
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 05 March 2025
Sec. Nephrology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1552539
Introduction: Contrast-induced acute kidney injury (CIAKI) is a common and serious complication following contrast administration in patients undergoing percutaneous coronary intervention (PCI). dapagliflozin, a sodium-glucose co-transporter 2 inhibitor (SGLT2i), has demonstrated renal protective effects in various clinical settings. However, the impact of dapagliflozin on the incidence of CIAKI in patients with type 2 diabetes mellitus (T2DM) undergoing PCI is not yet fully understood.
Objective: To evaluate the impact of dapagliflozin on CIAKI and long-term prognosis in T2DM patients undergoing PCI.
Methods: This retrospective cohort study included T2DM patients who underwent PCI at the Department of Cardiology, Tianjin University Chest Hospital, from January 2022 to June 2023. Patients were grouped based on dapagliflozin use (dapagliflozin vs. no dapagliflozin). Renal function was assessed before PCI, 48 h, and 1 week post-PCI, measuring serum creatinine, estimated glomerular filtration rate, cystatin C, and neutrophil gelatinase-associated lipocalin. All patients were followed for at least 1 year. The primary endpoint was CIAKI incidence, with secondary endpoints including renal function changes and major adverse cardiovascular events (MACE).
Results: CIAKI occurred less frequently in the dapagliflozin group compared to the control group (5.8% vs. 11.7%, χ2 = 4.494, p = 0.033). After adjusting for confounders, dapagliflozin was an independent predictor of reduced CIAKI risk (OR = 0.365, 95% CI: 0.176–0.767, p = 0.008). During a median 15-month follow-up, the dapagliflozin group had a lower incidence of MACE compared to the control group (Log-rank χ2 = 6.719, p = 0.009). Cox regression analysis showed that dapagliflozin reduced the risk of MACE (HR = 0.484, 95% CI: 0.246–0.955, p = 0.036).
Conclusion: Chronic administration of dapagliflozin can reduces the risk of CIAKI and improves long-term cardiovascular outcomes in T2DM patients undergoing PCI. These findings support its potential use as adjunctive therapy to mitigate kidney injury and improve prognosis in this high-risk population.
Contrast-induced acute kidney injury (CIAKI) is a known complication following the use of iodinated contrast agents in procedures like percutaneous coronary intervention (PCI). It is characterized by a sudden decline in renal function, typically within 48 to 72 h of contrast exposure, and is associated with increased morbidity, prolonged hospitalization, and higher mortality (1, 2). Patients with type 2 diabetes mellitus (T2DM) are particularly at risk due to factors such as pre-existing chronic kidney disease (CKD), endothelial dysfunction, and hyperglycemia, which impair renal autoregulation and exacerbate nephrotoxicity (3, 4).
To date, strategies to prevent CIAKI in patients undergoing PCI have focused on reducing contrast volume, optimizing hydration, and adjusting medications. However, no pharmacologic agents have been universally adopted, and many interventions show limited efficacy (5, 6). Recently, sodium-glucose co-transporter 2 inhibitors (SGLT2i), initially developed for managing T2DM, have gained attention for their renal protective and cardiovascular benefits (7, 8). Dapagliflozin, a widely used SGLT2i, has shown promise in slowing CKD progression, reducing heart failure risk, and improving survival in patients with diabetes and cardiovascular comorbidities (9, 10). The renoprotective effects of SGLT2i are thought to stem from mechanisms such as reduced intraglomerular pressure, inflammation, oxidative stress, and fibrosis (11, 12). These benefits, along with improved glycemic control, make SGLT2i a compelling option for patients at high risk of kidney injury.
Despite promising findings, early warnings from the U.S. Food and Drug Administration (FDA) and some studies raised concerns about an increased risk of acute kidney injury (AKI) with SGLT2i use (13, 14). These warnings have affected clinicians’ confidence in the renal safety of SGLT2i, particularly in T2DM patients undergoing contrast administration. Some experts recommend discontinuing SGLT2i before contrast exposure to reduce the risk of CIAKI. However, there is no consensus on whether SGLT2i should be stopped during the perioperative period of PCI, and the impact of dapagliflozin on CIAKI risk in T2DM patients remains unclear. This study aims to assess the effect of dapagliflozin on CIAKI incidence and its impact on the prognosis of T2DM patients undergoing PCI. The results may provide evidence to support the use of dapagliflozin in managing T2DM patients at risk for CIAKI after PCI.
This study adhered to the ethical guidelines of the Declaration of Helsinki. Informed consent was waived for enrolled participants, and patient confidentiality was strictly maintained. The study was approved by the Medical Ethics Committee of Tianjin University Chest Hospital (Approval no: 2023YS-1215-01). This retrospective cohort study included adult T2DM patients who underwent PCI at the Department of Cardiology, Tianjin University Chest Hospital, between January 2022 and June 2023. Patients were divided into two groups: those who received dapagliflozin and those who did not. Exclusion Criteria: (1) allergy to contrast agents or dapagliflozin, (2) emergency PCI, (3) cardiogenic shock with systolic blood pressure < 90 mmHg, (4) thyroid dysfunction, (5) estimated glomerular filtration rate (eGFR) < 30 mL/(min·1.73m2), (6) repeated contrast use within the past week, (7) malignant tumors, (8) previous kidney transplantation or nephrectomy, (9) incomplete follow-up. A consort flow diagram of the study is shown in Figure 1.
