- Department of Molecular Virology, Tokyo Medical and Dental University, Tokyo, Japan
Current human immunodeficiency virus type 1 pandemic is believed to originate from cross-species transmission of simian immunodeficiency virus (SIV) into human population. Such cross-species transmission, however, is not efficient in general, because viral replication is modulated by host cell factors, with the species-specificity of these factors affecting viral tropism. An understanding of those host cell factors that affect viral replication contributes to elucidation of the mechanism for determination of viral tropism. This review will focus an anti-viral effect of ApoB mRNA editing catalytic subunit, tripartite motif protein 5 alpha, and cyclophilins on SIV replication and provide insight into the mechanism of species-specific barriers against viral infection in human cells. It will then present our current understanding of the mechanism that may explain zoonotic transmission of retroviruses.
Introduction
There is significant evidence that the ongoing worldwide acquired immunodeficiency syndrome (AIDS) epidemic was caused by cross-species transmission of simian immunodeficiency viruses (SIVs) into the human population. Replication of primate lentiviruses in their natural hosts is generally non-pathogenic; however, cross-species transmission of these viruses can result in highly pathogenic phenotypes. How and when this transmission occurred is still debated but it is now generally accepted that HIV-2 originated from a sooty mangabeys strain of SIV (SIVsm; Hirsch et al., 1989; Chen et al., 1996) while HIV-1 appears to have originated from a chimpanzee strain of SIV (SIVcpz; Gao et al., 1999). Zoonotic transmission of SIVs, however, is not common and is controlled by host factors that generally prohibit SIV replication in human hosts and many human-derived cell lines.
Viral replication is modulated by host cell factors, with the species-specificity of these factors affecting viral tropism. Some of these host factors can restrict viral replication and the anti-viral systems mediated by such host restriction factors, termed intrinsic immunity, play an important role in determining species-specific barriers against viral infection. For instance, Fv-1 in mice is known to restrict replication of a murine leukemia virus (Rein et al., 1976; Gautsch et al., 1978; Towers et al., 2000) and tripartite interaction motif 5α (TRIM5α) recently has been found to be responsible for restricting HIV-1 but not SIV infection in Old World monkey (OWM) cells (Hatziioannou et al., 2004b; Keckesova et al., 2004; Stremlau et al., 2004; Yap et al., 2004; Song et al., 2005; Ylinen et al., 2005). Restriction of retroviral replication by these host cell factors takes place after viral entry, but before the integration step, and the viral determinants for this type of restriction have been mapped to the capsid (CA) protein (Gautsch et al., 1978; Kozak and Chakraborti, 1996; Towers et al., 2000; Goff, 2004; Stremlau et al., 2006). Two recent studies showed that the cellular protein SAMHD1 is myeloid-lineage cell-specific HIV-1 restriction factor counteracted by Vpx proteins from HIV-2 and SIVsm (Hrecka et al., 2011; Laguette et al., 2011). Restriction of lentivirus infection by SAMHD1 is likely to take place at the reverse transcription step. Another anti-retroviral protein, tetherin (also referred to as BST-2, CD317, or HM1.24) inhibits retrovirus release and is antagonized by HIV-1 Vpu protein, Nef protein of many SIVs, or Env protein of HIV-2 (Neil et al., 2008; Le Tortorec and Neil, 2009; Zhang et al., 2009). Understanding how host cell factors affect viral replication, positively or negatively, would contribute to elucidating the molecular mechanism that determines viral tropism. Here, we discuss an anti-viral effect of ApoB mRNA editing catalytic subunit (APOBEC), TRIM5α, and cyclophilins (Cyps) on SIV replication.
APOBEC: Enzymatic Restriction Factor that Target Retroviruses
Replication of HIV-1 in primary CD4+ T cells, monocyte, and some immortalized T cell lines depends on the presence of the HIV-1 accessory gene product, Vif (standing for virus infectivity factor; Fisher et al., 1987; Strebel et al., 1987), and it works in a host cell-specific manner. Vif is required for enhanced HIV-1 replication in some cell types called non-permissive cells. In contrast, HIV-1 replication is Vif-independent in permissive cells (Akari et al., 1992; Fan and Peden, 1992; Gabuzda et al., 1992; Blanc et al., 1993; Sakai et al., 1993; von Schwedler et al., 1993; Borman et al., 1995). Recently, some cytidine deaminases were identified as a new class of host restriction factors that target retroviruses such as HIV-1 or SIV (Harris and Liddament, 2004; Cullen, 2006). APOBEC3G (Apo3G), a member of the APOBEC family of cytidine deaminases, is the first identified enzymatic restriction factor and the determinant that makes cells permissive or non-permissive. Apo3G is also a host factor that restricts replication of human and simian lentiviruses in their respective target cells. Unlike TRIM5α or Fv-1, Apo3G does not exert its anti-viral activity by targeting the viral CA protein, but it has to be incorporated into a newly synthesized virion during a production step, and then inhibits virus replication by targeting single-stranded viral cDNA during a subsequent infection step. HIV-1 counteracts Apo3G with Vif expression. During the production of progeny virions, Vif binds to Apo3G and induces Apo3G’s proteasomal degradation, resulting in the decreased steady-state levels of human Apo3G (hApo3G; Yu et al., 2003).
There are several anti-retroviral mechanisms of Apo3G against HIV-1 infection. First, Apo3G-containing virus can accumulate in a large number of substitutions that register as cytidine (C) to deoxyuridine (dU) in a virus minus-strand during reverse transcription, resulting guanine (G) to adenine (A) mutations in a viral plus-strand, known as “G-to-A hypermutation” (Harris et al., 2003; Lecossier et al., 2003; Mangeat et al., 2003; Mariani et al., 2003; Zhang et al., 2003; Yu et al., 2004b). Second, Apo3G can inhibit tRNA annealing or tRNA processing during reverse transcription (Guo et al., 2006,2007; Mbisa et al., 2007). Third, Apo3G inhibits DNA strand transfer or integration (Li et al., 2007; Luo et al., 2007; Mbisa et al., 2007). Although Apo3G has the most potent anti-HIV-1 activity among the APOBEC family of proteins, another member of the family, APOBEC3F (Apo3F) was shown to inhibit HIV-1 infection in the absence of Vif (Bishop et al., 2004a; Liddament et al., 2004; Wiegand et al., 2004; Zheng et al., 2004), whereas APOBEC3B (Apo3B) can inhibit HIV-1 infection in both the presence and absence of Vif (Bishop et al., 2004a; Doehle et al., 2005; Rose et al., 2005).
