- 1 Center for Infectious Disease Research, Medical College of Wisconsin, Milwaukee, WI, USA
- 2 Department of Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, WI, USA
Pseudomonas aeruginosa possesses a type III secretion system (T3SS) to intoxicate host cells and evade innate immunity. This virulence-related machinery consists of a molecular syringe and needle assembled on the bacterial surface, which allows delivery of T3 effector proteins into infected cells. To accomplish a one-step effector translocation, a tip protein is required at the top end of the T3 needle structure. Strains lacking expression of the functional tip protein fail to intoxicate host cells. P. aeruginosa encodes a T3S that is highly homologous to the proteins encoded by Yersinia spp. The needle-tip proteins of Yersinia, LcrV, and P. aeruginosa, PcrV, share 37% identity and 65% similarity. Other known tip proteins are AcrV (Aeromonas), IpaD (Shigella), SipD (Salmonella), BipD (Burkholderia), EspA (EPEC, EHEC), Bsp22 (Bordetella), with additional proteins identified from various Gram-negative species, such as Vibrio and Bordetella. The tip proteins can serve as a protective antigen or may be critical for sensing host cells and evading innate immune responses. Recognition of the host microenvironment transcriptionally activates synthesis of T3SS components. The machinery appears to be mechanically controlled by the assemblage of specific junctions within the apparatus. These junctions include the tip and base of the T3 apparatus, the needle proteins and components within the bacterial cytoplasm. The tip proteins likely have chaperone functions for translocon proteins, allowing the proper assembly of translocation channels in the host membrane and completing vectorial delivery of effector proteins into the host cytoplasm. Multi-functional features of the needle-tip proteins appear to be intricately controlled. In this review, we highlight the functional aspects and complex controls of T3 needle-tip proteins with particular emphasis on PcrV and LcrV.
Introduction
The type III secretion system (T3SS) is likened to a molecular machine located on the surface and within the envelope of many Gram-negative bacteria. These systems are essential for virulence and act as a syringe and needle, called an injectisome, forming a channel in the eukaryotic membrane to facilitate the passage of bacterial effectors into infected cells (Galan and Collmer, 1999; Cornelis, 2006). The T3S apparatus has been identified in a variety of pathogens that infect animals, plants, and insects (Hueck, 1998; Mota and Cornelis, 2005; Troisfontaines and Cornelis, 2005). Functional attributes of T3SS effectors vary among bacterial genera, with cytotoxicity and anti-phagocytic activity being characteristic for Pseudomonas aeruginosa and Yersinia spp., invasion, intracellular survival, and the promotion of proinflammatory responses for Shigella and Salmonella spp., and profound effects on cytoskeletal structure for many other pathogens (reviewed in Hueck, 1998; Galan and Collmer, 1999; Mota and Cornelis, 2005).
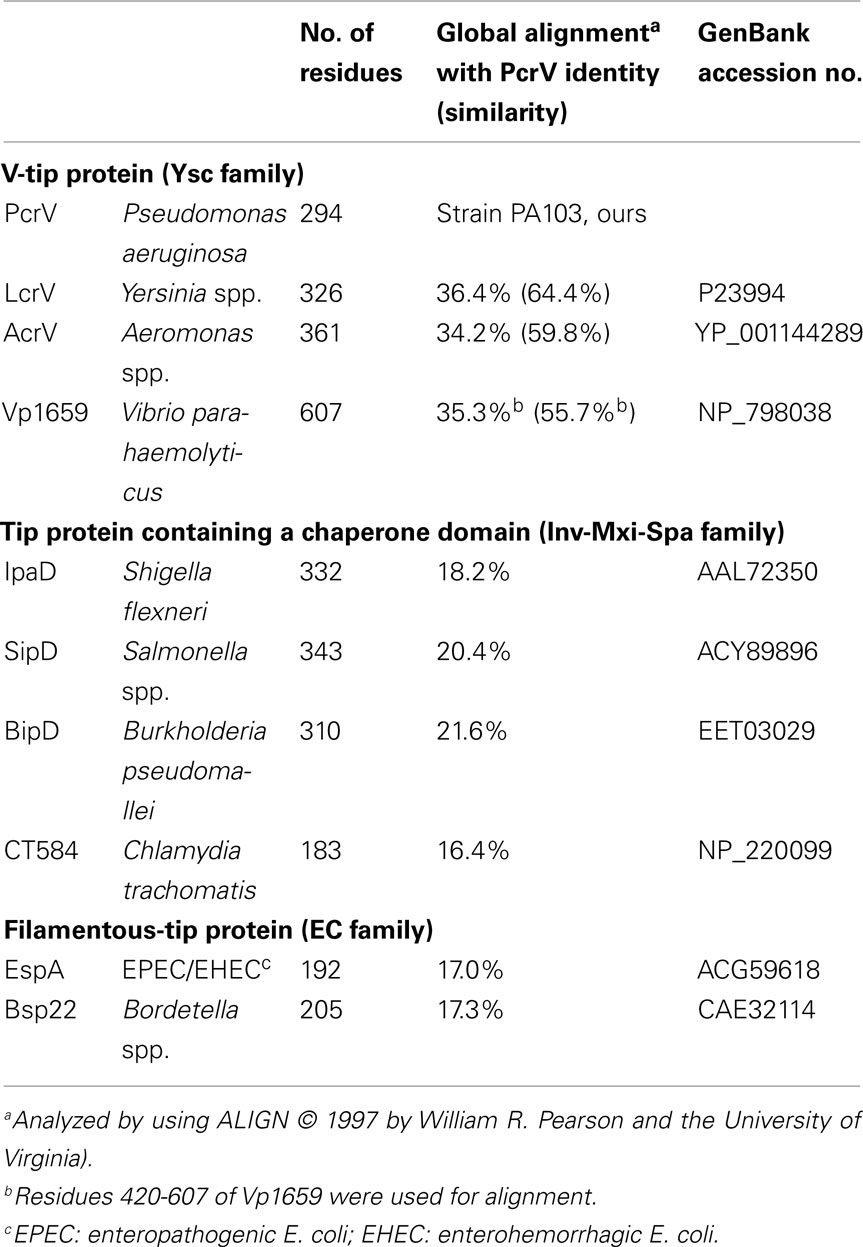
More than 20 T3S systems have been identified in 16S RNA based studies (Cornelis, 2002; Troisfontaines and Cornelis, 2005). In P. aeruginosa, 36 genes are involved in T3S (Frank, 1997). The structure of the assembled injectisome is complex and several components constitute the apparatus. An extracellular needle structure topped with a tip complex extrudes from the basal body that consists of multiple rings spanning the bacterial inner and outer membranes (Figure 1). The inner and outer rings of the basal body are connected by a neck domain. A T3S-associated ATPase, another component of the basal body located at the cytosolic face, is involved in the dissociation of secretion substrates from their chaperones as well as supplying energy for protein export (Figure 1; Cornelis, 2006; Galan and Wolf-Watz, 2006; Blocker et al., 2008; Moraes et al., 2008; Mueller et al., 2008). Bacteria have to complete the assembly of the basal body and polymerize the needle subunit proteins to an appropriate length prior to the formation of the tip complex on the distal end, which is controlled by a substrate-switching mechanism (Journet et al., 2003; Galan and Wolf-Watz, 2006). Other proteins necessary for a functional T3SS are intracellular regulators, specialized chaperones, translocators or translocases, and effectors (Cornelis, 2006; Galan and Wolf-Watz, 2006; Schroeder and Hilbi, 2008; Hauser, 2009; Parsot, 2009).
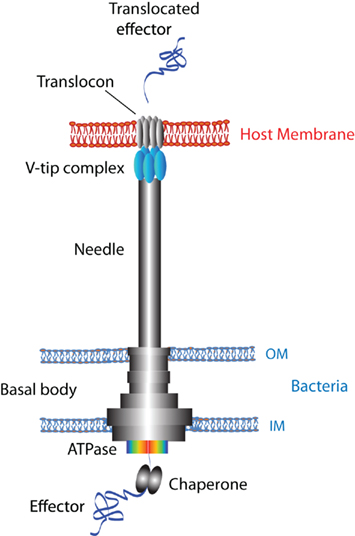
Figure 1. Schematic diagram of the type III apparatus and host membrane. The T3SS injectisome comprises an extracellular needle structure from the basal body that spans the bacterial inner and outer membranes (indicated as IM and OM), the tip complex topped at the distal end of the needle (shown in blue) and a T3S-associated ATPase located at the cytosolic face of the basal body (shown in a rainbow color). The ATPase is involved in the dissociation of unfolded substrates from the specialized chaperones in addition to supplying energy for protein export (Galan and Wolf-Watz, 2006). It is postulated that the structure and assembly of tip complexes are distinct for each protein family: V-tip proteins form a homo-pentamer complex, IpaD requires IpaB to assemble the hetero-pentamer complex, and EC family proteins assemble into a filamentous structure (Knutton et al., 1998; Veenendaal et al., 2007). The tip complex is required for the assembly of a translocon (PopB and PopD for Pseudomonas, YopB and YopD for Yersinia) in the host cell membrane (Cornelis, 2006; Hauser, 2009). An unfolded effector protein is transferred through the channel of the needle and translocon and eventually delivered into the host cytosolic compartment (blue ribbon).
Activation of the T3SS is postulated to occur upon sensing the presence of host cells. Bacteria respond by turning on the transcription of T3SS genes, inserting a translocation complex or translocon assembled with translocator proteins into eukaryotic membranes, and eventually delivering effector proteins into the host to result in manipulation of cell physiology (Figure 1; Deane et al., 2006; Veenendaal et al., 2007; Mueller et al., 2008; Hauser, 2009; Parsot, 2009). Structural and functional aspects of the T3SS and related proteins have been summarized in numerous reviews, thus this review focuses on the needle-tip proteins pertaining to the Ysc family, PcrV from P. aeruginosa and LcrV from Yersinia spp. The tip complex likely possesses multiple roles in T3SS-mediated modulation of host cellular physiology: (1) sensing the microenvironment of host cell membranes and propagating a signal back to the bacterial cell, (2) regulation of secretion and translocation at the level of the tip complex coordinated with transcriptional activation or repression of bacterial genes, (3) assembly and insertion of a translocon in eukaryotic membranes, (4) physically bridging the needle and translocon embedded in the host membrane during effector delivery, and (5) serving as a clinically important protective antigen. In this review, we will discuss these multi-functional properties of T3 needle-tip proteins.