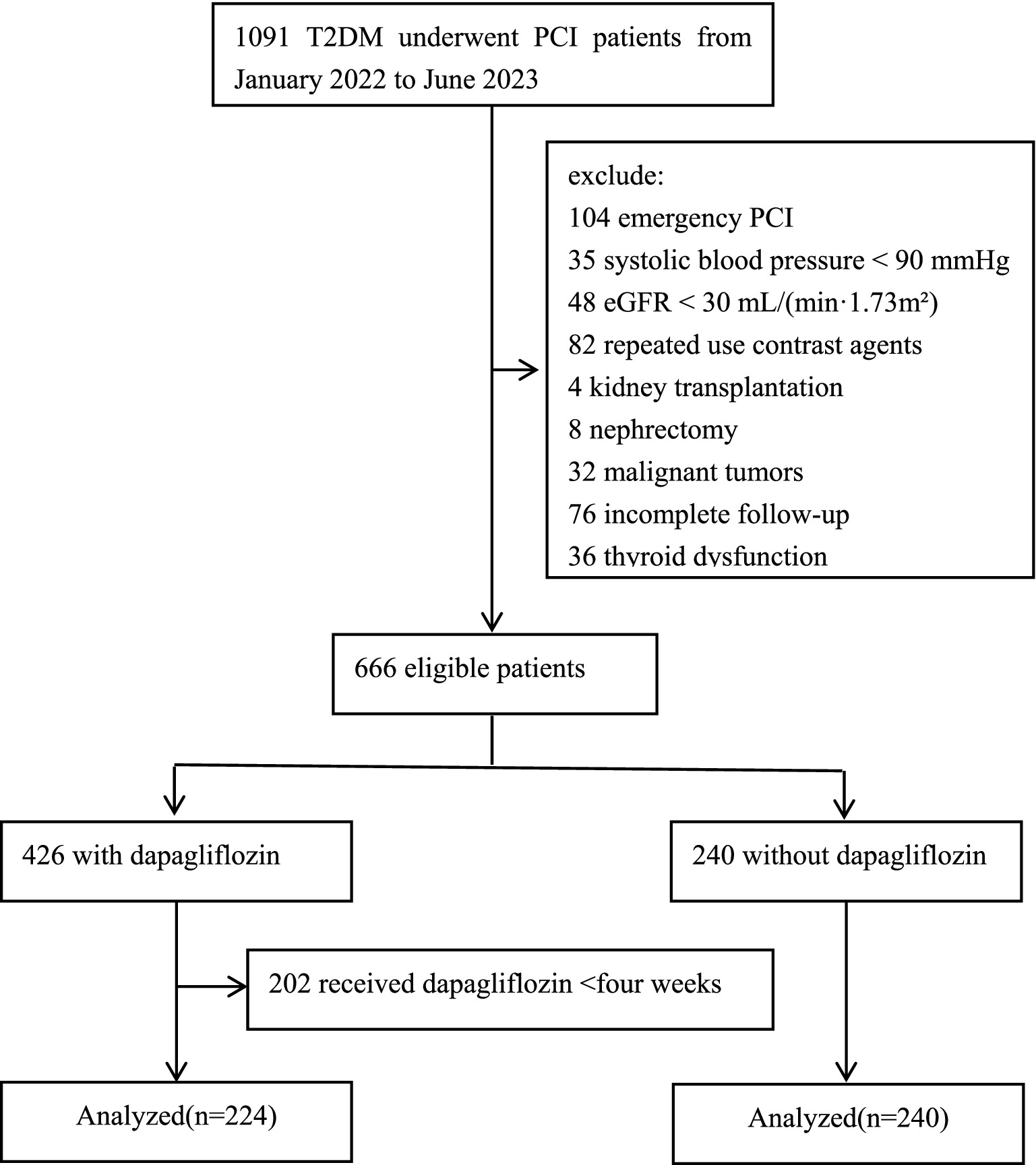
Figure 1. Consort flow diagram of the study. T2DM, type 2 diabetes mellitus; PCI, percutaneous coronary intervention; eGFR, estimated glomerular filtration rate.
To minimize the risk of CIAKI, all patients received hydration therapy with sodium chloride saline (Shanghai Baxter Medical Products Co., Ltd., Batch No.: H19993745) at a rate of 1 mL/(kg·h) for 6 ~ 12 h before and after PCI. For patients with heart failure or left ventricular ejection fraction (LVEF) < 45%, the hydration rate was reduced to 0.5 mL/(kg·h). All patients were also encouraged to drink plenty of water. Enrolled patients received either iodixanol (Yangtze River Pharmaceutical Group Co., Ltd., Batch No.: H20184002), an isotonic contrast agent, or iopamidol (Jiangsu Hengrui Medicine Co., Ltd., Batch No.: H20067896), a low-osmolar contrast agent. Patients in the dapagliflozin group received 10 mg of dapagliflozin (AstraZeneca Pharmaceuticals Co., Ltd., Batch No.: H20170119) orally once daily for at least 4 weeks prior to contrast administration and continued the same dose post-PCI.
The basic information and medical history of both patient groups were recorded, including medication history, dapagliflozin duration, type and dosage of contrast agent, and hydration volume. Renal function parameters were also assessed before PCI and at 48 h and 1 week post-treatment. These included serum creatinine (Scr), eGFR, serum cystatin C (Cys-C), and neutrophil gelatinase-associated lipocalin (NGAL).
