- 1Clinical Innovation & Business Unit, South Western Sydney Local Health District, Sydney, NSW, Australia
- 2South Western Sydney Nursing and Midwifery Research Alliance, Ingham Institute for Applied Medical Research, Sydney, NSW, Australia
- 3Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
- 4Faculty of Medicine, Western Sydney University, Sydney, NSW, Australia
- 5Research Directorate, South Western Sydney Local Health District, Liverpool, NSW, Australia
- 6Clinical Trial Support Unit, Ingham Institute for Applied Medical Research, Liverpool, NSW, Australia
- 7District Nursing and Midwifery Office, South Western Sydney Local Health District, Liverpool, NSW, Australia
- 8Emergency Department, Bankstown Hospital, South Western Sydney Local Health District, Liverpool, NSW, Australia
- 9Neurology Research Unit, South Western Sydney Local Health District, Liverpool, NSW, Australia
Background: There is increasing evidence that COVID-19 survivors are at increased risk of experiencing a wide range of cardiovascular complications post infection; however, there are no validated models or clear guidelines for remotely monitoring the cardiac health of COVID-19 survivors.
Objective: This study aims to test a virtual, in-home healthcare monitoring model of care for detection of clinical symptoms and impacts on COVID-19 survivors. It also aims to demonstrate system usability and feasibility.
Methods: This open label, prospective, descriptive study was conducted in South Western Sydney. Included in the study were patients admitted to the hospital with the diagnosis of COVID-19 between June 2021 and November 2021. Eligible participants after consent were provided with a pulse oximeter to measure oxygen saturation and a S-Patch EX to monitor their electrocardiogram (ECG) for a duration of 3 months. Data was transmitted in real-time to a mobile phone via Bluetooth technology and results were sent to the study team via a cloud-based platform. All the data was reviewed in a timely manner by the investigator team, for post COVID-19 related symptoms, such as reduction in oxygen saturation and arrhythmia.
Outcome measure: This study was designed for feasibility in real clinical setting implementation, enabling the study team to develop and utilise a virtual, in-home healthcare monitoring model of care to detect post COVID-19 clinical symptoms and impacts on COVID-19 survivors.
Results: During the study period, 23 patients provided consent for participation. Out of which 19 patients commenced monitoring. Sixteen patients with 81 (73.6%) valid tests were included in the analysis and amongst them seven patients were detected by artificial intelligence to have cardiac arrhythmias but not clinically symptomatic. The patients with arrhythmias had a higher occurrence of supraventricular ectopy, and most of them took at least 2 tests before detection. Notably, patients with arrhythmia had significantly more tests than those without [t-test, t (13) = 2.29, p < 0.05].
Conclusions: Preliminary observations have identified cardiac arrhythmias on prolonged cardiac monitoring in 7 out of the first 16 participants who completed their 3 months follow-up. This has allowed early escalation to their treating doctors for further investigations and early interventions.
Introduction
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. Some people may experience a wide range of ongoing health problems post COVID-19 infection which may last for weeks, months or years (1, 2). These health problems have been collectively termed as post-COVID conditions or long COVID (3, 4). The severity of symptoms vary between different individuals which can range from very mild to severe. The most commonly reported long COVID symptoms are fatigue, dyspnoea, depression, anxiety, memory loss, sleep disorder and concentration difficulties (5). The follow-up studies have reported that the symptoms could persist in patients up to 2 years or more after acute infection (6, 7). However the occurrence and duration of long COVID is still unclear due to heterogeneity in the studies with respect to follow-up duration, case definitions, study cohort, assessment tools and study design (6–9).
One of the common long term health issues faced by COVID-19 survivors is post-exertional malaise (PEM), a worsening of symptoms and reduction in function after physical or mental activity (10). In some cases, the symptoms are fairly constant, while others may experience fluctuation in their symptoms (11). Multiple types of triggers can lead to an extremal deterioration of the overall health condition (12). In an international online survey of 3,762 individuals with suspected or confirmed COVID-19 illness, more than 85% of individuals experienced relapses of their symptoms, with exercise, physical or mental activity, and stress being the main triggers (8). Vital signs such as heart rate, blood pressure, and respiratory rate are the most sensitive to the PEM triggers and thus measuring and monitoring these vitals could help identify and prevent any further deterioration in patient's condition (12–14).
Sensors and commercial wearable devices have been widely used to monitor vital signs in COVID-19 patients over long periods of time (15–19). Studies utilizing data from fitbit devices have shown modification in the Resting Heart Rate (RHR) for up to 3 months following symptom onset with substantial intraindividual variability. A pattern of bradycardia and tachycardia in the RHR were also observed in the patients over this period (17, 18). For patients with underlying heart conditions and respiratory disease, COVID-19 can have fatal consequences. There is increasing evidence that COVID-19 survivors are at increased risk of experiencing a wide range of cardiovascular complications regardless of age, race, sex, symptom severity, underlying chronic condition and care setting (20–24). Recent studies have shown a significant variation in parasympathetic modulation and Heart Rate Variability (HRV) in patients post COVID-19 infection (25–27), thus highlighting the need for early identification of patients at risk of clinical deterioration.