Needle-Tip Complexes of the T3SS
Families of Tip Proteins and Structural Considerations
In animal pathogens, there are three major classes of T3SS: (1) the Ysc family that include the so-called V-tip proteins, PcrV (P. aeruginosa), LcrV (Yersinia spp.), and AcrV from a fish pathogen, Aeromonas salmonicida; (2) the Inv-Mxi-Spa family members identified in Shigella flexneri and Salmonella typhimurium (belonging to the SPI-1 system), and (3) the Esc family of enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), and S. typhimurium (SPI-2 system; Cornelis, 2002, 2006). In the Ysc family, PcrV and LcrV share the highest overall homology (Table 1). Recently, another protein Vp1659 from Vibrio parahaemolyticus was identified as a member of this family (Zhou et al., 2010). The amino acid sequence of the C-terminus of Vp1659 (residues 420–607) is homologous to PcrV (residues 95–294) with 35% identity and 55% similarity (Table 1). The Inv-Mxi-Spa family, is comprised of IpaD (S. flexneri), SipD (Salmonella spp.), BipD (Burkholderia pseudomallei), and CT584 (Chlamydia trachomatis; Erskine et al., 2006; Espina et al., 2006; Johnson et al., 2007; Lara-Tejero and Galan, 2009). Although the proteins in this family contain less sequence identity to PcrV (Table 1), the structural similarity of IpaD, BipD, and SipD to the V-proteins has been well studied (Blocker et al., 2008; Mueller et al., 2008). CT584 is a late cycle gene product of C. trachomatis (Betts-Hampikian and Fields, 2010) and the tertiary structure of this protein could be modeled based on the structure of IpaD (Markham et al., 2009). Esc family members include EspA (EPEC and EHEC) and Bsp22 (Bordetella spp.; Table 1). These proteins polymerize and assemble into a distinct filamentous structure at the tip of the T3 needle (Knutton et al., 1998; Daniell et al., 2001; Crepin et al., 2005; Medhekar et al., 2009). Despite the little structural similarity to the other tip protein families, the polymerized-tip complex appears to function similarly in T3SS (Knutton et al., 1998; Crepin et al., 2005).
PcrV and LcrV tip proteins are located at the distal end of the needle structure and required for the pathogenic phenotype of host infection (Fields et al., 1999; Pettersson et al., 1999; Sato et al., 2011). Bacterial strains with a deletion of pcrV or lcrV are avirulent in mice (Carter et al., 1980; Price et al., 1991; Skrzypek and Straley, 1995; Sawa et al., 1999) and during infection of cultured epithelial or phagocytic cells (Pettersson et al., 1999; Sato et al., 2011). In cell culture systems, pcrV-deletion strains release effector proteins into the culture medium and are incapable of vectorial translocation of effectors into the host cytosol (Sawa et al., 1999; Goure et al., 2004; Sato et al., 2011). Complementation with wild-type pcrV restores translocation and cytotoxicity, suggesting the important role of the tip protein in the intoxication of eukaryotic cells.
The crystal structure of LcrV revealed an overall dumbbell shape with a “grip” formed by the coiled-coil interaction of two internal α-helices flanked by globular domains at each end (Derewenda et al., 2004). Other V-proteins have sufficient sequence similarity to construct structural models using LcrV as a template (1r6f chain A, deposited in PBD; Derewenda et al., 2004). The members of the Ysc family modeled by using the Swiss Model server1 (Guex and Peitsch, 1997) are shown in Figure 2. The coiled-coil structure of two long α-helices is conserved in all three families of the tip proteins (Derewenda et al., 2004; Yip et al., 2005; Johnson et al., 2007; Blocker et al., 2008; Mueller et al., 2008).
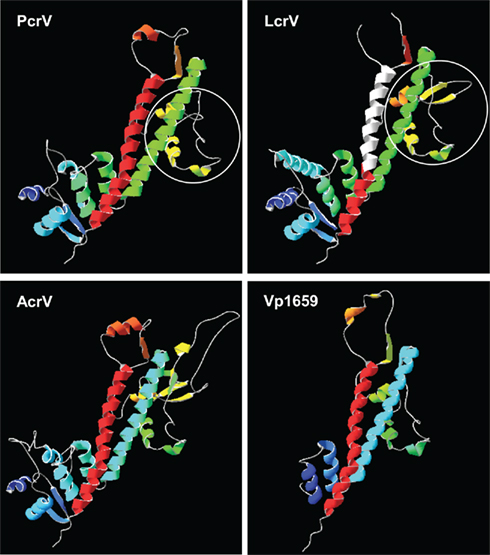
Figure 2. Structural models of the V-tip protein family. V-tip proteins are modeled using LcrV (PBD: 1r6f chain A) as a template (Swiss Model server, http://www.expasy.org). The models indicate the conserved coiled-coil structure of two α-helices flanked by globular domains at each end. One of the globular domains contains protective-epitope regions identified in PcrV and LcrV (a while circle; Frank et al., 2002; Quenee et al., 2010). The immunomodulatory region of LcrV (residues 271 to 300) is located in one of the coiled-coil helices with a connected coil structure (indicated in white; Overheim et al., 2005). This region is deleted in the rV10 subunit vaccine, by which the immunomodulatory responses are eliminated (Overheim et al., 2005).
Current structural studies indicate that tip proteins form an oligomer, possibly a pentamer, at the top end of the needle structure (Mueller et al., 2005, 2008; Deane et al., 2006; Broz et al., 2007; Caroline et al., 2008). Scanning transmission electron microscopy analyses visualized the oligomer structures of parental or chimeric V-tip proteins on the assembled needle expressed in the lcrV deletion strain of Y. enterocolitica (Mueller et al., 2005; Broz et al., 2007). The N-terminal globular domain of LcrV, PcrV, and AcrV form the base structure of the tip complex that interacts with the YscF needle (Broz et al., 2007). PcrV forms a complex with a narrower base than that of LcrV or AcrV (Mueller et al., 2005; Broz et al., 2007). Compared to LcrV translocons (YopB and YopD), PcrV expressed in Y. pseudotuberculosis assembles smaller translocation channels with YopB and YopD in infected erythrocyte membranes and slowly delivers effector proteins into host cells (Holmstrom et al., 2001; Broms et al., 2003). The AcrV oligomerized structure is larger in size and varied in shape (Mueller et al., 2005).
Structural studies of Inv-Mxi-Spa family members demonstrate that the N-terminal domain of IpaD, SipD, and BipD possesses structural similarity to common chaperones, suggesting a self-chaperoning function of the domain (Erskine et al., 2006; Johnson et al., 2007; Blocker et al., 2008; Wang et al., 2010b; Chatterjee et al., 2011; Rathinavelan et al., 2011). In contrast, the N-terminal structure of V-proteins is predicted to be globular and appears to have no intramolecular chaperoning domain (Blocker et al., 2008). PcrV and LcrV utilize cognate chaperones PcrG and LcrG in the bacterial cytoplasm. The chaperones and V-proteins are expressed from genes located within the same operon (Blocker et al., 2008; Lee et al., 2010). EspA is chaperoned by CesA (Yip et al., 2005).
The distinct coiled-coil structure in the center of the V-tip proteins is similar to the coiled-coil formation of monomeric needle proteins, such as MxiH from Shigella (Deane et al., 2006), suggesting a common mechanism of assembly and interaction of tip proteins and needle subunits (Blocker et al., 2008). One of the coiled-coil helices in LcrV is structurally homologous to a stabilizing helix of monomeric MxiH, which permits the modeling of the LcrV tip complex on the top of the MxiH needle without structural restraints (Deane et al., 2006). Also, it has been shown that the lower part of the coiled-coil structure of SipD interacts with the Salmonella needle protein PrgI (Rathinavelan et al., 2011).
Sensing the Microenvironment and Host Cells
Bacteria modulate gene expression according to growth conditions to maximize advantageous strategies for survival and continued replication. It has been suggested that as bacteria sense the growth environment, the clusters of T3SS genes are transcriptionally repressed or activated (reviewed in Motin et al., 1994; Hill et al., 1997; Sawa et al., 1999; Brubaker, 2003; Yahr and Wolfgang, 2006; Brutinel and Yahr, 2008; Urbanowski and Yahr, 2008; Baer et al., 2009). For P. aeruginosa, a low-calcium growth environment is one signal to derepress or induce T3SS transcription by the exsCEBA-control system with an additional regulatory protein, ExsD (McCaw et al., 2002; Dasgupta et al., 2004; Rietsch et al., 2005; Urbanowski et al., 2005, 2007; Brutinel et al., 2009). Translocator and effector proteins are secreted into the extracellular growth medium under low-calcium conditions (Yahr et al., 1997). When calcium concentrations are sufficiently high, secretion of effectors is inhibited (Frank, 1997; Yahr et al., 1997; McCaw et al., 2002). In addition to calcium depletion, other signals that are involved in ExsA-mediated regulation of the T3SS include host–cell contact, the contribution of two component system regulators, DNA damage, and osmotic and metabolic stresses (Rietsch et al., 2004; Rietsch and Mekalanos, 2006; Yahr and Wolfgang, 2006; Kang et al., 2009). In Yersinia, LcrQ was identified as a transcriptional regulator protein unique to Yersinia with no homolog found in Pseudomonas (Wulff-Strobel et al., 2002). T3SS transcriptional control in Yersinia also involves regulators, LcrH, SycH, YopD, and YscM1/2 (Rimpilainen et al., 1992; Pettersson et al., 1996; Brutinel and Yahr, 2008). In addition, the T3SS is regulated by changes in oxygen levels or by intracellular pH as observed in cellular infections with EHEC, Shigella, or Salmonella (SPI-2; Marteyn et al., 2010; Schuller and Phillips, 2010; Yu et al., 2010). Further, the T3SS mRNA levels are post-transcriptionally modulated by small RNAs (Bordi et al., 2010).