All patients were followed for at least 1 year post-PCI, with follow-up data collected via outpatient visits, hospital records, or telephone interviews. During follow-up, major adverse cardiac events (MACE)—including cardiac death, non-fatal myocardial infarction, non-fatal stroke, unplanned revascularization, and acute heart failure—were recorded. The primary endpoint was the incidence of CIAKI, defined as a Scr increase of >26.5 μmol/L (0.3 mg/dL) or > 50% from baseline within 48 h or 1 week of contrast administration, respectively (1). Secondary endpoints included changes in renal function and the occurrence of MACE during follow-up.
The eGFR was calculated using the formula: eGFR = 186 × Scr (mg/L)−1.154 × age (years)−0.203 × (female × 0.742). T2DM was defined by the presence of diabetes symptoms (e.g., polydipsia, polyphagia, polyuria, weight loss) along with any of the following blood glucose levels: postprandial glucose ≧ 11.1 mmol/L, fasting glucose ≧ 7.0 mmol/L, or glucose ≧ 11.1 mmol/L 2 h after a glucose tolerance test. Anemia was defined as hemoglobin <120 g/L or red blood cell count <4.5 × 1012/L for adult males, and hemoglobin <110 g/L or red blood cell count <4.0 × 1012/L for adult females.
Descriptive statistics summarize baseline characteristics. Continuous variables were expressed as mean ± standard deviation (SD), two-way ANOVA was used for comparing multiple groups, and pairwise comparisons between groups were performed using the SNK-q test. Categorical variables were presented as frequencies and percentages. The differences between the dapagliflozin and control groups were compared using independent t-tests or Mann–Whitney U tests for continuous variables, and chi-square or Fisher’s exact tests for categorical variables. Multivariate logistic regression identified independent predictors of CIAKI, adjusting for potential confounders. The Log-rank test compared MACE occurrence between groups, while Cox regression assessed the effect of dapagliflozin on prognosis. Kaplan–Meier curves were plotted using GraphPad Prism 9.0. A p-value <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 22.0 (IBM Corp., Armonk, NY, United States).
A total of 464 T2DM patients undergoing PCI were enrolled, with a mean age of 64.05 ± 15.36 years (340 males and 124 females). The dapagliflozin group included 224 patients, who had an average dapagliflozin treatment duration of 10.56 ± 2.62 weeks prior to PCI. The control group consisted of 240 patients. The dapagliflozin group had a significantly lower LVEF compared to the control group, 53.22% ± 10.24% vs. 55.46% ± 11.18% (p < 0.05). No other baseline characteristics showed significant differences between the groups (p > 0.05). This indicates that the general characteristics between the two groups of patients are comparable. As shown in Table 1. All patients completed the follow-up as scheduled.
Before PCI, no significant differences were found in Scr, eGFR, Cys-C, or NGAL levels between the two groups (p > 0.05). At 48 h post-PCI, Cys-C and NGAL levels increased in both groups compared to pre-PCI values. However, the dapagliflozin group had significantly lower Cys-C and NGAL levels than the control group (p < 0.05). By 1 week post-PCI, no significant differences in Scr, eGFR, Cys-C, or NGAL were observed between the two groups (p > 0.05), as shown in Table 2.
A total of 41 cases of CIAKI occurred across both groups, resulting in an overall incidence of 8.8%. In the dapagliflozin group, 13 cases (5.8% incidence) were reported, while the control group had 28 cases (11.7% incidence). The difference in CIAKI incidence between the two groups was statistically significant (5.8% VS. 11.7%, χ2 = 4.494, p = 0.033).Logistic regression analysis identified factors influencing CIAKI, including LVEF, LVEF ≤45%, eGFR <60 mL/(min·1.73m2), contrast agent dosage, iohexol, hydration volume, age > 75 years, anemia, and dapagliflozin. Dapagliflozin was found to be an independent protective factor against CIAKI (OR = 0.365, 95% CI: 0.176–0.767, p = 0.008), as shown in Table 3.
During a median follow-up of 15 months (range: 12.0–16.8 months), MACE events were recorded in both groups. In the dapagliflozin group, there were 2 cases of recurrent acute myocardial infarction, 3 cases of unplanned revascularization, 5 cases of acute heart failure, and 1 case of stroke. In the control group, 3 cases of recurrent acute myocardial infarction, 12 unplanned revascularizations, 12 acute heart failure cases, 2 strokes, and 1 sudden death occurred. Kaplan–Meier curves showed a significant benefit for dapagliflozin-treated patients (Log-rank χ2 = 6.719, p = 0.009), as shown in Figure 2.
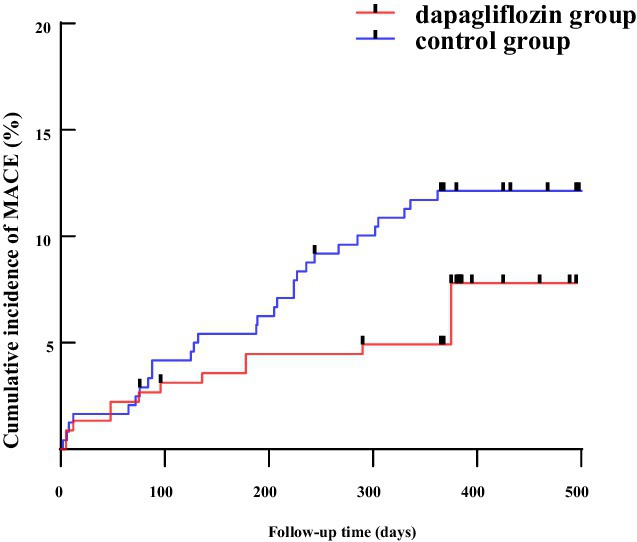
Figure 2. The Kaplan–Meier survival curve for dapagliflozin group and control group. MACE, major adverse cardiovascular events; CIAKI, contrast-induced acute kidney injury.
