
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 13 March 2015
Sec. Fungi and Their Interactions
Volume 6 - 2015 | https://doi.org/10.3389/fmicb.2015.00202
 Ludmila M. Baltazar1,2
Ludmila M. Baltazar1,2 Anjana Ray1,2
Anjana Ray1,2 Daniel A. Santos3
Daniel A. Santos3 Patrícia S. Cisalpino3
Patrícia S. Cisalpino3 Adam J. Friedman4,5
Adam J. Friedman4,5 Joshua D. Nosanchuk1,2*
Joshua D. Nosanchuk1,2*
Skin mycoses are caused mainly by dermatophytes, which are fungal species that primarily infect areas rich in keratin such as hair, nails, and skin. Significantly, there are increasing rates of antimicrobial resistance among dermatophytes, especially for Trichophyton rubrum, the most frequent etiologic agent worldwide. Hence, investigators have been developing new therapeutic approaches, including photodynamic treatment. Photodynamic therapy (PDT) utilizes a photosensitive substance activated by a light source of a specific wavelength. The photoactivation induces cascades of photochemicals and photobiological events that cause irreversible changes in the exposed cells. Although photodynamic approaches are well established experimentally for the treatment of certain cutaneous infections, there is limited information about its mechanism of action for specific pathogens as well as the risks to healthy tissues. In this work, we have conducted a comprehensive review of the current knowledge of PDT as it specifically applies to fungal diseases. The data to date suggests that photodynamic treatment approaches hold great promise for combating certain fungal pathogens, particularly dermatophytes.
Fungi are eukaryotic organisms and their similarities to mammalian cells have led to significant difficulties in the development of new antifungal drugs. Fungal infections are an important health problem worldwide, affecting both immunocompetent and immunocompromised individuals. Acquisition of fungal pathogens results in varied outcomes ranging from asymptomatic infection to rapidly lethal systemic disease (Cowen, 2008).
Though cutaneous mycoses are rarely life-threatening; they result in significant morbidity, causing discomfort, disfigurement, social isolation, and may predispose to bacterial diseases (Brown et al., 2012). These mycoses are frequently recurrent and chronic. Moreover, they are extremely common as it is estimated that 10–20% of the worldwide population may be affected (Drake et al., 1996; El-Gohary et al., 2014). The main fungal skin diseases are caused by Malassezia sp. and the dermatophytes (White et al., 2014).
Malassezia sp. are frequent commensal inhabitants of the skin and scalp that can cause a range of diseases, including pityriasis versicolor, dandruff, and seborrheic dermatitis (Gaitanis et al., 2013). These agents are associated with ∼50% of the dermal disorders in healthy humans and 70–75% of immunosuppressed individuals (White et al., 2014). The dermatophytes are a group of filamentous fungi that are the etiologic agent of dermatophytosis, diseases affecting skin, hair, and nails. The dermatophytes produce enzymes that digest keratin, which the fungi use as a food source, but also facilities their capacity to infect tissues containing keratin (Weitzman and Summerbell, 1995). Immunocompromised individuals are at increased risk for dermatophytoses, including progression to disseminated disease (Nenoff et al., 2014b). Although clinical resistance to current antifungal drugs has been well documented, clinical failures are most often associated with discontinuation of the treatment by the patient (Mukherjee et al., 2003). Although some diseases caused by Malassezia sp. and the dermatophytes can be eradicated with several days of antifungal therapy, months of therapy may be required for combating infections of the nails or diseases in the setting of immune deficiency. Typically administered antifungal drugs include azoles, allylamines, ciclopirox, and amorolfine (Gupta and Cooper, 2008).
Photodynamic therapy (PDT) is an alternative approach to these antifungal medications that primarily target ergosterol production. Antimicrobial photodynamic inhibition (aPI) or therapy (aPDT) combines a pharmacologically inert chromophore, termed a photosensitizer (PS), with a light corresponding to the chromophore’s specific absorption wavelength (Dai et al., 2012). This exposure of the chromophore to the specific light wavelength induces the production of harmful radicals, such as reactive species of oxygen (ROS) and nitrogen (RNS), which are capable of killing cells (Hamblin and Hasan, 2004). The ability of aPI to kill microbes has been described by several investigators, and the data suggests that aPI is potentially effective against bacterial, viral, fungal, and protozoal infections (reviewed in Hamblin and Hasan, 2004; Krausz and Friedman, 2014). Significantly, investigators have shown that aPDT effectively inactivates Trichophyton rubrum, the most common causative agent of dermatophytosis (Smijs and Pavel, 2011; Nenoff et al., 2014a). In this review, we provide a detailed overview of the promise of aPDT in the context of the fungal infections, describing in vitro, preclinical, and human studies.
The use of light combined with a photosensitive substance is actually an ancient approach for the treatment of skin diseases. There are documents from ∼1200–2000 BC showing that Egyptian and Chinese physicians as well as Indian Hindu Ayurvedic practitioners used combinations of plant extracts with exposure to sunlight to treat skin disorders (Pathak and Fitzpatrick, 1992; Craig et al., 2014). For example, the Egyptians used the application of an extract of Ammi majus, a furanocoumarin-containing plant, associated with sun exposure to topically treat vitiligo. In Ayurvedic traditional medicine, an extract of Psoralea corylifolia, which is a furanocoumarin, was similarly used for vitiligo (Pathak and Fitzpatrick, 1992).
However, the term PDT was coined in 1900 by Tappeiner and his co-workers in Germany (Tappeiner, 1900). The first detailed report of the observation that the combination of light and dye could be harmful to a cell was published by Raab (1900), a student of Tappeiner. Rabb observed that the protozoon Paramecium caudatum died after light exposure in the presence of an acridine dye and the amount of light exposure correlated with the killing efficiency of the system. Following this finding, in 1903, Tappeiner and the dermatologist Jesionek translated their findings from the bench to the bedside in a report that detailed how the topical application of eosin associated with exposure to white light effectively treated a skin tumor (Jesionek and Tappeiner, 1903). Significantly, Tappeiner and his colleague Jodlbauer also noted that the phototoxic effect did not occur in the absence of oxygen and they introduced the term “photodynamic action” in 1907 to describe this reaction (Tappeiner and Jodlbauer, 1904, 1907).
The first PS broadly used in the medicine was the porphyrin hematoporphyrin (Hp), obtained from dried blood after treatment with concentrated sulfuric acid. Hp was first tested in vitro by Hausman (1911) in Austria, where he demonstrated that the activated compound was effective against paramecia and erythrocytes (Scherer, 1841). Hausman (1911) described the phototoxic effect of Hp on murine skin after systemic application of Hp followed by exposing the mice to light (Hausman, 1911). Meyer-Betz (1913) pioneered the study of Hp as PS in humans when he self-injected 200 mg of Hp. After exposure to sunlight, Meyer–Betz suffered a painful phototoxic reaction that lasted for over 2 months. Policard (1924) described the affinity of endogenous porphyrins to tumors by using a Wood lamp to detect a red fluorescence in rat sarcoma after Hp application. Schwartz et al. (1955) in the USA, demonstrated that the phototoxic effect of Hp could be reduced by treatment of Hp with acetic acid and sulfuric acid, obtaining a mixture of porphyrin, a hematoporphyrin derivate (HpD). This improved photosensitizing compound had high affinity for tumors and the ability to detect tumors using HpD was demonstrated by Lipson and Baldes (1960). The first systematic trial of PDT was reported by Dougherty et al. (1978), in which 113 cutaneous and subcutaneous tumors were subjected to HpD and red light resulting in 111 partial or total responses to therapy. The first PS to gain federal approval for clinical use was Photofrin® in Canada in 1993, and other countries subsequently followed, including the U.S. Food and Drug Administration (FDA) in 1995 (Usuda et al., 2006). With the broader entry of PDT into clinical practice as a chemotherapeutic modality, investigators have increasingly explored PDT as an alternative approach to combat infectious diseases.