It is postulated that the T3 needle-tip complex functions as a sensor of the growth environment and the presence of host cells for infection (Deane et al., 2006; Veenendaal et al., 2007; Mueller et al., 2008; Parsot, 2009). A physiological signal for T3SS activation is the recognition of eukaryotic membranes upon contact (Menard et al., 1994; Pettersson et al., 1996). Contact of a pathogen to the host cell induces expression of T3SS proteins, leading to the polarized transfer of effectors into the target cell cytosol (Menard et al., 1994; Rosqvist et al., 1994; Cisz et al., 2008). Contact-mediated induction of ExoS expression requires host cell integrity (Cisz et al., 2008). For S. flexneri, interaction with epithelial cells or incubation with serum is required to release IpaB and IpaC proteins (Menard et al., 1994).
Low-calcium conditions, induced by the addition of a calcium chelater to the growth medium, activate the T3SS in Pseudomonas and Yersinia (Carter et al., 1980; Frank et al., 1994). Mutations in pcrV or lcrV lead to a calcium-insensitive phenotype (Carter et al., 1980; Bergman et al., 1991; Sato et al., 2011). In the Shigella T3SS, several inducers have been shown, such as the amphipathic dye Congo Red, fetal bovine serum, cholesterol-containing lipids, and bile salts (Bahrani et al., 1997; Blocker et al., 1999; van der Goot et al., 2004; Olive et al., 2007). The Shigella tip protein IpaD “senses” deoxycholate or other bile salts in the environment, resulting in a conformational change in IpaD and subsequent step-wise activation of the secretion apparatus by recruitment of IpaB to the tip (Stensrud et al., 2008; Dickenson et al., 2011). After completion of IpaB surface presentation, followed by interaction with cholesterol and sphingomyelin containing lipids, IpaC moves toward the surface of the needle tip (Epler et al., 2009). At this stage, the tip complex is poised for translocation of effectors (Epler et al., 2009). Salmonella SipD also interacts with bile salts (Wang et al., 2010b; Chatterjee et al., 2011). In V. parahaemolyticus, a bile acid was identified as a host-derived inducer for T3SS genes in a transcriptional regulator-dependent manner, yet the involvement of tip proteins is unknown (Gotoh et al., 2010).
Regulation of Type III Secretion and Translocation
In this section, we describe the post-translational control of the T3SS, which requires multiple levels of regulatory proteins and several types of signals (Pettersson et al., 1996; Wulff-Strobel et al., 2002; Agrain et al., 2005; Akeda and Galan, 2005; Galan and Wolf-Watz, 2006; Sorg et al., 2007; Botteaux et al., 2009). To complete the formation of a translocon and delivery of effectors into host cells, a functional injection apparatus, including the basal body, needle, and tip complex, must be assembled. This multistep process is postulated to be accomplished by a substrate-switching mechanism, a hierarchical and timely regulation of protein export, which will be discussed later. Post-translational regulation is organized into six classes based on the location of the regulatory or accessory proteins.
(1) The needle-tip complex of the injectisome: The tip complex is located at the distal end of the needle structure (Mueller et al., 2005; Broz et al., 2007; Blocker et al., 2008). V-tip proteins have been shown to be involved in the mechanical regulation of translocation and secretion (Sarker et al., 1998; Sato et al., 2011). Bacterial strains containing a deletion of a gene encoding a tip protein, pcrV or ipaD, are non-cytotoxic and possess a deregulated secretion phenotype as measured by the constitutive secretion of effector proteins (Menard et al., 1993; Frank, 1997; Sawa et al., 1999; McCaw et al., 2002; Goure et al., 2004; Picking et al., 2005; Rietsch et al., 2005; Sato et al., 2011). For pcrV and lcrV deletion strains, this is referred to as a calcium-blind or calcium independent phenotype (Carter et al., 1980; Bergman et al., 1991; Sato et al., 2011). Similarly, an ipaD-deletion mutant is insensitive to the inducer, Congo Red (Veenendaal et al., 2007). These data suggest that tip proteins are involved in the control of translocation and secretion of effectors by the type III apparatus.
(2) The needle protein: It has been postulated that the type III needle propagates signals between the needle-tip sensor and regulators in the bacterial cytoplasm (Kenjale et al., 2005; Torruellas et al., 2005; Deane et al., 2006). The needle subunit proteins are related to secretory regulation and multiple YscF mutants demonstrate a constitutive-secretion phenotype (Torruellas et al., 2005; Davis and Mecsas, 2007). A dominant-negative YscF mutant, L54V, blocks secretion of parental YscF and Yop effectors and fails to assemble the injectisome (Davis et al., 2010). Mutations in the Shigella needle protein MixH, lead to constitutive secretion of effectors, some of which are also not inducible (Kenjale et al., 2005). The conserved tertiary structure of both needle subunits and tip proteins is coiled-coil formed by two α-helices, suggesting a similar mechanism of oligomeric assembly (Kenjale et al., 2005; Torruellas et al., 2005; Blocker et al., 2008). Biophysical analyses of common α-helical coiled-coil structures demonstrated that an allosteric potential in this type of structure induces conformational amplification involved in mechanotransduction. In other words, these proteins appear to function as a nanoswitch (Yogurtcu et al., 2010). Thus, the environmental signals sensed by the tip complex may be amplified and transmitted to the cytoplasmic side through the needle proteins by conformational changes in coiled-coil structures.
(3) Inner membrane proteins: An inner membrane component in Yersinia, YscU, recognizes translocators for secretion and is involved in substrate switching. Amino acid substitutions at specific residues of YscU fail to autocleave the cytoplasmic C-terminal domain and abolish export of translocator proteins LcrV, YopB, and YopD but not Yop effectors (Sorg et al., 2007; Riordan and Schneewind, 2008).
(4) The ATPase complex in the basal body and cytoplasmic chaperones: The basal body consists of the outer and inner rings connected by a neck domain and the ATPase complex (Figure 1; reviewed in Galan and Wolf-Watz, 2006). Salmonella enterica ATPase, InvC, has a critical role in substrate recognition and release of cognate chaperones (Akeda and Galan, 2005). T3SS-specific chaperones are classified based on their cognate interacting partners, effectors, translocators, and needle proteins (Stebbins and Galan, 2003; Cornelis, 2006). Prior to export, chaperones interact with their secreted protein substrates to target this sub-assemblage to the ATPase-sorting complex associated with the basal body. At this specific locale, the effector protein is unfolded, dissociated from the effector-chaperone complex, and then presented to the sorting complex (Boyd et al., 2000; Luo et al., 2001; Birtalan et al., 2002; Galan and Wolf-Watz, 2006; Lara-Tejero et al., 2011). An alternative interface of the effector–chaperone complex induced by conformational changes may act as a recognition signal for the controlled export of effectors (Stebbins and Galan, 2003). In addition, the ATPase provides proton motive force for export of substrates (Eichelberg et al., 1994; Wilharm et al., 2004). Tip protein-specific chaperones PcrG and LcrG for PcrV and LcrV, respectively, possess a regulatory function in T3 protein secretion in the presence of calcium (Nilles et al., 1997; Matson and Nilles, 2001; McCaw et al., 2002; Sundin et al., 2004; Rietsch et al., 2005). In Yersinia, a deletion of lcrG leads to premature release of Yop proteins (DeBord et al., 2001). In the Pseudomonas system, the interaction between PcrV and its cognate chaperone PcrG facilitates the export of PcrV despite the absence of an influence on the secretory regulation of effectors (Lee et al., 2010).
(5) Cytoplasmic regulators: an intrabacterial regulator YopN in Yersinia complexes with the co-regulatory protein TyeA to control the entry of secreted proteins to the basal body (Forsberg et al., 1991; Iriarte et al., 1998; Ferracci et al., 2005; Schubot et al., 2005). MxiC, an ortholog of YopN in Shigella, interacts with the Spa47 ATPase (Botteaux et al., 2009). A knockout mutant of mxiC constitutively secretes effectors but the secretion of translocator IpaC is weak and delayed upon type III induction (Botteaux et al., 2009; Martinez-Argudo and Blocker, 2010). Another cytoplasmic regulator is a molecular ruler or timer, YscP in Yersinia and Spa32 in Shigella. These molecules determine the needle length and are also involved in the control of substrate specificity switching (Journet et al., 2003; Agrain et al., 2005).
(6) In both Pseudomonas and Yersinia systems, translocated effector proteins ExoS and YopE play some role in a negative regulatory loop. After delivery of ExoS and YopE into the host cytosol, additional cycles of effector translocation into the same host cell are blocked in a feedback fashion (Aili et al., 2008; Cisz et al., 2008; Urbanowski and Yahr, 2008). The mechanisms of regulation or feedback signal transduction have not been defined.
Translocon Assembly and Bridging to the Needle Structure
Activation of the T3 secretory activity initiates the assembly of a translocation complex, called a translocon, in eukaryotic membranes (Figure 1). P. aeruginosa requires the products of the pcrGVHpopBD operon to form the translocon (Frank, 1997; Yahr et al., 1997; Frithz-Lindsten et al., 1998). PcrV, PopB, and PopD are classified as translocators or translocases necessary to form the translocon structure in eukaryotic membranes (Lee et al., 2000; Schoehn et al., 2003; Goure et al., 2004; Caroline et al., 2008). PopB and PopD are hydrophobic translocators that insert into membrane lipids while a hydrophilic translocator, PcrV, is required for assembly and insertion of the functional PopB/PopD translocon into host membranes (Frithz-Lindsten et al., 1998; Goure et al., 2004, 2005). The translocon assembly is necessary for contact-dependent lysis of erythrocytes and the one-step delivery of effector proteins into the host cytosolic compartment (Hakansson et al., 1996; Lee et al., 2000; Schoehn et al., 2003; Goure et al., 2004; Cornelis, 2006; Galan and Wolf-Watz, 2006; Blocker et al., 2008; Caroline et al., 2008). Also, contact-dependent activation of T3SS requires translocon proteins PopB and PopD as well as host cell integrity (Cisz et al., 2008). Using a Yersinia co-infection system, YopB, YopD, and LcrV are sufficient for channel formation when these proteins are expressed and secreted by the same bacterium (Marenne et al., 2003).