Among the CIAKI group, there were 2 cases of recurrent acute myocardial infarction, 1 unplanned revascularization, 3 acute heart failure cases, 1 stroke, and 1 sudden death. In the non-CIAKI group, there were 3 recurrent myocardial infarctions, 14 unplanned revascularizations, 14 acute heart failure cases, 2 strokes, and no sudden deaths. Kaplan–Meier analysis revealed that CIAKI increased the risk of MACE (Log-rank χ2 = 5.804, p = 0.016), as shown in Figure 3.
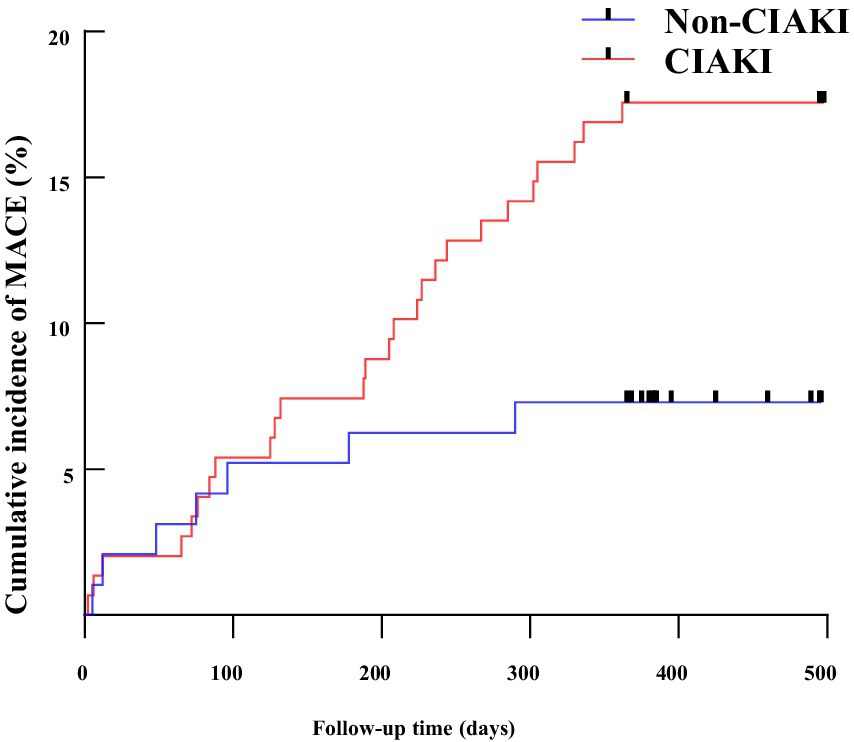
Figure 3. The Kaplan–Meier survival curve for CIAKI and Non CIAKI. MACE, major adverse cardiovascular events; CIAKI, contrast-induced acute kidney injury.
Cox regression analysis identified factors influencing MACE occurrence, with MACE as the dependent variable and age > 75, eGFR <60 mL/(min·1.73m2), LVEF ≦ 45%, anemia, AMI, CIAKI, and dapagliflozin as independent variables. The results showed that dapagliflozin improved the prognosis of T2DM patients after PCI (HR = 0.484, 95% CI: 0.246–0.955, p = 0.036), as shown in Table 4.
Our study found that dapagliflozin significantly reduced the incidence of CIAKI compared to the control group. Additionally, dapagliflozin was associated with improved long-term outcomes, showing fewer MACE events during at least 1 year of follow-up. Therefore, in T2DM patients on long-term dapagliflozin, clinicians need not worry about an increased risk of CIAKI, and discontinuing dapagliflozin before contrast administration is unnecessary.
Urine output and Scr are commonly used to diagnose acute kidney injury (AKI) (15, 16), but they have limitations. Scr is influenced by factors such as weight, muscle mass, age, diet, and gender, while urine output can be affected by fluid intake, diuretics, and cardiac function, reducing their accuracy for early CIAKI diagnosis. Scr levels typically peak 2–3 days after contrast agent administration and usually return to baseline levels within 7–10 days. In contrast, Cys-C and NGAL are expressed in large amounts and released into the blood and urine within hours after the onset of AKI, significantly increasing within a short time frame (6–12 h), and then gradually decreasing. Cys-C is a more sensitive biomarker for early renal impairment, as it is less influenced by factors like muscle mass, age, and gender (17). NGAL, a protein involved in regulating apoptosis in renal tubular cells, increases rapidly in response to AKI and can be detected in both blood and urine (18). Thus, Cys-C and NGAL are reliable early biomarkers for detecting CIAKI. In this study, there was no statistically significant difference in Scr levels between the two groups 48 h post-PCI. However, 48 h after PCI, the dapagliflozin group had significantly lower levels of Cys-C and NGAL compared to the control group. This difference in the levels of Cys-C and NGAL between the two groups at 48 h after PCI, and the 48-h elevation in scr and 1 week recovery rate were similar in both groups could be related to the sensitivity differences between Scr, Cys-C, and NGAL in the early diagnosis of AKI.