CardioVascular Disease (CVD) is one of our biggest health problems, killing an Australian every 12 min (28). Cardiac arrhythmias in particular, Atrial Fibrillation (AF) increase risk of stroke and heart failure, and are a common cause of death (29). COVID-19 can cause myocardial (heart muscle) damage and cardiac arrhythmias, increasing the risk of stroke and heart failure (30–32). Up to 30% of patients hospitalised with COVID-19 in 2019 had evidence of myocardial involvement and acute cardiac injury was associated with higher morbidity and mortality (21, 30). An in-depth analysis of federal health data indicated that the risk and 1-year burden of CVD in survivors of acute COVID-19 are substantial (23).
Most people with COVID-19 do not require admission to hospital as they are able to recover at home. With a disease as infectious as COVID-19, remaining in isolation plays a key role in preventing transmission however it is also necessary to ensure patients are receiving the care they need. Virtual, in-home digital healthcare programs for confirmed COVID-19 cases have been implemented globally and in Australia in response to the pandemic, however, more evidence is needed on their adaptation and implementation. There are no validated models or clear guidelines for remotely monitoring the cardiac health of COVID-19 survivors. Despite reasonably effective vaccines, COVID-19 continues to be a serious public health problem. The significant negative economic impact has driven government policy decisions to remove mask mandates and lift most COVID-19 restrictions. However, with the emergence of new variants and increasing surges in case numbers, the ultimate health impact is huge. Furthermore, there is no definite understanding of the chronic impacts of COVID-19 on heart, lungs, organs and brain. This warrants the development of innovative and alternate approaches to designing collaborative models of care. A validated model for digital health solution that detects cardiac health deterioration early and reduces the burden on hospital-based clinical care will benefit patients, primary and secondary health carers, and acute-care hospitals. Therefore, early screening of convalescing patients via a virtual, in-home healthcare program may reduce the population burden of long-term CVD from COVID-19.
Materials and methods
Study aim
This study aimed to demonstrate usability and feasibility of the implementation of an innovative, novel model of digital healthcare in the primary healthcare setting for vulnerable populations affected by COVID-19 survivors. We had combined two smart, wearable/portable biosensor technologies to remotely identify clinical signs and symptoms of CVD in patients who had returned home after hospitalisation due to COVID-19 infection.
Study design and intervention
This was an open label, prospective, descriptive study of a custom-designed virtual, in-home healthcare monitoring model of care to detect ongoing clinical symptom and impacts on COVID-19 survivors. The model of care utilised bio-instrumentation technologies, including pulse oximeter to measure oxygen saturation, and S-Patch EX to monitor the electrocardiogram (ECG) via mobile phone application which allowed clinicians to review the results via a cloud-based platform. These devices were approved for clinical use worldwide and have approval from the Therapeutic Goods Authority (TGA).
iHealth pulse oximeter
The iHealth wireless pulse oximeter worked by emitting beams of light through the finger and determining the blood oxygen levels based on light absorption (Figure 1). The participants were required to download the iHealth app on their smart phones which connected to their pulse oximeter to record their oxygen saturations, a minimum of 3 times a day.
S-Patch EX
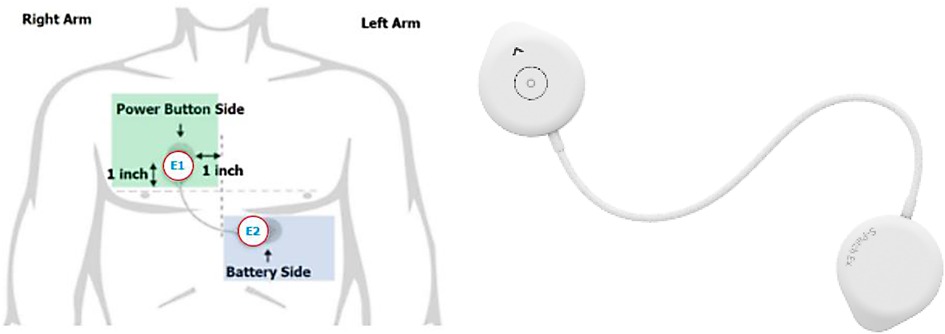
Wellysis, a Samsung SDS spinoff company, had developed a bio-processor; a single, compact chip that can measure Galvanic Skin Response (GSR) and PhotoPlethysmoGram (PPG) to sense pulsatile skin blood flow, ECG skin temperature, and body fat. This bio-processor is built into a device called the “S-Patch”, attachable to the chest with two electrode stickers (Figure 2); similar to a ward telemetry monitor that attaches to the chest via five electrode stickers. The bio-processor chip collected the data through the S-Patch device, which was transmitted in real-time to a mobile phone via Bluetooth technology. The participants were required to downloaded S Patch EX app onto their smartphones. The data was transmitted to the Cloud (e-server) for initial analysis and reporting, with this data being accessible with secure logon from desktops, tablets or other mobile devices. Our previous study had demonstrated the potential for the S-Patch wearable technology to be a clinically equivalent and improved solution for screening and diagnosis of arrhythmias whilst transforming into a more integrated and innovative model of care (33).