The formation of translocon channels is assessed by the release of hemoglobins from erythrocytes (Blocker et al., 1999; Neyt and Cornelis, 1999; Goure et al., 2005). Pore formation in eukaryotic cells can be identified by infection with effectorless strains of T3SS-competent bacteria (Frithz-Lindsten et al., 1998; Viboud and Bliska, 2001; Marenne et al., 2003; Roy et al., 2004; Sato et al., 2011). Even in the absence of known effector proteins, expression of PcrV and other type III components leads to cell damage (Roy et al., 2004; El Solh et al., 2008). V-protein deletion strains are non-cytotoxic and non-hemolytic due to the inability to assemble and insert the translocon in the host membranes (Sarker et al., 1998; Sawa et al., 1999; Goure et al., 2004; Sato et al., 2011). Complementation with a wild-type copy of pcrV restores cytotoxicity and hemolytic activity (Sawa et al., 1999; Goure et al., 2004; Sato et al., 2011). These results suggest that PcrV is involved in the mechanical control of translocon formation and insertion in lipid bilayers. It is believed that V-proteins act as a platform for assembly and insertion of translocons (Sarker et al., 1998; Goure et al., 2004; Broz et al., 2007). V-proteins may have a chaperone function for translocon assembly and possibly for self-oligomerization (Goure et al., 2005). The Inv-Mxi-Spa family tip proteins possess a chaperone domain in the N-terminus (reviewed in Blocker et al., 2008). It has also been shown that the C-terminal α-helix of BipD is involved in contacts with translocon proteins (Erskine et al., 2006).
In cell culture systems, effector proteins are translocated into the host cytoplasm in a polarized or vectorial manner (Rosqvist et al., 1994; Sory and Cornelis, 1994; Persson et al., 1995; Vallis et al., 1999a,b;). It has been suggested that the tip complex forms a physical bridge between the needle and translocon assembled within the host membrane (Knutton et al., 1998; Nilles et al., 1998; Daniell et al., 2001; Sato et al., 2011).The leakage of effector proteins in the cell culture medium is minimum and rarely detectable during infection in vitro (Vallis et al., 1999a,b; Sundin et al., 2004; Sato et al., 2011).
Immunization and Therapeutics Targeting T3SS
LcrV: A Protective Epitope of Yersinia and Determinant for Evasion of Host Innate Immunity
LcrV was first identified as an antigenic factor of Y. pestis and named “V-antigen” by Burrows and Bacon 1956. Active immunization with V-antigen protects against pneumonic and bubonic plague in mice (Lawton et al., 1963; Leary et al., 1995; Anderson et al., 1996). Polyclonal antibodies for LcrV provide passive protection against infection with Y. pestis and Y. pseudotuberculosis (Une and Brubaker, 1984; Motin et al., 1994). LcrV is located at the tip of needles and surface exposed on bacteria so that anti-LcrV antibodies can inhibit the translocon assembly, causing a failure of effector delivery into target cells (Fields et al., 1999; Pettersson et al., 1999; Mueller et al., 2005). LcrV possesses conformational epitopes reported by several groups (Hill et al., 1997; Vernazza et al., 2009; Quenee et al., 2010). In addition to conformational epitopes, a linear epitope was identified in LcrV (amino acid residues 195–225; Figure 2; Quenee et al., 2010). Several laboratories developed LcrV subunit vaccines (rV) alone or in combination with other Y. pestis proteins, such as the fraction 1 pilus capsular antigen or rF1, which are currently in clinical trials (Powell et al., 2005; Williamson et al., 2005; Quenee and Schneewind, 2009).
Another biological function of LcrV is blocking host inflammatory responses and triggering immunosuppression by the release of IL-10 (Nakajima et al., 1995; Nedialkov et al., 1997; Sing et al., 2002a; Brubaker, 2003; Overheim et al., 2005). IL-10 release was amplified in a TLR2/TLR6 and CD14-dependent manner, suggesting that Yersinia evades the host innate immune response by exploiting innate pattern recognition molecules (Sing et al., 2002b, (Sing et al. 2005; Depaolo et al., 2008). Additionally, LcrV inhibits the release of proinflammatory cytokines, IFN-γ and TNF-α (Nakajima and Brubaker, 1993; Nakajima et al., 1995). Therapeutically, the ability of LcrV to suppress IFN-γ and TNF-α release was utilized to postpone the inflammatory response in mouse skin allograft models (Motin et al., 1997). A deletion of residues 271–300 in LcrV (the position is indicated in the structural model in Figure 2) eliminated the immunomodulatory responses and this variant protein has been developed to the rV10 subunit vaccine (Overheim et al., 2005). Active immunization with rV10 alone or a combination with rF1 protects cynomolgus macaques from pneumonic plague, and the antibodies from macaques provide protection against bubonic plaque in mice (Cornelius et al., 2008). Active immunization with rV10 was also protective in rats and mice (DeBord et al., 2006; Anderson et al., 2009). The rV10 vaccine is undergoing the preclinical efficacy study (Cornelius et al., 2008) along with the FDA pre-investigational new drug authorization review (Quenee and Schneewind, 2009).
PcrV: A Protective Epitope of Pseudomonas
Among three translocator proteins, only PcrV is a potent protective antigen against T3SS-mediated Pseudomonas infection (Sawa et al., 1999; Holder et al., 2001; Frank et al., 2002). Active immunization with recombinant PcrV protects mice from lethal infection even under induced-leukocytopenia or immunosuppression induced by a burn injury (Sawa et al., 1999; Holder et al., 2001; Moriyama et al., 2009). Passive immunization with polyclonal antisera, affinity-purified antibodies, or F(ab′)2 were effective against cellular intoxication, lung injury, bacteremia, and sepsis in animal models (Sawa et al., 1999; Shime et al., 2001; Frank et al., 2002; Neely et al., 2005; Imamura et al., 2007; Baer et al., 2009). Polyclonal anti-PcrV antibodies block T3SS-mediated hemolysis of erythrocytes and cytotoxicity of macrophages in vitro (Goure et al., 2005; Sato et al., 2011) and reduce inflammatory response and lung injury in infected BALB/c mice (Sawa et al., 1999). In addition to the inhibition of the delivery of effector proteins, anti-PcrV antibodies decrease anti-phagocytic effects mediated by host cells (Sawa et al., 1999).
More than 80 monoclonal stable cell lines were screened by in vitro and in vivo assays to identify an antibody or combination of antibodies that neutralize the cytotoxic effect of P. aeruginosa infection (Frank et al., 2002). Mab166 possesses this property and was subsequently developed to a humanized single-chain antibody for immunotherapy. The F(ab′)2 single-chain antibody conjugated with polyethylene glycol was initially produced by InterMune (Frank et al., 2002; Baer et al., 2009; Moriyama et al., 2009) and subjected to a proprietary affinity maturation procedure by Kalobios to produce KB001. Phase 1 and 1/2 clinical studies of KB001 have been completed in mechanically ventilated ICU patients and cystic fibrosis patients. The results of clinical trials indicated the reduction in the number of inflammatory cells and markers and decreased pneumonia events (KaloBios website2).
The protective epitope in PcrV is conformational and located within a region between amino acids 144 and 257 (deletion mapping) or between 158 and 217 as determined by phage display (Frank et al., 2002). Only a conformational epitope has been identified in PcrV at this time (Frank et al., 2002). Mab166 is reactive to the epitope located in the globular region between the central and C-terminal helices (Figure 2). The location of the epitope in the PcrV-structure models overlaps the regions identified as the linear and conformational epitopes of LcrV (Vernazza et al., 2009; Quenee et al., 2010). There is no indication of PcrV-mediated immunomodulatory effects while LcrV increases IL-10 production and decreases the release of proinflammatory cytokines, IFN-γ and TNF-α (Nakajima and Brubaker, 1993; Nakajima et al., 1995; Sing et al., 2002a). PcrV has little homology to TLR2-binding motifs located in LcrV, which are responsible for IL-10 induction (Sing et al., 2005; Abramov et al., 2007). In contrast to LcrV, PcrV does not colocalize with TLR2 (Sing et al., 2002b; Overheim et al., 2005). T3SS-competent P. aeruginosa strains, but not the deletion mutants of pcrV or other translocator genes, recruit neutrophils to the lung, triggering inflammatory responses (Wangdi et al., 2010).
Pseudomonas aeruginosa is intrinsically resistant to a broad range of antibiotics. A high rate of ventilator-associated pneumonia and mortality in patients is caused by P. aeruginosa (Rello et al., 1993). PcrV is expressed in most of the type III positive clinical isolates from patients with acute lower respiratory tract infection and systemic infection (Roy-Burman et al., 2001). Infection with PcrV-positive isolates results in high mortality rate even in the absence of cytotoxin ExoU or ExoS (Roy-Burman et al., 2001; El Solh et al., 2008). These data indicate that there might be advantages to the use of PcrV as a therapeutic target. The neutralization of PcrV likely confers little selective pressure to bacteria for their development to a resistant phenotype as compared to selection of resistance through the use of current antibiotic therapies. Thus, passive or active immunization may be useful in combination therapies against intrinsically resistant P. aeruginosa isolates (El Solh and Alhajhusain, 2009).
Possible Therapeutics for Other Type III Targets
Immunotherapies targeting a needle-tip protein have been also studied for other type III-positive bacteria. Antibodies recognizing the N-terminus of IpaD neutralize the hemolytic activity of S. flexneri (Espina et al., 2006). Antibodies against SipD (SPI-1) protect epithelial cells from invasion by S. enterica serovar Enteritidis (Desin et al., 2010). In contrast, active immunization of mice with recombinant BipD is not protective against experimental melioidosis (Druar et al., 2008). CT584 does not induce antibody responses in humans infected with C. trachomatis (Wang et al., 2010a). It will be interesting to see the efficacy of a multivalent vaccine based on tip proteins IpaD, BipD, SipD, LcrV, and PcrV, which is in the initial stages of development (Markham et al., 2010). For filamentous-tip protein family, anti-EspA antibodies inhibit cytoskeletal changes in host cells in vitro (La Ragione et al., 2006). One monoclonal antibody specific to a linear epitope of EspA was protective against EHEC infection (Yu et al., 2011). Polyclonal sera reactive to Bsp22 protects mice against Bordetella infection (Medhekar et al., 2009). Interestingly, the needle protein YscF of Y. pestis, another surface-exposed type III protein, induces significant antibody responses and protective effects in mice upon active immunization (Matson et al., 2005).