Although we can imagine the broad range of anti-retroviral activity of APOBEC family because APOBEC proteins from non-human species can also inhibit HIV-1 infection (Mariani et al., 2003; Bishop et al., 2004a,2004b; Wiegand et al., 2004; Cullen, 2006), the Vif-Apo3G interaction is thought to be species-specific (Simon et al., 1998; Mariani et al., 2003). Accordingly, hApo3G is insensitive to SIVagm Vif while African green monkey Apo3G (agmApo3G) is insensitive to HIV-1 Vif and the determinant of this species-specificity depends on amino acid 128 of hApo3G and agmApo3G (Mariani et al., 2003; Bogerd et al., 2004; Mangeat et al., 2004; Schrofelbauer et al., 2004; Xu et al., 2004).
However, such species-specificity is not strictly controlled, for example, a report from the laboratory of Klaus Strebel demonstrated that SIVagm Vif supported replication of SIVagm virus in the hApo3G-positive human A3.01 T cell line (Takeuchi et al., 2005). Replication of vif-defective SIVagm in A3.01 cells was severely restricted, resulted in an accumulation of cytidine deaminase-induced G-to-A mutations in SIVagm genome (Takeuchi et al., 2005).
Moreover, two independent groups showed that the different APOBEC3 family members function to neutralize specific lentiviruses (Yu et al., 2004a; Dang et al., 2006). One report from the lab of Dr. Nathaniel R. Landau showed that APOBEC3B and APOBEC3C were potent inhibitors of SIV (Yu et al., 2004a). Both enzymes were efficiently encapsidated by HIV-1 and SIV. Another report from the lab of Dr. Yong-Hui Zheng demonstrated that APOBEC3DE blocked the replication of both HIV-1 and SIV but not that of MLV (Dang et al., 2006) and APOBEC3H inhibited the replication of HIV-1 by a cytidine deamination-independent mechanism (Dang et al., 2008). These findings raise the possibility that the various APOBEC3 family members protect against different lentiviruses and point to a possible role in the zoonotic transmission of SIV.
TRIM5α: Fv-1-Type Host Factor Restricting HIV-1 in Primate Cells
The host protein which dictates Ref1 activity was identified as an α-isoform of rhesus macaque TRIM5 protein by the laboratory of Dr. Joseph Sodroski (Stremlau et al., 2004). TRIM5 is a member of the TRIM family of proteins, and has RING, B-box 2, and coiled-coil as common and conserved domains among the family and B30.2 (PRYSPRY) domain on its C-terminal region (Nisole et al., 2005). Subsequently, the human and non-human primate homologs of TRIM5α were shown to restrict retroviruses, such as N-MLV, and equine infectious anemia virus (Hatziioannou et al., 2004b; Keckesova et al., 2004; Perron et al., 2004; Yap et al., 2004; Song et al., 2005; Ylinen et al., 2005; Si et al., 2006). Rhesus monkey TRIM5α (rhTRIM5α) has strong anti-HIV-1 activity but only modestly restricts SIV isolated from a macaque monkey (SIVmac) and does not block MLV infection, whereas its human homolog does not restrict HIV-1 infection.
TRIM5α recognizes incoming viral cores, but not a monomeric CA protein, thorough its B30.2 (PRYSPRY) domain. B-box 2 and coiled-coil domains are required for TRIM5α multimerization, and both coiled-coil and B30.2 (PRYSPRY) domains are essential for viral core binding (Reymond et al., 2001; Stremlau et al., 2006). TRIM5α captures HIV-1 core at a very early step(s) after infection, immediately after the release of the core into cytoplasm. To restrict HIV-1 infection and to recognize viral core, TRIM5α must oligomerize through its B-box 2 and coiled-coil domains (Mische et al., 2005; Li and Sodroski, 2008). Its RING domain has E3 ubiquitin ligase activity. It self-ubiquitination occurs TRIM5α is quickly degraded (Diaz-Griffero et al., 2006). This rapid degradation of TRIM5α is not required for post-entry restriction since replacement of TRIM5α RING domain with the corresponding domain of TRIM21, which has lower self-ubiquitination activity and a longer half-life than TRIM5α did not alter the anti-viral activity (Kar et al., 2008). Recently, the laboratory of Dr. Mark Yeager discussed a novel architecture made with dimers of TRIM5-21R. TRIM5α-21R forms a dimer through its B-box 2 and coiled-coil domains, and these dimers form six-sided rings on CA lattices to promote rapid core disassembly (Ganser-Pornillos et al., 2011). Overexpression of TRIM5α leads to the formation of cytoplasmic bodies and is believed to be required for its anti-viral activity (Stremlau et al., 2006; Campbell et al., 2008). During TRIM5α-mediated post-entry restriction, disassembly of viral cores is induced too quickly and the accumulation of viral RT-products is reduced (Stremlau et al., 2006). On the other hand, MG132 treatment inhibited quick-disassembly, yet HIV-1 infectivity was still restricted. Two reports showed that TRIM5α could block not only viral cDNA accumulation but also the nuclear import of viral cDNA (Berthoux et al., 2004; Wu et al., 2006). Thus, TRIM5α-mediated post-entry restriction is thought to have at least two phases: (i) TRIM5α induces rapid disassembly of viral core in a proteasome-dependent manner and (ii) TRIM5α degrades HIV-1 cDNAs in a proteasome-independent manner. The determinant of specificity and magnitude of the post-entry restriction lies on B30.2 (PRYSPRY) domain. Previous report showed that TRIM5α alleles did not cluster by species between rhesus macaques and sooty mangabeys and none of the alleles from either species restricted SIV, suggesting that there is little effect of rhTRIM5α on transmission of SIVsm within species (Newman et al., 2006). Recently, Pacheco et al. (2010) reported that New World monkey (NWM) TRIM5α restricts foamy virus infection. Another consideration is the clinical significance of TRIM5α against AIDS in human. Moreover, several reports showed that the efficacy of TRIM5α-mediated suppression of HIV-1 replication might interfere with disease progression of AIDS in humans (van Manen et al., 2008; Cagliani et al., 2010; Takeuchi et al., 2012). Thus, TRIM5α-mediated restriction may be a multi-step process in retrovirus replication with the relationship between other host factor(s).