Study setting
This study was conducted in South Western Sydney.
Participants
Since this was a feasibility study, the sample for this study consisted of patients enrolled over the specific time period. All the patients admitted to the hospitals in the South Western Sydney region during June 2021 to November 2021 with the diagnosis of COVID-19 were screened for eligibility. Individuals were suitable for inclusion if they were 18 years of age or above, had a smartphone with Wi-Fi Internet at home and provided informed consent. Excluded from the study were patients who were pacemaker dependent or had Implantable Cardiac Defibrillator (ICD) and did not provide consent.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of this research. A survey of feedback from the patients was planned for electronic collection at 1, 2 and 3 months. There were five responses including one incomplete response and no conclusive results could be drawn. A number of healthcare worker representatives were involved in the development of the research question and the research methodology. De-identified preliminary study results were presented to key healthcare workers.
Data collection
The list of admitted patients with COVID-19 diagnosis was obtained from the South Western Sydney Local Health District Emergency Operations Centre. All patients were screened and assessed for suitability against the inclusion and exclusion criteria. The identified eligible participants were called as per the telephone script. Interested participants were sent the Participant Information Sheet (PIS) and Consent Form (CF). Depending on the participants preferences the PISCF was sent electronically via the Veeva myconsent platform, email or post. Participants were given ample time to consider their participation in the study prior to signing of the consent form. They could also discuss the study with their doctor or family members before consenting. Participation in this study was strictly voluntary and all participants were required to provide informed consent prior to any study procedure. Follow up phone call will be made to the participant to answer any questions or if any escalation of their remote monitoring data. Participants have access to the phone number of the research team and are encouraged to make the calls if required.
Once the patients consented, they were provided with both the iHealth pulse oximeter and the S-Patch EX for up to 3 months before returning to the research team via a pre-paid envelope. Other consumables such as batteries and ECG dots required for S-Patch were also provided. No additional visit to hospital was required. The participant was then fitted with both the devices according to the Product Operations Manual, guided by an experienced nurse/researcher via teleconsultation. Data was transmitted in real-time to their mobile phone via Bluetooth technology and results were uploaded on a cloud-based platform accessible to the study team.
The data was reviewed daily by a study investigator (clinician) with any findings requiring escalations highlighted and forwarded to the research team's medical officers for further review and advice. Medical officers in the research team had access to the telemetry database to allow for real-time review of ECG strips. Based on the advice of the medical officer's telemetry review, the patient and their doctor were informed of the findings and recommendation of any interventions if required. These interventions included but were not limited to the following:
• If the participant was determined at risk for an adverse event such as AF, this information was shared with their doctor immediately, via phone call +/− fax of summary telemetry.
• If the patient was found to be experiencing a medical emergency, they were advised to present to the nearest hospital or call the ambulance for assistance.
• If no further action was required outside of ongoing monitoring, the summary was provided to the patient and their doctor for their records.
• Should the patients require specialist review, their local treating doctor was expected to organise specialist consultations.
Analysis
The data analysis was conducted with Pandas 1.3.5 (34), an open-source data analysis tool. The statistical test including t-test was conducted with Scipy 1.7.3 (35), an open-source software for mathematics, science, and engineering. The analysis results were visualized using Maplotlib 3.5.1 (36).
Results
During the study period, 23 patients provided consent for participation. Out of which four were considered non-compliant as they did not commence any monitoring. The 19 patients who commenced monitoring had a mean age of 49 years (SD = 12.27), 57.9% (n = 11) of them were female.
S-Patch EX
The data analysis for the ECG via the S-Patch Ex included 16 patients with 81 (73.6%) valid tests out of 19 patients (total of 110 tests). Amongst 29 tests, 13 were excluded due to lack of valid data (N = 13) and other 16 were ruled out as they were less than 30 min (N = 16). Seven patients were detected by the Artificial Intelligence (AI) algorithms from the S-patch EX monitoring system, to have cardiac arrhythmias, including Atrial Fibrillation (AF), SupraVentricular Tachycardia (SVT), and Ventricular Tachycardia (VT).
Data summary
Out of those 16 patients with 81 valid tests (16 days of data collected on average) study reported, 7 patients had cardiac arrhythmias, including AF, SVT, and VT (Patient 3, 6, 7, 8, 10, 12, 18).
Figures 3A,B shows the cumulative recording time of all the patients and the number of tests, and seven patients with arrhythmia were shown in dark colour. Most of the patients with arrhythmia took at least 2 tests before detection, except for Patient 6, who had abnormal rhythms in the first test. Notably, patients with arrhythmias had significantly more tests than those without [t-test, t (13) = 2.29, p < 0.05, Figure 3C].