Photosensitizers are dyes with the capacity of absorb energy from a light source and transfer this energy to another molecule (Plaetzer et al., 2009). An effective PS is typically characterized by water solubility, minimal dark toxicity, a low mutagenic potential, and highly chemically stable. The PS should have the ability to accumulate preferentially in the specific tissue/cell target and be rapidly eliminated after administration to avoid prolonged photosensitization (Nyman and Hynninen, 2004; Plaetzer et al., 2009). In addition, the waveband of absorption of the PS should be between 600 and 800 nm in order to avoid skin phototoxicity. This therapeutic window minimizes (1) absorption during exposure to typical daylight (wavelength 400–600 nm) and (2) its absorption by water molecules, which increases at wavelengths above 800 nm (Plaetzer et al., 2009; Sekkat et al., 2012). More recently, researchers have designed modern carriers such as liposomes, nanoparticles, and microspheres to reduce chromophore self-aggregation in fluid mediums and increase the selectivity of the PS (Nyman and Hynninen, 2004).
The major PSs used in modern clinical trials are the phenothiazine salts toluidine blue O (TBO) and methylene blue (MB), with wavelengths of absorption of 600–660 nm (Calzavara-Pinton et al., 2012). Both are clinically approved for human use and, notably, they can effectively act on the fungal membrane, causing structural damage (Plaetzer et al., 2009; Calzavara-Pinton et al., 2012; Dai et al., 2012). Other substances, such as porphyrins, phthalocyanines, 5-aminolevulinic acid (ALA) and curcumin, have also been used as PSs. Porphyrin dyes (absorption at 400–650 nm range), can cause alterations at cell membranes, allowing the penetration of the PS into the cell with consequent damage to intracellular targets (Cormick et al., 2009; Calzavara-Pinton et al., 2012). Phthalocyanines (absorption at 630–720 nm range) are similar to porphyrins compounds (Calzavara-Pinton et al., 2012; Sekkat et al., 2012); however, they are strongly hydrophobic, a characteristic that is usually balanced by modifications in its chemical structure to improve water solubility (Mantareva et al., 2011; Sekkat et al., 2012). ALA is not intrinsically photodynamically active, but irradiation of cells containing ALA produces a range of endogenous PS that generate reactive oxygen species (ROS), which damage mitochondria and plasma membranes (Harris and Pierpoint, 2012). Curcumin (absorption at 408–434 nm range) is a yellow dye (also known as the spice turmeric) isolated from Curcuma longa that is a well established PS (Dovigo et al., 2013) and PDT with curcumin generates high levels of ROS that cause cell death by apoptosis (Sharma et al., 2010).
Currently, both coherent (lasers) and non-coherent (diode emission of light – LED and lamps) light sources are used for PDT (Nyman and Hynninen, 2004; Calin and Parasca, 2009). Lasers are able to deliver light with high degrees of monochromaticity that can be focused into an optic fiber. However, the high cost and the difficulties to transport are some of the drawbacks for the use of lasers in PTD. LEDs are less expensive, easily transportable and, with the discovery of PSs with longer wavelengths, are increasingly being used in experimental and clinical applications of PDT (Hamblin et al., 1996; Nyman and Hynninen, 2004). For white or fluorescent lamps, it is very important to minimize ultra-violet emissions to avoid mutagenesis, as well as infrared, to minimize the risk of heating host tissues (Donnelly et al., 2008). In order to reduce damage to normal tissues, the light dose should not be higher than 200 mW/cm2 (Nyman and Hynninen, 2004; Donnelly et al., 2008). In addition, the light source should be chosen according to the targeted tissue, because the dose is dependent on the thickness of the tissue. For example, red light penetrates ∼3.0 nm whereas blue light penetrates ∼1.5 nm (Donnelly et al., 2008; Garland et al., 2009).
Photodynamic therapy typically induces the production of ROS and RNS (Hamblin and Hasan, 2004; Dai et al., 2012). The basic protocol of treatment involves PS administration followed by a wait time of varying duration to allow for the accumulation of the PS in the cells/tissue, after which the target tissue is irradiated with light source. The ground state of a PS is the singlet state (S0). Activation by irradiation results in the transit of electrons to a different orbital, exciting the PS to the form of an unstable molecule with a short half-life (first excited singlet-state, S1). In order to return to its stable ground state, the PS emits fluorescence or phosphorescence (by intersystem crossing; Nyman and Hynninen, 2004; Garland et al., 2009). Fluorescence emission does not alter the electron spin, phosphorescence changes in the spin rotation from an excited singlet-state to an excited triplet state, which has a long half-life (Hamblin and Hasan, 2004; Garland et al., 2009). The excited triplet state is the main mediator of the photodynamic reactions. The photophysical process is illustrated in Figure 1, using the energy levels, or Jablonski, diagram.
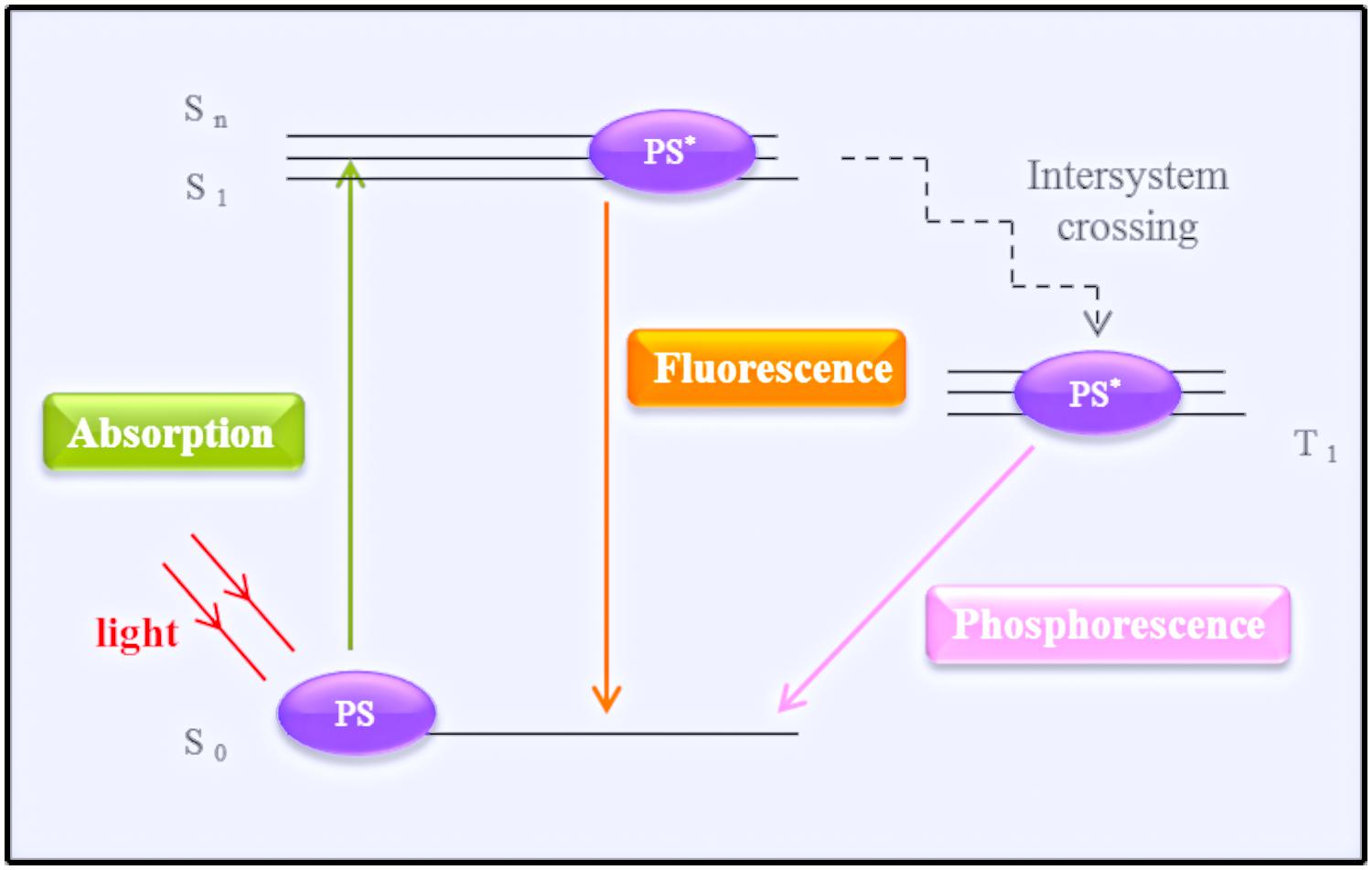
FIGURE 1. Simplified schematic representation of a Jablonski diagram. The photosensitizer (PS) at the ground state (S0) transitions after irradiation by a light source to its first single activate state (S1). To return to its ground state, the PS emits energy by fluorescence or phosphorescence (after reaching the triplet state – T1).
