- 1College of Pharmacy, The University of Texas at Austin, Austin, TX, United States
- 2College of Pharmacy, University of Iowa, Iowa, IA, United States
Three-dimensional (3D) printing or Additive manufacturing has paved the way for developing and manufacturing pharmaceuticals in a personalized manner for patients with high volume and rare diseases. The traditional pharmaceutical manufacturing process involves the utilization of various excipients to facilitate the stages of blending, mixing, pressing, releasing, and packaging. In some cases, these excipients cause serious side effects to the patients. The 3D printing of pharmaceutical manufacturing avoids the need for excessive excipients. The two major components of a 3D printed tablet or dosage form are polymer matrix and drug component alone. Hence the usage of the 3D printed dosage forms for disease treatment will avoid unwanted side effects and provide higher therapeutic efficacy. With respect to the benefits of the 3D printed pharmaceuticals, the present review was constructed by discussing the role of 3D printing in producing formulations of various dosage forms such as fast and slow releasing, buccal delivery, and localized delivery. The dosage forms are polymeric tablets, nanoparticles, scaffolds, and films employed for treating different diseases.
Introduction
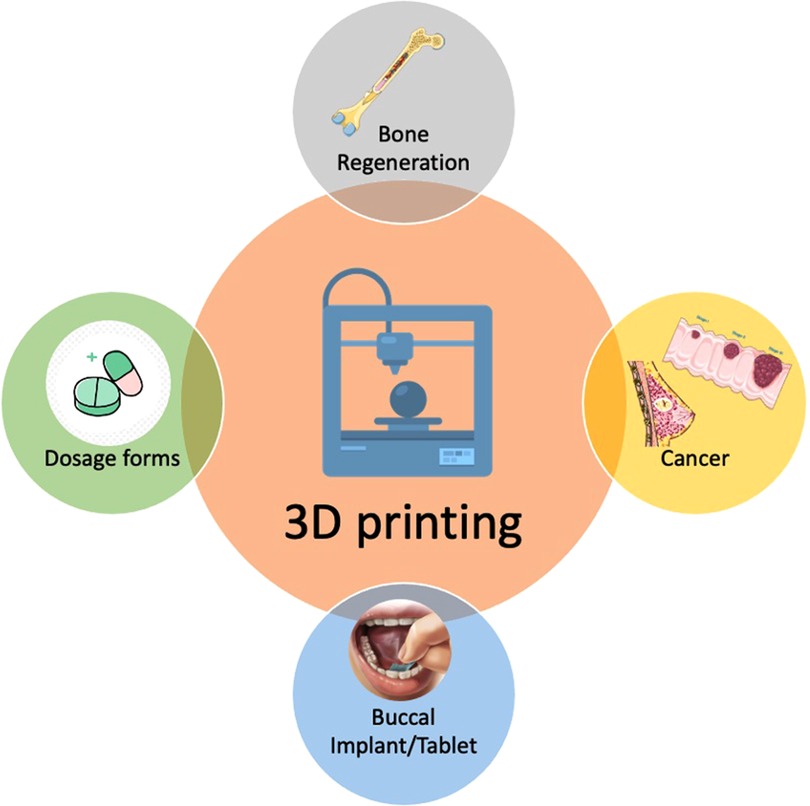
Three-dimensional (3D) printing has progressed the stagnant pharmaceutical industry to develop medical dosages beyond earth and space (1). Compared to traditional manufacturing methods, such as compacting, milling, and molding, 3D printing offers many advantages such as rapid and on-demand production, flexibility in terms of drug dosages, and complexity in terms of object geometry (2, 3). The tailoring of drug combinations and concentrations to individual requirements and the shape of any printed devices to specifically match one's anatomy (4). Researchers expressed their views regarding the stages of improvement needed for developing pharmaceuticals with 3D printing (5). The interest of healthcare professionals has increased by more than 60% for prescribing 3D printed tablets (6). In general, 3D printing has been used but not limited to bone tissue regeneration and implants for disease treatments such as cancer, oral dosage forms and buccal implants (Figure 1).
Technological advancements have changed the face of the earth with the first and second industrial revolutions. The rapid development of 3D printing and its application in product manufacturing has been considered the third industrial revolution. These advancements can ease a similar impact on the pharmaceutical industry. The pharmaceutical industry is very conserved for several decades if not centuries. The industry typically follows the established manufacturing processes to make dosage forms such as tablets, capsules, etc. With the power of novel technological advancements, the industry can revolutionize the way medicines are designed and manufactured. The three-dimensional (3D) printing technology has evolved from a do-it-yourself rudimentary stage to the highly advanced state-of-the-art developed stage. Established companies such as Stratasys, 3D systems, etc., are developing new and advanced 3D printers for manufacturing auto and aero parts. However, the adaptation of this technology in the pharma industry is still in its nascent stage. There are no major players in the pharma industry developing pharmaceuticals for everyday use. There are several startups like Teva and Aprecia pharmaceuticals that are exploring this opportunity. Aprecia Pharmaceuticals is the first company that produced and marketed the first FDA-approved 3D printed formulation called “Spritam” (Levetiracetam) for epilepsy in 2015 using their patented Zip dose technology (5).