The pathogenesis of CIAKI involves complex physiological and biochemical processes (19, 20). After contrast agent administration, renal vasoconstriction reduces renal perfusion and blood flow, leading to ischemia and hypoxia in renal tissues, most pronounced in the renal medulla. This triggers oxidative stress, characterized by increased production of reactive oxygen species (ROS), which damages renal tubular cells, causing apoptosis and necrosis. Ischemia and oxidative stress also activate inflammatory responses, releasing cytokines and chemokines that further aggravate tubular injury and promote cell death. The combined effects of ischemia, oxidative stress, and inflammation contribute to CIAKI, resulting in elevated serum creatinine levels and reduced urine output post-PCI. Thus, strategies that inhibit inflammation, reduce oxidative stress, and improve renal perfusion are crucial for preventing and treating CIAKI.
In recent years, SGLT2i, particularly dapagliflozin, have emerged as a promising class of medications for managing T2DM, with notable cardiovascular and renal protective effects. Dapagliflozin works by inhibiting glucose reabsorption in the renal tubules, promoting urinary glucose excretion, and effectively controlling blood glucose levels in T2DM patients (21, 22). Beyond glucose control, dapagliflozin improves cardiac energy metabolism, reduces myocardial fibrosis, and mitigates oxidative stress and inflammation. It also lowers blood pressure, reduces body weight, increases urinary sodium excretion, and decreases kidney hyperfiltration (23). Additionally, dapagliflozin can reduce urinary protein, improve renal function in T2DM patients, and delay the progression of chronic kidney disease (24). These combined effects contribute to improved cardiovascular and renal outcomes in T2DM patients.
Although the cardiovascular and renal benefits of dapagliflozin in T2DM are well-established, its effect on CIAKI remains unclear. Some studies suggest that SGLT2i may increase the risk of AKI (25) due to several factors: (1) SGLT2i induce osmotic diuresis by increasing urinary glucose excretion, which can reduce intravascular volume and renal perfusion, especially in vulnerable patients, potentially leading to prerenal AKI in cases of dehydration, low blood pressure, chronic kidney disease, heart failure, or concurrent diuretic use (26); (2) SGLT2i decrease sodium reabsorption in the proximal tubule, raising sodium levels in the macula densa, which constricts afferent arterioles and reduces renal blood flow (27); (3) Elevated glucose concentrations in the renal tubules can alter fructose metabolism, inducing oxidative stress, uric acid production, and the release of inflammatory mediators, leading to tubular damage (28). (4) SGLT-2 inhibitors exacerbate renal medullary ischemia and hypoxia (29). These factors can cause a significant decline in eGFR and increase the risk of AKI. Consequently, some clinicians recommend discontinuing SGLT2i before PCI to minimize the risk of CIAKI.
Recent clinical data suggests that dapagliflozin does not increase the risk of AKI; in fact, it may reduce its incidence and demonstrate favorable renal safety. A study from the Nuffield Department of Population Health, published in The Lancet, found that SGLT2i lower AKI risk by 23% (OR = 0.77, 95% CI: 0.70–0.84) (30). Bailey et al. reported that SGLT2i reduce AKI occurrence by inhibiting oxidative stress responses (31). Additionally, the DECLARE study showed that the dapagliflozin group had a lower incidence of AKI compared to the placebo group (1.5% vs. 2.0%, HR = 0.69, 95% CI: 0.55–0.87) (32).
Clinical data show that eGFR may transiently decline during the first 1–4 weeks of SGLT2i therapy, especially in the first 2 weeks (33–35), likely due to the diuretic effect of SGLT2i, which reduces blood pressure, decreases renal blood volume, and constricts afferent arterioles. However, eGFR typically returns to baseline or normal levels after 4 weeks of treatment. Therefore, in this study, patients were required to take dapagliflozin for at least 4 weeks prior to PCI. In this study, the average duration of dapagliflozin application before PCI in the dapagliflozin group was 10.56 ± 2.62 weeks. Additionally, during the perioperative period, patients received adequate hydration to dilate renal blood vessels, increase renal blood volume, and facilitate contrast agent excretion.
This study found that the incidence of CIAKI was lower in the dapagliflozin group compared to the control group. Logistic regression analysis identified dapagliflozin as an independent protective factor against CIAKI. At 48 h post-PCI, the dapagliflozin group showed lower levels of Cys-C and NGAL than the control group. These results suggest that, with adequate hydration, dapagliflozin does not increase the risk of CIAKI. At the same time, the latest meta-analysis showed that chronic treatment (with a minimum duration of 2 weeks to 6 months) using an SGLT2 inhibitor was associated with a significantly reduced risk of CI-AKI in T2DM patients undergoing coronary procedures, compared with the control group (RR = 0.48,95%CI:0.39–0.59, p < 0.001) (36). Additionally, Huang et al. (37) reported that dapagliflozin may improve CIAKI by inhibiting the HIF-1α/HE4/NF-κB signaling pathway. Yet, lower HIF 1 alpha in tubular cells could simply reflect improved cortical oxygenation, as shown by O’Neill et al. (38), and HIF-mediated gene expression might be renoprotective. Therefore, for T2DM patients on long-term SGLT2i therapy, there is no need to discontinue SGLT2i before angiography, as it is safe. Clinicians need not worry about an increased risk of CIAKI from SGLT2i and can continue its use during the PCI perioperative period.