Recently, the lab of Dr. Yasuhiro Ikeda reported that rhesus macaque TRIM5α also inhibits HIV-1 production by inducing the degradation of a viral precursor Gag protein (Sakuma et al., 2007). To restrict HIV-1 production, amino acid residues in B-box 2 and coiled-coil domains dictated the specificity of the restriction. In the late restriction, the accumulation of HIV-1 RNA was not affected but the accumulation of precursor Gag was inhibited in an ubiquitin–proteasome-independent manner. This TRIM5α-mediated late-restriction is still controversial (Zhang et al., 2008), yet it is conceivable that TRIM5α restricts HIV-1 infection and production in two distinct mechanisms. Although TRIM5α restricts HIV-1 infection in a broad range of cells, its late restriction involved transient overexpression (Sakuma et al., 2007).
Here is another notable class of the TRIM family called TRIM-Cyp isolated from NWM. A report from the laboratory of Dr. Jeremy Luban demonstrated that owl monkey cells express TRIM-Cyp that restricts HIV-1 infection (Sayah et al., 2004). Although TRIM-Cyp has a cyclophilin A (CypA) sequence in its C-terminal region instead of B30.2 (PRYSPRY) domain that dictates the specificity and the magnitude of post-entry restriction in OWM TRIM5α-mediated post-entry restriction, it recognizes incoming core structure and restricts HIV-1 infection (Stremlau et al., 2006). Recently, TRIM-Cyp mRNA was also detected in a rhesus macaque cell, and overexpressed rhesus TRIM-Cyp restricts HIV-1 infection and production (Newman et al., 2006; Brennan et al., 2008; Wilson et al., 2008; Dietrich et al., 2010).
Unlike other restriction factors, there is no known accessory gene product of HIV-1 to antagonize TRIM5α-mediated restrictions. Indeed, human TRIM5α has only a modest restriction activity against HIV-1 infection. TRIM5 proteins from several NWM species restrict infection by SIVmac and SIVagm (Song et al., 2005). This suggests that TRIM5α could be a key molecule of the species-species barrier.
Cyclophilins: Host Factors Involved in Retrovirus Replication
Cyclophilins are ubiquitous proteins and first identified as the target of cyclosporine A (CsA), an immunosuppressive reagent (Takahashi et al., 1989). CypA has proline-isomerase activity that catalyzes the cis–trans isomerization of proline residue (Fischer et al., 1989). The binding of CsA to CypA inhibits this isomerase activity (Takahashi et al., 1989). In retrovirus replication, CypA was found to bind HIV-1 CA in the yeast two-hybrid system (Luban et al., 1993). The sequence Ala88-Gly89-Pro90-Ile91 of CA protein is the major fragment bound to the active site of CypA (Franke et al., 1994; Gamble et al., 1996; Zhao et al., 1997). Interestingly, The peptidyl-prolyl bond between Gly89 and Pro90 of the CA fragment has a trans conformation, in contrast to the cis conformation observed in other known CypA–peptide complexes (Zhao et al., 1997; Bosco et al., 2002), and Gly89 preceding Pro90 has an unfavorable backbone formation usually only adopted by glycine, suggesting that special Gly89-Pro90 sequence but not other Gly-Pro motif is required for the binding of CA protein to CypA. Therefore, CypA might be likely to act as a molecular chaperone but not a cis–trans isomerase (Zhao et al., 1997). However, one report showed that CypA does not only bind CA protein but also catalyzes efficiently cis–trans isomerization of Gly89-Pro90 peptidyl-prolyl bond (Bosco et al., 2002). The relationship between the Gly89-Pro90 bond and catalysis of cis–trans isomerization by CypA remains unclear.
It has been well established that CypA promotes an early step of HIV-1 infection in human cells (Franke et al., 1994; Thali et al., 1994; Braaten et al., 1996a,1996c; Franke and Luban, 1996; Braaten and Luban, 2001; Sokolskaja et al., 2004; Hatziioannou et al., 2005). CypA is efficiently encapsidated into HIV-1 produced from infected cells through interaction with the CA domains of the Gag polyprotein and disruption of CypA incorporation into virions by CsA or HIV-1 Gag mutants caused a decrease in replication efficiency (Franke et al., 1994; Thali et al., 1994; Ott et al., 1995; Braaten et al., 1996a; Bukovsky et al., 1997; Ackerson et al., 1998; Braaten and Luban, 2001). It is still unclear how CypA is efficiently packaged into HIV-1 virion, but several reports showed that both dimerization of CA and multimerization of CypA are required for efficient interaction (Colgan et al., 1996; Javanbakht et al., 2007). Although CA-CypA interaction is required for infectivity, the important point is that CypA interacts with incoming HIV-1 cores in newly infected target cells rather than during HIV-1 budding from the virion producer cells, indicating that target cell CypA promotes HIV-1 infectivity (Kootstra et al., 2003; Towers et al., 2003; Sokolskaja et al., 2004).
CypA-dependent virus replication is only limited to retroviruses which encode CA that binds CypA. In fact, only those retroviruses are dependent upon CypA for replication (Luban et al., 1993; Franke et al., 1994; Thali et al., 1994; Braaten et al., 1996c; Franke and Luban, 1996). These observations suggested that CA–CypA interaction might contribute tropism determinants for retroviruses. HIV-1 infection in non-human primate cells is blocked prior to reverse transcription after virus entry (Shibata et al., 1995; Himathongkham and Luciw, 1996; Hofmann et al., 1999; Besnier et al., 2002; Cowan et al., 2002; Munk et al., 2002; Hatziioannou et al., 2003; Towers et al., 2003). This restriction is thought to be the same step in the retrovirus life cycle where CypA works (Braaten et al., 1996b). Indeed, analysis of CypA-binding region of CA with chimeric viruses of HIV-1 and SIV showed the viral determinant for species-specificity (Shibata et al., 1991,1995; Dorfman and Gottlinger, 1996; Bukovsky et al., 1997; Cowan et al., 2002; Kootstra et al., 2003; Owens et al., 2003,2004; Towers et al., 2003; Berthoux et al., 2004; Hatziioannou et al., 2004a,2006; Ikeda et al., 2004; Sayah et al., 2004; Stremlau et al., 2004; Kamada et al., 2006).
Human CypA is required for efficient HIV-1 infection but not SIV. There is no known role for CypA in SIV infection in human cells. Recently, the first report from the laboratory of Klaus Strebel showed that human CypA acts as restriction factor against the infection of two SIVs (SIVmac and SIVagm) in human cells, and Vif protein of two SIVs counteracts a CypA-imposed inhibition against the infection of two SIV strains with exclusion of CypA from SIV virion (Takeuchi et al., 2007). This phenomenon is different from the function of SIVagm Vif against hApo3G previously reported from the same laboratory (Takeuchi et al., 2005) because they used human cells lacking detectable deaminase activity.