Types of 3D printing
The technology differs based on the technique used to achieve the 3D shape. Several 3D printing methods are being explored by many researchers around the world. Fused Deposition Modeling, Extrusion based printing, Selective Laser ablation, Stereolithography, Binder jet printing, and powder-bed method are the widely used 3D printing methods across different disciplines. Each technique has its unique advantages owing to the kind of material and the field of application.
Fused Deposition Modeling (FDM) is an extremely popular 3D printing technique that is widely employed for manufacturing a wide variety of products. The adaptation of this technique in tablet manufacturing has opened new venues for the design of unique formulations (7). The hot-melt extrusion coupled dual nozzle printing of an FDM process delivered bilayer tablets for independent drug release profiles (8). Fixed-dose combinations are the new kind of formulations that achieve optimal goals for the treatment of disease with minimal side effects resulting in improved patient compliance (9). Chewable theophylline tablets for veterinary purposes were evaluated to meet an unmet need for precise off-label use of non-human products (10). Drug-loaded 3D films were developed for understanding the patient-centric effect of the drug release phenomenon (11). Recently, mathematical adaptations were employed to develop drug-loaded formulations to facilitate an optimized polymer and drug wt. percentages for a tailored drug release (12). Antibiotics are key for treating bacterial infections. However, the high dosage of antibiotics supplies bacterial resistance. To avoid that, consistent release of antibiotics avoids drug resistance. The 3D printed thermoplastic gentamycin-released PCL formulations avoided the growth of S. aureus in mice (13). The Key advantage is that the formulation development is faster, and the printing technology is well established giving it an easy fabrication process facilitating rapid production and scale up production lines. The major disadvantage of FDM is the high processing temperature not suitable for certain temperature sensitive drugs and biologics. Compatibility and stability are two important aspects of tablet evaluation for safety and efficacy (14). Semisolid extrusion is a 3D printing method carried out at room temperature that produces objects or devices out of gels or pastes. The re-dispersible formulations contain drug-loaded polymeric nano capsules inside a hydrogel for drug delivery applications (15). Effervescent tablets are another kind of medication that delivers the drug. The major advantage is that the formulation typically includes drug, acid, and alkali pastes which are printed in a localized manner to prevent any premature effervescence (16). The adaptability of the technology is still nascent in the pharmaceutical industry but highly popular with bioprinting for tissue regeneration applications. Selective laser sintering is another popular 3D printing technique used to prepare formulations and personalized implants. High volume of printing tablets can be carried out using this technique without the influence of personnel errors. The laser beam sinters a layer of the powder formulation which coalesce the particles together. The material with absorption of laser is key for improved resolution and tailoring drug release. This technique is being widely explored to print oral formulations (17–19). Blending of different drugs is very difficult with SLS. Binder jet printing is one of the earliest techniques that is used for automotive industry by has limited applications for drug delivery applications. Lu et al. has published a modular approach for releasing multiple drugs of anti-viral nature at a time. This work is very interesting and provided new ways of employing binder jet technology for pharmaceutical development (20). The binder jet printing is similar to selective laser sintering, but a high-resolution print head will deposit binder solution on to the powder bed resulting in a hardened 3D structure. However, this technology was adapted by Aprecia Pharmaceuticals and developed a revolutionary Zipdose technology and eventually succeed in launching Spritam (Levetiracetam) an Food and Drug Administration approved drug first time in the world. Stereolithography (SLA) has recently been used to fabricate tablets. SLA is also known as vat photopolymerization where the resin is hardened by the exposure to ultraviolet light resulting in a hardened structure. The resins used in this technique are highly toxic. The adaptation of existing resins for biomedical and pharmaceutical applications is a major limitation of this technique. However, recent reports showed the usage of biocompatible polytheyleneglycol diacrylate or other acrylates for tablet fabrication. The technology has a limitation of using only few materials that are biocompatible and UV curable. Development of new materials in future can accelerate the adaptation of this technology. Similarly, Immediate release tablets were fabricated using this technique to release Zolpidem tartrate (21). The same technique was also used to develop medical devices that have photothermal and shape memory functions (22).