However, there is some controversy regarding the impact of short-term SGLT2i use on CIAKI. Recently, only one study has reported on the association between short-term SGLT2i use and CIAKI in T2DM patients. This study showed that the incidence of CIAKI in patients using SGLT2i for a short term (1.7 ± 1.4 days) was significantly higher than in those using SGLT2i for a long term (191.5 ± 223.3 days) (20.5% vs. 3.4%, p = 0.018) (39). Therefore, the impact of short-term SGLT2i use on the incidence of CIAKI requires further investigation. And for patients who are planned for angiography and intend to start SGLT2i treatment, it is advisable to delay the initiation of SGLT2i until the contrast agent is fully cleared from the body, to avoid initiating SGLT2i during the PCI perioperative period.
Currently, several clinical trials (40, 41) have shown that long-term use of SGLT2 inhibitors is beneficial for cardiovascular outcomes in T2DM patients. In this study, patients in the dapagliflozin group had better long-term outcomes compared to the control group. Considering the impact of SGLT2i on CIAKI, it may be related to the duration of SGLT2i use and considering that there was no difference in the prevention of AKI or the time of recovery from CIAKI by SGLT2i.The difference in long-term outcomes could be related to the beneficial effects of the SGLT2 inhibitor itself rather than its effects on CIAKI.
Our study provides strong evidence that chronic administration of dapagliflozin significantly reduces the risk of CIAKI and improves prognosis in T2DM patients undergoing PCI. However, several limitations must be considered. First, this retrospective cohort study has a limited sample size and potential selection bias due to the absence of propensity score matching. Second, the non-randomized treatment allocation may have introduced unmeasured factors influencing the decision to prescribe dapagliflozin, which could affect the outcomes. Meanwhile, in this study, we did not measure the proteinuria/albuminuria parameters, and therefore, we were unable to observe changes in proteinuria/albuminuria or assess the impact of dapagliflozin on proteinuria/albuminuria. Third, notably, the lower LVEF in the dapagliflozin group may reflect clinicians’ preference for dapagliflozin in patients with heart failure and reduced LVEF, this selection bias may have a potential impact on the study results. Fourth, as is well known, NGAL can significantly increase 2 h after PCI, peak at 4 h, and then gradually decline (19). Our study only measured NGAL levels at 48 h post-PCI, which may not reflect the early peak levels. Additionally, the study does not assess the impact of short-term dapagliflozin use on CIAKI, and the exact mechanisms by which dapagliflozin reduces CIAKI remain unclear. Finally, since this study excluded high-risk populations for CIAKI, such as those with advanced CKD, hemodynamic instability, and emergent PCI, it cannot assess the impact of SGLT2i on the incidence of CIAKI in these high-risk groups, further research is needed to analyze the effects of SGLT2i on such high-risk populations. Therefore, further prospective studies are needed to confirm these findings and explore the underlying mechanisms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Medical Ethics Committee of Tianjin University Chest Hospital (Approval no: 2023YS-1215-01). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective cohort study that complies with the requirements of the declaration of Helsinki, with a waiver for obtaining informed consent from the included patients.
SY: Writing – original draft, Writing – review & editing. HH: Data curation, Investigation, Methodology, Writing – review & editing. XZ: Data curation, Methodology, Writing – review & editing. PZ: Data curation, Methodology, Writing – review & editing. NF: Data curation, Funding acquisition, Writing – review & editing, Validation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research funded by Special Fund for Key Disciplines of the Tianjin Municipal Health Commission (no. TJWJ2022XK033) and Tianjin Key Medical Discipline Construction (Cardiology, no. TJYXZDXK-055B).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vemireddy, L, and Bansal, S. Contrast-associated acute kidney injury: definitions, epidemiology, pathophysiology, and implications. Interv Cardiol Clin. (2023) 12:489–98. doi: 10.1016/j.iccl.2023.06.007
2. Guo, W, Liu, J, He, Y, Lei, L, Guo, Z, Song, F, et al. The impact of contrast-associated acute kidney injury on all-cause mortality in older patients after coronary angiography: a 7.5-year follow-up. Angiology. (2024) 75:434–40. doi: 10.1177/00033197231155610
3. Shuka, N, Hasimi, E, Kristo, A, Simoni, L, Gishto, T, Shirka, E, et al. Contrast-induced nephropathy in interventional cardiology: incidence, risk factors, and identification of high-risk patients. Cureus. (2023) 15:e51283. doi: 10.7759/cureus.51283