Two types of photodynamic reactions can occur, type 1 and type 2. In the type 1 reaction, the PS triplet directly transfers an electron or hydrogen to a biomolecule, producing reactive intermediates such as anion superoxide (O2-), hydrogen peroxide (H2O2), hydroxyl radials (OH-), nitric oxide (NO⋅), and peroxide nitrite (ONOO⋅; Hamblin and Hasan, 2004; Baltazar Lde et al., 2013; Figure 2). In the type 2 reaction, the PS transfers energy to molecular oxygen yielding the production of singlet oxygen (1O2), which is an extremely powerful oxidant with a very short life time, but it can react with several biomolecules, such as lipids and proteins (Hamblin and Hasan, 2004).
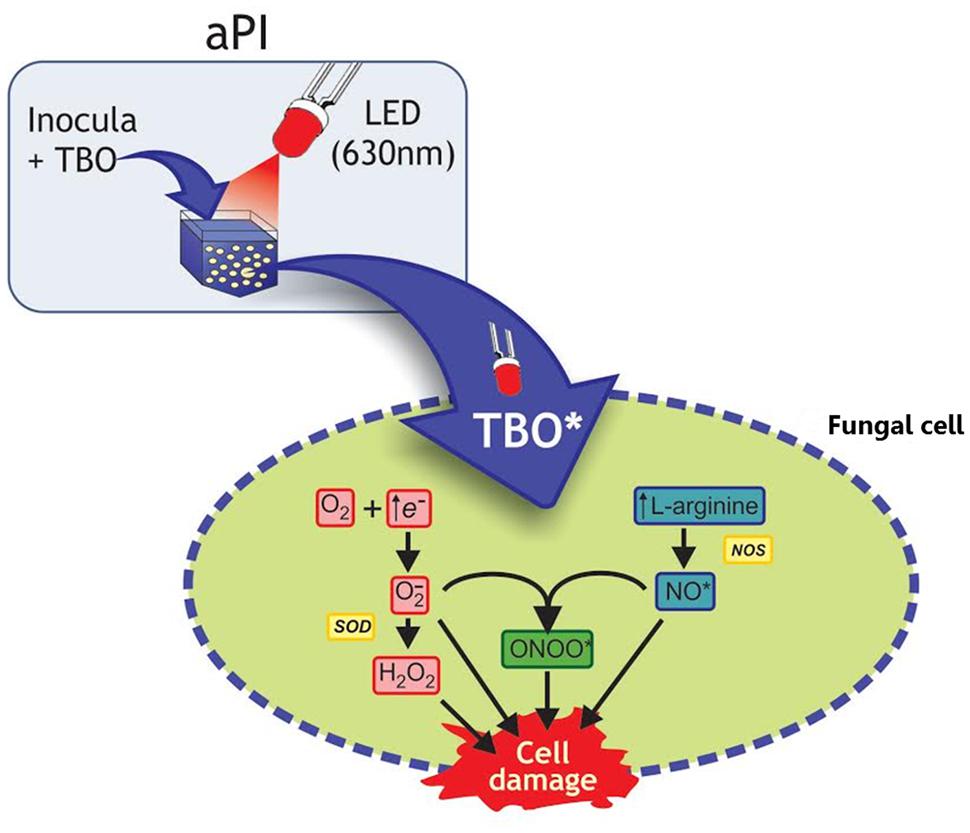
FIGURE 2. Schematic illustration of the aPI (TBO + LED 630 nm) effects on fungal cell. In this illustration with Trichophyton rubrum conidia, toluidine blue O (TBO) and LED light were used as example according to present the mechanism described by Baltazar Lde et al. (2013). Activation of TBO (both intra and extracellular) increases l-arginine levels, the substrate of oxide nitric synthase (NOS), which results in increasing NO• levels. The increased availability of free electrons increases H2O2 production. Moreover, NO• can react with O2-, generating ONOO•. In eukaryotic cells generation of NO• occurs by oxidation of l-arginine. All these toxic radicals can react with the cell membrane and cytosolic components, leading to cell damage.
An important aspect of the generation of oxidative and nitrosative stresses by this process for antimicrobial applications is that the diverse cellular targets of these radicals reduces the probability of the selection of resistant strains, which is the main problem faced by the current antifungal therapies (Calzavara-Pinton et al., 2005, 2012). The radicals generated by PDT have extremely short half-lives and they react only in their sites of formation, which reduces their toxicity to adjacent normal tissues (Nyman and Hynninen, 2004).
The generated radicals alter the structure of the fungal cell wall and membrane, which provides the further translocation of the PS into the cell. Subsequently, these ROS and RNS produced outside and within the fungal cell cause an imbalance in cellular homeostasis, including damaging cytoplasmic organelles and nucleic acids, resulting in cell death by apoptosis, necrosis, or autophagy (Mroz et al., 2011). Interestingly, treatment using high doses of light and high concentrations of PS leads to cell death by necrosis, while treatment with low doses tends to induce cell death by apoptosis (Lennon et al., 1991; Noodt et al., 1996; Mroz et al., 2011). Depending on the amount of ROS produced and degree damage, death by autophagy can also occur (Mroz et al., 2011).
The in vitro effect of aPI against fungal cells has been demonstrated using different treatment regimes (Table 1). Takahashi et al. (2014) recently reported that Malassezia furfur is effectively killed using TONS504, a cationic PS, and 670 nm LED. The cidal effect on M. furfur is dose dependent and a reduction >80% was achieved using 100 J/cm2 and 1 μg/mL of light and PS, respectively. Smijs and Schuitmaker (2003) reported that T. rubrum cells are killed (based on a cut off of two colonies) after treatment with porphyrins deuteroporphyrin monomethylester (DP mme) and 5,10,15-tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride (Sylsens B) in concentrations of 3 μg/mL or higher in combination with white light (1080 kJ/cm2). The study also highlights the possibility of using Sylsens B as a PS to treat tinea infections using red light to more deeply penetrate the skin. Kamp et al. (2005) showed that aPI using ALA (10 mmol l-1) as PS and quartz-halogen lamp (dose of 10 J) reduces T. rubrum growth by about 50% compared to untreated control conditions. Treating T. rubrum cells using a phenothiazine PS, Baltazar Lde et al. (2013), determined that aPI with TBO at a concentration of 10 μg/mL and an LED dose 48 J/cm2 is cidal to this microbe. Notably, this work also provides a description of the mechanism of action of aPI, which involves the production of ROS, NO⋅, and ONOO⋅. In addition, Baltazar et al. (2015) recently showed that curcumin (curc) and curcumin-nanoparticle (curc-np) aPI, at optimal conditions of 10 μg/mL of PS with 10 J/cm2 of blue light (417 ± 5 nm), completely inhibited T. rubrum growth via induction of ROS and RNS, which was associated with fungal death by apoptosis. Delivery of curc by nanoparticle enhanced apoptosis due to increased NO⋅ production. Romagnoli et al. (1998) reported that the combination of the thiophene 5-(4-OH-1-butinyl)-2,2′-bithienyl (BBTOH) with concentration of 50 μg/mL with UVA light (320–400 nm) over 90 min results in a >50% reduction in the growth of several dermatophytes, including T. rubrum, T. mentagrophytes, T. tonsurans, Microsporum cookei, M. gypseum, and Epidermophyton floccosum, with E. floccosum having the greatest susceptibility to this regimen.