Another aspect of immunotherapy studies concerns improvement in delivery methods and adjuvants. For example, various and effective delivery methods of vaccines against Yersinia infection include liposomal delivery of epitopes or virus-based delivery of vaccines (Chattopadhyay et al., 2008; Heurtault et al., 2009; Bhattacharya et al., 2010; Van Blarcom et al., 2010). Also, whole-cell vaccines, either killed or live and attenuated have been well studied (Bumann et al., 2010; Kamei et al., 2011 and reviewed in Quenee and Schneewind, 2009).
Small molecule inhibitors targeting T3SS have been pursued for the development of novel therapeutics. Inhibitors targeting the enzymatic activity of type III effector proteins, ExoU and ExoS, are effective in vitro (Lee et al., 2007; Arnoldo et al., 2008). Inhibitors specific to a Yersinia transcription factor, LcrF (VirF), were effective against Y. pseudotuberculosis-mediated cytotoxicity in vitro and bacterial burden in the lungs in mice of pneumonia, increasing their survival (Garrity-Ryan et al., 2010). Moreover, numerous small molecules have been screened for antivirulence inhibitors targeting the T3SS in several genera of bacteria (Veenendaal et al., 2009; Baron, 2010).
Concluding Remarks
The T3SS is a potent part of the virulence machinery expressed by P. aeruginosa and multiple Gram-negative pathogens. Assembly of type III components and subsequent execution of this intoxication system are intricately regulated and coordinated at multiple levels. Having a comprehensive understanding of these systems as well as working out the mechanistic steps of each stage in the process will give investigators a clue toward the development of novel therapeutics. These therapeutics could target the assembly of the injectisome, the sensing of the microenvironment followed by signal transduction, transcriptional activation, translocon formation in host membranes, and effector delivery into the host cytoplasmic compartment. The PcrV tip complex is located at the distal end of the needle and responsible for several important functions. pcrV-deletion strains are unable to intoxicate host target cells due to a failure in the formation of the translocon channel in host plasma membranes. Thus, the structural and functional mechanism of the tip proteins and translocon proteins is particularly relevant to the design of vaccines and therapeutics targeted to neutralize T3SS-mediated intoxication (Motin et al., 1994; Hill et al., 1997; Sawa et al., 1999; Brubaker, 2003; Baer et al., 2009).
Conflict of Interest Statement
D. W. Frank is a co-inventor of Mab 166 and its humanized derivative molecules.
Acknowledgments
This work was supported by NIH grant AI49577 from the National Institute of Allergy and Infectious Diseases, the Center for Infectious Disease Research, and the Advancing a Healthier Wisconsin Foundation.
Footnotes
References
Abramov, V. M., Khlebnikov, V. S., Vasiliev, A. M., Kosarev, I. V., Vasilenko, R. N., Kulikova, N. L., Khodyakova, A. V., Evstigneev, V. I., Uversky, V. N., Motin, V. L., Smirnov, G. B., and Brubaker, R. R. (2007). Attachment of LcrV from Yersinia pestis at dual binding sites to human TLR-2 and human IFN-gamma receptor. J. Proteome Res. 6, 2222–2231.
Agrain, C., Callebaut, I., Journet, L., Sorg, I., Paroz, C., Mota, L. J., and Cornelis, G. R. (2005). Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol. Microbiol. 56, 54–67.
Aili, M., Isaksson, E. L., Carlsson, S. E., Wolf-Watz, H., Rosqvist, R., and Francis, M. S. (2008). Regulation of Yersinia Yop-effector delivery by translocated YopE. Int. J. Med. Microbiol. 298, 183–192.
Akeda, Y., and Galan, J. E. (2005). Chaperone release and unfolding of substrates in type III secretion. Nature 437, 911–915.
Anderson, D. M., Ciletti, N. A., Lee-Lewis, H., Elli, D., Segal, J., DeBord, K. L., Overheim, K. A., Tretiakova, M., Brubaker, R. R., and Schneewind, O. (2009). Pneumonic plague pathogenesis and immunity in Brown Norway rats. Am. J. Pathol. 174, 910–921.
Anderson, G. W. Jr., Leary, S. E., Williamson, E. D., Titball, R. W., Welkos, S. L., Worsham, P. L., and Friedlander, A. M. (1996). Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64, 4580–4585.
Arnoldo, A., Curak, J., Kittanakom, S., Chevelev, I., Lee, V. T., Sahebol-Amri, M., Koscik, B., Ljuma, L., Roy, P. J., Bedalov, A., Giaever, G., Nislow, C., Merrill, A. R., Lory, S., and Stagljar, I. (2008). Identification of small molecule inhibitors of Pseudomonas aeruginosa exoenzyme S using a yeast phenotypic screen. PLoS. Genet. 4, e1000005. doi: 10.1371/journal.pgen.1000005
Baer, M., Sawa, T., Flynn, P., Luehrsen, K., Martinez, D., Wiener-Kronish, J. P., Yarranton, G., and Bebbington, C. (2009). An engineered human antibody fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infect. Immun. 77, 1083–1090.
Bahrani, F. K., Sansonetti, P. J., and Parsot, C. (1997). Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect. Immun. 65, 4005–4010.
Baron, C. (2010). Antivirulence drugs to target bacterial secretion systems. Curr. Opin. Microbiol. 13, 100–105.
Bergman, T., Hakansson, S., Forsberg, A., Norlander, L., Macellaro, A., Backman, A., Bolin, I., and Wolf-Watz, H. (1991). Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173, 1607–1616.
Betts-Hampikian, H. J., and Fields, K. A. (2010). The chlamydial type III secretion mechanism: revealing cracks in a tough nut. Front. Microbiol. 2:114. doi: 10.3389/fmicb.2010.00114
Bhattacharya, D., Mecsas, J., and Hu, L. T. (2010). Development of a vaccinia virus based reservoir-targeted vaccine against Yersinia pestis. Vaccine 28, 7683–7689.
Birtalan, S. C., Phillips, R. M., and Ghosh, P. (2002). Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell 9, 971–980.
Blocker, A., Gounon, P., Larquet, E., Niebuhr, K., Cabiaux, V., Parsot, C., and Sansonetti, P. (1999). The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147, 683–693.
Blocker, A. J., Deane, J. E., Veenendaal, A. K., Roversi, P., Hodgkinson, J. L., Johnson, S., and Lea, S. M. (2008). What’s the point of the type III secretion system needle? Proc. Natl. Acad. Sci. U.S.A. 105, 6507–6513.
Bordi, C., Lamy, M. C., Ventre, I., Termine, E., Hachani, A., Fillet, S., Roche, B., Bleves, S., Mejean, V., Lazdunski, A., and Filloux, A. (2010). Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol. Microbiol. 76, 1427–1443.
Botteaux, A., Sory, M. P., Biskri, L., Parsot, C., and Allaoui, A. (2009). MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 71, 449–460.
Boyd, A. P., Lambermont, I., and Cornelis, G. R. (2000). Competition between the Yops of Yersinia enterocolitica for delivery into eukaryotic cells: role of the SycE chaperone binding domain of YopE. J. Bacteriol. 182, 4811–4821.
Broms, J. E., Sundin, C., Francis, M. S., and Forsberg, A. (2003). Comparative analysis of type III effector translocation by Yersinia pseudotuberculosis expressing native LcrV or PcrV from Pseudomonas aeruginosa. J. Infect. Dis. 188, 239–249.
Broz, P., Mueller, C. A., Muller, S. A., Philippsen, A., Sorg, I., Engel, A., and Cornelis, G. R. (2007). Function and molecular architecture of the Yersinia injectisome tip complex. Mol. Microbiol. 65, 1311–1320.
Brubaker, R. R. (2003). Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71, 3673–3681.
Brutinel, E. D., Vakulskas, C. A., and Yahr, T. L. (2009). Functional domains of ExsA, the transcriptional activator of the Pseudomonas aeruginosa type III secretion system. J. Bacteriol. 191, 3811–3821.
Brutinel, E. D., and Yahr, T. L. (2008). Control of gene expression by type III secretory activity. Curr. Opin. Microbiol. 11, 128–133.
Bumann, D., Behre, C., Behre, K., Herz, S., Gewecke, B., Gessner, J. E., von Specht, B. U., and Baumann, U. (2010). Systemic, nasal and oral live vaccines against Pseudomonas aeruginosa: a clinical trial of immunogenicity in lower airways of human volunteers. Vaccine 28, 707–713.
Burrows, T. W., and Bacon, G. A. (1956). The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br. J. Exp. Pathol. 37, 481–493.
Caroline, G., Eric, F., Bohn, Y. S., Sylvie, E., and Attree, I. (2008). Oligomerization of PcrV and LcrV, protective antigens of Pseudomonas aeruginosa and Yersinia pestis. J. Biol. Chem. 283, 23940–23949.
Carter, P. B., Zahorchak, R. J., and Brubaker, R. R. (1980). Plague virulence antigens from Yersinia enterocolitica. Infect. Immun. 28, 638–640.
Chatterjee, S., Zhong, D., Nordhues, B. A., Battaile, K. P., Lovell, S., and De Guzman, R. N. (2011). The crystal structures of the Salmonella type III secretion system tip protein SipD in complex with deoxycholate and chenodeoxycholate. Protein Sci. 20, 75–86.
Chattopadhyay, A., Park, S., Delmas, G., Suresh, R., Senina, S., Perlin, D. S., and Rose, J. K. (2008). Single-dose, virus-vectored vaccine protection against Yersinia pestis challenge: CD4( cells are required at the time of challenge for optimal protection. Vaccine 26, 6329–6337.
Cisz, M., Lee, P. C., and Rietsch, A. (2008). ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J. Bacteriol. 190, 2726–2738.
Cornelis, G. R. (2002). The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell Biol. 3, 742–752.
