- 1Department of Epidemiology and Medical Informatics, Graduate School of Public Health, Korea University, Seoul, South Korea
- 2Graduate School of Interdisciplinary Management, Ulsan National Institute of Science and Technology (UNIST), Ulsan, South Korea
- 3Department of Clinical Pharmacy, Graduate School, Cha University, Seoul, South Korea
Background: Bibliometric analyses are used to provide information on trends within a specific research field, along with indicators of the impact of a publication. With such an analysis, we map the scientific landscape of chimeric antigen receptor T-cell (CAR-T) research to see the emerging topics and infer directions the field might take.
Methods: We extracted the 100 most-cited articles, published all periods (from 2008 to 2019) by the Web of Science Core Collection. Using their bibliographic details, including year of publication, country of author, research organization, author information, and keywords, we graph the networks created between the articles.
Results: Of the 100 papers identified, the majority (93%) were written in the USA. Notable was that 34 papers were published from the University of Pennsylvania. Regarding authors, Carl H. June participated in 29 researches, followed by Bruce L. Levine who participated in 12. As for journals, Blood (n = 19) published the most papers, followed by Science Translational Medicine (n = 9) and Cancer Research (n = 9). Lastly, the most frequently used keywords were “adoptive immunotherapy” (n = 47), “lymphocytes” (n = 27), and “antitumor activity” (n = 22).
Conclusion: By evaluating the top 100 most-cited papers in the CAR-T field, this study provides insight into the direction of the scientific growth and its trends, as well as information on the field's network structure.
Introduction
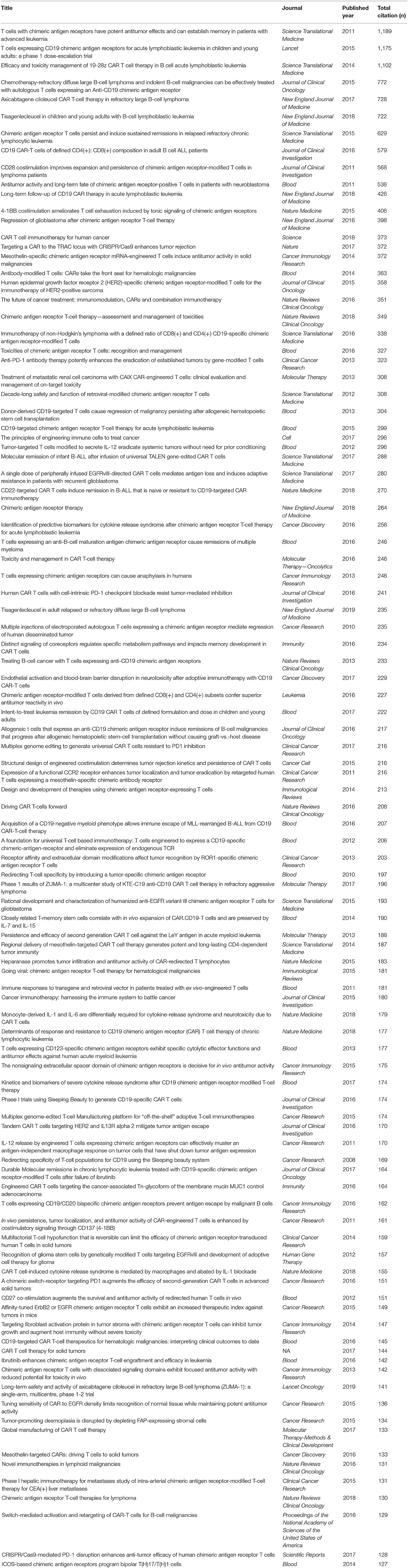
Since chimeric antigen receptors (CARs) were genetically engineered to express on T-cells three decades ago (1), they have become one of the most promising targeted immunotherapy research interests. These CAR T-cells (CAR-Ts) are modified to express cancer antigen-recognizing CARs and to stimulate the immune system (2–4). To produce CAR-Ts, leukapheresis is performed in the patient's blood: first, the T-cell is extracted (selection and activation phase) with the virus vector (retrovirus/lentivirus vector), and then the host T-cell is injected with the unique cancer-specific CAR DNA (CAR transduction) for cell proliferation (expansion). It is critical that these methods, such as the handling of the virus and the quality of the CAR-T, are properly verified as these manufactured CAR-Ts are reinfused into the patient, and any errors in selection may compromise their health. The success of these early researches and trials sets the basis for a larger clinical trial in a CD19-targeted CAR-T therapy called tisagenlecleucel (Kymriah™) for children and adolescents with acute lymphoblastic leukemia. Based on the clinical trial results, the Food and Drug Administration (FDA) approved tisagenlecleucel as the first treatment in August 2017. Currently, only two multinational pharmaceutical companies (Novartis and Gilead) have FDA approval for the treatment of some hematologic disorder (Table 1).