In addition to the dermatophytes, aPI is effective against several yeast species. Candida albicans is a common microorganism used as model to study aPI. Munin et al. (2007) reported that aPI using MB (concentrations of 0.027–0.27 mM) and laser (683 nm, 28 J/cm2) reduced the growth of C. albicans in 40% or more, depending on the PS concentration. In addition, germ tube formation (the transition from a yeast cell to the hyphal form) was reduced by more than 75% using MB in the concentrations 0.013 and 0.134 mM with the same light dose. This work was the first to show the ability of aPI to inhibit the transition from yeast to hyphae cells, a step essential to the virulence of this species, suggesting that aPI could decrease the ability of C. albicans cells to cause disease. Similarly, Giroldo et al. (2009) found that MB (0.05 mg/mL) and laser (684 nm, dose of 28 J/cm2) reduced the viability of C. albicans by 50%. The results were associated with the permeabilization of the cells by MB, which damaged the plasma membrane. In addition, Carvalho et al. (2009) reported that aPI using MB (0.05 mg/mL) and TBO (0.1 mg/mL) and two different LED lights (dose of 28 J/cm2) with wavelengths of 684 and 660 nm, respectively, effectively decreased fungal viability by 80–90%. Notably, the phototoxic effect of MB was calcium dependent, a fact not observed with TBO, suggesting that they have different mechanisms of action against C. albicans. For MB, toxicity was related to alterations in plasma membrane calcium channels and the generation of ROS (Carvalho et al., 2009). Using TBO (25 μM) and LED (dose of 180 J/cm2), Soares et al. (2009) found that aPI reduced cell growth, reducing the median to Log10 3.41 and adhesion ∼55% of different clinical isolates of Candida (C. albicans, C. tropicalis, and C. parapsilosis) to buccal epithelial cells. The study also reported that isolates that were resistant to fluconazole were susceptible to aPI.
Antimicrobial photodynamic inhibition efficacy may be impacted by cellular resistance strategies. Prates et al. (2011) reported that a C. albicans mutant overexpressing an ATP-binding cassette (ABC), a multidrug efflux system (MES), were not significantly damaged by MB-aPI due to the reduced accumulation of the PS in the cytoplasm. However, they showed that the combination of aPI with verapamil (an ABC inhibitor) increased MB uptake and enhanced the killing of C. albicans. Using curcumin (concentration of 20 μM) as PS, Dovigo et al. (2011) showed complete inactivation of planktonic C. albicans after irradiation by blue LED (440–460 nm) of 5.28 J/cm2. The study suggested that curcumin could either bind to or be taken up by the planktonic yeast cells. However, the efficacy of this approach was lower in the setting of biofilm growth as the same light dose. Nevertheless, higher concentration of curcumin (40 μM) with different pre-incubation times (of 5 or 20 min) reduced viability to 68 and 87%, respectively. Additionally, the conditions used for planktonic C. albicans cells were also toxic to macrophages, which limits the systemic clinical application of this approach.
Antimicrobial photodynamic inhibition is also effective against Cryptococcus sp. Fuchs et al. (2007) showed that Cryptococcus neoformans was susceptible (killing at a Log10 of 2) to a polycationic conjugate of polyethyleneimine and the PS chlorin (e6; concentration of 10 μM) and LED 665 nm. The importance of cell wall integrity in the outcome of aPI was demonstrated in this work using a C. neoformans mutant rom2 (with alterations in cell wall integrity) in which they found that the rom2 mutant accumulated higher amounts of the PS inside the cell cytoplasm compared to wild-type. Using TBO (25 μM) and LED (dose of 54 J/cm2), Soares et al. (2011) reported the efficacy of aPI in a set of C. gattii isolates with different susceptibility profiles to antifungal drugs, suggesting that aPI could be an alternative tool to inhibit C. gattii growth. The pattern of reduction was variable among the strains which showed reduction of viability in the range of 1.78 Log10 to 6.45 Log10. The study also reported that aPI induced massive production of ROS/ONOO⋅, which was correlated to its killing effect; however, higher catalase and peroxidase activities were related with lower susceptibility to aPI.
Supporting the role of cell wall integrity in modifying the efficacy of aPI, Rodrigues et al. (2012) found that melanized C. neoformans cells were killed (up to 6 Logs) by aPI with 4.5 μM of CIAIPc in nanoemulsion (CIAIPc/NE) and light 675 nm (dose of 10 J/cm2). The study also showed that using lower CIAIPc/NE concentration (0.045 μM) and lower light dose (5 J/cm2) melanized cells had slightly reduced susceptibility compared non-melanized cells. Similar results were found by Prates et al. (2013) using five different PSs [MB, rose Bengal, selenium derivative of a Nile blue (EtNBSe), tris-cationic fullerene (BB6), and conjugate between poly-l-lysine and chlorin (e6)] and an appropriate light source. The presence of cell wall, laccase (the enzyme responsible for melanization of C. neoformans) and melanin protected the cells from the harmful effects related with aPI, but cidality could nevertheless be achieved with certain combinations of PS and light dose.
Similar to the work with dermatophytes, aPI has been successfully employed against agents of subcutaneous mycosis such as Sporothrix schenckii, Fonsecaea pedrosoi, and Cladosporium carrionii. Gilaberte et al. (2014) described the success of aPI against S. schenckii, obtaining a 6 Log10 fungicidal effect using different phenothiazinium PSs [MB, new methylene blue (NMB), or 1,9-dimethylmethylene blue (DMMB)] and LED (639.8 ± 10 nm) light dose of 37 J/cm2. Lyon et al. (2013) reported that MB (32 μg/mL) combined with LED (200 mW/cm2) was effective in killing F. pedrosoi and C. carrionii. These two pathogens are etiological agents of chromoblastomycosis, which is a disease that is severely resistant to standard antifungal treatment. Hence, these results indicate that aPI could be a promising approach to chromoblastomycosis. Hu et al. (2015) reported that ALA-PDT and LED (635 nm, 10 J) also reduced the viability of F. monophora.
