 111–118. doi: 10.1016/j.ijantimicag.2003.12.019
Tada, T., Miyoshi-Akiyama, T., Dahal, R. K., Mishra, S. K., Shimada, K., Ohara, H., et al. (2014). Identification of a novel 6′-N-aminoglycoside acetyltransferase, AAC(6′)-Iak, from a multidrug-resistant clinical isolate of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 58, 6324–6327. doi: 10.1128/AAC.03354-14
Talfan, A., Mounsey, O., Charman, M., Townsend, E., and Avison, M. B. (2013). Involvement of mutation in ampD I, mrcA, and at least one additional gene in beta-lactamase hyperproduction in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 57, 5486–5491. doi: 10.1128/AAC.01446-13
Tan, R., Liu, J., Li, M., Huang, J., Sun, J., and Qu, H. (2014). Epidemiology and antimicrobial resistance among commonly encountered bacteria associated with infections and colonization in intensive care units in a university affiliated hospital in Shanghai. J. Microbiol. Immunol. Infect. 47, 87–94. doi: 10.1016/j.jmii.2012.11.006
Tekçe, Y. T., Erbay, A., Cabadak, H., and Sen, S. (2012). Tigecycline as a therapeutic option in Stenotrophomonas maltophilia infections. J. Chemother. 24, 150–154. doi: 10.1179/1120009X12Z.00000000022
Toleman, M. A., Bennett, P. M., Bennett, D. M., Jones, R. N., and Walsh, T. R. (2007). Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg. Infect. Dis. 13, 559–565. doi: 10.3201/eid1304.061378
Valdezate, S., Vindel, A., Loza, E., Baquero, F., and Cantón, R. (2001). Antimicrobial susceptibilities of unique Stenotrophomonas maltophilia clinical strains. Antimicrob. Agents Chemother. 45, 1581–1584. doi: 10.1128/AAC.45.5.1581-1584.2001
Valdezate, S., Vindel, A., Saéz-Nieto, J. A., Baquero, F., and Cantón, R. (2005). Preservation of topoisomerase genetic sequences during in vivo and in vitro development of high-level resistance to ciprofloxacin in isogenic Stenotrophomonas maltophilia strains. J. Antimicrob. Chemother. 56, 220–223. doi: 10.1093/jac/dki182
Valenza, G., Tappe, D., Turnwald, D., Frosch, M., König, C., Hebestreit, H., et al. (2008). Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 7, 123–127. doi: 10.1016/j.jcf.2007.06.006
Vartivarian, S., Anaissie, E., Bodey, G., Sprigg, H., and Rolston, K. (1994). A changing pattern of susceptibility of Xanthomonas maltophilia to antimicrobial agents: implications for therapy. Antimicrob. Agents Chemother. 38, 624–627. doi: 10.1128/AAC.38.3.624
Vartivarian, S. E., Anaissie, E. J., Kiwan, E. N., and Papadakis, K. A. (2000). The clinical spectrum of Stenotrophomonas (Xanthomonas) maltophilia respiratory infection. Semin. Respir. Crit. Care Med. 21, 349–355. doi: 10.1055/s-2000-9859
Vartivarian, S. E., Papadakis, K. A., and Anaissie, E. J. (1996). Stenotrophomonas (Xanthomonas) maltophilia urinary tract infection. A disease that is usually severe and complicated. Arch. Intern. Med. 156, 433–435. doi: 10.1001/archinte.1996.00440040111012
Victor, M. A., Arpi, M., Bruun, B., Jønsson, V., and Hansen, M. M. (1994). Xanthomonas maltophilia bacteremia in immunocompromised hematological patients. Scand. J. Infect. Dis. 26, 163–170. doi: 10.3109/00365549409011780
Visalli, M. A., Jacobs, M. R., and Appelbaum, P. C. (1998). Activities of three quinolones, alone and in combination with extended-spectrum cephalosporins or gentamicin, against Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 42, 2002–2005.
Walkty, A., Adam, H., Baxter, M., Denisuik, A., Lagacé-Wiens, P., Karlowsky, J. A., et al. (2014). In vitro activity of plazomicin against 5,015 gram-negative and gram-positive clinical isolates obtained from patients in canadian hospitals as part of the CANWARD study, 2011-2012. Antimicrob. Agents Chemother. 58, 2554–2563. doi: 10.1128/AAC.02744-13
Wang, C. H., Lin, J. C., Lin, H. A., Chang, F. Y., Wang, N. C., Chiu, S. K., et al. (2014a). Comparisons between patients with trimethoprim-sulfamethoxazole-susceptible and trimethoprim-sulfamethoxazole-resistant Stenotrophomonas maltophilia monomicrobial bacteremia: A 10-year retrospectivestudy. J. Microbiol. Immunol. Infect. doi: 10.1016/j.jmii.2014.06.005. [Epub ahead of print].