Moreover, a recent report showed a species-specific effect of CsA, a peptidyl-prolyl cis–trans isomerase (PPIase) inhibitor, on SIV replication, implying a possible contribution of Cyps to the determination of SIV tropism (Figure 1; Takeuchi et al., 2012). They demonstrated a host species-specific effect of CypA on SIV replication: CypA affects the replication of two SIVs (SIVmac and SIVagm) negatively in human cells but positively in macaque cells (Figure 1). Further analysis indicated that the infection of two SIVs was not significantly affected by CypA but inhibited by cyclophilin B (CypB), another PPIase, in human cells (Figure 2A; Takeuchi et al., 2012). In contrast, CypA is likely to have positive effects on the infection of two SIVs in macaque cells (Figure 2B; Takeuchi et al., 2012). These results suggest that Cyps might have a host species-specific effect of Cyps on SIV replication and provide insight into the mechanism of species-specific barriers against viral infection.
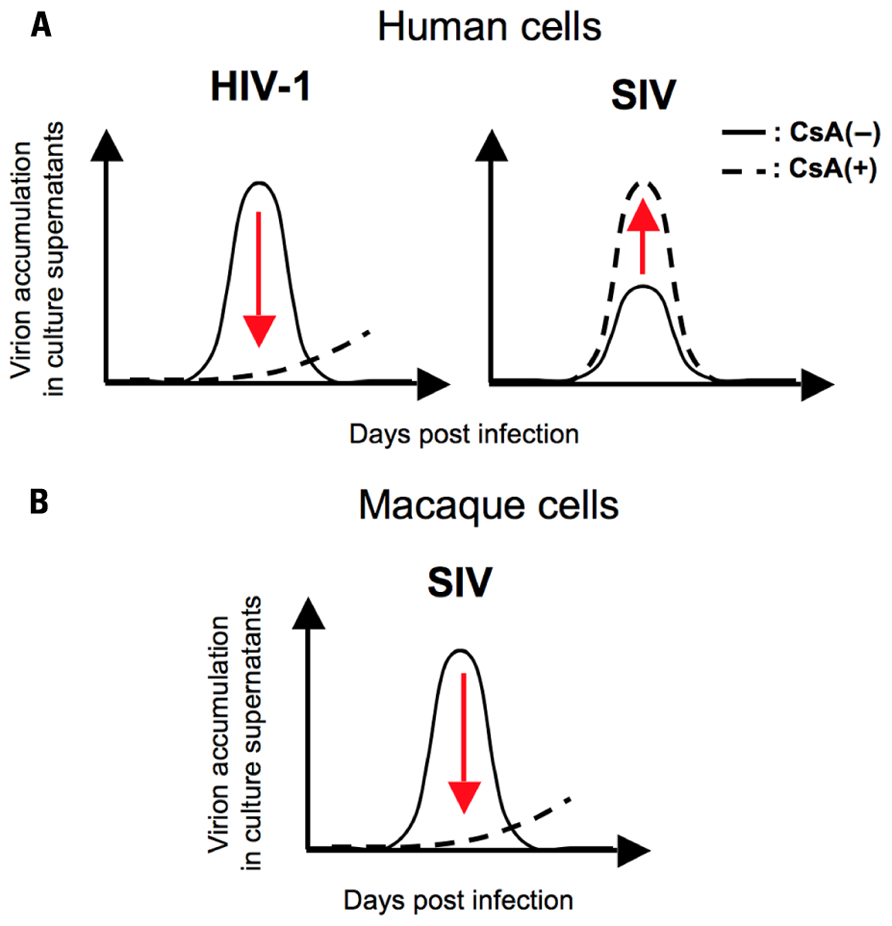
FIGURE 1. A schema for the effect of CsA on HIV/SIV replication in human/macaque cells. (A) CsA treatment impairs the replication of HIV-1 (left panel) but enhances SIV replication (right panel) in human cells. (B) CsA treatment inhibits SIV replication in macaque cells. The solid line indicates virion accumulation of culture supernatant in the absence of CsA and the broken line indicates that of culture supernatant in the presence of CsA.
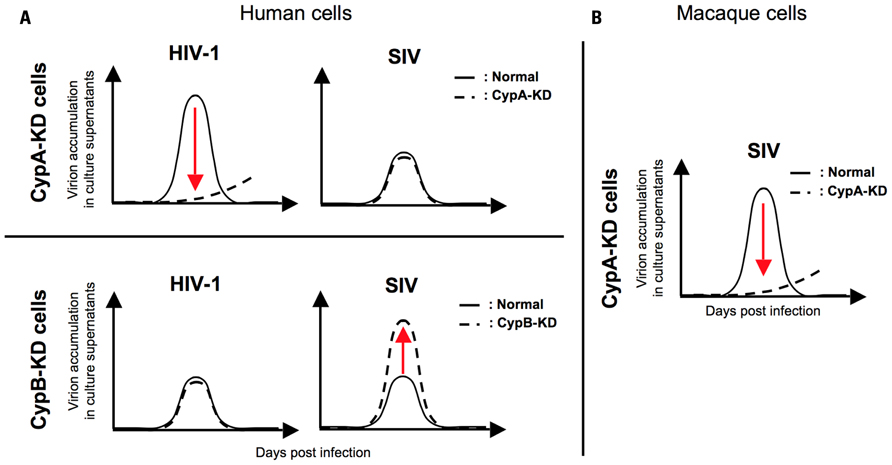
FIGURE 2. A schema for the effect of cyclophilin A and cyclophilin B on HIV/SIV replication in human/macaque cells. (A) CypA knock-down (CypA-KD) impairs the replication of HIV-1 (upper left panel). In contrast, SIV replication is not reduced but rather enhanced by CypA knock-down (upper right panel). CypB knock-down (CypB-KD) shows no significant effect on HIV-1 replication (lower left panel) but enhances the replication of SIV (lower right panel). (B) CypA-KD inhibits SIV infection. The solid line indicates virion accumulation of culture supernatant produced from normal cells and the broken line indicates that of culture supernatant produced from CypA or CypB knock-down cells.