Table 1. Status of chimeric antigen receptor T-cell (CAR-T) approved by the Food and Drug Administration (FDA) or in Phase III clinical trials (as of July 1, 2019).
The CAR-T studies registered at ClinicalTrials.gov as ongoing (i.e., not yet recruiting, recruiting, enrolling by invitation, and active but not recruiting) number over 300. Among them are studies that look into the development of new combination therapeutic options for malignant blood cancer, but also solid tumors, and other immunotherapies (5–7). In many therapeutic areas, immunotherapy using CD19-targeted CAR-T therapy is being introduced as a new and promising treatment for systemic lupus erythematosus (SLE), regulatory rheumatoid factor (regRF) bring into being lymphocytes also use therapeutic targets in rheumatoid arthritis (8–10).
Not only has the number of CAR-T experiments been growing, but over 3,000 articles regarding CAR-Ts have been published according to Clarivate Analytics' Web of Science Core Collection (WoSCC; www.webofknowledge.com). The WoSCC online database provides systematic literature information, including data under the scope of the Science Citation Index (SCI) and the Science Citation Index Expanded (SCIE), Social Science Citation Index (SSCI), Arts and Humanity Citation Index (A&HCI), and the Emerging Sources Citation Index (ESCI) (11).
Typically, citations express an author's consent to a study's presented insights, findings, and interpretations presented. Thus, citation analysis, as a quantitative bibliometric method, can be used to provide information on a study's trends, as well as an objective index of the scientific effect of publications through their citation frequency within a specific field (9–11). Consequently, the most-cited articles are analyzed through their bibliometrics to understand the direction of scientific growth and flow of the study area (12–14).
In this study, a bibliometric network analysis will be conducted on the 100 most-cited publications in the CAR-T area. Using their bibliographic information (i.e., year of publication, country, funding, institution, author information, and keywords), we can provide insights on the target cells and genes typically studied in the field, indications for major studies, and any hot topics regarding CAR-Ts after analyzing the simultaneous exposure and frequency of the core keywords. Also, the information yielded may allow us to determine potential new study fields, the opportunities available to researchers, and leading funding organizations.
Methods
Using “chimeric antigen receptor T cell,” “CAR-T,” or “CAR T” as keywords, we found a total of 3,871 articles published from January 1, 2008, to December 31, 2019. All of them were located using the WoSCC as of May 8, 2020. Based on the target period, the top 100 cited articles were reviewed, and there were excellent techniques and quantitative growth such as immuno-oncology and gene therapy area. The most highly cited papers are listed in Table 2.
All publication information (i.e., journal name, country, enhanced-organization information, author, title, publisher, keyword, PubMed ID and citation frequency, and references) was downloaded as Bib-Text files and then converted into the XML format. The network graph was constructed using VOSviewer 1.6.13, a software tool for visualizing and exploring network data. The VOSviewer manual by Van Eck and Waltman states that VOSviewer is a program for building and visualizing networks of scientific publications, journals, researchers, research institutions, countries, keywords, or terms. It can be used to analyze bibliographic and other types of networks (15). In this study, we used the function of the VOSviewer 1.6.13 version to design the network graph. For clearer network visualization, keywords can be marked more than five times and author networks more than two times. For brevity of presentation, only information on the top 10 is included in the tables. This noninterference study conducted a bibliometric analysis not involving any human subjects; thus, no approval was required from any institutional review board or ethics committee.
Result
The publication rate and the citation frequency of the 100 most-cited publications are shown in Figure 1.