Cornelius, C. A., Quenee, L. E., Overheim, K. A., Koster, F., Brasel, T. L., Elli, D., Ciletti, N. A., and Schneewind, O. (2008). Immunization with recombinant V10 protects cynomolgus macaques from lethal pneumonic plague. Infect. Immun. 76, 5588–5597.
Crepin, V. F., Shaw, R., Abe, C. M., Knutton, S., and Frankel, G. (2005). Polarity of enteropathogenic Escherichia coli EspA filament assembly and protein secretion. J. Bacteriol. 187, 2881–2889.
Daniell, S. J., Takahashi, N., Wilson, R., Friedberg, D., Rosenshine, I., Booy, F. P., Shaw, R. K., Knutton, S., Frankel, G., and Aizawa, S. (2001). The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell. Microbiol. 3, 865–871.
Dasgupta, N., Lykken, G. L., Wolfgang, M. C., and Yahr, T. L. (2004). A novel anti-anti-activator mechanism regulates expression of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 53, 297–308.
Davis, A. J., Diaz, D. A., and Mecsas, J. (2010). A dominant-negative needle mutant blocks type III secretion of early but not late substrates in Yersinia. Mol. Microbiol. 76, 236–259.
Davis, A. J., and Mecsas, J. (2007). Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells. J. Bacteriol. 189, 83–97.
Deane, J. E., Roversi, P., Cordes, F. S., Johnson, S., Kenjale, R., Daniell, S., Booy, F., Picking, W. D., Picking, W. L., Blocker, A. J., and Lea, S. M. (2006). Molecular model of a type III secretion system needle: Implications for host-cell sensing. Proc. Natl. Acad. Sci. U.S.A. 103, 12529–12533.
DeBord, K. L., Anderson, D. M., Marketon, M. M., Overheim, K. A., Depaolo, R. W., Ciletti, N. A., Jabri, B., and Schneewind, O. (2006). Immunogenicity and protective immunity against bubonic plague and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 74, 4910–4914.
DeBord, K. L., Lee, V. T., and Schneewind, O. (2001). Roles of LcrG and LcrV during type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 183, 4588–4598.
Depaolo, R. W., Tang, F., Kim, I., Han, M., Levin, N., Ciletti, N., Lin, A., Anderson, D., Schneewind, O., and Jabri, B. (2008). Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host. Microbe 4, 350–361.
Derewenda, U., Mateja, A., Devedjiev, Y., Routzahn, K. M., Evdokimov, A. G., Derewenda, Z. S., and Waugh, D. S. (2004). The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure 12, 301–306.
Desin, T. S., Mickael, C. S., Lam, P. K., Potter, A. A., and Koster, W. (2010). Protection of epithelial cells from Salmonella enterica serovar Enteritidis invasion by antibodies against the SPI-1 type III secretion system. Can. J. Microbiol. 56, 522–526.
Dickenson, N. E., Zhang, L., Epler, C. R., Adam, P. R., Picking, W. L., and Picking, W. D. (2011). Conformational changes in IpaD from Shigella flexneri upon binding bile salts provide insight into the second step of type III secretion. Biochemistry 50, 172–180.
Druar, C., Yu, F., Barnes, J. L., Okinaka, R. T., Chantratita, N., Beg, S., Stratilo, C. W., Olive, A. J., Soltes, G., Russell, M. L., Limmathurotsakul, D., Norton, R. E., Ni, S. X., Picking, W. D., Jackson, P. J., Stewart, D. I., Tsvetnitsky, V., Picking, W. L., Cherwonogrodzky, J. W., Ketheesan, N., Peacock, S. J., and Wiersma, E. J. (2008). Evaluating Burkholderia pseudomallei Bip proteins as vaccines and Bip antibodies as detection agents. FEMS Immunol. Med. Microbiol. 52, 78–87.
Eichelberg, K., Ginocchio, C. C., and Galan, J. E. (1994). Molecular and functional characterization of the Salmonella typhimurium invasion genes invB and invC: homology of InvC to the F0F1 ATPase family of proteins. J. Bacteriol. 176, 4501–4510.
El Solh, A. A., Akinnusi, M. E., Wiener-Kronish, J. P., Lynch, S. V., Pineda, L. A., and Szarpa, K. (2008). Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 178, 513–519.
El Solh, A. A., and Alhajhusain, A. (2009). Update on the treatment of Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 64, 229–238.
Epler, C. R., Dickenson, N. E., Olive, A. J., Picking, W. L., and Picking, W. D. (2009). Liposomes recruit IpaC to the Shigella type III secretion apparatus needle as a final step in secretion induction. Infect. Immun. 77, 2754–2761.
Erskine, P. T., Knight, M. J., Ruaux, A., Mikolajek, H., Sang, N. W. F., Withers, J., Gill, R., Wood, S. P., Wood, M., Fox, G. C., and Cooper, J. B. (2006). High resolution structure of BipD: an invasion protein associated with the type III secretion system of Burkholderia pseudomallei. J. Mol. Biol. 363, 125–136.
Espina, M., Olive, A. J., Kenjale, R., Moore, D. S., Ausar, S. F., Kaminski, R. W., Oaks, E. V., Middaugh, C. R., Picking, W. D., and Picking, W. L. (2006). IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 74, 4391–4400.
Ferracci, F., Schubot, F. D., Waugh, D. S., and Plano, G. V. (2005). Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57, 970–987.
Fields, K. A., Nilles, M. L., Cowan, C., and Straley, S. C. (1999). Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67, 5395–5408.
Forsberg, A., Viitanen, A. M., Skurnik, M., and Wolf-Watz, H. (1991). The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5, 977–986.
Frank, D. W. (1997). The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26, 621–629.
Frank, D. W., Nair, G., and Schweizer, H. P. (1994). Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect. Immun. 62, 554–563.
Frank, D. W., Vallis, A., Wiener-Kronish, J. P., Roy-Burman, A., Spack, E. G., Mullaney, B. P., Megdoud, M., Marks, J. D., Fritz, R., and Sawa, T. (2002). Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186, 64–73.
Frithz-Lindsten, E., Holmstrom, A., Jacobsson, L., Soltani, M., Olsson, J., Rosqvist, R., and Forsberg, A. (1998). Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol. Microbiol. 29, 1155–1165.
Galan, J. E., and Collmer, A. (1999). Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322–1328.
Galan, J. E., and Wolf-Watz, H. (2006). Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573.
Garrity-Ryan, L. K., Kim, O. K., Balada-Llasat, J. M., Bartlett, V. J., Verma, A. K., Fisher, M. L., Castillo, C., Songsungthong, W., Tanaka, S. K., Levy, S. B., Mecsas, J., and Alekshun, M. N. (2010). Small molecule inhibitors of LcrF, a Yersinia pseudotuberculosis transcription factor, attenuate virulence and limit infection in a murine pneumonia model. Infect. Immun. 78, 4683–4690.
Gotoh, K., Kodama, T., Hiyoshi, H., Izutsu, K., Park, K. S., Dryselius, R., Akeda, Y., Honda, T., and Iida, T. (2010). Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS ONE 5, e13365. doi: 10.1371/journal.pone.0013365
Goure, J., Broz, P., Attree, O., Cornelis, G. R., and Attree, I. (2005). Protective anti-V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J. Infect. Dis. 192, 218–225.
Goure, J., Pastor, A., Faudry, E., Chabert, J., Dessen, A., and Attree, I. (2004). The V antigen of Pseudomonas aeruginosa is required for assembly of the functional PopB/PopD translocation pore in host cell membranes. Infect. Immun. 72, 4741–4750.
Guex, N., and Peitsch, M. C. (1997). SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723.
Hakansson, S., Schesser, K., Persson, C., Galyov, E. E., Rosqvist, R., Homble, F., and Wolf-Watz, H. (1996). The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15, 5812–5823.
Hauser, A. R. (2009). The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7, 654–665.
Heurtault, B., Gentine, P., Thomann, J. S., Baehr, C., Frisch, B., and Pons, F. (2009). Design of a liposomal candidate vaccine against Pseudomonas aeruginosa and its evaluation in triggering systemic and lung mucosal immunity. Pharm. Res. 26, 276–285.
Hill, J., Leary, S. E., Griffin, K. F., Williamson, E. D., and Titball, R. W. (1997). Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 65, 4476–4482.
Holder, I. A., Neely, A. N., and Frank, D. W. (2001). PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect. Immun. 69, 5908–5910.
Holmstrom, A., Olsson, J., Cherepanov, P., Maier, E., Nordfelth, R., Pettersson, J., Benz, R., Wolf-Watz, H., and Forsberg, A. (2001). LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39, 620–632.
Hueck, C. J. (1998). Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62, 379–433.
Imamura, Y., Yanagihara, K., Fukuda, Y., Kaneko, Y., Seki, M., Izumikawa, K., Miyazaki, Y., Hirakata, Y., Sawa, T., Wiener-Kronish, J. P., and Kohno, S. (2007). Effect of anti-PcrV antibody in a murine chronic airway Pseudomonas aeruginosa infection model. Eur. Respir. J. 29, 965–968.
Iriarte, M., Sory, M. P., Boland, A., Boyd, A. P., Mills, S. D., Lambermont, I., and Cornelis, G. R. (1998). TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17, 1907–1918.
Johnson, S., Roversi, P., Espina, M., Olive, A., Deane, J. E., Birket, S., Field, T., Picking, W. D., Blocker, A. J., Galyov, E. E., Picking, W. L., and Lea, S. M. (2007). Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J. Biol. Chem. 282, 4035–4044.
Journet, L., Agrain, C., Broz, P., and Cornelis, G. R. (2003). The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302, 1757–1760.
Kamei, A., Coutinho-Sledge, Y. S., Goldberg, J. B., Priebe, G. P., and Pier, G. B. (2011). Mucosal vaccination with a multivalent, live-attenuated vaccine induces multifactorial immunity against Pseudomonas aeruginosa acute lung infection. Infect. Immun. 79, 1289–1299.
Kang, Y., Lunin, V. V., Skarina, T., Savchenko, A., Schurr, M. J., and Hoang, T. T. (2009). The long-chain fatty acid sensor, PsrA, modulates the expression of rpoS and the type III secretion exsCEBA operon in Pseudomonas aeruginosa. Mol. Microbiol. 73, 120–136.