Concluding Remarks
Viral replication is modulated by host cell factors. Many of these factors function in a species-specific manner. On the other hand, there exist host factors that restrict viral replication. The anti-viral system mediated by some of these restriction factors, termed intrinsic immunity, which is distinguished from the conventional innate and adaptive immunity has been indicated to play an important role in making species-specific barriers against viral infection. As discussed in this review, we describe the current progress in understanding of such restriction factors against retroviral replication, especially focusing on TRIM5α and APOBEC whose anti-retroviral effects have recently been recognized. Additionally, we mentioned a host species-specific effect of Cyps including CypA and CypB on SIV replication. Such restriction factors would play an important role in determining species-specific barriers against viral infection.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any
Acknowledgments
This work supported by a grant for Young Scientists of HIV/AIDS research from the Ministry of Health, Labor, and Welfare of Japan.
References
Ackerson, B., Rey, O., Canon, J., and Krogstad, P. (1998). Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A. J. Virol. 72, 303–308.
Akari, H., Sakuragi, J., Takebe, Y., Tomonaga, K., Kawamura, M., Fukasawa, M., Miura, T., Shinjo, T., and Hayami, M. (1992). Biological characterization of human immunodeficiency virus type 1 and type 2 mutants in human peripheral blood mononuclear cells. Arch. Virol. 123, 157–167.
Berthoux, L., Sebastian, S., Sokolskaja, E., and Luban, J. (2004). Lv1 inhibition of human immunodeficiency virus type 1 is counteracted by factors that stimulate synthesis or nuclear translocation of viral cDNA. J. Virol. 78, 11739–11750.
Besnier, C., Takeuchi, Y., and Towers, G. (2002). Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. U.S.A. 99, 11920–11925.
Bishop, K. N., Holmes, R. K., Sheehy, A. M., Davidson, N. O., Cho, S. J., and Malim, M. H. (2004a). Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 14, 1392–1396.
Bishop, K. N., Holmes, R. K., Sheehy, A. M., and Malim, M. H. (2004b). APOBEC-mediated editing of viral RNA. Science 305, 645.
Blanc, D., Patience, C., Schulz, T. F., Weiss, R., and Spire, B. (1993). Transcomplementation of VIF- HIV-1 mutants in CEM cells suggests that VIF affects late steps of the viral life cycle. Virology 193, 186–192.
Bogerd, H. P., Doehle, B. P., Wiegand, H. L., and Cullen, B. R. (2004). A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc. Natl. Acad. Sci. U.S.A. 101, 3770–3774.
Borman, A. M., Quillent, C., Charneau, P., Dauguet, C., and Clavel, F. (1995). Human immunodeficiency virus type 1 Vif- mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J. Virol. 69, 2058–2067.
Bosco, D. A., Eisenmesser, E. Z., Pochapsky, S., Sundquist, W. I., and Kern, D. (2002). Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A. Proc. Natl. Acad. Sci. U.S.A. 99, 5247–5252.
Braaten, D., Aberham, C., Franke, E. K., Yin, L., Phares, W., and Luban, J. (1996a). Cyclosporine A-resistant human immunodeficiency virus type 1 mutants demonstrate that Gag encodes the functional target of cyclophilin A. J. Virol. 70, 5170–5176.
Braaten, D., Franke, E. K., and Luban, J. (1996b). Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70, 3551–3560.
Braaten, D., Franke, E. K., and Luban, J. (1996c). Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIV(CPZ)GAB but not group O HIV-1 or other primate immunodeficiency viruses. J. Virol. 70, 4220–4227.
Braaten, D., and Luban, J. (2001). Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20, 1300–1309.
Brennan, G., Kozyrev, Y., and Hu, S. L. (2008). TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl. Acad. Sci. U.S.A. 105, 3569–3574.
Bukovsky, A. A., Weimann, A., Accola, M. A., and Gottlinger, H. G. (1997). Transfer of the HIV-1 cyclophilin-binding site to simian immunodeficiency virus from Macaca mulatta can confer both cyclosporin sensitivity and cyclosporin dependence. Proc. Natl. Acad. Sci. U.S.A. 94, 10943–10948.
Cagliani, R., Fumagalli, M., Biasin, M., Piacentini, L., Riva, S., Pozzoli, U., Bonaglia, M. C., Bresolin, N., Clerici, M., and Sironi, M. (2010). Long-term balancing selection maintains trans-specific polymorphisms in the human TRIM5 gene. Hum. Genet. 128, 577–588.
Campbell, E. M., Perez, O., Anderson, J. L., and Hope, T. J. (2008). Visualization of a proteasome-independent intermediate during restriction of HIV-1 by rhesus TRIM5alpha. J. Cell Biol. 180, 549–561.
Chen, Z., Telfier, P., Gettie, A., Reed, P., Zhang, L., Ho, D. D., and Marx, P. A. (1996). Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 70, 3617–3627.
Colgan, J., Yuan, H. E., Franke, E. K., and Luban, J. (1996). Binding of the human immunodeficiency virus type 1 Gag polyprotein to cyclophilin A is mediated by the central region of capsid and requires Gag dimerization. J. Virol. 70, 4299–4310.
Cowan, S., Hatziioannou, T., Cunningham, T., Muesing, M. A., Gottlinger, H. G., and Bieniasz, P. D. (2002). Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. U.S.A. 99, 11914–11919.
Cullen, B. R. (2006). Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J. Virol. 80, 1067–1076.
Dang, Y., Siew, L. M., Wang, X., Han, Y., Lampen, R., and Zheng, Y. H. (2008). Human cytidine deaminase APOBEC3H restricts HIV-1 replication. J. Biol. Chem. 283, 11606–11614.
Dang, Y., Wang, X., Esselman, W. J., and Zheng, Y. H. (2006). Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J. Virol. 80, 10522–10533.
Diaz-Griffero, F., Li, X., Javanbakht, H., Song, B., Welikala, S., Stremlau, M., and Sodroski, J. (2006). Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology 349, 300–315.
Dietrich, E. A., Jones-Engel, L., and Hu, S. L. (2010). Evolution of the antiretroviral restriction factor TRIMCyp in Old World primates. PLoS ONE 5, e14019. doi: 10.1371/journal.pone.0014019
Doehle, B. P., Schafer, A., and Cullen, B. R. (2005). Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology 339, 281–288.
Dorfman, T., and Gottlinger, H. G. (1996). The human immunodeficiency virus type 1 capsid p2 domain confers sensitivity to the cyclophilin-binding drug SDZ NIM 811. J. Virol. 70, 5751–5757.
Fischer, G., Wittmann-Liebold, B., Lang, K., Kiefhaber, T., and Schmid, F. X. (1989). Cyclophilin and peptidyl-prolyl cis–trans isomerase are probably identical proteins. Nature 337, 476–478.
