- 1Department of Microbial Drugs, Helmholtz Centre for Infection Research, Braunschweig, Germany
- 2Department of Pharmaceutical Microbiology, Hans Knöll Institute, Friedrich Schiller Universität Jena, Germany
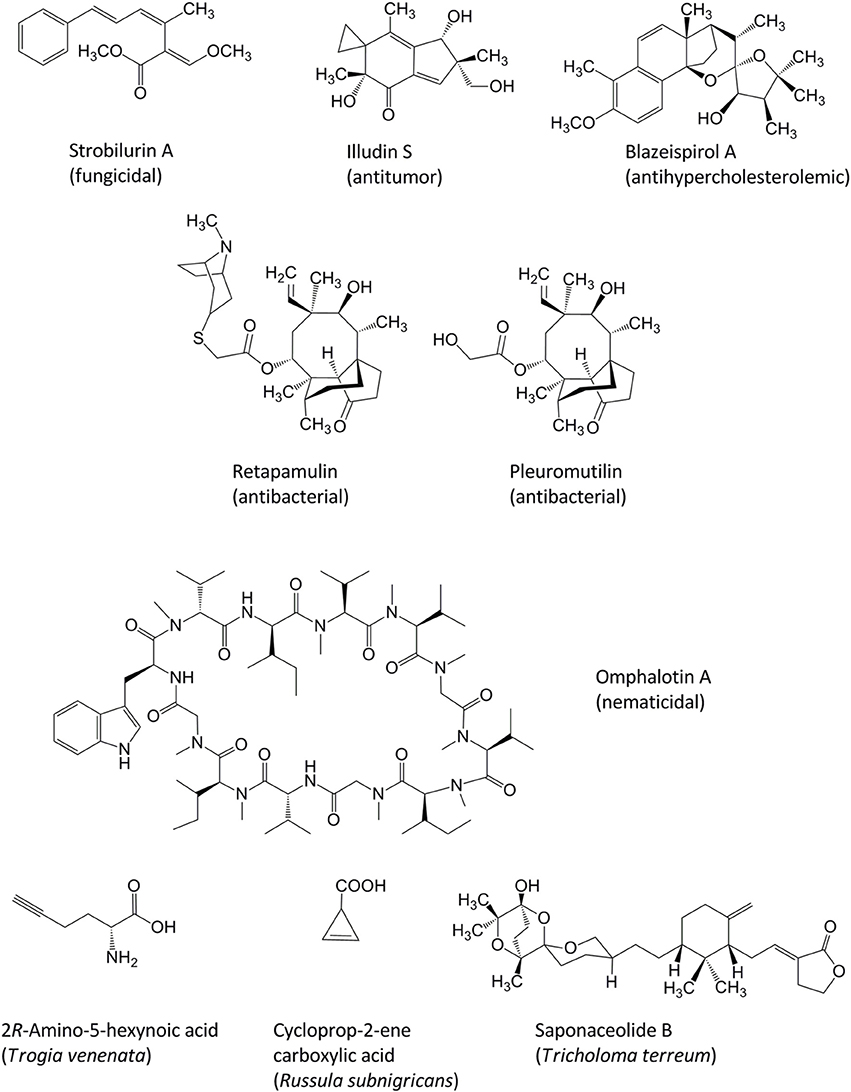
Among the first documented studies on the chemistry of fungal natural products were descriptions of quinoid pigments, i.e., the L-tyrosine- and L-phenylalanine-derived terphenylquinones atromentin and polyporic acid, respectively. The isolation of these compounds from mushroom fruiting bodies (basidiomes) was published around 1877 by Stahlschmidt and Thörner. Ever since, organic chemists embraced basidiomycetes as a prolific source of bioactive compounds and investigated these fungi with regard to compound isolation, structure elucidation, and synthesis (Gill and Steglich, 1987; Zhou and Liu, 2010; De Silva et al., 2013 and previous reviews referenced therein, Lorenzen and Anke, 1998; Richter et al., in press). Mushrooms seem to be particularly talented in producing unique terpenoids, and the molecular background behind the biosynthesis of some of those compounds has only recently been elucidated (Quin et al., 2014). Prominent examples of basidiomycete metabolites for lead structures in agrochemistry and drug research are, among others, the strobilurins, i.e., agriculturally used ß-methoxyacrylate fungicides from cultures of Mycena, Oudemansiella, Strobilurus, Xerula and several other basidiomycete genera (Sauter et al., 1999, Figure 1). Other examples are the pleuromutilins, the illudins, and the omphalotins (Figure 1). The pleuromutilins from cultures of species that are now placed in the genera Clitopilus and Omphalina served as scaffold for the development of the semisynthetic antibacterial antibiotic retapamulin (Kirst, 2013) which is clinically used for topical treatment of infections with Staphylococcus aureus. The illudins from Lampteromyces and Omphalotus species (Omphalotaceae) are sesquiterpenes featuring an unusual cyclopropane ring and are currently developed as anticancer drugs (Tanasova and Sturla, 2012). The omphalotins are cyclopeptides with pronounced nematicidal activites against root knot nematodes (Büchel et al., 1998), which are also exclusively found in the Omphalotaceae. Recently, the blazeispirols from Agaricus subrufescens were discovered as strong and selective agonists of the Liver X receptor (LXR alpha). Concurrently, relevant in vivo effects of blazeispirols in a mouse model were observed which might give rise to the development of a new anti-hypercholesterolemic agent from cultures of a medicinal mushroom (Grothe et al., 2011).