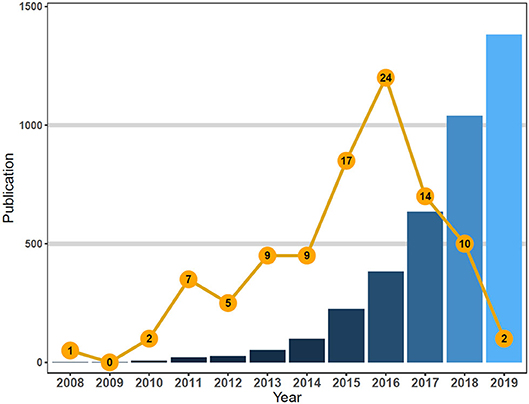
Figure 1. Total chimeric antigen receptor T-cell (CAR-T)-related publications vs. top-cited publications from 2008 to 2019.
The total citation frequencies of the articles ranged from 1,189 to 127 (μ = 277; Md = 213). The top 30% (>34 rank) were referenced an average of 468.3 times. Of the 100 articles published across the decade, 58% were published in 2015 (n = 17), 2016 (n = 24), and 2017 (n = 14), with the most referenced publications found in 2016 (Figure 1).
We observed that majority of the publications were original articles (80%), while the remaining were reviews (20%); all publications were released in English across 28 different journals. The top 100 most-cited articles were published by co-authors from 19 countries—most of who were from the USA (n = 93), Germany (n = 11), Italy (n = 6), the UK (n = 5), and Canada (n = 5) (Table 3).
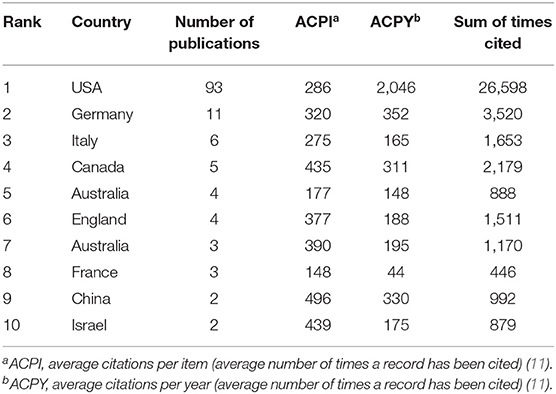
Table 3. Status of countries/funding organizations that top 100 cited publications related to chimeric antigen receptor T-cell (CAR-T).
The results are able to note that the USA and Germany showed the most pronounced activity (Figure 2). The thickness of the link is expressed according to the number of connected nodes.
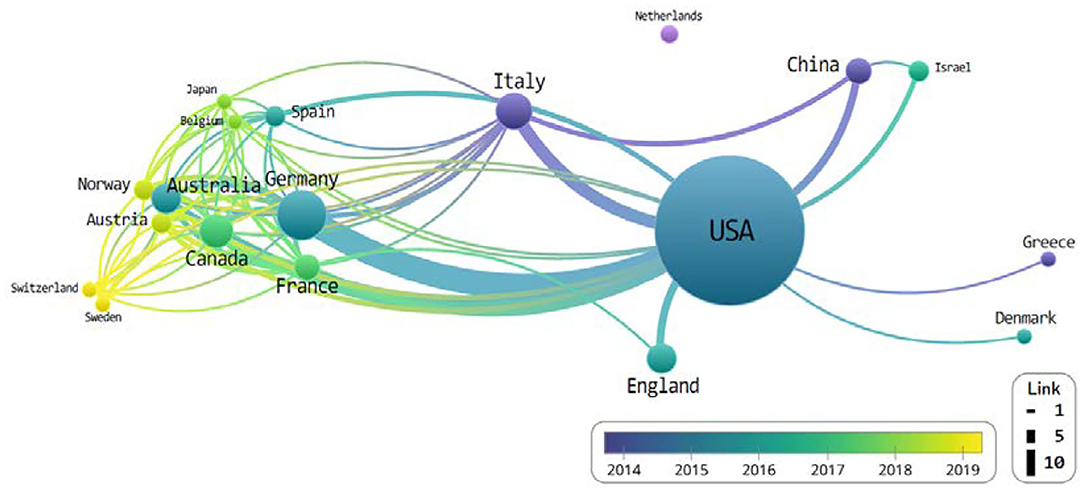
Figure 2. The network of countries that co-published related to chimeric antigen receptor T-cell (CAR-T) from 2018 to June 2019.