Fisher, A. G., Ensoli, B., Ivanoff, L., Chamberlain, M., Petteway, S., Ratner, L., Gallo, R. C., and Wong-Staal, F. (1987). The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science 237, 888–893.
Franke, E. K., and Luban, J. (1996). Inhibition of HIV-1 replication by cyclosporine A or related compounds correlates with the ability to disrupt the Gag-cyclophilin A interaction. Virology 222, 279–282.
Franke, E. K., Yuan, H. E., and Luban, J. (1994). Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372, 359–362.
Gabuzda, D. H., Lawrence, K., Langhoff, E., Terwilliger, E., Dorfman, T., Haseltine, W. A., and Sodroski, J. (1992). Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J. Virol. 66, 6489–6495.
Gamble, T. R., Vajdos, F. F., Yoo, S., Worthylake, D. K., Houseweart, M., Sundquist, W. I., and Hill, C. P. (1996). Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87, 1285–1294.
Ganser-Pornillos, B. K., Chandrasekaran, V., Pornillos, O., Sodroski, J. G., Sundquist, W. I., and Yeager, M. (2011). Hexagonal assembly of a restricting TRIM5alpha protein. Proc. Natl. Acad. Sci. U.S.A. 108, 534–539.
Gao, F., Bailes, E., Robertson, D. L., Chen, Y., Rodenburg, C. M., Michael, S. F., Cummins, L. B., Arthur, L. O., Peeters, M., Shaw, G. M., Sharp, P. M., and Hahn, B. H. (1999). Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397, 436–441.
Gautsch, J. W., Elder, J. H., Schindler, J., Jensen, F. C., and Lerner, R. A. (1978). Structural markers on core protein p30 of murine leukemia virus: functional correlation with Fv-1 tropism. Proc. Natl. Acad. Sci. U.S.A. 75, 4170–4174.
Goff, S. P. (2004). Genetic control of retrovirus susceptibility in mammalian cells. Annu. Rev. Genet. 38, 61–85.
Guo, F., Cen, S., Niu, M., Saadatmand, J., and Kleiman, L. (2006). Inhibition of formula-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication. J. Virol. 80, 11710–11722.
Guo, F., Cen, S., Niu, M., Yang, Y., Gorelick, R. J., and Kleiman, L. (2007). The interaction of APOBEC3G with human immunodeficiency virus type 1 nucleocapsid inhibits tRNA3Lys annealing to viral RNA. J. Virol. 81, 11322–11331.
Harris, R. S., Bishop, K. N., Sheehy, A. M., Craig, H. M., Petersen-Mahrt, S. K., Watt, I. N., Neuberger, M. S., and Malim, M. H. (2003). DNA deamination mediates innate immunity to retroviral infection. Cell 113, 803–809.
Harris, R. S., and Liddament, M. T. (2004). Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 4, 868–877.
Hatziioannou, T., Cowan, S., Goff, S. P., Bieniasz, P. D., and Towers, G. J. (2003). Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22, 385–394.
Hatziioannou, T., Cowan, S., von Schwedler, U. K., Sundquist, W. I., and Bieniasz, P. D. (2004a). Species-specific tropism determinants in the human immunodeficiency virus type 1 capsid. J. Virol. 78, 6005–6012.
Hatziioannou, T., Perez-Caballero, D., Yang, A., Cowan, S., and Bieniasz, P. D. (2004b). Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc. Natl. Acad. Sci. U.S.A. 101, 10774–10779.
Hatziioannou, T., Perez-Caballero, D., Cowan, S., and Bieniasz, P. D. (2005). Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells. J. Virol. 79, 176–183.
Hatziioannou, T., Princiotta, M., Piatak, M. Jr., Yuan, F., Zhang, F., Lifson, J. D., and Bieniasz, P. D. (2006). Generation of simian-tropic HIV-1 by restriction factor evasion. Science 314, 95.
Himathongkham, S., and Luciw, P. A. (1996). Restriction of HIV-1 (subtype B) replication at the entry step in rhesus macaque cells. Virology 219, 485–488.
Hirsch, V. M., Olmsted, R. A., Murphey-Corb, M., Purcell, R. H., and Johnson, P. R. (1989). An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339, 389–392.
Hofmann, W., Schubert, D., Labonte, J., Munson, L., Gibson, S., Scammell, J., Ferrigno, P., and Sodroski, J. (1999). Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73, 10020–10028.
Hrecka, K., Hao, C., Gierszewska, M., Swanson, S. K., Kesik-Brodacka, M., Srivastava, S., Florens, L., Washburn, M. P., and Skowronski, J. (2011). Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661.
Ikeda, Y., Ylinen, L. M., Kahar-Bador, M., and Towers, G. J. (2004). Influence of gag on human immunodeficiency virus type 1 species-specific tropism. J. Virol. 78, 11816–11822.
Javanbakht, H., Diaz-Griffero, F., Yuan, W., Yeung, D. F., Li, X., Song, B., and Sodroski, J. (2007). The ability of multimerized cyclophilin A to restrict retrovirus infection. Virology 367, 19–29.
Kamada, K., Igarashi, T., Martin, M. A., Khamsri, B., Hatcho, K., Yamashita, T., Fujita, M., Uchiyama, T., and Adachi, A. (2006). Generation of HIV-1 derivatives that productively infect macaque monkey lymphoid cells. Proc. Natl. Acad. Sci. U.S.A. 103, 16959–16964.
Kar, A. K., Diaz-Griffero, F., Li, Y., Li, X., and Sodroski, J. (2008). Biochemical and biophysical characterization of a chimeric TRIM21-TRIM5alpha protein. J. Virol. 82, 11669–11681.
Keckesova, Z., Ylinen, L. M., and Towers, G. J. (2004). The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc. Natl. Acad. Sci. U.S.A. 101, 10780–10785.
Kootstra, N. A., Munk, C., Tonnu, N., Landau, N. R., and Verma, I. M. (2003). Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. U.S.A. 100, 1298–1303.
Kozak, C. A., and Chakraborti, A. (1996). Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology 225, 300–305.
Laguette, N., Sobhian, B., Casartelli, N., Ringeard, M., Chable-Bessia, C., Segeral, E., Yatim, A., Emiliani, S., Schwartz, O., and Benkirane, M. (2011). SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657.
Lecossier, D., Bouchonnet, F., Clavel, F., and Hance, A. J. (2003). Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 300, 1112.
Le Tortorec, A., and Neil, S. J. (2009). Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83, 11966–11978.
Li, X., and Sodroski, J. (2008). The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J. Virol. 82, 11495–11502.