4. Li, Y, and Wang, J. Contrast-induced acute kidney injury: a review of definition, pathogenesis, risk factors, prevention and treatment. BMC Nephrol. (2024) 25:140. doi: 10.1186/s12882-024-03570-6
5. Macdonald, DB, Hurrell, CD, Costa, AF, McInnes, MDF, O'Malley, M, Barrett, BJ, et al. Canadian Association of Radiologists Guidance on contrast-associated acute kidney injury. Can J Kidney Health Dis. (2022) 9:1–15. doi: 10.1177/20543581221097455
6. Somkereki, C, Palfi, R, and Scridon, A. Prevention of contrast-associated acute kidney injury in an era of increasingly complex interventional procedures. Front Med (Lausanne). (2024) 10:1180861. doi: 10.3389/fmed.2023.1180861
7. Mentz, RJ, Brunton, SA, and Rangaswami, J. Sodium-glucose cotransporter-2 inhibition for heart failure with preserved ejection fraction and chronic kidney disease with or without type 2 diabetes mellitus: a narrative review. Cardiovasc Diabetol. (2023) 22:316. doi: 10.1186/s12933-023-02023-y
8. Preda, A, Montecucco, F, Carbone, F, Camici, GG, Lüscher, TF, Kraler, S, et al. SGLT2 inhibitors: from glucose-lowering to cardiovascular benefits. Cardiovasc Res. (2024) 120:443–60. doi: 10.1093/cvr/cvae047
9. Murphy, DP, Wolfson, J, Reule, S, Johansen, KL, Ishani, A, and Drawz, PE. Kidney outcomes with sodium-glucose Cotransporter-2 inhibitor initiation after AKI among veterans with diabetic kidney disease. Kidney360. (2024) 5:335–43. doi: 10.34067/KID.0000000000000375
10. Santulli, G, Varzideh, F, Forzano, I, Wilson, S, Salemme, L, de Donato, A, et al. Functional and clinical importance of SGLT2-inhibitors in frailty: from the kidney to the heart. Hypertension. (2023) 80:1800–9. doi: 10.1161/HYPERTENSIONAHA.123.20598
11. Iordan, L, Gaita, L, Timar, R, Avram, V, Sturza, A, and Timar, B. The Renoprotective mechanisms of sodium-glucose Cotransporter-2 inhibitors (SGLT2i)-a narrative review. Int J Mol Sci. (2024) 25:7057. doi: 10.3390/ijms25137057
12. Lytvyn, Y, Bjornstad, P, Udell, JA, Lovshin, JA, and Cherney, DZI. Sodium glucose Cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. (2017) 136:1643–58. doi: 10.1161/CIRCULATIONAHA.117.030012
13. Perlman, A, Heyman, SN, Matok, I, Stokar, J, Muszkat, M, and Szalat, A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. (2017) 27:1108–13. doi: 10.1016/j.numecd.2017.10.011
14. Tang, H, Li, D, Zhang, J, Li, Y, Wang, T, Zhai, S, et al. Sodium-glucose co-transporter-2 inhibitors and risk of adverse renal outcomes among patients with type 2 diabetes: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. (2017) 19:1106–15. doi: 10.1111/dom.12917
15. Almazmomi, MA, Esmat, A, and Naeem, A. Acute kidney injury: definition, management, and promising therapeutic target. Cureus. (2023) 15:e51228. doi: 10.7759/cureus.51228
16. Ricci, Z, and Romagnoli, S. Acute kidney injury: diagnosis and classification in adults and children. Contrib Nephrol. (2018) 193:1–12. doi: 10.1159/000484956
17. Lei, L, Li, LP, Zeng, Z, Mu, JX, Yang, X, Zhou, C, et al. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep. (2018) 8:7962. doi: 10.1038/s41598-018-26226-6
18. Platt, E, Robertson, F, Al-Rashed, A, Klootwijk, R, Hall, A, Quaglia, A, et al. NGAL in the development of acute kidney injury in a murine model of remote Ischaemic preconditioning and liver Ischaemia reperfusion. Int J Mol Sci. (2024) 25:5061. doi: 10.3390/ijms25105061
19. Stoll, E, Monedero, P, Martin-Moreno, PL, and Garcia-Fernandez, N. Biomarkers of oxidative stress and inflammation in contrast-associated acute kidney injury. An Sist Sanit Navar. (2021) 47:e1081. doi: 10.23938/ASSN.1081
20. Yang, Z, Qiao, Y, Wang, D, Yan, G, and Tang, C. Association between inflammatory biomarkers and contrast-induced acute kidney injury in ACS patients undergoing percutaneous coronary intervention: a cross-sectional study. Angiology. (2024) 75:831–40. doi: 10.1177/00033197231185445
21. Cox, ZL, Collins, SP, Hernandez, GA, McRae, AT, Davidson, BT, Adams, K, et al. Efficacy and safety of dapagliflozin in patients with acute heart failure. J Am Coll Cardiol. (2024) 83:1295–306. doi: 10.1016/j.jacc.2024.02.009
22. Chatur, S, Vaduganathan, M, Claggett, BL, Mc Causland, FR, Desai, AS, Jhund, PS, et al. Dapagliflozin in patients with heart failure and deterioration in renal function. J Am Coll Cardiol. (2021) 82:1854–63. doi: 10.1016/j.jacc.2023.08.026
23. Wu, Q, Yao, Q, Hu, T, Yu, J, Jiang, K, Wan, Y, et al. Dapagliflozin protects against chronic heart failure in mice by inhibiting macrophage-mediated inflammation, independent of SGLT2. Cell Rep Med. (2023) 4:101334. doi: 10.1016/j.xcrm.2023.101334