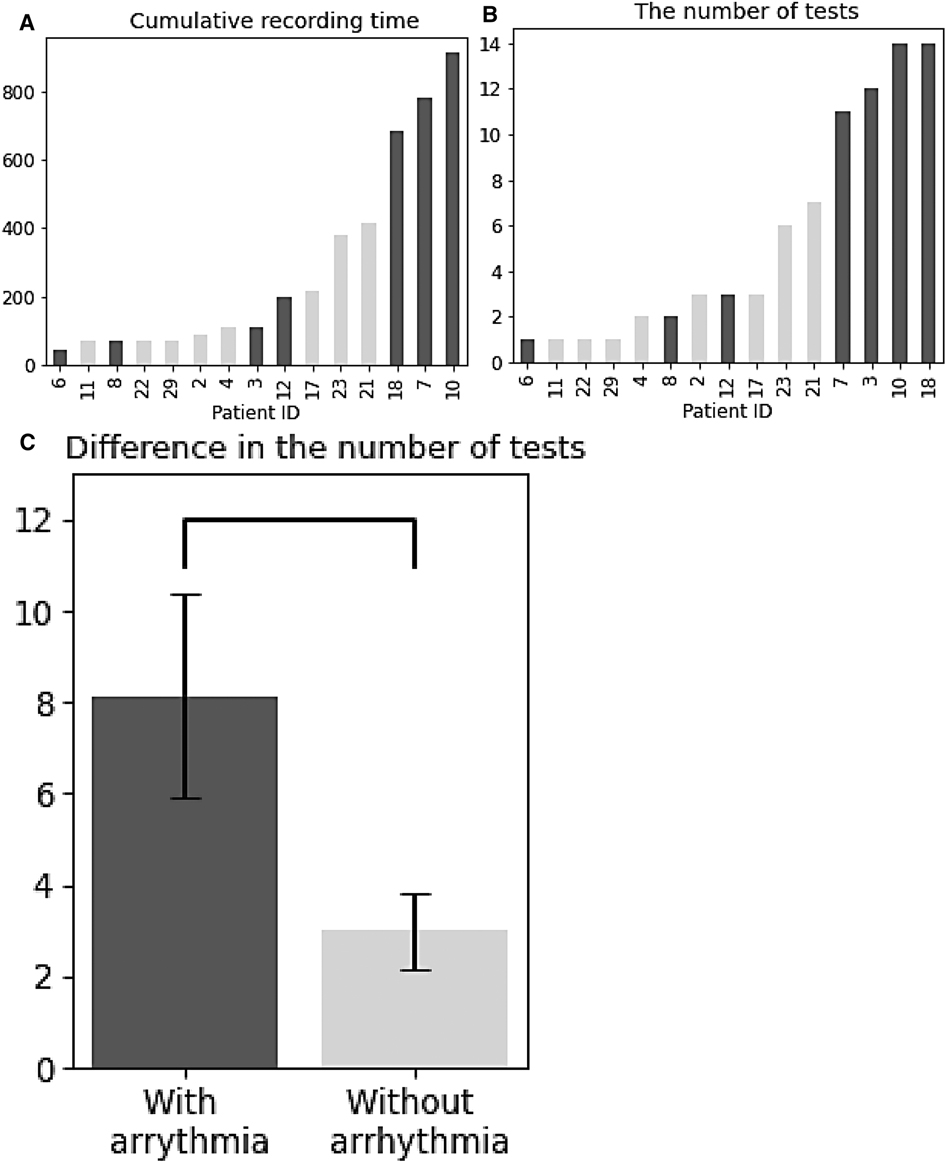
Figure 3. Overall data summary. (A) Cumulative recording time for patients. (B) The number of tests for patients. (C) Difference in the number of tests between patients with and without arrythmia. (A–C) Patients with arrhythmia are shown in dark colour.
Mean of QRS complexes, SVE%, and VE% for all patients
The percentage (%) of total QRS in relation to the total recording time per patient was analysed (Figure 4A) and it did not show any trend between the number of QRS complexes and cardiac arrhythmias occurrence. The average of SVE% (the ratio of SVE to the number of QRS) by patients was observed and the patients with arrhythmias had a higher occurrence of SVE on the tests they performed (Figure 4B). The means of SVE% for the patients (especially Patient 18, 3, 7) tended to be higher than for the other patients (Figure 4B). In summary, the % occurrence of SVE per test might be correlated to cardiac arrhythmias. In contrast to SVE, the average of VE% (the ratio of VE to the number of QRS) was not correlated to cardiac arrhythmias since no trend was observed (Figure 4C).