The workflow of three-dimensional printing is the deposition, polymerization, or binding of the material of choice in a layer-by-layer fashion resulting in a final three-dimensional structure. The general process is identified as ‘3 Ds of 3D printing. These are (i) Design, (ii) Develop, and (iii) Dispense. Any 3D printing process start with the development of a computer-generated 3D model using software called computer-aided design (CAD). The model is a 3D projection with defined parameters based on the type of structure. These pre-defined parameters of the shape will be reflected in the final 3D printed structure. This CAD is exported as the widely popular.stl file extension. The slicing software will read this.stl file and process it into several layers and convert this information into a.gcode file. Depending on the printer type and the CAD design employed, the commands and information will be different, but all 3D printers, in general, can only read this .gcode file format. This stage is to identify the method for the development of dosage forms using the 3D printing technique, print parameters, suitable materials, and drugs to work with for a certain application. The users must ensure material compatibility, drug compatibility, and material-drug interactions based on the API properties to achieve an active, desirable dosage form. The 3D printer can then be filled with the drug-loaded feedstock. Formulations are prepared in a layer-by-layer fashion, which is then ready for “dispensing”. This method of production varies depending on the printing platform selected. This process can benefit industries and researchers to produce dosage forms for several clinical applications and a greater advancement in creating personalized medicines.
3D Printing pharmaceutics
Fast releasing tablets
The fused deposition modeling (FDM) 3D printing technique has considerable potential for patient-specific dosage forms. Design approach where the caplets with perforated channels accelerate drug release from 3D printed tablets (23). The fabrication of ready-to-use immediate-release tablets via 3D printing provides a powerful tool for on-demand individualization of the dosage form. Fused deposition modeling (FDM) involves passing a filament based on thermoplastic polymers through a hot nozzle, where the temperature is elevated above its glass transition temperature (Tg). The major limitation of FDM 3D printing renders it unsuitable for the production of immediate-release tablets, which count for approximately 70% of all oral dosage forms (24). Alhan's group reported the fabrication of immediate-release tablet based on positively charged methacrylic polymers (25). Same group tested the release of Theophylline and Dipyridamole using polyvinylpyrrolidone polymer delivering a 100% release in 70 min (26).
Buccal formulations
The 3D printing of oral formulations has been the most extensively investigated. Drug delivery across buccal mucosa is one of the promising alternative delivery approaches to deliver drug compounds that are degraded either due to high first-pass metabolism or due to harsh conditions in the GI tract. Considering the rich vasculature of buccal mucosa, the drug compounds can be directly delivered into the systemic circulation. In specific, buccal and sublingual mucosa of the oral cavity have been studied to be the most efficient and alternative delivery route for several drug compounds for both systemic and local delivery.
Thin-film systems constituting both mucoadhesive and or dispersible thin films are the most effective dosage forms suitable for oral mucosal administration. Currently, solvent casting technique and hot-melt extrusion are the most commonly used techniques for the preparation of oro-mucosal films or patches. However, several limitations have been identified for each of these processes including (1) long processing and drying times due to solvent evaporation for solvent casting technique. Also, solvent casting techniques are not suitable for APIs that are prone to hydrolysis (27), (2) hot-melt extrusion could improve the solubility of the API but is not suitable for thermo-labile drugs (27, 28). They also provide limited opportunities for patient-tailored dosing regimens and the incorporation of multiple functional materials targeting different drug release characteristics. Recently, 3D printing technology has been identified to be a successful approach for the design and development of various dosage forms intended for buccal administration. More specifically, 3D printing manufacturing techniques are very promising to develop personalized doses/various combinations, and films with robust mechanical and drug release characteristics (29, 30). Various 3D printing technologies have been evaluated for the manufacture of oro-mucosal films (31, 32) including fused deposition modeling (FDM) (33), inkjet printing (34), flexographic printing (35), and direct ink writing (robocasting/pressure-assisted 3D printing) (36). Below details summarize the recent studies that have evaluated various 3D printing technologies for the fabrication of buccal dosage forms.