Kenjale, R., Wilson, J., Zenk, S. F., Saurya, S., Picking, W. L., Picking, W. D., and Blocker, A. (2005). The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J. Biol. Chem. 280, 42929–42937.
Knutton, S., Rosenshine, I., Pallen, M. J., Nisan, I., Neves, B. C., Bain, C., Wolff, C., Dougan, G., and Frankel, G. (1998). A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17, 2166–2176.
La Ragione, R. M., Patel, S., Maddison, B., Woodward, M. J., Best, A., Whitelam, G. C., and Gough, K. C. (2006). Recombinant anti-EspA antibodies block Escherichia coli O157:H7-induced attaching and effacing lesions in vitro. Microbes Infect. 8, 426–433.
Lara-Tejero, M., and Galan, J. E. (2009). Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect. Immun. 77, 2635–2642.
Lara-Tejero, M., Kato, J., Wagner, S., Liu, X., and Galan, J. E. (2011). A Sorting Platform Determines the Order of Protein Secretion in Bacterial Type III Systems. Science 1188, 1188–1191.
Lawton, W. D., Erdman, R. I., and Surgalla, M. J. (1963). Biosynthesis and purification of V and W antigen in Pasteurella pestis. J. Immunol. 91, 179–184.
Leary, S. E., Williamson, E. D., Griffin, K. F., Russell, P., Eley, S. M., and Titball, R. W. (1995). Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 63, 2854–2858.
Lee, P. C., Stopford, C. M., Svenson, A. G., and Rietsch, A. (2010). Control of effector export by the P. aeruginosa type III secretion proteins PcrG and PcrV. Mol. Microbiol. 75, 924–941.
Lee, V. T., Pukatzki, S., Sato, H., Kikawada, E., Kazimirova, A. A., Huang, J., Li, X., Arm, J. P., Frank, D. W., and Lory, S. (2007). Pseudolipasin A is a specific inhibitor for phospholipase A2 activity of Pseudomonas aeruginosa cytotoxin ExoU. Infect. Immun. 75, 1089–1098.
Lee, V. T., Tam, C., and Schneewind, O. (2000). LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275, 36869–36875.
Luo, Y., Bertero, M. G., Frey, E. A., Pfuetzner, R. A., Wenk, M. R., Creagh, L., Marcus, S. L., Lim, D., Sicheri, F., Kay, C., Haynes, C., Finlay, B. B., and Strynadka, N. C. (2001). Structural and biochemical characterization of the type III secretion chaperones CesT and SigE. Nat. Struct. Biol. 8, 1031–1036.
Marenne, M. N., Journet, L., Mota, L. J., and Cornelis, G. R. (2003). Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35, 243–258.
Markham, A. P., Barrett, B. S., Esfandiary, R., Picking, W. L., Picking, W. D., Joshi, S. B., and Middaugh, C. R. (2010). Formulation and immunogenicity of a potential multivalent type III secretion system-based protein vaccine. J. Pharm. Sci. 99, 4497–4509.
Markham, A. P., Jaafar, Z. A., Kemege, K. E., Middaugh, C. R., and Hefty, P. S. (2009). Biophysical characterization of Chlamydia trachomatis CT584 supports its potential role as a type III secretion needle tip protein. Biochemistry 48, 10353–10361.
Marteyn, B., West, N. P., Browning, D. F., Cole, J. A., Shaw, J. G., Palm, F., Mounier, J., Prevost, M. C., Sansonetti, P., and Tang, C. M. (2010). Modulation of Shigella virulence in response to available oxygen in vivo. Nature 465, 355–358.
Martinez-Argudo, I., and Blocker, A. J. (2010). The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol. Microbiol. 78, 1365–1378.
Matson, J. S., Durick, K. A., Bradley, D. S., and Nilles, M. L. (2005). Immunization of mice with YscF provides protection from Yersinia pestis infections. BMC Microbiol. 5, 38. doi: 10.1186/1471-2180-5-38
Matson, J. S., and Nilles, M. L. (2001). LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J. Bacteriol. 183, 5082–5091.
McCaw, M. L., Lykken, G. L., Singh, P. K., and Yahr, T. L. (2002). ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46, 1123–1133.
Medhekar, B., Shrivastava, R., Mattoo, S., Gingery, M., and Miller, J. F. (2009). Bordetella Bsp22 forms a filamentous type III secretion system tip complex and is immunoprotective in vitro and in vivo. Mol. Microbiol. 71, 492–504.
Menard, R., Sansonetti, P., and Parsot, C. (1994). The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13, 5293–5302.
Menard, R., Sansonetti, P. J., and Parsot, C. (1993). Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175, 5899–5906.
Moraes, T. F., Spreter, T., and Strynadka, N. C. (2008). Piecing together the type III injectisome of bacterial pathogens. Curr. Opin. Struct. Biol. 18, 258–266.
Moriyama, K., Wiener-Kronish, J. P., and Sawa, T. (2009). Protective effects of affinity-purified antibody and truncated vaccines against Pseudomonas aeruginosa V-antigen in neutropenic mice. Microbiol. Immunol. 53, 587–594.
Mota, L. J., and Cornelis, G. R. (2005). The bacterial injection kit: type III secretion systems. Ann. Med. 37, 234–249.
Motin, V. L., Kutas, S. M., and Brubaker, R. R. (1997). Suppression of mouse skin allograft rejection by protein A-Yersiniae V antigen fusion peptide. Transplantation 63, 1040–1042.
Motin, V. L., Nakajima, R., Smirnov, G. B., and Brubaker, R. R. (1994). Passive immunity to Yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62, 4192–4201.
Mueller, C. A., Broz, P., and Cornelis, G. R. (2008). The type III secretion system tip complex and translocon. Mol. Microbiol. 68, 1085–1095.
Mueller, C. A., Broz, P., Muller, S. A., Ringler, P., Erne-Brand, F., Sorg, I., Kuhn, M., Engel, A., and Cornelis, G. R. (2005). The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310, 674–676.
Nakajima, R., and Brubaker, R. R. (1993). Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61, 23–31.
Nakajima, R., Motin, V. L., and Brubaker, R. R. (1995). Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63, 3021–3029.
Nedialkov, Y. A., Motin, V. L., and Brubaker, R. R. (1997). Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65, 1196–1203.
Neely, A. N., Holder, I. A., Wiener-Kronish, J. P., and Sawa, T. (2005). Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns 31, 153–158.
Neyt, C., and Cornelis, G. R. (1999). Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33, 971–981.
Nilles, M. L., Fields, K. A., and Straley, S. C. (1998). The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J. Bacteriol. 180, 3410–3420.
Nilles, M. L., Williams, A. W., Skrzypek, E., and Straley, S. C. (1997). Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J. Bacteriol. 179, 1307–1316.
Olive, A. J., Kenjale, R., Espina, M., Moore, D. S., Picking, W. L., and Picking, W. D. (2007). Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect. Immun. 75, 2626–2629.
Overheim, K. A., Depaolo, R. W., DeBord, K. L., Morrin, E. M., Anderson, D. M., Green, N. M., Brubaker, R. R., Jabri, B., and Schneewind, O. (2005). LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 73, 5152–5159.
Parsot, C. (2009). Shigella type III secretion effectors: how, where, when, for what purposes? Current Opinion in Microbiology 12, 110–116.
Persson, C., Nordfelth, R., Holmstrom, A., Hakansson, S., Rosqvist, R., and Wolf-Watz, H. (1995). Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18, 135–150.
Pettersson, J., Holmstrom, A., Hill, J., Leary, S., Frithz-Lindsten, E., Euler-Matell, A., Carlsson, E., Titball, R., Forsberg, A., and Wolf-Watz, H. (1999). The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32, 961–976.
Pettersson, J., Nordfelth, R., Dubinina, E., Bergman, T., Gustafsson, M., Magnusson, K. E., and Wolf-Watz, H. (1996). Modulation of virulence factor expression by pathogen target cell contact. Science 273, 1231–1233.
Picking, W. L., Nishioka, H., Hearn, P. D., Baxter, M. A., Harrington, A. T., Blocker, A., and Picking, W. D. (2005). IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect. Immun. 73, 1432–1440.
Powell, B. S., Andrews, G. P., Enama, J. T., Jendrek, S., Bolt, C., Worsham, P., Pullen, J. K., Ribot, W., Hines, H., Smith, L., Heath, D. G., and Adamovicz, J. J. (2005). Design and testing for a nontagged F1-V fusion protein as vaccine antigen against bubonic and pneumonic plague. Biotechnol. Prog. 21, 1490–1510.
Price, S. B., Cowan, C., Perry, R. D., and Straley, S. C. (1991). The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+ -dependent growth and maximal expression of low-Ca2+ response virulence genes. J. Bacteriol. 173, 2649–2657.
Quenee, L. E., Berube, B. J., Segal, J., Elli, D., Ciletti, N. A., Anderson, D., and Schneewind, O. (2010). Amino acid residues 196-225 of LcrV represent a plague protective epitope. Vaccine 28, 1870–1876.
Quenee, L. E., and Schneewind, O. (2009). Plague vaccines and the molecular basis of immunity against Yersinia pestis. Hum. Vaccin. 5, 817–823.
Rathinavelan, T., Tang, C., and De Guzman, R. N. (2011). Characterization of the interaction between the Salmonella type III secretion system tip protein SipD and the needle protein PrgI by paramagnetic relaxation enhancement. J. Biol. Chem. 286, 4922–4930.
Rello, J., Ausina, V., Ricart, M., Castella, J., and Prats, G. (1993). Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest 104, 1230–1235.
Rietsch, A., and Mekalanos, J. J. (2006). Metabolic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 59, 807–820.
Rietsch, A., Vallet-Gely, I., Dove, S. L., and Mekalanos, J. J. (2005). ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 102, 8006–8011.
Rietsch, A., Wolfgang, M. C., and Mekalanos, J. J. (2004). Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect. Immun. 72, 1383–1390.
Rimpilainen, M., Forsberg, A., and Wolf-Watz, H. (1992). A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J. Bacteriol. 174, 3355–3363.