Hartmann et al. (16) corroborates this lead the USA possesses as he cites their overwhelming percentage of next-generation development and research in the rapidly developing field of CAR-T, similar to their development of new technologies and cost-intensive immunotherapies.
The articles were published across 28 journals, focusing on subjects such as blood cancer, chemotherapy research, and immune-chemotherapies; all of them were located in the USA (Table 3). Notably, it was the journals Blood (n = 19), Cancer Research (n = 9), and Science Translational Medicine (n = 9) that accounted for 37% of the publications, 14 of which had only published one paper (Table 4).
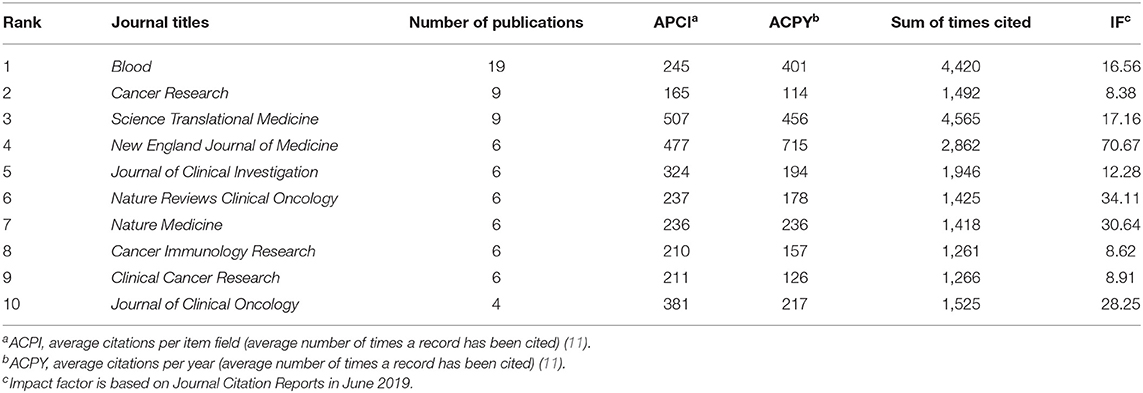
Table 4. Status of journal titles that top 100 cited publications related to chimeric antigen receptor T-cell (CAR-T).
The authorial organizations and affiliations of the top 10 are summarized in Table 5. We found that the most-cited publications were authored in the University of Pennsylvania (n = 34), followed by the University of Texas (n = 15). However, the difference in citation frequency between the two was more than double. These are then proceeded by the Memorial Sloan Kettering Cancer Center (n = 15), University of Washington (n = 12), and the Fred Hutchinson Cancer Center (n = 11; Table 5). The University of Pennsylvania is the top-cited publication institution, along with the most-cited author (C. H. June; n = 29). Subsequently, authors B. L. Levine (University of Pennsylvania) and S. R. Riddell (Fred Hutchinson Cancer Research Center) published 12 articles each.
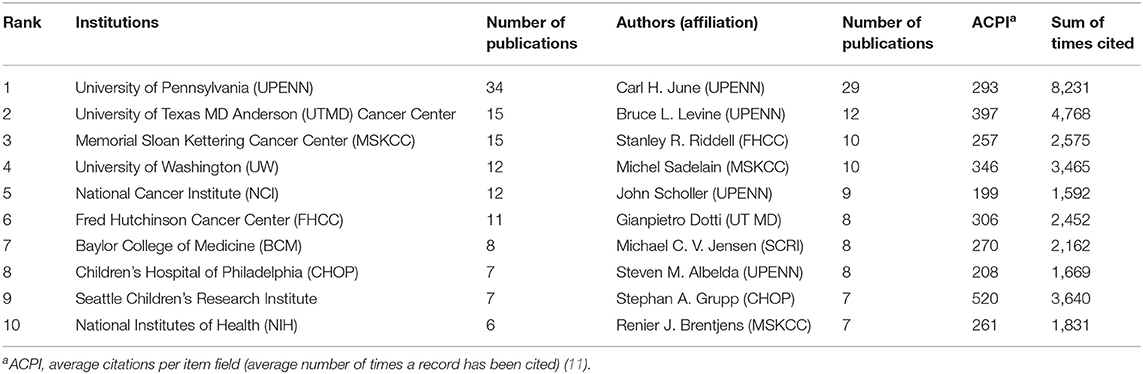
Table 5. Status of institutions/authors (affiliations) organizations that top 100 cited publications related to chimeric antigen receptor T-cell (CAR-T) from 2009 to 2019.