Buccal films
Mucoadhesive buccal films are one of the attractive approaches considering their enhanced muco-retention properties along with the permeation-enhancing ability for either local or systemic delivery options. Mucoadhesive films involve the usage of various functional excipients including mucoadhesive agents, permeation enhancers, and film formers attached to different backing layers. The drug compound is incorporated in different regions of the film based on the desired drug release characteristics. Spatial distribution of these components becomes important in designing buccal thin films since the functionality of these excipients plays a critical role in defining attachment of the film based on the site of administration, the direction of drug release, and the rate of drug release, followed by absorption of drug compounds across the buccal mucosa. In recent work, the effect of FDM, 3D printing technology was used in the manufacturing of Diclofenac Sodium multi-layered mucoadhesive layers (33). Their studies used poly(vinyl alcohol) (mucoadhesive) chitosan (mucoadhesive and permeation enhancer) along with ethyl cellulose and other commonly used commercial wafers as backing layers. Their results showed uniform content across all the 6 different formulation types indicating the FDM printing process was reproducible. Based on the SEM observations, the filament diameter was adjusted in the 3D printer software, resulting in avoiding excessive molten blend and producing buccal films that are more homogenous with a smooth external surface and no internal porous structures. Tensile strength measurements showed that backing layers exhibited poor mechanical properties. However, without backing layers, the flexibility of the 3D printed films tested by folding endurance resulted in a folding endurance of at least 300 times without breakage. Other in vitro testing also showed superior film properties including, unidirectional release and similar drug release behavior up to first 15 min from formulations loaded with either ethyl cellulose or wafer-containing backing layers. Chitosan-loaded films exhibited superior mucoadhesive strength (∼52%) and higher permeation-enhancing ability (>3 fold) across the porcine buccal mucosa compared to other formulations. Overall, their studies indicated that 3D printing of mucoadhesive buccal films produced acceptable film-forming properties with sufficient mechanical properties that can withstand the force at the site of administration (33). Also, the authors concluded that 3D printing could be a versatile approach for the manufacture of multilayered buccal films. A combination of FDM and ink-jet printing was investigated as an alternative manufacturing process in order to overcome the temperature-sensitive limitations of the FDM technique. Dual drug-loaded films were fabricated by utilizing both methods. FDM was used to fabricate HPMC-based mucoadhesive films loaded with NSAID (ketoprofen) and ink jet printing was used for the deposition of Lidocaine Hcl (LIH) along with l-menthol (permeation enhancer) on the film (37). Inkjet printing generated optimal design with an 80:20 v/v sample loaded with 300 mg/mL of Lidocaine HCL. SEM images showed the presence of some crystalline matter on the 3D printed HPMC substrates and ink-jet printed films. Based on DSC studies, an endotherm at 165°C was identified, which references plain HPMC, indicating that the recrystallization could be attributed to raw HPMC material. XRD studies showed few characteristic peaks that are representative of HPMC, LiH, and menthol indicating the existence of crystalline forms of both the polymer and LiH on the ink-jet printed film systems. Even though ink deposition effected onto the mucoadhesive HPMC substrate resulted in some alterations in the overall morphology of the films, no change in drug release characteristics or drug absorption across the buccal epithelial cell layers (both porcine mucosa and TR146 cell layers) was observed. Overall, this study demonstrated proof of concept of the use of the FDM/ink-jet dual printing process for the incorporation of temperature-sensitive drug compounds in mucoadhesive films (37). Another recent study demonstrated the use of direct ink writing (DIW) technology for the fabrication of more complex and unique design features containing saquinavir (antiretroviral drug compound) with pHm (microenvironment) modifying buccal patches. This study used the integration of three inks on a sub-millimeter scale (38). Printing materials include acidic saquinavir-loaded HPMC ink and alkaline sodium-carbonate ink incorporated on a methylcellulose backing layer resulting in buccal patches with a mesh design that exhibited superior stretchable mechanical properties (38).
Oral dispensable films using 3D printing
Oral dispensable films are thin polymeric systems that are dissolved readily within the oral cavity. Oro dispersible films rapidly disintegrate in presence of saliva, and the active drug compounds released are either absorbed through the mucosal route within the oral cavity or ingested through saliva into the gastrointestinal tract. FDM was first explored to evaluate Aripriprazole-PVA-based dispersible films (39). 3D printed films were compared to the films prepared by solvent casting technique for physical and mechanical properties. DSC and XRD studies confirmed that the 3D printing process has transitioned the crystalline nature of Aripiprazole to the amorphous state resulting in an increase in dissolution rate where 3D printed films released 95% Aripiprazole within 15 min whereas, 75% of aripiprazole was released within 15 min from solvent cast films (39).