Riordan, K. E., and Schneewind, O. (2008). YscU cleavage and the assembly of Yersinia type III secretion machine complexes. Mol. Microbiol. 68, 1485–1501.
Rosqvist, R., Magnusson, K. E., and Wolf-Watz, H. (1994). Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13, 964–972.
Roy, D., Liston, D. R., Idone, V. J., Di, A., Nelson, D. J., Pujol, C., Bliska, J. B., Chakrabarti, S., and Andrews, N. W. (2004). A process for controlling intracellular bacterial infections induced by membrane injury. Science 304, 1515–1518.
Roy-Burman, A., Savel, R. H., Racine, S., Swanson, B. L., Revadigar, N. S., Fujimoto, J., Sawa, T., Frank, D. W., and Wiener-Kronish, J. P. (2001). Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183, 1767–1774.
Sarker, M. R., Neyt, C., Stainier, I., and Cornelis, G. R. (1998). The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180, 1207–1214.
Sato, H., Hunt, M. L., Weiner, J. J., Hansen, A. T., and Frank, D. W. (2011). Modified needle-tip PcrV proteins reveal distinct phenotypes relevant to the control of type III secretion and intoxication by Pseudomonas aeruginosa. PLoS ONE 6, e18356.
Sawa, T., Yahr, T. L., Ohara, M., Kurahashi, K., Gropper, M. A., Wiener-Kronish, J. P., and Frank, D. W. (1999). Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5, 392–398.
Schoehn, G., Di Guilmi, A. M., Lemaire, D., Attree, I., Weissenhorn, W., and Dessen, A. (2003). Oligomerization of type III secretion proteins PopB and PopD precedes pore formation in Pseudomonas. EMBO J. 22, 4957–4967.
Schroeder, G. N., and Hilbi, H. (2008). Molecular pathogenesis of Shigella spp.: Controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 21, 134–156.
Schubot, F. D., Jackson, M. W., Penrose, K. J., Cherry, S., Tropea, J. E., Plano, G. V., and Waugh, D. S. (2005). Three-dimensional structure of a macromolecular assembly that regulates type III secretion in Yersinia pestis. J. Mol. Biol. 346, 1147–1161.
Schuller, S., and Phillips, A. D. (2010). Microaerobic conditions enhance type III secretion and adherence of enterohaemorrhagic Escherichia coli to polarized human intestinal epithelial cells. Environ. Microbiol. 12, 2426–2435.
Shime, N., Sawa, T., Fujimoto, J., Faure, K., Allmond, L. R., Karaca, T., Swanson, B. L., Spack, E. G., and Wiener-Kronish, J. P. (2001). Therapeutic administration of anti-PcrV F(ab′)(2) in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 167, 5880–5886.
Sing, A., Reithmeier-Rost, D., Granfors, K., Hill, J., Roggenkamp, A., and Heesemann, J. (2005). A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. U.S.A. 102, 16049–16054.
Sing, A., Roggenkamp, A., Geiger, A. M., and Heesemann, J. (2002a). Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168, 1315–1321.
Sing, A., Rost, D., Tvardovskaia, N., Roggenkamp, A., Wiedemann, A., Kirschning, C. J., Aepfelbacher, M., and Heesemann, J. (2002b). Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196, 1017–1024.
Skrzypek, E., and Straley, S. C. (1995). Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J. Bacteriol. 177, 2530–2542.
Sorg, I., Wagner, S., Amstutz, M., Muller, S. A., Broz, P., Lussi, Y., Engel, A., and Cornelis, G. R. (2007). YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J. 26, 3015–3024.
Sory, M. P., and Cornelis, G. R. (1994). Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14, 583–594.
Stebbins, C. E., and Galan, J. E. (2003). Priming virulence factors for delivery into the host. Nat. Rev. Mol. Cell Biol. 4, 738–743.
Stensrud, K. F., Adam, P. R., La Mar, C. D., Olive, A. J., Lushington, G. H., Sudharsan, R., Shelton, N. L., Givens, R. S., Picking, W. L., and Picking, W. D. (2008). Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J. Biol. Chem. 283, 18646–18654.
Sundin, C., Thelaus, J., Broms, J. E., and Forsberg, A. (2004). Polarisation of type III translocation by Pseudomonas aeruginosa requires PcrG, PcrV and PopN. Microb. Pathog. 37, 313–322.
Torruellas, J., Jackson, M. W., Pennock, J. W., and Plano, G. V. (2005). The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol. Microbiol. 57, 1719–1733.
Troisfontaines, P., and Cornelis, G. R. (2005). Type III secretion: more systems than you think. Physiology 20, 326–339.
Une, T., and Brubaker, R. R. (1984). Roles of V antigen in promoting virulence and immunity in Yersiniae. J. Immunol. 133, 2226–2230.
Urbanowski, M. L., Brutinel, E. D., and Yahr, T. L. (2007). Translocation of ExsE into Chinese hamster ovary cells is required for transcriptional induction of the Pseudomonas aeruginosa type III secretion system. Infect. Immun. 75, 4432–4439.
Urbanowski, M. L., Lykken, G. L., and Yahr, T. L. (2005). A secreted regulatory protein couples transcription to the secretory activity of the Pseudomonas aeruginosa type III secretion system. Proc. Natl. Acad. Sci. U.S.A. 102, 9930–9935.
Urbanowski, M. L., and Yahr, T. L. (2008). Limiting too much of a good thing: a negative feedback mechanism prevents unregulated translocation of type III effector proteins. J. Bacteriol. 190, 2643–2644.
Vallis, A. J., Finck-Barbancon, V., Yahr, T. L., and Frank, D. W. (1999a). Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67, 2040–2044.
Vallis, A. J., Yahr, T. L., Barbieri, J. T., and Frank, D. W. (1999b). Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect. Immun. 67, 914–920.
Van Blarcom, T. J., Sofer-Podesta, C., Ang, J., Boyer, J. L., Crystal, R. G., and Georgiou, G. (2010). Affinity maturation of an anti-V antigen IgG expressed in situ through adenovirus gene delivery confers enhanced protection against Yersinia pestis challenge. Gene Ther. 17, 913–921.
van der Goot, F. G., Tran, v. N., Allaoui, A., Sansonetti, P., and Lafont, F. (2004). Rafts can trigger contact-mediated secretion of bacterial effectors via a lipid-based mechanism. J. Biol. Chem. 279, 47792–47798.
Veenendaal, A. K., Hodgkinson, J. L., Schwarzer, L., Stabat, D., Zenk, S. F., and Blocker, A. J. (2007). The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol. Microbiol. 63, 1719–1730.
Veenendaal, A. K., Sundin, C., and Blocker, A. J. (2009). Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton. J. Bacteriol. 191, 563–570.
Vernazza, C., Lingard, B., Flick-Smith, H. C., Baillie, L. W., Hill, J., and Atkins, H. S. (2009). Small protective fragments of the Yersinia pestis V antigen. Vaccine 27, 2775–2780.
Viboud, G. I., and Bliska, J. B. (2001). A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20, 5373–5382.
Wang, J., Zhang, Y., Lu, C., Lei, L., Yu, P., and Zhong, G. (2010a). A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J. Immunol. 185, 1670–1680.
Wang, Y., Nordhues, B. A., Zhong, D., and De Guzman, R. N. (2010b). NMR characterization of the interaction of the Salmonella type III secretion system protein SipD and bile salts. Biochemistry 49, 4220–4226.
Wangdi, T., Mijares, L. A., and Kazmierczak, B. I. (2010). In vivo discrimination of type 3 secretion system-positive and -negative Pseudomonas aeruginosa via a caspase-1-dependent pathway. Infect. Immun. 78, 4744–4753.
Wilharm, G., Lehmann, V., Krauss, K., Lehnert, B., Richter, S., Ruckdeschel, K., Heesemann, J., and Trulzsch, K. (2004). Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB. Infect. Immun. 72, 4004–4009.
Williamson, E. D., Flick-Smith, H. C., Lebutt, C., Rowland, C. A., Jones, S. M., Waters, E. L., Gwyther, R. J., Miller, J., Packer, P. J., and Irving, M. (2005). Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 73, 3598–3608.
Wulff-Strobel, C. R., Williams, A. W., and Straley, S. C. (2002). LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion. Mol. Microbiol. 43, 411–423.
Yahr, T. L., Mende-Mueller, L. M., Friese, M. B., and Frank, D. W. (1997). Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179, 7165–7168.
Yahr, T. L., and Wolfgang, M. C. (2006). Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62, 631–640.
Yip, C. K., Finlay, B. B., and Strynadka, N. C. J. (2005). Structural characterization of a type III secretion system filament protein in complex with its chaperone. Nat. Struct. Mol. Biol. 12, 75–81.
Yogurtcu, O. N., Wolgemuth, C. W., and Sun, S. X. (2010). Mechanical response and conformational amplification in alpha-helical coiled coils. Biophys. J. 99, 3895–3904.
Yu, S., Gu, J., Wang, H. G., Wang, Q. X., Luo, P., Wu, C., Zhang, W. J., Guo, G., Tong, W. D., Zou, Q. M., and Mao, X. H. (2011). Identification of a novel linear epitope on EspA from enterohemorrhagic E. coli using a neutralizing and protective monoclonal antibody. Clin. Immunol. 138, 77–84.
Yu, X. J., McGourty, K., Liu, M., Unsworth, K. E., and Holden, D. W. (2010). pH sensing by intracellular Salmonella induces effector translocation. Science 328, 1040–1043.
Keywords: type III secretion, needle-tip proteins, PcrV, LcrV, Pseudomonas aeruginosa, Yersinia, protective antigen
Citation: Sato H and Frank DW (2011) Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other Gram-negative bacteria. Front. Microbio. 2:142. doi: 10.3389/fmicb.2011.00142
Received: 05 March 2011; Paper pending published: 02 June 2011;
Accepted: 15 June 2011; Published online: 04 July 2011.
Edited by:
Michael J. Schurr, University of Colorado, USACopyright: © 2011 Sato and Frank. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Dara W. Frank, Center for Infectious Disease Research, Department of Microbiology and Molecular Genetics, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA. e-mail:ZnJhbmtkQG1jdy5lZHU=