The in vitro efficacy of aPI against different fungal pathogens has been confirmed in vivo using various animal models (Table 2). In a murine cutaneous C. albicans infection model, Dai et al. (2011) evaluated aPDT using NMB and red light (at 635 ± 15 nm or 660 ± 15 nm delivered at 78 J/cm2 for “prophylaxis” at 30 min or 120 J/cm2 at 24 h for treatment post-infection). A luciferase-expressing strain of C. albicans was used to allow real-time monitoring through bioluminescence imaging. aPDT initiated either at 30 min or at 24 h post-infection significantly reduced the C. albicans burden 95.4 and 97.4%, respectively, compared to controls. Teichert et al. (2002) evaluated the efficacy of MB-mediated PDT to treat oral candidiasis in an immunosuppressed murine model, in an attempt to mimic thrush in patients. Mice with severe immunodeficiency disease were inoculated orally with C. albicans by swab three times a week for a 4-week period. Before treatment, mice were cultured for baseline fungal growth and received a topical oral cavity administration of 0.05 mL MB solution at different concentrations (250, 275, 300, 350, 400, 450, or 500 μg/mL). After of 10 min of MB solution treatment, mice were irradiated with light at 664 nm using a diode laser light with a cylindrical diffuser. MB aPDT had a dose-dependent effect as concentrations from 250 to 400 μg/mL reduced fungal growth but did not eliminate C. albicans while concentrations of 450 and 500 μg/mL totally eradicated the fungus from the oral cavity. Junqueira et al. (2009) evaluated the effects of aPDT on buccal candidiasis using a rat model. After inducing candidiasis on the dorsal aspect of rat tongues, aPDT was achieved using a laser and MB, and Candida colonization, epithelial alterations, and chronic inflammation were analyzed using histology. The effect was more visible on day 5 after treatment; at day 5 treated rats had fewer epithelial alterations (pathological score 1.00 treated and 1.50 in control group) and less chronic inflammation (pathological score 1.00 treated and 1.50 in control) than control animals. Mima et al. (2010) conducted an in vivo oral candidiasis study in immunosuppressed mice to evaluate the efficacy of aPDT of oral candidiasis using Photogem, a hematoporphyrin derivative, at 400, 500, or 1000 mg/L which was followed 30 min later by illumination with LED light (305 J/cm2) at 455 or 630 nm. aPDT resulted in 1.05, 1.59, and 1.40 log10 reductions, respectively, in tongue C. albicans colony counts; however, there was no difference in fungal burden between the concentrations of Photogem and LED light wavelengths used. Notably, histological evaluation of the tongue revealed that aPDT did not cause any significant adverse effects to the local mucosa. A murine oral candidiasis model was also utilized to explore the efficacy of curcumin as a PS (Dovigo et al., 2013). Five days after C. albicans infection, mice received topical curcumin (20, 40, and 80 μM) and illumination with LED light at 455 nm. This treatment significantly reduced the C. albicans viability in a dose dependent manner with 80 μM of curcumin associated with light leading to the highest reduction, 4 logs, in colony counts.
Mitra et al. (2011) investigated the efficacy of aPDT for the treatment of C. albicans ear pinna infection using a mouse model. They selected TMP-1363 as the PS after showing its efficacy for killing C. albicans in vitro. The intradermal space of the ear pinna was inoculated with C. albicans. After 2 days, 0.3 mg/mL TMP-1363 was administered topically and the ears were irradiated at 514 nm using a fluence of 90 J/cm2 delivered at an irradiance of 50 mW/cm2. aPDT with TMP-1363 resulted in a 50-fold reduction of C. albicans CFU/ear compared to untreated controls, and the infected ears subjected to aPDT completely healed over time without any residual damage to the pinna. Machado-de-Sena et al. (2014) evaluated the efficacy of aPDT for treatment of C. albicans vaginal infection using MB and red light. Mice were inoculated intravaginally with C. albicans, and then were treated with aPDT 5 days later using MB and red laser. This approach significantly reduced C. albicans growth 1.66 log CFU/mL and percentages of inflammatory area were significantly reduced with just two sessions of aPDT.
Recently, we reported the in vivo application of aPDT against T. rubrum (Baltazar et al., 2014). C57BL/6 mice were cutaneously infected with T. rubrum and treated with aPDT for 7 days every 24 h using a TBO 0.2% gel formulation and an LED 630 nm dose of 42 J/cm2. aPDT was compared to treatment with the antifungal cyclopiroxolamine (CPX, 0.65 mg/mice) administered topically every 48 h for 7 days. aPDT was 64% more efficient than CPX in reducing the fungal burden, and both treatments reduced the damage caused by the fungus in the skin. aPDT also reduced myeloperoxidase (MPO) levels, but not the activity of N-acetylglucosaminidase (NAG), suggesting that there was a reduction in neutrophils but not macrophages in the affected tissues. Furthermore, the study associated the effective production of ROS with aPDT efficacy.
Antimicrobial photodynamic inhibition of C. albicans was also studied using the non-vertebrate host Galleria mellonella, the wax moth (Chibebe Junior et al., 2013). aPDT MB with red light significantly reduced the fungal burden and prolonged the survival of C. albicans infected G. mellonella larvae compared to controls. A fluconazole-resistant C. albicans strain was also used to test the combination of aPDT and fluconazole, and this combined approach significantly prolonged the survival of the larvae compared to each individual treatment alone.
The increased incidence of drug resistant pathogens has led investigators to explore innovative approaches to infectious diseases in clinical studies, including using aPI to combat fungal infections. In this section, we highlight the human studies for the treatment of fungal infection using aPDT (Table 3).
Kim and Kim (2007) using ALA-PDT (two sessions) and red light (70–100 J/cm2) showed the efficacy of this approach for treating pityriasis versicolor. The study reported that there were no hyphae or spores found in the infected area at 10 days after treatment. Piraccini et al. (2008) described the success of onychomycosis (caused by T. rubrum) treatment using aPDT in a patient with had failed to respond to the treatments with conventional topical antifungal drugs. Before each session (three sessions at 15 day intervals between treatments), the patient’s nail was first coated with a 40% urea ointment that was then kept under occlusion for 7 days to soften the plate and then the diseased nail was subjected to aPDT using ALA (160 mg/g) and red light (630 nm, 37 J/cm2). After the three aPDT sessions during a period of 45 days, the patient was evaluated every 3 months for 24 months. Cultures were positive at the third aPDT session, but became negative 3 months after the last treatment. At the 12th month visit, cultures were still negative and the toenails were considered clinically cured and disease had not recurred at the 24th month evaluation. In another clinical trial of 30 patients with onychomycosis, patients that received aPDT therapy combining ALA and red light (570–670 nm, dose of 40 J/cm2) had a 43% cure rate at 12 months after the treatment and 37% remained disease free at 18 months (Sotiriou et al., 2010). Lee et al. (2010) reported that four of six patients with recalcitrant Malassezia folliculitis demonstrated significant improvement after treatment with methyl 5-ALA (MAL)-PDT and red LED light (630 nm, light dose of 37 J/cm2).
Gilaberte et al. (2011), reported treatment of two patients with fingernail onychomycosis unresponsive to standard antifungals with disease caused by the Fusarium oxysporum or Aspergillus terreus. In both cases, the nail plate was first softened with 40% urea ointment under occlusion for 12 h. aPDT was performed using MAL 16% cream and illumination using a 635-nm LED (dose of 37 J/cm2). A single treatment clinically improved the nail appearance and cultures were thereafter negative. Two additional treatments were administered and both patients remained disease free during subsequent follow up evaluations.
Mima et al. (2012) compared aPDT with topical antifungal cream for the treatment of denture stomatitis (DS) caused by Candida species. Patients were randomly assigned (n = 20 each) to receive either nystatin (NYT) or aPDT. In the NYT group, patients received topical treatment with nystatin (100 000 IU) four times daily during 15 days. The aPDT group each had 500 mg/L of Photogem® applied to their dentures and palates. After 30 min of incubation, the Photogem coated surfaces were illuminated with LED light (455 nm, doses of 37.5 and 122 J/cm2, respectively) three times a week for 15 days. Mycological cultures were taken from dentures at baseline (day 0), at the end of the treatment (day 15) and at the follow-up time intervals (days 30, 60, and 90). Both treatments significantly reduced the fungal burden at the end of the treatments and on day 30 of the follow-up period; however, there were no significant differences between the two treatment modalities (53 vs. 45% for NYT and PDT, respectively). The study highlighted that fewer sessions of aPDT were necessary to achieve the same result that NYT achieved, albeit the NYT approach did not require clinic visits.