Transdermal drug delivery applications
3D printing has been identified as a successful approach for the fabrication of pharmaceutical drug product dosage forms of various geometries for transdermal delivery applications. Based on the therapeutic requirement, 3D printing was successfully used for the development of several dosage forms like microneedles, patches, and implants for both systemic and local delivery of drug compounds. In one study, Allen et al. piezoelectric inkjet printing process was used for the fabrication of vaccine-loaded dissolvable microneedles (40). They were fabricated by dispensing liquid formulation using the drop-on-drop deposition technique onto Polydimethylsiloxane (PDMS) microneedle molds. As a part of their study, it was observed that the stable precise piezoelectric dispensing process was primarily dependent on formulation attributes (viscosity, wettability) and actuation setting. Their initial design of experiments demonstrated that a 1% PVA-based formulation with a viscosity of 4–8 cP generated a more precise piezoelectric dispensing system with optimal PDMS mold wetting (contact angle <100°) and stable drop formation. After dispensing, the biological integrity of the vaccine was tested at a range of voltages (30 V, 50 V, and 80 V) and it was observed that the biological integrity of the vaccine was maintained only low voltage setting (30 V). Overall, their results demonstrated successful usage of the piezoelectric inkjet 3D printing technique for the fabrication of vaccine-loaded microneedle systems. Another study also used the inkjet printing technique to coat the 3D microneedle arrays with three anticancer components including curcumin, cisplatin, and 5-fluorouracil, for transdermal drug delivery (41). It was reported that the piezoelectric dispensing technique produced uniform and reproducible drug coating on the microneedles. In another approach, Economidou et al. prepared 3D printed microneedle arrays using stereolithography technique containing biocompatible resin for insulin delivery via transdermal application. It has been identified that a uniform printing process developed microneedles with enhanced penetration capacity with minimally applied forces (2–5 N). PK studies have also demonstrated faster insulin action resulting in adequate hypoglycemic action within 60 min (42).
3D printable implants
3D printable drug eluting implants has received considerable attention due to various printing options, biocompatible printing materials and several customized and personalized implants. 3D printed implants for several cancer categories have been evaluated, and few of them are presented in Table 1. Few Studies showed the application of 3D printed implant for cancer immunotherapy (43, 44). Mao et al., developed a 3D printed nanogel implant for the eradication of residual glioblastoma cells from the tumor cavities after the surgery. In their study, 3D printed therapeutic device was engineered that could match the post-surgery tumor cavity and release oncolytic DNA nanocomplexes to kill glioblastoma cells. In order to test this, 3D printed implant was inserted into a subcutaneous glioblastoma xenograft and identified that the release of DNA nanocomplexes resulted in a significant delay of glioblastoma cells and efficiently delays the tumor recurrence. Thus, this study provided a proof- of concept of 3D printed Implant delivery system for glioblastoma therapy (43). Culp et al. recently demonstrated the therapeutic efficacy of microsphere eluting polymeric implant for hepatocellular neoplasia (45). A standard chemotherpay treatment involve multiple drugs. Tsai et al. developed an implantable microdevice to deliver 12 chemotherapeutic drugs to patients for treating non-small cell lung carcinoma (46).
3D printing for other local delivery applications
3D printing technologies have been used for several local delivery applications. A few of them are summarized here. In one study, Goyanes et al. designed and developed a personalized nose mask that can deliver anti-acne drug (salicylic acid) by topical delivery. 3D model of a nose scan of the person was obtained from 3D scanning technology and a nose mask was fabricated using Flex EcoPLA™ (FPLA) and polycaprolactone (PCL) polymer material incorporated with salicylic acid by FDM hot-melt extrusion (HME) technique. The in vitro diffusion test data showed that a 3D printed nose mask released <187 μg/cm2 of salicylic acid within 3 h (48). Another study showed the usage of FMD-based 3D printing techniques to develop patient-specific anti-microbial wound dressings (49). This study developed various antimicrobial wound dressings with different shapes were generated using an FDA approved polymer (polycaprolactone- PCL) filaments incorporated with various concentrations of metals (Ag (10% w/w)-PCL, Zn (10% w/w)-PCL, Zn (25% w/w)-PCL, Cu (10% w/w)-PCL and Cu (25% w/w)-PCL)). It was observed that the fabricated wound dressings showed fast drug release (up to 24 h) followed by slow release (up to 72 h). Overall, their study demonstrated that personalized customizable wound dressings with respect to shape, size, and type of antimicrobial agents can be developed using 3D printing techniques. Another study demonstrated the usage of a 3D printed biodegradable patch for local delivery of 5-fluorouracil at the tumor site (50). Polycaprolactone and poly (lactic-co-glycolic acid) were used as polymeric compounds and fabricated systems exhibited prolonged drug release characteristics for up to 4 weeks. It was also understood that the drug release properties were highly dependent on the size of the patch. Thus, the results conclude that 3D printing could be a promising approach for local delivery applications.
Future directions
Deviating conventional pharmaceutical manufacturing has opened all new possibilities for exploring the development of dosage forms in terms of design, materials, and processing. The advancements in 3D printing of pharmaceuticals are progressed towards realizing the end goal of personalized medicine with on-demand manufacturing capabilities. Aprecia Pharmaceuticals is partnering with Cycle Pharmaceuticals to print other approved, orphan drugs using this technology. The regulatory changes for 3D printing technology is being actively undergoing in Food and Drug Administration and everal guidelines were published in their website. The inception of new Good Manufacturing Practices grade 3D printers might be a key in unleashing the potential of the 3D printing technology as a mainstream tablet manufacturing in pharma industry.