All of the organizations and authors of the top 10 most-cited publications were all from the USA. Figure 3 then illustrates the overall institution network graph. The network is centered around the University of Pennsylvania, Memorial Sloan Kettering Cancer Center, and University of Texas. Novartis is located close to the University of Pennsylvania and Kite Pharma to the University of Texas. On the other hand, the Fred Hutchinson Cancer Research Center collaborated primarily with Washington University, Juno Therapeutics, and the Seattle Children Research Institution. Looking at the co-authors. H. Lee Moffitt Cancer Center and Research Institute has published the most recent cited articles than both the University of Pennsylvania and MD Anderson. Nevertheless, no relationship is expressed in thick, or it is located as an independent organization.
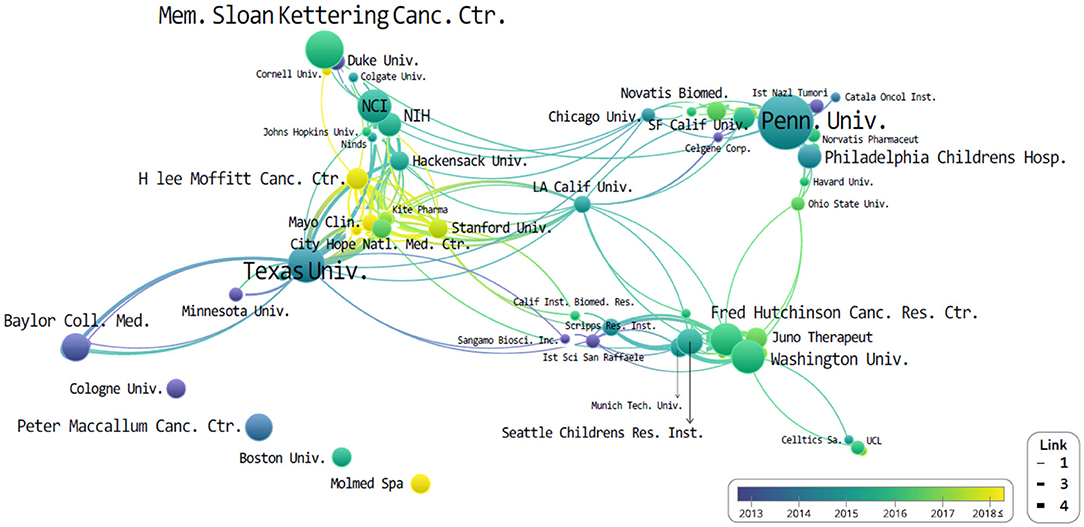
Figure 3. The network of institutions that co-published related to chimeric antigen receptor T-cell (CAR-T) from 2008 to 2019.
The University of Pennsylvania is the top-cited publication, along with the most-cited author (C. H. June; n = 29). Subsequently, B. L. Levine (University of Pennsylvania) and S. R. Riddell (Fred Hutchinson Cancer Research Center) published 12 and 11 articles each, from the top 100 most-cited papers. Also notable was that the organizations and authors of the top 10 most-cited papers were all from the USA. L. A. Johnson connected between the J. N. Kochenderfer and C. H. June groups as a bridge role. In addition, M. Sadelain, G. Dotti, and S. R. Riddell have no clear connection bridge. It seems that each study has been conducted in small groups separately.
Finally, keywords from the most-cited 100 publications were extracted using “Keywords Plus” function from WoSCC. “KeyWords Plus are words or phrases that frequently appear in the titles of an article's references, but do not appear in the title of the article itself. Based upon a special algorithm that is unique to Clarivate Analytics databases, KeyWords Plus enhances the power of cited-reference searching by searching across disciplines for all the articles that have cited references in common” (11). We analyzed natural languages such as “treatment,” “Cell,” and “CAR” through independent reviewers by classifying them into the meaningless words. The most frequently used keywords were “adoptive immunotherapy” (n = 47), “lymphocytes” (n = 27), and “antitumor activity” (n = 22; Table 6).