Wang, Y. L., Scipione, M. R., Dubrovskaya, Y., and Papadopoulos, J. (2014b). Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob. Agents Chemother. 58, 176–182. doi: 10.1128/AAC.01324-13
Waters, V. (2012). New treatments for emerging cystic fibrosis pathogens other than Pseudomonas. Curr. Pharm. Des. 18, 696–725. doi: 10.2174/138161212799315939
Wood, G. C., Underwood, E. L., Croce, M. A., Swanson, J. M., and Fabian, T. C. (2010). Treatment of recurrent Stenotrophomonas maltophilia ventilator-associated pneumonia with doxycycline and aerosolized colistin. Ann. Pharmacother. 44, 1665–1668. doi: 10.1345/aph.1P217
Wu, H., Wang, J. T., Shiau, Y. R., Wang, H. Y., Lauderdale, T. L., Chang, S. C., et al. (2012). A multicenter surveillance of antimicrobial resistance on Stenotrophomonas maltophilia in Taiwan. J. Microbiol. Immunol. Infect. 45, 120–126. doi: 10.1016/j.jmii.2011.09.028
Wu, K., Yau, Y. C., Matukas, L., and Waters, V. (2013). Biofilm compared to conventional antimicrobial susceptibility of Stenotrophomonas maltophilia Isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 57, 1546–1548. doi: 10.1128/AAC.02215-12
Wu, Y., and Shao, Z. (2014). High-dosage tigecycline for Stenotrophomonas maltophilia bacteremia. Chin. Med. J. (Engl.) 127:3199. doi: 10.3760/cma.j.issn.0366-6999.20140364
Yang, Q., Wang, H., Chen, M., Ni, Y., Yu, Y., Hu, B., et al. (2010). Surveillance of antimicrobial susceptibility of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in China: the 2002-2009 Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 36, 507–512. doi: 10.1016/j.ijantimicag.2010.09.001
Yang, T. C., Huang, Y. W., Hu, R. M., Huang, S. C., and Lin, Y. T. (2009). AmpDI is involved in expression of the chromosomal L1 and L2 beta-lactamases of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 53, 2902–2907. doi: 10.1128/AAC.01513-08
Yu, V. L., Felegie, T. P., Yee, R. B., Pasculle, A. W., and Taylor, F. H. (1980). Synergistic interaction in vitro with use of three antibiotics simultaneously against Pseudomonas maltophilia. J. Infect. Dis. 142, 602–607. doi: 10.1093/infdis/142.4.602
Zhanel, G. G., Adam, H. J., Baxter, M. R., Fuller, J., Nichol, K. A., Denisuik, A. J., et al. (2013). Antimicrobial susceptibility of 22746 pathogens from Canadian hospitals: results of the CANWARD 2007-11 study. J. Antimicrob. Chemother. 68(Suppl. 1), i7–i22. doi: 10.1093/jac/dkt022
Zhanel, G. G., Adam, H. J., Low, D. E., Blondeau, J., Decorby, M., Karlowsky, J. A., et al. (2011). Antimicrobial susceptibility of 15,644 pathogens from Canadian hospitals: results of the CANWARD 2007-2009 study. Diagn. Microbiol. Infect. Dis. 69, 291–306. doi: 10.1016/j.diagmicrobio.2010.10.025
Zhanel, G. G., Decorby, M., Adam, H., Mulvey, M. R., Mccracken, M., Lagacé-Wiens, P., et al. (2010). Prevalence of antimicrobial-resistant pathogens in Canadian hospitals: results of the Canadian Ward Surveillance Study (CANWARD 2008). Antimicrob. Agents Chemother. 54, 4684–4693. doi: 10.1128/AAC.00469-10
Zhanel, G. G., Decorby, M., Nichol, K. A., Wierzbowski, A., Baudry, P. J., Karlowsky, J. A., et al. (2008). Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagn. Microbiol. Infect. Dis. 62, 67–80. doi: 10.1016/j.diagmicrobio.2008.04.012
Keywords: Stenotrophomonas maltophilia, prevalence, susceptibility, surveillance, treatment
Citation: Chang Y-T, Lin C-Y, Chen Y-H and Hsueh P-R (2015) Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 6:893. doi: 10.3389/fmicb.2015.00893
Received: 03 March 2015; Accepted: 17 August 2015;
Published: 02 September 2015.
Edited by:
Giovanni Di Bonaventura, Università degli Studi “G. d'Annunzio” Chieti e Pescara, ItalyReviewed by:
Deborah R. Yoder-Himes, University of Louisville, USACopyright © 2015 Chang, Lin, Chen and Hsueh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yen-Hsu Chen, Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100, Tzyou 1st Road, Kaohsiung 807, Taiwan,aW5mY2hlbkBnbWFpbC5jb20=;
Po-Ren Hsueh, Departments of Laboratory Medicine and Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, No. 7, Chung-Shan South Road, Taipei 100, Taiwan,aHNwb3JlbkBudHUuZWR1LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.