Author contributions
Both authors contributed equally to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Seoane-Viaño I, Ong JJ, Basit AW, Goyanes A. To infinity and beyond: strategies for fabricating medicines in outer space. Int J Pharm X. (2022) 4:100121. doi: 10.1016/j.ijpx.2022.100121
2. Lim SH, Kathuria H, Tan JJY, Kang L. 3D printed drug delivery and testing systems - a passing fad or the future? Adv Drug Deliv Rev. (2018) 132:139–68. doi: 10.1016/j.addr.2018.05.006
3. Norman J, Madurawe RD, Moore CMV, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. (2017) 108:39–50. doi: 10.1016/j.addr.2016.03.001
4. Trenfield SJ, Awad A, Goyanes A, Gaisford S, Basit AW. 3D Printing pharmaceuticals: drug development to frontline care. Trends Pharmacol Sci. (2018) 39(5):440–51. doi: 10.1016/j.tips.2018.02.006
5. Liang K, Brambilla D, Leroux J-C. Is 3D printing of pharmaceuticals a disruptor or enabler? Adv Mater. (2019) 31(5):1805680. doi: 10.1002/adma.201805680
6. Goh O, Goh WJ, Lim SH, Hoo GS, Liew R, Ng TM. Preferences of healthcare professionals on 3D-printed tablets: a pilot study. Pharmaceutics. (2022) 14(7):1521. doi: 10.3390/pharmaceutics14071521
7. Zhang J, Thakkar R, Kulkarni VR, Zhang Y, Lu A, Maniruzzaman M. Investigation of the Fused Deposition Modeling Additive Manufacturing I: Influence of Process Temperature on the Quality and Crystallinity of the Dosage Forms. AAPS PharmSciTech. (2021) 22(8):258. doi: 10.1208/s12249-021-02094-8
8. Zhang P, Xu P, Chung S, Bandari S, Repka MA. Fabrication of bilayer tablets using hot melt extrusion-based dual-nozzle fused deposition modeling 3D printing. Int J Pharm. (2022) 624:121972. doi: 10.1016/j.ijpharm.2022.121972
9. Alzahrani A, Narala S, Adel Ali Youssef A, Nyavanandi D, Bandari S, Mandati P, et al. Fabrication of a shell-core fixed-dose combination tablet using fused deposition modeling 3D printing. Eur J Pharm Biopharm. (2022) 177:211–23. doi: 10.1016/j.ejpb.2022.07.003
10. Sjöholm E, Mathiyalagan R, Wang X, Sandler N. Compounding tailored veterinary chewable tablets close to the point-of-care by means of 3D printing. Pharmaceutics. (2022) 14(7):1339. doi: 10.3390/pharmaceutics14071339
11. Lee J-H, Park C, Song I-O, Lee B-J, Kang C-Y, Park J-B. Investigation of patient-centric 3D-printed orodispersible films containing amorphous aripiprazole. Pharm Basel Switz. (2022) 15(7):895. doi: 10.3390/ph15070895
12. Barberis ME, Palma SD, Gonzo EE, Bermúdez JM, Lorier M, Ibarra M, et al. Mathematical and pharmacokinetic approaches for the design of new 3D printing inks using ricobendazole. Pharm Res. (2022) 39:2277–90. doi: 10.1007/s11095-022-03320-z
13. Guarch-Pérez C, Shaqour B, Riool M, Verleije B, Beyers K, Vervaet C, et al. 3D-Printed Gentamicin-Releasing poly-ε-caprolactone composite prevents fracture-related Staphylococcus Aureus infection in mice. Pharmaceutics. (2022) 14(7):1363. doi: 10.3390/pharmaceutics14071363
14. Silva IA, Lima AL, Gratieri T, Gelfuso GM, Sa-Barreto LL, Cunha-Filho M. Compatibility and stability studies involving polymers used in fused deposition modeling 3D printing of medicines. J Pharm Anal. (2022) 12(3):424–35. doi: 10.1016/j.jpha.2021.09.010
15. de Oliveira TV, de Oliveira RS, Dos Santos J, Funk NL, Petzhold CL, Beck RCR. Redispersible 3D printed nanomedicines: an original application of the semisolid extrusion technique. Int J Pharm. (2022) 624:122029. doi: 10.1016/j.ijpharm.2022.122029
16. Dong X, Zhang W, Wang X, Liu S, Liang J, Liufu C, et al. A novel preparation method for effervescent tablets of Xianganfang containing fresh juice using a semi-solid extrusion 3D printer with three cartridge holders. AAPS PharmSciTech. (2022) 23(6):193. doi: 10.1208/s12249-022-02336-3
17. Oral preparations with tunable dissolution behavior based on selective laser sintering technique - ScienceDirect. Available at: https://www.sciencedirect.com/science/article/pii/S0378517320311121 (Accessed 2022-09-07).