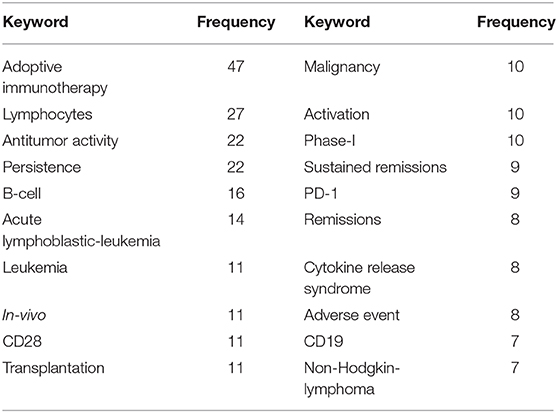
Table 6. Frequency of keyword that top 100 cited publications related to chimeric antigen receptor T-cell (CAR-T) from 2008 to 2019.
The density of the keywords is also accounted for and determined by their frequency of appearance (Figure 5). Higher-density keywords are represented in yellow, and lower densities are represented in blue; shorter distances between keyword nodes indicate frequent expression as co-occurring keywords.
Discussion
We extracted the top 100 cited articles in the CAR-T field from the WoSCC database to analyze the field's network characteristics. In this study, we tried to visually express the research trends and mainstream structure through the network mapping of the simultaneous exposure of countries, funding bodies, researchers' affiliations and organizations, and keywords. Our network analysis found the USA and Germany possessed the most nodes, followed by Italy, Canada, and China. While an overwhelming amount of studies have been conducted in the USA, there appears to be an exchange with the vast majority of other countries (Figure 2).
The study of CAR-Ts is led mainly by three institutions: first is the University of Pennsylvania, then there is University of Texas MD Anderson (UTMD) Cancer Center, and Memorial Sloan Kettering Cancer Center (MSKCC) (Figure 3). When looking at the authorial connection and citation relationships, there seems to be a network centered around the works of C. H. June and B. L. Levine, with M. Sadelain acting as a bridging node. Another cluster of nodes centers around S. R. Riddell, and one around G. Dotti, but neither has a bridging node between them (Figure 4). This may imply that each group is being led by their respective institution directing the research and that there is minimal to no research network cooperation in the researches listed in ClinicalTrials.gov (7). Finally, the most used keyword is “adoptive immunotherapy,” which lies at the center, while other keywords like “lymphocytes,” “antitumor activity,” “persistence,” and “B-cell” were connected through multi-frequency simultaneous exposures (Figure 5).
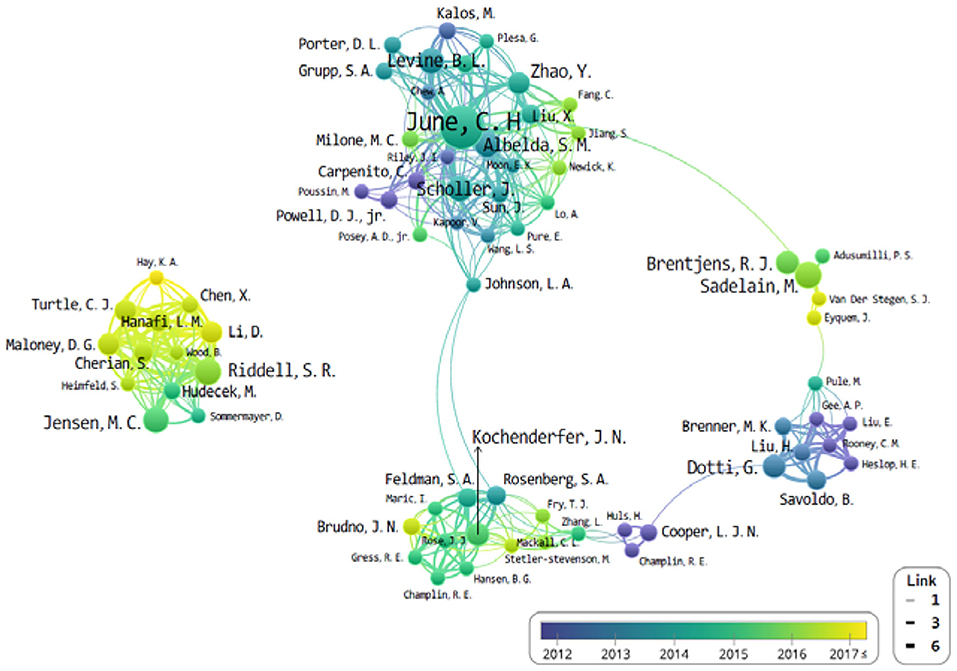
Figure 4. The network of authors that co-published related to chimeric antigen receptor T-cell (CAR-T) from 2008 to June 2019.