Gilaberte et al. (2014) used aPDT in a patient with recalcitrant cutaneous sporotrichosis. They used intralesional applications of 1% MB and LED light at 635 nm to administer 37 J/cm2 to each lesion in combination with low doses of itraconazole (100 mg/day). The aPDT was performed three times every other week and this approach resulted in a complete clinical and microbiological cure (Gilaberte et al., 2014). In a clinical trial, Lyon et al. (2011) treated 10 patients with chromoblastomycosis with a combination of MB and red LED (660 nm, dose of 28 J/cm2). The patients underwent six treatment sessions (every week) and, although the treatment did not result in complete healing of the lesions, aPDT resulted in clear reductions of the volume and cicatrization of 80–90% of the lesions.
The significant burden of dermatophytoses and the worldwide increase in fungal strains resistant to the current antifungals (Cowen, 2008) increases the urgency for the development of new therapeutic strategies, such as aPDT. In this review we described in vitro and in vivo studies as well as the few, small human experiences and trials that support the development of the aPDT either as adjuvant or as a primary therapeutic approach against cutaneous mycoses.
The in vitro mechanism of action described thus far demonstrate that aPI induces the generation of ROS and RNS, which effectively damage a range of fungal cellular structures and induce cell death. There are no reports of mutagenic or genotoxic effects to the fungal or human cells. However, there is an ongoing need for deeper study of the mechanisms of aPDT to facilitate the expanded clinical use of this promising therapeutic approach. Although the majority of aPDT studies have focused on Candida biofilms, it is important to also investigate the application of this approach using other major fungal pathogens, including Cryptococcus species or Coccidioides species where biofilm formation contributes to disease severity in the central nervous system (Sardi Jde et al., 2014). The incorporation of PSs into liposomes, micelles, or nanoparticles is a promising approach to reduce the PS self-aggregation and to enhance the targeted delivery of the PS. The development of these vehicles is particularly important for the potential expansion of aPDT for the treatment of deep fungal infections using fiber optic lasers, applied endoscopically, or interstitially.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
LMB was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors thank Laboratório de Bioengenharia (LABBIO), Departamento de Engenharia Mecânica, UFMG, for assistance with the creation of Figure 2.
Baltazar, L. M., Krausz, A. E., Souza, A. C. O., Adler, B. L., Landriscina, A., Musaev, T.,et al. (2015). Trichophyton rubrum is inhibited by free and nanoparticle encapsulated curcumin by induction of nitrosative stress after photodynamic activation. PLoS ONE (in press).
Baltazar Lde, M., Soares, B. M., Carneiro, H. C., Avila, T. V., Gouveia, L. F., Souza, D. G.,et al. (2013). Photodynamic inhibition of Trichophyton rubrum: in vitro activity and the role of oxidative and nitrosative bursts in fungal death. J. Antimicrob. Chemother. 68, 354–361. doi: 10.1093/jac/dks414
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baltazar, L. M., Werneck, S. M., Carneiro, H. C., Gouveia, L. F., De Paula, T. P., Byrro, R. M.,et al. (2014). photodynamic therapy efficiently controls dermatophytosis caused by Trichophyton rubrum in a murine model. Br. J. Dermatol. doi: 10.1111/bjd.13494 [Epub ahead of print].
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brown, G. D., Denning, D. W., Gow, N. A., Levitz, S. M., Netea, M. G., and White, T. C. (2012). Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13. doi: 10.1126/scitranslmed.3004404
Calin, M. A., and Parasca, S. V. (2009). Light sources for photodynamic inactivation of bacteria. Lasers Med. Sci. 24, 453–460. doi: 10.1007/s10103-008-0588-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Calzavara-Pinton, P., Rossi, M. T., Sala, R., and Venturini, M. (2012). Photodynamic antifungal chemotherapy. Photochem. Photobiol. 88, 512–522. doi: 10.1111/j.1751-1097.2012.01107.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Calzavara-Pinton, P. G., Venturini, M., and Sala, R. (2005). A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J. Photochem. Photobiol. B Biol. 78, 1–6. doi: 10.1016/j.jphotobiol.2004.06.006.
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carvalho, G. G., Felipe, M. P., and Costa, M. S. (2009). The photodynamic effect of methylene blue and toluidine blue on Candida albicans is dependent on medium conditions. J. Microbiol. 47, 619–623. doi: 10.1007/s12275-009-0059-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chibebe Junior, J., Sabino, C. P., Tan, X., Junqueira, J. C., Wang, Y., Fuchs, B. B.,et al. (2013). Selective photoinactivation of Candida albicans in the non-vertebrate host infection model Galleria mellonella. BMC Microbiol. 13:217. doi: 10.1186/1471-2180-13-217
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cormick, M. P., Alvarez, M. G., Rovera, M., and Durantini, E. N. (2009). Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 44, 1592–1599. doi: 10.1016/j.ejmech.2008.07.026
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cowen, L. E. (2008). The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 6, 187–198. doi: 10.1038/nrmicro1835
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Craig, R. A., Mccoy, C. P., Gorman, S. P., and Jones, D. S. (2014). Photosensitisers – the progression from photodynamic therapy to anti-infective surfaces. Expert. Opin. Drug. Deliv. 12, 85–101. doi: 10.1517/17425247.2015.962512
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dai, T., Bil De Arce, V. J., Tegos, G. P., and Hamblin, M. R. (2011). Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 55, 5710–5717. doi: 10.1128/AAC.05404-11
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dai, T., Fuchs, B. B., Coleman, J. J., Prates, R. A., Astrakas, C., St Denis, T. G.,et al. (2012). Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 3:120. doi: 10.3389/fmicb.2012.00120
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Donnelly, R. F., Mccarron, P. A., and Tunney, M. M. (2008). Antifungal photodynamic therapy. Microbiol. Res. 163, 1–12. doi: 10.1016/j.micres.2007.08.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dougherty, T. J., Kaufman, J. E., Goldfarb, A., Weishaupt, K. R., Boyle, D., and Mittleman, A. (1978). Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 38, 2628–2635.
Dovigo, L. N., Carmello, J. C., De Souza Costa, C. A., Vergani, C. E., Brunetti, I. L., Bagnato, V. S.,et al. (2013). Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med. Mycol. 51, 243–251. doi: 10.3109/13693786.2012.714081
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dovigo, L. N., Pavarina, A. C., Ribeiro, A. P., Brunetti, I. L., Costa, C. A., Jacomassi, D. P.,et al. (2011). Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 87, 895–903. doi: 10.1111/j.1751-1097.2011.00937.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drake, L. A., Dinehart, S. M., Farmer, E. R., Goltz, R. W., Graham, G. F., Hordinsky, M. K.,et al. (1996). Guidelines of care for superficial mycotic infections of the skin: tinea capitis and tinea barbae. Guidelines/Outcomes Committee. American Academy of Dermatology. J. Am. Acad. Dermatol. 34, 290–294. doi: 10.1016/S0190-9622(96)80137-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
El-Gohary, M., Van Zuuren, E. J., Fedorowicz, Z., Burgess, H., Doney, L., Stuart, B.,et al. (2014). Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst. Rev. 8, CD009992. doi: 10.1002/14651858.CD009992.pub2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fuchs, B. B., Tegos, G. P., Hamblin, M. R., and Mylonakis, E. (2007). Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob. Agents Chemother. 51, 2929–2936. doi: 10.1128/AAC.00121-07
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gaitanis, G., Velegraki, A., Mayser, P., and Bassukas, I. D. (2013). Skin diseases associated with Malassezia yeasts: facts and controversies. Clin. Dermatol. 31, 455–463. doi: 10.1016/j.clindermatol.2013.01.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Garland, M. J., Cassidy, C. M., Woolfson, D., and Donnelly, R. F. (2009). Designing photosensitizers for photodynamic therapy: strategies, challenges and promising developments. Future Med.Chem. 1, 667–691. doi: 10.4155/fmc.09.55
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gilaberte, Y., Aspiroz, C., Alejandre, M. C., Andres-Ciriano, E., Fortuno, B., Charlez, L.,et al. (2014). Cutaneous sporotrichosis treated with photodynamic therapy: an in vitro and in vivo study. Photomed. Laser Surg. 32, 54–57. doi: 10.1089/pho.2013.3590
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gilaberte, Y., Aspiroz, C., Martes, M. P., Alcalde, V., Espinel-Ingroff, A., and Rezusta, A. (2011). Treatment of refractory fingernail onychomycosis caused by nondermatophyte molds with methylaminolevulinate photodynamic therapy. J. Am. Acad. Dermatol. 65, 669–671. doi: 10.1016/j.jaad.2010.06.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Giroldo, L. M., Felipe, M. P., De Oliveira, M. A., Munin, E., Alves, L. P., and Costa, M. S. (2009). Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers Med. Sci. 24, 109–112. doi: 10.1007/s10103-007-0530-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, A. K., and Cooper, E. A. (2008). Update in antifungal therapy of dermatophytosis. Mycopathologia 166, 353–367. doi: 10.1007/s11046-008-9109-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hamblin, M. R., and Hasan, T. (2004). Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem.Photobiol. Sci. 3, 436–450. doi: 10.1039/b311900a
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hamblin, M. R., Miller, J. L., and Hasan, T. (1996). Effect of charge on the interaction of site-specific photoimmunoconjugates with human ovarian cancer cells. Cancer Res. 56, 5205–5210.