The above examples illustrate that basidiomycete secondary metabolomes merit further exploration. Perhaps fortunately for coming generations of Ph.D. students, the realm of basidiomycete metabolites is still underexplored, even after decades of intensive research to isolate and structurally elucidate compounds. This is also evident by the fact that toxic principles of mushrooms which repeatedly led to poisonings were identified only recently (Figure 1). Recent advances pertain to Trogia venenata fruiting bodies, in which the toxic 2R-amino-5-hexynoic acid and related compounds were found (Zhou et al., 2012). Cycloprop-2-ene carboxylic acid causing rhabdomyolysis was isolated from Russula subnigricans, a toxic mushroom native to East Asia (Matsuura et al., 2009). Furthermore, saponaceolide toxins with their unusual molecular skeleton were discovered in Tricholoma terreum (Yin et al., 2014).
For basidiomycetes, the genomic era set in later than for ascomycetes, and in numbers of genome projects the former are still lagging behind the latter. Still, the available genomic data impacted natural product research as it reveals a stimulating disparity: the number of natural product genes, best reflected by the number of genes for polyketide synthases and peptide synthetases exceeds the number of known compounds by far—even after decades of chemical research. The “house eater” fungus Serpula lacrymans encodes 21 PKS and NRPS genes (Eastwood et al., 2011), the average number of PKS genes per basidiomycete genome is four, according to a survey of 35 mostly saprotrophic species (Lackner et al., 2012).
The wealth of natural product biosynthesis genes in a given species contrasts the few compounds known from the same species. This situation is reminiscent of what was found for ascomycete genomes years ago, e.g., for the genera Aspergillus, Penicillium, Fusarium, and others (Keller et al., 2005; Desjardins and Proctor, 2007; Sanchez et al., 2012). However, the course research has taken (and will be taking) to make as much sense as possible out of the genomic data is quite different with basidiomycetes. This is due to a number of reasons that contrast the situation with ascomycetes.
(a) Basidiomycetes are mostly dikaryotic and hence little suitable for reverse genetics, although some species grow as monokarya in vitro.
(b) Basidiomycetes are little amenable, if at all, to transformation and genetic manipulation, as only a very modest number of genetic tools and procedures are in place. Notable exceptions with regard to producers of pharmacologically active metabolites pertain to the honey mushroom Armillaria mellea (Baumgartner et al., 2010) which produces the melleolides, i.e., unusual sesquiterpene ester antibiotics (Bohnert et al., 2014). Also, a transformation procedure was established for the pleuromutilin producer Clitopilus passeckerianus (Kilaru et al., 2009).
(c) The ecological preferences of numerous basidiomycetes have so far remained obscure and can only now be established by using modern methods of molecular ecology. For instance, some species that were hitherto regarded as saprotrophs, such as Hygrocybe virginea, are now suspected to possess hitherto unknown associations with plants, illustrating in-depth studies on their ecology might in future be rewarding (Tello et al., 2014).
(d) Many basidiomycetes, in particular the biotrophic pathogens, are difficult or virtually impossible to grow in axenic culture. This applies e.g., to the entire subdivision Pucciniomycotina, (“rust fungi”) and to the obligate mycorrhizal taxa, comprising important families, such as the Russulaceae and Cortinariaceae, from whose basidiomes numerous unique secondary metabolites have already been obtained. Even in some other “saprotrophic” genera, such as Pluteus, the basidiospores do not readily germinate, and stable cultures can hardly be established from sterile mycelial plugs taken out of the basidome tissues using standard methodology. Protocols to culture rust fungi or mycorrhizal symbionts have been elaborated by competent mycologists several decades ago. We encourage the community to also emphasize teaching classical mycological techniques, to educate the coming generation of mycologists and prevent these valuable methods from slowly being forgotten. In fact, such techniques could be useful to facilitate work on the genomics and metabolomics of these organisms since stable cultures could be used for propagation of sufficient biomass and a number of other interesting tasks.