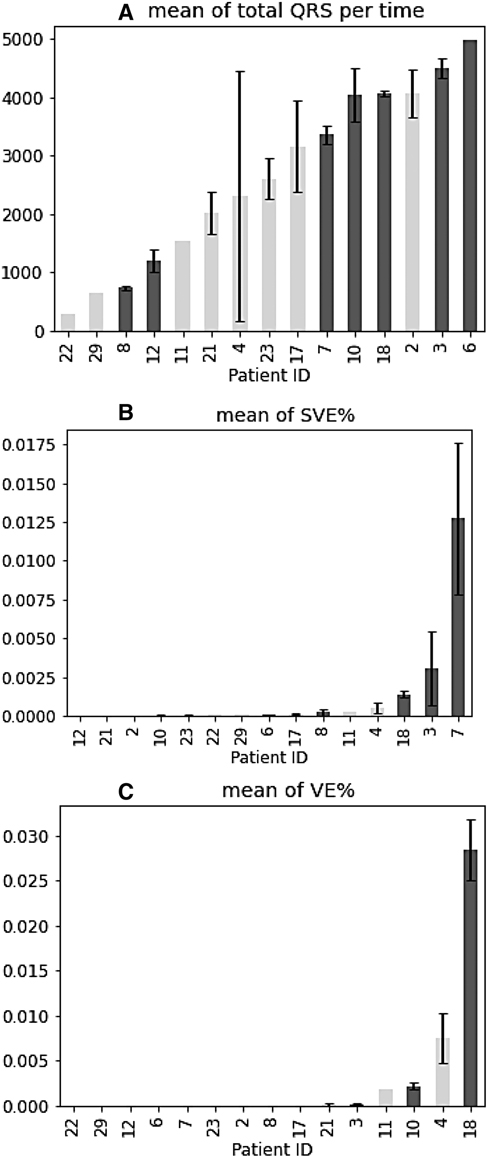
Figure 4. Mean of QRS complexes, SVE%, and VE% for all patients. (A–C) Patients with arrhythmia are shown in blue. Error bars indicate ± standard error of the mean.
Change of QRS complexes, SVE%, and VE% across tests within arrhythmia patients
After examining overall trend across all patients and investigating the change of these statistics across tests within the patients with cardiac arrhythmias (Patient 3, 6, 7, 8, 10, 12, 18). There was no trend between the number of QRS complexes and arrhythmias occurrence throughout the tests. They did not increase or decrease across the tests, and the test with arrhythmias did not show particularly high or low number of QRS (Figure 5-1).
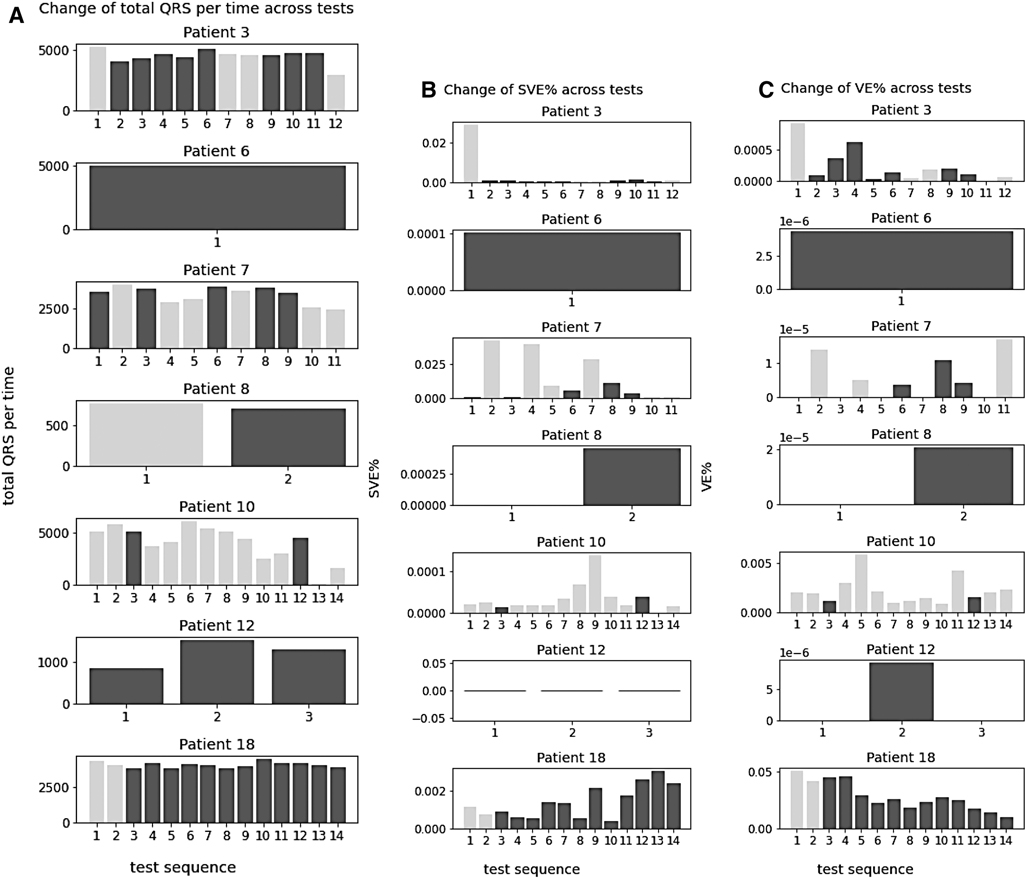
Figure 5. Change of QRS complexes, SVE%, and VE% across tests within arrhythmia patients. (A–C) Tests with arrythmia detected are shown in blue.