18. Thakkar R, Jara MO, Swinnea S, Pillai AR, Maniruzzaman M. Impact of laser speed and drug particle size on selective laser sintering 3d printing of amorphous solid dispersions. Pharmaceutics. (2021) 13(8):1149. doi: 10.3390/pharmaceutics13081149
19. Thakkar R, Davis Jr, DA, Williams III, RO. Maniruzzaman, M. Selective laser sintering of a photosensitive drug: impact of processing and formulation parameters on degradation, solid state, and quality of 3D-printed dosage forms. Mol Pharm. (2021) 18(10):3894–908. doi: 10.1021/acs.molpharmaceut.1c00557
20. Lu A, Zhang J, Jiang J, Zhang Y, Giri BR, Kulkarni VR, et al. Novel 3D printed modular tablets containing multiple anti-viral drugs: a case of high precision drop-on-demand drug deposition. Pharm Res. (2022) 39(11):2905–18. doi: 10.1007/s11095-022-03378-9
21. Adamov I, Stanojević G, Medarević D, Ivković B, Kočović D, Mirković D, et al. Formulation and characterization of immediate-release oral dosage forms with zolpidem tartrate fabricated by digital light processing (DLP) 3D printing technique. Int J Pharm. (2022) 624:122046. doi: 10.1016/j.ijpharm.2022.122046
22. Paunović N, Marbach J, Bao Y, Berger V, Klein K, Schleich S, et al. Digital light 3D printed bioresorbable and NIR-responsive devices with photothermal and shape-memory functions. Adv Sci. (2022) 9(27):2200907. doi: 10.1002/advs.202200907
23. Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Controlled Release. (2018) 269:355–63. doi: 10.1016/j.jconrel.2017.11.022
24. Oral Drug Delivery Market Report. Contract Pharma. Available at: https://www.contractpharma.com/issues/2012-06/view_features/oral-drug-delivery-market-report/ (Accessed 2022-09-07).
25. Pietrzak K, Isreb A, Alhnan MA. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur J Pharm Biopharm. (2015) 96:380–7. doi: 10.1016/j.ejpb.2015.07.027
26. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets | SpringerLink. Available at: https://link.springer.com/article/10.1007/s11095-016-1995-0 (Accessed 2022-09-07).
27. Uddin MJ, Scoutaris N, Economidou SN, Giraud C, Chowdhry BZ, Donnelly RF, et al. 3D Printed microneedles for anticancer therapy of skin tumours. Mater Sci Eng C. (2020) 107:110248. doi: 10.1016/j.msec.2019.110248
28. Zhang J, Zhao S, Zhu M, Zhu Y, Zhang Y, Liu Z, et al. 3D-printed Magnetic Fe 3 O 4 /MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermi . J Mater Chem B. (2014) 2:7583–95. doi: 10.1039/C4TB01063A.
29. Ma H, Luo J, Sun Z, Xia L, Shi M, Liu M, et al. 3D Printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials. (2016) 111:138–48. doi: 10.1016/j.biomaterials.2016.10.005
30. Magnetically-actuated drug delivery device (MADDD) for minimally invasive treatment of prostate cancer: An in vivo animal pilot study - Struss - 2017 - The Prostate - Wiley Online Library. Available at: https://onlinelibrary.wiley.com/doi/full/10.1002/pros.23395 (Accessed 2022-09-07).
31. Wang Y, Sun L, Mei Z, Zhang F, He M, Fletcher C, et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater Des. (2020) 186:108336. doi: 10.1016/j.matdes.2019.108336
32. E-Jet 3D-Printed Scaffolds as Sustained Multi-Drug Delivery Vehicles in Breast Cancer Therapy | SpringerLink. Available at: https://link.springer.com/article/10.1007/s11095-019-2687-3 (Accessed 2022-09-07).