The typical approach to explore metabolic pathways includes gene inactivation, combined with chemical characterization of the resulting phenotype. Whereas for model species/genera such as Aspergillus and other ascomycetes, numerous procedures and protocols were in place for reverse genetics, to manipulate expression of silent natural product genes, and to harness -omics technologies, this is only modestly (if at all) the case for basidiomycetes. Hence, the above reasons add more complexity to research which aims at functionally characterizing individual genes and basidiomycete secondary metabolomes. Consequently, despite chemically intriguing and unique features of their natural products, and also for the lack of robust biotechnological expression systems, basidiomycetes have not become the objects of choice. As long as the respective genes, enzymes, and mechanisms are present elsewhere, e.g., in Aspergillus or Fusarium species, or in streptomycetes, these will be preferred organisms. Projects including these organisms will be sooner finished and sooner published. On the other hand, one new basidiomycete genome after the other is currently released and sequence data are made available at a much faster pace than biochemists and natural product chemists can keep up with. Hence, an increasing amount of (natural product gene) sequence data is produced and because verification by wet-bench work cannot keep pace the amount of hypothetical and misannotated natural produce genes is ever-increasing.
Despite all these challenges there are three encouraging reasons why basidiomycetes advance mycology and natural product chemistry. Firstly, unique structures, e.g., the ones mentioned in the introduction, deserve elucidation of the biochemically and mechanistically intriguing basis behind their biogenesis. Secondly, fungi as such are a widely unexplored source for novel biotechnological products in general (cf. Rambold et al., 2013), and this especially holds true for the basidiomycetes. These two reasons alone justify new genomes to be sequenced. Finally, basidiomycetes are of outstanding ecological significance and may be key to answer the question as to why natural products exist. Due to their ability to form mycorrhizae with conifers and deciduous trees, they are key elements of temperate and boreal climax vegetations. They efficiently degrade lignocellulose which makes them indispensable to keep the global carbon cycles going. The basidiomycetes and the existing genomes represent a good opportunity to follow a different approach, but still contributing substantially to natural product research: with perhaps a dozen of carefully chosen symbiotic, parasitic, and saprotrophic species and a concerted effort of mycologists, chemists, ecologists, biochemists and bioinformaticians we may come to a more profound understanding why these magnificent small molecules were evolved, beyond the established examples that they serve as defense agents and to compete with other microbes in their ecological niche.
Conflict of Interest Statement
The Guest Associate Editor Nancy Keller declares that, despite having collaborated with author Dirk Hoffmeister, the review process was handled objectively. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Baumgartner, K., Fujiyoshi, P., Foster, G. D., and Bailey, A. M. (2010). Agrobacterium tumefaciens-mediated transformation for investigation of somatic recombination in the fungal pathogen Armillaria mellea. Appl. Environ. Microbiol. 76, 7990–7996. doi: 10.1128/AEM.01049-10
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bohnert, M., Nützmann, H. W., Schroeckh, V., Horn, F., Dahse, H. M., Brakhage, A. A., et al. (2014). Cytotoxic and antifungal activities of melleolide antibiotics follow dissimilar structure-activity relationships. Phytochemistry 105, 101–108. doi: 10.1016/j.phytochem.2014.05.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Büchel, E., Mayer, A., Martini, U., Anke, H., and Sterner, O. (1998). Structure elucidation of omphalotin, a cyclic dodecapeptide with potent nematicidal activity isolated from Omphalotus olearius. Pesticide Sci. 54, 309–311.
De Silva, D. D., Rapior, S., Sudarman, E., Stadler, M., Xu, J., Alias, S. A., et al. (2013). Bioactive metabolites from macrofungi: ethnopharmacology, biological activities and chemistry. Fungal Divers. 62, 1–40. doi: 10.1007/s13225-013-0265-2
Desjardins, A. E., and Proctor, R. H. (2007). Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 119, 47–50. doi: 10.1016/j.ijfoodmicro.2007.07.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eastwood, D. C., Floudas, D., Binder, M., Majcherczyk, A., Schneider, P., Aerts, A., et al. (2011). The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 333, 762–765. doi: 10.1126/science.1205411
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gill, M., and Steglich, W. (1987). Pigments of fungi (Macromycetes). Prog. Chem. Org. Nat. Prod. 51, 1–317.
Grothe, T., Stadler, M., Köpcke, B., Roemer, E., Bitzer, J., and Wabnitz, P. (2011). Terpenoid Spiro Ketal Compounds with LXR Agonists Activity, Their Use and Formulations with Them. World Patent WO/2012/079721.