Harris, F., and Pierpoint, L. (2012). Photodynamic therapy based on 5-aminolevulinic acid and its use as an antimicrobial agent. Med. Res. Rev. 32, 1292–1327. doi: 10.1002/med.20251
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hu, Y., Huang, X., Lu, S., Hamblin, M. R., Mylonakis, E., Zhang, J.,et al. (2015). Photodynamic therapy combined with terbinafine against chromoblastomycosis and the effect of PDT on Fonsecaea monophora in vitro. Mycopathologia 179, 103–119. doi: 10.1007/s11046-014-9828-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jesionek, H., and Tappeiner, H. V. (1903). Therapeutische Versuche mit Fluoreszierenden Stoffen. Muench. Med. Wochneshr. 47, 2024–2044.
Junqueira, J. C., Martins Jda, S., Faria, R. L., Colombo, C. E., and Jorge, A. O. (2009). Photodynamic therapy for the treatment of buccal candidiasis in rats. Lasers Med. Sci. 24, 877–884. doi: 10.1007/s10103-009-0673-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kamp, H., Tietz, H. J., Lutz, M., Piazena, H., Sowyrda, P., Lademann, J.,et al. (2005). Antifungal effect of 5-aminolevulinic acid PDT in Trichophyton rubrum. Mycoses 48, 101–107. doi: 10.1111/j.1439-0507.2004.01070.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, Y. J., and Kim, Y. C. (2007). Successful treatment of pityriasis versicolor with 5-aminolevulinic acid photodynamic therapy. Arch. Dermatol. 143, 1218–1220. doi: 10.1001/archderm.143.9.1218
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Krausz, A., and Friedman, A. J. (2014). News, views, & reviews: antimicrobial photodynamic therapy: applications beyond skin cancer. J. Drugs Dermatol. 13, 624–626.
Lee, J. W., Kim, B. J., and Kim, M. N. (2010). Photodynamic therapy: new treatment for recalcitrant Malassezia folliculitis. Lasers Surg. Med. 42, 192–196. doi: 10.1002/lsm.20857
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lennon, S. V., Martin, S. J., and Cotter, T. G. (1991). Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 24, 203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lipson, R. L., and Baldes, E. J. (1960). The photodynamic properties of a particular hematoporphyrin derivative. Arch. Dermatol. 82, 508–516. doi: 10.1001/archderm.1960.01580040026005
Lyon, J. P., Moreira, L. M., De Carvalho, V. S., Dos Santos, F. V., De Lima, C. J., and De Resende, M. A. (2013). In vitro photodynamic therapy against Fonsecaea pedrosoi and Cladophialophora carrionii. Mycoses 56, 157–161. doi: 10.1111/j.1439-0507.2012.02226.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lyon, J. P., Pedroso e Silva Azevedo Cde, M., Moreira, L. M., De Lima, C. J., and De Resende, M. A. (2011). Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia 172, 293–297. doi: 10.1007/s11046-011-9434-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Machado-de-Sena, R. M., Correa, L., Kato, I. T., Prates, R. A., Senna, A. M., Santos, C. C.,et al. (2014). Photodynamic therapy has antifungal effect and reduces inflammatory signals in Candida albicans-induced murine vaginitis. Photodiagnosis Photodyn. Ther. 11, 275–282. doi: 10.1016/j.pdpdt.2014.03.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mantareva, V., Angelov, I., Kussovski, V., Dimitrov, R., Lapok, L., and Wohrle, D. (2011). Photodynamic efficacy of water-soluble Si(IV) and Ge(IV) phthalocyanines towards Candida albicans planktonic and biofilm cultures. Eur. J. Med. Chem. 46, 4430–4440. doi: 10.1016/j.ejmech.2011.07.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Meyer-Betz, F. (1913). Untersuchungen uber die Biologische (photodynamische) Wirkung des Hamatoporphyrins und anderer Derivate des Blut – und Galenfarbstoffs. Dtsch. Arch. Klin. Med. 112, 475–503.
Mima, E. G., Pavarina, A. C., Dovigo, L. N., Vergani, C. E., Costa, C. A., Kurachi, C.,et al. (2010). Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109, 392–401. doi: 10.1016/j.tripleo.2009.10.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mima, E. G., Vergani, C. E., Machado, A. L., Massucato, E. M., Colombo, A. L., Bagnato, V. S.,et al. (2012). Comparison of photodynamic therapy versus conventional antifungal therapy for the treatment of denture stomatitis: a randomized clinical trial. Clin. Microbiol. Infect. 18, E380–E388. doi: 10.1111/j.1469-0691.2012.03933.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mitra, S., Haidaris, C. G., Snell, S. B., Giesselman, B. R., Hupcher, S. M., and Foster, T. H. (2011). Effective photosensitization and selectivity in vivo of Candida Albicans by meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate. Lasers Surg. Med. 43, 324–332. doi: 10.1002/lsm.21049
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mroz, P., Yaroslavsky, A., Kharkwal, G. B., and Hamblin, M. R. (2011). Cell death pathways in photodynamic therapy of cancer. Cancers (Basel) 3, 2516–2539. doi: 10.3390/cancers3022516
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mukherjee, P. K., Leidich, S. D., Isham, N., Leitner, I., Ryder, N. S., and Ghannoum, M. A. (2003). Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob. Agents Chemother. 47, 82–86. doi: 10.1128/AAC.47.1.82-86.2003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Munin, E., Giroldo, L. M., Alves, L. P., and Costa, M. S. (2007). Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J. Photochem. Photobiol. B Biol. 88, 16–20. doi: 10.1016/j.jphotobiol.2007.04.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nenoff, P., Grunewald, S., and Paasch, U. (2014a). Laser therapy of onychomycosis. J. Dtsch. Dermatol. Ges. 12, 33–38. doi: 10.1111/ddg.12251
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nenoff, P., Kruger, C., Ginter-Hanselmayer, G., and Tietz, H. J. (2014b). Mycology – an update. Part 1: Dermatomycoses: causative agents, epidemiology and pathogenesis. J. Dtsch. Dermatol. Ges. 12, 188–209; quiz 210, 188–211; quiz 212. doi: 10.1111/ddg.12245
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Noodt, B. B., Berg, K., Stokke, T., Peng, Q., and Nesland, J. M. (1996). Apoptosis and necrosis induced with light and 5-aminolaevulinic acid-derived protoporphyrin IX. Br. J. Cancer 74, 22–29. doi: 10.1038/bjc.1996.310
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nyman, E. S., and Hynninen, P. H. (2004). Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 73, 1–28. doi: 10.1016/j.jphotobiol.2003.10.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pathak, M. A., and Fitzpatrick, T. B. (1992). The evolution of photochemotherapy with psoralens and UVA (PUVA): 2000 BC to 1992 AD. J. Photochem. Photobiol. B Biol. 14, 3–22. doi: 10.1016/1011-1344(92)85080-E
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Piraccini, B. M., Rech, G., and Tosti, A. (2008). Photodynamic therapy of onychomycosis caused by Trichophyton rubrum. J. Am. Acad. Dermatol. 59, S75–S76. doi: 10.1016/j.jaad.2008.06.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Plaetzer, K., Krammer, B., Berlanda, J., Berr, F., and Kiesslich, T. (2009). Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med. Sci. 24, 259–268. doi: 10.1007/s10103-008-0539-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Policard, A. (1924). Etudes sur les aspects offerts par des tumeurs experimentales examinees a la lumiere de Wood. Cr. Soc. Biol. 91, 1423–1428.