24. Cho, J, Doo, SW, Song, N, Lee, M, Lee, H, Kim, H, et al. Dapagliflozin reduces urinary kidney injury biomarkers in chronic kidney disease irrespective of albuminuria level. Clin Pharmacol Ther. (2024) 115:1441–9. doi: 10.1002/cpt.3237
25. Szalat, A, Perlman, A, Muszkat, M, Khamaisi, M, Abassi, Z, and Heyman, SN. Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. (2018) 41:239–52. doi: 10.1007/s40264-017-0602-6
26. Gilbert, RE, and Thorpe, KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: a meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab. (2019) 21:1996–2000. doi: 10.1111/dom.13754
27. Menne, J, Dumann, E, Haller, H, and Schmidt, BMW. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med. (2019) 16:e1002983. doi: 10.1371/journal.pmed.1002983
28. Baer, PC, Koch, B, Freitag, J, Schubert, R, and Geiger, H. No cytotoxic and inflammatory effects of Empagliflozin and dapagliflozin on primary renal proximal tubular epithelial cells under diabetic conditions in vitro. Int J Mol Sci. (2020) 21:391. doi: 10.3390/ijms21020391
29. Heyman, SN, Aronson, D, and Abassi, Z. SGLT2 inhibitors and the risk of contrast-associated nephropathy following angiographic intervention: contradictory concepts and clinical outcomes. Int J Mol Sci. (2024) 25:10759. doi: 10.3390/ijms251910759
30. Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. (2022) 400:1788–801. doi: 10.1016/S0140-6736(22)02074-8
31. Bailey, CJ, Day, C, and Bellary, S. Renal protection with SGLT2 inhibitors: effects in acute and chronic kidney disease. Curr Diab Rep. (2022) 22:39–52. doi: 10.1007/s11892-021-01442-z
32. Cahn, A, Mosenzon, O, Wiviott, SD, Rozenberg, A, Yanuv, I, Goodrich, EL, et al. Efficacy and safety of dapagliflozin in the elderly: analysis from the DECLARE-TIMI 58 study. Diabetes Care. (2020) 43:468–75. doi: 10.2337/dc19-1476
33. Adamson, C, Docherty, KF, HJL, H, de Boer, RA, Damman, K, Inzucchi, SE, et al. Initial decline (dip) in estimated glomerular filtration rate after initiation of dapagliflozin in patients with heart failure and reduced ejection fraction: insights from DAPA-HF. Circulation. (2022) 146:438–49. doi: 10.1161/CIRCULATIONAHA.121.058910
34. Jongs, N, Chertow, GM, Greene, T, McMurray, JJV, Langkilde, AM, Correa-Rotter, R, et al. Correlates and consequences of an acute change in eGFR in response to the SGLT2 inhibitor dapagliflozin in patients with CKD. J Am Soc Nephrol. (2022) 33:2094–107. doi: 10.1681/ASN.2022030306
35. Kraus, BJ, Weir, MR, Bakris, GL, Mattheus, M, Cherney, DZI, Sattar, N, et al. Characterization and implications of the initial estimated glomerular filtration rate 'dip' upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. (2021) 99:750–62. doi: 10.1016/j.kint.2020.10.031
36. Dimitriadis, K, Vakka, A, Pyrpyris, N, Apostolos, A, Beneki, E, Stathopoulou, E, et al. Efficacy of chronic use of sodium-glucose co-transporter 2 inhibitors on the prevention of contrast-induced acute kidney injury in patients with type 2 diabetes mellitus following coronary procedures: a systematic review and Meta-analysis. Am J Cardiovasc Drugs. (2025) 25:57–69. doi: 10.1007/s40256-024-00684-y
37. Huang, X, Guo, X, Yan, G, Zhang, Y, Yao, Y, Qiao, Y, et al. Dapagliflozin attenuates contrast-induced acute kidney injury by regulating the HIF-1α/HE4/NF-κB pathway. J Cardiovasc Pharmacol. (2022) 79:904–13. doi: 10.1097/FJC.0000000000001268
38. O'Neill, J, Fasching, A, Pihl, L, Patinha, D, Franzén, S, and Palm, F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol. (2015) 309:F227–34. doi: 10.1152/ajprenal.00689.2014
39. Zang, J, Liang, J, Zhang, X, Sang, D, Duan, X, Wang, Z, et al. Short term sodium glucose transport protein 2 inhibitors are associated with post contrast acute kidney injury in patients with diabetes. Sci Rep. (2024) 14:22937. doi: 10.1038/s41598-024-74233-7
40. Wiviott, SD, Raz, I, Bonaca, MP, Mosenzon, O, Kato, ET, Cahn, A, et al. The design and rationale for the dapagliflozin effect on cardiovascular events (DECLARE)-TIMI 58 trial. Am Heart J. (2018) 200:83–9. doi: 10.1016/j.ahj.2018.01.012
Keywords: sodium-glucose co-transporter 2 inhibitor, dapagliflozin, type 2 diabetes mellitus, contrast-induced acute kidney injury, prognosis
Citation: Yang S, Hao H, Zhai X, Zhang P and Fu N (2025) Effect of sodium-glucose co-transporter 2 inhibitor on contrast-induced acute kidney injury and prognosis in type 2 diabetes patients undergoing percutaneous coronary intervention. Front. Med. 12:1552539. doi: 10.3389/fmed.2025.1552539
Received: 28 December 2024; Accepted: 18 February 2025;
Published: 05 March 2025.
Edited by:
Zaid A. Abassi, Technion Israel Institute of Technology, IsraelReviewed by:
Samuel Heyman, Hadassah Hebrew University Hospitals, IsraelCopyright © 2025 Yang, Hao, Zhai, Zhang and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naikuan Fu, Y2RyZm5rQDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.