Keller, N. P., Turner, G., and Bennett, J. W. (2005). Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 3, 937–947. doi: 10.1038/nrmicro1286
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kilaru, S., Collins, C. M., Hartley, A. J., Bailey, A. M., and Foster, G. D. (2009). Establishing molecular tools for genetic manipulation of the pleuromutilin-producing fungus Clitopilus passeckerianus. Appl. Environ. Microbiol. 75, 7196–7204. doi: 10.1128/AEM.01151-09
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kirst, H. A. (2013). Developing new antibacterials through natural product research. Expert. Opin. Drug Discov. 8, 479–493. doi: 10.1517/17460441.2013.779666
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lackner, G., Misiek, M., Braesel, J., and Hoffmeister, D. (2012). Genome mining reveals the evolutionary origin and biosynthetic potential of basidiomycete polyketide synthases. Fungal Genet. Biol. 49, 996–1003. doi: 10.1016/j.fgb.2012.09.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lorenzen, K., and Anke, T. (1998). Basidiomycetes as a source for new bioactive natural products. Curr. Org. Chem. 2, 329–364.
Matsuura, M., Saikawa, Y., Inui, K., Nakae, K., Igarashi, M., Hashimoto, K., et al. (2009). Identification of the toxic trigger in mushroom poisoning. Nat. Chem. Biol. 5, 465–467. doi: 10.1038/nchembio.179
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Quin, M. B., Flynn, C. M., and Schmidt-Dannert, C. (2014). Traversing the fungal terpenome. Nat. Prod. Rep. 10, 1449–1473. doi: 10.1039/c4np00075g
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rambold, G., Stadler, M., and Begerow, D. (2013). Mycology should be recognized as a field in biology at eye level with other major disciplines—a memorandum. Mycol. Progr. 12, 455–463. doi: 10.1007/s11557-013-0902-x
Richter, C., Wittstein, K., Kirk, P. M., and Stadler, M. (in press). An assessment of the taxonomy and chemotaxonomy of Ganoderma. Fungal Divers.
Sanchez, J. F., Somoza, A. D., Keller, N. P., and Wang, C. C. (2012). Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 29, 351–371. doi: 10.1039/c2np00084a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sauter, H., Steglich, W., and Anke, T. (1999). Strobilurins: evolution of a new class of active substances. Angew. Chem. Int. Ed. 38, 1328–1349.
Tanasova, M., and Sturla, S. J. (2012). Chemistry and biology of acylfulvenes: sesquiterpene-derived antitumor agents. Chem. Rev. 112, 3578–3610. doi: 10.1021/cr2001367
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tello, S. A., Silva-Flores, P., Agerer, R., Halbwachs, H., Beck, A., and Persoh, D. (2014). Hygrocybe virginea is a systemic endophyte of Plantago lanceolata. Mycol. Prog. 13, 471–475. doi: 10.1007/s11557-013-0928-0
Yin, X., Feng, T., Shang, J. H., Zhao, Y. L., Wang, F., Li, Z. H., et al. (2014). Chemical and toxicological investigations of a previously unknown poisonous European mushroom Tricholoma terreum. Chemistry 20, 7001–7009. doi: 10.1002/chem.201400226
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhou, Z.-Y., and Liu, J.-K. (2010). Pigments of fungi (macromycetes). Nat. Prod. Rep. 27, 1531–1570. doi: 10.1039/c004593d
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhou, Z. Y., Shi, G. Q., Fontaine, R., Wei, K., Feng, T., Wang, F., et al. (2012). Evidence for the natural toxins from the mushroom Trogia venenata as a cause of sudden unexpected death in Yunnan Province, China. Angew. Chem. Int. Ed. 51, 2368–2370. doi: 10.1002/anie.201106502
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: Basidiomycete, natural product, secondary metabolism, bioactivity
Citation: Stadler M and Hoffmeister D (2015) Fungal natural products—the mushroom perspective. Front. Microbiol. 6:127. doi: 10.3389/fmicb.2015.00127
Received: 25 October 2014; Accepted: 03 February 2015;
Published online: 18 February 2015.
Edited by:
Nancy Keller, University of Wisconsin-Madison, USAReviewed by:
Nancy Keller, University of Wisconsin-Madison, USAClaudia Schmidt-Dannert, University of Minnesota, USA
Copyright © 2015 Stadler and Hoffmeister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:bWFyYy5zdGFkbGVyQGhlbG1ob2x0ei1oemkuZGU=;ZGlyay5ob2ZmbWVpc3RlckBoa2ktamVuYS5kZQ==
 Marc Stadler
Marc Stadler Dirk Hoffmeister
Dirk Hoffmeister