The change of SVE % (the ratio of SVE to the number of QRS) and VE % (the ratio of VE to the number of QRS) was also analysed and no trend was found in relation to the arrhythmias across tests. They did not increase or decrease across the tests, and the test with arrhythmias did not show particularly high or low SVE% and VE%.
iHealth pulse oximeter
Analysis of the data from the iHealth pulse oximeter was incomplete due to the lack of consistent and/or valid data as required for the study. Participants were required to download the app and take pulse oximeter readings a minimum of three times a day and send the data via the app. While most of the participants were focused on the S-Patch EX and its app, they failed to record and send their oxygen saturations despite of reminder and/or compliant with the frequency and the reporting. Participants may have recorded their data at various times during the study however this could not be determined as there was limited iHealth data (n = 2) received by the study team. Incidentally, the S-Patch EX also had the capability to collect heart rate data. All the readings collected and submitted were within the normal range.
Refinement of the protocol and communication platform
The study team met weekly to evaluate and refine the protocol, Standard Operation Procedure (SOP) process of care pathway and communication platform, for the proposed larger study. The processes of care pathway included (i) patients' selection to determine eligibility, (ii) onboarding of patients to the remote in-home monitoring service (provision of information to patient and/or carer on monitoring process), (iii) monitoring (including recording of observations, communication of the information, assessment of the information by the project and medical team), (iv) escalation (if required), and (v) discharge from the pathway.
The results of the experience and feedback of this preliminary group of participants provided valuable information for the study protocol amendments, which were submitted and approval by the Human Research Ethics Committee for further refinement. Some of the key amendments included:
• Instead of using the Bluetooth connection for the iHealth app to record their pulse oximetry readings, future participants will record their readings into the S-Patch Ex app. This information will then be uploaded with the S-Patch EX information automatically.
• Instead of requirement for 24/7 monitoring for a period of up to 3 months, those participants who were doing reasonably well and were unable to monitor every day, an option was given to monitor during weekends OR 2–3 consecutive days per week OR only during their “bad days” when they experience any symptom (extreme fatigue, shortness of breath, heart palpitations, chest pain or tightness problems with memory and concentration changes to taste and smell joint and muscle pain).
Discussion
Various remote home monitoring models have been designed and implemented for COVID-19 patients who were stable enough to be monitored at home providing early detection of deterioration, manage care escalation, avoid unwarranted hospital visit and further reduce strain on the hospitals (14, 37). Most of the models mainly focused on monitoring patients during their infectious period. Our study aimed to co-design and implement an innovative, novel model of virtual, in-home healthcare monitoring using advanced technologies and AI for vulnerable populations affected by long-term COVID symptoms and assessing its impacts on COVID-19 survivors. The project team had also developed and utilised a communications platform to connect all the healthcare professionals in the cardiac care pathway to ensure the patients received early screening via virtual, in-home healthcare program and activate appropriate interventions if required. This study identified recruitment barriers and developed guided strategies to overcome these so as to determine the most feasible and usable monitoring protocol.
Arrhythmias, such as AF are associated with an increased risk of stroke and heart failure. Recent studies (23, 24) have shown increased risk of arrhythmia related disorders among COVID survivors. In our study, the continuous data from S-Patch EX and its AI technology has enabled further investigation on the changes of cardiac physiology for the study participants. In particular, it showed that patients detected to have arrhythmias were tested significantly more than those who were not. Although it should be interpreted with caution whether more tests led to more detection of cardia arrhythmia or whether more tests were performed in patients with discomfort, this result suggested that a sufficient number of tests might help to detect cardiac arrhythmias. The patients with arrhythmias had a higher occurrence of SVE, while the number of QRS complexes and VE did not seem relevant to the abnormal heart rhythms. With the change of these statistics across tests within patients, no trend in SVE was observed. According to Figure 4B, patients with arrhythmias showed higher SVE% than other patients, but individual tests did not show any trend in relation to arrhythmias. This might indicate that although the average number of SVEs is important, the change over time or the frequency at the time of arrhythmias onset could not be significant.
Potential implications for policy and communications
An important finding from this study is the co-design and deployment of an innovative virtual care to fill the current gap, leveraging advancements in technologies to expedite the development and implementation of a digital intelligence model of cardiac health screening and monitoring. It has also improved patients' access to early screening and facilitation of early diagnostic and intervention from their primary healthcare partners.
Limitations of study
There are a number of limitations of this study. The limitation of available and approved technologies in Australia was a barrier to optimise the co-design and option for the solution. The inability to integrate both the S-Patch Ex and the iHealth pulse oximeter into a single app was a barrier which gave rise to technical challenges and issues with compliance resulting in lack of consistent and valid data from the pulse oximeter. The full function of the technologies had not been utilised, for example, the S-Patch EX devices have some benefits suitable for long-term and patient-engaged test processes. Patients can conveniently wear the S-Patch EX on their bodies themselves, so it was possible to start tests easily when patients feel symptoms as in the study. Patients also can remotely test their ECG at home by directly transmitting the ECG data to medical staff without frequent hospital visits.
While this study has demonstrated feasibility for the solution in a real clinical setting and further adaptation is currently in progress, a well-designed prospective study with larger sample will be required. A non-randomised interventional study has been designed to further evaluate this virtual healthcare solution. This has the potential to make the in-home service more accessible, affordable and effective to boost patients' satisfaction with our healthcare system and reduce the burden of healthcare through efficiency gains.