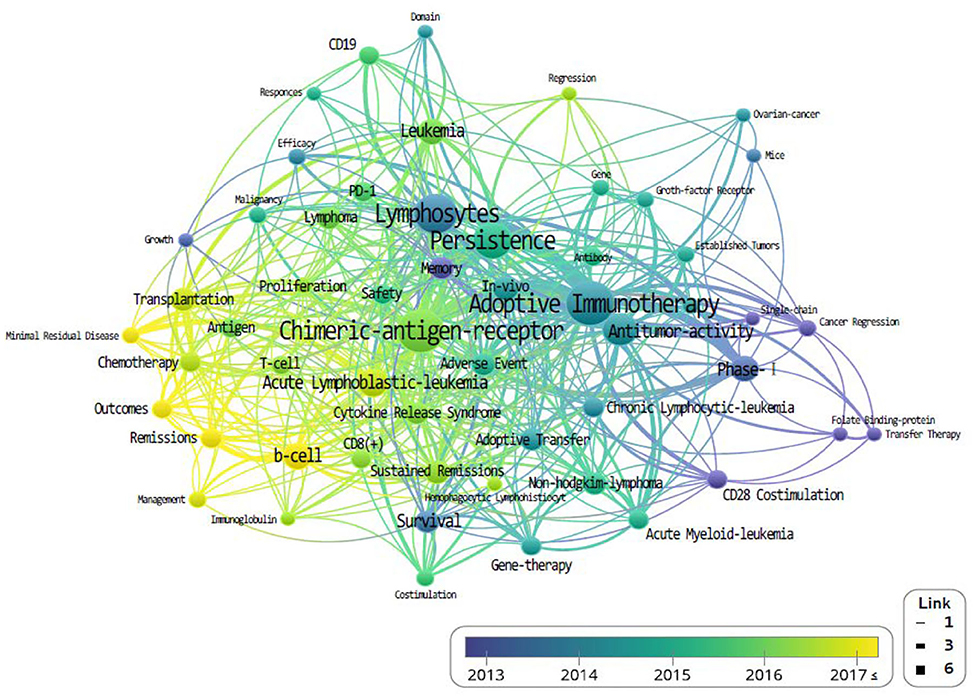
Figure 5. The co-occurrence density of keyword that co-published related to chimeric antigen receptor T-cell (CAR-T) from 1999 to June 2019.
In our determination of effect and value, we only consider the citation frequency. As such, we also only consider the number of co-authorships, regardless of an author's actual achievements and contributions. Future studies may consider the use of high quality databases that considers both qualitative and quantitative evidence for their objective analysis. Should consideration be placed on a study's phase and design, and the author's achievement and contribution for their analytical database, more robust results may be gained. Even now, there may be studies in press or in the process of completion that can contribute to the development of the field in spite of their low citation frequency.
CAR-T is a highly technological and high-cost method of cancer therapy; and the top funding bodies, journals, organizations, and authors were based or from the USA. The USA is objectively leading the development and research of the CAR-T field that is growing at an incredibly rapid rate (17–19). However, these advancements are not limited only to those dealing with CAR-T research and general cost-intensive treatments but also with overall immuno-chemotherapy research (20). In addition, CAR-T is a highly advanced next-generation treatment that has been studied in various fields such as lymphoma, solid cancer, rheumatoid arthritis, and autoimmune treatment. However, we have to consider the side effects of all treatments. Multiple cardiovascular adverse events are frequently stated after CAR-T therapy associated with mortality (21).