Li, X. Y., Guo, F., Zhang, L., Kleiman, L., and Cen, S. (2007). APOBEC3G inhibits DNA strand transfer during HIV-1 reverse transcription. J. Biol. Chem. 282, 32065–32074.
Liddament, M. T., Brown, W. L., Schumacher, A. J., and Harris, R. S. (2004). APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr. Biol. 14, 1385–1391.
Luban, J., Bossolt, K. L., Franke, E. K., Kalpana, G. V., and Goff, S. P. (1993). Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73, 1067–1078.
Luo, K., Wang, T., Liu, B., Tian, C., Xiao, Z., Kappes, J., and Yu, X. F. (2007). Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J. Virol. 81, 7238–7248.
Mangeat, B., Turelli, P., Caron, G., Friedli, M., Perrin, L., and Trono, D. (2003). Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424, 99–103.
Mangeat, B., Turelli, P., Liao, S., and Trono, D. (2004). A single amino acid determinant governs the species-specific sensitivity of APOBEC3G to Vif action. J. Biol. Chem. 279, 14481–14483.
Mariani, R., Chen, D., Schrofelbauer, B., Navarro, F., Konig, R., Bollman, B., Munk, C., Nymark-Mcmahon, H., and Landau, N. R. (2003). Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114, 21–31.
Mbisa, J. L., Barr, R., Thomas, J. A., Vandegraaff, N., Dorweiler, I. J., Svarovskaia, E. S., Brown, W. L., Mansky, L. M., Gorelick, R. J., Harris, R. S., Engelman, A., and Pathak, V. K. (2007). Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J. Virol. 81, 7099–7110.
Mische, C. C., Javanbakht, H., Song, B., Diaz-Griffero, F., Stremlau, M., Strack, B., Si, Z., and Sodroski, J. (2005). Retroviral restriction factor TRIM5alpha is a trimer. J. Virol. 79, 14446–14450.
Munk, C., Brandt, S. M., Lucero, G., and Landau, N. R. (2002). A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. U.S.A. 99, 13843–13848.
Neil, S. J., Zang, T., and Bieniasz, P. D. (2008). Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430.
Newman, R. M., Hall, L., Connole, M., Chen, G. L., Sato, S., Yuste, E., Diehl, W., Hunter, E., Kaur, A., Miller, G. M., and Johnson, W. E. (2006). Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5alpha. Proc. Natl. Acad. Sci. U.S.A. 103, 19134–19139.
Nisole, S., Stoye, J. P., and Saib, A. (2005). TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3, 799–808.
Ott, D. E., Coren, L. V., Johnson, D. G., Sowder, R. C. II, Arthur, L. O., and Henderson, L. E. (1995). Analysis and localization of cyclophilin A found in the virions of human immunodeficiency virus type 1 MN strain. AIDS Res. Hum. Retroviruses 11, 1003–1006.
Owens, C. M., Song, B., Perron, M. J., Yang, P. C., Stremlau, M., and Sodroski, J. (2004). Binding and susceptibility to postentry restriction factors in monkey cells are specified by distinct regions of the human immunodeficiency virus type 1 capsid. J. Virol. 78, 5423–5437.
Owens, C. M., Yang, P. C., Gottlinger, H., and Sodroski, J. (2003). Human and simian immunodeficiency virus capsid proteins are major viral determinants of early, postentry replication blocks in simian cells. J. Virol. 77, 726–731.
Pacheco, B., Finzi, A., Mcgee-Estrada, K., and Sodroski, J. (2010). Species-specific inhibition of foamy viruses from South American monkeys by New World Monkey TRIM5{alpha} proteins. J. Virol. 84, 4095–4099.
Perron, M. J., Stremlau, M., Song, B., Ulm, W., Mulligan, R. C., and Sodroski, J. (2004). TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl. Acad. Sci. U.S.A. 101, 11827–11832.
Rein, A., Kashmiri, S. V., Bassin, R. H., Gerwin, B. L., and Duran-Troise, G. (1976). Phenotypic mixing between N- and B-tropic murine leukemia viruses: infectious particles with dual sensitivity to Fv-1 restriction. Cell 7, 373–379.
Reymond, A., Meroni, G., Fantozzi, A., Merla, G., Cairo, S., Luzi, L., Riganelli, D., Zanaria, E., Messali, S., Cainarca, S., Guffanti, A., Minucci, S., Pelicci, P. G., and Ballabio, A. (2001). The tripartite motif family identifies cell compartments. EMBO J. 20, 2140–2151.
Rose, K. M., Marin, M., Kozak, S. L., and Kabat, D. (2005). Regulated production and anti-HIV type 1 activities of cytidine deaminases APOBEC3B, 3F, and 3G. AIDS Res. Hum. Retroviruses 21, 611–619.
Sakai, H., Shibata, R., Sakuragi, J., Sakuragi, S., Kawamura, M., and Adachi, A. (1993). Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J. Virol. 67, 1663–1666.
Sakuma, R., Noser, J. A., Ohmine, S., and Ikeda, Y. (2007). Rhesus monkey TRIM5alpha restricts HIV-1 production through rapid degradation of viral Gag polyproteins. Nat. Med. 13, 631–635.
Sayah, D. M., Sokolskaja, E., Berthoux, L., and Luban, J. (2004). Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430, 569–573.
Schrofelbauer, B., Chen, D., and Landau, N. R. (2004). A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif). Proc. Natl. Acad. Sci. U.S.A. 101, 3927–3932.
Shibata, R., Kawamura, M., Sakai, H., Hayami, M., Ishimoto, A., and Adachi, A. (1991). Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J. Virol. 65, 3514–3520.
Shibata, R., Sakai, H., Kawamura, M., Tokunaga, K., and Adachi, A. (1995). Early replication block of human immunodeficiency virus type 1 in monkey cells. J. Gen. Virol. 76(Pt 11), 2723–2730.
Si, Z., Vandegraaff, N., O’huigin, C., Song, B., Yuan, W., Xu, C., Perron, M., Li, X., Marasco, W. A., Engelman, A., Dean, M., and Sodroski, J. (2006). Evolution of a cytoplasmic tripartite motif (TRIM) protein in cows that restricts retroviral infection. Proc. Natl. Acad. Sci. U.S.A. 103, 7454–7459.
Simon, J. H., Miller, D. L., Fouchier, R. A., Soares, M. A., Peden, K. W., and Malim, M. H. (1998). The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 17, 1259–1267.
Sokolskaja, E., Sayah, D. M., and Luban, J. (2004). Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J. Virol. 78, 12800–12808.