Conclusion
Implementation of the S-Patch device is anticipated to result in a reduction of healthcare costs through more efficient medical monitoring/data collection via the utilisation of new technology; as well as being able to improve patient outcomes by encouraging closer patient and health professional relationships through collaborative data monitoring and open discussion approaches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by South Western Sydney Local Health District Human Ethics Research Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JC, SM, and MP developed the idea and methodology of the study, provided governance and oversight. AD, MF, SS, JR, RT, and NM helped conduct the study and recruited participants. RT and AP reviewed clinical data and escalation. JC, NM, and MF provided advice about study planning. JC and NM drafted the protocol and manuscript. All co-authors reviewed draft manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Han Q, Zheng B, Daines L, Sheikh A. Long-Term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. (2022) 11(2):269. doi: 10.3390/pathogens11020269
2. Yang T, Yan MZ, Li X, Lau EHY. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: a systematic review and meta-analysis. Infection. (2022) 50(5):1067–109. doi: 10.1007/s15010-022-01862-3
3. Brodin P, Casari G, Townsend L, O'Farrelly C, Tancevski I, Löffler-Ragg J, et al. Studying severe long COVID to understand post-infectious disorders beyond COVID-19. Nat Med. (2022) 28:879–82. doi: 10.1038/s41591-022-01766-7
4. Ziauddeen N, Gurdasani D, O'Hara ME, Hastie C, Roderick P, Yao G, et al. Characteristics and impact of long COVID: findings from an online survey. PLoS One. (2022) 17(3):e0264331. doi: 10.1371/journal.pone.0264331
5. Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. (2021) 114(9):428–42. doi: 10.1177/01410768211032850
6. Helmsdal G, Hanusson KD, Kristiansen MF, Foldbo BM, Danielsen ME, Steig BÁ, et al. Long COVID in the long run-23-month follow-up study of persistent symptoms. Open Forum Infect Dis. (2022) 9(7):ofac270. doi: 10.1093/ofid/ofac270
7. Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. (2022) 10:863–76. doi: 10.1016/S2213-2600(22)00126-6
8. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
9. Petersen MS, Kristiansen MF, Hanusson KD, Foldbo BM, Danielsen ME, Steig B Á, et al. Prevalence of long COVID in a national cohort: longitudinal measures from disease onset until 8 months’ follow-up. Int J Infect Dis. (2022) 122:437–41. doi: 10.1016/j.ijid.2022.06.031
10. Centers for Disease Control and Prevention. Treating the Most Disruptive Symptoms First and Preventing Worsening of Symptoms (2019). Available at: https://www.cdc.gov/mecfs/healthcare-providers/clinical-care-patients-mecfs/treating-most-disruptive-symptoms.htm
11. Shepherd C. Post COVID-19 fatigue, post/long covid-19 syndromes and post-covid ME/CFS (2020). Available at: https://meassociation.org.uk/wp-content/uploads/Post-Covid-Fatigue-Syndrome-and-MECFS-September-2020.pdf
12. Felix W, Wiede C, Seidl K. Vital signs and sensors for post-exertional malaise prevention. Curr Dir Biomed Eng. (2021) 7(2):371–74. doi: 10.1515/cdbme-2021-2094
13. Jackson S. Using a heart rate monitor to prevent post-exertional malaise in ME/CFS (2019). Available at: https://www.daniellewis.com.au/health-information/chronic-fatigue/using-a-heart-rate-monitor-to-prevent-post-exertional-malaise-in-me-cfs/
14. Aalam AA, Hood C, Donelan C, Rutenberg A, Kane EM, Sikka N. Remote patient monitoring for ED discharges in the COVID-19 pandemic. Emerg Med J. (2021) 38(3):229–31. doi: 10.1136/emermed-2020-210022
15. Zhang G, Vahia IV, Liu Y, Yang Y, May R, Cray HV, et al. Contactless in-home monitoring of the long-term respiratory and behavioral phenotypes in older adults with COVID-19: a case series. Front Psychiatry. (2021) 12:754169. doi: 10.3389/fpsyt.2021.754169
16. Mekhael M, Lim CH, El Hajjar AH, Noujaim C, Pottle C, Makan N, et al. Studying the effect of long COVID-19 infection on sleep quality using wearable health devices: observational study. J Med Internet Res. (2022) 24(7):e38000. doi: 10.2196/38000
17. Radin JM, Quer G, Ramos E, Baca-Motes K, Gadaleta M, Topol EJ, et al. Assessment of prolonged physiological and behavioral changes associated with COVID-19 infection. JAMA Netw Open. (2021) 4(7):e2115959. doi: 10.1001/jamanetworkopen.2021.15959
18. Natarajan A, Su HW, Heneghan C. Occurrence of relative bradycardia and relative tachycardia in individuals diagnosed with COVID-19. Front Physiol. (2022) 13:898251. doi: 10.3389/fphys.2022.898251