Conclusion
Using the 100 most-cited papers in the CAR-T field, we attempted to provide insight into the direction of the scientific growth and core study areas and trends, and opportunity to understand information on the main network structure of those studies. What we observed was that CAR-T engineering is a developing technology- and cost-intensive form of immunotherapy, with most of its studies funded and led by US-based institutions and researchers.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors were involved in the design of the study. JK was responsible for critically reviewed, approved by all authors, and supervising. SK was responsible for data curation and visualization and contributed to data interpretation and critically reviewed all manuscript terms. BS originally draft writing, critically reviewed all manuscript version, and led this project. All authors agreed upon the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. (2017) 168:724–40. doi: 10.1016/j.cell.2017.01.016
2. Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. (2016) 13:370–83. doi: 10.1038/nrclinonc.2016.36
3. Park JH, Brentjens RJ. Adoptive immunotherapy for B-cell malignancies with autologous chimeric antigen receptor modified tumor targeted T cells. Discov Med. (2010) 9:277–88.
4. National Cancer Institute. Available online at: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
5. Strohl WR, Naso M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies. (2019) 8:41. doi: 10.3390/antib8030041
6. Newick K, O'Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. (2017) 68:139–52. doi: 10.1146/annurev-med-062315-120245
7. Clinicaltrials.gov information. Available online at: https://clinicaltrials.gov/ct2/results?cond=CAR-T&Search=Apply&age_v=&gndr=&type=&rslt= (accessed July 1, 2019).
8. Stolyarova E, Beduleva L, Menshikov I, Sidorov A, Khramova T. T lymphocyte dependence of the immune response to immunosuppressive neoantigen-exposing Fc fragments of IgG. J Biol Regul Homeost Agents. (2019) 33:869–76.
9. Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. (2019) 10:128. doi: 10.3389/fimmu.2019.00128
10. McHugh, J. CAR T cells drive out B cells in SLE. Nat Rev Rheumatol. (2019) 15:249. doi: 10.1038/s41584-019-0214-x
11. WoSCC detailed information. Available online at: https://images.webofknowledge.com/images/help/WOS/hp_citation_report.html (accessed July 1, 2019).
12. Brandt JS, Downing AC, Howard DL, Kofinas JD, Chasen ST. Citation classics in obstetrics and gynecology: the 100 most frequently cited journal articles in the last 50 years. Am J Obstet Gynecol. (2010) 203:355.e1–7. doi: 10.1016/j.ajog.2010.07.025
13. Ponce FA, Lozano AM. Highly cited works in neurosurgery. Part I: the 100 top-cited papers in neurosurgical journals. J Neurosurg. (2010) 112:223–32. doi: 10.3171/2009.12.JNS091599
14. Van Noorden R, Maher B, Nuzzo R. The top 100 papers. Nature. (2014) 514:550–3. doi: 10.1038/514550a
16. Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. (2017) 9:1183–97. doi: 10.15252/emmm.201607485
17. Hoos A. Development of immuno-oncology drugs-from CTLA4 to PD1 to the next generations. Nature Rev Drug Discov. (2016) 15:235–47. doi: 10.1038/nrd.2015.35
18. Shi H, Sun M, Liu L, Wang Z. Chimeric antigen receptor for adoptive immunotherapy of cancer: latest research and future prospects. Mol Cancer. (2014) 13:219. doi: 10.1186/1476-4598-13-219
19. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. (2017) 29:84–91. doi: 10.1093/annonc/mdx755
20. Jürgens B, Clarke NS. Evolution of CAR T-cell immunotherapy in terms of patenting activity. Nat Biotechnol. (2019) 37:370–5. doi: 10.1038/s41587-019-0083-5
Keywords: chimeric antigen receptors, chimeric antigen receptor T-cell, citation classic, bibliometric, Web of Science, VOSviewer, CAR-T
Citation: Seo B, Kim S and Kim J (2020) The 100 Most Influential Studies in Chimeric Antigen Receptor T-Cell: A Bibliometric Analysis. Front. Med. Technol. 2:3. doi: 10.3389/fmedt.2020.00003
Received: 24 December 2019; Accepted: 24 July 2020;
Published: 22 September 2020.
Edited by:
Maria A. Deli, Biological Research Centre, HungaryReviewed by:
Pio Conti, University of Studies G. d'Annunzio Chieti and Pescara, ItalyAttila Gacser, University of Szeged, Hungary
Copyright © 2020 Seo, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeeyoon Kim, Y2hlemplZXlAbmF2ZXIuY29t
 Beomjun Seo
Beomjun Seo Seungwook Kim
Seungwook Kim Jeeyoon Kim
Jeeyoon Kim