33. Cho H, Jammalamadaka U, Tappa K, Egbulefu C, Prior J, Tang R, et al. 3D Printing of poloxamer 407 nanogel discs and their applications in adjuvant ovarian cancer therapy. Mol Pharm. (2019) 16(2):552–60. doi: 10.1021/acs.molpharmaceut.8b00836
34. Montenegro-Nicolini M, Morales JO. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech. (2017) 18(1):3–14. doi: 10.1208/s12249-016-0525-z
35. Morales JO, McConville JT. Manufacture and characterization of mucoadhesive buccal films. Eur J Pharm Biopharm. (2011) 77(2):187–99. doi: 10.1016/j.ejpb.2010.11.023
36. Repka MA, Gutta K, Prodduturi S, Munjal M, Stodghill SP. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur J Pharm Biopharm. (2005) 59(1):189–96. doi: 10.1016/j.ejpb.2004.06.008
37. Alomari M, Mohamed FH, Basit AW, Gaisford S. Personalised dosing: printing a dose of one's own medicine. Int J Pharm. (2015) 494(2):568–77. doi: 10.1016/j.ijpharm.2014.12.006
38. Seoane-Viaño I, Trenfield SJ, Basit AW, Goyanes A. Translating 3D printed pharmaceuticals: from hype to real-world clinical applications. Adv Drug Deliv Rev. (2021) 174:553–75. doi: 10.1016/j.addr.2021.05.003
39. Krampe R, Visser JC, Frijlink HW, Breitkreutz J, Woerdenbag HJ, Preis M. Oromucosal film preparations: points to consider for patient centricity and manufacturing processes. Expert Opin Drug Deliv. (2016) 13(4):493–506. doi: 10.1517/17425247.2016.1118048
40. Preis M, Woertz C, Kleinebudde P, Breitkreutz J. Oromucosal film preparations: classification and characterization methods. Expert Opin Drug Deliv. (2013) 10(9):1303–17. doi: 10.1517/17425247.2013.804058
41. Eleftheriadis GK, Ritzoulis C, Bouropoulos N, Tzetzis D, Andreadis DA, Boetker J, et al. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: in vitro and ex vivo evaluation. Eur J Pharm Biopharm. (2019) 144:180–92. doi: 10.1016/j.ejpb.2019.09.018
42. Economidou SN, Pere CPP, Reid A, Uddin MJ, Windmill JFC, Lamprou DA, et al. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater Sci Eng C. (2019) 102:743–55. doi: 10.1016/j.msec.2019.04.063
43. Yang Y, Du T, Zhang J, Kang T, Luo L, Tao J, et al. A 3D-engineered conformal implant releases DNA nanocomplexs for eradicating the postsurgery residual glioblastoma. Adv Sci. (2017) 4(8):1600491. doi: 10.1002/advs.201600491
44. Goldberg MS. Immunoengineering: how nanotechnology can enhance cancer immunotherapy. Cell. (2015) 161(2):201–4. doi: 10.1016/j.cell.2015.03.037
45. Culp WTN, Johnson EG, Giuffrida MA, Rebhun RB, Cawthra JK, Schwanz HA, et al. Evaluation of the use of a novel bioabsorbable polymer drug-eluting microsphere for transarterial embolization of hepatocellular neoplasia in dogs. PloS One. (2022) 17(8):e0269941. doi: 10.1371/journal.pone.0269941
46. Tsai LL, Phillips WW, Hung YP, Dominas C, Deans K, Ahn S, et al. First-in-Human intrathoracic implantation of multidrug-eluting microdevices for in situ chemotherapeutic sensitivity testing as proof of concept in non-small cell lung cancer. Ann Surg. (2022). doi: 10.1097/SLA.0000000000005385
47. Lu Y, Mantha SN, Crowder DC, Chinchilla S, Shah KN, Yun YH, et al. Microstereolithography and characterization of poly(propylene fumarate)-based drug-loaded microneedle arrays. Biofabrication. (2015) 7(4):045001. doi: 10.1088/1758-5090/7/4/045001
48. Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. (2016) 234:41–8. doi: 10.1016/j.jconrel.2016.05.034
49. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings - ScienceDirect. Available at: https://www.sciencedirect.com/science/article/pii/S0378517317303873 (Accessed 2022-09-07).
Keywords: 3D printing (3DP), cancer, implants — artificial, pharmaceuical, buccal activity
Citation: Chakka LRJ and Chede S (2023) 3D printing of pharmaceuticals for disease treatment. Front. Med. Technol. 4:1040052. doi: 10.3389/fmedt.2022.1040052
Received: 8 September 2022; Accepted: 22 November 2022;
Published: 10 January 2023.
Edited by:
Christian Celia, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Nikolaos Bouropoulos, University of Patras, Greece© 2023 Chakka and Chede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: L. R. Jaidev Chakka amNAYXVzdGluLnV0ZXhhcy5lZHU=
Specialty Section: This article was submitted to Nano-Based Drug Delivery, a section of the journal Frontiers in Medical Technology
 L. R. Jaidev Chakka
L. R. Jaidev Chakka Shanthi Chede2
Shanthi Chede2