Song, B., Javanbakht, H., Perron, M., Park, D. H., Stremlau, M., and Sodroski, J. (2005). Retrovirus restriction by TRIM5alpha variants from Old World and New World primates. J. Virol. 79, 3930–3937.
Strebel, K., Daugherty, D., Clouse, K., Cohen, D., Folks, T., and Martin, M. A. (1987). The HIV ‘A’ (sor) gene product is essential for virus infectivity. Nature 328, 728–730.
Stremlau, M., Owens, C. M., Perron, M. J., Kiessling, M., Autissier, P., and Sodroski, J. (2004). The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853.
Stremlau, M., Perron, M., Lee, M., Li, Y., Song, B., Javanbakht, H., Diaz-Griffero, F., Anderson, D. J., Sundquist, W. I., and Sodroski, J. (2006). Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. U.S.A. 103, 5514–5519.
Takahashi, N., Hayano, T., and Suzuki, M. (1989). Peptidyl-prolyl cis–trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337, 473–475.
Takeuchi, H., Buckler-White, A., Goila-Gaur, R., Miyagi, E., Khan, M. A., Opi, S., Kao, S., Sokolskaja, E., Pertel, T., Luban, J., and Strebel, K. (2007). Vif counteracts a cyclophilin A-imposed inhibition of simian immunodeficiency viruses in human cells. J. Virol. 81, 8080–8090.
Takeuchi, H., Ishii, H., Kuwano, T., Inagaki, N., Akari, H., and Matano, T. (2012). Host cell species-specific effect of cyclosporine A on simian immunodeficiency virus replication. Retrovirology 9, 3.
Takeuchi, H., Kao, S., Miyagi, E., Khan, M. A., Buckler-White, A., Plishka, R., and Strebel, K. (2005). Production of infectious SIVagm from human cells requires functional inactivation but not viral exclusion of human APOBEC3G. J. Biol. Chem. 280, 375–382.
Thali, M., Bukovsky, A., Kondo, E., Rosenwirth, B., Walsh, C. T., Sodroski, J., and Gottlinger, H. G. (1994). Functional association of cyclophilin A with HIV-1 virions. Nature 372, 363–365.
Towers, G., Bock, M., Martin, S., Takeuchi, Y., Stoye, J. P., and Danos, O. (2000). A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. U.S.A. 97, 12295–12299.
Towers, G. J., Hatziioannou, T., Cowan, S., Goff, S. P., Luban, J., and Bieniasz, P. D. (2003). Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 9, 1138–1143.
van Manen, D., Rits, M. A., Beugeling, C., Van Dort, K., Schuitemaker, H., and Kootstra, N. A. (2008). The effect of Trim5 polymorphisms on the clinical course of HIV-1 infection. PLoS Pathog. 4, e18. doi: 10.1371/journal.ppat.0040018
von Schwedler, U., Song, J., Aiken, C., and Trono, D. (1993). Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J. Virol. 67, 4945–4955.
Wiegand, H. L., Doehle, B. P., Bogerd, H. P., and Cullen, B. R. (2004). A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 23, 2451–2458.
Wilson, S. J., Webb, B. L., Ylinen, L. M., Verschoor, E., Heeney, J. L., and Towers, G. J. (2008). Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 105, 3557–3562.
Wu, X., Anderson, J. L., Campbell, E. M., Joseph, A. M., and Hope, T. J. (2006). Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. U.S.A. 103, 7465–7470.
Xu, H., Svarovskaia, E. S., Barr, R., Zhang, Y., Khan, M. A., Strebel, K., and Pathak, V. K. (2004). A single amino acid substitution in human APOBEC3G antiretroviral enzyme confers resistance to HIV-1 virion infectivity factor-induced depletion. Proc. Natl. Acad. Sci. U.S.A. 101, 5652–5657.
Yap, M. W., Nisole, S., Lynch, C., and Stoye, J. P. (2004). TRIM5alpha protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. U.S.A. 101, 10786–10791.
Ylinen, L. M., Keckesova, Z., Wilson, S. J., Ranasinghe, S., and Towers, G. J. (2005). Differential restriction of human immunodeficiency virus type 2 and simian immunodeficiency virus SIVmac by TRIM5alpha alleles. J. Virol. 79, 11580–11587.
Yu, Q., Chen, D., Konig, R., Mariani, R., Unutmaz, D., and Landau, N. R. (2004a). APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J. Biol. Chem. 279, 53379–53386.
Yu, Q., Konig, R., Pillai, S., Chiles, K., Kearney, M., Palmer, S., Richman, D., Coffin, J. M., and Landau, N. R. (2004b). Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat. Struct. Mol. Biol. 11, 435–442.
Yu, X., Yu, Y., Liu, B., Luo, K., Kong, W., Mao, P., and Yu, X. F. (2003). Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302, 1056–1060.
Zhang, F., Perez-Caballero, D., Hatziioannou, T., and Bieniasz, P. D. (2008). No effect of endogenous TRIM5alpha on HIV-1 production. Nat. Med. 14, 235–236; author reply 236–238.
Zhang, F., Wilson, S. J., Landford, W. C., Virgen, B., Gregory, D., Johnson, M. C., Munch, J., Kirchhoff, F., Bieniasz, P. D., and Hatziioannou, T. (2009). Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6, 54–67.
Zhang, H., Yang, B., Pomerantz, R. J., Zhang, C., Arunachalam, S. C., and Gao, L. (2003). The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424, 94–98.
Zhao, Y., Chen, Y., Schutkowski, M., Fischer, G., and Ke, H. (1997). Cyclophilin A complexed with a fragment of HIV-1 gag protein: insights into HIV-1 infectious activity. Structure 5, 139–146.
Keywords: HIV-1, SIV, APOBEC3G, TRIM5α, cyclophilin A, cyclophilin B
Citation: Sakuma R and Takeuchi H (2012) SIV replication in human cells. Front. Microbio. 3:162. doi: 10.3389/fmicb.2012.00162
Received: 01 February 2012; Accepted: 10 April 2012;
Published online: 27 April 2012.
Edited by:
Akio Adachi, The University of Tokushima Graduate School, JapanReviewed by:
Vanessa Hirsch, National Institute of Allergy and Infectious Diseases, USATomoyuki Miura, Kyoto University, Japan
Klaus Strebel, National Institutes of Health, USA
Copyright: © 2012 Sakuma and Takeuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Hiroaki Takeuchi, Department of Molecular Virology, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8519, Japan. e-mail:aHRha2UubW9sdkB0bWQuYWMuanA=