Prates, R. A., Fuchs, B. B., Mizuno, K., Naqvi, Q., Kato, I. T., Ribeiro, M. S.,et al. (2013). Effect of virulence factors on the photodynamic inactivation of Cryptococcus neoformans. PLoS ONE 8:e54387. doi: 10.1371/journal.pone.0054387
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prates, R. A., Kato, I. T., Ribeiro, M. S., Tegos, G. P., and Hamblin, M. R. (2011). Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J. Antimicrob. Chemother. 66, 1525–1532. doi: 10.1093/jac/dkr160.
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rodrigues, G. B., Primo, F. L., Tedesco, A. C., and Braga, G. U. (2012). In vitro photodynamic inactivation of Cryptococcus neoformans melanized cells with chloroaluminum phthalocyanine nanoemulsion. Photochem. Photobiol. 88, 440–447. doi: 10.1111/j.1751-1097.2011.01055.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Romagnoli, C., Mares, D., Sacchetti, G., and Bruni, A. (1998). The photodynamic effect of 5-(4-hydroxy-1-butinyl)-2,2-bithienyl on dermatophytes. Mycol. Res. 102, 1519–1524. doi: 10.1017/S0953756298006637
Sardi Jde, C., Pitangui Nde, S., Rodriguez-Arellanes, G., and Taylor, M. L., Fusco-Almeida, A. M., and Mendes-Giannini, M. J. (2014). Highlights in pathogenic fungal biofilms. Rev. Iberoam. Micol. 31, 22–29. doi: 10.1016/j.riam.2013.09.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scherer, H. (1841). Chemisch-physiologische untersuchungen. Ann. Chem. Pharm. 40, 1–64. doi: 10.1002/jlac.18410400102
Schwartz, S. K., Absolon, K., and Vernund, H. (1955). Some relationships of porphyrins, X-rays, and tumours. Univ. Minn. Med. Bull. 27, 7–8.
Sekkat, N., Van Den Bergh, H., Nyokong, T., and Lange, N. (2012). Like a bolt from the blue: phthalocyanines in biomedical optics. Molecules 17, 98–144. doi: 10.3390/molecules17010098
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sharma, M., Manoharlal, R., Puri, N., and Prasad, R. (2010). Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 30, 391–404. doi: 10.1042/BSR20090151
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Smijs, T. G., and Pavel, S. (2011). The susceptibility of dermatophytes to photodynamic treatment with special focus on Trichophyton rubrum. Photochem. Photobiol. 87, 2–13. doi: 10.1111/j.1751-1097.2010.00848.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Smijs, T. G., and Schuitmaker, H. J. (2003). Photodynamic inactivation of the dermatophyte Trichophyton rubrum. Photochem. Photobiol. 77, 556–560. doi: 10.1562/0031-8655(2003)077<0556:PIOTDT>2.0.CO;2
Soares, B. M., Alves, O. A., Ferreira, M. V., Amorim, J. C., Sousa, G. R., Silveira Lde, B.,et al. (2011). Cryptococcus gattii: in vitro susceptibility to photodynamic inactivation. Photochem. Photobiol. 87, 357–364. doi: 10.1111/j.1751-1097.2010.00868.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Soares, B. M., Da Silva, D. L., Sousa, G. R., Amorim, J. C., De Resende, M. A., Pinotti, M.,et al. (2009). In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J. Photochem. Photobiol. B Biol. 94, 65–70. doi: 10.1016/j.jphotobiol.2008.07.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sotiriou, E., Koussidou-Eremonti, T., Chaidemenos, G., Apalla, Z., and Ioannides, D. (2010). Photodynamic therapy for distal and lateral subungual toenail onychomycosis caused by Trichophyton rubrum: preliminary results of a single-centre open trial. Acta Derm. Venereol. 90, 216–217. doi: 10.2340/00015555-0811
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takahashi, H., Nakajima, S., Sakata, I., and Iizuka, H. (2014). Antifungal effect of TONS504-photodynamic therapy on Malassezia furfur. J. Dermatol. 41, 895–897. doi: 10.1111/1346-8138.12615
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tappeiner, H. V. (1900). Über die Wirkung fluoreszierender Stoffe auf Infusorien nach Versuchen von O. Raab. Muench. Med. Wochenschr. 47, 5–7.
Tappeiner, H. V., and Jodlbauer, A. (1904). Über Wirkung der photodynamischen (fluoreszierenden) Stoffe auf Protozoan und Enzyme. [On the effect of photodynamic (fluorescent) substances on protozoa and enzymes]. Dtsch. Arch. Klin. Med. 80, 427–487.
Tappeiner, H. V., and Jodlbauer, A. (1907). Die sensibilisierende wirkung fluorieszierender substanzer. Gesammte Untersuchungen uber die photodynamische Erscheinung. Leipzig: FCW Vogel.
Teichert, M. C., Jones, J. W., Usacheva, M. N., and Biel, M. A. (2002). Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 93, 155–160. doi: 10.1067/moe.2002.120051
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Usuda, J., Kato, H., Okunaka, T., Furukawa, K., Tsutsui, H., Yamada, K.,et al. (2006). Photodynamic therapy (PDT) for lung cancers. J. Thorac. Oncol. 1, 489–493. doi: 10.1097/01243894-200606000-00018
White, T. C., Findley, K., Dawson, T. L. Jr., Scheynius, A., Boekhout, T., Cuomo, C. A.,et al. (2014). Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 4, a019802. doi: 10.1101/cshperspect.a019802
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: photodynamic inhibition, fungal cells, treatment, photosensitizer, light source, photochemicals and photobiological events
Citation: Baltazar LM, Ray A, Santos DA, Cisalpino PS, Friedman AJ and Nosanchuk JD (2015) Antimicrobial photodynamic therapy: an effective alternative approach to control fungal infections. Front. Microbiol. 6:202. doi: 10.3389/fmicb.2015.00202
Received: 29 January 2015; Paper pending published: 20 February 2015;
Accepted: 25 February 2015; Published online: 13 March 2015
Edited by:
Luis R. Martinez, New York Institute of Technology College of Osteopathic Medicine, USAReviewed by:
Leonardo Nimrichter, Federal University of Rio de Janeiro, BrazilCopyright © 2015 Baltazar, Ray, Santos, Cisalpino, Friedman and Nosanchuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joshua D. Nosanchuk, Department of Microbiology and Immunology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USAam9zaC5ub3NhbmNodWtAZWluc3RlaW4ueXUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.