19. Stewart C, Ranjan Y, Conde P, Sun S, Rashid Z, Sankesara H, et al. Presentation of long COVID and associated risk factors in a mobile health study. Br Med J (2022). doi: 10.1101/2022.09.27.22280404
20. Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post-COVID syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. Br Med J. (2021) 372:n693. doi: 10.1136/bmj.n693
21. Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. Br Med J. (2021) 373:n1098. doi: 10.1136/bmj.n1098
22. Sidik SM. Heart disease after COVID: what the data say. Nature. (2022) 608(7921):26–8. doi: 10.1038/d41586-022-02074-3
23. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. (2022) 28(3):583–90. doi: 10.1038/s41591-022-01689-3
24. Wang W, Wang CY, Wang SI, Wei JC. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. (2022) 53:101619. doi: 10.1016/j.eclinm.2022.101619
25. Asarcikli LD, Mİ H, Osken A, Keskin K, Kolak Z, Aksu T. Heart rate variability and cardiac autonomic functions in post-COVID period. J Interv Card Electrophysiol. (2022) 63(3):715–21. doi: 10.1007/s10840-022-01138-8
26. Marques KC, Silva CC, Trindade SDS, Santos MCS, Rocha RSB, Vasconcelos PFDC, et al. Reduction of cardiac autonomic modulation and increased sympathetic activity by heart rate variability in patients with long COVID. Front Cardiovasc Med. (2022) 9:862001. doi: 10.3389/fcvm.2022.862001
27. Soliński M, Pawlak A, Petelczyc M, Buchner T, Aftyka J, Gil R, et al. Heart rate variability comparison between young males after 4-6 weeks from the end of SARS-CoV-2 infection and controls. Sci Rep. (2022) 12(1):8832. doi: 10.1038/s41598-022-12844-8
28. Heart Foundation. Key Statistics: Coronary Heart Disease (2021). Available at: https://www.heartfoundation.org.au/bundles/for-professionals/coronary-heart-disease-key-stats
29. Cleveland Clinic. Atrial Fibrillation (2021). Available at: https://my.clevelandclinic.org/departments/heart/depts/atrial-fibrillation-center
30. Caforio A. COVID-19: Evaluation and management of cardiac disease in adults. UpToDate (2020). Available at: https://www.uptodate.com/contents/covid-19-evaluation-and-management-of-cardiac-disease-in-adults
31. Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, et al. Arrhythmias and COVID-19: a review. JACC Clin Electrophysiol. (2020) 6(9):1193–204. doi: 10.1016/j.jacep.2020.08.002
32. Ingul CB, Grimsmo J, Mecinaj A, Trebinjac D, Berger Nossen M, Andrup S, et al. Cardiac dysfunction and arrhythmias 3 months after hospitalization for COVID-19. J Am Heart Assoc. (2022) 11(3):e023473. doi: 10.1161/JAHA.121.023473
33. Chow J, Hopkins A, Dimitri H, Tie H, Williams R, Rajaratnam R, et al. An integrated care solution for the electrocardiogram monitoring. J Integr Care. (2021) 29(1):61–71. doi: 10.1108/JICA-04-2020-0018
34. McKinney W. Data structures for statistical computing in python. Proceedings of the 9th python in science conference (2010). Vol. 445: p 51–6.
35. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. Scipy 1.0: fundamental algorithms for scientific computing in python. Nat Methods. (2020) 17(3):261–72. doi: 10.1038/s41592-019-0686-2
36. Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng. (2007) 9(03):90–5. doi: 10.1109/MCSE.2007.55
37. Georghiou T, Sherlaw-Johnson C, Massou E, Morris S, Crellin NE, Herlitz L, et al. The impact of post-hospital remote monitoring of COVID-19 patients using pulse oximetry: a national observational study using hospital activity data. EClinicalMedicine. (2022) 48:101441. doi: 10.1016/j.eclinm.2022.101441
Keywords: COVID survivors, long COVID, post-COVID clinical symptom, virtual monitoring, model of care
Citation: Chow JSF, D’Souza A, Ford M, Marshall S, San Miguel S, Parameswaran A, Parsons M, Ramirez J, Teramayi R and Maurya N (2023) A descriptive study of the clinical impacts on COVID-19 survivors using telemonitoring (The TeleCOVID Study). Front. Med. Technol. 5:1126258. doi: 10.3389/fmedt.2023.1126258
Received: 17 December 2022; Accepted: 2 March 2023;
Published: 20 March 2023.
Edited by:
Jun Zhang, Griffith University, AustraliaReviewed by:
Silvio Marcello Pagliara, University of Warwick, United KingdomMaria De Fátima Paulino, University of Coimbra, Portugal
© 2023 Chow, D'Souza, Ford, Marshall, San Miguel, Parameswaran, Parsons, Ramirez, Teramayi and Maurya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josephine Sau Fan Chow am9zZXBoaW5lLmNob3dAaGVhbHRoLm5zdy5nb3YuYXU=
Specialty Section: This article was submitted to Diagnostic and Therapeutic Devices, a section of the journal Frontiers in Medical Technology
 Josephine Sau Fan Chow
Josephine Sau Fan Chow Annamarie D’Souza5
Annamarie D’Souza5 Mark Parsons
Mark Parsons Nutan Maurya
Nutan Maurya